- 1School of Clinical Medicine, Weifang Medical University, Weifang, China
- 2Central Laboratory, Linyi People’s Hospital, Linyi, China
- 3Key Laboratory of Combined Multi-Organ Transplantation, Ministry of Public Health, First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 4Department of Hepatobiliary and Pancreatic Surgery, First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 5Linyi Key Laboratory of Tumor Biology, Linyi, China
The galectin family of proteins has high affinity with β-galactoside-containing glycans. These proteins participate in cell growth and differentiation, cell adhesion, cell signal transduction, cell apoptosis, and other cellular activities. In recent years, a large number of studies have described the expression and correlation of galectins in different tumors. Each member of the family plays a vital role in tumor growth, progression, angiogenesis, adhesion, and tumor immune escape. Studies on the roles of galectins in lymphoma have mainly involved galectin-1, -3, -7, and -9. The results suggest that galectins may become novel targets for precise tumor treatment. This article reviews current research progress regarding galectins in lymphoma and provides new ideas for exploring them as novel targets for treating lymphoma and other important medical issues.
Introduction
Lymphoma is the most common malignant tumor, originating from the lymphoid hematopoietic system (1). According to the latest global cancer statistics, the number of new cases of lymphoma worldwide in 2020 was 627,439, and the number of deaths was 283,169 (1). The main treatments for lymphoma are chemotherapy, radiotherapy, hematopoietic stem cell transplantation, molecular targeted therapy and immunotherapy (2, 3). A variety of emerging immunotherapeutic strategies, including monoclonal antibodies, antibody-drug conjugates, immunomodulatory drugs, immune checkpoint inhibitors, and CAR-T cell therapy, have been approved by the United States Food and Drug Administration for the treatment of lymphoma (3). However, improved therapies are needed.
Galectins belong to an endogenous lectin family and play important roles in cell differentiation, proliferation, apoptosis, adhesion, and migration (4). They have one or two carbohydrate recognition domains (CRDs) and high affinity for β-galactosides (5). To date, 16 members of the galectin family have been discovered and classified into three types: “proto-type“ galectins (including galectin-1, 2, 5, 7, 10, 11, 13, 14, 15,16) (6), “tandem-repeat” galectins (including galectin-4, 6, 8, 9, 12), and “chimera -type” galectin, galectin-3 (5). Galectins are widely expressed in various cells and recognize glycoconjugates containing β-galactosides on the cell surface, extracellular matrix, and intracellular vesicle cavities (4).
Galectins are expressed in various tumors; Table 1 summarizes the functions and clinical significance of these proteins in different tumors. The galectin family also plays key roles in lymphoma by promoting tumor cell growth, survival, and tumor immune escape (88). Intervention with galectin inhibitors is emerging as an attractive treatment option for lymphoma (88). In subsequent sections, we summarize the latest research on galectins in lymphoma.
Galectin-1
Galectin-1 has a molecular weight of 14.7 kDa and is encoded by the LGALS1 gene located at 22q12 (89). Galectin-1 exists and functions as a homodimer and is a typical cytoplasmic protein with an acetylated N-terminal (89). Galectin-1 is mainly expressed in the cytoplasm, shuttles between the cytoplasm and nucleus and is transferred to the cell membrane or extracellular matrix (89, 90). Galectin-1 has vital roles in tumorigenesis and tumor development. Overexpression of galectin-1 activates oncogenes, promotes the transformation of normal cells into malignant cells, and accelerates the growth and development of tumors by regulating the cell cycle (91). Galectin-1 promotes tumor migration, invasion, and angiogenesis through epithelial-mesenchymal transition (92), mediates the adhesion of tumor cells, and enhances the adhesion of cells to the extracellular matrix through glycoproteins in the basement membrane (93). Galectin-1 also accelerates the growth of tumor cells by promoting angiogenesis and the activation and proliferation of vascular endothelial cells (94).
Tumor cells weaken the function of immune cells by secreting galectin-1. This induces the tumor microenvironment to shift to the direction of immunosuppression and leads to immune escape of tumor cells (95). In addition, galectin-1 selectively reduces the viability of Th1 cells and participates in the immunosuppressive microenvironment by promoting the production of Th2 cytokines and the expansion of regulatory T cells (96).
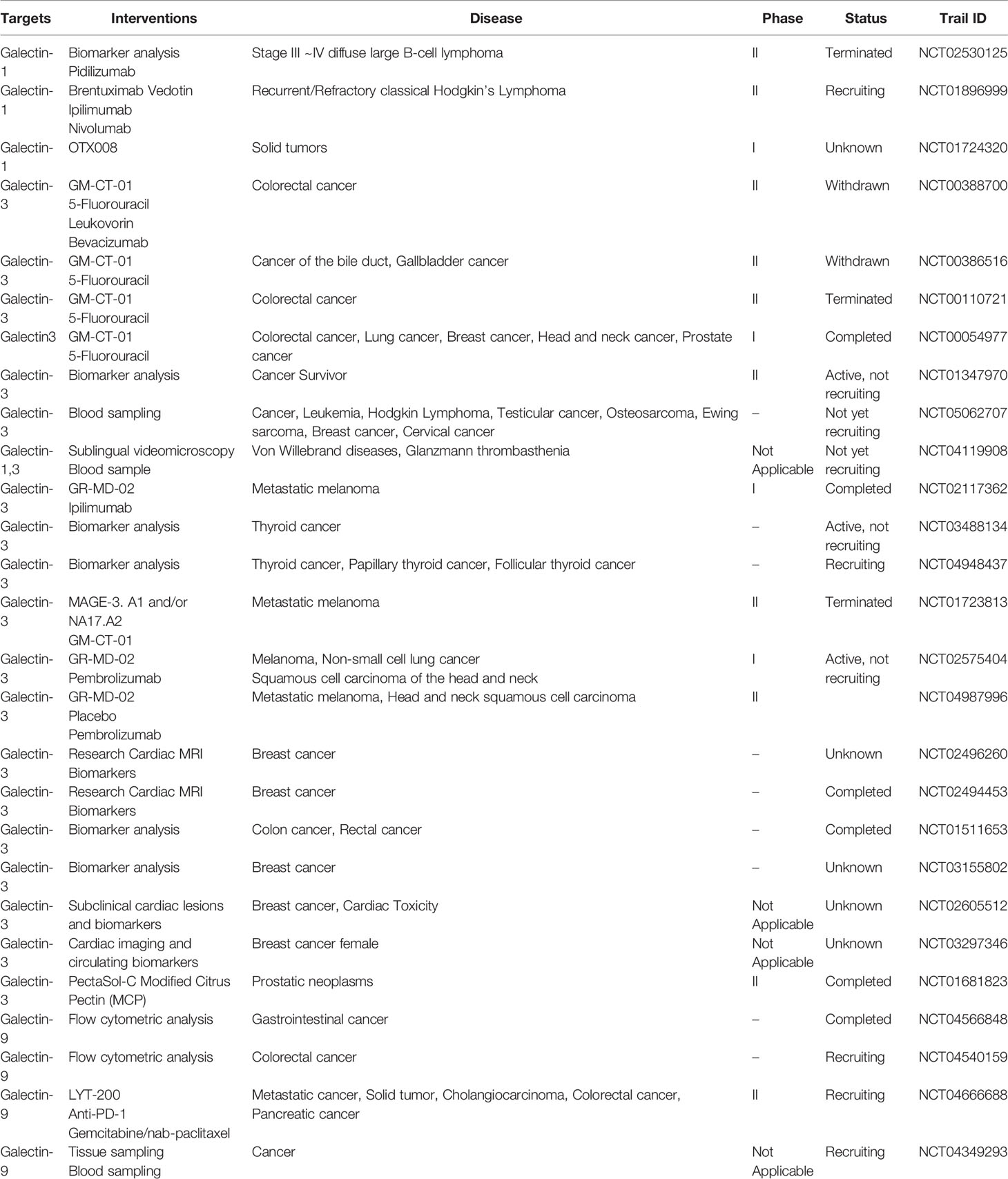
Galectin-1 is overexpressed in lymphoma and plays important roles in this cancer. A possible mechanism of action of galectin-1 in lymphoma is shown in Figure 1. Galectin-1 is overexpressed in patients with classical Hodgkin’s lymphoma (cHL), particularly in Reed-Sternberg (R-S) cells (97). It is regulated by an activator protein-1 (AP-1)-dependent enhancer. This is a construct with a GC-rich regulatory element with an AP-1-binding site on R-S cells that selectively upregulates galectin-1 expression in cHL (96). Galectin-1 overexpression in R-S cells is a negative regulator of Epstein-Barr virus-specific T cell immunity and induces R-S cells to evade immune attack in cHL (98). It was also demonstrated that serum galectin-1 levels reflect the tumor burden and adverse clinical characteristics of cHL (99). Proteomics confirmed that galectin-1 expression in the tumor microenvironment is associated with poor clinical outcomes of cHL (100). Therefore, galectin-1 may be used as a prognostic biomarker for relapsed/refractory cHL (100).
