- 1Department of Breast Surgery and Oncology, the Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2The Key Laboratory of Cancer Prevention and Intervention, China National Ministry of Education, Zhejiang University School of Medicine, Hangzhou, China
- 3Department of General Surgery, Longquan People’s Hospital, Longquan, China
- 4Cancer Center, Zhejiang University, Hangzhou, China
Purpose: Postmastectomy radiotherapy (PMRT) after neoadjuvant chemotherapy (NAC) in breast cancer patients with initial clinical stage cT1-2N+, especially for those who achieved ypT1-2N0, is still controversial. This study was to evaluate the survival prognosis of cT1-2N+ patients after NAC with or without PMRT, and to discuss the selection of patients who may omit PMRT.
Patients and Methods: From January 2005 to December 2017, 3055 female breast cancer patients underwent mastectomy in our medical center, among whom 215 patients of cT1-2N+ stage, receiving NAC with or without PMRT were finally analyzed. The median follow-up duration was 72.6 months. The primary endpoint was disease-free survival (DFS), and secondary endpoint was overall survival (OS). Comparison was conducted between PMRT and non-PMRT subgroups.
Results: Of the 215 eligible patients, 35.8% (77/215) cT1-2N+ patients achieved ypT0-2N0 after NAC while 64.2% (138/215) of the patients remained nodal positive (ypT0-2N+). The 5-year DFS of ypT0-2N0 non-PMRT was 79.5% (95% confidence interval [CI] 63.4-95.6%). No statistically significant difference was observed between the ypT0-2N0 PMRT and non-PMRT subgroups for the 5-year DFS (78.5% vs 79.5%, p = 0.673) and OS (88.8% vs 90.8%, p = 0.721). The 5-years DFS didn’t obviously differ between the ypT0-2N0 non-PMRT subgroup and cT1-2N0 subgroup (79.5% vs 93.3%, p = 0.070). By using Cox regression model in multivariate analyses of prognosis in ypT0-2N+ PMRT subgroup, HER2 overexpression and triple-negative breast cancer were significantly poor predictors of DFS and OS, while ypN stage was significant independent predictors of OS.
Conclusion: An effective response to NAC (ypT0-2N0) indicates a sufficiently favorable prognosis, and PMRT might be omitted for cT1-2N+ breast cancer patients with ypT0-2N0 after NAC.
Introduction
Neoadjuvant chemotherapy (NAC) is increasingly used to treat patients with breast cancer, especially those with locally advanced disease (1). NAC can facilitate surgery by converting an inoperable tumor to an operable tumor or by converting a patient requiring mastectomy to one who can be treated with breast-conserving surgery (2). NAC usually alters tumor stage and has been found to downsize primary tumors in 70–80% of patients (3, 4). In addition, NAC was reported to downstage axillary lymph nodes status in 20–40% of patients (4, 5).
Postmastectomy radiotherapy (PMRT) has been shown to reduce the risk of locoregional recurrence and enhance overall survival in patients with breast cancer. PMRT is recommended for patients with tumors ≥ 5 cm size or those with at least four positive lymph nodes (6, 7). In contrast, the role of PMRT in T1-2 tumors with 1–3 positive lymph nodes remain unclear, with this treatment usually recommended for patients with T1-2N1 tumors and high-risk features. These guidelines for PMRT guidance, however, were formulated for patients in the absence of NAC, making the indications for PMRT unclear for patients who receive NAC. Moreover, the potential downstaging associated with NAC can alter the standard indications for PMRT.
Indications for PMRT following NAC remain unclear, particularly in patients initially diagnosed with stage cT1-2N+ breast tumors. Because increasing numbers of patients undergo breast reconstruction after surgery, determining indications for PMRT has become more important, as PMRT can adversely affect the incidence of complications and aesthetic outcomes of immediate breast reconstructions (8). Moreover, it has not yet been determined whether an effective response to NAC (e.g., achieve ypT0-2N0) can allow the omission of PMRT.
The present study evaluated the efficacy of PMRT after NAC and surgery in breast cancer patients with initial clinical stage cT1-2N+M0. Three questions were addressed: 1) whether patients presenting with cT1-2N+ disease who achieve ypT0-2N0 after NAC require PMRT; 2) the comparative prognosis in the absence of PMRT of patients with cT1-2N+ and cT1-2N0 who achieve ypT0-2N0 after NAC; and 3) the correlations between clinical variables and prognosis in patients with residual nodal disease after NAC. These findings may help determine the indications for PMRT after NAC.
Methods
Patient Population
Of the 3055 women diagnosed with breast cancer who underwent mastectomy at the Second Affiliated Hospital, Zhejiang University School of Medicine from January 2005 to December 2017, 456 (14.9%) received NAC before mastectomy. Patients who received fewer than two cycles of NAC (n=5), those with ypT3-4 stage after NAC (n=6), those with undetermined ypT stage (n=19) and ypN stage (n=1), and those with unknown radiotherapy (n=62) were excluded. This retrospective analysis therefore included 215 patients diagnosed with cT1-2N+ stage breast before NAC followed by mastectomy. The study protocol was approved by the Ethics Committee of our hospital (Approval No: 2020-363).
The medical records of all included patients were extracted from the computerized database of the Second Affiliated Hospital, Zhejiang University School of Medicine. Follow-up information on all patients was obtained by clinic visits and telephone contact. Clinical tumor size (cT) was determined by ultrasound, mammography or magnetic resonance imaging. Patients classified as cN+ were defined as those with clinically diagnosed metastatic lymph nodes, including palpable lymph nodes that were fixed or matted, metastatic lymph nodes diagnosed by imaging, or lymph node metastases pathologically confirmed after biopsy. Of the 215 cT1-2N+ patients in the study cohort, 138 (64.2%) were pathologically confirmed as having lymph node metastases, either by biopsy before NAC or by intraoperative sentinel lymph node biopsy or axillary lymph node dissection. TNM staging was performed in accordance with American Joint Committee on Cancer guidelines (version 8, 2017). Estrogen receptor (ER) and progesterone receptor (PR) status were evaluated by immunohistochemistry (IHC), with cutoffs of 1% to dichotomize tumors as positive or negative (9). Human epidermal receptor 2 (HER2) status was evaluated by IHC, with tumors having IHC scores of 2+, defined as indeterminate, further analyzed by IHC and fluorescence in situ hybridization (FISH) (10).
Treatment
The patients with cT1-2N+ breast cancer were divided into two groups, the ypT0-2N+ and the ypT0-2N0 groups, based on the pathological lymph node status of surgical specimens removed after NAC. Each of these groups was divided into two subgroups, those who did and did not receive PMRT (Figure 1). Of the patients in this study, 97.2% underwent axillary lymph node dissection. All hormone receptor-positive patients received adjuvant endocrine therapy, and 47 (49.5%) of the 95 patients with HER2-positive breast cancer were treated with Trastuzumab.

Figure 1 Study design. NAC, neoadjuvant chemotherapy; PMRT, postmastectomy radiotherapy; cT, clinical tumor size; cN, clinical lymph node; ypT, pathologic tumor size after neoadjuvant therapy; ypN, pathologic lymph node after neoadjuvant therapy.
