
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 29 July 2022
Sec. Gastrointestinal Cancers: Gastric and Esophageal Cancers
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.882900
 Yun Luo1,2†
Yun Luo1,2† Xue-Fen Weng1†
Xue-Fen Weng1† Jia-Tao Huang1,2
Jia-Tao Huang1,2 Xue-Hao Hu3
Xue-Hao Hu3 Lai-Feng Wei1,2
Lai-Feng Wei1,2 Yi-Wei Lin1,2,4
Yi-Wei Lin1,2,4 Tian-Yan Ding1,2
Tian-Yan Ding1,2 Biao Zhang1,2
Biao Zhang1,2 Ling-Yu Chu1,2
Ling-Yu Chu1,2 Can-Tong Liu1,2,4
Can-Tong Liu1,2,4 Yu-Hui Peng1,2,4
Yu-Hui Peng1,2,4 Yi-Wei Xu1,2,4*
Yi-Wei Xu1,2,4* Fang-Cai Wu5*
Fang-Cai Wu5*Objectives: At present, esophageal squamous cell carcinoma (ESCC) patients accepting neoadjuvant chemoradiotherapy (nCRT) plus surgery lack corresponding prognostic indicators. This study aimed to construct a prognostic prediction model for ESCC patients undergoing nCRT and surgery based on immune and inflammation-related indicators.
Methods: We retrospectively analyzed the levels of serum immune- and inflammation-related indicators of ESCC patients before receiving nCRT plus surgery in the training cohort (99 patients) and validation cohort (67 patients), which were collected from 2007 to 2020. Univariate and multivariate Cox survival analyses were conducted to evaluate the indicators to set up a nomogram associated with the patients’ overall survival (OS). The prediction accuracy and discriminative ability of the nomogram were measured by the concordance index (C-index), decision curve, calibration curve, integrated discrimination improvement (IDI), and net reclassification improvement (NRI).
Results: Univariate and multivariate Cox analyses demonstrated that immune globin A (IgA) and C-reactive protein (CRP) were independent risk factors. A nomogram based on IgA, CRP, and cTNM stage was established for predicted OS in the training cohort and validated in the validation cohort. The C-index of the nomogram was 0.820 (95% CI: 0.705–0.934), which was higher than that of the cTNM stage (0.655 (95% CI: 0.546–0.764), p < 0.05) in the training cohort, and similar results were observed in the validation cohort (0.832 (95% CI: 0.760–0.903 vs 0.635 (95% CI: 0.509–0.757), p < 0.001). Furthermore, the prediction accuracy and net benefit of the nomogram verified by the calibration curve, decision curve, NRI, and IDI were satisfactory in the training and validation cohorts.
Conclusion: The newly constructed nomogram concluding serum IgA, CRP, and cTNM stage might be helpful in the prognosis prediction for ESCC patients receiving nCRT plus surgery.
Esophageal cancer is the seventh most common malignant cancer all over the world and the sixth leading cause of cancer mortality (1). In 2018, 508,585 esophageal cancer-related deaths occurred globally (2). Approximately 90% of the pathological types of esophageal cancer are esophageal squamous cell carcinoma (ESCC) (3, 4). For the treatment of ESCC, traditional curative esophagectomy was a major selection. However, in patients with locally advanced esophageal cancer (T3–4aN0–1M0), only radical resection is accompanied by a high recurrence rate and mortality rate of 3- to 5-year overall survival (OS) (5, 6). Therefore, combined modality therapy is necessary. Some studies indicated that when compared with preoperative chemotherapy or surgery alone in patients with locoregional esophageal cancer, neoadjuvant chemoradiotherapy was connected with improved OS and disease-free survival (DFS) (7–11). Several markers (such as Rad51, osteopontin, plasma fibrinogen, and soluble interleukin-6 receptor) have also been reported to be associated with the prognosis of ESCC patients who received preoperative chemoradiotherapy (12–16). However, the treatment effect and prognostic indicator before neoadjuvant chemoradiotherapy still need further research.
This is a broad consensus that TNM staging is pivotal and irreplaceable for the diagnosis and treatment of tumors including esophageal carcinoma. Clinical studies indicated that the TNM stage is an independent prognostic factor for OS (17, 18). However, the classification of the cTNM stage mainly depends on computed tomography (CT) and endoscopic ultrasonography (EUS). Studies reported that the sensitivity of EUS (usually less than 50% sensitivity) for detecting metastatic nodes was low. The specificity to discriminate the N0 from a node-positive disease of CT was just 38.7%, and CT was unreliable for local staging (19–21). Based on these findings, only depending on a single cTNM stage may lead to deviations in the accuracy of prediction when establishing a prognostic model for ESCC patients with neoadjuvant chemoradiotherapy (nCRT) plus surgery. Therefore, it is necessary to construct a multivariate prognostic model.
It is increasingly recognized that cancer-related inflammation (CRI) is related to tumor progression. Inflammation in tumor microenvironments can promote cell proliferation and inhibit the adaptive immune response (22). Systemic inflammation score (SIS) has played an independent role in the prognosis of ESCC patients (23). In cancer-related immunology, immune cells exerted anti-tumor capacity through different mechanisms such as binding ligands on the surface of tumor cells through self-surface receptors; meanwhile, pro-inflammatory and immunomodulatory cytokines such as IL-12, TNF-α, IFN-γ, and IL-6 were released during the process. These cytokines would cause changes in other inflammatory substances such as C-reactive protein (CRP). In the cancer immunoediting process, antibodies are involved in the event of antibody-dependent cell-mediated cytotoxicity. Moreover, the level of different antibodies was variable in tumor location or blood (22, 24). The connection between cancer-related inflammation and immunology is tight. The antibody, inflammatory factor, and cytokine generated the re-sculpting of the tumor immune and inflammatory microenvironment (25). However, the relationship between serum immune and inflammation indicators and prognosis with ESCC patients who received nCRT plus surgery is indistinct. The target of the study was to construct a nomogram constructed with immune and inflammatory indicators. Through this nomogram, we can macroscopically recognize the benefit that ESCC patients could obtain after nCRT plus surgery.
In the study, 166 ESCC patients who were treated with nCRT followed by esophagectomy in the Cancer Hospital of Shantou University Medical College were enrolled from 2007 to 2020. A total of 99 patients were included in the training cohort from 2007 to 2020, and 67 patients were included in the validation cohort from 2013 to 2019. All cases were diagnosed as esophageal squamous cell carcinoma by pathological biopsy. Pathological and clinical data were collected through case history records. Basic information includes weight, height, age, gender, body mass index (BMI), and tumor location. Hematology indexes included IgA, IgG, IgM, CRP, CRP/albumin (CRP/ALB), complement 3 (C3), C4, lymphocyte ratio (LY%), lymphocytes count (LY#), monocyte ratio (MO%), monocyte count (MO#), neutrophil ratio (NE%), and neutrophil count (NE#). In order to obtain a consistent tumor cTNM stage, the depth of tumor invasion (T), lymph node metastasis (N), and distant organ metastasis (M) were recorded by the examination results of computed tomography and endoscopic ultrasound. The cTNM stage was assessed according to the standards of the American Joint Committee on Cancer (AJCC) Staging Manual of Esophageal Cancer (8th edition) (26). Patients who had a history of other cancers or suffered from inflammatory diseases such as chronic gastritis, inflammatory bowel disease, autoimmune diseases, and infectious diseases, which may influence the levels of the above-mentioned pretreatment serum markers, were excluded.
