
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 11 August 2022
Sec. Surgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.891710
This article is part of the Research Topic Reviews in Surgical Oncology View all 18 articles
 Benjamin Paik1,2,3
Benjamin Paik1,2,3 Chin Jin Seo1,2,4
Chin Jin Seo1,2,4 Joey Wee-Shan Tan1,2,5
Joey Wee-Shan Tan1,2,5 Wen Kai Darryl Juan1,2
Wen Kai Darryl Juan1,2 Khee Chee Soo1,2
Khee Chee Soo1,2 Chin-Ann Johnny Ong1,2,4,5,6,7
Chin-Ann Johnny Ong1,2,4,5,6,7 Claramae Shulyn Chia1,2,4,6
Claramae Shulyn Chia1,2,4,6 Jolene Si Min Wong1,2,4,6*
Jolene Si Min Wong1,2,4,6*Retroperitoneal liposarcomas (RPLPSs) are a rare tumor group for which current guidelines recommend aggressive en bloc resection to attain microscopically negative (R0) margins. To ensure R0 margins, resection of adherent or adjacent organs is often required. However, it is still unclear if R0 margins confer any additional benefit to patients over a grossly negative but microscopically positive (R1) margin. We performed a systematic search of PubMed and Embase databases for studies including patients receiving R0 or R1 resection for RPLPS. Nine retrospective cohort studies, one prospective cohort study, and 49 case reports/case series were included. A total of 552 patients with RPLPS were evaluated: 346 underwent R0 resection and 206 underwent R1 resection. In the R0 group, 5-year overall survival (OS) ranged from 58.3% to 85.7%; local recurrence (LR) ranged from 45.5% to 52.3%. In the R1 group, 5-year OS ranged from 35% to 55.3%; LR ranged from 66.7% to 91.7%. Among cohort studies, OS, disease-free survival (DFS), LR rate, and LR-free survival (LRFS) were significantly associated with R0 resections. Assessment of case series and reports suggested that the R0 margin led to a slightly higher morbidity than that of R1. In conclusion, this review found the R0 margin to be associated with reductions in LR rates and improved OS when compared with the R1 margins, though accompanied by slight increases in morbidity. The roles of tumor histotype and perioperative chemotherapy or radiotherapy were not well-elucidated in this review.
Retroperitoneal soft tissue sarcomas are uncommon and affect less than 0.1% of the population (1). Among them, a multitude of histological subtypes exist, with liposarcomas (LPSs) representing the most common histotype (2). Favorable survival profiles and lower propensity for distant metastases in LPS, especially in the well-differentiated (WDLPS) and low-grade dedifferentiated (DDLPS) patients (3), have generated great interest among sarcoma surgeons. For once, when tumor biology is “favorable,” the surgeon is now at the helm to possibly dictate patient outcomes via strategies to lower local recurrence (LR) rates.
Up-front extended resection (ER) to achieve microscopically clear (R0) margins was introduced by Gronchi et al. (4) and has been shown to significantly lower rates of LR with acceptable morbidity and mortality profile. While adopted by most of Europe and the Trans-Atlantic group (TARPSWG) (5), differing opinions continue to exist regarding the utility of such an aggressive surgical approach in the management of retroperitoneal sarcomas (RPS). Few would argue for the preservation of involved or encased organs; as such, the debate lies mainly in the en bloc removal of adherent or adjacent organs in which the suspicion of histological invasion is low (6, 7).
The addition of perioperative radiation therapy (RT) to the armamentarium of tools aimed at minimizing LR rates further adds complexity to the subject matter (8). It is unknown if a planned R1 (microscopically positive) margin in the context of neoadjuvant RT is of equivalence to the R0 margin. In the subset of patients with LPS, however, exploratory analysis from the STRASS trial appears to suggest a potential benefit of preoperative RT (9).
To date, data on the optimal surgical margins in retroperitoneal LPS (RPLPS) have been limited to retrospective cohort studies or case series/reports. As such, our study aims to provide a summative analysis on patients with RPLPS in an attempt to shed light on the effects of margins status, RT, chemotherapy (CT), and histotype on survival and recurrence outcomes.
A literature search of PubMed, OvidSP, Embase, and Cochrane databases was conducted for studies reporting on the surgical management of RPLPS up to March 2020. The medical search headings (MeSH) “retroperitoneal liposarcoma,” “well-differentiated liposarcoma,” “de-differentiated liposarcoma,” “R0,” “R1,” “resection,” “extended resection,” “microscopic,” and “margin” were used. Additional relevant studies were identified by screening the references cited in shortlisted articles. This study was conducted in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Figure 1) (10).
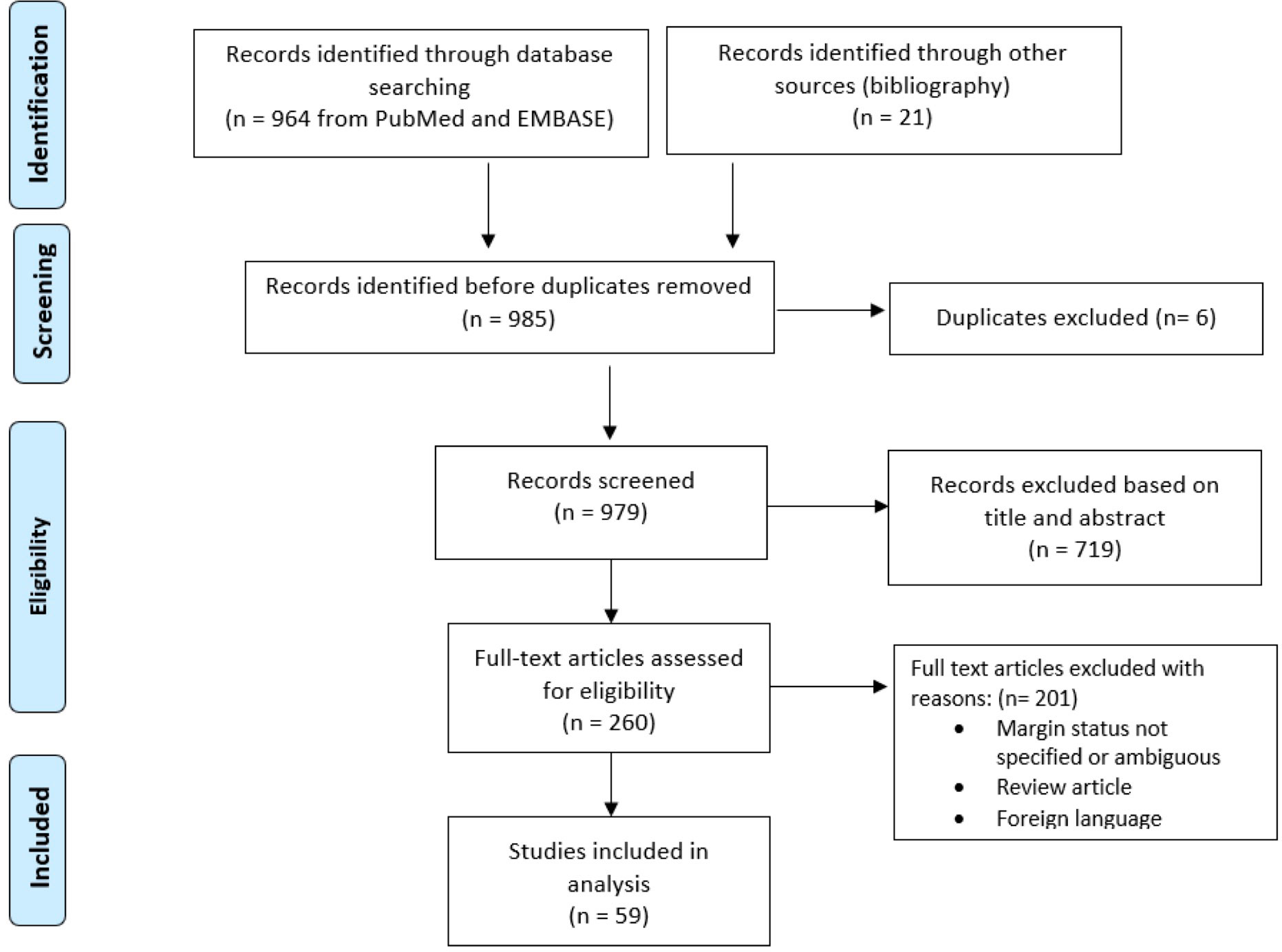
Figure 1 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of selection of eligible studies.
