
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 03 August 2022
Sec. Radiation Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.887525
This article is part of the Research Topic Rising stars in Radiation Oncology 2022 View all 7 articles
Background: Radiotherapy (RT)/Chemoradiotherapy (CRT) are important treatments for all stages of esophageal cancer (EC). The combination of immune checkpoint inhibitors (ICIs) with RT/CRT seems to be promising avenue for the treatment of EC. Therefore, a systematic review and meta-analysis was performed in order to assess the safety and efficacy of RT/CRT and ICI combination therapy for EC patients.
Methods: PubMed and several other databases were searched (according to specific criteria) to find relevant studies published prior to the 31st of December 2021.
Results: 1962 articles were identified for screening, and six trials containing 668 patients were identified and pooled to determine the one- and two-year overall survival (OS), which were 84.5% (95% confidence interval (CI): 69.9%-100%) and 68.3% (95% CI: 49.0%-95.1%), respectively. Additionally, the rate of pooled grade 3-5 adverse reactions was 41.0% (95% CI: 31.2%-51.2%). The rate of specific grade 3-5 adverse reactions are as follows: lymphopenia (36.8%-60%), esophagitis (20%), anastomotic leakage (18%), esophageal fistula (10%), pain (10%), leukopenia (5.3%-10%), esophageal hemorrhage (2.5%-5%), chyle leakage (3%), fatigue (5%), cough (2.7%-5%), diarrhea (2.7%), pulmonary embolism (2.5%) and allergic reaction (2.5%). The pooled rate of pneumonitis of grade 3-5 and grade 1-5 was 0.8% (95% CI: 0.1%-0.16%, I2: 0%) and 5.4% (95% CI: 2.0%-14.2%, I2: 82%). For thoracic complication, esophagitis was 63.6% (95% CI: 42.4%-80.6%), which appeared to be more frequent with the combination of ICIs to RT/CRT (12%-37.7%). Other thoracic complications include esophageal hemorrhage (2.5%-10%), esophageal fistula (6%-10%) and anastomotic leakage (6%-21%). Additionally, some of the trials did not report cardiac related adverse reactions. The subgroup analyses also revealed that the pooled rate patients with grade 3-5 pneumonitis was higher for CRT/RT with concurrent and sequential ICI treatment (1.9%) than other groups (0.8%).
Conclusion: This study suggests that the addition of ICIs to RT/CRT for EC patients may be both safe and feasible. However, larger randomized studies are needed to confirm these results.
Esophageal cancer (EC) ranks seventh among all malignant tumors in terms of morbidity. It was also the sixth leading cause of cancer related death worldwide due to its aggressive nature (1). At present, the prognosis for EC is relatively poor and is predominantly treated with surgery, radiotherapy and chemotherapy (2). Moreover, limited progress has been made in the treatment of advanced esophageal cancer, for which the prognosis remains poor with a five-year survival rate of 5% for stage IV EC cases (3).
Radiation therapy (RT)/chemoradiotherapy (CRT) has been an important treatment in all stages of EC. Since the 1990s, the Radiation Therapy Oncology Group (RTOG) 85-01 trial indicated that chemoradiotherapy should be the standard care for unresectable, locally advanced EC (4). This notion was also supported by the Chemoradiotherapy for Oesophageal Cancer Followed by Surgery Study (CROSS), Shapiro et al. concluded that neoadjuvant CRT (compared with surgery alone) could improve survival for resectable EC (5).
Immunotherapy has rapidly become one of the most promising sources of novel anti-cancer drugs. It has considerably improved the prognosis for patients with various types of cancers. Immune checkpoint inhibitors (ICIs) enable the reversion of T cell suppression and enhance anti-tumor immune responses by blocking programmed cell death 1 (PD-1, PDCD1)/programmed death-ligand 1 (PD-L1, CD274) signaling (6). Numerous ICI clinical trials have reported promising anti-tumor activity of for the treatment of EC (7–9). Some attention has been given to the clinical efficacy and safety of combination RT/CRT and ICIs for EC, but further investigation is still needed (10–15).
This is particularly important given the lack of consensus on the utility of RT/CRT and ICIs combination therapy for other cancers. Some studies have demonstrated that RT/CRT plus ICIs enabled an increased anti-tumor efficacy for non-small cell lung cancer (NSCLC) (16, 17). However, Cho et al. reported that RT-induced lymphopenia reduced the efficacy of ICIs (18). Additionally, other studies have observed increased toxicity (especially pulmonary toxicity) when ICIs were used in combination with RT/CRT (19, 20). Pre-clinical data suggested that RT generated oxidative damage to DNA and proteins in lung tissue, causing pulmonary injuries. This contributes to the release of tumor antigens and inflammatory factors, which activate T cells. ICIs also activate T cells and promote inflammation, which may damage otherwise healthy tissues when used the combination with RT. Thereby exacerbating pulmonary toxicity in addition to the amplification of anti-tumor effects (21, 22). The post hoc analysis from the phase I KEYNOTE-001 trial noted a significant increase in combination treatment related pulmonary toxicities (13% vs. 1%, P=0.046), and a borderline increase in the incidence of all pulmonary toxicities (63% vs. 40%, P=0.052), including dyspnea, cough, pneumonitis, and respiratory failure (23). Botticella et al. also observed the occurrence of grade ≥ 3 pneumonitis in 16.7% of the patients receiving combination therapy compared to 2.4% of the patients receiving ICIs alone (P=<0.001) (24). Moreover, the use of this combination therapy in trials may not reflect the real-world data. For example, patients with a history of interstitial lung disease (ILD) were excluded from the aforementioned trials. Indeed, Suresh et al. found a higher incidence of immune-associated pneumonia in real-world setting (25). For these reasons, the efficacy and safety of RT/CRT and ICIs combination therapy remains controversial. Therefore, we performed a systematic review and meta-analysis herein to elucidate the safety and efficacy of RT/CRT and ICIs combination therapy for EC.
This systematic review and meta-analysis was conducted using a Preferred Reporting Items for Systematic Review (PRISMA) and Meta-analysis statement (26). Ethical approval was not required for this study because all the data is derived from previously published sources.
PubMed, ISI Web of Science and the Cochrane Library were searched to identify literature in English language journals. This study utilized articles published prior to the 31st of December 2021, without a lower date boundary. The following search terms were used: 1) “o) esophageal neoplasm (s)/cancer (s)/carcinoma (s)/adenocarcinoma (s)/squamous cell carcinoma (s)” or “(o) esophagus neoplasm (s)/cancer (s)/carcinoma (s)/adenocarcinoma (s)/squamous cell carcinoma (s)” or “gastro esophageal neoplasm (s)/cancer (s)/carcinoma (s)” or “Barrett (s) (o) esophagus” or “(o) esophageal squamous dysplasia”. 2) “radiotherapy” or “radiation therapy” or “radiation treatment” or “radio-chemotherapy” or “chemoradiotherapy”. 3) “immunotherapy” or “immune checkpoint inhibitors” or “programmed cell death 1 receptor” or “programmed cell death 1 ligand 1” or “cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) antigen” or “anti-CTLA-4” or “anti-PD-1” or “anti-PD-L1” or “Durvalumab” or “Atezolizumab” or “Pembrolizumab” or “Nivolumab” or “Toripalima” or “Tislelizumab” or “Camrelizumab” or “Sintilimab” or “Tremelimumab” or “Ipilimumab” or “PDCD1” or “CD274”.
Studies were included in the meta-analysis if they met the following criteria: 1) describe participants with histologically confirmed esophageal cancer; 2) immune checkpoint inhibitors were used in combination with radiotherapy or chemoradiotherapy, radiotherapy with sequential or concurrent ICIs therapy, or radiotherapy alone (including conventional radiotherapy and stereotactic radiotherapy); 3) utilized a prospective or retrospective study design; 4) outcomes included clinical efficacy and treatment safety; 5) published in English.
Conference abstracts, case reports, comments, reviews, studies in animals, and mechanistic studies were excluded. Studies without sufficient data (missing clinical outcomes data) or unclear descriptions (the description of the trial was not clear or have not precisely measured or described the outcomes of trial) were also excluded. When articles described the same study population, only the most recent or the most complete analysis was included. Disagreements related to article selection were resolved during group discussions with all the authors of this study.
The following data was extracted by two independent researchers: the first author’s name, time of publication, country, number of cases, position of the tumor, pathological subgroups, treatment regimens, radiotherapy type and dose, drugs used, anti-PD-1/PD-L1 therapy dosage, time of publication. Data related to the study outcomes was also extracted, such as the overall survival (OS), progression free survival (PFS) and the number of patients who experienced adverse reaction. Any discrepancies were resolved by group discussion until a consensus was reached. The revised Cochrane Risk of Bias tool for randomized trials (RoB2) (27) and Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool (28) were used for the quality assessment of randomized controlled trials (RCTs) and non-randomized trials respectively.
