
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 06 October 2022
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.879341
This article is part of the Research TopicReviews in Thoracic OncologyView all 17 articles
Tyrosine kinase inhibitors (TKIs) are a significant treatment strategy for the management of non-small cell lung cancer (NSCLC) with epidermal growth factor receptor (EGFR) mutation status. Currently, EGFR mutation status is established based on tumor tissue acquired by biopsy or resection, so there is a compelling need to develop non-invasive, rapid, and accurate gene mutation detection methods. Non-invasive molecular imaging, such as positron emission tomography/computed tomography (PET/CT), has been widely applied to obtain the tumor molecular and genomic features for NSCLC treatment. Recent studies have shown that PET/CT can precisely quantify EGFR mutation status in NSCLC patients for precision therapy. This review article discusses PET/CT advances in predicting EGFR mutation status in NSCLC and their clinical usefulness.
Lung cancer has the highest incidence and mortality worldwide (1), with non-small cell lung cancer (NSCLC) accounting for approximately 85% of all lung cancer cases and adenocarcinoma (ADC) being the most prevalent pathological type (2). The emergence of targeted therapy of epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) paradigms has radically changed advanced NSCLC treatment and improved patient survival rates, especially for advanced lung adenocarcinoma (3). Accurate and rapid quantification of EGFR mutation status in NSCLC patients is crucial to selecting the most effective management strategy for individualized therapy and precision medicine to improve patient prognosis.
The gold standard assessment of EGFR mutation status is based on tumor tissue acquired by fine-needle aspiration, biopsy, or resection (4). However, acquiring a representative biopsy is not necessarily feasible with inherent limitations, including sampling bias due to the intratumoral heterogeneous tissue samples that are not readily available, and the invasive methods have low repeatability, may cause patient discomfort, and are time-consuming and costly, with inadequate samples or poor-quality tissue samples leading to inconclusive results (5). Despite liquid biopsy’s convenience, rapidity, and affordability, its sensitivity and stability are not ideal (6). Therefore, it is critical to develop a high-throughput and ideally non-invasive longitudinal method for EGFR mutation detection in NSCLC.
Image-based phenotyping is a promising clinical method for precision medicine, as it provides a non-invasive approach to visualizing tumor phenotypic characteristics (7). CT imaging combined with clinical characteristics has been systematically analyzed to predict EGFR mutations in NSCLC (8), with positron emission tomography/computed tomography (PET/CT) now widely applied to assess NSCLC patients undergoing targeted treatment. PET images capture the molecular tumor phenotypes indicating somatic mutations (9); thus, there is increasing interest in whether PET/CT can predict EGFR mutation status in NSCLC patients to develop individualized treatment. This review article discusses PET/CT advances in predicting EGFR mutation status in NSCLC and their clinical usefulness.
The EGFR signaling pathway maintains aerobic glycolysis in EGFR-mutated lung cancer cells, and EGFR TKIs have an early and profound influence on aerobic glycolysis, as they activate and promote increased oxidative phosphorylation (10), consequently indicating that EGFR mutation status is closely related to glucose metabolism in lung cancer cells. 18F-FDG PET/CT is increasingly used for cancer diagnosis and image-guided therapy, as it can characterize tumor cell proliferation and glucose metabolism. Accordingly, 18F-FDG metabolic parameters, for instance, maximum standardized uptake value (SUVmax), total lesion glycolysis (TLG), and metabolic tumor volume (MTV) may, in part, reflect EGFR mutation status in NSCLC. Numerous studies have assessed the association between 18F-FDG uptake and EGFR mutation status in NSCLC (Figure 1) but have conflicting results (Table 1).
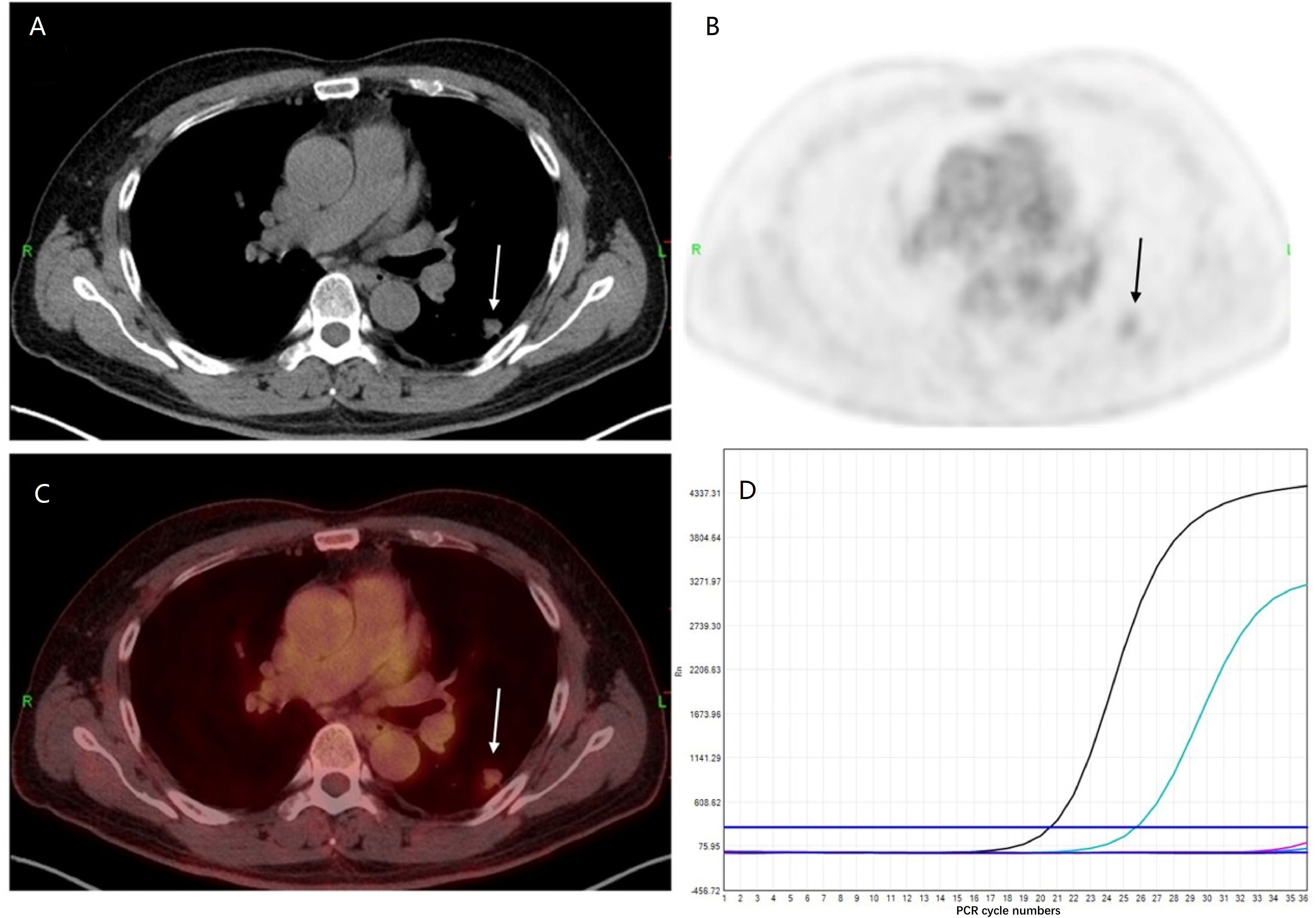
Figure 1 Representative epidermal growth factor receptor (EGFR) status and 18F-FDG PET/CT finding. A 53-year-old man with EGFR wild-type lung adenocarcinoma. (A) CT, (B) PET, and (C) PET/CT fusion images show a 1.0-cm-sized mild 18F-FDG uptake mass in the dorsal segment of the left lower lobe (SUVmax = 2.3) (arrow). (D) Genetic testing demonstrates wild-type EGFR status.
