- 1Department of Surgical Oncology, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Department of General Surgery, The Children’s Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 3Department of Medical Oncology, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 4Department of Nuclear Medicine, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 5Department of Pathology, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 6Department of Surgery, New York Presbyterian Hospital and Weill Medical College of Cornell University, New York, NY, United States
Background: Thyroid autoimmunity is common in papillary thyroid carcinoma (PTC) and was believed to confer a better prognosis; however, controversy still remains. This study aimed to investigate the prognostic value of chronic lymphocytic thyroiditis (CLT) and preoperative thyroid peroxidase antibody (TPOAb) in PTC patients.
Methods: A retrospective analysis was performed on 5,770 PTC patients who underwent surgical treatment with pathologically confirmed PTC in our institution between 2012 to 2016. The patients were divided into groups with respect to the coexistence of CLT or preoperative TPOAb levels. The clinicopathological characteristics and disease-free survival (DFS) rates were compared between the groups.
Results: The coexistence of CLT was likely to have bilateral, multifocal tumors. Particularly, PTC patients with TPOAb++ (>1,000 IU/L) had a larger tumor size (p = 0.007) and higher rates of bilaterality and multifocality than those with TPOAb− (TPOAb< 100 IU/L), while for lymph node metastasis and extrathyroidal extension, there is no statistical difference. Tumor recurrence was found in 15 of 425 (3.5%), 9 of 436 (2.1%), and 56 of 3,519 (1.6%) patients with TPOAb++, TPOAb+, and TPOAb−, respectively (p = 0.017). On univariate analysis, TPOAb++ was correlated with tumor recurrence, with a hazard ratio of 2.20 [95% confidence interval (CI), 1.25–3.89], which remained as an independent risk factor at 1.98 (95% CI, 1.10–3.55) on multivariate analysis. PTC patients with TPOAb++ had the lowest DFS rates (96.5 vs. 97.9 vs. 98.4%, p = 0.020).
Conclusion: CLT is not a protective factor in PTC patients. We provide initial evidence that the preoperative TPOAb instead predicts recurrence in papillary thyroid carcinoma.
Introduction
Thyroid cancer ranks as the most common endocrine malignancy, and its overall incidence increases annually (1, 2). Papillary thyroid carcinoma (PTC) is the predominant histological type, representing more than 90% of all thyroid cancer cases (3). Chronic lymphocytic thyroiditis (CLT) is an autoimmune disease accompanied by the presence of thyroid autoantibody in the blood plasma (4). The prevalence of CLT in PTC has been reported to range from 5 to 38% (5–7). However, the relationship between CLT and PTC remains in dispute. Early studies suggested that preexisting CLT promoted the tumorigenesis of PTC (8–10), but it appeared to confer a better prognosis (8, 11–15). In contrast, some other studies showed that CLT did not interfere with prognosis or even predicted poorer outcomes (16–18).
Thyroid peroxidase antibody (TPOAb) is a serological marker of CLT (19). Its levels can be measured preoperatively and quantitatively and may represent the degree of thyroid inflammation (19, 20). An elevated TPOAb level has been reported to be potentially associated with the development of thyroid cancer (21). However, whether preoperative TPOAb levels correlate with recurrence in PTC patients remains unclear.
In the current study, we aimed to investigate the clinical value of coexistent CLT in a cohort of 5,770 consecutive PTC patients. We also evaluated the clinicopathologic and prognostic significance of preoperative TPOAb levels in these cases. The large sample size provided us with an opportunity to study the prognostic effect of TPOAb levels in PTCs.
Materials and Methods
Subjects
The medical records of 6,723 patients who underwent total or hemithyroidectomy with a final histopathological diagnosis of PTC at the First Affiliated Hospital, Zhejiang University School of Medicine (Hangzhou, China), from 2012 to 2016, were retrospectively reviewed. In total, 190 patients with a history of thyroidectomy, 141 patients with a history of thyroid disease or radiation exposure, and 622 patients lacking preoperative TPOAb levels were excluded. A total of 5,770 patients were finally included in the retrospective study (Figure 1). Among them, 2,775 (48.1%) cases underwent hemi-thyroidectomy, and 2,995 (51.9%) cases underwent total thyroidectomy. This study was approved by the Institutional Review Board of the First Affiliated Hospital, Zhejiang University School of Medicine.
