- 1Department of Nuclear Medicine, The First Medical Centre, Chinese the People's Liberation Army (PLA) General Hospital, Beijing, China
- 2Key Laboratory of Digital Hepetobiliary Surgery, Faculty of Hepato-Pancreato-Biliary Surgery, Chinese People's Liberation Army (PLA) General Hospital, Institute of Hepatobiliary Surgery of Chinese People's Liberation Army (PLA), Beijing, China
- 3Department of Oncology, The Fifth Medical Centre, Chinese People's Liberation Army (PLA) General Hospital, Beijing, China
- 4Graduate School, Medical School of Chinese People's Liberation Army (PLA), Beijing, China
- 5Department of Pathology, The First Medical Centre, Chinese People's Liberation Army (PLA) General Hospital, Beijing, China
- 6General Electric (GE) Healthcare China, Shanghai, China
Objectives: This study aimed to assess the pretreatment 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) as a predictor of the pathological treatment response (PTR) of hepatocellular carcinoma (HCC) patients treated with PD-1 inhibitors and lenvatinib as a conversion therapy in BCLC stage C.
Methods: All patients (n=20) underwent pretreatment 18F-FDG PET/CT and were treated with conversion therapy and surgery. Patients were categorized into responders (n=9) and non-responders (n=11) according to PTR. The parameters of PET/CT, including lesion size, SUVmean (mean standard uptake value), MTV (metabolic tumor volume), TLG (total lesion glycolysis), SUVpeak (peak standard uptake value), and TLR (tumor-to-normal liver standardized uptake value ratio), were calculated. The diagnostic efficacy was evaluated by receiver operating characteristic analysis (ROC). PTR was compared with pretreatment PET/CT parameters by using Spearman correlation analysis. The patients were followed up.
Results: There was significant difference in TLR (5.59 ± 1.90 vs. 2.84 ± 1.70, respectively; P=0.003) between responders and non-responders, with the largest area under the curve (sensitivity=100%, specificity=72.7%, AUC=0.899, 95%CI: 0.759-1.000, optimal diagnostic threshold of 3.09). The relationship between 18F-FDG PET/CT parameters and PTR indicated TLR was moderately and positively correlated with pathological treatment response, with correlation coefficients (rs) of 0.69 (P<0.01). During the follow-up, no patients died, and tumor recurrence was found in one of the responders (11.1%). In all 11 non-responders, tumor recurrence was found in six patients (54.5%) and four patients (36.4%) died.
Conclusions: TLR may be a powerful marker to predict PTR of HCC patients with BCLC stage C who were treated with conversion therapy.
Introduction
Hepatocellular carcinoma (HCC) is the most common pathological type of liver cancer, accounting for 90% of primary liver cancers (1). Most HCC patients are diagnosed as local advanced or metastatic diseases, and patients may lose the opportunity for a radical cure with a poor prognosis (2). According to the criteria for the diagnosis and treatment of Barcelona Clinic Liver Cancer (BCLC) system (3) and the majority of international guidelines, patients with portal thrombosis or extrahepatic spread (BCLC stage C) should be treated with systemic therapies.
The current systemic therapy for patients with advanced HCC consists of tyrosine kinase inhibitors (TKI) and immune checkpoint inhibitors (ICI) (4). TKI, lenvatinib, as an emerging first-line systemic therapy for HCC, has been approved in China, USA, EU, Japan, and other countries for advanced HCC (5). Recent clinical studies have found that the combination of ICI and TKI was more effective. For example, the combination of lenvatinib with pembrolizumab or nivolumab showed objective response rates (ORRs) of 36% and 54.2%, respectively (6, 7). Another surprising discovery was that some patients who received ICI plus TKI as a conversion therapy retrieved the opportunity of surgery (8–14).
18F-fluorodeoxyglucose positron emission tomography (18F-FDG PET) is generally used for diagnosis, staging, and monitoring treatment of cancers, but shows poor sensitivity for the detection of HCC compared with other solid tumors (15). The higher 18F-FDG uptake appears to present in more undifferentiated tumors (16), and 18F-FDG uptake positive HCC is associated with a poor response to various treatments (17, 18). However, some studies found that 18F-FDG uptake is correlated with PD-1/PD-L1 status (19), and high 18F-FDG uptake may be a useful predictor of a rapid lenvatinib treatment response (20). Based on the findings above, we hypothesized that metabolic parameters of 18F-FDG PET may predict the patients who benefit from conversion therapy and may facilitate the choice of optimal therapy.
The main aim of this study was to evaluate the ability of metabolic parameters of pretreatment 18F-FDG PET to predict the pathological treatment response of HCC patients with BCLC stage C who were treated with PD-1 inhibitors plus lenvatinib.
Materials and Methods
Patients
Between July 2019 and April 2021, a total of 62 HCC patients with BCLC stage C who underwent pretreatment 18F-FDG PET/CT in Department of Nuclear Medicine, Chinese PLA General Hospital were retrospectively recruited.
The inclusion criteria were as follows:
1. All patients were aged >18 years without a history of other malignancies.
2. The diagnosis of HCC was confirmed by fine-needle biopsy pathology or in accordance with the clinical diagnosis criteria of the American Association for the Study of Liver Diseases (AASLD) (21).
3. All patients were diagnosed as HCC of BCLC stage C.
4. 18F-FDG PET/CT was performed within 2 weeks prior to treatment.
5. All patients were treated with PD-1 inhibitors plus lenvatinib after clinical evaluation in our hospital (Faculty of Hepato-Pancreato-Biliary Surgery, Chinese PLA General Hospital).
6. No other anti-tumor treatment was given during the treatment of PD-1 inhibitors plus lenvatinib, and the drugs were not stopped or changed during the treatment.
7. All patients received surgical treatment, and postoperative pathological examination was performed.
Finally, a total of 20 patients were included in this study (Figure 1). This single-center retrospective study was approved by the institutional ethics committee of the General Hospital of the People’s Liberation Army. All patients signed the informed consent before 18F-FDG PET/CT. The study was performed in accordance with the Declaration of Helsinki.
