- 1Merck & Co., Inc., Rahway, NJ, United States
- 2Richard M. Fairbanks School of Public Health, Indiana University, Indianapolis, IN, United States
- 3Division of Neurosurgery, Huntington Hospital, Northwell Health, Huntington, NY, United States
- 4Seagen Inc., Bothell, WA, United States
- 5Integrative Precision Health, Limited Liability Company (LLC), Carmel, IN, United States
Background: Up to 60% of melanoma patients develop melanoma brain metastases (MBM), which traditionally have a poor diagnosis. Current treatment strategies include immunotherapies (IO), targeted therapies (TT), and stereotactic radiosurgery (SRS), but there is considerable heterogeneity across worldwide consensus guidelines.
Objective: To summarize current treatments and compare worldwide guidelines for the treatment of MBM.
Methods: Review of global consensus treatment guidelines for MBM patients.
Results: Substantial evidence supported that concurrent IO or TT plus SRS improves progression-free survival (PFS) and overall survival (OS). Guidelines are inconsistent with regards to recommendations for surgical resection of MBM, since surgical resection of symptomatic lesions alleviates neurological symptoms but does not improve OS. Whole-brain radiation therapy is not recommended by all guidelines due to negative effects on neurocognition but can be offered in rare palliative scenarios.
Conclusion: Worldwide consensus guidelines consistently recommend up-front combination IO or TT with or without SRS for the treatment of MBM.
1 Introduction
The global incidence of melanoma is increasing, accounting for 73% of skin cancer-related deaths (1, 2). Despite melanoma being the least common type of skin cancer, 60% of patients develop melanoma brain metastases (MBM), with a dismal median survival of 3 to 6 months (3, 4). Immunotherapy (IO), including anti-programmed cell death protein 1 (anti-PD1) and anti-cytotoxic T lymphocyte-associated protein 4 (anti-CTLA-4) therapies (5), and targeted therapy (TT) against BRAF V600 E/K mutations (BRAFi) and MEK/MAPK signaling pathways (MEKi) (6), have improved progression-free survival (PFS) and overall survival (OS) of patients with metastatic melanoma and reached median OS of up to 24.3 months (7). Delivery of precise doses of radiation using stereotactic radiosurgery (SRS) to discrete MBM and adjuvant doses of radiation to the post-surgical resection cavity have also significantly improved local intracranial disease control (8, 9).
In this review, we conducted a targeted literature review by focusing on the current modalities for the treatment of MBM as outlined in the consensus guidelines from the European Society for Medical Oncology (ESMO) (10), European Organization for Research and Treatment of Cancer (EORTC) (11), National Comprehensive Cancer Network (NCCN) (12), Cancer Council of Australia (CCA) (13), and Japanese Dermatological Association (JDA) (14). We further offered a comprehensive comparison of the consensus guidelines for each modality.
2 Methods
A targeted literature review for the treatment of MBM and the most recent international guidelines on the treatment of cutaneous melanoma with respect to MBM was performed. Guidelines reviewed included: 1) The National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology, Cutaneous Melanoma, version 2.2021 (12); 2) The 2019 European Organization for Research and Treatment of Cancer (EORTC) recommendations on cutaneous melanoma diagnosis and treatment (11); 3) The European Society for Medical Oncology (ESMO) consensus conference guidelines on melanoma (10); 4) Evidence-based clinical practice guidelines for the management of MBM put forth by Cancer Council Australia (CCA) in 2020 (13); and 5) The 2019 melanoma guidelines of the Japanese Dermatological Association (14). The American Society of Clinical Oncology (ASCO) is currently preparing guidelines for the treatment of MBM but has not yet been published (15). References mentioned throughout this manuscript pertaining to the treatment of MBM were directly drawn from studies that were reviewed and referenced within the consensus guidelines themselves.
3 Results
3.1 Current Modalities for the Treatment of Melanoma Brain Metastases as Outlined in the Global Consensus Guidelines
3.1.1 Role of Surgery
Surgery is recommended in the setting of large symptomatic lesions (> 3 cm diameter) presenting with mass effect, hemorrhage, or obstructive hydrocephalus. Patients with a single MBM, functional independence, limited or absent extracranial disease, should be offered surgery with palliative benefits (16, 17). MBM patients treated with immunotherapy and surgery achieve excellent local control rates (18). Similarly, patients with a single MBM treated with surgery plus whole brain radiotherapy (WBRT) have longer survival than WBRT alone (19, 20). Response to IO is associated with prolonged survival in patients who underwent resection of their MBM, while adjuvant WBRT does not (21).
3.1.2 SRS and WBRT
SRS delivers a high dose of radiation to a focused target with high three-dimensional conformality and has proven efficacy at controlling a small number (< 4) of MBM lesions (with a total cerebral tumor volume of < 5 cubic centimeters) (8, 22–24). It has been suggested that multiple lesions, failure to treat with IO or TT, poorly controlled systemic disease, and intratumoral hemorrhage are predictors of poor response to SRS (23). A phase III randomized clinical trial (RCT) showed that adjuvant SRS boost to the surgical cavity significantly lowers local recurrence but does not improve OS (25).