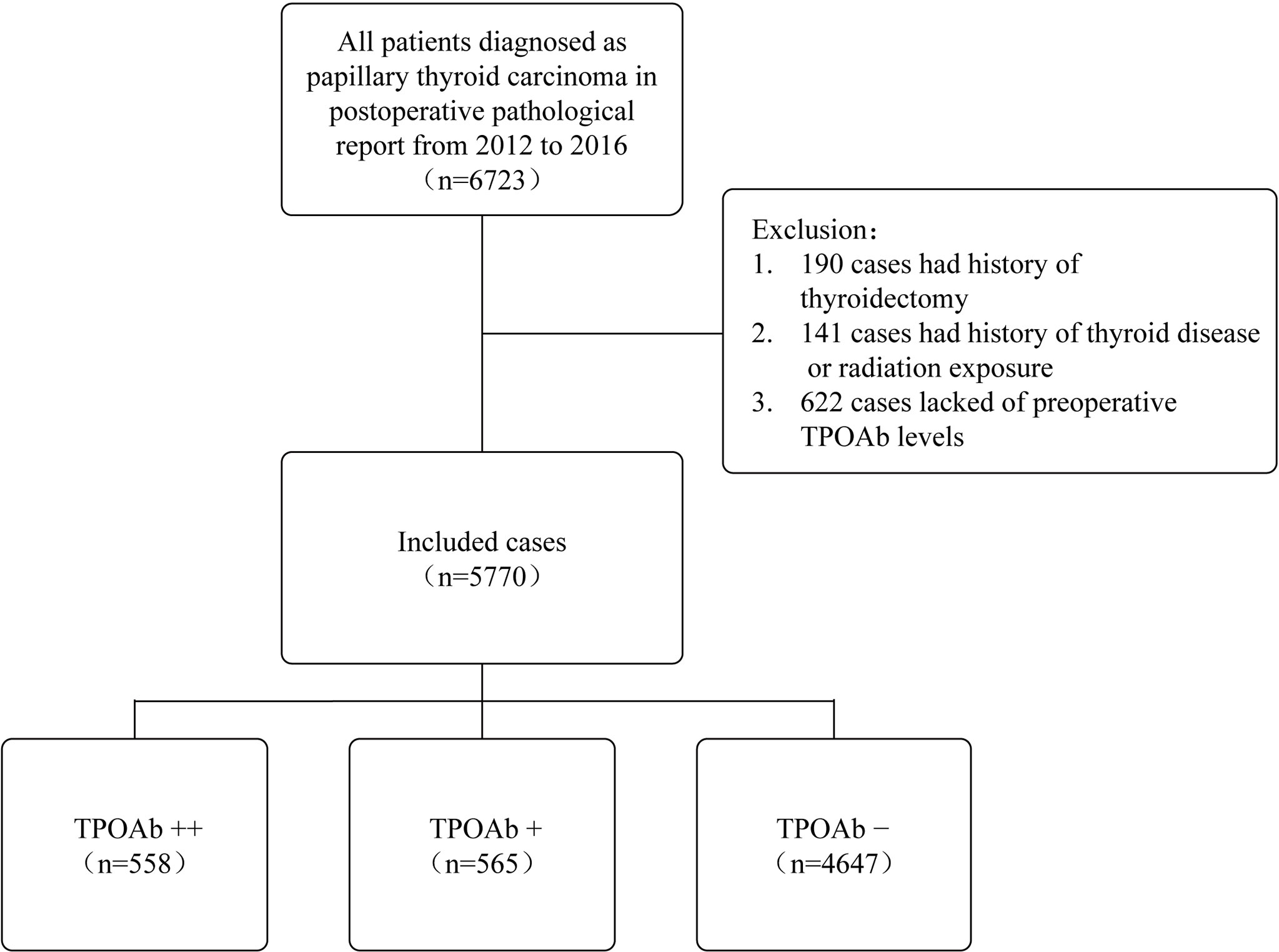
Figure 1 Case selection process for this study. TPOAb, thyroid peroxidase antibody; TPOAb−, 0 < TPOAb ≤ 100 IU/L; TPOAb+, 100 < TPOAb ≤ 1,000 IU/L; TPOAb++, TPOAb > 1,000 IU/L.
Data Collection
The following clinical variables were collected from the registry: age, gender, preoperative serum levels of free triiodothyronine (FT3), free thyroxine (FT4), thyrotrophin (TSH), and TPOAb. TPOAb was measured by an automated chemiluminescent immunoassay system (Advia Centaur; Siemens, Munich, Germany) at 1 to 2 days before surgery. The postoperative pathological reports, including tumor size, bilaterality, multifocality, extrathyroidal extension (ETE), lymph node metastasis (LNM), and tumor stage (AJCC 8th), were also studied. According to previous studies, CLT was diagnosed based on either preoperative TPOAb higher than 100 IU/L or the presence of diffuse lymphocytic infiltration in the surrounding thyroid tissue (12, 22). For multifocal tumors, the maximum diameter was recognized as the tumor size. Additionally, we defined TPOAb+ when its serum level fell in between 100 and 1,000 IU/L (100 < TPOAb ≤ 1,000 IU/L) and defined TPOAb++ when the serum level was 10 times higher than the normal value (TPOAb > 1,000 IU/L). The normal ranges for the serum levels of FT4, FT3, TSH, and TPOAb were 10.45–24.38 pmol/L, 2.77–6.31 pmol/L, 0.380–4.340 mIU/L, and 0–100.0 IU/L, respectively, at our institution.
At the end of the study, 4,380 patients were available for survival analysis. These patients were followed postoperatively with measurements of serum thyroglobulin and thyroglobulin antibody, neck ultrasound, and iodine-131 whole-body scans to monitor for disease recurrence and survival. The mean follow-up was 3.5 years (range, 1–5 years). A total of 696 (15.9%) cases received adjuvant radioactive iodine (RAI) treatment. Moreover, 80 of 4,380 patients (1.8%) were diagnosed with recurrent disease or metastases, including 17 in the thyroid bed, 60 in the cervical lymph nodes, and 3 with lung metastases. In most patients, recurrence was confirmed by pathologic examination, while 10 patients were diagnosed based on increased thyroglobulin levels and imaging evidence from iodine-131 scans.
Groups and Comparisons
The clinicopathological features and disease outcomes were assessed between the 2 groups according to the coexistence of CLT or not. Based on the preoperative TPOAb levels, we also divided all PTC patients into those with TPOAb−, TPOAb+, and TPOAb++ to investigate the prognostic values of different preoperative TPOAb levels.