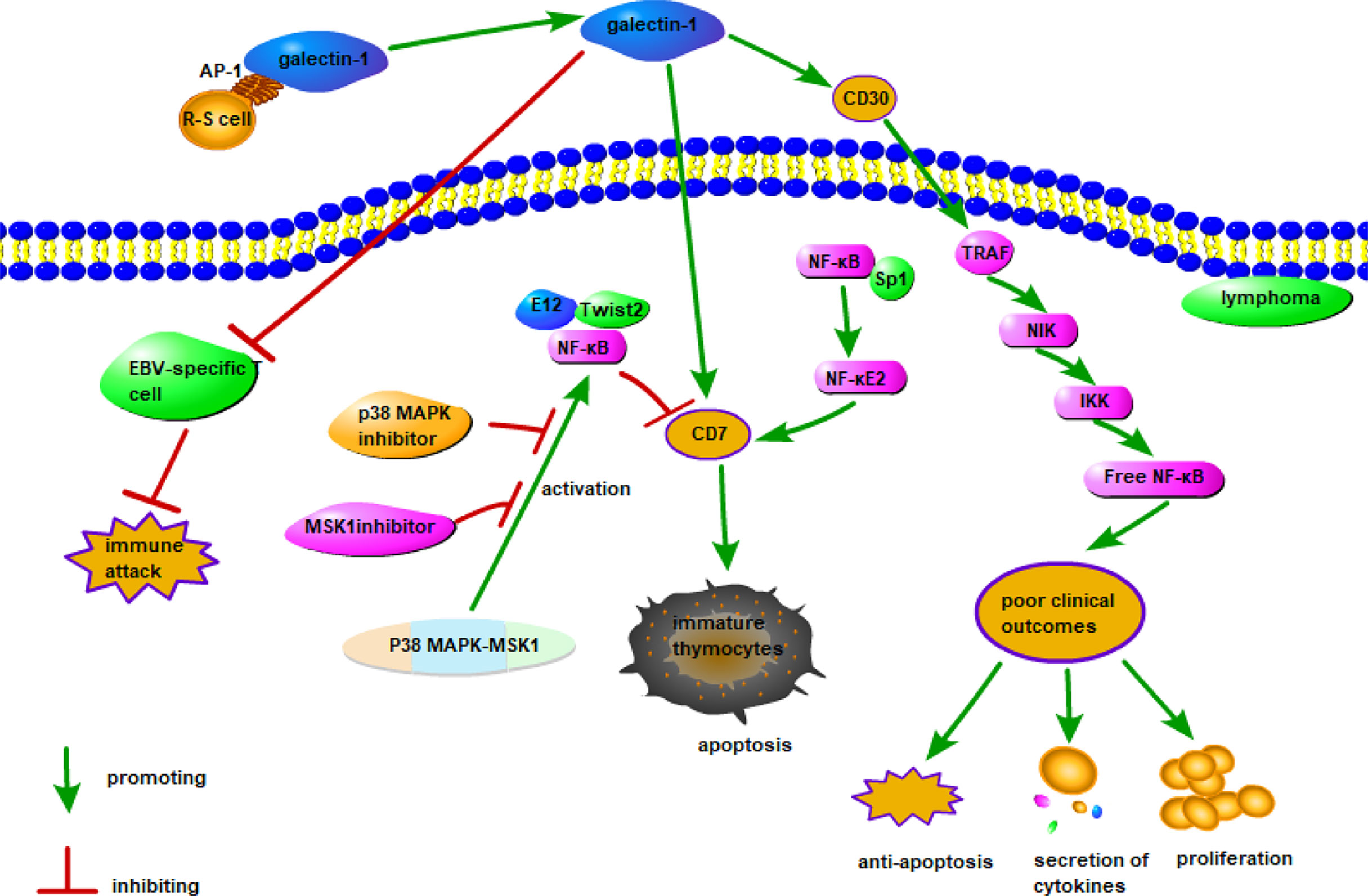
Figure 1 Possible mechanism of galectin-1 in lymphoma. The combination of AP-1 on the surface of R-S cells and galectin-1 promotes the expression of galectin-1, the combination of overexpressed galectin-1 and CD30 stimulates tumor necrosis factor–associated factor and activates the NF-κB signaling pathway to produce poor clinical outcomes. The combination of galectin-1 and CD7 induces apoptosis of immature thymocytes, the combination of NF-κB and Sp1 promotes the expression of CD7, while the combination of E12 and Twist2 with NF-κB inhibits the expression of CD7. Since the p38 MAPK-MSK1 pathway regulates CD7 expression by activating NF-κB, inhibitors of the p38 MAPK and MSK1 pathways can directly reduce CD7 expression. In addition, EBV-specific T cells binding to galectin-1 may inhibit immune attack.
Anaplastic large cell lymphoma (ALCL) overexpresses galectin-1, and the expression level is strongly correlated with c-Jun in the AP-1 transcription complex (101). Because most non-mediastinal diffuse large B-cell lymphomas (DLBCL) and mediastinal large B-cell lymphomas do not express galectin-1 and c-Jun, the combination of galectin-1 and c-Jun can be used as a diagnostic biomarker to distinguish other lymphomas with the same morphological or molecular characteristics as cHL and ALCL.
Two-thirds of cutaneous T cell lymphoma (CTCL) patients overexpress galectin-1, and this protein induces T cell apoptosis by binding to the T cell surface glycoprotein, CD7 (9). In CTCL, tumor-secreted galectin-1 inhibits the viability, proliferation, and Th1 response of non-malignant T cells and promotes the Th2 response that is conducive to tumor survival (102). Furthermore, galectin-1 is a key regulator of early CTCL keratinocyte proliferation (103). Therefore, inhibiting the secretion and expression of galectin-1 might be an effective strategy to delay the progression of CTCL (103).
A lack of CD7 expression in Sezary cells reduces their sensitivity to galectin-1-induced apoptosis and provides these cells with a survival advantage (104). It has been demonstrated that galectin-1 in the tumor microenvironment weakens the sensitivity of lymphomas to CD20 immunotherapy (105). The prognosis of peripheral T-cell lymphoma patients is significantly poor, and high intratumoral galectin-1 expression before treatment was associated with adverse outcomes in a cohort of patients with CD30+ and ALK− peripheral T-cell lymphoma (106). HIV infection reduces the expression of highly soluble galectin-1, which leads to a pro-inflammatory but ineffective T cell response that ultimately promotes HIV-associated lymphoma. However, there are different opinions regarding the role of galectin-1. In HIV-associated DLBCL, patients with a higher intratumoral galectin-1 expression level have a higher survival rate (107). In addition, galectin-1 induced the death of ALCL cells, and this effect was more obvious when combined with CD30 pre-stimulation (108). Other studies have shown that galectin-1 promoted cell death by inhibiting the activity of CD45 protein tyrosine phosphatase (109). Although galectin-1 inhibitors and antibodies have been developed (Table 2), further studies are needed to explore their clinical effectiveness.
Galectin-3
The molecular weight of galectin-3 is 29-35 kDa, and it is encoded by the LGALS3 gene located on chromosome 14 (37). Galectin-3 is the only single chimeric protein of the galectin family, consisted of three structurally distinct domains: a short amino terminal, collagen-like structures, and a COOH-terminal CRD (C-CRD) containing the NWGR anti-death motif from the CRD and B-cell lymphoma-2 (Bcl-2) family (110). Galectin-3 is a multifunctional protein mainly located in the cytoplasm. It is shuttled between the cytoplasm and nucleus and transported to the cell membrane and extracellular environment through non-classical secretory pathways (110). In the cytoplasm, galectin-3 inhibits cell apoptosis by binding to ligands including Bcl-2, CD95, and Alix/AIP1 (111). In the nucleus, galectin-3 acts as a splicing factor for pre-mRNA and functions in spliceosome assembly (111). Galectin-3 in cell membranes and the extracellular matrix mediates cell adhesion, migration, and growth by binding with its ligands (laminin and fibronectin) (112).
Galectin-3 is overexpressed in many tumors and positively correlates with the degree of tumor malignancy. It promotes the formation, progression, metastasis, and recurrence of tumors (110). Galectin-3 also suppresses tumor cell apoptosis via competing for a conserved structure with Bcl-2, suppressing cyclin, and increasing cell cycle inhibitors (113, 114). Galectin-3 regulates the phosphoinositide 3-kinase/Akt signaling pathway and enhances the activity of the anti-apoptotic factor, NF-κB (115). It stimulates early angiogenesis, accelerates the infiltration of tumor cells into the basement membrane and matrix, enhances vascular permeability, and promotes tumor cell extravasation (116). Galectin-3 also has important roles in tumor immunity. It interferes with the binding of natural killer cells to tumor cells, thereby evading the ability of natural killer cells to kill tumor cells (117). Extracellular galectin-3 binds to glycoproteins on the surface of T cells to induce T cell apoptosis (118). The possible mechanism of action of galectin-3 in lymphoma is shown in Figure 2.
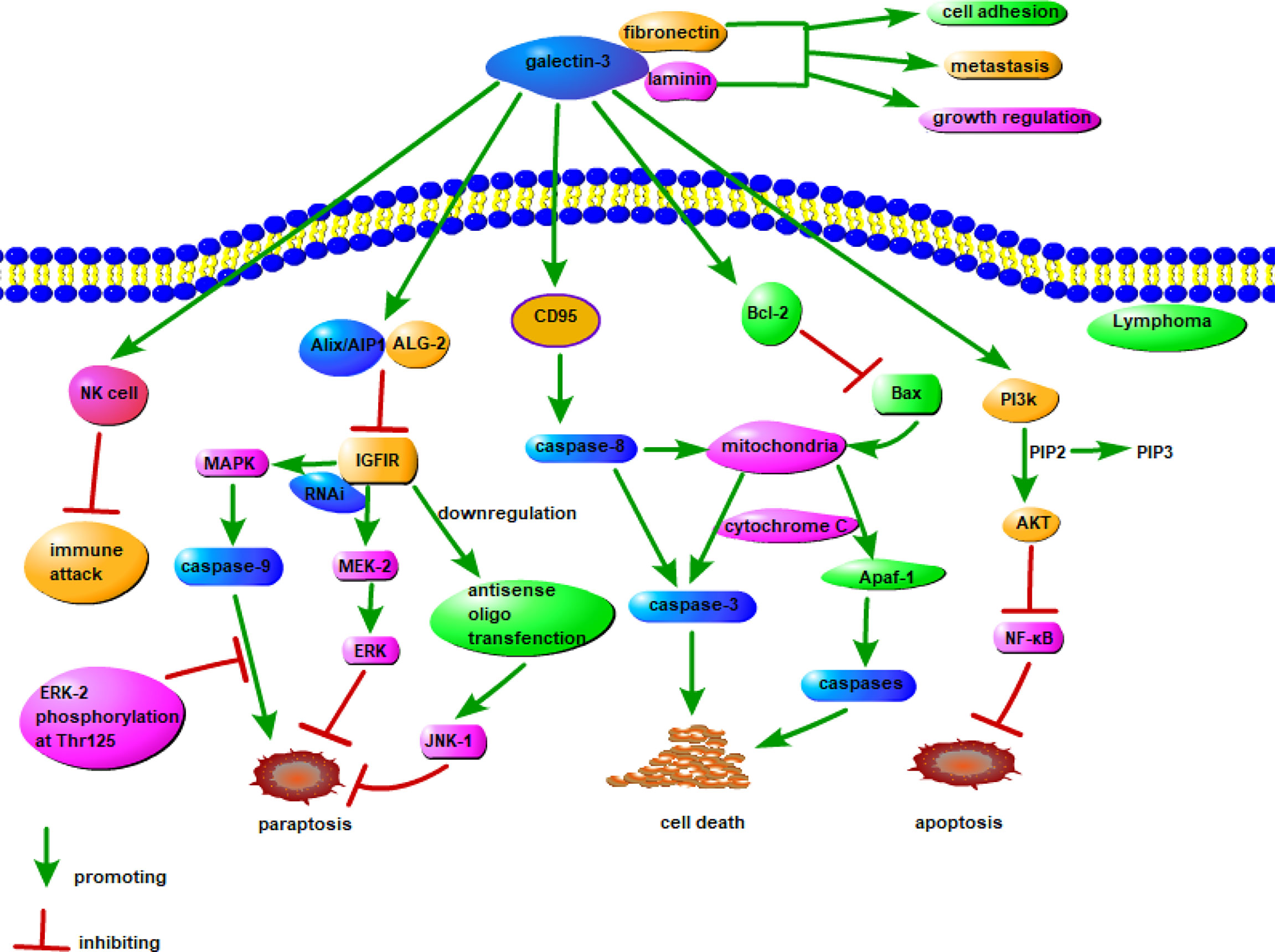
Figure 2 Possible mechanism of galectin-3 in lymphoma. Insulin-like growth factor I receptor (IGFIR) triggers the involvement of at least two signaling pathways, namely the MAPK/ERK and JNK pathways, leads to paraptosis. Both pharmacological inhibition of MAPK and downregulation of MEK-2 by RNAi, as well as downregulation of JNK1 by antisense oligo transfection, inhibites paraptosis. Among them, caspase-9 is a direct target of MAPK, and the phosphorylation of ERK-2 to Thr125 inhibits the pro-apoptotic activity of caspase-9. Galectin-3 inhibits IGFIR in combination with Alix/AIP1, thereby modulating paraptosis. The combination of galectin-3 and CD95 stimulates the activation of caspase-8 and interferes with the apoptotic signaling pathway from caspase-8 to mitochondria, and it can also combine with Bcl-2 to stimulate Bax and interfere with the apoptosis signaling pathway of mitochondrial Apaf-1. Galectin-3 can also inhibit apoptosis through PI3K/AKT/NF-κB signaling pathway. The combination of galectin-3 with NK cells may have the effect of suppressing immune attack, and the combination with fibronectin and laminin can promote tumorigenesis in lymphoma.