WBRT was traditionally used to treat patients with multiple MBMs but only affords a small increase in median survival of 3.5 months, albeit before recent systemic therapy advances (26, 27). A pooled analyses of trials comparing WBRT to WBRT plus surgery showed no significant difference in OS (28) and patients treated with WBRT had decreased neurocognitive function (29). Furthermore, a multicenter RCT comparing WBRT plus surgery with surgery alone in 215 MBM patients did not demonstrate any clinical benefit for adjuvant WBRT and therefore adjuvant WBRT is no longer offered to patients (30, 31).
3.1.3 Systemic Therapies Including IO and TT
Combination IO (anti-CTLA4 and anti-PD-1) and TT that inhibit BRAF V600 E/K and MEK (known to be mutated in approximately 40-50% of melanoma patients) are effective at treating MBM and prolonging PFS (5, 7, 32–34). The open-label, multicenter, single-arm phase II study CheckMate 204 suggested that combination IO nivolumab (nivo) plus ipilimumab (ipi) had clinically meaningful intracranial efficacy, concordant with extracranial activity in patients with at least one asymptomatic, measurable, non-irradiated BM (5). The anti-PD1 brain collaboration (ABC) trial also demonstrated clinically meaningful intracranial efficacy of combination IO nivo plus ipi (33). Similarly, the phase II multicentered COMBI-MB trial of combination TT dabrafenib plus trametinib in patients with BRAF V600 E/K mutant asymptomatic MBM demonstrated clinical safety with manageable symptoms (7). A recent systematic review and meta-analysis of combination IO, TT, and mono-agent IO in combination with radiotherapy for the treatment of MBM patients revealed that combination IO and TT had a similar intracranial response rate, while combination IO was associated with increased PFS and OS compared to mono-agent IO and combination TT (32).
3.1.4 Combination of SRS Plus IO or TT
Multiple systematic reviews and meta-analyses have demonstrated a survival benefit of combining SRS with concurrent IO or TT compared to SRS alone (9, 35–40). As such, combination IO or TT are now recommended as upfront treatments followed by SRS and/or surgical resection of MBM. When combining SRS with TT, there should be a washout period of 3 to 5 days prior to commencement of SRS (41).
3.2 Review and Comparison of Worldwide Consensus Guidelines
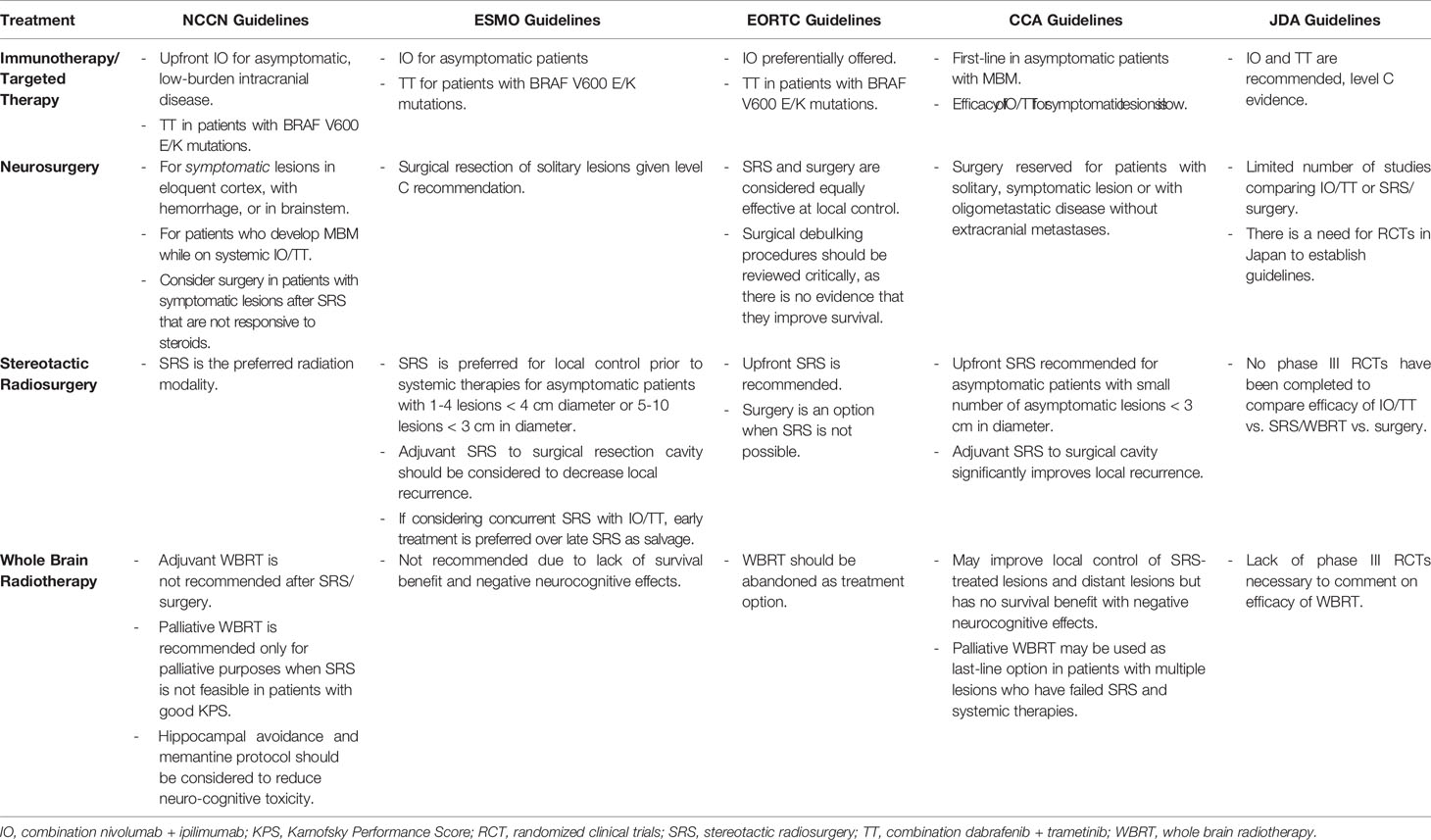
In the second segment of this review, we summarize and compare the most recent global consensus guidelines published by ESMO, EORTC, NCCN, CCA, and JDA (Table 1). Of note, Canadian guidelines were omitted because they do not discuss the treatment of MBM. Comparison of guideline recommendations are subcategorized according to treatment modalities with the understanding that all current consensus guidelines state that most MBM patients will likely require multimodal combination therapies throughout their treatment course.
3.2.1 Upfront and/or Subsequent Surgical Resection of MBM
Guidelines are inconsistent with regards to recommendations for surgical resection of MBM. The EORTC guidelines consider surgical resection as an option when SRS is not possible and that SRS is equally effective at achieving local brain control while being non-invasive, applicable to several lesions, repeatable, and provides early local control compared to surgical resection (11, 42). The NCCN (12) and CCA (13) guidelines state that patients with symptomatic lesions > 1 cm in diameter in non-eloquent cortex, resectable locations, should be offered surgical resection.
