
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 17 June 2022
Sec. Genitourinary Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.891623
This article is part of the Research Topic Penile Cancer in Genitourinary Oncology View all 17 articles
Purpose: The aim of this study is to investigate the trends in incidence and mortality, and explore any change in survival of penile cancer in the United States.
Methods: We obtained data from the Surveillance, Epidemiology, and End Results (SEER) database (2000–2018) utilizing the SEER Stat software. The joinpoint regression was used to analyze the secular trend of incidence and incidence-based mortality (IBM) stratified by age, race, and summary stage. The 5-year relative survival rate was also calculated.
Result: The age-adjusted rates of penile cancer patients were 0.38 (0.37–0.39) and 0.21 (0.2–0.21) for overall incidence and IBM, respectively. The 5-year relative survival rates were 67.7%, 66.99%, and 65.67% for the calendar periods of 2000–2004, 2005–2009, and 2010–2014, respectively. No significant changes in incidence by era were observed from 2000 to 2018 [annual percentage change (APC) = 0.5%, p = 0.064]. The IBM rate of penile cancer showed an initial significant increase from 2000 to 2002 (APC = 78.6%, 95% CI, −1.7–224.6) followed by a deceleration rate of 4.6% (95% CI, 3.9–5.3) during 2002 to 2018. No significant improvement in 5-year relative survival was observed. The trends by age, race, and summary stage in incidence and IBM were significantly different.
Conclusion: This study, using population-level data from the SEER database, showed an increasing trend in IBM and no significant improvement in the 5-year relative survival rate. Meanwhile, the incidence of penile cancer exhibited a relatively stable trend during the study period. These results might be due to the lack of significant progress in the treatment and management of penile cancer patients in the United States in recent decades. More efforts, like increasing awareness among the general population and doctors, and centralized management, might be needed in the future to improve the survival of this rare disease.
Penile cancer is a relatively rare neoplasm in Western countries, presenting an incidence rate of less than 1 per 100,000 men (1). However, a prominent geographic variation in incidence can be observed, and it may be due to different socioeconomic status, hygiene, religious, and cultural conditions (2, 3). For example, incidence rates in some developing countries like India (up to 2.3 per 100,000) and Eastern and Southern Africa (up to 2.7 per 100,000) were significantly higher than 1 per 100,000 men (1, 4). Brazil is one of the countries with the highest penile cancer incidence rates, which reached up to 3.3 per 100,000 based on record. A relatively higher mortality and a gradually increasing trend with an annual percent change (APC) of 1.4% are also reported in these countries during 1996 to 2010 (1, 5). Relative survival rates also showed a geographic variation between countries. For instance, the relative 5-year survival rate in Norway is 80%, while this value is only 62% in Finland from 1999 to 2003 (6). Risk factors confirmed to be associated with penile cancer include human papillomavirus (HPV) infection, smoking, circumcision status, and lower socioeconomic status (7–9). Although the exact pathogenesis is still unclear, some studies suggested that inflammation may play an essential role in tumor development or progression because tumors may likely arise from sites of penile infection and chronic irritation (10, 11).
Significant differences in incidence and mortality rate trends of penile cancer existed among different countries. For example, the trend in the incidence of penile cancer has been presented as increasing in Denmark during 1978–2008. However, in the United States, the trend of penile cancer incidence showed a significant decrease with a rate of 0.84, 0.69, and 0.58 per 100,000 for 1973 −1982, 1982−1992, and 1993−2002, respectively (12).
The trend of incidence rate and mortality rate of a disease can reflect the prevention, treatment, and management level of the disease, thereby deepening the understanding of disease and making recommendations for disease guidelines. As a developed country, America’s advanced medical technology and disease management strategy are often explored and used for reference by other countries. To our knowledge, there has been a lack of studies describing the trend of incidence, mortality, and survival of penile cancer in the United States over the past 10 years. In addition, a comparative study to explore the association between incidence, mortality, and survival rate has not been performed. This analysis, based on the Surveillance, Epidemiology, and End Results (SEER) database (2000–2018), aims to explore the up-to-date epidemiology of penile cancer in the United States. The trends in incidence, mortality, and survival of penile cancer by age, race, and summary stage are investigated according to the up-to-date information of epidemiology.
We obtained penile cancer patients from the SEER Program of the National Cancer Institute (ID: 20420-Nov2020). Patients diagnosed with penile cancer as their first malignancy according to the list of Site Recode the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) and cases who were coded with penis were enrolled in our study.
Incident cases were obtained from the database of incidence–SEER 18 registries of the US National Cancer Institute from 2000 to 2018, which collected data on cancer incidence and mortality involving approximately 26.4% of the U.S. population.
Incidence-based mortality (IBM) cases were obtained from the database of IBM–SEER 18 registries of the US National Cancer Institute from 2000 to 2018.
Survival cases were obtained from the database of incidence–SEER 18 registries of the U.S. National Cancer Institute from 2000 to 2014. We failed to acquire more data considering the 5-year relative survival rate was not recorded after 2014 in the SEER database. The study period was averagely divided into three time periods (2000–2004, 2005–2009, and 2010–2014) to observe prominent survival rate disparities.
Three primary outcomes were calculated in this study: incidence, IBM, and 5-year relative survival rate. Incidence and IBM rates were adjusted to the 2000 U.S. standard population and calculated by 100,000 person-years. We calculated IBM rates as the number of all-cause death cases diagnosed with penile cancer among cases diagnosed over person-time at risk among people in areas of the SEER. In the registries of population-based SEER cancer, the incidence of individuals was linked to their mortality outcomes. It could calculate mortality rates by variables like the stage at diagnosis. This special mortality measure was defined as IBM (13, 14). Relative survival estimates were defined as the ratio of the observed survival of penile cancer patients and the expected survival of the underlying general population (15).
Then, we analyzed the annual percentage change (APC) of incidence and IBM rates stratified by age (15–44, 45–54, 55–64, 65–74, and 75+), race [White, Black, American/Indian/Alaska/Native (AIAN), and Asian/Pacific Islander (API)], and summary stage (localized, regional, and distant). Localized stage referred to an invasive neoplasm confined entirely to the penis (mainly including T1-4N0M0) and tumor staged as regional was defined as extending to surrounding organs, tissues, or regional lymph nodes (mainly including T4N0M0 or T1-4N1-3M0). Distant disease referred to the tumor that had spread to remote sites of the body (mainly including M1).
