- Department of Blood Transfusion, The Affiliated Hospital of Qingdao University, Qingdao, China
Colorectal cancer (CRC) is one of the most common malignant tumors. The morbidity and mortality rates have been increasing all over the world. It is critical to elucidate the mechanism of CRC occurrence and development. However, tumor microenvironment (TME) includes immune cells, fibroblasts, endothelial cells, cytokines, chemokines and other components that affect the progression of CRC and patients’ prognosis. Non-coding RNAs (ncRNAs) including microRNAs (miRNAs), long non-coding RNAs (lncRNAs), circular RNAs (circRNAs) without protein-coding ability have been shown to engage in tumor microenvironment-mediated angiogenesis and metastasis. Therefore, clarifying the mechanism of ncRNAs regulating the microenvironment is very important to develop the therapeutic target of CRC and improve the survival time of patients. This review focuses on the role and mechanism of ncRNAs in the CRC microenvironment and puts forward possible clinical treatment strategies.
1 Introduction
Colorectal cancer (CRC) is the most common malignant tumor, which seriously threatens human health (1). The incidence rate of CRC occupies the third position in all cancers worldwide (2). In addition, CRC is the second leading cause of death in malignant tumors due to the poor prognosis and high postoperative metastasis rate (3). Therefore, it is significant to clarify the mechanism of CRC progression and find treatment targets.
Tumor microenvironment (TME) includes immune cells, fibroblasts, endothelial cells, and other components that affect the occurrence and development of CRC (4). Cancer cells can functionally shape their microenvironment by secreting various cytokines, chemokines, and other factors (5). Existing evidence confirms that there are complex and continuous interactions between tumor cells and their microenvironment (6). Targeting TME can regulate multiple stages and processes in tumorigenesis and development (7). In recent years, many studies have shown that some ncRNAs are involved in the progression of CRC as an oncogene or tumor suppressor (8–10). Various types of ncRNAs are abnormally regulated in many cancer types (10, 11). They regulate proliferation, differentiation, apoptosis, necrosis, and autophagy of tumor cells by affecting the components of the TME (12). Therefore, the regulatory relationship between ncRNAs and TME has attracted more and more attention.
In this review, we describe the roles of abnormal expression of ncRNAs in the TME to promote CRC progression. Besides, we discuss the promising clinical treatment of ncRNAs in TME of CRC.
2 TME
TME is the internal environment in which tumor cells produce and live (5). It is mainly composed of tumor cells and their surrounding immune and inflammatory cells, tumor related fibroblasts, nearby interstitial tissues, capillaries, as well as various cytokines and chemokines (12, 13). The dynamic changes of the above components and other factors related to TME, such as hypoxia, chronic inflammation and immunosuppression promote the tumor progression and patients’ prognosis (14). The development of the tumorigenic microenvironment in CRC is driven by the genetic instability of its cancer cells and epigenetic factors in response to exogenous and endogenous stress stimuli (15–18). When intestinal inflammation occurs, various cells such as neutrophils, macrophages and fibroblasts are recruited (19). Fibroblasts and inflammatory cells will infiltrate into inflamed tissues and play a role in TME. These recruited inflammatory cells interact with CRC cells by producing various cytokines and chemokines to promote tumor growth and progression (20, 21). Actually, TME varies depending on the location and type of tumors. Its heterogeneity and dynamic changes lead to the therapeutic drug resistance of tumors (22). Therefore, an in-depth understanding of TME may provide important clues for finding new treatment options and enhancing treatment effects (23, 24).
3 Roles and Mechanism of ncRNAs in TME
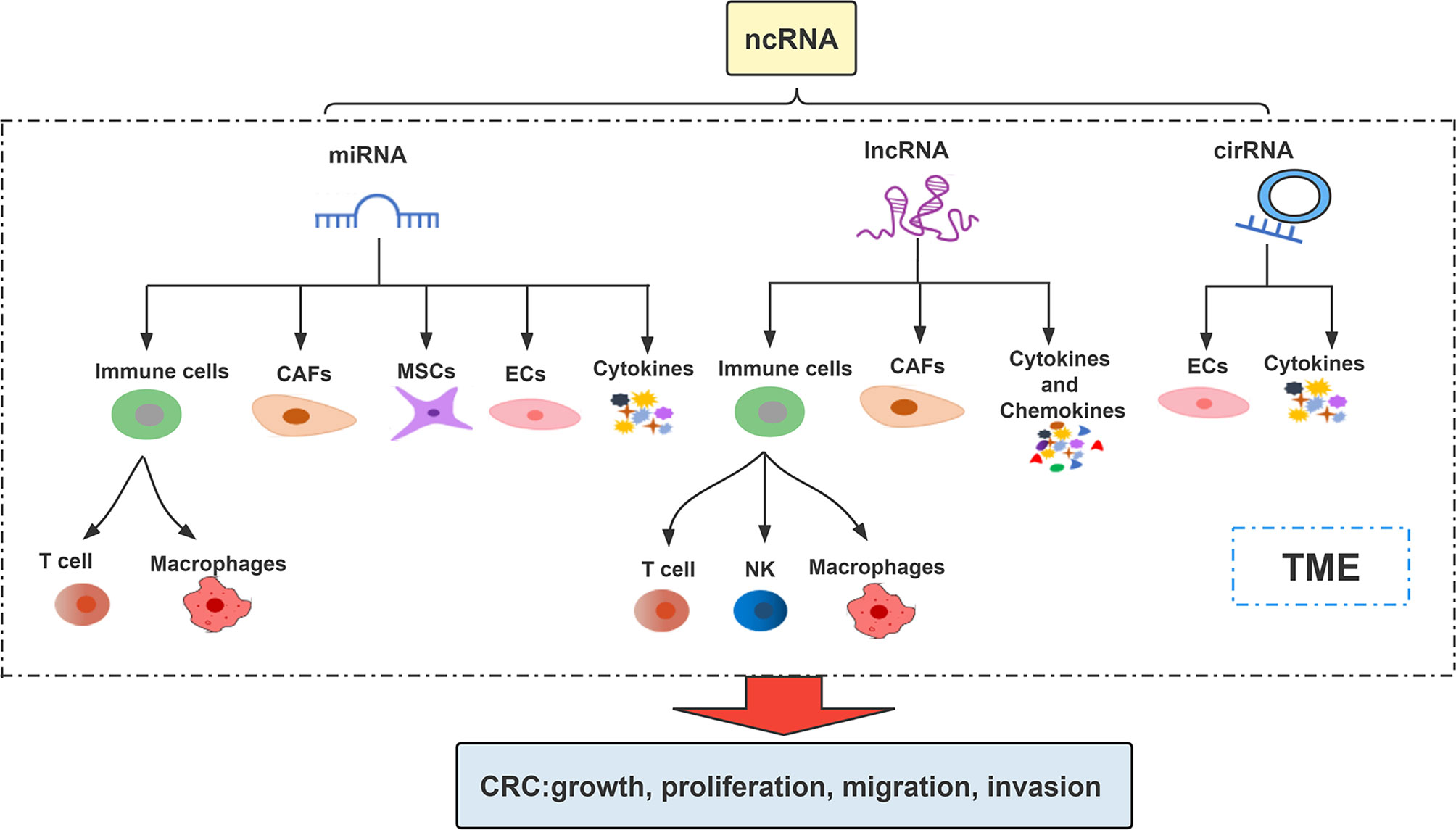
ncRNAs are involved in the regulation of multiple biological processes by regulating gene expression in multi-level and multi-channel (25). They affect and participate in the regulation of CRC progression by affecting different cells or intercellular matrix, microvessels and various factors in TME (26, 27). The interaction among ncRNA, TME components and cancer cells increases the cancer progression (28) (Figure 1). Association between them makes ncRNAs (including miRNAs, lncRNAs, circRNAs) mediated TME as current potential treatment strategies for cancer treatment (29, 30).
3.1 miRNAs in TME
miRNA is a type of small ncRNA that can bind to 3’-untranslated regions (3’-UTRs) or amino acid coding sequences to regulate mRNA expression (31). A large number of studies have shown that miRNA imbalance can play a role in many biological processes such as tumor proliferation, angiogenesis, metastasis, immune response and drug resistance through the interaction among malignant cells, non-malignant stromal cells and non-cellular components in TME (32, 33). It is worth noting that studies have confirmed that some miRNAs are abnormally expressed in CRC and are involved in the regulation of CRC immune escape (34, 35). Next, we summarize some miRNAs that influence the development of CRC through the different components of TME (Figure 2).
3.1.1 Immune Cells
Immune cells are important parts of the TME (36). Inflammation and infiltration of immune cells into tumor tissues have been proven to support tumor growth, invasion and metastasis. Tumor progression can promote TME remodeling through the communication between tumor and immune cells (37). Different immune cells have the property of inhibiting tumors or promoting tumors, which depends on the stimulation of the cell and tissue environment (20, 37–39). In particular, the functional inactivation of tumor-reactive T cells play an important role (40–42). As an immunomodulatory enzyme, IDO1 can catalyze the degradation of tryptophan (Trp) to canine purine (Kyn) to induce apoptosis/dysfunction of effector T cells and generate immunosuppressive regulatory T cells (43–45). Lou et al. found that miR-448, which has a tumor-suppressive effect that can inhibiting the apoptosis of CD8+ T cells by inhibiting IDO1 expression in TME of CRC. That enables to exert cytotoxic T lymphocyte effector function and inducing tumor cells apoptosis (45). Conversely, miR-148a-3p affects CRC progression by inhibiting CANX/MHC-I axis and significantly attenuating CD8+ T cell-mediated immune attack in vitro and in vivo (46).