A meta-analysis was performed using the random-effects model by “meta” package implemented in R (version 4.1.2, R Foundation for Statistical Computing). A 95% confidence interval (CI) was adopted. Heterogeneity among studies was assessed using Cochran’s Q and I2 statistics. I2 values of 0%, 25%, 50% and 75% representing no, low, moderate and high heterogeneity, respectively. A meta-regression was considered to be inappropriate due to the insufficient study volume (<10). Subgroup and sensitivity analyses were conducted to examine sources of study heterogeneity and determine the influence of each individual study. The possibility of publication bias was estimated using the Begg’s and Egger’s test. A threshold of P=<0.05 was used when considering the statistical significance.
The systematic study search process (Figure 1) enabled the identification of six trials (Table 1) for this systematic review and meta-analysis (10–15). The six trials consisted of five non-randomized trials (10–13, 15) and one randomized controlled trial (14). Two trials were conducted in the United States of America (14, 15), three in China (10, 12, 13), and one in the Netherlands (11). Of the six studies included, two were phase I/Ib trials (12, 13), three were phase II trials (10, 11, 15), one was a phase III trial (14). The drug camrelizumab was used in two studies (12, 13), whereas pembrolizumab (10), durvalumab (15), atezolizumab (11) and nivolumab (14) were used in one study each. ICIs were administered after CRT in two studies (14, 15), concurrently with CRT in one study (10); whereas three studies examined both concurrent and sequential administration of CRT/RT with ICIs (11–13). ICIs were administered for less than six months in two trials (10, 11), up to 12 months in two trials (14, 15) and up to 32 weeks in two other trials (12, 13). The total radiation dose was 41.4 Gy in two studies (10, 11) and 60 Gy in two other studies (12, 13). The RCT (14) was deemed to be at low overall risk of bias. Three non-randomized trials (10, 12, 13) were judged to be at moderate risk of bias, and two (11, 15) had a low overall risk of bias (Supplementary Materials Figures 1–4). A total of 668 patients were included in the aforementioned trials.
The trials included in this study had different primary efficacy variables, which prevented the performance of an efficacy based meta-analysis. The pathological complete response (pCR) rate was 30.3-55.6% for patients treated with neoadjuvant CRT and ICIs (10, 11), with a major pathological response (mPR) of 89% (10), and a 94%-100% R0 resection rate (10, 11). For locally advanced EC cases, the rate of complete remission (CR) was 10%-10.5% (12, 13), partial remission (PR) was 55%-63.2% (12, 13), with an objective response rate (ORR) of 65%-73.7% (12, 13).
The pooled two-year PFS was 63.2% (95% CI: 37.5%-83.1%, I2: 73%) (11–13), with a one-year and two-year OS of 84.5% (95% CI: 69.9%-100%, I2: 66%) (12, 13, 15) and 68.3% (95% CI: 49.0%-95.1%, I2: 78%), respectively (11–13, 15) (Figure 2).
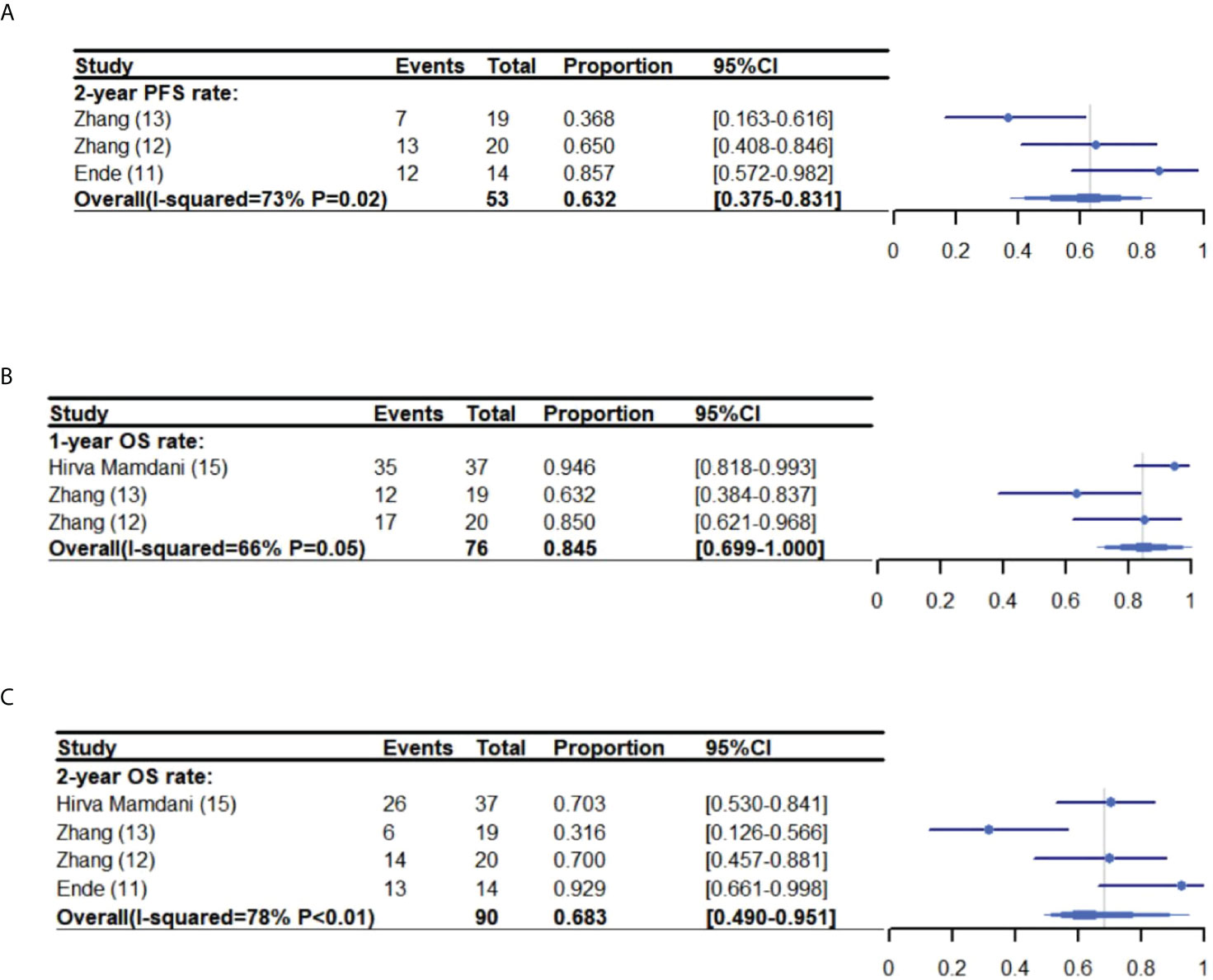
Figure 2 One-year PFS meta-analysis and forest plot (A), one-year OS (B) and two-year OS (C) for esophageal cancer patients treated with ICIs and CRT/RT. PFS, Progression free survival; OS, Overall survival; ICIs, Immune checkpoint inhibitors; CRT, Chemoradiotherapy; RT, Radiotherapy.
Mamdani, et al. (15) reported that patients who received sequential CRT and ICIs treatment had a one-year recurrence free survival rate (RFS) of 73%, a two-year RFS of 51.4%. Whereas, Kelly, et al. (14) reported a one-year disease-free survival rate (DFS) of 62%, and a one-year distant metastasis free survival rate (DMFS) of 92.1%. For locally advanced EC, Zhang, et al. reported the rate of locoregional recurrence-free survival as 62.7% at 12 months and 48.8% at 24 months (13). Additionally, Zhang, et al. found that the one-year PFS was 47.4%-80% (12, 13).
The pooled rate of grade 3-5 adverse reactions from all the studies was 41.0% (95% CI: 31.2%-51.2%, I2: 57%) (10–15) (Figure 3). The pooled rate of the other grades were not analyzed due to excessive heterogeneity, with few trials reporting related side effects. The rate of specific adverse reactions are as follows: 36.8%-60% of patients experienced Lymphopenia (10, 13), 20% radiation esophagitis (12), 18% anastomotic leakage (11), 10% esophageal fistula (12), 10% pain (12), 5.3%-10% leukopenia (10, 12, 13), 2.5%-5% esophageal hemorrhage (10, 11), 3% chyle leakage (11), 5% fatigue (12), 2.7%-5% cough (10, 12, 13, 15), 2.7% diarrhea (15), 2.5% pulmonary embolism (11) and 2.5% allergic reaction (11).