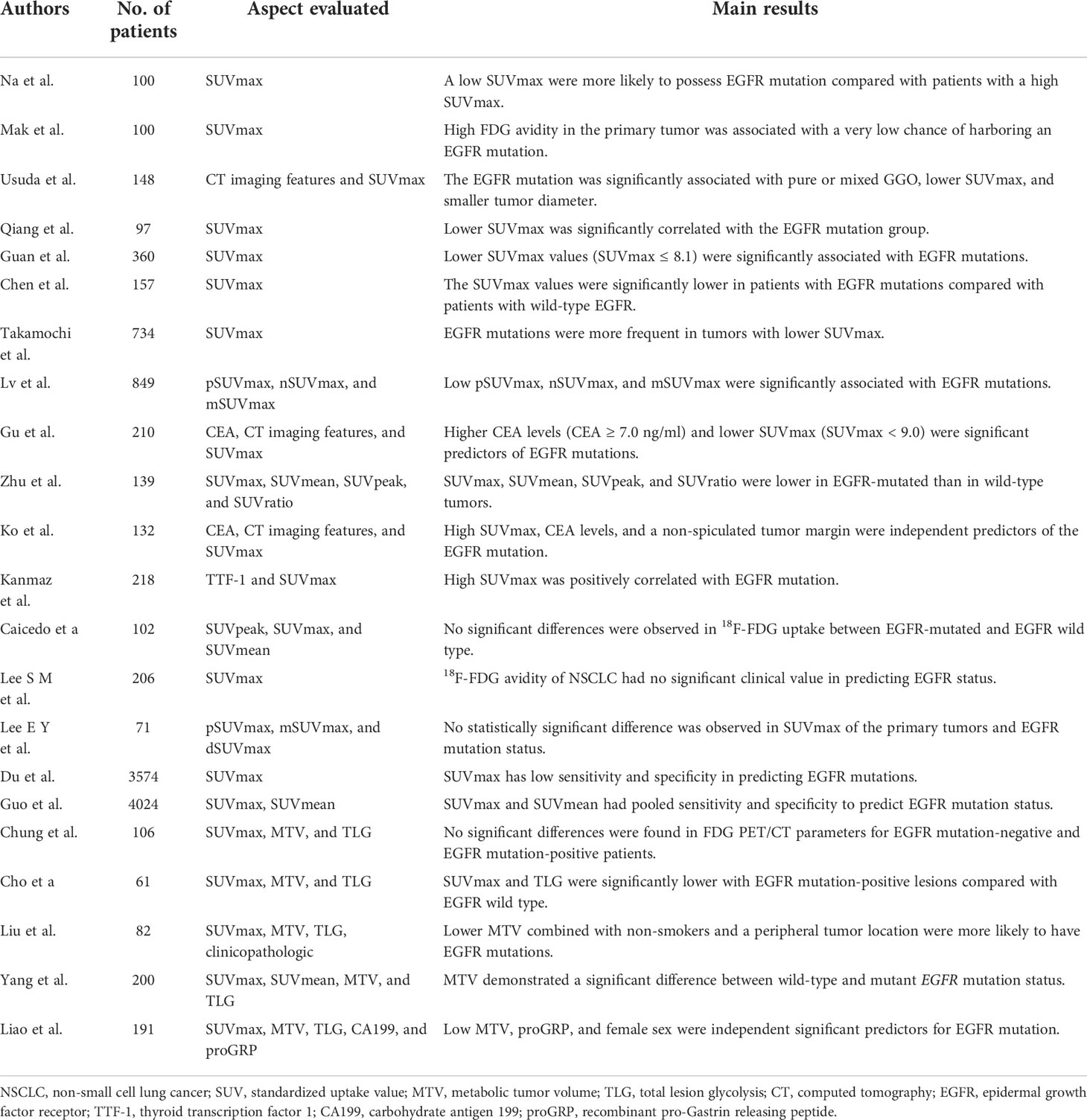
Table 1 Recent publications about the association of 18F-FDG metabolic parameters of PET/CT with epidermal growth factor receptor mutation status in non-small cell lung cancer.
Na et al. evaluated the relationship between the EGFR mutation status and the SUVmax of 18F-FDG uptake by reviewing 100 patients with NSCLC (11), reporting that patients with a low SUVmax were more likely to have an EGFR mutation as compared to patients with a high SUVmax. Mak et al. (12) assessed 100 patients with NSCLC (24 EGFR mutants and 76 wild types), demonstrating that high FDG uptake in the primary tumor is related to a very low risk of an EGFR mutation. Subsequently, increasing evidence demonstrated that EGFR mutation status is associated with a lower SUVmax in NSCLC (9, 13). Chen et al. (14) showed that patients with an EGFR mutation showed decreased SUVmax values and subsequently reported that decreased FDG uptake associated with EGFR mutation status was via NOX4/ROS/GLUT1 axis. Yang et al. (15) analyzed 200 patients with lung adenocarcinoma, demonstrating that MTV of wild-type and mutant EGFR was significantly different. Furthermore, a study by Liao et al. (16) demonstrated that low primary MTV (pMTV) (<8.13 cm) was a strong and independent predictor and could be combined with female sex and gastrin-releasing peptide levels (proGRP, ≥38.44 pg/ml) to determine EGFR mutation status. In addition, decreased FDG uptake was shown to be a significant predictor of EGFR mutation status (17–22). Interestingly, EGFR mutation status was reported to be associated with a higher SUVmax (23, 24). Ko et al. (23) demonstrated a tendency of higher SUVmax in NSCLC patients with an EGFR mutation, and higher SUVmax could be combined with never smoking, carcinoma embryonic antigen (CEA) level, and a non-spiculated tumor margin to obtain a higher area under the receiver operating characteristic (ROC) curve for EGFR mutation status. A similar conclusion was reached by Kanmaz et al. (24).
However, multiple studies have shown no association between 18F-FDG uptake and EGFR mutation status. Chung et al. found no significant differences in 18F-FDG PET/CT parameters (SUVmax, MTV, and TLG) of EGFR mutation-positive and mutation-negative lung adenocarcinoma cases (25). Other studies confirmed that 18F-FDG metabolic parameters of PET/CT in NSCLC had no significant clinical value in predicting EGFR mutation status (26–29). The low diagnostic OR and the likelihood ratio scatter plot indicated that 18F-FDG PET/CT might be useless for predicting EGFR mutation status in NSCLC as indicated by a meta-analysis of Du et al. (30). According to a recent meta-analysis (31), SUVmax of the primary tumor had a moderate predictive value for EGFR mutation status in NSCLC. Due to this dispute, further high-quality studies are required to explore the predictive value of EGFR mutation status in NSCLC.
Radiomics texture is an emerging field of interest in medical imaging and is a high-throughput and quantitative extraction of imaging features based on a computational approach (32). The rapid advance of emerging radiomics analysis could help discriminate the disease type, predict survival, and monitor the response to therapy using large datasets and artificial intelligence techniques (33). Radiomics also has various logistic advantages, for instance, offering nearly real-time results and being non-invasive (34). Additionally, compared with standard biopsy, radiomics can provide a comprehensive analysis of one lesion and multiple lesions within the examined area (35). The growing applications of 18F-FDG PET/CT radiomics have therefore attracted extensive interest in recent years, especially in lung cancer (36). The radiomics analysis of 18F-FDG PET/CT data comprises five steps: 1) data acquisition, 2) image segmentation, 3) feature extraction, 4) feature selection, and 5) model construction (Figure 2). Indeed, 18F-FDG PET/CT radiomics estimates of the tumor imaging phenotype extracted from PET/CT images facilitate the management of lung cancer, including differential diagnosis of benign/malignant solitary pulmonary nodules, NSCLC subtypes, lymph node metastasis, and distant metastases, as well as response evaluation and survival prediction (34, 37, 38). Increasing studies have confirmed the feasibility and potential superiority of 18F-FDG PET/CT radiomics to predict EGFR mutation status in NSCLC (Table 2).
