- Perlmutter Cancer Center, New York University (NYU) Langone Health, New York, NY, United States
Many decades in the making, immunotherapy has demonstrated its ability to produce durable responses in several cancer types. In the last decade, immunotherapy has shown itself to be a viable therapeutic approach for non-small cell lung cancer (NSCLC). Several clinical trials have established the efficacy of immune checkpoint blockade (ICB), particularly in the form of anti-programmed death 1 (PD-1) antibodies, anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) antibodies and anti-programmed death 1 ligand (PD-L1) antibodies. Many trials have shown progression free survival (PFS) and overall survival (OS) benefit with either ICB alone or in combination with chemotherapy when compared to chemotherapy alone. The identification of biomarkers to predict response to immunotherapy continues to be evaluated. The future of immunotherapy in lung cancer continues to hold promise with the development of combination therapies, cytokine modulating therapies and cellular therapies. Lastly, we expect that innovative advances in technology, such as artificial intelligence (AI) and machine learning, will begin to play a role in the future care of patients with lung cancer.
Introduction
Immune modulation has long been studied as a potential therapeutic target in the battle against disease. In 1798, Edward Jenner coined the term “vaccination” as he popularized and spread the concept of inoculation with cowpox to prevent smallpox across Europe (1). Almost a hundred years later, across the Atlantic, William Coley began injecting bacterial products known as Coley’s Toxins into patients with sarcomas (2). This was perhaps modern medicine’s first foray into immuno-oncology.
In 1909, Paul Ehrlich proposed the hypothesis that the aberrant transformation of cells was an inherent and common process, but host defense mechanisms prevent them from turning into cancer (3). Paul Ehrlich’s hypothesis was further refined in the 1950s and 1960s by Lewis Thomas and Frank Burnet, who hypothesized that an antigenic response by the immune system against tumor cells formed the basis of the cancer immunosurveillance hypothesis (4). Around the turn of the century, this hypothesis was further molded into the cancer immunoediting hypothesis, largely known by its three “E”s: elimination, equilibrium, and escape (5). Normal cells undergo malignant transformation due to any number of factors, which induces the expression of tumor-associated antigens that promote immune-related elimination of malignancy cells, classically referred to as immunosurveillance. In a subset of these situations, the immune system will not be able to completely eradicate a malignant group of cells and these cells will exist in equilibrium. Lastly, some of these cells will undergo immune escape to ultimately develop into clinically significant cancers (4–7).
The discovery and description of the programmed death (PD) pathway as mediators of tumor immune evasion changed the trajectory of immuno-oncology (8–12). The process of T cell activation begins when the T cell receptor engages with antigen presented by a major histocompatibility protein (MHC). However, T cell activation does not occur unless a second co-stimulatory signal is received. On the other hand, inhibitory checkpoints, such as CTLA-4 and PD-1, lead to T cell anergy or apoptosis when engaged. In normal states, these checkpoints are crucial in controlling immune activation and preventing collateral tissue damage from autoimmunity. In states of chronic inflammation or cancer, exhausted T cells are found to upregulate expression of inhibitory CTLA-4 and PD-1 molecules. Monoclonal antibodies against CTLA-4, PD-1/PD-L1 are designed to reinvigorate exhausted T cells and restore immune-mediated elimination of malignant cells, a strategy that has highly successful against multiple tumor subtypes including NSCLC (13–19).
The 2018 Nobel Prize in Physiology or Medicine was awarded to Drs. James Allison and Tasuku Honjo for their work in describing the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and PD-1 pathways as checkpoints and further showing the inhibition of these checkpoints improve T-cell mediated anti-tumor effects (20). Ipilimumab, an anti-CTLA-4 antibody, gained widespread attention when it was shown to improve survival in advanced melanoma (21). Nivolumab, the first monoclonal antibody (mAb) against PD-1, first demonstrated its utility in the treatment of metastatic melanoma, non-small cell lung cancer (NSCLC), and advanced renal cell carcinoma (RCC) and further showed survival benefit in a variety of malignancies such as head and neck squamous cell carcinoma (HNSCC) amongst others (22–27). Another well-known PD-1 mAb, pembrolizumab, generated similar excitement. Pembrolizumab has shown positive activity in many cancers, including melanoma, NSCLC, triple negative breast cancer (TNBC), and HNSCC (28–34). Aside from nivolumab and pembrolizumab, many other PD-1 and PD-L1 modulators have been developed and have shown activity in small cell lung cancer (SCLC), cutaneous squamous cell carcinoma, bladder cancer, and TNBC (35–42).
The current immunotherapy landscape for the treatment of NSCLC
Immunotherapy in early stage NSCLC
Adjuvant immunotherapy in resected NSCLC
Until recently, chemotherapy with a platinum doublet has been the mainstay of adjuvant therapy after curative resection of early stage NSCLC. The IMpower010 study is the first clinical trial to demonstrate meaningful improvements in disease free survival (DFS) with immunotherapy in stage IB-IIIA NSCLC. Patients enrolled in this study recent standard of care chemotherapy were randomized to adjuvant atezolizumab, a PD-L1 inhibitor, for one year or best supportive care. At interim analysis, patients with PD-L1 positive (≥1%), stage II-IIIA disease who received atezolizumab had a superior median DFS compared to best supportive care (stratified HR 0.66, 95%CI 0.50-0.88, p=0.004) (43). Although the data on overall survival is still immature at this point, the improvements in DFS is felt to be clinically meaningful. It is important to note that immunotherapy is an addition and not a replacement for adjuvant chemotherapy. In addition, patients with resected EGFR mutant NSCLC should receive an adjuvant tyrosine kinase inhibitor (TKI) (44) rather than immunotherapy. Although there is no direct comparison, the effectiveness of immunotherapy in EGFR mutant NSCLC is low in advanced disease based on pooled analysis of several trials including KEYNOTE-010 and CheckMate 057 (45–48) and the use of adjuvant EGFR TKIs demonstrated impressive improvements in DFS in the phase 3 ADAURA trial of 682 patients with stage IB to IIIA EGFR mutant NSCLC. This trial showed a 2 year DFS of 89% vs 53% patients treated with osimertinib compared to those treated with placebo (44). Based on interim results of the IMpower010 study, atezolizumab received approval from the US Food and Drug Administration (FDA) for use as adjuvant therapy in resected stage II-IIIA NSCLC with PD-L1≥1%. It is the first study to demonstrate that immunotherapy can improve the cure rates of patients with early stage lung cancer. The phase 3 KEYNOTE-091 trial further supported the use of immunotherapy in the adjuvant study. This study, presented at ESMO in 2022, evaluated the use of pembrolizumab versus placebo in stage IB to IIIA NSCLC after resection and adjuvant chemotherapy (if indicated) and found improved median DFS (53.6 months vs 42.0 months; HR 0.76; 95% CI 0.63-0.91; p<0.01) in patients receiving pembrolizumab. It is important to note that pembrolizumab was well tolerated in this trial, with a median number of doses of 17 in the investigational arm compared to 18 in the placebo arm. Not surprisingly, patients who received pembrolizumab experienced more grade ≥3 adverse events than those who did not (34.1% vs 25.8%). There was also a treatment discontinuation rate of 19.8% in the pembrolizumab arm compared to 5.9% in the placebo arm (49), suggesting that the DFS benefit must be considered in context of the relatively favorable safety and tolerability profile of this agent.
Neoadjuvant immunotherapy in NSCLC
Immunotherapy has also been explored in the neoadjuvant setting. Neoadjuvant therapy has several potential benefits: 1) downstage tumor prior to definitive surgery, 2) early treatment of micrometastatic disease, 3) improved T cell priming from neoantigens derived from the primary tumor and 4) provide prognostic information based on tumor pathological response to treatment.
Forde et al., published the first pilot study investigating two preoperative doses of nivolumab, a PD-1 inhibitor, in patients with stage I, II, or IIIA NSCLC in the CheckMate-159 trial (50). Although the number of patients was small (n=21), they reported a major pathological response (MPR) in 9 of 20 resected tumors (45%). They also showed that this treatment was associated with few side effects and no delays to surgery. The Lung Cancer Mutation Consortium (LCMC) 3 trial is the largest study investigating the use of single agent ICI as neoadjuvant therapy in patients with resectable stage IB-IIIB NSCLC. A total of 181 patients were enrolled. Patients were treated with 2 cycles of neoadjuvant atezolizumab followed by surgery, then completion of adjuvant atezolizumab for one year. The MPR rate, defined as <10% viable tumor cells, was 21%, with a 7% complete pathologic response rate, supporting the findings from the smaller studies. Notably, in 10% of patients, surgery occurred outside of the protocol window and 11% of patients ultimately did not undergo surgery (51). Encouragingly, the exploratory analyses on DFS and OS, stratified by stage, were similar to historical controls.
PD-1/PD-L1 inhibitors in combination with CTLA-4 inhibitors or chemotherapy have also been investigated as neoadjuvant therapy. The phase 2 NEOSTAR trial evaluated MPR in patients receiving neoadjuvant nivolumab alone or nivolumab and ipilimumab. Of the 44 patients enrolled, there was a 24% MPR rate in the nivolumab arm and 50% in the nivolumab and ipilimumab arm. 22% of patient had surgery after 42 days of completion of neoadjuvant treatment (52). In a single-arm phase 2 trial evaluating combination chemotherapy with atezolizumab in resectable NSCLC, 57% of patients achieved major pathologic response (53). The NADIM trial evaluated the role of neoadjuvant nivolumab in combination with platinum doublet chemotherapy in resectable stage IIIA NSCLC (54). In this trial, patients were treated with paclitaxel and carboplatin plus nivolumab for 3 cycles prior to surgery followed by 1 year of adjuvant nivolumab. Progression-free survival was found to be 77.1% at 24 months.
The CheckMate-816 is the only phase 3 trial in this space investigating neoadjuvant chemotherapy with or without nivolumab in patients with resectable stage IB-IIIA NSCLC. The primary endpoint of pathological complete response (pCR) was significant higher in patients who received nivolumab and chemotherapy compared to chemotherapy alone (24% vs 2.2%, OR 13.94, 99% CI, 3.49-55.75, p<0.0001). Benefit was seen in both PD-L1 <1% and PD-L1≥1% subgroups (55). MPR rates were 36.9% in the nivolumab-containing group and 8.9% in patients receiving chemotherapy alone. There was no increase in grades 3 or 4 drug-related adverse events and no safety signals on surgical complications or outcomes (56).
Neoadjuvant immunotherapy has strong mechanistic rationale to improve anticancer T cell mediated immunity. This can be due to expansion of tumor infiltrating lymphocytes via PD-L1 and PD-L2 expressing dendritic cells. Furthermore, these dendritic cells (after treatment with PD-(L)1 blockade) can better present tumor associated antigens in regional lymph nodes, further enhancing antitumor immunity (57).
As of March 2022, neoadjuvant immunotherapy (nivolumab) was approved by the FDA in combination with platinum-doublet chemotherapy based on the CheckMate-816 trial. Improved Event Free Survival (EFS) was recently reported in this trial – 31.6 months with nivolumab plus chemotherapy versus 20.8 months with chemotherapy alone (HR for progression, recurrence or death: 0.63, 97.38% CI: 0.43-0.91, p=0.005) (58). While the above-mentioned trials demonstrate benefit, it is important to await data maturity to ensure that benefits in pathologic response translates to similar benefits in survival. Additionally, it is important to exercise caution given that an important limitation of neoadjuvant therapy stems from the inherent delay to definitive surgical resection that neoadjuvant treatment creates for patients. To this end, investigators have proposed the integration of surgical endpoints and metrics to help further clarify this issue (56). Lastly, just as in the metastatic setting, complete genetic profiling of early stage tumors is critical prior to initiation of therapy, as recurrence of tumors harboring driver mutations have been described (59).
Immunotherapy in locally advanced NSCLC
The PACIFIC trial is a randomized phase 3 trial of 713 patients with unresectable stage III NSCLC who were treated with concurrent chemoradiotherapy, followed by durvalumab, a PD-L1 inhibitor, consolidation versus placebo. The trial showed PFS benefit in patients treated with durvalumab as compared to placebo (PFS 16.8 months vs 5.6 months, HR 0.52, 95% CI 4.6-7.8). In addition, an OS benefit was demonstrated with durvalumab (HR 0.68; 99.73% CI, 0.47-0.997; p=0.0025) with a 24-month OS rate of 66.3% compared to 55.6% in the placebo group (36). An updated 5-year data analysis showed persistent benefit with a 5-year OS rate of 42.9% in the group that received durvalumab compared to 33.4% in the placebo group (HR 0.72, 95% CI 0.59-0.89), supporting the use of durvalumab after CRT as standard of care in this setting (60). The rates of severe adverse events were similar. In addition, quality of life, assessed prospectively by patient reported outcomes, was similar even with an additional year of active therapy (61).
Building upon the success of the PACIFIC trial. Investigators have also evaluated the efficacy immunotherapy combinations. The KEYNOTE-799 study is a phase II trial that investigated chemo-immunotherapy with pembrolizumab, a PD-1 inhibitor, concurrently with radiation (62). The results demonstrated a promising response rate of approximately 70% although survival data remained immature. The use of pembrolizumab concurrently with radiation did not appear to affect the rates of severe pneumonitis (~8%). The PACIFIC-2 trial moves the use of durvalumab earlier in the treatment course of patients with stage III NSCLC and is attempting to answer whether concurrent chemoradiation and immunotherapy is superior to chemoradiation with consolidative immunotherapy (63). Given that many patients are not candidates for concurrent chemoradiation, the phase 2 PACIFIC-6 trial is assessing the safety of durvalulmab after sequential chemoradiation. Data presented in 2022 showed similar safety to that seen in the PACIFIC trial suggesting that this may also represent a reasonable treatment strategy in patients unable to undergo concurrent chemoradiation (64). In the phase II COAST study, durvalumab was combined with olecumab (anti-CD73 mAb) or monalizumab (anti-NKG2A mAb) as consolidative treatment of unresectable stage III NSCLC after concurrent chemoradiation. Both the durvalumab plus olecumab and the durvalumab plus monalizumab arms showed improved ORR compared to durvalumab alone (38.3%, 37.1%, and 25.5% respectively) as well as improved median PFS (not reached, 15.1 months, and 6.3 months, respectively). Toxicity was similar across all three arms, suggesting that combinatorial approaches to consolidation treatment can improve outcomes (65).
