- Fujian Medical University Cancer Hospital, Fujian Cancer Hospital, Fuzhou, China
Background: This study was conducted to determine risk factors for developing brain metastasis (BM) and to predict brain metastasis free survival (BMFS) and overall survival (OS) by combining several clinical parameters and inflammatory indexes.
Materials and Methods: A nomogram and risk stratification were developed based on multivariate analysis results. The prognostic index (PI) predicting the high risk of BM was calculated by multiplying the weighted factor (β coefficient) with each variable.
Results: Thirty-two of one hundred patients (32.0%) developed BM. Multivariate cox regression analysis revealed that concurrent chemoradiotherapy (CCRT; hazard ratio (HR), 3.356; p = 0.020), monocyte–lymphocyte ratio (MLR; HR, 4.511; p = 0.002), neutrophil–lymphocyte ratio (NLR; HR, 4.023; p = 0.033), and prognostic-nutrition index (PNI; HR, 2.902; p = 0.018) were independent prognostic factors of BMFS. The nomogram has good accuracy in predicting BMFS, and the C-index was 0.73. The ROC curve showed that these risk factors have good discriminant ability. Similarly, tumor location (HR, 1.675; p = 0.035) and MLR (HR, 2.076; p = 0.013) were independent prognostic factors of OS. In the subgroup analysis of OS, the good group had a better prognosis than the other groups. Risk stratification by PI: the high-risk group had worse BMFS than the low-risk group, which also has certain practical significance for clinical practice in OS.
Conclusion: We developed a nomogram and corresponding risk stratification in stage III SCLC patients who developed BM. This model and risk stratification can help clinicians improve patient treatment management and better deliver personalized therapy.
Introduction
Small cell lung cancer (SCLC) is an aggressive neuroendocrine malignant tumor characterized by rapid doubling time and poor prognosis, with only one-third of patients in limited-stage (1). Chemoradiotherapy (CRT) is the primary treatment of limited-stage SCLC (LS-SCLC) (2, 3). Compared with chemotherapy alone, the multimodality treatment improves survival rates significantly. Although the primary tumor is sensitive to chemotherapy and radiotherapy, local recurrence or metastatic spread is common shortly after treatment (4). The brain is considered a refuge for relapse because the blood–brain barrier blocks the entry of most chemotherapy drugs. Therefore, the brain is a common metastatic site of SCLC, and more than 50% of patients with LS-SCLC still develop intracranial metastasis after completion of CRT (5). Autopsy studies have demonstrated that one-half to two-thirds of patients with SCLC develop brain metastasis (BM) at death (6, 7).
Even though prophylactic cranial irradiation (PCI) reduced the occurrence of BM, the incidence of BM in patients with SCLC remains high. The effect of PCI on the overall survival (OS) of patients with SCLC is still controversial. The randomized trial data showed that PCI reduced the rate of BM from about 60 to 30% and increased the 3-year OS by about 5% (8). Another multicenter and randomized trial result demonstrated that PCI reduced the rate of BM, but failed to improve the OS of the patient (9). However, about 20–40% of patients are diagnosed with BM even after PCI (10). Additionally, PCI can cause discomfort such as dizziness, lethargy, and loss of appetite, and some patients even have serious side effects such as neurocognitive impairment and hormone deregulation (11). These symptoms significantly affect the quality of life of patients, which our clinicians must consider, particularly in SCLC. Besides, not all patients with LS-SCLC receive PCI treatment in reality. A study indicated that patients with BM at the time of initial diagnosis had significantly higher survival than those diagnosed with BM after completion of CRT (12). Therefore, early identification of risk factors related to particular patients with BM would be valuable for therapeutic management and improving SCLC prognosis.
SCLC patients with BM usually have a poor prognosis with a median survival time of 2–14 months (13). It has been proved that the factors correlated with the prognosis of SCLC patients with BM include age, performance status, number of brain lesions, and so on (14). However, few studies have investigated the prognostic impact of inflammation markers in LS-SCLC patients diagnosed with BM after receiving CRT. Inflammatory and immune responses are indispensable to the development and metastasis of tumors (15). For example, several studies have linked increased platelet counts or decreased lymphocyte counts to poor prognosis in lung cancer patients (15, 16). Neutrophil–lymphocyte ratio (NLR) has been identified as a risk factor associated with BM in SCLC (17). Interestingly, the neutrophils, lymphocytes, monocytes, platelets, and albumin could be easily measured during treatment. Therefore, we carried out this study to explore the risk factors of developing BM after initial treatment in patients with stage III LS-SCLC through the inflammatory index and clinical factors.
Materials and Methods
Patients and Follow-Up
We reviewed patients with stage III LS-SCLC who received CRT at the Fujian Provincial Cancer Hospital from 2007 to 2019. All patients received a standardized evaluation before antineoplastic treatment, namely, thoracic and abdominal computed tomography (CT) with contrast medium, radionuclide bone scanning, and cranial magnetic resonance imaging (MRI) or CT with contrast medium, or position emission tomography/computerized tomography (PET/CT). The patients were staged according to the TNM classification of the eighth edition of the American Joint Commission on Cancer (AJCC, 8th edition) and the two-stage system based on version 1.2016 of the National Comprehensive Cancer Network Guidelines for SCLC (NCCN2016). Inclusion criteria were (1): pathology and imaging proved LS-SCLC; (2) without BM at first diagnosis; (3) availability of clinical data and peripheral blood cell counts; and (4) receiving CRT. Patients who received PCI were excluded. Finally, 100 stage III LS-SCLC patients were enrolled. The Ethics Committee approved this study at the Fujian Provincial Cancer Hospital.
Patients were generally reexamined at the end of every 2 or 3 cycles of chemotherapy and at the beginning and end of radiotherapy. After completing the initial treatment, patients were usually revisited every three months for the first two years, every six months for 3 to 5 years, and yearly thereafter. However, this follow-up time was not permanently fixed. Thoracic CT or PET/CT images were usually obtained at each follow-up. Brain MRI or CT should be performed at each follow-up despite the variation.
Chemotherapy and Radiotherapy
All patients received individualized CRT. Chemotherapy regimens included etoposide, paclitaxel, or irinotecan with cisplatin, carboplatin, nedaplatin, or lobaplatin. The median cycle of chemotherapy was five cycles. Thoracic radiotherapy was performed using 3-dimensional conformal radiotherapy (3D-CRT) or intensity-modulated radiotherapy (IMRT). Most of the patients received conventional fractionated radiotherapy (CFRT), while a few patients received hyper-fractionated radiotherapy (HFRT). Individual radiation was performed with CFRT with 42–69 Gy in 20–33 daily fractions or HFRT with 45–48 Gy in 15–16 fractions. Radiotherapy (RT) used a 6MV medical linear accelerator. The gross tumor volume (GTV) included the primary lung tumor and elective or involved lymph nodes. Considering the microscopic spread, the clinical tumor volume (CTV) comprised the GTV with an edge of 5 mm in all directions. On the basis of CTV, the planned tumor volume (PTV) expanded 5–8 mm in all directions.
Inflammatory and Nutritionl Index
The systemic immune-inflammation index (SII), monocyte–lymphocyte ratio (MLR), neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR), prognostic-nutrition index (PNI), and platelet–albumin ratio (PAR) were calculated as follows: SII = absolute neutrophil count times absolute platelet count divided by absolute lymphocyte count; MLR = absolute monocyte count divided by absolute lymphocyte count. NLR = absolute neutrophil count divided by absolute lymphocyte count. PLR = absolute platelet count divided by absolute lymphocyte count. PNI = serum albumin level plus five times the absolute lymphocyte count. PAR = absolute platelet count divided by serum albumin level. These inflammatory indexes were calculated using the blood biochemical data collected within five days before therapy.
Developing Prognostic Index for Brain Metastasis
The prognostic index (PI) predicting a high risk of brain metastases was calculated in the study population using the Cox regression model. Each independent prognostic factor is multiplied by a β coefficient. Then, the prognostic index was generated by summing. Finally, the study population was divided into the high-risk and low-risk groups according to the risk score calculated from the PI.