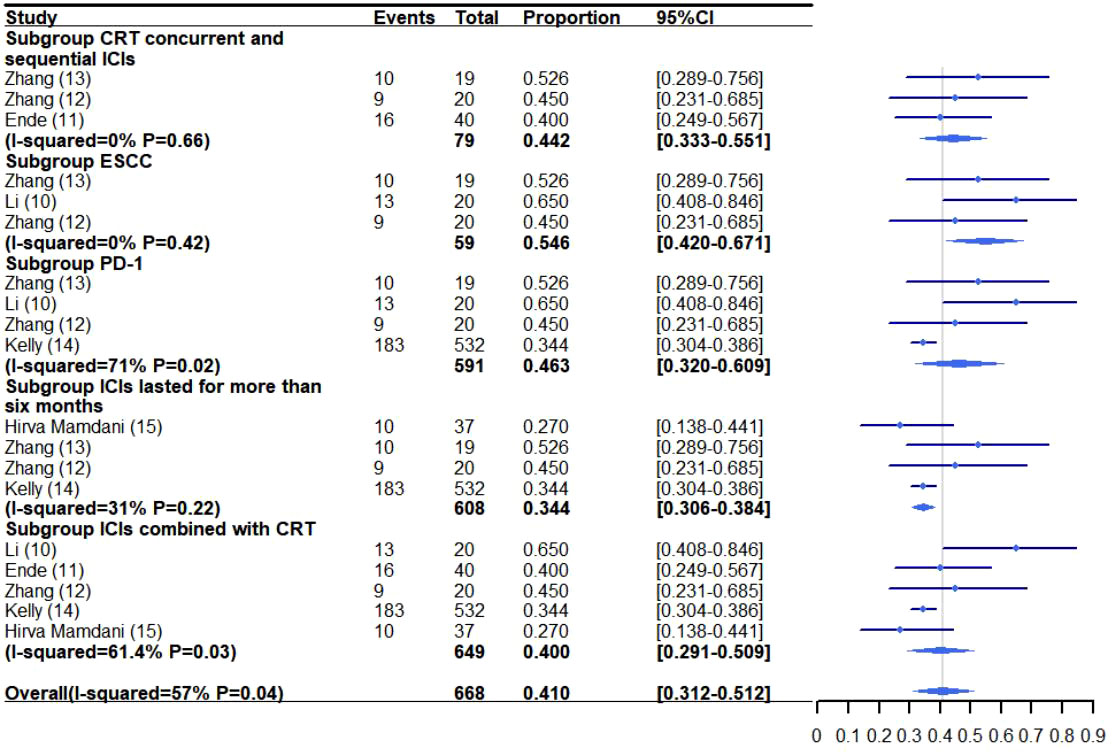
Figure 3 Grade 3-5 adverse reaction meta-analysis and forest plot for esophageal cancer patients treated with CRT/RT and ICIs. CRT, Chemoradiotherapy; RT, Radiotherapy; ICIs, Immune checkpoint inhibitors; ESCC, Esophageal squamous cell carcinoma; PD-1, Programmed death-1.
The pooled rate of grade 3-5 and grade 1-5 pneumonitis was 0.8% (95% CI: 0.1%-0.16%, I2: 0%) (10–15) and 5.4% (95% CI: 2.0%-14.2%, I2: 82%) (10–15), respectively. The incidence of grade 1-5 cough was 16.3% (95% CI: 8.3%-26.0%, I2: 56%) (12–15) (Figure 4).
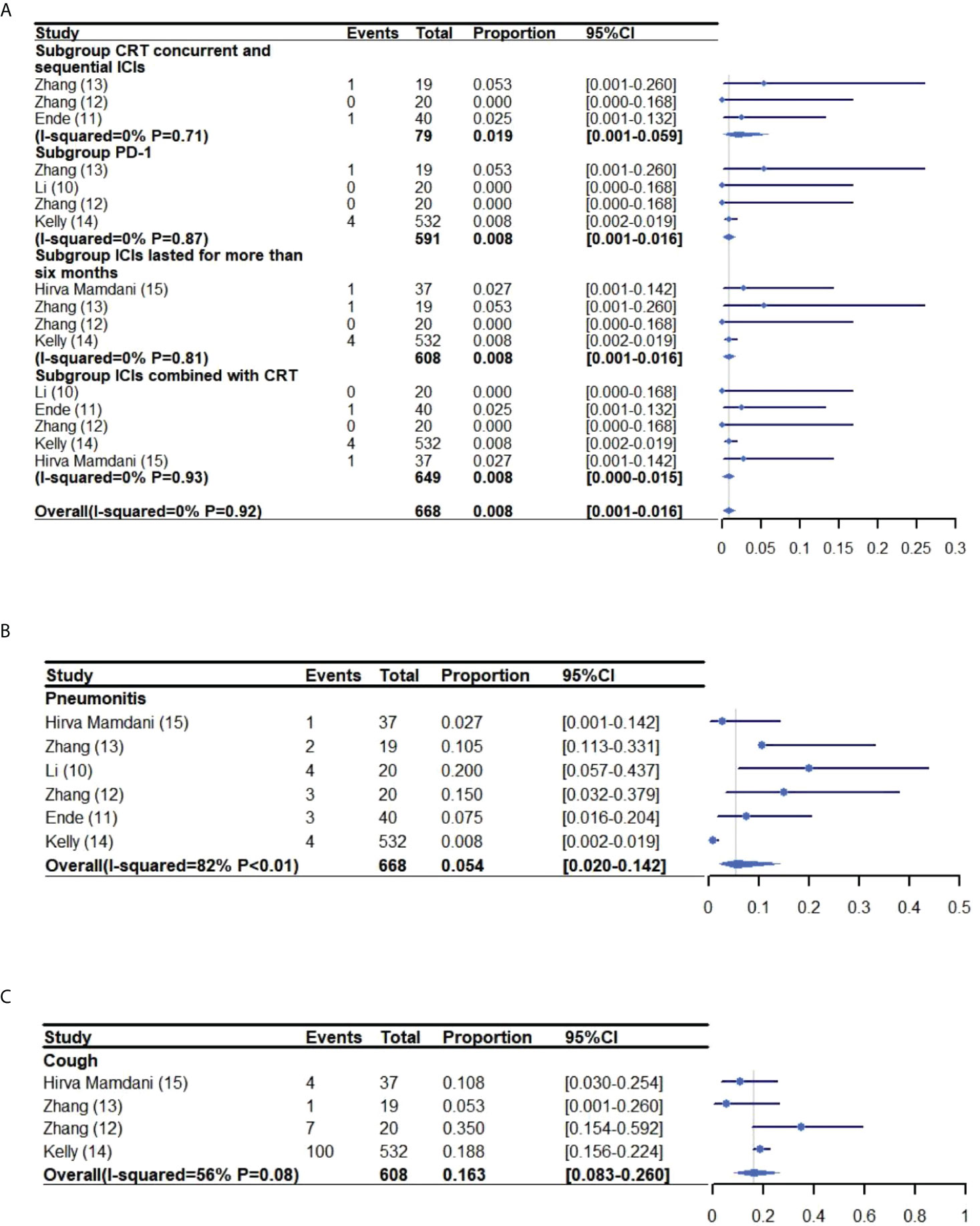
Figure 4 Meta-analysis and forest plot for esophageal cancer patients treated with CRT/RT and ICIs who experienced grade 3-5 Pneumonitis (A), grade 1-2 pneumonitis (B) and cough (C). CRT, Chemoradiotherapy; RT, Radiotherapy; ICIs, Immune checkpoint inhibitors; PD-1, Programmed death-1.
The incidence of esophagitis was 63.6% (95% CI: 42.4%-80.6%, I2: 66%) (10, 12, 13) (Figure 5). Other thoracic effects were not assessed specifically because few trials reported these side effects. However, some thoracic effects were reported as follows: anastomotic leakage (6.0%-21.0%) (10, 11), chyle leakage (15.0%) (11), esophageal fistula (6%-10%) (10, 12), pulmonary embolism (8%) (12), esophageal stenosis (5%) (11, 12), esophageal hemorrhage (2.5%-10%) (10, 11) and chylothorax (2.5%) (11). Additionally, some of the trials did not report cardiac related adverse reactions.
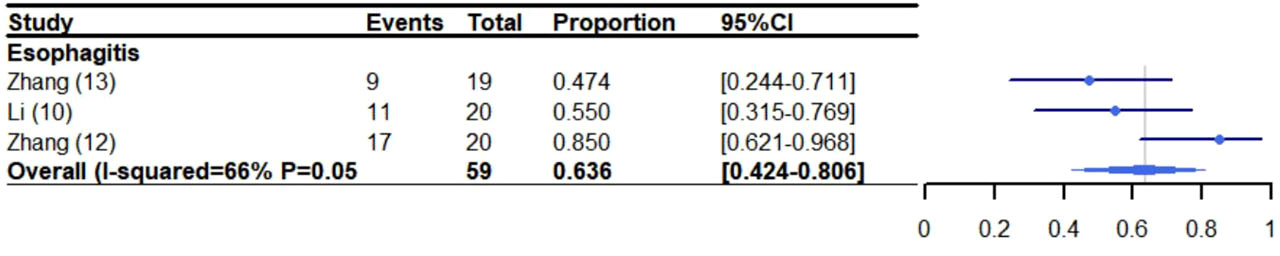
Figure 5 Meta-analysis and forest plot for patients who experienced esophagitis receiving CRT/RT and ICIs. CRT: Chemoradiotherapy; RT: Radiotherapy; ICIs: Immune checkpoint inhibitors.