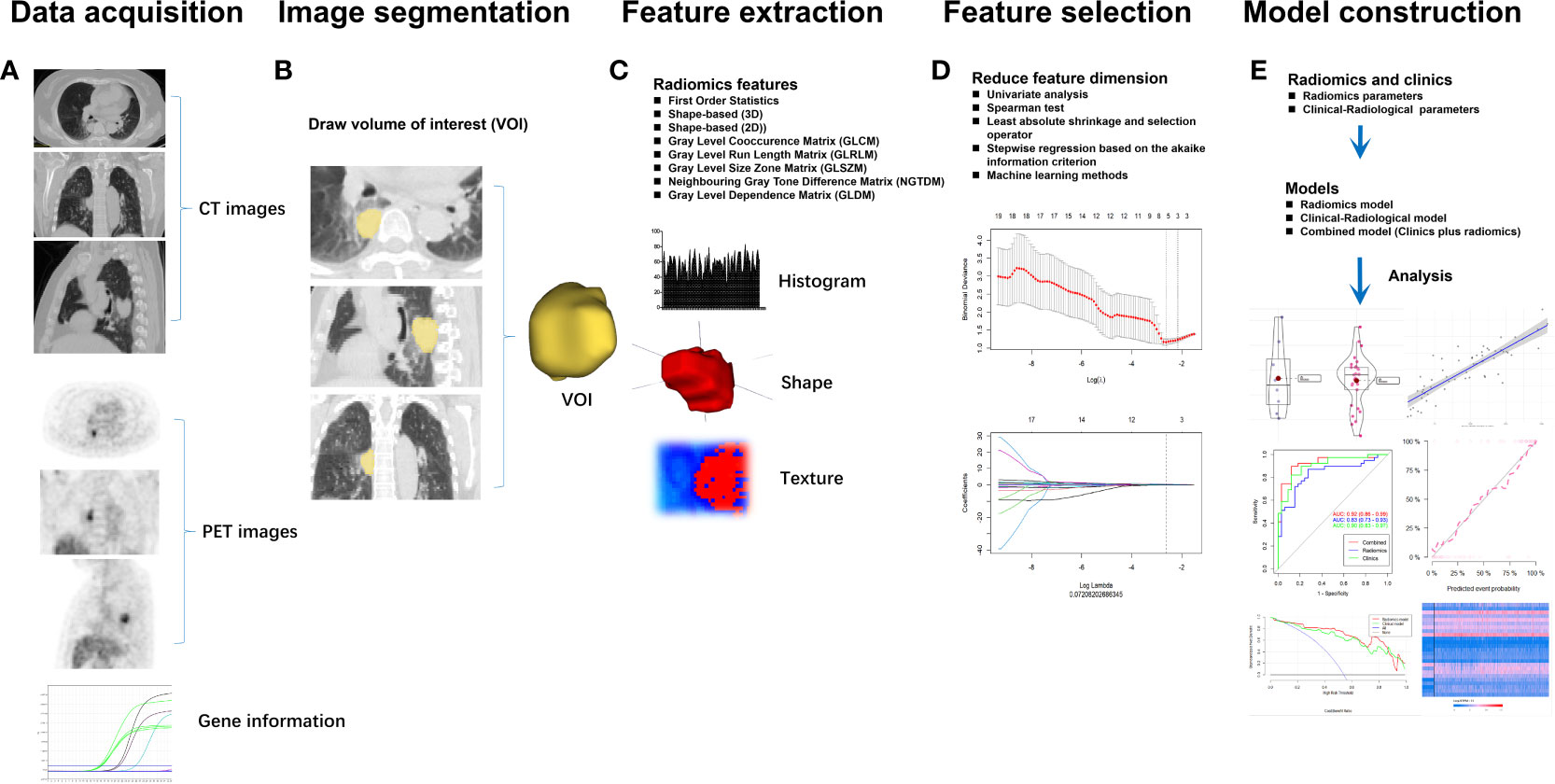
Figure 2 The workflow for radiomics analysis of 18F-FDG PET/CT data comprises five steps: (A) data acquisition, (B) image segmentation, (C) feature extraction, (D) feature selection, and (E) model construction.
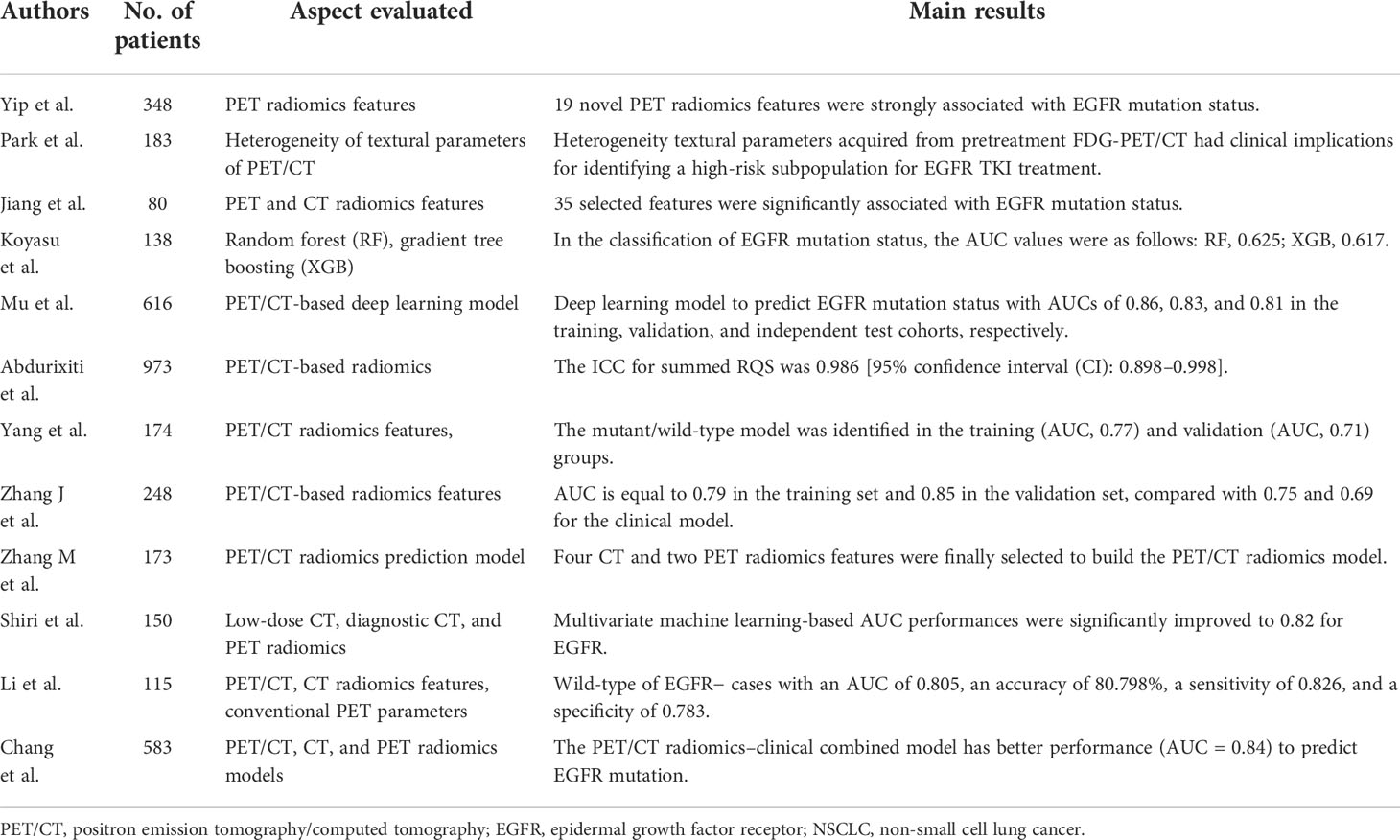
Table 2 Recent publications about the predictive value of 18F-FDG PET/CT-derived radiomics with epidermal growth factor receptor mutation status in non-small cell lung cancer.