Statistical Analysis
The SPSS 25.0 software was used for all statistical analysis. Statistical significance was defined as a 2-tailed p-value of less than 0.05. Continuous variables were presented as mean ± SD, and categorical variables were presented as the number of cases, with percentage (%). Pearson’s chi-square test was used for categorical variables, and Student’s t-test or one-way analysis of variance was used for continuous variables. The variables associated with clinical outcomes were evaluated using Cox proportional hazard models in univariate/multivariate analyses. Disease-free survival (DFS) curves were constructed using the Kaplan–Meier method, and the log-rank test was used to compare DFS.
Results
Coexistent CLT Did Not Predict a Better Outcome in PTC Patients
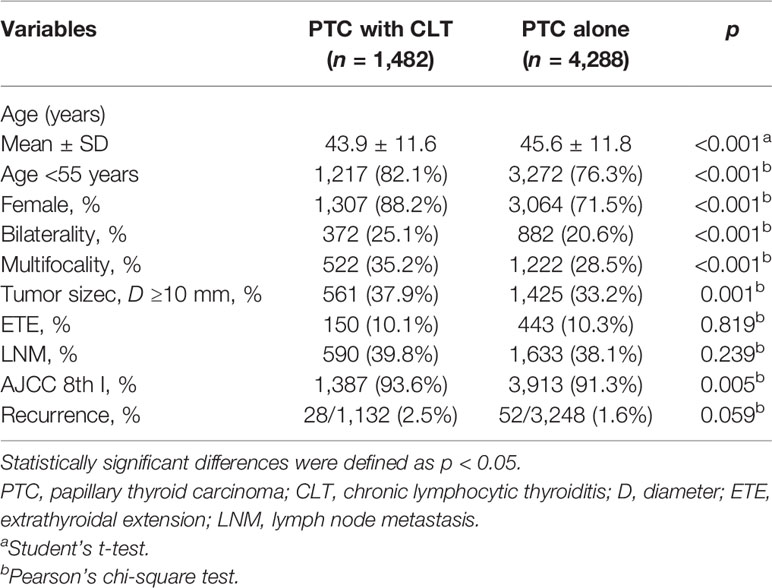
CLT was present in 1,482 of 5,770 (25.7%) PTC patients, and their clinicopathologic characteristics are shown in Table 1. The PTC patients with CLT were significantly correlated with a younger age (43.9 ± 11.6 vs. 45.6 ± 11.8, p < 0.001), female gender (88.2 vs. 71.5%, p < 0.001), more bilateral (25.1 vs. 20.6%, p < 0.001) and multifocal (35.2 vs. 28.5%, p < 0.001) tumors as well as a higher proportion of early stage (AJCC 8th stage I: 93.6 vs. 91.3%, p = 0.005). No difference was observed between the 2 groups regarding the prevalence of ETE (10.1 vs. 10.3%, p = 0.819) and the frequency of LNM (39.8 vs. 38.1%, p = 0.239). For disease recurrence, however, we even found that PTC patients with CLT had a relatively higher recurrence rate than those without CLT, with borderline significance (2.5 vs. 1.6%, p = 0.059).
Preoperative TPOAb Levels Correlate With Aggressive Clinicopathological Features
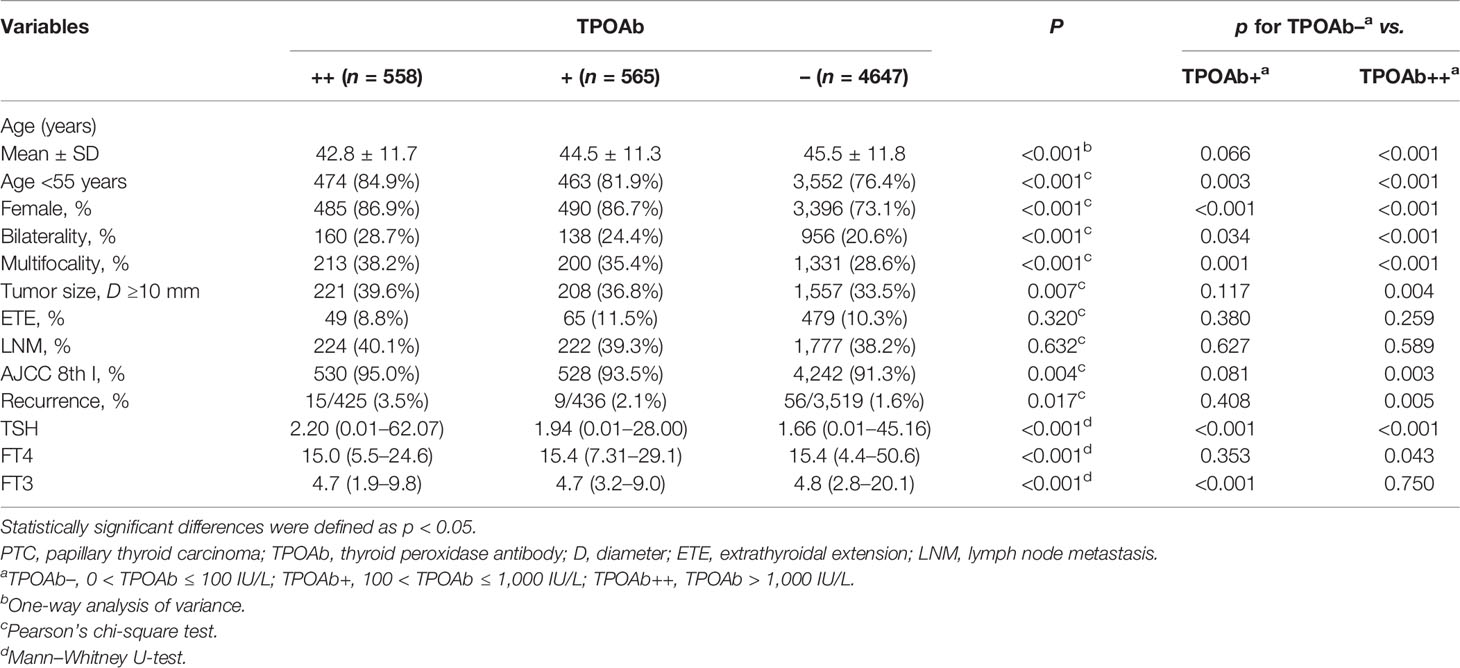