Macrophages are generally considered as the most common leukocytes in TME, which can be divided into M1-like macrophages (M1 macrophages) and M2-like macrophages (M2 macrophages) (47). Macrophages in TME are usually called tumor-associated macrophages (TAMs) (48) and most of them are M2-like (49). Programmed tumor cells can secrete mediators to activate tumor-associated macrophages (50). They respond to various factors produced by tumor cells in TME. These factors can induce epithelial mesenchymal transition (EMT) and play an important role in tumorigenesis and metastasis (51). For example, tumor suppressor miR-195-5p inhibits GATA3-mediated secretion of IL-4 in CRC cells and ultimately M2-like tumor-associated macrophages polarization by suppressing the NOTCH2 expression (52). Zhao et al. verified that exosomal miR-934-induced M2 macrophage polarization promotes CRC liver metastasis through activation of the CXCL13/CXCR5 axis in CRC cells (53). Similarly, Wang et al. found that exosomal miRNAs (miR-25-3p, miR-130b-3p, miR-425-5p) promoted CXCL12/CXCR4-induced CRC liver metastasis by enhancing M2 polarization of macrophages (54). Conversely, TAMs promote CRC cells proliferation and invasion by secreting transforming growth factor-β (TGF-β) that inhibits miR-34a expression and upregulates VEGF (55). Based on these, miRNAs that interact with immune cells also provide a new perspective for us to understand the progress of CRC.
3.1.2 Cancer Associated Fibroblasts
Cancer associated fibroblasts (CAFs) are also one important component of TME, which is usually an important part of the tumor matrix (56). A large amount of evidence shows that many miRNAs are related to CAFs in CRC (57). CAFs can affect the TME of CRC by secreting a variety of chemokines and cytokines, to promote the metastasis and diffusion of cancer cells (57). Yang et al. confirmed that miR-31 can regulate the proliferation, metastasis and radiosensitivity of CRC cells by inhibiting autophagy of CAFs (58). In addition, HIF-1α up-regulates miR-210 affects the expression of VMP1, thereby improving the migration and invasion of CAFs. Metastasis of cancer associated fibroblasts from the CRC primary promotes tumor metastasis (59).
It is worth noting that CAFs can also mediate the expression level of miRNA by transferring extracellular vesicles directly to CRC cells (60, 61). Zheng et al. found that CAFs-derived extracellular vesicles can transfer SLC4A4-targeted miR-224-5p to CRC cells and promote the proliferation, migration, invasion and anti-apoptosis of CRC cells (62). Exosomes are another form of extracellular vesicles (63). Hu et al. found that the exosomes secreted by CAFs led to the increased expression of miR-92a-3p in CRC through the Wnt/β-Catenin signaling pathway to promote cell stem, EMT and metastasis in CRC (64). These suggest that CAFs are an important part of the TME that promotes the development of CRC.
3.1.3 Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) are one of the main components of TME and can promote tumor angiogenesis and metastasis (65, 66). MSC-derived extracellular vesicles may play a role in the progression of CRC. For example, Li et al. have shown that MSC-exosomal miR-3940-5p inhibits invasion and EMT of CRC cells as well as growth and metastasis of tumors through targeting ITGA6 and TGF-β1 inactivation (67). Zhao et al. found that MSC-derived extracellular vesicles transmitting miR-34a-5p suppress tumorigenesis of CRC through c-MYC/DNMT3a/PTEN axis (68). Zhang et al. found that inhibiting miR-424 in bone marrow MSC-derived exosomes suppresses tumor growth in CRC by up-regulating TGFBR3 (69).
3.1.4 Endothelial Cells
When the nutrition and oxygen provided by existing blood vessels cannot meet the needs of tumor growth and invasion, tumors will produce angiogenesis-related factors to change the local microenvironment and form new blood vessels (70). Endothelial cells (ECs) are the basic components of blood vessels (71). Previous data suggest that certain miRNA are key determinants of endothelial cells behavior during angiogenesis (72, 73).
Vascular endothelial growth factor A (VEGFA), which controls the proliferation, survival and migration of endothelial cells, is the main regulatory factor for the germination of new blood vessels (71). miR-125a-5p inhibits the ability of human umbilical vein endothelial cell (HUVEC) tube formation through VEGFA/VEGFR2 signaling pathway, and inhibits CRC progression, which has been further verified by mouse model (74). Moreover, miR-150-5p in CRC can inhibit HUVEC tube-forming ability and inhibit CRC tumor progression by targeting VEGFA in CRC through VEGFA/VEGFR2/Akt/mTOR signaling pathway (75).
Exosomal miR-25-3p regulates endothelial cells by targeting KLF2 and KLF4 to promote vascular permeability and angiogenesis, which can promote CRC metastasis (76). In addition, cancer cells-derived exosomal miR-27b-3p can be transported to vascular endothelial cells, where targeted p120 and vascular endothelial cadherin (VE-Cad) destroy the integrity of vascular endothelial cell junction. It also increases vascular permeability and result in promoting circulating tumor cell-mediated CRC metastasis (77).
3.1.5 Cytokines
Differential expression of cytokines, chemokines and ncRNAs can regulate TME in the process of tumor progression (78). Pro-inflammatory cytokines play significant roles in CRC-related cachexia, including tumor necrosis factor α (TNFα), which is involved in the maintenance and homeostasis of immune system, inflammation and host defense (79). The overexpression of miR-19a can enhance the ability of TNF-α to induce spindle-like morphological characteristics and significantly upregulation the expression of N-cadherin, Fibronectin and Vimentin induced by TNF-α, and induce EMT to promote the invasion of CRC cells (80).
The interleukin-17 (IL-17) family is a Th17 cell-derived pro-inflammatory cytokine family involved in numerous human diseases (81). IL-17C has been shown to promote tumor progression by increasing epithelial cell survival and tumor angiogenesis in CRC (82, 83). Lee et al. demonstrated that IL-17C produced VEGF via the STAT3/miR-23a-3p/SEMA6D axis can cause intestinal endothelial cells to promote angiogenesis in the tumor environment (84).
3.2 LncRNAs in TME
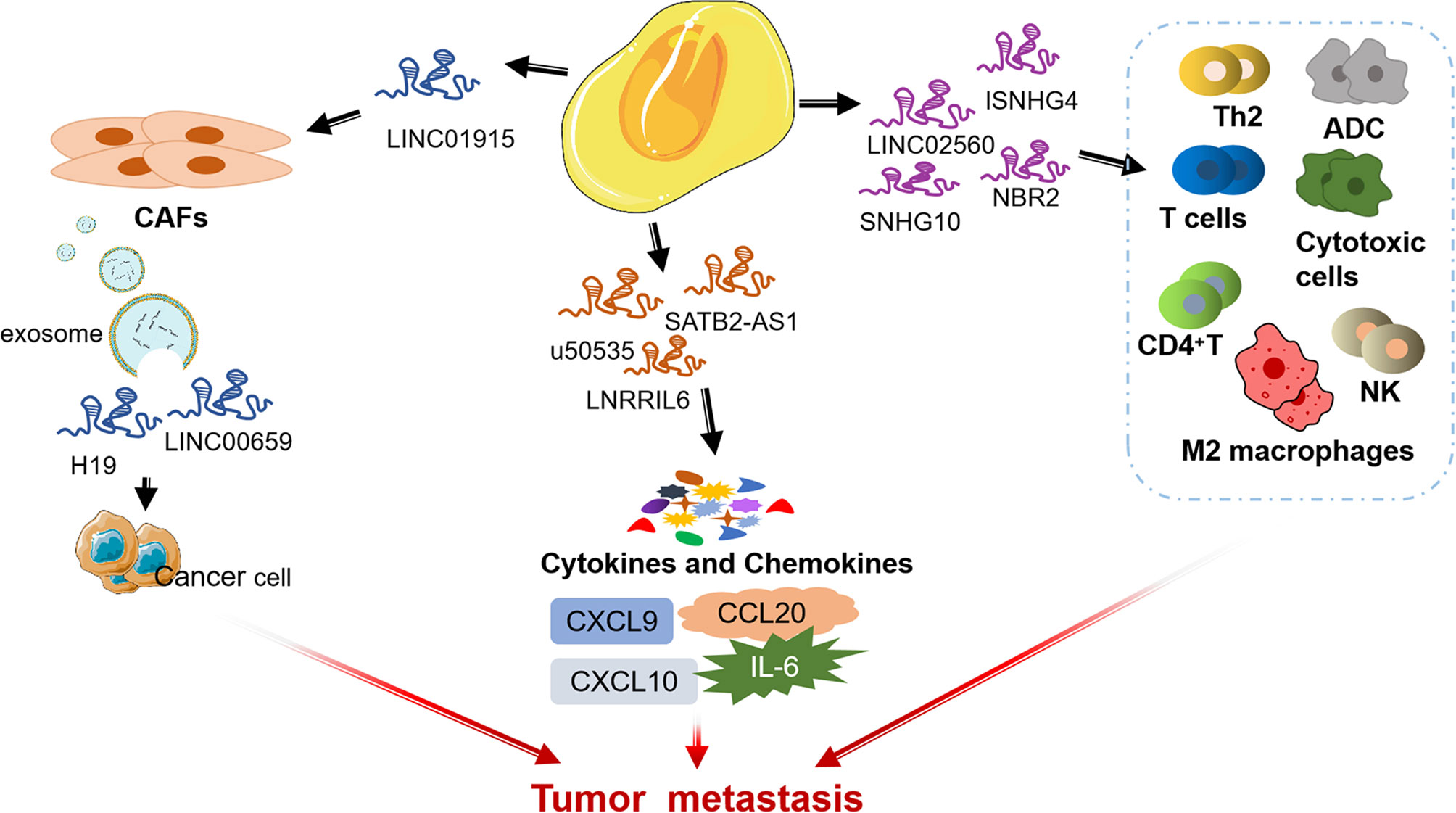
LncRNA is a kind of RNA with a length of more than 200 nt and no protein coding ability (85, 86). Recent studies have shown that lncRNAs play a vital role in CRC by affecting TME (87, 88). Next, we summarize some lncRNAs that influence CRC progression through regulating the different components of TME (Figure 3).