In patients undergoing PMRT, 50 Gy (range 45–50 Gy) radiation in 25 fractions (range 25–28) of 2 Gy each was delivered to the chest wall and/or regional lymph nodes (i.e., the supraclavicular/infraclavicular and/or internal mammary lymph node regions). Of the patients who received PMRT, 86.4% received intensity-modulated radiation therapy and 13.6% received 3D conformal radiation therapy. The chest wall and supraclavicular/infraclavicular nodal regions were generally irradiated with photons, and the internal mammary lymph node regions were irradiated with electrons.
Study Endpoints
Patients were followed-up until September 16, 2021, with the median follow-up time being 72.6 months (5.96 years). The primary endpoint was disease-free survival (DFS), defined as the time from the date of diagnosis to the date of first locoregional recurrence (LR), distant metastasis (DM), new primary tumors in the breast, death from any cause, or last follow-up (11). The secondary endpoint was overall survival (OS), defined as the time from the date of diagnosis to the date of death due from any cause or last follow-up (11). LR included clinical, radiographic, or pathological evidence of recurrence in the ipsilateral chest wall and/or regional lymph nodes, whereas DM included recurrences at all other sites (i.e., contralateral breast, bone, lung, liver, or brain).
Statistical Analysis
Categorical variables in two groups of patients were compared using the Pearson’s χ2 test or Fisher’s exact test, as appropriate, whereas continuous variables were compared by t-test. Survival, including DFS and OS, was analyzed by the Kaplan–Meier method, with differences compared by log-rank tests. Patients who were lost to follow-up were censored at the last contact. Factors associated with survival in NAC patients with residual nodal disease were assessed by univariate and multivariate Cox proportional hazards analyses, with variables found to be significant (P<0.10) on univariate analysis were included in the multivariate model. ER status and triple-negative breast cancer were excluded from the multivariate model due to their collinearity with molecular subtypes. All tests were two-sided, with p values < 0.05 considered statistically significant. Statistical analyses were performed using SPSS version 20.0 software (IBM Institute, Chicago, IL, USA) and GraphPad Prism version 8 software (GraphPad Inc, San Diego, CA, USA).
Results
Patient and Tumor Characteristics
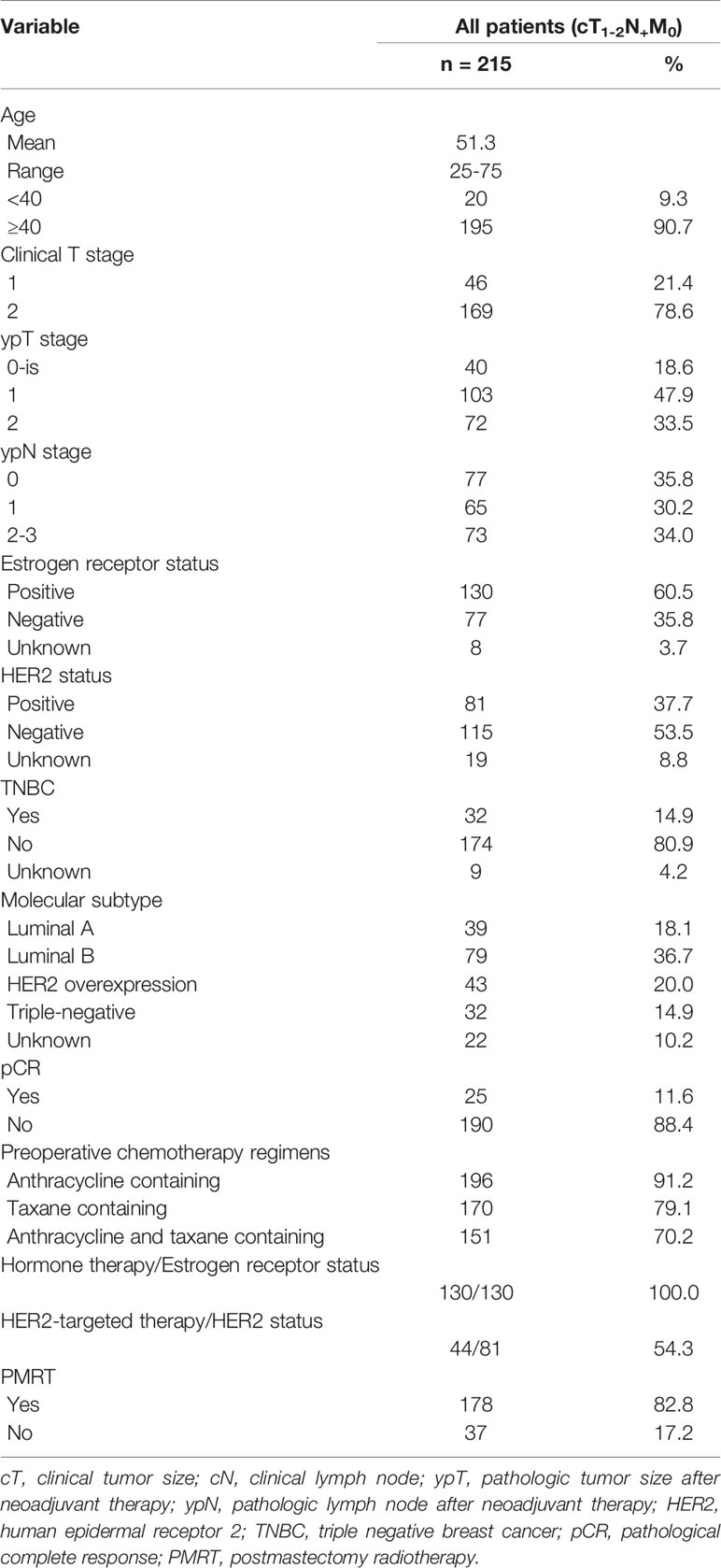
The clinical characteristics of the 215 patients with cT1-2N+ breast cancer included in this study are illustrated in Table 1. Median age at diagnosis was 51.2 years (range: 25–75 years), with 188 (87.4%) of these patients diagnosed with invasive ductal carcinomas. Of these 215 patients, 46 (21.4%) and 169 (78.6%) were in clinical T1 and T2 stages, respectively. After NAC followed by mastectomy, the primary tumor staging was ypT0 in 40 (18.6%) patients, ypT1 in 103 (47.9%), and ypT2 in 72 (33.5%), with 77 (35.8%), 65 (30.2%), and 73 (34.0%) having lymph node stages ypN0, ypN1, and ypN2-3, respectively. Thirty-two (14.9%) patients had triple negative breast cancer (TNBC), and 130 (60.5%) were ER-positive, with all of the latter receiving endocrine therapy. HER2 was positive in 95 (44.1%) of patients, with 47 (49.5%) of the latter being treated with trastuzumab. Anthracycline-containing chemotherapy regimens were administered to 196 (91.2%) patients, taxane-containing regimens to 170 (79.1%), and combined anthracycline and taxane regimens to 151 (70.2%). PMRT was administered to 178 (82.8%) patients; of the latter, eight (4.5%) received irradiation to chest wall alone, 133 (74.5%) received irradiation to both the chest wall and supraclavicular/infraclavicular lymph node regions; and two (1.1%) received irradiation to chest wall, supraclavicular/infraclavicular and internal mammary lymph node regions, with the irradiation fields not specified for the remaining 35 (19.7%) patients who received PMRT.