The main regimen of chemotherapy was cisplatin combined with paclitaxel, vinorelbine, docetaxel, or fluorouracil. Depending on the patient’s condition, at least two cycles of chemotherapy were conducted. Concurrent radiotherapy began on the first day of chemotherapy. The gross tumor volume included the primary tumor and enlarged regional lymph nodes. The total planned dose for the planning target volume was 40–50 Gy in 20–25 fractions within 5 weeks. Esophagectomy was performed 4 to 6 weeks after the completion of nCRT.
The time from the date of diagnosis to any form of death or the last follow-up was defined as OS. The study was allowed by the ethics committee of the Cancer Hospital of Shantou University Medical College.
Categorical variables were adopted for all continuous variables in both cohorts, according to the best cutoff values determined by the X-tile program (27). Combined with independent prognostic factors determined by multiple Cox regression analysis, a nomogram was established to predict 1-year and 3-year OS of ESCC patients in the training cohort, and the prediction accuracy and applicability of the nomogram were verified in the validation cohort. The discrimination of the nomogram was assessed by the concordance index (C-index). The accuracy and benefits of the new model were assessed by integrated discrimination improvement (IDI), net reclassification improvement (NRI), and decision curve analysis (DCA). The goodness-of-fit model was determined by the calibration curve. Based on the nomogram, the total points were calculated, and the survival curve was plotted for risk stratification.
IBM SPSS statistical software was used for statistical analysis, version 19.0 (IBM Corp., Chicago, IL), and the R software, version 4.0.4, for Windows. R packages including ggplot2, ggpubr, survminer, survival, rms, pec, magrittr, dplyr, survIDINRI, and Hmisc were used in the analysis. Included indicators of a significant level of p ≤ 0.05 in the univariate Cox analysis were used in the multivariate Cox analysis. In the multivariate Cox analysis, indicators with a significant level of p ≤ 0.05 were determined as an independent prognostic factor. The Kaplan–Meier curve was carried out to obtain the survival rate, and the log-rank test was used to test it.
Table 1 shows the clinical characteristics of ESCC patients receiving nCRT plus surgery in the training and validation cohorts. The cutoff values of continuous variables were as follows: age (64 years), IgA (3 g/L), IgG (12.9 g/L), IgM (0.9 g/L), CRP (4.4 mg/L), CRP/ALB (0.1), C3 (1.3 g/L), BMI (19.6), C4 (0.3 g/L), LY% (23.9), LY# (1.7 * 109/L), MO% (8.0), MO# (0.6 * 109/L), NE% (66.1), and NE# (5.1 * 109/L). The cutoff values in the validation cohort were consistent with the training cohort.
Univariate analysis demonstrated that the cTNM stage (p = 0.049), IgA (p = 0.007), CRP (p = 0.001), CRP/ALB (p = 0.009), MO# (p = 0.011), and NE# (p = 0.014) were statistically significant for OS in the training cohort. Based on the cTNM stage, CRP, CRP/ALB, IgA, MO#, and NE#, the Kaplan–Meier curves of OS were significantly different through the log-rank test (p < 0.05) as shown in Figure 1. The collinearity diagnosis of these six variables suggested that collinearity existed between CRP and CRP/ALB. After the multivariate Cox analysis by the forward stepwise method, which was adopted to exclude the variables with collinearity, the IgA (p = 0.017, hazard ratio (HR) = 3.498, 95% CI: 1.255–9.748) and CRP (p = 0.002, HR = 4.936, 95% CI: 1.828–13.330) were evaluated as independent factors for OS in the training cohort (Table 2).
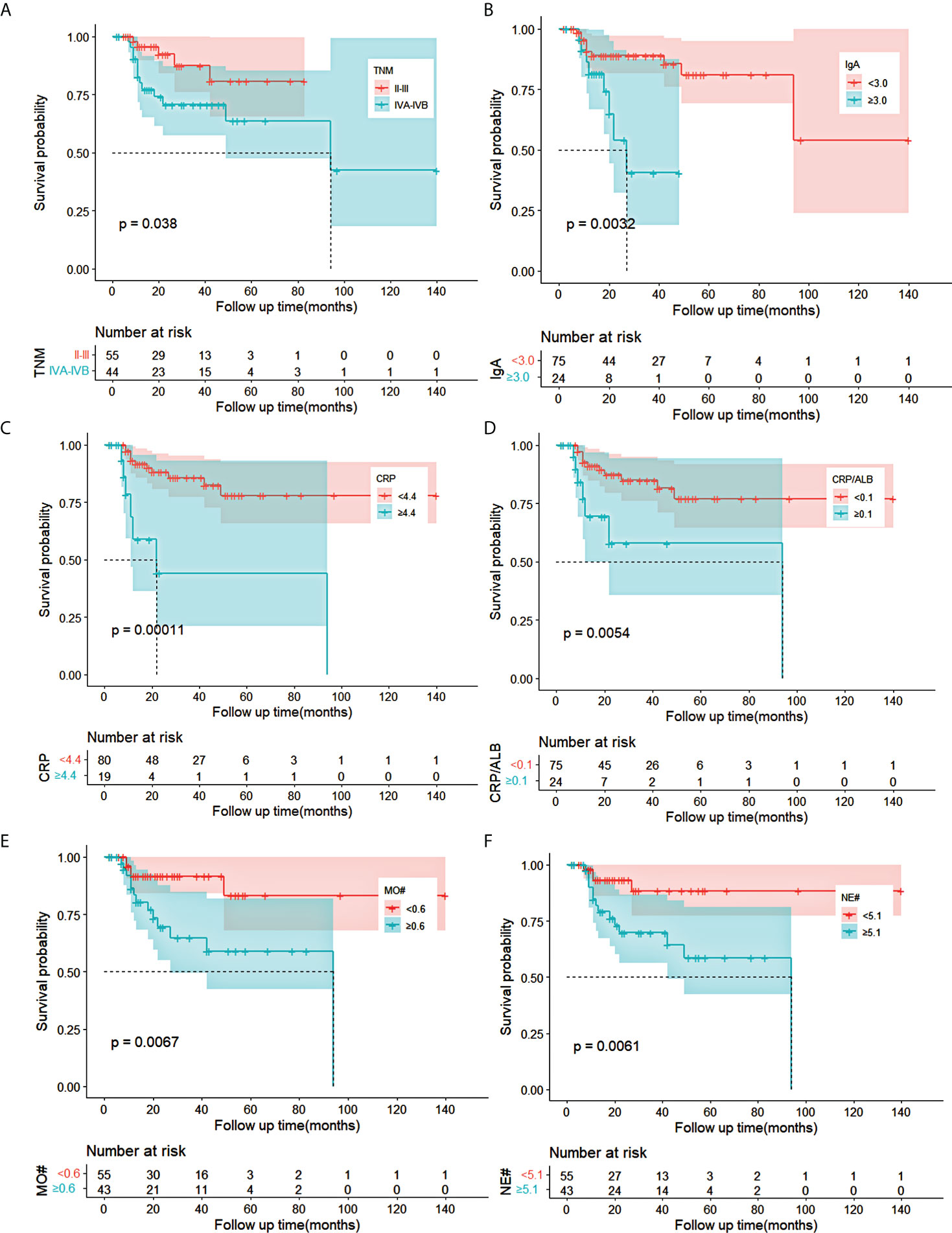
Figure 1 Kaplan–Meier curves for OS in ESCC patients receiving nCRT plus surgery in training cohort. (A–F) The survival curves of clinical TNM stage, IgA, CRP, CRP/ALB, MO#, and NE# in ESCC patients. OS, overall survival; ESCC, esophageal squamous cell carcinoma; nCRT, neoadjuvant chemoradiotherapy; IgA, immune globin A; CRP, C-reactive protein; ALB, albumin; MO#, absolute count of monocytes; NE#, absolute count of neutrophils.