SEER_Stat version 8.3.2 was used to calculate incidence, mortality rates, and 5-year relative survival rate of penile cancer. Then, joinpoint regression was performed to identify the best-fitting log-linear regression model, which appropriately demonstrated the incidence and mortality rate trend by era. The National Cancer Institute’s Joinpoint Regression Program, Version 4.6.0.0, was utilized to calculate the APC and 95% confidence intervals (95% CIs) (16). The Joinpoint Regression software utilized t-tests to confirm whether statistical differences existed between APC and zero, and p < 0.05 was considered statistically significant. All statistical results were two-sided. Notably, we excluded the data not recorded from the joinpoint regression because no cases were reported at a certain year.
Finally, 6,397 patients diagnosed with penile cancer, who were from 18 SEER registries from 2000 to 2018, were enrolled in our study. Table 1 demonstrates the characteristics of patients for incidence and IBM analysis. For all cases, the most common age group was 75+ years [2,132 (33.33%)], and White cases accounted for the most significant proportion in the study population [5,017 (83.55%)]. Compared to patients with other stages, patients with localized stage were more commonly seen [3,032 (47.4%)]. Of the eligible patients, 3,348 patients with penile cancer died during the study period. Of all the deaths, 1,399 (45.85%) patients were observed to be aged 75+ years, and 2,572 (84.3%) cases were White patients. A total of 1,495 (49%) patients who were recorded as dead were diagnosed with localized stage.
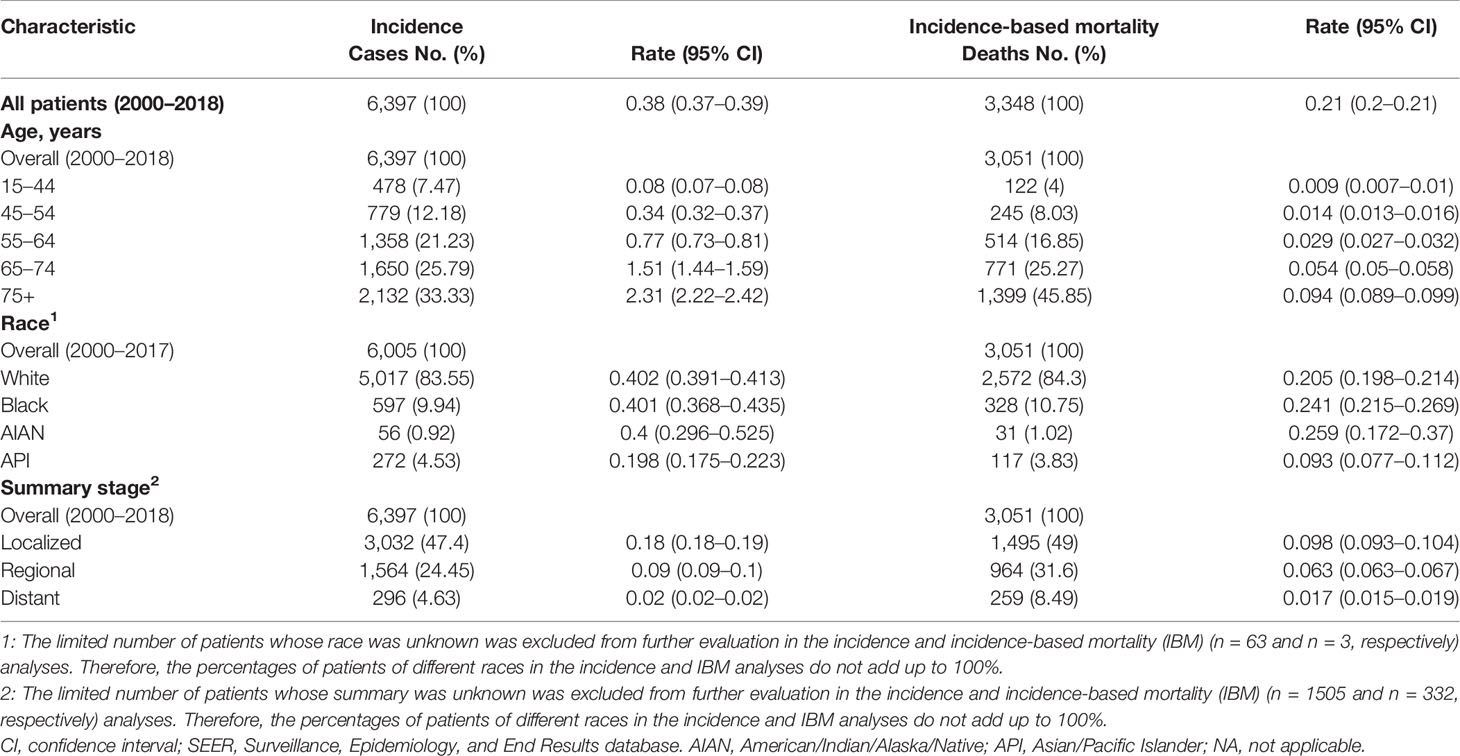
Table 1 Penile cancer incidence (2000–2018) and incidence-based mortality (2000–2018): the SEER-18 registry database.
Of all study populations, the age-adjusted rates of penile cancer patients were 0.38 (0.37–0.39) and 0.21 (0.2–0.21) for incidence and IBM rate, respectively (Table 1). The incidence of penile cancer had no significant change from 2000 to 2018 (APC = 0.5%, 95% CI = −1.1–2.0; p = 0.064) (Figure 1A and Table 2). The IBM rate of penile cancer showed an initial significant increase from 2000 to 2002 (APC = 78.6%, 95% CI, −1.7–224.6) followed by a deceleration rate of 4.6% (95% CI, 3.9–5.3) during 2002 to 2018 (Figure 1B and Table 2).