Immunotherapy in advanced NSCLC
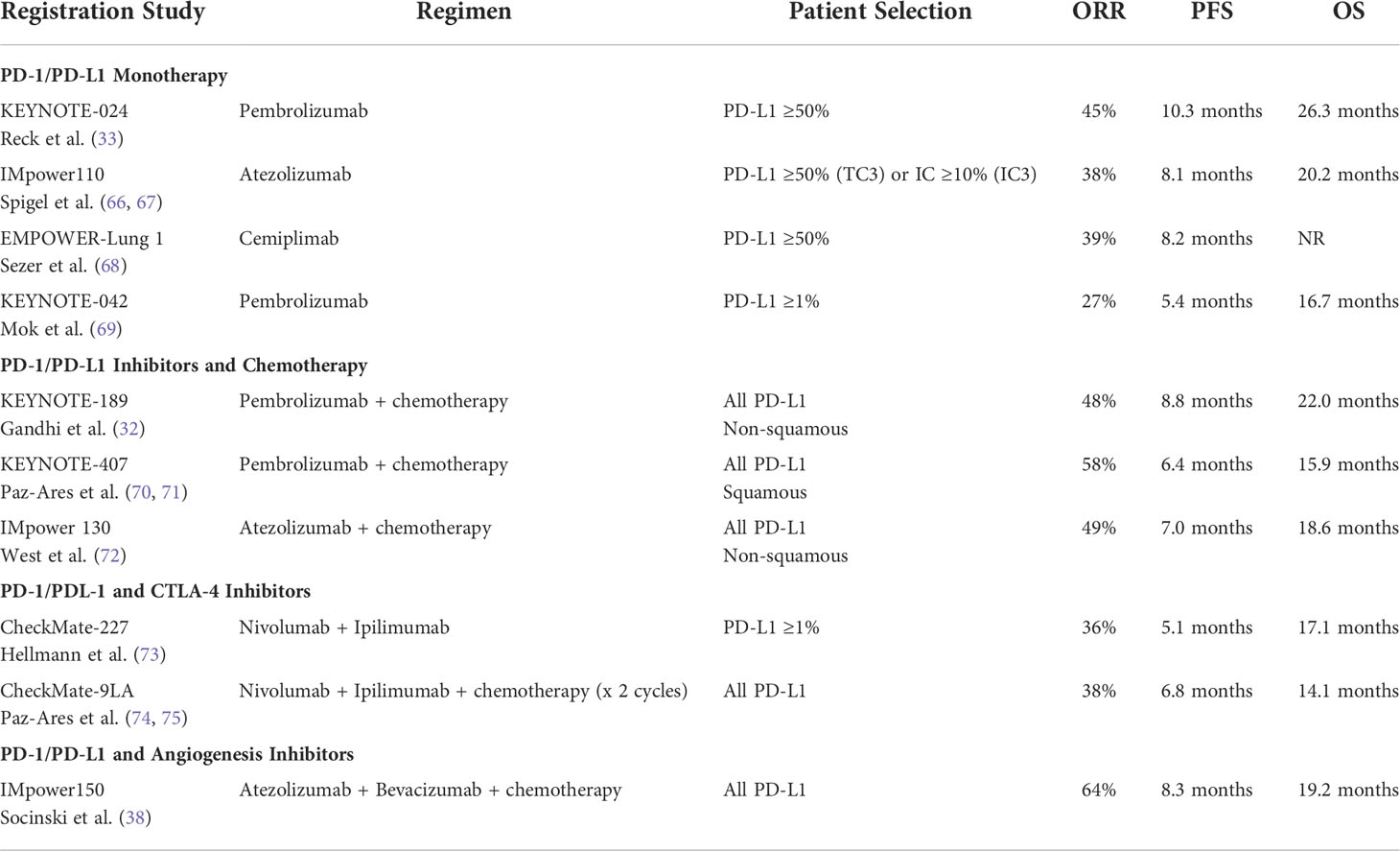
There are now several FDA approved regimens for the treatment of metastatic NSCLC, giving patients and physicians options to individualize therapy given specific characteristics. There are options to avoid chemotherapy when appropriate. The approved first line immunotherapy regimens and their efficacy data are summarized in Table 1.
Checkpoint inhibitor monotherapy
First line treatment
CheckMate-012, published in 2016, was a multicenter phase 1 trial of 52 patients that studied first-line nivolumab in advanced NSCLC and yielded positive safety data as well as durable response rates (76). This trial effectively brought nivolumab into the front-line setting and set the stage for further trials. Following this, CheckMate-026 selected patients with stage IV or recurrent NSCLC with PD-L1 expression of ≥5% to receive first-line nivolumab or platinum-based chemotherapy in this phase 3 trial. While this trial did not show a significant PFS or survival benefit, it did show significantly fewer treatment-related grade 3 and 4 adverse events (18% of patients who received nivolumab vs 51% of patients who received chemotherapy) (77). Possible explanations for why this trial did not demonstrate PFS or OS benefits include imbalances between groups that conferred better prognoses and imbalances in patients with PD-L1 ≥ 50%.
The KEYNOTE-024 study was a phase 3 trial in which 305 patients whose tumors had a PD-L1 tumor proportion score (TPS) ≥50% and lacked EGFR or ALK alterations were randomized to single agent pembrolizumab or chemotherapy in the first-line setting. Median PFS was 10.3 months versus 6 months in the chemotherapy group (p <0.001). Additionally, ORR was higher in the pembrolizumab group (44.8% versus 27.8%) compared to the chemotherapy group as well as duration of response (not reached versus 6.3 months). Updated analysis confirmed the value of single-agent pembrolizumab in this setting (OS of 30 months versus 14.2 months with HR of 0.63 (95% CI, 0.47-0.86), and when adjusted for crossover, the HR was 0.49 (95% CI, 0.34-0.69). Serious TRAEs were lower in patients treated with pembrolizumab compared to chemotherapy (26.6% versus 53.3%) (33). This trial led to the first approval of an ICI for patients with treatment naïve metastatic NSCLC (78). A subsequent study of atezolizumab compared to chemotherapy demonstrated similar efficacy in metastatic NSCLC with PD-L1 expression ≥50% or immune cell (IC) PD-L1 ≥10% (median OS 20.2 months versus 13.1 months, HR 0.59, p=0.01) (66). Cemiplimab is the newest PD-1 inhibitor approved in this setting, demonstrating improvements in PFS (8.2 months vs 5.7 months, HR 0.54, 0.43-0.68, p<0.0001) and OS (median NR vs 14.2 months, HR 0.57, 0.42-0.77, p=0.0002) compared to chemotherapy in PD-L1≥50% disease (79). The similarities in efficacy across different trials suggest that this is a class effect of PD-1/PD-L1 inhibitors. Adverse events profiles are also similar and each drug has demonstrated improvements in quality of life (80–82).
Given initial success in the PD-L1 high population, single agent ICIs was investigated in tumors with any PD-L1 expression ≥1%, with mixed results. The CheckMate-026 study compared nivolumab to chemotherapy in NSCLC with PD-L1 expression of ≥5%, but failed to demonstrate improvements in PFS or OS (77). An updated analysis of atezolizumab also failed to demonstrate an improvement in OS over chemotherapy (67). However, the KEYNOTE-042 study, which compared pembrolizumab to chemotherapy in tumors with PD-L1 ≥1%, demonstrated statistically improved OS across all PD-L1 expression levels: TPS ≥50% (HR 0.69, 95% CI 0.56-0.85 p=0.0003), TPS ≥20% (HR 0.77, 95% CI 0.64-0.92, p=0.002), and TPS>1% (HR 0.81, 95% CI 0.71-0.93, p= 0.0018) (69). There is no obvious explanation for differences in efficacy across the different drugs, but it is clear that PD-L1 is a predictive biomarker for single agent ICIs and efficacy is generally lower among patients with low PD-L1 expression. Pembrolizumab is the only ICI approved as monotherapy in the first line setting in NSCLC with low PD-L1 expression (PD-L1≥1%).
Second line and beyond treatment
In patients who have not received ICIs in the first line setting, several ICIs are approved as monotherapy for subsequent treatment. Several pivotal studies demonstrated improved overall survival with nivolumab compared to docetaxel in previous treated NSCLC (45, 83–85). CheckMate-017 randomized 272 patients with progressive squamous NSCLC to either nivolumab or docetaxel and reported improved overall survival (OS) in the nivolumab arm compared to the docetaxel arm (9.2 months vs 6 months, HR 0.59, 95% CI 0.44-0.79, p< 0.001) as well as an improved ORR (20% vs 9%) (85). The CheckMate-057 trial, designed similarly to CheckMate-017 but for patients with nonsquamous histology, also randomized patients to either nivolumab or docetaxel. The trial met its primary endpoint of OS, in which the nivolumab arm was found to have improved OS compared to the docetaxel arm (12.2 months vs 9.4 months, HR 0.73, 96% CI 0.59-0.89, p = 0.002). Two year follow up showed that nivolumab continued to demonstrate OS benefit compared to docetaxel (86).
Historically, the use of pembrolizumab emerged as a key immunotherapy agent in NSCLC based on the early KEYNOTE trials. In the phase 1 KEYNOTE-001 trial, published in 2015, 495 patients with advanced NSCLC were treated with pembrolizumab. This study demonstrated the safety of pembrolizumab as well as an anti-tumor effect in this population. Furthermore, this trial showed that PD-L1 expression of >50% was associated with greater efficacy compared to PD-L1 expression of <1% and PD-L1 expression of between 1% and 49% (30).
The KEYNOTE-010 trial, published in Lancet in 2016, was a randomized phase 2/3 study in which over a thousand patients with previously treated advanced NSCLC with PD-L1 expression of at least 1% were randomized to either pembrolizumab (2mg/kg or 10mg/kg) or docetaxel. Patients treated with pembrolizumab experienced improved OS (10.4 months with pembrolizumab 2mg/kg HR 0.71, 95% CI 0.58-0.88, p=0.0008], 12.7 months with pembrolizumab 10mg/kg [HR 0.61, 95% CI 0.49-0.75, p<0.0001]) compared to docetaxel (8.5 months). PFS was not significantly different between these three groups. In patients with PD-L1 expression of ≥50%, OS was more pronounced at 14.9 or 17.3 months compared to 8.2 months with docetaxel (pembrolizumab 2mg/kg: 14.9 months, HR 0.54, 95% CI 0.38-0.77, p=0.0002; pembrolizumab 10mg/kg; 17.3 months, HR 0.50, 95% CI 0.36-0.70, p<0.0001). Furthermore, fewer severe treatment-related adverse events (TRAEs) were reported with pembrolizumab (13% with 2mg/kg and 16% with 10mg/kg) compared to docetaxel (35%) (45). Long-term follow up, at 43 months, presented in 2018 showed durable responses with pembrolizumab (87).
The OAK study comparing atezolizumab and chemotherapy demonstrated similar findings in patients with all PD-L1 expressions (median OS 13.8 vs 9.6 months) (83). In addition, durvalumab was studied in the third line and beyond setting. In these heavily pre-treated patients, there was still a statistically significant and clinically meaningful improvement in survival of patients with PD-L1≥25% (median OS 11.7 vs. 6.8 months), underscoring the importance of ICIs as a backbone therapy in NSCLC (88).
Checkpoint inhibitor combined with chemotherapy
The addition of chemotherapy to PD-1/PD-L1 inhibitors is thought to potentiate the anti-tumor immune response by enhancing neoantigen presentation after destruction. In clinical trials, the combination demonstrated significant activity and is a standard first line treatment option in metastatic NSCLC.
The efficacy of first-line pembrolizumab in combination with a platinum doublet followed by chemo-immunotherapy maintenance was established in the KEYNOTE-189 trial for nonsquamous metastatic NSCLC. The rate of OS at 12 months was improved in the pembrolizumab group (69.2% versus 49.4%, HR 0.49, 95% CI 0.38-0.64, p<0.001) as was median PFS (8.8 months versus 4.9 months, HR 0.52, 95% CI 0.43-0.64). Grade 3 or higher adverse events between the two groups were comparable (67.2% versus 65.8%) (32). Updated data showed continued OS benefit in the pembrolizumab group (22 months versus 10.7 months, HR 0.56, 95% CI 0.45-0.70). OS and PFS benefits were seen regardless of PD-L1 expression and presence of liver or brain metastases (89). This update further supports the role of pembrolizumab with platinum doublet-based therapy in the treatment of patients with metastatic nonsquamous NSCLC. For metastatic squamous NSCLC, the KEYNOTE-407 trial established the role of first-line pembrolizumab in combination with a platinum-based drug combined with a taxane, followed by immunotherapy maintenance. Combination pembrolizumab and chemotherapy demonstrated significant improvements in OS (17.1 months versus 11.6 months, HR 0.71 95% CI 0.58-0.88) and PFS (6.4 months versus 4.8 months, HR 0.56, 95% CI 0.45-0.70, p<0.001) (70, 71). Benefit was also seen regardless of PD-L1 expression. Rates of severe adverse events were also comparable between the two groups (69.8% versus 68.2%) (71).
The combination of cemiplimab and chemotherapy also demonstrated preliminary efficacy in a phase 3 study over chemotherapy alone across all PD-L1 levels (median OS 21.9 months versus 13 months, HR 0.71, p = 0.014) (90).