Requirement for PMRT After NAC in Patients With cT1-2N+ Disease Who Achieve ypT0-2N0
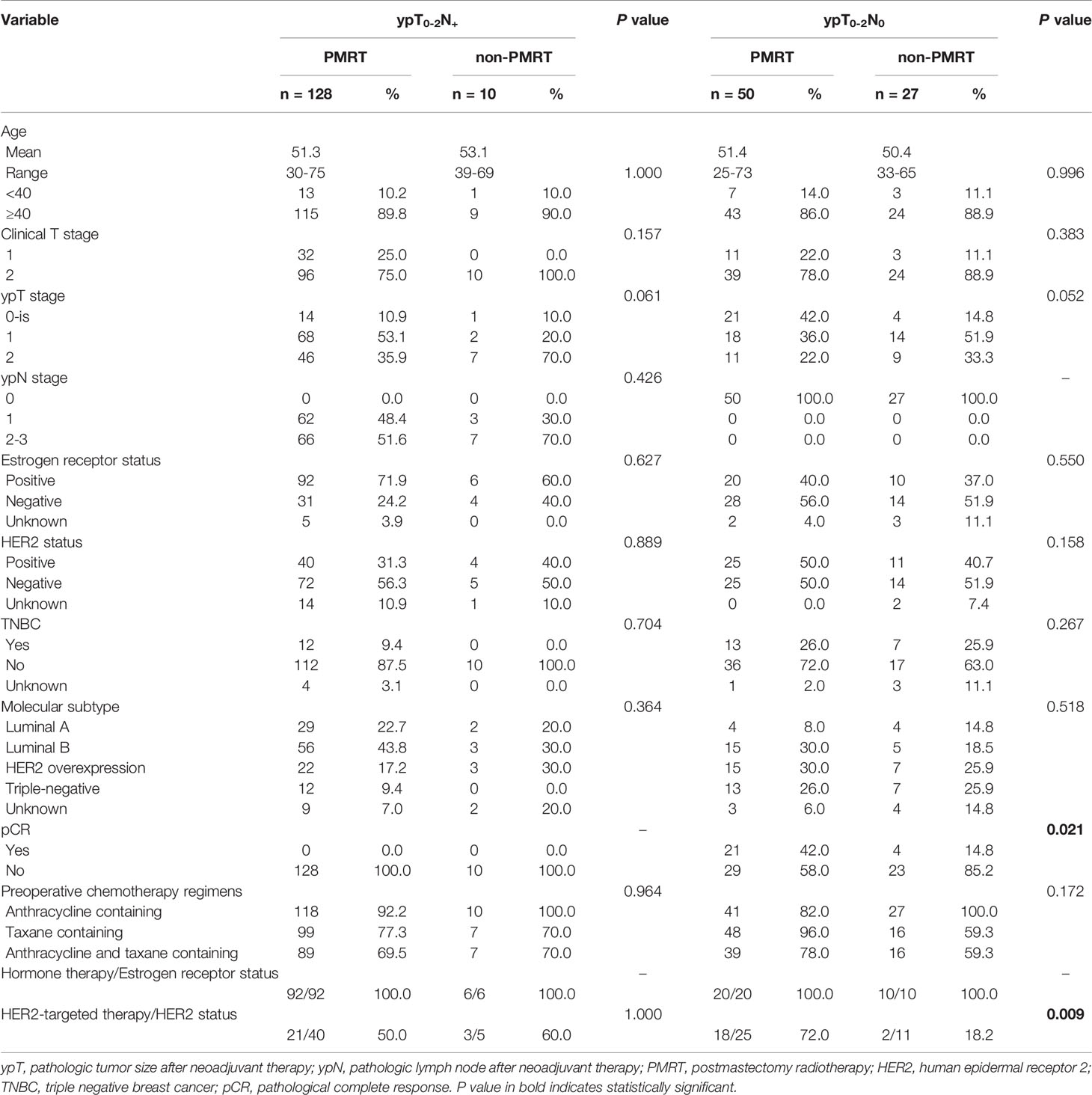
After NAC, 77 (35.8%) of the 215 cT1-2N+ patients achieved ypT0-2N0. Of these 77 patients, 50 (64.9%) received PMRT, whereas 27 (35.1%) did not. A comparison of clinical and treatment characteristics in these two subgroups found that only the rates of pathological complete response (pCR; 42% vs. 14.8%, p=0.021) and trastuzumab treatment of HER2+ patients (72.0% vs. 18.2%, p=0.009) differed significantly (Table 2).
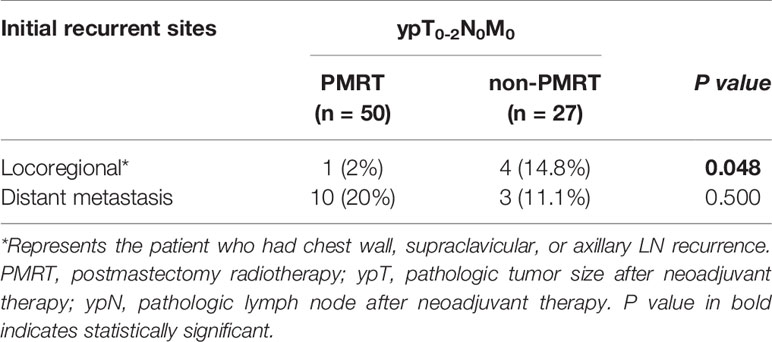
Eleven (22%) of the 50 patients in the ypT0-2N0 PMRT subgroup and seven (25.9%) of the 27 patients in the ypT0-2N0 non-PMRT subgroup had LR or DM. Analysis of the recurrence patterns in these subgroups showed that the LR rate was significantly lower in the ypT0-2N0 PMRT than in the ypT0-2N0 non-PMRT subgroup (2% vs. 14.8%, p=0.048), whereas the DM rate did not differ in these two subgroups (Table 3).
The 5-year DFS rates were similar in the ypT0-2N0 PMRT and ypT0-2N0 non-PMRT subgroups (78.5% vs. 79.5%, p = 0.673) (Figure 2A). Similarly, the 5-year OS rate was 88.8% (95% CI 79.6–98.0%) in the ypT0-2N0 PMRT subgroup and 90.8% (95% CI 78.6–103%) in the ypT0-2N0 non-PMRT subgroup, a difference that was not statistically significant (p = 0.721) (Figure 2B).