Through the multivariate Cox analysis, CRP and IgA were identified to meaningfully influence the OS. Meanwhile, the cTNM stage is a significant factor in the evaluation of cancer treatment decision-making and prognosis before any cancer-related therapy. Thus, the cTNM stage was included in the nomogram model. Hence, the nomogram was established using the cTNM stage, CRP, and IgA to forecast the 1- and 3-year OS in the training cohort Figure 2). In the nomogram, CRP had a greater effect on OS as compared with IgA or the cTNM stage. Risk points related to different levels of each predictive indicator were obtained by drawing a straight line to the “Points” line according to the corresponding indicator value and then summing up these risk points to obtain total points. A straight line was painted down from the total point to the straight line of 1- and 3-year OS, and the intersection point is the 1- or 3-year survival rate.
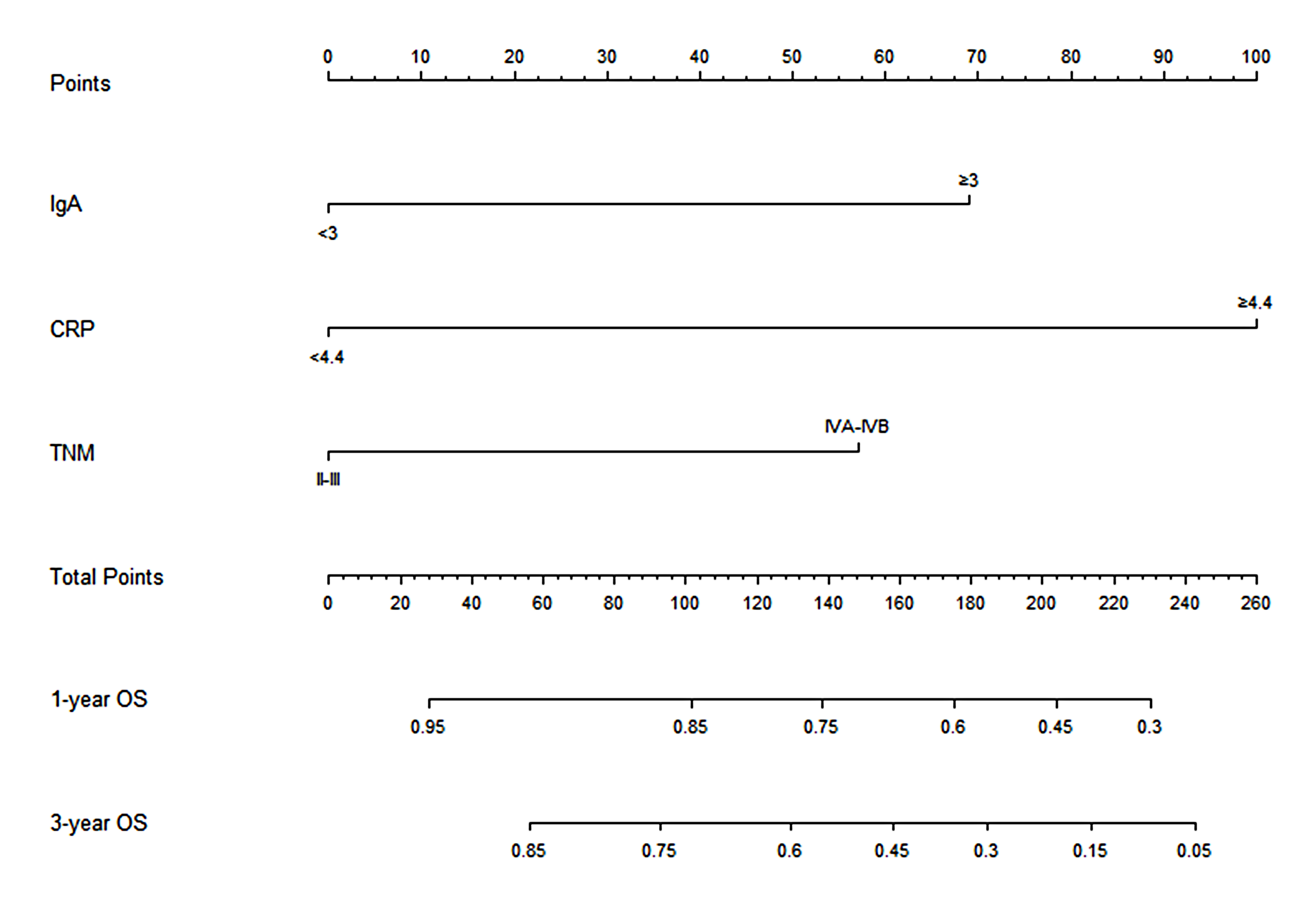
Figure 2 Nomogram based on CRP, IgA, and clinical TNM stage for predicting the 1- and 3-year OS in ESCC patients who received nCRT plus surgery, by summing up the points identified on the points scale for each variable. The total points projected on the bottom scales determined the probability of 1- and 3-year survival. CRP, C-reactive protein; IgA, immune globin A; OS, overall survival; ESCC, esophageal squamous cell carcinoma; nCRT, neoadjuvant chemoradiotherapy.
Calibration curves of this nomogram to predict the 1- and 3-year OS were drawn in both cohorts (Figure 3). The calibration curve demonstrated that the predicted OS based on the nomogram excellently fitted the actual OS in either the training cohort or the validation cohort. Moreover, the C-index indicating prediction accuracy of the nomogram was 0.820 (95% CI: 0.705–0.934), higher than that of the cTNM stage, CRP, and IgA (0.655 (95% CI: 0.546–0.764), 0.656 (95% CI: 0.540–0.773), and 0.624 (95% CI: 0.501–0.747), respectively, p < 0.05) in the training cohort. Similar results were identified in the validation cohort, with the C-index of 0.832 (95% CI: 0.760–0.903), better than those of the cTNM stage, CRP, and IgA (0.635 (95% CI: 0.509–0.757), 0.679 (95% CI: 0.557–0.792), and 0.678 (95% CI: 0.549–0.800)) (Table 3). The C-index curves under the time distribution of 3 years to predict OS of ESCC patients who received nCRT plus surgery in the training and validation cohorts are shown in Figures 4A, C, and the C-index of the nomogram was contrasted with every single variable. In Figures 4B, D, the internal verification by the Bootstrap algorithm was calculated to obtain a more reliable C-index in both cohorts. Both of them revealed that the C-index of the nomogram was good.
