- 1Department of Pharmacy, Traditional Chinese Medical Hospital of Zhuji, Zhuji, China
- 2Department of Gastroenterology, Traditional Chinese Medical Hospital of Zhuji, Zhuji, China
- 3Department of Pharmacy, Zhuji People’s Hospital of Zhejiang Province, Zhuji, China
Objective: Meta analysis was used to compare the efficacy and safety of immune checkpoint inhibitor and docetaxel in the treatment of non-small cell lung cancer.
Methods: CNKI, CBM, PubMed, EMBASE, Cochrane Library, web of science and other databases were searched by computer, and the randomized controlled trials of immune checkpoint inhibitors and docetaxel in the treatment of NSCLC published as of February 2022 were collected. Two researchers searched independently, screened the literature and extracted the data according to the nanodischarge criteria, and used Revman5.4. The included studies were statistically analyzed, and publication bias was analyzed with Egger test in Stata12.
Results: A total of 8 RCTs were included, including 2444 cases treated with immune checkpoint inhibitors and 2097 cases treated with docetaxel. Compared with docetaxel, the overall survival (HR = 1.40, 95%CI: 1.30-1.50, P < 0.00001) and progression free survival (HR = 1.22, 95%CI: 1.13-1.32, P < 0.00001) of NSCLC treated with ICIs were longer. The risk ratio of any grade of adverse reactions (HR = 0.41, 95%CI: 0.32-0.52, P < 0.00001) and above grade III adverse reactions (HR = 0.27, 95%CI: 0.18-0.41, P < 0.00001) in the treatment of NSCLC with ICIs was lower. There was no publication bias in Egger test.
Conclusion: Compared with docetaxel, immune checkpoint inhibitor treatment can improve the clinical efficacy of NSCLC patients and has a lower incidence of adverse reactions. This treatment may be a promising treatment for NSCLC patients.
Introduction
Lung cancer is one of the most common malignant tumors in China. Its incidence rate and mortality rate are the first in all tumors. Lung cancer is mainly divided into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), of which NSCLC accounts for 85%. Non-small cell lung cancer includes squamous cell carcinoma, adenocarcinoma and large cell carcinoma. The etiology of lung cancer is complex. At present, it is considered that it is mainly related to smoking, air pollution, occupational factors and changes in molecular genetics. The early symptoms of lung cancer are not obvious. Later, there are often symptoms such as cough, blood in sputum, chest pain and so on (1, 2). Due to the limited diagnostic tools currently used, 75% of patients were found to be in advanced stage. The prognosis of most patients is poor. Based on the stage of the disease at the time of diagnosis, the 5-year survival rate of patients is 4% - 17% (3). At present, docetaxel chemotherapy is one of the most commonly used second-line treatments for NSCLC (2, 3). Its mechanism is to increase the polymerization of tubulin, and then inhibit the depolymerization of microtubules, and thus inhibit the division and growth of tumor cells (4). However, docetaxel has poor efficacy and high toxicity in the treatment of NSCLC. Therefore, it is necessary to explore new treatment methods to prolong the survival time and improve the quality of life of patients.
In recent years, with the rapid development of tumor immunology, immunotherapy has become another new tumor treatment method besides surgery, chemotherapy and radiotherapy. Great breakthroughs have been made in the research of immune checkpoint inhibitors, and the role of inhibitory antibodies in clinical treatment trials of malignant tumors has also been recognized. PD-1 is a cell surface receptor, which is highly expressed on activated T cells and is considered to be a marker of T cell failure. It can regulate the activity of T cells, activate the apoptosis of tumor specific T cells and inhibit the apoptosis of regulatory T cells, so as to inhibit immune response and promote self tolerance (5). PD-L1 is expressed on some types of tumor cells and antigen-presenting cells and is considered to be a co suppressor of immune response. It can be bind to PD-1, activate PD-1/PD-L1 pathway, inhibit downstream signal transduction and T cell biological function, lead to tumor specific T cell failure and apoptosis, and make tumor cells escape immune surveillance (6). PD-1/PD-L1 pathway induces and maintains immune tolerance in tumor microenvironment and promotes tumor development. In this study, randomized controlled trials (RCTs) of ICIs and docetaxel monotherapy in the treatment of NSCLC were searched and efficacy and safety were evaluated by meta-analysis. The results obtained can provide a reference for clinical treatment.