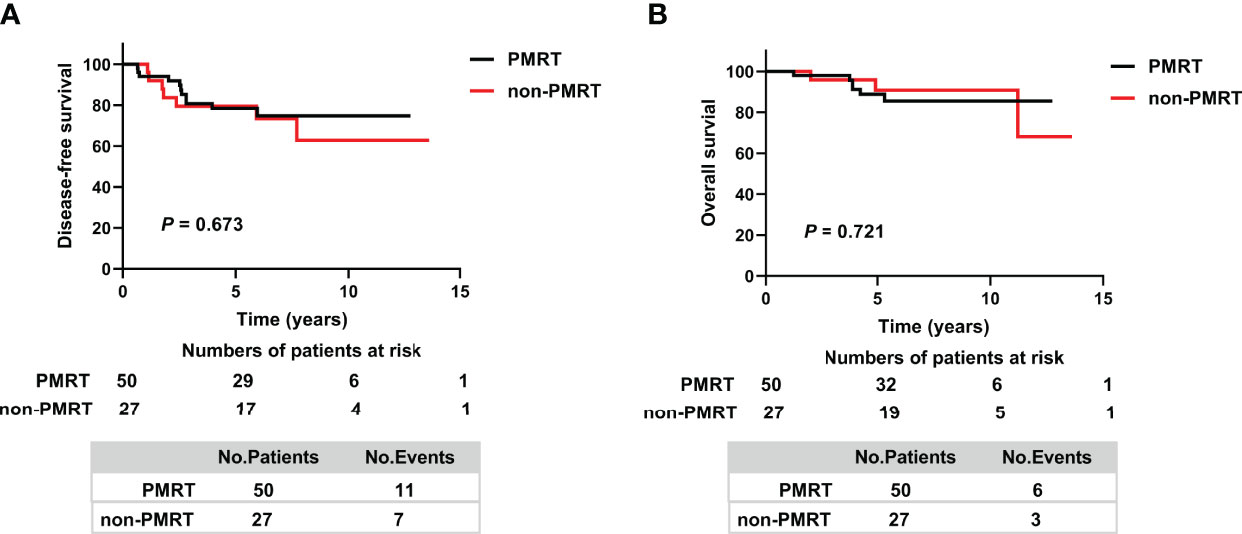
Figure 2 Kaplan–Meier analysis of (A) Disease-free survival and (B) Overall survival in patients who achieved ypT0-2N0 after NAC and in the PMRT and non-PMRT subgroups. PMRT, postmastectomy radiotherapy.
Comparative Prognoses of cT1-2N+ Patients Who Achieve ypT0-2N0 Without PMRT and cT1-2N0 Patients?
Because the prognosis of patients in the ypT0-2N0 non-PMRT subgroup was non-inferior to that of patients in the ypT0-2N0 PMRT subgroup, the survival of ypT0-2N0 non-PMRT patients was compared with that of patients with cT1-2N0 stage tumors before NAC. The clinical and treatment characteristics of these two groups did not differ significantly (Supplementary Table 1), nor did their 5-year DFS rates (79.5% vs. 93.3%, p = 0.070) (Figure 3A). By the date of the last follow-up, no patient in the cT1-2N0 group had died. The 5-year OS rates in the ypT0-2N0 non-PMRT and cT1-2N0 group also did not differ significantly (p = 0.063) (Figure 3B).
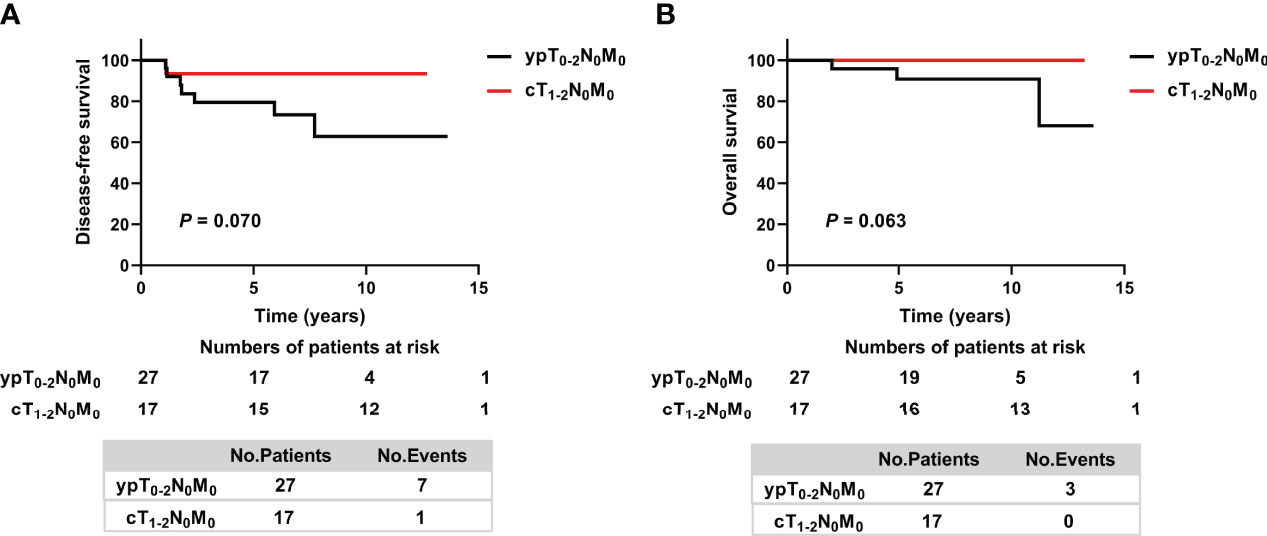
Figure 3 Kaplan–Meier analysis of (A) Disease-free survival and (B) Overall survival in cT1-2N+ patients who achieved ypT0-2N0 after NAC without PMRT and cT1-2N0 non-PMRT patients. ypT, pathologic tumor size after neoadjuvant therapy; ypN, pathologic lymph node after neoadjuvant therapy; cT, clinical tumor size; cN, clinical lymph node.
