- 1Department of Urology, Yichun People’s Hospital, Yichun, China
- 2Department of Nursing, Wanzai County Traditional Chinese Medicine Hospital, Yichun, China
Background: To investigate the potential prognostic role of C-reactive protein to albumin ratio (CAR) in patients with urinary cancers, including renal cell carcinoma (RCC), bladder cancer (BC), and prostate cancer (PC).
Methods: We searched and screened literatures with PubMed, Embase, Cochrane Library, and Web of Science in January 2022. We applied combined hazard ratios (HRs) and 95% confidence intervals (CIs) to assess the associations.
Results: Thirteen studies including 2,941 cases were analyzed in our study. Merged results indicated that highly pretreated CAR was associated with inferior overall survival (HR 2.21, 95% CI 1.86-2.62, p < 0.001) and progression-free survival (HR 1.85, 95% CI 1.36-2.52, p < 0.001) for urinary cancers. In a subgroup analysis of OS by tumor type, CAR can be a predictor in RCC (HR 2.10, 95% CI 1.72-2.56), BC (HR 3.35, 95% CI 1.94-5.80), and PC (HR 2.20, 95% CI 1.43-3.37). In a subgroup analysis of PFS by tumor type, CAR can be a predictor in BC (HR 1.76, 95% CI 1.03-3.02), and RCC (HR 1.90, 95% CI 1.25-2.89). The reliability and robustness of results were confirmed.
Conclusions: High pretreated CAR was effective predictor of poor survival in patients with urinary cancers and can act as prognostic factor for these cases.
Systematic Review Registration: PROSPERO (CRD42022306414).
Introduction
Urinary neoplasms, including renal cell carcinoma (RCC), bladder cancer (BC) and prostate cancer (PC), are usual cancers with increased morbidity and mortality. These three tumors were among the 10 most common malignancies in the United States in 2019 (1). In general, most cancers of the urinary system are found at a local stage. Tumor excision is the preferred management and can achieve well-pleasing outcomes. Nevertheless, some patients may develop metastasis at initial diagnosis, and most local tumors eventually progress to relapsing or metastatic disease. With the development of molecular targeted drugs (2, 3) and immunotherapy (4), the survival of urinary tumors has been greatly improved. However, the long-term survival of these tumors remains disappointing. Therefore, it is of interest to study the prognostic biomarkers in these cases in order to better understand their underlying mechanisms and contribute to the optimal treatment of urinary tumors.
There was increasing evidence of a link between tumor-caused inflammatory response and tumorigenesis and disease progression (5). Kinds of inflammatory and immune response factors have been reported as survival biomarkers for various cancers (6). The C-reactive protein to albumin ratio (CAR) is a measure of serum C-reactive protein (CRP) and albumin and is generated as the CRP level divided by the albumin level. The ratio was originally suggested to forecast the prognosis of acute hospitalized patients (7). Recently, various centers have explored the application of CAR as a prognostic biomarker for kinds of cancers, including gastric cancer (8), colorectal cancer (9), non-small-cell lung cancer (10), gallbladder cancer (11), etc. As for urinary neoplasms, a lot of studies have examined the prognostic value of CAR in RCC. After combining data from these studies, a meta-analysis confirmed that high pretreated CAR was effective predictor of poor survival in cases with RCC (12). Nevertheless, data with potential bias from univariable analysis was included, and the latest studies could not be included (13). Moreover, several studies focusing on the prognostic role of CAR in BC and PC have been published (14–17). This situation encouraged us to conduct the present study to present an integrated review of all related evidence exploring the value of CAR on outcomes in urological cancer patients.
Methods
Literature Searching
Our study was performed and presented following the PRISMA guidelines. The protocol has been presented on PROSPERO (No. CRD42022306414). A comprehensive literature search was conducted with MEDLINE, Embase, Cochrane Library, and Web of Science electronic databases. We searched and screened the potential literatures up to January 2022. The key words embraced: “C-reactive protein to albumin ratio” (e.g., “CAR”, “C-reactive protein to albumin ratio”, “C-reactive protein-to-albumin ratio”, “C-reactive protein/albumin ratio”), “urinary cancer” (e.g., “renal cell carcinoma”, “bladder cancer”, “prostate cancer”, “urothelial carcinoma”, “testicular cancer”, “penile cancer”) and “prognosis” (e.g., “survival”, “prognosis”, “outcome”, “progression”, “recurrence”, “metastasis”, “mortality”). A manual screen of study references was also conducted to obtain possibly relevant literatures. The language of included studies was restricted to English.
Study Inclusion and Exclusion
To be eligible for inclusion, studies must meet the following criteria: (1) studies focusing on the association between pretreated CAR and prognosis of urological cancers, including renal cell carcinoma (RCC), bladder cancer (BC), prostate cancer (PC); (2) studies reported the correlation of pretreated CAR with overall survival (OS), and progression-free survival (PFS); (3) studies directly offered hazard ratios (HRs) with 95% confidence intervals (CIs) in the multivariable cox analyses; (4) studies published in peer-reviewed journals. Exclusion studies according to exclusion criteria: (1) studies didn’t provided HRs with 95% CIs for pretreated CAR in the multivariable cox analyses; (2) duplicated studies; (3) not original articles, such as conference abstracts, reviews, letters or case reports. Two researchers (QSC, ZLY) screened and assessed the literatures independently. The dispute was settled through discussion.
Data Extraction
In the light of a pre-designed standardized form, data were extracted by two researchers (QSC, ZLY) as following: study features (author’s name, year of publication, region, study design, and number of study cases); patient and tumor characteristics (age, cancer type [renal cell carcinoma, bladder cancer, prostate cancer], cancer stage, detailed treatment strategy, follow-up duration, endpoints of outcome); characteristics of study methodology (detailed value of cut-off, determine method of cut-off, statistical methods of cox analyses, adjusted factors); the detailed HRs with 95% CIs of each study and endpoint.