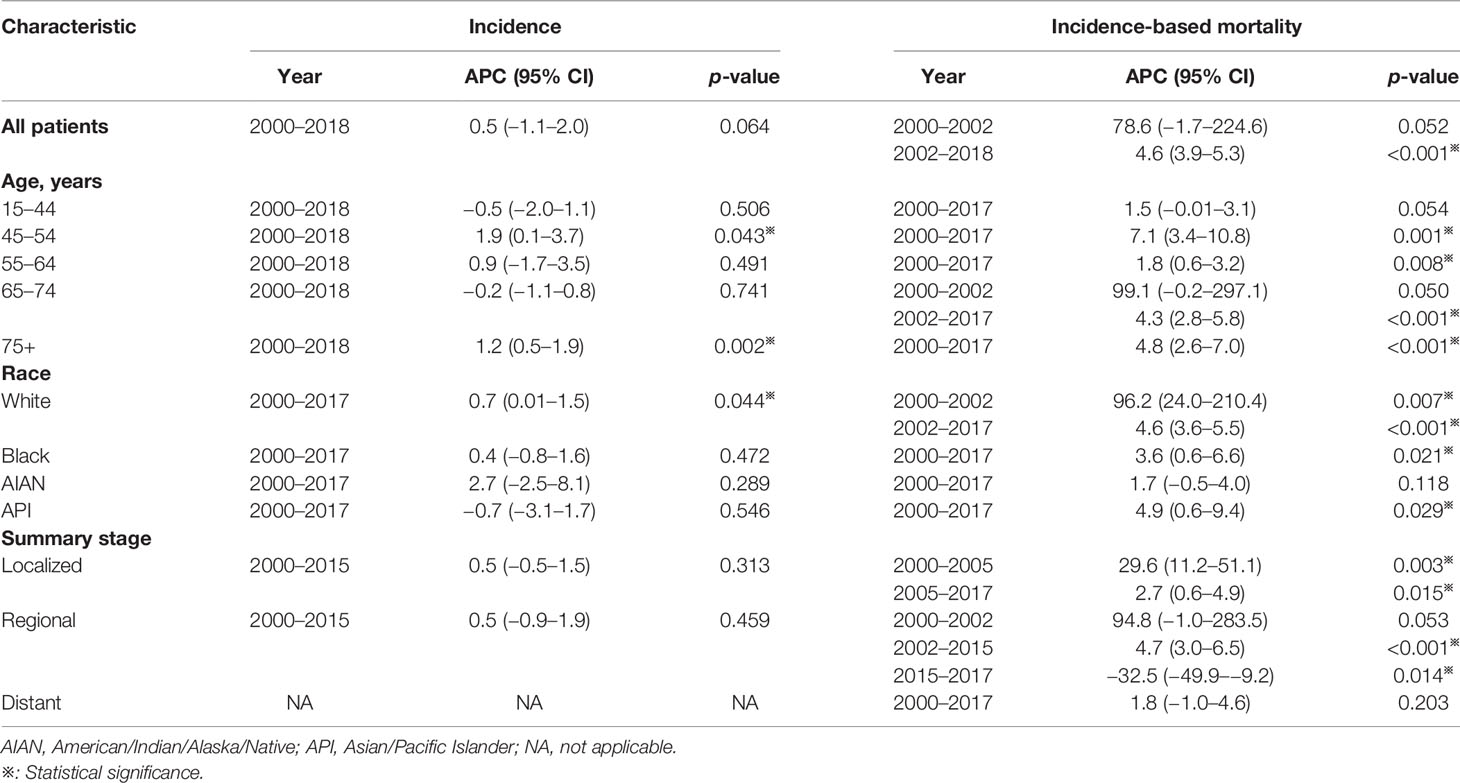
Table 2 Trends in the incidence rates and incidence-based mortality of penile cancer (2000–2018): the SEER-18 registry database.
The penile cancer incidence rates were highest among cases aged over 75 years (2.31, 95% CI, 2.22–2.42), White patients (0.402, 95% CI, 0.391–0.413), and patients diagnosed with localized stage (0.18, 95% CI, 0.18–0.19) (Table 1). Similarly, the IBM rates of penile cancer were highest among patients aged 75+ years (0.094, 95% CI, 0.089–0.099), AIAN patients (0.259, 95% CI, 0.172–0.37), and patients with localized stage (0.098, 95% CI, 0.093–0.104) (Table 1).
The incidence rates among penile cancer patients in the age group of 45–54 and 75+ years exhibited a slight increase with an APC of 1.9% (95% CI, 0.1–3.7, p = 0.043) and 1.2% (95% CI, 0.5–1.9, p = 0.002), respectively, for the period of 2000–2018 (Figures 2B, E and Table 2). We did not obtain statistically significant trends in incidence rates in other age groups. For IBM rate analysis by age, patients diagnosed at ages 45–54, 55–64, and 75+ years exhibited an increasing trend at the rate of 7.1% (95% CI, 3.4–10.8, p = 0.001), 1.8% (95% CI, 0.6–3.2, p = 0.008), and 4.8% (95% CI, 2.6–7.0, p = 0.001), respectively, in 2000 to 2017 (Figures 2G, H, J and Table 2). In addition, for those aged 65–74 years, the trend of IBM presented a rapid initial increase (APC = 99.1%, 95% CI, −0.2–297.1) and then showed a deceleration for 2002–2017 (APC = 4.3%, 95% CI, 2.8–5.8, p < 0.001). (Figure 2I and Table 2).