Articles were included if they 1) were original articles published in English in peer-reviewed journals; 2) included patients with RPLPS identified via imaging modalities such as computed tomography or magnetic resonance imaging scans; 3) included patients with biopsy-proven RPLPS; 4) unambiguously reported margin status, patient survival, and morbidity outcomes.
Articles were excluded if they 1) did not report the margin status of the resections; 2) included patients presenting with metastatic disease at initial diagnosis; 3) reported all outcomes for R0 and R1 resections collectively. Studies that presented data on RPS patients with other non-LPS histotypes were included only if data of patients with RPLPS could be extracted independently. For example, the retrospective cohort study of RPS patients from the Memorial Sloan-Kettering Cancer Center could not be included because survival and recurrence data for R1 or R0 resections were merged with other non-LPS histotypes (11). Similarly, the TAPRSWG 2020 study evaluating a large cohort of RPS patients was excluded, as outcomes for R1 and R0 resections were reported together (12).
Data were extracted using standardized forms, which recorded patient and study characteristiCJS, the radicality of resection performed (R0 or R1), histologic subtype (well-differentiated, dedifferentiated, pleomorphic, or myxoid), tumor grade (FNCLCC), postoperative morbidity and mortality, the use of neoadjuvant and/or adjuvant chemotherapy (CT) or radiotherapy (RT), number of additional organs removed and other perioperative outcomes, by two independent reviewers. Where appropriate, data that were reported for R0 and R1 collectively were extracted but were not considered in further analysis.
All studies were assessed for their level of evidence using the Oxford Centre for Evidence-Based Medicine Levels of Evidence (13). The authors elected to perform a descriptive review of the data as opposed to a meta-analysis due to the heterogeneity of the studies assessed.
In accordance with residual tumor classification (R-classification) guidelines laid out by the American Joint Committee on Cancer (AJCC), an R1 resection was defined as microscopic tumor cells present at the border of the specimen, while an R0 resection was defined as the absence of tumor cells at the inked resection surface (14).
An “R+1” margin was defined as having >1 mm of normal tissue between the tumor and the inked resection margin (15).
The search identified 59 relevant articles published between 1996 and 2019 (Tables 1A, B).
A total of 52 studies reported on the outcomes of RPLPS patients who received R0 resection (Tables 2A, 3A).
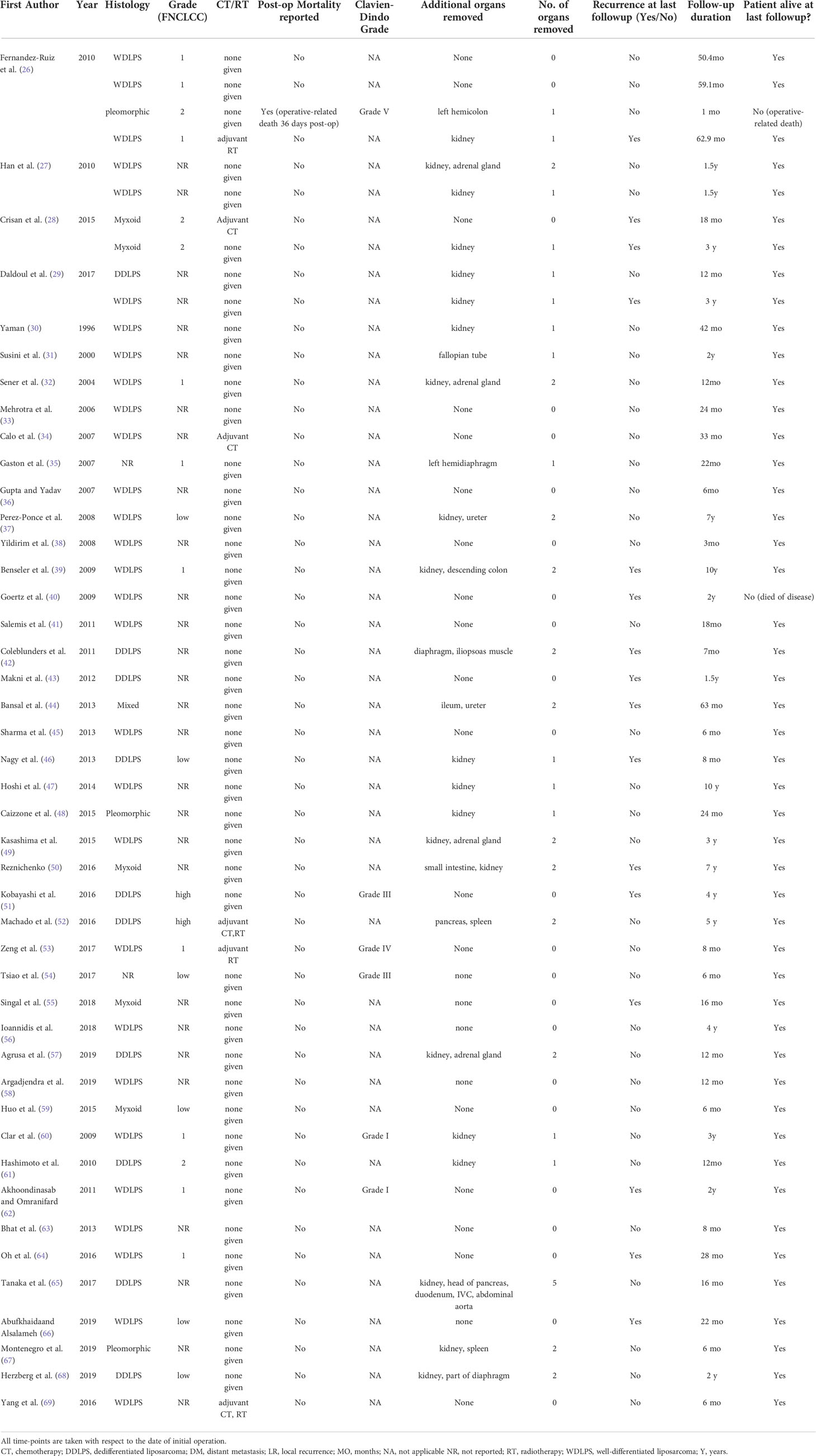
Table 3A Summary of 4 case series and 40 case reports which included patients receiving R0-margin resection.
Eight were retrospective cohort studies evaluating the relationship between margin status and recurrence and survival outcomes (16–23). R0-margin patients receiving adjuvant or neoadjuvant CT/RT were included in these studies, but data on their recurrence and survival outcomes were reported together with R1-margin patients and hence could not be extracted. Of note, three studies (16, 20, 21) adopted the stricter R+1 margin classification system that classifies margins as R0 only if the resection margins are surrounded by >1 mm of tumor-negative tissue.
Of the remaining 44 studies, 4 were case series (26–29) and 40 were case reports (30–69), both documenting the recurrence and survival outcomes of RPLPS patients receiving R0-margin resection for RPLPS.
A total of 16 studies reported on the outcomes of RPLPS patients who received R1 resection (Tables 2B, 3B). R1-margin patients receiving adjuvant or neoadjuvant CT/RT were included in these studies, but data on their recurrence and survival outcomes were reported together with R0-margin patients and hence could not be extracted.
