
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 07 July 2022
Sec. Breast Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.891619
This article is part of the Research Topic Diagnostic, Prognostic and Predictive Factors of Response in the Era of Precision Oncology in Breast Cancer View all 33 articles
 Jiayi Zhang1
Jiayi Zhang1 Gang Wu2
Gang Wu2 Hailong Zhu2
Hailong Zhu2 Fengyuan Yang2
Fengyuan Yang2 Shuman Yang3
Shuman Yang3 Ann M. Vuong4
Ann M. Vuong4 Jincheng Li2
Jincheng Li2 Demiao Zhu2
Demiao Zhu2 Yiyan Sun2
Yiyan Sun2 Wei Tao2*
Wei Tao2*Introduction: Epidemiological studies investigating the association between carnitine and breast cancer are scarce.
Materials and Methods: This 1:1 age-matched retrospective case-control study identified 991 female breast cancer cases and 991 female controls without breast cancer using pathological testing. We used targeted metabolomics technology to measure 16 types of whole blood carnitine compounds, such as free carnitine (C0) and octadecanoylcarnitine (C18).
Results: The average age for cases and controls was approximately 50 ± 8.7 years. After adjusting for covariates, each standard deviation (SD) increase in malonylcarnitine (C3DC; OR 0.91; 95% CI 0.83-1.00), decenoylcarnitine (C10:1; OR 0.87; 95% CI 0.79-0.96), and decadienoylcarnitine (C10:2; OR 0.90; 95% CI 0.82-0.99) level was associated with decreased odds of breast cancer. However, higher butyrylcarnitine (C4) levels were associated with increased odds of breast cancer (OR 1.12; 95% CI 1.02-1.23). No statistically significant relationship was noted between other carnitine compounds and breast cancer. The false discovery rates for C3DC, C4, C10:1 and C10:2 were 0.172, 0.120, 0.064 and 0.139, respectively.
Conclusions: Higher levels of C3DC, C10:1, and C10:2 were protective factors for breast cancer, whereas increased C4 levels were a risk factor for the disease.
Breast cancer remains the most common type of malignancy in females not only in China, but also among other countries in the world (1). The age-standardized incidence rate of breast cancer among females was 28.51 per 100,000 in 2014 in China (2). Females between the ages of 35-69 years are projected to have a higher age-standardized incidence rate for breast cancer in 2021, with a rate of 85 per 100,000 (3).
The major challenges with breast cancer survival are delayed and inaccurate diagnoses, which subsequently affect treatment timing, options, and success rates (4, 5). Identifying specific metabolites contributing to breast cancer development and diagnosis may improve prognosis. Park et al. and Rashed et al. found that plasma metabolites, including l-octanoylcarnitine, 5-oxoproline, hypoxanthine, and docosahexaenoic acid, could be potential biomarkers for diagnosing breast cancer (6, 7). Because metabolites are suggested to be related to the progression of breast cancer, they may be useful for preventing and treating breast cancer (8–11).
Carnitine is an amino acid derivative that is comprised of free carnitine and various forms of short-, medium- and long-chain acylcarnitines in its endogenous form. Carnitine has many metabolic functions (Additional file 1), including stimulating hematopoiesis, inhibiting collagen-induced platelet aggregation, preventing programmed cell death in immune cells, and modulating fatty acid oxidation (12, 13). Carnitine is also able to preserve membrane integrity (14), stabilize the physiological coenzyme A (CoASH)/acetyl-CoA ratio in the mitochondria, and reduce lactate production (12, 15). Carnitine may be involved in the pathogenesis of cancer development. There is evidence suggesting that carnitine-induced fatty acid oxidation plays a critical role in the production of NADH, FADH2, NADPH and ATP, which could contribute to the development of tumors (16–18). Carnitine palmitoyltransferase I (CPTI) was reported to be overexpressed in numerous tumors, suggesting it may play an important role in tumor neovascularization (19).
Although carnitine may be related to breast cancer development, existing epidemiological studies are scarce and those that have examined this potential relationship are limited in their sample size (6, 20). A Turkish case-control study, consisting of 58 breast cancer cases and 30 healthy controls, reported that serum carnitine levels in cases after radiotherapy were lower than in controls (20). Another Korean case-control study of 30 breast cancer cases and 16 healthy controls found that higher plasma l-octanoylcarnitine levels were associated with lower breast cancer risk (6). Thus, we used a 1:1 matched case-control study with a relatively large sample size to examine the association between circulating carnitine levels and breast cancer in females.
We recruited participants from The First Affiliated Hospital of Jinzhou Medical University located in Jinzhou, Liaoning, China. This hospital provides medical services to approximately 11.2 million people residing in west Liaoning.
The participants were recruited from the Department of Breast Surgery between November 2015 and September 2020. Eligibility criteria included the following: 1) have a clear diagnosis for either breast cancer or non-breast cancer that was confirmed with a pathological test (Additional file 2); 2) have a valid carnitine measurement; and 3) have complete and valid covariate data. Women with current or past carnitine related treatments (i.e., l-carnitine) were excluded. All controls were individually matched to cases by age ( ± 1 year) at a 1:1 ratio. This research was approved by the Institutional Review Board (IRB; Project #: 202007) at the hospital and written informed consent was obtained from all participants.
Pathological stage of diagnosis (21) and the histological tumor grades (22) for breast cancer cases were obtained. We also assessed surrogate subtypes of breast cancer; these included human epidermal growth factor receptor-2 [HER2]-positive, Luminal A-like, Luminal B-like (HER2-positive or negative), and Triple negative (22, 23).
Participants were asked to fast for at least 8 hours prior to blood collection. Nurses collected whole blood samples using vacutainer tubes (red cap) in the morning. We used dried blood filter paper to produce dried blood spot samples for carnitine assays. These samples were stored at -80 °C until assays were completed.