Gene chip detection has demonstrated that galectin-3 is expressed in DLBCL patients but not in low-grade follicular lymphoma (FL) patients, providing one of the best means to distinguish DLBCL from FL (119). Histochemical staining confirmed the high expression levels of galectin-3 in DLBCL patients, and further research showed that galectin-3 protected B cells against Fas-induced apoptosis (120). Galectin-3 is also highly expressed in patients and cell lines of primary exudative lymphoma but not Burkitt’s lymphoma, marginal zone lymphoma, and small B-cell lymphoma (120). The expression level of galectin-3 is lowest in germinal center B cells and highest in primitive B cells (CD17−/IgD+) and memory B cells (CD10−/CD27+/IgD−) (120, 121). Galectin-3 combines with 90K to form a galectin-3/90K complex that promotes cell adhesion. It was demonstrated that high levels of 90K and galectin-3 were directly related to a poor response to therapy, high invasiveness, and short survival in patients with DLBCL (122).
A tissue chip assay was used to detect the expression of galectin-3 in 259 cases of primary DLBCL. The results showed that galectin-3 was localized to several subcellular sites and cell surfaces (34). In that study, after galectin-3 glycan inhibitor GCS-100 was used to remove galectin-3 from the surface of DLBCL cells, the cells were sensitive to apoptosis induced by dextran, rituximab, and etoposide (34). An immunoprecipitation assay confirmed that CD45 was the main counterreceptor of galectin-3 on the cell surface. In addition, removing galectin-3 from cell surface CD45 enhanced the phosphorylation activity, thereby increasing the sensitivity of DLBCL cells to chemotherapeutic drug-induced death. In contrast, galectin-3 can bind to specific O-glycans on CD45, reducing tyrosine phosphatase activity and thereby having anti-apoptotic effects in DLBCL (34). Additional studies found that the anti-apoptotic activity of galectin-3 in DLBCL mainly occurred on the cell surface. One study demonstrated that galectin-3 was overexpressed in all cases of Ki-1+ ALCL and might be a potential marker of this lymphoma (123).
Mitteldorf et al. compared the expression levels of galectin-3 in primary cutaneous anaplastic large cell lymphoma and lymphoid papulosis and found no difference, except for a different localization (35). The presence of endothelial hyperplasia and overexpression of galectin-3 in endothelial cells were considered prognostic factors for a poor primary central nervous system lymphoma outcome with normal immune function (124). Interestingly, the expression levels of galectin-3 in sera of non-Hodgkin’s lymphoma patients were related to cardiovascular events, and serum galectin-3 might be a prognostic biomarker for cumulative cardiovascular events (36).
Galectin-3 is widely expressed in stromal cells of adult T cells/lymphoma (ATLL) (125). Galectin-3 binding to CD7 induced tumor cell apoptosis, while lymphoma cells resisted exogenous galectin-3-induced apoptosis, resulting in a poor prognosis in ATLL (125). Therefore, galectin-3 may be used as an indicator of poor prognosis of lymphoma. Overall, research on the function of galectin-3 in lymphoma requires further exploration.
Galectin-7
Galectin-7 has a molecular weight of 15 kDa and is encoded by the LGALS7 gene located on chromosome 19 (126). Galectin-7 is localized in the cytoplasm and nucleus and is secreted extracellularly via a non-classical secretion pathway (126). Galectin-7 has a high degree of tissue specificity, and its expression is mostly restricted to stratified epithelial cells (127). The expression of galectin-7 is regulated by a variety of transcription factors. In addition, the P53 gene induces the expression of galectin-7 in colorectal cancer (128).
Intracellular galectin-7 promotes cell apoptosis by increasing the activity of caspase-3 (129), accelerating the release of cytochrome C, and enhancing the activity of amino-terminal kinases that play important roles in maintaining epidermal homeostasis (129, 130). Galectin-7 is also involved in cell adhesion and migration and functions in wound healing, cancer progression, embryonic development, allergic inflammation, autoimmune diseases, and transplant rejection (131). Galectin-7 increases the expression levels of matrix metalloproteinase (MMP)-9, which has vital roles in tumorigenesis, metastasis, migration, and invasion via regulating extracellular signal-regulated kinase, c-Jun N-terminal kinase, and p38 mitogen activated protein kinase signaling pathways (132, 133).
Overexpression of galectin-7 inhibits the formation of new blood vessels, resulting in significant inhibition of the growth of colon cancer cells in mice (64). Galectin-7 acts similarly to galectin-1 in reducing the growth of neuroblastoma cells, without involving classical apoptosis, thereby playing a key role in spontaneous regression of neuroblastoma (54). DNA methylation induced galectin-7 and is usually related to the evolution of lymphoma cells into highly aggressive tumor cells (134). It was reported that high expression of galectin-7 in 164T2 lymphoma cells was associated with an increased recurrence rate and poor prognosis (55). Subsequent studies showed that the expression of galectin-7 was related to the DNA hypomethylation of its promoter (55).
Galectin-7 accelerates the development of lymphoma cells and increases the metastatic behavior of low metastatic lymphoma cells via MMP-9 (135). The specific mechanism of action of galectin-7 in lymphoma has not been elucidated, but based on its general role in cancer, the mechanism is summarized in Figure 3. Galectin-7 is overexpressed in mature neoplastic B-cells rather than normal B cells (136). Galectin-7 cDNA transfection significantly suppresses the dissemination and invasion of lymphoma cells and increases the survival of mice. Inhibition of galectin-7 in aggressive lymphoma cells is related to reduced invasion by tumor cells and decreased expression of MMP-9 (136). Overall, the positive regulatory effect of galectin-7 on lymphoma provides us with a new therapeutic direction. Furthermore, the ability to inhibit galectin-7 to decrease tumor invasion and metastasis may become a new therapeutic strategy for lymphoma.
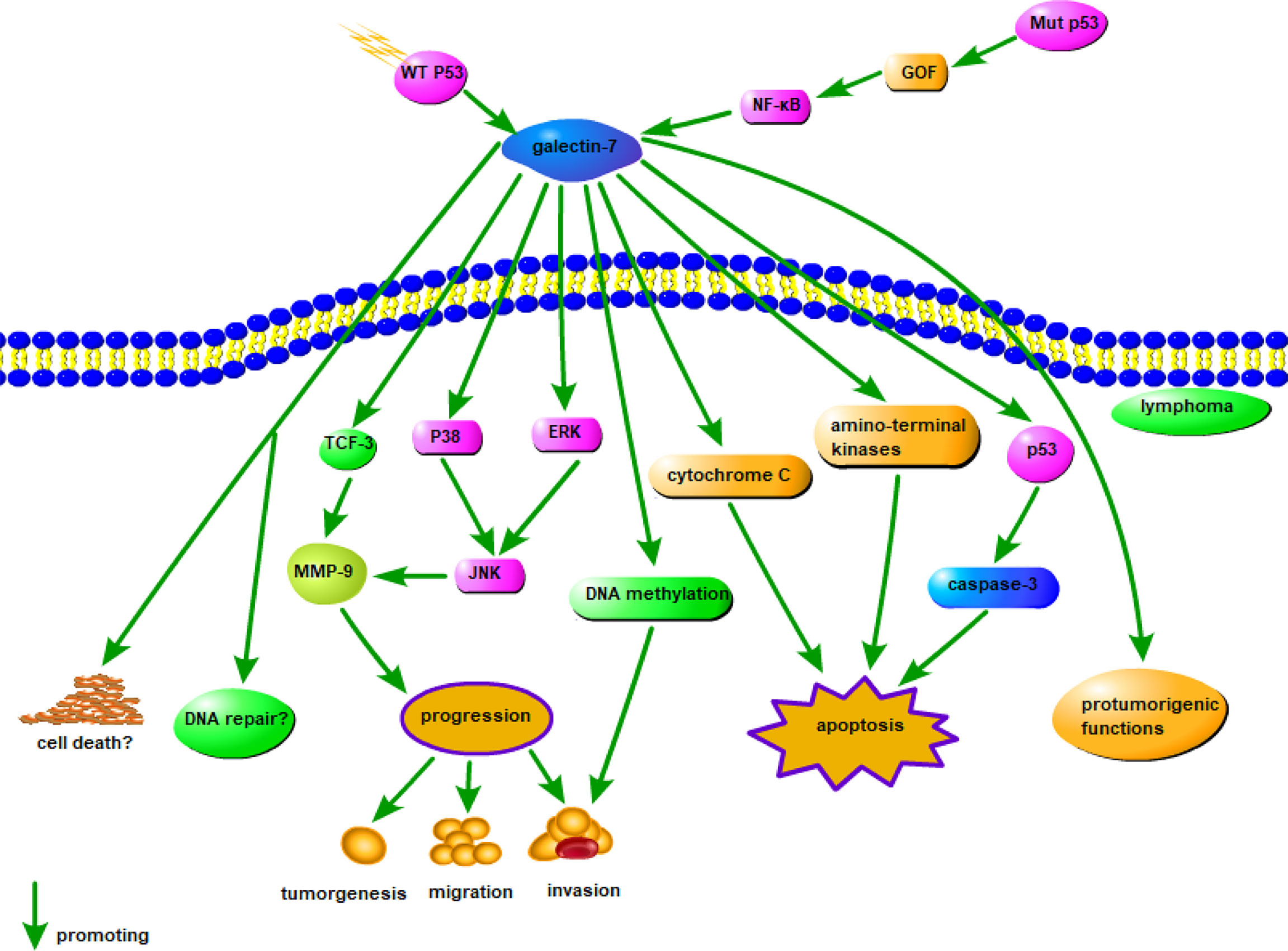
Figure 3 Possible mechanism of galectin-7 in lymphoma. MMP-9 overexpression is significantly related to the aggressive progression of lymphoma, and intracellular galectin-7 increases MMP-9 expression by TCF-3, while extracellular increases MMP-9 expression through P38, ERK, and JNK pathways. WT p53-induced galectin-7 expression induced by post-stress signaling can regulate cell death and/or DNA repair, and in cancer cells, galectin-7 can be induced by mutation p53 by a gain-of-function (GOF) mechanism, shifting balance to pro-tumor effects. In addition, DNA methylation, cytochrome C and amino-terminal kinases may also cause apoptosis by the action of galectin-7.