Quality Evaluation
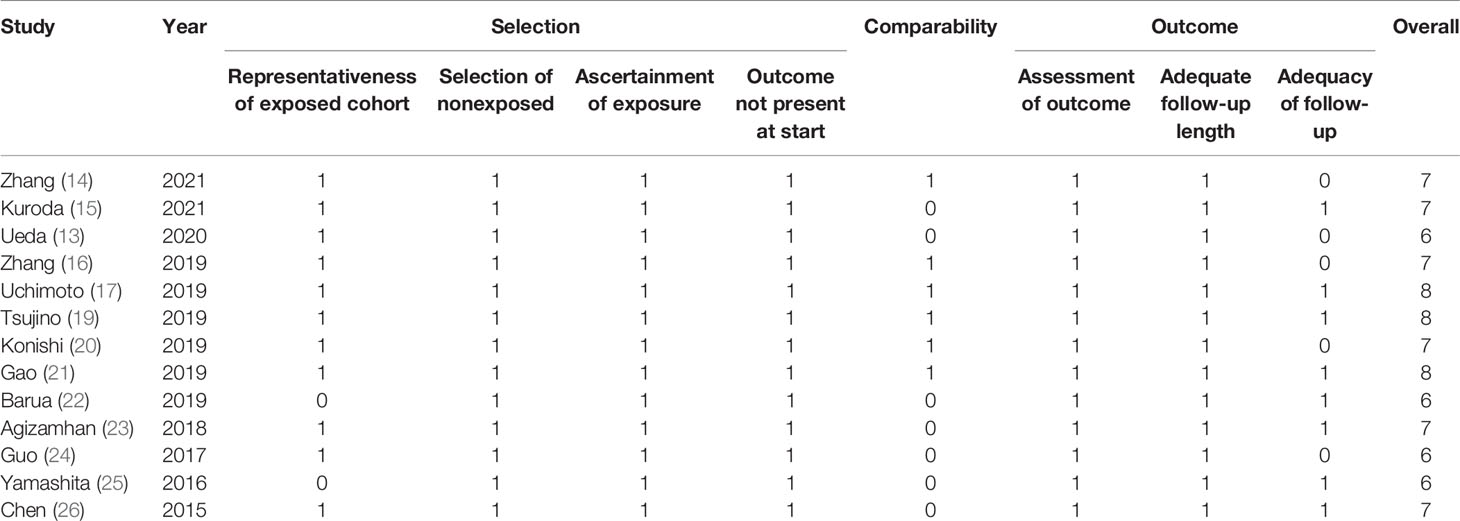
The New-castle-Ottawa Scale (NOS) was applied to assess the quality of the included studies. There were 9 items in total (18). The value of NOS higher than 6 was believed as high quality.
Statistical Analysis
HRs with related 95% CI in each qualified study were combined to assess the prognostic role of pretreated CAR in cases with urinary tumors. In each meta-analysis, Higgins I-Squared statistics and Cochran’s Q test were used to evaluate heterogeneity among the enrolled literature. I2 > 50% and/or P<0.1 were believed as measures of significant heterogeneity. In the case of significant heterogeneity, the random-effects model was applied to calculate the aggregate HRs and 95% CI. Otherwise, the fixed effects model was applied. Subgroup analyses of overall survival and progression-free survival were also conducted. Publication bias was evaluated by funnel plots, checked by Egger’s and Begg’s tests. We also conducted sensitivity analysis to examine the stability of the findings. Stata 12.0 (Stata Corporation, College Station) was applied for all statistical analyses.
Results
Included Literature
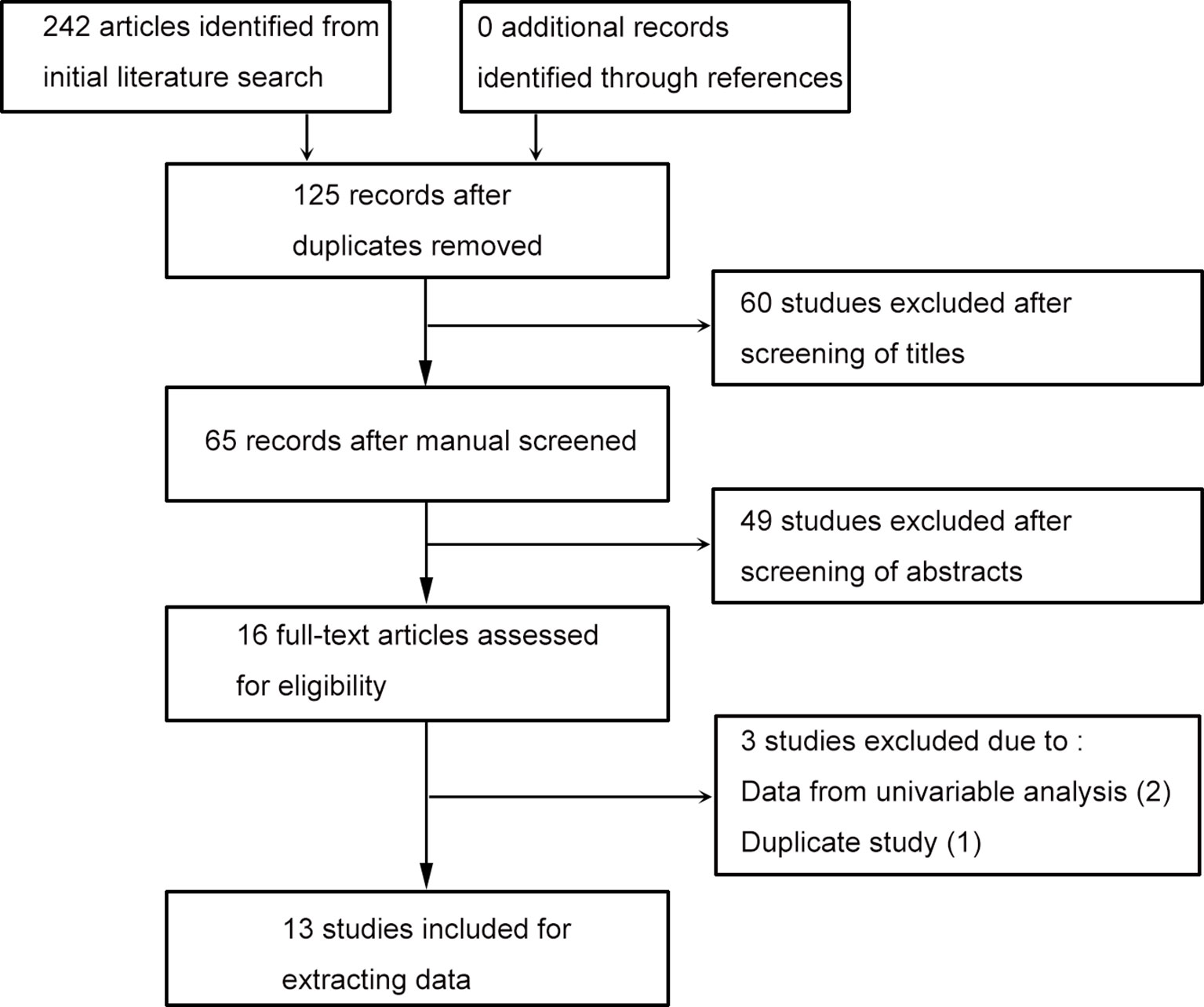
Literature search identified 242 studies, and no article was discovered through reference screening. As shown in the flow chart of the literature searching (Figure 1), 125 records retained after excluding duplicated studies. After screening literature titles and abstracts, 16 full-text papers retained for next evaluation. Two papers were removed due to lacking data from multivariable analysis, one article was excluded due to duplicate study. Finally, 13 literatures were included for evidence synthesis (13–17, 19–26).
