- 1Department of Hepatobiliary Surgery, The People’s Hospital of Leshan, Leshan, China
- 2Department of Medical Oncology, The People’s Hospital of Leshan, Leshan, China
Background: Combining two immune checkpoint inhibitors (ICIs) instead of using one can effectively improve the prognosis of advanced malignant tumors. At present, ipilimumab alongside nivolumab is the most widely used combinatorial regimen of ICIs. However, the risk of treatment-related adverse events is higher in combinatorial regimens than in single-drug regimens. Thus, this study aimed to evaluate the risks of common adverse events associated with the combinatorial regimen of ipilimumab and nivolumab by using meta-analysis.
Methods: We searched Pubmed, Medline, EMBASE, and Cochrane Library for reports published by 30 September 2021. A randomized controlled study was developed and analyzed using the statistical software R to determine the efficacy of the combinatorial treatment. Risk estimates (hazard ratios, RR) and 95% confidence intervals for various common serious adverse events were used.
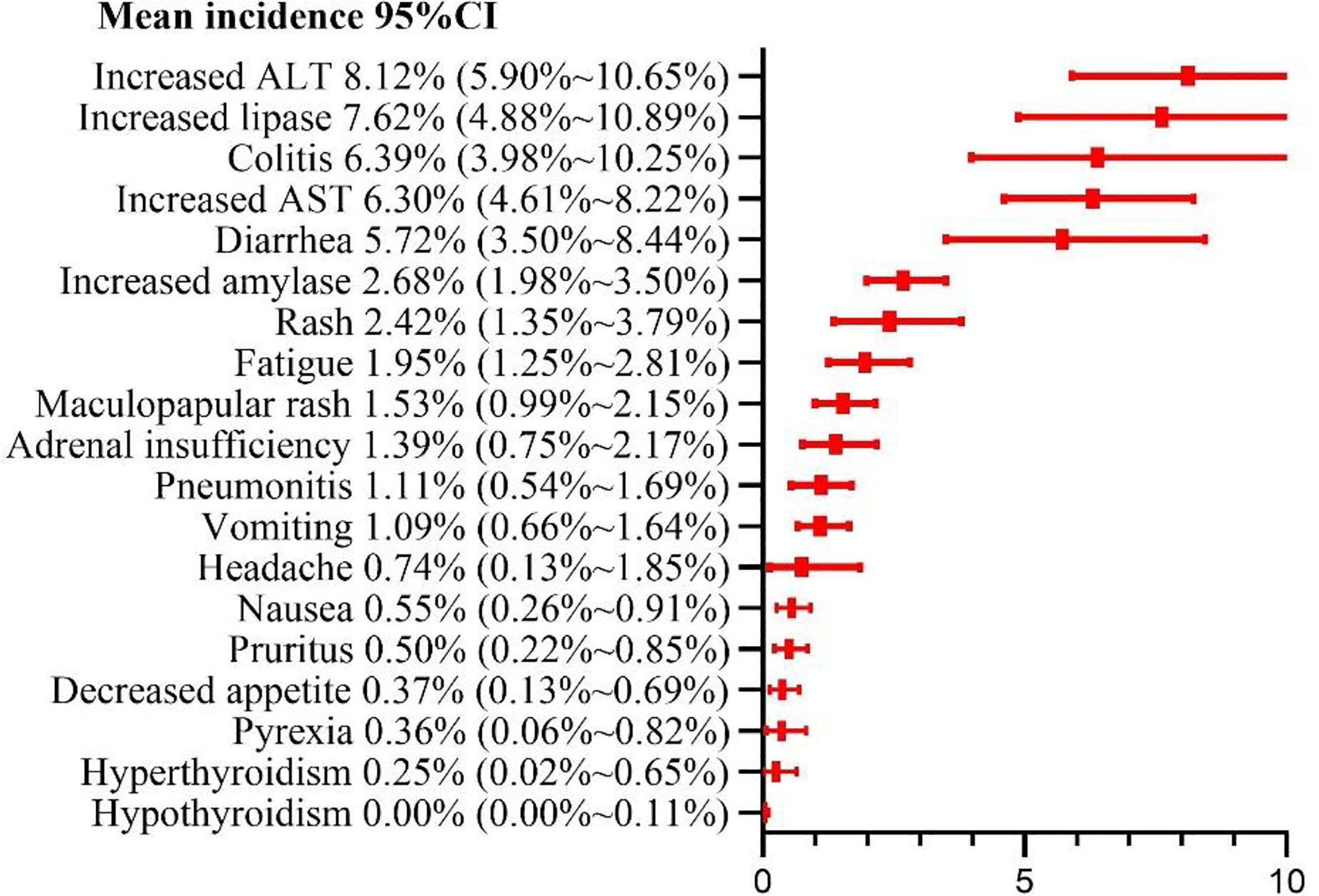
Results: A total of 23 randomized control trials (n = 3970 patients) were included. Our meta-analysis indicated the risks of adverse events of any grade and grade ≥ 3 as 90.42% (95%CI: 85.91% ~ 94.18%) and 46.46% (95%CI: 39.37% ~ 53.69%), respectively; the risks of treatment-related death and adverse events leading to discontinuation were estimated at 0.42% (95% CI, 0.18% ~ 0.72%) and 19.11% (95% CI, 14.99% ~ 24.38%), respectively. Classification of 19 common adverse events. The top 5 grade 1-2 adverse events were found to be fatigue (30.92%, 95% CI: 24.59% ~ 37.62%), pruritus (26.05%, 95%CI: 22.29%~29.99%), diarrhea (23.58%, 95% CI: 20.62% ~ 26.96%), rash (19.90%, 95%CI: 15.75% ~ 25.15%), and nausea (17.19%, 95% CI:13.7% ~ 21.57%). The top 5 grade ≥ 3 adverse events were identified as increased alanine aminotransferase(8.12%, 95% CI: 5.90%~10.65%), increased lipase(7.62%, 95% CI: 4.88% ~ 10.89%), and colitis (6.39%, 95%CI: 3.98% ~ 10.25%), increased aspartate aminotransferase (6.30%, 95% CI: 4.61% ~ 8.22%), and diarrhea(5.72%, 95%CI: 3.50% ~ 8.44%). Subgroup analysis revealed some differences in the adverse events between the N1-I3 and N3-I1 subgroups and between subgroups of different cancer types.
Conclusion: This study summarized the risks of common adverse events in the co-treatment of malignant-tumor patients with ipilimumab and nivolumab and identified the impacts of various initial administration schemes on the risks of such events, thereby providing an important reference for the toxicity of co-treatment with ipilimumab and nivolumab.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier: CRD42020181350.