A pooled meta-analysis of other adverse reactions was presented in Supplementary Materials Figure 5. 16.1% (95% CI: 13.2%-16.1%, I2: 0%) of patients experienced vomiting (10, 11, 14), 22.6% (95% CI: 13.7%-32.9%, I2: 0%) constipation (11–13), 16.7% (95% CI: 13.8%-19.7%, I2: 0%) diarrhea (10, 11, 14, 15), 0.9% (95% CI: 0.0%-3.1%, I2: 33%) gastric bleeding (10, 11, 14), (95% CI: 7.6%-12.5%, I2: 8%) hypothyroidism (12–15) 9.7% and 9.0% (95% CI: 6.7%-11.5%, I2: 0%) skin rash (11, 14, 15).
A subgroup analysis was performed to investigate the incidence of grade 3-5 adverse reactions for patients receiving concurrent and sequential CRT/RT and ICI treatment (11–13) (Figures 3–4A). The rate of grade 3-5 adverse reactions was 44.2% (95% CI: 33.3%-55.1%, I2: 0%) and grade 3-5 pneumonitis was 1.9% (95% CI: 0.1%-5.9%, I2: 0%).
The incidence of grade 3-5 adverse reactions for ESCC patients treated with combination therapy was reported in three trials (10, 12, 13) (Figures 3–4A). The pooled rate of grade 3-5 adverse reactions was 54.6% (95% CI: 42.0%-67.1%, I2: 0%).
The rate of grade 3-5 adverse reactions in patients receiving CRT/RT and PD-1 inhibitors was 46.3% (95% CI: 32.0%-60.9%, I2: 71%) and 0.8% (95% CI: 0.1%-1.5%, I2: 0%) for grade 3-5 pneumonitis (10, 12–14) (Figures 3–4A).
ICIs were administered for more than six months in four trials (12–15). The pooled incidence of grade 3-5 adverse reactions was 34.4% (95% CI: 30.6%-38.4%, I2: 31%) and grade 3-5 pneumonitis was 0.8% (95% CI: 0.1%-1.6%, I2: 0%) (Figures 3–4A).
The rate of grade 3-5 adverse reactions in patients receiving CRT and ICIs was 40.0% (95% CI: 29.1%-50.9%, I2: 61.4%) and 0.8% (95% CI: 0.1%-1.5%, I2: 0%) for grade 3-5 pneumonitis (10–12, 14, 15) (Figures 3–4A).
There was some publication bias for the of grade 3-5 pneumonitis (ESCC) subgroup analysis. So, the grade 3-5 pneumonitis subgroup analysis was not conducted. The Begg’s and Egger’s tests found that there was no publication bias in the other analyses (Supplementary Materials Table 1).
The study contained herein provides an overview of published trials that focus upon the use of RT/CRT with ICIs in EC patients. This review and meta-analysis, systematically, quantitatively and comprehensively analyzes the clinical efficacy and safety of RT/CRT when combined with ICIs for the treatment of EC. However, it does have some limitations that are mostly related to the availability of data/studies in this field. This prevented the exploration of some details surrounding the efficacy and safety of RT/CRT plus ICIs (such as the influence of different types of chemotherapeutics, radiotherapy doses, fractions, and target volumes. Additionally, most of the studies included in the meta-analysis were single arm clinical trials, which prevents a comparison between the advantages and disadvantages of CRT/RT with ICIs and CRT/RT based upon a balanced baseline.
The primary efficacy variable published by each study was also different. Therefore, the analysis could only cover the one-year OS (84.5%: 95% CI: 69.9%-100%, I2: 66%), two-year OS (60.0%: 95% CI: 41.2%-87.5%, I2: 62%) and two-year PFS (63.2%: 95% CI: 37.5%-83.1%, I2: 73%). Nevertheless, this enabled the estimation that the 2-year OS rate was 36.4%-61.5% for patients with locally advanced EC patients treated CRT (29–32). EC patients treated neoadjuvant CRT and ICIs had a pCR rate of 30.3% in the PERFECT trial (11) and 55.6% in the PALACE-1 trial (10). The pCR rate was higher in PALACE-1 trial than that reported by the other trials, which only administered neoadjuvant CRT (33–36). Except for the CheckMate-577 trial, which evaluated the adjuvant use of nivolumab for patients that were administered neoadjuvant CRT and patients post resection with residual pathologic tissue. In 2021, the US Food and Drug Administration (FDA) (37) and the European Medicines Agency (EMA) (38) approved the use of nivolumab for EC patients experiencing disease progression following CRT, which was associated with superior DFS when compared with a placebo (median DFS, 22.4 vs. 11 months, HR 0.69; 95% CI, 0.56–0.86, P=<0.001).
Additionally, this meta-analysis suggests that the rate of grade 3-5 adverse reactions was similar for patients receiving/RT and ICIs when compared to RT/CRT alone (27%-61.5%) (29, 33, 39, 40). The incidence of patients experiencing grade 3-5 adverse reactions ranged from 27% to 65%, with an overall rate of 41.0% (95% CI: 31.2%-51.2%) in the pooled analysis. The rate of grade 3-5 pneumonitis was 0.0%-5.3%, with a rate of 0.8% (95% CI: 0.1%-0.16%) in the pooled analysis. Cardiac related adverse reactions were not reported by the trials, with the exception of CheckMate 577 (14), which reported the death of one patient due to cardiac arrest. However, that event was not thought to be related to ICIs by the investigators. Therefore, it appears that the combination of RT/CRT with ICIs is a safe treatment option, although further study may be warranted.
It is notable that few trials reported esophageal related side effects, which prevented the performance of a pooled analysis. But, it appears that combined treatment did not increase the occurrence of esophageal hemorrhage (2.5%-10% vs 0.5-8%) (33, 34), esophageal fistula (6%-10% vs 1.1%–22%) (33, 41, 42) and anastomotic leakage (6%-21% vs 8.6-22%) (33, 34). Nevertheless, future trials should probably pay attention to the reporting of esophageal events, which as the site of this cancer could be at risk of perforation and bleeding. Indeed, one patient with grade III lymphopenia experienced significant esophageal hemorrhage after the second dose of chemotherapy and died while awaiting surgery (10). The NEOCRTEC5010 (33) and CROSS trials (43) also reported the death of patients due to esophageal hemorrhage. This notion is also supported by the pooled esophagitis analysis, since 63.6% (95% CI: 42.4%-80.6%, I2: 66%) (10, 12, 13) of patients experienced this event when administered an ICI and CRT combination, which was more frequent when compared to patients receiving CRT alone (12%-37.7%) (30, 33, 34, 44, 45). However, this finding should be interpreted with caution due to the relatively small number of cases reported.
The subgroup analyses revealed that the pooled rate of grade 3-5 pneumonitis was higher for patients treated with CRT/RT and concurrent/sequential ICIs (1.9%) when compared to patients receiving other regimens (0.8%). However, it remains unknown whether there are toxicity differences between concurrent and sequential administration of CRT/RT with ICIs for EC patients. This notion may require further investigation because Zhang, et al. (46) reported that patients treated with ICIs before or during thoracic radiotherapy developed radiation pneumonia at a higher rate than patients treated with ICIs after radiotherapy (27/45 vs 14/50, 60% vs 28%, P=0.01). Hence, EC patients may also experience an increased incidence of adverse reactions when treated with ICIs before or during. However, we also think the addition of ICIs to RT/CRT for EC patients may be both safe and feasible.
Esophageal adenocarcinomas (EAC) and esophageal squamous cell carcinomas (ESCC) have distinct histopathology, epidemiology, and molecular characteristics. A comprehensive molecular analysis showed that ESCC is more similar to squamous cell carcinoma located in other organs, whereas EAC is more similar to the chromosomal instability subtype of gastric cancer (47). Positive PD-L1 expression seems higher in ESCC than EAC (48–51). A meta-analysis evaluating the prognostic value of PD-L1 in ESCC showed a correlation of high PD-L1 expression with distant metastasis and poor OS (49). However, PD-L1 expression did not seem to affect survival in EAC (51). Interestingly, KEYNOTE-180 evaluated pembrolizumab in a third and further- setting for patients with advanced and metastatic esophageal cancer (52), the ORR in the whole population was 9.9% (95% CI, 5.2–16.7), 14.3% (95% CI, 6.7–25.4) among ESCC, and 5.2% (95% CI, 1.1–14.4) among EAC. Based on these positive results, the KEYNOTE-181 trial, investigating pembrolizumab versus chemotherapy in patients with advanced/metastatic SCC or AC of the esophagus, which progressed after one prior therapy session, was initiated. Although pembrolizumab showed promising results in the overall cohort, the most significant benefit was seen in ESCC (8.2 versus 7.1 months; HR 0.78; 95% CI 0.63–0.96; p = 0.0095) (53). Additionally, the combination of the CROSS regimen with adjuvant nivolumab in the aforementioned CheckMate 577 (14) trial showed a greater disease-free survival benefit in the ESCC subgroup (AC: placebo 11.1 months (95% CI 8.3–16.8) versus nivolumab after 19.4 months (95% CI 15.9–29.4); SCC: placebo 11.0 months (95% CI 7.6–17.8) and versus nivolumab after 29.7 months (95%CI 14.4–not estimated).