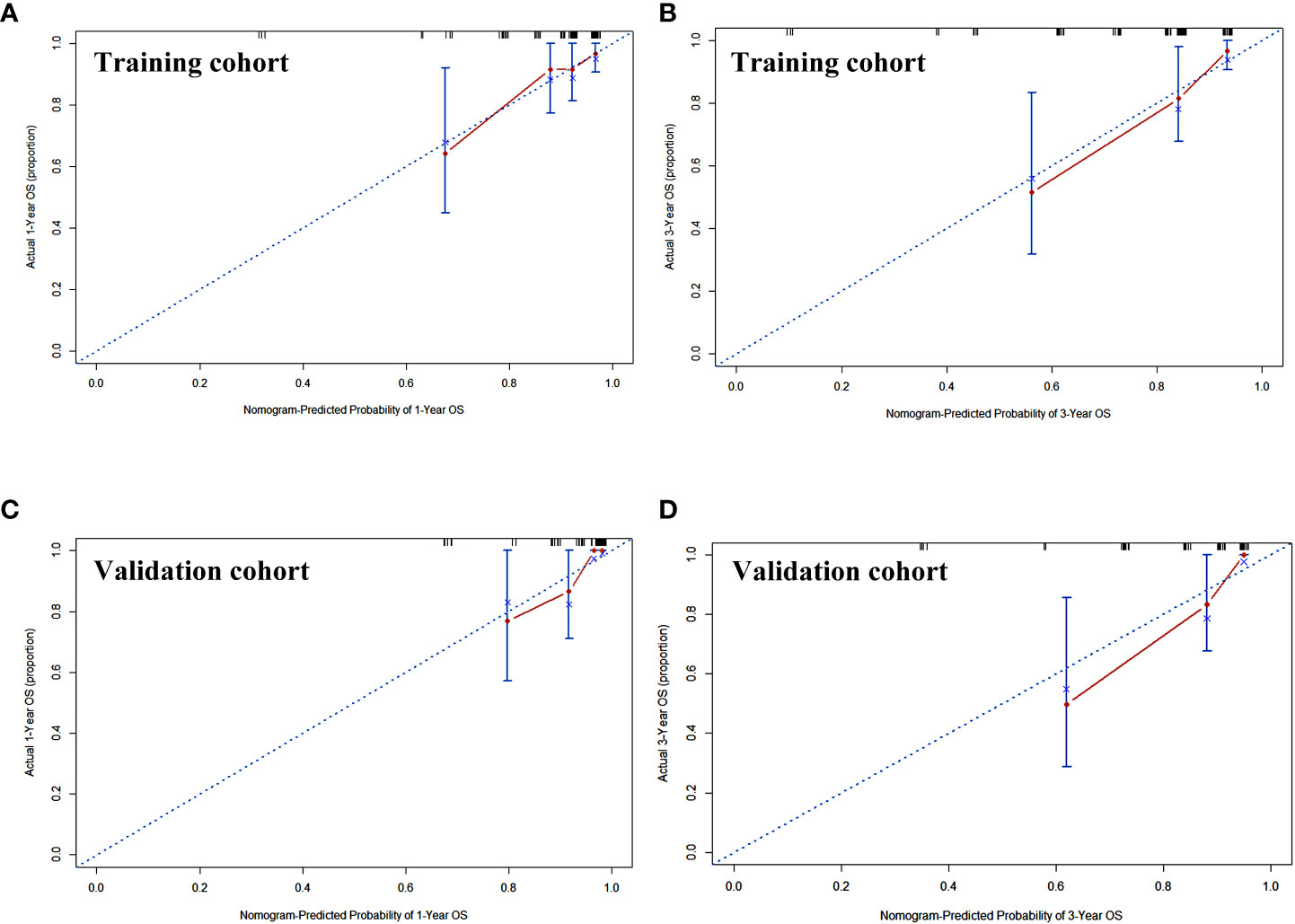
Figure 3 The calibration curve of the nomogram to predict the overall survival rate of 1 and 3 years in training cohort (A, B) and validation cohort (C, D). x-Axis was the nomogram-predicted probability of 1 or 3 years of OS. y-Axis was the actual OS of the patients included in the study. OS, overall survival.
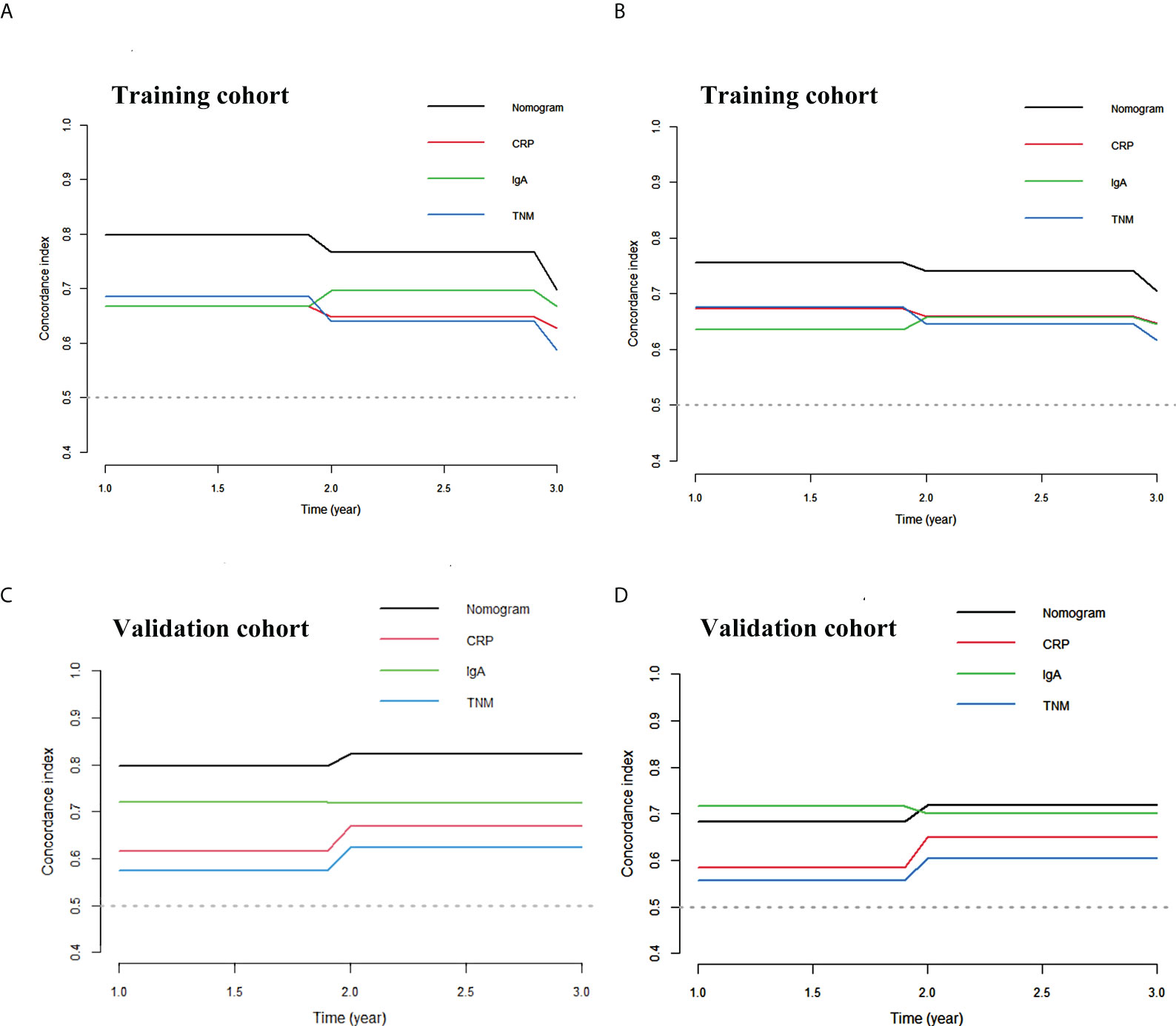
Figure 4 The C-index curve of the nomogram. (A) The C-index curve under the time distribution of 3 years to predict OS in training cohort. (B) The internal verification by Bootstrap algorithm of 3 years to predict OS in training cohort. (C) The C-index curve under the time distribution of 3 years to predict OS in validation cohort. (D) The internal verification by Bootstrap algorithm of 3 years to predict OS in validation cohort. OS, overall survival.