Methods and Materials
Search Strategy
We used computers to search PubMed, EMBASE, Cochrane Library, Web of science database, etc. Chinese search terms: immune checkpoint inhibitor, docetaxel, non-small cell lung cancer, randomized controlled trial; English search terms: ICIs, docetaxel, non small cell lung cancer, NSCLC, randomized controlled trials, RCTs. The search deadline is February 2022.
Study Selection
Inclusion criteria: ① Literature: retrospective study, prospective study; ② The subjects were patients with NSCLC diagnosed by clinicopathological examination; ③ Intervention measures: patients in the experimental group treated with ICI monotherapy and patients in the control group treated with docetaxel monotherapy; ④ The primary clinical outcome measures were overall survival (OS) and progression free survival (PFS). The secondary outcome measures were adverse reactions at any level and adverse reactions above grade 3.
Exclusion criteria: ① repeatedly published literature; ② Documents that cannot obtain original data or contact the author to obtain the original text; ③ Abstract, review, meta-analysis, case report and animal experiment; ④ Non Chinese and English literature.
Literature Screening and Data Extraction
Two researchers independently read the initial literature titles and abstracts according to the inclusion and exclusion criteria, screened the literature that may meet the inclusion criteria by reading the full text, and extracted the data according to the pre-designed table, including the first author, year of publication, number of patients, OS, PFS and adverse reactions. In case of differences, the two researchers shall discuss and solve them.
Bias Risk Assessment