Studies of atezolizumab in combination with chemotherapy had mixed results. In the Impower130 study, atezolizumab, carboplatin and nab-paclitaxel was compared to chemotherapy in non-squamous NSCLC and demonstrated improvements in PFS (7.0 vs 5.5 months; HR 0.64; p<0.0001) and OS (18.6 vs 13.9 months; HR 0.69; p=0.03) (72). Based on these results, the combination was granted regulatory approval. However, the subsequent IMPower132 study, which included the same patient population, failed to improve OS when atezolizumab was combined with carboplatin/cisplatin and pemetrexed (7.6 months versus 5.2 months, HR 0.60, 95% CI 0.49-0.72, p<0.0001) (91). In squamous NSCLC, the Impower131 study, atezolizumab, carboplatin and nab-paclitaxel (A+CP) improved PFS (6.3 months versus 5.6 months, HR 0.71, 95% CI 0.60-0.85, p = 0.0001) but not OS (14.2 vs 13.5 months; HR 0.88; p=0.16) (92). There are several potential explanations for these discrepancies including the type of chemotherapy that was used and the complexity of study designs with co-primary endpoints. It also highlights the limitations of surrogate endpoints, such as PFS, which may not always translate into a survival advantage.
Dual checkpoint inhibitors
The CheckMate 227 study evaluated nivolumab plus ipilimumab, and nivolumab alone to chemotherapy in treatment naïve, PD-L1≥1%, metastatic NSCLC. In this trial, patients were randomized to either nivolumab plus ipilimumab, nivolumab, or chemotherapy. This trial was amended to add a co-primary endpoint of PFS in TMB high regardless of PD-L1 expression. The combination of nivolumab and ipilimumab demonstrated an improvement in OS compared to chemotherapy alone (17.1 vs 14.9 months, p=0.007). In a secondary analysis the benefit of nivolumab plus ipilimumab was seen even in patients with PD-L1<1%. Rates of serious adverse events were 32.8% and 36% in patients receiving nivolumab plus ipilimumab and chemotherapy, respectively (93). In an updated analysis, 4-year survival rates in patients with PD-L1≥50% were 37% (nivolumab plus ipilimumab), 26% (nivolumab alone) and 20% (chemotherapy), and 4-year survival rates in patients with PD-L1<1% were 24% (nivolumab plus ipilimumab), 13% (nivolumab), and 10% (chemotherapy), with a HR for OS for nivolumab plus ipilimumab of 0.64 (95% CI 0.51-0.81) suggesting durable benefit in a subset of patients (94).
The CheckMate 9LA trial explored the role of first-line nivolumab plus ipilimumab in combination with two cycles of chemotherapy (74). In this trial, patients were randomized to either nivolumab plus ipilimumab plus two cycles of platinum doublet chemotherapy or chemotherapy alone. Interim analysis showed increased OS in the experimental group (median 14.1 months vs 10.7 months, 95% CI 9.5 -12.6, HR 0.66, 95% CI 0.55-0.80). Notably, any-grade treatment-related adverse events occurred in 30% of the experimental group and in 18% of the control group. On the basis of this trial, fit patients who have significant disease burden are suggested to benefit from upfront chemotherapy in combination with dual checkpoint blockade. Importantly this trial did not show an early decrease in PFS compared to other trials. Furthermore, with only two cycles of chemotherapy, there is potential to avoid long-term chemotherapy-related toxicity, thus presenting a unique treatment option. This trial established the role of dual-checkpoint blockade as a reasonable first-line treatment option in metastatic NSCLC. The two-year update presented at ASCO 2021 reported that patients who received the experimental combination treatment derived OS benefit compared to the control arm, with OS of 15.8 months vs 11.0 months (HR 0.72, 95% CI 0.61-0.86), PFS benefit of 6.7 months vs 5.3 months (HR 0.67, 95% CI 0.56-0.79), and improved ORR (38% vs 25%), with similar benefits being seen across varying PD-L1 expression levels (75). Importantly, a post hoc analysis of the 14% of patients with brain metastases showed that this chemo-immunotherapy combination was associated with benefit this patient in this subset of patients (95).
A similar approach of first-line dual checkpoint blockade in metastatic NSCLC was explored in the phase 3 POSEIDON trial and was presented at WCLC in 2021. In this trial, durvalumab (anti-PD-L1 mAb) and tremelimumab (anti-CTLA4 mAb) plus chemotherapy was shown to be superior to chemotherapy alone with a PFS benefit (6.2 months vs 4.8 months, HR 0.72, 95% CI 0.60-0.86, p = 0.00031) as well as an OS benefit (14.0 months vs 11.7 months, 95% CI 11.7-16.1, p = 0.00304). Notably this trial reported a 51.8% grade ¾ treatment-related adverse effect rate with 15.5% of patients requiring discontinuation of study treatments. Interestingly, the addition of tremelimumab was not associated with a large difference in treatment discontinuation rates compared to the durvalumab plus chemotherapy arm (14.1%) (96). This finding in particular supports the notion that dual checkpoint blockade with PD-1/PD-L1 and CTLA-4 blockade may not necessarily be more toxic than single agent PD-1/PD-L1 blockade and could represent yet another first-line option in the treatment of metastatic NSCLC.
Checkpoint inhibitors combined with angiogenesis inhibitors
The Impower150 trial randomized patients with nonsquamous metastatic NSCLC to receive either atezolizumab plus carboplatin and paclitaxel (ACP), or bevacizumab plus carboplatin and paclitaxel (BCP), or atezolizumab plus bevacizumab plus carboplatin and paclitaxel (ABCP), followed by atezolizumab or bevacizumab maintenance, or both. This trial found improved PFS (8.3 months vs 6.8 months, HR 0.62, 95% CI 0.52-0.74, p<0.001) and improved OS (19.2 months vs 14.7 months, HR 0.78, 95% CI, 0.64-0.96, p=0.02) with the addition of atezolizumab to bevacizumab plus chemotherapy regardless of PD-L1 expression and EGFR/ALK status (38).
Many immunotherapy trials have historically excluded patients with untreated brain metastases and thus the efficacy of immunotherapy for CNS control has not been fully elucidated. Cemiplimab monotherapy was examined in patients with PD-L1 ≥ 50% in a subgroup analysis of the EMPOWER-Lung 1 trial. This trial showed improved PFS (10.4 months vs 5.3 months, HR 0.45, 0.22-0.92, p=0.0231) and OS (18.7 months vs 11.7 months, HR 0.17, 0.04-0.76, p=0.0091) compared to chemotherapy alone in patients with treated, clinically stable brain metastases (79). To further study CNS disease, the ATEZO-BRAIN study was a phase II trial of atezolizumab with carboplatin and pemetrexed in patient with metastatic nonsquamous NSCLC and untreated brain metastases. This study evaluated 40 patients and noted a median PFS of 8.9 months and a median OS of 13.6 months. Importantly 36 of 40 patients had concordant response between CNS disease and systemic disease suggesting that immunotherapy with chemotherapy can be utilized in patients with brain metastases without necessitation of local CNS therapy (97). Yet another sub group analysis evaluating brain metastases in the CheckMate 9LA trial showed that immunotherapy led to improved survival compared to chemotherapy alone and showed similar 2-year OS rates in patients who received nivolumab, ipilimumab, and chemotherapy with brain metastases and those without (25% vs 39%) (98). These data together strongly suggest the utility of immunotherapy alone or in combination with chemotherapy in patients with CNS involvement.
Oncogene driven NSCLC
Despite successes of ICIs in patients with wild type NSCLC, their efficacy in EGFR and ALK mutant NSCLC is disappointing. Their low efficacy was first observed in second line studies where single agent PD-1 inhibitors had no benefit over docetaxel in subgroup analysis. Real world data from the ImmunoTarget Global registry also shows that patients without actionable mutations and smokers are more likely to respond to immunotherapy. Specifically, evaluation of this registry showed that patients with KRAS, BRAF, and MET alterations responded more favorably than patients with EGFR, ALK, or RET alterations (99, 100). A phase 2 trial of pembrolizumab in TKI naïve, PD-L1-positive EGFR-mutant advanced NSCLC was stopped due to poor efficacy, providing clinical data discouraging the use of immunotherapy in this patient population, despite otherwise encouraging PD-L1 levels (47). Translational studies have demonstrated that EGFR-mutant NSCLC tend to have an immune excluded phenotype where anti-tumor T cells are unable to traffic into the tumor microenvironment (101, 102). Efforts to combine ICI inhibitors and targeted TKIs were disastrous with significant increases in severe toxicity such as pneumonitis and hepatitis (103, 104). This area remains an unmet need and is topic of active investigation and highlights the necessity of genomic profiling in order to guide treatment choices.
Biomarkers in immuno-oncology
PD-L1 tumor proportion score
The most commonly reported and used biomarker for immunotherapy is PD-L1 expression. There are various PD-L1 assays that are currently being utilized. The companion diagnostic for nivolumab utilizes an assay developed by Dako (5H1 or 28-8 assay), whereas pembrolizumab uses the Dako 22C3 assay. Many of the IMpower trials investigating atezolizumab utilized the Ventana SP263 assay and assessed PD-L1 expression on both tumor cells and immune cells (105). Higher PD-L1 expression is predictive of response, particularly for ICI monotherapy as demonstrated by many of the first line studies discussed. The Blueprint PD-L1 IHC Assay Comparison Project evaluated comparability of the different IHC assays and found that the Ventana SP263 assay stained fewer tumor cells. Furthermore, the study noted that 37% of tumors received different classifications depending on which IHC assay was being utilized (106). In addition to the Blueprint Project, there is additional data available to suggest that PD-L1 expression is heterogenous within tumors and thus, random biopsy may not be indicative of a true immunosuppressed state (107–110). These inherent flaws in IHC evaluation of PD-L1 expression may, at least in part, explain the variability in response rates seen in tumors with similar PD-L1 expression. The role of PD-L1 expression as a biomarker in early stage NSCLC is controversial. A study reported in 2015 showed that PD-L1 expression was a favorable prognostic indicator (111), however other studies suggested no prognostic value (112) questioning the utility of this biomarker especially in early stage disease.
Tumor Mutational Burden (TMB)
Tumor mutational burden (TMB) has been investigated as a potential biomarker for immunotherapy response however results have been conflicting. The initial interest in TMB stemmed from a study conducted by Rizvi et al, who conducted whole-exome sequencing on NSCLC specimens that had been treated with pembrolizumab. Investigators found improved objective response, durable clinical benefit, and PFS in tumors which had higher nonsynonymous mutation burdens. Furthermore, higher mutation burden was associated with higher neoantigen burden and DNA repair pathway mutations (113).
In the CheckMate-026 trial, TMB analysis using whole exome sequencing (WES) was conducted. This analysis showed that patients with high TMB and treated with nivolumab had higher ORR (47% vs 28%) and median PFS (9.7 months vs 5.8 months, HR 0.62, 95% CI 0.38-1.00) compared to those treated with chemotherapy. Notably, there was no correlation seen between TMB and PD-L1 expression. Lastly, ORR was increased when selecting for patients with both high TMB and PD-L1≥50%, however increased TMB level did not translate into better OS (77).
The CheckMate-227 trial demonstrated PFS benefit with combination nivolumab and ipilimumab compared to chemotherapy in first-line treatment of NSCLC with high TMB (≥10 mutations per megabase) (114). Further exploratory analyses combining both PD-L1 expression and TMB failed to reveal a subgroup that had an increased benefit from combination nivolumab and ipilimumab. Given these data, the utility of TMB as a biomarker has been brought into question.
A novel assay developed by Foundation Medicine allowed assessment of blood TMB (bTMB), and was retrospectively studied using samples from the phase 2 POPLAR and phase 3 OAK trials to identify predictive thresholds. In this study, patients with bTMB ≥ 16 mutations (16 mut/Mb) who were treated with atezolizumab had improved PFS as compared to patients with the same bTMB but treated with docetaxel (HR 0.65, 95% CI 0.47-0.92, p=0.013) (115). This assay is currently being studied in two prospective trials: Blood First Assay Screening Trial (BFAST) and in the Blood First-Line Ready Screening Trial (B-F1RST). Interim results of the B-F1RST trial showed that bTMB has value as a predictive biomarker as it relates to PFS (PFS for bTMB high was 9.5 months vs 2.8 months for bTMB low, HR 0.49, 90% CI, 0.23-1.04, p=0.11) and ORR (ORR for bTMB high was 36.4% versus 6.4% for bTMB low, OR 8.38, 90% CI 2.02-34.79, p = 0.02) (116). Final efficacy results showed an ORR of 17%, median PFS of 4.1 months, and median OS of 14.8 months. In bTMB of ≥ 16 mutations compared to <16, median OS was 23.9 months vs 13.4 months (117).
The MYSTIC trial was a lesson in patient selection. In this trial, patients with metastatic NSCLC without EGFR or ALK alterations were stratified based on tumor PD-L1 expression of ≥25% or ≤25% and histological classification. In this trial, 1118 patients were randomized to either durvalumab, durvalumab plus tremelimumab (anti-CTLA-4 mAb), or platinum-doublet chemotherapy and did not meet its primary endpoint of improved OS in the experimental arms (118, 119). PD-L1 expression was determined based on the VENTANA PD-L1 (SP262) assay. Perhaps more interesting is that an exploratory analysis showed that patients who had a high bTMB (≥20) and were treated with durvalumab and tremelimumab had OS of 21.9 months compared to 10 months in patients treated with chemotherapy (HR 0.49, 95% CI, 0.32-0.74) (119, 120).
The cancer gene panel (CGP), NCC-GP150, that serves as an estimation of blood TMB, was found to be a potentially useful tool in the treatment of NSCLC. Published in JAMA Oncology in 2019, Wang et al, demonstrated in two independent cohorts of patients, that the NCC-GP150 correlated to tissue TMB as determined by WES. bTMB of ≥ 6 (units) was associated with better PFS (HR 0.39; 95% CI 0.18-0.84, p = 0.01). ORR was also higher in tumors with bTMB ≥6 at 39.3% (95% CI 23.9%-56.5%) compared to 9.1% in tumors with bTMB <6 (95% CI, 1.6%-25.9%) (121).