TPOAb is the best serological marker of CLT. To further investigate the role of CLT in PTC, we stratified the PTC patients into TPOAb++, TPOAb+, and TPOAb− groups according to the preoperative TPOAb levels (Table 2). PTCs with higher TPOAb levels tended to exhibit a younger age (42.8 ± 11.7 vs. 44.5 ± 11.3 vs. 45.5 ± 11.8, p < 0.001), female preponderance (86.9% vs. 86.7% vs. 73.1%, p < 0.001), higher rates of bilaterality (28.7% vs. 24.4% vs. 20.6%, p < 0.001) and multifocality (38.2% vs. 35.4% vs. 28.6%, p < 0.001). However, there was no difference among the 3 groups regarding the prevalence of ETE (8.8% vs. 11.5% vs. 10.3%, p = 0.320) or LNM (40.1% vs. 39.3% vs. 38.2%, p = 0.632). Furthermore, we found that TPOAb ++ group was significantly characterized by younger age, female preponderance and more aggressive features: higher rates of bilaterality, multifocality, tumor size ≥ 10mm and recurrence. Additionally, we compared the levels of TPOAb with the pathological features on inflammation degree. Oxyphilic metaplasia, follicular atrophy or follicular disruption, which indicate high degree of thyroid inflammation (19), were more frequently found in TPOAb++ group. Accompanied higher TSH levels indicated more severe destruction of thyroid follicular cells in these patients. (Supplementary Table S1).
Preoperative TPOAb ++ Was an Independent Risk Factor for Disease Recurrence
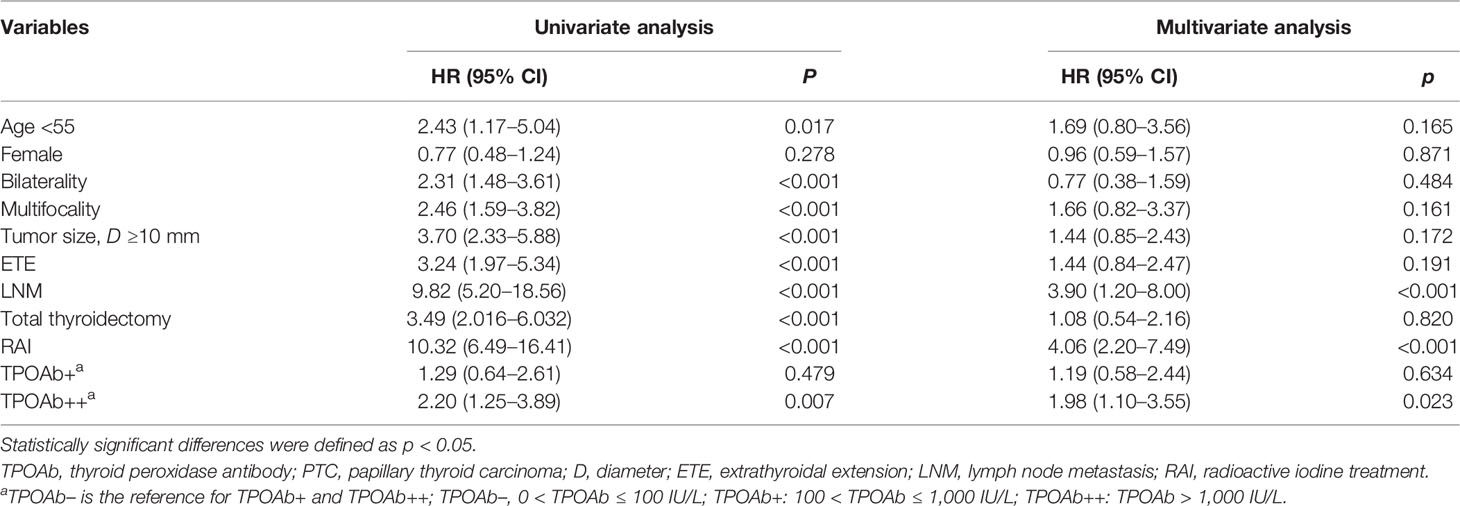
We then investigated the prognostic value of preoperative TPOAb levels. Tumor recurrence was found in 15 of 425 (3.5%), 9 of 436 (2.1%), and 56 of 3,519 (1.6%) PTC patients in the TPOAb++, TPOAb+, and TPOAb– groups, respectively (p = 0.017, Table 2). Univariate analysis revealed that age <55, bilaterality, multifocality, tumor size ≥10 mm, ETE, LNM, total thyroidectomy, RAI, and TPOAb++ were significantly associated with tumor recurrence (Table 3). By multivariate Cox analysis, LNM [hazard ratio (HR), 3.90; 95% CI, 1.20–8.00; p < 0.001], RAI (HR, 4.06; 95% CI, 2.20–7.49; p < 0.001), and TPOAb++ (HR, 1.98; 95% CI, 1.10–3.55; p = 0.023) were identified as independent risk factors for tumor recurrence.
