- 1Department of Obstetrics and Gynecology, Jiaxing University Affiliated Maternity and Child Hospital, Jiaxing, China
- 2Department of Gynecology, Women’s Hospital, School of Medicine, Zhejiang University, Hangzhou, China
Background: Myometrial invasion (MI), lymphovascular space invasion (LVSI), and lymph node metastasis (LNM) have been found to have independent prognostic factors in endometrial cancer. Tumor size has practical advantages in endometrial cancer. The cutoff values for tumor size conformed with current literature. More and more studies inferred that tumor size >20 mm showed a strong correlation. However, the relationship between tumor size >20 mm and MI, LVSI, LNM, recurrence, and overall survival (OS) remains controversial, and no meta-analysis has been conducted. Therefore, a systematic review and meta-analysis should be performed to discuss this issue later on.
Methods: Relevant articles were collected from PubMed, EMBASE, and Cochrane Library databases from January 1990 to June 2021. The predictive value of tumor size >20 mm in endometrial cancer was studied, and data were pooled for meta-analysis using Review Manager 5.1. Additionally, the odds ratio (OR) was analyzed, and cumulative analyses of hazard ratio (HR) and their corresponding 95% CI were conducted.
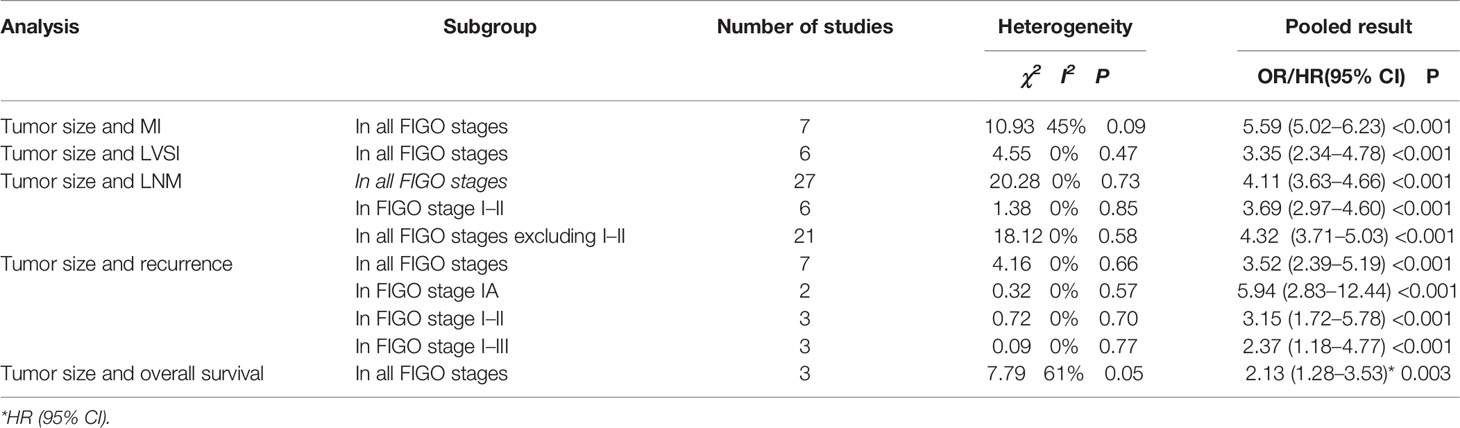
Results: A total of 40 articles with 53,276 endometrial cancer patients were included in the meta-analysis. It contained 7 articles for MI, 6 for LVSI, 21 for LNM, 7 for recurrence, and 3 for OS. Primary tumor size >20 mm was significantly associated with depth of MI (OR = 5.59, 95% CI [5.02, 6.23], p < 0.001), positive LVSI (OR = 3.35, 95% CI [2.34, 4.78], p < 0.001), positive LNM (OR = 4.11, 95% CI [3.63, 4.66], p < 0.001), and recurrence (OR = 3.52, 95% CI [2.39, 5.19], p < 0.001). Tumor size >20 mm was also related to OS via meta-synthesis of HR in univariate survival (HR 2.13, 95% CI [1.28, 3.53], p = 0.003). There was no significant publication bias in this study by funnel plot analysis.
Conclusion: Primary tumor size >20 mm was an independent predictive factor for the depth of MI, positive LVSI, positive LNM, recurrence, and poor OS. Therefore, it is more important to take into account the value of tumor size in the clinicopathological staging of endometrial carcinoma. Tumor size >20 mm should be integrated into the intraoperative algorithm for performing a full surgical staging. Well-designed and multicenter studies, with a larger sample size, are still required to verify the findings.
Introduction
Endometrial cancer is the sixth most common neoplasm in women worldwide, and the incidence rate is increasing rapidly (1). The International Federation of Gynecology and Obstetrics (FIGO) mandated that the treatment of endometrial cancer was surgical staging, which includes hysterectomy, bilateral salpingo-oophorectomy, or pelvic and para-aortic lymphadenectomy (2). A gynecologic oncology group study identified some risk factors, such as stage, histological subtype, depth of myometrial invasion (MI), lymphovascular space invasion (LVSI), grade, and lymph node metastasis (LNM), which could predict recurrence and survival (3).
A gynecologic oncology group study in 1987 proposed that primary tumor size was not considered a risk factor for lymphatic metastasis (4). Some published studies indicated tumor size was not a risk associated with recurrence in women with endometrial cancer (5, 6). However, other literature showed that tumor size seemed to be a significant risk factor for endometrial cancer (7, 8). Recent data suggested that primary tumor size was an important parameter in predicting the clinicopathological outcomes for endometrial cancer patients, but it seemed to be controversial. Gusberg et al. firstly implied that it came out to be a poor prognosis with a tumor size of >10 cm (9). Riggs et al. analyzed the optimal tumor diameter that can predict LNM and was noted to be 35 mm (10). The Mayo Criteria, which included the FIGO grade 1 or 2 endometrioid cancer, with tumor size <20 mm, MI < 50%, and no intraoperative evidence of macroscopic disease, was used to guide lymphadenectomy assessment (11). Milwaukee Model suggested that primary tumor size >50 mm and MI > 33% identifies possible lymphatic dissemination in low-risk endometrial cancer patients (12). The cutoff values for tumor size conformed with current literature, which varies from 20 to 50 mm (12, 13). Kilt et al. explored that cutoff of tumor size increasing from 20 to 30 and 50 mm had a lower at-risk rate of lymph node dissection but an unacceptably high false-negative rate (14). Tumor sizes <20 mm for low-risk endometrial cancer remained more sensitive than those with tumor sizes <30 mm for identifying lymphatic dissemination (14). Recently, more and more studies inferred that a tumor size of 20 mm remains clinically significant in relation to the risk of recurrence (7, 8). Therefore, we should focus on the relationship between the tumor size of 20 mm and MI, LVSI, LNM, recurrence, and OS.
There was no meta-analysis about the relationship between tumor size >20 mm and MI, LVSI, LNM, recurrence, and OS. The aim of our study was to investigate the relationship between primary tumor size of 20 mm and clinicopathological parameters, recurrence, and OS.
Methods
Literature Search Strategy
A rigorous search of the PubMed, EMBASE, and Cochrane Library databases from January 1990 to June 2021 was undertaken to identify relevant articles. The key search terms were drafted as follows: “tumor size,” “tumor diameter,” “uterine cancer,” “uterine carcinoma,” “endometrial cancer,” “endometrial carcinoma,” “prognosis,” “prognostic factor,” “risk,” “myometrial invasion,” “lymphovascular space invasion,” “lymph node metastasis,” “recurrence,” and “overall survival.” The literature search was performed by two authors independently.
Criteria for Inclusion and Exclusion
The inclusion criteria included the following: 1) the patients were only diagnosed with endometrial cancer; 2) tumor size, which was defined as a cutoff of 20 mm; 3) one or more main clinicopathological factors included MI, LVSI, LNM, recurrence, and OS; and 4) article was published in English. The exclusion criteria included the following terms: 1) letters, editorials, expert opinions, reviews, and animal studies; 2) preoperative tumor size at MRI and PET/CT or ultrasound; and 3) studies of data were insufficient.
Data Extraction
The data from the selected trials were extracted and assessed by two authors independently. Any disagreements in data extraction were resolved by further discussion and consensus. Three categories of data extraction in each study are the following: baseline patient characteristics, clinicopathological outcomes, and survival outcomes. Baseline characteristics of the included studies need the first author’s name, study publication year, country, and sample size. Clinicopathological outcomes included MI, LVSI, and LNM. Survival outcomes included recurrence and OS.