3.2.1 mmune Cells
There is increasing evidence that lncRNAs are indispensable in immune responses by regulating immune cells (89–92). LINC02560 expression in CRC was significantly and negatively correlated with the infiltration of four immune cells (Th2 cells, T cells, ADCs, and Cytotoxic cells). Tumor with high LINC02560 expression reduced cellular immunity and antigen-presenting capacity in the microenvironment and participated in the progression of CRC (93). Ning et al. found that lncRNA SNHG4 induces CACO2 cell-CD4+ T cell interaction by targeting miR-144-3p and induces CD4+T cell apoptosis through the PD-1/PD-L1 immune checkpoint (94). In addition, exosomal lncRNA SNHG10 derived from CRC cells inhibits the activity and cytotoxicity of NK cells and promotes the immune escape of CRC cells by up-regulating the expression of INHBC (95).
LncRNAs mediate the regulation of TME by TAMs to affect tumor progression (96, 97). One study showed CRC cell-derived exosomes transport RPPH1 to macrophages, mediate the M2 polarization of macrophages and affect TME, thereby promoting the metastasis of CRC cells (98). In addition, Lai et al. found that up-regulation of lncRNA NBR2 in macrophages could inhibit M2 macrophages polarization and thus prevent the proliferation and metastasis of CRC cells (99).
3.2.2 Cancer Associated Fibroblasts
LncRNA can affect crosstalk between malignant cells and CAFs during tumorigenesis (100, 101). LINC00659 metastasized from CAFs to cancer cells through exosomes and promoted CRC cell proliferation, invasion, migration, and EMT progression through the miR-342-3p/ANXA2 axis (102). Zhou et al. revealed that LINC01915 can inhibit the conversion of normal fibroblasts (NFs) into CAFs via the miR-92a-3p/KLF4/CH25H axis, and inhibit angiogenesis, thus preventing tumor growth (103). Moreover, CAFs contribute to promoting the stemness and chemoresistance of CRC by transferring exosomal H19 act as a competitive endogenous RNA sponge for miR-141 to activate the β-catenin pathway in CRC (104). In a separate study, CAFs can induce the up-regulation of lncRNA UCA1 and activate the proto-oncogene mTOR, thereby significantly stimulating the proliferation and migration of CRC cells (105).
3.2.3 Cytokines and Chemokines
Interleukin-6 (IL-6) in TME is a pleiotropic cytokine, which plays an important role in the regulation of the immune system (106). As an important mediator of inflammatory reaction and activator of STAT3, IL-6 can reduce the apoptosis of cancer cells (107). LNRRIL6 can be used as an IL-6 activator to activate STAT3 by up-regulating IL-6 in the microenvironment, protecting CRC cells and promoting their proliferation (108).
LncRNA can regulate CRC tumor metastasis and affect tumor immune microenvironment by targeting some chemokines in TME of CRC (such as CC chemokine subfamily and CXC chemokine subfamily) (109, 110). For example, lncRNA SATB2-AS1 inhibits the expression of TH1 chemokines CXCL9 and CXCL10 affects the tumor immune cell microenvironment in CRC by regulating SATB2 (111). In addition, others found that CCL20 secreted by tumor cells is one of the main chemokines in TME, and lncRNA u50535 can regulate CCL20 expression and affect CCL20/CCR6/ERK signaling, leading to CRC tumorigenesis (112, 113).
In summary, lncRNA mediates the process of CRC by acting on cytokines in TME, and new insights on the function of cytokines in CRC help to promote the effective application of cytokine regulatory therapy in CRC.
3.3 CircRNAs in TME
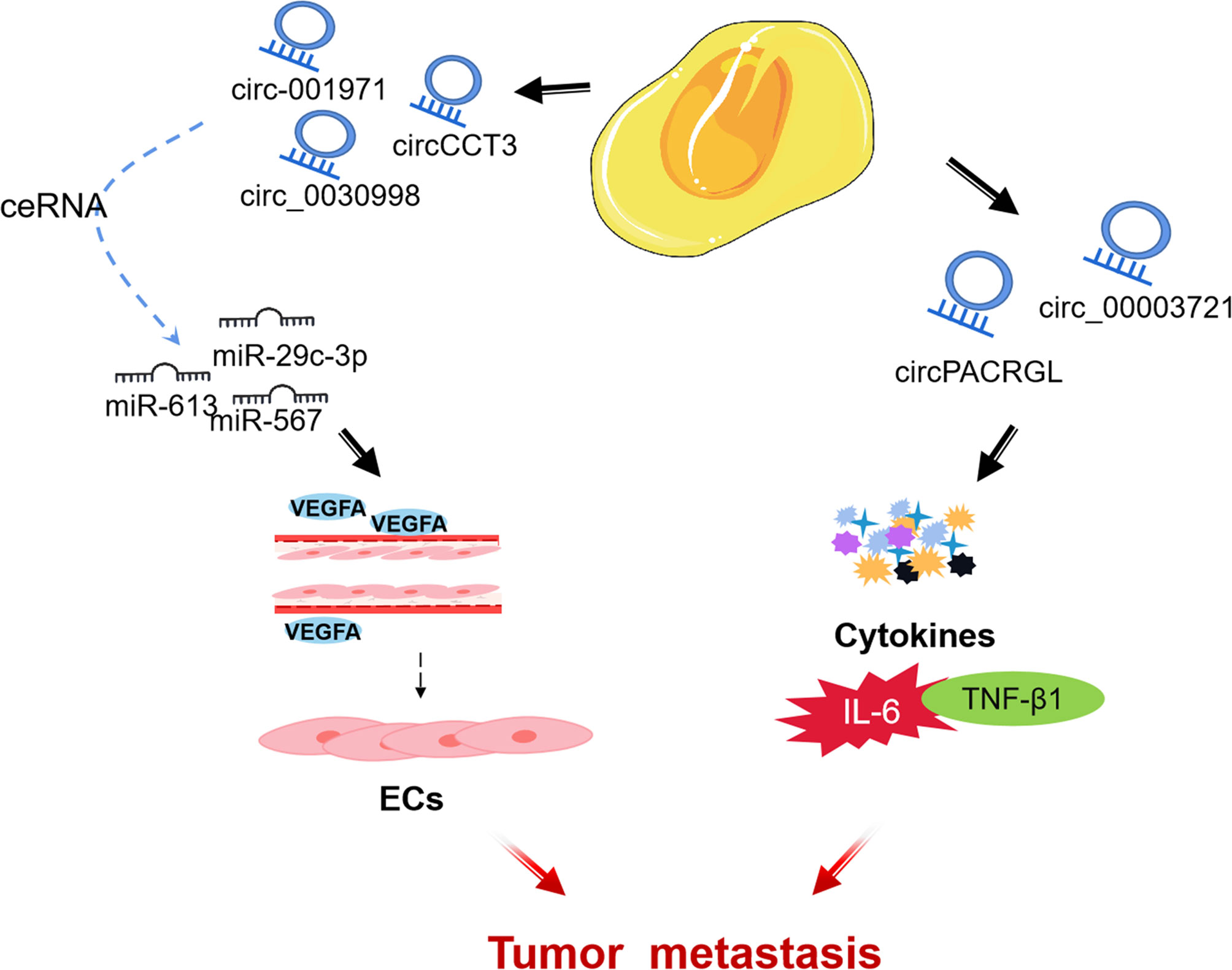
CircRNA is a subgroup of endogenous ncRNA (114). Some circRNAs are rich in microRNA (miRNA) response elements and can act as competing endogenous RNAs, binding miRNAs and thereby inhibiting miRNA functions and regulating gene expression (115, 116). With the advent of high-throughput sequencing, many circular RNAs have been successfully identified and have been confirmed to be involved in many biological regulations in the TME of CRC (117). Nonetheless, the biological functions of circRNAs in CRC are largely still intricate and unclear (118). Accordingly, understanding the mechanism of circRNAs in TME of CRC may be used as a diagnostic and prognostic biomarker, as well as a potential therapeutic target for CRC. Next, we review some circRNAs that influence CRC progression through regulating the different components of TME (Figure 4).
3.3.1 Endothelial Cell
CircRNA can be used as an important process for ceRNA to affect the angiogenesis of ECs and mediate the growth and metastasis of CRC tumors by regulating miRNA. Chen et al. found that circ-001971 acts as a ceRNA to attenuate miR-29c-3p induced VEGFA inhibition, and through TME affects HUVEC tube formation, thereby exacerbating the proliferation, invasion, and angiogenesis of CRC (119). Similarly, Li et al. found that circCCT3 is highly expressed in human clinical CRC tumors, and regulates VEGFA expression and enhances the metastasis of CRC by sponging miR-613 (120). In addition, located in the cytoplasm of CRC cells, circ_0030998 was found to regulate VEGFA in CRC by sponging miR-567, promoting HUVECs formation and CRC cells proliferation (121).
3.3.2 Cytokines
TGF-β1 (transforming growth factor -β1), a subfamily of TGF-β, inhibits N1 neutrophil in TME but promotes N2 neutrophil differentiation and cancer development (122). Exosomal circPACRGL promotes CRC cell proliferation, migration, and invasion, as well as N1-N2 neutrophil differentiation progression of CRC via the miR-142-3p/miR-506-3p-TGF-β1 axis (123). In addition, Liu et al. found that circ_0000372 up-regulated the expression of IL6 through spongiform miR-495, and activated the STAT3 pathway to promote the proliferation, migration and invasion of CRC (124).