Galectin-9
Galectin-9 has a molecular weight of 36 kDa and is encoded by the LGALS9 gene located on chromosome 17 (137). Galectin-9 was first isolated from mouse embryonic kidney tissue in 1997 and was cloned from the tumor tissue of nodular sclerosing Hodgkin’s lymphoma (137). Galectin-9 contains two different but homologous CRDs (N-CRD and C-CRD) that differ in inducing T cell death and activating dendritic cells. The C-CRD of galectin-9 mainly determines receptor recognition and T cell death pathway signaling, while the N-CRD mainly activates dendritic cells (138). Previous studies have shown that galectin-9 is widely distributed in the liver, spleen, stomach, colon, lymph nodes, appendix, gallbladder, bone marrow, lung, and bladder and various cells, including eosinophils, epithelial cells, endothelial cells, T lymphocytes, dendritic cells, and macrophages (137).
Intra- and extracellular galectin-9 interacts with ligands to regulate biological functions. A variety of galectin-9 surface-binding ligands have been reported, such as T cell immunoglobulin mucin-3 (Tim-3), cell surface protein disulfide isomerase, CD44, 4-1BB (CD137), glucose transport protein-2, Forssman glycosphingolipid, IgE, and IgM (137, 139). When combined with its ligands, galectin-9 is implicated in the occurrence and development of various autoimmune diseases, transplant rejection, allergic diseases, infections, and tumors (137). The most characteristic ligand of galectin is Tim-3. This ligand is widely expressed on the surface of immune cells and induces Th1 and Th17 cell apoptosis after binding with galectin-9 (140). Activating the galectin-9/Tim-3 pathway suppresses the immune response by inducing the proliferation of bone marrow-derived suppressive cells and leads to the failure of T cells (141, 142). Moreover, Tim-3 plays important roles in the process of anti-programmed cell death 1 (PD1)/programmed cell death ligand 1 (PD-L1) treatment resistance (143). The galectin-9/Tim-3 signaling pathway was shown to be a key mechanism of resistance to anti-PD1 immunotherapy (77). Therefore, galectin-9/Tim-3 inhibitors may be an effective treatment to enhance the efficacy of PD1/PD-L1 antibodies.
The expression of galectin-9 is far less extensive than that of galectin-1 and galectin-3 in lymphoma. Primarily, galectin-9 is increased in patients with various infectious diseases and allergies (144). The possible mechanism of galectin-9 in lymphoma is shown in Figure 4. In ATL/ATLL, increased plasma galectin-9 level indicates the tumor burden and reflects opportunistic infections resembling the immune reconstitution inflammatory syndrome due to mogamulizumab therapy (144). Therefore, increased galectin-9 level might reflect immune-related adverse effects of lymphoma biotherapy (144).
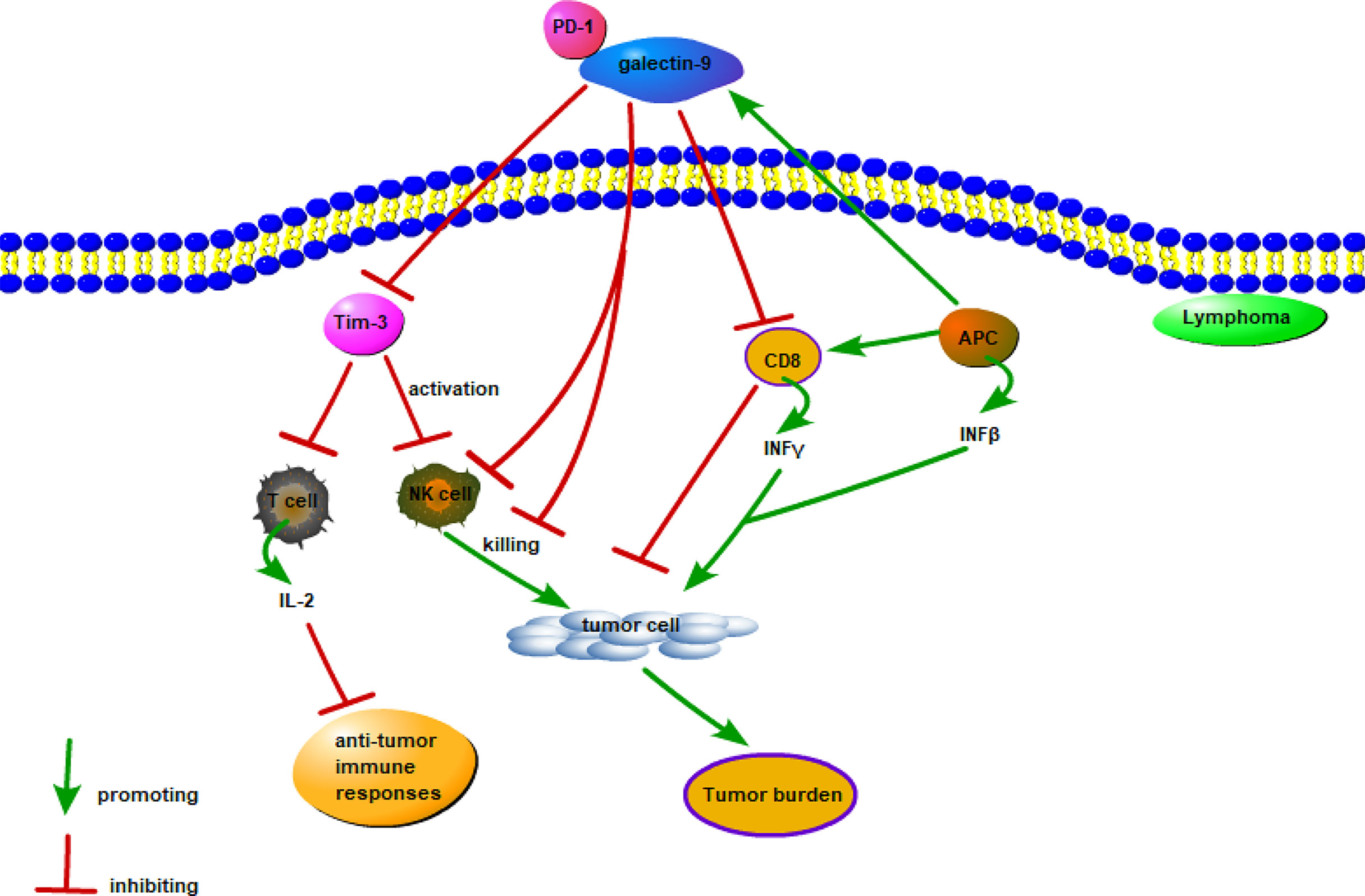
Figure 4 Possible mechanism of galectin-9 in lymphoma. Galectin-9 mainly exerts a pro-tumor effect by binding to Tim-3, Tim-3 inhibits the cytotoxic IL-2 secreted by T cells and inhibits the lethality of NK cells, and the combination of PD-1 and galectin-9 weakens the work of Gal-9/Tim-3. Interferon-induced expression and secretion of galectin-9 is a potential mechanism for tumor-acquired immune resistance. INFβ produced by APC and tumor cells and INFγ produced by activated CD8 T cells induce APC and tumor cells to express and secrete galectin-9. However, galcectin-9 induces T cell death and inhibits the anti-tumor immune response.
Galectin-9 is overexpressed on tumor cells in lesional skin of CTCL (7). The expression levels correlate with reduced CD8+ T-cell infiltration and disease severity markers (7). Galectin-9 promotes CTCL cell death via activating caspase-3 and caspase-9, which elicits apoptosis and inhibits the growth of CTCL cells (7). An anti-Tim-3 blocking antibody combined with galectin-9 strengthens the suppression of CTCL growth (7). Galectin-9/Tim-3 co-blockade has been studied extensively in other tumors (143) and may be developed as a new therapy against PD1/PD-L1-resistant lymphoma.
Conclusion
In summary, the widespread expression of galectin family proteins in tissues is inseparable from the occurrence, development, invasion, and metastasis of tumors. Importantly, the different galectin expression levels in normal and tumor tissues create the possibility of this family functioning as biomarkers for detecting cancer progression and serving as targets for improving the clinical prognosis. Overall, using galectin as a novel target provides new approaches for improving the diagnosis, treatment, and prognosis of lymphoma. Preclinical experiments have shown that inhibiting galectins effectively decreases tumor progression. However, the clinical exploration of galectin inhibitors is still in the preliminary stage, and whether they can be used in cancer treatment requires further research. Nevertheless, in recent decades, research into the roles of galectins in tumors has made significant progress and led to a number of galectin inhibitors entering clinical trials. Clinical studies investigating the use of galectin inhibitors in tumors, recognized by the National Institutes of Health (https://clinicaltrials.gov/), are shown in Table 2. These clinical studies mainly focus on the detection of biomarkers and the application of galectin inhibitors and monoclonal antibodies. However, due to the lack of clinical trials of galectin inhibitors, the efficacy and side effects of galectin inhibitors in the human body have not been systematically elucidated, so the clinical application of galectin inhibitors is challenging. In the future, further researches are needed on the role and mechanism of galectins in lymphoma and tumors, so as to provide new solutions for the treatment of lymphoma and other cancers.