Endpoints
The primary endpoint of interest was brain metastasis-free survival (BMFS), defined as the period from pathological diagnosis to the date of discovery of BM. Overall survival (OS) was calculated as the time from pathological diagnosis to death or the last follow-up. The median follow-up time was 35.8 months (9.2–153.8 months).
Statistical Analysis
Statistical analyses were performed using SPSS software (version 25.0) and R software (version 4.0.2). The optimal cut-off values of time from initial treatment to radiotherapy, cycle of chemotherapy before radiotherapy, cycle of chemotherapy before BM, RT dose, SII, MLR, NLR, PLR, PNI, PAR, and PI were calculated using the X-tile software (version 3.6.1), which is essential for generating the best cut-off point with the minimum p-value. The Chi-squared tests or Fisher’s exact tests compared the categorical variables. The plot survival curve was drawn using the Kaplan–Meier method, and the log-rank test was used to compare the difference in the survival curve. Variables with p <0.2 in univariate analysis were incorporated into multivariate Cox regression analysis. Multivariate Cox regression analysis was used to determine independent risk factors related to survival. Based on BMFS multivariate analysis, only the factors with a p-value of <0.05 were included in the nomogram. The performance of the nomogram was evaluated by the calibration curve (with 1,000 bootstrap resamples) and the C-index. The larger the C-index, the more accurate the prediction. The clinical value of risk factors for BM was analyzed by receiver operating characteristic (ROC) curves. All tests were double-tailed, and a p-value less than 0.05 was considered statistically significant.
Results
Patient Characteristics
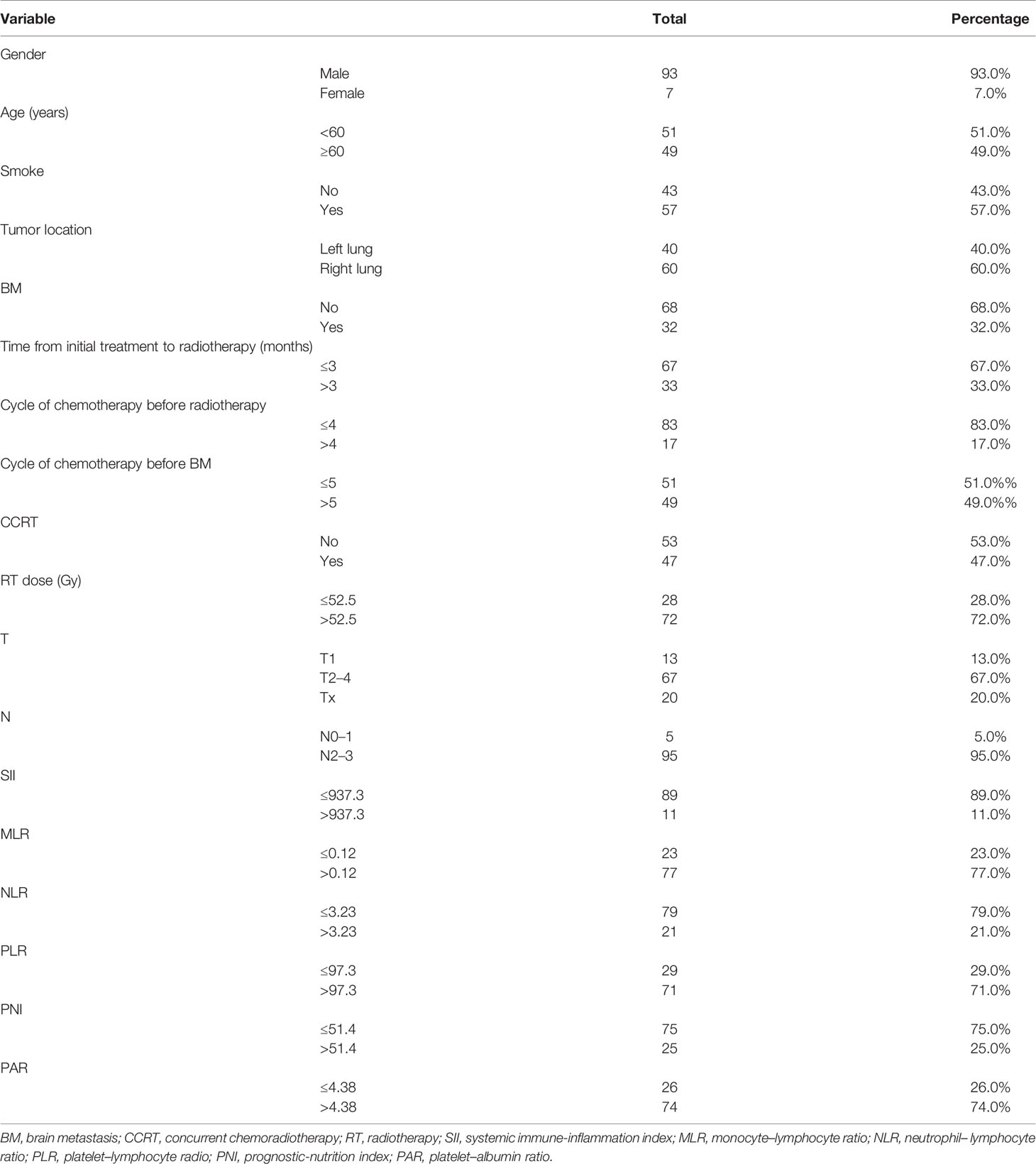
The medical records of 100 patients with stage III SCLC were collected and analyzed. Table 1 summarizes the clinical characteristics of the patients. Among them, 93 (93.0%) were men, 49 (49.0%) were ≥60 years old, 57 (57.0%) had a history of smoking, 60 (60.0%) were located in the right lung, 47 (47.0%) received concurrent chemoradiotherapy, and 32 (32.0%) had BM. The best cut-off points of time from initial treatment to radiotherapy, cycle of chemotherapy before radiotherapy, cycle of chemotherapy before BM, RT dose, SII, MLR, NLR, PLR, PNI, PAR, and PI were 3, 4, 5, 52.5, 937.3, 0.12, 3.23, 97.3, 51.4, 4.38, and 2.46, respectively.