4 Perspective: ncRNAs as Potential Targets for Colorectal Cancer
To date, the role of ncRNAs in radiotherapy and chemotherapy suggests that they can be used as new therapeutic targets for CRC (125–128). Over the past few decades, the treatment of CRC patients has changed significantly. In addition to traditional radiotherapy and chemotherapy, therapeutic targeting of TME has become a promising cancer treatment due to the critical role of TME in regulating tumor progression (4, 129). The dynamically complex TME provides favorable conditions for tumor growth, and tumor evolution and resistance to anticancer therapy are mediated through dynamic interaction with TME (130–133). The study of ncRNAs and TME also provides reliable evidence for their potential application in the clinical treatment of CRC.
A large number of studies have confirmed that ncRNAs can have multiple biological functions in regulating the onset and progression of cancer by affecting components or being affected in the TME (32, 114, 134–138). Regulatory T cells (Tregs) are known for their immunosuppressive effects, and targeting Tregs are effective method to increase chemical sensitivity (139–141). Studies have shown that miR-208b secreted by CRC cells is sufficiently delivered to receptor T cells to promote Treg amplification, tumor growth and oxaliplatin resistance by targeting programmed cell death factor 4 (PDCD4) (142). miRNA-124-3p inhibits the expression of PD-L1 by regulating STAT3 signaling pathway, and then promotes the anti-cancer response of CRC cells mediated by Tregs to inhibit tumorigenesis (143). Crosstalk between CAFs and cancer cells in the TME, making CAFs potentially important targets for stroma-based therapy in CRC treatment (4, 144). For example, Hu et al. confirmed that CAF-derived exosomal miR-92a-3p could be directly transferred to CRC cells to promote its metastasis and resistance to 5-FU/L-OHP (64). Moreover, exosomal miR-24-3p can be transferred from CAFs to colon cancer cells and down-regulate CDX2/HEPH axis, resulting in significantly enhanced resistance of colon cancer cells to methotrexate (MTX) (145). Deng et al. found that lncRNA CCAL transferred from CAFs to cancer cells through exosomes promoted the resistance of CRC cells to oxaliplatin (146). Yang et al. creatively found that the circEIF3K/miR-214/PD-L1 axis mediated the progression of CRC induced by hypoxia through CAF, providing a theoretical basis for the development of new targeted therapies for CRC (147). Furthermore, Zhou et al. found that LncRNA MIR155HG induced polarization of CRC cell M2 macrophages by regulating ANXA2, promoted CRC progression, and enhanced CRC cell resistance to oxaliplatin (148). Above evidences suggest that ncRNAs can play a role in the development of drug resistance in CRC, and the promotion of drug resistance can significantly reduce the efficacy of drugs and increase the failure rate of treatment with anticancer drugs such as oxaliplatin and 5FU (149, 150). This indicates that various ncRNAs in TME can be potential therapeutic targets to overcome drug resistance.
Actually, exosomes are widespread in all body fluids and can be detected and used as markers for the early CRC diagnosis (151). In addition, exosome ncRNAs are protected by lipid bilayer encapsulation and can be prevented from being degraded by ribonucleases in blood, which has a great possibility of being used as a carrier of therapeutic drugs (152, 153). Relevant exosome engineering technologies have been used to treat tumors by delivering tumor-inhibiting exosome ncRNAs (154–156). For example, modified exosome is used for co-delivery of 5-FU and miR-21 inhibitors (miR-21i) targeting CRC cells, which effectively reverses the drug resistance of tumor cells and remarkably enhances the toxicity of 5-FU-resistant cancer cells (157). These suggest exosomes can be used as carriers of therapeutic drugs and may guide changes in clinical practice.
In summary, above evidences suggest that ncRNAs play an important role in CRC therapy by modulating TME to enhance potential antitumor therapeutic targets. The function and potential clinical application value of ncRNAs in TME are worthy of affirmation. Their research will provide new ideas for targeted tumor therapy in the future and may be further explored in future research.
5 Conclusion
In conclusion, this review comprehensively discussed the roles of ncRNAs such as miRNAs, lncRNAs, circRNAs in TME of CRC and involved multiple biological processes. In this study, we affirmed that the aberrant expression of ncRNAs in the TME can directly or indirectly promote the proliferation, migration and drug resistance of CRC tumor cells. Although research on TME in ncRNAs and CRC is very limited to date, it offers broad prospects for cancer diagnostic and therapeutic applications. Further exploring the great potential and unresolved problems of ncRNAs and TME as potential therapeutic targets in clinical applications, we believe that more CRC patients will benefit in the future.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
ZW and QJ were involved in the conception of the study. ZW were involved in writing the article. QJ critically revised the manuscript. All authors have read and approved the final manuscript.
Funding
This study was supported by the Clinical Medicine + X Project of Affiliated Hospital of Qingdao University (grant no. 66 to QJ).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors sincerely thank all participants involved in this study.
References
1. Yuan J, Wei Z, Xu X, Ocansey DKW, Cai X, Mao F. The Effects of Mesenchymal Stem Cell on Colorectal Cancer. Stem Cells Int (2021) 2021:9136583. doi: 10.1155/2021/9136583
2. Wong MCS, Huang J, Lok V, Wang J, Fung F, Ding H, et al. Differences in Incidence and Mortality Trends of Colorectal Cancer Worldwide Based on Sex, Age, and Anatomic Location. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc (2021) 19:955–966.e61. doi: 10.1016/j.cgh.2020.02.026
3. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
4. Bejarano L, Jordāo MJC, Joyce JA. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov (2021) 11:933–59. doi: 10.1158/2159-8290.CD-20-1808
5. Hinshaw DC, Shevde LA. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res (2019) 79:4557–66. doi: 10.1158/0008-5472.CAN-18-3962
6. AlMusawi S, Ahmed M, Nateri AS. Understanding Cell-Cell Communication and Signaling in the Colorectal Cancer Microenvironment. Clin Transl Med (2021) 11:e308. doi: 10.1002/ctm2.308
7. Hui L, Chen Y. Tumor Microenvironment: Sanctuary of the Devil. Cancer Lett (2015) 368:7–13. doi: 10.1016/j.canlet.2015.07.039
8. Esteller M. Non-Coding RNAs in Human Disease. Nat Rev Genet (2011) 12:861–74. doi: 10.1038/nrg3074
9. Galamb O, Barták BK, Kalmár A, Nagy ZB, Szigeti KA, Tulassay Z, et al. Diagnostic and Prognostic Potential of Tissue and Circulating Long non-Coding RNAs in Colorectal Tumors. World J Gastroenterol (2019) 25:5026–48. doi: 10.3748/wjg.v25.i34.5026
10. Anastasiadou E, Jacob LS, Slack FJ. Non-Coding RNA Networks in Cancer. Nat Rev Cancer (2018) 18:5–18. doi: 10.1038/nrc.2017.99
11. Mohapatra S, Pioppini C, Ozpolat B, Calin GA. Non-Coding RNAs Regulation of Macrophage Polarization in Cancer. Mol Cancer (2021) 20:24. doi: 10.1186/s12943-021-01313-x
12. Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell (2010) 140:883–99. doi: 10.1016/j.cell.2010.01.025
13. Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the Tumor Immune Microenvironment (TIME) for Effective Therapy. Nat Med (2018) 24:541–50. doi: 10.1038/s41591-018-0014-x
14. Maacha S, Bhat AA, Jimenez L, Raza A, Haris M, Uddin S, et al. Extracellular Vesicles-Mediated Intercellular Communication: Roles in the Tumor Microenvironment and Anti-Cancer Drug Resistance. Mol Cancer (2019) 18:55. doi: 10.1186/s12943-019-0965-7
15. Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
16. Han D, Wang M, Ma N, Xu Y, Jiang Y, Gao X. Long Noncoding RNAs: Novel Players in Colorectal Cancer. Cancer Lett (2015) 361:13–21. doi: 10.1016/j.canlet.2015.03.002