3.2.2 Use of SRS
The NCCN currently recommends 15-24 Gy SRS in a single fraction to small tumors < 3 cm (43). SRS is typically not recommended for lesions > 4 cm, which may be treated with fractionated stereotactic radiotherapy (SRT), 24-27 Gy in 3 fractions or 25-35 Gy in 5 fractions (44, 45). Adjuvant SRS at 12-20 Gy may be applied to resection cavities < 5 cm (44) with fractionated SRT for larger cavities. TT should be held for ≥ 3 days before and after fractionated SRT and for ≥ 1 day before and after SRS to avoid toxicities associated with concurrent TT and SRS/SRT treatment (41, 46–50). EORTC considers SRS to asymptomatic MBM lesions < 3 cm (solitary or up to 5 lesions) to achieve superior early local control compared to surgical resection (42). ESMO recommends SRS for the treatment of limited asymptomatic MBMs (up to 4 lesions) with a maximum diameter of 4 cm or 5-10 lesions with the largest tumor < 10 mL in volume, < 3 cm in diameter, and a total cumulative volume of ≤ 15 mL (10, 51). The Australian guidelines recommend SRS in patients with a single or a small number of lesions (52–56). All guidelines except for the JDA recommend adjuvant SRS to the post-resection cavity based on two randomized trials evaluating effects of SRS to the resection cavity of multiple types of BM (25, 57). The JDA refrained from providing strong recommendations for adjuvant SRS to the resection cavity again due a lack of phase III randomized trials comparing SRS to local brain directed therapies (14).
3.2.3 Use of WBRT
NCCN recommends considering palliative WBRT when SRS/SRT is not feasible in patients who have failed systemic therapy or in patients with signs and symptoms of leptomeningeal carcinomatosis. Hippocampal avoidance and memantine therapy should be considered to patients receiving WBRT to reduce neurocognitive toxicity (58). Adjuvant WBRT after resection or SRS/SRT is not recommended due to worsening cognitive decline following WBRT with no benefit in OS (57, 59). EORTC and EMSO guidelines recommend restricting WBRT to those few patients who have exhausted all systemic, SRS, and other local brain therapy options. All guidelines do not recommend treating patients with WBRT after surgical resection or SRS treatment for MBM.
3.2.4 Use of IO and TT
The NCCN, ESMO, EORTC, and CCA recommend upfront combination IO (nivo + ipi) as the preferred initial treatment in patients with asymptomatic MBM < 3 cm, not requiring corticosteroids and who have not received prior systemic therapies. This recommendation is based on the study reporting high intracranial response rates using nivo + ipi in patients with previously untreated asymptomatic MBM (5). Anti-PD-1 monotherapy is not recommended, and systemic corticosteroids may negatively affect the efficacy of nivo + ipi and should be avoided in MBM patients (60). For patients with BRAF V600E mutations, combination BRAFi + MEKi should be considered. Brain-directed therapy is preferred in patients with symptomatic MBM as limited evidence exists supporting the effectiveness of upfront systemic therapies for symptomatic MBM (7, 60–62). In contrast, the JDA currently provides conditional recommendations for using IO or TT for the treatment of MBM patients due to the lack of phase III clinical trials comparing the efficacy of IO, TT, SRS, or surgery for the treatment of MBM, and that the existing phase II studies are limited by selection bias and small sample size (5, 33).