Furthermore, we compared the Kaplan–Meier DFS curves with respect to the risk factors identified above. The 3.5-year DFS rates of those patients with tumor size ≥10 mm (96.5 vs. 99.1%, p < 0.001, shown in Figure 2A), ETE (95.4 vs. 98.5%, p < 0.001, shown in Figure 2B), and LNM (96.0 vs. 99.6%, p < 0.001, shown in Figure 2C) were significantly lower than those of the control group. It is noteworthy that the lowest DFS curve was documented in the TPOAb++ group compared with the TPOAb+ and TPOAb– groups (96.5 vs. 97.9% vs. 98.4%, p = 0.020, shown in Figure 2D).
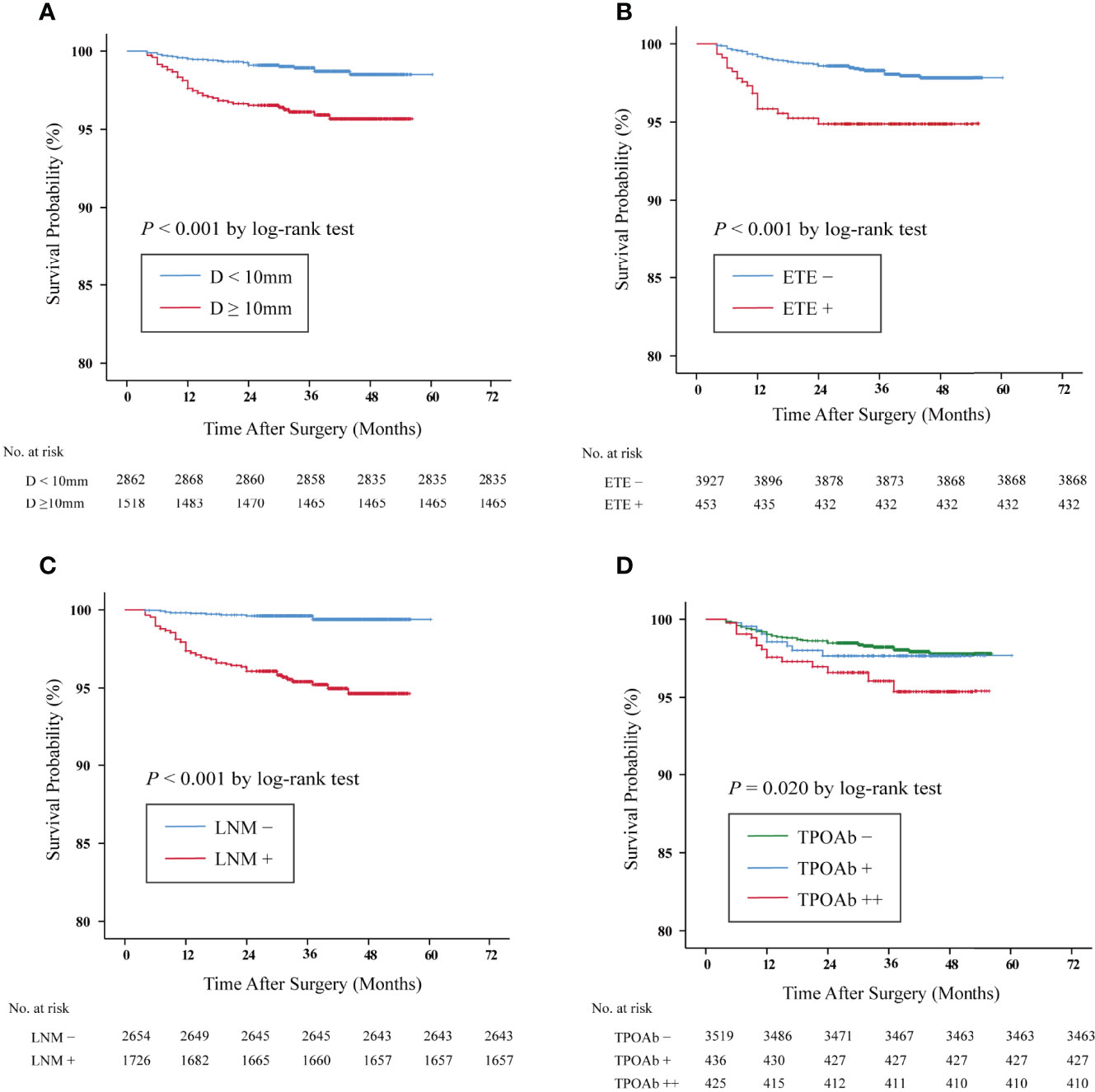
Figure 2 Kaplan–Meier survival curves of disease-free survival (DFS) in papillary thyroid carcinoma (PTC) patients. The DFS rates of PTC patients with (A) tumor size ≥10 mm vs. <10 mm: 96.5 vs. 99.1%, p < 0.001. (B) Extrathyroidal extension (ETE) + vs. ETE–, 95.4 vs. 98.5%, p < 0.001. (C) Lymph node metastasis (LNM)+ vs. LNM–: 96.0 vs. 99.6%, p < 0.001. (D) The DFS rates of the TPOAb++ group were significantly lower than those of the TPOAb– group (p = 0.020 by log-rank test, TPOAb++ vs. TPOAb −, p = 0.005; TPOAb++ vs. TPOAb+, p = 0.195; TPOAb+ vs. TPOAb−, p = 0.476). TPOAb−, 0 < TPOAb ≤ 100 IU/L; TPOAb+, 100 < TPOAb ≤ 1,000 IU/L; TPOAb++, TPOAb > 1,000 IU/L.
