
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 14 June 2022
Sec. Surgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.888739
 Mingqing Zhang1,2,3,4†
Mingqing Zhang1,2,3,4† Yongdan Zhang2,3†
Yongdan Zhang2,3† Haoren Jing2,3
Haoren Jing2,3 Lizhong Zhao2,3
Lizhong Zhao2,3 Mingyue Xu2,3
Mingyue Xu2,3 Hui Xu2,3
Hui Xu2,3 Siwei Zhu1,2,3,4*
Siwei Zhu1,2,3,4* Xipeng Zhang2,3,4*
Xipeng Zhang2,3,4*Aim: Transanal endoscopic microsurgery (TEM) is widely performed in early rectal cancer. This technique offers greater organ preservation and decreases the risk of subsequent surgery. However, postoperative local recurrence and distant metastasis remain challenges for patients with high-risk pathological factors. This single-center study reports the prognosis of early rectal cancer patients over 60 years old after TEM.
Methods: The data of the patients over 60 years old who underwent local anal resection were collected retrospectively. Moreover, the 5-year follow-up data were analyzed to determine the 5-year DFS and OS.
Results: 47 early rectal cancer patients over 60 years old underwent TEM. There were 27 patients with high-risk factors and 20 patients without high-risk factors. Two patients underwent radical surgery after TEM and ten patients received adjuvant treatment. Local recurrence occurred in 7 patients, of which 4 underwent salvage surgery. The 5-year progression-free survival rate was 75.6%, which was lower in the high-risk patients group (69.6%) than in the non-high-risk patients group (83.3%) (P>0.05). The 5-year OS was 90.2%, but there was no statistically significant difference between the two groups (high-risk patients 87.0%, non-high-risk patients 94.4%). Furthermore, there was no significant difference in DFS and OS between people over and under 70 years old.
Conclusion: Some high-risk factor patients over 60 years old do not have inferior 5-year DFS and OS to the non-high-risk patients. TEM is an option for old patients with high surgical risks. Even if postoperative pathology revealed high-risk factors, timely surgical treatment after local recurrence would be beneficial to improve the 5-year DFS and OS.
Transanal local resection is one of the commonly employed surgical approaches for early rectal cancer. It is also recommended by many clinical guidelines for the treatment of early rectal cancer. Among these therapies, transanal endoscopic microsurgery (TEM) is the most commonly performed. Currently, TEM is mainly performed on patients with T1 low-risk rectal cancer (1). Generally speaking, the following criteria are favorable for TEM (2): lesions accounting for less than 30% of the rectal circumference; largest diameter of the tumor less than 3cm; distance between the margin and the tumor greater than 3mm; mobile tumor (not fixed); less than 8cm from the anal margin; T1 tumor; no vascular, lymphatic or nerve infiltration; highly to moderately differentiated. Other criteria where TEM can be considered include: no signs of lymph node metastasis in imaging examination; polyps resected under endoscopy showed cancerous infiltration, or the pathological examination results were uncertain; additional extended local resection was required; full-thickness resection was achieved.
With its excellent oncological and surgical safety (3), TEM allows for full-thickness resection and suture under direct vision (4, 5). Compared with radical surgery, TEM has been widely used for its superior anal function protection, low operation risk and fast postoperative recovery (6–9). The technique is especially popular with elderly patients and patients requesting anus preservation (10, 11). However, the risk of local recurrence and distant metastasis after TEM is still present (4). Clinical studies indicated that the local recurrence rate of T1stage rectal cancer after TEM was about 10% (12), and the 5-year local recurrence rate of the patients with high risk or incomplete resection exceeded 30% (13). The patients with pathological high-risk factors were generally recommended to pursue additional radical surgery after TEM (14). For those high-risk patients who failed to undergo additional radical surgery, the prognosis after TEM deserved further evaluation (15). This study aimed to investigate the prognosis of the patients over 60 years old who underwent TEM in our center, regardless of the presence or absence of postoperative pathological high-risk factors.
A retrospective analysis was conducted based on the collected data of the early rectal cancer patients over 60 years old who underwent TEM at the department of colorectal surgery of Tianjin Union Medical Center from January 2011 to January 2016. Patients with a histopathological diagnosis of malignancy were enrolled. Cases of severe dysplasia and carcinoma in situ and neoadjuvant chemoradiotherapy were excluded. All patients underwent preoperative digital rectal examination and colonoscopy. The local tumor stage and lymph node status were assessed by pelvic MRI or transrectal ultrasound, and distant metastasis was assessed by chest and abdominal CT scan. There was no evidence of lymph node involvement and distant metastasis in preoperative imaging.
The operation was performed by three surgeons with extensive experience in TEM surgery. All procedures were performed under general anesthesia, as previously described (16, 17). Resection margins were marked to incorporate a 1-cm cuff of normal mucosa around the location where cancer was known or suspected. In most patients, a full-thickness resection was performed. Closure of the rectal wall defect was routinely performed.
All patients were diagnosed with early rectal cancer (pT1-T2) according to the UICC/AJCC T Staging System (Seventh Edition) for pathological staging. pT1 tumors were evaluated according to the Kikuchi submucosal staging system (Sm1-3). The resection margin was considered positive when the cancer was located within 1 mm of the specimen’s resection margin.
The collected data included demographic information, preoperative staging, intrarectal ultrasonography, MRI, adjuvant therapy, surgical details, complications, tumor histopathology, postoperative management (additional radical surgery, radiotherapy or monitoring), recurrence and metastasis. The present study was approved by the Ethics Committee of Tianjin Union Medical Center.
Periodical re-examination was recommended in accordance with the clinical guidelines: The patients should be tested once every 3-6 months in the first 2 years, and once every 6 months in the 3rd to 5th years with a digital-anal examination, proctoscopy, transrectal ultrasound or MRI and CEA. Colonoscopy was performed within 1 year after surgery. Patients with progression-free survival of more than 5 years were followed up by telephone. All patients completed the LARS scoring questionnaire (18) to evaluate postoperative anal defecation function.
The main outcome indicator was the 5-year overall survival (OS). The secondary outcome indicators included the 5-year disease-free survival time (DFS) and the 5-year recurrence and metastasis rate. OS and DFS were both measured from the initial TEM date. The observation endpoint of OS was the date of death or the time of the last follow-up; the observation endpoint of DFS was the date of the first diagnosis of recurrence or metastasis after the operation or the time of the last follow-up. The follow-up period ended on August 27, 2021.
Statistical analysis was performed using SPSS 23.0. The count data were represented by the number of cases (composition ratio), while the measurement data conformed to a normal distribution and were described by (x̄±s). Continuous variables not suiting a normal distribution were described as median (interquartile range [IQR]) and compared between groups using the non-parametric Wilcoxon rank-sum test. The Kaplan-Meier method was employed to draw the survival curve of the patients, and the Log-rank test was adopted to compare the survival curves. P<0.05 was considered statistically significant throughout this study.
During the 5-year period from January 2011 to January 2016, 265 TEM procedures were recorded. 47 early rectal cancer patients over 60 years old underwent TEM, including 20 males and 27 females, with an average age of 69.9 years (ranging from 60 to 88 years old). Adenocarcinoma was confirmed by preoperative pathological biopsy in 25 patients. The mean distance between the lesion and the anal verge was 5.4cm (range 4-12cm), and the preoperative staging evaluated by transrectal ultrasound/MRI was no later than cT1N0M0 in all patients. The detailed screening workflow is shown in Figure 1, and the demographic data and tumor characteristics are displayed in Table 1.