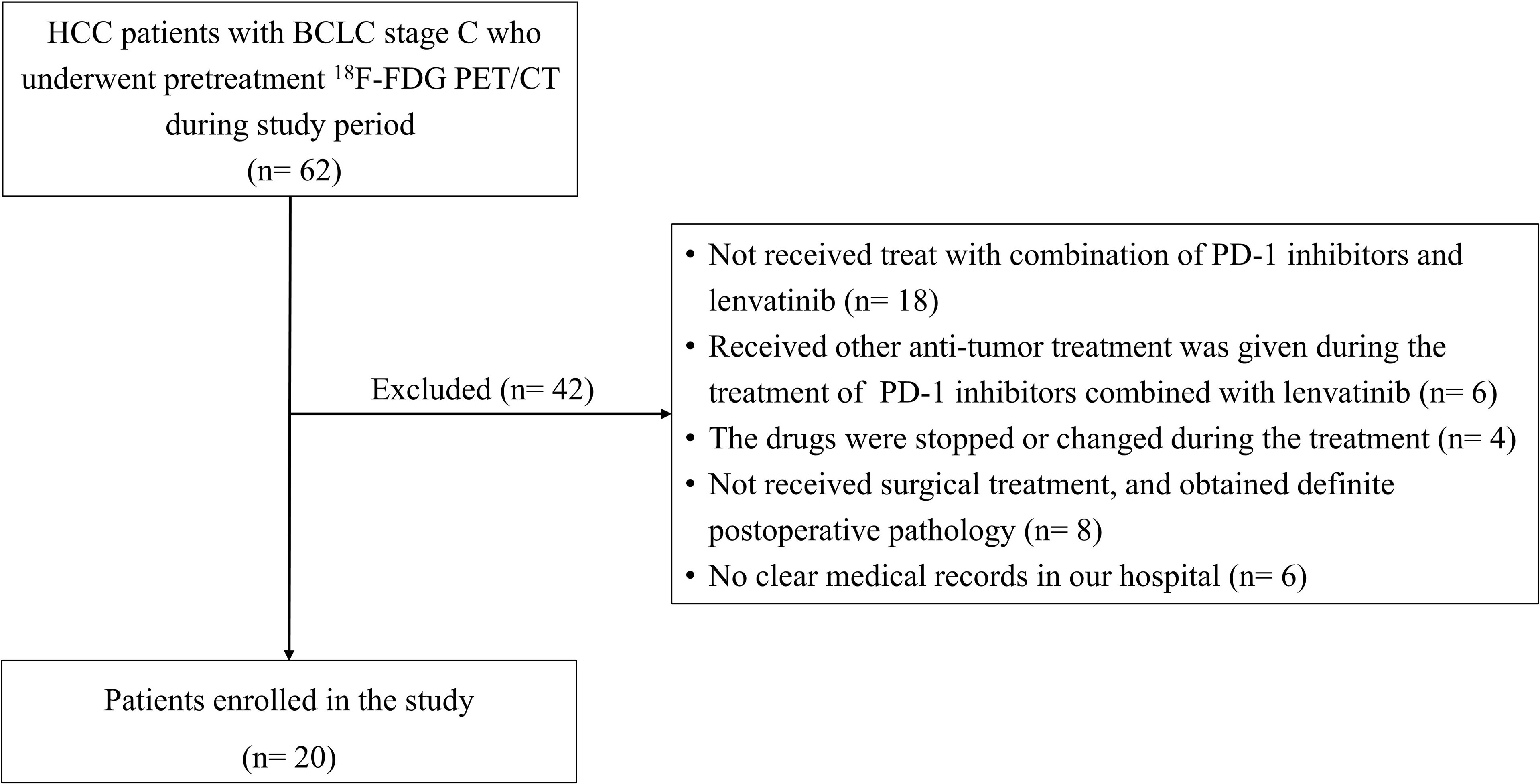
Figure 1 Patient in- and exclusion flow diagram. HCC, Hepatocellular carcinoma; BCLC, Barcelona Clinic Liver Cancer; 18F-FDG PET/CT, 18F-fluorodeoxyglucose positron emission tomography/computed tomography.
PET/CT Scanning
All patients were scanned with 18F-FDG PET/CT (Biograph 64, GE Healthcare). Patients were fasted for 6 hours with plasma glucose levels < 11.1 mmol/L, and rested for at least 20 min in a quiet waiting room before intravenous administration of 18F-FDG (18F-FDG; Manufactured by our department, radiochemical purity of >95%). The patients were injected with 18F-FDG at a dose of 3.70-4.44 MBq/kg (0.10-0.12mCi/kg). PET/CT scan was performed after 60 min, beginning from the skull base to the upper femur in free-breathing mode. The low dose CT (LDCT) parameters: voltage=120kV, current=100mAs, rotation=0.8, layer thickness=5mm, pitch=1. The parameters of PET included three-dimensional mode, 2 min/bed (30% overlap), 4-5 beds/person, three iterations, 21 subsets, Gaussian filter half-height width=4.0 mm. The images were reconstructed with CT attenuation correction (AC) by using the ordered subset expectation maximization algorithm (OSEM).
Image Analysis
Multiparametric Analysis prototype (GE Healthcare, Germany), a dedicated prototype post-processing tool, was used for imaging analysis. Quantitative analyses were performed by two experienced physicians of nuclear medicine blinded to the clinical information of the patients. If the views were inconsistent between the two physicians, the process would be repeated two weeks later until a consensus was reached. Areas with abnormal uptake of 18F-FDG on PET and/or abnormal density on CT were defined as lesions. A two-dimensional region of interest (ROI) was delineated manually according to the boundary of the tumor lesion on each layer of transaxial CT images to form a three-dimensional volume of interest (VOI). Sometimes contrast-enhanced magnetic resonance imaging (MRI)/CT were used to help determine the VOI. We applied the VOI to the corresponding PET images, which were registered to CT images. To measure normal liver activity, three non-overlapping spherical 1-cm3-sized VOIs were drawn in the normal liver on the axial PET images, avoiding the HCC areas on dynamic CT. MTV and TLG were obtained based on a threshold SUV of≥ 2.5.
The parameters of PET/CT, including lesion size (diameters, mm), SUVmean (mean standard uptake value), MTV (metabolic tumor volume), TLG (total lesion glycolysis, SUVmean×MTV), SUVpeak (peak standard uptake value), and TLR (tumor-to-normal liver standardized uptake value ratio, SUVmax of the tumor/SUVmean of the normal liver parenchyma), were calculated.
Systemic Therapy
Conversion therapy mainly includes PD-1 inhibitors and lenvatinib. Lenvatinib was taken orally (8 or 12 mg/day, depending on patients’ weight<60kg or ≥60kg). The patients were treated with intravenous infusion of anti-PD-1 antibodies (dose, 200-240mg), and the great majority of the data collected were associated with four treatment regimens (pembrolizumab 200 mg/q3w, sintilimab 200 mg/q3w, toripalimab 240 mg/q3w, and tislelizumab 200mg/q3w), as shown in Table S1.
Follow-Up During Systemic Therapy and Radiological Assessment
All patients were treated regularly and should be monitored to assess their response to systemic therapy. The complete blood count, thyroid, cardiac, liver, renal, adrenal functions, and tumor markers were examined prior to each cycle of PD-1 treatment. Before and after the conversion treatment protocol, imaging examinations were performed. For patients with multiple lesions, we defined the largest lesion as the main lesion by pretreatment contrast-enhanced MRI/CT and 18F-FDG PET/CT. Tumor response [according to RECIST v1.1 (22) and mRECIST criteria (23)] and the resectability of liver cancer were evaluated by contrast-enhanced MRI/CT, chest CT, and other liver function assessments. Adverse events were assessed using the National Cancer Institute’s Common Terminology Criteria for Adverse Events v4.0.
Criteria for Successful Conversion Therapy
The criteria for successful conversion were below:
1. Child-Pugh grade A.
2. ECOG PS score ≤ 1.
3. Metastatic lymph nodes shrink or disappear, and the remaining lymph nodes can be removed.
4. No new extrahepatic metastases.
5. Intact vascular structure (including inflow and outflow) of the reserved liver.
6. The expected ratio of future liver remnant volume to standard liver volume (FLR/SLV) after resection of tumor-bearing liver is ≥40% in the compromised liver and 35% in the normal liver.
All patients who met the criteria of successful conversion were informed of the benefits and risks of surgery.
Histopathologic Assessment of Tumor Regression
Surgical specimens were analyzed by two experienced pathologists who were blinded to the treatment and outcomes. The pathological treatment response was classified based on the tumor cellularity, and only the primary tumors were analyzed. Major pathologic response (MPR, <10% residual viable tumor cellularity) after conversion immunotherapy was used as an endpoint in the great majority of clinical trials (24). Hence, we divided all patients into two groups: responders (<10% cellularity), and non-responders (≥10% cellularity).