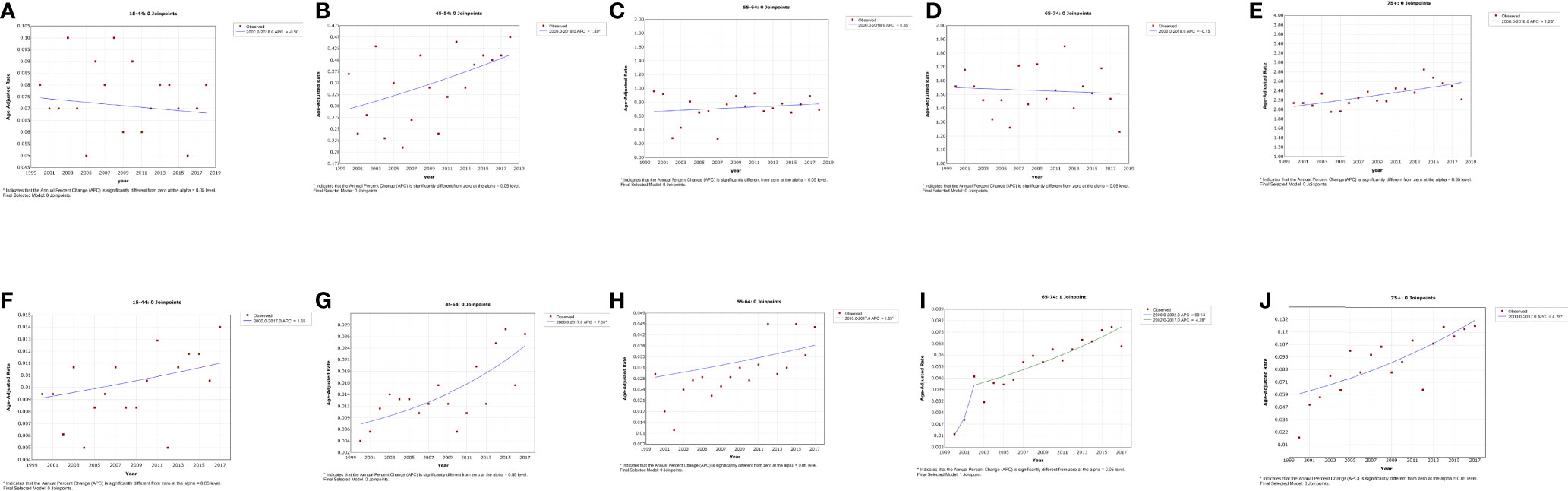
Figure 2 Trends in the annual incidence (A–E) and incidence-based mortality (F–J) of penile cancer in patients stratified by age at diagnosis.
A slightly increased incidence trend was observed from 2000 to 2017 with an APC of 0.7% (95% CI, 0.01–1.5, p = 0.044) among White penile cancer patients (Figure 3A and Table 2). No noticeable change in incidence was observed in other races. Of Black and API patients, the trend of IBM rates demonstrated a slowly rising trend with an APC of 4.6% (95% CI, 3.6–5.5, p < 0.001) and 4.9% (95% CI, 0.6–9.4, p = 0.029), respectively (Figures 3F, H and Table 2). The IBM rate in White patients increased sharply at the initial time of 2000 to 2002 (APC = 96.2%, 95% CI 24.0–210.4, p = 0.007), and the increasing trend had slowed down in 2002 (APC = 4.6%, 95% CI 3.6–5.5, p < 0.001). (Figure 3E and Table 2).
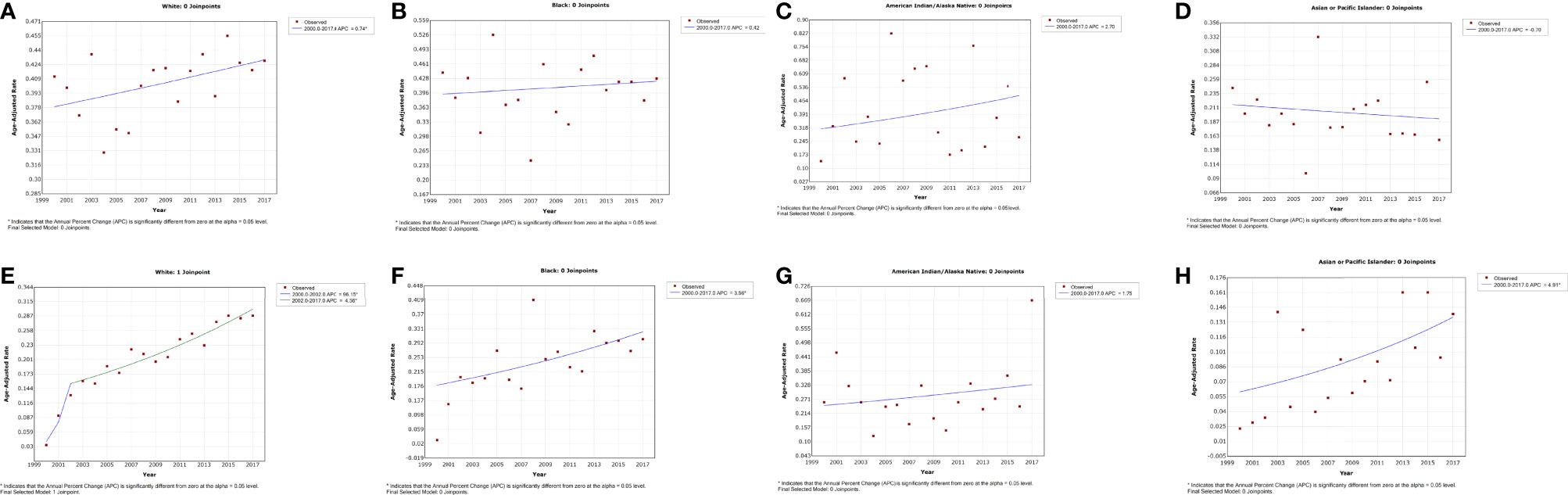
Figure 3 Trends in the annual incidence (A–D) and incidence-based mortality (E–H) of penile cancer in patients stratified by race.
No significant changes were observed in the incidence trend by summary stage from 2000 to 2015. We failed to obtain a best-fitting line and APC for patients with distant stage due to the relatively low incidence and the lack of regular variation (Figure 4F). Of patients diagnosed with localized stage, the trend of IBM rates showed an initial prominent increase during 2000 to 2005 (APC = 29.6%, 95% CI = 11.2–51.1, p = 0.003), followed by a deceleration thereafter (APC = 2.7%, 95% CI, 0.6–4.9, p = 0.015) (Figure 4A and Table 2). For patients with regional stage, a continuous increasing IBM rate was observed from 2002 to 2015 (APC = 4.7%, 95% CI, 3.0–6.5; p < 0.001), but a steep decline in the trend of IBM rates was exhibited after 2015 (APC = −32.5%, 95% CI, −49.9–−9.2, p = 0.014) (Figure 4B and Table 2).