Prognostic Factors for BMFS
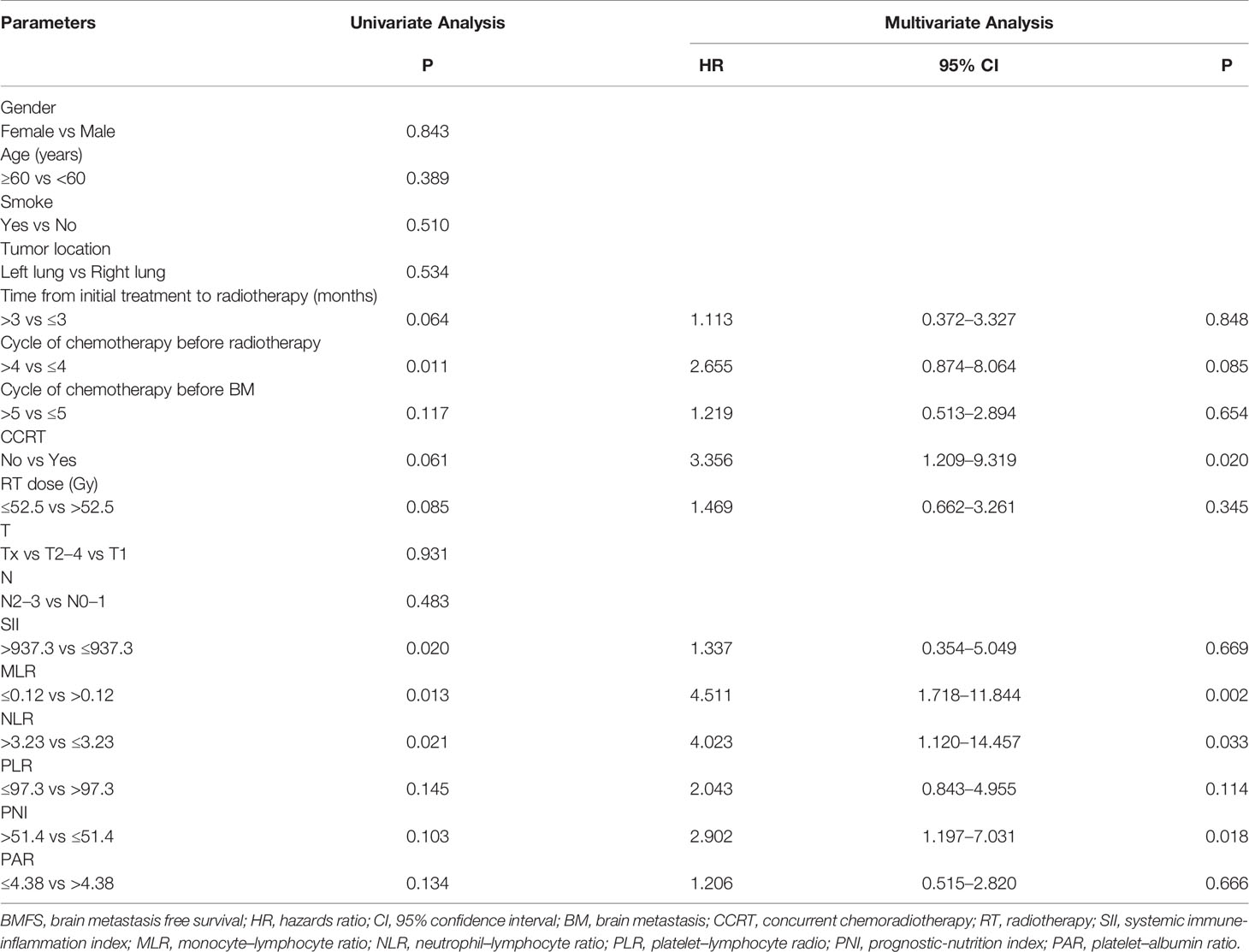
The median time of BMFS was 27.6 months. Table 2 shows the results of the univariate and multivariate Cox analyses of BMFS. Univariate analyses showed that time from initial treatment to radiotherapy, the cycle of chemotherapy before radiotherapy, the cycle of chemotherapy before BM, concurrent chemotherapy (CCRT), RT dose, MLR, NLR, PLR, PNI, and PAR were associated with BMFS. Multivariate analyses showed that CCRT [hazard ratio (HR) = 3.356, p = 0.020], MLR (HR = 4.511, p = 0.002), NLR (HR = 4.023, p = 0.033), and PNI (HR = 2.902, p = 0.018) were independent prognosis factors of BMFS.
Risk Prediction Model for BMFS
Based on the results of BMFS multivariate analysis, the variables included in the nomogram were as follows: CCRT, MLR, NLR, and PNI. The ROC of CCRT, MLR, NLR, PNI, and complex (CCRT, MLR, NLR, and PNI) are shown in Figures 1A, B. The complex’s area under the curve (AUC) was 0.708, which was higher than each independent risk factor. The nomogram results were reported as 1-year and 2-year BMFS (Figure 2A). The C-index of the nomogram was 0.73. The 1-year and 2-year BMFS calibration curves displayed favorable consistency between the actual observations and predicted values (Figures 2B, C). PI for predicting BM was obtained by multiplying the weighted factor (β coefficient) with each variable (Figure 3A). The patients with a PI higher than 2.46 were in the high-risk groups (n = 29), while those with a PI lower than 2.46 were in the low-risk group (n = 71). The high-risk group had a poorer BMFS than the low-risk group (Figure 3B). The high-risk group had poorer OS than the low-risk group, but it was not statistically significant (p = 0.093) (Figure 3C).
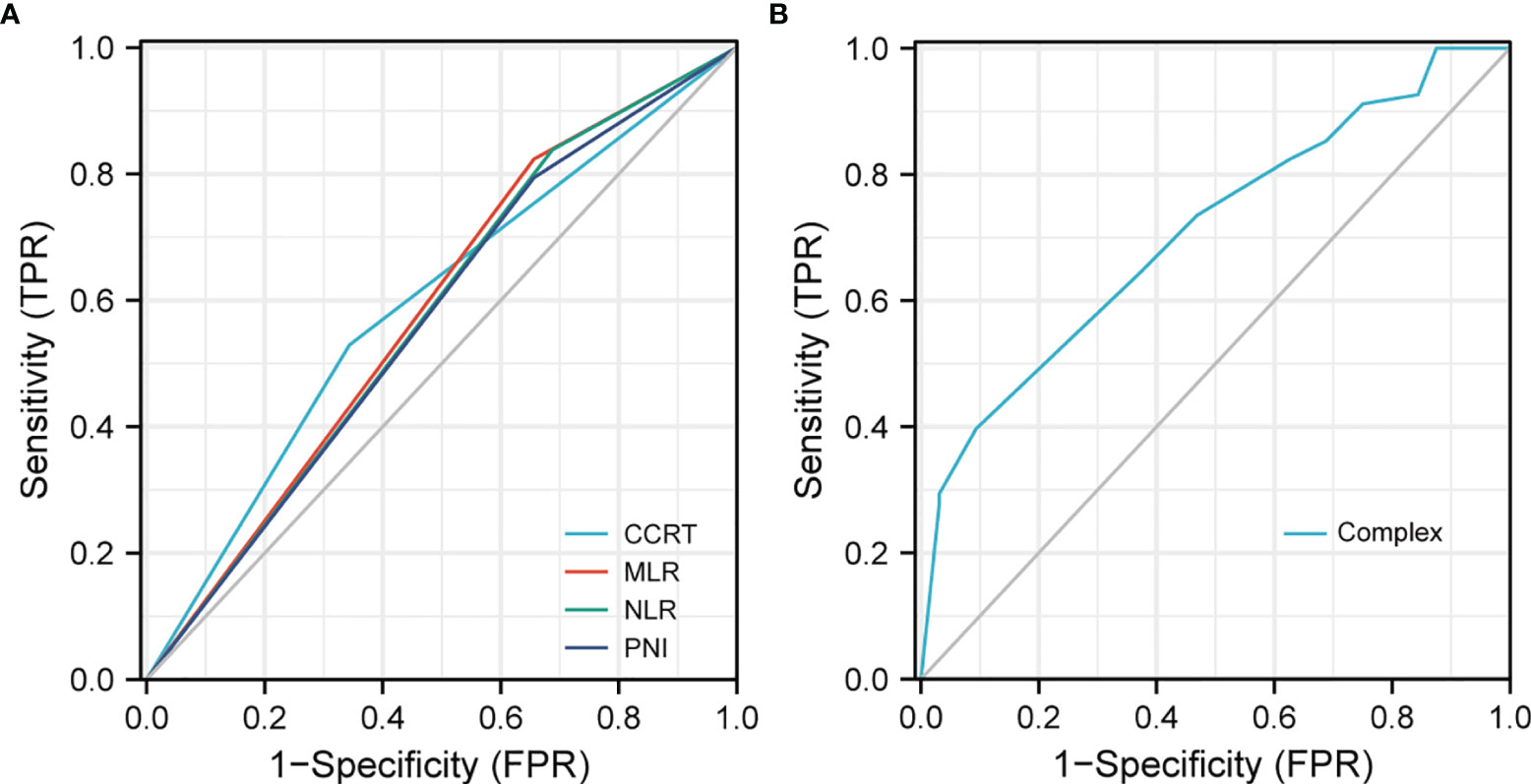
Figure 1 ROC to predict BM. (A) The area under of the curve (AUC) of CCRT, MLR, NLR, and PNI was 0.593, 0.584, 0.575, and 0.569. (B) AUC of the complex (CCRT, MLR, NLR, and PNI) was 0.708.