The postoperative pathological staging was as follows: 37 cases (78.7%) were T1 and 10 cases (21.3%) were T2. There were 41 cases (87.2%) of adenocarcinoma, 4 cases (8.6%) of mucinous adenocarcinoma, 1 case (2.1%) of signet ring cell carcinoma, and 1 case of micropapillary carcinoma (2.1%). Considering of pathological risk factors, only one case (2.1%) demonstrated moderate-poor differentiation. A vascular tumor thrombus was found in 3 (6.4%) cases, and positive tumor margins were found in 4 (8.5%) cases. In addition, retrospective analysis of the pathological sections showed that 15 patients (31.9%) with stage T1 were Sm3. There were 27 patients (collectively called high-risk patients, 57.4%) with pathological risk factors (poor differentiation, positive resection margin, T2 stage and above, vascular invasion, Sm3 etc.) and 20 non-high-risk patients (42.6%). There was no significant difference in age and gender between the two groups (Table 2).
According to the postoperative pathological results, pT1N0 patients with pathological risk factors and pT2 patients were recommended to undergo radical rectal cancer surgery or conventional fractional concurrent chemoradiotherapy (radiation therapy of 50-54Gy for 25-30 times and/or capecitabine or other chemotherapy). Among them, 2 patients agreed to undergo radical surgery and 10 patients received adjuvant therapy.
Two patients with pathological factors (both T2 stage) underwent early radical surgery in the first week following TEM as per the doctor’s recommendation. 1 case of low anterior rectal resection had anastomotic recurrence 4 months after radical surgery and died of other causes 28 months after TEM. The other case (rectal abdominoperineal resection) had no disease recurrence within the 111 months of follow-up after TEM. These two patients underwent radical surgery within a short period of time after TEM, which is not reflective of TEM surgery efficacy. Thus, they were excluded from the subsequent analysis of local recurrence, metastasis and survival.
Ten patients received adjuvant treatment after TEM, in which 1 case received radiotherapy combined with chemotherapy (2.1%), 3 cases received chemotherapy (6.4%), 3 cases received radiotherapy (6.4%), and 3 cases received other treatment (traditional Chinese medicine, etc., 6.4%). The patient receiving combined radiotherapy and chemotherapy developed local recurrence and a secondary malignant tumor in the perineum 32 months after TEM surgery. Rectal abdominoperineal resection was then performed, and the patient was still alive at the end of the follow-up period. Among the 3 patients receiving chemotherapy, 2 patients survived, while the other patient died 55 months after TEM. Furthermore, 2 of the 3 patients who received radiotherapy were alive, whereas 1 case died 61 months after TEM. Among the 3 patients who received other treatments, 2 were alive; 1 case had a local recurrence 4 months after TEM, underwent low anterior rectal resection, and died 28 months after TEM.
Among the 45 patients who underwent TEM, a total of 7 (15.6%) patients had recurrence and metastasis at the end of the follow-up period, including 6 cases of local recurrence (2 cases with lung metastasis, 1 case with perineal metastasis) and 1 case with isolated liver metastasis. The median time of recurrence and metastasis after TEM was 32 months (ranging from 13 to 48 months). According to the Kaplan-Meier statistical analysis, the 5-year recurrence and metastasis rate was 18.4% (7/38) (95% CI: 50.972-58.502). The 5-year recurrence rate of T2 patients was 28.6% higher than that of T1 patients at 16.1%, but the difference was not statistically significant (χ2 = 0.657, P=0.418). A higher 5-year recurrence and metastasis rate was observed in the high-risk population (23.8%) than in the non-high-risk population (11.8%), but the difference was not statistically significant (χ2 = 0.997, P = 0.318) (Figure 2).
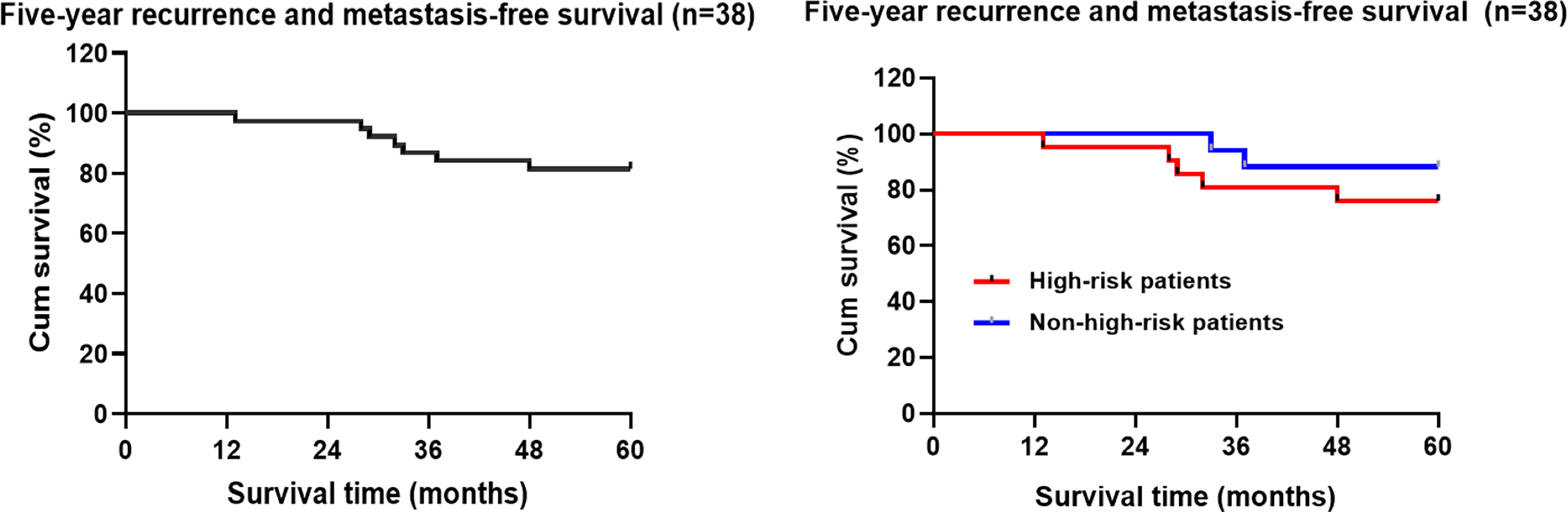
Figure 2 Kaplan-Meier analysis of the follow-up five-year recurrence and metastasis free-survival rate of the patients with early rectal cancer undergoing TEM.
Among the 7 patients with recurrence and metastasis, 4 cases underwent rectal abdominoperineal resection after local rectal recurrence. The median time of operation was 36.0 months (ranging from 32.0 to 48.0 months), and the pathology after additional surgery revealed a negative surgical margin. After the recurrence, 1 patient received radiotherapy and chemotherapy before the surgery. One year after the surgery, a secondary malignant tumor was found in the lung, and chemotherapy with mFOLFOX6 was given. Besides, 1 patient with recurrence was not given treatment after the surgery, but no new recurrence or metastasis occurred. 1 patient underwent abdominoperineal resection for rectal cancer after the recurrence, a secondary malignant tumor appeared in the perineum 8 months after the operation, and surgical resection was performed again. 1 patient underwent a second TEM after the recurrence, and abdominoperineal resection for rectal cancer was performed due to local recurrence 3 months after the surgery, and postoperative chemotherapy was given. The other 3 patients with recurrence and metastasis did not undergo further surgery.
During the 5-year follow-up, pathological features of adenocarcinoma were identified in 4 pT1 patients, with moderately differentiated, non-lymphovascular invasion, and negative margin; two of the patients were Sm3 and two of them received postoperative radiotherapy. None of the patients had abnormal anal function.