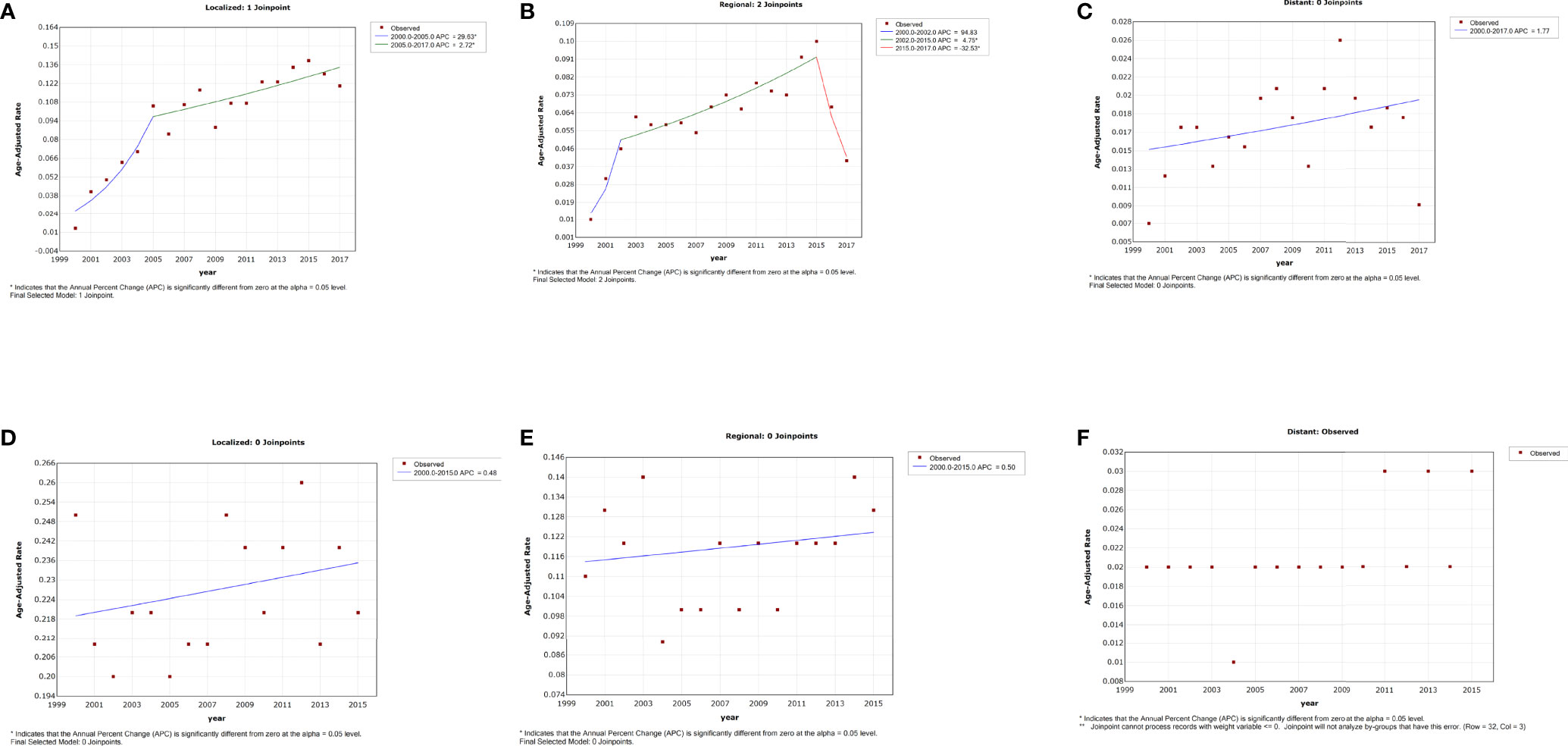
Figure 4 Trends in the annual incidence (D–F) and incidence-based mortality (A–C) of penile cancer in patients stratified by summary stage.
The overall 5-year relative survival rates showed a slight decrease with a rate of 67.7% (SE = 1.76%), 66.99% (SE = 1.7%), and 65.67% (SE = 1.66%) for the time periods 2000–2004, 2005–2009, and 2010–2014, respectively (Table 3). However, this change was not statistically significant (p = 0.12). The descending trend in relative survival rates was observed in White and Black patients, and it was relatively prominent in Black patients (change −8.08%, p < 0.001) (Table 3). For patients diagnosed with localized stage, the 5-year relative survival rates exhibited an increasing trend with a rate of 76.6% (SE = 2.2%), 79.57% (SE = 2.11%), and 81.55% (SE = 2.1%) for time periods 2000–2004, 2005–2009, and 2010–2014, respectively (change 4.95%, p < 0.001) (Table 3). For API, the 5-year relative survival rates increased from 72.62% in 2000–2004 to 91.52% in 2005–2009, and then dropped to 65.72% in 2010–2014. Similar trends were observed in patients diagnosed with regional stage and diagnosed at 15–44, 45–54, and 75+ years. However, none of these trends was regular and easily to explain.
This study comprehensively explored the trend of incidence, IBM, and 5-year relative survival rate of penile cancer in the United States during 2000–2018, and further examined the trend by stratifying age, race, and tumor stage. There were no significant changes in the trend of incidence of penile cancer from 2000 to 2018. However, we found that the IBM rate of penile cancer significantly increased and that there was no significant improvement in the 5-year relative survival rate over the study period.
The incidence rate of penile cancer, at 0.38 per 100,000 over 2000 to 2018, was relatively lower than the result from a previous study based on the SEER database (12). They found an incidence rate of 0.84, 0.69, and 0.58 per 100,000 for the calendar periods 1973−1982, 1982−1992, and 1993−2002, respectively, and the data were collected from 9 SEER registries, which cover approximately 9.4% of the U.S. population. Compared to the previous incidence, the incidence in this study had still decreased. However, considering the geographical variation, we should seriously explain this discrepancy considering that our data came from 18 registries.