4 Discussion
The current iterations of consensus guidelines are limited to evidence gathered largely from relatively small, phase I and II clinical trials, retrospective case series, and observational studies (52–54, 63). CheckMate 204 was a phase II study evaluating the efficacy and safety of nivo + ipi in asymptomatic MBM patients with a relatively small sample size (n = 101 patients) and median follow-up of 14.0 months (5). Similarly, the phase II ABC study enrolled only 79 patients in 3 cohorts of patients treated with nivo or nivo+ipi, with considerable heterogeneity amongst the cohorts (33).
It is important to keep in mind when reviewing consensus practice guidelines that physicians in real-world practice may not always follow consensus guidelines. This may be due to a multitude of reasons, such as the availability of certain treatments or approval for their use by insurance providers. Studies using real-world evidence and observational data are being performed in an attempt to gain further understanding of actual treatment patterns (64). A recent study using the National Cancer Database (NCDB) of 3008 cases of MBM between 2011 to 2015 reported real-world outcomes of combination and the timing of IO with radiotherapy for MBM and showed longer survival in patients treated with combination IO with SRS/WBRT compared to SRS/WBRT alone and in patients receiving concurrent SRS and IO compared to non-concurrent therapy (40).
Limitations of this study included: The use of a retrospective database, precluding the ability to assess the benefit of IO given as second-line treatment since only IO given as first-line systemic therapy was recorded; And the exclusion of sociodemographic factors, disease factors, and treatment locations that could have limited a patient’s access to a specific treatment modality, which could have affected their outcomes (40). Thus, the ability to reference studies using real-world data could therefore serve as complimentary information to consensus guidelines for treating physicians.
Investigators are also now exploring novel combinations of multimodal therapies in MBM patients. These ongoing trials are mostly combining triplet therapy consisting of IO and TT with other novel small molecule inhibitors (65, 66). Current ongoing trials include: EMBRAIN-MEL (NCT03898908) combining Encorafenib plus Binimetinib before SRS; RadioCoBRIM (NCT03430947) combining vemurafenib plus cobimetinib after SRS; The phase III NIBIT-M2 study (NCT02460068) comparing the chemotherapy agent fotemustine alone versus combination fotemustine plus ipi alone or combination fotemustine plus ipi and nivo; And the phase II study combining vemurafenib and combimetinib with azetolizumab (NCT03625141). Ongoing trials are also evaluating the toxicity of SRS in combination with IO or TT, as previous studies have shown statistically significant differences in radiation necrosis and brain edema among patients receiving the combination, although data are inconsistent (34).
In summary, the evidence used to compile the current versions of the worldwide consensus guidelines show promise for improving the survival of patients with MBM who receive upfront concurrent combination IO or TT with SRS. The emergence of studies using real-world evidence could serve to further compliment consensus guidelines for the treatment of MBM.
Author Contributions
X-LT, AL, FL, and JH contributed to conception and design of the review and wrote portions of the manuscript. All authors contributed to manuscript critique and revision and approved the submitted version.
Conflict of Interest
X-LT, ES, RJ, SJD, and IMS are employees of Merck & Co., Inc. JH and AL are employed by Integrative Precision Health LLC. ES was also employed by Seagen Inc.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gershenwald JE, Guy GP Jr. Stemming the Rising Incidence of Melanoma: Calling Prevention to Action. J Natl Cancer Inst (2016) 108(1):djv381. doi: 10.1093/jnci/djv381
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
3. Davies MA, Liu P, McIntyre S, Kim KB, Papadopoulos N, Hwu WJ, et al. Prognostic Factors for Survival in Melanoma Patients With Brain Metastases. Cancer (2011) 117(8):1687–96. doi: 10.1002/cncr.25634
4. Kohler BA, Ward E, McCarthy BJ, Schymura MJ, Ries LA, Eheman C, et al. Annual Report to the Nation on the Status of Cancer, 1975-2007, Featuring Tumors of the Brain and Other Nervous System. J Natl Cancer Inst (2011) 103(9):714–36. doi: 10.1093/jnci/djr077
5. Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi FS, Moschos SJ, et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N Engl J Med (2018) 379(8):722–30. doi: 10.1056/NEJMoa1805453
6. Schreuer M, Jansen Y, Planken S, Chevolet I, Seremet T, Kruse V, et al. Combination of Dabrafenib Plus Trametinib for BRAF and MEK Inhibitor Pretreated Patients With Advanced BRAF(V600)-mutant Melanoma: An Open-Label, Single Arm, Dual-Centre, Phase 2 Clinical Trial. Lancet Oncol (2017) 18(4):464–72. doi: 10.1016/S1470-2045(17)30171-7
7. Davies MA, Saiag P, Robert C, Grob JJ, Flaherty KT, Arance A, et al. Dabrafenib Plus Trametinib in Patients With BRAF(V600)-mutant Melanoma Brain Metastases (COMBI-MB): A Multicentre, Multicohort, Open-Label, Phase 2 Trial. Lancet Oncol (2017) 18(7):863–73. doi: 10.1016/S1470-2045(17)30429-1
8. Kelley KD, Marrero M, Knisely JP. Principles of Image-Guided Hypofractionated Stereotactic Radiosurgery for Brain Tumors. In: Sahgal A, Lo SS, Ma L, Sheehan JP, editors. Imaged-Guided Hypofractionated Stereotactic Radiosurgery - A Practical Approach to Guide Treatment of Brain and Spine Tumors. Boca Raton: CRC Press (2016). p. 117–27.