Correlations Between Clinical Variables and Prognosis in NAC Patients With Residual Nodal Disease
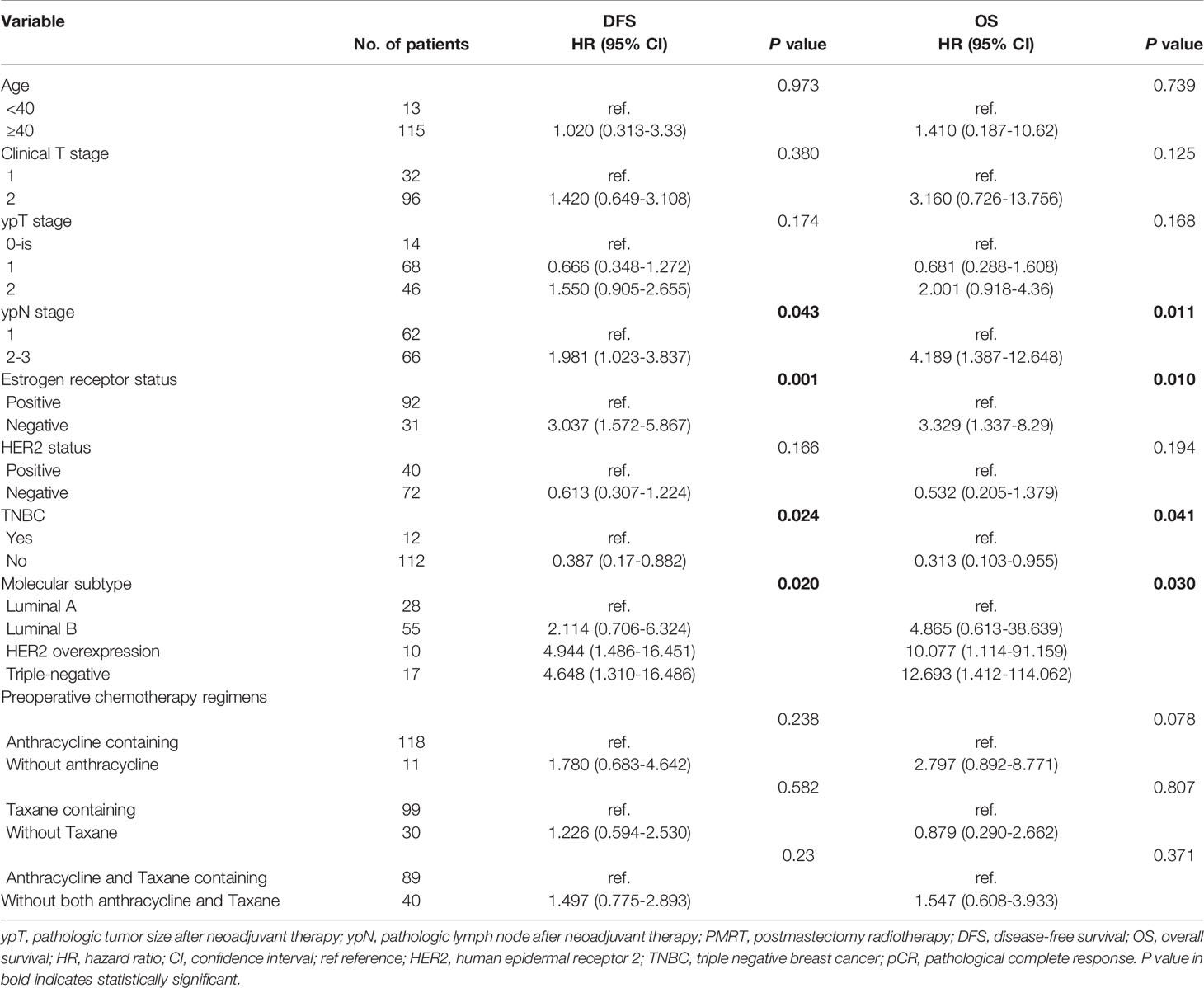
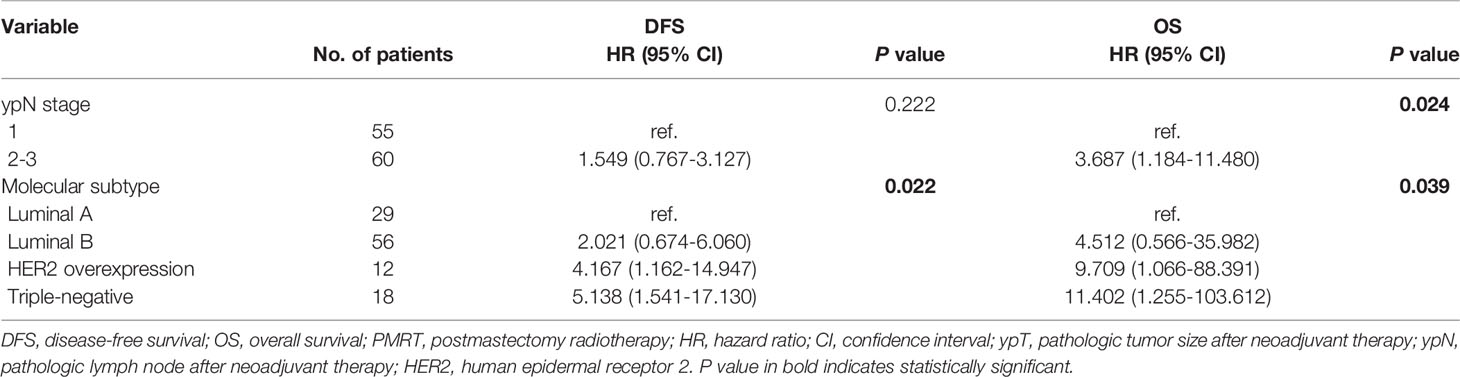
Based on National Cancer Institute guidelines (12), 128 (92.8%) of the 138 patients with residual nodal disease after NAC and surgery (ypT0-2N+) received PMRT. Analysis of the correlations between clinical variables and prognosis in these patients showed that various prognostic factors correlated with DFS and OS (Table 4). Univariate analyses showed that ypN stage, ER status and molecular subtypes, including HER2 overexpression and TNBC were significantly associated with both DFS and OS. Distant recurrence and all-cause mortality rates were was 1.98 and 4.19 times higher, respectively, in patients with ypN2-3 than in those with ypN1. Multivariate analyses showed that HER2 overexpression and TNBC were significant predictors of poorer DFS and OS, whereas ypN stage was a significant independent predictor of OS in the ypT0-2N+ PMRT subgroup (Table 5).