There is a relatively big difference between the trend in the incidence rate of different countries. For instance, the trend in the incidence of penile cancer was increasing for Denmark over 1978–2008 and England over 1979–2009 (17, 18), whereas this tendency was inverse in Finland during 1971–1995 and the United States during 1973–2002 (19, 20). In this study, we found a stable trend in the incidence of penile cancer in the United States during 2000–2018, although we obtained a slight upward best-fitting line (p > 0.05, Figure 1A). Although we observed a slight increase in incidence rate for patients aged 45–54 and 75+ years and White patients, the extent of this change was quite small. Previous studies usually explain the decreasing incidence rate with improved sanitation, declining smoking rate, and newborn male circumcision (21–23). For example, several data-based studies suggested that the rate of male circumcision ranged from 42% to 80% in the United States, and the procedure is commonly performed during the newborn period (24). The available evidence proved that male circumcision had special benefits in preventing urinary tract infection, HIV infection, the transmission of some sexually transmitted infections, and penile cancer (25). A relatively higher rate of male circumcision was considered a protective factor for penile cancer, and it might be a crucial reason for the stable incidence in the United States. Another notable reason was chronic inflammation, which was considered as a significant pathogenic pathway of penile cancer (25–28). A relatively perfect healthcare system and universal sex education might account for the lower rate of chronic inflammation than those in developing countries. These results showed that the prevention of penile cancer in the United States had a good performance.
There were relatively few studies focusing on the trend of penile cancer mortality. A retrospective study, whose data were from the Netherlands during 1989–2006, suggested a slight decrease in mortality (11, 29). Similarly, a decrease in mortality was also observed in England for 1979–2009 (30). Nevertheless, we found a prominent increase in the IBM rate in the United States for the period of 2000–2018. Interestingly, a rapid increase of IBM was observed at the initial period of 2000–2002 (APC = 78.6%), but it failed to obtain a statistically significant p-value due to the relatively short study period. Similarly, of patients aged 65–74 years, White cases, and patients with regional stage, we also observed a sharp increase in IBM in the initial period of 2000–2002 (Figures 2I, 3A, 4B). In addition, we also did not observe any improvement in the 5-year relative survival rate of penile cancer in the United States. The phenomenon of no significant improvement in the 5-year survival rate and increased mortality of penile cancer might be due to the lack of significant progress in the treatment and management of penile cancer (31).
A likely explanation for these results was difficult to make. A recent study suggested that penile sparing surgery had been increasingly adopted, and no prominent differences in survival were observed between patients undergoing sparing and complete surgery (32). This improved surgical approach might lead to a better quality of life. Still, its contribution to high-risk patients, especially those with positive lymph nodes and distant metastasis, was not remarkable. In the past two decades, the most significant progress in the treatment of penile cancer was treating primary lesions, modified lymphadenectomy, and identifying and treating occult regional lymph node metastasis with the help of sentinel lymph node biopsy (SNB) (33, 34). About 80% of patients with one or two lymph nodes involved can be cured by lymphadenectomy. Even patients with pelvic lymph node involvement can still be cured by surgery.
The main goal of SNB was to reduce mortality and improve survival in clinical lymph node-negative (cN0) patients. Reported data showed that about 20% to 25% of the cN0 penile cancer patients had occult lymph node metastases at diagnosis, and early surgical resection of these occult lymph nodes could obtain better survival than those with clinically apparent nodes (35). The introduction of SNB might thus have improved survival, especially those with occult lymph nodes. An unpublished study from the Netherlands does show that cancer-specific survival in cN0 patients had improved since the introduction of SNB. However, we did not observe an improvement in 5-year relative survival, and even an increase in mortality in patients with penile cancer was obtained in this study. This result might account for the relatively low referral rate to hospitals specializing in the treatment of penile cancer, or the improvement of penile cancer treatment had not been fully implemented in hospitals.
For the management of penile cancer, several European countries have centralized management of penile cancer. The interval between diagnosis and treatment was significantly shortened, and compliance with the guidelines for patients with penile cancer was improved through this method (36, 37). Notably, the major delay in diagnosis of penile cancer was the time between the first symptom and diagnostic confirmation considering that patients and doctors might misinterpret the symptoms of penile cancer as condyloma, benign phimosis, or benign skin disease. This centralized management strategy could shorten this interval. In addition, this strategy was proved to work in improving survival and reducing mortality in the long run. Verhoeven et al. compared the 5-year relative survival rate of penile cancer patients between Europe and the United States over 1985–2007, and they found an increase from 65% to 70% and a decrease from 72% to 63% in the 5-year relative survival rate for Europe and the United States, respectively (38). For Norway and Denmark, the 5-year relative survival increased from 61% to 80% and 63% to 74%, respectively (6). However, the United States had not fully adopted this centralized management system, which might be an important explanation for the condition.
Another possible explanation for this result was that the main population of penile cancer patients was aging. For example, previous studies showed that the most common age of penile cancer patients was between 50 and 70 years (29, 39). However, patients aged 75+ years were the main population age group in our study. A higher proportion of elderly patients might lead to higher mortality and poor survival in penile cancer patients.
This is the first study that comprehensively explored the epidemiology of a rare disease from incidence, IBM, and 5-year relative survival for the period of 2000 to 2018 in the United States. The condition of penile cancer patients seemed to not have a noticeable improvement and progression considering the increasing IBM and the lack of significant change in the 5-year relative survival rate. Multiple comprehensive factors like changes in treatment and demographics, increase in exposure to HPV, and variation of cancer should be considered when interpreting results (22, 23, 40).
Several limitations should be noted when interpreting the results of this study. First, we selected a data list that collected epidemic information of approximately 26.4% of the U.S. population. Meanwhile, a relatively shorter study period was also chosen compared to previous similar studies. In addition, except for the low case numbers resulting in high standard errors of incidence, IBM, and survival estimates, essential pieces of information such as HPV infection, smoking, diagnosis, and follow-up treatment were not obtained in the SEER database. Finally, similar to the limitations of most epidemiological studies, our study has revealed a phenomenon in a period but cannot provide a definite explanation for the condition (6, 23, 38, 41). Therefore, more evidence was needed to explain these results.
The current study, using population-level data from the SEER database, provides valuable data on penile cancer. It shows an increasing trend in IBM and no significant improvement in the 5-year relative survival rate among penile cancer patients for the period of 2000 to 2018 in the United States. Meanwhile, the incidence of penile cancer exhibited a relatively stable trend during the study period. These results indicate the lack of significant progress in the treatment and management of penile cancer patients in the United States in recent decades. More efforts, like increasing awareness among the general population and doctors, and centralized management, may be needed in the future to improve the survival of this rare disease.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
The data from SEER is publicly available and de-identified. This study was approved by the institution of the First Affiliated Hospital of Nanchang University. No informed consent was needed.