To our knowledge, studies demonstrating the relationship between 18F-FDG PET/CT imaging textures and EGFR mutation status are limited. However, they have proved that prediction models based on 18F-FDG PET/CT imaging features can help differentiate EGFR mutation status in NSCLC, which is crucial in clinical practice to identify candidates for targeted therapy (39–44). Yang et al. (45) used 18F-FDG PET/CT-based radiomics features integrated with clinical features and 18F-FDG PET/CT metabolic parameters (MTV, TLG, SUVmax, and SUVmean) of 174 lung adenocarcinoma patients to establish prediction models and achieved an area under the curve (AUC) of 0.71–0.77. Shiri et al. (46), Zhang et al. (47), and Zhang et al. (48) reached a similar conclusion.
Li et al. (49) showed that radiomics signatures derived from 18F-FDG PET/CT images were significantly more predictive of EGFR mutations than those derived from CT or conventional PET images. In addition, a recent study found that PET/CT radiomics model has a better capability (AUC = 0.76) to predict EGFR mutation status than the PET radiomics model (AUC = 0.71) and the CT radiomics model (AUC = 0.74) in NSCLC (50). A meta-analysis by Abdurixiti et al. (51) revealed that PET/CT-based radiomics signatures could be used as a diagnostic index for EGFR mutation status in patients with NSCLC.
The reachable results in the literature are definitely promising; 18F-FDG PET/CT-based radiomics has the potential to replace classic approaches based on biopsy and histopathology to detect EGFR mutation status in NSCLC. However, the results should be interpreted with caution, as there is a lack of reproducibility and a basic deficiency of normalization methods and settings (52), so further studies are essential to establish a consistent approach. Furthermore, a high-quality predictive model depends on a large amount of data, so additional studies involving larger multicenter cohorts will be needed to develop this method into a clinical tool.
18F-FDG metabolic parameters associated with EGFR mutation status in NSCLC reflect the tumor cell glucose metabolism of tumor cells, which have poor sensitivity and are limited by many factors. Therefore, the targeting moiety or ligand must be attached with an applicable labeling agent for the imaging modality to accurately evaluate EGFR mutation status or guide EGFR-TKI treatment. Antibodies are often used due to their sufficient high-affinity specific EGFR (wild and mutated) binding. Currently, the molecular imaging modalities employed for detecting EGFR mutations are SPECT, PET, and PET/CT. Isotopic labeling substances may be combined with monoclonal antibodies to EGFR or EGFR-TKI molecular probes to reflect EGFR mutation status according to radioactive uptake in PET/CT images. Previous studies mainly used radioactive nuclides such as 86Y, 64Cu, and 89Zr to label anti-EGFR monoclonal antibodies (including cetuximab and panitumumab) and 11C and 18F to label EGFR-TKI (involved PD153035, gefitinib, erlotinib, and afatinib). However, current research focuses on cell and animal experiments with little clinical application (Table 3).
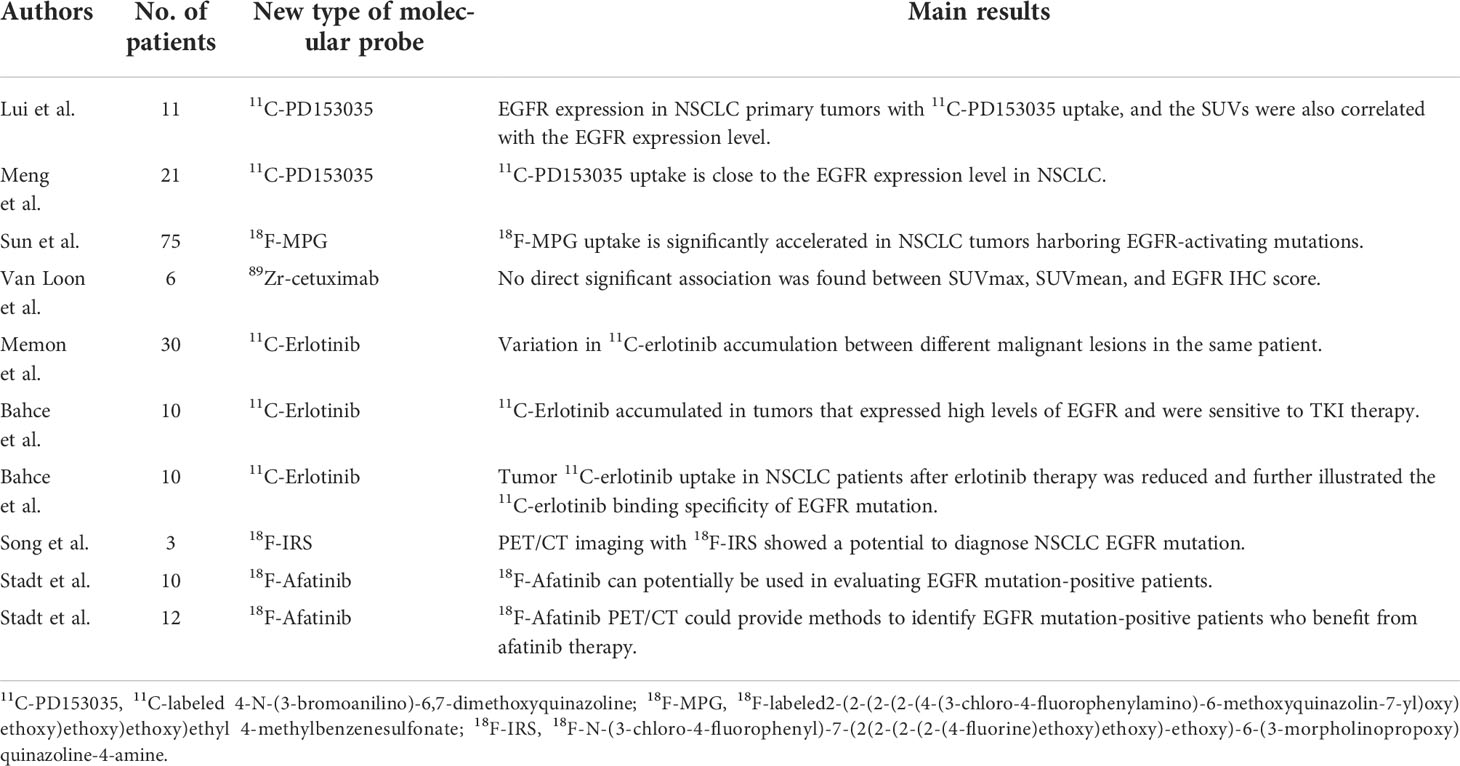
Table 3 Recent publications about the new type of molecular probe of PET/CT in use for the detection of epidermal growth factor receptor mutation status in non-small cell lung cancer.