Furthermore, decision curve analysis was applied to evaluate the net benefit of the nomogram in both cohorts. As shown in Figure 5, the nomogram has a higher net benefit than that of other indicators to predict overall survival rates of 1 and 3 years. Moreover, as shown in Table 4, the NRI demonstrated that the prediction accuracy of the nomogram was better than CRP, IgA, and cTNM stage (NRI > 0), and the IDI suggested that the accuracy of the nomogram to predict 1- and 3-year OS was increased by 8.7% and 23.2%, respectively, as compared with the cTNM stage in the training cohort. Similarly, the NRI and IDI revealed that the prediction accuracy of the nomogram was better than IgA and the cTNM stage. To conclude, the prediction accuracy and net benefit of nomogram were higher than those of other assessment systems, which were identified through calibration curve, C-index, DCA, IDI, and NRI in the training cohort. Moreover, the prediction accuracy and applicability of the nomogram were verified in the validation cohort. Therefore, the model was reliable.
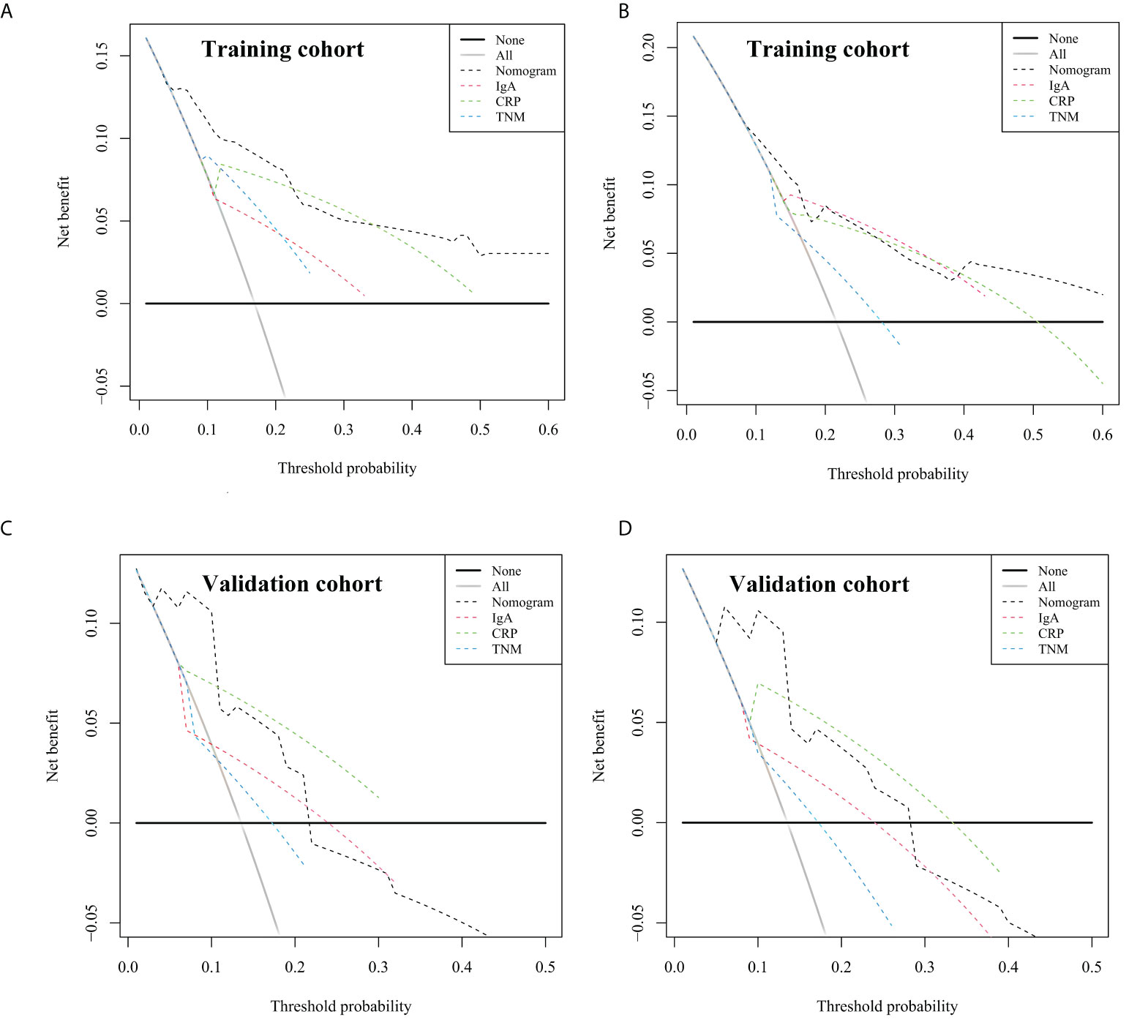
Figure 5 Decision curve analysis of the nomogram for overall survival (OS), compared with CRP, IgA, and clinical TNM stage. (A) The decision curve for 1-year OS in training cohort. (B) The decision curve for 3-year OS in training cohort. (C) The decision curve for 1-year OS in validation cohort. (D) The decision curve for 3-year OS in validation cohort. The straight black line represents the assumption that all patients die, and the horizontal line represents the assumption that no deaths happened. CRP, C-reactive protein; IgA, immune globin A.