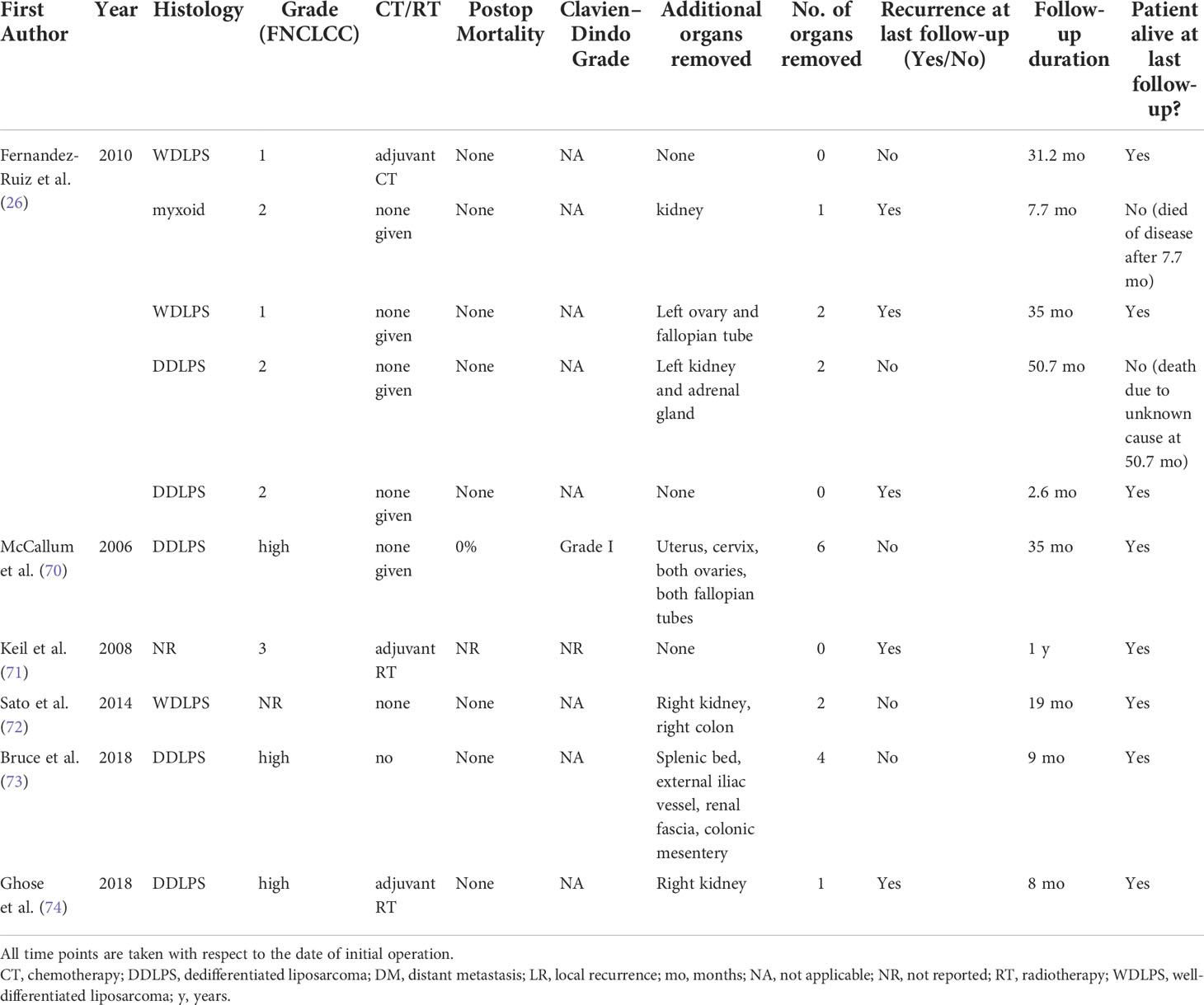
Table 3B Summary of 1 case series and 5 case reports that included patients receiving R1-margin resection.
Ten were retrospective cohort studies evaluating the relationship between margin status (R0/R1) with recurrence and survival outcomes (16–25). One study (19) was a prospective cohort study examining the effect of preoperative irradiation by high-dose helical tomotherapy with a total dose of 54 Gy over 30 fractions.
Of the remaining six studies, one was a retrospective case series (26) and five were case reports (70–74), both documenting the recurrence and survival outcomes of RPLPS patients receiving R1-margin resection for RPLPS.
In total, our systematic review evaluated a total of 552 patients with RPLPS of whom 346 underwent R0-margin resection and 206 underwent R1-margin resection.
A total of 346 patients achieved R0 resections, of whom 296 patients came from cohort studies and 50 from case series/case reports.
A total of 296 patients from eight cohort studies received R0-margin resection (Table 2A). The rates of LR ranged from 45.5% to 52.3%. The 3-year OS and DFS ranged from 87% to 87.5% and 22.2% to 62.5%, respectively. The 5-year OS ranged from 58.3% to 85.7%. From the study by Sargos et al. (19), the recurrence rate among R0-margin patients who received preoperative RT was 0%.
Due to the heterogeneity of the data, there is little basis for comparison between studies that adopted an “R+1” margin definition (16, 20, 22) vs. studies using the “R” margin definition. For example, Lee et al. (20) who used the “R+1” definition reported a lower 5-year OS (58.3%) than Milone et al. (21) (85.7%) who used the “R” definition.
A total of 50 patients from 44 case series/case reports received R0-margin resection. The data extracted from the case series and case reports for RPLPS patients receiving R0 resection are shown in Table 3A and are summarized as follows. The median follow-up duration was 22 months. The histological distribution was as follows: 58% WDLPS (n = 29), 20% DDLPS (n = 10), 10% myxoid (n = 5), 6% pleomorphic (n = 3), and 6% mixed or unreported (n = 3). Moreover, 32% (n = 16) of tumors were low-grade (G1), 12% (n = 5) were high-grade (G2/G3), and 56% (n = 28) did not report tumor grade. In addition, 54% of patients (n = 27) received multivisceral resection, of whom 28% (n = 14) of patients had one additional organ resected, 24% (n = 12) had two additional organs resected, and 2% (n = 1) had five additional organs resected. The most common organ removed was the kidney (78%, n = 21) followed by the adrenal gland (15%, n = 4), diaphragm (11%, n = 3), colon (8%, n = 2), and pancreas (8%, n = 2). Regarding adjuvant CT and RT, two patients had adjuvant CT and RT, two patients had adjuvant RT, two patients had adjuvant CT, and 44 patients had neither adjuvant CT nor RT.
The postoperative outcomes are presented as follows. The median follow-up time was 22 (range 1–120 months), and two out of 50 patients demised at the end of follow-up. Cause of the two mortalities were tumor recurrence (40) and septic shock secondary to burst abdomen (26). The recurrence rate ranged from 0% to 100%. No distant metastases were reported during the duration of follow-up. Furthermore, 12% of patients (n = 6) (26, 51, 53, 54, 60, 62) experienced postoperative complications, of which 50% were Clavien–Dindo Grade 3 and above (75).
LR among WDLPS patients was 24% (n = 7/29) while that among DDLPS patients was 40% (n = 4/10).
LR among patients who received no CT or RT, only adjuvant CT, only adjuvant RT, and adjuvant CT and RT was 31% (n = 14), 50% (n = 1), 50% (n = 1), and 0% (n = 0), respectively.
A total of 206 patients in this review received resections leading to an R1 margin, of whom 196 patients came from cohort studies and 10 from case series or case reports.
A total of 196 patients from 10 cohort studies received R1-margin resection. The rates of LR ranged from 66.7% to 91.7% (Table 2B). The 3-year OS ranged from 70% to 88.9%. The 5-year OS ranged from 35% to 55.3%. The 3-year LRFS was 50%, and the 5-year LRFS was 47%.