Kaplan-Meier statistical analysis indicated that the 5-year progression-free survival rate of the patients was 75.6% (31/41) (95%CI: 50.832-57.94) (Figure 3). Further analysis of the clinicopathological characteristics of the patients revealed differences in gender, age, postoperative adjuvant treatment, pathological T staging, pathological type, and Sm3, but the differences were not statistically significant. This may be related to the small sample size of this group. In contrast, there were statistically significant differences in tumor diameter. A lower 5-year DFS was observed in high-risk patients (69.6%) compared to non-high-risk patients (83.3%) (P>0.05) (Table 3 and Figure 3).
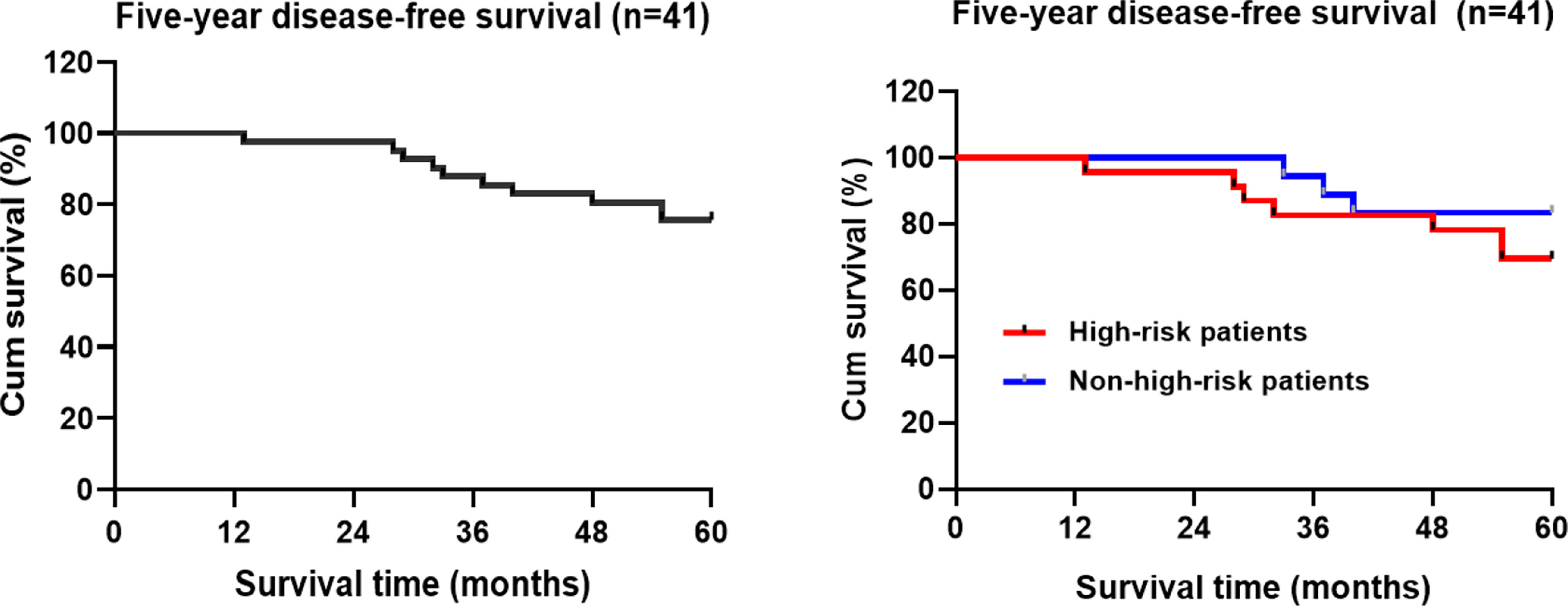
Figure 3 Kaplan-Meier analysis of the five-year disease-free survival rate of TEM patients with early rectal cancer.
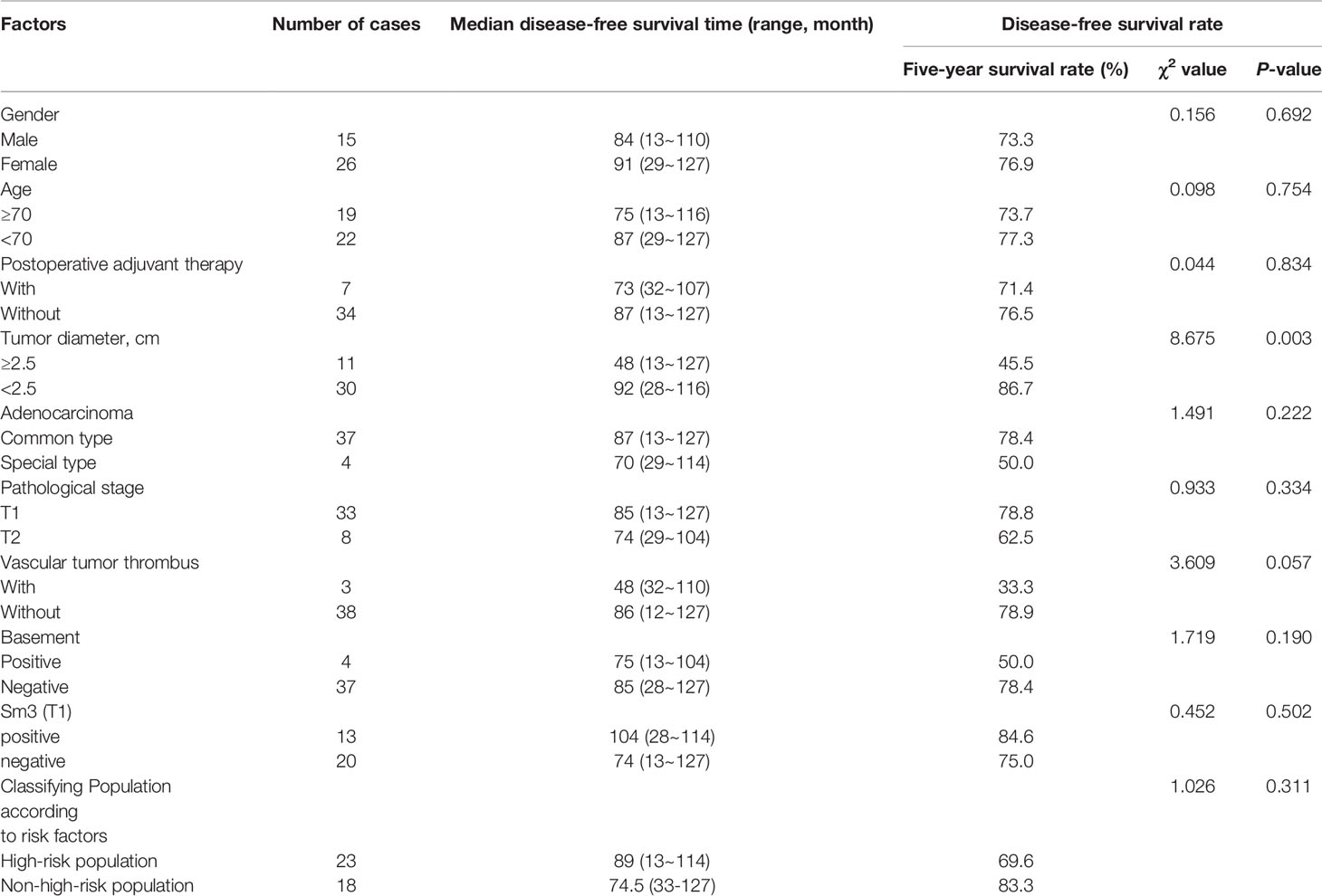
Table 3 Analysis of clinically related factors affecting the disease-free survival time of the patients with early rectal cancer.