Somewhat unexpectedly, higher TMB has been associated with earlier stages of disease. In a study of 197 samples, TMB was higher in stage I and II than in stage III and IV tumors (67% vs 47.5%, p=0.01) (122). The clinical value of this finding is not clear, but it highlights the fact that broad statements about TMB cannot be made without the context of related variables such as stage. Despite the confusing and conflicting results discussed, there is likely value of TMB as biomarker. Recently, pembrolizumab received regulatory approval for management of TMB-High solids tumors (≥10 mut/Mb) based on a biomarker directed analysis of 10 cohorts of commonly occurring solid tumors, but which did not include NSCLC (123). Given that the current use of ICIs in NSCLC is mainly driven by PD-L1 expression, which is supported by clinical trial data, the role of TMB as a biomarker for NSCLC remains yet to be determined.
Future directions for immunotherapy in NSCLC
The overall response rate of immunotherapy in NSCLC is about 20-30%, which means that a significant proportion of patients do not have meaningful responses. Why patients, who on the surface have similar tumors, can have very different responses to immunotherapy is a complicated question, one without clear answers. In some part, lack of response to immunotherapy can be blamed on the lack of activated immune cells within the tumor microenvironment (TME).
The future of immuno-oncology for the treatment of lung cancer lies in two approaches – the first is by improving the efficacy of treatment by combining it with modalities that enhance the immune response or through novel therapies (Table 2). The second is by improving patient selection by further developing biomarkers that can identify patients most likely to benefit from immunotherapy and integrating new technology into this process of patient selection. Here we discuss several novel treatment approaches which are in development (Figure 1).

Table 2 Select clinical trials currently underway which utilize investigational agents in combination with checkpoint inhibitors.
Figure 1 Select emerging immunotherapies currently in clinical development. Created with BioRender.com.
Other immune checkpoints
Tiragolumab, monoclonal antibody against the inhibitory checkpoint TIGIT, in combination with atezolizumab has been given breakthrough therapy designation by the FDA for the first-line treatment of PD-L1 positive metastatic NSCLC. This designation was granted on the basis of the CITYSCAPE trial. In this phase II trial, the ORR was 37.3% in the combination arm, compared to 20.6% in the atezolizumab only arm. Furthermore, PFS was 5.6 vs 3.9 months in the combination arm and atezolizumab arm, respectively (124). The phase 3, SKYSCRAPER-01 trial is currently underway, however interim results released in May 2022 showed that the trial did not meet its co-primary end point of PFS (NCT04294810, Roche News Release 5 11 2022).
Lymphocyte activation gene 3 (LAG-3) has emerged as another co-inhibitory checkpoint receptor in the regulation of T cells and evidence suggests that dual expression of LAG-3 and PD-1 is associated with T cell anergy and subsequent immunosuppression within the TME. Preclinical data shows that LAG-3 inhibition alongside PD-1 inhibition can have strong anti-tumor activity (125). Study of REGN3767 (an anti-LAG-3 ab) as monotherapy and in combination with cemiplimab (anti-PD1 ab) is currently underway (126, 127). Importantly, there appears to further ligands to LAG-3, specifically fibrinogen-like protein 1 (FGL1) (128), which may have implications for further drug discovery.
4-1BB/CD137 is a costimulatory receptor and is part of the TNF receptor superfamily. Engagement of this receptor enhances T and NK cell activity and has been shown to produce anti-tumor effects. Utolimumab (PF-05082566), a 4-1BB/CD137 agonist was studied in a phase 1 clinical trial in patients with advanced malignancies as a single agent (129) and in combination with pembrolizumab (130) and was shown to be well tolerated and had positive preliminary clinical activity. Utolimumab is currently in trials in combination with an OX40 agonist, another co-stimulatory checkpoint, (NCT02315066) and in combination with avelumab (anti-PD-L1 mAb) (NCT02554812).
Data from a phase 1 trial utilizing an anti-T-cell immunoglobulin domain and mucin domain–containing molecule-3 (TIM-3) (LY3321367) antibody as a single agent or in combination with LY3300054 (anti-PD-L1 mAb) was presented at the 2019 ASCO-SITC meeting and showed positive safety results (131). Such combination therapies have great potential to improve responses in patients already responding to immunotherapy and induce responses in those whose tumors are inherently less responsive.
Cytokines
Combination therapy of cytokines with immunotherapy has been the focus of much attention for many years as it attempts to solve a central problem in tumor immune-escape, namely the immunosuppressive quality of the TME. Herein we discuss several important compounds and treatment strategies currently under development.
The PIVOT trial (NCT02983045) is a phase 1/2 study of combination therapy with NKTR-214 (bempegaldesleukin) and nivolumab in advanced malignancies including NSCLC (132, 133). NKTR-214 is a CD122-biased agonist (CD122 is also known as the interleukin-2 receptor beta subunit) that was developed on the basis of prior experience with IL-2 in the treatment of malignancy. This compound functions to increase proliferation of CD8+ effector and natural killer (NK) cells within the TME (134). As discussed previously, there is a direct correlation between TIL concentration and response to immunotherapy (135–137). Encouraging ORR and DCR responses have been seen (132). The currently underway phase I/II PROPEL trial (NCT03138889) includes first-line NSCLC patients for treatment with bempegaldesleukin and pembrolizumab with or without chemotherapy.
IL-15 has long been studied for its potential anti-tumor effects for its T and NK cell stimulatory effects (without stimulation of Tregs). A phase 1 dose-escalation trial utilized subcutaneous recombinant IL-15 (rhIL-15) to achieve much higher doses than possible when given intravenously. Nineteen total patients were treated, including 6 with NSCLC (138). While this study did not detect objective responses, a clinical trial is underway (NCT0338863) that is testing rhIL-15 with nivolumab, with ipilimumab, and with both ipilimumab and nivolumab in advanced solid malignancies. ALT-803 is an IL-15/IL-15Rα complex fused to an IgG1 Fc, referred to as an IL-15 superagonist. In this compound, the IL-15 is mutated to increase agonism of IL-2 and 15βγ receptors. A phase 1b trial of ALT-803 in combination with nivolumab in advanced NSCLC showed adequate safety and encouraging clinical activity with an ORR of 29% (139).
IL-8 has been known to have protumoral effects, through multiple proposed mechanisms (140). Interestingly, Sanmamed et al, showed that low IL-8 levels in melanoma and NSCLC are associated with better response to immunotherapy (141). A phase 1 trial of BMS-986253, an anti-IL-8 antibody, has been completed and showed that monotherapy is well tolerated (142). NCT03400332 is a trial that is currently underway in which BMS-986253 is being evaluated in combination with nivolumab (143).
M7824, developed by EMD Serono, is a unique bifunctional protein which contains anti-PD-L1 1gG1 mAb that is fused with 2 extracellular domains of TGF-B receptor II to form a so called “TGF-B trap.” This compound attempts to circumvent a classic problem with ICB, namely tumor immunosuppression. This molecule has been shown to impede TGF-B related signaling in the TME and, in animal models, to be more effective than either TGF-B inhibition or PD-L1 inhibition (144). A second line trial of 80 patients with NSCLC showed impressive efficacy with ORR of 85.7% in patients with tumors with PD-L1 ≥ 80% and 36% in PD-L1 ≥ 1% at the recommended phase 2 dose (145).
STING agonists
In an attempt to counteract this dearth of immune cells in the TME, several therapeutic avenues are being evaluated. Intratumoral injection of a STING (stimulator of interferon genes) agonist (cGAMP) has been shown in non-inflamed lung cancer models to increase intra-tumoral CD8+ T cells, increase PD-1, PD-L1, IFN concentrations, and synergistically suppressed tumor growth in cells otherwise resistant to immune checkpoint inhibitors (ICIs) when combined with anti-PD-L1 ab (146). Preliminary results from a phase 1 trial of MK-1454 (a STING agonist) showed encouraging safety and efficacy when combined with pembrolizumab (147). Another example of this approach is with sitravatinib, a tyrosine kinase inhibitor which targets TAM and VEGFR2, reducing immunosuppressive MDSCs and Tregs but increasing M1/M2-polarized macrophages and thereby overcoming the immunosuppressive TME. A phase 2 trial was presented at ESMO 2021 which showed favorable survival data when used in combination with nivolumab in the second and third line treatment of advanced NSCLC (148). A plethora of approaches are currently underway which utilize intratumoral administration of various forms of immunotherapy, including gene therapies, cellular therapies, and immunostimulatory compounds are currently being evaluated (149).
Adenosine antagonists
Adenosine is a unique compound in regard to its role in tumorigenesis. The TME is a hypoxic environment which stimulates the release of ATP which subsequently activates an inflammatory response (150). In order to counterbalance this effect, ATP is converted to adenosine, which produces a profound anti-inflammatory response via inhibition of many cells including mast cells, endothelial cells, macrophages, neutrophils, NK cells, dendritic cells, and lymphocytes (151); adenosine has a potent effect on inducing T cell anergy and promotes the differentiation of CD4 cells into Foxp3+ Tregs (151, 152). Furthermore, hypoxia within the TME results in generation of HIF1α (hypoxia inducible factor 1α) which further induces adenosine receptor A2BR (A2AR and A2BR primarily have immunosuppressive qualities) (153, 154). Overexpression of these molecules has been associated with metastatic disease and worse clinical outcomes in various tumor types (155, 156). As mentioned previously, it is theorized that this immunosuppressed state within the TME results in poor responses to immunotherapy. CPI-444, developed by Corvus Pharmaceuticals, is an orally available A2AR antagonist which was shown in a preclinical mouse model to work synergistically with anti-PD-L1 therapy or anti-CTLA therapy and produce complete responses in 90% of mice; furthermore, this therapy was shown to induce a memory response in treated mice (157). PBF-509 (NIR178) is another A2AR antagonist that has shown promising results in a murine model by restoring TIL activity (158). A phase 1/2 trial in advanced NSCLC suggests adequate safety as well as clinical benefit (159). This study also plans to treat patients with PBF-509 in combination with PDR001 (anti PD-1 mAb). This unique direct modulation of the immune response by adenosine allows for improvement of the anti-tumor immune response using well-studied checkpoint inhibitors (PD-1, PD-L1, CTLA4 inhibitors) in combination with metabolically active agents. As adenosinergic molecules are further developed, we expect to see them more widely tested in combination with currently approved therapies.
Cellular therapies
Cellular therapies are another type of therapeutic approach that is being evaluated in the treatment of NSCLC. There are several different kinds of cellular therapies, the most well studied in solid malignancies are chimeric antigen receptor-modified T (CAR-T) therapy, adoptive cell therapy (ACT) and cancer vaccine therapy. A phase 1 trial of CAR-T cell technology directed to EGFR in NSCLC was published in 2016 and showed adequate safety (160). There have been many studies that have suggested the use of various targets in NSCLC for further CAR-T development such as EphA2, EGFR, HER2, MSLN, MUC1, ROR1, PD-L1, and PSCA (161–163). A form of T cell therapy developed by Adaptimmune is ADP-A2M10 which utilizes affinity-enhanced autologous T cells directed against the MAGE-A10c796 tumor antigen. Initial safety data from phase 1 clinical trials, presented at ASCO in 2018,were promising (164). A phase 1 trial of BI 1361849, an mRNA vaccine which contains several NSCLC antigens (MUC1, survivin, NY-ESO-1, 5T4, MAGE-C2, and MAGE-C1) is currently underway in combination with durvalumab (anti-PD-L1 ab) or in combination with both durvalumab and tremelimumab (anti-CTLA-4 mAb) (165). Tumor infiltrating lymphocyte (TIL) therapy as a form of adoptive cellular therapy has shown encouraging results in 20 patients with advanced NSCLC (NCT03215810). In this trial, autologous TILs were surgically harvested from patient tumors and were expanded ex vivo. After lymphodepleting and infusion of TILs, the cells were expanded in vivo utilizing IL-2 (166). This approach garners much attention as it an example of personalized medicine at its best and has the potential to induce durable immune responses. In fact, the trial mentioned had 2 complete responders (166). Another form of cellular therapy, NK cells, and in this case, NK cells manufactured from induced pluripotent stem cells, have been shown to increase T cell recruitment within tumors and synergize with anti-PD-1 antibodies in preclinical models (167). This form of therapy has entered clinical trials (NCT05069935).
Vaccination against PD-L1 is an interesting approach to decrease immunosuppression within the tumor microenvironment that deserves attention. IO102-IO103 is a cancer vaccine targeting IDO and PD-L1 that has shown encouraging safety and efficacy data in metastatic melanoma (168) and is currently in Phase 2 clinical trials in combination with pembrolizumab in patients with NSCLC (NCT05077709).
Novel formulations of immune checkpoint inhibitors
While still early in development, orally available small molecule PD-L1 inhibitors, such as CA-170, have been developed which show preclinical efficacy and hold promise of easy administration and improved pharmacokinetic properties (169). Probody therapeutics (Pb-tx) are a form of antibody prodrugs which are activated by proteases. The mechanistic underpinning of this approach is that antibody can be better delivered to the tumor microenvironment as is it reliant on protease activation. These therapies have preclinically been shown to have similar activity as their traditional anti-PD-1 and anti-PD-L1 mAbs, however with less systemic toxicity (170). CX-072, an anti-PD-L1 Pb-Tx is currently in the clinic and is associated with good tolerability as well as antitumor efficacy (171). De-fucosylated (or non-fucosylated) antibodies have long been studied as means to improve antibody efficacy. De-fucosylated anti-PD-L1 antibodies have been shown to have increased antibody dependent cellular cytotoxicity via improved FcγRIIIa binding (172). We anticipate further study and optimization of these novel formulations of immune checkpoint inhibitors and are hopeful that they will improve the effectiveness of these agents.
Other emerging therapies
Serine proteases have also been suggested as a potential avenue to increase antigen presentation. Serine proteases within the TME have been shown to increase HLA-1 expression of peptides by lung cancer cells (173). While this approach is still in its infancy, we expect this approach and other similar ones to be further studied and enter the clinics soon.