17. Martinez-Outschoorn UE, Peiris-Pagés M, Pestell RG, Sotgia F, Lisanti MP. Cancer Metabolism: A Therapeutic Perspective. Nat Rev Clin Oncol (2017) 14:11–31. doi: 10.1038/nrclinonc.2016.60
18. Fridman WH, Zitvogel L, Sautès-Fridman C, Kroemer G. The Immune Contexture in Cancer Prognosis and Treatment. Nat Rev Clin Oncol (2017) 14:717–34. doi: 10.1038/nrclinonc.2017.101
19. Mizuno R, Kawada K, Sakai Y. Prostaglandin E2/EP Signaling in the Tumor Microenvironment of Colorectal Cancer. Int J Mol Sci (2019) 20:6254 doi: 10.3390/ijms20246254
20. Joyce JA, Pollard JW. Microenvironmental Regulation of Metastasis. Nat Rev Cancer (2009) 9:239–52. doi: 10.1038/nrc2618
21. Mizuno R, Kawada K, Itatani Y, Ogawa R, Kiyasu Y, Sakai Y. The Role of Tumor-Associated Neutrophils in Colorectal Cancer. Int J Mol Sci (2019) 20:529. doi: 10.3390/ijms20030529
22. Wu T, Dai Y. Tumor Microenvironment and Therapeutic Response. Cancer Lett (2017) 387:61–8. doi: 10.1016/j.canlet.2016.01.043
23. Pasqualini C, Kozaki T, Bruschi M, Nguyen THH, Minard-Colin V, Castel D, et al. Modeling the Interaction Between the Microenvironment and Tumor Cells in Brain Tumors. Neuron (2020) 108:1025–44. doi: 10.1016/j.neuron.2020.09.018
24. Catalano V, Turdo A, Di Franco S, Dieli F, Todaro M, Stassi G. Tumor and its Microenvironment: A Synergistic Interplay. Semin Cancer Biol (2013) 23:522–32. doi: 10.1016/j.semcancer.2013.08.007
25. Toden S, Zumwalt TJ, Goel A. Non-Coding RNAs and Potential Therapeutic Targeting in Cancer. Biochim Biophys Acta - Rev Cancer (2021) 1875:188491. doi: 10.1016/j.bbcan.2020.188491
26. Ragusa M, Barbagallo C, Statello L, Condorelli AG, Battaglia R, Tamburello L, et al. Non-Coding Landscapes of Colorectal Cancer. World J Gastroenterol (2015) 21:11709–39. doi: 10.3748/wjg.v21.i41.11709
27. Wang J, Song Y-X, Ma B, Wang J-J, Sun J-X, Chen X-W, et al. Regulatory Roles of Non-Coding RNAs in Colorectal Cancer. Int J Mol Sci (2015) 16:19886–919. doi: 10.3390/ijms160819886
28. Drak Alsibai K, Meseure D. Tumor Microenvironment and Noncoding RNAs as Co-Drivers of Epithelial-Mesenchymal Transition and Cancer Metastasis. Dev Dyn an Off Publ Am Assoc Anat (2018) 247:405–31. doi: 10.1002/dvdy.24548
29. Beermann J, Piccoli MT, Viereck J, Thum T. Non-Coding Rnas in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol Rev (2016) 96:1297–325. doi: 10.1152/physrev.00041.2015
30. Taft RJ, Pang KC, Mercer TR, Dinger M, Mattick JS. Non-Coding RNAs: Regulators of Disease. J Pathol (2010) 220:126–39. doi: 10.1002/path.2638
31. Baer C, Squadrito ML, Laoui D, Thompson D, Hansen SK, Kiialainen A, et al. Suppression of microRNA Activity Amplifies IFN-γ-Induced Macrophage Activation and Promotes Anti-Tumour Immunity. Nat Cell Biol (2016) 18:790–802. doi: 10.1038/ncb3371
32. Lee SS, Cheah YK. The Interplay Between MicroRNAs and Cellular Components of Tumour Microenvironment (TME) on Non-Small-Cell Lung Cancer (NSCLC) Progression. J Immunol Res (2019) 2019:3046379. doi: 10.1155/2019/3046379
33. Kloosterman WP, Plasterk RHA. The Diverse Functions of microRNAs in Animal Development and Disease. Dev Cell (2006) 11:441–50. doi: 10.1016/j.devcel.2006.09.009
34. Shen Z, Zhou R, Liu C, Wang Y, Zhan W, Shao Z, et al. MicroRNA-105 is Involved in TNF-α-Related Tumor Microenvironment Enhanced Colorectal Cancer Progression. Cell Death Dis (2017) 8:3213. doi: 10.1038/s41419-017-0048-x
35. Zhou X, Mao Y, Zhu J, Meng F, Chen Q, Tao L, et al. TGF-β1 Promotes Colorectal Cancer Immune Escape by Elevating B7-H3 and B7-H4 via the miR-155/miR-143 Axis. Oncotarget (2016) 7:67196–211. doi: 10.18632/oncotarget.11950
36. Lei X, Lei Y, Li J-K, Du W-X, Li R-G, Yang J, et al. Immune Cells Within the Tumor Microenvironment: Biological Functions and Roles in Cancer Immunotherapy. Cancer Lett (2020) 470:126–33. doi: 10.1016/j.canlet.2019.11.009
37. Smith HA, Kang Y. The Metastasis-Promoting Roles of Tumor-Associated Immune Cells. J Mol Med (Berl) (2013) 91:411–29. doi: 10.1007/s00109-013-1021-5
38. Ostrand-Rosenberg S. Immune Surveillance: A Balance Between Protumor and Antitumor Immunity. Curr Opin Genet Dev (2008) 18:11–8. doi: 10.1016/j.gde.2007.12.007
39. Quail DF, Joyce JA. Microenvironmental Regulation of Tumor Progression and Metastasis. Nat Med (2013) 19:1423–37. doi: 10.1038/nm.3394
40. Friese C, Harbst K, Borch TH, Westergaard MCW, Pedersen M, Kverneland A, et al. CTLA-4 Blockade Boosts the Expansion of Tumor-Reactive CD8(+) Tumor-Infiltrating Lymphocytes in Ovarian Cancer. Sci Rep (2020) 10:3914. doi: 10.1038/s41598-020-60738-4
41. O’Connor RA, Chauhan V, Mathieson L, Titmarsh H, Koppensteiner L, Young I, et al. T Cells Drive Negative Feedback Mechanisms in Cancer Associated Fibroblasts, Promoting Expression of Co-Inhibitory Ligands, CD73 and IL-27 in non-Small Cell Lung Cancer. Oncoimmunology (2021) 10:1940675. doi: 10.1080/2162402X.2021.1940675
42. Eisel D, Das K, Dickes E, König R, Osen W, Eichmüller SB. Cognate Interaction With CD4(+) T Cells Instructs Tumor-Associated Macrophages to Acquire M1-Like Phenotype. Front Immunol (2019) 10:219. doi: 10.3389/fimmu.2019.00219
43. Zhai L, Ladomersky E, Lenzen A, Nguyen B, Patel R, Lauing KL, et al. IDO1 in Cancer: A Gemini of Immune Checkpoints. Cell Mol Immunol (2018) 15:447–57. doi: 10.1038/cmi.2017.143
44. Chen B, Alvarado DM, Iticovici M, Kau NS, Park H, Parikh PJ, et al. Interferon-Induced IDO1 Mediates Radiation Resistance and Is a Therapeutic Target in Colorectal Cancer. Cancer Immunol Res (2020) 8:451–64. doi: 10.1158/2326-6066.CIR-19-0282
45. Lou Q, Liu R, Yang X, Li W, Huang L, Wei L, et al. MiR-448 Targets IDO1 and Regulates CD8+ T Cell Response in Human Colon Cancer. J Immunother Cancer (2019) 7:210, 1–14. doi: 10.1186/s40425-019-0691-0
46. Zheng J, Yang T, Gao S, Cheng M, Shao Y, Xi Y, et al. miR-148a-3p Silences the CANX/MHC-I Pathway and Impairs CD8(+) T Cell-Mediated Immune Attack in Colorectal Cancer. FASEB J Off Publ Fed Am Soc Exp Biol (2021) 35:e21776. doi: 10.1096/fj.202100235R
47. Mantovani A, Locati M. Tumor-Associated Macrophages as a Paradigm of Macrophage Plasticity, Diversity, and Polarization: Lessons and Open Questions. Arterioscler Thromb Vasc Biol (2013) 33:1478–83. doi: 10.1161/ATVBAHA.113.300168
48. Yang L, Zhang Y. Tumor-Associated Macrophages: From Basic Research to Clinical Application. J Hematol Oncol (2017) 10:58. doi: 10.1186/s13045-017-0430-2
49. Kashfi K, Kannikal J, Nath N. Macrophage Reprogramming and Cancer Therapeutics: Role of iNOS-Derived No. Cells (2021) 10:3194. doi: 10.3390/cells10113194
50. Chen D, Zhang X, Li Z, Zhu B. Metabolic Regulatory Crosstalk Between Tumor Microenvironment and Tumor-Associated Macrophages. Theranostics (2021) 11:1016–30. doi: 10.7150/thno.51777
51. Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L. Macrophages and Metabolism in the Tumor Microenvironment. Cell Metab (2019) 30:36–50. doi: 10.1016/j.cmet.2019.06.001
52. Lin X, Wang S, Sun M, Zhang C, Wei C, Yang C, et al. MiR-195-5p/NOTCH2-Mediated EMT Modulates IL-4 Secretion in Colorectal Cancer to Affect M2-Like TAM Polarization. J Hematol Oncol (2019) 12:1–14. doi: 10.1186/s13045-019-0708-7
53. Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu Y, et al. Tumor-Derived Exosomal miR-934 Induces Macrophage M2 Polarization to Promote Liver Metastasis of Colorectal Cancer. J Hematol Oncol (2020) 13:156. doi: 10.1186/s13045-020-00991-2
54. Wang D, Wang X, Si M, Yang J, Sun S, Wu H, et al. Exosome-Encapsulated miRNAs Contribute to CXCL12/CXCR4-Induced Liver Metastasis of Colorectal Cancer by Enhancing M2 Polarization of Macrophages. Cancer Lett (2020) 474:36–52. doi: 10.1016/j.canlet.2020.01.005
55. Zhang D, Qiu X, Li J, Zheng S, Li L, Zhao H. TGF-β Secreted by Tumor-Associated Macrophages Promotes Proliferation and Invasion Of Colorectal Cancer via miR-34a-VEGF Axis. Cell Cycle (2018) 17:2766–78. doi: 10.1080/15384101.2018.1556064
56. Mao X, Xu J, Wang W, Liang C, Hua J, Liu J, et al. Crosstalk Between Cancer-Associated Fibroblasts and Immune Cells in the Tumor Microenvironment: New Findings and Future Perspectives. Mol Cancer (2021) 20:131. doi: 10.1186/s12943-021-01428-1