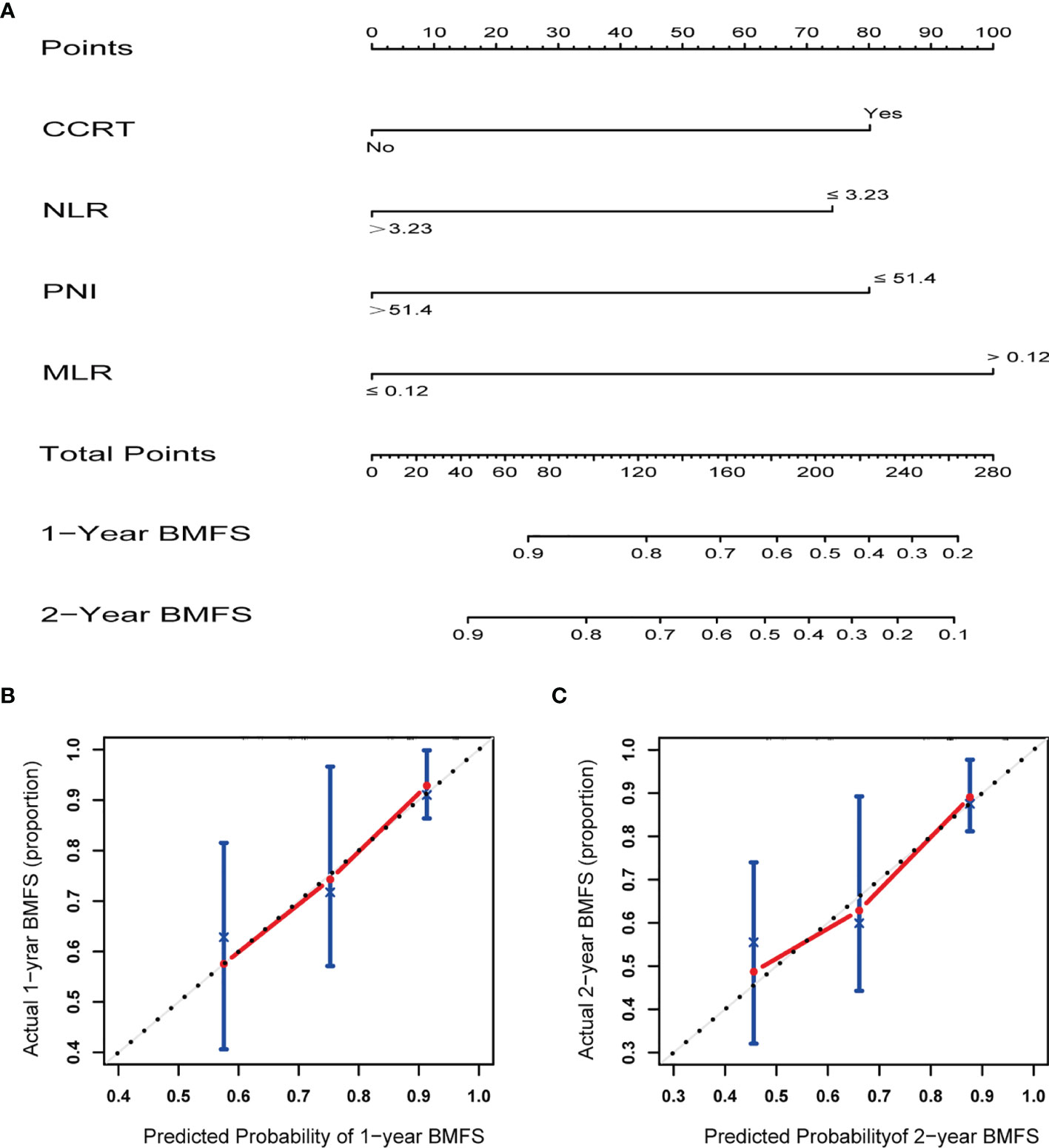
Figure 2 (A) Nomogram for prediction 1- and 2-year brain metastasis free survival of stage III small cell lung cancer. (B) Calibration curves demonstrating the probability of 1-year BMFS between the prediction and the actual observation. (C) Calibration curves demonstrating the probability of 2-year BMFS between the prediction and the actual observation. X-axis represents the nomogram predicted probability, and Y-axis represents the actual observation.
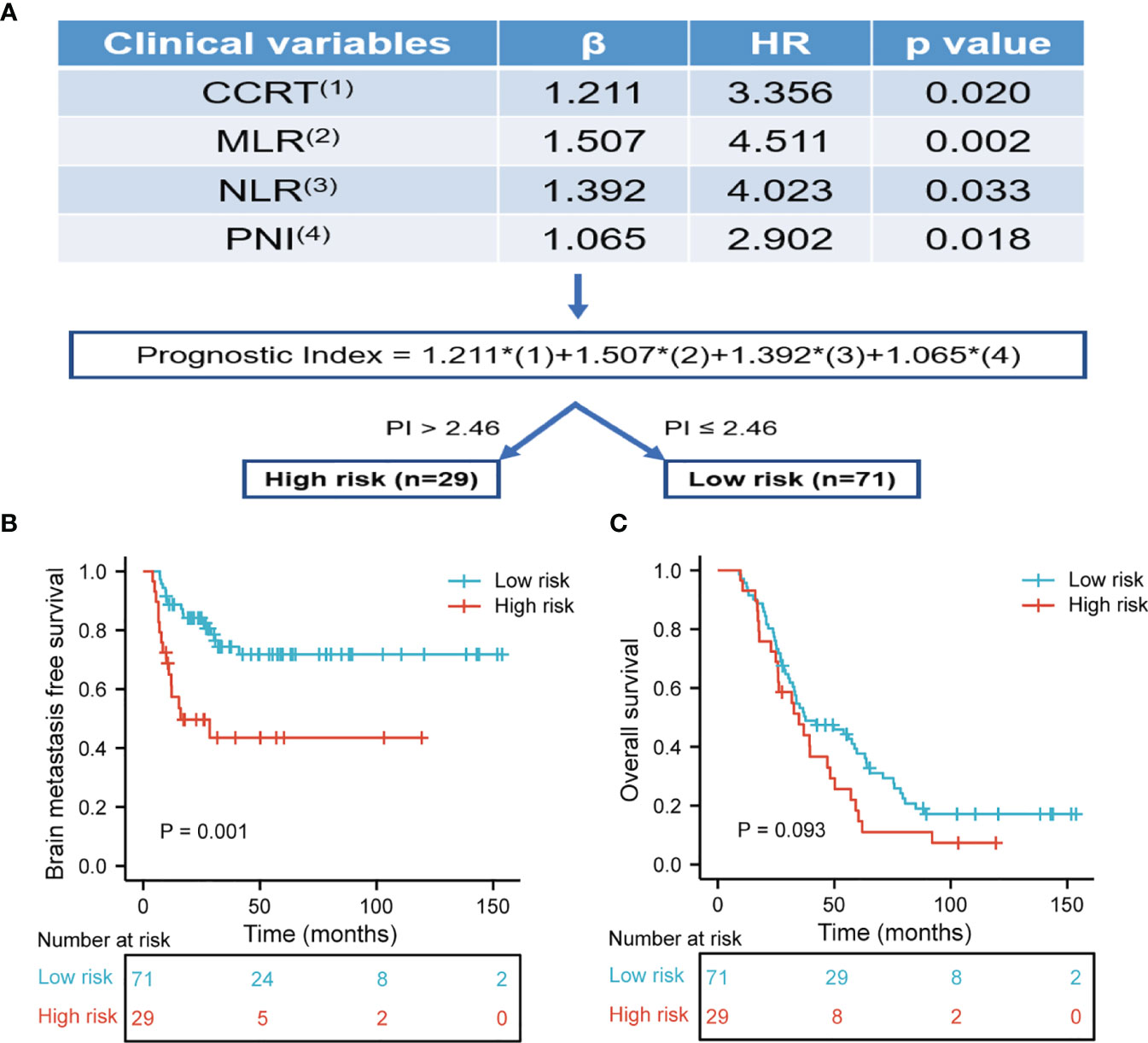
Figure 3 (A) The risk prediction model of BM. The prognostic index for predicting BMFS was calculated by multiplying the weighted factor (β coefficient) with four statistically significant variables. Each factor is one for existence and zero in the absence. The prognostic index was divided into high-risk and low-risk subset with a boundary of 2.46. (B) Brain metastasis free survival was risk stratification according to prognostic index. The high-risk group had poor BMFS than the low-risk group (p = 0.001). (C) Overall survival was risk stratification according to prognostic index. The high-risk group had poor OS than the low-risk group, but it was not statistically significant (p = 0.093).