Data Analysis
All statistical analyses were performed by using the Cochrane Collaboration’s Review Manager Software 5.1. Clinicopathological outcomes and recurrence were pooled as odds ratio (OR) and 95% CI. Pooled hazard ratio (HR) and corresponding 95% CI were used to analyze the association between tumor size and OS. Fixed- or random-effects meta-analysis models were varied according to the existence of heterogeneity among the included studies. It appeared that heterogeneity with chi-square p > 0.1 and/or I2 > 50%, publication bias was evaluated by the shape of the funnel plot. The test for funnel plot asymmetry was applied only when at least 10 studies were included in a meta-analysis. A significant statistical difference was pointed out when a p-value was less than 0.05.
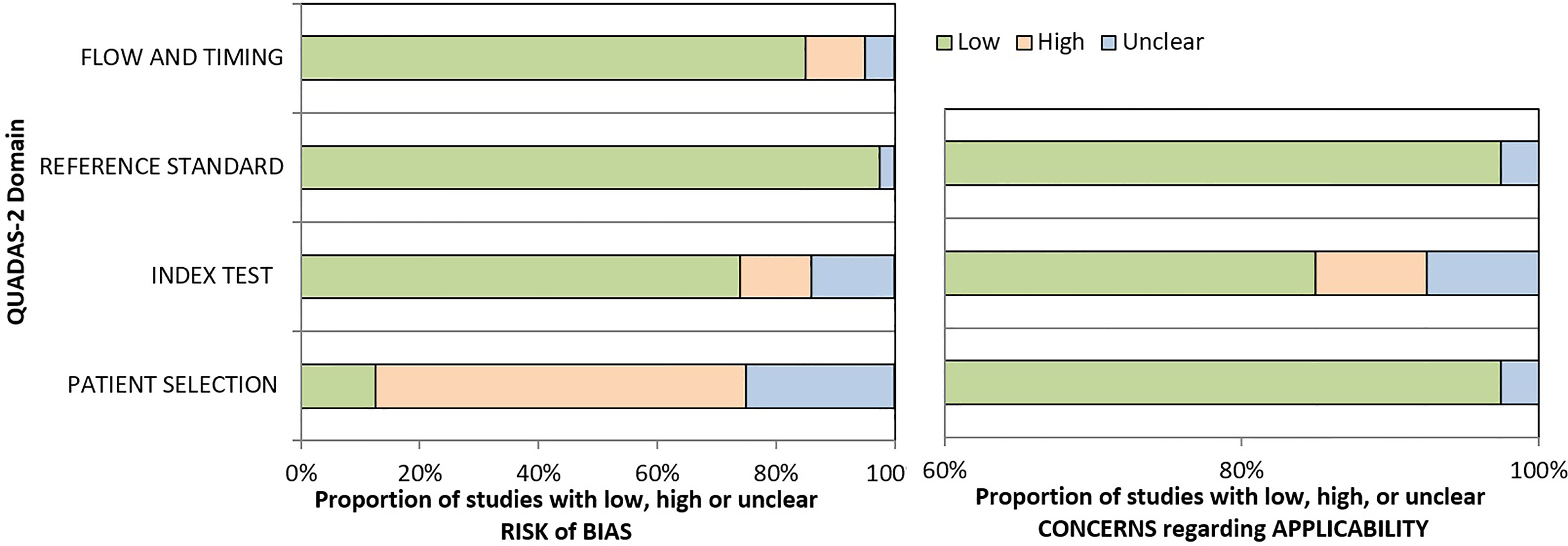
The quality of the included studies was assessed by the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2), which is essential to evaluate the risk of bias for included studies.
Results
Study Characteristics
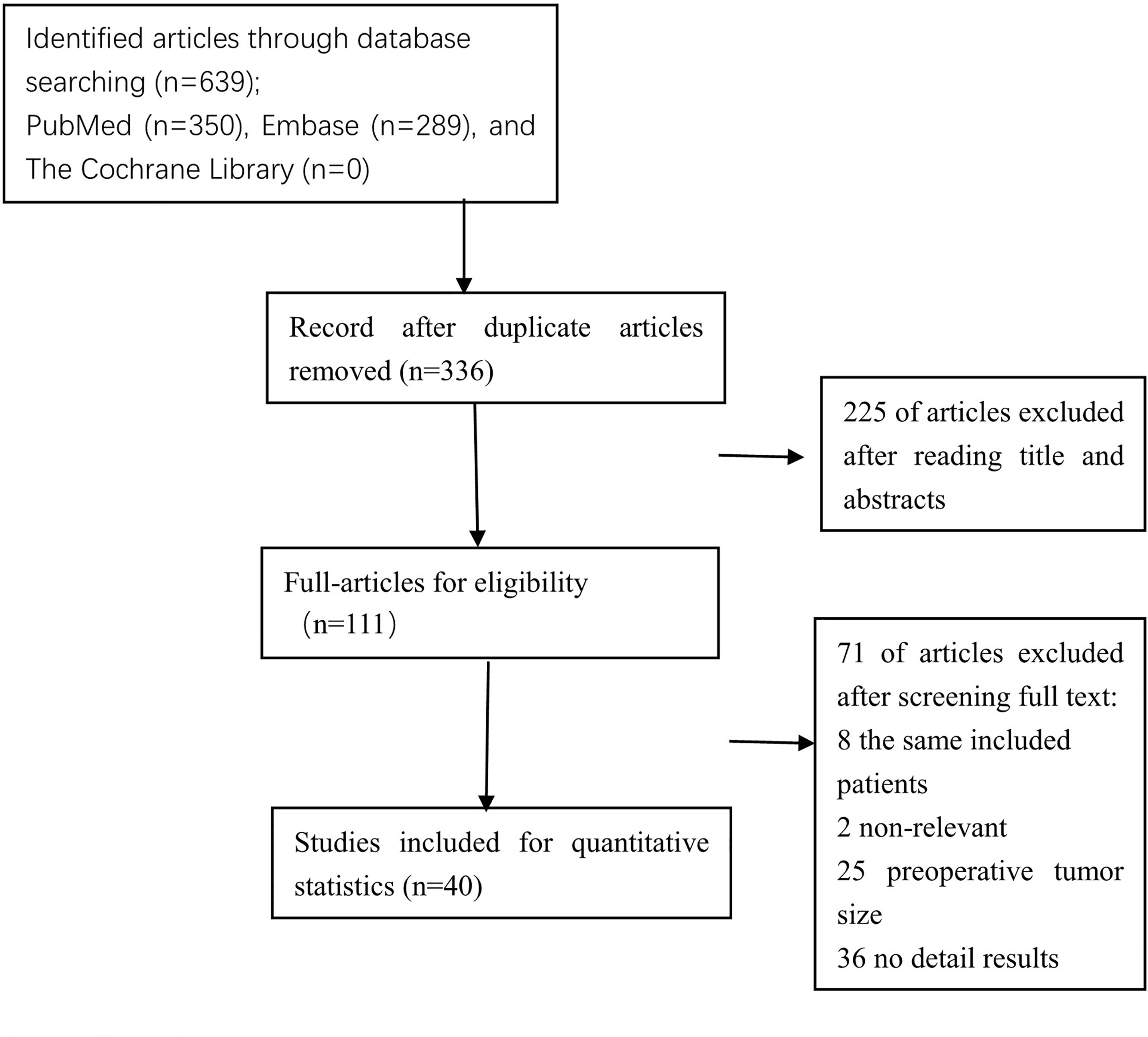
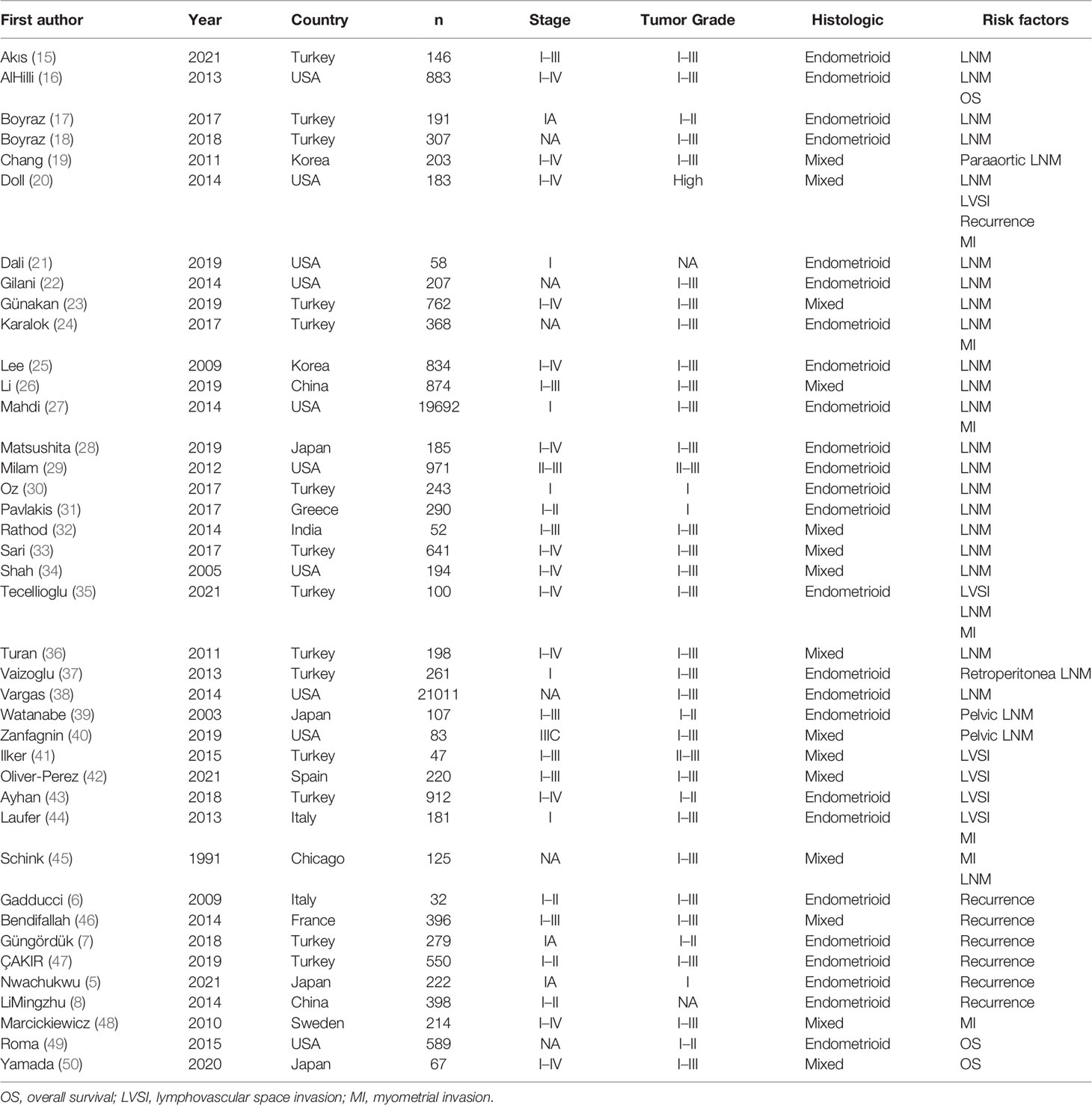
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram was shown in Figure 1. After titles and abstracts were screened, 225 records were excluded, including 97 that indicated that the cutoff tumor size was not 20 mm, 100 that indicated the preoperative tumor size, 21 without original data, and 7 without relevant outcome. A full text of 111 articles was assessed, 71 records were excluded, including studies with the same included patients, 2 that indicated HRs from univariate survival analyses not available, 25 that indicated preoperative tumor size, 36 that have no detailed results, and