Study Features
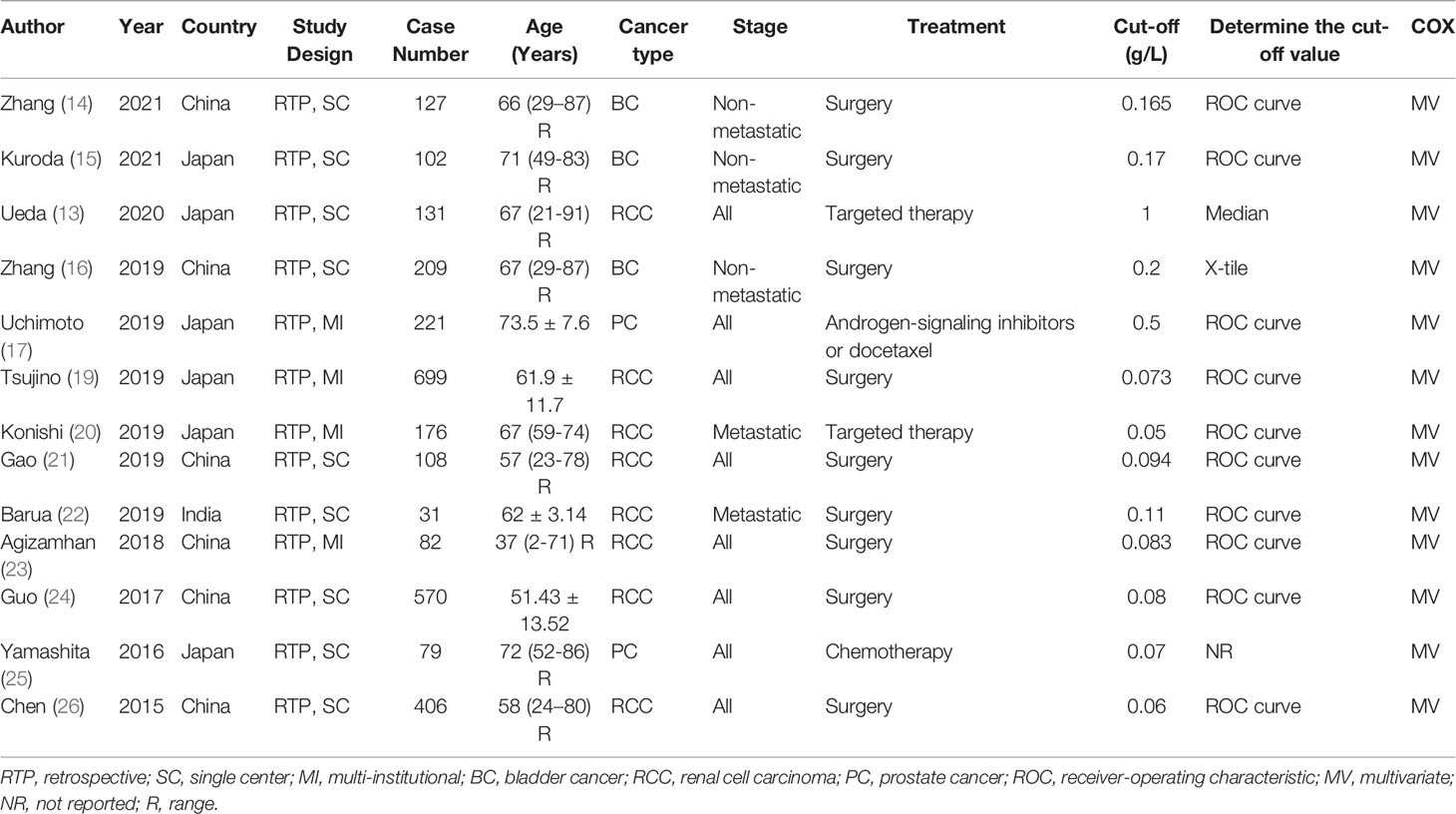
Only data on RCC, BC and PC was identified, studies about the prognostic role of CAR in other urinary cancers were lacking. Table 1 outlined the main features of all enrolled studies. All studies were published recently (2015–2021) by Asian (Chinese, Japanese and India) researchers. All studies retrospectively analyzed data, nine of them based on single-center patients, four of them based on multi-institutional patients. The median or mean age of subjects ranged from 37 to 73.5 years, and the sample size ranged from 31 to 699. Of all studies, three looked at BC (14–16), eight looked at RCC (13, 19–24, 26), and two looked at PC (17, 25). Most patients were in various stages of the disease and undergo surgery. All studies provided the results of a multivariate Cox analysis. The adjusted factors included patient and tumor characteristics, as detailed in Table 2. All studies had high quality, with NOS scores ranging from 6 to 8, as shown in Table 3.