Indications for PMRT After NAC: Current Literature Review
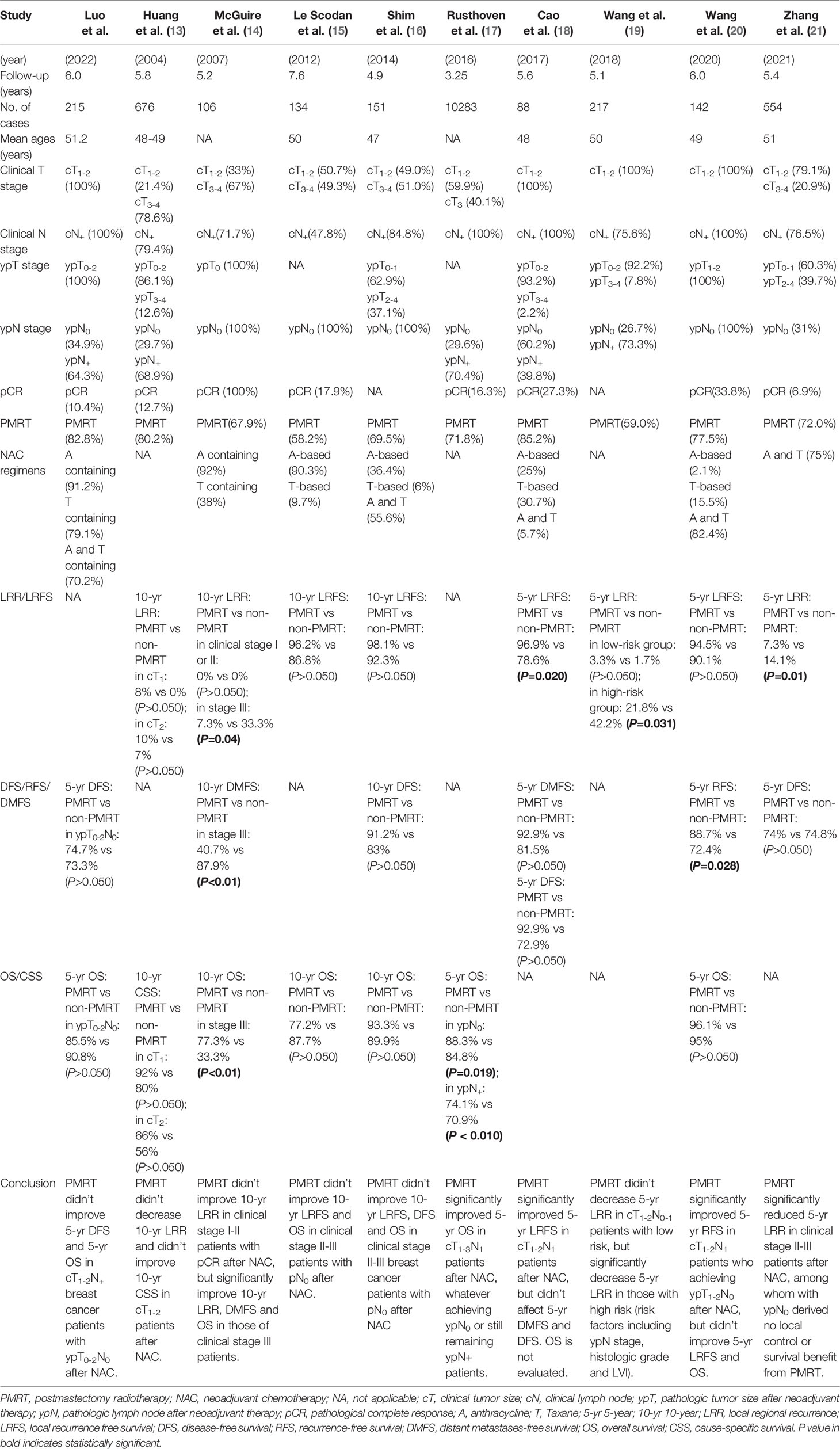
To further assess whether PMRT can benefit patients after NAC, the PubMed database was systematically searched for using the search terms, “postmastectomy radiation therapy”, “neoadjuvant chemotherapy” and “breast cancer”, for studies of PMRT after NAC published from March 1993 to November 2021. Of the 184 articles identified, nine (13–21) report results similar to ours (Table 6). Eight of these studies reported the results of event-free survival analysis, such as locoregional recurrence (LRR), local recurrence free survival (LRFS), DFS, recurrence-free survival (RFS), and distant metastasis-free survival (DMFS) (13–16, 18–21), whereas six studies reported OS and cause-specific survival (CSS) rates (13–17, 20).
Discussion
Both prospective and retrospective studies have provided evidence for recommending PMRT after NAC for breast cancer patients with cT3-4, cN2-3 or residual lymph node disease, as well as omitting PMRT for cT1-2N0-1 patients who develop a pCR (22). However, the benefits of PMRT after NAC in breast cancer patients with initial clinical stage cT1-2N+, especially for those who achieved ypT1-2N0, remain largely unknown. The present study retrospectively analyzed outcomes of PMRT after NAC in cT1-2N+ breast cancer patients in our medical center. These findings suggest that an effective response to NAC (ypT0-2N0) is indicative of a sufficiently favorable prognosis and that PMRT may be unnecessary for cT1-2N+ patients who achieve ypT0-2N0 after NAC.
Most of the clinical and treatment characteristics of patients in the ypT0-2N0 PMRT and ypT0-2N0 non-PMRT groups did not differ significantly, except for pCR rate and the percentages of HER2+ patients treated with trastuzumab. The pCR rate was lower in patients in the ypT0-2N0 non-PMRT group, which may have been due to the lower percentage of patients in this group who had completed NAC regimens (33.3% vs. 64%, p = 0.010) (Supplementary Table 2). The inclusion of patients who had received adjuvant chemotherapy in this analysis resulted in similar percentages of patients in the ypT0-2N0 PMRT and ypT0-2N0 PMRT non-PMRT groups who had completed both neoadjuvant and adjuvant chemotherapy regimens (96% vs. 92.6%, p = 0.609). The findings in this study suggest that the chemotherapy completion rate was more related to patient prognosis than the completion of NAC alone.
Of the HER2+ patients in the ypT0-2N0 group, only 55.6% were treated with trastuzumab, perhaps because this agent was not approved for local medical insurance until September 2017. The percentage of HER2+ patients receiving trastuzumab was lower in the ypT0-2N0 non-PMRT than in the ypT0-2N0 PMRT group, resulting in inadequate treatment of the former. Even under all these circumstances, however, the 5-year DFS and OS rates did not differ significantly in these two subgroups, providing further evidence that ypT0-2N0 patients have a favorable prognosis, even without PMRT.
Interestingly survival outcomes did not differ significantly in cT1-2N+ patients who achieved ypT0-2N0 without PMRT and cT1-2N0 patients before NAC. Although there were concerns that the lymph node status of cT1-2N0 might be downstaged, all the cT1-2N0 patients remained ypT1-2N0 after NAC. Based on PMRT guidelines, T1-2N0 stage patients without NAC do not need PMRT (6, 7). None of the patients in the present study with cT1-2N0 received PMRT after NAC, while having favorable 5-year DFS and OS rates. Thus, the comparable survival of cT1-2N+ patients who achieved ypT0-2N0 without PMRT and cT1-2N0 patients suggested the former can show favorable survival outcomes, even in the absence of PMRT. However, due to the limited sample size in ypT0-2N0 and cT0-2N0 subgroup, there is a possibility of increasing type I error caused by limited sample size.
Two ongoing prospective trials are addressing the need for PMRT in cT1-2N+ patients who achieve nodal pCR after NAC. The NSABP51 trial is a randomized phase III clinical trial evaluating PMRT in pathologically proven cT1-3N1 patients who convert to pN0 after NAC (www.nsabp.pitt.edu/B-51.asp). The RAPCHEM trial is prospective observational study evaluating the 5-year LRR rate in cT1-2N0-1 patients after NAC, breast surgery and radiotherapy, with protocols based on ypTNM stage (https://clinicaltrials.gov/ct2/show/study/NCT01279304). The protocol of the RAPCHEM trial resulted in the stratification of patients with ypN0 to a low risk group, with these patients not receiving PMRT.