9. Petrelli F, De Stefani A, Trevisan F, Parati C, Inno A, Merelli B, et al. Combination of Radiotherapy and Immunotherapy for Brain Metastases: A Systematic Review and Meta-Analysis. Crit Rev Oncol Hematol (2019) 144:102830. doi: 10.1016/j.critrevonc.2019.102830
10. Keilholz U, Ascierto PA, Dummer R, Robert C, Lorigan P, van Akkooi A, et al. ESMO Consensus Conference Recommendations on the Management of Metastatic Melanoma: Under the Auspices of the ESMO Guidelines Committee. Ann Oncol (2020) 31(11):1435–48. doi: 10.1016/j.annonc.2020.07.004
11. Garbe C, Amaral T, Peris K, Hauschild A, Arenberger P, Bastholt L, et al. European Consensus-Based Interdisciplinary Guideline for Melanoma. Part 2: Treatment - Update 2019. Eur J Cancer (2020) 126:159–77. doi: 10.1016/j.ejca.2019.11.015
12. Swetter SM, Thompson JA, Albertini MR, Barker CA, Baumgartner J, Boland G, et al. Melanoma: Cutaneous (Version 2.2021). National Comprehensive Cancer Network (2021). Available at: https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf.
13. Hong AM, Waldstein C, Shivalingam B, Carlino MS, Atkinson V, Kefford RF, et al. Management of Melanoma Brain Metastases: Evidence-based Clinical Practice Guidelines by Cancer Council Australia. Eur J Cancer (2021) 142:10–7. doi: 10.1016/j.ejca.2020.10.013
14. Nakamura Y, Asai J, Igaki H, Inozume T, Namikawa K, Hayashi A, et al. Japanese Dermatological Association Guidelines: Outlines of Guidelines for Cutaneous Melanoma 2019. J Dermatol (2020) 47(2):89–103. doi: 10.1111/1346-8138.15151
15. Seth R, Messersmith H, Kaur V, Kirkwood JM, Kudchadkar R, McQuade JL, et al. Systemic Therapy for Melanoma: Asco Guideline. J Clin Oncol (2020) 38(33):3947–70. doi: 10.1200/JCO.20.00198
16. Kalkanis SN, Kondziolka D, Gaspar LE, Burri SH, Asher AL, Cobbs CS, et al. The Role of Surgical Resection in the Management of Newly Diagnosed Brain Metastases: A Systematic Review and Evidence-Based Clinical Practice Guideline. J Neurooncol (2010) 96(1):33–43. doi: 10.1007/s11060-009-0061-8
17. Wronski M, Arbit E. Surgical Treatment of Brain Metastases From Melanoma: A Retrospective Study of 91 Patients. J Neurosurg (2000) 93(1):9–18. doi: 10.3171/jns.2000.93.1.0009
18. Lonser RR, Song DK, Klapper J, Hagan M, Auh S, Kerr PB, et al. Surgical Management of Melanoma Brain Metastases in Patients Treated With Immunotherapy. J Neurosurg (2011) 115(1):30–6. doi: 10.3171/2011.3.JNS091107
19. Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A Randomized Trial of Surgery in the Treatment of Single Metastases to the Brain. N Engl J Med (1990) 322(8):494–500. doi: 10.1056/NEJM199002223220802
20. Vecht CJ, Haaxma-Reiche H, Noordijk EM, Padberg GW, Voormolen JH, Hoekstra FH, et al. Treatment of Single Brain Metastasis: Radiotherapy Alone or Combined With Neurosurgery? Ann Neurol (1993) 33(6):583–90. doi: 10.1002/ana.410330605
21. Goyal S, Silk AW, Tian S, Mehnert J, Danish S, Ranjan S, et al. Clinical Management of Multiple Melanoma Brain Metastases: A Systematic Review. JAMA Oncol (2015) 1(5):668–76. doi: 10.1001/jamaoncol.2015.1206
22. Mathieu D, Kondziolka D, Cooper PB, Flickinger JC, Niranjan A, Agarwala S, et al. Gamma Knife Radiosurgery in the Management of Malignant Melanoma Brain Metastases. Neurosurgery (2007) 60(3):471–81; discussion 81-2. doi: 10.1227/01.NEU.0000255342.10780.52
23. Liew DN, Kano H, Kondziolka D, Mathieu D, Niranjan A, Flickinger JC, et al. Outcome Predictors of Gamma Knife Surgery for Melanoma Brain Metastases. Clin Art J Neurosurg (2011) 114(3):769–79. doi: 10.3171/2010.5.JNS1014
24. DiLuna ML, King JT Jr., Knisely JP, Chiang VL. Prognostic Factors for Survival After Stereotactic Radiosurgery Vary With the Number of Cerebral Metastases. Cancer (2007) 109(1):135–45. doi: 10.1002/cncr.22367