CAR and OS in Urological Cancers
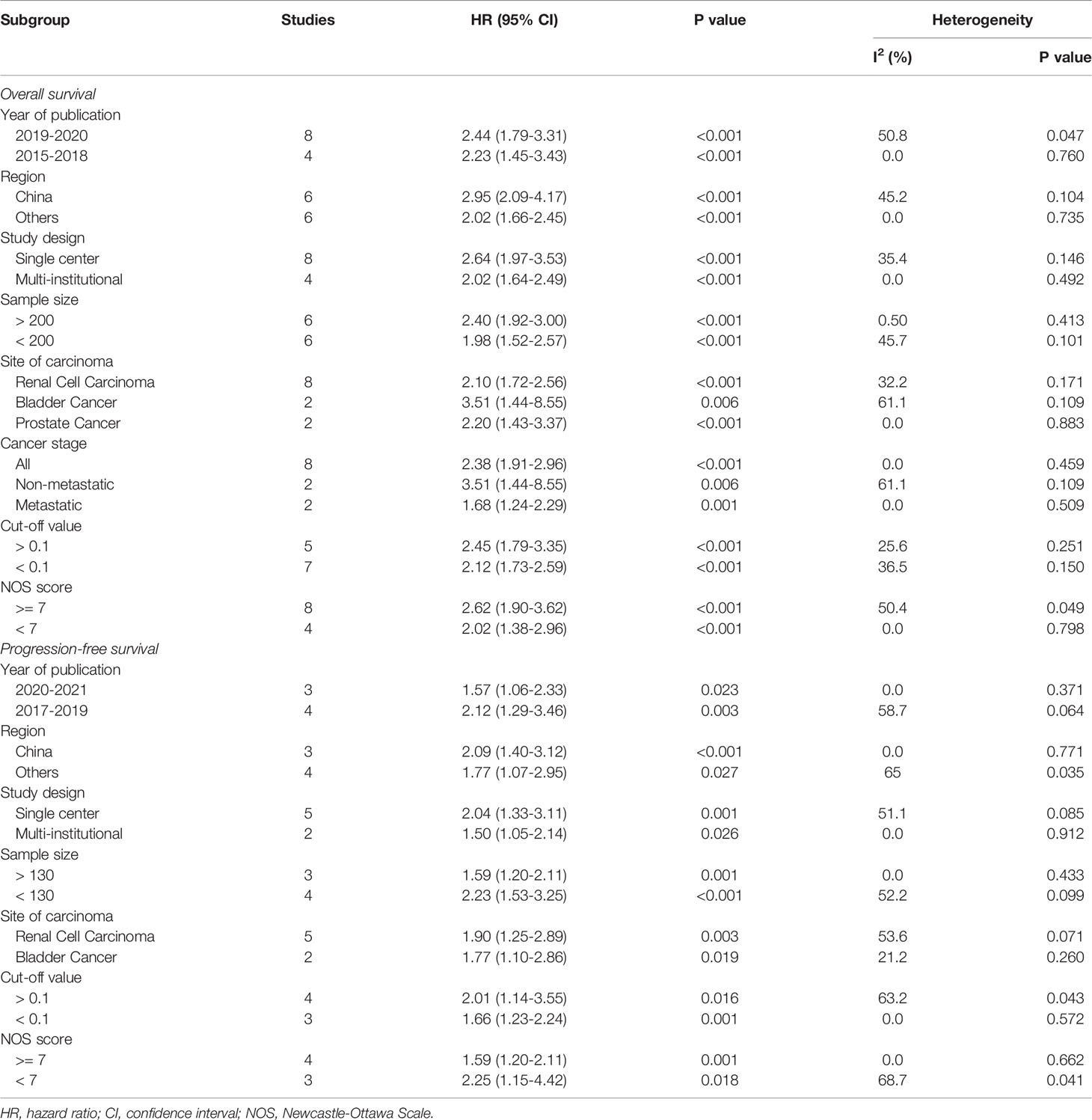
There were 11 literatures on OS of urological cancers (13, 16, 17, 19–26). Since there was no significant heterogeneity among these literatures (I2 = 28.6%, p = 0.165), a fixed model was used for analysis. Pooled results showed that higher levels of CAR were associated with poorer OS for urological cancers (HR 2.21, 95% CI 1.86-2.62, p < 0.001). Subgroup analysis by cancer type showed that higher CAR levels were associated with poorer OS in RCC (HR 2.10, 95% CI 1.72-2.56, p < 0.001), BC (HR 3.35, 95% CI 1.94-5.80, p < 0.001), and PC (HR 2.20, 95% CI 1.43-3.37, p < 0.001) (Figure 2A). In addition to cancer type, a subgroup analysis of OS included many other variables, including year of publication, region, study design, sample size, cancer stage, and so on. The results for these grouping variables were still significant (Table 4).