Prognostic Factors for OS
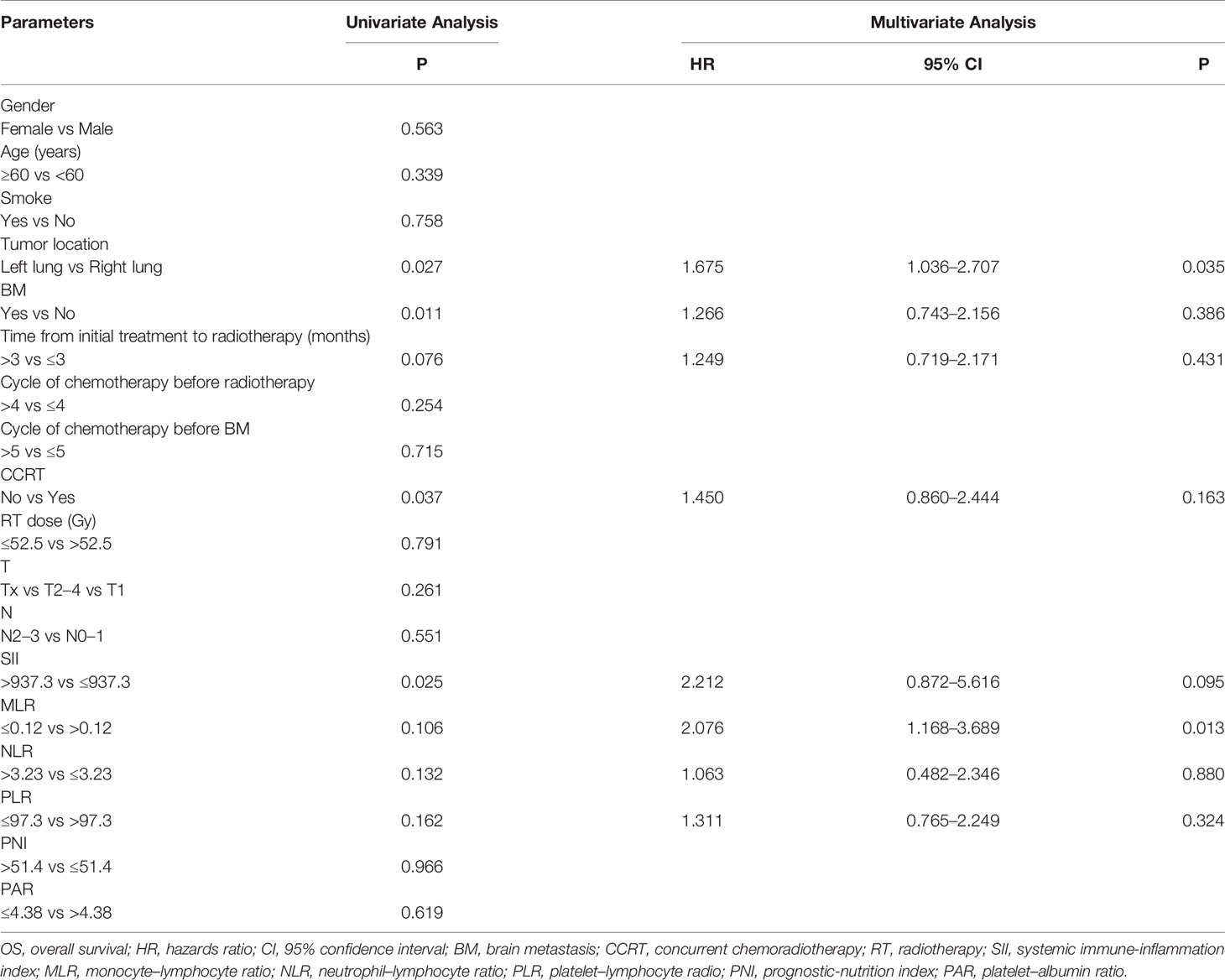
The median OS was 35.8 months. Table 3 shows the results of univariate and multivariate Cox analyses of OS. In Table 3, univariate analyses revealed that tumor location, BM (Figure 4A), time from initial treatment to radiotherapy, CCRT, MLR, NLR, and PLR were potential risk factors for OS. On multivariate analysis, tumor location (HR = 1.675, p = 0.035) and MLR (HR = 2.076, p = 0.013) were independently related to worse prognosis. The chemotherapy regimen showed no statistical difference in OS.
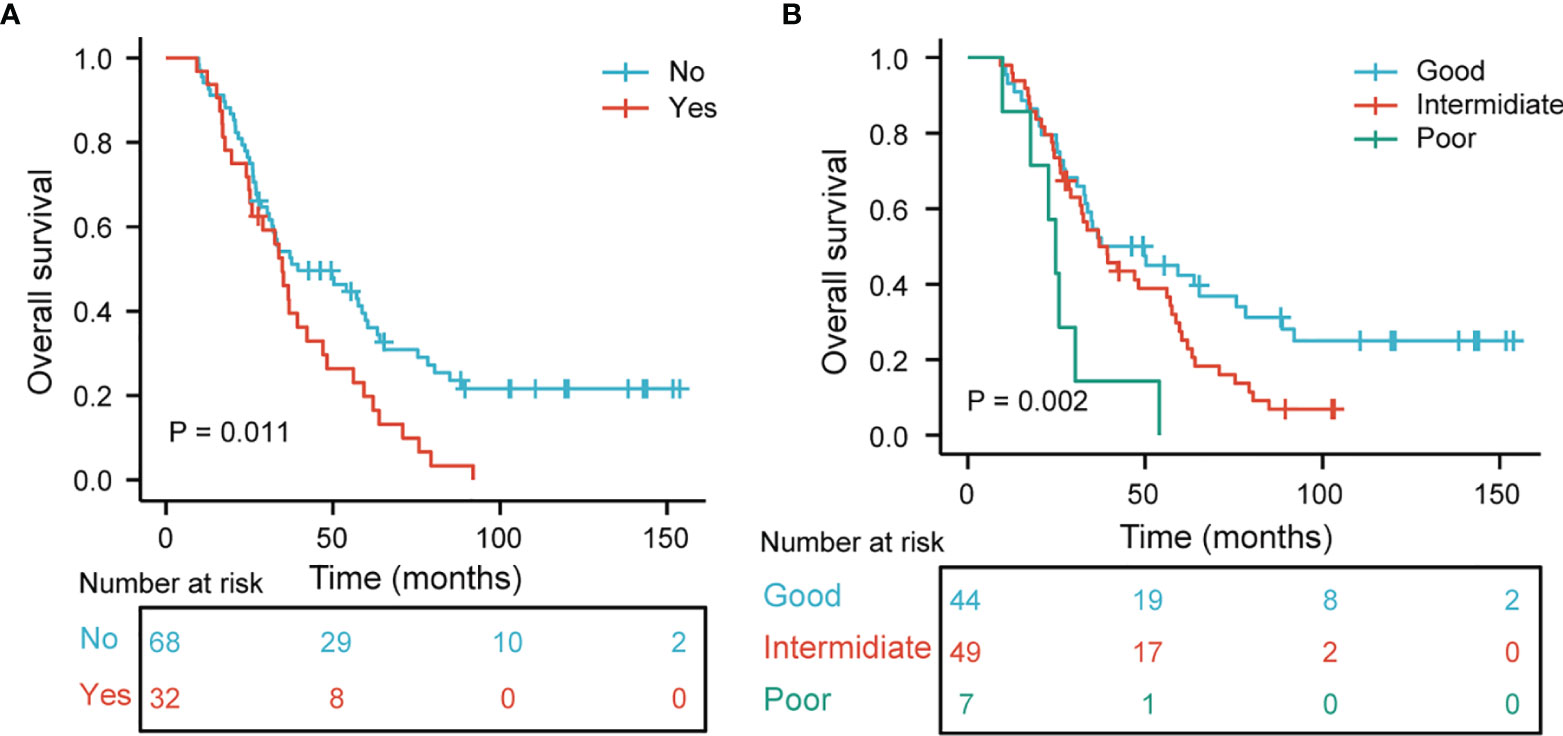
Figure 4 (A) Kaplan–Meier curves and log-ranks tests of overall survival in stage III SCLC patients according to brain metastasis. (B) Overall survival subgroup analysis according to tumor location and MLR (p = 0.002).