Discussion
CLT is frequently found in PTC, but its prognostic implication in PTC remains an active focus of research and is still under debate. Previous studies displayed that coexistent CLT was associated with a lower rate of ETE and lymph node metastasis in PTC patients (12, 23), while very recently, Lee et al. found more frequent multifocality and ETE in PTC patients coexisting with CLT, but with a similar recurrence rate than those without CLT (17). In the present study with 5,770 cases, we demonstrated that CLT was not a protective factor for PTC patients. Coexistent CLT was instead attributed to a larger tumor size, bilaterality, multifocality, and a relatively lower DFS rate. Notably, a high preoperative TPOAb level (>1,000 IU/L) was identified as an independent risk factor for tumor recurrence in PTC patients. Our findings may be partially explained by a recent study which implied that lymphovascular and perineural invasion was more common in the PTCs with CLT group (24).
TPOAb is measurable preoperatively and regarded as a sensitive marker of CLT or thyroid autoimmunity (25). The discrepancy concerning the role of coexistent CLT in predicting the prognosis of PTC could partially result from the different degree of thyroid inflammation. Previous studies showed that an elevated TPOAb level might contribute to the development of thyroid cancer (26, 27), while a recent meta-analysis found that although positive TPOAb was associated with an increased risk of thyroid cancer, this association did not exist in some subgroups (28). They argued that the relation between positive TPOAb and the risk of thyroid cancer remained to be elucidated (28). On the other hand, elevated TPOAb appeared as a protective factor for lymph node metastasis (21, 29). However, Shen et al. argued that TPOAb positivity was a risk indicator for more metastatic cervical lymph nodes (30). Recently, Song et al. demonstrated that positive TPOAb was associated with less tumor recurrence in PTC patients (31). Not conducting a subgroup analysis according to the TPOAb values might account in part for these inconsistent conclusions. The levels of TPOAb have been demonstrated to correlate with the degree of thyroid inflammation in autoimmune thyroiditis (19, 20). A higher level of TPOAb may represent a severe degree of inflammation. Here we graded the severity of autoimmune inflammation by preoperative TPOAb levels and found that TPOAb++ was an independent risk factor for disease recurrence. PTC patients with TPOAb++ exhibited the shortest DFS, indicating that a higher degree of thyroid inflammation could predict tumor recurrence in PTC patients.
The extent of initial thyroidectomy is a major concern when treating PTC patients, and it is usually determined by multiple factors. Here we found a much higher incidence of bilaterality and multifocality in patients with CLT (25.1 vs. 20.6 and 35.2 vs. 28.5%, respectively). Particularly, in patients with TPOAb++, the possibility of bilaterality and multifocality was as high as 28.7 and 38.2%. Therefore, total thyroidectomy may be favored in these PTC patients. However, it seems not rational to perform a more aggressive prophylactic cervical lymph node dissection for PTCs with CLT since the rate for lymph node metastasis was almost the same as that of patients without CLT (39.8 vs.38.1%).
The current study has some certain limitations. Primarily, it is a retrospective, single-institution study. In addition, because of the favorable prognosis of PTC, our follow-up period is not long enough to uncover the true prognostic significance. We plan to continue this study to obtain longer outcomes. Another limitation is that we do not have thyroglobulin antibody data, which could serve as another clinical marker for CLT (32). Lastly, the current study lacks information of genetic alterations like BRAF and RET/PTC rearrangements, which correlate with CLT and may impact the patients’ outcomes.