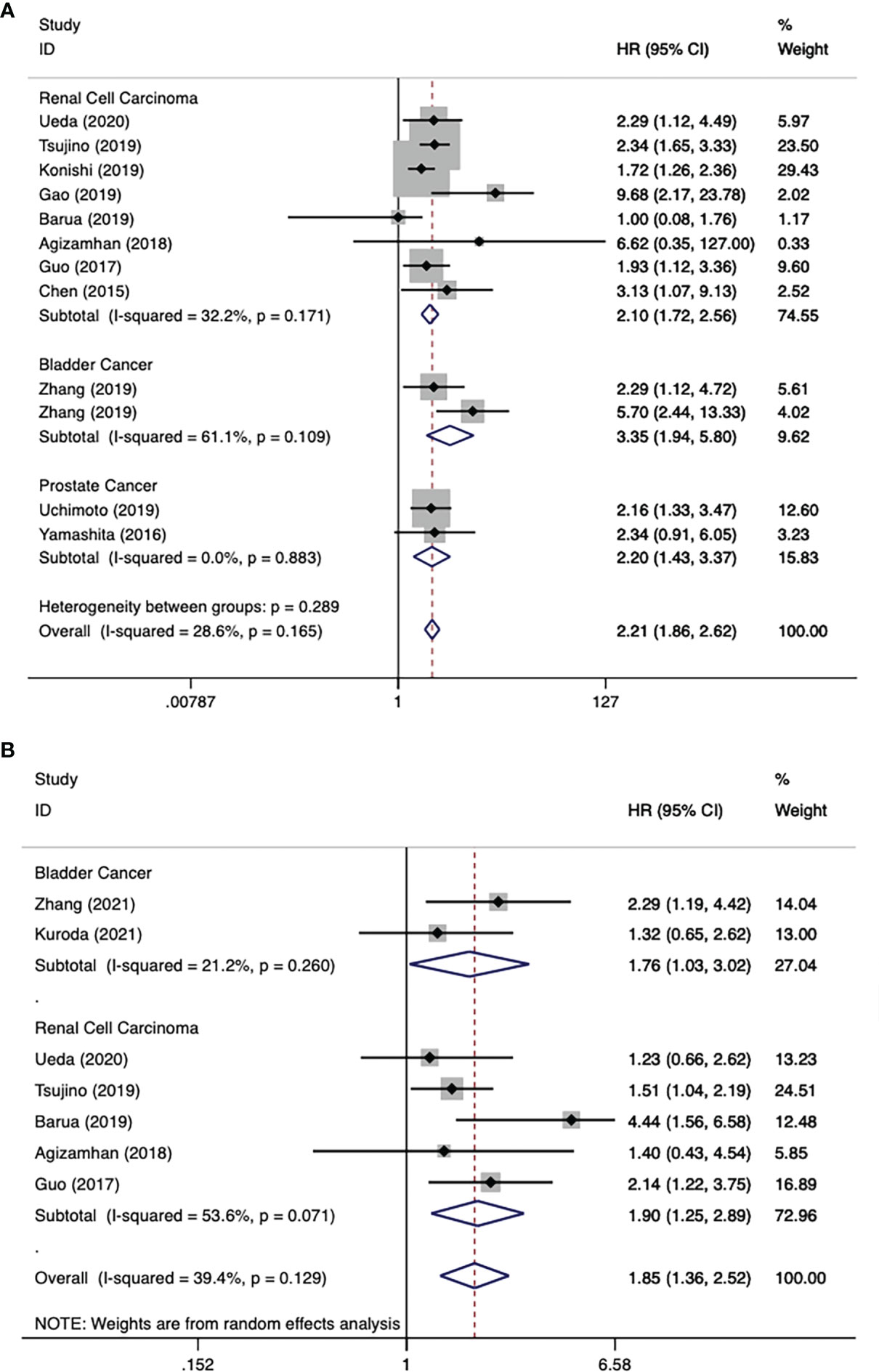
Figure 2 Forest plot reflects the association between CAR and OS/PFS for urological cancers. (A) CAR and OS; (B) CAR and PFS.
CAR and PFS in Urological Cancers
There were 7 literatures on PFS of urological cancers (13–15, 19, 22–24). Pooled results showed that higher levels of CAR were associated with poorer PFS for urological cancers (HR 1.85, 95% CI 1.36-2.52, p < 0.001). Subgroup analysis by cancer type showed that higher CAR levels were associated with poorer PFS in BC (HR 1.76, 95% CI 1.03-3.02, p = 0.039), RCC (HR 1.90, 95% CI 1.25-2.89, p = 0.003) (Figure 2B). In addition to cancer type, a subgroup analysis of PFS included many other variables, including year of publication, region, study design, sample size, and so on. The results for these grouping variables were still significant (Table 4).
Publication Bias
The funnel diagram of OS and PFS was shown in Figure 3, and the two were visually symmetric. Begg’s and Egger’s quantitative tests also showed that there was a low probability of publication bias for OS (P = 0.104 and 0.182) and PFS (P = 0.368 and 0.659).
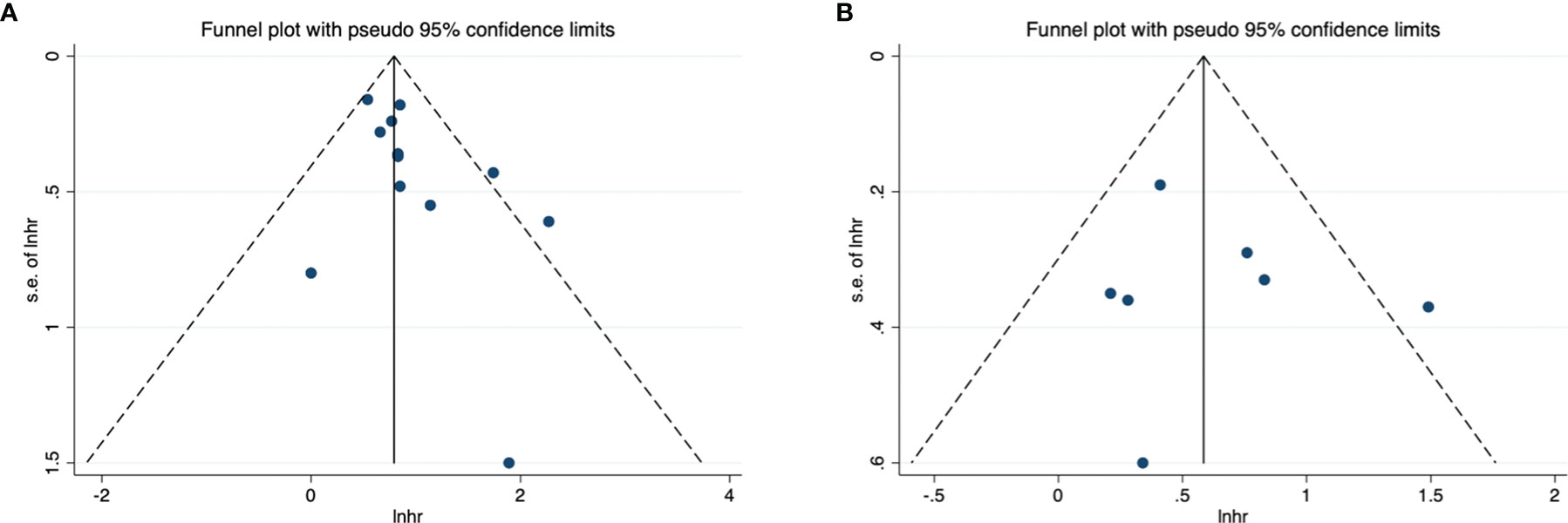
Figure 3 Funnel plot for publication bias. (A) correlation of CAR with OS in urological cancers; (B) correlation of CAR with PFS in urological cancers.