These findings indicate that the combination of systemic and radiotherapy acts by sensitizing SCC cells, and thereby leads to a major survival benefit compared to other strategies. Three of the studies included herein explored the efficacy and adverse events associated with RT/CRT and ICIs combination therapy for esophageal squamous-cell carcinoma patients, and only one study explored the efficacy and adverse event of RT/CRT and ICIs combination for esophageal adenocarcinoma patients. Due to these differing primary efficacy variables, we were unable to prevente the performance/efficacy based upon ESCC and EAC status. An AE subgroup analysis was performed for ESCC, which found that the incidence of grade 3-5 adverse reactions was 54.6% (95% CI: 42.0%-67.1%, I2: 0%) for ESCC. The difference between ESCC and EAC in the efficacy of RT/CRT with ICIs is likely worth further exploration.
In our study, the rate of grade 3-5 adverse reactions and pneumonitis in patients receiving CRT/RT and PD-1 inhibitors was 46.3% (95% CI: 32.0%-60.9%, I2: 71%) and 0.8% (95% CI: 0.1%-1.5%, I2: 0%). Drugs against PD-L1 were used in two studies and PD-1 in four studies. However, the limited number of studies prevented the further exploration of the differences between PD-1 and PD-L1. A previous study stated that the toxicity profiles of PD-1 and PD-L1 inhibitors in NSCLC patients are similar (54). Gu et al. found that pneumonitis was more frequent when using PD-1 inhibitors for lung cancer, but hepatitis, rash and lipase elevation were more frequent in PD-L1 inhibitors (55). At present, there is no head-to-head study to compare the difference in AEs between PD-1 and PD-L1 inhibitors combined with RT. Although PD-1 inhibitors have been associated with a significantly higher incidence of high-grade immune-related pneumonitis (55–58). The potential mechanism involved in the higher incidence of pneumonitis may be the blockage of PD-1-PD-L2 and induced by PD-1 inhibitors. This blockage assists in the release of cytokines and proliferation of self-reactive T cells, leading to the enhancement of the antitumor effect and AEs (59). Li et al. (58) thought that RT rather than ICIs might be the leading reason for the similar incidence of PD-1 and PD-L1 inhibitors when combined with RT. Thus, the selection of candidate ICIs is recommended, primarily depending on their efficacy rather than the toxicity.
Few studies have explored the optimal schedule for the administration of RT/CRT with ICIs for EC. In our study, we found the incidence of grade 3-5 adverse reactions was 44.2% (95% CI: 33.3%-55.1%, I2: 0%) and grade 3-5 pneumonitis was 1.9% (95% CI: 0.1%-5.9%, I2: 0%) for patients receiving concurrent and sequential CRT/RT and ICI treatment. The limited number of studies prevented a subgroup analysis for ICIs when used concurrently with RT/CRT and ICIs with sequential RT/CRT. Sihag et al. (60) investigated the safety and feasibility ICIs treatment prior to CRT (followed by surgery after 6 to 8 weeks and then adjuvant ICI therapy for 6 months). However, their primary end point for major complications was 30-days, therefore further is analysis needed to explore the sequencing of CRT and ICIs for EC. There remains no definite conclusion regarding this factor. However, OS was found to be significantly (P=0.01) worse for metastatic cancer (80% were lung cancer and 20% were other cancers) patients receiving stereotactic body radiotherapy (SBRT/SRT) after completing immunotherapy (3.6 months) when compared to patients that either received SRT before or concurrently with immunotherapy ICIs (13.0 months) (61). Price, et al. (62) also suggested that treatment with ICIs prior to RT was associated with greater PFS (compared to RT after ICIs) for metastatic NSCLC. But, Lesueur, et al. suggested that there may not be OS or PFS differences based upon whether RT was administered before or during/after ICIs for metastatic NSCLC (63).
It is interesting that some preclinical studies have suggested that the optimal timing may depend on the type of ICI. Anti-CTLA-4 therapies were found to be most effective when given prior to radiation therapy, whereas the optimal timing of anti-OX40 delivery was one day following RT during the post-radiation window of increased antigen presentation (64). Recently, Anscher et al. (65) assessed whether there was an increased risk of serious AEs associated with RT when given within 90 days prior to an ICI. The study utilized 16,835 patients from 68 prospective trials for ICIs that were submitted in initial or supplemental licensing applications in the US Food and Drug Administration (FDA) databases through December 2019. In this pooled analysis, they found that the administration of an ICI within 90 days following RT did not appear to be associated with an increased risk of serious AEs. The RT ≤ 90 patients had slightly numerically higher rates of fatigue, endocrinopathies, and pneumonitis vs the no-RT group. These differences were due to low-grade (grade 1-2) AEs, as there was no difference in grade 3 to 4 AEs between the RT and no-RT groups. Thus, it does appear to be safe to administer an ICI within 90 days of receiving RT.
Based upon the current evidence, the use of RT/CRT combined with ICIs appears promising. Nevertheless, a consensus has yet to be reached on many other features that could contribute to the optimal combination strategy (segmentation mode, dose of radiotherapy, selection of chemotherapy regimen and applicability of biomarkers, etc.). A number of EC clinical trials are ongoing, which investigate the use of with CRT/RT with ICIs, some of which are summarized in Table 2.
Different radiotherapy dosages and segmentation modes have been reported to have different effects upon the tumor associated immune system. Many pre-clinical studies have suggested that 8-10 Gy in a single fraction appears to generate are more effective anti-tumor when response compared to 2.0 Gy in a single fraction (66–68). A pooled analysis of the PEMBRO-RT and MDACC trials by Welsh et al. found that pembrolizumab when combined with ablative RT (24Gy/3 fractions and 50Gy/4 fractions) had significantly (P=<0.05) better ORRs (48% and 54%, respectively) when compared to non-ablative RT (18% ORR with 45Gy/15 fractions) and pembrolizumab alone (20%). It is possible that the higher response rate to ablative RT (compared to non-ablative RT) was due to detrimental effects of non-ablative RT on absolute lymphocyte counts (69).
Other studies have explored the use of 0.5 to 2.0 Gy (with 1 or a few fractions) low-dose radiation therapy (LDRT) to enhance the abscopal response of distant tumors, and to increase the immunogenicity of “cold tumors” (70, 71).The use of hypo-fractionated radiation therapy (HFRT) in combination with ICIs has also been investigated for the induction of antitumor T cell responses. Bilateral mouse tumor models and patients with stage IV NSCLC have demonstrated that a better systemic antitumor response is possible using a triple treatment consisting of LDRT, HFRT and ICIs. Of the nine patients (with metastatic NSCLC) treated with this triple therapy, PR was achieved for three patients and stable disease (SD) for two patients (72). It has been established that it is not feasible to irradiate large segments with HFRT to treat EC. Therefore, LDRT has been proposed for the treatment of advanced EC (metastatic foci), which may warrant further study.
Further consideration should be given to selection of elective nodal irradiation (ENI) because lymph nodes can be a repository for lymphocyte clones against specific antigens, which might affect the curative effects of immunotherapy. It is thought that EC, lung cancer and other thoracic tumor might be more susceptible to radiotherapy due to its effects on circulating lymphocytes that receive different radiation doses as they pass through large blood vessels, the heart and pulmonary circulation. In a preclinical model, Marciscano, et al. showed that stereotactic radiation therapy (SRT) with ENI restrained the adaptive immune response (compared to SRT alone). This effect was associated with the modulation of the chemoattractant and chemokine signature, which led to the reduction of tumor-specific effector T-cell intra-tumoral infiltration and an unfavorable balance between effector T cells and regulatory T cells. Furthermore, ENI was shown to attenuate the combined efficacy of RT and anti-CTLA-4 therapy (73). Another study suggested that tumor draining lymph nodes were enriched with PD-1+ T cells, which was associated with prognosis in melanoma following the selective targeting of PD-L1 via the induction of effective anti-tumor T-cell responses (74). Therefore, the benefit of omitting ENI could be tested when combining RT/CRT with ICIs for localized/locally advanced disease. However, there is a risk that the omission of ENI could be deleterious for patients with micro-metastases.
There are still some difficulties associated with the identification of patients who would derive the most survival benefit from combination therapy or patients who are more likely to suffer from adverse reactions. PD-L1 expression is a potential biomarker for checkpoint inhibitors in clinical practice. In esophageal cancers, PD-L1 expression and its use for the prediction of immunotherapy efficacy is still controversial. In the KEYNOTE-180 (52) and KEYNOTE-181 (53) trails, patients with CPS PD-L1≥10 seemed to be associated with a slight tumor response improvement when compared to PD-L1 negative patients. Whereas, CheckMate-032 found PD-L1 expression did not correlate with tumor response (75). This indicates that PD-L1 expression is not a perfect marker for immunotherapy outcome.