57. Savardashtaki A, Shabaninejad Z, Movahedpour A, Sahebnasagh R, Mirzaei H, Hamblin MR. miRNAs Derived From Cancer-Associated Fibroblasts in Colorectal Cancer. Epigenomics (2019) 11:1627–45. doi: 10.2217/epi-2019-0110
58. Yang X, Xu X, Zhu J, Zhang S, Wu Y, Wu Y, et al. miR-31 Affects Colorectal Cancer Cells by Inhibiting Autophagy in Cancer-Associated Fibroblasts. Oncotarget (2016) 7:79617–28. doi: 10.18632/oncotarget.12873
59. Yang Y, Gu J, Li X, Xue C, Ba L, Gao Y, et al. HIF-1α Promotes the Migration and Invasion of Cancer-Associated Fibroblasts by miR-210. Aging Dis (2021) 12:1794–807. doi: 10.14336/AD.2021.0315
60. Desmond BJ, Dennett ER, Danielson KM. Circulating Extracellular Vesicle MicroRNA as Diagnostic Biomarkers in Early Colorectal Cancer-A Review. Cancers (Basel) (2019) 12:52. doi: 10.3390/cancers12010052
61. Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J, et al. Exosomes: Key Players in Cancer and Potential Therapeutic Strategy. Signal Transduct Target Ther (2020) 5:145. doi: 10.1038/s41392-020-00261-0
62. Zheng Y, Zeng J, Lin D, Xia H, Wang X, Chen L, et al. Extracellular Vesicles Derived From Cancer-Associated Fibroblast Carries miR-224-5p Targeting SLC4A4 to Promote the Proliferation, Invasion and Migration of Colorectal Cancer Cells. Carcinogenesis (2021) 42:1143–53. doi: 10.1093/carcin/bgab055
63. Raposo G, Stoorvogel W. Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J Cell Biol (2013) 200:373–83. doi: 10.1083/jcb.201211138
64. Hu JL, Wang W, Lan XL, Zeng ZC, Liang YS, Yan YR, et al. CAFs Secreted Exosomes Promote Metastasis and Chemotherapy Resistance by Enhancing Cell Stemness and Epithelial-Mesenchymal Transition in Colorectal Cancer. Mol Cancer (2019) 18:91. doi: 10.1186/s12943-019-1019-x
65. Whiteside TL. Exosome and Mesenchymal Stem Cell Cross-Talk in the Tumor Microenvironment. Semin Immunol (2018) 35:69–79. doi: 10.1016/j.smim.2017.12.003
66. Shojaei S, Hashemi SM, Ghanbarian H, Salehi M, Mohammadi-Yeganeh S. Effect of Mesenchymal Stem Cells-Derived Exosomes on Tumor Microenvironment: Tumor Progression Versus Tumor Suppression. J Cell Physiol (2019) 234:3394–409. doi: 10.1002/jcp.27326
67. Li T, Wan Y, Su Z, Li J, Han M, Zhou C. Mesenchymal Stem Cell-Derived Exosomal microRNA-3940-5p Inhibits Colorectal Cancer Metastasis by Targeting Integrin α6. Dig Dis Sci (2021) 66:1916–27. doi: 10.1007/s10620-020-06458-1
68. Zhao J, Lin H, Huang K. Mesenchymal Stem Cell-Derived Extracellular Vesicles Transmitting MicroRNA-34a-5p Suppress Tumorigenesis of Colorectal Cancer Through C-MYC/DNMT3a/PTEN Axis. Mol Neurobiol (2022) 59:47–60. doi: 10.1007/s12035-021-02431-9
69. Zhang N, Li L, Luo J, Tan J, Hu W, Li Z, et al. Inhibiting microRNA-424 in Bone Marrow Mesenchymal Stem Cells-Derived Exosomes Suppresses Tumor Growth in Colorectal Cancer by Upregulating TGFBR3. Arch Biochem Biophys (2021) 709:108965. doi: 10.1016/j.abb.2021.108965
70. Herbert SP, Stainier DYR. Molecular Control of Endothelial Cell Behaviour During Blood Vessel Morphogenesis. Nat Rev Mol Cell Biol (2011) 12:551–64. doi: 10.1038/nrm3176
71. Schaaf MB, Houbaert D, Meçe O, Agostinis P. Autophagy in Endothelial Cells and Tumor Angiogenesis. Cell Death Differ (2019) 26:665–79. doi: 10.1038/s41418-019-0287-8
72. Small EM, Olson EN. Pervasive Roles of microRNAs in Cardiovascular Biology. Nature (2011) 469:336–42. doi: 10.1038/nature09783
73. Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, et al. The Endothelial-Specific microRNA miR-126 Governs Vascular Integrity and Angiogenesis. Dev Cell (2008) 15:261–71. doi: 10.1016/j.devcel.2008.07.002
74. Yang X, Qiu J, Kang H, Wang Y, Qian J. miR-125a-5p Suppresses Colorectal Cancer Progression by Targeting VEGFA. Cancer Manag Res (2018) 10:5839–53. doi: 10.2147/CMAR.S161990
75. Chen X, Xu X, Pan B, Zeng K, Xu M, Liu X, et al. miR-150-5p Suppresses Tumor Progression by Targeting VEGFA in Colorectal Cancer. Aging (Albany NY) (2018) 10:3421–37. doi: 10.18632/aging.101656
76. Zeng Z, Li Y, Pan Y, Lan X, Song F, Sun J, et al. Cancer-Derived Exosomal miR-25-3p Promotes Pre-Metastatic Niche Formation by Inducing Vascular Permeability and Angiogenesis. Nat Commun (2018) 9:5395. doi: 10.1038/s41467-018-07810-w
77. Dou R, Liu K, Yang C, Zheng J, Shi D, Lin X, et al. EMT-Cancer Cells-Derived Exosomal miR-27b-3p Promotes Circulating Tumour Cells-Mediated Metastasis by Modulating Vascular Permeability in Colorectal Cancer. Clin Transl Med (2021) 11:e595. doi: 10.1002/ctm2.595
78. Arora S, Khan S, Zaki A, Tabassum G, Mohsin M, Bhutto HN, et al. Integration of Chemokine Signaling With non-Coding RNAs in Tumor Microenvironment and Heterogeneity in Different Cancers. Semin Cancer Biol (2022) 4:S1044-579X(22)00059-1. doi: 10.1016/j.semcancer.2022.03.002
79. Balkwill F. TNF-Alpha in Promotion and Progression of Cancer. Cancer Metastasis Rev (2006) 25:409–16. doi: 10.1007/s10555-006-9005-3
80. Huang L, Wang X, Wen C, Yang X, Song M, Chen J, et al. Hsa-miR-19a is Associated With Lymph Metastasis and Mediates the TNF-α Induced Epithelial-to-Mesenchymal Transition in Colorectal Cancer. Sci Rep (2015) 5:13350. doi: 10.1038/srep13350
81. Li H, Chen J, Huang A, Stinson J, Heldens S, Foster J, et al. Cloning and Characterization of IL-17B and IL-17C, Two New Members of the IL-17 Cytokine Family. Proc Natl Acad Sci USA (2000) 97:773–8. doi: 10.1073/pnas.97.2.773
82. Song X, Gao H, Lin Y, Yao Y, Zhu S, Wang J, et al. Alterations in the Microbiota Drive Interleukin-17C Production From Intestinal Epithelial Cells to Promote Tumorigenesis. Immunity (2014) 40:140–52. doi: 10.1016/j.immuni.2013.11.018
83. Liu J, Duan Y, Cheng X, Chen X, Xie W, Long H, et al. IL-17 is Associated With Poor Prognosis and Promotes Angiogenesis via Stimulating VEGF Production of Cancer Cells in Colorectal Carcinoma. Biochem Biophys Res Commun (2011) 407:348–54. doi: 10.1016/j.bbrc.2011.03.021
84. Lee Y, Kim SJ, Choo J, Heo G, Yoo J-W, Jung Y, et al. miR-23a-3p is a Key Regulator of IL-17c-Induced Tumor Angiogenesis in Colorectal Cancer. Cells (2020) 9:1363. doi: 10.3390/cells9061363
85. Bridges MC, Daulagala AC, Kourtidis A. LNCcation: lncRNA Localization and Function. J Cell Biol (2021) 220:e202009045. doi: 10.1083/jcb.202009045
86. Qian X, Zhao J, Yeung PY, Zhang QC, Kwok CK. Revealing lncRNA Structures and Interactions by Sequencing-Based Approaches. Trends Biochem Sci (2019) 44:33–52. doi: 10.1016/j.tibs.2018.09.012
87. Botti G, Scognamiglio G, Aquino G, Liguori G, Cantile M. LncRNA HOTAIR in Tumor Microenvironment: What Role? Int J Mol Sci (2019) 20:2279. doi: 10.3390/ijms20092279
88. Rizk NI, Abulsoud AI, Kamal MM, Kassem DH, Hamdy NM. Exosomal-Long non-Coding RNAs Journey in Colorectal Cancer: Evil and Goodness Faces Of Key Players. Life Sci (2022) 292:120325. doi: 10.1016/j.lfs.2022.120325
89. Jiang R, Tang J, Chen Y, Deng L, Ji J, Xie Y, et al. The Long Noncoding RNA lnc-EGFR Stimulates T-Regulatory Cells Differentiation Thus Promoting Hepatocellular Carcinoma Immune Evasion. Nat Commun (2017) 8:15129. doi: 10.1038/ncomms15129
90. Huang D, Chen J, Yang L, Ouyang Q, Li J, Lao L, et al. NKILA lncRNA Promotes Tumor Immune Evasion by Sensitizing T Cells to Activation-Induced Cell Death. Nat Immunol (2018) 19:1112–25. doi: 10.1038/s41590-018-0207-y
91. Zheng Y, Tian X, Wang T, Xia X, Cao F, Tian J, et al. Long Noncoding RNA Pvt1 Regulates the Immunosuppression Activity of Granulocytic Myeloid-Derived Suppressor Cells in Tumor-Bearing Mice. Mol Cancer (2019) 18:61. doi: 10.1186/s12943-019-0978-2
92. Zhou P, Lu Y, Zhang Y, Wang L. Construction of an Immune-Related Six-lncRNA Signature to Predict the Outcomes, Immune Cell Infiltration, and Immunotherapy Response in Patients With Hepatocellular Carcinoma. Front Oncol (2021) 11:661758. doi: 10.3389/fonc.2021.661758
93. Luo C, Liu F, Su W, Long P, Liang J, Hou W, et al. Prognostic Value of LINC02560 in Colorectal Cancer Correlates With Tumor Microenvironment Immunity. J Cancer (2021) 12:7507–17. doi: 10.7150/jca.64940
94. Zhou N, Chen Y, Yang L, Xu T, Wang F, Chen L, et al. LncRNA SNHG4 Promotes Malignant Biological Behaviors and Immune Escape of Colorectal Cancer Cells by Regulating the miR-144-3p/MET Axis. Am J Transl Res (2021) 13:11144–61.