The 5-year overall survival rate of the patients in this group was 90.9% (4/41) (95%CI:58.066-60.129) (Figure 4). Kaplan-Meier statistical analysis of the clinicopathological characteristics of the patients demonstrated no significant difference in gender, age, postoperative adjuvant treatment, tumor diameter, T stage, pathological type, presence of the tumor thrombus, Sm3, and positive basal lamina. A lower 5-year OS was observed in the high-risk population (87.0%) than in the non-high-risk population (94.4%), but the difference was not statistically significant (Table 4 and Figure 4).
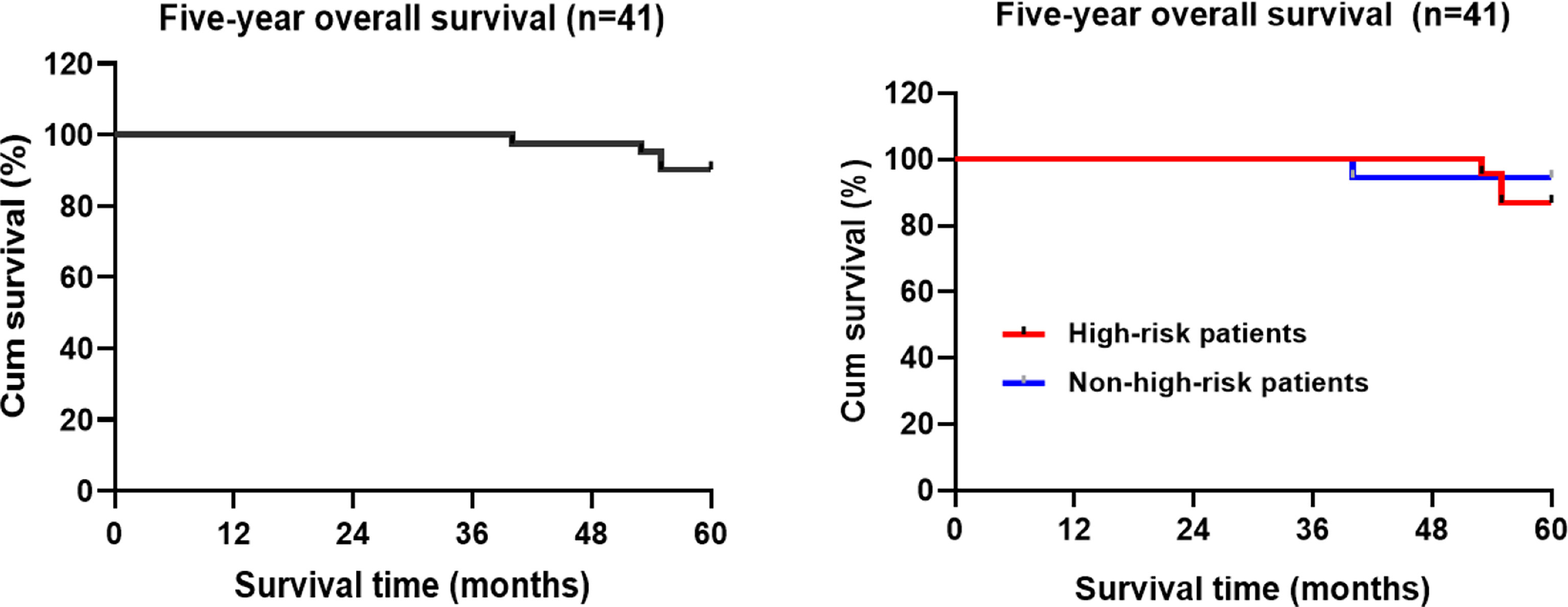
Figure 4 Kaplan-Meier estimated the overall survival rate of the patients with rectal cancer during the 5-year follow-up period.
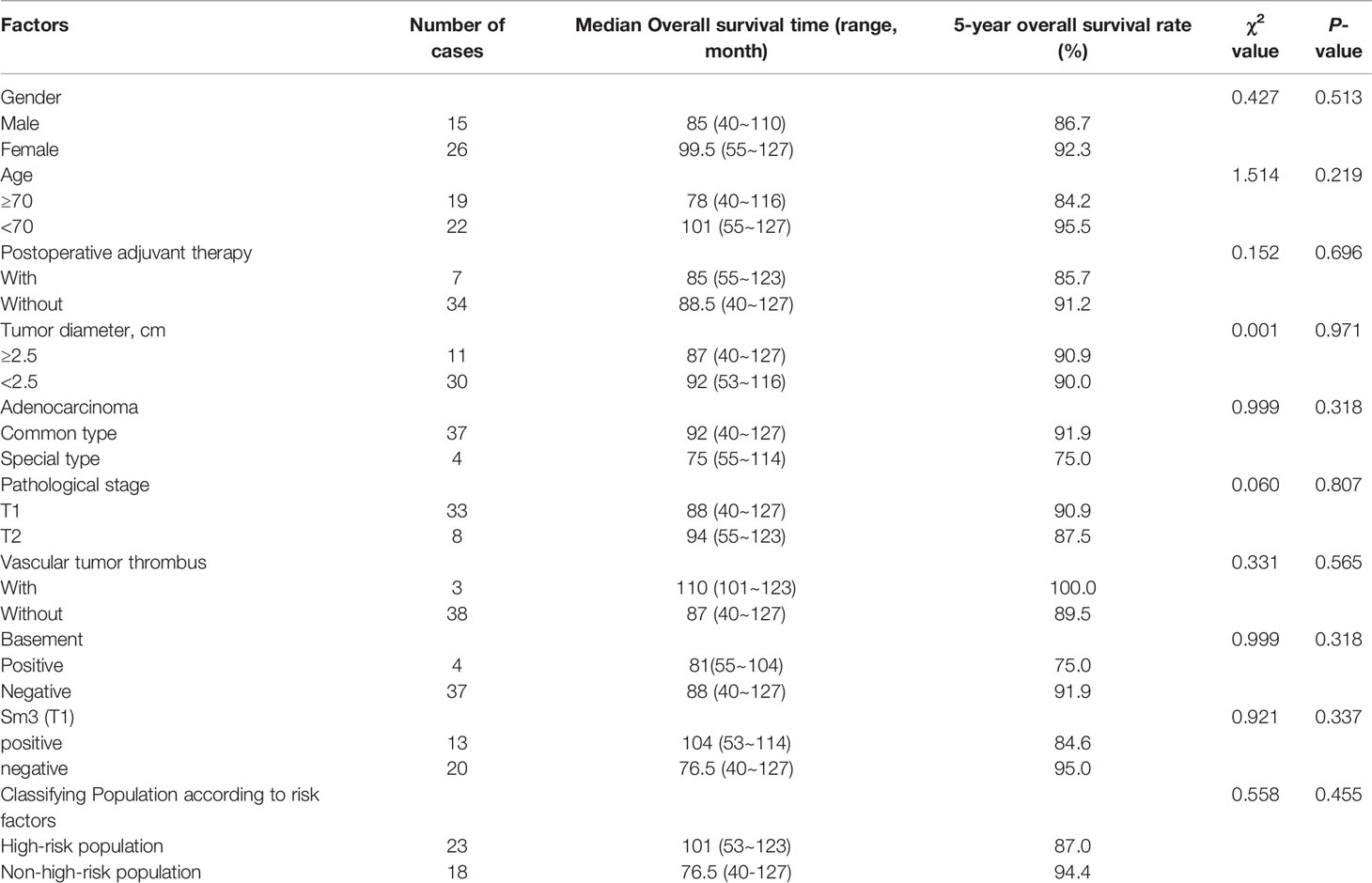
Table 4 Analysis of clinically related factors affecting the overall survival time of the patients with early rectal cancer.