Author Contributions
All authors participated in the development, writing, and editing of the review article. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by grants from the key research project program of Shandong Province (2018GSF118035), the Medical Health Science and Technology Development Plan of Shandong Province (2017–462), the Affiliated Hospital Development Fund of Xuzhou Medical University (XYFM2020016) and Zhejiang Provincial Natural Science Foundation of China (LZ22H030003).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ALCL, anaplastic large cell lymphoma; AP-1, activator protein-1; ATL/ATLL, adult T-cell leukemia/adult T cells lymphoma; Bcl-2, B-cell lymphoma-2; cHL, classical Hodgkin’s Lymphoma; c-Jun, a transcriptional regulator of leucine zipper family; CRD, carbohydrate recognition domains; CTCL, cutaneous T cell lymphoma; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; NF-κB, nuclear factor kappa-B; MMP, matrix metalloproteinase; PI3K/Akt, a proto-oncogene and a signaling pathway related to phosphatidylinositol; R-S cells, Reed-Sternberg cells; Tim-3,T cell immunoglobulin mucin-3.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Tanaka J. Recent Advances in Cellular Therapy for Malignant Lymphoma. Cytotherapy (2021) 23(8):662–71. doi: 10.1016/j.jcyt.2020.12.007
3. Neelapu SS, Adkins S, Ansell SM, Brody J, Cairo MS, Friedberg JW, et al. Society for Immunotherapy of Cancer (Sitc) Clinical Practice Guideline on Immunotherapy for the Treatment of Lymphoma. J Immunother Cancer (2020) 8(2):e001235. doi: 10.1136/jitc-2020-001235
4. Bänfer S, Jacob R. Galectins in Intra- and Extracellular Vesicles. Biomolecules (2020) 10(9):1232. doi: 10.3390/biom10091232
5. Chou FC, Chen HY, Kuo CC, Sytwu HK. Role of Galectins in Tumors and in Clinical Immunotherapy. Int J Mol Sci (2018) 19(2):430. doi: 10.3390/ijms19020430
6. Kaminker JD, Timoshenko AV. Expression, Regulation, and Functions of the Galectin-16 Gene in Human Cells and Tissues. Biomolecules (2021) 11(12):1909. doi: 10.3390/biom11121909
7. Nakajima R, Miyagaki T, Kamijo H, Oka T, Shishido-Takahashi N, Suga H, et al. Possible Therapeutic Applicability of Galectin-9 in Cutaneous T-Cell Lymphoma. J Dermatol Sci (2019) 96(3):134–42. doi: 10.1016/j.jdermsci.2019.09.004
8. Zheng Y, Feng W, Wang YJ, Sun Y, Shi G, Yu Q. Galectins as Potential Emerging Key Targets in Different Types of Leukemia. Eur J Pharmacol (2019) 844:73–8. doi: 10.1016/j.ejphar.2018.11.019
9. Wollina U, Graefe T, Feldrappe S, André S, Wasano K, Kaltner H, et al. Galectin Fingerprinting by Immuno- and Lectin Histochemistry in Cutaneous Lymphoma. J Cancer Res Clin Oncol (2002) 128(2):103–10. doi: 10.1007/s00432-001-0304-3
10. Paz H, Joo EJ, Chou CH, Fei F, Mayo KH, Abdel-Azim H, et al. Treatment of B-Cell Precursor Acute Lymphoblastic Leukemia With the Galectin-1 Inhibitor Ptx008. J Exp Clin Cancer Res CR (2018) 37(1):67. doi: 10.1186/s13046-018-0721-7
11. Juszczynski P, Rodig SJ, Ouyang J, O'Donnell E, Takeyama K, Mlynarski W, et al. Mll-Rearranged B Lymphoblastic Leukemias Selectively Express the Immunoregulatory Carbohydrate-Binding Protein Galectin-1. Clin Cancer (2010) 16(7):2122–30. doi: 10.1158/1078-0432.Ccr-09-2765
12. Luo W, Song L, Chen XL, Zeng XF, Wu JZ, Zhu CR, et al. Identification of Galectin-1 as a Novel Mediator for Chemoresistance in Chronic Myeloid Leukemia Cells. Oncotarget (2016) 7(18):26709–23. doi: 10.18632/oncotarget.8489
13. Croci DO, Morande PE, Dergan-Dylon S, Borge M, Toscano MA, Stupirski JC, et al. Nurse-Like Cells Control the Activity of Chronic Lymphocytic Leukemia B Cells Via Galectin-1. Leukemia (2013) 27(6):1413–6. doi: 10.1038/leu.2012.315
14. Kostic M, Dzopalic T, Marjanovic G, Urosevic I, Milosevic I. Immunomodulatory Effects of Galectin-1 in Patients With Chronic Lymphocytic Leukemia. Cent Eur J Immunol (2021) 46(1):54–62. doi: 10.5114/ceji.2021.105246
15. Storti P, Marchica V, Giuliani N. Role of Galectins in Multiple Myeloma. Int J Mol Sci (2017) 18(12):2740. doi: 10.3390/ijms18122740
16. Nambiar DK, Aguilera T, Cao H, Kwok S, Kong C, Bloomstein J, et al. Galectin-1-Driven T Cell Exclusion in the Tumor Endothelium Promotes Immunotherapy Resistance. J Clin Invest (2019) 129(12):5553–67. doi: 10.1172/jci129025
17. Salunkhe V, Mahajan A, Prakash N, Pradeep GL, Patil R, Gajdhar SK. Galectin-1 Expression in Oral Squamous Cell Carcinoma: An Immunohistochemical Study. J Oral Maxillofac Pathol JOMFP (2020) 24(1):186. doi: 10.4103/jomfp.JOMFP_240_19
18. Alves PM, Godoy GP, Gomes DQ, Medeiros AM, de Souza LB, da Silveira EJ, et al. Significance of Galectins-1, -3, -4 and -7 in the Progression of Squamous Cell Carcinoma of the Tongue. Pathol Res Pract (2011) 207(4):236–40. doi: 10.1016/j.prp.2011.02.004
19. Saussez S, Decaestecker C, Lorfevre F, Chevalier D, Mortuaire G, Kaltner H, et al. Increased Expression and Altered Intracellular Distribution of Adhesion/Growth-Regulatory Lectins Galectins-1 and -7 During Tumour Progression in Hypopharyngeal and Laryngeal Squamous Cell Carcinomas. Histopathology (2008) 52(4):483–93. doi: 10.1111/j.1365-2559.2008.02973.x
20. Pasmatzi E, Papadionysiou C, Monastirli A, Badavanis G, Tsambaos D. Galectin 1 in Dermatology: Current Knowledge and Perspectives. Acta Dermatovenerol Alp Pannonica Adriat (2019) 28(1):27–31. doi: 10.15570/actaapa.2019.6
21. Martínez-Bosch N, Navarro P. Galectins in the Tumor Microenvironment: Focus on Galectin-1. Adv Exp Med Biol (2020) 1259:17–38. doi: 10.1007/978-3-030-43093-1_2
22. Bacigalupo ML, Manzi M, Rabinovich GA, Troncoso MF. Hierarchical and Selective Roles of Galectins in Hepatocarcinogenesis, Liver Fibrosis and Inflammation of Hepatocellular Carcinoma. World J Gastroenterol (2013) 19(47):8831–49. doi: 10.3748/wjg.v19.i47.8831
23. Wang L, Zhao Y, Wang Y, Wu X. The Role of Galectins in Cervical Cancer Biology and Progression. BioMed Res Int (2018) 2018:2175927. doi: 10.1155/2018/2175927
24. Wu H, Song S, Yan A, Guo X, Chang L, Xu L, et al. Rack1 Promotes the Invasive Activities and Lymph Node Metastasis of Cervical Cancer Via Galectin-1. Cancer Lett (2020) 469:287–300. doi: 10.1016/j.canlet.2019.11.002
25. Sun XF, Dai SY. The Significance of Galectin-1 and Galectin-9 Expression in Endometrial Carcinoma. Gynecol Obstet Invest (2020) 85(1):34–40. doi: 10.1159/000502787
26. Langbein S, Brade J, Badawi JK, Hatzinger M, Kaltner H, Lensch M, et al. Gene-Expression Signature of Adhesion/Growth-Regulatory Tissue Lectins (Galectins) in Transitional Cell Cancer and Its Prognostic Relevance. Histopathology (2007) 51(5):681–90. doi: 10.1111/j.1365-2559.2007.02852.x
27. Wang J, Liu Y, Yang Y, Xu Z, Zhang G, Liu Z, et al. High Expression of Galectin-7 Associates With Poor Overall Survival in Patients With Non-Metastatic Clear-Cell Renal Cell Carcinoma. Oncotarget (2016) 7(27):41986–95. doi: 10.18632/oncotarget.9749
28. Shih TC, Liu R, Wu CT, Li X, Xiao W, Deng X, et al. Targeting Galectin-1 Impairs Castration-Resistant Prostate Cancer Progression and Invasion. Clin Cancer Res (2018) 24(17):4319–31. doi: 10.1158/1078-0432.Ccr-18-0157
29. Barrow H, Guo X, Wandall HH, Pedersen JW, Fu B, Zhao Q, et al. Serum Galectin-2, -4, and -8 Are Greatly Increased in Colon and Breast Cancer Patients and Promote Cancer Cell Adhesion to Blood Vascular Endothelium. Clin Cancer Res (2011) 17(22):7035–46. doi: 10.1158/1078-0432.Ccr-11-1462
30. Li H, Zhao L, Lau YS, Zhang C, Han R. Genome-Wide Crispr Screen Identifies Lgals2 as an Oxidative Stress-Responsive Gene With an Inhibitory Function on Colon Tumor Growth. Oncogene (2021) 40(1):177–88. doi: 10.1038/s41388-020-01523-5
31. Hu K, Gu Y, Lou L, Liu L, Hu Y, Wang B, et al. Galectin-3 Mediates Bone Marrow Microenvironment-Induced Drug Resistance in Acute Leukemia Cells Via Wnt/B-Catenin Signaling Pathway. J Hematol Oncol (2015) 8:1. doi: 10.1186/s13045-014-0099-8
32. Yamamoto-Sugitani M, Kuroda J, Ashihara E, Nagoshi H, Kobayashi T, Matsumoto Y, et al. Galectin-3 (Gal-3) Induced by Leukemia Microenvironment Promotes Drug Resistance and Bone Marrow Lodgment in Chronic Myelogenous Leukemia. Proc Natl Acad Sci USA (2011) 108(42):17468–73. doi: 10.1073/pnas.1111138108
33. Tarighat SS, Fei F, Joo EJ, Abdel-Azim H, Yang L, Geng H, et al. Overcoming Microenvironment-Mediated Chemoprotection Through Stromal Galectin-3 Inhibition in Acute Lymphoblastic Leukemia. Int J Mol Sci (2021) 22(22):12167. doi: 10.3390/ijms222212167
34. Clark MC, Pang M, Hsu DK, Liu FT, de Vos S, Gascoyne RD, et al. Galectin-3 Binds to Cd45 on Diffuse Large B-Cell Lymphoma Cells to Regulate Susceptibility to Cell Death. Blood (2012) 120(23):4635–44. doi: 10.1182/blood-2012-06-438234
35. Mitteldorf C, Robson A, Tronnier M, Pfaltz MC, Kempf W. Galectin-3 Expression in Primary Cutaneous Cd30-Positive Lymphoproliferative Disorders and Transformed Mycosis Fungoides. Dermatol (Basel Switzerland) (2015) 231(2):164–70. doi: 10.1159/000431313
36. Samura B. Galectin-3 As A Prognostic Biomarker In Patients With Non-Hodgkin Lymphoma. In Nino Mikaberidze, editor. Georgian Medical News. Georgia, FL: Assotsiatsiia delovoi pressy Gruzii Press (2015). p.7–11