Postoperative Therapy and Follow-Up
Patients continued to receive therapy according to the pathological results and personal conditions 4-6 weeks after surgery, and clinical evaluation. Serum tumor biomarkers were examined every cycle, and imaging examinations (contrast-enhanced MRI/CT or abdominal ultrasound) were performed every 3 months to monitor tumor recurrence. HCC recurrence was defined as the presence of radiological evidence of new intra- and/or extrahepatic tumors (25). According to the guidelines (26), post-recurrence treatments were administered.
Statistical Analysis
Qualitative data were expressed as number of cases and percentage [n (%)]. Quantitative data were expressed as medians (interquartile ranges) or mean ± SD. The student t test or Mann-Whitney test was used to compare pretreatment 18F-FDG PET/CT parameters between responders and non-responders. The optimal cut-off values for continuous variables were estimated using receiver operating characteristic (ROC) curve analysis with the area under the curve (AUC), and we calculated sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV), respectively. Pathological treatment response was compared with pretreatment 18F-FDG PET/CT parameters by using Spearman correlation analysis. The statistical analysis was performed by using commercially available software (IBM SPSS Statistics 24, IBM, Armonk, NY; and R software program, version 4.0.2, Bell Laboratories, USA). All statistical tests were two-sided, and the significance level was set at P=0.05.
Results
Patients’ Characteristics
Patient characteristics before treatment are shown in Table 1. Eventually, 20 patients underwent surgical excision after successful conversion therapy, who were enrolled in our study (18 male; median age, 54.0 years; IQR, 42.3–60.3 years). Eleven patients had a history of liver diseases (including 11 HBV, three HCV, and one alcoholic liver disease). The ECOG PS score of all cases was 0, and their liver function was Child-Pugh grade A before treatment.
Sixteen of the included patients were treated with 3-6 cycles of PD-1 inhibitors plus lenvatinib, two patients received seven cycles, one patient received nine cycles, and one patient received 20 cycles. Nineteen patients received anatomic resection, and their surgical margins were all negative for malignancy (R0).
The Value of Pretreatment 18F-FDG PET/CT Parameters in Prediction of Pathological Treatment Response
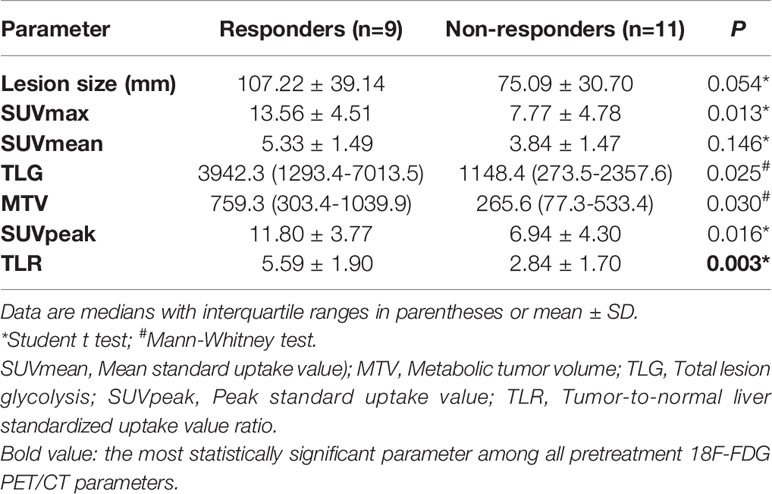
Among 20 patients, nine patients (45.0%) were considered responders. Pretreatment 18F-FDG PET/CT parameters were compared between responders and non-responders, and it was found that pretreatment TLR (5.59 ± 1.90 vs. 2.84 ± 1.70, respectively; P=0.003), SUVmax (13.56 ± 4.51 vs. 7.77 ± 4.78, respectively; P=0.013), SUVpeak (11.80 ± 3.77 vs. 6.94 ± 4.30, respectively; P=0.016), TLG [3942.3 (1293.4-7013.5) vs. 1148.4 (273.5-2357.6), respectively; P=0.025] and MTV [759.3 (303.4-1039.9) vs. 265.6 (77.3-533.4), respectively; P=0.030] were higher in the responder group than in the non-responder group. However, there was no significant difference in SUVmean between responder and non-responder groups (Table 2).
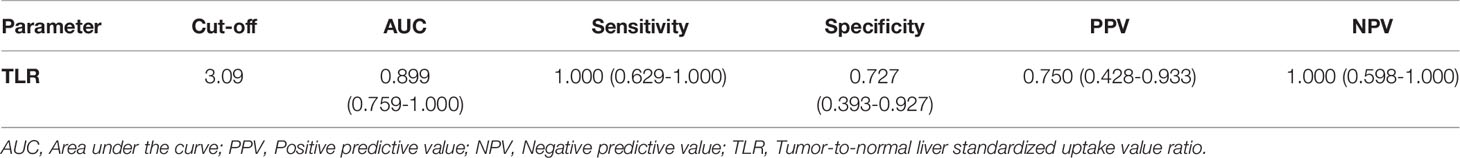
The efficiencies of lesion size and 18F-FDG PET/CT parameters for predicting pathological treatment response were evaluated. TLR showed the largest area under the curve (AUC=0.899, 95% confidence interval [CI]: 0.759-1.000), with a Youden index of 0.727, and optimal diagnostic threshold of 3.09. The positive predictive value (PPV) and negative predictive value (NPV) were 0.750 (0.428-0.933) and 1.000 (0.598-1.000), respectively. Details are shown in Table 3 and Figure 2.
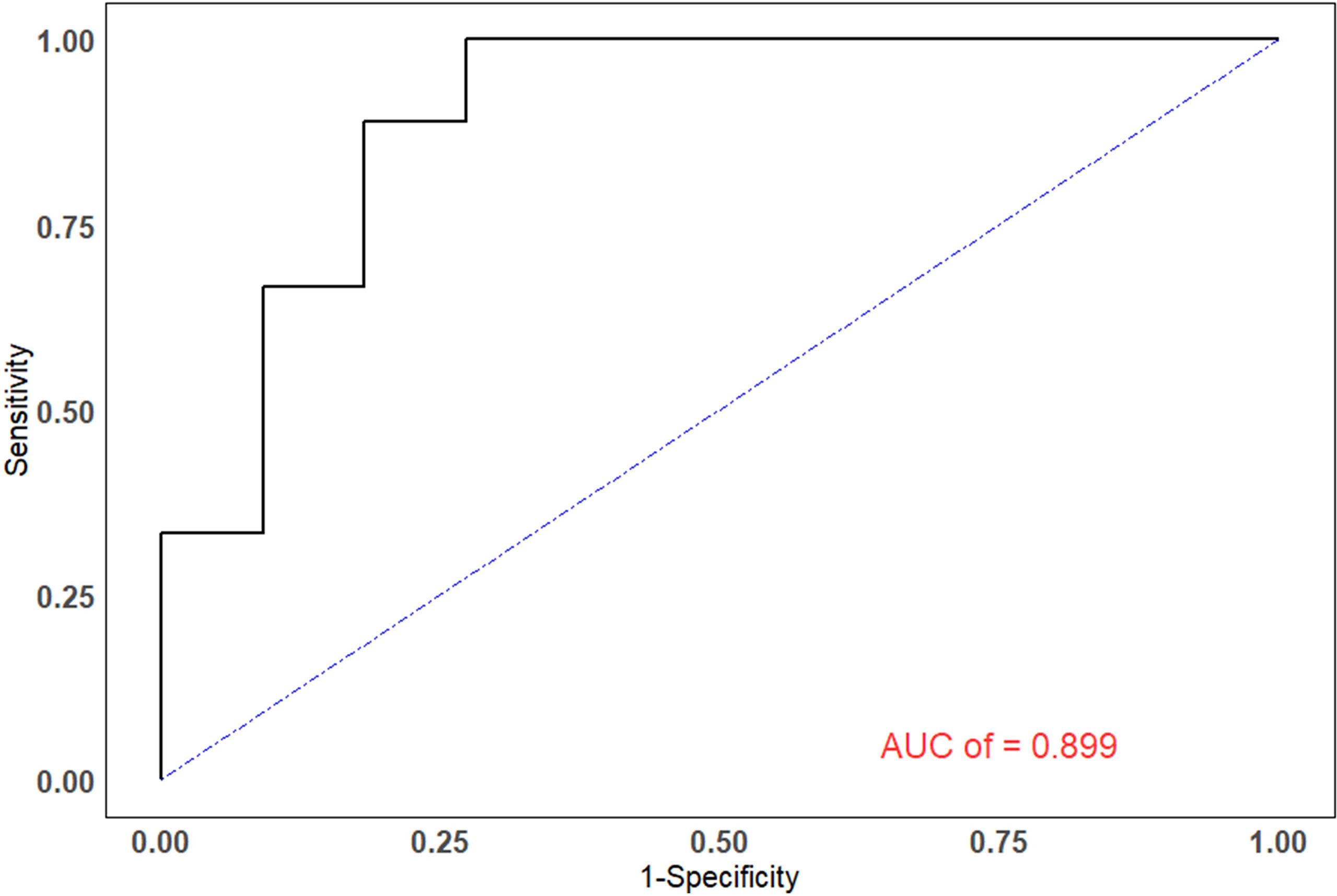
Figure 2 The ROC curves of TLR. The areas under the ROC curves for the ability to predict pathological treatment response for TLR was 0.899.