Emerging biomarkers: Gene expression signatures
Data suggest that patients with advanced NSCLC whose tumors have high PD-L1 expression but low cytotoxic T (CD8+ T) cell tumor infiltrating lymphocytes (TILs) have a poor prognosis with median OS of 3.7 months compared to 8 months for patients with tumors not exhibiting this fatal combination (p=0.02). In this study, investigators identified a CD8A/CD274 gene signature (evaluation of mRNA by RNA sequencing) that was able to predict tumors that would respond to ICB (174). These results are in concordance with prior studies which demonstrate that in NSCLC, increased TIL concentrations are associated with better prognoses (135, 136, 175, 176). TIL concentrations have been evaluated as a potential predictive biomarker (137, 177), however the previously stated findings support the notion that TIL concentration in combination with PD-L1 expression can be used as a predictive biomarker for response to immunotherapy (174).
An exploratory analysis of the RATIONALE-307 was presented at WCLC 2021. In this biomarker evaluation, investigators found an association between tumor inflammation signature (TIS, a 18-gene panel representing inflammatory genes) and benefit of PD-1 inhibition with tislelizumab (anti-PD-1 mAb) in combination with chemotherapy compared to chemotherapy alone in the first-line treatment of advanced squamous NSCLC (178). These data suggest the utility of TIS as a biomarker more so than even PD-L1 and tTMB.
Given the potential pitfalls associated with the above-mentioned biomarkers, continued study is currently underway to identify more efficient and more accurate biomarkers, some of which are discussed here. Interferon gamma (IFN-γ) expression, in recent studies, has been evaluated as a potential biomarker that is suggested to predict response to immunotherapy (179–181). The intestinal microbiome has been shown to influence the efficacy of immunotherapy and has subsequently been suggested as a potential predictive biomarker for response to checkpoint blockade (182–184). Janus Kinase (JAK) 1 and 2 mutations as well as beta-2-microglobulin (B2M) aberrations and T cell immunoglobulin mucin-3 (TIM-3) upregulation have also been suggested as potential biomarkers that are currently undergoing study but have yet to be validated (185, 186).
Radiomics as a biomarker
Patient selection can be improved upon by utilizing improved biomarkers as well as artificial intelligence (AI) based approaches to treatment of NSCLC. As previously discussed in this review, high concentrations of TILs are associated with improved prognoses and better responses to immunotherapy. A recent study by Corredor et al, was able to predict the likelihood of recurrence in stage I and II NSCLC by evaluating computer-extracted spatial TIL (SpaTIL) features from digital images of tumors (187). This concept, if broadened, may help identify patients who are most likely to respond to immunotherapy and may guide adjunctive therapy selection for patients who are deemed less likely to respond. Developments in deep learning and radiomics may also aide in prognosticating patients based on radiographic appearance (188). In a study published by Coudray et al. in 2018, investigators were able to train a deep convolutional neural network (CNN) on pathology images and was able to predict histology as well as identify 6 commonly mutated genes (STK11, EGFR, FAT1, SETBP1, KRAS and TP53) (189). Radiomics is quickly emerging as a field with great potential. Radiomic based approaches have been developed to predict the benefit of adjuvant chemotherapy in early-stage NSCLC (190). These approaches have been shown to predict survival and response to immunotherapy in advanced NSCLC (191). Quantitative vessel tortuosity (QVT), a set of features evaluating the quantity and quality of vessels as measured on CT scans, is also gaining popularity as a potential biomarker (192). As this technology is further developed, we can envision its applications into producing biomarkers for response and improving patient selection for immunotherapy.
Discussion
In this paper, we have reviewed the development of immunotherapy and its initial application to NSCLC. We have also reviewed many of the landmark clinical trials that have changed practice and led to FDA approvals. Biomarkers play a large role in selecting patients most likely to respond to immunotherapy, but thus far, efforts remain imperfect as accuracy and efficiency are being sought after. Continued research into novel biomarkers will be paramount to the immunotherapy field. Despite all of these momentous advancements in the treatment of an otherwise dismal disease, there is still much potential for improvement. Several strategies have been employed to improve responses to immunotherapy, such as improving antigen presentation, combinations with cytokines, and cellular therapies. Lastly, we have reviewed up and coming science that may aide in improving patient selection and prediction of response to immunotherapy.
Author contributions
SP, ES, CG, SL, and VV contributed to writing of this manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Riedel S. Edward Jenner And the history of smallpox and vaccination. Proc (Bayl Univ Med Cent) (2005) 18(1):21–5. doi: 10.1080/08998280.2005.11928028
2. McCarthy EF. The toxins of William b. coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop J (2006) 26:154–8.
3. Ehrlich P. Ueber den jetzigen stand der karzinomforschung nederlandsch tijdschrift voor geneeskunde. Ned Tijdschr voor Geneeskd (1909) 5:273–90.
4. Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity (2004) 21(2):137–48. doi: 10.1016/j.immuni.2004.07.017
5. Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol (2004) 22(1):329–60. doi: 10.1146/annurev.immunol.22.012703.104803
6. Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases–elimination, equilibrium and escape. Curr Opin Immunol (2014) 27:16–25. doi: 10.1016/j.coi.2014.01.004
7. Hanahan D, Weinberg Robert A. Hallmarks of cancer: The next generation. Cell (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013
8. Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med (1999) 5(12):1365–9. doi: 10.1038/70932
9. Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol (2008) 8(6):467–77. doi: 10.1038/nri2326
10. Chen L, Han X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest (2015) 125(9):3384–91. doi: 10.1172/JCI80011
11. Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J (1992) 11(11):3887–95. doi: 10.1002/j.1460-2075.1992.tb05481.x
12. Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med (2000) 192(7):1027–34. doi: 10.1084/jem.192.7.1027
13. Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol (2020) 20(11):651–68. doi: 10.1038/s41577-020-0306-5
14. Sharpe AH, Pauken KE. The diverse functions of the PD1 inhibitory pathway. Nat Rev Immunol (2018) 18(3):153–67. doi: 10.1038/nri.2017.108
15. Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov (2018) 8(9):1069–86. doi: 10.1158/2159-8290.CD-18-0367
16. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer (2012) 12(4):252–64. doi: 10.1038/nrc3239
17. Kim JM, Chen DS. Immune escape to PD-L1/PD-1 blockade: seven steps to success (or failure). Ann Oncol (2016) 27(8):1492–504. doi: 10.1093/annonc/mdw217
19. Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev (2010) 236:219–42. doi: 10.1111/j.1600-065X.2010.00923.x
20. Wolchok J. Putting the immunologic brakes on cancer. Cell (2018) 175(6):1452–4. doi: 10.1016/j.cell.2018.11.006
21. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med (2010) 363(8):711–23. doi: 10.1056/NEJMoa1003466
22. Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol (2010) 28(19):3167–75. doi: 10.1200/JCO.2009.26.7609
23. Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med (2012) 366(26):2443–54. doi: 10.1056/NEJMoa1200690
24. Topalian SL, Sznol M, McDermott DF, Kluger HM, Carvajal RD, Sharfman WH, et al. Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol (2014) 32(10):1020–30. doi: 10.1200/JCO.2013.53.0105
25. Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med (2015) 372(4):320–30. doi: 10.1056/NEJMoa1412082
26. Rizvi NA, Mazieres J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial. Lancet Oncol (2015) 16(3):257–65. doi: 10.1016/S1470-2045(15)70054-9
27. Ferris RL, Blumenschein G Jr., Fayette J, Guigay J, Colevas AD, Licitra L, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med (2016) 375(19):1856–67. doi: 10.1056/NEJMoa1602252
28. Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med (2013) 369(2):134–44. doi: 10.1056/NEJMoa1305133
29. Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet (2014) 384(9948):1109–17. doi: 10.1016/S0140-6736(14)60958-2
30. Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med (2015) 372(21):2018–28. doi: 10.1056/NEJMoa1501824
31. Adams S, Schmid P, Rugo HS, Winer EP, Loirat D, Awada A, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort a of the phase II KEYNOTE-086 study. Ann Oncol (2019) 30(3):397–404. doi: 10.1093/annonc/mdy517
32. Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, et al. Pembrolizumab plus chemotherapy in metastatic non-Small-Cell lung cancer. N Engl J Med (2018) 378(22):2078–92. doi: 10.1056/NEJMoa1801005
33. Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-Positive non-Small-Cell lung cancer. N Engl J Med (2016) 375(19):1823–33. doi: 10.1056/NEJMoa1606774
34. Mehra R, Seiwert TY, Gupta S, Weiss J, Gluck I, Eder JP, et al. Efficacy and safety of pembrolizumab in recurrent/metastatic head and neck squamous cell carcinoma: pooled analyses after long-term follow-up in KEYNOTE-012. Br J Cancer (2018) 119(2):153–9. doi: 10.1038/s41416-018-0131-9
35. Migden MR, Rischin D, Schmults CD, Guminski A, Hauschild A, Lewis KD, et al. PD-1 blockade with cemiplimab in advanced cutaneous squamous-cell carcinoma. N Engl J Med (2018) 379(4):341–51. doi: 10.1056/NEJMoa1805131
36. Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med (2018) 379(24):2342–50. doi: 10.1056/NEJMoa1809697
37. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med (2018) 379(22):2108–21. doi: 10.1056/NEJMoa1809615
38. Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med (2018) 378(24):2288–301. doi: 10.1056/NEJMoa1716948
39. Horn L, Mansfield AS, Szczesna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med (2018) 379(23):2220–9. doi: 10.1056/NEJMoa1809064
40. Lee SM, Chow LQ. A new addition to the PD-1 checkpoint inhibitors for non-small cell lung cancer-the anti-PDL1 antibody-MEDI4736. Transl Lung Cancer Res (2014) 3(6):408–10. doi: 10.3978/j.issn.2218-6751.2014.11.10
41. Armand P, Nagler A, Weller EA, Devine SM, Avigan DE, Chen YB, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large b-cell lymphoma: results of an international phase II trial. J Clin Oncol (2013) 31(33):4199–206. doi: 10.1200/JCO.2012.48.3685
42. Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature (2014) 515(7528):558–62. doi: 10.1038/nature13904
43. Felip E, Altorki N, Zhou C, Csőszi T, Vynnychenko I, Goloborodko O, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet (2021) 398(10308):1344–57. doi: 10.1016/S0140-6736(21)02098-5
44. Wu Y-L, Tsuboi M, He J, John T, Grohe C, Majem M, et al. Osimertinib in resected EGFR-mutated non–Small-Cell lung cancer. New Engl J Med (2020) 383(18):1711–23. doi: 10.1056/NEJMoa2027071
45. Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet (2016) 387(10027):1540–50. doi: 10.1016/S0140-6736(15)01281-7
46. Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, et al. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-a meta-analysis. J Thorac Oncol (2017) 12(2):403–7. doi: 10.1016/j.jtho.2016.10.007
47. Lisberg A, Cummings A, Goldman JW, Bornazyan K, Reese N, Wang T, et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naïve patients with advanced NSCLC. J Thorac Oncol (2018) 13(8):1138–45. doi: 10.1016/j.jtho.2018.03.035
48. Lee CK, Man J, Lord S, Cooper W, Links M, Gebski V, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: A systematic review and meta-analysis. JAMA Oncol (2018) 4(2):210–6. doi: 10.1001/jamaoncol.2017.4427
49. Paz-Ares L, O'Brien M, Mauer M, Dafni U, Oselin K, Havel L, et al. VP3-2022: Pembrolizumab (pembro) versus placebo for early-stage non-small cell lung cancer (NSCLC) following complete resection and adjuvant chemotherapy (chemo) when indicated: Randomized, triple-blind, phase III EORTC-1416-LCG/ETOP 8-15 – PEARLS/KEYNOTE-091 study. Ann Oncol (2022) 33:451–3. doi: 10.1016/j.annonc.2022.02.224
50. Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. New Engl J Med (2018) 378(21):1976–86. doi: 10.1056/NEJMoa1716078
51. Lee JMCJ, Nicholas A eds. Abstract PS02.05: Surgical and clinical outcomes with neoadjuvant atezolizumab in resectable stage IB–IIIB NSCLC: LCMC3 trial primary analysis. In: 2020 world conference on lung cancer. (Singapore: International Association for the Study of Lung Cancer).