Sensitivity Analysis
Sensitivity analysis was further conducted to examine the effect of a single study on the overall results. There was no significant change in HRs associated with CAR and OS or PFS in patients with urinary cancers (Figure 4).
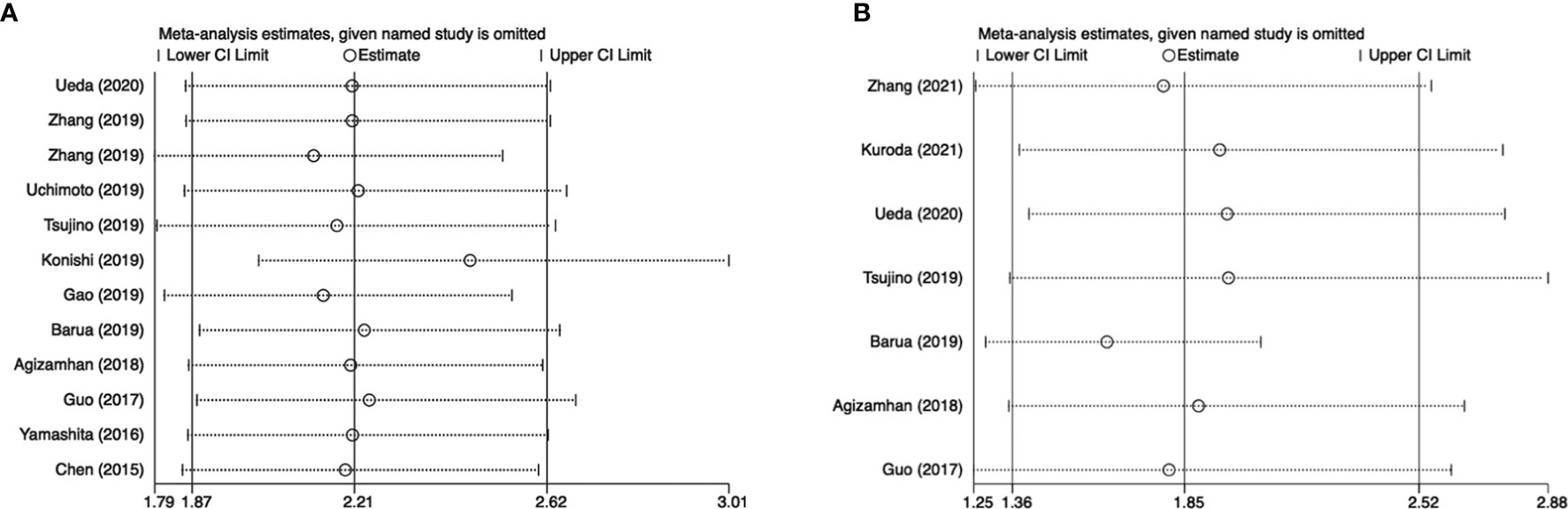
Figure 4 Results of sensitivity analysis. (A) correlation of CAR with OS in urological cancers; (B) correlation of CAR with PFS in urological cancers.
Discussion
The present study attempted to systematically review the available literatures and to evaluate the prognostic significance of CAR in urogenital malignancies using a meta-analysis. Based on high-quality studies, high level of pretreated CAR was found to be significantly correlated to poorer OS and PFS after combining the data. Subgroup analysis by cancer type remained significant for RCC, BC, and PC. Subgroup analyses of several variables of OS and PFS did not alter the direction of the findings. Publication bias checks and sensitivity analyses also confirmed the reliability and robustness of our findings. CRP and albumin are common hematologic indexes in clinic, which are easy to measure and low cost. Thus, CAR can be applied as a competent prognostic indicator for urinary tumors.
For urological tumors, a comprehensive meta-analysis initially examined CAR as a prognostic factor in cases with RCC. They identify that high pretreated CAR was correlated to poorer OS and PFS (12). The associations of CAR and clinicopathological characteristics were also explored. High level of pretreatment CAR was identified to associated with several adverse factors, such as higher Fuhrman grade, higher TNM stage, venous thrombus formation, lymph node invasion, distant metastasis. However, the data from univariable analysis was included, which may bring in potential bias (27). And newly published articles cannot be included. Moreover, several studies investigating the prognostic role of CAR in BC and PC have been published (14–17). In this case, we only included data from multivariable analyses. The present study provided the latest and most integrated evidence of the prognostic role of CAR in urinary tumors. Based on adjusted data, we have found similar results to study by Zhou et al. (12). More detailed results about BC and PC were reported in the present study. Pretreatment CAR remained to be an important prognosis predictor for patients with BC and PC.
Inflammatory reactions promote the development of tumors by influencing the microenvironment of urinary tumors. Cancer cell proliferation, necrosis, invasion, and hypoxia trigger immune responses in the tumor microenvironment, and in turn trigger the generation of various of inflammatory factors (28). Serum C-reactive protein and albumin are indexes of chronic inflammation and malnutrition in cancer patients (29, 30). C-reactive protein is an acute phase protein generated in the liver that stimulates cancer-related inflammatory factors such as IL-1, IL-6, and TNF-A, resulting in progression of malignance (31). The studies showed that high CRP level was associated with poorer survival outcomes in urological cancer cases (32). Serum albumin level reflects the nutritional status of patients. Low serum albumin levels indicate malnutrition. Hypoalbuminemia is caused by nutrient intakes and tumor overconsumption and induces stimulation of inflammatory factors such as IL-1, IL-6, and TNF-A (33). CAR was generated in the light of CRP and albumin levels. Thus, CAR provides a biological basis and is considered a promising prognostic tool for urinary tumors.
There was increasing evidence of a link between tumor-caused inflammatory response and tumorigenesis and disease progression (5). Kinds of inflammatory and immune response factors have been reported as survival biomarkers for various cancers (6). Many other inflammatory biomarkers that were also prognostic in urologic tumors, such as neutrophil-to-lymphocyte ratio (NLR) (34), platelet-to-lymphocyte ratio (PLR) (35), lymphocyte-to-monocyte ratio (LMR) (36), etc. Based on published studies, similar meta-analyses about the prognostic role of these biomarkers in urological tumors also have been performed by many researchers. They are common hematologic indexes in clinic, easily to measure and low cost, which can be widely used. However, which marker was the best predictor, the studies comparing these markers were inadequate. We believed that combining these markers for prediction and improving prediction efficiency may be the direction of future researches.