Correlation Between 18F-FDG PET/CT Parameters and Pathological Treatment Response
We performed Spearman correlation to explore the relationship between 18F-FDG PET/CT parameters and pathological treatment response. The results showed that TLR, SUVmean, and SUVpeak had moderate positive correlation with pathological treatment response, and the correlation coefficients (rs) were 0.69, 0.62, and 0.60, respectively (P<0.05). Lesion size, TLG, and MTV showed no significant correlation (r =0.42, r =0.51 and r =0.50, respectively; P>0.05). The results are displayed in Table 4 and Figure 3.
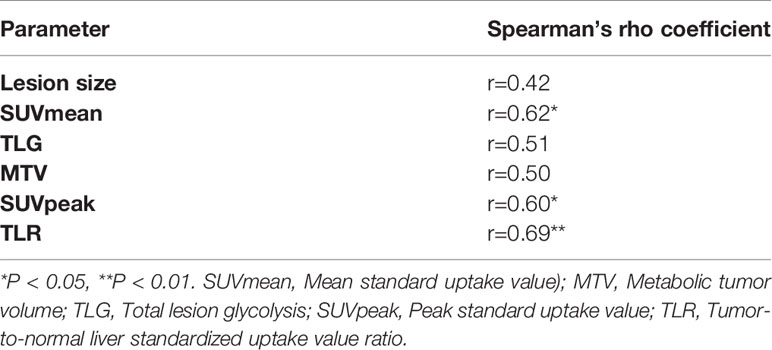
Table 4 The correlation between pretreatment 18F-FDG PET/CT parameters and pathological treatment response.
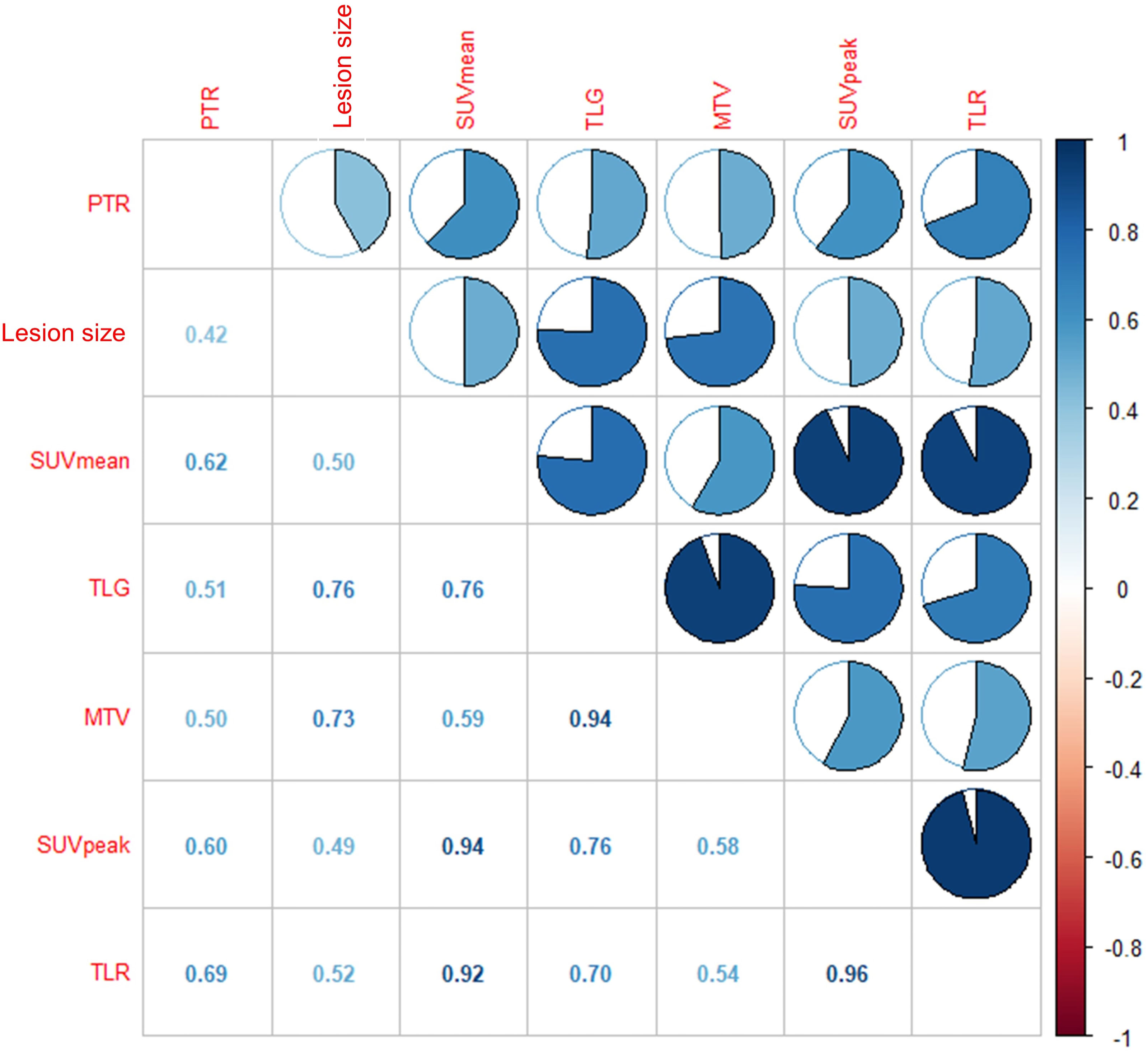
Figure 3 The results showed that moderate correlation between TLR and pathological treatment response (r = 0.69, P < 0.01), SUVmean and pathological treatment response (r = 0.62, P < 0.05), SUVpeak and pathological treatment response (r = 0.60, P < 0.05), respectively.