52. Cascone T, William WN, Weissferdt A, Leung CH, Lin HY, Pataer A, et al. Neoadjuvant nivolumab or nivolumab plus ipilimumab in operable non-small cell lung cancer: the phase 2 randomized NEOSTAR trial. Nat Med (2021) 27(3):504–14. doi: 10.1038/s41591-020-01224-2
53. Shu CA, Gainor JF, Awad MM, Chiuzan C, Grigg CM, Pabani A, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol (2020) 21(6):786–95. doi: 10.1016/S1470-2045(20)30140-6
54. Provencio M, Nadal E, Insa A, García-Campelo MR, Casal-Rubio J, Dómine M, et al. Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell lung cancer (NADIM): an open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol (2020) 21(11):1413–22. doi: 10.1016/S1470-2045(20)30453-8
55. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM. eds. Abstract CT003: Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo as neoadjuvant treatment (tx) for resectable (IB-IIIA) non-small cell lung cancer (NSCLC) in the phase 3 CheckMate 816 trial. In: AACR annual meeting. New York; 96-98: virtual; 178: virtua
56. Spicer J, Wang C, Tanaka F, Saylors GB, Chen K-N, Liberman M, et al. Surgical outcomes from the phase 3 CheckMate 816 trial: Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo alone as neoadjuvant treatment for patients with resectable non-small cell lung cancer (NSCLC). J Clin Oncol (2021) 39(15_suppl):8503. doi: 10.1200/JCO.2021.39.15_suppl.8503
57. Topalian SL, Taube JM, Pardoll DM. Neoadjuvant checkpoint blockade for cancer immunotherapy. Science (2020) 367(6477). doi: 10.1126/science.aax0182
58. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. New Engl J Med (2022) 386(21):1973–85. doi: 10.1056/NEJMoa2202170
59. Su P-L, Chen J-Y, Chu C-Y, Chen Y-L, Chen W-L, Lin K-Y, et al. The impact of driver mutation on the treatment outcome of early-stage lung cancer patients receiving neoadjuvant immunotherapy and chemotherapy. Sci Rep (2022) 12(1):3319. doi: 10.1038/s41598-022-07423-w
60. Spigel DR, Faivre-Finn C, Gray JE, Vicente D, Planchard D, Paz-Ares LG, et al. Five-year survival outcomes with durvalumab after chemoradiotherapy in unresectable stage III NSCLC: An update from the PACIFIC trial. J Clin Oncol (2021) 39(15_suppl):8511. doi: 10.1200/JCO.2021.39.15_suppl.8511
61. Hui R, Özgüroğlu M, Villegas A, Daniel D, Vicente D, Murakami S, et al. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study. Lancet Oncol (2019) 20(12):1670–80. doi: 10.1016/S1470-2045(19)30519-4
62. Jabbour SK, Lee KH, Frost N, Breder VV, Kowalski DM, Alawin IA, et al. KEYNOTE-799: Phase 2 trial of pembrolizumab plus platinum chemotherapy and radiotherapy for unresectable, locally advanced, stage 3 NSCLC. J Clin Oncol (2021) 39(15_suppl):8512. doi: 10.1200/JCO.2021.39.15_suppl.8512
63. Bradley JD, Nishio M, Okamoto I, Newton MD, Trani L, Shire NJ, et al. PACIFIC-2: Phase 3 study of concurrent durvalumab and platinum-based chemoradiotherapy in patients with unresectable, stage III NSCLC. J Clin Oncol (2019) 37(15_suppl):TPS8573–TPS. doi: 10.1200/JCO.2019.37.15_suppl.TPS8573
64. Garassino MC, Mazieres J, Reck M, Chouaid C, Bischoff H, Reinmuth N, et al. 108MO safety and efficacy outcomes with durvalumab after sequential chemoradiotherapy (sCRT) in stage III, unresectable NSCLC (PACIFIC-6). Ann Oncol (2022) 33:S81–S2. doi: 10.1016/j.annonc.2022.02.135
65. Martinez-Marti MM A, Barlesi F, Carcereny Costa E, Chu Q, Monnet I, Sanchez A, et al. LBA42 - COAST: An open-label, randomised, phase II platform study of durvalumab alone or in combination with novel agents in patients with locally advanced, unresectable, stage III NSCLC. Ann Oncol (2021) 32:S1283–S346. doi: 10.1016/j.annonc.2021.08.2121
66. Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for first-line treatment of PD-L1-Selected patients with NSCLC. N Engl J Med (2020) 383(14):1328–39. doi: 10.1056/NEJMoa1917346
67. Jassem J, de Marinis F, Giaccone G, Vergnenegre A, Barrios CH, Morise M, et al. Updated overall survival analysis from IMpower110: Atezolizumab versus platinum-based chemotherapy in treatment-naive programmed death-ligand 1–selected NSCLC. J Thorac Oncol (2021) 16(11):1872–82. doi: 10.1016/j.jtho.2021.06.019
68. Sezer A, Kilickap S, Gümüş M, Bondarenko I, Özgüroğlu M, Gogishvili M, et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: a multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet (2021) 397(10274):592–604. doi: 10.1016/s0140-6736(21)00228-2
69. Lopes G, Wu Y-L, Kudaba I, Kowalski D, Cho BC, Castro G, et al. Pembrolizumab (pembro) versus platinum-based chemotherapy (chemo) as first-line therapy for advanced/metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥ 1%: Open-label, phase 3 KEYNOTE-042 study. J Clin Oncol (2018) 36(18_suppl):LBA4–LBA. doi: 10.1200/JCO.2018.36.18_suppl.LBA4
70. Paz-Ares L, Vicente D, Tafreshi A, Robinson A, Soto Parra H, Mazières J, et al. A randomized, placebo-controlled trial of pembrolizumab plus chemotherapy in patients with metastatic squamous NSCLC: Protocol-specified final analysis of KEYNOTE-407. J Thorac Oncol (2020) 15(10):1657–69. doi: 10.1016/j.jtho.2020.06.015
71. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, et al. Pembrolizumab plus chemotherapy for squamous non-Small-Cell lung cancer. N Engl J Med (2018) 379(21):2040–51. doi: 10.1056/NEJMoa1810865
72. West H, McCleod M, Hussein M, Morabito A, Rittmeyer A, Conter HJ, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol (2019) 20(7):924–37. doi: 10.1016/S1470-2045(19)30167-6
73. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab plus ipilimumab in advanced non-Small-Cell lung cancer. N Engl J Med (2019) 381(21):2020–31. doi: 10.1056/NEJMoa1910231
74. Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol (2021) 22(2):198–211. doi: 10.1016/S1470-2045(20)30641-0
75. Reck M, Ciuleanu T-E, Cobo M, Schenker M, Zurawski B, JJd M, et al. First-line nivolumab (NIVO) plus ipilimumab (IPI) plus two cycles of chemotherapy (chemo) versus chemo alone (4 cycles) in patients with advanced non-small cell lung cancer (NSCLC): Two-year update from CheckMate 9LA. J Clin Oncol (2021) 39(15_suppl):9000. doi: 10.1200/JCO.2021.39.15_suppl.9000
76. Gettinger S, Rizvi NA, Chow LQ, Borghaei H, Brahmer J, Ready N, et al. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol (2016) 34(25):2980–7. doi: 10.1200/JCO.2016.66.9929
77. Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, Steins M, et al. First-line nivolumab in stage IV or recurrent non-Small-Cell lung cancer. N Engl J Med (2017) 376(25):2415–26. doi: 10.1056/NEJMoa1613493
78. Pai-Scherf L, Blumenthal GM, Li H, Subramaniam S, Mishra-Kalyani PS, He K, et al. FDA Approval summary: Pembrolizumab for treatment of metastatic non-small cell lung cancer: First-line therapy and beyond. Oncologist (2017) 22(11):1392–9. doi: 10.1634/theoncologist.2017-0078
79. Ozguroglu M, Sezer A, Kilickap S, Gumus M, Bondarenko I, Gogishvili M, et al. Cemiplimab monotherapy as first-line (1L) treatment of patients with brain metastases from advanced non-small cell lung cancer (NSCLC) with programmed cell death-ligand 1 (PD-L1) ≥ 50%: EMPOWER-lung 1 subgroup analysis. J Clin Oncol (2021) 39(15_suppl):9085–. doi: 10.1200/JCO.2021.39.15_suppl.9085
80. Brahmer JR, Rodríguez-Abreu D, Robinson AG, Hui R, Csőszi T, Fülöp A, et al. Health-related quality-of-life results for pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC (KEYNOTE-024): a multicentre, international, randomised, open-label phase 3 trial. Lancet Oncol (2017) 18(12):1600–9. doi: 10.1016/S1470-2045(17)30690-3
81. Marinis FD, Giaccone G, Herbst RS, Oprean C-M, Szczesna A, Boukovinas I, et al. Patient-reported outcomes (PROs) in the randomized, phase III IMpower110 study of atezolizumab (atezo) vs chemotherapy in 1L metastatic NSCLC. J Clin Oncol (2020) 38(15_suppl):9594. doi: 10.1200/JCO.2020.38.15_suppl.9594
82. Gumus M, Chen C-I, Ivanescu C, Kilickap S, Bondarenko I, Ozguroglu M, et al. Patient-reported symptoms, functioning, and quality of life (QoL) in patients treated with cemiplimab monotherapy for first-line treatment of advanced NSCLC with PD-L1 ≥ 50%: Results from EMPOWER-lung 1 study. J Clin Oncol (2021) 39(15_suppl):9078. doi: 10.1200/JCO.2021.39.15_suppl.9078
83. Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet (2017) 389(10066):255–65. doi: 10.1016/S0140-6736(16)32517-X
84. Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus docetaxel in advanced nonsquamous non-Small-Cell lung cancer. N Engl J Med (2015) 373(17):1627–39. doi: 10.1056/NEJMoa1507643
85. Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, et al. Nivolumab versus docetaxel in advanced squamous-cell non-Small-Cell lung cancer. N Engl J Med (2015) 373(2):123–35. doi: 10.1056/NEJMoa1504627
86. Barlesi F, Steins M, Horn L, Ready N, Felip E, Borghaei H, et al. Long-term outcomes with nivolumab (Nivo) vs docetaxel (Doc) in patients (Pts) with advanced (Adv) NSCLC: CheckMate 017 and CheckMate 057 2-y update. Ann Oncol (2016) 27(suppl_6). doi: 10.1093/annonc/mdw383.15
87. Herbst RS, Garon EB, Kim DW, Chul Cho B, Pérez Gracia JL, Han JY, et al. Long-term follow-up in the KEYNOTE-010 study of pembrolizumab (pembro) for advanced NSCLC, including in patients (pts) who completed 2 years of pembro and pts who received a second course of pembro. Ann Oncol (2018) 29(suppl_10):x42–x3. doi: 10.1093/annonc/mdy511.003
88. Planchard D, Reinmuth N, Orlov S, Fischer JR, Sugawara S, Mandziuk S, et al. ARCTIC: durvalumab with or without tremelimumab as third-line or later treatment of metastatic non-small-cell lung cancer. Ann Oncol (2020) 31(5):609–18. doi: 10.1016/j.annonc.2020.02.006
89. Gadgeel S, Rodriguez-Abreu D, Speranza G, Esteban E, Felip E, Domine M, et al. Updated analysis from KEYNOTE-189: Pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non-Small-Cell lung cancer. J Clin Oncol (2020) 38(14):1505–17. doi: 10.1200/JCO.19.03136
90. Gogishvili TM M, Makharadze3 T, Giorgadze D, Dvorkin M, Penkov KD, Laktionov K, et al. LBA51 - EMPOWER-lung 3: Cemiplimab in combination with platinum doublet chemotherapy for first-line (1L) treatment of advanced non-small cell lung cancer (NSCLC). Ann Oncol (2021) 32:S1283–S346. doi: 10.1016/j.annonc.2021.08.2130
91. Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, et al. Atezolizumab plus chemotherapy for first-line treatment of non-squamous non-small cell lung cancer: Results from the randomized phase III IMpower132 trial. J Thorac Oncol (2020) 91(16):653–64. doi: 10.1016/j.jtho.2020.11.025
92. Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodríguez-Abreu D, Hussein M, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): Results from a randomized phase III trial. J Thorac Oncol (2020) 15(8):1351–60. doi: 10.1016/j.jtho.2020.03.028
93. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim S-W, Carcereny Costa E, et al. Nivolumab plus ipilimumab in advanced non–Small-Cell lung cancer. New Engl J Med (2019) 381(21):2020–31. doi: 10.1056/NEJMoa1910231
94. Paz-Ares LG, Ciuleanu T-E, Lee J-S, Urban L, Caro RB, Park K, et al. Nivolumab (NIVO) plus ipilimumab (IPI) versus chemotherapy (chemo) as first-line (1L) treatment for advanced non-small cell lung cancer (NSCLC): 4-year update from CheckMate 227. J Clin Oncol (2021) 39(15_suppl):9016. doi: 10.1200/JCO.2021.39.15_suppl.9016
95. Carbone D, Ciuleanu T, Cobo M, Schenker M, Zurawski B, Menezes J, et al. OA09.01 first-line nivolumab + ipilimumab + chemo in patients with advanced NSCLC and brain metastases: Results from CheckMate 9LA. J Thorac Oncol (2021) 16(10):S862. doi: 10.1016/j.jtho.2021.08.061
96. Johnson M ed. PL02.01: Durvalumab + tremelimumab + chemotherapy as first-line treatment for mNSCLC: results from the phase 3 POSEIDON study. In: International association for the study of lung cancer 2021 world conference on lung cancer.
97. Nadal E ed. OA09.02: Single arm phase II study of atezolizumab plus chemotherapy in stage IV NSCLC with untreated brain metastases. In: International association for the study of lung cancer 2021 world conference on lung cancer.
98. Hui R ed. OA09.01 - first-line nivolumab + ipilimumab + chemo in patients with advanced NSCLC and brain metastases: Results from CheckMate 9LA. In: International association for the study of lung cancer 2021 world conference on lung cancer.