XD, YL and XZ contributed equally to this work. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This study was supported by the National Natural Science Foundation of China (Grant Nos. 81560419, 81960512, and 81760457) and Jiangxi Provincial “Double Thousand Plan” Fund Project (Grant No. jxsq2019201027).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Ferlay J, Laversanne M, Brewster DH, Gombe Mbalawa C, Kohler B, et al. Cancer Incidence in Five Continents: Inclusion Criteria, Highlights From Volume X and the Global Status of Cancer Registration. Int J Cancer (2015) 137(9):2060–71. doi: 10.1002/ijc.29670
2. Hernandez BY, Barnholtz-Sloan J, German RR, Giuliano A, Goodman MT, King JB, et al. Burden of Invasive Squamous Cell Carcinoma of the Penis in the United States, 1998-2003. Cancer (2008) 113(10 Suppl):2883–91. doi: 10.1002/cncr.23743
3. Maiche AG. Epidemiological Aspects of Cancer of the Penis in Finland. Eur J Cancer Prev (1992) 1(2):153–8. doi: 10.1097/00008469-199202000-00008
4. Misra S, Chaturvedi A, Misra NC. Penile Carcinoma: A Challenge for the Developing World. Lancet Oncol (2004) 5(4):240–7. doi: 10.1016/S1470-2045(04)01427-5
5. de Souza DL, Curado MP, Bernal MM, Jerez-Roig J, Boffetta P. Mortality Trends and Prediction of HPV-Related Cancers in Brazil. Eur J Cancer Prev (2013) 22(4):380–7. doi: 10.1097/CEJ.0b013e32835b6a43
6. Bray F, Klint A, Gislum M, Hakulinen T, Engholm G, Tryggvadottir L, et al. Trends in Survival of Patients Diagnosed With Male Genital Cancers in the Nordic Countries 1964-2003 Followed Up Until the End of 2006. Acta Oncol (2010) 49(5):644–54. doi: 10.3109/02841860903575315
7. Rubin MA, Kleter B, Zhou M, Ayala G, Cubilla AL, Quint WG, et al. Detection and Typing of Human Papillomavirus DNA in Penile Carcinoma: Evidence for Multiple Independent Pathways of Penile Carcinogenesis. Am J Pathol (2001) 159(4):1211–8. doi: 10.1016/S0002-9440(10)62506-0
8. Heideman DA, Waterboer T, Pawlita M, Delis-van Diemen P, Nindl I, Leijte JA, et al. Human Papillomavirus-16 is the Predominant Type Etiologically Involved in Penile Squamous Cell Carcinoma. J Clin Oncol (2007) 25(29):4550–6. doi: 10.1200/JCO.2007.12.3182
9. Bleeker MC, Heideman DA, Snijders PJ, Horenblas S, Dillner J, Meijer CJ. Penile Cancer: Epidemiology, Pathogenesis and Prevention. World J Urol (2009) 27(2):141–50. doi: 10.1007/s00345-008-0302-z
10. Dillner J, von Krogh G, Horenblas S, Meijer CJ. Etiology of Squamous Cell Carcinoma of the Penis. Scandinavian J Urol Nephrol Supplementum (2000) 205):189–93. doi: 10.1080/00365590050509913
11. Graafland NM, Verhoeven RH, Coebergh JW, Horenblas S. Incidence Trends and Survival of Penile Squamous Cell Carcinoma in the Netherlands. Int J Cancer (2011) 128(2):426–32. doi: 10.1002/ijc.25355
12. Barnholtz-Sloan JS, Maldonado JL, Pow-sang J, Giuliano AR. Incidence Trends in Primary Malignant Penile Cancer. Urol Oncol (2007) 25(5):361–7. doi: 10.1016/j.urolonc.2006.08.029
13. Chu KC, Miller BA, Feuer EJ, Hankey BF. A Method for Partitioning Cancer Mortality Trends by Factors Associated With Diagnosis: An Application to Female Breast Cancer. J Clin Epidemiol (1994) 47(12):1451–61. doi: 10.1016/0895-4356(94)90089-2
14. Guo F, Kuo YF, Shih YCT, Giordano SH, Berenson AB. Trends in Breast Cancer Mortality by Stage at Diagnosis Among Young Women in the United States. Cancer (2018) 124(17):3500–9. doi: 10.1002/cncr.31638
15. Ederer F, Axtell LM, Cutler SJ. The Relative Survival Rate: A Statistical Methodology. Natl Cancer Institute Monograph (1961) 6:101–21.
16. Joinpoint Regression Program, Version 4.6.0.0 -April 2018; Statistical Methodology and Applications Branch, Surveillance Research Program. National Cancer Institute.