Current candidate biomarkers also include (76) (1): Cell surface markers: FAS ligands and tumor antigen-specific T cells. (2) Tumor infiltrating lymphocytes (TIL): CD8+ TIL, PD-1+ CD8+ T cells, PD-L1high regulatory T cells (Tregs). (3) Immune related gene expression profiling (GEP): including genes involved with active gamma-interferon (γ-IFN) signaling, T cell cytolytic activity, antigen presentation, chemokine production and adaptive resistance. (4) Tumor mutational burden (TMB). (5) Liquid biopsies for circulating biomarkers: circulating tumor DNA (ctDNA), peripheral blood cells and lymphocyte ratios, such as white blood cells, neutrophil cells, NK cells, monocytes, platelet, lactate dehydrogenase (LDH), neutrophil to lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and lymphocyte to monocyte ratio (LMR). (6) Imaging biomarkers: standardized uptake value (SUV), metabolic tumor volume (MTV), and tumor lesion glycolysis (TLG) based upon [18F]- fluoro-2-deoxy-d-glucose (18F-FDG) positron emission tomography (PET). At present, only PD-L1 expression levels on tumor cells have been widely used as a standard predictor to drive anti-PD-1/PD-L1 treatment in the clinic, while multiple other markers detected by genomic, transcriptomic, proteomic and metabolomic analyses are still being investigated and validated.
The combination of RT/CRT and ICIs at any stage of EC (i.e., from early stage to both oligo- and poly-metastatic EC) is offering new hope for the treatment of patients with EC. However, given the dual effect of RT/CRT upon the host immune system the RT/CRT schedule must be optimized whenever a synergistic effect of the combination of RT/CRT and ICIs is expected. To reach this objective, several traditional dogmas about RT might need to be explored in this new therapeutic era, regarding dose, fractionation, target volumes, dose to organs at risk and dose delivery techniques. For EC, how can the timing of RT/CRT and ICIs be used and which chemotherapy regimen may be more effective? Is it better to combine with a PD-1 inhibitor or PD-L1 inhibitor? In the case of sufficient chemotherapy and immunotherapy, can a better therapeutic gain ratio be achieved via the reduction of radiation field or radiation dose? Can abscopal response for distant EC be induced by external irradiation of other metastases when combined with ICIs? Additionally, biomarkers to identify the most sensitive population are still being investigated and validated. Thus, both translational and clinical studies are necessary to better understand the mechanisms underlying the immune effects of RT and to provide a strong rationale for this combination.
Our review has some limitations. Firstly, because of the paucity of available data in this field, the number of included studies in our analysis was low. Secondly, most of the included studies in the meta-analysis were single arm clinical trials, we could not compare the advantages and disadvantages of CRT/RT + ICIs and CRT/RT based on a balanced baseline. Thirdly, certain results may contain a high amount of statistical heterogeneity, so subgroup analyze were conducted to examine sources of study heterogeneity. Finally, due to the available data in this field, we could not explore more details regarding the efficacy and safety of ICIs plus RT/CRT, such as the influence of different radiotherapy dose and fractionation, irradiated target volumes, chemotherapy regimen, etc. on the relationship of ICIs plus RT/CRT for EC patients.
In conclusion, this is the first systematic review and meta-analysis to explore the clinical efficacy and safety of immune checkpoint inhibitors when used in combination with radiotherapy/chemoradiotherapy for esophageal cancer patients. Based upon the study contained herein, this mode of therapy appears to be both safe and feasible. However, randomized studies with larger groups of patients need to performed to confirm these results.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
YZ conceptualized the study. JW, RD, TN, QZ and YL collected the data. JW, RD, TN and FT analyzed the data. JW and YZ wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the: (1) Science and Technology Fund Project of Guizhou Health Commission (grant no. gzwjkj2020-1-032); (2) Guizhou Province high-level Innovative Talents (grant no. GZSYQCC [2016] 003); (3) LIAN YUN GANG SHI HUI LAN PUBLIC FOUNDATION (HL-HS2020-33); (4) Clinical special of Science and Technology Department of Guizhou Province (grant no. Qiankehechengguo-LC [2021] 015); (5) Health Commission Science and Technology Foundation of Guizhou Province [gzwkj 294 2022-028].
This is a short text to acknowledge the contributions of specific colleagues, institutions, or agencies that aided the efforts of the authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.887525/full#supplementary-material
1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: Cancer J Clin (2015) 65(2):87–108. doi: 10.3322/caac.21262
2. Kitagawa Y, Uno T, Oyama T, Kato K, Kato H, Kawakubo H, et al. Esophageal cancer practice guidelines 2017 edited by the Japan esophageal society: Part 1. Esophagus (2019) 16(1):1–24. doi: 10.1007/s10388-018-0641-9
3. Thrift AP. Barrett's esophagus and esophageal adenocarcinoma: How common are they really? Digestive Dis Sci (2018) 63(8):1988–96. doi: 10.1007/s10620-018-5068-6
4. Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr., Al-Sarraf M, et al. Chemoradiotherapy of locally advanced esophageal cancer: Long-term follow-up of a prospective randomized trial (Rtog 85-01). radiation therapy oncology group. JAMA (1999) 281(17):1623–7. doi: 10.1001/jama.281.17.1623
5. Shapiro J, van Lanschot JJB, Hulshof M, van Hagen P, van Berge Henegouwen MI, Wijnhoven BPL, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (Cross): Long-term results of a randomised controlled trial. Lancet Oncol (2015) 16(9):1090–8. doi: 10.1016/s1470-2045(15)00040-6
6. Kurtulus S, Madi A, Escobar G, Klapholz M, Nyman J, Christian E, et al. Checkpoint blockade immunotherapy induces dynamic changes in pd-1(-)Cd8(+) tumor-infiltrating T cells. Immunity (2019) 50(1):181–94.e6. doi: 10.1016/j.immuni.2018.11.014
7. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (Checkmate 649): A randomised, open-label, phase 3 trial. Lancet (London England) (2021) 398(10294):27–40. doi: 10.1016/s0140-6736(21)00797-2
8. Sun JM, Shen L, Shah MA, Enzinger P, Adenis A, Doi T, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (Keynote-590): A randomised, placebo-controlled, phase 3 study. Lancet (London England) (2021) 398(10302):759–71. doi: 10.1016/s0140-6736(21)01234-4
9. Kato K, Cho BC, Takahashi M, Okada M, Lin CY, Chin K, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (Attraction-3): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2019) 20(11):1506–17. doi: 10.1016/s1470-2045(19)30626-6
10. Li C, Zhao S, Zheng Y, Han Y, Chen X, Cheng Z, et al. Preoperative pembrolizumab combined with chemoradiotherapy for oesophageal squamous cell carcinoma (Palace-1). Eur J Cancer (Oxford Engl 1990) (2021) 144:232–41. doi: 10.1016/j.ejca.2020.11.039
11. van den Ende T, de Clercq NC, van Berge Henegouwen MI, Gisbertz SS, Geijsen ED, Verhoeven RHA, et al. Neoadjuvant chemoradiotherapy combined with atezolizumab for resectable esophageal adenocarcinoma: A single-arm phase ii feasibility trial (Perfect). Clin Cancer Res (2021) 27(12):3351–9. doi: 10.1158/1078-0432.Ccr-20-4443
12. Zhang W, Yan C, Zhang T, Chen X, Dong J, Zhao J, et al. Addition of camrelizumab to docetaxel, cisplatin, and radiation therapy in patients with locally advanced esophageal squamous cell carcinoma: A phase 1b study. Oncoimmunology (2021) 10(1):1971418. doi: 10.1080/2162402x.2021.1971418
13. Zhang W, Yan C, Gao X, Li X, Cao F, Zhao G, et al. Safety and feasibility of radiotherapy plus camrelizumab for locally advanced esophageal squamous cell carcinoma. oncologist (2021) 26(7):e1110–e24. doi: 10.1002/onco.13797
14. Kelly RJ, Ajani JA, Kuzdzal J, Zander T, Van Cutsem E, Piessen G, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer. New Engl J Med (2021) 384(13):1191–203. doi: 10.1056/NEJMoa2032125