We assessed the quality of inclusion in clinical randomized controlled trials, met the criteria proposed in the Cochrane manual for systematic evaluation of interventions (5.1.0), and evaluated the generation of random sequences, allocation concealment, blinding of participants, blinding of outcome evaluation, and incomplete outcome data to ensure a low incidence of bias.
Statistical Analysis
We use Revman5.4 software to analyze the data of the included studies. Relative risk ratios (RR) and 95% confidence interval (95%CI) were used as effect indexes for counting data, and the difference was statistically significant (P < 0.05). I2 is used to evaluate the heterogeneity. If the heterogeneity test result I2 is less than 50%, it means that there is no statistical heterogeneity among the research results, and the fixed effect model is used; If the heterogeneity test result I2 > 50%, analyze the source of heterogeneity. If the heterogeneity still exists, select the random effect model to estimate the combined effect.
Results
Literature Search and Screening
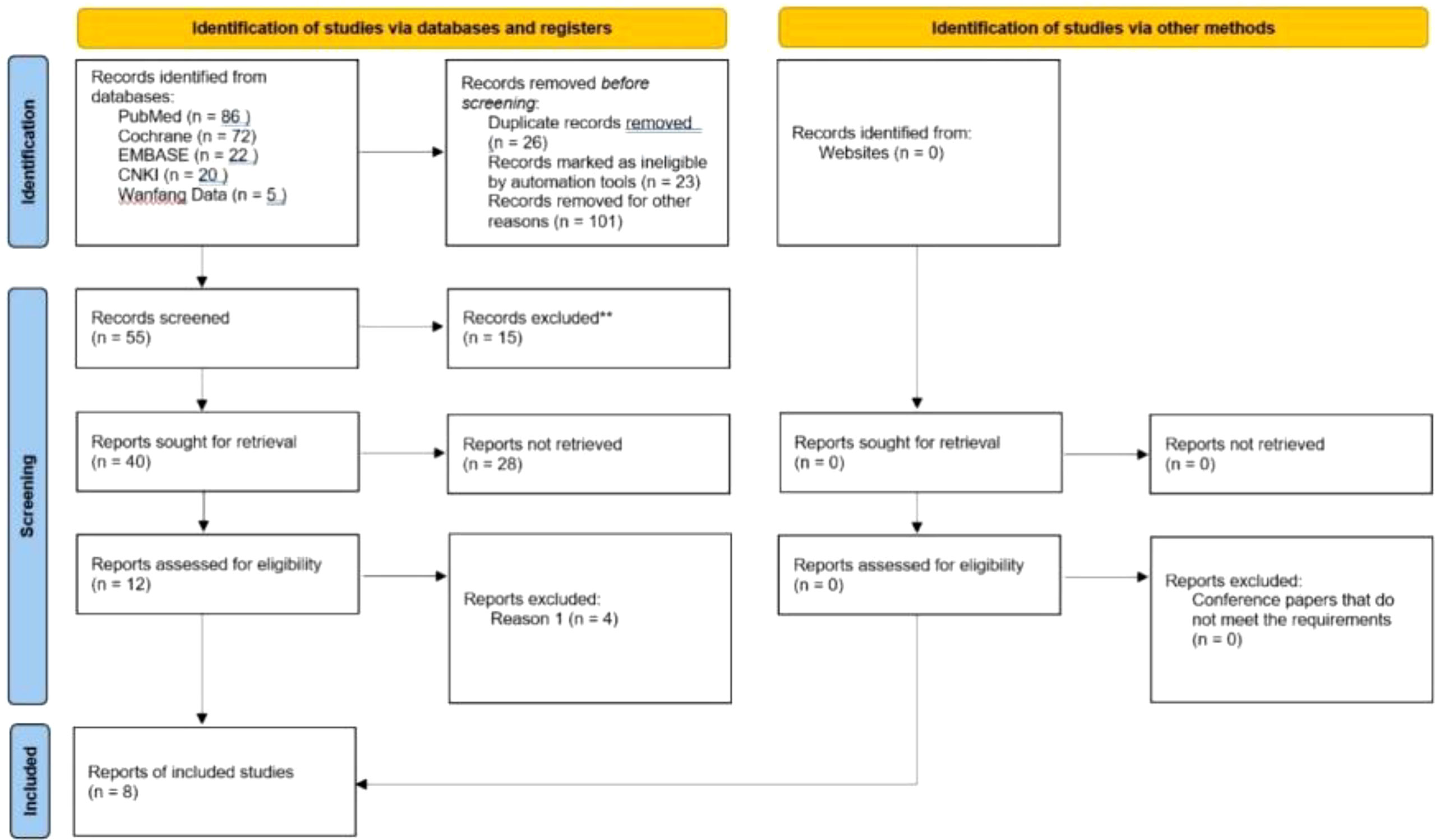
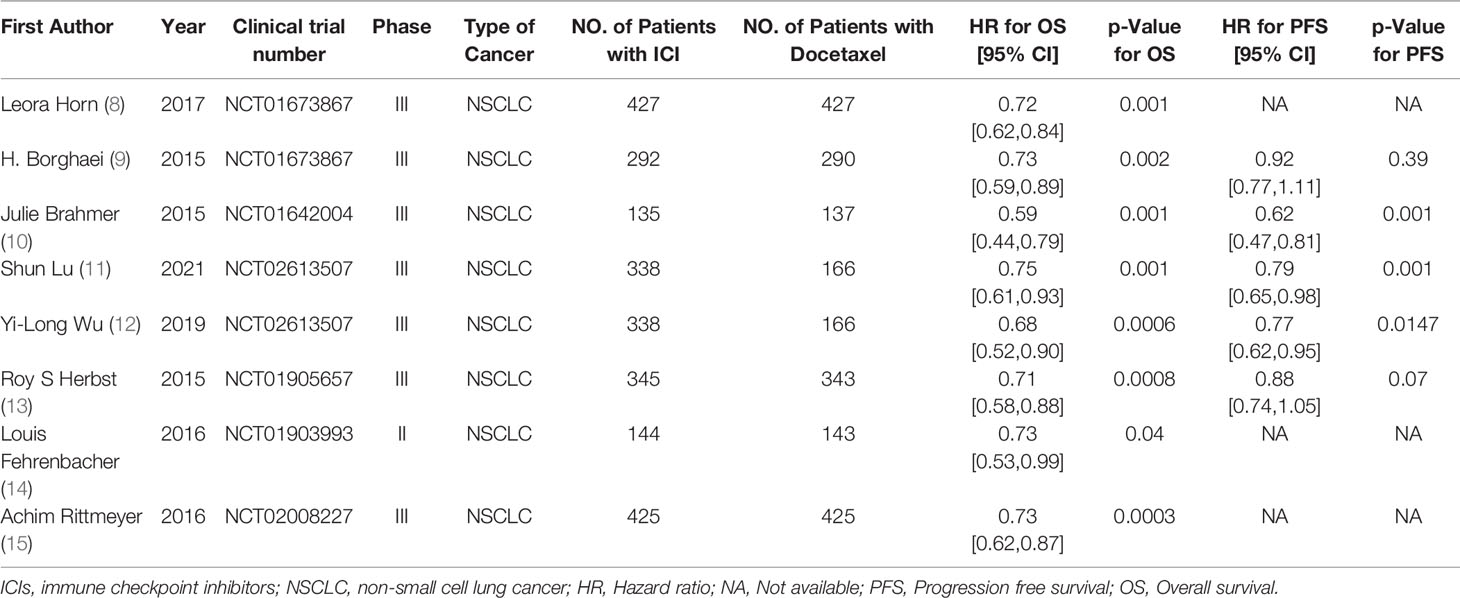
205 literatures (including 86 PubMed, 72 Cochrane, 22 Embase, 20 CNKI, and Wanfang VIP5) were searched by computer, and 55 were selected according to the title and abstract. After full-text analysis and evaluation, 47 literatures with abnormal data, incomplete information or unavailable due to non comparative research were excluded, and finally 8 (7–14) literatures were included for systematic evaluation and meta-analysis. The process of literature screening is shown in Figure 1. Among them, 2444 patients were treated with ICIs monotherapy and 2097 patients were treated with docetaxel monotherapy. Table 1 summarizes the basic characteristics and main evaluation indicators of the included research.