99. Gautschi O, Drilon A, Milia J, Lusque A, Mhanna L, Li B, et al. MA04.03 immunotherapy for non-small cell lung cancers (NSCLC) with oncogenic driver mutations: New results from the global IMMUNOTARGET registry. J Thorac Oncol (2018) 13(10):S367–S. doi: 10.1016/j.jtho.2018.08.342
100. Mazieres J, Drilon AE, Mhanna L, Milia J, Lusque A, Cortot AB, et al. Efficacy of immune-checkpoint inhibitors (ICI) in non-small cell lung cancer (NSCLC) patients harboring activating molecular alterations (ImmunoTarget). J Clin Oncol (2018) 36(15_suppl):9010. doi: 10.1200/JCO.2018.36.15_suppl.9010
101. Dong Z-Y, Zhang J-T, Liu S-Y, Su J, Zhang C, Xie Z, et al. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. OncoImmunology (2017) 6(11):e1356145. doi: 10.1080/2162402X.2017.1356145
102. Soo RA, Lim SM, Syn NL, Teng R, Soong R, Mok TSK, et al. Immune checkpoint inhibitors in epidermal growth factor receptor mutant non-small cell lung cancer: Current controversies and future directions. Lung Cancer (2018) 115:12–20. doi: 10.1016/j.lungcan.2017.11.009
103. Oxnard GR, Yang JC, Yu H, Kim SW, Saka H, Horn L, et al. TATTON: a multi-arm, phase ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann Oncol (2020) 31(4):507–16. doi: 10.1016/j.annonc.2020.01.013
104. Shaw AT, Lee S-H, Ramalingam SS, Bauer TM, Boyer MJ, Costa EC, et al. Avelumab (anti–PD-L1) in combination with crizotinib or lorlatinib in patients with previously treated advanced NSCLC: Phase 1b results from JAVELIN lung 101. J Clin Oncol (2018) 36(15_suppl):9008. doi: 10.1200/JCO.2018.36.15_suppl.9008
105. Buttner R, Gosney JR, Skov BG, Adam J, Motoi N, Bloom KJ, et al. Programmed death-ligand 1 immunohistochemistry testing: A review of analytical assays and clinical implementation in non-Small-Cell lung cancer. J Clin Oncol (2017) 35(34):3867–76. doi: 10.1200/JCO.2017.74.7642
106. Hirsch FR, McElhinny A, Stanforth D, Ranger-Moore J, Jansson M, Kulangara K, et al. PD-L1 immunohistochemistry assays for lung cancer: Results from phase 1 of the blueprint PD-L1 IHC assay comparison project. J Thorac Oncol (2017) 12(2):208–22. doi: 10.1016/j.jtho.2016.11.2228
107. Munari E, Zamboni G, Lunardi G, Marchionni L, Marconi M, Sommaggio M, et al. PD-L1 expression heterogeneity in non-small cell lung cancer: Defining criteria for harmonization between biopsy specimens and whole sections. J Thorac Oncol (2018) 13(8):1113–20. doi: 10.1016/j.jtho.2018.04.017
108. Bassanelli M, Sioletic S, Martini M, Giacinti S, Viterbo A, Staddon A, et al. Heterogeneity of PD-L1 expression and relationship with biology of NSCLC. Anticancer Res (2018) 38(7):3789–96. doi: 10.21873/anticanres.12662
109. Munari E, Zamboni G, Marconi M, Sommaggio M, Brunelli M, Martignoni G, et al. PD-L1 expression heterogeneity in non-small cell lung cancer: evaluation of small biopsies reliability. Oncotarget (2017) 8(52):90123–31. doi: 10.18632/oncotarget.21485
110. Gniadek TJ, Li QK, Tully E, Chatterjee S, Nimmagadda S, Gabrielson E. Heterogeneous expression of PD-L1 in pulmonary squamous cell carcinoma and adenocarcinoma: implications for assessment by small biopsy. Mod Pathol (2017) 30(4):530–8. doi: 10.1038/modpathol.2016.213
111. Cooper WA, Tran T, Vilain RE, Madore J, Selinger CI, Kohonen-Corish M, et al. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer (2015) 89(2):181–8. doi: 10.1016/j.lungcan.2015.05.007
112. Tsao MS, Le Teuff G, Shepherd FA, Landais C, Hainaut P, Filipits M, et al. PD-L1 protein expression assessed by immunohistochemistry is neither prognostic nor predictive of benefit from adjuvant chemotherapy in resected non-small cell lung cancer. Ann Oncol (2017) 28(4):882–9. doi: 10.1093/annonc/mdx003
113. Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Cancer Immunol Sci (2015) 348(6230):124–8. doi: 10.1126/science.aaa1348
114. Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med (2018) 378(22):2093–104. doi: 10.1056/NEJMoa1801946
115. Gandara DR, Paul SM, Kowanetz M, Schleifman E, Zou W, Li Y, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med (2018) 24(9):1441–8. doi: 10.1038/s41591-018-0134-3
116. Velcheti V, Kim ES, Mekhail T, Dakhil C, Stella PJ, Shen X, et al. Prospective clinical evaluation of blood-based tumor mutational burden (bTMB) as a predictive biomarker for atezolizumab (atezo) in 1L non-small cell lung cancer (NSCLC): Interim b-F1RST results. J Clin Oncol (2018) 36(15_suppl):12001. doi: 10.1200/JCO.2018.36.15_suppl.12001
117. Socinski M, Velcheti V, Mekhail T, Chae YK, Leal TA, Dowell JE, et al. LBA83 - final efficacy results from b-F1RST, a prospective phase II trial evaluating blood-based tumour mutational burden (bTMB) as a predictive biomarker for atezolizumab (atezo) in 1L non-small cell lung cancer (NSCLC). Ann Oncol (2019) 30:v919–v20. doi: 10.1093/annonc/mdz394.081
118. Rizvi NA, Chul Cho B, Reinmuth N, Lee KH, Ahn MJ, Luft A, et al. Durvalumab with or without tremelimumab vs platinum-based chemotherapy as first-line treatment for metastatic non-small cell lung cancer: MYSTIC. Ann Oncol (2018) 29(suppl_10):x40–x1. doi: 10.1093/annonc/mdy511.005
119. Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn MJ, et al. Durvalumab with or without tremelimumab vs standard chemotherapy in first-line treatment of metastatic non-small cell lung cancer: The MYSTIC phase 3 randomized clinical trial. JAMA Oncol (2020) 6(5):661–74. doi: 10.1001/jamaoncol.2020.0237
120. Peters S, Cho BC, Reinmuth N, Lee KH, Luft A, Ahn M-J, et al. CT074: Tumor mutational burden (TMB) as a biomarker of survival in metastatic non-small cell lung cancer (mNSCLC): Blood and tissue TMB analysis from MYSTIC, a phase III study of first-line durvalumab ± tremelimumab vs chemotherapy. Abstract Cancer Res (2019) 79(13 Supplement):CT074–CT. doi: 10.1158/1538-7445.AM2019-CT074
121. Wang Z, Duan J, Cai S, Han M, Dong H, Zhao J, et al. Assessment of blood tumor mutational burden as a potential biomarker for immunotherapy in patients with non-small cell lung cancer with use of a next-generation sequencing cancer gene panel. JAMA Oncol (2019) 5(5):696–702. doi: 10.1001/jamaoncol.2018.7098
122. Heuvel G, Kroeze L, Ligtenberg M, Grunberg K, Rhein D, Jansen E, et al. 3MO mutational signatures in the perspective of tumour mutational burden in patients with non-small cell lung cancer. Ann Oncol (2020) 31:S1217–S8. doi: 10.1016/j.annonc.2020.08.2162
123. Marabelle A, Fakih M, Lopez J, Shah M, Shapira-Frommer R, Nakagawa K, et al. Association of tumour mutational burden with outcomes in patients with advanced solid tumours treated with pembrolizumab: prospective biomarker analysis of the multicohort, open-label, phase 2 KEYNOTE-158 study. Lancet Oncol (2020) 21(10):1353–65. doi: 10.1016/S1470-2045(20)30445-9
124. Rodriguez-Abreu D, Johnson ML, Hussein MA, Cobo M, Patel AJ, Secen NM, et al. Primary analysis of a randomized, double-blind, phase II study of the anti-TIGIT antibody tiragolumab (tira) plus atezolizumab (atezo) versus placebo plus atezo as first-line (1L) treatment in patients with PD-L1-selected NSCLC (CITYSCAPE). J Clin Oncol (2020) 38(15_suppl):9503. doi: 10.1200/JCO.2020.38.15_suppl.9503
125. Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer Res (2012) 72(4):917–27. doi: 10.1158/0008-5472.CAN-11-1620
126. Papadopoulos KP, Lakhani NJ, Johnson ML, Park H, Wang D, Yap TA, et al. A study of REGN3767, an anti-LAG-3 antibody, alone and in combination with cemiplimab (REGN2810), an anti-PD1 antibody, in advanced cancers. J Clin Oncol (2018) 36(15_suppl):TPS3127–TPS. doi: 10.1200/JCO.2018.36.15_suppl.TPS3127
127. Papadopoulos KP, Lakhani NJ, Johnson ML, Park H, Wang D, Yap TA, et al. First-in-human study of REGN3767 (R3767), a human LAG-3 monoclonal antibody (mAb), ± cemiplimab in patients (pts) with advanced malignancies. J Clin Oncol (2019) 37(15_suppl):2508. doi: 10.1200/JCO.2019.37.15_suppl.2508
128. Wang J, Sanmamed MF, Datar I, Su TT, Ji L, Sun J, et al. Fibrinogen-like protein 1 is a major immune inhibitory ligand of LAG-3. Cell (2019) 176(1-2):334–47.e12. doi: 10.1016/j.cell.2018.11.010
129. Segal NH, He AR, Doi T, Levy R, Bhatia S, Pishvaian MJ, et al. Phase I study of single-agent utomilumab (PF-05082566), a 4-1BB/CD137 agonist, in patients with advanced cancer. Clin Cancer Res (2018) 24(8):1816–23. doi: 10.1158/1078-0432.CCR-17-1922
130. Tolcher AW, Sznol M, Hu-Lieskovan S, Papadopoulos KP, Patnaik A, Rasco DW, et al. Phase ib study of utomilumab (PF-05082566), a 4-1BB/CD137 agonist, in combination with pembrolizumab (MK-3475) in patients with advanced solid tumors. Clin Cancer Res (2017) 23(18):5349–57. doi: 10.1158/1078-0432.CCR-17-1243
131. James J. Harding APVMMSAMJNVdMZTLMKEC. a phase Ia/Ib study of an anti-TIM-3 antibody (LY3321367) monotherapy or in combination with an anti-PD-L1 antibody (LY3300054): Interim safety, efficacy, and pharmacokinetic findings in advanced cancers. J Clin Oncol (2019) 37(suppl 8; abstr 12). doi: 10.1200/JCO.2019.37.8_suppl.12
132. Diab A, Hurwitz ME, Cho DC, Papadimitrakopoulou V, Curti BD, Tykodi SS, et al. NKTR-214 (CD122-biased agonist) plus nivolumab in patients with advanced solid tumors: Preliminary phase 1/2 results of PIVOT. J Clin Oncol (2018) 36(15_suppl):3006. doi: 10.1200/JCO.2018.36.15_suppl.3006
133. Diab A, Tannir NM, Bentebibel S-E, Hwu P, Papadimitrakopoulou V, Haymaker C, et al. Bempegaldesleukin (NKTR-214) plus nivolumab in patients with advanced solid tumors: Phase I dose-escalation study of safety, efficacy, and immune activation (PIVOT-02). Cancer Discov (2020) 10(8):1158–73. doi: 10.1158/2159-8290.CD-19-1510
134. Charych DH, Hoch U, Langowski JL, Lee SR, Addepalli MK, Kirk PB, et al. NKTR-214, an engineered cytokine with biased IL2 receptor binding, increased tumor exposure, and marked efficacy in mouse tumor models. Clin Cancer Res (2016) 22(3):680–90. doi: 10.1158/1078-0432.CCR-15-1631
135. Donnem T, Hald SM, Paulsen EE, Richardsen E, Al-Saad S, Kilvaer TK, et al. Stromal CD8+ T-cell density-a promising supplement to TNM staging in non-small cell lung cancer. Clin Cancer Res (2015) 21(11):2635–43. doi: 10.1158/1078-0432.CCR-14-1905
136. Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund LT. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res (2008) 14(16):5220–7. doi: 10.1158/1078-0432.CCR-08-0133
137. Mazzaschi G, Madeddu D, Falco A, Bocchialini G, Goldoni M, Sogni F, et al. Low PD-1 expression in cytotoxic CD8(+) tumor-infiltrating lymphocytes confers an immune-privileged tissue microenvironment in NSCLC with a prognostic and predictive value. Clin Cancer Res (2018) 24(2):407–19. doi: 10.1158/1078-0432.CCR-17-2156
138. Miller JS, Morishima C, McNeel DG, Patel MR, Kohrt HEK, Thompson JA, et al. A first-in-Human phase I study of subcutaneous outpatient recombinant human IL15 (rhIL15) in adults with advanced solid tumors. Clin Cancer Res (2018) 24(7):1525–35. doi: 10.1158/1078-0432.CCR-17-2451
139. Wrangle JM, Velcheti V, Patel MR, Garrett-Mayer E, Hill EG, Ravenel JG, et al. ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol (2018) 19(5):694–704. doi: 10.1016/S1470-2045(18)30148-7
140. Berraondo P, Sanmamed MF, Ochoa MC, Etxeberria I, Aznar MA, Perez-Gracia JL, et al. Cytokines in clinical cancer immunotherapy. Br J Cancer (2019) 120(1):6–15. doi: 10.1038/s41416-018-0328-y
141. Sanmamed MF, Perez-Gracia JL, Schalper KA, Fusco JP, Gonzalez A, Rodriguez-Ruiz ME, et al. Changes in serum interleukin-8 (IL-8) levels reflect and predict response to anti-PD-1 treatment in melanoma and non-small-cell lung cancer patients. Ann Oncol (2017) 28(8):1988–95. doi: 10.1093/annonc/mdx190
142. Collins JM, Heery CR, Donahue RN, Palena C, Madan RA, Strauss J, et al. Phase I trial of BMS-986253, an anti-IL-8 monoclonal antibody, in patients with metastatic or unresectable solid tumors. J Clin Oncol (2018) 36(15_suppl):3091. doi: 10.1200/JCO.2018.36.15_suppl.3091
143. Melero Bermejo I, Jaffee EM, Davar D, Cardarelli J, Williams D, Phillips P, et al. Phase 1b/2 study of nivolumab in combination with an anti–IL-8 monoclonal antibody, BMS-986253, in a biomarker-enriched population of patients with advanced cancer. J Clin Oncol (2018) 36(15_suppl):TPS3109–TPS. doi: 10.1200/JCO.2018.36.15_suppl.TPS3109
144. Knudson KM, Hicks KC, Luo X, Chen JQ, Schlom J, Gameiro SR. M7824, a novel bifunctional anti-PD-L1/TGFbeta trap fusion protein, promotes anti-tumor efficacy as monotherapy and in combination with vaccine. Oncoimmunology (2018) 7(5):e1426519. doi: 10.1080/2162402X.2018.1426519