We punched the dried blood spot papers into 3-mm discs (24), which were extracted with ethanol and centrifuged (2 min at 1500g) to collect the supernatant. The supernatant was filtered and moved to 96-well plates. Standard carnitine (catalog number: NSK-A; Cambridge Isotope Laboratory, Tewksbury, MA) solution samples served as quality controls. These plates were dried under 50 °C pure nitrogen flow, incubated with a 1-butanol/acetyl chloride mixture, and dried again at 50°C under pure nitrogen flow. Finally, mobile phase solution (80% acetonitrile aqueous solution) was used to dissolve dried samples, which were measured with liquid chromatography coupled with mass spectrometry (high performance liquid chromatography detector LC-20A [Shimadze, Japan] and tandem mass spectrometry detection system AB Sciex 4000 QTrap [AB Sciex, Framinham MA]); this platform is suggested to be able to sensitively detect biomolecules (25). Absolute concentrations (μmol/L) of each carnitine compound was obtained by using a standard curve. We measured circulating levels of the following carnitine compounds: free carnitine [C0], acetylcarnitine [C2], propionylcarnitine [C3], malonylcarnitine [C3DC], butyrylcarnitine [C4], isovalerylcarnitine [C5], tiglylcarnitine [C5:1], hexanoylcarnitine [C6], octanoylcarnitine [C8], decanoylcarnitine [C10], decenoylcarnitine [C10:1], decadienoylcarnitine [C10:2], dodecanoylcarnitine [C12], myristoylcarnitine [C14], palmitoylcarnitine [C16], and octadecanoylcarnitine [C18].
Demographics (age and body mass index [BMI]), lifestyle factors (smoking and alcohol consumption), and medical history (age at menarche, postmenopausal status, hypertension diagnosis, type 2 diabetes diagnosis, personal history of cancer, family history of cancer, and parity) were considered as covariates. BMI was calculated using the formula: measured weight (kg)/(measured height [m])2. An individual was considered to be hypertensive if the measured systolic blood pressure was ≥140 mmHg or if the diastolic blood pressure measurement was ≥90 mmHg (26). Participants were considered to have type 2 diabetes based on standard clinical definitions using results from fasting plasma glucose testing, glucose tolerance testing, and glycated hemoglobin (A1C) testing (27). Information on age at menarche, history of cancer, smoking status, alcohol consumption, family history of cancer, postmenopausal status, and parity (subsequently categorized as 0, 1, 2, and 3+) was extracted from medical records.
Characteristics and carnitine levels were descriptively analyzed for cases and controls. We used conditional logistic regression models to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for the association between one standard deviation (SD) increase in carnitine level and breast cancer. The final conditional logistic regression models were adjusted for postmenopausal status and parity, because only these two factors were significantly associated with breast cancer at alpha = 0.05 under bivariate analysis. To address the issue of multiple testing, we also calculated the false discovery rates (FDRs) for all carnitines.
Carnitine compounds that were statistically significant in the logistic regression analysis were subsequently analyzed with multiple linear regression models to identify whether baseline characteristics are associated with circulating levels in the blood. Subgroup analyses by pathological stage of diagnosis, tumor grades, and surrogate subtypes were also performed with unconditional logistic regression models, in which breast cancer in each subgroup was the dependent variable and each carnitine compound (per 1-SD increase) was the independent variable. These models were adjusted for age, BMI, age at menarche, hypertension diagnosis, type 2 diabetes diagnosis, history of cancer, smoking status, alcohol consumption, family history of cancer, postmenopausal status, and parity. We tested for dose response by pathological stage of diagnosis and tumor grades among cases using multiple linear regression models, whereby continuous measures of carnitine (per 1-SD increase) were the independent variables, and pathological stages of diagnosis and tumor grades were dependent variables. P for interaction between surrogate subtypes and carnitine levels were tested using the interaction term (surrogate subtypes* carnitine levels [per 1-SD increase]) among cases in unconditional logistic regression models. All models were adjusted for the same covariates described above. All statistical analyses were performed using SPSS (version: 24.0; SPSS, Chicago, IL) and R (Version 3.5.3, R Foundation for Statistical Computing).
We identified 991 cases and 991 controls, with a mean age of 50.0 years (SD: 8.7 years) and 49.5 years (SD: 8.7 years), respectively (Table 1). Among cases, a total of 332 (33.5%), 481 (48.5%), 164 (16.5%), and 14 (1.4%) had a pathologically confirmed diagnosis stage of I, II, III, and IV, respectively. A majority of the cases had a tumor grade of II (61.5%), followed by grade III (12.9%) and grade 1 (5.5%). Approximately 20% of cases (n=199) did not have information on tumor grade. There were 143 (14.4%), 192 (19.3%), 155 (15.6%), and 471 (47.5%) cases with Triple negative, HER2-positive, Luminal A-like, and Luminal B-like (HER2-positive or negative) surrogate subtypes, respectively. surrogate subtype was not available for 3% of the cases. A majority of the controls (96.5%) had a benign breast lump, while others had either a mastitis (2.1%), a benign accessory breast lump (0.7%), hyperplasia of the mammary glands (0.5%), a benign axillary lump (0.1%), or a lipoma of the breast (0.1%).
Postmenopausal status and parity were significantly associated with breast cancer status (Table 1). Age, BMI, age at menarche, hypertension diagnosis, type 2 diabetes diagnosis, history of cancer, smoking status, alcohol consumption, and family history of cancer were not significantly different between cases and controls.
Levels of C3DC, C10:1 and C10:2 in cases were lower than in controls, whereas C4 levels were higher in cases than in controls (Table 1). We noted decreased odds of breast cancer with increasing levels of C3DC (OR 0.91; 95% CI 0.83-1.00), C10:1 (OR 0.87; 95% CI 0.79-0.96), and C10:2 (OR 0.90; 95% CI 0.82-0.99) (Table 2). However, higher C4 levels were associated increased risk of breast cancer (OR 1.12; 95% CI 1.02-1.23). The FDRs for C3DC, C4, C10:1, and C10:2 were 0.172, 0.120, 0.064, and 0.139, respectively. No other carnitine compounds were associated with breast cancer. The association of C3DC, C4, C10:1, and C10:2 with breast cancer did not differ with stage of diagnosis or tumor grade (all P for trend > 0.1; Figures 1–4). Subgroup analysis by surrogate subtype suggests that the impact of C4 on breast cancer was higher for individuals with the luminal B-like (HER2-positive or negative) subtype (P for interaction = 0.013; Figure 1). However, we did not observe any evidence to suggest that surrogate subtype modifies the association of C4, C10:1, or C10:2 with breast cancer (Figures 2–4).