Follow-Up
The follow-up ended on January 1, 2022. No patient was lost to follow-up and the median follow-up time was 15.8 months (range, 5.0-32.9 months). During the follow-up, all nine responders remained alive, and tumor recurrence was found in two patients (2.2%; at 6.7 months and 9.9 months, respectively). However, in the non-responder group, tumor recurrence was found in six patients [54.5%; a median of 5.9 months (range, 4.6-14.4 months)] and four patients [36.4%; a median of 10.3 months (range: 8.2-12.9 months)] died. Follow-up information of the patients is shown in Table S2.
Discussion
This is the first study that utilized the metabolic parameters of pretreatment 18F-FDG PET to predict the pathological treatment response of HCC patients with BCLC stage C who were treated with PD-1 inhibitors and lenvatinib. Our findings showed that the metabolic parameters of pretreatment 18F-FDG PET could distinguish responders from non-responders, and a high TLR (TLR≥3.09) was likely to predict pathological treatment response. This result may suggest the pretreatment 18F-FDG PET can help HCC patients of BCLC stage C stratified before treatment of PD-1 inhibitors and lenvatinib.
Surgery has always been the most effective treatment for liver cancer. However, patients with advanced HCC (BCLC stage C) often have lost the opportunity for surgery, and systemic treatment is recommended by the guidelines (1, 27). TKI, such as sorafenib and lenvatinib, has been proven effective in the first-line systemic therapy of advanced HCC patients (5, 28). In recent years, ICI (anti-PD-1, anti-PD-L1, and anti-CTLA-4 antibodies) has exhibited potential therapeutic effects for advanced HCC (29). Although the previously discussed approaches provide novel opportunities for the treatment of advanced HCC, satisfactory clinical results cannot be obtained using single therapy. Some studies have shown that the combination of PD-1/PD-L1 inhibitors and anti-angiogenesis targeted drugs showed a better curative effect (6, 7, 30–33). For example, lenvatinib combined with pembrolizumab or nivolumab showed promising antitumor activity with a tolerable safety profile in patients with advanced HCC (6, 7). Of note, Ho et al. reported a case of successful conversion of locally advanced hepatocellular carcinoma to resectable tumor after treatment with TKI plus immunotherapy (11). Since then, Ho et al. pursued research, and it was found that cabozantinib and nivolumab converted locally advanced hepatocellular carcinoma into resectable disease (12). Of 20 patients enrolled, 20 (100%) underwent successful margin-negative resection (R0) and nine out of 20 (45%) had MPR. Zhu et al. found the combination of TKI/anti-PD-1 antibodies was a feasible conversion therapy for patients with unresectable HCC to become resectable (13). A prospective clinical registration study (ChiCTR1900023914) also found that patients who were treated with PD-1 inhibitors plus TKI were successfully converted and subsequently underwent surgery (8–10). Therefore, we conducted a preliminary study on the relationship between parameters of pretreatment 18F-FDG PET and pathological treatment response.
Pretreatment FDG PET can provide useful information on the expression of immune checkpoints and the metabolic state of the tumor microenvironment (34), and 18F-FDG uptake is correlated with the expression of PD-1/PD-L1 in lung cancer and bladder cancer (35–37). However, HCC is a very heterogeneous tumor from a genetic standpoint with different oncogenic pathways (38). Immunotherapy based on checkpoint inhibitors in HCC is not associated with increased or decreased efficacy based on PD-1 or PD-L1 assessment (39), and only serum alpha-fetoprotein (AFP) levels might play a clinical role in patients treated by TKI (40). In our study, the metabolic parameters of pretreatment 18F-FDG PET could distinguish responders from non-responders. One explanation is that 18F-FDG uptake and tumor infiltrative lymphocytes (TILs), especially T cells, might be the causes of this phenomenon (41, 42). Itoh et al. found that SUVmax values of HCC were associated with intratumoral CD8+ T cell counts (P=0.0044) (43). It was further found that immune cells, including T cells and B cells, were significantly enriched in tumor cells of locally advanced hepatocellular carcinoma patients responding to the novel combination of cabozantinib and nivolumab (11, 12). Moreover, Kawamura et al. reported that high TLR≥2 in HCC may be a useful predictor of an extremely rapid response to lenvatinib (20). The evidence above may suggest that high 18F-FDG uptake of tumors is related to the effectiveness of PD-1 and TKI treatment. Our results suggest that a high TLR (TLR≥3.09) can be used as a powerful indicator to predict the pathological treatment response of HCC patients of BCLC stage C who were treated with PD-1 inhibitors and lenvatinib as a conversion therapy (Figures 4A, B). It is noteworthy that, although high TLR may suggest a response to the combination of PD-1 inhibitors and lenvatinib, 18F-FDG PET positive HCC is highly associated with poor histological differentiation and poor prognosis (20, 44). But other exploratory analyses showed that higher expression of PD-L1 in tumor tissues, higher expression of vascular endothelial growth factor (VEGF) receptor 2, and higher T-regulatory cells immune phenotype may be associated with longer survival of patients treated by atezolizumab plus bevacizumab (45). It is suggested that 18F-FDG PET may direct systemic treatment of patients with advanced HCC (1). However, biomarkers related to the treatment of advanced HCC are still very limited, and this conjecture remains to be proven by more larger studies.
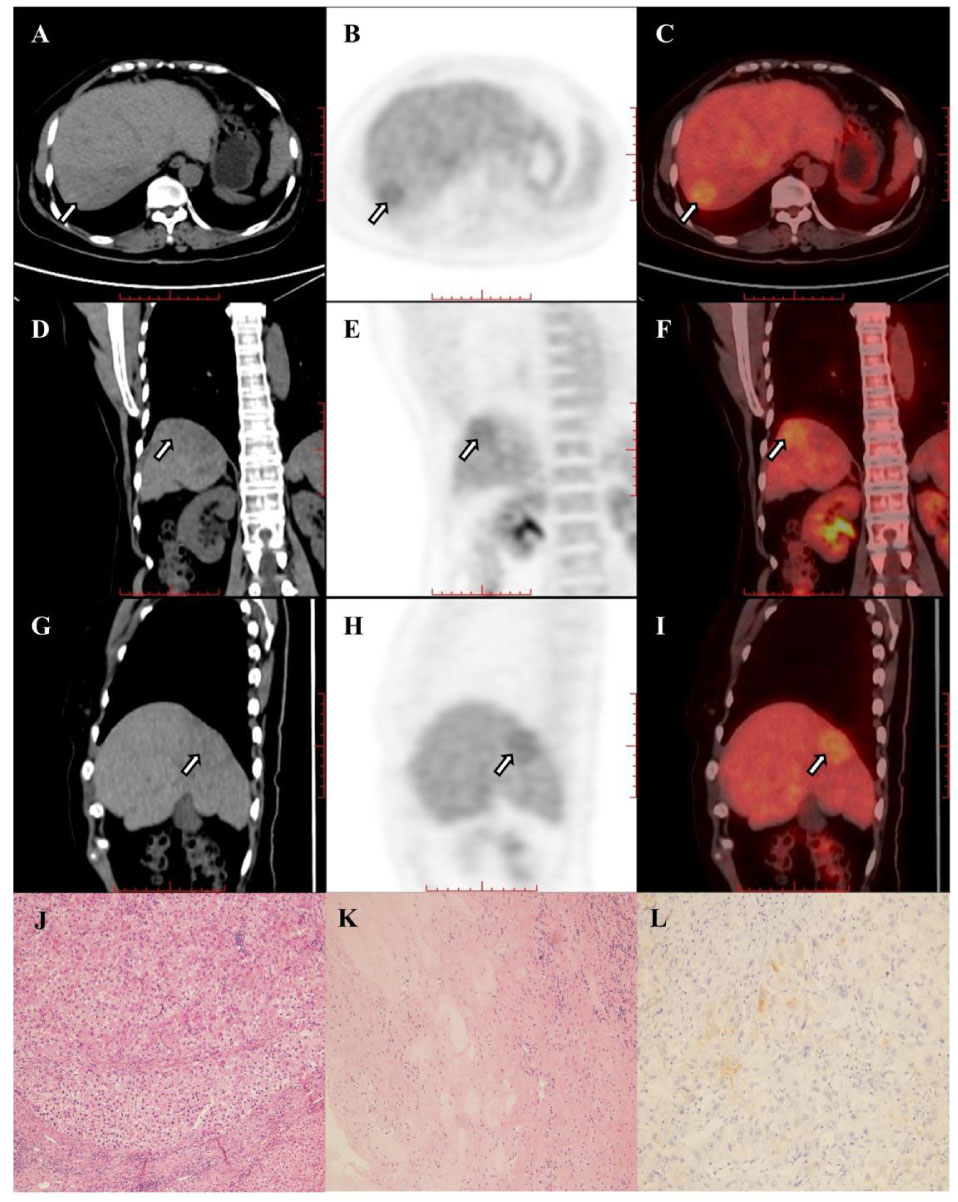
Figure 4A (A–I) Images in 67-year-old woman with hepatocellular carcinoma in the right hepatic lobe (arrow), and patient was accompanied by portal vein tumor thrombus and hilar lymph node metastasis. The hepatic lesion showed low uptake (TLR=1.31). The baseline AFP was 2.19 and the Child-Pugh score was 6. The patient underwent right hemihepatectomy after 4 cycles of combination of pembrolizumab plus lenvatinib therapy. Histopathologic evaluation of response revealed bad response to therapy (residual viable tumor cellularity>90%, J–L).