Then we calculated the predicted total points according to the established nomogram in both cohorts, utilized the X-tile program to obtain the best cutoff value (100 for OS), and subdivided patients into the low-risk or high-risk groups according to the cutoff value. We used Kaplan–Meier survival analysis to evaluate survival. As indicated in Figure 6, the OS of the high-risk group was shorter than that of the low-risk group in both cohorts (p < 0.05). This revealed that the nomogram was feasible for risk stratification and OS prediction for patients before receiving nCRT plus surgery.
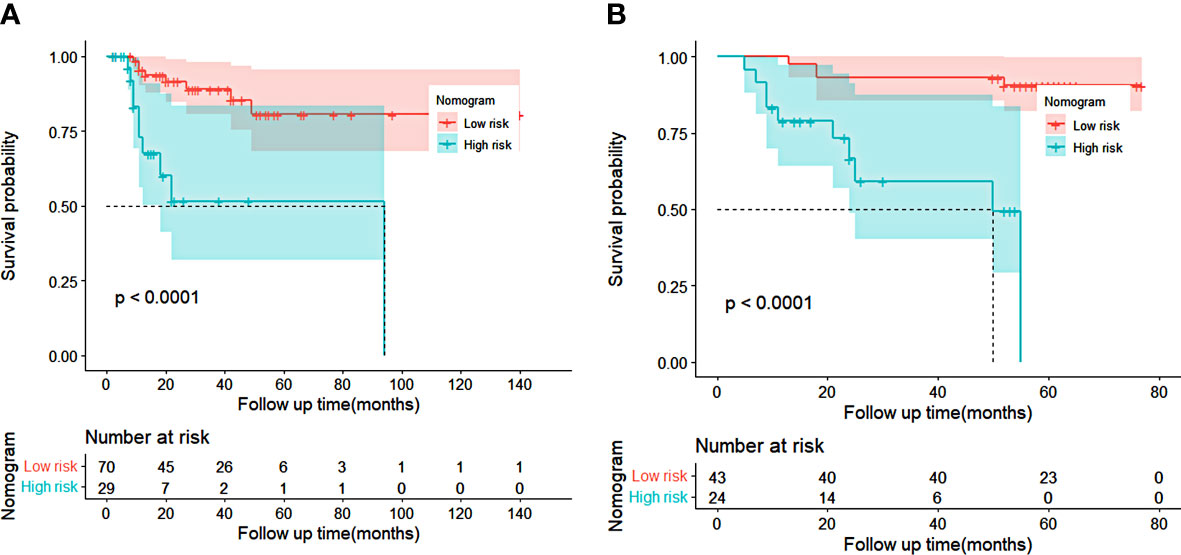
Figure 6 Kaplan–Meier curves of risk stratification for overall survival (OS) based on the predictions of the nomogram in training cohort (A) and validation cohort (B). Low risk: total points <100 for OS. High risk: total points ≥100 for OS.
Esophageal cancer as the sixth leading cause of cancer death causes huge economic pressure, especially for developing countries, and many of the tumors will have progressed to advanced stages during the diagnosis. Moreover, the benefit rate of surgery alone in advanced patients was low (1, 3, 4). Neoadjuvant chemoradiation is recommended for advanced esophageal cancer patients. Chemotherapy medicines such as carboplatin, paclitaxel, cisplatin, and fluorouracil were frequently selected in combination therapy with radiation, and the pathological complete response and overall survival rate were improved as compared with surgery alone (28, 29). Nevertheless, the benefit rate of nCRT was closely related to the individual. Therefore, it was necessary to establish a prognostic model for ESCC patients who received nCRT. As research reported, the DFS was shorter for the patients with preoperative hyperfibrinogenemia (fibrinogen > 350 mg/dl), and the plasma fibrinogen level was a biomarker for predicting postoperative recurrence (14). Although some prognostic indicators were confirmed as independent risk factors for OS or DFS, most of them were single indicators. This meant the predicted bias may exist for prognosis. Further, the significance of the model based on combined indicators deserved to evaluate. Here, we established the nomogram model according to the preoperative cTNM stage, serum CRP, and IgA for predicting the prognosis of ESCC patients receiving nCRT plus surgery and verified the nomogram in the validation cohort. Our nomogram showed improved prediction accuracy for prognosis, compared with the cTNM stage.
The connection between cancer-related inflammation and protective tumor immunity is dynamic. Consequently, evaluating the immune and inflammation status of tumor patients was of positive significance for prognosis. CRP, which existed in the form of pentamer in plasma, was synthesized mainly by hepatocytes (30–33). As an acute reaction protein, under normal circumstances, the content of CRP in human serum was low, but the CRP concentration even increased 1,000-fold in response to inflammation or tissue damage (34). Several studies reported that the high level of CRP as an independent prognostic factor was connected with OS in gastric, lung, ovarian, and esophageal cancers (35–40), and the high preoperative or postoperative CRP levels were related to worse survival prognosis in ESCC patients (35, 40–42). These results were consistent with the finding of serum CRP observed in our study. Survival analysis showed that a high CRP level (≥4.4 mg/L) before nCRT plus surgery was associated with worse OS. In our nomogram, CRP had a strong effect on predicting OS. IgA, which was produced by plasma cells, existed in the mucous membrane, tissues, and blood. Furthermore, IgA was the second most abundant immunoglobulin isotype in serum. IgA drove passive immunity-related functions. Recently, IgA-induced inflammation in diseases has been discussed (43, 44). Furthermore, as reported by Shalapour et al., IgA+ cells induced by an inflammatory environment dismantled anti-liver tumor immunity (45). IgA, which effectuated an important role in the diagnosis of chronic lymphocytic leukemia (CLL), was an independent prognostic factor for disease progression, survival, and infection in CLL (46). However, studies about the prognostic prediction ability of IgA on solid tumors were insufficient. In our work, we revealed that IgA was an independent risk factor for OS of ESCC patients who received nCRT plus surgery, and survival analysis revealed that high IgA level (≥3.0 g/L) was related to worse 1- or 3-year OS.
In our study, the prediction accuracy of the nomogram was satisfactory, as the C-index of the nomogram adopting internal verification through the Bootstrap algorithm was 0.820 (95% CI: 0.705–0.934) in the training cohort and 0.832 (95% CI: 0.760–0.903) in the validation cohort, which was higher than those of any other single indicators including the cTNM stage. DCA, IDI, NRI, and calibration curve consistently certified that the net benefit, model fit, and accuracy of nomogram were satisfactory in both cohorts. These consequences confirmed that the prognostic prediction of the nomogram established with the levels of immunity and inflammation-related indicators was reliable.
However, the shortcomings of our study still existed. Firstly, the sample size was relatively small in our study. This may cause a bias in the results. For example, the TNM stage was generally used to evaluate cancer patient survival in clinical practice. However, in our study, the cTNM stage was statistically related to patient survival, but not an independent prognostic factor in multivariate Cox analysis. This is mainly attributed to the small sample size and the majority of enrolled patients with advanced stage. In the future, expanding the samples is important to address this issue. Secondly, both the training cohort and the validation cohort were from the same hospital. Arrangement of the verification in external cohorts was required in the future. Thirdly, the retrospective nature of analyses was another limitation of this work. Thus, further research with prospective design is warranted to validate our constructed nomogram.