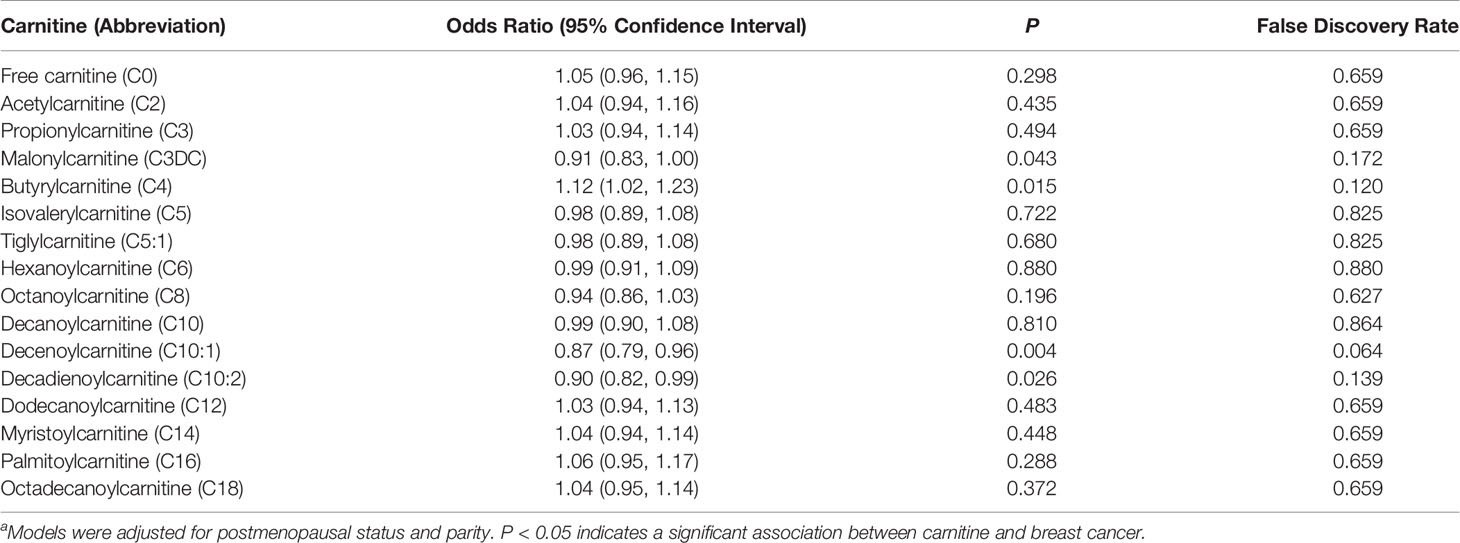
Table 2 Multivariable logistic regression analysisa of the association between carnitine levels (per 1-SD increase) and breast cancer.
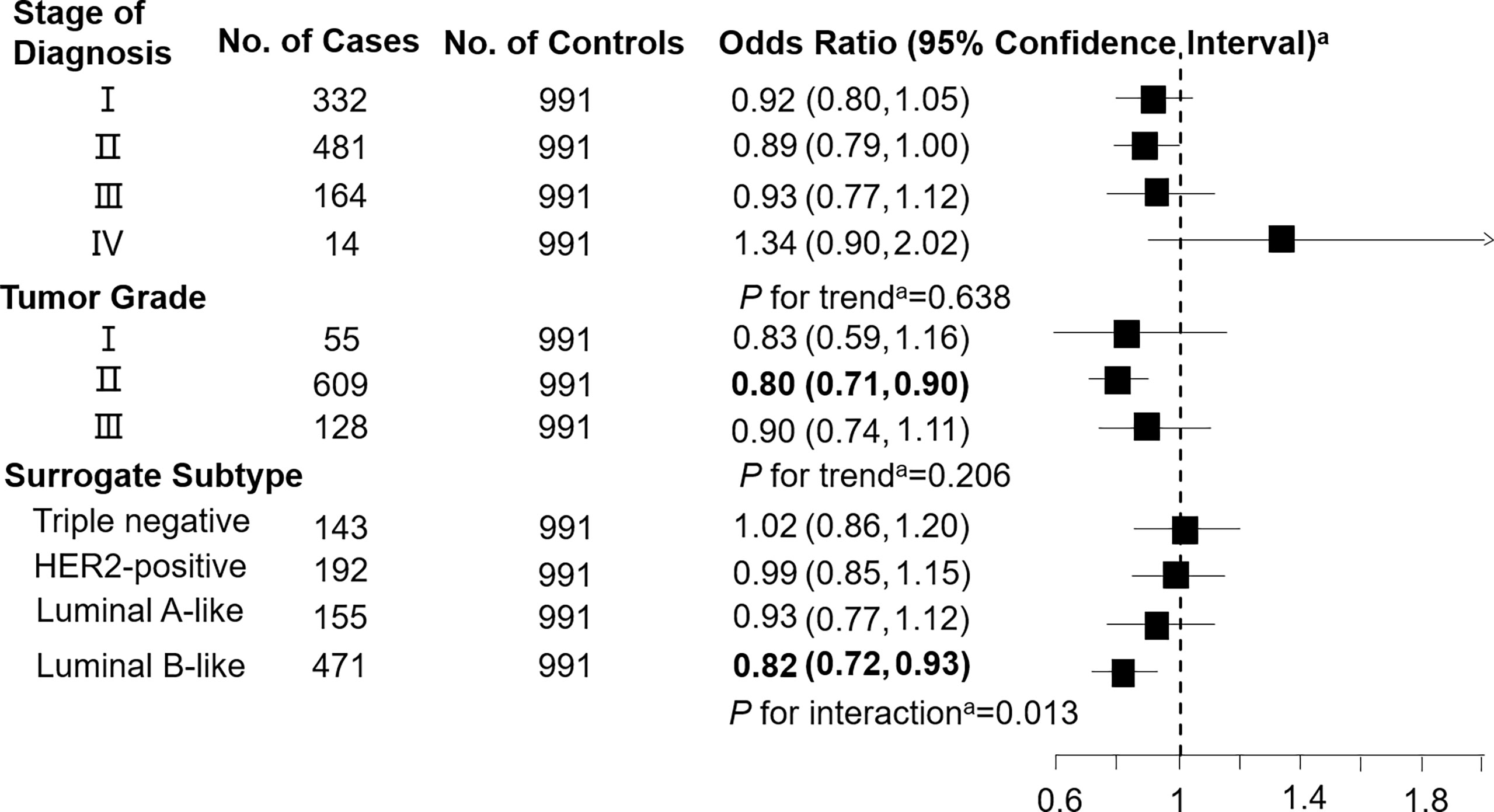
Figure 1 Association between malonylcarnitine (C3DC; per 1-SD increase) and breast cancer by pathological stage of diagnosis, tumor grade, and surrogate subtype. aAll models were adjusted for age, body mass index, age at menarche, hypertension diagnosis, type 2 diabetes diagnosis, history of cancer, smoking status, alcohol consumption, family history of cancer, postmenopausal status, and parity. Luminal B-like included HER2-positive and negative. Bold values are statistically significant at α = 0.05.