Quality Assessment Results
The quality assessment results are shown in Figures 2A, B. All included studies had a low risk of bias.
Meta Analysis Results of Effectiveness of ICIs and Docetaxel
OS comparison 8 RCTs reported the OS of patients, and there were no statistically significant differences between studies. The results of meta-analysis showed that the OS of ICIS treatment group was longer than that of docetaxel chemotherapy group, and the difference was statistically significant (HR = 1.40, 95% CI: 1.30-1.50, P < 0.00001), indicating that the efficacy of ICIs in the treatment of NSCLC was better than that of docetaxel chemotherapy (as shown in Figure 3).
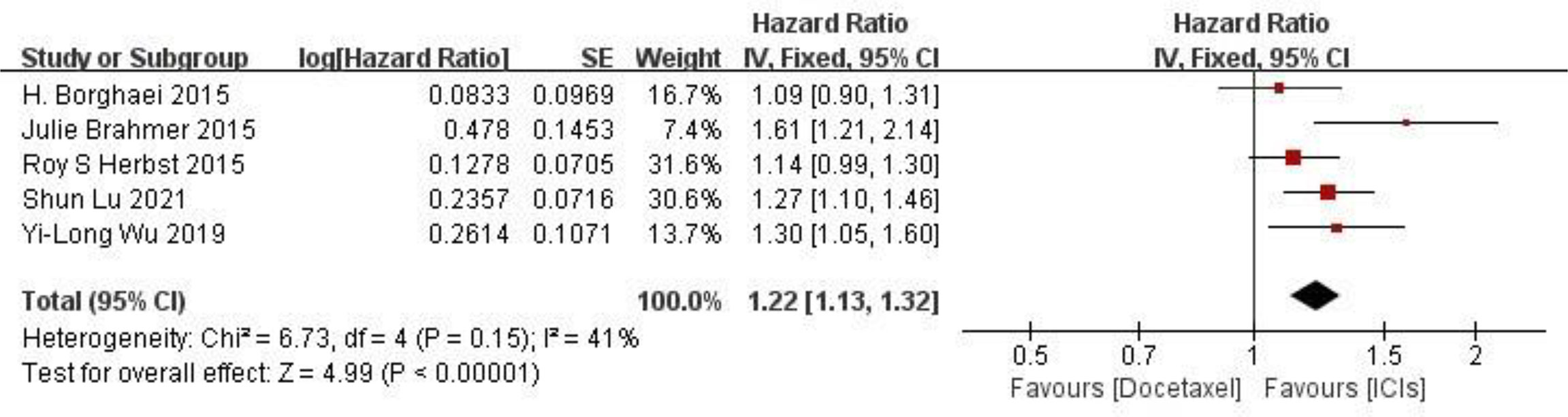
PFS comparison Five RCTs reported PFS of patients, and there were no statistically significant differences between studies. The results of meta-analysis showed that the PFS of ICIS treatment group was longer than that of docetaxel chemotherapy group, and the difference was statistically significant (HR = 1.22, 95%CI: 1.13-1.32, P < 0.00001), indicating that the efficacy of ICIs in the treatment of NSCLC was better than that of docetaxel chemotherapy (as shown in Figure 4).
Results of Safety Meta-Analysis of ICIs and Docetaxel
Adverse reactions at any level Five RCTs reported typical adverse reactions at any level (including fatigue, nausea, ashenia, diarrhea and anemia). There were no statistically significant differences between studies. The results of meta-analysis showed that the risk of adverse reactions at any level in the ICIs treatment group was lower than that in the docetaxel chemotherapy group, and the difference was statistically significant (HR = 0.41, 95%CI: 0.32-0.52, P < 0.00001), indicating that the safety of ICIs is superior to docetaxel monotherapy in NSCLC (as shown in Figure 5).