Introduction
According to the estimates of the World Cancer Center and the American Cancer Center, there were 9 million cancer-related deaths worldwide in 2020 and 600000 in the United States in 2021 (1, 2). Surgical treatment, radiotherapy, chemotherapy, and targeted drug treatment are the common treatment strategies for malignant tumors. However, these approaches have limited effects on some advanced malignant tumors. The in-depth studies on immune checkpoint inhibitors (ICIs) in recent years have provided a good prospect for the treatment of advanced malignant tumors (3, 4). ICIs are monoclonal antibodies that can activate the immune system to enhance antitumor immunity. The results of many large-scale multicenter randomized control trials (RCTs) have shown that immunotherapy can effectively prolong the survival of patients with advanced malignant tumors, and some immunotherapeutic drugs have become the first-line antitumor therapeutics (5, 6). At present, common ICIs include ipilimumab, tremelimumab, nivolumab, pembrolizumab, atezolizumab, and durvalumab. Studies have shown that the efficacy of single-drug therapies is limited, and thus combinatorial immunotherapy is gradually becoming the focus of cancer research worldwide (7, 8). Multi-phase clinical trials on combinatorial therapies involving immune-targeted therapy, chemoradiotherapy, or two ICIs have yielded gratifying results. Ipilimumab alongside nivolumab is the most common combination of two ICIs in cancer treatment and has been successfully applied to malignant tumors, such as advanced malignant melanoma and lung and kidney cancers (9). Combinatorial immunotherapy can have a good curative effect but lacks selectivity and specificity, inhibits both normal and abnormal immune responses, and is often accompanied by some adverse events. Although several meta-analysis studies have reported the risk of adverse events associated with some combinatorial immunotherapy regimens, the plausible combinations of immunotherapy drugs are extensive, and no such study has been reported on the combinatorial use of ipilimumab and nivolumab (10–12). Therefore, this study aimed to evaluate the risk of various common adverse events associated with the combinatorial use of ipilimumab and nivolumab, thereby providing an evidence-based basis for the management of such events in the clinic.