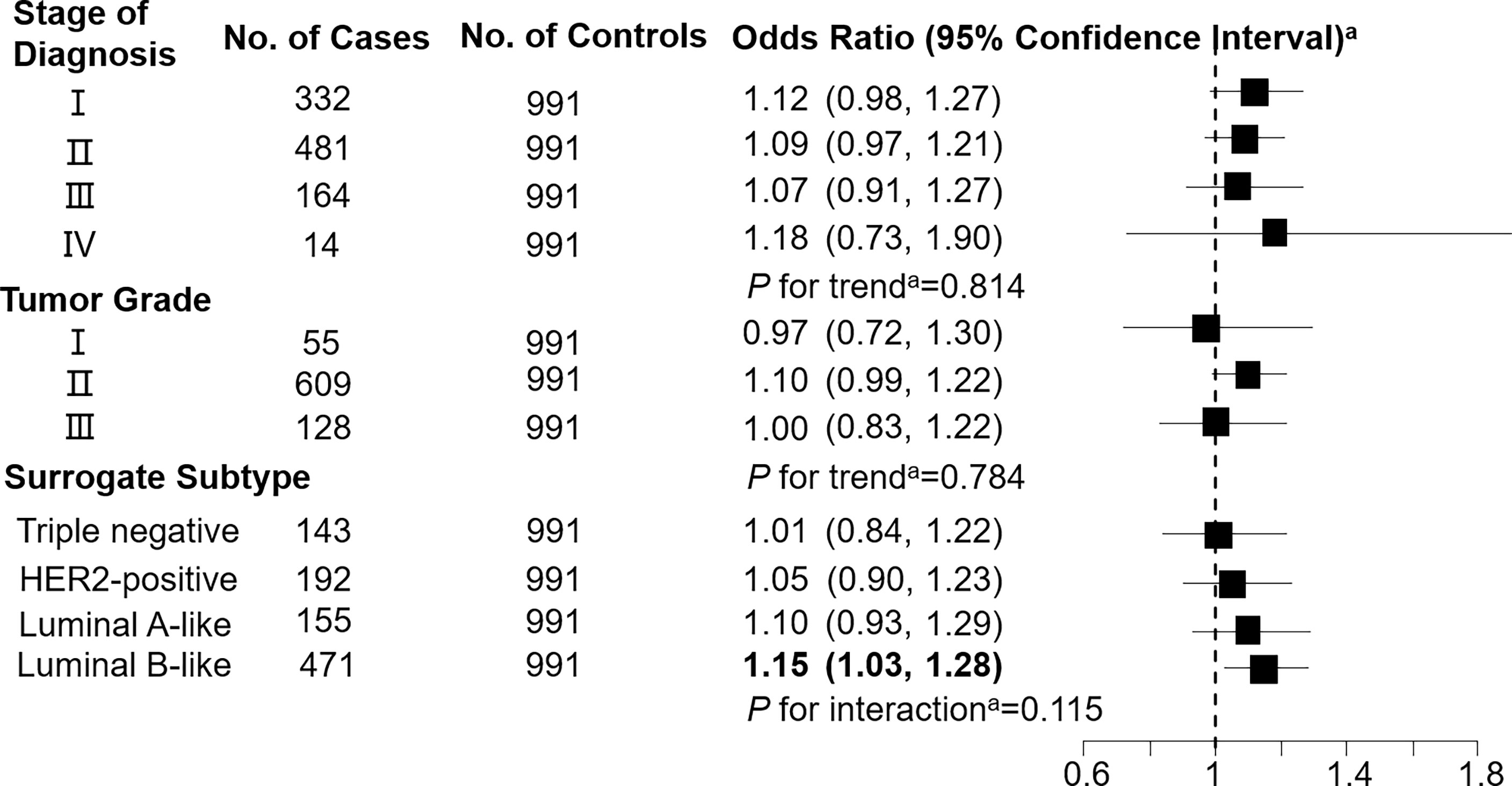
Figure 2 Association between butyrylcarnitine (C4; per 1-SD increase) and breast cancer by pathological stage of diagnosis, tumor grade, and surrogate subtype. aAll models were adjusted for age, body mass index, age at menarche, hypertension diagnosis, type 2 diabetes diagnosis, history of cancer, smoking status, alcohol consumption, family history of cancer, postmenopausal status, and parity. Luminal B-like included HER2-positive and negative. Bold values are statistically significant at α = 0.05.