25. Mahajan A, Ahmed S, McAleer MF, Weinberg JS, Li J, Brown P, et al. Post-Operative Stereotactic Radiosurgery Versus Observation for Completely Resected Brain Metastases: A Single-Centre, Randomised, Controlled, Phase 3 Trial. Lancet Oncol (2017) 18(8):1040–8. doi: 10.1016/S1470-2045(17)30414-X
26. Sampson JH, Carter JH Jr., Friedman AH, Seigler HF. Demographics, Prognosis, and Therapy in 702 Patients With Brain Metastases From Malignant Melanoma. J Neurosurg (1998) 88(1):11–20. doi: 10.3171/jns.1998.88.1.0011
27. Fife KM, Colman MH, Stevens GN, Firth IC, Moon D, Shannon KF, et al. Determinants of Outcome in Melanoma Patients With Cerebral Metastases. J Clin Oncol (2004) 22(7):1293–300. doi: 10.1200/JCO.2004.08.140
28. Tsao H, Sober AJ. Melanoma Treatment Update. Dermatol Clin (2005) 23(2):323–33. doi: 10.1016/j.det.2004.09.005
29. Tsao MN, Xu W, Wong RK, Lloyd N, Laperriere N, Sahgal A, et al. Whole Brain Radiotherapy for the Treatment of Newly Diagnosed Multiple Brain Metastases. Cochrane Database Syst Rev (2018) 1:CD003869. doi: 10.1002/14651858.CD003869.pub4
30. Fogarty G, Morton RL, Vardy J, Nowak AK, Mandel C, Forder PM, et al. Whole Brain Radiotherapy After Local Treatment of Brain Metastases in Melanoma Patients–a Randomised Phase III Trial. BMC Cancer (2011) 11:142. doi: 10.1186/1471-2407-11-142
31. Fogarty GB, Hong A, Thompson JF. Should Patients With Melanoma Brain Metastases Receive Adjuvant Whole-Brain Radiotherapy? Lancet Oncol (2015) 16(5):e195–6. doi: 10.1016/S1470-2045(15)70183-X
32. Rulli E, Legramandi L, Salvati L, Mandala M. The Impact of Targeted Therapies and Immunotherapy in Melanoma Brain Metastases: A Systematic Review and Meta-Analysis. Cancer (2019) 125(21):3776–89. doi: 10.1002/cncr.32375
33. Long GV, Atkinson VG, Lo S, Sandhu SK, Brown M, Gonzalez M, et al. Long-Term Outcomes From the Randomized Phase II Study of Nivolumab (Nivo) or Nivo + Ipilimumab (Ipi) in Patients (Pts) With Melanoma Brain Metastases (Mets): Anti-PD1 Brain Collaboration (ABC). Ann Oncol (2019) 305):v534. doi: 10.1093/annonc/mdz255.001
34. Becco P, Gallo S, Poletto S, Frascione MPM, Crotto L, Zaccagna A, et al. Melanoma Brain Metastases in the Era of Target Therapies: An Overview. Cancers (Basel) (2020) 12(6):1640. doi: 10.3390/cancers12061640
35. Lu VM, Goyal A, Rovin RA, Lee A, McDonald KL. Concurrent Versus non-Concurrent Immune Checkpoint Inhibition With Stereotactic Radiosurgery for Metastatic Brain Disease: A Systematic Review and Meta-Analysis. J Neurooncol (2019) 141(1):1–12. doi: 10.1007/s11060-018-03020-y
36. Lehrer EJ, Peterson J, Brown PD, Sheehan JP, Quinones-Hinojosa A, Zaorsky NG, et al. Treatment of Brain Metastases With Stereotactic Radiosurgery and Immune Checkpoint Inhibitors: An International Meta-Analysis of Individual Patient Data. Radiother Oncol (2019) 130:104–12. doi: 10.1016/j.radonc.2018.08.025
37. Pin Y, Paix A, Todeschi J, Antoni D, Proust F, Noel G. Brain Metastasis Formation and Irradiation by Stereotactic Radiation Therapy Combined With Immunotherapy: A Systematic Review. Crit Rev Oncol Hematol (2020) 149:102923. doi: 10.1016/j.critrevonc.2020.102923
38. van Opijnen MP, Dirven L, Coremans IEM, Taphoorn MJB, Kapiteijn EHW. The Impact of Current Treatment Modalities on the Outcomes of Patients With Melanoma Brain Metastases: A Systematic Review. Int J Cancer (2020) 146(6):1479–89. doi: 10.1002/ijc.32696
39. Weaver BD, Goodman JR, Jensen R. Concurrent Radiosurgery and Systemic Therapies for Melanoma Brain Metastases: A Systematic Review. Cureus (2019) 11(11):e6147. doi: 10.7759/cureus.6147
40. Moyers JT, Chong EG, Peng J, Tsai HHC, Sufficool D, Shavlik D, et al. Real World Outcomes of Combination and Timing of Immunotherapy With Radiotherapy for Melanoma With Brain Metastases. Cancer Med (2021) 10(4):1201–11. doi: 10.1002/cam4.3716