17. Baldur-Felskov B, Hannibal CG, Munk C, Kjaer SK. Increased Incidence of Penile Cancer and High-Grade Penile Intraepithelial Neoplasia in Denmark 1978-2008: A Nationwide Population-Based Study. Cancer Causes Control (2012) 23(2):273–80. doi: 10.1007/s10552-011-9876-7
18. Daubisse-Marliac L, Colonna M, Tretarre B, Defossez G, Molinie F, Jehannin-Ligier K, et al. Long-Term Trends in Incidence and Survival of Penile Cancer in France. Cancer Epidemiol (2017) 50(Pt A):125–31. doi: 10.1016/j.canep.2017.08.014
19. Pukkala E, Weiderpass E. Socio-Economic Differences in Incidence Rates of Cancers of the Male Genital Organs in Finland, 1971-95. Int J Cancer (2002) 102(6):643–8. doi: 10.1002/ijc.10749
20. Backes DM, Kurman RJ, Pimenta JM, Smith JS. Systematic Review of Human Papillomavirus Prevalence in Invasive Penile Cancer. Cancer Causes Control (2009) 20(4):449–57. doi: 10.1007/s10552-008-9276-9
21. Larke NL, Thomas SL, dos Santos Silva I, Weiss HA. Male Circumcision and Penile Cancer: A Systematic Review and Meta-Analysis. Cancer Causes Control (2011) 22(8):1097–110. doi: 10.1007/s10552-011-9785-9
22. Schoffer O, Neumann A, Stabenow R, Schulein S, Bohm WD, Gonsior A, et al. Penile Cancer - Incidence, Mortality, and Survival in Saxony, Germany. Urol Oncol (2019) 37(4):295 e1– e8. doi: 10.1016/j.urolonc.2018.12.003
23. Hansen BT, Orumaa M, Lie AK, Brennhovd B, Nygard M. Trends in Incidence, Mortality and Survival of Penile Squamous Cell Carcinoma in Norway 1956-2015. Int J Cancer (2018) 142(8):1586–93. doi: 10.1002/ijc.31194
24. American Academy of Pediatrics Task Force on C. Male Circumcision. Pediatrics (2012) 130(3):e756–85. doi: 10.1542/peds.2012-1990
25. Hakenberg OW, Drager DL, Erbersdobler A, Naumann CM, Junemann KP, Protzel C. The Diagnosis and Treatment of Penile Cancer. Dtsch Arztebl Int (2018) 115(39):646–52. doi: 10.3238/arztebl.2018.0646
26. Attalla K, Paulucci DJ, Blum K, Anastos H, Moses KA, Badani KK, et al. Demographic and Socioeconomic Predictors of Treatment Delays, Pathologic Stage, and Survival Among Patients With Penile Cancer: A Report From the National Cancer Database. Urol Oncol (2018) 36(1):14 e7– e24. doi: 10.1016/j.urolonc.2017.09.014
27. Hakenberg OW, Comperat EM, Minhas S, Necchi A, Protzel C, Watkin N. EAU Guidelines on Penile Cancer: 2014 Update. Eur Urol (2015) 67(1):142–50. doi: 10.1016/j.eururo.2014.10.017
28. Marchioni M, Berardinelli F, De Nunzio C, Spiess P, Porpiglia F, Schips L, et al. New Insight in Penile Cancer. Minerva Urol Nefrol (2018) 70(6):559–69. doi: 10.23736/S0393-2249.18.03215-0
29. Qi F, Wei X, Zheng Y, Ren X, Li X, Zhao E. Incidence Trends and Survival Outcomes of Penile Squamous Cell Carcinoma: Evidence From the Surveillance, Epidemiology and End Results Population-Based Data. Ann Transl Med (2020) 8(21):1428. doi: 10.21037/atm-20-1802
30. Arya M, Li R, Pegler K, Sangar V, Kelly JD, Minhas S, et al. Long-Term Trends in Incidence, Survival and Mortality of Primary Penile Cancer in England. Cancer Causes Control (2013) 24(12):2169–76. doi: 10.1007/s10552-013-0293-y
31. Verhoeven RH, Janssen-Heijnen ML, Saum KU, Zanetti R, Caldarella A, Holleczek B, et al. Population-Based Survival of Penile Cancer Patients in Europe and the United States of America: No Improvement Since 1990. Eur J Cancer (2013) 49(6):1414–21. doi: 10.1016/j.ejca.2012.10.029
32. Kamel MH, Bissada N, Warford R, Farias J, Davis R. Organ Sparing Surgery for Penile Cancer: A Systematic Review. J Urol (2017) 198(4):770–9. doi: 10.1016/j.juro.2017.01.088
33. McDougal WS. Advances in the Treatment of Carcinoma of the Penis. Urology (2005) 66(Suppl 5):114–7. doi: 10.1016/j.urology.2005.06.007
34. Kroon BK, Horenblas S, Nieweg OE. Contemporary Management of Penile Squamous Cell Carcinoma. J Surg Oncol (2005) 89(1):43–50. doi: 10.1002/jso.20170
35. Kroon BK, Horenblas S, Lont AP, Tanis PJ, Gallee MP, Nieweg OE. Patients With Penile Carcinoma Benefit From Immediate Resection of Clinically Occult Lymph Node Metastases. J Urol (2005) 173(3):816–9. doi: 10.1097/01.ju.0000154565.37397.4d
36. Jakobsen JK, Jensen JB. DaPeCa-2: Implementation of Fast-Track Clinical Pathways for Penile Cancer Shortens Waiting Time and Accelerates the Diagnostic Process – A Comparative Before-And-After Study in a Tertiary Referral Centre in Denmark. Scandinavian J Urol (2015) 50(1):80–7. doi: 10.3109/21681805.2015.1077472
37. Kirrander P, Sherif A, Friedrich B, Lambe M, Hakansson U. Steering Committee of the Swedish National Penile Cancer R. Swedish National Penile Cancer Register: Incidence, Tumour Characteristics, Management and Survival. BJU Int (2016) 117(2):287–92. doi: 10.1111/bju.12993
38. Koh WJ, Greer BE, Abu-Rustum NR, Apte SM, Campos SM, Chan J, et al. Uterine Neoplasms, Version 1.2014. J Natl Compr Canc Netw (2014) 12(2):248–80. doi: 10.6004/jnccn.2014.0025
39. Clark PE, Spiess PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE, et al. Penile Cancer: Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Network (2013) 11(5):594–615. doi: 10.6004/jnccn.2013.0075
40. Rippentrop JM, Joslyn SA, Konety BR. Squamous Cell Carcinoma of the Penis: Evaluation of Data From the Surveillance, Epidemiology, and End Results Program. Cancer (2004) 101(6):1357–63. doi: 10.1002/cncr.20519
Keywords: penile cancer, incidence, mortality, survival, SEER, epidemiology, trend
Citation: Deng X, Liu Y, Zhan X, Chen T, Jiang M, Jiang X, Chen L and Fu B (2022) Trends in Incidence, Mortality, and Survival of Penile Cancer in the United States: A Population-Based Study. Front. Oncol. 12:891623. doi: 10.3389/fonc.2022.891623
Received: 08 March 2022; Accepted: 18 May 2022;
Published: 17 June 2022.
Edited by:
Susan F. Slovin, Memorial Sloan Kettering Cancer Center, United StatesReviewed by:
Oliver Walther Hakenberg, University Hospital Rostock, GermanyCopyright © 2022 Deng, Liu, Zhan, Chen, Jiang, Jiang, Chen and Fu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bin Fu, dXJvZmJpbkAxNjMuY29t; Luyao Chen, Y2hlbmx1eWFvMzAxQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.