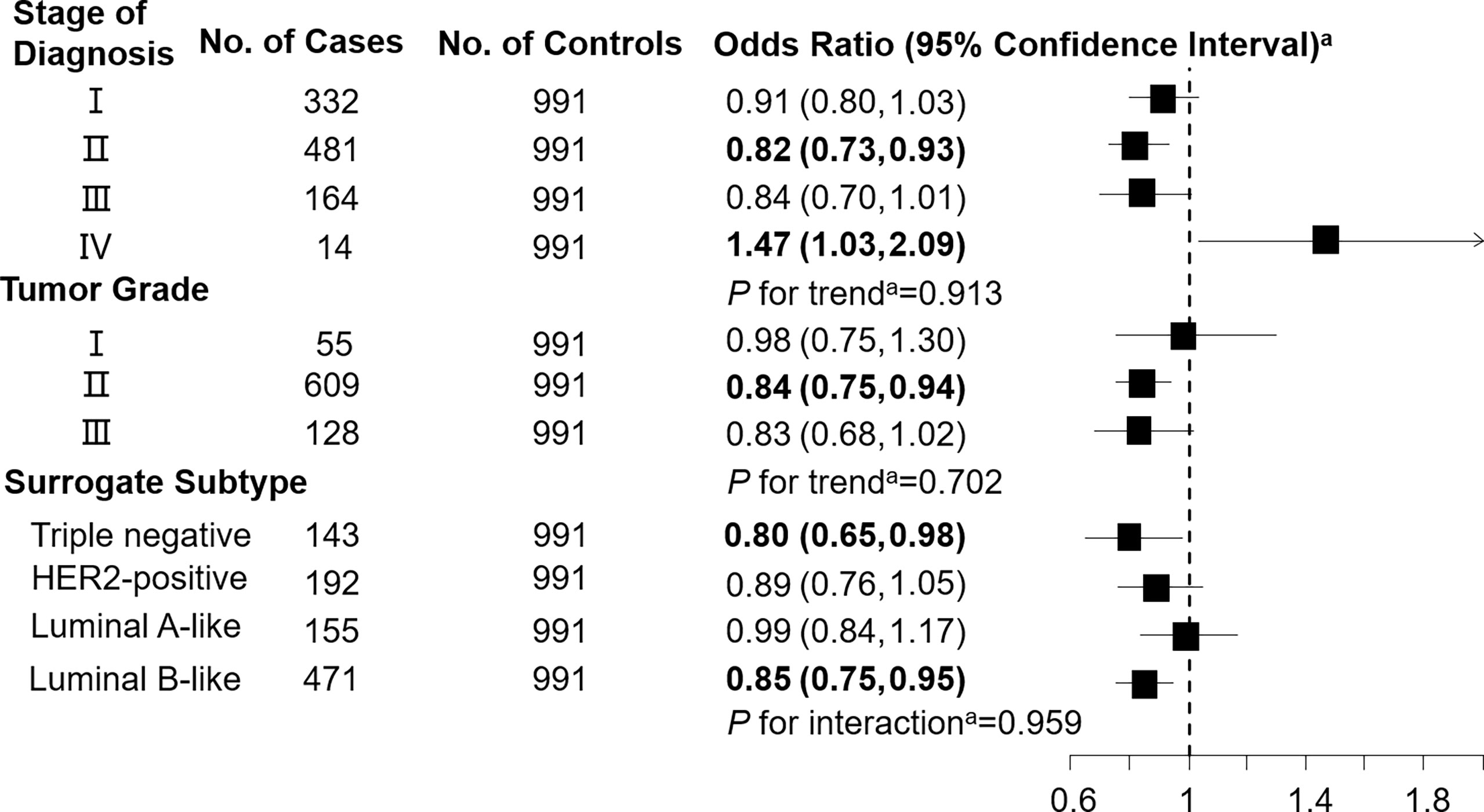
Figure 3 Association between decenoylcarnitine (C10:1; per 1-SD increase) and breast cancer by pathological stage of diagnosis, tumor grade, and surrogate subtype. aAll models were adjusted for age, body mass index, age at menarche, hypertension diagnosis, type 2 diabetes diagnosis, history of cancer, smoking status, alcohol consumption, family history of cancer, postmenopausal status, and parity. Luminal B-like included HER2-positive and negative. Bold values are statistically significant at α = 0.05.