41. Kroeze SG, Fritz C, Hoyer M, Lo SS, Ricardi U, Sahgal A, et al. Toxicity of Concurrent Stereotactic Radiotherapy and Targeted Therapy or Immunotherapy: A Systematic Review. Cancer Treat Rev (2017) 53:25–37. doi: 10.1016/j.ctrv.2016.11.013
42. Churilla TM, Chowdhury IH, Handorf E, Collette L, Collette S, Dong Y, et al. Comparison of Local Control of Brain Metastases With Stereotactic Radiosurgery vs Surgical Resection: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol (2019) 5(2):243–7. doi: 10.1001/jamaoncol.2018.4610
43. Shaw E, Scott C, Souhami L, Dinapoli R, Kline R, Loeffler J, et al. Single Dose Radiosurgical Treatment of Recurrent Previously Irradiated Primary Brain Tumors and Brain Metastases: Final Report of RTOG Protocol 90-05. Int J Radiat Oncol Biol Phys (2000) 47(2):291–8. doi: 10.1016/S0360-3016(99)00507-6
44. Minniti G, D'Angelillo RM, Scaringi C, Trodella LE, Clarke E, Matteucci P, et al. Fractionated Stereotactic Radiosurgery for Patients With Brain Metastases. J Neurooncol (2014) 117(2):295–301. doi: 10.1007/s11060-014-1388-3
45. Rajakesari S, Arvold ND, Jimenez RB, Christianson LW, Horvath MC, Claus EB, et al. Local Control After Fractionated Stereotactic Radiation Therapy for Brain Metastases. J Neurooncol (2014) 120(2):339–46. doi: 10.1007/s11060-014-1556-5
46. Bang A, Wilhite TJ, Pike LRG, Cagney DN, Aizer AA, Taylor A, et al. Multicenter Evaluation of the Tolerability of Combined Treatment With PD-1 and CTLA-4 Immune Checkpoint Inhibitors and Palliative Radiation Therapy. Int J Radiat Oncol Biol Phys (2017) 98(2):344–51. doi: 10.1016/j.ijrobp.2017.02.003
47. Barker CA, Postow MA, Khan SA, Beal K, Parhar PK, Yamada Y, et al. Concurrent Radiotherapy and Ipilimumab Immunotherapy for Patients With Melanoma. Cancer Immunol Res (2013) 1(2):92–8. doi: 10.1158/2326-6066.CIR-13-0082
48. Anker CJ, Ribas A, Grossmann AH, Chen X, Narra KK, Akerley W, et al. Severe Liver and Skin Toxicity After Radiation and Vemurafenib in Metastatic Melanoma. J Clin Oncol (2013) 31(17):e283–7. doi: 10.1200/JCO.2012.44.7755
49. Peuvrel L, Ruellan AL, Thillays F, Quereux G, Brocard A, Saint-Jean M, et al. Severe Radiotherapy-Induced Extracutaneous Toxicity Under Vemurafenib. Eur J Dermatol (2013) 23(6):879–81. doi: 10.1684/ejd.2013.2193
50. Anker CJ, Grossmann KF, Atkins MB, Suneja G, Tarhini AA, Kirkwood JM. Avoiding Severe Toxicity From Combined BRAF Inhibitor and Radiation Treatment: Consensus Guidelines From the Eastern Cooperative Oncology Group (Ecog). Int J Radiat Oncol Biol Phys (2016) 95(2):632–46. doi: 10.1016/j.ijrobp.2016.01.038
51. Yamamoto M, Serizawa T, Shuto T, Akabane A, Higuchi Y, Kawagishi J, et al. Stereotactic Radiosurgery for Patients With Multiple Brain Metastases (JLGK0901): A Multi-Institutional Prospective Observational Study. Lancet Oncol (2014) 15(4):387–95. doi: 10.1016/S1470-2045(14)70061-0
52. Nieder C, Grosu AL, Gaspar LE. Stereotactic Radiosurgery (SRS) for Brain Metastases: A Systematic Review. Radiat Oncol (2014) 9:155. doi: 10.1186/1748-717X-9-155
53. Bernard ME, Wegner RE, Reineman K, Heron DE, Kirkwood J, Burton SA, et al. Linear Accelerator Based Stereotactic Radiosurgery for Melanoma Brain Metastases. J Cancer Res Ther (2012) 8(2):215–21. doi: 10.4103/0973-1482.98973
54. Christ SM, Mahadevan A, Floyd SR, Lam FC, Chen CC, Wong ET, et al. Stereotactic Radiosurgery for Brain Metastases From Malignant Melanoma. Surg Neurol Int (2015) 6(Suppl 12):S355–65. doi: 10.4103/2152-7806.163315
55. Bates JE, Youn P, Usuki KY, Walter KA, Huggins CF, Okunieff P, et al. Brain Metastasis From Melanoma: The Prognostic Value of Varying Sites of Extracranial Disease. J Neurooncol (2015) 125(2):411–8. doi: 10.1007/s11060-015-1932-9
56. Rades D, Sehmisch L, Huttenlocher S, Blank O, Hornung D, Terheyden P, et al. Radiosurgery Alone for 1-3 Newly-Diagnosed Brain Metastases From Melanoma: Impact of Dose on Treatment Outcomes. Anticancer Res (2014) 34(9):5079–82.