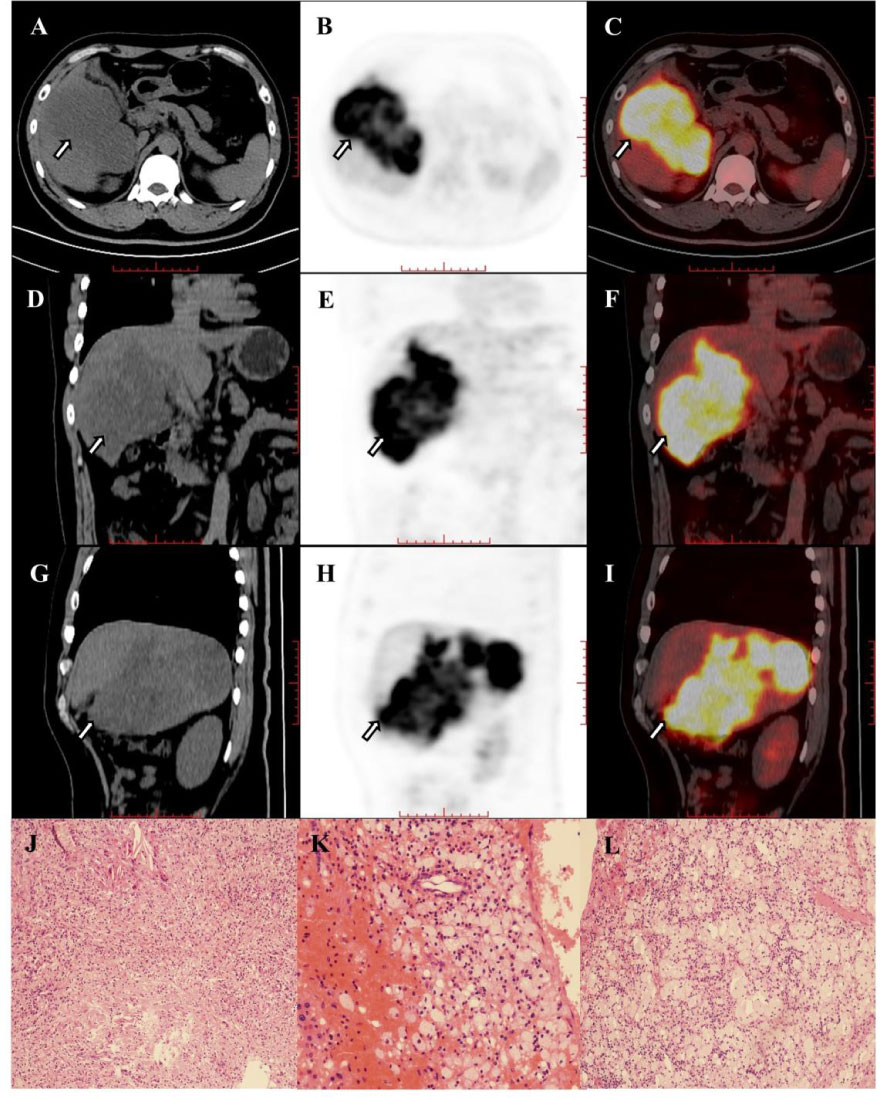
Figure 4B (A–I) Images in 42-year-old man with hepatocellular carcinoma in the right hepatic lobe (arrow) and hilar lymph node metastasis. The hepatic lesion showed high uptake (TLR=7.66). The baseline AFP was >60500 and the Child-Pugh score was 6. The patient underwent right hemihepatectomy after 4 cycles of combination of pembrolizumab plus lenvatinib therapy. Histopathologic evaluation of response revealed good response to therapy (complete disappearance of the tumor cells, J–L).
There are some limitations in this preliminary study. First, the number of patients was small in this retrospective and single-center cohort study. Second, only pathological treatment response of the primary tumor without venous thrombosis and peripheral lymph nodes was evaluated. However, our finding remains to be verified in the tumor with lymph node metastasis or distal metastasis. Third, we investigated the correlation between PET parameters and histopathological indexes, but due to insufficient number of patients, the correlation between PET parameters and progression-free survival or overall survival was not evaluated. Using the surgical specimen of the treated tumor, it is feasible to assess treatment efficacy and potentially predict disease-free survival and overall survival (24), and our limited follow-up results also indicate that responders had a better prognosis. Our study may provide evidence for the treatment of advanced HCC patients with ICI plus TKI. However, our findings remain to be further verified by a prospective study with a larger cohort and comprehensive evaluation of pathological treatment response and prognosis.
Conclusion
The metabolic parameters of pretreatment 18F-FDG PET, especially a high TLR, may be a powerful marker to predict the pathological treatment response of HCC patients of BCLC stage C who were treated with PD-1 inhibitors and lenvatinib as a conversion therapy. The parameters of pretreatment 18F-FDG PET might be useful for making treatment strategies for HCC patients.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
Conception and design: GW, WZ, SL, JT, GM, and BX; Data collation: GW, WZ, JC, XL, and ZW; Statistical Analysis: GW, YW, and ZG; Article writing: GW, WZ, JC, XX, and ZG; Article revision: YW, SY, SL, JT, GM, and BX. All authors contributed to the article and approved the submitted version.