Due to the heterogeneity of the data, there is little basis for comparison between studies that adopted an “R+1” margin definition (16, 20, 22) vs. studies using the “R” margin definition.
A total of 10 patients from six case series/case reports received R1-margin resection. The data extracted from the case series and case reports for RPLPS patients receiving R1 resection are shown in Table 3B and are summarized as follows. The median follow-up duration was 15.5 months. The histological distribution was as follows: 30% WDLPS (n = 3), 50% DDLPS (n = 5), 10% myxoid (n = 1), and 10% unreported (n = 1). In addition, 20% (n = 2) of tumors were low-grade (G1) and 70% (n = 7) were high-grade (G2/G3), with 10% (n = 1) unreported grade. Moreover, 70% of patients (n = 7) received multivisceral organ resection, of whom 20% (n = 2) had one additional organ resected, 30% (n = 3) had two additional organs resected, 10% (n = 1) had four additional organs resected, and 10% (n = 1) had six additional organs resected. Of the patients who received multivisceral resection, the most common organ removed was the kidney (58%, n = 4), followed by the ovary (29%, n = 2). Regarding adjuvant CT/RT, seven patients had neither CT nor RT, one patient had adjuvant CT, and two patients had adjuvant RT.
At a median follow-up of 15.5 months (range 2.6–50.7), two out of 50 patients had demised (26). Only one patient (70) experienced minor Clavien–Dindo Grade 1 postoperative complications.
LR among WDLPS patients was 33% (n = 1/3) while that among DDLPS patients was 40% (n = 2/5).
LR among patients who received neither CT nor RT was 43% (three out of seven patients), LR among patients who received only CT was 0% (zero out of one patient), and LR among patients who received only RT was 100% (two out of two patients).
In the cohort studies, survival and recurrence outcomes of patients receiving neoadjuvant or adjuvant CT/RT were reported collectively as R0/R1 and could not be extracted independently for aggregation across studies. However, three retrospective cohort studies individually reported on the effects of neoadjuvant or adjuvant CT/RT upon univariate or multivariate analysis, with differing results. Sánchez-Hidalgo et al. (16) reported that administering adjuvant CT or RT to patients with dedifferentiated tumor histology neither improved DFS/OS nor reduced LR rates. Similarly, Nathenson et al. (17) reported that none of adjuvant CT, neoadjuvant RT, or adjuvant RT had a significant influence on OS and PFS, regardless of tumor histology and grade. Zhao et al. (18) reported a lower median survival for patients receiving adjuvant therapy (intraoperative/postoperative RT or CT) than those who did not undergo adjuvant therapy (p = 0.03) but acknowledged selection bias due to adjuvant therapy being arranged only for patients with high-grade tumors.
RPS accounts for 15% of all soft tissue sarcomas and represents a rare class of tumors occurring in approximately 5 per 100,000 people in Europe (76). To date, the impact of microscopic margin status (R0 vs. R1 margin) has never been validated in RPS. While few would defend the preservation of involved or encased organs, much of the debate lies in whether an en bloc approach to remove all adjacent or adherent organs should override intraoperative assessment of suspected histopathologic organ invasion (HOI). To further complicate the matter, it has been shown that up to 26% of patients in whom there was no suspicion of organ involvement actually demonstrate pathologically identified HOI; this underscores the need for a more aggressive and extended resection regardless of intraoperative assessment (7). Hence, while groups like the TARPSWG (77) and EORTC-STBSG (78) recommend en bloc resection to maximize the chances of achieving an R0 margin, so far, there is limited evidence to conclude if the elusive R0 margin even makes a difference to patient outcomes. As such, the role of the R0-margin status is controversial in RPS.
The results of our systematic review provide some clarity on this matter. As shown in Tables 2A, B, although the numerical values for OS and DFS vary considerably between cohort studies, the R0 margin demonstrated benefits over the R1 margin with regard to these outcomes in most individual studies. For OS, the R0 margin was prognostic for increased OS in the studies by Nathenson et al. (17), Zhao et al. (18), Milone et al. (21), Singer et al. (22), and Linehan et al. (23), while studies by Sánchez-Hidalgo et al. (16) and Lee et al. (20) did not find a statistically significant correlation between the R0/R1 margin and OS. For DFS, the R0 margin was prognostic for increased DFS in studies by Sánchez-Hidalgo et al. (16) and Nathenson et al. (17), but the study by Lee et al. (20) did not find a statistically significant correlation between the R0/R1 margin and DFS. Among the case series and case reports included in our review, the follow-up duration varied tremendously and follow-up data were limited, hence preventing any formal assessment of the benefits of the R0 margin on survival outcomes.
Additionally, while different studies adopted the R+1 classification system that requires at least 1 mm of healthy tissue around the tumor margin to qualify as R0 (in essence, an R0+1 margin), there was no obvious superiority over the standard R0 margin.
One of the biggest arguments for aggressive surgical approaches, such as frontline extended resection, is the reduction in the LR rate and hence an increase in local control. Gronchi et al. (79) showed that the 5-year LR rate was lower at 28% with the frontline extended approach compared to 48% with standard less aggressive approaches. The French Sarcoma Group (80) also cited a 3.29-fold reduced LR rate for an aggressive extended approach compared to patients who underwent simple complete resection.
The studies included in our analysis showed that the R0 margin led to a lower LR rate compared to that of the R1 margin. The LR rate for the R0 margin ranged from 45.5% to 52.3%, lower than the LR rate of the R1 margin that ranged from 66.7% to 91.7%. In particular, Sánchez-Hidalgo et al. (16) found that the R1 margin was strongly correlated with early recurrence (<12 months) on univariate analysis, and in the series by Milone et al. (21), the R0-margin LR rate was lower than the R1-margin LR rate, although no statistical significance analysis was done to reinforce these findings. The limited data for LRFS appear to corroborate the above findings. Only the studies by Singer et al. (22) and Linehan et al. (23) presented LRFS data for the R0 and R1 margin separately for comparison between R0 and R1 to be done. While Singer et al. (22) reported that the R0 margin led to longer LRFS and longer distant recurrence-free survival, the benefit over the R1 margin was not statistically significant. On the other hand, Linehan et al. (23) reported that the R1 margin paradoxically led to a longer LRFS (albeit not statistically significant).
From our analysis, there were hardly any extractable data from the cohort studies concerning survival and LR data stratified by RPLPS subtypes (WDLPS/DDLPS), although the case series and reports suggest that the R0 margin benefits LR in WDLPS patients (R0, 24%; R1, 33%) but offers no additional benefit in DDLPS patients (R0, 40%; R1, 40%). At the same time, while a more aggressive multivisceral resection would increase the chance of attaining R0 margins (77, 78), the final margin status attained potentially also depends on underlying tumor biology because more dedifferentiated RPLPS tends to be more locally invasive (6) and hence has a higher inherent tendency to invade the tumor capsule to increase the chance of margins being positive on final histopathology. It is therefore possible that despite a multivisceral resection, the margin status may end up as R1. In our dataset, out of the R0 patients, majority were WDLPS histotype, whereas of the R1 patients, majority were DDLPS histotype. Yet, the more common margin status attained in each of the WDLPS and DDLPS was still R0, suggesting that R1-margin cases are grossly underrepresented in the available literature. Hence, it is challenging to conclude regarding the extent that tumor biology and extent of resection contribute to the margin status attained just based on these limited data from case series and reports. The patients with pleomorphic and myxoid RPLPS were too few to be adequately represented, and no further analysis on their outcomes was done.
That being said, proponents of aggressive resection argue that it offers the best chances of local control that in turn drives oncologic outcomes in WDLPS and <G2 DDLPS. However, aggressive resection does not offer further benefit in high-grade DDLPS patients in whom distant metastases are the main driver of outcomes (3).