T1 rectal cancers without pathological risk factors may be effectively treated with TEM without jeopardizing long-term oncologic outcomes (19). In contrast, radical surgery is recommended in high-risk pathological stage T1 (pT1) or pT2 rectal cancer. However, older patients with significant comorbidity may not be viable candidates for radical surgery (20). The patients in this group were generally older (the average age was 69.9 years) and some elderly patients could not tolerate radical surgery or refused to undergo combined abdominal and perineal radical surgery, even in the presence of pathological risk factors. Therefore, some patients with larger tumors (the maximum diameter was 6.0cm) opted for TEM. Through follow-up, it was found that the 5-year OS of the patients reached 90.2%, with a favorable overall prognosis, demonstrating that TEM is a worthwhile option for elderly patients with early rectal cancer.
Significant differences in the recurrence rate between T1 and T2 patients after TEM surgery have been reported. The 5-year local recurrence rate of T2 patients was as high as 29.3 ~ 47% (21), and the lymph node involvement rate of T2 tumors was also about twice higher than that of the T1 stage (22, 23). Therefore, T2 patients should receive more active surgical treatment (24). This was also consistent with our findings. Although the results were not statistically significant, the 5-year recurrence and metastasis rate in T2 patients (28.6%) higher than that in T1 patients (16.1%) (25). Meanwhile, T1 rectal cancer also carries the risk of lymph node metastasis (26, 27). Signs of lymph node metastasis before surgery would also directly affect the treatment program (19, 28, 29). In the present study, all patients underwent preoperative MRI or intrarectal ultrasound, chest and abdominal CT scan, and no evidence of lymph node involvement and distant metastasis was found.
A detailed pathological examination should be conducted for the tissue specimens resected by TEM. For the patients with poor differentiation, large tumors, vascular invasion and other risk factors (30), an increased local recurrence rate was observed, ranging from 20% to 30% (31). Therefore, if the postoperative pathological examination showed poor histological differentiation, positive margins, lymphatic and vascular invasion, T2 and above stages, etc., additional radical surgery is recommended (32). For patients with the above-mentioned high-risk factors who refused to undergo secondary surgical resection and had unclear lymph node status, the subsequent treatment plan should be determined after multidisciplinary discussion (33).
Complete tumor resection greatly reduces the risk of local recurrence (13). Previous studies indicated that the low risk of local recurrence of rectal cancer after TEM was mostly caused by the tumor residues from the previous resection rather than from undetermined lymph node metastasis (34). Therefore, full-thickness resection, adequate resection margins and en-bloc resection are performed to achieve adequate oncological safety (35). When the tumor cannot be completely removed, such as positive margins, additional radical surgery is currently recommended (36). Studies have shown no difference in outcomes with primary TME surgery (37). Considering the relationship between the depth of invasion and lymph node metastasis and local recurrence, most scholars recommend radical surgery for patients with stage T1 rectal cancer with deep submucosal invasion (Sm3 or > 1 mm) (30), despite other studies reporting Sm3 was not related to lymph node metastasis and survival (38). In this study, Sm3 was not included in the treatment guidelines at the time of treatment for some patients. We performed a retrospective analysis and found that the DFS and OS of Sm3 patients in T1 patients were similar to those of other T1 patients. However, this finding may be related to the small sample size.
Currently, the optimal treatment measures for patients with pathological high-risk factors are controversial (15, 24, 39). Most experts still recommend additional radical surgery, but other treatment measures are also worth exploring, such as adjuvant radiotherapy (40, 41)or extended local resection for patients with a high risk of recurrence (42). However, the previous studies pointed out that even if the patients were treated with total mesorectal excision (TME), the efficacy of the radical surgery would be lower than that of direct TME due to the effects of the initial TEM (43). Nevertheless, it is not associated with increased morbidity or mortality. Immediate laparoscopic TME after TEM for rectal cancer may result in a significantly increased risk of APR (44). Furthermore, the fibrotic scar caused by full-thickness TEM hinders the maintenance of proper surgical planes during TME surgery (45). Therefore, some researchers suggest that TME surgery following TEM excision is best delayed for 6 to 12 weeks in order to reduce postsurgical inflammation (29).
In this data group, the 5-year OS of the patients with recurrence after TEM and additional radical surgery were not inferior to those of low-risk patients without recurrence. The results indicated that the additional radical surgery after the recurrence with TEM surgery could improve the prognosis of the patients. This is consistent with some other observations and conclusions (37, 46, 47). Consequently, TEM can be used in exceptional cases with high-risk factors when the patient is not fit for radical surgery (45).
In one study including 33 patients who received adjuvant radiotherapy due to poor histopathological characteristics with a median follow-up of 3.2 years, the results indicated that 3 patients (9.1%) had local recurrence, and the estimated 1-year and 3-year local recurrence rates were 0% and 6.9%, respectively (40). The above protocol was also offered to the patients in the present study.
In another study, additional TEM was performed on part of the patients. It was found that the safety and radical effect of the additional TEM was nearly the same as that of the first operation (48, 49). In this study, four patients had positive margins; all of the tumors were greater than 2 cm in diameter (2.5-4 cm) and were close to the anus, increasing the risk of impaired anal function for additional surgery. The patients refused the recommended repeat TEM surgery or radical surgery and received postoperative adjuvant therapy. Although the anorectal function after repeated TEM is preserved (50), many factors including malignant lesions (51, 52), excessive circumferential mucosal defects (53), and closer proximity of the tumor to the anal verge suggest a higher risk of impaired anal function after TEM.
In addition, this study found no statistically significant differences in recurrent metastases and overall survival with or without adjuvant therapy after TEM. These findings are similar to the results of a recent study, concluding that survival after transanal local excision with or without chemoradiotherapy is comparable to that of TME, while TEM allows better bowel function and postoperative quality of life (54).
The follow-up program should be determined according to the risk of tumor recurrence and metastasis. Clinical studies indicated that the recurrence in T1 patients with local resection mostly occurred within 1 to 2 years after the surgery. Recurrence was usually located at the site of the primary lesions, and more than one-third of the patients recurred at the site outside the intestinal cavity (55). Therefore, most studies limit the duration of close follow-up to the initial 1 to 2 years (35, 56). It is generally believed that the patients should be reviewed every 3 months in the first 2 years (57). The examination included digital rectal examination, rigid sigmoidoscopy or proctoscope, ERUS and pelvic MRI, and monitoring carcinoembryonic antigen levels (58). Some scholars suggested that a CT examination of the chest and abdomen should be performed every 6 months to exclude distant metastasis (59). The postoperative follow-up of patients was performed according to guideline recommendations. Most of the patients who developed local recurrences had no obvious clinical symptoms. As a consequence, it is essential to optimize the follow-up program and closely monitor for local recurrence. The routine application of ERUS and pelvic MRI should be recommended (60).
This study has several main limitations, including the retrospective design and small sample size. Furthermore, due to various factors, some patients did not comply with the recommendations to receive postoperative adjuvant therapy, which may affect the conclusion of the study.