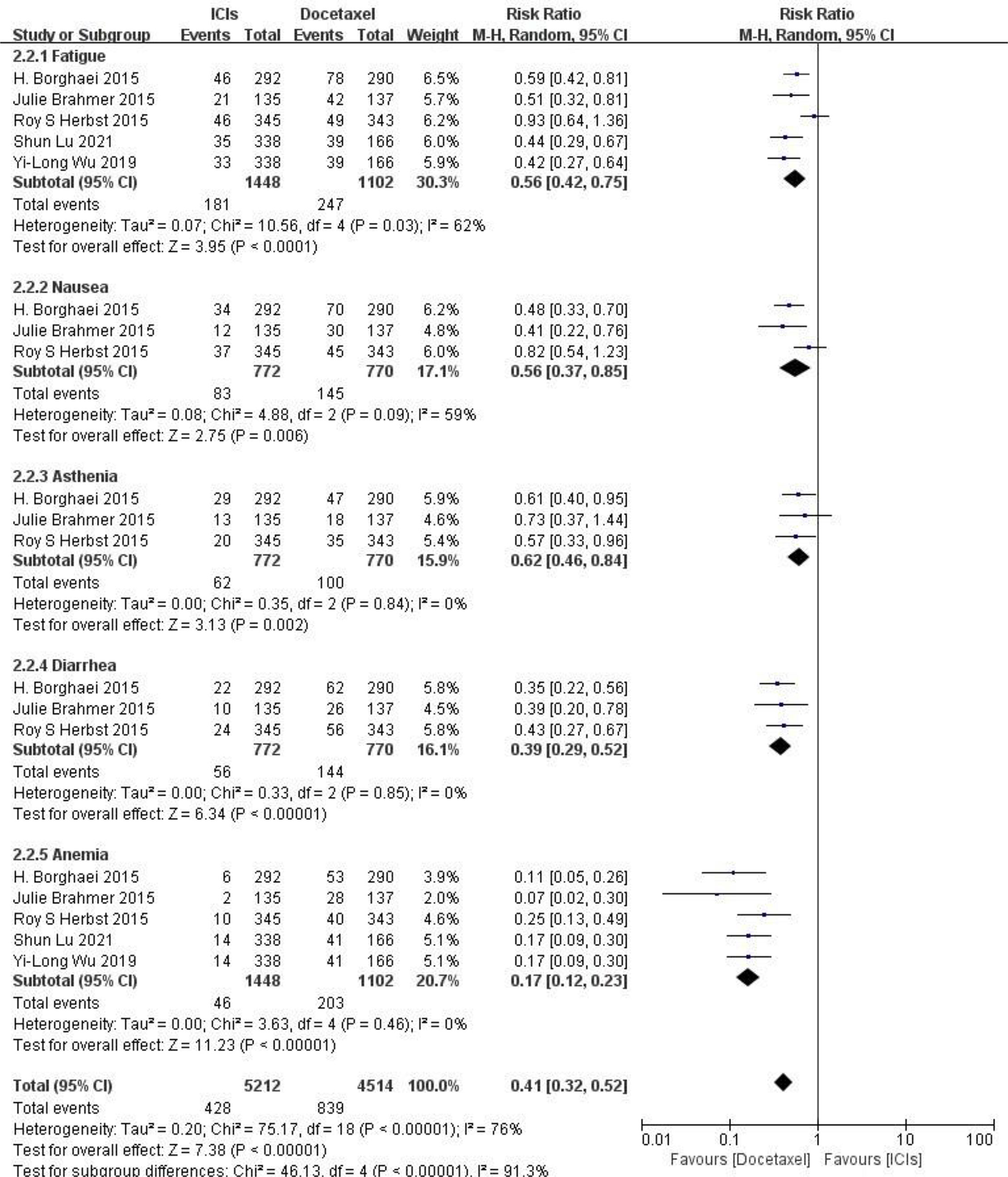
Figure 5 Meta-analysis results of adverse reactions of any grade between ICIs group and docetaxel group.