Existing large-scale studies on RPS in general are not unanimous on whether aggressive resections increase morbidity and mortality. While studies by Gronchi et al. (79) argue that aggressive resections do not increase morbidity and mortality, this is refuted by groups such as the TARPSWG (81) that argues that the removal of major organs when resecting aggressively puts patients at 1.5 times greater risk of morbidity.
In our analysis, postoperative morbidity/mortality data could only be extracted from case series and case reports; where it was extractable from cohort studies, the morbidity rate for R0 and R1 was equal (Tables 2A, B). Among the case series and reports, although there were more incidences of morbidity among R0 than R1 patients, the percentage morbidity in both patient groups was roughly equal (R0 = 12% vs. R1 = 10%) due to the different total numbers of patients. It is however valid to say that R0 has slightly higher morbidity as evidenced by the presence of Clavien–Dindo Grade 3, 4, and 5 complications. Postoperative mortality was low in both R0 and R1 patients, with there being only one case of mortality in R0 and none among R1 patients. On the whole, our analysis suggests that postoperative morbidity and mortality are only slightly higher for the R0 margin than those for the R1 margin in the context of RPLPS.
While the precise role of each of CT and RT in survival and LR outcomes in RPLPS is not well-established due to most studies being conducted on RPS in general, it has been reported elsewhere that standard chemotherapy has a marginal role in WDLPS due to the very low mitotic rate (82), and its use is therefore limited to metastatic and recurrent tumors (83). Furthermore, within the retroperitoneal space, the presence of radiosensitive organs, such as the pancreas, and kidney, in close proximity to the primary tumor limits the effectiveness and delivery of radiotherapy (be it neoadjuvant or adjuvant) (84).
Among the studies included in our review, analysis in studies performed by Sánchez-Hidalgo et al. (16), Nathenson et al. (17), and Zhao et al. (18) failed to find any statistically significant influence of CT/RT on survival and LR outcomes. As these are retrospective studies, there is expected to be some selection bias, since CT/RT would be offered more to patients with high-grade tumors or inherently aggressive tumor biology. Furthermore, the regimen of CT and RT was not standardized among the cohort studies and, in some instances, not specified at all. The limited follow-up data from case series and reports do not show any improvement of CT/RT to survival and LR in both R0 and R1 patients nor is there any definitive proof to address the question of whether R1 with CT/RT is of equivalence to the R0 margin.
The findings of our systematic review support and allude to the latest general consensus management guidelines for RPS published by the TARPSWG in 2021 (85). Our review showed that the R0-margin resection for RPLPS increased OS and reduced LR. Indeed, the TARPSWG recommends an extended approach to resect adherent organs regardless of expected microscopic infiltration, with removal of all ipsilateral retroperitoneal fat, especially for well-differentiated histotypes that are harder to distinguish from normal adipose tissue. For this reason, obtaining intraoperative frozen sections will not add further value to guide the extent of resection.
Our review showed that WDLPS histotypes could potentially stand to benefit more from the R0 resection than DDLPS in terms of LR. While the TARPSWG suggests that the same aggressive strategy be used for both WDLPS and DDLPS, it acknowledges that more data are required to guide operative strategies for DDLPS (especially the high-grade type); data from the ongoing STRASS2 trial will shed further light on this matter.
While the studies included in our review seems to suggest that perioperative CT/RT has no significant effect on survival and LR, the TARPSWG recommends preoperative CT to downsize the primary tumor in order to facilitate grossly complete resection. Preoperative RT should be considered only for WDLPS and low-grade DDLPS that have high risks of LR, whereas high-grade DDLPS does not benefit from preoperative RT. There is still no proven benefit of postoperative CT or RT after grossly complete resection.
Our review highlighted that the majority of available studies on this topic are retrospective in nature. Outcome data for R0 were not always reported separately from those of R1, and if it was reported separately, there was also heterogeneity in the patient populations included under the R0 and R1 groups, and each study had varying proportions of WDLPS and DDLPS patients. The heterogeneity of the data limited the authors’ ability to perform a formal meta-analysis; as such, the authors elected to perform a systematic review of the available evidence.
Inconsistencies in the definitions of margin status among the cohort studies also limited the extent to which the results could be analyzed. For example, in the case of resections that had less than 1 mm of healthy tissue around the margin, this would be classified in papers adopting the “R+1” system as R1 but classified in papers adopting the “R” system as R0. Among the case reports and case series, some of the papers used did not categorically specify if the margins were R0 or R1 but described resections as “margin-positive” or “margin-negative.”
Although the numbers of R0 and R1 patients from cohort studies are fairly equal, there were much more case series and case reports of R0 patients than those of R1 patients, possibly stemming from publication bias. As such, data for short case series and case reports were simply presented in a descriptive manner. Therefore, it is difficult to make definitive conclusions regarding the effect of CT/RT, tumor histotype, or extent of resection on survival or recurrence outcomes.
Among the publications included in our systematic review, there was unanimity that the R0 margin delivered statistically significant improvements to OS, and there was fairly strong evidence that the R0 margin led to increased DFS. Data heterogeneity and collective reporting of R0 and R1 outcomes prevented a direct comparison of the differences in LRFS and RR, but the evidence points toward decreased RR from R0-margin resection. A modest amount of evidence points to a roughly equal postoperative morbidity rate between R0 and R1 resection.
To date, there have been no systematic reviews on the impact of the R0 margin in the treatment of RPLPS or even RPS for that matter. On the whole, our review suggests that the R0 margin benefits survival and LR in RPLPS patients without compromising postoperative morbidity. The role of other factors such as tumor biology and the role of CT/RT, while important, have yet to be delineated. At this juncture, our review emphasizes the need for larger-scale multicenter cohort studies assessing the effect of histotype, CT/RT, and extent of resection on survival and recurrence outcomes.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Conceptualization: JSMW. Data curation: BP, JSMW. Formal analysis: BP, CJS, JW-ST, WKDJ, JSMW. Funding acquisition: JSMW. Investigation: BP, CJS, JW-ST, WKDJ, JSMW. Methodology: JSMW. Project administration: CC, CO. Resources: KCS, CC, C-AJO, JSMW. Supervision: KCS, C-AJO, CC, JSMW. Validation: BP, CS JW-ST, WKDJ, JSMW. Visualization: BP, CS. Roles/Writing - original draft: BP, JSMW. Writing - review and editing: BP, CJS, JW-ST, WKDJ, KCS, CC, C-AJO, JSMW. All authors contributed to the article and approved the submitted version.