TEM surgery provides prominent advantages in the treatment of early rectal cancer, preserving anal function and imposing lower surgical risk and surgical pain. The results of the present study suggest a favorable overall prognosis for early rectal cancer patients over 60 years old who underwent TEM. For patients with postoperative pathological high-risk factors, additional radical surgery after local recurrence could also effectively improve the prognosis of the patients. Moreover, the stratified analysis demonstrated no difference in DFS and OS in patients above 70 years. TEM surgery may be an option for older patients with high surgical risk. With the promotion of neoadjuvant therapy, the application of TEM in patients with T2 or T3 stage tumors is being explored (21, 61–63). Further research on this topic will enable a broader application of TEM, which will play an essential role in the treatment of rectal cancer.
TEM is widely performed in early rectal cancer and also can be used in patients with high-risk factors who are not fit for radical surgery. Elderly patients tend to opt for TEM as it allows organ preservation and is a relatively safe surgery. However, these data remain to be confirmed. We report the prognosis of early rectal cancer patients over 60 years old. As an alternative for elderly rectal cancer patients with or without pathological high-risk factors, TEM is a reliable and effective therapeutic option.
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Ethics Committee of Tianjin Union Medical Center. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
SZ, XZ and MZ conceived the idea for the study. XZ and MZ were involved in planning and supervised data collection. HJ, YZ and LZ performed data collection. MZ, YZ and LZ conducted data analysis. MZ, YZ drafted the manuscript. MX and HX contributed to writing of manuscript. All authors have discussed and decided that this manuscript is the final version and agreed to publish it.
This study was funded by Foundation of Tianjin Union Medical Center (grant number: 2016YJZD002 and 2016RMNK002). This work was funded by Tianjin Key Medical Discipline (Specialty) Construction Project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Halverson AL, Morris AM, Cleary RK, Chang GJ. For Patients With Early Rectal Cancer, Does Local Excision Have an Impact on Recurrence, Survival, and Quality of Life Relative to Radical Resection? Ann Surg Oncol (2019) 26(8):2497–506. doi: 10.1245/s10434-019-07328-5
2. You YN, Hardiman KM, Bafford A, Poylin V, Francone TD, Davis K, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Rectal Cancer. Dis Colon Rectum (2020) 63(9):1191–222. doi: 10.1097/DCR.0000000000001762
3. Serra-Aracil X, Labró-Ciurans M, Rebasa P, Mora-López L, Pallisera-Lloveras A, Serra-Pla S, et al. Morbidity After Transanal Endoscopic Microsurgery: Risk Factors for Postoperative Complications and the Design of a 1-Day Surgery Program. Surg Endosc (2019) 33(5):1508–17. doi: 10.1007/s00464-018-6432-5
4. Al-Najami I, Rancinger CP, Larsen MK, Thomassen N, Buch N, Baatrup G. Transanal Endoscopic Microsurgery for Advanced Polyps and Early Cancers in the Rectum-Long-Term Outcome: A STROBE Compliant Observational Study. Med (Baltimore) (2016) 95(36):e4732. doi: 10.1097/MD.0000000000004732
5. Moore JS, Cataldo PA, Osler T, Hyman NH. Transanal Endoscopic Microsurgery Is More Effective Than Traditional Transanal Excision for Resection of Rectal Masses. Dis Colon Rectum (2008) 51(7):1026–30. doi: 10.1007/s10350-008-9337-x
6. Biviano I, Balla A, Badiali D, Quaresima S, D'Ambrosio G, Lezoche E, et al. Anal Function After Endoluminal Locoregional Resection by Transanal Endoscopic Microsurgery and Radiotherapy for Rectal Cancer. Colorectal Dis (2017) 19(6):O177–O85. doi: 10.1111/codi.13656
7. Lu JY, Lin GL, Qiu HZ, Xiao Y, Wu B, Zhou JL. Comparison of Transanal Endoscopic Microsurgery and Total Mesorectal Excision in the Treatment of T1 Rectal Cancer: A Meta-Analysis. PLoS One (2015) 10(10):e0141427. doi: 10.1371/journal.pone.0141427
8. Dimitriou N, Michail O, Moris D, Griniatsos J. Low Rectal Cancer: Sphincter Preserving Techniques-Selection of Patients, Techniques and Outcomes. World J Gastrointest Oncol (2015) 7(7):55–70. doi: 10.4251/wjgo.v7.i7.55
9. Hompes R, Ashraf SQ, Gosselink MP, van Dongen KW, Mortensen NJ, Lindsey I, et al. Evaluation of Quality of Life and Function at 1 Year After Transanal Endoscopic Microsurgery. Colorectal Dis (2015) 17(2):O54–61. doi: 10.1111/codi.12858
10. Xiong X, Wang C, Wang B, Shen Z, Jiang K, Gao Z, et al. Can Transanal Endoscopic Microsurgery Effectively Treat T1 or T2 Rectal Cancer?A Systematic Review and Meta-Analysis. Surg Oncol (2021) 37:101561. doi: 10.1016/j.suronc.2021.101561
11. Serra-Aracil X, Serra-Pla S, Mora-Lopez L, Pallisera-Lloveras A, Labro-Ciurans M, Navarro-Soto S. Transanal Endoscopic Micro-Surgery in Elderly and Very Elderly Patients: A Safe Option? Observational Study With Prospective Data Collection. Surg Endosc (2019) 33(1):184–91. doi: 10.1007/s00464-018-6292-z
12. Tsai BM, Finne CO, Nordenstam JF, Christoforidis D, Madoff RD, Mellgren A. Transanal Endoscopic Microsurgery Resection of Rectal Tumors: Outcomes and Recommendations. Dis Colon Rectum (2010) 53(1):16–23. doi: 10.1007/DCR.0b013e3181bbd6ee
13. Junginger T, Goenner U, Hitzler M, Trinh TT, Heintz A, Wollschlaeger D, et al. Long-Term Oncologic Outcome After Transanal Endoscopic Microsurgery for Rectal Carcinoma. Dis Colon Rectum (2016) 59(1):8–15. doi: 10.1097/DCR.0000000000000509
14. Gollins S, Moran B, Adams R, Cunningham C, Bach S, Myint AS, et al. Association of Coloproctology of Great Britain & Ireland (ACPGBI): Guidelines for the Management of Cancer of the Colon, Rectum and Anus (2017) - Multidisciplinary Management. Colorectal Dis (2017) 19 Suppl 1:37–66. doi: 10.1111/codi.13705
15. Javed MA, Shamim S, Slawik S, Andrews T, Montazeri A, Ahmed S. Long-Term Outcomes of Patients With Poor Prognostic Factors Following Transanal Endoscopic Microsurgery for Early Rectal Cancer. Colorectal Dis (2021) 23(8):1953–60. doi: 10.1111/codi.15693