OS Stratified by Tumor Location and MLR
We further analyzed the relationship between tumor location and MLR to OS. Patients with the right lung and MLR >0.12 were considered a good group. Patients with a left lung and MLR ≤0.12 were considered the poor group. The remaining combinations were considered the intermediate group. Figure 4B demonstrates that the good group, rather than the intermediate and poor groups, had a better OS (p = 0.002 for all).
Discussion
As an invasive tumor, SCLCs are prominent with high prevalence rates of BM. The current research results have reached a consensus that PCI can reduce the incidence of BM, but whether it can improve the prognosis of patients remains controversial (9, 10). Moreover, a considerable proportion of LS-SCLC patients do not receive PCI treatment because they are worried about side effects. Additionally, the changes in chronic neurotoxicity and quality of life caused by PCI have been reported (18). A retrospective study demonstrated that 44.7% of eligible LS-SCLC patients were without PCI, and the most frequent reason for the recorded omission of PCI was that patients refused to undergo PCI because of potential toxicity (19). The outcomes of SCLC patients with BM are generally poor. If PCI is used to treat specific patients prone to BM, it will improve the prognosis. Therefore, it is helpful for clinicians to improve the treatment strategy of SCLC by identifying the risk of BM in patients who do not receive PCI.
There have been limited studies on the treatment and risk factors in patients with LS-SCLC and BM. There are no reported risk factors for stage III LS-SCLC patients who developed BM after receiving chemoradiotherapy without PCI. In this study, we established and verified a nomogram and risk stratification for predicting the prognosis of stage III LS-SCLC patients who develop BM. The predictors included CCRT, MLR, NLR, and PNI. Different statistical methods verified the model. PI divided the patients into two subgroups, and the high-risk group had worse BMFS than the low-risk group, which also has certain significance for clinical practice in OS. Therefore, this result is helpful for clinicians to improve the treatment management of patients in the high-risk group, such as elective PCI for this group. Besides, the results showed that tumor location and MLR were correlated with OS. In the subgroup analysis of OS, the good group had a better prognosis than the other groups.
CCRT is the main treatment pattern for patients with LS-SCLC. Although CCRT can improve the prognosis, its potential side effects (myelosuppression and radiation-related inflammation responses) cannot be ignored by every clinician (20, 21). Therefore, according to the nutritional status and treatment tolerance condition of each patient, about 47% of the patients in this study received CCRT, and 53% received sequential chemoradiotherapy. Additionally, previous studies have demonstrated that CCRT has better progression-free survival than sequential chemoradiotherapy (SCRT) in SCLC or other cancers, reducing the risk of distant metastasis (22–24). The fundamental principle for uniting chemotherapy and radiotherapy is to integrate the benefits of chemotherapy in reducing the risk of metastatic disease with the benefits of radiotherapy in local-regional control. Interestingly, CCRT has better BMFS than SCRT in our study. CCRT usually has a better response rate than SCRT, chemotherapy, or radiotherapy alone. Farkhad et al. investigated the association between the treatment response of primary tumor and BMFS, and results showed that the incidence of BM in poor responders to chemoradiotherapy (stable disease/local progression) was significantly increased compared with partial and complete responders (25). Clinical trials have demonstrated that distant control can be corrected by improving local treatment (22, 26). Importantly, patients treated with early CCRT had a lower incidence of subsequent BM (27, 28). Therefore, CCRT can directly improve the therapeutic effect of the primary tumor and indirectly reduce the incidence of distant metastasis in patients.
Inflammation and immune response play a crucial role in tumor development, immune surveillance, and therapeutic response (29–31). Some inflammatory indexes associated with blood components promote cancer progression, such as NLR and platelets (32, 33). Furthermore, two previous studies have reported that high NLR was associated with BM in SCLC patients without PCI (17, 34). This conclusion is consistent with our results that high NLR and low MLR have poor BMFS. The plausible explanation is that the tumor response mediated by increased neutrophils and decreased monocytes may play a role. From a clinical perspective, cancer therapy can trigger a significant tumor-associated inflammatory response. For example, chemoradiotherapy can cause massive necrosis of the surrounding tissues and cancer cells, leading to an inflammatory response. Treatment-induced inflammation may have a tumor-promoting effect (35). Non-steroidal anti-inflammatory drugs reduce cancer risk (36) and may prevent tumor metastasis (37).
Our study found that MLR and tumor location were closely related to OS. The myeloid lineage cells (monocytes, macrophages, and neutrophils) gradually accumulate in tumors, where they construct an inflammatory tumor microenvironment (31). It has been suggested that tumor-associated macrophages, which result from monocytic precursors, play a crucial role in the inflammatory microenvironment of cancer progression (38). In many studies (39, 40), MLR represents relative levels of monocytes and lymphocytes in the peripheral blood, and its prognostic value has been observed. Although some studies have shown that tumor location impacts prognosis, different studies have different classification standards for tumor location. Hyun et al. demonstrated that upper lobe tumors were related to better survival than lower lobe tumors (41). Yang et al. reported that the primary tumor site had a significant influence on lung adenocarcinoma prognosis, with the main bronchus site associated with poorer prognosis and more lymph node involvement (42). Li et al. showed that the left lung had a poorer outcome than the right lung, but the difference was statistically insignificant (p = 0.071) (43). Interestingly, this is consistent with our finding that the left lung has a worse prognosis than the right lung. However, the biological mechanisms underlying the relationship between tumor location and prognosis remain unclear. There is an association between the lung tumor site and lymph node metastasis. Tsuguo et al. examined lymph node metastasis in 1,815 patients with lung cancer. They found that lymph node metastasis in patients with left lung cancer was significantly higher than in patients with right lung cancer (44). It is well known that the higher the lymph node metastasis rate of lung cancer, the worse the prognosis. Another theory is that the same treatment could have different effects depending on the location of the tumor. The left lung is anatomically adjacent to the heart, so clinicians have to consider the effects of dose and other factors on the heart when designing and delineating target volume. Further multicenter, prospective studies are needed to confirm our results and elucidate the underlying biological mechanisms.
The limitations of this study must be acknowledged. First, this was a retrospective study of one institution, which had its limitations, such as inevitable selection bias. Secondly, the number of patients enrolled in this study was relatively small. Thirdly, our study was limited to patients with stage III SCLC. Since patients with stage III SCLC account for most of the data, we excluded patients in other stages to reduce the differences between groups caused by staging. Whether other stages can reach the same conclusion is debatable. Further validation is required in a large cohort of patients. Additionally, some known prognostic factors of LS-SCLC, such as tumor markers and tumor size, were not incorporated into the study. Despite these limitations, this study first developed a nomogram model to predict the development of BM in stage III SCLC patients.
Conclusion
We developed a nomogram and corresponding risk stratification for predicting BMFS in stage III SCLC patients. Various statistical methods to prove that the model had satisfactory performance. According to the risk stratification system, the high-risk group had a worse BMFS than the low-risk group, which also has certain practical significance for clinical practice in OS. This model and risk stratification can help clinicians improve patient treatment management and better deliver personalized therapy. Of course, further studies must confirm this model and the prognostic index for BMFS in stage III SCLC.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Author Contributions
JL and JQ designed this study. JQ and DK contributed to the data collection. HaL, HuL, and YY analyzed the data. JL supervised the study. JQ, DK, HZ, HaL, YY, QZ, LL, HuL, YW, ZW, and TL wrote the manuscript. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The project was supported by the National Clinical Key Specialty Construction Program and Fujian provincial Clinical Research Center for Cancer Radiotherapy and Immunotherapy (Grant number 2020Y2012).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank all the researchers and patients, and also thank JL for his work, which greatly improved the quality of this study.