Monoclonal antibodies directly target the extracellular domain of EGFR to prevent the binding of EGFR to ligands, thus blocking downstream signal transduction pathways. Monoclonal antibodies are all large molecules that need to be labeled with radionuclides with a long half-life, such as 64Cu, 11C, and 89Zr, as they infiltrate tissue very slowly. PET/CT using 89Zr-cetuximab allowed the visualization and quantification of tumor 89Zr-cetuximab uptake in cells and animals (53) or other malignancies (54) with EGFR mutations. Van Loon et al. studied head and neck cancer (NHC) and NSCLC patients using 89Zr-cetuximab PET/CT but showed that SUVmax and SUVmean had no direct relationship between EGFR immunohistochemistry (IHC) score and tumor-to-background ratio (TBR) (55). 89Zr-DFO-panitumumab PET/CT imaging assessed EGFR expression at a cellular level and in animals (56, 57).
Radiolabeled EGFR-TKI can bind specifically to the tyrosine kinase domain of the mutant protein, and the uptake levels can reflect EGFR expression and mutation status. Therefore, EGFR-TKI molecular probes have many obvious advantages over monoclonal antibodies. EGFR-TKI molecular probes are labeled with radionuclides of short circulating half-life, such as 11C and 18F, which can penetrate tissues quickly because they are small molecules.
4-N-[3-bromoanilino]-6,7-dimethoxyquinazoline (PD153035) is a reversible inhibitor of EGFR tyrosine kinase and a potent ATP-competitive TKI of EGFR (58). Additionally, 11C-labeled PD153035 has been assessed in vivo as a PET/CT agent to estimate EGFR expression in multiple tumors (59). Liu et al. studied the distribution of 11C-PD153035 in PET/CT imaging of 11 patients with NSCLC, finding that SUVs were correlated with expression levels of EGFR (60). Meng et al. analyzed 11C-PD153035 PET/CT images of 21 NSCLC patients revealing that 11C-PD153035 uptake is closely related to EGFR expression (61). Dai et al. demonstrated that 11C-PD153035 PET/CT imaging can be used as a simple and efficient method to detect NSCLC patients who are sensitive to EGFR-TKIs (62). Furthermore, the synthesis of polyethylene glycol (PEG)-modified (PEGylated) anilinoquinazoline derivative, 2-(2-(2-(2-(4-(3-chloro-4-fluorophenylamino)-6-methoxyquinazolin-7 yl)oxy)ethoxy)ethoxy)ethoxy)ethyl 4-methylbenzenesulfonate (T-MPG) derived from the known EGFR-TKI PD153035 has been reported by Sun et al. (63). Not only their preclinical research but also clinical research that involved 75 NSCLC patients has suggested that 18F-MPG uptake is dramatically accelerated in EGFR-mutated NSCLC.
11C-Erlotinib is a PET imaging tracer with great promise for evaluating EGFR expression in NSCLC patients and has been reported in animal models and human subjects, but only a limited number of clinical PET/CT studies have been conducted. Bahce et al. illustrated that 11C-erlotinib accumulated in tumors that highly expressed EGFR by reviewing 11C-erlotinib PET/CT images of 10 patients with NSCLC (64). A study by Bachce et al. analyzed 10 NSCLC patients with EGFR mutation status, demonstrating that 11C-erlotinib uptake in tumors reduces after erlotinib therapy (65). However, Petrulli et al. showed a lack of association between EGFR mutation status and 11C-erlotinib uptake in an analysis of 10 NSCLC patients via dynamic multi-bed PET/CT scan using 11C-erlotinib, suggesting disease heterogeneity and low tracer uptake for the lack of association (66).
Gefitinib is a small-molecule EGFR kinase inhibitor that binds to the intracellular tyrosine kinase domain and disrupts EGFR kinase activity with nanomolar affinity (67). 11C- and 18F-radiolabeled gefitinib could be applied to image EGFR expression and pharmacokinetics non-invasive study of gefitinib in patients. However, a few studies have been conducted at the cell and animal levels, and human tumor xenografts have not shown EGFR-specific concentrations (68). However, a novel radiotracer, 18F-N-(3-chloro-4-fluorophenyl)-7-(2(2-(2-(2-(4-fluorine)ethoxy)ethoxy)-ethoxy)-6-(3-morpholinopropoxy)quinazoline-4-amine (18F-IRS) based on gefitinib has been designed and synthesized, with 18F-IRS PET/CT showing potential to diagnose NSCLC EGFR mutation according to higher 18F-IRS uptake in NSCLC with EGFR mutations (69).
Afatinib is a second-generation irreversible 4-anilinoquinazoline EGFR kinase inhibitor (70). In mouse models bearing NSCLC xenografts [EGFR-mutated (HCC827 and H1975) xenografts and EGFR wild-type (A549)], Slobbe et al. suggested accumulation of 18F-afatinib in NSCLC tumors with EGFR mutation status (71, 72), justifying the further evaluation of NSCLC tumor EGFR mutations. Stadt et al. (73) quantified 18F-afatinib tumor uptake in NSCLC patients, suggesting that 18F-afatinib could potentially be used to evaluate EGFR mutation-positive patients. Furthermore, Stadt et al. (74) also evaluated whether 18F-afatinib uptake could predict the response to afatinib therapy by evaluating 18F-afatinib PET/CT images of 12 patients with NSCLC, showing that 18F-afatinib PET/CT could serve as a method for precise quantification of EGFR mutation status in NSCLC patients who would benefit from afatinib therapy.
The possibilities of protein molecular probes targeting EGFR have been demonstrated in in vivo imaging cell, animal, and clinical studies, especially EGFR-TKI-type molecular probes. Although these studies showed that molecular probes targeting EGFR for PET/CT imaging can identify EGFR mutation status in NSCLC, they tend to produce high background noise because of high lipophilicity, which leads to poor imaging quality. The short half-life of 11C also limits its widespread use in clinical practice, and 18F labeling requires many procedures to label the TKIs.
EGFR is a significant target for lung cancer diagnosis and treatment; thus, non-invasive, accurate, and rapid methods for EGFR mutation detection should be developed in NSCLC. Due to recent advances in molecular imaging and analytic platforms, PET/CT may play a crucial role in identifying EGFR mutation status. The relatively new 18F-FDG PET/CT-derived radiomics to predict EGFR mutations has attracted much attention, with studies revealing promising results. PET/CT imaging with radiolabeled monoclonal antibodies and EGFR TKIs is particularly attractive and may be better than 18F-FDG PET/CT-derived radiomics in detecting EGFR mutation status in NSCLC because it can be repeatedly operate and reflect receptor status in real-time. However, since most of the research to date has been performed at the cellular level or in animals, further clinical studies are needed in the future.
Conceptualization: NH, PGL, and PL. Writing (original draft preparation): NH, GY, YHW, and PGL. Writing (review and editing): PGL, YW, LW, and YNX. All the authors have read the manuscript and have approved it before submission.
This work was supported partly by the Science and Technology Projects of Guizhou Province (Qiankehe Support [2020]4Y193, Qiankehe Basic-ZK[2022]General 422) and the National Natural Science Foundation of China (81960338).