37. Funasaka T, Raz A, Nangia-Makker P. Nuclear Transport of Galectin-3 and Its Therapeutic Implications. Semin Cancer Biol (2014) 27:30–8. doi: 10.1016/j.semcancer.2014.03.004
38. Asgarian-Omran H, Forghani P, Hojjat-Farsangi M, Roohi A, Sharifian RA, Razavi SM, et al. Expression Profile of Galectin-1 and Galectin-3 Molecules in Different Subtypes of Chronic Lymphocytic Leukemia. Cancer Invest (2010) 28(7):717–25. doi: 10.3109/07357907.2010.494319
39. Kim SJ, Chun KH. Non-Classical Role of Galectin-3 in Cancer Progression: Translocation to Nucleus by Carbohydrate-Recognition Independent Manner. BMB Rep (2020) 53(4):173–80. doi: 10.5483/BMBRep.2020.53.4.020
40. Wang L, Li YS, Yu LG, Zhang XK, Zhao L, Gong FL, et al. Galectin-3 Expression and Secretion by Tumor-Associated Macrophages in Hypoxia Promotes Breast Cancer Progression. Biochem Pharmacol (2020) 178:114113. doi: 10.1016/j.bcp.2020.114113
41. Vuong L, Kouverianou E, Rooney CM, McHugh BJ, Howie SEM, Gregory CD, et al. An Orally Active Galectin-3 Antagonist Inhibits Lung Adenocarcinoma Growth and Augments Response to Pd-L1 Blockade. Cancer Res (2019) 79(7):1480–92. doi: 10.1158/0008-5472.Can-18-2244
42. Zhang J, Deng G, Qiao L, Luo H, Liu Q, Liang N, et al. Effect of Galectin-3 on Vasculogenic Mimicry in Esophageal Cancer Cells. Oncol Lett (2019) 17(1):719. doi: 10.3892/ol.2018.9643
43. Kang HG, Kim WJ, Kang HG, Chun KH, Kim SJ. Galectin-3 Interacts With C/Ebpβ and Upregulates Hyaluronan-Mediated Motility Receptor Expression in Gastric Cancer. Mol Cancer Res MCR (2020) 18(3):403–13. doi: 10.1158/1541-7786.Mcr-19-0811
44. Dietlmeier S, Ye Y, Kuhn C, Vattai A, Vilsmaier T, Schröder L, et al. The Prostaglandin Receptor Ep2 Determines Prognosis in Ep3-Negative and Galectin-3-High Cervical Cancer Cases. Sci Rep (2020) 10(1):1154. doi: 10.1038/s41598-020-58095-3
45. Ji J, Cheng X, Wang W, Zhang J. Vitamin D Regulates Cell Viability, Migration and Proliferation by Suppressing Galectin-3 (Gal-3) Gene in Ovarian Cancer Cells. J Biosci (2020) 45:69. doi: 10.1007/s12038-020-00038-1
46. Boutas I, Kontogeorgi A, Dimitrakakis C, Kalantaridou SN. The Expression of Galectin-3 in Endometrial Cancer: A Systematic Review of the Literature. Mol Biol Rep (2021) 48(7):5699–705. doi: 10.1007/s11033-021-06536-1
47. Caputo S, Grioni M, Brambillasca CS, Monno A, Brevi A, Freschi M, et al. Galectin-3 in Prostate Cancer Stem-Like Cells Is Immunosuppressive and Drives Early Metastasis. Front Immunol (2020) 11:1820. doi: 10.3389/fimmu.2020.01820
48. Hayashi T, Saito T, Fujimura T, Hara K, Takamochi K, Mitani K, et al. Galectin-4, A Novel Predictor for Lymph Node Metastasis in Lung Adenocarcinoma. PloS One (2013) 8(12):e81883. doi: 10.1371/journal.pone.0081883
49. Shao Q, He J, Chen Z, Wu C. Prognostic Role of Galectins Expression in Patients With Hepatic Cancer: A Meta-Analysis. Medicine (2020) 99(15):e19622. doi: 10.1097/md.0000000000019622
50. Hu D, Ansari D, Zhou Q, Sasor A, Said Hilmersson K, Andersson R. Galectin 4 Is a Biomarker for Early Recurrence and Death After Surgical Resection for Pancreatic Ductal Adenocarcinoma. Scand J Gastroenterol (2019) 54(1):95–100. doi: 10.1080/00365521.2018.1561937
51. Michalak M, Warnken U, Schnölzer M, Gabius HJ, Kopitz J. Detection of Malignancy-Associated Phosphoproteome Changes in Human Colorectal Cancer Induced by Cell Surface Binding of Growth-Inhibitory Galectin-4. IUBMB Life (2019) 71(3):364–75. doi: 10.1002/iub.1987
52. Rodia MT, Solmi R, Pasini F, Nardi E, Mattei G, Ugolini G, et al. Lgals4, Ceacam6, Tspan8, and Col1a2: Blood Markers for Colorectal Cancer-Validation in a Cohort of Subjects With Positive Fecal Immunochemical Test Result. Clin Colorect Cancer (2018) 17(2):e217–28. doi: 10.1016/j.clcc.2017.12.002
53. Ding Y, Cao Q, Wang C, Duan H, Shen H. Lgals4 as a Prognostic Factor in Urothelial Carcinoma of Bladder Affects Cell Functions. Technol Cancer Res Treat (2019) 18:1533033819876601. doi: 10.1177/1533033819876601
54. Kopitz J, André S, von Reitzenstein C, Versluis K, Kaltner H, Pieters RJ, et al. Homodimeric Galectin-7 (P53-Induced Gene 1) Is a Negative Growth Regulator for Human Neuroblastoma Cells. Oncogene (2003) 22(40):6277–88. doi: 10.1038/sj.onc.1206631
55. Demers M, Couillard J, Giglia-Mari G, Magnaldo T, St-Pierre Y. Increased Galectin-7 Gene Expression in Lymphoma Cells Is Under the Control of DNA Methylation. Biochem Biophys Res Commun (2009) 387(3):425–9. doi: 10.1016/j.bbrc.2009.07.015
56. Tzeng SF, Tsai CH, Chao TK, Chou YC, Yang YC, Tsai MH, et al. O-Glycosylation-Mediated Signaling Circuit Drives Metastatic Castration-Resistant Prostate Cancer. FASEB J (2018) 32:fj201800687. doi: 10.1096/fj.201800687
57. Chen YS, Chang CW, Tsay YG, Huang LY, Wu YC, Cheng LH, et al. Hsp40 Co-Chaperone Protein Tid1 Suppresses Metastasis of Head and Neck Cancer by Inhibiting Galectin-7-Tcf3-Mmp9 Axis Signaling. Theranostics (2018) 8(14):3841–55. doi: 10.7150/thno.25784
58. Kaur M, Kaur T, Kamboj SS, Singh J. Roles of Galectin-7 in Cancer. Asian Pac J Cancer Prev APJCP (2016) 17(2):455–61. doi: 10.7314/apjcp.2016.17.2.455
59. Saussez S, Cucu DR, Decaestecker C, Chevalier D, Kaltner H, André S, et al. Galectin 7 (P53-Induced Gene 1): A New Prognostic Predictor of Recurrence and Survival in Stage Iv Hypopharyngeal Cancer. Ann Surg Oncol (2006) 13(7):999–1009. doi: 10.1245/aso.2006.08.033
60. Duray A, De Maesschalck T, Decaestecker C, Remmelink M, Chantrain G, Neiveyans J, et al. Galectin Fingerprinting in Naso-Sinusal Diseases. Oncol Rep (2014) 32(1):23–32. doi: 10.3892/or.2014.3213
61. Zhu X, Ding M, Yu ML, Feng MX, Tan LJ, Zhao FK. Identification of Galectin-7 as a Potential Biomarker for Esophageal Squamous Cell Carcinoma by Proteomic Analysis. BMC Cancer (2010) 10:290. doi: 10.1186/1471-2407-10-290
62. Higareda-Almaraz JC, Ruiz-Moreno JS, Klimentova J, Barbieri D, Salvador-Gallego R, Ly R, et al. Systems-Level Effects of Ectopic Galectin-7 Reconstitution in Cervical Cancer and Its Microenvironment. BMC Cancer (2016) 16(1):680. doi: 10.1186/s12885-016-2700-8
63. Menkhorst E, Griffiths M, Van Sinderen M, Rainczuk K, Niven K, Dimitriadis E. Galectin-7 Is Elevated in Endometrioid (Type I) Endometrial Cancer and Promotes Cell Migration. Oncol Lett (2018) 16(4):4721–8. doi: 10.3892/ol.2018.9193
64. Ueda S, Kuwabara I, Liu FT. Suppression of Tumor Growth by Galectin-7 Gene Transfer. Cancer Res (2004) 64(16):5672–6. doi: 10.1158/0008-5472.Can-04-0985
65. Matsui Y, Ueda S, Watanabe J, Kuwabara I, Ogawa O, Nishiyama H. Sensitizing Effect of Galectin-7 in Urothelial Cancer to Cisplatin Through the Accumulation of Intracellular Reactive Oxygen Species. Cancer Res (2007) 67(3):1212–20. doi: 10.1158/0008-5472.Can-06-3283
66. Ghasemi M, Vahedi Larijani L, Yazdani-Charati J, Kamali Hakim E. Reduced Expression of Galectin-8 May Contribute in Carcinogenic Pathway of Head and Neck Squamous Cell Carcinoma. Iran J Pathol (2021) 16(2):195–204. doi: 10.30699/ijp.2021.121140.2318
67. Savin S, Cvejić D, Janković M, Isić T, Paunović I, Tatić S. Evaluation of Galectin-8 Expression in Thyroid Tumors. Med Oncol (Northw Lond Engl) (2009) 26(3):314–8. doi: 10.1007/s12032-008-9122-7
68. Ferragut F, Cagnoni AJ, Colombo LL, Sánchez Terrero C, Wolfenstein-Todel C, Troncoso MF, et al. Dual Knockdown of Galectin-8 and Its Glycosylated Ligand, the Activated Leukocyte Cell Adhesion Molecule (Alcam/Cd166), Synergistically Delays in Vivo Breast Cancer Growth. Biochim Biophys Acta Mol Cell Res (2019) 1866(8):1338–52. doi: 10.1016/j.bbamcr.2019.03.010
69. Wu S, Liu H, Zhang H, Lin C, Li R, Cao Y, et al. Galectin-8 Is Associated With Recurrence and Survival of Patients With Non-Metastatic Gastric Cancer After Surgery. Tumour Biol (2016) 37(9):12635–42. doi: 10.1007/s13277-016-5175-y
70. Nagy N, Bronckart Y, Camby I, Legendre H, Lahm H, Kaltner H, et al. Galectin-8 Expression Decreases in Cancer Compared With Normal and Dysplastic Human Colon Tissue and Acts Significantly on Human Colon Cancer Cell Migration as a Suppressor. Gut (2002) 50(3):392–401. doi: 10.1136/gut.50.3.392
71. Schulz H, Kuhn C, Hofmann S, Mayr D, Mahner S, Jeschke U, et al. Overall Survival of Ovarian Cancer Patients Is Determined by Expression of Galectins-8 and -9. Int J Mol Sci (2018) 19(1):323. doi: 10.3390/ijms19010323
72. Kramer MW, Waalkes S, Serth J, Hennenlotter J, Tezval H, Stenzl A, et al. Decreased Galectin-8 Is a Strong Marker for Recurrence in Urothelial Carcinoma of the Bladder. Urol Int (2011) 87(2):143–50. doi: 10.1159/000328439
73. Gentilini LD, Jaworski FM, Tiraboschi C, Pérez IG, Kotler ML, Chauchereau A, et al. Stable and High Expression of Galectin-8 Tightly Controls Metastatic Progression of Prostate Cancer. Oncotarget (2017) 8(27):44654–68. doi: 10.18632/oncotarget.17963
74. Bidon-Wagner N, Le Pennec JP. Human Galectin-8 Isoforms and Cancer. Glycoconj J (2002) 19(7-9):557–63. doi: 10.1023/b:Glyc.0000014086.38343.98
75. Yasinska IM, Meyer NH, Schlichtner S, Hussain R, Siligardi G, Casely-Hayford M, et al. Ligand-Receptor Interactions of Galectin-9 and Vista Suppress Human T Lymphocyte Cytotoxic Activity. Front Immunol (2020) 11:580557. doi: 10.3389/fimmu.2020.580557