finally, forty studies with a total of 53,276 eligible patients. Baseline characteristics of the included studies are shown in Table 1. All of the included studies were retrospectively designed, including 7 for MI (20, 24, 27, 35, 44, 45, 48), 6 for LVSI (20, 35, 41–44), 27 for LNM (15–40, 45), 7 for recurrence (5–8, 20, 46, 47), and 3 for OS (16, 49, 50). Included studies consisted of 2 large-scale retrospective cohort studies (27, 38). The results of the meta-analysis are summarized in Table 2.
Literature Quality
The QUADAS-2 was used to evaluate the quality of the included studies. Two reviewers independently evaluated the quality of the included 40 studies. The outcome is shown in Figure 2.
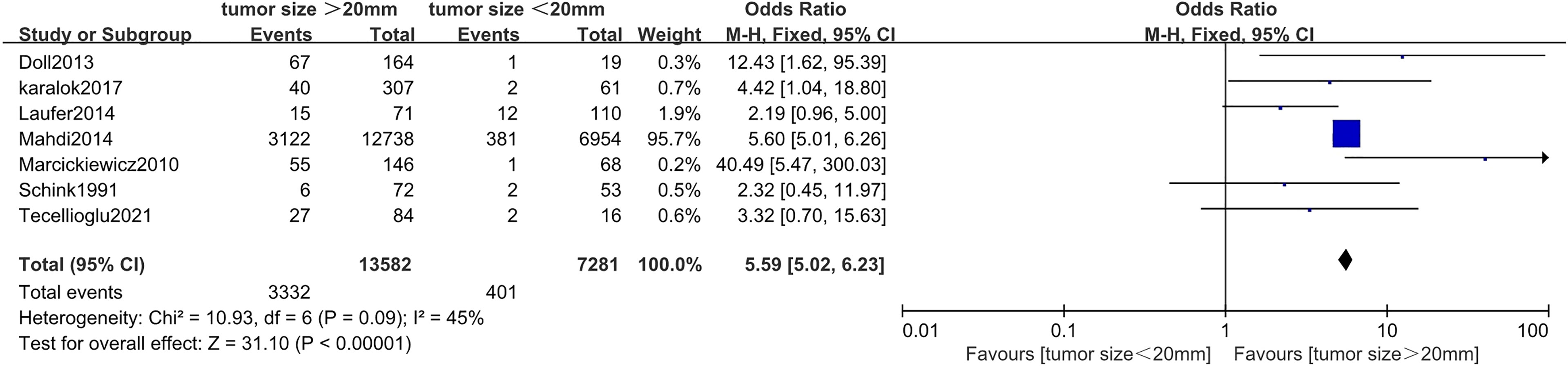
Correlation Between Tumor Size and Myometrial Invasion in Endometrial Cancer
Seven studies (20, 24, 27, 35, 44, 45, 48) including 20,863 endometrial cancer patients were eligible to analyze the association between tumor size and MI in endometrial cancer. Pooled analysis showed that tumor size >20 mm was significantly associated with incidences of depth of MI (>50%) (OR = 5.59, 95% CI [5.02, 6.23], p < 0.001, I2 = 45%, p = 0.09) (Figure 3).
Correlation Between Tumor Size and Lymphovascular Space Invasion in Endometrial Cancer
Six studies (20, 35, 41–44) with a total of 1,643 endometrial cancer patients were included for this analysis. The results of the pooled analysis revealed that tumor size >20 mm was significantly associated with positive LVSI (OR = 3.35, 95% CI [2.34, 4.78], p < 0.001, I2 = 0%, p = 0.47) (Figure 4).
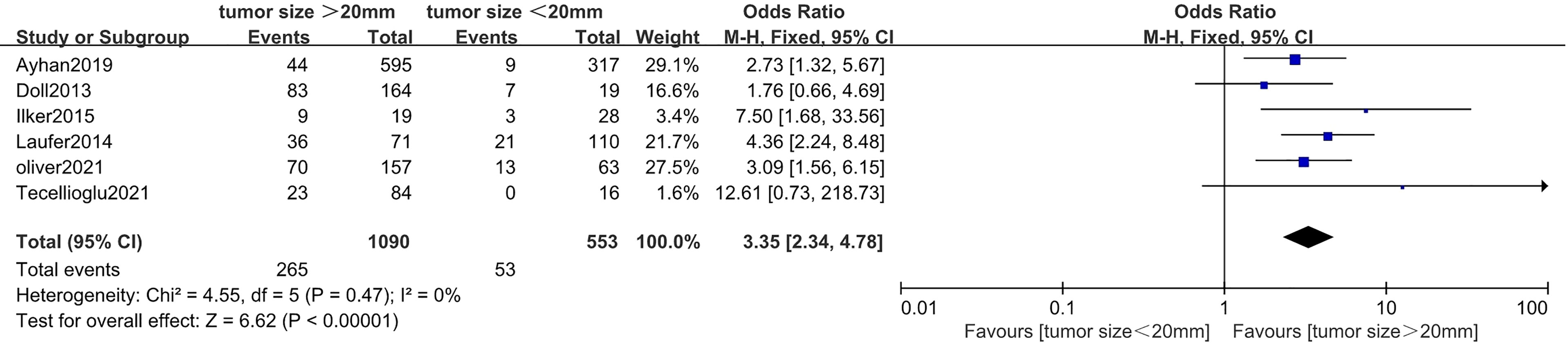
Figure 4 Forest plots showing the correlation between tumor size and lymphovascular space invasion (LVSI).