16. Bach SP, Hill J, Monson JRT, Simson JNL, Lane L, Merrie A, et al. A Predictive Model for Local Recurrence After Transanal Endoscopic Microsurgery for Rectal Cancer. Br J Surg (2009) 96(3):280–90. doi: 10.1002/bjs.6456
17. Buess G, Theiss R, Gunther M, Hutterer F, Pichlmaier H. Endoscopic Surgery in the Rectum. Endoscopy (1985) 17(1):31–5. doi: 10.1055/s-2007-1018451
18. Emmertsen KJ, Laurberg S. Low Anterior Resection Syndrome Score: Development and Validation of a Symptom-Based Scoring System for Bowel Dysfunction After Low Anterior Resection for Rectal Cancer. Ann Surg (2012) 255(5):922–8. doi: 10.1097/SLA.0b013e31824f1c21
19. Allaix ME, Arezzo A, Morino M. Transanal Endoscopic Microsurgery for Rectal Cancer: T1 and Beyond? An Evidence-Based Review. Surg Endosc (2016) 30(11):4841–52. doi: 10.1007/s00464-016-4818-9
20. Xu K, Liu Y, Yu P, Shang W, Zhang Y, Jiao M, et al. Oncological Outcomes of Transanal Endoscopic Microsurgery Plus Adjuvant Chemoradiotherapy for Patients With High-Risk T1 and T2 Rectal Cancer. J Laparoendosc Adv Surg Tech A (2021) 31(9):1006–13. doi: 10.1089/lap.2020.0706
21. Garcia-Aguilar J, Renfro LA, Chow OS, Shi Q, Carrero XW, Lynn PB, et al. Organ Preservation for Clinical T2N0 Distal Rectal Cancer Using Neoadjuvant Chemoradiotherapy and Local Excision (ACOSOG Z6041): Results of an Open-Label, Single-Arm, Multi-Institutional, Phase 2 Trial. Lancet Oncol (2015) 16(15):1537–46. doi: 10.1016/S1470-2045(15)00215-6
22. Chang HC, Huang SC, Chen JS, Tang R, Changchien CR, Chiang JM, et al. Risk Factors for Lymph Node Metastasis in Pt1 and Pt2 Rectal Cancer: A Single-Institute Experience in 943 Patients and Literature Review. Ann Surg Oncol (2012) 19(8):2477–84. doi: 10.1245/s10434-012-2303-9
23. Mathew R. Radical Surgery Versus Organ Preservation for Early-Stage Rectal Cancer. Lancet Gastroenterol Hepatol (2021) 6(4):263. doi: 10.1016/S2468-1253(21)00015-7
24. van Oostendorp SE, Smits LJH, Vroom Y, Detering R, Heymans MW, Moons LMG, et al. Local Recurrence After Local Excision of Early Rectal Cancer: A Meta-Analysis of Completion TME, Adjuvant (Chemo)Radiation, or No Additional Treatment. Br J Surg (2020) 107(13):1719–30. doi: 10.1002/bjs.12040
25. Stornes T, Wibe A, Nesbakken A, Myklebust TÅ, Endreseth BH. National Early Rectal Cancer Treatment Revisited. Dis Colon Rectum (2016) 59(7):623–9. doi: 10.1097/DCR.0000000000000591
26. Saitoh Y, Inaba Y, Sasaki T, Sugiyama R, Sukegawa R, Fujiya M. Management of Colorectal T1 Carcinoma Treated by Endoscopic Resection. Dig Endosc (2016) 28(3):324–9. doi: 10.1111/den.12503
27. Beaton C, Twine CP, Williams GL, Radcliffe AG. Systematic Review and Meta-Analysis of Histopathological Factors Influencing the Risk of Lymph Node Metastasis in Early Colorectal Cancer. Colorectal Dis (2013) 15(7):788–97. doi: 10.1111/codi.12129
28. Yan S, Ding H, Zhao X, Wang J, Deng W. Development and Validation of a Nomogram for Further Decision of Radical Surgery in Pt1 Colorectal Cancer After Local Resection. Int J Colorectal Dis (2021) 36(7):1499–506. doi: 10.1007/s00384-021-03928-4
29. Ondhia M, Tamvakeras P, O'Toole P, Montazerri A, Andrews T, Farrell C, et al. Transanal Endoscopic Microsurgery for Rectal Lesions in a Specialist Regional Early Rectal Cancer Centre: The Mersey Experience. Colorectal Dis (2019) 21(10):1164–74. doi: 10.1111/codi.14730
30. Oh BY, Yun HR, Kim SH, Yun SH, Kim HC, Lee WY, et al. Features of Late Recurrence Following Transanal Local Excision for Early Rectal Cancer. Dis Colon Rectum (2015) 58(11):1041–7. doi: 10.1097/DCR.0000000000000456
31. Serra-Aracil X, Pallisera-Lloveras A, Mora-Lopez L, Rebasa P, Serra-Pla S, Navarro S. Perforation in the Peritoneal Cavity During Transanal Endoscopic Microsurgery for Rectal Tumors: A Real Surgical Complication With a Challenging Prognosis? Surg Endoscopy (2019) 33(6):1870–9. doi: 10.1007/s00464-018-6466-8
32. van Groningen JT, van Hagen P, Tollenaar R, Tuynman JB, de Mheen PJM, Doornebosch PG, et al. Evaluation of a Completion Total Mesorectal Excision in Patients After Local Excision of Rectal Cancer: A Word of Caution. J Natl Compr Canc Netw (2018) 16(7):822–8. doi: 10.6004/jnccn.2018.7026
33. Beets GL, Figueiredo NF, Beets-Tan RG. Management of Rectal Cancer Without Radical Resection. Annu Rev Med (2017) 68:169–82. doi: 10.1146/annurev-med-062915-021419
34. Junginger T, Goenner U, Hitzler M, Trinh TT, Heintz A, Roth W, et al. Analysis of Local Recurrences After Transanal Endoscopic Microsurgery for Low Risk Rectal Carcinoma. Int J Colorectal Dis (2017) 32(2):265–71. doi: 10.1007/s00384-016-2715-2
35. Serra-Aracil X, Ruiz-Edo N, Casalots-Casado A, Mora-Lopez L, Pallisera-Lloveras A, Serra-Pla S, et al. Importance of Resection Margins in the Treatment of Rectal Adenomas by Transanal Endoscopic Surgery. J Gastrointestinal Surg (2019) 23(9):1874–83. doi: 10.1007/s11605-018-3980-x
36. Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2019 for the Treatment of Colorectal Cancer. Int J Clin Oncol (2020) 25(1):1–42. doi: 10.1007/s10147-019-01485-z
37. Levic Souzani K, Bulut O, Kuhlmann TP, Gogenur I, Bisgaard T. Completion Total Mesorectal Excision Following Transanal Endoscopic Microsurgery Does Not Compromise Outcomes in Patients With Rectal Cancer. Surg Endosc (2022) 36(2):1181–90. doi: 10.1007/s00464-021-08385-2
38. Debove C, Svrcek M, Dumont S, Chafai N, Tiret E, Parc Y, et al. Is the Assessment of Submucosal Invasion Still Useful in the Management of Early Rectal Cancer? A Study of 91 Consecutive Patients. Colorectal Dis (2017) 19(1):27–37. doi: 10.1111/codi.13405
39. Balyasnikova S, Read J, Tait D, Wotherspoon A, Swift I, Cunningham D, et al. The Results of Local Excision With or Without Postoperative Adjuvant Chemoradiotherapy for Early Rectal Cancer Among Patients Choosing to Avoid Radical Surgery. Colorectal Dis (2017) 19(2):139–47. doi: 10.1111/codi.13477
40. Jones HJS, Goodbrand S, Hompes R, Mortensen N, Cunningham C. Radiotherapy After Local Excision of Rectal Cancer may Offer Reduced Local Recurrence Rates. Colorectal Dis (2019) 21(4):451–9. doi: 10.1111/codi.14546