76. Kikushige Y, Miyamoto T, Yuda J, Jabbarzadeh-Tabrizi S, Shima T, Takayanagi S, et al. A Tim-3/Gal-9 Autocrine Stimulatory Loop Drives Self-Renewal of Human Myeloid Leukemia Stem Cells and Leukemic Progression. Cell Stem Cell (2015) 17(3):341–52. doi: 10.1016/j.stem.2015.07.011
77. Limagne E, Richard C, Thibaudin M, Fumet JD, Truntzer C, Lagrange A, et al. Tim-3/Galectin-9 Pathway and Mmdsc Control Primary and Secondary Resistances to Pd-1 Blockade in Lung Cancer Patients. Oncoimmunology (2019) 8(4):e1564505. doi: 10.1080/2162402x.2018.1564505
78. Kocibalova Z, Guzyova M, Borovska I, Messingerova L, Copakova L, Sulova Z, et al. Development of Multidrug Resistance in Acute Myeloid Leukemia Is Associated With Alterations of the Lphn1/Gal-9/Tim-3 Signaling Pathway. Cancers (2021) 13(14):3629. doi: 10.3390/cancers13143629
79. Lee BH, Park Y, Kim JH, Kang KW, Lee SJ, Kim SJ, et al. Prognostic Value of Galectin-9 Relates to Programmed Death-Ligand 1 in Patients With Multiple Myeloma. Front Oncol (2021) 11:669817. doi: 10.3389/fonc.2021.669817
80. Qi Y, Chang Y, Wang Z, Chen L, Kong Y, Zhang P, et al. Tumor-Associated Macrophages Expressing Galectin-9 Identify Immunoevasive Subtype Muscle-Invasive Bladder Cancer With Poor Prognosis But Favorable Adjuvant Chemotherapeutic Response. Cancer Immunol Immunother CII (2019) 68(12):2067–80. doi: 10.1007/s00262-019-02429-2
81. He Y, Jia K, Dziadziuszko R, Zhao S, Zhang X, Deng J, et al. Galectin-9 in Non-Small Cell Lung Cancer. Lung Cancer (Amsterdam Netherlands) (2019) 136:80–5. doi: 10.1016/j.lungcan.2019.08.014
82. Zhang L, Tian S, Pei M, Zhao M, Wang L, Jiang Y, et al. Crosstalk Between Histone Modification and DNA Methylation Orchestrates the Epigenetic Regulation of the Costimulatory Factors, Tim−3 and Galectin−9, in Cervical Cancer. Oncol Rep (2019) 42(6):2655–69. doi: 10.3892/or.2019.7388
83. Wang Y, Zhao E, Zhang Z, Zhao G, Cao H. Association Between Tim−3 and Gal−9 Expression and Gastric Cancer Prognosis. Oncol Rep (2018) 40(4):2115–26. doi: 10.3892/or.2018.6627
84. Okura R, Fujihara S, Iwama H, Morishita A, Chiyo T, Watanabe M, et al. Microrna Profiles During Galectin-9-Induced Apoptosis of Pancreatic Cancer Cells. Oncol Lett (2018) 15(1):407–14. doi: 10.3892/ol.2017.7316
85. Jafari SM, Nazri A, Shabani M, Balajam NZ, Aghaei M. Galectin-9 Induces Apoptosis in Ovcar-3 Ovarian Cancer Cell Through Mitochondrial Pathway. Res Pharm Sci (2018) 13(6):557–65. doi: 10.4103/1735-5362.245967
86. Zhou X, Sun L, Jing D, Xu G, Zhang J, Lin L, et al. Galectin-9 Expression Predicts Favorable Clinical Outcome in Solid Tumors: A Systematic Review and Meta-Analysis. Front Physiol (2018) 9:452. doi: 10.3389/fphys.2018.00452
87. Peng F, Huang Y, Li MY, Li GQ, Huang HC, Guan R, et al. Dissecting Characteristics and Dynamics of Differentially Expressed Proteins During Multistage Carcinogenesis of Human Colorectal Cancer. World J Gastroenterol (2016) 22(18):4515–28. doi: 10.3748/wjg.v22.i18.4515
88. Giordano M, Croci DO, Rabinovich GA. Galectins in Hematological Malignancies. Curr Opin Hematol (2013) 20(4):327–35. doi: 10.1097/MOH.0b013e328362370f
89. Camby I, Le Mercier M, Lefranc F, Kiss R. Galectin-1: A Small Protein With Major Functions. Glycobiology (2006) 16(11):137r–57r. doi: 10.1093/glycob/cwl025
90. Cousin JM, Cloninger MJ. The Role of Galectin-1 in Cancer Progression, and Synthetic Multivalent Systems for the Study of Galectin-1. Int J Mol Sci (2016) 17(9):1566. doi: 10.3390/ijms17091566
91. Paz A, Haklai R, Elad-Sfadia G, Ballan E, Kloog Y. Galectin-1 Binds Oncogenic H-Ras to Mediate Ras Membrane Anchorage and Cell Transformation. Oncogene (2001) 20(51):7486–93. doi: 10.1038/sj.onc.1204950
92. You X, Liu Q, Wu J, Wang Y, Dai J, Chen D, et al. Galectin-1 Promotes Vasculogenic Mimicry in Gastric Cancer by Upregulating Emt Signaling. J Cancer (2019) 10(25):6286–97. doi: 10.7150/jca.33765
93. You X, Wu J, Zhao X, Jiang X, Tao W, Chen Z, et al. Fibroblastic Galectin-1-Fostered Invasion and Metastasis Are Mediated by Tgf-B1-Induced Epithelial-Mesenchymal Transition in Gastric Cancer. Aging (2021) 13(14):18464–81. doi: 10.18632/aging.203295
94. Thijssen VL. Galectins in Endothelial Cell Biology and Angiogenesis: The Basics. Biomolecules (2021) 11(9):1386. doi: 10.3390/biom11091386
95. Laderach DJ, Compagno D. Unraveling How Tumor-Derived Galectins Contribute to Anti-Cancer Immunity Failure. Cancers (2021) 13(18):4529. doi: 10.3390/cancers13184529
96. Juszczynski P, Ouyang J, Monti S, Rodig SJ, Takeyama K, Abramson J, et al. The Ap1-Dependent Secretion of Galectin-1 by Reed Sternberg Cells Fosters Immune Privilege in Classical Hodgkin Lymphoma. Proc Natl Acad Sci USA (2007) 104(32):13134–9. doi: 10.1073/pnas.0706017104
97. Plattel WJ, Alsada ZN, van Imhoff GW, Diepstra A, van den Berg A, Visser L. Biomarkers for Evaluation of Treatment Response in Classical Hodgkin Lymphoma: Comparison of Sgalectin-1, Scd163 and Scd30 With Tarc. Br J haematol (2016) 175(5):868–75. doi: 10.1111/bjh.14317
98. Gandhi MK, Moll G, Smith C, Dua U, Lambley E, Ramuz O, et al. Galectin-1 Mediated Suppression of Epstein-Barr Virus Specific T-Cell Immunity in Classic Hodgkin Lymphoma. Blood (2007) 110(4):1326–9. doi: 10.1182/blood-2007-01-066100
99. Ouyang J, Plütschow A, Pogge von Strandmann E, Reiners KS, Ponader S, Rabinovich GA, et al. Galectin-1 Serum Levels Reflect Tumor Burden and Adverse Clinical Features in Classical Hodgkin Lymphoma. Blood (2013) 121(17):3431–3. doi: 10.1182/blood-2012-12-474569
100. Kamper P, Ludvigsen M, Bendix K, Hamilton-Dutoit S, Rabinovich GA, Møller MB, et al. Proteomic Analysis Identifies Galectin-1 as a Predictive Biomarker for Relapsed/Refractory Disease in Classical Hodgkin Lymphoma. Blood (2011) 117(24):6638–49. doi: 10.1182/blood-2010-12-327346
101. Rodig SJ, Ouyang J, Juszczynski P, Currie T, Law K, Neuberg DS, et al. Ap1-Dependent Galectin-1 Expression Delineates Classical Hodgkin and Anaplastic Large Cell Lymphomas From Other Lymphoid Malignancies With Shared Molecular Features. Clin Cancer Res (2008) 14(11):3338–44. doi: 10.1158/1078-0432.Ccr-07-4709
102. Cedeno-Laurent F, Watanabe R, Teague JE, Kupper TS, Clark RA, Dimitroff CJ. Galectin-1 Inhibits the Viability, Proliferation, and Th1 Cytokine Production of Nonmalignant T Cells in Patients With Leukemic Cutaneous T-Cell Lymphoma. Blood (2012) 119(15):3534–8. doi: 10.1182/blood-2011-12-396457
103. Thode C, Woetmann A, Wandall HH, Carlsson MC, Qvortrup K, Kauczok CS, et al. Malignant T Cells Secrete Galectins and Induce Epidermal Hyperproliferation and Disorganized Stratification in a Skin Model of Cutaneous T-Cell Lymphoma. J Invest Dermatol (2015) 135(1):238–46. doi: 10.1038/jid.2014.284
104. Roberts AA, Amano M, Felten C, Galvan M, Sulur G, Pinter-Brown L, et al. Galectin-1-Mediated Apoptosis in Mycosis Fungoides: The Roles of Cd7 and Cell Surface Glycosylation. Modern Pathol (2003) 16(6):543–51. doi: 10.1097/01.Mp.0000071840.84469.06
105. Lykken JM, Horikawa M, Minard-Colin V, Kamata M, Miyagaki T, Poe JC, et al. Galectin-1 Drives Lymphoma Cd20 Immunotherapy Resistance: Validation of a Preclinical System to Identify Resistance Mechanisms. Blood (2016) 127(15):1886–95. doi: 10.1182/blood-2015-11-681130
106. Holst JM, Ludvigsen M, Hamilton-Dutoit SJ, Bendix K, Plesner TL, Nørgaard P, et al. High Intratumoural Galectin-1 Expression Predicts Adverse Outcome in Alk(-) Alcl and Cd30(+) Ptcl-Nos. Hematological Oncol (2020) 38(1):59–66. doi: 10.1002/hon.2702
107. Vase M, Ludvigsen M, Bendix K, Dutoit SH, Hjortebjerg R, Petruskevicius I, et al. Predictive Value of Galectin-1 in the Development and Progression of Hiv-Associated Lymphoma. AIDS (Lond Engl) (2017) 31(16):2311–3. doi: 10.1097/qad.0000000000001622
108. Suzuki O, Hirsch B, Abe M, Dürkop H, Stein H. Galectin-1-Mediated Cell Death Is Increased by Cd30-Induced Signaling in Anaplastic Large Cell Lymphoma Cells But Not in Hodgkin Lymphoma Cells. Lab Invest J Tech Methods Pathol (2012) 92(2):191–9. doi: 10.1038/labinvest.2011.151
109. Suzuki O, Abe M. Galectin-1-Mediated Cell Adhesion, Invasion and Cell Death in Human Anaplastic Large Cell Lymphoma: Regulatory Roles of Cell Surface Glycans. Int J Oncol (2014) 44(5):1433–42. doi: 10.3892/ijo.2014.2319
110. Nangia-Makker P, Hogan V, Raz A. Galectin-3 and Cancer Stemness. Glycobiology (2018) 28(4):172–81. doi: 10.1093/glycob/cwy001
111. Dumic J, Dabelic S, Flögel M. Galectin-3: An Open-Ended Story. Biochim Biophys Acta (2006) 1760(4):616–35. doi: 10.1016/j.bbagen.2005.12.020
112. Nangia-Makker P, Balan V, Raz A. Regulation of Tumor Progression by Extracellular Galectin-3. Cancer Microenviron (2008) 1(1):43–51. doi: 10.1007/s12307-008-0003-6
113. Nakahara S, Oka N, Raz A. On the Role of Galectin-3 in Cancer Apoptosis. Apoptosis an Int J programmed Cell Death (2005) 10(2):267–75. doi: 10.1007/s10495-005-0801-y
114. Kim HR, Lin HM, Biliran H, Raz A. Cell Cycle Arrest and Inhibition of Anoikis by Galectin-3 in Human Breast Epithelial Cells. Cancer Res (1999) 59(16):4148–54.