15. Mamdani H, Schneider B, Perkins SM, Burney HN, Kasi PM, Abushahin LI, et al. A phase ii trial of adjuvant durvalumab following trimodality therapy for locally advanced esophageal and gastroesophageal junction adenocarcinoma: A big ten cancer research consortium study. Front Oncol (2021) 11:736620. doi: 10.3389/fonc.2021.736620
16. Gray JE, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Three-year overall survival with durvalumab after chemoradiotherapy in stage iii nsclc-update from pacific. J Thorac Oncol (2020) 15(2):288–93. doi: 10.1016/j.jtho.2019.10.002
17. Lin SH, Lin Y, Yao L, Kalhor N, Carter BW, Altan M, et al. Phase ii trial of concurrent atezolizumab with chemoradiation for unresectable nsclc. J Thorac Oncol (2020) 15(2):248–57. doi: 10.1016/j.jtho.2019.10.024
18. Cho Y, Park S, Byun HK, Lee CG, Cho J, Hong MH, et al. Impact of treatment-related lymphopenia on immunotherapy for advanced non-small cell lung cancer. Int J Radiat oncology biology Phys (2019) 105(5):1065–73. doi: 10.1016/j.ijrobp.2019.08.047
19. Li M, Gan L, Song A, Xue J, Lu Y. Rethinking pulmonary toxicity in advanced non-small cell lung cancer in the era of combining anti-Pd-1/Pd-L1 therapy with thoracic radiotherapy. Biochim Biophys Acta Rev Cancer (2019) 1871(2):323–30. doi: 10.1016/j.bbcan.2019.02.004
20. Bang A, Wilhite TJ, Pike LRG, Cagney DN, Aizer AA, Taylor A, et al. Multicenter evaluation of the tolerability of combined treatment with pd-1 and ctla-4 immune checkpoint inhibitors and palliative radiation therapy. Int J Radiat oncology biology Phys (2017) 98(2):344–51. doi: 10.1016/j.ijrobp.2017.02.003
21. Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-Pd-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest (2014) 124(2):687–95. doi: 10.1172/jci67313
22. Hwang WL, Pike LRG, Royce TJ, Mahal BA, Loeffler JS. Safety of combining radiotherapy with immune-checkpoint inhibition. Nat Rev Clin Oncol (2018) 15(8):477–94. doi: 10.1038/s41571-018-0046-7
23. Shaverdian N, Lisberg AE, Bornazyan K, Veruttipong D, Goldman JW, Formenti SC, et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: A secondary analysis of the keynote-001 phase 1 trial. Lancet Oncol (2017) 18(7):895–903. doi: 10.1016/s1470-2045(17)30380-7
24. Botticella A, Ibrahim T, Mezquita L, Hendriks L, Le Pavec J, Ferrara R, et al. P1.01-07 immune-related pneumonitis in nsclc patients treated with immune checkpoint inhibitors (Ici): Impact of previous thoracic radiotherapy. J Thorac Oncol (2018) 13(10):S461–2. doi: 10.1016/j.jtho.2018.08.563
25. Suresh K, Voong KR, Shankar B, Forde PM, Ettinger DS, Marrone KA, et al. Pneumonitis in non-small cell lung cancer patients receiving immune checkpoint immunotherapy: Incidence and risk factors. J Thorac Oncol (2018) 13(12):1930–9. doi: 10.1016/j.jtho.2018.08.2035
26. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The prisma statement. PLoS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
27. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: A revised tool for assessing risk of bias in randomised trials. BMJ (Clinical Res ed) (2019) 366:l4898. doi: 10.1136/bmj.l4898
28. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. Robins-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Clinical Res ed) (2016) 355:i4919. doi: 10.1136/bmj.i4919
29. Chen Y, Ye J, Zhu Z, Zhao W, Zhou J, Wu C, et al. Comparing paclitaxel plus fluorouracil versus cisplatin plus fluorouracil in chemoradiotherapy for locally advanced esophageal squamous cell cancer: A randomized, multicenter, phase iii clinical trial. J Clin Oncol (2019) 37(20):1695–703. doi: 10.1200/jco.18.02122
30. Suntharalingam M, Winter K, Ilson D, Dicker AP, Kachnic L, Konski A, et al. Effect of the addition of cetuximab to paclitaxel, cisplatin, and radiation therapy for patients with esophageal cancer: The nrg oncology rtog 0436 phase 3 randomized clinical trial. JAMA Oncol (2017) 3(11):1520–8. doi: 10.1001/jamaoncol.2017.1598
31. Bedenne L, Michel P, Bouché O, Milan C, Mariette C, Conroy T, et al. Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: Ffcd 9102. J Clin Oncol (2007) 25(10):1160–8. doi: 10.1200/jco.2005.04.7118
32. Rawat S, Kumar G, Kakria A, Sharma MK, Chauhan D. Chemoradiotherapy in the management of locally advanced squamous cell carcinoma esophagus: Is surgical resection required? J Gastrointestinal Cancer (2013) 44(3):277–84. doi: 10.1007/s12029-013-9477-7
33. Yang H, Liu H, Chen Y, Zhu C, Fang W, Yu Z, et al. Neoadjuvant chemoradiotherapy followed by surgery versus surgery alone for locally advanced squamous cell carcinoma of the esophagus (Neocrtec5010): A phase iii multicenter, randomized, open-label clinical trial. J Clin Oncol (2018) 36(27):2796–803. doi: 10.1200/jco.2018.79.1483
34. Lorenzen S, Brücher B, Zimmermann F, Geinitz H, Riera J, Schuster T, et al. Neoadjuvant continuous infusion of weekly 5-fluorouracil and escalating doses of oxaliplatin plus concurrent radiation in locally advanced oesophageal squamous cell carcinoma: Results of a phase I/Ii trial. Br J Cancer (2008) 99(7):1020–6. doi: 10.1038/sj.bjc.6604659
35. Mariette C, Dahan L, Mornex F, Maillard E, Thomas PA, Meunier B, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and ii esophageal cancer: Final analysis of randomized controlled phase iii trial ffcd 9901. J Clin Oncol (2014) 32(23):2416–22. doi: 10.1200/jco.2013.53.6532
36. Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, et al. Phase iii trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: Calgb 9781. J Clin Oncol (2008) 26(7):1086–92. doi: 10.1200/jco.2007.12.9593
37. Food and Drug Administration. Fda approves nivolumab for resected esophageal or gej cancer (Accessed 10 August 2021).
38. Bristol Myers Squibb. Bristol Myers Squibb Receives positive chmp opinion for opdivo (Nivolumab) as adjuvant treatment for esophageal or gastroesophageal junction cancer patients with residual pathologic disease following chemoradiotherapy (Accessed 15 August 2021).
39. Zhou XL, Yu CH, Wang WW, Ji FZ, Xiong YZ, Zhu WG, et al. Concurrent chemoradiotherapy with s-1 compared with concurrent chemoradiotherapy with docetaxel and cisplatin for locally advanced esophageal squamous cell carcinoma. Radiat Oncol (London England) (2021) 16(1):94. doi: 10.1186/s13014-021-01821-6
40. Barbetta A, Hsu M, Tan KS, Stefanova D, Herman K, Adusumilli PS, et al. Definitive chemoradiotherapy versus neoadjuvant chemoradiotherapy followed by surgery for stage ii to iii esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg (2018) 155(6):2710–21.e3. doi: 10.1016/j.jtcvs.2018.01.086
41. Hu B, Jia F, Zhou H, Zhou T, Zhao Q, Chen Y, et al. Risk factors associated with esophageal fistula after radiotherapy for esophageal squamous cell carcinoma. J Cancer (2020) 11(12):3693–700. doi: 10.7150/jca.39033
42. Tsushima T, Mizusawa J, Sudo K, Honma Y, Kato K, Igaki H, et al. Risk factors for esophageal fistula associated with chemoradiotherapy for locally advanced unresectable esophageal cancer: A supplementary analysis of Jcog0303. Medicine (2016) 95(20):e3699. doi: 10.1097/md.0000000000003699
43. van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med (2012) 366(22):2074–84. doi: 10.1056/NEJMoa1112088
44. Park SR, Yoon DH, Kim JH, Kim YH, Kim HR, Lee HJ, et al. A randomized phase iii trial on the role of esophagectomy in complete responders to preoperative chemoradiotherapy for esophageal squamous cell carcinoma (Esopresso). Anticancer Res (2019) 39(9):5123–33. doi: 10.21873/anticanres.13707
45. de Castro Junior G, Segalla JG, de Azevedo SJ, Andrade CJ, Grabarz D, de Araújo Lima França B, et al. A randomised phase ii study of chemoradiotherapy with or without nimotuzumab in locally advanced oesophageal cancer: Nice trial. Eur J Cancer (Oxford Engl 1990) (2018) 88:21–30. doi: 10.1016/j.ejca.2017.10.005
46. Zhang N, Zhu X, Kong C, Song X, Chen C, Jiang N, et al. 1907p application of anti-Pd1 drugs before or during thoracic radiotherapy increases the incidence of radiation pneumonia compared to the application after radiotherapy. Ann Oncol (2020) 31:S1081. doi: 10.1016/j.annonc.2020.08.1450