57. Brown PD, Ballman KV, Cerhan JH, Anderson SK, Carrero XW, Whitton AC, et al. Postoperative Stereotactic Radiosurgery Compared With Whole Brain Radiotherapy for Resected Metastatic Brain Disease (NCCTG N107C/CEC3): A Multicentre, Randomised, Controlled, Phase 3 Trial. Lancet Oncol (2017) 18(8):1049–60. doi: 10.1016/S1470-2045(17)30441-2
58. Brown PD, Gondi V, Pugh S, Tome WA, Wefel JS, Armstrong TS, et al. Hippocampal Avoidance During Whole-Brain Radiotherapy Plus Memantine for Patients With Brain Metastases: Phase Iii Trial Nrg Oncology Cc001. J Clin Oncol (2020) 38(10):1019–29. doi: 10.1200/JCO.19.02767
59. Hong AM, Fogarty GB, Dolven-Jacobsen K, Burmeister BH, Lo SN, Haydu LE, et al. Adjuvant Whole-Brain Radiation Therapy Compared With Observation After Local Treatment of Melanoma Brain Metastases: A Multicenter, Randomized Phase III Trial. J Clin Oncol (2019) 37(33):3132–41. doi: 10.1200/JCO.19.01414
60. Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, et al. Combination Nivolumab and Ipilimumab or Nivolumab Alone in Melanoma Brain Metastases: A Multicentre Randomised Phase 2 Study. Lancet Oncol (2018) 19(5):672–81. doi: 10.1016/S1470-2045(18)30139-6
61. Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, et al. Ipilimumab in Patients With Melanoma and Brain Metastases: An Open-Label, Phase 2 Trial. Lancet Oncol (2012) 13(5):459–65. doi: 10.1016/S1470-2045(12)70090-6
62. Dummer R, Goldinger SM, Turtschi CP, Eggmann NB, Michielin O, Mitchell L, et al. Vemurafenib in Patients With BRAF(V600) Mutation-Positive Melanoma With Symptomatic Brain Metastases: Final Results of an Open-Label Pilot Study. Eur J Cancer (2014) 50(3):611–21. doi: 10.1016/j.ejca.2013.11.002
63. Ahmed KA, Abuodeh YA, Echevarria MI, Arrington JA, Stallworth DG, Hogue C, et al. Clinical Outcomes of Melanoma Brain Metastases Treated With Stereotactic Radiosurgery and anti-PD-1 Therapy, anti-CTLA-4 Therapy, BRAF/MEK Inhibitors, BRAF Inhibitor, or Conventional Chemotherapy. Ann Oncol (2016) 27(12):2288–94. doi: 10.1093/annonc/mdw417
64. Collins R, Bowman L, Landray M, Peto R. The Magic of Randomization Versus the Myth of Real-World Evidence. N Engl J Med (2020) 382(7):674–8. doi: 10.1056/NEJMsb1901642
65. Tawbi H. The Standard of Care for Brain Metastases in Melanoma. Clin Adv Hematol Oncol (2020) 18(1):28–31.
Keywords: melanoma, brain metastasis, immunotherapy, targeted therapy, treatment guidelines
Citation: Tan X-L, Le A, Lam FC, Scherrer E, Kerr RG, Lau AC, Han J, Jiang R, Diede SJ and Shui IM (2022) Current Treatment Approaches and Global Consensus Guidelines for Brain Metastases in Melanoma. Front. Oncol. 12:885472. doi: 10.3389/fonc.2022.885472
Received: 28 February 2022; Accepted: 08 April 2022;
Published: 05 May 2022.
Edited by:
David Kaul, Charité Universitätsmedizin Berlin, GermanyReviewed by:
Stefano Vagge, San Martino Hospital (IRCCS), ItalyBrian Jeremy Williams, University of Louisville, United States
Copyright © 2022 Tan, Le, Lam, Scherrer, Kerr, Lau, Han, Jiang, Diede and Shui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang-Lin Tan, eGlhbmdsaW4udGFuQG1lcmNrLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Xiang-Lin Tan1*†
Xiang-Lin Tan1*† Amy Le
Amy Le Fred C. Lam
Fred C. Lam