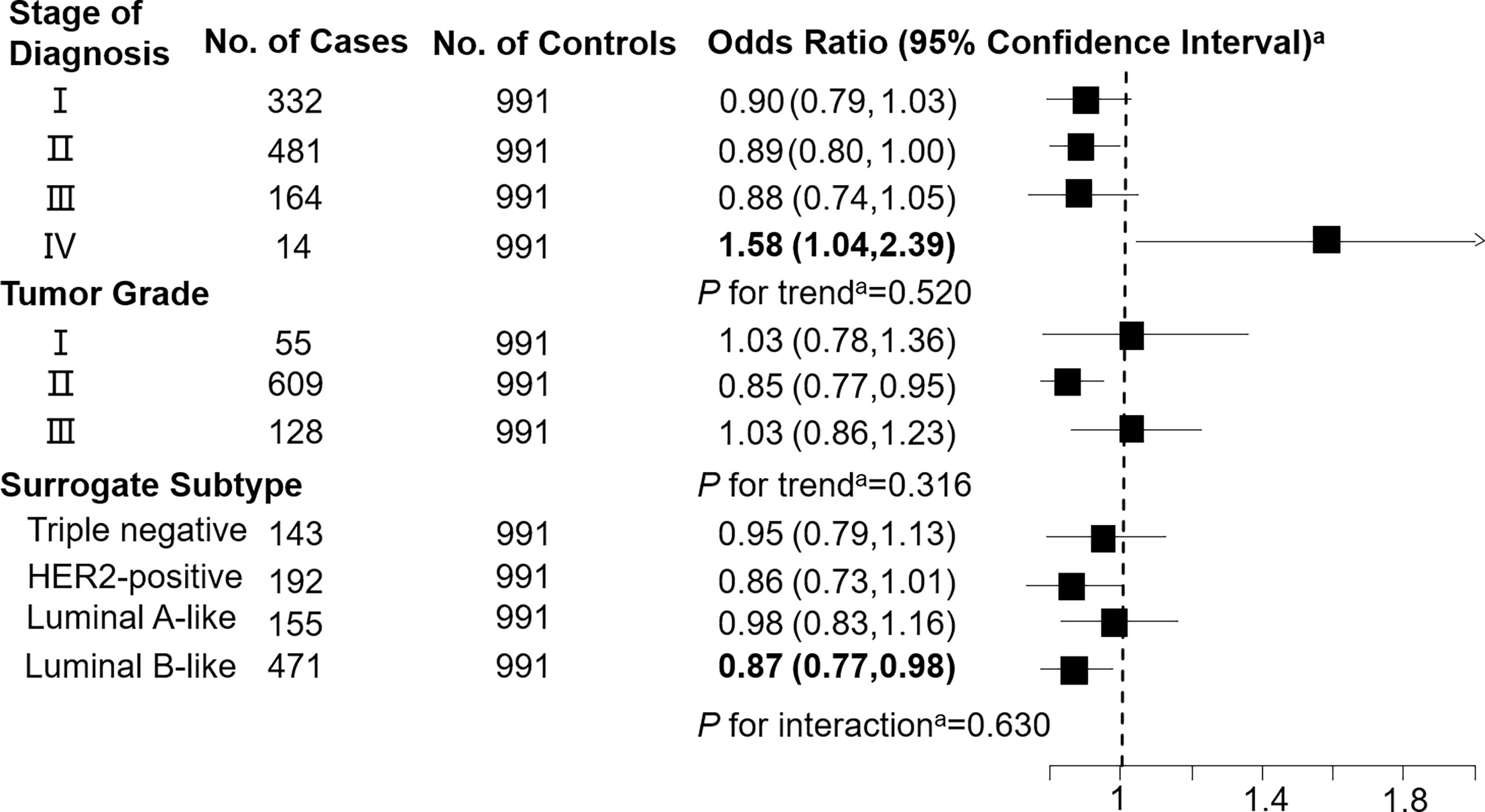
Figure 4 Association between decadienoylcarnitine (C10:2; per 1-SD increase) and breast cancer by pathological stage of diagnosis, tumor grade, and surrogate subtype. aAll models were adjusted for age, body mass index, age at menarche, hypertension diagnosis, type 2 diabetes diagnosis, history of cancer, smoking status, alcohol consumption, family history of cancer, postmenopausal status, and parity. Luminal B-like included HER2-positive and negative. Bold values are statistically significant at α = 0.05.
In the multivariable models, C3DC, C4 and C10:1 were positively associated with age among cases (Additional file 3). C3DC was inversely associated with postmenopausal status and parity. C4 was positively associated with BMI, but inversely associated with family history of cancer. However, C10:2 was positively associated with family history of cancer. C10:1 was inversely associated with postmenopausal status. With regard to controls, C3DC and C10:1 were positively associated with parity (Additional file 4). Lastly, C4 was positively associated with age, whereas no relationship was observed between baseline characteristics and C10:2.
This 1:1 individually matched case-control study found that C3DC, C4, C10:1, and C10:2 were significantly associated with increased risk of breast cancer. Stage of diagnosis, tumor grade, and surrogate subtype did not modify the association of C3DC, C10:1, and C10:2 with breast cancer. However, C4 was more strongly associated with breast cancer among individuals with a luminal B-like (HER2-positive or negative) subtype than other surrogate subtypes.
To the best of our knowledge, this is the first large epidemiological study examining the impact of 16 types of carnitine on breast cancer in females. We identified four types of carnitine, including C3DC, C4, C10:1 and C10:2, to be significantly associated with breast cancer, though the directionality of the association differed by the carnitine compound itself. In particular, we observed negative association of C3DC, C10:1, and C10:2 with breast cancer, which is partly consistent with a previous study where serum carnitine levels were lower in cases than in controls after radiotherapy (20). In contrast, a previous case-control study identified l-octanoylcarnitine as a candidate biomarker for breast cancer (6) - a conclusion that does not align with our finding of a null association between C8 and breast cancer. The underlying reasons for this discrepancy remains unclear. However, it may be partially attributed to the differences in the study population (Korean vs. Chinese), sample size (40 cases and 30 controls vs. 991 cases and 991 controls) and design (unmatched case-control study vs. matched case-control study) between the studies.
Given the high prevalence of breast cancer in females, circulating levels of C3DC, C4, C10:1, and C10:2 may have large clinical implications for breast cancer diagnosis as well as potentially risk assessment and treatment. Similar conclusions regarding study implications have also been conveyed by previous studies (6, 7, 9, 10). Further, carnitine levels can be modified via the consumption of animal-based products and l-carnitine supplements (28, 29). L-carnitine has been suggested to treat many oxidative stress related conditions, such as heart failure, angina and weight loss (30). Supplementation of specific carnitine compounds (e.g., C3DC, C10:1, and C10:2) can be potential therapeutic and/or prevention strategies for breast cancer following validation of our findings in human trials.
We report inconsistent association between various types of carnitine and breast cancer. The specific biological mechanisms for the relationships identified between C3DC, C4, C10:1, and C10:2 with breast cancer are still uncertain. It is postulated that higher levels of acylcarnitine indicate higher rates of mitochondrial fatty acid oxidation (13), which fuels breast cancer growth (16–18, 31). This mechanism is likely responsible for the positive association between C4 and breast cancer. In contrast, carnitine balances the CoASH/acetyl-CoA ratio (12, 15). A balanced CoASH/acetyl-CoA ratio removes excessive short- and medium-chain fatty acids from the mitochondria, which can potentially be toxic (32). This supports the negative association of C3DC, C10:1, and C10:2 with breast cancer. The physiological mechanism in the pathogenesis of breast cancer is likely to vary by different carnitine compounds (13).