41. Sideris M, Donaldson AN, Hanrahan J, Grunwald M, Papagrigoriadis S. Radiotherapy May Offer a Recurrence and Survival Benefit in Rectal Cancers Treated Surgically With Transanal Endoscopic Microsurgery: A Systematic Review and Meta-Analysis. Anticancer Res (2018) 38(4):1879–95. doi: 10.21873/anticanres.12425
42. de Jong GM, Hugen N. Minimally Invasive Transanal Surgery Is Safe After Incomplete Polypectomy of Low Risk T1 Rectal Cancer: A Systematic Review. Colorectal Dis (2019) 21(10):1112–9. doi: 10.1111/codi.14659
43. Coton C, Lefevre JH. Does Transanal Local Resection Increase Morbidity for Subsequent Total Mesorectal Excision for Early Rectal Cancer? Colorectal Dis (2019) 21(1):15–22. doi: 10.1111/codi.14445
44. Morino M, Allaix ME, Arolfo S, Arezzo A. Previous Transanal Endoscopic Microsurgery for Rectal Cancer Represents a Risk Factor for an Increased Abdominoperineal Resection Rate. Surg Endosc (2013) 27(9):3315–21. doi: 10.1007/s00464-013-2911-x
45. Dulskas A, Atkociunas A, Kilius A, Petrulis K, Samalavicius NE. Is Previous Transanal Endoscopic Microsurgery for Early Rectal Cancer a Risk Factor of Worse Outcome Following Salvage Surgery A Case-Matched Analysis. Visc Med (2019) 35(3):151–5. doi: 10.1159/000493281
46. O'Neill CH, Platz J, Moore JS, Callas PW, Cataldo PA. Transanal Endoscopic Microsurgery for Early Rectal Cancer: A Single-Center Experience. Dis Colon Rectum (2017) 60(2):152–60. doi: 10.1097/DCR.0000000000000764
47. Ortenzi M, Ghiselli R, Gesuita R, Guerrieri M. Transanal Endoscopic Microsurgery: Indications, Tips and Long-Term Results. A Single Center Exp Minerva Chir (2020) 75(3):129–40. doi: 10.23736/S0026-4733.20.08201-2
48. Ramkumar J, Letarte F, Karimuddin AA, Phang PT, Raval MJ, Brown CJ. Assessing the Safety and Outcomes of Repeat Transanal Endoscopic Microsurgery. Surg Endosc (2019) 33(6):1976–80. doi: 10.1007/s00464-018-6501-9
49. Stipa F, Giaccaglia V, Burza A. Management and Outcome of Local Recurrence Following Transanal Endoscopic Microsurgery for Rectal Cancer. Dis Colon Rectum (2012) 55(3):262–9. doi: 10.1097/DCR.0b013e318241ef22
50. Zhang HW, Han XD, Wang Y, Zhang P, Jin ZM. Anorectal Functional Outcome After Repeated Transanal Endoscopic Microsurgery. World J Gastroenterol (2012) 18(40):5807–11. doi: 10.3748/wjg.v18.i40.5807
51. Valsdottir EB, Yarandi SS, Marks JH, Marks GJ. Quality of Life and Fecal Incontinence After Transanal Endoscopic Microsurgery for Benign and Malignant Rectal Lesions. Surg Endosc (2014) 28(1):193–202. doi: 10.1007/s00464-013-3155-5
52. van Heinsbergen M, Leijtens JW, Slooter GD, Janssen-Heijnen ML, Konsten JL. Quality of Life and Bowel Dysfunction After Transanal Endoscopic Microsurgery for Rectal Cancer: One Third of Patients Experience Major Low Anterior Resection Syndrome. Dig Surg (2020) 37(1):39–46. doi: 10.1159/000496434
53. Ran L, Chuanwang Y, Wei S, Wenguang Y, Liang H, Jiancheng Z, et al. Risk Factors and Treatment of Rectal Stenosis After Transanal Endoscopic Microsurgery. Colorectal Dis (2022) 24(1):85–92. doi: 10.1111/codi.15904
54. Pacevicius J, Petrauskas V, Pilipavicius L, Dulskas A. Local Excision +/- Chemoradiotherapy vs. Total Mesorectal Excision for Early Rectal Cancer: Case-Matched Analysis of Long-Term Results. Front Surg (2021) 8:746784. doi: 10.3389/fsurg.2021.746784
55. You YN, Roses RE, Chang GJ, Rodriguez-Bigas MA, Feig BW, Slack R, et al. Multimodality Salvage of Recurrent Disease After Local Excision for Rectal Cancer. Dis Colon Rectum (2012) 55(12):1213–9. doi: 10.1097/DCR.0b013e318270837f
56. Keeping AC, Johnson PM, Kenyon CR, Neumann K. Timing of Recurrences of TEM Resected Rectal Neoplasms Is Variable as Per the Surveillance Practices of One Tertiary Care Institution. Sci Rep (2021) 11(1):6509. doi: 10.1038/s41598-021-85885-0
57. Rouleau-Fournier F, Brown CJ. Can Less be More? Organ Preservation Strategies in the Management of Rectal Cancer. Curr Oncol (Toronto Ont) (2019) 26(Suppl 1):S16–s23. doi: 10.3747/co.26.5841
58. Smith RK, Fry RD, Mahmoud NN, Paulson EC. Surveillance After Neoadjuvant Therapy in Advanced Rectal Cancer With Complete Clinical Response can Have Comparable Outcomes to Total Mesorectal Excision. Int J Colorectal Dis (2015) 30(6):769–74. doi: 10.1007/s00384-015-2165-2
59. Stijns RCH, de Graaf EJR, Punt CJA, Nagtegaal ID, Nuyttens J, van Meerten E, et al. Long-Term Oncological and Functional Outcomes of Chemoradiotherapy Followed by Organ-Sparing Transanal Endoscopic Microsurgery for Distal Rectal Cancer: The CARTS Study. JAMA Surg (2019) 154(1):47–54. doi: 10.1001/jamasurg.2018.3752
60. Hupkens BJP, Maas M. MRI Surveillance for the Detection of Local Recurrence in Rectal Cancer After Transanal Endoscopic Microsurgery Eur Radiol (2017) 27(12):4960–9. doi: 10.1007/s00330-017-4853-5
61. Guerrieri M, Gesuita R, Ghiselli R, Lezoche G, Budassi A, Baldarelli M. Treatment of Rectal Cancer by Transanal Endoscopic Microsurgery: Experience With 425 Patients. World J Gastroenterol (2014) 20(28):9556–63. doi: 10.3748/wjg.v20.i28.9556
62. Bach SP, Gilbert A, Brock K, Korsgen S, Geh I, Hill J, et al. Radical Surgery Versus Organ Preservation via Short-Course Radiotherapy Followed by Transanal Endoscopic Microsurgery for Early-Stage Rectal Cancer (TREC): A Randomised, Open-Label Feasibility Study. Lancet Gastroenterol Hepatol (2021) 6(2):92–105. doi: 10.1016/S2468-1253(20)30333-2
Keywords: early rectal cancer, local recurrence, adjuvant therapy, transanal endoscopic microsurgery, 5-year overall survival
Citation: Zhang M, Zhang Y, Jing H, Zhao L, Xu M, Xu H, Zhu S and Zhang X (2022) Prognosis of Patients Over 60 Years Old With Early Rectal Cancer Undergoing Transanal Endoscopic Microsurgery – A Single-Center Experience. Front. Oncol. 12:888739. doi: 10.3389/fonc.2022.888739
Received: 03 March 2022; Accepted: 12 May 2022;
Published: 14 June 2022.
Edited by:
Marco Scarpa, University Hospital of Padua, ItalyReviewed by:
Nidal Issa, Rabin Medical Center, IsraelCopyright © 2022 Zhang, Zhang, Jing, Zhao, Xu, Xu, Zhu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Siwei Zhu, c2l3ZWl6QG5hbmthaS5lZHUuY24=; Xipeng Zhang, emh4cDEwMTFAMTYzLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.