Conflict of Interest
YW was employed by the company GE Healthcare.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors were appreciative to the staffs at Department of Nuclear Medicine and Faculty of Hepato-Pancreato-Biliary Surgery (The First Medical Centre, Chinese PLA General Hospital, Beijing, China) for their assistance in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.884372/full#supplementary-material
References
1. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular Carcinoma. Nat Rev Dis Primers (2021) 7(1):6. doi: 10.1038/s41572-020-00240-3
2. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ, et al. Global Patterns of Hepatocellular Carcinoma Management From Diagnosis to Death: The BRIDGE Study. Liver Int (2015) 35(9):2155–66. doi: 10.1111/liv.12818
3. Llovet JM, Bru C, Bruix J. Prognosis of Hepatocellular Carcinoma: The BCLC Staging Classification. Semin Liver Dis (1999) 19(3):329–38. doi: 10.1055/s-2007-1007122
4. Zhang T, Zhang L, Xu Y, Lu X, Zhao H, Yang H, et al. Neoadjuvant Therapy and Immunotherapy Strategies for Hepatocellular Carcinoma. Am J Cancer Res (2020) 10(6):1658–67.
5. Zhao Y, Zhang YN, Wang KT, Chen L. Lenvatinib for Hepatocellular Carcinoma: From Preclinical Mechanisms to Anti-Cancer Therapy. Biochim Biophys Acta Rev Cancer (2020) 1874(1):188391. doi: 10.1016/j.bbcan.2020.188391
6. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, et al. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol (2020) 38(26):2960–70. doi: 10.1200/JCO.20.00808
7. Kudo M, Ikeda M, Motomura K, Kobayashi M. A Phase Ib Study of Lenvatinib (LEN) Plus Nivolumab (NIV) in Patients (Pts) With Unresectable Hepatocellular Carcinoma (uHCC): Study 117. J Clin Oncol (2020) 38(Suppl 4):513. doi: 10.1200/JCO.2020.38.4_suppl.513
8. Zhang W, Hu B, Han J, Wang H, Wang Z, Ye H, et al. A Real-World Study of PD-1 Inhibitors Combined With TKIs for HCC With Major Vascular Invasion as the Conversion Therapy: A Prospective, Non-Randomized, Open-Label Cohort Study. Ann Oncol (2020) 31(suppl_6):S1287–318. doi: 10.1016/j.annonc.2020.10.195
9. Zhang W, Lu S, Hu B, Wan T, Wang H, Han J, et al. PD-1 Inhibitor Combined With Lenvatinib for Unresectable Liver Cancer as the Conversion Therapy: An Open-Label, non-Randomized, Phase IV Study. J Clin Oncol (2021) 39(suppl 15):abstr:e16173. doi: 10.1200/JCO.2021.39.15_suppl.e16173
10. Zhang W, Hu B, Han J, Wang Z, Ma G, Ye H, et al. Surgery After Conversion Therapy With PD-1 Inhibitors Plus Tyrosine Kinase Inhibitors Are Effective and Safe for Advanced Hepatocellular Carcinoma: A Pilot Study of Ten Patients. Front Oncol (2021) 11(4082). doi: 10.3389/fonc.2021.747950
11. Ho WJ, Sharma G, Zhu Q, Stein-O'Brien G, Durham J, Anders R, et al. Integrated Immunological Analysis of a Successful Conversion of Locally Advanced Hepatocellular Carcinoma to Resectability With Neoadjuvant Therapy. J Immunother Cancer (2020) 8(2):e000932. doi: 10.1136/jitc-2020-000932
12. Ho WJ, Zhu Q, Durham J, Popovic A, Xavier S, Leatherman J, et al. Neoadjuvant Cabozantinib and Nivolumab Convert Locally Advanced Hepatocellular Carcinoma Into Resectable Disease With Enhanced Antitumor Immunity. Nat Cancer 2. (2021), 891–903. doi: 10.1038/s43018-021-00234-4
13. Zhu XD, Huang C, Shen YH, Ji Y, Ge NL, Qu XD, et al. Downstaging and Resection of Initially Unresectable Hepatocellular Carcinoma With Tyrosine Kinase Inhibitor and Anti-PD-1 Antibody Combinations. Liver Cancer (2021) 10(4):320–9. doi: 10.1159/000514313
14. Zhao L, Zhao H. Conversion Surgery for Hepatocellular Carcinoma in the New Era of Targeted and Immune Checkpoint Inhibitor Therapies. Hepatobiliary Surg Nutr (2020) 9(6):809–11. doi: 10.21037/hbsn-20-693
15. Hong CM, Ahn BC, Jang YJ, Jeong SY, Lee SW, Lee J. Prognostic Value of Metabolic Parameters of 18F-FDG PET/CT and Apparent Diffusion Coefficient of MRI in Hepatocellular Carcinoma. Clin Nucl Med (2017) 42(2):95–9. doi: 10.1097/RLU.0000000000001478
16. Goh V, Sarker D, Osmany S, Cook GJ. Functional Imaging Techniques in Hepatocellular Carcinoma. Eur J Nucl Med Mol Imaging (2012) 39(6):1070–9. doi: 10.1007/s00259-012-2096-x
17. Jreige M, Mitsakis P, Van DerGucht A, Pomoni A, Silva-Monteiro M, Gnesin S, et al. (18)F-FDG PET/CT Predicts Survival After (90)Y Transarterial Radioembolization in Unresectable Hepatocellular Carcinoma. Eur J Nucl Med Mol Imaging (2017) 44(7):1215–22. doi: 10.1007/s00259-017-3653-0
18. Kitamura K, Hatano E, Higashi T, Seo S, Nakamoto Y, Yamanaka K, et al. Preoperative FDG-PET Predicts Recurrence Patterns in Hepatocellular Carcinoma. Ann Surg Oncol (2012) 19(1):156–62. doi: 10.1245/s10434-011-1990-y
19. Decazes P, Bohn P. Immunotherapy by Immune Checkpoint Inhibitors and Nuclear Medicine Imaging: Current and Future Applications. Cancers (Basel) (2020) 12(2):371. doi: 10.3390/cancers12020371
20. Kawamura Y, Kobayashi M, Shindoh J, Kobayashi Y, Kasuya K, Sano T, et al. (18)F-Fluorodeoxyglucose Uptake in Hepatocellular Carcinoma as a Useful Predictor of an Extremely Rapid Response to Lenvatinib. Liver Cancer (2020) 9(1):84–92. doi: 10.1159/000503577
21. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology (2018) 68(2):723–50. doi: 10.1002/hep.29913
22. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
23. Lencioni R, Llovet JM. Modified RECIST (mRECIST) Assessment for Hepatocellular Carcinoma. Semin Liver Dis (2010) 30(1):52–60. doi: 10.1055/s-0030-1247132
24. Stein JE, Lipson EJ, Cottrell TR, Forde PM, Anders RA, Cimino-Mathews A, et al. Pan-Tumor Pathologic Scoring of Response to PD-(L)1 Blockade. Clin Cancer Res (2020) 26(3):545–51. doi: 10.1158/1078-0432.CCR-19-2379
25. Lim C, Salloum C, Chalaye J, Lahat E, Costentin CE, Osseis M, et al. 18f-FDG PET/CT Predicts Microvascular Invasion and Early Recurrence After Liver Resection for Hepatocellular Carcinoma: A Prospective Observational Study. HPB (Oxford) (2019) 21(6):739–47. doi: 10.1016/j.hpb.2018.10.007
26. Zhou J, Sun H, Wang Z, Cong W, Wang J, Zeng M, et al. Guidelines for the Diagnosis and Treatment of Hepatocellular Carcinoma (2019 Edition). Liver Cancer (2020) 9(6):682–720. doi: 10.1159/000509424