However, some of the limitations of this systematic review should be explained. First of all, only 13 studies have been enrolled in the meta-analysis, which may lack statistical power, especially for BC and PC. Second, all enrolled studies were performed in Asia. Therefore, we should be careful to apply the results of the present study in patients from western countries. Third, all the included studies were cohort studies with retrospective design, which may result in selection bias. Fourthly, different cut-off values of CAR in different studies may lead to inconsistent outcome thresholds. Lastly, due to inadequate literatures, other urinary cancers were not analyzed in our study, and the endpoint cancer-specific survival was not studied.
Taken together, the present study identified that pretreatment CAR level could be a likely predictor for cases with urinary tumors. Nevertheless, well-designed prospective studies also are wanted to validate these results.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
Protocol/project development: MW and YZ. Data collection or management: QC, ZY, and HG. Data analysis: PL, YL, and CL. Manuscript writing/editing: MW and YZ. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. CA: Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
2. Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative Effectiveness of Axitinib Versus Sorafenib in Advanced Renal Cell Carcinoma (AXIS): A Randomised Phase 3 Trial. Lancet (2011) 378(9807):1931–9. doi: 10.1016/S0140-6736(11)61613-9
3. Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in Advanced Clear-Cell Renal-Cell Carcinoma. N Engl J Med (2007) 356(2):125–34. doi: 10.1056/NEJMoa060655
4. Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. N Engl J Med (2010) 363(5):411–22. doi: 10.1056/NEJMoa1001294
5. Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-Related Inflammation and Treatment Effectiveness. Lancet Oncol (2014) 15(11):e493–503. doi: 10.1016/S1470-2045(14)70263-3
6. Fang Y, Zheng T, Zhang C. Prognostic Role of the C-Reactive Protein/Albumin Ratio in Patients With Gynecological Cancers: A Meta-Analysis. Front Oncol (2021) 11:737155. doi: 10.3389/fonc.2021.737155
7. Fairclough E, Cairns E, Hamilton J, Kelly C. Evaluation of a Modified Early Warning System for Acute Medical Admissions and Comparison With C-Reactive Protein/Albumin Ratio as a Predictor of Patient Outcome. Clin Med (Lond) (2009) 9(1):30–3. doi: 10.7861/clinmedicine.9-1-30
8. Yu Q, Li KZ, Fu YJ, Tang Y, Liang XQ, Liang ZQ, et al. Clinical Significance and Prognostic Value of C-Reactive Protein/Albumin Ratio in Gastric Cancer. Ann Surg Treat Res (2021) 100(6):338–46. doi: 10.4174/astr.2021.100.6.338
9. Tamai K, Okamura S, Makino S, Yamamura N, Fukuchi N, Ebisui C, et al. C-Reactive Protein/Albumin Ratio Predicts Survival After Curative Surgery in Elderly Patients With Colorectal Cancer. Updates Surg (2021) 74(1):153–62. doi: 10.1007/s13304-021-01011-9
10. Araki T, Tateishi K, Sonehara K, Hirota S, Komatsu M, Yamamoto M, et al. Clinical Utility of the C-Reactive Protein:Albumin Ratio in non-Small Cell Lung Cancer Patients Treated With Nivolumab. Thorac Cancer (2021) 12(5):603–12. doi: 10.1111/1759-7714.13788
11. Bao Y, Yang J, Duan Y, Chen Y, Chen W, Sun D. The C-Reactive Protein to Albumin Ratio is an Excellent Prognostic Predictor for Gallbladder Cancer. Biosci Trends (2021) 14(6):428–35. doi: 10.5582/bst.2020.03326
12. Zhou W, Zhang GL. C-Reactive Protein to Albumin Ratio Predicts the Outcome in Renal Cell Carcinoma: A Meta-Analysis. PloS One (2019) 14(10):e0224266. doi: 10.1371/journal.pone.0224266
13. Ueda K, Ogasawara N, Yonekura S, Matsunaga Y, Hoshino R, Kurose H, et al. The Prognostic Value of Systemic Inflammatory Markers in Advanced Renal Cell Carcinoma Patients Treated With Molecular Targeted Therapies. Anticancer Res (2020) 40(3):1739–45. doi: 10.21873/anticanres.14127
14. Zhang W, Yang F, Kadier A, Chen Y, Yu Y, Zhang J, et al. Development of Nomograms Related to Inflammatory Biomarkers to Estimate the Prognosis of Bladder Cancer After Radical Cystectomy. Ann Transl Med (2021) 9(18):1440. doi: 10.21037/atm-21-4097
15. Kuroda K, Tasaki S, Horiguchi A, Ito K. Postoperative C-Reactive Protein-to-Albumin Ratio Predicts Poor Prognosis in Patients With Bladder Cancer Undergoing Radial Cystectomy. Mol Clin Oncol (2021) 14(3):54. doi: 10.3892/mco.2021.2216
16. Zhang W, Wang R, Ma W, Wu Y, Maskey N, Guo Y, et al. Systemic Immune-Inflammation Index Predicts Prognosis of Bladder Cancer Patients After Radical Cystectomy. Ann Transl Med (2019) 7(18):431. doi: 10.21037/atm.2019.09.02
17. Uchimoto T, Komura K, Fujiwara Y, Saito K, Tanda N, Matsunaga T, et al. Prognostic Impact of C-Reactive Protein-Albumin Ratio for the Lethality in Castration-Resistant Prostate Cancer. Med Oncol (2019) 37(1):9. doi: 10.1007/s12032-019-1332-7
18. Stang A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