This study is supported by the NCCS Cancer Fund (Research) and SingHealth Duke-NUS Academic Medicine Centre, facilitated by Joint Office of Academic Medicine (JOAM). C-AJO is supported by the National Research Council Clinician Scientist-Individual Research Grant (CIRG21jun-0038). All the funding sources had no role in the study design, data interpretation or writing of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Seinen J, Almquist M, Styring E, Rydholm A, Nilbert M. Delays in the management of retroperitoneal sarcomas. Sarcoma (2010) 2010:702573. doi: 10.1155/2010/702573
2. Kirane A, Crago AM. The importance of surgical margins in retroperitoneal sarcoma. J Surg Oncol (2016) 113:270–6. doi: 10.1002/jso.24135
3. Dingley B, Fiore M, Gronchi A. Personalizing surgical margins in retroperitoneal sarcomas: An update. Expert Rev Anticancer Ther (2019) 19:613–31. doi: 10.1080/14737140.2019.1625774
4. Gronchi A, Miceli R, Colombo C, Stacchiotti S, Collini P, Mariani L, et al. Frontline extended surgery is associated with improved survival in retroperitoneal low- to intermediate-grade soft tissue sarcomas. Ann Oncol (2011) 23:1067–73. doi: 10.1093/annonc/mdr323
5. van Houdt WKDJ, Raut CP, Bonvalot S, Swallow CJ, Haas R, Gronchi A. New research strategies in retroperitoneal sarcoma. the case of TARPSWG, STRASS and RESAR: making progress through collaboration. . Curr Opin Oncol (2019) 31:310–6. doi: 10.1097/CCO.0000000000000535
6. Strauss DC, Renne SL, Gronchi A. Adjacent, adherent, invaded: A spectrum of biologic aggressiveness rather than a rationale for selecting organ resection in surgery of primary retroperitoneal sarcomas. Ann Surg Oncol (2018) 25:13–6. doi: 10.1245/s10434-017-6137-3
7. Fairweather M, Wang J, Jo VY, Baldini EH, Bertagnolli MM, Raut CP. Surgical management of primary retroperitoneal sarcomas: Rationale for selective organ resection. Ann Surg Oncol (2018) 25:98–106. doi: 10.1245/s10434-017-6136-4
8. Molina G, Hull MA, Chen Y-L, DeLaney TF, De Amorim Bernstein K, Choy E, et al. Preoperative radiation therapy combined with radical surgical resection is associated with a lower rate of local recurrence when treating unifocal, primary retroperitoneal liposarcoma. J Surg Oncol (2016) 114:814–20. doi: 10.1002/jso.24427
9. Bonvalot S, Gronchi A, Le Pechoux C, Swallow CJ, Strauss DC, Meeus P, et al. STRASS (EORTC 62092): A phase III randomized study of preoperative radiotherapy plus surgery versus surgery alone for patients with retroperitoneal sarcoma. J Clin Oncol (2019) 37:11001. doi: 10.1200/JCO.2019.37.15_suppl.11001
10. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ (2009) 339:b2700–0. doi: 10.1136/bmj.b2700
11. Tan MCB, Brennan MF, Kuk D, Agaram NP, Antonescu CR, Qin LX, et al. Histology-based classification predicts pattern of recurrence and improves risk stratification in primary retroperitoneal sarcoma. Ann Surgery Lippincott Williams Wilkins (2016) 263(3):593–600. doi: 10.1097/SLA.0000000000001149
12. Callegaro D, Raut CP, Ng D, Strauss DC, Honoré C, Stoeckle E, et al. Has the outcome for patients who undergo resection of primary retroperitoneal sarcoma changed over time? A study of time trends during the past 15 years. Ann Surg Oncol (2021) 28:1700–9. doi: 10.1245/s10434-020-09065-6
13. OCEBM levels of evidence (2016). Available at: https://www.cebm.net/2016/05/ocebm-levels-of-evidence/ (Accessed December 26, 2019).
14. Gundle KR, Kafchinski L, Gupta S, Griffin AM, Dickson BC, Chung PW, et al. Analysis of margin classification systems for assessing the risk of local recurrence after soft tissue sarcoma resection. J Clin Oncol Am Soc Clin Oncol (2018) 36(7):704–9. doi: 10.1200/JCO.2017.74.6941
15. Wittekind C, Compton CC, Greene FL, Sobin LH. TNM residual tumor classification revisited. Cancer (2002) 94:2511–6. doi: 10.1002/cncr.10492
16. Sánchez-Hidalgo JM, Rufián-Peña S, Durán-Martínez M, Arjona-Sánchez Á, Salcedo-Leal I, Lopez-Cillero P, et al. Risk factors of early recurrence in retroperitoneal liposarcoma. Cirugia Espanola (2018) 96:568–76. doi: 10.1016/j.ciresp.2018.06.002
17. Nathenson MJ, Barysauskas CM, Nathenson RA, Regine WF, Hanna N, Sausville E. Surgical resection for recurrent retroperitoneal leiomyosarcoma and liposarcoma. World J Surg Oncol (2018) 16:203. doi: 10.1186/s12957-018-1505-4
18. Zhao X, Li P, Huang X, Chen L, Liu N, She Y. Prognostic factors predicting the postoperative survival period following treatment for primary retroperitoneal liposarcoma. Chin Med J (2015) 128:85–90. doi: 10.4103/0366-6999.147822
19. Sargos P, Dejean C, de Figueiredo BH, Brouste V, Nguyen Bui B, Italiano A, et al. High-dose pre-operative helical tomotherapy (54 gy) for retroperitoneal liposarcoma. Radiat Oncol (London England) (2012) 7:214. doi: 10.1186/1748-717X-7-214
20. Lee SY, Goh BKP, Teo MCC, Chew MH, Chow PKH, Wong WK, et al. Retroperitoneal liposarcomas: The experience of a tertiary Asian center. World J Surg Oncol (2011) 9:12. doi: 10.1186/1477-7819-9-12
21. Milone M, Pezzullo LS, Salvatore G, Pezzullo MG, Leongito M, Esposito I, et al. Management of high-grade retroperitoneal liposarcomas: Personal experience. Updates Surg (2011) 63:119–24. doi: 10.1007/s13304-011-0061-z
22. Singer S, Antonescu CR, Riedel E, Brennan MF, Pollock RE. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann Surg (2003) 238(3):358–71. doi: 10.1097/01.sla.0000086542.11899.38
23. Linehan DC, Lewis JJ, Leung D, Brennan MF. Influence of biologic factors and anatomic site in completely resected liposarcoma. J Clin Oncol (2000) 18:1637–43. doi: 10.1200/JCO.2000.18.8.1637
24. Wu Y-X, Liu J-Y, Liu J-J, Yan P, Tang B, Cui Y-H, et al. A retrospective, single-center cohort study on 65 patients with primary retroperitoneal liposarcoma. Oncol Lett (2018) 15:1799–810. doi: 10.3892/ol.2017.7533
25. Rhu J, Cho CW, Lee KW, Park H, Park JB, Choi Y-L, et al. Comparison of retroperitoneal liposarcoma extending into the inguinal canal and inguinoscrotal liposarcoma. Can J Surg J Canadien Chirurgie (2017) 60:399–407. doi: 10.1503/cjs.005917
26. Fernandez-Ruiz M, Rodriguez-Gil Y, Guerra-Vales JM, Manrique-Municio A, Moreno-Gonzalez E, Colina-Ruizdelgado F. Primary retroperitoneal liposarcoma: Clinical and histological analysis of ten cases. Gastroenterologia y Hepatologia (2010) 33:370–6. doi: 10.1016/j.gastrohep.2009.12.010
27. Han HH, Choi KH, Kim DS, Jeong WKDJ, Yang SC, Jang SJ, et al. Retroperitoneal giant liposarcoma. Korean J Urol (2010) 51:579–82. doi: 10.4111/kju.2010.51.8.579
28. Crisan N, Ivan CS, Bungardean C, Cebotaru C, Coman I. Retroperitoneal perirenal myxoid liposarcoma. J Surg Case Rep (2015) 2015:rju127–7. doi: 10.1093/jscr/rju127
29. Daldoul S, Zahaf B, Ghassen EH, Marwa B, Ben SY, Ben MM. Retro-peritoneal liposarcoma: Diagnostic difficulties and therapeutic attitudes. Surg Curr Res (2017) 07. doi: 10.4172/2161-1076.1000302
30. Yaman. Renal liposarcoma of the sinus renalis. Int Urol Nephrol (1996) 28:477–80. doi: 10.1007/bf02550953
31. Susini T, Taddei G, Massi D, Massi G. Giant pelvic retroperitoneal liposarcoma. Obstetrics Gynecol (2000) 95:1002–4. doi: 10.1016/s0029-7844(00)00818-8
32. Sener A, Wehrli B, Bramwell V, Bella AJ, Izawa JI. Retroperitoneal liposarcoma and Li-fraumeni syndrome: A case report and review of management options. UroOncology (2004) 4:161–5. doi: 10.1080/15610950400006660
33. Mehrotra PK, Ramachandran CS, Goel D, Arora V. Inflammatory variant of a well-differentiated retroperitoneal liposarcoma: Case report of a rare giant variety. Indian J Cancer (2006) 43:36–8. doi: 10.4103/0019-509x.25774
34. Calo PG, Farris S, Tatti A, Tuveri M, Catani G, Nicolosi A. Primary mesenteric liposarcoma. Rep case. Il Giornale di Chirurgia (2007) 28:318–20.