Several previous retrospective studies have analyzed the need for PMRT in subjects who received NAC. For example, a study of 676 patients with locally advanced breast cancer, including 145 with cT1-2 stage tumors, who were treated with NAC and mastectomy, found that PMRT did not reduce LRR or improve 10-years CSS in patients with cT1-2 stage tumors (13). Although only 29.7% of patients achieved ypN0 and 68.9% had residual nodal disease after NAC, PMRT did not benefit survival. Another study of 106 cT1-4N+ breast cancer patients who achieved pCR after NAC also found that PMRT did not improve 10-year LRR in clinical stage I-II patients, but significantly improved 10-year LRR, DMFS and OS in clinical stage III patients (14), suggesting that PMRT may be more likely to improve survival in patients with more advanced stage tumors. Similarly, two other studies found that PMRT did not improve 10-year LRFS and OS in clinical stage II-III patients who achieved pN0 after NAC (15, 16). A large study in 10,283 cN+ patients found that PMRT significantly improved 5-year OS in cT1-3N1 patients after NAC, whether they achieved ypN0 or remained ypN+ (17). However, 40.1% of these patients had stage cT3 at diagnosis, a class of patients shown to benefit from PMRT, with clinical consensus indicating that PMRT after NAC can improve survival in patients with clinical stage III breast cancer (i.e., T3N1) (12). A study of 88 cT1-2N+ patients who developed ypN0 after NAC found that PMRT significantly improved 5-year LRFS, but had no effect on DMFS and DFS (18). Because 39.8% of these patients remained ypN1, a class that can gain survival benefits from PMRT, the effect of PMRT on cT1-2N+ patients who achieve ypN0 after NAC has not yet been clarified. Interestingly, a study of 217 cT1-2N0-1 patients found that PMRT significantly reduced 5-year LRR in high risk, but not in low risk, patients (19). Risk factors in that study included ypN stage, histologic grade and lymphatic vessel invasion. The cT1-2N+ patients who achieved ypN0 after NAC in our study may comparable to the low risk cT1-2N0-1 population in the previous study (19), in which PMRT did not decrease 5-year LRR. An analysis of 142 cT1-2N1 breast cancer patients found that PMRT did not improve OS in cT1-2N1 patients who achieved ypT1-2N0 after NAC, results consistent with the findings of the present study (20). That study, however, found that PMRT significantly improved RFS but not LRFS, whereas rates of locoregional recurrence and distant metastases did not difference in patients who did and did not receive PMRT. A recent study of 554 clinical stage II-III patients found that PMRT after NAC significantly reduced 5-year LRR in clinical stage II-III patients, but had no local control or survival benefit in patients with ypN0 after NAC (21).
To date, no Grade 1 evidence has shown that PMRT can be omitted in cT1-2N+ patients who achieve ypT0-2N0 after NAC. Although the present study suggests that PMRT can be omitted, this study had several limitations. First, this was a retrospective study, with selection bias between the PMRT and non-PMRT groups being the most important inherent shortcoming. Secondly, the sample size and number of events in this study are limited, which may lead to the statistical type I error. With interest, we will continue to follow up the outcomes of ypT0-2N0 PMRT and non-PMRT patients in our hospital, and may conduct a prospective study to investigate the possibility of exempting radiotherapy in cT1-2N+ breast cancer patients with ypT0-2N0 after NAC. Thirdly, data collection was subject to information bias due to the loss of individual data and limited follow-up time. Fourthly, the percentage of HER2+ patients treated with trastuzumab was low, resulting in inadequate treatment. Fifthly, incomplete neoadjuvant chemotherapy in ypT0-2N0 non-PMRT subgroup may cause lower lymph node complete response rate (ypN0), which might lead to fewer patients enrolled in ypT1-2N0 group. In addition, initial axillary lymph node status in a small proportion of patients (16.3%) was not determined by pathology.
In conclusion, this study found that PMRT did not affect survival in cT1-2N+ breast cancer patients who achieved ypT0-2N0 after NAC. Without PMRT, the prognosis of cT1-2N+ patients who achieved ypT0-2N0 after NAC did not differ significantly from that of cT1-2N0 patients, suggesting that PMRT may be safely omitted for cT1-2N+ breast cancer patients who achieve ypT0-2N0 after NAC. Prospective studies in larger patient populations are warranted.
Data Availability Statement
In this study, all the patients’ data were from the Second Affiliated Hospital, Zhejiang University School of Medicine, which are not publicly available in order to protect patient privacy, but can be accessed from the corresponding author, Dr. Jiaojiao Zhou (emhvdWpqQHpqdS5lZHUuY24=), on reasonable request.
Ethics Statement
This study was approved by the Ethics Committee of our hospital (Approval No: 2020-363) with waiver of informed consent.
Author Contributions
JZ, YC and SZ planned and designed this study. JZ, YC drew the outline of this study. JZ, ML and HC wrote the manuscript. ML, HC, HD and YJ collected the all the clinical data. ML and HC performed the data analysis. HD, YJ, KZ and HM did the follow-up of patients. ML, HC and GW participated in searching relevant literatures. All authors have read and approved the final manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 82172344, 81702866), the funding of the Key Program of the Natural Science Foundation of Zhejiang Province (Grant No. LZ16H160002), the funding of Medical Science and Technology Project of Zhejiang Province (Grant No. 2022RC174), the Fundamental Research Funds for the Central Universities (Grant No. 2021FZZX002-09)
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We gratefully thank Wenhong Xu for her valuable assistance in radiation therapy related data collection and analysis. An earlier version of this manuscript was posted as a preprint on ResearchSquare. This preprint is available at https://www.researchsquare.com/article/rs-1201823/v1 for download.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.881047/full#supplementary-material
Abbreviations
PMRT, postmastectomy radiotherapy; NAC, neoadjuvant chemotherapy; cT, clinical tumor size; cN, clinical lymph node; ypT, pathologic tumor size after neoadjuvant therapy; ypN, pathologic lymph node after neoadjuvant therapy; DFS, disease-free survival; OS, overall survival; CI, confidence interval; ER, estrogen receptor; PR, progesterone receptor; IHC, immunohistochemistry; HER2, human epidermal receptor 2; LR, locoregional recurrence; DM, distant metastasis; TNBC, triple negative breast cancer; pCR, pathological complete response; LRR, local regional recurrence; LRFS, local recurrence free survival; RFS, recurrence-free survival; DMFS, distant metastasesfreesurvival; CSS cause-specific survival.