Correlation Between Tumor Size and Lymph Node Metastasis in Endometrial Cancer
Twenty-seven studies with a total of 49,169 endometrial cancer patients were presented on the debate of association between tumor size and LNM (15–40, 45). The results of the pooled analysis revealed that tumor size >20 mm was significantly associated with LNM (OR = 4.11, 95% CI [3.63, 4.66], p < 0.001, I2 = 0%, p = 0.73). A total of 20,735 patients in FIGO stage I–II endometrial cancer that were based on 6 studies (17, 21, 27, 30, 31, 37) were enrolled in our meta-analysis. The pooled result showed that tumor size >20 mm was correlated with high LNM, and the pooled OR was 3.69 (95% CI [2.97, 4.60], p < 0.001), with heterogeneity (I2 = 0%, p = 0.85). A total of 28,434 patients had FIGO stage III–IV endometrial cancer, based on 21 studies that were enrolled in our meta-analysis (15, 16, 18–20, 22–26, 28, 29, 32–36, 38–40, 45). The pooled result showed that tumor size >20 mm was correlated with high LNM, and the pooled OR was 4.32 (95% CI [3.71, 5.03], p < 0.001), with heterogeneity (I2 = 0%, p = 0.58) (Figure 5).
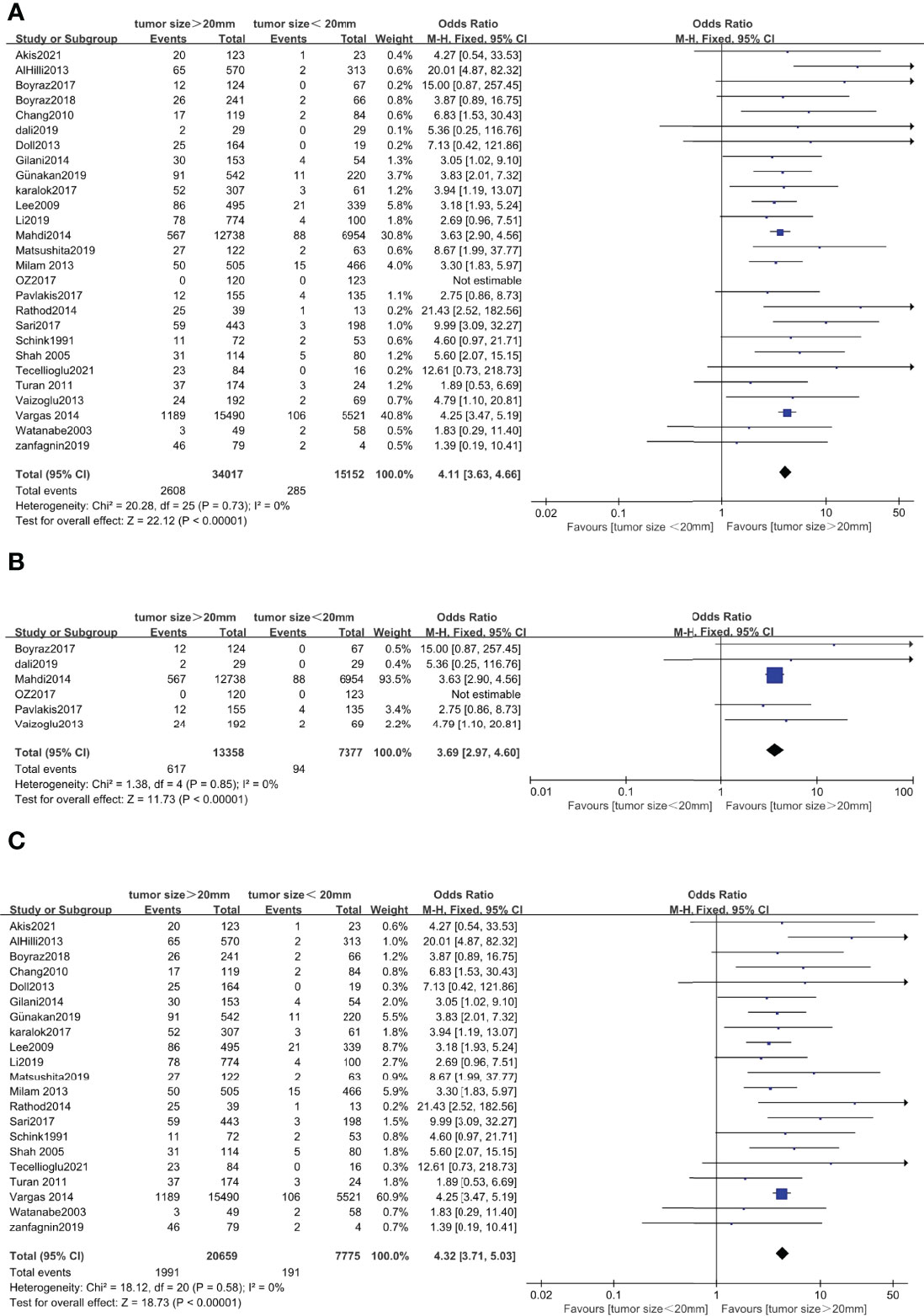
Figure 5 Forest plots showing the correlation between tumor size and lymph node metastasis (LNM). (A) All International Federation of Gynecology and Obstetrics (FIGO) stages. (B) FIGO stage I–II. (C) FIGO stage I–IV excluding stage I–II.
Correlation Between Tumor Size and Recurrence in Endometrial Cancer
Seven studies (5–8, 20, 46, 47) with a total of 2,060 endometrial cancer patients were eligible for analysis of the association between tumor size and recurrence. The pooled analysis revealed that tumor size >20 mm was significantly associated with recurrence (OR = 3.52, 95% CI [2.39, 5.19], p < 0.001, I2 = 0%, p = 0.66). A total of 501 patients in FIGO stage IA endometrial cancer, based on 2 studies, were enrolled in our meta-analysis (5, 7). The pooled result showed that tumor size >20 mm was correlated with high recurrence, and the pooled OR was 5.94 (95% CI [2.83, 12.44], p < 0.001), with heterogeneity (I2 = 0%, p = 0.57). A total of 980 patients in FIGO stage I–II endometrial cancer, based on 3 studies, were enrolled in our meta-analysis (6, 8, 47). The pooled result showed that tumor size >20 mm was also correlated with high recurrence, and OR was 3.15 (95% CI [1.72, 5.78], p < 0.001), with heterogeneity (I2 = 0%, p = 0.70). A total of 579 patients in FIGO stage I–III endometrial cancer, based on 2 studies, were enrolled in our meta-analysis (20, 46). The pooled result showed that tumor size >20 mm was also correlated with high recurrence, and OR was 2.37 (95% CI [1.18, 4.77], p < 0.001), with heterogeneity (I2 = 0%, p = 0.77) (Figure 6).