95. Huang Y, Luo Y, Ou W, Wang Y, Dong D, Peng X, et al. Exosomal lncRNA SNHG10 Derived From Colorectal Cancer Cells Suppresses Natural Killer Cell Cytotoxicity by Upregulating INHBC. Cancer Cell Int (2021) 21:528. doi: 10.1186/s12935-021-02221-2
96. Ye Y, Xu Y, Lai Y, He W, Li Y, Wang R, et al. Long non-Coding RNA Cox-2 Prevents Immune Evasion and Metastasis of Hepatocellular Carcinoma by Altering M1/M2 Macrophage Polarization. J Cell Biochem (2018) 119:2951–63. doi: 10.1002/jcb.26509
97. Chen Y, Li H, Ding T, Li J, Zhang Y, Wang J, et al. Lnc-M2 Controls M2 Macrophage Differentiation via the PKA/CREB Pathway. Mol Immunol (2020) 124:142–52. doi: 10.1016/j.molimm.2020.06.006
98. Liang Z-X, Liu H-S, Wang F-W, Xiong L, Zhou C, Hu T, et al. LncRNA RPPH1 Promotes Colorectal Cancer Metastasis by Interacting With TUBB3 and by Promoting Exosomes-Mediated Macrophage M2 Polarization. Cell Death Dis (2019) 10:829. doi: 10.1038/s41419-019-2077-0
99. Lai F, Zhang H, Xu B, Xie Y, Yu H. Long non-Coding RNA NBR2 Suppresses the Progress of Colorectal Cancer In Vitro and In Vivo by Regulating the Polarization of TAM. Bioengineered (2021) 12:5462–75. doi: 10.1080/21655979.2021.1958558
100. Shoucair I, Weber Mello F, Jabalee J, Maleki S, Garnis C. The Role of Cancer-Associated Fibroblasts and Extracellular Vesicles in Tumorigenesis. Int J Mol Sci (2020) 21:6837. doi: 10.3390/ijms21186837
101. Zhou L, Zhu Y, Sun D, Zhang Q. Emerging Roles of Long non-Coding RNAs in The Tumor Microenvironment. Int J Biol Sci (2020) 16:2094–103. doi: 10.7150/ijbs.44420
102. Zhou L, Li J, Tang Y, Yang M. Exosomal LncRNA LINC00659 Transferred From Cancer-Associated Fibroblasts Promotes Colorectal Cancer Cell Progression via miR-342-3p/ANXA2 Axis. J Transl Med (2021) 19:8. doi: 10.1186/s12967-020-02648-7
103. Zhou M, Wang S, Liu D, Zhou J. LINC01915 Facilitates the Conversion of Normal Fibroblasts Into Cancer-Associated Fibroblasts Induced by Colorectal Cancer-Derived Extracellular Vesicles Through the miR-92a-3p/KLF4/CH25H Axis. ACS Biomater Sci Eng (2021) 7:5255–68. doi: 10.1021/acsbiomaterials.1c00611
104. Ren J, Ding L, Zhang D, Shi G, Xu Q, Shen S, et al. Carcinoma-Associated Fibroblasts Promote the Stemness and Chemoresistance of Colorectal Cancer by Transferring Exosomal lncRNA H19. Theranostics (2018) 8:3932–48. doi: 10.7150/thno.25541
105. Jahangiri B, Khalaj-Kondori M, Asadollahi E, Sadeghizadeh M. Cancer-Associated Fibroblasts Enhance Cell Proliferation and Metastasis of Colorectal Cancer SW480 Cells by Provoking Long Noncoding RNA Uca1. J Cell Commun Signal (2019) 13:53–64. doi: 10.1007/s12079-018-0471-5
106. Yao X, Huang J, Zhong H, Shen N, Faggioni R, Fung M, et al. Targeting Interleukin-6 in Inflammatory Autoimmune Diseases and Cancers. Pharmacol Ther (2014) 141:125–39. doi: 10.1016/j.pharmthera.2013.09.004
107. Hodge DR, Hurt EM, Farrar WL. The Role of IL-6 and STAT3 in Inflammation and Cancer. Eur J Cancer (2005) 41:2502–12. doi: 10.1016/j.ejca.2005.08.016
108. Wang J, Zhou J, Jiang C, Zheng J, Namba H, Chi P, et al. LNRRIL6, a Novel Long Noncoding RNA, Protects Colorectal Cancer Cells by Activating The IL-6-STAT3 Pathway. Mol Oncol (2019) 13:2344–60. doi: 10.1002/1878-0261.12538
109. Balkwill F. Cancer and the Chemokine Network. Nat Rev Cancer (2004) 4:540–50. doi: 10.1038/nrc1388
110. Huarte M. The Emerging Role of lncRNAs in Cancer. Nat Med (2015) 21:1253–61. doi: 10.1038/nm.3981
111. Xu M, Xu X, Pan B, Chen X, Lin K, Zeng K, et al. LncRNA SATB2-AS1 Inhibits Tumor Metastasis and Affects the Tumor Immune Cell Microenvironment in Colorectal Cancer by Regulating SATB2. Mol Cancer (2019) 18:135. doi: 10.1186/s12943-019-1063-6
112. Yu X, Yuan Z, Yang Z, Chen D, Kim T, Cui Y, et al. The Novel Long Noncoding RNA U50535 Promotes Colorectal Cancer Growth and Metastasis by Regulating CCL20. Cell Death Dis (2018) 9:751. doi: 10.1038/s41419-018-0771-y
113. McLean MH, Murray GI, Stewart KN, Norrie G, Mayer C, Hold GL, et al. The Inflammatory Microenvironment in Colorectal Neoplasia. PloS One (2011) 6:e15366. doi: 10.1371/journal.pone.0015366
114. Zhang Q, Wang W, Zhou Q, Chen C, Yuan W, Liu J, et al. Roles of circRNAs in the Tumour Microenvironment. Mol Cancer (2020) 19:14. doi: 10.1186/s12943-019-1125-9
115. Taulli R, Loretelli C, Pandolfi PP. From pseudo-ceRNAs to circ-ceRNAs: A Tale of Cross-Talk and Competition. Nat Struct Mol Biol (2013) 20:541–3. doi: 10.1038/nsmb.2580
116. Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, et al. Natural RNA Circles Function as Efficient microRNA Sponges. Nature (2013) 495:384–8. doi: 10.1038/nature11993
117. Bachmayr-Heyda A, Reiner AT, Auer K, Sukhbaatar N, Aust S, Bachleitner-Hofmann T, et al. Correlation of Circular RNA Abundance With Proliferation–Exemplified With Colorectal and Ovarian Cancer, Idiopathic Lung Fibrosis, and Normal Human Tissues. Sci Rep (2015) 5:8057. doi: 10.1038/srep08057
118. Qu S, Yang X, Li X, Wang J, Gao Y, Shang R, et al. Circular RNA: A New Star of Noncoding RNAs. Cancer Lett (2015) 365:141–8. doi: 10.1016/j.canlet.2015.06.003
119. Chen C, Huang Z, Mo X, Song Y, Li X, Li X, et al. The Circular RNA 001971/miR-29c-3p Axis Modulates Colorectal Cancer Growth, Metastasis, and Angiogenesis Through VEGFA. J Exp Clin Cancer Res (2020) 39:1–15. doi: 10.1186/s13046-020-01594-y
120. Li W, Xu Y, Wang X, Cao G, Bu W, Wang X, et al. Circcct3 Modulates Vascular Endothelial Growth Factor A and Wnt Signaling to Enhance Colorectal Cancer Metastasis Through Sponging miR-613. DNA Cell Biol (2020) 39:118–25. doi: 10.1089/dna.2019.5139
121. Jin L, Han C, Zhai T, Zhang X, Chen C, Lian L. Circ_0030998 Promotes Tumor Proliferation and Angiogenesis by Sponging miR-567 to Regulate VEGFA in Colorectal Cancer. Cell Death Discovery (2021) 7:160. doi: 10.1038/s41420-021-00544-7
122. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-Beta: “N1” Versus “N2” TAN. Cancer Cell (2009) 16:183–94. doi: 10.1016/j.ccr.2009.06.017
123. Shang A, Gu C, Wang W, Wang X, Sun J, Zeng B, et al. Exosomal circPACRGL Promotes Progression of Colorectal Cancer via the miR-142-3p/miR-506-3p- TGF-β1 Axis. Mol Cancer (2020) 19:117. doi: 10.1186/s12943-020-01235-0
124. Liu X, Qin Y, Tang X, Wang Y, Bian C, Zhong J. Circular RNA Circ_0000372 Contributes to the Proliferation, Migration and Invasion Of Colorectal Cancer by Elevating IL6 Expression via Sponging miR-495. Anticancer Drugs (2021) 32:296–305. doi: 10.1097/CAD.0000000000001002
125. Yang Y, Yan X, Li X, Ma Y, Goel A. Long non-Coding RNAs in Colorectal Cancer: Novel Oncogenic Mechanisms and Promising Clinical Applications. Cancer Lett (2021) 504:67–80. doi: 10.1016/j.canlet.2021.01.009
126. Li X, Nie J, Mei Q, Han W-D. MicroRNAs: Novel Immunotherapeutic Targets in Colorectal Carcinoma. World J Gastroenterol (2016) 22:5317–31. doi: 10.3748/wjg.v22.i23.5317