In conclusion, our large retrospective cohort study demonstrated that CLT is not a protective factor in PTC patients. We instead provide new evidence that a high TPOAb level (>1,000 IU/L) correlates aggressive features, including larger tumor size, bilaterality, and multifocality, and is an independent risk factor for tumor recurrence. Hence, a preoperative evaluation of the TPOAb level is worthwhile for risk stratification and post-treatment surveillance in PTC patients.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by The Clinical Research Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
WW, LW, SC, LT, and ZL designed the current study and wrote the manuscript. WW, LW, ZM, and YD conducted the statistical analyses. WW, LW, SC, XS, ZM, YD, ZC, YC, JR, JY, JZ, and XT created the original databases to collect the clinicopathological data. TF, ZL, and LT reviewed and revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by the National Natural Science Foundation of China (numbers 81772853, 81972495, 81902719 and 82102758), the National Natural Science Foundation of Zhejiang (number LY18H160012), and the Key Project of Scientific and Technological Innovation of Zhejiang Province (number 2015C03031).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.881024/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global Cancer Statistics 2018: Globocan Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in Thyroid Cancer Incidence and Mortality in the United States, 1974-2013. JAMA (2017) 317(13):1338–48. doi: 10.1001/jama.2017.2719
3. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
4. Ahmed R, Al-Shaikh S, Akhtar M. Hashimoto Thyroiditis: A Century Later. Adv Anat Pathol (2012) 19(3):181–6. doi: 10.1097/PAP.0b013e3182534868
5. Dailey ME, Lindsay S, Skahen R. Relation of Thyroid Neoplasms to Hashimoto Disease of the Thyroid Gland. AMA Arch Surg (1955) 70(2):291–7. doi: 10.1001/archsurg.1955.01270080137023
6. Matsubayashi S, Kawai K, Matsumoto Y, Mukuta T, Morita T, Hirai K, et al. The Correlation Between Papillary Thyroid Carcinoma and Lymphocytic Infiltration in the Thyroid Gland. J Clin Endocrinol Metab (1995) 80(12):3421–4. doi: 10.1210/jcem.80.12.8530576
7. Kashima K, Yokoyama S, Noguchi S, Murakami N, Yamashita H, Watanabe S, et al. Chronic Thyroiditis as a Favorable Prognostic Factor in Papillary Thyroid Carcinoma. Thyroid (1998) 8(3):197–202. doi: 10.1089/thy.1998.8.197
8. Lee JH, Kim Y, Choi JW, Kim YS. The Association Between Papillary Thyroid Carcinoma and Histologically Proven Hashimoto's Thyroiditis: A Meta-Analysis. Eur J Endocrinol (2013) 168(3):343–9. doi: 10.1530/EJE-12-0903
9. de Paiva CR, Gronhoj C, Feldt-Rasmussen U, von Buchwald C. Association Between Hashimoto's Thyroiditis and Thyroid Cancer in 64,628 Patients. Front Oncol (2017) 7:53. doi: 10.3389/fonc.2017.00053
10. Paparodis RD, Karvounis E, Bantouna D, Chourpiliadis C, Chourpiliadi H, Livadas S, et al. Incidentally Discovered Papillary Thyroid Microcarcinomas Are More Frequently Found in Patients With Chronic Lymphocytic Thyroiditis Than With Multinodular Goiter or Graves' Disease. Thyroid (2020) 30(4):531–5. doi: 10.1089/thy.2019.0347
11. Kwak HY, Chae BJ, Eom YH, Hong YR, Seo JB, Lee SH, et al. Does Papillary Thyroid Carcinoma Have a Better Prognosis With or Without Hashimoto Thyroiditis? Int J Clin Oncol (2015) 20(3):463–73. doi: 10.1007/s10147-014-0754-7
12. Dvorkin S, Robenshtok E, Hirsch D, Strenov Y, Shimon I, Benbassat CA. Differentiated Thyroid Cancer Is Associated With Less Aggressive Disease and Better Outcome in Patients With Coexisting Hashimotos Thyroiditis. J Clin Endocrinol Metab (2013) 98(6):2409–14. doi: 10.1210/jc.2013-1309
13. Kim EY, Kim WG, Kim WB, Kim TY, Kim JM, Ryu JS, et al. Coexistence of Chronic Lymphocytic Thyroiditis Is Associated With Lower Recurrence Rates in Patients With Papillary Thyroid Carcinoma. Clin Endocrinol (Oxf) (2009) 71(4):581–6. doi: 10.1111/j.1365-2265.2009.03537.x
14. Ieni A, Vita R, Magliolo E, Santarpia M, Di Bari F, Benvenga S, et al. One-Third of an Archivial Series of Papillary Thyroid Cancer (Years 2007-2015) Has Coexistent Chronic Lymphocytic Thyroiditis, Which Is Associated With a More Favorable Tumor-Node-Metastasis Staging. Front Endocrinol (Lausanne) (2017) 8:337. doi: 10.3389/fendo.2017.00337
15. Kim SK, Woo JW, Lee JH, Park I, Choe JH, Kim JH, et al. Chronic Lymphocytic Thyroiditis and Braf V600e in Papillary Thyroid Carcinoma. Endocr Relat Cancer (2016) 23(1):27–34. doi: 10.1530/ERC-15-0408
16. Singh B, Shaha AR, Trivedi H, Carew JF, Poluri A, Shah JP. Coexistent Hashimoto's Thyroiditis With Papillary Thyroid Carcinoma: Impact on Presentation, Management, and Outcome. Surgery (1999) 126(6):1070–6. doi: 10.1067/msy.2099.101431