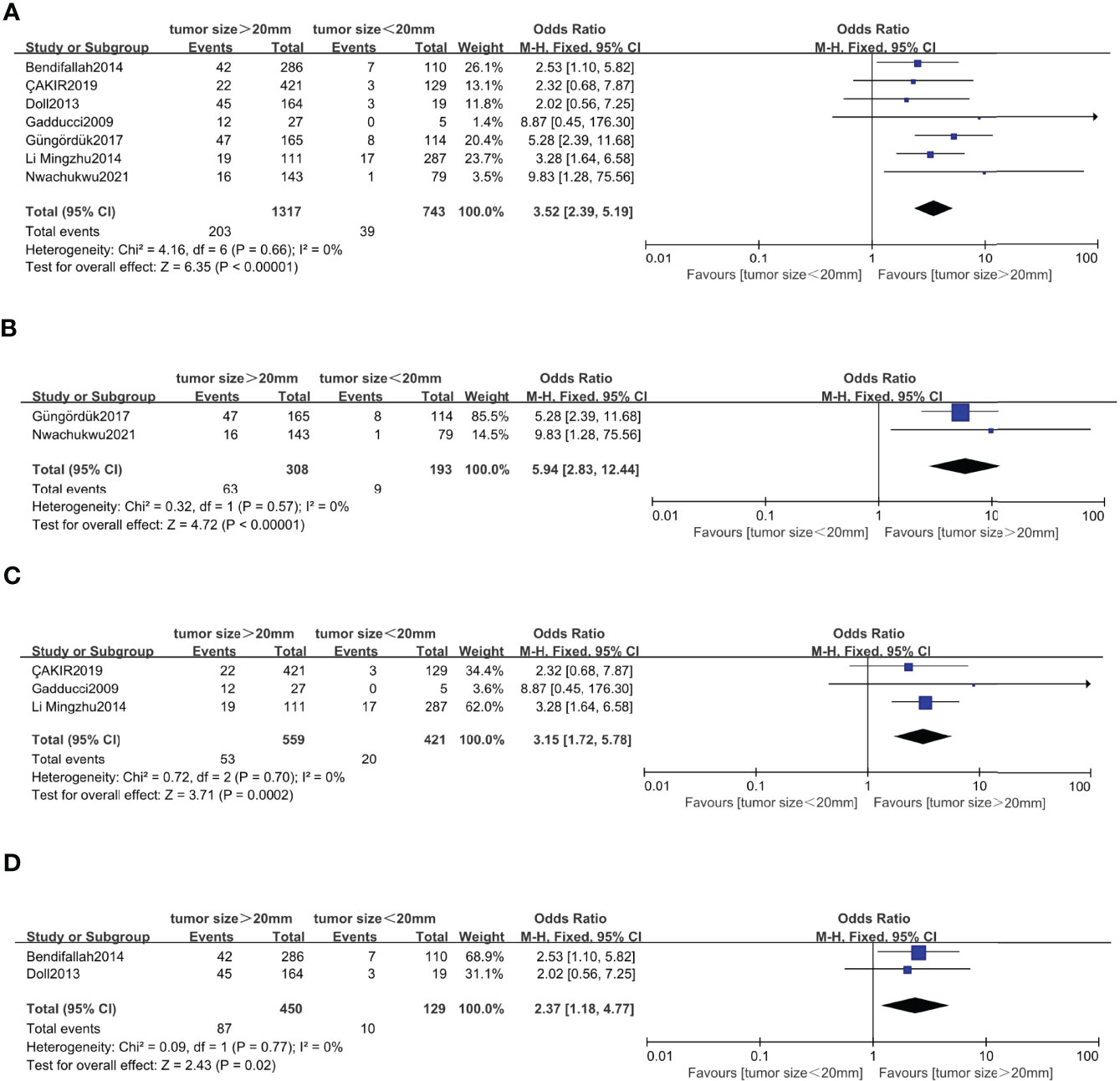
Figure 6 Forest plots showing the correlation between tumor size and recurrence. (A) All International Federation of Gynecology and Obstetrics (FIGO) stage. (B) FIGO IA. (C) FIGO stage I–II. (D) FIGO stage I–III.
Correlation Between Tumor Size and Overall Survival in Endometrial Cancer
Three studies (16, 49, 50) with a total number of 1,937 endometrial cancer patients were presented on the debate of tumor size >20 mm and OS. The random-effects model was applied for the significant heterogeneity. The pooled HRs of OS for univariate analyses were 2.13 (95% CI [1.28, 3.53], p = 0.003), with heterogeneity (I2 = 61%, p = 0.05) (Figure 7).
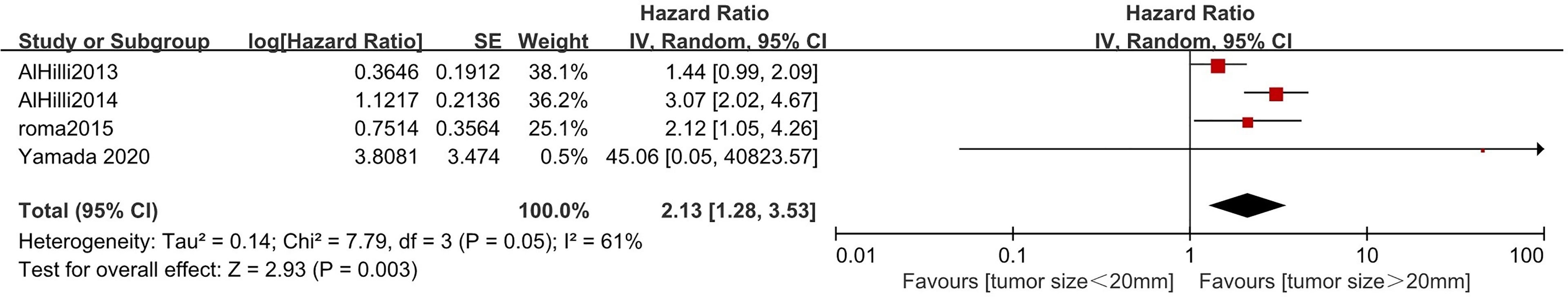
Figure 7 Meta-analysis of the association between tumor size and overall survival in endometrial cancer patients according to hazard ratio (HR) from univariate survival analyses.
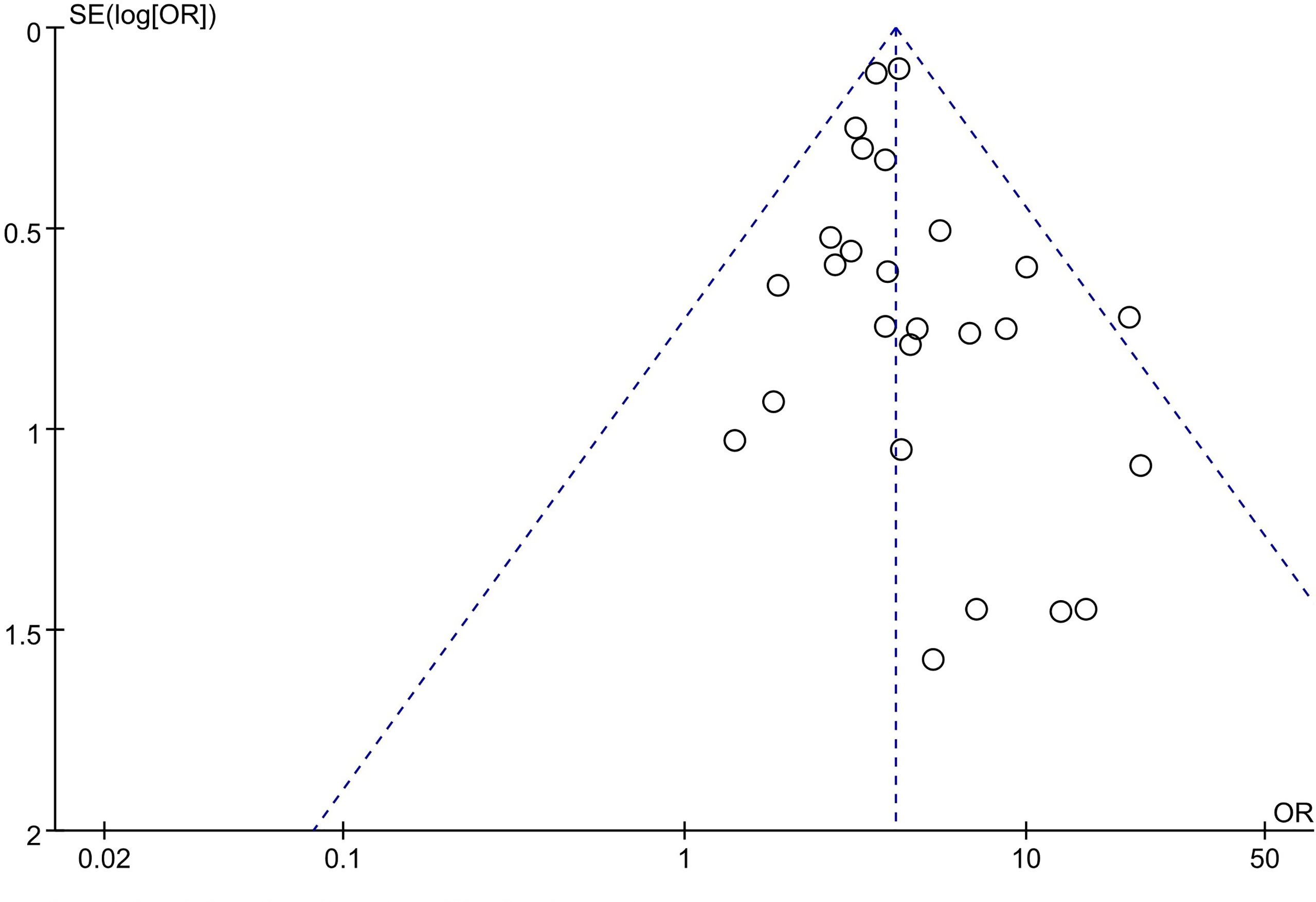
Publication Bias of Included Studies
A funnel plot was applied for the assessment of publication bias in the literature. The funnel plot for the included 27 studies on the association between tumor size and LNM was relatively symmetrical. Thus, there was no significant publication bias risk in all included studies investigating the association between tumor size and LNM (Figure 8).