Adverse reactions above grade III Five RCTs reported typical adverse reactions above grade III (including fatigue, nausea, ashenia, diarrhea and anemia), and there were no statistically significant differences between studies. The results of meta-analysis showed that the risk of grade III and above adverse reactions in the ICIs treatment group was lower than that in the docetaxel chemotherapy group, and the difference was statistically significant (HR = 0.27, 95%CI: 0.18-0.41, P < 0.00001), indicating that the safety of ICIs is superior to docetaxel monotherapy in NSCLC(as shown in Figure 6).

Figure 6 Meta-analysis results of adverse reactions above grade 3 between ICIs group and docetaxel group.
Sensitivity Analysis and Publication Bias Assessment
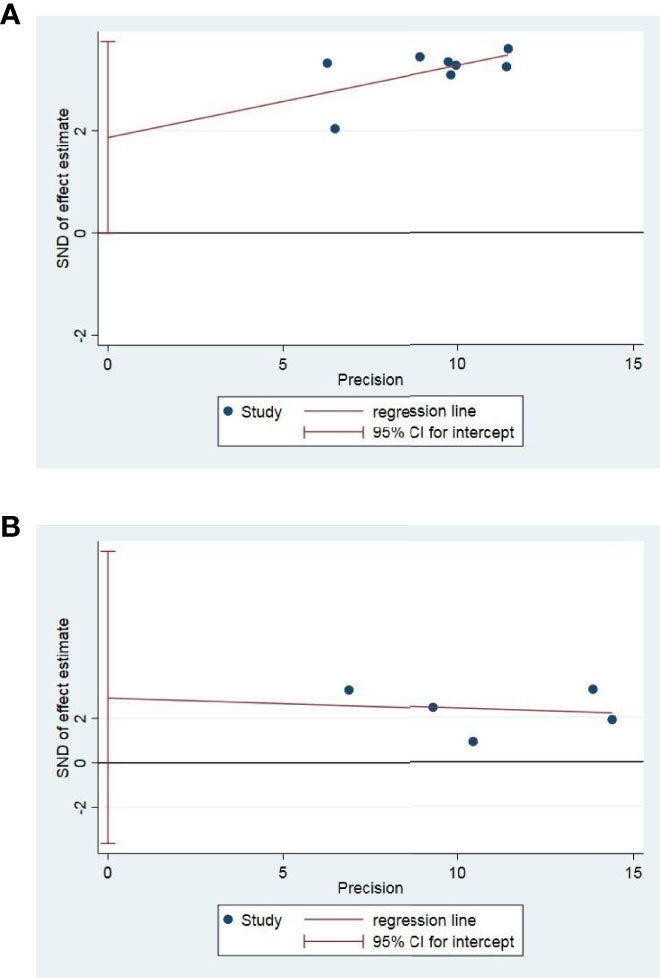
Publication bias assessment was performed only in OS and PFS. Egger test in Stata12 software was used for publication bias test. In a total of 8 studies with OS and PFS as outcome indicators, the results of publication bias test indicated that there was no publication bias OS(P = 0.051) and PFS(P = 0.255), as shown in Figure 7.
Discussion
In recent years, the choice of standard treatment for NSCLC patients has gradually changed from routine first-line drugs to immune checkpoint inhibitor (ICIs) therapy, or as a monotherapy, or combined with chemotherapy, anti angiogenic antibodies or other forms of ICIs. ICIs, an antibody against PD-1 or PD-L1, was approved for second-line and third-line treatment in patients with metastatic NSCLC without treatable driver mutations in 2015 (15). Since then, ICIs has been approved for first-line treatment, or for tumors with PD-L1 expression ≥ 50% alone, or in combination with chemotherapy independent of receptor status (16). Some patients treated with ICIs have particularly long-lasting response and survival. The study confirmed that up to 16% of patients with stage IV non-small cell lung cancer received second-line treatment with PD-1 inhibitor nivolumab and 31.9% received first-line treatment with PD-1 inhibitor pembrolizumab, with a survival time of 5 years (17). Another study showed that 4-year OS rates in POPLAR were 14.8% and 8.1% (and those in OAK were 15.5%and 8.7% for atezolizumab and docetaxel, respectively. However, it is worth noting that some patients with low PD-L1 expression may have poor efficacy in the treatment of tumors with immune checkpoint inhibitors. Therefore, it is very important to select biomarkers that can effectively predict the efficacy of PD-1/PD-L1 inhibitors, which is also an urgent problem to be solved in immunotherapy at this stage (18). At the same time, immune checkpoint inhibitors may cause immune related adverse reactions and infusion related reactions in the process of clinical application, which still needs further research (19).