The authors gratefully thank all the participants at Guizhou Medical University. They are also thankful to StudyForBetter Team who contributed their best research skills to the area of radiobioinformatics.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sharma R. Descriptive epidemiology of incidence and mortality of primary liver cancer in 185 countries: evidence from globocan 2018. Jpn J Clin Oncol (2020) 50:1370–9. doi: 10.1093/jjco/hyaa130
2. Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol (2016) 893:1–19. doi: 10.1007/978-3-319-24223-1_1
3. Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, et al. Erlotinib versus standard chemotherapy as first-line treatment for european patients with advanced egfr mutation-positive non-small-cell lung cancer (eurtac): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol (2012) 13:239–46. doi: 10.1016/S1470-2045(11)70393-X
4. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, et al. Non-small cell lung cancer, version 5.2017, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw (2017) 15:504–35. doi: 10.6004/jnccn.2017.0050
5. Taniguchi K, Okami J, Kodama K, Higashiyama M, Kato K. Intratumor heterogeneity of epidermal growth factor receptor mutations in lung cancer and its correlation to the response to gefitinib. Cancer Sci (2008) 99:929–35. doi: 10.1111/j.1349-7006.2008.00782.x
6. Goldman JW, Noor ZS, Remon J, Besse B, Rosenfeld N. Are liquid biopsies a surrogate for tissue egfr testing? Ann Oncol (2018) 29:i38–46. doi: 10.1093/annonc/mdx706
7. Aerts HJ. The potential of radiomic-based phenotyping in precision medicine: a review. JAMA Oncol (2016) 2:1636–42. doi: 10.1001/jamaoncol.2016.2631
8. Zhang H, Cai W, Wang Y, Liao M, Tian S. Ct and clinical characteristics that predict risk of egfr mutation in non-small cell lung cancer: a systematic review and meta-analysis. Int J Clin Oncol (2019) 24:649–59. doi: 10.1007/s10147-019-01403-3
9. Cho A, Hur J, Moon YW, Hong SR, Suh YJ, Kim YJ, et al. Correlation between egfr gene mutation, cytologic tumor markers, 18f-fdg uptake in non-small cell lung cancer. BMC Cancer. (2016) 16:224. doi: 10.1186/s12885-016-2251-z
10. De Rosa V, Iommelli F, Monti M, Fonti R, Votta G, Stoppelli MP, et al. Reversal of warburg effect and reactivation of oxidative phosphorylation by differential inhibition of egfr signaling pathways in non-small cell lung cancer. Clin Cancer Res (2015) 21:5110–20. doi: 10.1158/1078-0432.CCR-15-0375
11. Na II, Byun BH, Kim KM, Cheon GJ, Choe DH, Koh JS, et al. 18f-fdg uptake and egfr mutations in patients with non-small cell lung cancer: a single-institution retrospective analysis. Lung Cancer. (2010) 67:76–80. doi: 10.1016/j.lungcan.2009.03.010
12. Mak RH, Digumarthy SR, Muzikansky A, Engelman JA, Shepard JA, Choi NC, et al. Role of 18f-fluorodeoxyglucose positron emission tomography in predicting epidermal growth factor receptor mutations in non-small cell lung cancer. Oncologist (2011) 16:319–26. doi: 10.1634/theoncologist.2010-0300
13. Usuda K, Sagawa M, Motono N, Ueno M, Tanaka M, Machida Y, et al. Relationships between egfr mutation status of lung cancer and preoperative factors - are they predictive? Asian Pac J Cancer Prev (2014) 15:657–62. doi: 10.7314/apjcp.2014.15.2.657
14. Chen L, Zhou Y, Tang X, Yang C, Tian Y, Xie R, et al. Egfr mutation decreases fdg uptake in nonsmall cell lung cancer via the nox4/ros/glut1 axis. Int J Oncol (2019) 54:370–80. doi: 10.3892/ijo.2018.4626
15. Yang B, Wang QG, Lu M, Ge Y, Zheng YJ, Zhu H, et al. Correlations study between (18)f-fdg pet/ct metabolic parameters predicting epidermal growth factor receptor mutation status and prognosis in lung adenocarcinoma. Front Oncol (2019) 9:589. doi: 10.3389/fonc.2019.00589
16. Liao X, Cui Y, Chen X, Di L, Tong Z, Liu M, et al. Primary metabolic tumor volume from 18f-fdg pet/ct associated with epidermal growth factor receptor mutation in lung adenocarcinoma patients. Nucl Med Commun (2020) 41:1210–7. doi: 10.1097/MNM.0000000000001274
17. Takamochi K, Mogushi K, Kawaji H, Imashimizu K, Fukui M, Oh S, et al. Correlation of egfr or kras mutation status with 18f-fdg uptake on pet-ct scan in lung adenocarcinoma. PloS One (2017) 12:e175622. doi: 10.1371/journal.pone.0175622
18. Lv Z, Fan J, Xu J, Wu F, Huang Q, Guo M, et al. Value of (18)f-fdg pet/ct for predicting egfr mutations and positive alk expression in patients with non-small cell lung cancer: a retrospective analysis of 849 chinese patients. Eur J Nucl Med Mol Imaging. (2018) 45:735–50. doi: 10.1007/s00259-017-3885-z
19. Gu J, Xu S, Huang L, Li S, Wu J, Xu J, et al. Value of combining serum carcinoembryonic antigen and pet/ct in predicting egfr mutation in non-small cell lung cancer. J Thorac Dis (2018) 10:723–31. doi: 10.21037/jtd.2017.12.143
20. Zhu L, Yin G, Chen W, Li X, Yu X, Zhu X, et al. Correlation between egfr mutation status and f(18) -fluorodeoxyglucose positron emission tomography-computed tomography image features in lung adenocarcinoma. Thorac Cancer. (2019) 10:659–64. doi: 10.1111/1759-7714.12981
21. Guan J, Xiao NJ, Chen M, Zhou WL, Zhang YW, Wang S, et al. 18f-fdg uptake for prediction egfr mutation status in non-small cell lung cancer. Med (Baltimore). (2016) 95:e4421. doi: 10.1097/MD.0000000000004421
22. Qiang G, Huang W, Liang C, Xu R, Yan J, Xu Y, et al. Association between histopathological subtype, (18)f-fluorodeoxyglucose uptake and epidermal growth factor receptor mutations in lung adenocarcinoma. Oncol Lett (2016) 11:1769–77. doi: 10.3892/ol.2016.4154
23. Ko KH, Hsu HH, Huang TW, Gao HW, Shen DH, Chang WC, et al. Value of (1)(8)f-fdg uptake on pet/ct and cea level to predict epidermal growth factor receptor mutations in pulmonary adenocarcinoma. Eur J Nucl Med Mol Imaging. (2014) 41:1889–97. doi: 10.1007/s00259-014-2802-y
24. Kanmaz ZD, Aras G, Tuncay E, Bahadır A, Kocatürk C, Yaşar ZA, et al. Contribution of 18fluorodeoxyglucose positron emission tomography uptake and ttf-1 expression in the evaluation of the egfr mutation in patients with lung adenocarcinoma. Cancer biomark (2016) 16:489–98. doi: 10.3233/CBM-160588
25. Chung HW, Lee KY, Kim HJ, Kim WS, So Y. Fdg pet/ct metabolic tumor volume and total lesion glycolysis predict prognosis in patients with advanced lung adenocarcinoma. J Cancer Res Clin Oncol (2014) 140:89–98. doi: 10.1007/s00432-013-1545-7