127. Chen B, Xia Z, Deng Y-N, Yang Y, Zhang P, Zhu H, et al. Emerging microRNA Biomarkers for Colorectal Cancer Diagnosis and Prognosis. Open Biol (2019) 9:180212. doi: 10.1098/rsob.180212
128. Zhang Y, Zheng L, Huang J, Gao F, Lin X, He L, et al. MiR-124 Radiosensitizes Human Colorectal Cancer Cells by Targeting PRRX1. PloS One (2014) 9:e93917. doi: 10.1371/journal.pone.0093917
129. Zhang Q-Y, Wang F-X, Jia K-K, Kong L-D. Natural Product Interventions for Chemotherapy and Radiotherapy-Induced Side Effects. Front Pharmacol (2018) 9:1253. doi: 10.3389/fphar.2018.01253
130. Balkwill FR, Capasso M, Hagemann T. The Tumor Microenvironment at a Glance. J Cell Sci (2012) 125:5591–6. doi: 10.1242/jcs.116392
131. Metcalf KJ, Alazzeh A, Werb Z, Weaver VM. Leveraging Microenvironmental Synthetic Lethalities to Treat Cancer. J Clin Invest (2021) 131:e143765. doi: 10.1172/JCI143765
132. Sadeghi Rad H, Monkman J, Warkiani ME, Ladwa R, O’Byrne K, Rezaei N, et al. Understanding the Tumor Microenvironment for Effective Immunotherapy. Med Res Rev (2021) 41:1474–98. doi: 10.1002/med.21765
133. Shu Y, Cheng P. Targeting Tumor-Associated Macrophages for Cancer Immunotherapy. Biochim Biophys Acta Rev Cancer (2020) 1874:188434. doi: 10.1016/j.bbcan.2020.188434
134. Liu Q-P, Lin J-Y, An P, Chen Y-Y, Luan X, Zhang H. LncRNAs in Tumor Microenvironment: The Potential Target for Cancer Treatment With Natural Compounds and Chemical Drugs. Biochem Pharmacol (2021) 193:114802. doi: 10.1016/j.bcp.2021.114802
135. Weng Y-S, Tseng H-Y, Chen Y-A, Shen P-C, Al Haq AT, Chen L-M, et al. MCT-1/miR-34a/IL-6/IL-6R Signaling Axis Promotes EMT Progression, Cancer Stemness and M2 Macrophage Polarization in Triple-Negative Breast Cancer. Mol Cancer (2019) 18:42. doi: 10.1186/s12943-019-0988-0
136. Otmani K, Lewalle P. Tumor Suppressor miRNA in Cancer Cells and the Tumor Microenvironment: Mechanism of Deregulation and Clinical Implications. Front Oncol (2021) 11:708765. doi: 10.3389/fonc.2021.708765
137. Mu Q, Lv Y, Luo C, Liu X, Huang C, Xiu Y, et al. Research Progress on the Functions and Mechanism of circRNA in Cisplatin Resistance in Tumors. Front Pharmacol (2021) 12:709324. doi: 10.3389/fphar.2021.709324
138. Ma Z, Shuai Y, Gao X, Wen X, Ji J. Circular RNAs in the Tumour Microenvironment. Mol Cancer (2020) 19:8. doi: 10.1186/s12943-019-1113-0
139. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T Cells and Immune Tolerance. Cell (2008) 133:775–87. doi: 10.1016/j.cell.2008.05.009
140. Sakaguchi S, Mikami N, Wing JB, Tanaka A, Ichiyama K, Ohkura N. Regulatory T Cells and Human Disease. Annu Rev Immunol (2020) 38:541–66. doi: 10.1146/annurev-immunol-042718-041717
141. Khan U, Ghazanfar H. T Lymphocytes and Autoimmunity. Int Rev Cell Mol Biol (2018) 341:125–68. doi: 10.1016/bs.ircmb.2018.05.008
142. Ning T, Li J, He Y, Zhang H, Wang X, Deng T, et al. Exosomal miR-208b Related With Oxaliplatin Resistance Promotes Treg Expansion in Colorectal Cancer. Mol Ther (2021) 29:2723–36. doi: 10.1016/j.ymthe.2021.04.028
143. Roshani Asl E, Rasmi Y, Baradaran B. MicroRNA-124-3p Suppresses PD-L1 Expression and Inhibits Tumorigenesis of Colorectal Cancer Cells via Modulating STAT3 Signaling. J Cell Physiol (2021) 236:7071–87. doi: 10.1002/jcp.30378
144. Augsten M. Cancer-Associated Fibroblasts as Another Polarized Cell Type of the Tumor Microenvironment. Front Oncol (2014) 4:62. doi: 10.3389/fonc.2014.00062
145. Zhang H-W, Shi Y, Liu J-B, Wang H-M, Wang P-Y, Wu Z-J, et al. Cancer-Associated Fibroblast-Derived Exosomal microRNA-24-3p Enhances Colon Cancer Cell Resistance to MTX by Down-Regulating CDX2/HEPH Axis. J Cell Mol Med (2021) 25:3699–713. doi: 10.1111/jcmm.15765
146. Deng X, Ruan H, Zhang X, Xu X, Zhu Y, Peng H, et al. Long Noncoding RNA CCAL Transferred From Fibroblasts by Exosomes Promotes Chemoresistance of Colorectal Cancer Cells. Int J Cancer (2020) 146:1700–16. doi: 10.1002/ijc.32608
147. Yang K, Zhang J, Bao C. Exosomal Circeif3k From Cancer-Associated Fibroblast Promotes Colorectal Cancer (CRC) Progression via miR-214/PD-L1 Axis. BMC Cancer (2021) 21:933. doi: 10.1186/s12885-021-08669-9
148. Zhou L, Li J, Liao M, Zhang Q, Yang M. LncRNA MIR155HG Induces M2 Macrophage Polarization and Drug Resistance of Colorectal Cancer Cells by Regulating ANXA2. Cancer Immunol Immunother (2021) 71:1075–91. doi: 10.1007/s00262-021-03055-7
149. Dy GK, Hobday TJ, Nelson G, Windschitl HE, O’Connell MJ, Alberts SR, et al. Long-Term Survivors of Metastatic Colorectal Cancer Treated With Systemic Chemotherapy Alone: A North Central Cancer Treatment Group Review of 3811 Patients, N0144. Clin Colorectal Cancer (2009) 8:88–93. doi: 10.3816/CCC.2009.n.014
150. Qi F-F, Yang Y, Zhang H, Chen H. Long non-Coding RNAs: Key Regulators in Oxaliplatin Resistance of Colorectal Cancer. BioMed Pharmacother (2020) 128:110329. doi: 10.1016/j.biopha.2020.110329
151. Cheng L, Sharples RA, Scicluna BJ, Hill AF. Exosomes Provide a Protective and Enriched Source of miRNA for Biomarker Profiling Compared to Intracellular and Cell-Free Blood. J Extracell vesicles (2014) 3:10. doi: 10.3402/jev.v3.23743
152. Zickler AM, El Andaloussi S. Functional Extracellular Vesicles Aplenty. Nat BioMed Eng (2020) 4:9–11. doi: 10.1038/s41551-019-0507-z
153. Chen Q, Li Y, Liu Y, Xu W, Zhu X. Exosomal Non-Coding RNAs-Mediated Crosstalk in the Tumor Microenvironment. Front Cell Dev Biol (2021) 9:646864. doi: 10.3389/fcell.2021.646864
154. Tan S, Xia L, Yi P, Han Y, Tang L, Pan Q, et al. Exosomal miRNAs in Tumor Microenvironment. J Exp Clin Cancer Res (2020) 39:67. doi: 10.1186/s13046-020-01570-6
155. Solé C, Lawrie CH. MicroRNAs in Metastasis and the Tumour Microenvironment. Int J Mol Sci (2021) 22(9):4859. doi: 10.3390/ijms22094859
156. Alzhrani R, Alsaab HO, Petrovici A, Bhise K, Vanamala K, Sau S, et al. Improving the Therapeutic Efficiency of Noncoding RNAs in Cancers Using Targeted Drug Delivery Systems. Drug Discovery Today (2020) 25:718–30. doi: 10.1016/j.drudis.2019.11.006
Keywords: colorectal cancer, tumor microenvironment, non-coding RNAs, mechanism, clinical study
Citation: Wu Z and Ju Q (2022) Non-Coding RNAs Implicated in the Tumor Microenvironment of Colorectal Cancer: Roles, Mechanisms and Clinical Study. Front. Oncol. 12:888276. doi: 10.3389/fonc.2022.888276
Received: 02 March 2022; Accepted: 04 April 2022;
Published: 28 April 2022.
Edited by:
Zhijie Xu, Central South University, ChinaReviewed by:
Jianbo Tian, Huazhong University of Science and Technology, ChinaYiming Zhao, Fudan University, China
Copyright © 2022 Wu and Ju. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiang Ju, anVxaWFuZzg4QDE2My5jb20=
 Zhaoxu Wu
Zhaoxu Wu Qiang Ju
Qiang Ju