47. The Cancer Genome Atlas Research Network, Analysis Working Group: Asan University, BC Cancer Agency, Brigham and Women’ s Hospital, Broad Institute, Brown University, et al. Integrated genomic characterization of oesophageal carcinoma. Nature (2017) 541(7636):169–75. doi: 10.1038/nature20805
48. Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, et al. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res (2005) 11(8):2947–53. doi: 10.1158/1078-0432.Ccr-04-1469
49. Guo W, Wang P, Li N, Shao F, Zhang H, Yang Z, et al. Prognostic value of pd-L1 in esophageal squamous cell carcinoma: A meta-analysis. Oncotarget (2018) 9(17):13920–33. doi: 10.18632/oncotarget.23810
50. Kelly RJ. Immunotherapy for esophageal and gastric cancer. Am Soc Clin Oncol Educ book Am Soc Clin Oncol Annu Meeting (2017) 37:292–300. doi: 10.1200/edbk_175231
51. Derks S, Nason KS, Liao X, Stachler MD, Liu KX, Liu JB, et al. Epithelial pd-L2 expression marks barrett's esophagus and esophageal adenocarcinoma. Cancer Immunol Res (2015) 3(10):1123–9. doi: 10.1158/2326-6066.Cir-15-0046
52. Shah MA, Kojima T, Hochhauser D, Enzinger P, Raimbourg J, Hollebecque A, et al. Efficacy and safety of pembrolizumab for heavily pretreated patients with advanced, metastatic adenocarcinoma or squamous cell carcinoma of the esophagus: The phase 2 keynote-180 study. JAMA Oncol (2019) 5(4):546–50. doi: 10.1001/jamaoncol.2018.5441
53. Kojima T, Shah MA, Muro K, Francois E, Adenis A, Hsu CH, et al. Randomized phase iii keynote-181 study of pembrolizumab versus chemotherapy in advanced esophageal cancer. J Clin Oncol (2020) 38(35):4138–48. doi: 10.1200/jco.20.01888
54. Pillai RN, Behera M, Owonikoko TK, Kamphorst AO, Pakkala S, Belani CP, et al. Comparison of the toxicity profile of pd-1 versus pd-L1 inhibitors in non-small cell lung cancer: A systematic analysis of the literature. Cancer (2018) 124(2):271–7. doi: 10.1002/cncr.31043
55. Gu Y, Zhang H, Liu Z, Xia Y, Liang B, Liang L. Different patterns of treatment-related adverse events of programmed cell death-1 and its ligand-1 inhibitors in different cancer types: A meta-analysis and systemic review of clinical trials. Asia-Pacific J Clin Oncol (2020) 16(5):e160–78. doi: 10.1111/ajco.13385
56. Khunger M, Rakshit S, Pasupuleti V, Hernandez AV, Mazzone P, Stevenson J, et al. Incidence of pneumonitis with use of programmed death 1 and programmed death-ligand 1 inhibitors in non-small cell lung cancer: A systematic review and meta-analysis of trials. Chest (2017) 152(2):271–81. doi: 10.1016/j.chest.2017.04.177
57. Zhou C, Li M, Wang Z, An D, Li B. Adverse events of immunotherapy in non-small cell lung cancer: A systematic review and network meta-analysis. Int Immunopharmacol (2022) 102:108353. doi: 10.1016/j.intimp.2021.108353
58. Li B, Jiang C, Pang L, Zou B, Ding M, Sun X, et al. Toxicity profile of combining pd-1/Pd-L1 inhibitors and thoracic radiotherapy in non-small cell lung cancer: A systematic review. Front Immunol (2021) 12:627197. doi: 10.3389/fimmu.2021.627197
59. Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. Pd-L2 is a second ligand for pd-1 and inhibits T cell activation. Nat Immunol (2001) 2(3):261–8. doi: 10.1038/85330
60. Sihag S, Ku GY, Tan KS, Nussenzweig S, Wu A, Janjigian YY, et al. Safety and feasibility of esophagectomy following combined immunotherapy and chemoradiotherapy for esophageal cancer. J Thorac Cardiovasc Surg (2021) 161(3):836–43.e1. doi: 10.1016/j.jtcvs.2020.11.106
61. Ju AW, Woody S, Hegde AM, Arastu HH, Walker P. Survival is worse in patients completing immunotherapy prior to Sbrt/Srs compared to those receiving it concurrently or after. Int J Radiat OncologyBiologyPhysics (2020) 108(3):e174–5. doi: 10.3389/fonc.2022.785350
62. Price JG, Shantzer L, Jacobs CD, Singh A, Torok JA. Radiation therapy and immune checkpoint inhibition in metastatic non-small cell lung cancer: Determining toxicity and efficacy of combination treatment. Int J Radiat OncologyBiologyPhysics (2020) 108(3):e149–50. doi: 10.1016/j.ijrobp.2020.07.1320
63. Lesueur P, Escande A, Thariat J, Vauléon E, Monnet I, Cortot A, et al. Safety of combined pd-1 pathway inhibition and radiation therapy for non-small-cell lung cancer: A multicentric retrospective study from the gfpc. Cancer Med (2018) 7(11):5505–13. doi: 10.1002/cam4.1825
64. Young KH, Baird JR, Savage T, Cottam B, Friedman D, Bambina S, et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS One (2016) 11(6):e0157164. doi: 10.1371/journal.pone.0157164
65. Anscher MS, Arora S, Weinstock C, Amatya A, Bandaru P, Tang C, et al. Association of radiation therapy with risk of adverse events in patients receiving immunotherapy: A pooled analysis of trials in the us food and drug administration database. JAMA Oncol (2022) 8(2):232–40. doi: 10.1001/jamaoncol.2021.6439
66. Morisada M, Moore EC, Hodge R, Friedman J, Cash HA, Hodge JW, et al. Dose-dependent enhancement of T-lymphocyte priming and ctl lysis following ionizing radiation in an engineered model of oral cancer. Oral Oncol (2017) 71:87–94. doi: 10.1016/j.oraloncology.2017.06.005
67. Golden EB, Frances D, Pellicciotta I, Demaria S, Helen Barcellos-Hoff M, Formenti SC. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology (2014) 3:e28518. doi: 10.4161/onci.28518
68. Morisada M, Clavijo PE, Moore E, Sun L, Chamberlin M, Van Waes C, et al. Pd-1 blockade reverses adaptive immune resistance induced by high-dose hypofractionated but not low-dose daily fractionated radiation. Oncoimmunology (2018) 7(3):e1395996. doi: 10.1080/2162402x.2017.1395996
69. Welsh JW, Chen D, Baas P, Chang JY, Verma V, Comeaux N, et al. Radiotherapy to augment pembrolizumab responses and outcomes in metastatic non-small cell lung cancer: Pooled analysis of two randomized trials. J Clin Oncol (2020) 38(15_suppl):9548. doi: 10.1200/JCO.2020.38.15_suppl.9548
70. Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, et al. Low-dose irradiation programs macrophage differentiation to an Inos+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell (2013) 24(5):589–602. doi: 10.1016/j.ccr.2013.09.014
71. Shin SC, Lee KM, Kang YM, Kim K, Kim CS, Yang KH, et al. Alteration of cytokine profiles in mice exposed to chronic low-dose ionizing radiation. Biochem Biophys Res Commun (2010) 397(4):644–9. doi: 10.1016/j.bbrc.2010.05.121
72. Yin L, Xue J, Li R, Zhou L, Deng L, Chen L, et al. Effect of low-dose radiation therapy on abscopal responses to hypofractionated radiation therapy and anti-Pd1 in mice and patients with non-small cell lung cancer. Int J Radiat oncology biology Phys (2020) 108(1):212–24. doi: 10.1016/j.ijrobp.2020.05.002
73. Marciscano AE, Ghasemzadeh A, Nirschl TR, Theodros D, Kochel CM, Francica BJ, et al. Elective nodal irradiation attenuates the combinatorial efficacy of stereotactic radiation therapy and immunotherapy. Clin Cancer Res (2018) 24(20):5058–71. doi: 10.1158/1078-0432.Ccr-17-3427
74. Dammeijer F, van Gulijk M, Mulder EE, Lukkes M, Klaase L, van den Bosch T, et al. The pd-1/Pd-L1-Checkpoint restrains T cell immunity in tumor-draining lymph nodes. Cancer Cell (2020) 38(5):685–700.e8. doi: 10.1016/j.ccell.2020.09.001
75. Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, et al. Checkmate-032 study: Efficacy and safety of nivolumab and nivolumab plus ipilimumab in patients with metastatic esophagogastric cancer. J Clin Oncol (2018) 36(28):2836–44. doi: 10.1200/jco.2017.76.6212
Keywords: immune checkpoint inhibitors, radiation therapy, esophageal cancer, efficacy, safety, meta-analysis
Citation: Wu J, Deng R, Ni T, Zhong Q, Tang F, Li Y and Zhang Y (2022) Efficacy and safety of radiotherapy/chemoradiotherapy combined with immune checkpoint inhibitors for locally advanced stages of esophageal cancer: A systematic review and meta-analysis. Front. Oncol. 12:887525. doi: 10.3389/fonc.2022.887525
Received: 01 March 2022; Accepted: 12 July 2022;
Published: 03 August 2022.
Edited by:
Thomas FitzGerald, University of Massachusetts Boston, United StatesReviewed by:
Andrew Wilks, University of Massachusetts Medical School, United StatesCopyright © 2022 Wu, Deng, Ni, Zhong, Tang, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Zhang, c2t5bGluZV96eXVAMTYzLmNvbQ==
†These authors have contributed equally to this manuscript
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.