19. Tsujino T, Komura K, Hashimoto T, Muraoka R, Satake N, Matsunaga T, et al. C-Reactive Protein-Albumin Ratio as a Prognostic Factor in Renal Cell Carcinoma - A Data From Multi-Institutional Study in Japan. Urol Oncol (2019) 37(11):812.e1–8. doi: 10.1016/j.urolonc.2019.04.002
20. Konishi S, Hatakeyama S, Tanaka T, Ikehata Y, Tanaka T, Hamano I, et al. C-Reactive Protein/Albumin Ratio is a Predictive Factor for Prognosis in Patients With Metastatic Renal Cell Carcinoma. Int J Urol (2019) 26(10):992–8. doi: 10.1111/iju.14078
21. Gao J, Agizamhan S, Zhao X, Jiang B, Qin H, Chen M, et al. Preoperative C-Reactive Protein/Albumin Ratio Predicts Outcome of Surgical Papillary Renal Cell Carcinoma. Future Oncol (2019) 15(13):1459–68. doi: 10.2217/fon-2018-0611
22. Barua SK, Singh Y, Baruah SJ, PR T, PK B, Sarma D, et al. Predictors of Progression-Free Survival and Overall Survival in Metastatic Non-Clear Cell Renal Cell Carcinoma: A Single-Center Experience. World J Oncol (2019) 10(2):101–11. doi: 10.14740/wjon1188
23. Agizamhan S, Qu F, Liu N, Sun J, Xu W, Zhang L, et al. Preoperative Neutrophil-to-Lymphocyte Ratio Predicts the Surgical Outcome of Xp11.2 Translocation/TFE3 Renal Cell Carcinoma Patients. BMC Urol (2018) 18(1):60. doi: 10.1186/s12894-018-0374-z
24. Guo S, He X, Chen Q, Yang G, Yao K, Dong P, et al. The C-Reactive Protein/Albumin Ratio, a Validated Prognostic Score, Predicts Outcome of Surgical Renal Cell Carcinoma Patients. BMC Cancer (2017) 17(1):171. doi: 10.1186/s12885-017-3119-6
25. Yamashita S, Kohjimoto Y, Iguchi T, Koike H, Kusumoto H, Iba A, et al. Prognostic Factors and Risk Stratification in Patients With Castration-Resistant Prostate Cancer Receiving Docetaxel-Based Chemotherapy. BMC Urol (2016) 16:13. doi: 10.1186/s12894-016-0133-y
26. Chen Z, Shao Y, Fan M, Zhuang Q, Wang K, Cao W, et al. Prognostic Significance of Preoperative C-Reactive Protein: Albumin Ratio in Patients With Clear Cell Renal Cell Carcinoma. Int J Clin Exp Pathol (2015) 8(11):14893–900.
27. Komura K, Hashimoto T, Tsujino T, Muraoka R, Tsutsumi T, Satake N, et al. The CANLPH Score, an Integrative Model of Systemic Inflammation and Nutrition Status (SINS), Predicts Clinical Outcomes After Surgery in Renal Cell Carcinoma: Data From a Multicenter Cohort in Japan. Ann Surg Oncol (2019) 26(9):2994–3004. doi: 10.1245/s10434-019-07530-5
28. Kinoshita A, Onoda H, Imai N, Iwaku A, Oishi M, Tanaka K, et al. The C-Reactive Protein/Albumin Ratio, a Novel Inflammation-Based Prognostic Score, Predicts Outcomes in Patients With Hepatocellular Carcinoma. Ann Surg Oncol (2015) 22(3):803–10. doi: 10.1245/s10434-014-4048-0
29. McMillan DC. Systemic Inflammation, Nutritional Status and Survival in Patients With Cancer. Curr Opin Clin Nutr Metab Care (2009) 12(3):223–6. doi: 10.1097/MCO.0b013e32832a7902
30. Coussens LM, Werb Z. Inflammation and Cancer. Nature (2002) 420(6917):860–7. doi: 10.1038/nature01322
31. Asegaonkar SB, Asegaonkar BN, Takalkar UV, Advani S, Thorat AP. C-Reactive Protein and Breast Cancer: New Insights From Old Molecule. Int J Breast Cancer (2015) 2015:145647. doi: 10.1155/2015/145647
32. Zhou L, Cai X, Liu Q, Jian ZY, Li H, Wang KJ. Prognostic Role of C-Reactive Protein In Urological Cancers: A Meta-Analysis. Sci Rep (2015) 5:12733. doi: 10.1038/srep12733
33. Almasaudi AS, Dolan RD, Edwards CA, McMillan DC. Hypoalbuminemia Reflects Nutritional Risk, Body Composition and Systemic Inflammation and Is Independently Associated With Survival in Patients With Colorectal Cancer. Cancers (Basel) (2020) 12(7):1986. doi: 10.3390/cancers12071986
34. Mjaess G, Chebel R, Karam A, Moussa I, Pretot D, Abi Tayeh G, et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio (NLR) in Urological Tumors: An Umbrella Review of Evidence From Systematic Reviews and Meta-Analyses. Acta Oncol (2021) 60(6):704–13. doi: 10.1080/0284186X.2021.1886323
35. Li DY, Hao XY, Ma TM, Dai HX, Song YS. The Prognostic Value of Platelet-To-Lymphocyte Ratio in Urological Cancers: A Meta-Analysis. Sci Rep (2017) 7(1):15387. doi: 10.1038/s41598-017-15673-2
Keywords: urological cancer, C-reactive protein, albumin, prognosis, meta-analysis
Citation: Wu M, Zhou Y, Chen Q, Yu Z, Gu H, Lin P, Li Y and Liu C (2022) Prognostic Role of Pretreatment C-Reactive Protein to Albumin Ratio in Urological Cancers: A Systematic Review and Meta-Analysis. Front. Oncol. 12:879803. doi: 10.3389/fonc.2022.879803
Received: 20 February 2022; Accepted: 14 March 2022;
Published: 11 April 2022.
Edited by:
Michele Marchioni, University of Studies G. d’Annunzio Chieti and Pescara, ItalyReviewed by:
Georges Mjaess, Free University of Brussels, BelgiumLiangyou Gu, People’s Liberation Army General Hospital, China
Copyright © 2022 Wu, Zhou, Chen, Yu, Gu, Lin, Li and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Minhong Wu, d3VtaW5ob25nMzAxQDEyNi5jb20=
†These authors share first authorship
 Minhong Wu
Minhong Wu Yan Zhou2†
Yan Zhou2† Pengxiu Lin
Pengxiu Lin