26. Caicedo C, Garcia-Velloso MJ, Lozano MD, Labiano T, Vigil DC, Lopez-Picazo JM, et al. Role of [(1)(8)f]fdg pet in prediction of kras and egfr mutation status in patients with advanced non-small-cell lung cancer. Eur J Nucl Med Mol Imaging. (2014) 41:2058–65. doi: 10.1007/s00259-014-2833-4
27. Lee EY, Khong PL, Lee VH, Qian W, Yu X, Wong MP. Metabolic phenotype of stage iv lung adenocarcinoma: relationship with epidermal growth factor receptor mutation. Clin Nucl Med (2015) 40:e190–5. doi: 10.1097/RLU.0000000000000684
28. Lee SM, Bae SK, Jung SJ, Kim CK. Fdg uptake in non-small cell lung cancer is not an independent predictor of egfr or kras mutation status: a retrospective analysis of 206 patients. Clin Nucl Med (2015) 40:950–8. doi: 10.1097/RLU.0000000000000975
29. Liu A, Han A, Zhu H, Ma L, Huang Y, Li M, et al. The role of metabolic tumor volume (mtv) measured by [18f] fdg pet/ct in predicting egfr gene mutation status in non-small cell lung cancer. Oncotarget (2017) 8:33736–44. doi: 10.18632/oncotarget.16806
30. Du B, Wang S, Cui Y, Liu G, Li X, Li Y. Can (18)f-fdg pet/ct predict egfr status in patients with non-small cell lung cancer? a systematic review and meta-analysis. BMJ Open (2021) 11:e44313. doi: 10.1136/bmjopen-2020-044313
31. Guo Y, Zhu H, Yao Z, Liu F, Yang D. The diagnostic and predictive efficacy of 18f-fdg pet/ct metabolic parameters for egfr mutation status in non-small-cell lung cancer: a meta-analysis. Eur J Radiol (2021) 141:109792. doi: 10.1016/j.ejrad.2021.109792
32. Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology (2016) 278:563–77. doi: 10.1148/radiol.2015151169
33. Rizzo S, Botta F, Raimondi S, Origgi D, Fanciullo C, Morganti AG, et al. Radiomics: the facts and the challenges of image analysis. Eur Radiol Exp (2018) 2:36. doi: 10.1186/s41747-018-0068-z
34. Kirienko M, Cozzi L, Antunovic L, Lozza L, Fogliata A, Voulaz E, et al. Prediction of disease-free survival by the pet/ct radiomic signature in non-small cell lung cancer patients undergoing surgery. Eur J Nucl Med Mol Imaging. (2018) 45:207–17. doi: 10.1007/s00259-017-3837-7
35. Castiglioni I, Gilardi MC. Radiomics: is it time to compose the puzzle? Clin Transl Imaging. (2018) 6:411–3. doi: 10.1007/s40336-018-0302-y
36. Bianconi F, Palumbo I, Spanu A, Nuvoli S, Fravolini ML, Palumbo B. Pet/ct radiomics in lung cancer: an overview. Appl Sci (2020) 10:1718. doi: 10.3390/app10051718
37. Kang F, Mu W, Gong J, Wang S, Li G, Li G, et al. Integrating manual diagnosis into radiomics for reducing the false positive rate of (18)f-fdg pet/ct diagnosis in patients with suspected lung cancer. Eur J Nucl Med Mol Imaging. (2019) 46:2770–9. doi: 10.1007/s00259-019-04418-0
38. Bianconi F, Palumbo I, Fravolini ML, Chiari R, Minestrini M, Brunese L, et al. Texture analysis on [(18)f]fdg pet/ct in non-small-cell lung cancer: correlations between pet features, ct features, and histological types. Mol Imaging Biol (2019) 21:1200–9. doi: 10.1007/s11307-019-01336-3
39. Yip SS, Kim J, Coroller TP, Parmar C, Velazquez ER, Huynh E, et al. Associations between somatic mutations and metabolic imaging phenotypes in non-small cell lung cancer. J Nucl Med (2017) 58:569–76. doi: 10.2967/jnumed.116.181826
40. Park S, Ha S, Lee SH, Paeng JC, Keam B, Kim TM, et al. Intratumoral heterogeneity characterized by pretreatment pet in non-small cell lung cancer patients predicts progression-free survival on egfr tyrosine kinase inhibitor. PloS One (2018) 13:e189766. doi: 10.1371/journal.pone.0189766
41. Jiang M, Zhang Y, Xu J, Ji M, Guo Y, Guo Y, et al. Assessing egfr gene mutation status in non-small cell lung cancer with imaging features from pet/ct. Nucl Med Commun (2019) 40:842–9. doi: 10.1097/MNM.0000000000001043
42. Koyasu S, Nishio M, Isoda H, Nakamoto Y, Togashi K. Usefulness of gradient tree boosting for predicting histological subtype and egfr mutation status of non-small cell lung cancer on (18)f fdg-pet/ct. Ann Nucl Med (2020) 34:49–57. doi: 10.1007/s12149-019-01414-0
43. Liu Q, Sun D, Li N, Kim J, Feng D, Huang G, et al. Predicting egfr mutation subtypes in lung adenocarcinoma using (18)f-fdg pet/ct radiomic features. Transl Lung Cancer Res (2020) 9:549–62. doi: 10.21037/tlcr.2020.04.17
44. Mu W, Jiang L, Zhang J, Shi Y, Gray JE, Tunali I, et al. Non-invasive decision support for nsclc treatment using pet/ct radiomics. Nat Commun (2020) 11:5228. doi: 10.1038/s41467-020-19116-x
45. Yang B, Ji HS, Zhou CS, Dong H, Ma L, Ge YQ, et al. (18)f-fluorodeoxyglucose positron emission tomography/computed tomography-based radiomic features for prediction of epidermal growth factor receptor mutation status and prognosis in patients with lung adenocarcinoma. Transl Lung Cancer Res (2020) 9:563–74. doi: 10.21037/tlcr-19-592
46. Shiri I, Maleki H, Hajianfar G, Abdollahi H, Ashrafinia S, Hatt M, et al. Next-generation radiogenomics sequencing for prediction of egfr and kras mutation status in nsclc patients using multimodal imaging and machine learning algorithms. Mol Imaging Biol (2020) 22:1132–48. doi: 10.1007/s11307-020-01487-8
47. Zhang J, Zhao X, Zhao Y, Zhang J, Zhang Z, Wang J, et al. Value of pre-therapy (18)f-fdg pet/ct radiomics in predicting egfr mutation status in patients with non-small cell lung cancer. Eur J Nucl Med Mol Imaging. (2020) 47:1137–46. doi: 10.1007/s00259-019-04592-1
48. Zhang M, Bao Y, Rui W, Shangguan C, Liu J, Xu J, et al. Performance of (18)f-fdg pet/ct radiomics for predicting egfr mutation status in patients with non-small cell lung cancer. Front Oncol (2020) 10:568857. doi: 10.3389/fonc.2020.568857
49. Li X, Yin G, Zhang Y, Dai D, Liu J, Chen P, et al. Predictive power of a radiomic signature based on (18)f-fdg pet/ct images for egfr mutational status in nsclc. Front Oncol (2019) 9:1062. doi: 10.3389/fonc.2019.01062
50. Chang C, Zhou S, Yu H, Zhao W, Ge Y, Duan S, et al. A clinically practical radiomics-clinical combined model based on pet/ct data and nomogram predicts egfr mutation in lung adenocarcinoma. Eur Radiol (2021) 31:6259–68. doi: 10.1007/s00330-020-07676-x
51. Abdurixiti M, Nijiati M, Shen R, Ya Q, Abuduxiku N, Nijiati M. Current progress and quality of radiomic studies for predicting egfr mutation in patients with non-small cell lung cancer using pet/ct images: a systematic review. Br J Radiol (2021) 94:20201272. doi: 10.1259/bjr.20201272