References
1. Ikeda T, Jinno H, Matsu A, Masamura S, Kitajima M. The Role of Neoadjuvant Chemotherapy for Breast Cancer Treatment. Breast Cancer (Tokyo Japan) (2002) 9(1):8–14. doi: 10.1007/BF02967540
2. Hayes DF, Schott AF. Neoadjuvant Chemotherapy: What Are the Benefits for the Patient and for the Investigator? J Natl Cancer Inst Monogr (2015) 2015(51):36–9. doi: 10.1093/jncimonographs/lgv004
3. Group EBCTC. Long-Term Outcomes for Neoadjuvant Versus Adjuvant Chemotherapy in Early Breast Cancer: Meta-Analysis of Individual Patient Data From Ten Randomised Trials. Lancet Oncol (2018) 19(1):27–39. doi: 10.1016/S1470-2045(17)30777-5
4. Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, et al. Effect of Preoperative Chemotherapy on Local-Regional Disease in Women With Operable Breast Cancer: Findings From National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol (1997) 15(7):2483–93. doi: 10.1200/JCO.1997.15.7.2483
5. Kuerer HM, Sahin AA, Hunt KK, Newman LA, Breslin TM, Ames FC, et al. Incidence and Impact of Documented Eradication of Breast Cancer Axillary Lymph Node Metastases Before Surgery in Patients Treated With Neoadjuvant Chemotherapy. Ann Surg (1999) 230(1):72–8. doi: 10.1097/00000658-199907000-00011
6. Taylor ME, Haffty BG, Rabinovitch R, Arthur DW, Halberg FE, Strom EA, et al. Acr Appropriateness Criteria on Postmastectomy Radiotherapy Expert Panel on Radiation Oncology-Breast. Int J Radiat Oncol Biol Phys (2009) 73(4):997–1002. doi: 10.1016/j.ijrobp.2008.10.080
7. Recht A, Comen EA, Fine RE, Fleming GF, Hardenbergh PH, Ho AY, et al. Postmastectomy Radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline Update. J Clin Oncol (2016) 34(36):4431–42. doi: 10.1200/JCO.2016.69.1188
8. Kronowitz SJ, Robb GL. Breast Reconstruction With Postmastectomy Radiation Therapy: Current Issues. Plast Reconstr Surg (2004) 114(4):950–60. doi: 10.1097/01.prs.0000133200.99826.7f
9. Hammond MEH, Hayes DF, Wolff AC, Mangu PB, Temin S. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. J Oncol Pract (2010) 6(4):195–7. doi: 10.1200/JOP.777003
10. Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol (2018) 36(20):2105–22. doi: 10.1200/JCO.2018.77.8738
11. Huo D, Hu H, Rhie SK, Gamazon ER, Cherniack AD, Liu J, et al. Comparison of Breast Cancer Molecular Features and Survival by African and European Ancestry in the Cancer Genome Atlas. JAMA Oncol (2017) 3(12):1654–62. doi: 10.1001/jamaoncol.2017.0595
12. Buchholz TA, Lehman CD, Harris JR, Pockaj BA, Khouri N, Hylton NF, et al. Statement of the Science Concerning Locoregional Treatments After Preoperative Chemotherapy for Breast Cancer: A National Cancer Institute Conference. J Clin Oncol (2008) 26(5):791–7. doi: 10.1200/JCO.2007.15.0326
13. Huang EH, Tucker SL, Strom EA, McNeese MD, Kuerer HM, Buzdar AU, et al. Postmastectomy Radiation Improves Local-Regional Control and Survival for Selected Patients With Locally Advanced Breast Cancer Treated With Neoadjuvant Chemotherapy and Mastectomy. J Clin Oncol (2004) 22(23):4691–9. doi: 10.1200/JCO.2004.11.129
14. McGuire SE, Gonzalez-Angulo AM, Huang EH, Tucker SL, Kau S-WC, Yu T-K, et al. Postmastectomy Radiation Improves the Outcome of Patients With Locally Advanced Breast Cancer Who Achieve a Pathologic Complete Response to Neoadjuvant Chemotherapy. Int J Radiat Oncol Biol Phys (2007) 68(4):1004–9. doi: 10.1016/j.ijrobp.2007.01.023
15. Le Scodan R, Selz J, Stevens D, Bollet MA, de la Lande B, Daveau C, et al. Radiotherapy for Stage Ii and Stage Iii Breast Cancer Patients With Negative Lymph Nodes After Preoperative Chemotherapy and Mastectomy. Int J Radiat Oncol Biol Phys (2012) 82(1):e1–7. doi: 10.1016/j.ijrobp.2010.12.054
16. Shim SJ, Park W, Huh SJ, Choi DH, Shin KH, Lee NK, et al. The Role of Postmastectomy Radiation Therapy After Neoadjuvant Chemotherapy in Clinical Stage Ii-Iii Breast Cancer Patients With Pn0: A Multicenter, Retrospective Study (Krog 12-05). Int J Radiat Oncol Biol Phys (2014) 88(1):65–72. doi: 10.1016/j.ijrobp.2013.09.021
17. Rusthoven CG, Rabinovitch RA, Jones BL, Koshy M, Amini A, Yeh N, et al. The Impact of Postmastectomy and Regional Nodal Radiation After Neoadjuvant Chemotherapy for Clinically Lymph Node-Positive Breast Cancer: A National Cancer Database (Ncdb) Analysis. Ann Oncol (2016) 27(5):818–27. doi: 10.1093/annonc/mdw046
18. Cao L, Ou D, Shen KW, Cai G, Cai R, Xu F, et al. Outcome of Postmastectomy Radiotherapy After Primary Systemic Treatment in Patients With Clinical T1-2n1 Breast Cancer. Cancer Radiotherapie (2018) 22(1):38–44. doi: 10.1016/j.canrad.2017.07.051
19. Wang X, Xu L, Yin Z, Wang D, Wang Q, Xu K, et al. Locoregional Recurrence-Associated Factors and Risk-Adapted Postmastectomy Radiotherapy for Breast Cancer Staged in Ct1-2n0-1 After Neoadjuvant Chemotherapy. Cancer Manag Res (2018) 10:4105–12. doi: 10.2147/CMAR.S173628
20. Wang Q, Zhao J, Han X, Er P, Meng X, Shi J, et al. Is There a Role for Post-Mastectomy Radiotherapy for T1-2n1 Breast Cancers With Node-Positive Pathology After Patients Become Node-Negative Pathology Following Neoadjuvant Chemotherapy? Front Oncol (2020) 10:892. doi: 10.3389/fonc.2020.00892
21. Zhang Y, Zhang Y, Liu Z, Qin Z, Li Y, Zhao J, et al. Impact of Postmastectomy Radiotherapy on Locoregional Control and Disease-Free Survival in Patients With Breast Cancer Treated With Neoadjuvant Chemotherapy. J Oncol (2021) 2021:6632635. doi: 10.1155/2021/6632635
Keywords: postmastectomy radiotherapy, disease-free survival, overall survival, breast cancer, neoadjuvant chemotherapy
Citation: Luo M, Chen H, Deng H, Jin Y, Wang G, Zhang K, Ma H, Chen Y, Zhang S and Zhou J (2022) Postmastectomy Radiotherapy After Neoadjuvant Chemotherapy in cT1-2N+ Breast Cancer Patients: A Single Center Experience and Review of Current Literature. Front. Oncol. 12:881047. doi: 10.3389/fonc.2022.881047
Received: 22 February 2022; Accepted: 18 April 2022;
Published: 17 May 2022.
Edited by:
San-Gang Wu, First Affiliated Hospital of Xiamen University, ChinaReviewed by:
Chang Ik Yoon, The Catholic University of Korea, South KoreaViola Salvestrini, Careggi University Hospital, Italy
Copyright © 2022 Luo, Chen, Deng, Jin, Wang, Zhang, Ma, Chen, Zhang and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiaojiao Zhou, emhvdWpqQHpqdS5lZHUuY24=; Suzhan Zhang, enVjaUB6anUuZWR1LmNu; Yiding Chen, eWRjaGVuQHpqdS5lZHUuY24=
†These authors have contributed equally to this work
 Meng Luo
Meng Luo Huihui Chen
Huihui Chen Hao Deng1
Hao Deng1 Yiding Chen
Yiding Chen Jiaojiao Zhou
Jiaojiao Zhou