In this meta-analysis, we evaluated the efficacy of ICIs drugs and docetaxel in patients with NSCLC, and selected OS and PFS as the primary outcomes. The results showed that ICIs improved the HR and p of OS and PFS in terms of effectiveness, indicating that patients receiving immunotherapy had better OS and PFS than patients receiving docetaxel. In terms of safety, the risk ratio of adverse reactions at any level and above in the ICIs treatment group was significantly lower than that in the docetaxel group, suggesting that the safety of ICIs treatment was higher than that of docetaxel, and it is not easy to produce common and typical adverse reactions (fatigue, nauesa, ashenia, diarrhea, anemia). Many international researches also show similar results, Khan M et al. (20) shows that compared with chemotherapy drugs, ICI therapy (nivolumab, pembrolizumab, atezolizumab) leads to better OS (HR 0.72 [95% CI 0.63, 0.82; P <. 00001]), PFS (HR 0.84 [95% CI 0.72), 0.97; P <. 02]) and ORR (odds ratio [OR] 1.52 [95% CI 1.08, 2.14; P <. 02]). At the same time, higher safety was observed with ICI therapy (OR 0.31 [95% CI 0.26, 0.38; P <. 00001]). The results of this study are basically consistent with those of previous international studies.
This study also has some limitations: ① after systematic retrieval and screening, only 8 literatures were included for systematic evaluation and meta-analysis, and the sample size is too small; ② The heterogeneity of individual statistical results may affect the credibility of the research results; ③ Different types of NSCLC in different studies may increase heterogeneity and affect the reliability of the results. However, in the study, in order to better reduce the above bias, when implementing retrieval and data consolidation, this study will report scientifically and objectively as much as possible in accordance with the Cochrane system evaluation guidance manual.
In conclusion, compared with docetaxel, ICIs can prolong the OS and PFS of patients with NSCLC, with better clinical efficacy. This therapy may be a promising treatment. However, it still needs to be further confirmed by studies with multiple centers, larger sample size and higher quality.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
WY and BX searched the database and analysed the data. MC, XL, JH and HS selected the study and extracted the data. WY and BX wrote the manuscript. YZ reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the clinical fund project of Zhejiang Medical Association in 2021 (2021ZYC-A193).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mao Y, Yang D, He J, Krasna MJ. Epidemiology of Lung Cancer. Surg Oncol Clin N Am (2016) 25(3):439–45. doi: 10.1016/j.soc.2016.02.001
2. Rivera GA, Wakelee H. Lung Cancer In Never Smokers. Adv Exp Med Biol (2016) 893:43–57. doi: 10.1007/978-3-319-24223-1_3
3. Gavin S Jones DRB. Recent Advances in the Management of Lung Cancer. Clin Med (Lond) (2018) 18(Suppl 2):s41–6. doi: 10.7861/clinmedicine.18-2-s41
4. Ishida M, Morimoto K, Yamada T, Shiotsu S, Chihara Y, Yamada T, et al. Impact of Docetaxel Plus Ramucirumab in a Second-Line Setting After Chemoimmunotherapy in Patients With Non-Small-Cell Lung Cancer: A Retrospective Study[J]. Thorac Cancer (2022) 13(2):173–81. doi: 10.1111/1759-7714.14236
5. Lei Q, Wang D, Sun K, Wang L, Zhang Y. Resistance Mechanisms of Anti-PD1/PDL1 Therapy in Solid Tumors. Front Cell Dev Biol (2020) 8:672. doi: 10.3389/fcell.2020.00672
6. Bylicki O, Paleiron N, Rousseau-Bussac G, Chouaïd C. New PDL1 Inhibitors for Non-Small Cell Lung Cancer: Focus on Pembrolizumab. Onco Targets Ther (2018) 11:4051–64. doi: 10.2147/OTT.S154606
7. Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, et al. Nivolumab Versus Docetaxel in Previously Treated Patients With Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes From Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J Clin Oncol (2017) 35(35):3924–33. doi: 10.1200/JCO.2017.74.3062
8. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab Versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643
9. Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, et al. Nivolumab Versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med (2015) 373(2):123–35. doi: 10.1056/NEJMoa1504627
10. Lu S, Wang J, Cheng Y, Mok T, Chang J, Zhang L, et al. Nivolumab Versus Docetaxel in a Predominantly Chinese Patient Population With Previously Treated Advanced Non-Small Cell Lung Cancer: 2-Year Follow-Up From a Randomized, Open-Label, Phase 3 Study (CheckMate 078). Lung Cancer (2021) 152:7–14. doi: 10.1016/j.lungcan.2020.11.013
11. Wu YL, Lu S, Cheng Y, Zhou C, Wang J, Mok T, et al. Nivolumab Versus Docetaxel in a Predominantly Chinese Patient Population With Previously Treated Advanced NSCLC: CheckMate 078 Randomized Phase III Clinical Trial. J Thorac Oncol (2019) 14(5):867–75. doi: 10.1016/j.jtho.2019.01.006
12. Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han JY, et al. Pembrolizumab Versus Docetaxel for Previously Treated, PD-L1-Positive, Advanced Non-Small-Cell Lung Cancer (KEYNOTE-010): A Randomised Controlled Trial. Lancet (2016) 387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7
13. Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, et al. Atezolizumab Versus Docetaxel for Patients With Previously Treated Non-Small-Cell Lung Cancer (POPLAR): A Multicentre, Open-Label, Phase 2 Randomised Controlled Trial. Lancet (2016) 387(10030):1837–46. doi: 10.1016/S0140-6736(16)00587-0
14. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab Versus Docetaxel in Patients With Previously Treated Non-Small-Cell Lung Cancer (OAK): A Phase 3, Open-Label, Multicentre Randomised Controlled Trial. Lancet (2017) 389(10066):255–65. doi: 10.1016/S0140-6736(16)32517-X
15. Guilleminault L, Carmier D, Heuze-Vourc'h N, Diot P, Pichon E. [Immunotherapy in Non-Small Cell Lung Cancer: Inhibition of PD1/PDL1 Pathway]. Rev Pneumol Clin (2015) 71(1):44–56. doi: 10.1016/j.pneumo.2014.11.004
16. Ma X, Zhang Y, Wang S, Wei H, Yu J. Immune Checkpoint Inhibitor (ICI) Combination Therapy Compared to Monotherapy in Advanced Solid Cancer: A Systematic Review. J Cancer (2021) 12(5):1318–33. doi: 10.7150/jca.49174
17. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J Clin Oncol (2019) 37(7):537–46. doi: 10.1200/JCO.18.00149
18. Ai L, Xu A, Xu J. Roles of PD-1/PD-L1 Pathway: Signaling, Cancer, and Beyond. Adv Exp Med Biol (2020) 1248:33–59. doi: 10.1007/978-981-15-3266-5_3
19. Doroshow DB, Sanmamed MF, Hastings K, Politi K, Rimm DL, Chen L, et al. Immunotherapy in Non-Small Cell Lung Cancer: Facts and Hopes. Clin Cancer Res (2019) 25(15):4592–602. doi: 10.1158/1078-0432.CCR-18-1538
20. Khan M, Lin J, Liao G, Tian Y, Liang Y, Li R, et al. Comparative Analysis of Immune Checkpoint Inhibitors and Chemotherapy in the Treatment of Advanced Non-Small Cell Lung Cancer: A Meta-Analysis of Randomized Controlled Trials. Med (Baltimore) (2018) 97(33):e11936. doi: 10.1097/MD.0000000000011936
Keywords: immune checkpoint inhibitors, docetaxel, non small cell lung cancer, overall survival, progression free survival, security
Citation: Yang W, Xuan B, Chen M, Li X, He J, Si H and Zhang Y (2022) Comparison of Efficacy and Safety Between Immunotherapy and Docetaxel Monotherapy in NSCLC Patients. Front. Oncol. 12:883514. doi: 10.3389/fonc.2022.883514
Received: 25 February 2022; Accepted: 13 June 2022;
Published: 10 August 2022.
Edited by:
Jun Zhang, University of Kansas Medical Center, United StatesReviewed by:
Lei Xian, Second Affiliated Hospital of Guangxi Medical University, ChinaPatricia Iranzo, Vall d’Hebron Institute of Oncology, Spain
Mingming Wu, Harbin Medical University, China
Yan Chen, Sichuan Cancer Hospital, China
Copyright © 2022 Yang, Xuan, Chen, Li, He, Si and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yefei Zhang, emhhbmd5ZWZlaTg4QDEyNi5jb20=
†These authors have contributed equally to this work
 Wenchao Yang1†
Wenchao Yang1† Yefei Zhang
Yefei Zhang