52. Palumbo B, Bianconi F, Fravolini ML, Palumbo I, Palumbo B, Bianconi F, et al. Shape and texture analysis of radiomic data for computer-assisted diagnosis and prognostication: an overview. (2020). doi: 10.1007/978-3-030-31154-4_1
53. Aerts HJ, Dubois L, Perk L, Vermaelen P, van Dongen GA, Wouters BG, et al. Disparity between in vivo egfr expression and 89zr-labeled cetuximab uptake assessed with pet. J Nucl Med (2009) 50:123–31. doi: 10.2967/jnumed.108.054312
54. Makris NE, van Velden FH, Huisman MC, Menke CW, Lammertsma AA, Boellaard R. Validation of simplified dosimetry approaches in 89zr-pet/ct: the use of manual versus semi-automatic delineation methods to estimate organ absorbed doses. Med Phys (2014) 41:102503. doi: 10.1118/1.4895973
55. van Loon J, Even A, Aerts H, Öllers M, Hoebers F, van Elmpt W, et al. Pet imaging of zirconium-89 labelled cetuximab: a phase i trial in patients with head and neck and lung cancer. Radiother Oncol (2017) 122:267–73. doi: 10.1016/j.radonc.2016.11.020
56. Bhattacharyya S, Kurdziel K, Wei L, Riffle L, Kaur G, Hill GC, et al. Zirconium-89 labeled panitumumab: a potential immuno-pet probe for her1-expressing carcinomas. Nucl Med Biol (2013) 40:451–7. doi: 10.1016/j.nucmedbio.2013.01.007
57. Chang AJ, De Silva RA, Lapi SE. Development and characterization of 89zr-labeled panitumumab for immuno-positron emission tomographic imaging of the epidermal growth factor receptor. Mol Imaging. (2013) 12:17–27. doi: 10.2310/7290.2012.00016
58. Bos M, Mendelsohn J, Kim YM, Albanell J, Fry DW, Baselga J. Pd153035, a tyrosine kinase inhibitor, prevents epidermal growth factor receptor activation and inhibits growth of cancer cells in a receptor number-dependent manner. Clin Cancer Res (1997) 3:2099–106. doi: 10.2310/7290.2012.00016
59. Yu J, Liu N, Yang G, Guo H, Ma L, Zhao S, et al. Novel carbon-11 labeled 4-dimethylamino-but-2-enoic acid [4-(phenylamino)-quinazoline-6-yl]-amides: potential pet bioprobes for molecular imaging of egfr-positive tumors. Nucl Med Biol (2004) 31:469–76. doi: 10.1016/j.nucmedbio.2003.12.005
60. Yu J, Liu N, Yang G, Guo H, Ma L, Zhao S, et al. 11c-pd153035 pet/ct for molecular imaging of egfr in patients with non-small cell lung cancer (nsclc). J Clin Oncol (2008) 26:3503. doi: 10.1200/jco.2008.26.15_suppl.3503
61. Meng X, Loo BJ, Ma L, Murphy JD, Sun X, Yu J. Molecular imaging with 11c-pd153035 pet/ct predicts survival in non-small cell lung cancer treated with egfr-tki: a pilot study. J Nucl Med (2011) 52:1573–9. doi: 10.2967/jnumed.111.092874
62. Dai D, Li XF, Wang J, Liu JJ, Zhu YJ, Zhang Y, et al. Predictive efficacy of (11)c-pd153035 pet imaging for egfr-tyrosine kinase inhibitor sensitivity in non-small cell lung cancer patients. Int J Cancer. (2016) 138:1003–12. doi: 10.1002/ijc.29832
63. Sun X, Xiao Z, Chen G, Han Z, Liu Y, Zhang C, et al. A pet imaging approach for determining egfr mutation status for improved lung cancer patient management. Sci Transl Med (2018) 10:eaan8840. doi: 10.1126/scitranslmed.aan8840
64. Bahce I, Smit EF, Lubberink M, van der Veldt AA, Yaqub M, Windhorst AD, et al. Development of [(11)c]erlotinib positron emission tomography for in vivo evaluation of egf receptor mutational status. Clin Cancer Res (2013) 19:183–93. doi: 10.1158/1078-0432.CCR-12-0289
65. Bahce I, Yaqub M, Errami H, Schuit RC, Schober P, Thunnissen E, et al. Effects of erlotinib therapy on [(11)c]erlotinib uptake in egfr mutated, advanced nsclc. Ejnmmi Res (2016) 6:10. doi: 10.1186/s13550-016-0169-8
66. Petrulli JR, Zheng M, Huang Y, Nabulsi NB, Goldberg SB, Contessa JN, et al. Evaluation of quantitative modeling methods in whole-body, dynamic [(11)c]-erlotinib pet. Am J Nucl Med Mol Imaging. (2021) 11:143–53.
67. Wakeling AE, Guy SP, Woodburn JR, Ashton SE, Curry BJ, Barker AJ, et al. Zd1839 (iressa): an orally active inhibitor of epidermal growth factor signaling with potential for cancer therapy. Cancer Res (2002) 62:5749–54.
68. Su H, Seimbille Y, Ferl GZ, Bodenstein C, Fueger B, Kim KJ, et al. Evaluation of [(18)f]gefitinib as a molecular imaging probe for the assessment of the epidermal growth factor receptor status in malignant tumors. Eur J Nucl Med Mol Imaging. (2008) 35:1089–99. doi: 10.1007/s00259-007-0636-6
69. Song Y, Xiao Z, Wang K, Wang X, Zhang C, Fang F, et al. Development and evaluation of (18)f-irs for molecular imaging mutant egf receptors in nsclc. Sci Rep (2017) 7:3121. doi: 10.1038/s41598-017-01443-7
70. Solca F, Dahl G, Zoephel A, Bader G, Sanderson M, Klein C, et al. Target binding properties and cellular activity of afatinib (bibw 2992), an irreversible erbb family blocker. J Pharmacol Exp Ther (2012) 343:342–50. doi: 10.1124/jpet.112.197756
71. Slobbe P, Windhorst AD, Stigter-van WM, Schuit RC, Smit EF, Niessen HG, et al. Development of [18f]afatinib as new tki-pet tracer for egfr positive tumors. Nucl Med Biol (2014) 41:749–57. doi: 10.1016/j.nucmedbio.2014.06.005
72. Slobbe P, Windhorst AD, Stigter-van WM, Smit EF, Niessen HG, Solca F, et al. A comparative pet imaging study with the reversible and irreversible egfr tyrosine kinase inhibitors [(11)c]erlotinib and [(18)f]afatinib in lung cancer-bearing mice. Ejnmmi Res (2015) 5:14. doi: 10.1186/s13550-015-0088-0
73. van de Stadt EA, Yaqub M, Lammertsma AA, Poot AJ, Schober PR, Schuit RC, et al. Quantification of [(18)f]afatinib using pet/ct in nsclc patients: a feasibility study. Ejnmmi Res (2020) 10:97. doi: 10.1186/s13550-020-00684-4
Keywords: PET/CT, prediction model, epidermal growth factor receptor, non-small cell lung cancer, radiogenomics
Citation: Hu N, Yan G, Wu Y, Wang L, Wang Y, Xiang Y, Lei P and Luo P (2022) Recent and current advances in PET/CT imaging in the field of predicting epidermal growth factor receptor mutations in non-small cell lung cancer. Front. Oncol. 12:879341. doi: 10.3389/fonc.2022.879341
Received: 19 February 2022; Accepted: 20 September 2022;
Published: 06 October 2022.
Edited by:
Sally Lau, Grossman School of Medicine, New York University, United StatesReviewed by:
Kun Zheng, Peking Union Medical College Hospital (CAMS), ChinaCopyright © 2022 Hu, Yan, Wu, Wang, Wang, Xiang, Lei and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pinggui Lei, pingguilei@foxmail.com; Peng Luo, luopeng@gmc.edu.cn
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.