Discussion
A few published studies indicated that tumor size >20 mm could provide important prognostic outcomes for endometrial cancer (27, 45, 51, 52), but others showed that tumor size of 20 mm was not a prognostic factor in endometrial cancer (20, 47). In the current study, we performed a meta-analysis to roundly evaluate the prognostic value of tumor size. Our conclusion showed tumor size >20 mm was characterized by the presence of MI, which has 50% of patients with all FIGO stages in endometrial cancer. MI is vitally important in the development of endometrial cancer and a well-recognized predictor of extra-uterine spread (4, 53). MI is quite an early action of cancer cells, which classifies patients with initial stages as low-risk or high-risk patients for surgical planning (53). Depth of MI (>50%) definitely correlated to LVSI, LNM, recurrence, and OS (53).
Six studies with a total of 1,643 endometrial cancer patients were eligible for analysis, and the results demonstrated that tumor size >20 mm has a significant prognostic implication for positive LVSI. A retrospective analysis reported the impact on positive LVSI was more relevant than MI > 50% for predicting survival in stage I endometrial cancer (43). Positive LVSI should be emphasized in early-stage endometrial cancer (54). Moreover, these as well as other studies substantiated the fact that positive LVSI patients had lower recurrence-free survival and OS rates (55). The European Society of Gynaecological Oncology (ESGO) guidelines introduced that positive LVSI should recommend lymphadenectomy (56). Unfortunately, it is usually not possible to diagnose LVSI status on the frozen section, until the final pathology report. So tumor size may be a useful tool for predicting markers of LVSI in a preoperative or intraoperative surgical stage.
We have reached an agreement that LNM was one of the most important prognostic factors. Lymphadenectomy is the most component of the surgical procedure, providing survival benefits in the early stages of endometrial cancer (57). However, it could increase morbidity and postoperative complications (58). Yet it is important to emphasize that there is usually a more difficult procedure to readily evaluate MI, LVSI, and LNM on a frozen diagnosis. Thus, it is liable to measure tumor diameter macroscopically. In addition, it is more feasible to measure the tumor size before surgery. Our pooling data have shown that tumor size >20 mm was significantly correlated with higher incidences of LNM, whether in surgically FIGO stage I or FIGO stage I–IV. Based on our results, tumor size from intraoperative and preoperative could plan the surgery strategy, which may minimize the risk of complications, lower the burden of operation, and decrease morbidity or mortality.
Han et al. investigated different prognostic factors for the recurrence in stage IA and IB endometrial cancer. MI was the prognostic factor in stage IA, whereas the grade was the prognostic factor in stage IB (59). Our findings disclosed that the prevalence of tumor size >20 mm increased the risk of recurrence in FIGO IA endometrial cancer. We also found out that tumor size >20 mm significantly predicted higher recurrence in FIGO I–II/I–III endometrial cancer. Multivariate analysis showed that LVSI and depth of MI were independent risks for recurrence (49). Our pooled analysis also showed that tumor size >20 mm was a risk associated with LVSI and depth of MI, as well as higher recurrence. As it turned out, tumor size >20 mm was related to a greater risk of OS based on univariate survival analysis. Furthermore, we discovered that tumor size >20 mm could predict poorer OS in endometrial cancer.
Currently, gynecologists usually do not attach great importance to tumor size. In the evaluation criteria for the surgical–pathological staging, treatment, and prognosis of endometrial cancer, tumor size was rarely covered, and thereby its role may be underestimated. The relationship between tumor size and MI, LVSI, LNM, recurrence, and OS remains controversial. Therefore, we conducted this meta-analysis to investigate the relationship between primary tumor size of 20 mm and clinicopathological parameters, recurrence, and OS. The results showed that tumor size >20 mm was an independent predictive factor for the depth of MI, positive LVSI, positive LNM, recurrence, and poor OS, indicating the importance of tumor size. Tumor size >20 mm may provide additional information before surgery. Therefore, it is more important to take into account the value of tumor size in the clinicopathological staging of endometrial carcinoma.
The strength of the study was the first meta-analysis to discuss the value of tumor size >20 mm to predict clinicopathological outcomes and recurrence in patients with endometrial cancer. Nonetheless, the limitations of this meta-analysis included retrospective and non-randomized studies. In addition, the different cutoffs of tumor size will directly affect the association with the outcome. Other tumor sizes were not studied in the meta-analysis. A standardized cutoff of tumor size for future trials and studies should be highlighted.
Conclusion
The meta-analysis showed that tumor size >20 mm was an independent predictive factor for the depth of MI, positive LVSI, positive LNM, recurrence, and poor OS, indicating the importance of tumor size in endometrial cancer. Therefore, it is more important to take into account the value of tumor size in the clinicopathological staging of endometrial carcinoma. Tumor size >20 mm should be integrated into the intraoperative algorithm for performing a full surgical staging.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author Contributions
CS and XJ contributed equally to this work. CS and XJ: conceptualization, literature retrieval, data acquisition, and writing of the manuscript. XY and YY: statistical analysis. JW and XC: manuscript review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (Grant numbers: 81802591 and 81974225) and the Science and Technology Bureau of Jiaxing city (Grant number: 2021AY30004).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International Patterns and Trends in Endometrial Cancer Incidence, 1978-2013. J Natl Cancer Inst (2018) 110(4):354–61. doi: 10.1093/jnci/djx214
2. Barnes MN, Kilgore LC. Complete Surgical Staging of Early Endometrial Adenocarcinoma: Optimizing Patient Outcomes. Semin Radiat Oncol (2000) 10(1):3–7. doi: 10.1016/S1053-4296(00)80014-4
3. Morrow CP, Bundy BN, Kurman RJ, Creasman WT, Heller P, Homesley HD, et al. Relationship Between Surgical-Pathological Risk Factors and Outcome in Clinical Stage I and II Carcinoma of the Endometrium: A Gynecologic Oncology Group Study. Gynecol Oncol (1991) 40(1):55–65. doi: 10.1016/0090-8258(91)90086-K
4. Creasman WT, Morrow CP, Bundy BN, Homesley HD, Graham JE, Heller PB. Surgical Pathologic Spread Patterns of Endometrial Cancer. A Gynecol Oncol Group Study Cancer (1987) 60(8 Suppl):2035–41. doi: 10.1002/1097-0142(19901015)60:8+<2035::aid-cncr2820601515>3.0.co;2-8