Our study has several strengths. We had a relatively large sample size. Secondly, all cases and controls were confirmed with pathological testing, reducing concerns of outcome misclassification bias. The individually 1:1 matched case-control study design alleviates concerns of potential confounding by age. We further excluded individuals with carnitine related treatments to rule out the influence of the potential medical effects on circulating carnitine levels. We collected fasting blood samples to limit the influence of diet on carnitine measurements. Lastly, all blood samples were drawn in the morning to control for the potential impact of the circadian rhythm on carnitine levels (33).
Our findings should be interpreted within the context of a number of limitations. First, due to the case-control study design, we cannot make a causal inference about the relationship between C3DC, C4, C10:1, or C10:2 with breast cancer. Second, this study did not have data on a number of risk factors that have been identified for breast cancer, such as physical activity, hormone replacement therapy, and diethylstilbestrol use. Potential residual confounding cannot be completely ruled out. Third, a majority of the controls (96.5%) in this study had a benign breast lump. Whether similar conclusions can be drawn using healthy controls is still unclear. Our findings may not be generalizable as we used a hospital-based population, which may be influenced by selection bias. Lastly, we only investigated carnitines in this study, because the targeted approach could only measure a limited set of metabolites and funding was limited (34).
Higher levels of C3DC, C10:1, and C10:2 were found be protective factors for breast cancer. In contrast, higher C4 levels were identified as a risk factor for breast cancer. These findings substantially expand our understandings about the relationship between carnitine and breast cancer. Whether these carnitine compounds have an impact on breast cancer development requires further examination using high quality prospective cohort studies.
The datasets generated and/or analysed during the current study are not publicly available due to ethical reasons but are available from the corresponding author on reasonable request.
The studies involving human participants were reviewed and approved by This research was approved by the Institutional Review Board (IRB; Project #: 202007) at The First Affiliated Hospital of Jinzhou Medical University. The patients/participants provided their written informed consent to participate in this study.
Conception, design, and analysis (WT, SY and JZ). Interpretation of data (all authors). Drafting the article (JZ). Critically revising the article for important intellectual content (all authors). Final approval of the version to be published (all authors), and agreement to be accountable for all aspects of the work (all authors).
This work is supported by a research grant from the Jinzhou Science and Technology Bureau (Grant Number: JZ2022B039).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found onlineat: https://www.frontiersin.org/articles/10.3389/fonc.2022.891619/full#supplementary-material
1. Henley SJ, Ward EM, Scott S, Ma J, Anderson RN, Firth AU, et al. Annual Report to the Nation on the Status of Cancer, Part I: National Cancer Statistics. Cancer (2020) 126(20):2225–49. doi: 10.1002/cncr.32802
2. Jia M, Zheng R, Zhang S, Zeng H, Zou X, Chen W. Female Breast Cancer Incidence and Mortality in 2011, China. J Thorac Dis (2015) 7(7):1221–6. doi: 10.3978/j.issn.2072-1439.2015.05.15
3. Ziegler RG, Anderson WF, Gail MH. Increasing Breast Cancer Incidence in China: The Numbers Add Up. J Natl Cancer Inst (2008) 100(19):1339–41. doi: 10.1093/jnci/djn330
4. Unger-Saldana K. Challenges to the Early Diagnosis and Treatment of Breast Cancer in Developing Countries. World J Clin Oncol (2014) 5(3):465–77. doi: 10.5306/wjco.v5.i3.465
5. Borgquist S, Hall P, Lipkus I, Garber JE. Towards Prevention of Breast Cancer: What Are the Clinical Challenges? Cancer Prev Res (Phila) (2018) 11(5):255–64. doi: 10.1158/1940-6207.CAPR-16-0254
6. Park J, Shin Y, Kim TH, Kim DH, Lee A. Plasma Metabolites as Possible Biomarkers for Diagnosis of Breast Cancer. PloS One (2019) 14(12):e0225129. doi: 10.1371/journal.pone.0225129
7. Rashed R, Darwish H, Omran M, Belal A, Zahran F. A Novel Serum Metabolome Score for Breast Cancer Diagnosis. Br J BioMed Sci (2020) 77(4):196–201. doi: 10.1080/09674845.2020.1784568
8. Buentzel J, Klemp HG, Kraetzner R, Schulz M, Dihazi GH, Streit F, et al. Metabolomic Profiling of Blood-Derived Microvesicles in Breast Cancer Patients. Int J Mol Sci (2021) 22(24):13540. doi: 10.3390/ijms222413540
9. Zhao H, Shen J, Moore SC, Ye Y, Wu X, Esteva FJ, et al. Breast Cancer Risk in Relation to Plasma Metabolites Among Hispanic and African American Women. Breast Cancer Res Treat (2019) 176(3):687–96. doi: 10.1007/s10549-019-05165-4
10. Fan Y, Zhou X, Xia TS, Chen Z, Li J, Liu Q, et al. Human Plasma Metabolomics for Identifying Differential Metabolites and Predicting Molecular Subtypes of Breast Cancer. Oncotarget (2016) 7(9):9925–38. doi: 10.18632/oncotarget.7155