35. Gaston KE, White RLJ, Homsi S, Teigland C. Nephron-sparing radical excision of a giant perirenal liposarcoma involving a solitary kidney. Am Surgeon (2007) 73:377–80. doi: 10.1177/000313480707300413
36. Gupta NP, Yadav R. Renal sparing surgery for perirenal liposarcoma: 24 months recurrence free follow up. Int Braz J urol: Off J Braz Soc Urol (2007) 33:188–91. doi: 10.1590/s1677-55382007000200009
37. Pérez-Ponce Y, Castellanos-Alejandre R, Guerrero-Romero JF, Estrada-León F, Torres-Lobatón A. Retroperitoneal liposarcoma as etiology of abdominal pain. A Case Rep Literature Rev Cirugia y Cirujanos (2008) 76:77–82.
38. Yildirim O, Namdaroglu OB, Menekse E, Albayrak AL. Giant well-differentiated liposarcoma of retroperitoneum. Bratislavske lekarske listy (2008) 109:418–20.
39. Benseler V, Obed A, Schubert T, Schlitt H-J, Bolder U. [Case report–surgical therapy of a retroperitoneal liposarcoma weighing 45 kg]. Zentralblatt fur Chirurgie (2009) 134:174–7. doi: 10.1055/s-2008-1076878
40. Goertz RS, Lenfers BH, Goertz GH. Huge liposarcoma of the left retroperitoneum. Am J Surg (2009) 197:e59–60. doi: 10.1016/j.amjsurg.2008.07.061
41. Salemis NS, Nisotakis K, Patouras P, Karagkiouzis G, Gourgiotis S. Retroperitoneal liposarcoma extending into the thigh. Am J Surg (2011) 201:e38–40. doi: 10.1016/j.amjsurg.2010.04.019
42. Colebunders B, Colpaert SDM, Mertens M, Willemsen P. Thoraco-abdominal wall reconstruction after surgical debulking of a giant retroperitoneal liposarcoma: A case report. Acta chirurgica Belgica (2011) 111:250–2. doi: 10.1080/00015458.2011.11680749
43. Makni A, Triki A, Fetirich F, Ksantini R, Chebbi F, Jouini M, et al. Giant retroperitoneal liposarcoma. Rep 5 Cases. Annali Italiani di Chirurgia (2012) 83:161–6.
44. Bansal VK, Misra MC, Sharma A, Chabbra A, Murmu LR. Giant retroperitoneal liposarcoma- renal salvage by autotransplantation. Indian J Surg (2013) 75:159–61. doi: 10.1007/s12262-012-0474-z
45. Sharma M, Mannan R, Bhasin TS, Manjari M, Punj R. Giant inflammatory variant of well differentiated liposarcoma: A case report of a rare entity. J Clin Diagn Research: JCDR (2013) 7:1720–1. doi: 10.7860/JCDR/2013/5998.3267
46. Nagy V, Bober J, Zavacky P, Brandebur OJ, Svajdler M. The recurrent primary retroperitoneal liposarcoma. Bratislavske lekarske listy (2013) 114:662–7. doi: 10.4149/bll_2013_141
47. Hoshi S, Hayashi N, Yagi M, Ookubo T, Muto A, Sugano O, et al. Long term survival in a case of concurrent retroperitoneal liposarcoma and renal cell carcinoma: A case report. BMC Res Notes (2014) 7:538. doi: 10.1186/1756-0500-7-538
48. Caizzone A, Saladino E, Fleres F, Paviglianiti C, Iaropoli F, Mazzeo C, et al. Giant retroperitoneal liposarcoma: Case report and review of the literature. Int J Surg Case Rep (2015) 9:23–6. doi: 10.1016/j.ijscr.2015.02.019
49. Kasashima H, Yamasaki Y, Morimoto Y, Akamaru Y, Yasumasa K, Kasugai T, et al. A case of retroperitoneal liposarcoma after delivery with expression of estrogen receptor: Report of a case. Int J Surg Case Rep (2015) 7C:99–103. doi: 10.1016/j.ijscr.2015.01.002
50. Reznichenko AA. Simultaneous renal cell carcinoma and giant retroperitoneal liposarcoma involving small intestine. Case Rep Surg (2016) 2016:6021909. doi: 10.1155/2016/6021909
51. Kobayashi T, Miura K, Ishikawa H, Soma D, Zhang Z, Yuza K, et al. Successful re-resection for locally recurrent retroperitoneal liposarcoma at four years after ex vivo tumor resection and autotransplantation of the liver: A case report. Transplant Proc (2016) 48:1215–7. doi: 10.1016/j.transproceed.2016.01.026
52. Machado MCC, Fonseca GM, de Meirelles LR, Zacchi FFS, Bezerra ROF. Primary liposarcoma of the pancreas: A review illustrated by findings from a recent case. Pancreatology: Off J Int Assoc Pancreatol (IAP) . [et al] (2016) 16:715–8. doi: 10.1016/j.pan.2016.07.003
53. Zeng X, Liu W, Wu X, Gao J, Zhang P, Shuai X, et al. Clinicopathological characteristics and experience in the treatment of giant retroperitoneal liposarcoma: A case report and review of the literature. Cancer Biol Ther (2017) 18:660–5. doi: 10.1080/15384047.2017.1345388
54. Tsiao S, Aydin N, Misra S. Neuropraxia following resection of a retroperitoneal liposarcoma. Int J Surg Case Rep (2017) 36:170–4. doi: 10.1016/j.ijscr.2017.05.032
55. Singal R, Trehan S, Mittal A, Roy M, Joshi K. A giant retroperitoneal sarcoma: Current pathology and successful surgically. Acta Gastroenterologica Latinoamericana (2018) 48:52–5.
56. Ioannidis A, Koutserimpas C, Konstantinidis M, Drikos I, Voulgaris P, Economou N. Dyspnea caused by a giant retroperitoneal liposarcoma: A case report. Oncol Lett (2018) 16:1539–42. doi: 10.3892/ol.2018.8791
57. Agrusa A, Di Buono G, Buscemi S, Randisi B, Gulotta L, Sorce V, et al. Dedifferentiated retroperitoneal large liposarcoma and laparoscopic treatment: Is it possible and safe? the first literature case report. Int J Surg Case Rep (2019) 57:113–7. doi: 10.1016/j.ijscr.2019.03.023
58. Argadjendra M, Napitupulu R, Yudadi R, Hoetama S, Wibowo HS. Kidney sparing giant retroperitoneal liposarcoma: Case report and literature review. Int J Surg Case Rep (2019) 56:70–3. doi: 10.1016/j.ijscr.2019.02.008
59. Huo D, Liu L, Tang Y. Giant retroperitoneal liposarcoma during pregnancy: A case report. World J Surg Oncol (2015) 13:145. doi: 10.1186/s12957-015-0555-0
60. Clar H, Leithner A, Gruber G, Werkgartner G, Beham A, Windhager R. Interdisciplinary resection of a giant retroperitoneal liposarcoma of 25 kg. ANZ J Surg (2009) 79:957. doi: 10.1111/j.1445-2197.2009.05160.x
61. Hashimoto Y, Hatakeyama S, Tachiwada T, Yoneyama T, Koie T, Kamimura N, et al. Surgical treatment of a giant liposarcoma in a Japanese man. Adv Urol (2010) 2010:943073. doi: 10.1155/2010/943073