27. Villanueva A. Hepatocellular Carcinoma. N Engl J Med (2019) 380(15):1450–62. doi: 10.1056/NEJMra1713263
28. Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic Factors and Predictors of Sorafenib Benefit in Patients With Hepatocellular Carcinoma: Analysis of Two Phase III Studies. J Hepatol (2017) 67(5):999–1008. doi: 10.1016/j.jhep.2017.06.026
29. Zongyi Y, Xiaowu L. Immunotherapy for Hepatocellular Carcinoma. Cancer Lett (2020) 470:8–17. doi: 10.1016/j.canlet.2019.12.002
30. Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, et al. Atezolizumab Plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med (2020) 382(20):1894–905. doi: 10.1056/NEJMoa1915745
31. Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J, et al. Camrelizumab in Combination With Apatinib in Patients With Advanced Hepatocellular Carcinoma (RESCUE): A Nonrandomized, Open-Label, Phase II Trial. Clin Cancer Res (2021) 27(4):1003–11. doi: 10.1158/1078-0432.CCR-20-2571
32. Qin S, Ren Z, Feng YH, Yau T, Wang B, Zhao H, et al. Atezolizumab Plus Bevacizumab Versus Sorafenib in the Chinese Subpopulation With Unresectable Hepatocellular Carcinoma: Phase 3 Randomized, Open-Label IMbrave150 Study. Liver Cancer (2021) 10(4):296–308. doi: 10.1159/000513486
33. Wang Y, Lu LC, Guan Y, Ho MC, Lu S, Spahn J, et al. Atezolizumab Plus Bevacizumab Combination Enables an Unresectable Hepatocellular Carcinoma Resectable and Links Immune Exclusion and Tumor Dedifferentiation to Acquired Resistance. Exp Hematol Oncol (2021) 10(1):45. doi: 10.1186/s40164-021-00237-y
34. Aide N, Hicks RJ, Le Tourneau C, Lheureux S, Fanti S, Lopci E. FDG PET/CT for Assessing Tumour Response to Immunotherapy: Report on the EANM Symposium on Immune Modulation and Recent Review of the Literature. Eur J Nucl Med Mol Imaging (2019) 46(1):238–50. doi: 10.1007/s00259-018-4171-4
35. Chen R, Zhou X, Liu J, Huang G. Relationship Between the Expression of PD-1/PD-L1 and (18)F-FDG Uptake in Bladder Cancer. Eur J Nucl Med Mol Imaging (2019) 46(4):848–54. doi: 10.1007/s00259-018-4208-8
36. Hashimoto K, Kaira K, Yamaguchi O, Mouri A, Shiono A, Miura Y, et al. Potential of FDG-PET as Prognostic Significance After Anti-PD-1 Antibody Against Patients With Previously Treated Non-Small Cell Lung Cancer. J Clin Med (2020) 9(3):725. doi: 10.3390/jcm9030725
37. Surov A, Meyer HJ, Wienke A. Standardized Uptake Values Derived From (18)F-FDG PET May Predict Lung Cancer Microvessel Density and Expression of KI 67, VEGF, and HIF-1alpha But Not Expression of Cyclin D1, PCNA, EGFR, PD L1, and P53. Contrast Media Mol Imaging (2018) 2018:9257929. doi: 10.1155/2018/9257929
38. Llovet JM, Montal R, Sia D, Finn RS. Molecular Therapies and Precision Medicine for Hepatocellular Carcinoma. Nat Rev Clin Oncol (2018) 15(10):599–616. doi: 10.1038/s41571-018-0073-4
39. Paré L, Pascual T, Seguí E, Teixidó C, Gonzalez-Cao M, Galván P, et al. Association Between PD1 mRNA and Response to Anti-PD1 Monotherapy Across Multiple Cancer Types. Ann Oncol (2018) 29(10):2121–8. doi: 10.1093/annonc/mdy335
40. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and Safety of Sorafenib in Patients in the Asia-Pacific Region With Advanced Hepatocellular Carcinoma: A Phase III Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Oncol (2009) 10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7
41. Lopci E, Toschi L, Grizzi F, Rahal D, Olivari L, Castino GF, et al. Correlation of Metabolic Information on FDG-PET With Tissue Expression of Immune Markers in Patients With non-Small Cell Lung Cancer (NSCLC) Who are Candidates for Upfront Surgery. Eur J Nucl Med Mol Imaging (2016) 43(11):1954–61. doi: 10.1007/s00259-016-3425-2
42. Wofford JA, Wieman HL, Jacobs SR, Zhao Y, Rathmell JC. IL-7 Promotes Glut1 Trafficking and Glucose Uptake via STAT5-Mediated Activation of Akt to Support T-Cell Survival. Blood (2008) 111(4):2101–11. doi: 10.1182/blood-2007-06-096297
43. Itoh S, Yoshizumi T, Kitamura Y, Yugawa K, Iseda N, Shimagaki T, et al. Impact of Metabolic Activity in Hepatocellular Carcinoma: Association With Immune Status and Vascular Formation. Hepatol Commun (2021) 5(7):1278–89. doi: 10.1002/hep4.1715
44. Hatano E, Ikai I, Higashi T, Teramukai S, Torizuka T, Saga T, et al. Preoperative Positron Emission Tomography With Fluorine-18-Fluorodeoxyglucose is Predictive of Prognosis in Patients With Hepatocellular Carcinoma After Resection. World J Surg (2006) 30(9):1736–41. doi: 10.1007/s00268-005-0791-5
45. Zhu AX, Guan Y, Abbas AR, Koeppen H, Lu S, Hsu CH, et al. Genomic Correlates of Clinical Benefits From Atezolizumab Combined With Bevacizumab vs. Atezolizumab Alone in Patients With Advanced Hepatocellular Carcinoma (HCC). AACR, in: Genomic Correlates in HCC (2020). Available at: https://bit.ly/2yDnjKZ (Accessed 29 April 2020).
Keywords: hepatocellular carcinoma, BCLC stage C, conversion therapy, pathological treatment response, positron emission tomography
Citation: Wang G, Zhang W, Chen J, Luan X, Wang Z, Wang Y, Xu X, Yao S, Guan Z, Tian J, Lu S, Xu B and Ma G (2022) Pretreatment Metabolic Parameters Measured by 18F-FDG PET to Predict the Pathological Treatment Response of HCC Patients Treated With PD-1 Inhibitors and Lenvatinib as a Conversion Therapy in BCLC Stage C. Front. Oncol. 12:884372. doi: 10.3389/fonc.2022.884372
Received: 26 February 2022; Accepted: 03 May 2022;
Published: 03 June 2022.
Edited by:
Egesta Lopci, University of Milan, ItalyReviewed by:
Soňa Balogová, Comenius University, SlovakiaMohei-Eldin Abouzied, King Faisal Specialist Hospital & Research Centre, Saudi Arabia
Copyright © 2022 Wang, Zhang, Chen, Luan, Wang, Wang, Xu, Yao, Guan, Tian, Lu, Xu and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guangyu Ma, bWd5MzAxQDE2My5jb20=; Baixuan Xu, eGJ4MzAxQDE2My5jb20=; Shichun Lu, bHVzY18zMDFAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Guanyun Wang
Guanyun Wang Wenwen Zhang
Wenwen Zhang Jiaxin Chen3,4
Jiaxin Chen3,4 Yanmei Wang
Yanmei Wang Zhiwei Guan
Zhiwei Guan Baixuan Xu
Baixuan Xu Guangyu Ma
Guangyu Ma