145. Paz-Ares L, Kim TM, Vicente D, Felip E, Lee DH, Lee KH, et al. Bintrafusp Alfa, a bifunctional fusion protein targeting TGF-β and PD-L1, in second-line treatment of patients with NSCLC: Results from an expansion cohort of a phase 1 trial. J Thorac Oncol (2020) 15(7):1210–22. doi: 10.1016/j.jtho.2020.03.003
146. Chon H. Effect of STING agonist on tumor immune microenvironment of non-inflamed lung cancer and efficacy of immune checkpoint blockade. J Clin Oncol (2018) 36(5_suppl):178. doi: 10.1200/JCO.2018.36.5_suppl.178
147. Harrington KJ, Brody J, Ingham M, Strauss J, Cemerski S, Wang M, et al. Preliminary results of the first-in-human (FIH) study of MK-1454, an agonist of stimulator of interferon genes (STING), as monotherapy or in combination with pembrolizumab (pembro) in patients with advanced solid tumors or lymphomas. Ann Oncol (2018) 29(suppl_8). doi: 10.1093/annonc/mdy424.015
148. Leal DB TA, Rybkin I, Iams WT, Bruno D, Blakely C, Spira A, et al. 1191O - MRTX-500: Phase II trial of sitravatinib (sitra) + nivolumab (nivo) in patients (pts) with non-squamous (NSQ) non-small cell lung cancer (NSCLC) progressing on or after prior checkpoint inhibitor (CPI) therapy. Ann Oncol (2021) 32:S949–S1039. doi: 10.1016/j.annonc.2021.08.1796
149. DeMaio A, Sterman D. Bronchoscopic intratumoural therapies for non-small cell lung cancer. Eur Respir Rev (2020) 29(156). doi: 10.1183/16000617.0028-2020
150. Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature (2006) 440(7081):228–32. doi: 10.1038/nature04515
151. Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene (2010) 29(39):5346–58. doi: 10.1038/onc.2010.292
152. Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med (2007) 204(6):1257–65. doi: 10.1084/jem.20062512
153. Kong T, Westerman KA, Faigle M, Eltzschig HK, Colgan SP. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J (2006) 20(13):2242–50. doi: 10.1096/fj.06-6419com
154. Tak E, Jung DH, Kim SH, Park GC, Jun DY, Lee J, et al. Protective role of hypoxia-inducible factor-1alpha-dependent CD39 and CD73 in fulminant acute liver failure. Toxicol Appl Pharmacol (2017) 314:72–81. doi: 10.1016/j.taap.2016.11.016
155. Allard B, Longhi MS, Robson SC, Stagg J. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol Rev (2017) 276(1):121–44. doi: 10.1111/imr.12528
156. Mittal D, Sinha D, Barkauskas D, Young A, Kalimutho M, Stannard K, et al. Adenosine 2B receptor expression on cancer cells promotes metastasis. Cancer Res (2016) 76(15):4372–82. doi: 10.1158/0008-5472.CAN-16-0544
157. Willingham SB, Ho PY, Hotson A, Hill C, Piccione EC, Hsieh J, et al. A2AR antagonism with CPI-444 induces antitumor responses and augments efficacy to anti-PD-(L)1 and anti-CTLA-4 in preclinical models. Cancer Immunol Res (2018) 6(10):1136–49. doi: 10.1158/2326-6066.CIR-18-0056
158. Mediavilla-Varela M, Castro J, Chiappori A, Noyes D, Hernandez DC, Allard B, et al. A novel antagonist of the immune checkpoint protein adenosine A2a receptor restores tumor-infiltrating lymphocyte activity in the context of the tumor microenvironment. Neoplasia (2017) 19(7):530–6. doi: 10.1016/j.neo.2017.02.004
159. Chiappori A, Williams CC, Creelan BC, Tanvetyanon T, Gray JE, Haura EB, et al. Phase I/II study of the A2AR antagonist NIR178 (PBF-509), an oral immunotherapy, in patients (pts) with advanced NSCLC. J Clin Oncol (2018) 36(15_suppl):9089. doi: 10.1200/JCO.2018.36.15_suppl.9089
160. Feng K, Guo Y, Dai H, Wang Y, Li X, Jia H, et al. Chimeric antigen receptor-modified T cells for the immunotherapy of patients with EGFR-expressing advanced relapsed/refractory non-small cell lung cancer. Sci China Life Sci (2016) 59(5):468–79. doi: 10.1007/s11427-016-5023-8
161. Li N, Liu S, Sun M, Chen W, Xu X, Zeng Z, et al. Chimeric antigen receptor-modified T cells redirected to EphA2 for the immunotherapy of non-small cell lung cancer. Transl Oncol (2018) 11(1):11–7. doi: 10.1016/j.tranon.2017.10.009
162. Kiesgen S, Chicaybam L, Chintala NK, Adusumilli PS. Chimeric antigen receptor (CAR) T-cell therapy for thoracic malignancies. J Thorac Oncol (2018) 13(1):16–26. doi: 10.1016/j.jtho.2017.10.001
163. Li H, Huang Y, Jiang DQ, Cui LZ, He Z, Wang C, et al. Antitumor activity of EGFR-specific CAR T cells against non-small-cell lung cancer cells in vitro and in mice. Cell Death Dis (2018) 9(2):177. doi: 10.1038/s41419-017-0238-6
164. Lam VK, Hong DS, Heymach J, Blumenschein GR, Creelan BC, Bradbury PA, et al. Initial safety assessment of MAGE-A10c796TCR T-cells in two clinical trials. J Clin Oncol (2018) 36(15_suppl):3056. doi: 10.1200/JCO.2018.36.15_suppl.3056
165. Sabari J, Ramirez KA, Schwarzenberger P, Ricciardi T, Macri M, Ryan A, et al. eds. Abstract B209: Phase 1/2 study of mRNA vaccine therapy + durvalumab (durva) ± tremelimumab (treme) in patients with metastatic non-small cell lung cancer (NSCLC)2019/02//. In: American Association for cancer research. New York AACR Journals (2019)
166. Creelan BC, Wang C, Teer JK, Toloza EM, Yao J, Kim S, et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial. Nat Med (2021) 27(8):1410–8. doi: 10.1038/s41591-021-01462-y
167. Cichocki F, Bjordahl R, Gaidarova S, Mahmood S, Abujarour R, Wang H, et al. iPSC-derived NK cells maintain high cytotoxicity and enhance in vivo tumor control in concert with T cells and anti-PD-1 therapy. Sci Transl Med (2020) 12(568). doi: 10.1126/scitranslmed.aaz5618
168. Kjeldsen JW, Lorentzen CL, Martinenaite E, Ellebaek E, Donia M, Holmstroem RB, et al. A phase 1/2 trial of an immune-modulatory vaccine against IDO/PD-L1 in combination with nivolumab in metastatic melanoma. Nat Med (2021) 27(12):2212–23. doi: 10.1038/s41591-021-01544-x
169. Sasikumar PG, Sudarshan NS, Adurthi S, Ramachandra RK, Samiulla DS, Lakshminarasimhan A, et al. PD-1 derived CA-170 is an oral immune checkpoint inhibitor that exhibits preclinical anti-tumor efficacy. Commun Biol (2021) 4(1):699. doi: 10.1038/s42003-021-02191-1
170. Assi HH, Wong C, Tipton KA, Mei L, Wong K, Razo J, et al. Conditional PD-1/PD-L1 probody therapeutics induce comparable antitumor immunity but reduced systemic toxicity compared with traditional anti-PD-1/PD-L1 agents. Cancer Immunol Res (2021) 9(12):1451–64. doi: 10.1158/2326-6066.CIR-21-0031
171. Naing A, Thistlethwaite F, De Vries EGE, Eskens FALM, Uboha N, Ott PA, et al. CX-072 (pacmilimab), a probody® PD-L1 inhibitor, in advanced or recurrent solid tumors (PROCLAIM-CX-072): an open-label dose-finding and first-in-human study. J ImmunoTher Cancer (2021) 9(7):e002447. doi: 10.1136/jitc-2021-002447
172. Goletz C, Lischke T, Harnack U, Schiele P, Danielczyk A, Rühmann J, et al. Glyco-engineered anti-human programmed death-ligand 1 antibody mediates stronger CD8 T cell activation than its normal glycosylated and non-glycosylated counterparts. Front Immunol (2018) 9. doi: 10.3389/fimmu.2018.01614
173. Peters HL, Tripathi SC, Kerros C, Katayama H, Garber HR, St John LS, et al. Serine proteases enhance immunogenic antigen presentation on lung cancer cells. Cancer Immunol Res (2017) 5(4):319–29. doi: 10.1158/2326-6066.CIR-16-0141
174. Fumet JD, Richard C, Ledys F, Klopfenstein Q, Joubert P, Routy B, et al. Prognostic and predictive role of CD8 and PD-L1 determination in lung tumor tissue of patients under anti-PD-1 therapy. Br J Cancer (2018) 119(8):950–60. doi: 10.1038/s41416-018-0220-9
175. Tokito T, Azuma K, Kawahara A, Ishii H, Yamada K, Matsuo N, et al. Predictive relevance of PD-L1 expression combined with CD8+ TIL density in stage III non-small cell lung cancer patients receiving concurrent chemoradiotherapy. Eur J Cancer (2016) 55:7–14. doi: 10.1016/j.ejca.2015.11.020
176. Schalper KA, Brown J, Carvajal-Hausdorf D, McLaughlin J, Velcheti V, Syrigos KN, et al. Objective measurement and clinical significance of TILs in non-small cell lung cancer. J Natl Cancer Inst (2015) 107(3). doi: 10.1093/jnci/dju435
177. Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, et al. T-Cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature (2017) 545(7652):60–5. doi: 10.1038/nature22079
178. Wang J ed. FP04.02 - RATIONALE-307: Updated biomarker analysis of phase 3 study of tislelizumab plus chemo vs chemo alone for 1L advanced sq-NSCLC. In: International association for the study of lung cancer 2021 world conference on lung cancer.
179. Liu C, He H, Li X, Su MA, Cao Y. Dynamic metrics-based biomarkers to predict responders to anti-PD-1 immunotherapy. Br J Cancer (2019) 120(3):346–55. doi: 10.1038/s41416-018-0363-8
180. Ayers M, Lunceford J, Nebozhyn M, Murphy E, Loboda A, Kaufman DR, et al. IFN-gamma-related mRNA profile predicts clinical response to PD-1 blockade. J Clin Invest (2017) 127(8):2930–40. doi: 10.1172/JCI91190
181. Karachaliou N, Gonzalez-Cao M, Crespo G, Drozdowskyj A, Aldeguer E, Gimenez-Capitan A, et al. Interferon gamma, an important marker of response to immune checkpoint blockade in non-small cell lung cancer and melanoma patients. Ther Adv Med Oncol (2018) 10:1758834017749748. doi: 10.1177/1758834017749748
182. Routy B, Le Chatelier E, Derosa L, Duong CPM, Alou MT, Daillere R, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science (2018) 359(6371):91–7. doi: 10.1126/science.aan3706
183. Adachi K, Tamada K. Microbial biomarkers for immune checkpoint blockade therapy against cancer. J Gastroenterol (2018) 53(9):999–1005. doi: 10.1007/s00535-018-1492-9
184. Ocáriz-Díez M, Cruellas M, Gascón M, Lastra R, Martínez-Lostao L, Ramírez-Labrada A, et al. Microbiota and lung cancer. opportunities and challenges for improving immunotherapy efficacy. Front Oncol (1945) 2020:10. doi: 10.3389/fonc.2020.568939
185. Zaretsky JM, Garcia-Diaz A, Shin DS, Escuin-Ordinas H, Hugo W, Hu-Lieskovan S, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med (2016) 375(9):819–29. doi: 10.1056/NEJMoa1604958
186. Chen Q, Li T, Yue W. Drug response to PD-1/PD-L1 blockade: based on biomarkers. Onco Targets Ther (2018) 11:4673–83. doi: 10.2147/OTT.S168313
187. Corredor G, Wang X, Zhou Y, Lu C, Fu P, Syrigos K, et al. Spatial architecture and arrangement of tumor-infiltrating lymphocytes for predicting likelihood of recurrence in early-stage non-small cell lung cancer. Clin Cancer Res (2019) 25(5):1526–34. doi: 10.1158/1078-0432.CCR-18-2013
188. Hosny A, Parmar C, Coroller TP, Grossmann P, Zeleznik R, Kumar A, et al. Deep learning for lung cancer prognostication: A retrospective multi-cohort radiomics study. PLoS Med (2018) 15(11):e1002711. doi: 10.1371/journal.pmed.1002711
189. Coudray N, Ocampo PS, Sakellaropoulos T, Narula N, Snuderl M, Fenyo D, et al. Classification and mutation prediction from non-small cell lung cancer histopathology images using deep learning. Nat Med (2018) 24(10):1559–67. doi: 10.1038/s41591-018-0177-5
190. Vaidya P, Bera K, Gupta A, Wang X, Corredor G, Fu P, et al. CT derived radiomic score for predicting the added benefit of adjuvant chemotherapy following surgery in stage I, II resectable non-small cell lung cancer: a retrospective multi-cohort study for outcome prediction. Lancet Digit Health (2020) 2(3):e116–e28. doi: 10.1016/S2589-7500(20)30002-9
191. Khorrami M, Prasanna P, Gupta A, Patil P, Velu PD, Thawani R, et al. Changes in CT radiomic features associated with lymphocyte distribution predict overall survival and response to immunotherapy in non-small cell lung cancer. Cancer Immunol Res (2020) 8(1):108–19. doi: 10.1158/2326-6066.CIR-19-0476
Keywords: Immunotherapy, immune checkpoint blockade, PD-1, PD-L1, CTLA-4, biomarkers, NSCLC
Citation: Punekar SR, Shum E, Grello CM, Lau SC and Velcheti V (2022) Immunotherapy in non-small cell lung cancer: Past, present, and future directions. Front. Oncol. 12:877594. doi: 10.3389/fonc.2022.877594
Received: 16 February 2022; Accepted: 24 June 2022;
Published: 02 August 2022.
Edited by:
Lizza E.L. Hendriks, Maastricht University Medical Centre, NetherlandsReviewed by:
Kaushal Parikh, Hackensack University Medical Center, United StatesChunxia Su, Shanghai Pulmonary Hospital, China
Copyright © 2022 Punekar, Shum, Grello, Lau and Velcheti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Salman R. Punekar, c2FsbWFuLnB1bmVrYXJAbnl1bGFuZ29uZS5vcmc=
 Salman R. Punekar
Salman R. Punekar Elaine Shum
Elaine Shum Cassandra Mia Grello
Cassandra Mia Grello Sally C. Lau
Sally C. Lau Vamsidhar Velcheti
Vamsidhar Velcheti