62. Akhoondinasab MR, Omranifard M. Huge retroperitoneal liposarcoma. J Res Med Sci (2011) 16:565–7.
63. Bhat SP, Prasad KHL, Shetty R, Ballal R, Kumar SY, Hegde P. Giant inflammatory variant of well-differentiated liposarcoma of retroperitoneum: A rare case report. Indian J Surg Oncol (2013) 4:272–4. doi: 10.1007/s13193-013-0239-6
64. Oh SD, Oh SJ, Suh BJ, Shin JY, Oh CK, Park JK, et al. A giant retroperitoneal liposarcoma encasing the entire left kidney and adherent to adjacent structures: A case report. Case Rep Oncol (2016) 9:368–72. doi: 10.1159/000447488
65. Tanaka M, Kawahara T, Nishikoshi T, Hagiwara M, Imai K, Hasegawa K, et al. Successful surgical treatment for huge retroperitoneal liposarcoma involving the pancreas, right kidney, abdominal aorta and inferior vena cava. J Surg Case Rep (2017) 2017:rjx200. doi: 10.1093/jscr/rjx200
66. Abufkhaida BS, Alsalameh BK. Recurrent abdominal liposarcoma presenting with intestinal obstruction. J Surg Case Rep (2019) 2019:rjz188. doi: 10.1093/jscr/rjz188
67. Montenegro A, Varas M, Sanchez-Vizcaino E, Naval J, Loras C, Abad R. A giant retroperitoneal liposarcoma with renal involvement: A case report and literature review. Gastroenterologia y Hepatologia (2019) 42:490–1. doi: 10.1016/j.gastrohep.2019.01.003
68. Herzberg J, Niehaus K, Holl-Ulrich K, Honarpisheh H, Guraya SY, Strate T. Giant retroperitoneal liposarcoma: A case report and literature review. J Taibah Univ Med Sci (2019) 14:466–71. doi: 10.1016/j.jtumed.2019.08.005
69. Yang J, Zhao Y, Zheng CH, Wang Q, Pang XY, Wang T, et al. Huge retroperitoneal liposarcoma with renal involvement requires nephrectomy: A case report and literature review. Mol Clin Oncol (2016) 5:607–9. doi: 10.3892/mco.2016.1017
70. McCallum OJ, Burke JJ 2nd, Childs AJ, Ferro A, Gallup DG. Retroperitoneal liposarcoma weighing over one hundred pounds with review of the literature. Gynecologic Oncol (2006) 103:1152–4. doi: 10.1016/j.ygyno.2006.08.005
71. Keil S, Bruners P, Brehmer B, Mahnken AH. Percutaneous radiofrequency ablation for treatment of recurrent retroperitoneal liposarcoma. Cardiovasc Interventional Radiol (2008) 31 Suppl 2:S213–6. doi: 10.1007/s00270-007-9263-7
72. Sato Y, Yamamoto S, Fujita S. Retroperitoneal liposarcoma with colonic involvement: a case report. Japanese J Clin Oncol (2014) 44:374–8. doi: 10.1093/jjco/hyu009
73. Bruce NR, Tilley ZW, Carlson JT, Barreto Andrade JC. Management of T-cell large granular lymphocyte leukemia and concurrent retroperitoneal liposarcoma. J Surg Case Rep (2018) 2018) 7:1–3. doi: 10.1093/jscr/rjy142
74. Ghose J, Bhamre R, Mehta N, Desouza A, Patkar S, Dhareshwar J, et al. Resection of the inferior vena cava for retroperitoneal sarcoma: Six cases and a review of literature. Indian J Surg Oncol (2018) 9:538–46. doi: 10.1007/s13193-018-0796-9
75. Clavien PA, Barkun J, De Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The clavien-dindo classification of surgical complications: Five-year experience. Ann Surg (2009) 250:187–96. doi: 10.1097/SLA.0b013e3181b13ca2
76. Stiller CA, Trama A, Serraino D, Rossi S, Navarro C, Chirlaque MD, et al. Descriptive epidemiology of sarcomas in Europe: Report from the RARECARE project. Eur J Cancer (2013) 49:684–95. doi: 10.1016/j.ejca.2012.09.011
77. Trans-Atlantic RPS Working Group. Management of primary retroperitoneal sarcoma (RPS) in the adult: A consensus approach from the trans-Atlantic RPS working group. Ann Surg Oncol (2015) 22:256–63. doi: 10.1245/s10434-014-3965-2
78. Bonvalot S, Raut CP, Pollock RE, Rutkowski P, Strauss DC, Hayes AJ, et al. Technical considerations in surgery for retroperitoneal sarcomas: Position paper from e-surge, a master class in sarcoma surgery, and EORTC-STBSG. Ann Surg Oncol (2012) 19:2981–91. doi: 10.1245/s10434-012-2342-2
79. Gronchi A, Lo Vullo S, Fiore M, Mussi C, Stacchiotti S, Collini P, et al. Aggressive surgical policies in a retrospectively reviewed single-institution case series of retroperitoneal soft tissue sarcoma patients. J Clin Oncol (2009) 27:24–30. doi: 10.1200/JCO.2008.17.8871
80. Bonvalot S, Rivoire M, Castaing M, Stoeckle E, Le Cesne A, Blay JY, et al. Primary retroperitoneal sarcomas: A multivariate analysis of surgical factors associated with local control. J Clin Oncol (2009) 27:31–7. doi: 10.1200/JCO.2008.18.0802
81. Macneill AJ, Gronchi A, Miceli R, Bonvalot S, Swallow CJ, Hohenberger P, et al. Postoperative morbidity after radical resection of primary retroperitoneal sarcoma. Ann Surg (2018) 267:959–64. doi: 10.1097/SLA.0000000000002250
82. Callegaro D, Fiore M, Gronchi A. Personalizing surgical margins in retroperitoneal sarcomas. Expert Rev Anticancer Ther (2015) 15:553–67. doi: 10.1586/14737140.2015.1028375
83. Gronchi A, Strauss DC, Miceli R, Bonvalot S, Swallow CJ, Hohenberger P, et al. Variability in patterns of recurrence after resection of primary retroperitoneal sarcoma (RPS). A report on 1007 patients from the multi-institutional collaborative RPS working group. Ann Surg (2016) 263:1002–9. doi: 10.1097/SLA.0000000000001447
84. Lochan R, French J, Manas D. Surgery for retroperitoneal soft tissue sarcomas: aggressive re-resection of recurrent disease is possible. Ann R Coll Surgeons Engl (2011) 93:39–43. doi: 10.1308/003588410X12771863936729
85. Swallow CJ, Strauss DC, Bonvalot S, Rutkowski P, Desai A, Gladdy RA, et al. Management of primary retroperitoneal sarcoma (RPS) in the adult: An updated consensus approach from the transatlantic Australasian RPS working group. Ann Surg Oncol (2021) 28(12):7873–88. doi: 10.1245/s10434-021-09654-z
Keywords: liposarcoma, margin, retroperitoneal, sarcoma, R0
Citation: Paik B, Seo CJ, Tan JW-S, Juan WKD, Soo KC, Ong C-AJ, Chia CS and Wong JSM (2022) A systematic review of margin status in retroperitoneal liposarcomas: Does the R0 margin matter? Front. Oncol. 12:891710. doi: 10.3389/fonc.2022.891710
Received: 08 March 2022; Accepted: 18 July 2022;
Published: 11 August 2022.
Edited by:
Giuseppe Brisinda, Agostino Gemelli University Polyclinic (IRCCS), ItalyCopyright © 2022 Paik, Seo, Tan, Juan, Soo, Ong, Chia and Wong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jolene Si Min Wong, am9sZW5lLndvbmcucy5tQHNpbmdoZWFsdGguY29tLnNn
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.