5. Nwachukwu C, Baskovic M, Von Eyben R, Fujimoto D, Giaretta S, English D, et al. Recurrence Risk Factors in Stage IA Grade 1 Endometrial Cancer. J Gynecol Oncol (2021) 32(2):e22. doi: 10.3802/jgo.2021.32.e22
6. Gadducci A, Cavazzana A, Cosio S, DIC C, Tana R, Fanucchi A, et al. Lymph-Vascular Space Involvement and Outer One-Third Myometrial Invasion Are Strong Predictors of Distant Haematogeneous Failures in Patients With Stage I-II Endometrioid-Type Endometrial Cancer. Anticancer Res (2009) 29(5):1715–20. doi: 10.31838/srp.2020.5.26
7. Güngördük K, Firat Cüylan Z, Kahramanoglu I, Oge T, Akbayir O, Dede M, et al. Risk Factors for Recurrence in Low-Risk Endometrial Cancer: A Case-Control Study. Oncol Res Treat (2018) 41(7-8):466–70. doi: 10.1159/000488112
8. Li M, Wang Z, Zhao L, Li X, Wang J, Zhang C, et al. Predictors of Recurrence and Prognosis in Patients With Stage I and II Endometrial Carcinoma. Zhonghua Fu Chan Ke Za Zhi (2014) 49(6):455–9. doi: 10.26226/morressier.599bdc79d462b80296ca1138
9. Gusberg SB, Jones HC Jr., Tovell HM. Selection of Treatment for Corpus Cancer. Am J Obstet Gynecol (1960) 80:374–80. doi: 10.1016/0002-9378(60)90141-1
10. Riggs MJ, Cox Bauer CM, Miller CR, Aden JK, Kamelle SA. Validation of an Endometrial Tumor Diameter Model for Risk Assessment in the Absence of Lymph Node Mapping. J Patient Cent Res Rev (2020) 7(4):323–8. doi: 10.17294/2330-0698.1768
11. Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC. Low-Risk Corpus Cancer: Is Lymphadenectomy or Radiotherapy Necessary? Am J Obstet Gynecol (2000) 182(6):1506–19. doi: 10.1067/mob.2000.107335
12. Cox Bauer CM, Greer DM, Kram JJF, Kamelle SA. Tumor Diameter as a Predictor of Lymphatic Dissemination in Endometrioid Endometrial Cancer. Gynecol Oncol (2016) 141(2):199–205. doi: 10.1016/j.ygyno.2016.02.017
13. Pollom EL, Conklin CM, von Eyben R, Folkins AK, Kidd EA. Nomogram to Predict Risk of Lymph Node Metastases in Patients With Endometrioid Endometrial Cancer. Int J Gynecol Pathol (2016) 35(5):395–401. doi: 10.1097/PGP.0000000000000246
14. Kilts TP, Glaser GE, Langstraat CL, Kumar A, Weaver AL, Mc Gree ME, et al. Comparing Risk Stratification Criteria for Predicting Lymphatic Dissemination in Endometrial Cancer. Gynecol Oncol (2019) 155(1):21–6. doi: 10.1016/j.ygyno.2019.08.005
15. Akar S, Çelik ZE, Fındık S, İlhan TT, Ercan F, Çelik Ç. Prognostic Significance of Solid Growth in Endometrioid Endometrial Adenocarcinoma. Int J Clin Oncol (2020) 25(1):195–202. doi: 10.1007/s10147-019-01529-4
16. AlHilli MM, Mariani A, Bakkum-Gamez JN, Dowdy SC, Weaver AL, Peethambaram PP, et al. Risk-Scoring Models for Individualized Prediction of Overall Survival in Low-Grade and High-Grade Endometrial Cancer. Gynecol Oncol (2014) 133(3):485–93. doi: 10.1016/j.ygyno.2014.03.567
17. Boyraz G, Salman MC, Gultekin M, Basaran D, Cagan M, Ozgul N, et al. Incidence of Lymph Node Metastasis in Surgically Staged FIGO IA G1/G2 Endometrial Cancer With a Tumor Size of More Than 2 Cm. Int J Gynecol Cancer (2017) 27(3):486–92. doi: 10.1097/IGC.0000000000000919
18. Boyraz G, Atalay FO, Salman MC, Usubutun A, Erturk A, Gultekin M, et al. Comparison of Mayo and Milwaukee Risk Stratification Models for Predicting Lymph Node Metastasis in Endometrial Cancer. Int J Gynecol Cancer (2018) 28(5):869–74. doi: 10.1097/IGC.0000000000001261
19. Chang SJ, Kong TW, Kim WY, Yoo SC, Yoon JH, Chang KH, et al. Lymph-Vascular Space Invasion as a Significant Risk Factor for Isolated Para-Aortic Lymph Node Metastasis in Endometrial Cancer: A Study of 203 Consecutive Patients. Ann Surg Oncol (2011) 18(1):58–64. doi: 10.1245/s10434-010-1206-x
20. Doll KM, Tseng J, Denslow SA, Fader AN, Gehrig PA. High-Grade Endometrial Cancer: Revisiting the Impact of Tumor Size and Location on Outcomes. Gynecol Oncol (2014) 132(1):44–9. doi: 10.1016/j.ygyno.2013.10.023
21. Al-Dali F, Puig M, Lopez C, Fernandez M, Salanas G, Ojeda F. Tumor Size as Prognostic Factor of Lymph Node Involvement in Endometrial Cancer. Int J Gynecol Cancer (2019) 29:A297. doi: 10.1136/ijgc-2019-ESGO.527
22. Gilani S, Anderson I, Fathallah L, Mazzara P. Factors Predicting Nodal Metastasis in Endometrial Cancer. Arch Gynecol Obstet (2014) 290(6):1187–93. doi: 10.1007/s00404-014-3330-5
23. Günakan E, Atan S, Haberal AN, Küçükyıldız İA, Gökçe E, Ayhan A. A Novel Prediction Method for Lymph Node Involvement in Endometrial Cancer: Machine Learning. Int J Gynecol Cancer (2019) 29(2):320–4. doi: 10.1136/ijgc-2018-000033
24. Karalok A, Turan T, Basaran D, Turkmen O, Comert Kimyon G, Tulunay G, et al. Lymph Node Metastasis in Patients With Endometrioid Endometrial Cancer: Overtreatment Is the Main Issue. Int J Gynecol Cancer (2017) 27(4):748–53. doi: 10.1097/IGC.0000000000000937
25. Lee KB, Ki KD, Lee JM, Lee JK, Kim JW, Cho CH, et al. The Risk of Lymph Node Metastasis Based on Myometrial Invasion and Tumor Grade in Endometrioid Uterine Cancers: A Multicenter, Retrospective Korean Study. Ann Surg Oncol (2009) 16(10):2882–7. doi: 10.1245/s10434-009-0535-0
26. Li M, Wu S, Xie Y, Zhang X, Wang Z, Zhu Y, et al. Cervical Invasion, Lymphovascular Space Invasion, and Ovarian Metastasis as Predictors of Lymph Node Metastasis and Poor Outcome on Stages I to III Endometrial Cancers: A Single-Center Retrospective Study. World J Surg Oncol (2019) 17(1):193. doi: 10.1186/s12957-019-1733-2
27. Mahdi H, Munkarah AR, Ali-Fehmi R, Woessner J, Shah SN, Moslemi-Kebria M. Tumor Size is an Independent Predictor of Lymph Node Metastasis and Survival in Early Stage Endometrioid Endometrial Cancer. Arch Gynecol Obstet (2015) 292(1):183–90. doi: 10.1007/s00404-014-3609-6
28. Matsushita C, Fujiwara H, Takei Y, Saga Y, Machida S, Taneichi A, et al. New Criteria for the Omission of Lymphadenectomy in Endometrioid Carcinoma. Int J Gynecol Cancer (2019) 29(3):541–6. doi: 10.1136/ijgc-2018-000044
29. Milam MR, Java J, Walker JL, Metzinger DS, Parker LP, Coleman RL. Nodal Metastasis Risk in Endometrioid Endometrial Cancer. Obstet Gynecol (2012) 119(2 Pt 1):286–92. doi: 10.1097/AOG.0b013e318240de51
30. Oz M, Korkmaz V, Meydanli MM, Sari ME, Cuylan ZF, Gungor T. Is Tumor Size Really Important for Prediction of Lymphatic Dissemination in Grade 1 Endometrial Carcinoma With Superficial Myometrial Invasion? Int J Gynecol Cancer (2017) 27(7):1393–8. doi: 10.1097/IGC.0000000000001025
31. Pavlakis K, Rodolakis A, Vagios S, Voulgaris Z, Messini I, Yiannou P, et al. Identifiable Risk Factors for Lymph Node Metastases in Grade 1 Endometrial Carcinoma. Int J Gynecol Cancer (2017) 27(8):1694–700. doi: 10.1097/IGC.0000000000001070
32. Rathod PS, Shakuntala PN, Pallavi VR, Kundaragi R, Shankaranand B, Vijay CR, et al. The Risk and Pattern of Pelvic and Para Aortic Lymph Nodal Metastasis in Patients With Intermediate and High Risk Endometrial Cancer. Indian J Surg Oncol (2014) 5(2):109–14. doi: 10.1007/s13193-014-0303-x
33. Sari ME, Yalcin İ, Sahin H, Meydanli MM, Gungor T. Risk Factors for Paraaortic Lymph Node Metastasis in Endometrial Cancer. Int J Clin Oncol (2017) 22(5):937–44. doi: 10.1007/s10147-017-1139-5