In our study, we established a nomogram based on serum IgA, CRP, and cTNM stage to predict the prognosis of ESCC patients receiving nCRT plus surgery. The predicted ability, accuracy, and applicability were satisfactory, which were verified in a validation cohort.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Hospital Ethics Committee in the Cancer Hospital of Shantou University Medical College. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YL and X-FW designed the study, collected and analyzed the patient samples and clinical data, and wrote the manuscript. J-TH and X-HH collected patient samples and clinical data, analyzed and interpreted the clinical data, and revised the manuscript. L-FW, Y-WL, and T-YD collected patient samples and clinical data, analyzed the data, and revised the manuscript. BZ, L-YC, and C-TL collected patient samples and clinical data, analyzed the clinical data, and revised the manuscript. Y-HP, Y-WX, and F-CW conceptualized and designed the study, supervised the project, and revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was funded by grants from Science and Technology Planning Project of Shantou City (No. 220506106490650), the Natural Science Foundation of China (No. 81972801), Guangdong Basic and Applied Basic Research Foundation (No. 2019A1515011873), and 2020 Li Ka Shing Foundation Cross-Disciplinary Research Grant (No. 2020LKSFG01B).
The authors thank the Department of Clinical Laboratory Medicine of the Cancer Hospital of Shantou University Medical College for their contributions to data collection and analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Huang J, Koulaouzidis A, Marlicz W, Lok V, Chu C, Ngai CH, et al. Global burden, risk factors, and trends of esophageal cancer: An analysis of cancer registries from 48 countries. Cancers (Basel) (2021) 13(1):141. doi: 10.3390/cancers13010141
3. Abnet CC, Arnold M, Wei WQ. Epidemiology of esophageal squamous cell carcinoma. Gastroenterology (2018) 154(2):360–73. doi: 10.1053/j.gastro.2017.08.023
4. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin (2011) 61(2):69–90. doi: 10.3322/caac.20107
5. Liu H-C, Hung S-K, Huang C-J, Chen C-C, Chen M-J, Chang C-C, et al. Esophagectomy for locally advanced esophageal cancer, followed by chemoradiotherapy and adjuvant chemotherapy. World J Gastroenterol (2005) 11(34):5367–72. doi: 10.3748/wjg.v11.i34.5367
6. Kelsen DP, Ginsberg R, Pajak TF, Sheahan DG, Gunderson L, Mortimer J, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. New Engl J Med (1998) 339(27):1979–84. doi: 10.1056/NEJM199812313392704
7. Babic B, Fuchs HF, Bruns CJ. Neoadjuvant chemoradiotherapy or chemotherapy for locally advanced esophageal cancer? Chirurg (2020) 91(5):379–83. doi: 10.1007/s00104-020-01150-6
8. Ma HF, Lv GX, Cai ZF, Zhang DH. Comparison of the prognosis of neoadjuvant chemoradiotherapy treatment with surgery alone in esophageal carcinoma: A meta-analysis. Onco Targets Ther (2018) 11:3441–7. doi: 10.2147/OTT.S145063
9. Sjoquist KM, Burmeister BH, Smithers BM, Zalcberg JR, Simes RJ, Barbour A, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: An updated meta-analysis. Lancet Oncol (2011) 12(7):681–92. doi: 10.1016/s1470-2045(11)70142-5
10. Zhao X, Ren Y, Hu Y, Cui N, Wang X, Cui Y. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or the gastroesophageal junction: A meta-analysis based on clinical trials. PLoS One (2018) 13(8):e0202185. doi: 10.1371/journal.pone.0202185
11. Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, et al. Esophageal and esophagogastric junction cancers, version 2.2019, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw (2019) 17(7):855–83. doi: 10.6004/jnccn.2019.0033
12. Chiu TJ, Lu HI, Chen CH, Huang WT, Wang YM, Lin WC, et al. Osteopontin expression is associated with the poor prognosis in patients with locally advanced esophageal squamous cell carcinoma receiving preoperative chemoradiotherapy. BioMed Res Int (2018) 2018:9098215. doi: 10.1155/2018/9098215
13. Makuuchi Y, Honda K, Osaka Y, Kato K, Kojima T, Daiko H, et al. Soluble interleukin-6 receptor is a serum biomarker for the response of esophageal carcinoma to neoadjuvant chemoradiotherapy. Cancer Sci (2013) 104(8):1045–51. doi: 10.1111/cas.12187
14. Matsuda S, Takeuchi H, Fukuda K, Nakamura R, Takahashi T, Wada N, et al. Clinical significance of plasma fibrinogen level as a predictive marker for postoperative recurrence of esophageal squamous cell carcinoma in patients receiving neoadjuvant treatment. Dis Esophagus (2014) 27(7):654–61. doi: 10.1111/dote.12115
15. Nakanoko T, Saeki H, Morita M, Nakashima Y, Ando K, Oki E, et al. Rad51 expression is a useful predictive factor for the efficacy of neoadjuvant chemoradiotherapy in squamous cell carcinoma of the esophagus. Ann Surg Oncol (2014) 21(2):597–604. doi: 10.1245/s10434-013-3220-2
16. Nasierowska-Guttmejer A, Szawłowski A, Jastrzębska M, Jeziorski K, Radziszewski J. P53 protein accumulation as a prognostic marker of preoperative radiotherapy and/or chemotherapy in advanced squamous cell esophageal carcinoma – preliminary report *. Dis Esophagus (1999) 12(2):128–31. doi: 10.1046/j.1442-2050.1999.00030.x
17. Chen SB, Weng HR, Wang G, Yang JS, Yang WP, Liu DT, et al. Prognostic factors and outcome for patients with esophageal squamous cell carcinoma underwent surgical resection alone: Evaluation of the seventh edition of the American joint committee on cancer staging system for esophageal squamous cell carcinoma. J Thorac Oncol (2013) 8(4):495–501. doi: 10.1097/JTO.0b013e3182829e2c
18. Wang J, Wu N, Zheng QF, Yan S, Lv C, Li SL, et al. Evaluation of the 7th edition of the tnm classification in patients with resected esophageal squamous cell carcinoma. World J Gastroenterol (2014) 20(48):18397–403. doi: 10.3748/wjg.v20.i48.18397
19. Jeong DY, Kim MY, Lee KS, Choi JY, Kim SJ, Chung MJ, et al. Surgically resected T1- and T2-stage esophageal squamous cell carcinoma: T and n staging performance of eus and Pet/Ct. Cancer Med (2018) 7(8):3561–70. doi: 10.1002/cam4.1617
20. Foley KG, Lewis WG, Fielding P, Karran A, Chan D, Blake P, et al. N-staging of oesophageal and junctional carcinoma: Is there still a role for eus in patients staged N0 at Pet/Ct? Clin Radiol (2014) 69(9):959–64. doi: 10.1016/j.crad.2014.04.023
21. Choi JY, Lee KH, Shim YM, Lee KS, Kim JJ, Kim SE, et al. Improved detection of individual nodal involvement in squamous cell carcinoma of the esophagus by fdg pet. J Nucl Med (2000) 41(5):808.
22. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature (2008) 454(7203):436–44. doi: 10.1038/nature07205
23. Fu X, Li T, Dai Y, Li J. Preoperative systemic inflammation score (Sis) is superior to neutrophil to lymphocyte ratio (Nlr) as a predicting indicator in patients with esophageal squamous cell carcinoma. BMC Cancer (2019) 19(1):721. doi: 10.1186/s12885-019-5940-6
24. Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity's roles in cancer suppression and promotion. Science (2011) 331(6024):1565–70. doi: 10.1126/science.1203486
25. Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res (2019) 79(18):4557–66. doi: 10.1158/0008-5472.CAN-18-3962
26. Rice TW, Ishwaran H, Ferguson MK, Blackstone EH, Goldstraw P. Cancer of the esophagus and esophagogastric junction: An eighth edition staging primer. J Thorac Oncol (2017) 12(1):36–42. doi: 10.1016/j.jtho.2016.10.016
27. Camp RL, Dolled-Filhart M, Rimm DL. X-Tile. Clin Cancer Res (2004) 10(21):7252. doi: 10.1158/1078-0432.CCR-04-0713
28. Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, et al. Phase iii trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: Calgb 9781. J Clin Oncol (2008) 26(7):1086–92. doi: 10.1200/jco.2007.12.9593
29. van Hagen P, Hulshof MCCM, van Lanschot JJB, Steyerberg EW, Henegouwen M, Wijnhoven BPL, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. New Engl J Med (2012) 366(22):2074–84. doi: 10.1056/NEJMoa1112088
30. Weinhold B, Rüther U. Interleukin-6-Dependent and -independent regulation of the human c-reactive protein gene. Biochem J (1997) 327(Pt 2):425–9. doi: 10.1042/bj3270425
31. Zhang D, Jiang SL, Rzewnicki D, Samols D, Kushner I. The effect of interleukin-1 on c-reactive protein expression in Hep3b cells is exerted at the transcriptional level. Biochem J (1995) 310(1):143–8. doi: 10.1042/bj3100143
32. Tucci A, Goldberger G, Whitehead AS, Kay RM, Woods DE, Colten HR. Biosynthesis and postsynthetic processing of human c-reactive protein. J Immunol (1983) 131(5):2416.
33. Diehl EE, Haines GK, Radosevich JA, Potempa LA. Immunohistochemical localization of modified c-reactive protein antigen in normal vascular tissue. Am J Med Sci (2000) 319(2):79–83. doi: 10.1016/s0002-9629(15)40692-5
34. Black S, Kushner I, Samols D. C-reactive protein. J Biol Chem (2004) 279(47):48487–90. doi: 10.1074/jbc.R400025200
35. Huang W, Wu L, Liu X, Long H, Rong T, Ma G. Preoperative serum c-reactive protein levels and postoperative survival in patients with esophageal squamous cell carcinoma: A propensity score matching analysis. J Cardiothorac Surg (2019) 14(1):167. doi: 10.1186/s13019-019-0981-0
36. Nozoe T, Iguchi T, Adachi E, Matsukuma A, Ezaki T. Preoperative elevation of serum c-reactive protein as an independent prognostic indicator for gastric cancer. Surg Today (2011) 41(4):510–3. doi: 10.1007/s00595-009-4297-x
37. Park D-S, Kim D, Hwang K-E, Hwang Y-R, Park C, Seol C-H, et al. Diagnostic value and prognostic significance of pleural c-reactive protein in lung cancer patients with malignant pleural effusions. Yonsei Med J (2013) 54(2):396–402. doi: 10.3349/ymj.2013.54.2.396
38. Powell A, Eley C, Chin C, Coxon AH, Christian A, Lewis WG, et al. Prognostic significance of serum inflammatory markers in esophageal cancer. Esophagus (2021) 18(2):267–77. doi: 10.1007/s10388-020-00772-3
39. Toriola AT, Grankvist K, Agborsangaya CB, Lukanova A, Lehtinen M, Surcel HM. Changes in pre-diagnostic serum c-reactive protein concentrations and ovarian cancer risk: A longitudinal study. Ann Oncol (2011) 22(8):1916–21. doi: 10.1093/annonc/mdq694
40. Zhang H, Guo XW, Yin XX, Liu YC, Ji SJ. Nomogram-integrated c-reactive Protein/Albumin ratio predicts efficacy and prognosis in patients with thoracic esophageal squamous cell carcinoma receiving chemoradiotherapy. Cancer Manag Res (2019) 11:9459–68. doi: 10.2147/CMAR.S228113
41. Huang Y, Liu JS, Feng JF. The combination of preoperative serum c-reactive protein and carcinoembryonic antigen is a useful prognostic factor in patients with esophageal squamous cell carcinoma: A combined roc analysis. Onco Targets Ther (2015) 8:795–803. doi: 10.2147/OTT.S77378
42. Katsurahara K, Shiozaki A, Fujiwara H, Konishi H, Kudou M, Shoda K, et al. Relationship between postoperative crp and prognosis in thoracic esophageal squamous cell carcinoma. Anticancer Res (2018) 38(11):6513–8. doi: 10.21873/anticanres.13016
43. Woof JM, Kerr MA. The function of immunoglobulin a in immunity. J Pathol (2006) 208(2):270–82. doi: 10.1002/path.1877
44. Hansen IS, Baeten DLP, den Dunnen J. The inflammatory function of human iga. Cell Mol Life Sci (2019) 76(6):1041–55. doi: 10.1007/s00018-018-2976-8
45. Shalapour S, Lin XJ, Bastian IN, Brain J, Burt AD, Aksenov AA, et al. Inflammation-induced iga+ cells dismantle anti-liver cancer immunity. Nature (2017) 551(7680):340–5. doi: 10.1038/nature24302
Keywords: esophageal squamous cell carcinoma, prognosis, IgA, CRP, neoadjuvant chemoradiotherapy
Citation: Luo Y, Weng X-F, Huang J-T, Hu X-H, Wei L-F, Lin Y-W, Ding T-Y, Zhang B, Chu L-Y, Liu C-T, Peng Y-H, Xu Y-W and Wu F-C (2022) Nomogram constructed by immunological and inflammatory indicators for predicting prognosis of patients with esophageal squamous cell carcinoma treated with neoadjuvant chemoradiotherapy plus surgery. Front. Oncol. 12:882900. doi: 10.3389/fonc.2022.882900
Received: 24 February 2022; Accepted: 11 July 2022;
Published: 29 July 2022.
Edited by:
Zhendong Jin, Second Military Medical University, ChinaReviewed by:
Jing Wen, Sun Yat-sen University Cancer Center, ChinaCopyright © 2022 Luo, Weng, Huang, Hu, Wei, Lin, Ding, Zhang, Chu, Liu, Peng, Xu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang-Cai Wu, MjgwNTUwMTA5QHFxLmNvbQ==; Yi-Wei Xu, eWl3ZWk1MTJAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.