11. Yoo HJ, Kim M, Kim M, Kang M, Jung KJ, Hwang SM, et al. Analysis of Metabolites and Metabolic Pathways in Breast Cancer in a Korean Prospective Cohort: The Korean Cancer Prevention Study-Ii. Metabolomics (2018) 14(6):85. doi: 10.1007/s11306-018-1382-4
12. Karlic H, Lohninger A. Supplementation of L-Carnitine in Athletes: Does It Make Sense? Nutrition (2004) 20(7-8):709–15. doi: 10.1016/j.nut.2004.04.003
13. Wolf M, Chen S, Zhao X, Scheler M, Irmler M, Staiger H, et al. Production and Release of Acylcarnitines by Primary Myotubes Reflect the Differences in Fasting Fat Oxidation of the Donors. J Clin Endocrinol Metab (2013) 98(6):E1137–42. doi: 10.1210/jc.2012-3976
14. Siliprandi N, Di Lisa F, Menabo R. Clinical Use of Carnitine. Past, Present and Future. Adv Exp Med Biol (1990) 272:175–81. doi: 10.1007/978-1-4684-5826-8_11
15. Friolet R, Hoppeler H, Krahenbuhl S. Relationship Between the Coenzyme A and the Carnitine Pools in Human Skeletal Muscle at Rest and After Exhaustive Exercise Under Normoxic and Acutely Hypoxic Conditions. J Clin Invest (1994) 94(4):1490–5. doi: 10.1172/JCI117488
16. Carracedo A, Cantley LC, Pandolfi PP. Cancer Metabolism: Fatty Acid Oxidation in the Limelight. Nat Rev Cancer (2013) 13(4):227–32. doi: 10.1038/nrc3483
17. Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, et al. Inhibition of Fatty Acid Oxidation Modulates Immunosuppressive Functions of Myeloid-Derived Suppressor Cells and Enhances Cancer Therapies. Cancer Immunol Res (2015) 3(11):1236–47. doi: 10.1158/2326-6066.CIR-15-0036
18. Ricciardi MR, Mirabilii S, Allegretti M, Licchetta R, Calarco A, Torrisi MR, et al. Targeting the Leukemia Cell Metabolism by the CPT1a Inhibition: Functional Preclinical Effects in Leukemias. Blood (2015) 126(16):1925–9. doi: 10.1182/blood-2014-12-617498
19. Qu Q, Zeng F, Liu X, Wang QJ, Deng F. Fatty Acid Oxidation and Carnitine Palmitoyltransferase I: Emerging Therapeutic Targets in Cancer. Cell Death Dis (2016) 7:e2226. doi: 10.1038/cddis.2016.132
20. Ozmen HK, Erdemci B, Askin S, Sezen O. Carnitine and Adiponectin Levels in Breast Cancer After Radiotherapy. Open Med (Wars) (2017) 12:189–94. doi: 10.1515/med-2017-0028
21. Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann Surg Oncol (2010) 17(6):1471–4. doi: 10.1245/s10434-010-0985-4
22. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer. Arch Pathol Lab Med (2007) 131(1):18–43. doi: 10.1043/1543-2165(2007)131[18:ASOCCO]2.0.CO;210.5858/2007-131-18-ASOCCO
23. Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. J Oncol Pract (2010) 6(4):195–7. doi: 10.1200/JOP.777003
24. Jing F, Hu X, Cao Y, Xu M, Wang Y, Jing Y, et al. Discriminating Gastric Cancer and Gastric Ulcer Using Human Plasma Amino Acid Metabolic Profile. IUBMB Life (2018) 70(6):553–62. doi: 10.1002/iub.1748
25. Huang Q, Tan Y, Yin P, Ye G, Gao P, Lu X, et al. Metabolic Characterization of Hepatocellular Carcinoma Using Nontargeted Tissue Metabolomics. Cancer Res (2013) 73(16):4992–5002. doi: 10.1158/0008-5472.CAN-13-0308
26. Lacruz ME, Kluttig A, Hartwig S, Loer M, Tiller D, Greiser KH, et al. Prevalence and Incidence of Hypertension in the General Adult Population: Results of the CARLA-Cohort Study. Med (Baltimore) (2015) 94(22):e952. doi: 10.1097/MD.0000000000000952
27. Armstrong C. ADA Updates Standards of Medical Care for Patients With Diabetes Mellitus. Am Fam Physician (2017) 95(1):40–3. doi: 10.1002
28. Reuter SE, Evans AM. Carnitine and Acylcarnitines: Pharmacokinetic, Pharmacological and Clinical Aspects. Clin Pharmacokinet (2012) 51(9):553–72. doi: 10.2165/11633940-000000000-0000010.1007/BF03261931
29. Marx W, Teleni L, Opie RS, Kelly J, Marshall S, Itsiopoulos C, et al. Efficacy and Effectiveness of Carnitine Supplementation for Cancer-Related Fatigue: A Systematic Literature Review and Meta-Analysis. Nutrients (2017) 9(11):1224. doi: 10.3390/nu9111224
30. Pekala J, Patkowska-Sokola B, Bodkowski R, Jamroz D, Nowakowski P, Lochynski S, et al. L-Carnitine–Metabolic Functions and Meaning in Humans Life. Curr Drug Metab (2011) 12(7):667–78. doi: 10.2174/138920011796504536
31. Monaco ME. Fatty Acid Metabolism in Breast Cancer Subtypes. Oncotarget (2017) 8(17):29487–500. doi: 10.18632/oncotarget.15494
32. Pietrzak I, Opala G. [The Role of Carnitine in Human Lipid Metabolism]. Wiad Lek (1998) 51(1-2):71–5.
33. De Palo E, Gatti R, Crivellaro C, De Palo C, Scandellari C. Plasma Carnitine and Acetyl-Carnitine Levels at Different Times of the Day. Clin Physiol Biochem (1987) 5(2):95–102.
Keywords: carnitine, breast cancer, women, metabolites, risk assessment
Citation: Zhang J, Wu G, Zhu H, Yang F, Yang S, Vuong AM, Li J, Zhu D, Sun Y and Tao W (2022) Circulating Carnitine Levels and Breast Cancer: A Matched Retrospective Case-Control Study. Front. Oncol. 12:891619. doi: 10.3389/fonc.2022.891619
Received: 08 March 2022; Accepted: 09 June 2022;
Published: 07 July 2022.
Edited by:
Francesco Schettini, Institut de Recerca Biomèdica August Pi i Sunyer (IDIBAPS), SpainReviewed by:
Yaping Shao, Dalian Medical University, ChinaCopyright © 2022 Zhang, Wu, Zhu, Yang, Yang, Vuong, Li, Zhu, Sun and Tao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Tao, dGFvd2VpMjgxMUBzaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.