17. Lee I, Kim HK, Soh EY, Lee J. The Association Between Chronic Lymphocytic Thyroiditis and the Progress of Papillary Thyroid Cancer. World J Surg (2020) 44(5):1506–13. doi: 10.1007/s00268-019-05337-9
18. Dobrinja C, Makovac P, Pastoricchio M, Cipolat Mis T, Bernardi S, Fabris B, et al. Coexistence of Chronic Lymphocytic Thyroiditis and Papillary Thyroid Carcinoma. Impact on Presentation, Management, and Outcome. Int J Surg (2016) 28 Suppl 1:S70–4. doi: 10.1016/j.ijsu.2015.12.059
19. Rho MH, Kim DW, Hong HP, Park YM, Kwon MJ, Jung SJ, et al. Diagnostic Value of Antithyroid Peroxidase Antibody for Incidental Autoimmune Thyroiditis Based on Histopathologic Results. Endocrine (2012) 42(3):647–52. doi: 10.1007/s12020-012-9695-y
20. Guan H, de Morais NS, Stuart J, Ahmadi S, Marqusee E, Kim MI, et al. Discordance of Serological and Sonographic Markers for Hashimoto's Thyroiditis With Gold Standard Histopathology. Eur J Endocrinol (2019) 181(5):539–44. doi: 10.1530/EJE-19-0424
21. Paparodis R, Imam S, Todorova-Koteva K, Staii A, Jaume JC. Hashimoto's Thyroiditis Pathology and Risk for Thyroid Cancer. Thyroid (2014) 24(7):1107–14. doi: 10.1089/thy.2013.0588
22. Grani G, Carbotta G, Nesca A, D'Alessandri M, Vitale M, Del Sordo M, et al. A Comprehensive Score to Diagnose Hashimoto's Thyroiditis: A Proposal. Endocrine (2015) 49(2):361–5. doi: 10.1007/s12020-014-0441-5
23. Loh KC, Greenspan FS, Dong F, Miller TR, Yeo PP. Influence of Lymphocytic Thyroiditis on the Prognostic Outcome of Patients With Papillary Thyroid Carcinoma. J Clin Endocrinol Metab (1999) 84(2):458–63. doi: 10.1210/jcem.84.2.5443
24. Sakiz D, Sencar ME, Calapkulu M, Unsal IO, Aktas L, Ucan B, et al. The Effects of Chronic Lymphocytic Thyroiditis on Clinicopathologic Factors in Papillary Thyroid Cancer. Endocr Pract (2021) 27(12):1199–204. doi: 10.1016/j.eprac.2021.07.011
25. Carvalho GA, Perez CL, Ward LS. The Clinical Use of Thyroid Function Tests. Arq Bras Endocrinol Metabol (2013) 57(3):193–204. doi: 10.1590/s0004-27302013000300005
26. Radetti G, Loche S, D'Antonio V, Salerno M, Guzzetti C, Aversa T, et al. Influence of Hashimoto Thyroiditis on the Development of Thyroid Nodules and Cancer in Children and Adolescents. J Endocr Soc (2019) 3(3):607–16. doi: 10.1210/js.2018-00287
27. Wu X, Lun Y, Jiang H, Gang Q, Xin S, Duan Z, et al. Coexistence of Thyroglobulin Antibodies and Thyroid Peroxidase Antibodies Correlates With Elevated Thyroid-Stimulating Hormone Level and Advanced Tumor Stage of Papillary Thyroid Cancer. Endocrine (2014) 46(3):554–60. doi: 10.1007/s12020-013-0121-x
28. Xiao Y, Zhou Q, Xu Y, Yuan SL, Liu QA. Positive Thyroid Antibodies and Risk of Thyroid Cancer: A Systematic Review and Meta-Analysis. Mol Clin Oncol (2019) 11(3):234–42. doi: 10.3892/mco.2019.1886
29. Wen X, Wang B, Jin Q, Zhang W, Qiu M. Thyroid Antibody Status Is Associated With Central Lymph Node Metastases in Papillary Thyroid Carcinoma Patients With Hashimoto's Thyroiditis. Ann Surg Oncol (2019) 26(6):1751–8. doi: 10.1245/s10434-019-07256-4
30. Shen CT, Zhang XY, Qiu ZL, Sun ZK, Wei WJ, Song HJ, et al. Thyroid Autoimmune Antibodies in Patients With Papillary Thyroid Carcinoma: A Double-Edged Sword? Endocrine (2017) 58(1):176–83. doi: 10.1007/s12020-017-1401-7
31. Song E, Oh H-S, Jeon MJ, Chung KW, Hong SJ, Ryu JS, et al. The Value of Preoperative Antithyroidperoxidase Antibody as a Novel Predictor of Recurrence in Papillary Thyroid Carcinoma. Int J Cancer (2019) 144(6):1414–20. doi: 10.1002/ijc.31944
Keywords: thyroid peroxidase antibody, papillary thyroid carcinoma, recurrence, chronic lymphocytic thyroiditis, autoimmune thyroiditis
Citation: Wang W, Wen L, Chen S, Su X, Mao Z, Ding Y, Chen Z, Chen Y, Ruan J, Yang J, Zhou J, Teng X, Fahey TJ III, Li Z and Teng L (2022) Preoperative Thyroid Peroxidase Antibody Predicts Recurrence in Papillary Thyroid Carcinoma: A Consecutive Study With 5,770 Cases. Front. Oncol. 12:881024. doi: 10.3389/fonc.2022.881024
Received: 22 February 2022; Accepted: 08 April 2022;
Published: 09 May 2022.
Edited by:
Oded Cohen, Yale University, United StatesReviewed by:
Sharon Tzelnick, Toronto General Hospital, CanadaHadar Duskin-Bitan, Rabin Medical Center, Israel
Copyright © 2022 Wang, Wen, Chen, Su, Mao, Ding, Chen, Chen, Ruan, Yang, Zhou, Teng, Fahey, Li and Teng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lisong Teng, bHN0ZW5nQHpqdS5lZHUuY24=; Zhongqi Li, bGl6cTA0MDVAemp1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Weibin Wang
Weibin Wang Liping Wen1,2†
Liping Wen1,2† Yongfeng Ding
Yongfeng Ding Zhendong Chen
Zhendong Chen Yiran Chen
Yiran Chen Xiaodong Teng
Xiaodong Teng Thomas J. Fahey III
Thomas J. Fahey III Lisong Teng
Lisong Teng