Abbreviations
SCLC, small cell lung cancer; CRT, chemotherapy; LS-SCLC, limited-stage small cell lung cancer; TRT, thoracic radiotherapy; BM, brain metastasis; PCI, prophylactic cranial irradiation (PCI); OS, overall survival; NLR, neutrophil-lymphocyte ratio; PLR, platelet-lymphocyte ratio. CT, computed tomography; MRI, magnetic resonance imaging (MRI); PET/CT, position emission tomography/computerized tomography; AJCC, American joint commission on cancer; NCCN, national comprehensive cancer network guidelines; 3D-CRT, 3-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; CFRT, conventional fractionated radiotherapy; HFRT, hyper-fractionated radiotherapy; RT, radiotherapy; GTV, gross tumor volume; CTV, clinical tumor volume; PTV, planned tumor volume; SII, systemic immune-inflammation index; MLR, monocyte-lymphocyte ratio; PNI, prognostic-nutrition index; PAR, platelets-albumin ratio; BMFS, brain metastasis free survival; PI, prognostic index; ROC, receiver operating characteristic; CCRT, concurrent chemotherapy; AUC, the area under the curve; SCRT, sequential chemoradiotherapy.
References
1. Gazdar AF, Bunn PA, Minna JD. Small-Cell Lung Cancer: What We Know, What We Need to Know and the Path Forward. Nat Rev Cancer (2017) 17(12):725–37. doi: 10.1038/nrc.2017.87
2. Jackman DM, Johnson BE. Small-Cell Lung Cancer. Lancet (2005) 366(9494):1385–96. doi: 10.1016/S0140-6736(05)67569-1
3. Socinski MA, Bogart JA. Limited-Stage Small-Cell Lung Cancer: The Current Status of Combined-Modality Therapy. J Clin Oncol (2007) 25(26):4137–45. doi: 10.1200/JCO.2007.11.5303
4. Altan M, Chiang AC. Management of Small Cell Lung Cancer: Progress and Updates. Cancer J (2015) 21(5):425–33. doi: 10.1097/PPO.0000000000000148
5. Arriagada R, Le Chevalier T, Borie F, Riviere A, Chomy P, Monnet I, et al. Prophylactic Cranial Irradiation for Patients With Small-Cell Lung Cancer in Complete Remission. J Natl Cancer Inst (1995) 87(3):183–90. doi: 10.1093/jnci/87.3.183
6. Hirsch FR, Paulson OB, Hansen HH, Vraa-Jensen J. Intracranial Metastases in Small Cell Carcinoma of the Lung: Correlation of Clinical and Autopsy Findings. Cancer (1982) 50(11):2433–7. doi: 10.1002/1097-0142(19821201)50:11<2433::aid-cncr2820501131>3.0.co;2-e
7. Nugent JL, Bunn PA Jr., Matthews MJ, Ihde DC, Cohen MH, Gazdar A, et al. CNS Metastases in Small Cell Bronchogenic Carcinoma: Increasing Frequency and Changing Pattern With Lengthening Survival. Cancer (1979) 44(5):1885–93. doi: 10.1002/1097-0142(197911)44:5<1885::aid-cncr2820440550>3.0.co;2-f
8. Suwinski R. Prophylactic Cranial Irradiation in SCLC. Transl Lung Cancer Res (2021) 10(4):2071–8. doi: 10.21037/tlcr-2020-rtm-05
9. Takahashi T, Yamanaka T, Seto T, Harada H, Nokihara H, Saka H, et al. Prophylactic Cranial Irradiation Versus Observation in Patients With Extensive-Disease Small-Cell Lung Cancer: A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2017) 18(5):663–71. doi: 10.1016/S1470-2045(17)30230-9
10. Putora PM, Fischer GF, Fruh M, Califano R, Faivre-Finn C, Van Houtte P, et al. Treatment of Brain Metastases in Small Cell Lung Cancer: Decision-Making Amongst a Multidisciplinary Panel of European Experts. Radiother Oncol (2020) 149:84–8. doi: 10.1016/j.radonc.2020.04.015
11. Giordano FA, Welzel G, Abo-Madyan Y, Wenz F. Potential Toxicities of Prophylactic Cranial Irradiation. Transl Lung Cancer Res (2012) 1(4):254–62. doi: 10.3978/j.issn.2218-6751.2012.10.03
12. van Oosterhout AG, van de Pol M, ten Velde GP, Twijnstra A. Neurologic Disorders in 203 Consecutive Patients With Small Cell Lung Cancer. Results of a Longitudinal Study. Cancer (1996) 77(8):1434–41. doi: 10.1002/(SICI)1097-0142(19960415)77:8<1434::AID-CNCR3>3.0.CO;2-C
13. Quan AL, Videtic GM, Suh JH. Brain Metastases in Small Cell Lung Cancer. Oncol (Williston Park) (2004) 18(8):961–72; discussion 974, 979-80, 987.
14. Seute T, Leffers P, ten Velde GP, Twijnstra A. Neurologic Disorders in 432 Consecutive Patients With Small Cell Lung Carcinoma. Cancer (2004) 100(4):801–6. doi: 10.1002/cncr.20043
15. Cho O, Oh YT, Chun M, Noh OK, Lee HW. Radiation-Related Lymphopenia as a New Prognostic Factor in Limited-Stage Small Cell Lung Cancer. Tumour Biol (2016) 37(1):971–8. doi: 10.1007/s13277-015-3888-y
16. Xie D, Marks R, Zhang M, Jiang G, Jatoi A, Garces YI, et al. Nomograms Predict Overall Survival for Patients With Small-Cell Lung Cancer Incorporating Pretreatment Peripheral Blood Markers. J Thorac Oncol (2015) 10(8):1213–20. doi: 10.1097/JTO.0000000000000585
17. Zheng Y, Wang L, Zhao W, Dou Y, Lv W, Yang H, et al. Risk Factors for Brain Metastasis in Patients With Small Cell Lung Cancer Without Prophylactic Cranial Irradiation. Strahlenther Onkol (2018) 194(12):1152–62. doi: 10.1007/s00066-018-1362-7
18. Wolfson AH, Bae K, Komaki R, Meyers C, Movsas B, Le Pechoux C, et al. Primary Analysis of a Phase II Randomized Trial Radiation Therapy Oncology Group (RTOG) 0212: Impact of Different Total Doses and Schedules of Prophylactic Cranial Irradiation on Chronic Neurotoxicity and Quality of Life for Patients With Limited-Disease Small-Cell Lung Cancer. Int J Radiat Oncol Biol Phys (2011) 81(1):77–84. doi: 10.1016/j.ijrobp.2010.05.013
19. Lok BH, Ma J, Foster A, Perez CA, Shi W, Zhang Z, et al. Factors Influencing the Utilization of Prophylactic Cranial Irradiation in Patients With Limited-Stage Small Cell Lung Cancer. Adv Radiat Oncol (2017) 2(4):548–54. doi: 10.1016/j.adro.2017.08.001
20. Wang Z, Wan J, Liu C, Li L, Dong X, Geng H. Sequential Versus Concurrent Thoracic Radiotherapy in Combination With Cisplatin and Etoposide for N3 Limited-Stage Small-Cell Lung Cancer. Cancer Control (2020) 27(1):1073274820956619. doi: 10.1177/1073274820956619
21. Huang EX, Bradley JD, El Naqa I, Hope AJ, Lindsay PE, Bosch WR, et al. Modeling the Risk of Radiation-Induced Acute Esophagitis for Combined Washington University and RTOG Trial 93-11 Lung Cancer Patients. Int J Radiat Oncol Biol Phys (2012) 82(5):1674–9. doi: 10.1016/j.ijrobp.2011.02.052