34. Shah C, Johnson EB, Everett E, Tamimi H, Greer B, Swisher E, et al. Does Size Matter? Tumor Size and Morphology as Predictors of Nodal Status and Recurrence in Endometrial Cancer. Gynecol Oncol (2005) 99(3):564–70. doi: 10.1016/j.ygyno.2005.06.011
35. Tecellioglu FS, Akpolat N, Sahin N. Mmp-9 and Fascin-1 Expression in Endometrioid-Type Endometrial Carcinoma and Their Prognostic Value. Indian J Gynecol Oncol (2021) 19(1):24–30. doi: 10.1007/s40944-020-00492-7
36. Turan T, Hizli D, Sarici S, Boran N, Gundogdu B, Karadag B, et al. Is it Possible to Predict Para-Aortic Lymph Node Metastasis in Endometrial Cancer? Eur J Obstet Gynecol Reprod Biol (2011) 158(2):274–9. doi: 10.1016/j.ejogrb.2011.04.031
37. Vaizoglu F, Yuce K, Salman MC, Basaran D, Calis P, Ozgul N, et al. Lymphovascular Space Involvement is the Sole Independent Predictor of Lymph Node Metastasis in Clinical Early Stage Endometrial Cancer. Arch Gynecol Obstet (2013) 288(6):1391–7. doi: 10.1007/s00404-013-2913-x
38. Vargas R, Rauh-Hain JA, Clemmer J, Clark RM, Goodman A, Growdon WB, et al. Tumor Size, Depth of Invasion, and Histologic Grade as Prognostic Factors of Lymph Node Involvement in Endometrial Cancer: A SEER Analysis. Gynecol Oncol (2014) 133(2):216–20. doi: 10.1016/j.ygyno.2014.02.011
39. Watanabe M, Aoki Y, Kase H, Fujita K, Tanaka K. Low Risk Endometrial Cancer: A Study of Pelvic Lymph Node Metastasis. Int J Gynecol Cancer (2003) 13(1):38–41. doi: 10.1136/ijgc-00009577-200301000-00007
40. Zanfagnin V, Huang Y, Mc Gree ME, Weaver AL, Casarin J, Multinu F, et al. Predictors of Extensive Lymphatic Dissemination and Recurrences in Node-Positive Endometrial Cancer. Gynecol Oncol (2019) 154(3):480–6. doi: 10.1016/j.ygyno.2019.07.006
41. Ilker S, Nilufer C, Firat CZ, Bulent O, Hatice B, Tayfun G. Predicting Lympho-Vascular Space Invasion in Endometrial Cancers With Mucinous Carcinomatous Components. Asian Pac J Cancer Prev (2015) 16(10):4247–50. doi: 10.7314/APJCP.2015.16.10.4247
42. Oliver-Perez MR, Magriña J, Villalain-Gonzalez C, Jimenez-Lopez JS, Lopez-Gonzalez G, Barcena C, et al. Lymphovascular Space Invasion in Endometrial Carcinoma: Tumor Size and Location Matter. Surg Oncol (2021) 37:101541. doi: 10.1016/j.suronc.2021.101541
43. Ayhan A, Şahin H, Sari ME, Yalçin I, Haberal A, Meydanli MM. Prognostic Significance of Lymphovascular Space Invasion in Low-Risk Endometrial Cancer. Int J Gynecol Cancer (2019) 29(3):505–12. doi: 10.1136/ijgc-2018-000069
44. Laufer J, Scasso S, Papadia A, Sosa C, Cirillo F, Raspagliesi F. Association Between Tumor Diameter and Lymphovascular Space Invasion Among Women With Early-Stage Endometrial Cancer. Int J Gynaecol Obstet (2013) 123(2):142–5. doi: 10.1016/j.ijgo.2013.05.012
45. Schink JC, Rademaker AW, Miller DS, Lurain JR. Tumor Size in Endometrial Cancer. Cancer (1991) 67(11):2791–4. doi: 10.1002/1097-0142(19910601)67:11<2791::AID-CNCR2820671113>3.0.CO;2-S
46. Bendifallah S, Canlorbe G, Huguet F, Coutant C, Hudry D, Graesslin O, et al. A Risk Scoring System to Determine Recurrence in Early-Stage Type 1 Endometrial Cancer: A French Multicentre Study. Ann Surg Oncol (2014) 21(13):4239–45. doi: 10.1245/s10434-014-3864-6
47. Çakır C, Kılıç İ, Yüksel D, Karyal YA, Üreyen I, Boyraz G, et al. Does Tumor Size Have Prognostic Value in Patients Undergoing Lymphadenectomy in Endometrioid-Type Endometrial Cancer Confined to the Uterine Corpus? Turk J Med Sci (2019) 49(5):1403–10. doi: 10.3906/sag-1902-224
48. Marcickiewicz J, Sundfeldt K. Accuracy of Intraoperative Gross Visual Assessment of Myometrial Invasion in Endometrial Cancer. Acta Obstet Gynecol Scand (2011) 90(8):846–51. doi: 10.1111/j.1600-0412.2011.01166.x
49. Roma AA, Rybicki LA, Barbuto D, Euscher E, Djordjevic B, Frauenhoffer E, et al. Risk Factor Analysis of Recurrence in Low-Grade Endometrial Adenocarcinoma. Hum Pathol (2015) 46(10):1529–39. doi: 10.1016/j.humpath.2015.06.015
50. Yamada S, Tsuyoshi H, Yamamoto M, Tsujikawa T, Kiyono Y, Okazawa H, et al. Prognostic Value of 16α-(18)F-Fluoro-17β-Estradiol PET as a Predictor of Disease Outcome in Endometrial Cancer: A Prospective Study. J Nucl Med (2021) 62(5):636–42. doi: 10.2967/jnumed.120.244319
51. Numazaki R, Miyagi E, Konnai K, Ikeda M, Yamamoto A, Onose R, et al. Analysis of Stage IVB Endometrial Carcinoma Patients With Distant Metastasis: A Review of Prognoses in 55 Patients. Int J Clin Oncol (2009) 14(4):344–50. doi: 10.1007/s10147-009-0878-3
52. AlHilli MM, Podratz KC, Dowdy SC, Bakkum-Gamez JN, Weaver AL, McGree ME, et al. Preoperative Biopsy and Intraoperative Tumor Diameter Predict Lymph Node Dissemination in Endometrial Cancer. Gynecol Oncol (2013) 128(2):294–9. doi: 10.1016/j.ygyno.2012.10.009
53. Wang J, Xu P, Yang X, Yu Q, Xu X, Zou G, et al. Association of Myometrial Invasion With Lymphovascular Space Invasion, Lymph Node Metastasis, Recurrence, and Overall Survival in Endometrial Cancer: A Meta-Analysis of 79 Studies With 68,870 Patients. Front Oncol (2021) 11:762329. doi: 10.3389/fonc.2021.762329
54. Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. A Phase III Trial of Surgery With or Without Adjunctive External Pelvic Radiation Therapy in Intermediate Risk Endometrial Adenocarcinoma: A Gynecologic Oncology Group Study. Gynecol Oncol (2004) 92(3):744–51. doi: 10.1016/j.ygyno.2003.11.048
55. dos Reis R, Burzawa JK, Tsunoda AT, Hosaka M, Frumovitz M, Westin SN, et al. Lymphovascular Space Invasion Portends Poor Prognosis in Low-Risk Endometrial Cancer. Int J Gynecol Cancer (2015) 25(7):1292–9. doi: 10.1097/IGC.0000000000000490
56. Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, et al. ESMO-ESGO-ESTRO Consensus Conference on Endometrial Cancer: Diagnosis, Treatment and Follow-Up. Radiother Oncol (2015) 117(3):559–81. doi: 10.1016/j.radonc.2015.11.013
57. Kitchener H, Swart AM, Qian Q, Amos C, Parmar MK. Efficacy of Systematic Pelvic Lymphadenectomy in Endometrial Cancer (MRC ASTEC Trial): A Randomised Study. Lancet (2009) 373(9658):125–36. doi: 10.1016/s0140-6736(08)61766-3
58. Todo Y, Sakuragi N. Systematic Lymphadenectomy in Endometrial Cancer. J Obstet Gynaecol Res (2013) 39(2):471–7. doi: 10.1111/j.1447-0756.2012.02062.x
Keywords: endometrial cancer, tumor size, myometrial invasion, lymphovascular space invasion, lymph node metastasis, recurrence, overall survival
Citation: Jin X, Shen C, Yang X, Yu Y, Wang J and Che X (2022) Association of Tumor Size With Myometrial Invasion, Lymphovascular Space Invasion, Lymph Node Metastasis, and Recurrence in Endometrial Cancer: A Meta-Analysis of 40 Studies With 53,276 Patients. Front. Oncol. 12:881850. doi: 10.3389/fonc.2022.881850
Received: 23 February 2022; Accepted: 04 May 2022;
Published: 02 June 2022.
Edited by:
Shannon Neville Westin, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Christos Iavazzo, Metaxa Cancer Hospital, GreeceBi Cong Yan, Shanghai Jiao Tong University, China
Copyright © 2022 Jin, Shen, Yang, Yu, Wang and Che. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuan Che, Y2hleHVhbmp4ZnlAMTYzLmNvbQ==; Jianzhang Wang, amlhbnpoYW5nLndhbmdAemp1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Xiaoying Jin1†
Xiaoying Jin1† Xuan Che
Xuan Che