115. Mirandola L, Nguyen DD, Rahman RL, Grizzi F, Yuefei Y, Figueroa JA, et al. Anti-Galectin-3 Therapy: A New Chance for Multiple Myeloma and Ovarian Cancer? Int Rev Immunol (2014) 33(5):417–27. doi: 10.3109/08830185.2014.911855
116. Mammadova-Bach E, Gil-Pulido J, Sarukhanyan E, Burkard P, Shityakov S, Schonhart C, et al. Platelet Glycoprotein Vi Promotes Metastasis Through Interaction With Cancer Cell-Derived Galectin-3. Blood (2020) 135(14):1146–60. doi: 10.1182/blood.2019002649
117. Tsuboi S, Sutoh M, Hatakeyama S, Hiraoka N, Habuchi T, Horikawa Y, et al. A Novel Strategy for Evasion of Nk Cell Immunity by Tumours Expressing Core2 O-Glycans. EMBO J (2011) 30(15):3173–85. doi: 10.1038/emboj.2011.215
118. Stillman BN, Hsu DK, Pang M, Brewer CF, Johnson P, Liu FT, et al. Galectin-3 and Galectin-1 Bind Distinct Cell Surface Glycoprotein Receptors to Induce T Cell Death. J Immunol (Baltimore Md 1950) (2006) 176(2):778–89. doi: 10.4049/jimmunol.176.2.778
119. Shipp MA, Ross KN, Tamayo P, Weng AP, Kutok JL, Aguiar RC, et al. Diffuse Large B-Cell Lymphoma Outcome Prediction by Gene-Expression Profiling and Supervised Machine Learning. Nat Med (2002) 8(1):68–74. doi: 10.1038/nm0102-68
120. Hoyer KK, Pang M, Gui D, Shintaku IP, Kuwabara I, Liu FT, et al. An Anti-Apoptotic Role for Galectin-3 in Diffuse Large B-Cell Lymphomas. Am J Pathol (2004) 164(3):893–902. doi: 10.1016/s0002-9440(10)63177-x
121. D'Haene N, Maris C, Sandras F, Dehou MF, Remmelink M, Decaestecker C, et al. The Differential Expression of Galectin-1 and Galectin-3 in Normal Lymphoid Tissue and Non-Hodgkin's and Hodgkin's Lymphomas. Int J Immunopathol Pharmacol (2005) 18(3):431–43. doi: 10.1177/039463200501800304
122. Kim SJ, Lee SJ, Sung HJ, Choi IK, Choi CW, Kim BS, et al. Increased Serum 90k and Galectin-3 Expression Are Associated With Advanced Stage and a Worse Prognosis in Diffuse Large B-Cell Lymphomas. Acta Haematologica (2008) 120(4):211–6. doi: 10.1159/000193223
123. Konstantinov KN, Robbins BA, Liu FT. Galectin-3, a Beta-Galactoside-Binding Animal Lectin, Is a Marker of Anaplastic Large-Cell Lymphoma. Am J Pathol (1996) 148(1):25–30.
124. D'Haene N, Catteau X, Maris C, Martin B, Salmon I, Decaestecker C. Endothelial Hyperplasia and Endothelial Galectin-3 Expression Are Prognostic Factors in Primary Central Nervous System Lymphomas. Br J haematol (2008) 140(4):402–10. doi: 10.1111/j.1365-2141.2007.06929.x
125. Liu TY, Chen CY, Tien HF, Lin CW. Loss of Cd7, Independent of Galectin-3 Expression, Implies a Worse Prognosis in Adult T-Cell Leukaemia/Lymphoma. Histopathology (2009) 54(2):214–20. doi: 10.1111/j.1365-2559.2008.03199.x
126. Johannes L, Jacob R, Leffler H. Galectins at a Glance. J Cell Sci (2018) 131(9):jcs208884. doi: 10.1242/jcs.208884
127. St-Pierre Y. Towards a Better Understanding of the Relationships Between Galectin-7, P53 and Mmp-9 During Cancer Progression. Biomolecules (2021) 11(6):879. doi: 10.3390/biom11060879
128. Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. A Model for P53-Induced Apoptosis. Nature (1997) 389(6648):300–5. doi: 10.1038/38525
129. Kuwabara I, Kuwabara Y, Yang RY, Schuler M, Green DR, Zuraw BL, et al. Galectin-7 (Pig1) Exhibits Pro-Apoptotic Function Through Jnk Activation and Mitochondrial Cytochrome C Release. J Biol Chem (2002) 277(5):3487–97. doi: 10.1074/jbc.M109360200
130. Advedissian T, Deshayes F, Viguier M. Galectin-7 in Epithelial Homeostasis and Carcinomas. Int J Mol Sci (2017) 18(12):2760. doi: 10.3390/ijms18122760
131. Sewgobind NV, Albers S, Pieters RJ. Functions and Inhibition of Galectin-7, an Emerging Target in Cellular Pathophysiology. Biomolecules (2021) 11(11):1720. doi: 10.3390/biom11111720
132. Guo JP, Li XG. Galectin-7 Promotes the Invasiveness of Human Oral Squamous Cell Carcinoma Cells Via Activation of Erk and Jnk Signaling. Oncol Lett (2017) 13(3):1919–24. doi: 10.3892/ol.2017.5649
133. Park JE, Chang WY, Cho M. Induction of Matrix Metalloproteinase-9 by Galectin-7 Through P38 Mapk Signaling in Hela Human Cervical Epithelial Adenocarcinoma Cells. Oncol Rep (2009) 22(6):1373–9. doi: 10.3892/or_00000577
134. Moisan S, Demers M, Mercier J, Magnaldo T, Potworowski EF, St-Pierre Y. Upregulation of Galectin-7 in Murine Lymphoma Cells Is Associated With Progression Toward an Aggressive Phenotype. Leukemia (2003) 17(4):751–9. doi: 10.1038/sj.leu.2402870
135. Demers M, Magnaldo T, St-Pierre Y. A Novel Function for Galectin-7: Promoting Tumorigenesis by Up-Regulating Mmp-9 Gene Expression. Cancer Res (2005) 65(12):5205–10. doi: 10.1158/0008-5472.Can-05-0134
136. Demers M, Biron-Pain K, Hébert J, Lamarre A, Magnaldo T, St-Pierre Y. Galectin-7 in Lymphoma: Elevated Expression in Human Lymphoid Malignancies and Decreased Lymphoma Dissemination by Antisense Strategies in Experimental Model. Cancer Res (2007) 67(6):2824–9. doi: 10.1158/0008-5472.Can-06-3891
137. Moar P, Tandon R. Galectin-9 as a Biomarker of Disease Severity. Cell Immunol (2021) 361:104287. doi: 10.1016/j.cellimm.2021.104287
138. Li Y, Feng J, Geng S, Geng S, Wei H, Chen G, et al. The N- and C-Terminal Carbohydrate Recognition Domains of Galectin-9 Contribute Differently to Its Multiple Functions in Innate Immunity and Adaptive Immunity. Mol Immunol (2011) 48(4):670–7. doi: 10.1016/j.molimm.2010.11.011
139. Schaefer K, Webb NE, Pang M, Hernandez-Davies JE, Lee KP, Gonzalez P, et al. Galectin-9 Binds to O-Glycans on Protein Disulfide Isomerase. Glycobiology (2017) 27(9):878–87. doi: 10.1093/glycob/cwx065
140. Sabatos CA, Chakravarti S, Cha E, Schubart A, Sánchez-Fueyo A, Zheng XX, et al. Interaction of Tim-3 and Tim-3 Ligand Regulates T Helper Type 1 Responses and Induction of Peripheral Tolerance. Nat Immunol (2003) 4(11):1102–10. doi: 10.1038/ni988
141. Shahbaz S, Dunsmore G, Koleva P, Xu L, Houston S, Elahi S. Galectin-9 and Vista Expression Define Terminally Exhausted T Cells in Hiv-1 Infection. J Immunol (Baltimore Md 1950) (2020) 204(9):2474–91. doi: 10.4049/jimmunol.1901481
142. Zhang ZN, Yi N, Zhang TW, Zhang LL, Wu X, Liu M, et al. Myeloid-Derived Suppressor Cells Associated With Disease Progression in Primary Hiv Infection: Pd-L1 Blockade Attenuates Inhibition. J Acquir Immune Defic Syndr (1999) (2017) 76(2):200–8. doi: 10.1097/qai.0000000000001471
143. Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, et al. Adaptive Resistance to Therapeutic Pd-1 Blockade Is Associated With Upregulation of Alternative Immune Checkpoints. Nat Commun (2016) 7:10501. doi: 10.1038/ncomms10501
144. Mohammed TO, Chagan-Yasutan H, Ashino Y, Nakayama W, Takahashi Y, Shimomura T, et al. Galectin-9 as a Predictive Marker for the Onset of Immune-Related Adverse Effects Associated With Anti-Ccr4 Moab Therapy in Patients With Adult T Cell Leukemia. Tohoku J Exp Med (2017) 241(3):201–8. doi: 10.1620/tjem.241.201
Keywords: lymphoma, galectin-1, galectin-3, galectin-7, galectin-9
Citation: Shi Y, Tang D, Li X, Xie X, Ye Y and Wang L (2022) Galectin Family Members: Emerging Novel Targets for Lymphoma Therapy? Front. Oncol. 12:889034. doi: 10.3389/fonc.2022.889034
Received: 03 March 2022; Accepted: 14 April 2022;
Published: 23 May 2022.
Edited by:
Francesco Paolo Tambaro, AORN Santobono-Pausilipon, ItalyReviewed by:
Alexander Timoshenko, Western University, CanadaRalf Jacob, University of Marburg, Germany
Copyright © 2022 Shi, Tang, Li, Xie, Ye and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijuan Wang, d2FuZ2xqNzMwQDE2My5jb20=
†These authors have contributed equally to this work
 Yuanwei Shi
Yuanwei Shi Danting Tang
Danting Tang Xiaoqi Li
Xiaoqi Li Xiaoli Xie
Xiaoli Xie Yufu Ye3,4
Yufu Ye3,4 Lijuan Wang
Lijuan Wang