22. Takada M, Fukuoka M, Kawahara M, Sugiura T, Yokoyama A, Yokota S, et al. Phase III Study of Concurrent Versus Sequential Thoracic Radiotherapy in Combination With Cisplatin and Etoposide for Limited-Stage Small-Cell Lung Cancer: Results of the Japan Clinical Oncology Group Study 9104. J Clin Oncol (2002) 20(14):3054–60. doi: 10.1200/JCO.2002.12.071
23. Auperin A, Le Pechoux C, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-Analysis of Concomitant Versus Sequential Radiochemotherapy in Locally Advanced Non-Small-Cell Lung Cancer. J Clin Oncol (2010) 28(13):2181–90. doi: 10.1200/JCO.2009.26.2543
24. O'Rourke N, Roque IFM, Farre Bernado N, Macbeth F. Concurrent Chemoradiotherapy in Non-Small Cell Lung Cancer. Cochrane Database Syst Rev (2010) 6):CD002140. doi: 10.1002/14651858.CD002140.pub3
25. Manapov F, Klocking S, Niyazi M, Levitskiy V, Belka C, Hildebrandt G, et al. Primary Tumor Response to Chemoradiotherapy in Limited-Disease Small-Cell Lung Cancer Correlates With Duration of Brain-Metastasis Free Survival. J Neurooncol (2012) 109(2):309–14. doi: 10.1007/s11060-012-0894-4
26. Jeremic B, Shibamoto Y, Acimovic L, Milisavljevic S. Initial Versus Delayed Accelerated Hyperfractionated Radiation Therapy and Concurrent Chemotherapy in Limited Small-Cell Lung Cancer: A Randomized Study. J Clin Oncol (1997) 15(3):893–900. doi: 10.1200/JCO.1997.15.3.893
27. Coy P, Hodson DI, Murray N, Pater JL, Payne DG, Arnold A, et al. Patterns of Failure Following Loco-Regional Radiotherapy in the Treatment of Limited Stage Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys (1994) 28(2):355–62. doi: 10.1016/0360-3016(94)90058-2
28. Murray N, Coy P, Pater JL, Hodson I, Arnold A, Zee BC, et al. Importance of Timing for Thoracic Irradiation in the Combined Modality Treatment of Limited-Stage Small-Cell Lung Cancer. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol (1993) 11(2):336–44. doi: 10.1200/JCO.1993.11.2.336
29. Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell (2010) 140(6):883–99. doi: 10.1016/j.cell.2010.01.025
30. Mittal V, El Rayes T, Narula N, McGraw TE, Altorki NK, Barcellos-Hoff MH. The Microenvironment of Lung Cancer and Therapeutic Implications. Adv Exp Med Biol (2016) 890:75–110. doi: 10.1007/978-3-319-24932-2_5
31. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-Related Inflammation. Nature (2008) 454(7203):436–44. doi: 10.1038/nature07205
32. Placke T, Orgel M, Schaller M, Jung G, Rammensee HG, Kopp HG, et al. Platelet-Derived MHC Class I Confers a Pseudonormal Phenotype to Cancer Cells That Subverts the Antitumor Reactivity of Natural Killer Immune Cells. Cancer Res (2012) 72(2):440–8. doi: 10.1158/0008-5472.CAN-11-1872
33. Sitia G, Aiolfi R, Di Lucia P, Mainetti M, Fiocchi A, Mingozzi F, et al. Antiplatelet Therapy Prevents Hepatocellular Carcinoma and Improves Survival in a Mouse Model of Chronic Hepatitis B. Proc Natl Acad Sci U S A (2012) 109(32):E2165–72. doi: 10.1073/pnas.1209182109
34. Chung JH, Kang SY, Wu HG, Seo YS, Kim DW, Kang KW, et al. Risk Stratification of Symptomatic Brain Metastases by Clinical and FDG PET Parameters for Selective Use of Prophylactic Cranial Irradiation in Patients With Extensive Disease of Small Cell Lung Cancer. Radiother Oncol (2020) 143:81–7. doi: 10.1016/j.radonc.2020.01.009
35. Vakkila J, Lotze MT. Inflammation and Necrosis Promote Tumour Growth. Nat Rev Immunol (2004) 4(8):641–8. doi: 10.1038/nri1415
36. Rothwell PM, Fowkes FG, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of Daily Aspirin on Long-Term Risk of Death Due to Cancer: Analysis of Individual Patient Data From Randomised Trials. Lancet (2011) 377(9759):31–41. doi: 10.1016/S0140-6736(10)62110-1
37. Rothwell PM, Wilson M, Price JF, Belch JF, Meade TW, Mehta Z. Effect of Daily Aspirin on Risk of Cancer Metastasis: A Study of Incident Cancers During Randomised Controlled Trials. Lancet (2012) 379(9826):1591–601. doi: 10.1016/S0140-6736(12)60209-8
38. Galdiero MR, Garlanda C, Jaillon S, Marone G, Mantovani A. Tumor Associated Macrophages and Neutrophils in Tumor Progression. J Cell Physiol (2013) 228(7):1404–12. doi: 10.1002/jcp.24260
39. Ni XJ, Zhang XL, Ou-Yang QW, Qian GW, Wang L, Chen S, et al. An Elevated Peripheral Blood Lymphocyte-to-Monocyte Ratio Predicts Favorable Response and Prognosis in Locally Advanced Breast Cancer Following Neoadjuvant Chemotherapy. PLoS One (2014) 9(11):e111886. doi: 10.1371/journal.pone.0111886
40. Hu P, Shen H, Wang G, Zhang P, Liu Q, Du J. Prognostic Significance of Systemic Inflammation-Based Lymphocyte- Monocyte Ratio in Patients With Lung Cancer: Based on a Large Cohort Study. PLoS One (2014) 9(9):e108062. doi: 10.1371/journal.pone.0108062
41. Lee HW, Lee CH, Park YS. Location of Stage I-III Non-Small Cell Lung Cancer and Survival Rate: Systematic Review and Meta-Analysis. Thorac Cancer (2018) 9(12):1614–22. doi: 10.1111/1759-7714.12869
42. Yang L, Wang S, Gerber DE, Zhou Y, Xu F, Liu J, et al. Main Bronchus Location Is a Predictor for Metastasis and Prognosis in Lung Adenocarcinoma: A Large Cohort Analysis. Lung Cancer (2018) 120:22–6. doi: 10.1016/j.lungcan.2018.03.011
43. Li C, Liu J, Lin J, Li Z, Shang X, Wang H. Poor Survival of Non-Small-Cell Lung Cancer Patients With Main Bronchus Tumor: A Large Population-Based Study. Future Oncol (2019) 15(24):2819–27. doi: 10.2217/fon-2019-0098
Keywords: small cell lung cancer, brain metastasis, risk factor, risk stratification, nomogram
Citation: Qiu J, Ke D, Yu Y, Lin H, Zheng Q, Li H, Zheng H, Liu L, Wang Z, Wu Y, Liu T and Li J (2022) A New Nomogram and Risk Stratification of Brain Metastasis by Clinical and Inflammatory Parameters in Stage III Small Cell Lung Cancer Without Prophylactic Cranial Irradiation. Front. Oncol. 12:882744. doi: 10.3389/fonc.2022.882744
Received: 24 February 2022; Accepted: 27 May 2022;
Published: 07 July 2022.
Edited by:
Alessio Bruni, University Hospital of Modena, ItalyReviewed by:
Chengzhi Zhou, National Respiratory Medical Center, ChinaNagla Abdel Karim, Augusta University, United States
Copyright © 2022 Qiu, Ke, Yu, Lin, Zheng, Li, Zheng, Liu, Wang, Wu, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiancheng Li, jianchengli_jack@126.com
 Jianjian Qiu
Jianjian Qiu Yilin Yu
Yilin Yu Qunhao Zheng
Qunhao Zheng Zhiping Wang
Zhiping Wang Yahua Wu
Yahua Wu Jiancheng Li
Jiancheng Li