Materials and Methods
Literature Review and Study Identification
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) and Assessing the Methodological Quality of Systematic Reviews (AMSTAR) guidelines. This study was registered on Prospero (Registration number: CRD42020181350). Two independent researchers searched the PubMed, EMBASE, Cochrane Library, and MEDLINE databases for the relevant literature published between the beginning of database construction and September 30, 2021, and extracted the relevant data. The search keywords were “nivolumab”, “ipilimumab”, “CTLA-4”, and “PD-1”. We also manually checked the supplementary materials and list of references in each retrieved article to further identify any potential relevant RCT and searched the websites of the relevant regulatory agencies in the United States and Europe [The Federal Drug Administration and European Drug Administration, respectively]. We reported the basis of this systematic review and meta-analysis in accordance with Cochrane’s recommendations on preferred reporting items for systematic review and meta-analysis.
Article Selection
We included only phase I–IV RCTs on ipilimumab and nivolumab combinatorial therapy of patients with malignant tumors. We excluded non-randomized trials, and studies with malignant-tumor patients, additional regimens (e.g., radiotherapy, chemotherapy, and targeted therapy), incomplete data on adverse events, or high bias in risk assessment. If two reports corresponded to the same study of a research group, we included only the most complete and up-to-date study. Two reviewers independently screened all titles, abstracts, and full texts to assess whether the corresponding studies qualified. Any disagreement among these reviewers was judged by a third researcher and finally resolved by consensus.
Data Extraction and Quality Assessment
Two researchers repeatedly extracted data according to the preset extraction table. Any inconsistency in extracted data between the two researchers was resolved via discussion with a third researcher. The extracted data included the study registration number, first author, publication year, trial stage, tumor type, number of cases, treatment scheme, and initial dose scheme of each included study. We defined adverse events ≥ 10 literatures as common adverse events. The extracted analysis data included the total number of adverse events of any grade, grade 1-2, and grade ≥ 3, as well as adverse events leading to drug withdrawal and death. We evaluated the potential bias risk of each included RCT by using the bias-risk assessment tool of the Cochrane Center.
Statistical Analysis
The meta-analysis was performed using R statistical soft-ware (packages metafor and meta, R Foundation) (13). We used the R software to calculate the risk ratio and 95% confidence interval of each outcome index and to perform logarithmic, logit, anti-sinusoidal, and double anti-sinusoidal transformations on the analysis data to test the normal distribution of each transformation. For each set of data, we finally select the set of 4 transformed data that is closest to the normal distribution for meta-analysis. If there was significant heterogeneity (I2> 50%), the random effect model was selected, otherwise, the fixed-effect model was used. For subgroup analysis, we sub-grouped the patients into N1–I3 subgroup (nivolumab 1 mg/kg + ipilimumab 3 mg/kg) and N3–I1 subgroup (nivolumab 3 mg/kg + ipilimumab 1 mg/kg), according to the different initial administration schemes. Finally, we used the Graphpad (version 9.2) software to draw the classification summary of results and used Egger’s test to evaluate publication bias. The significance level of the bilateral test was set at p < 0.05.
Results
Eligible Studies and Characteristics
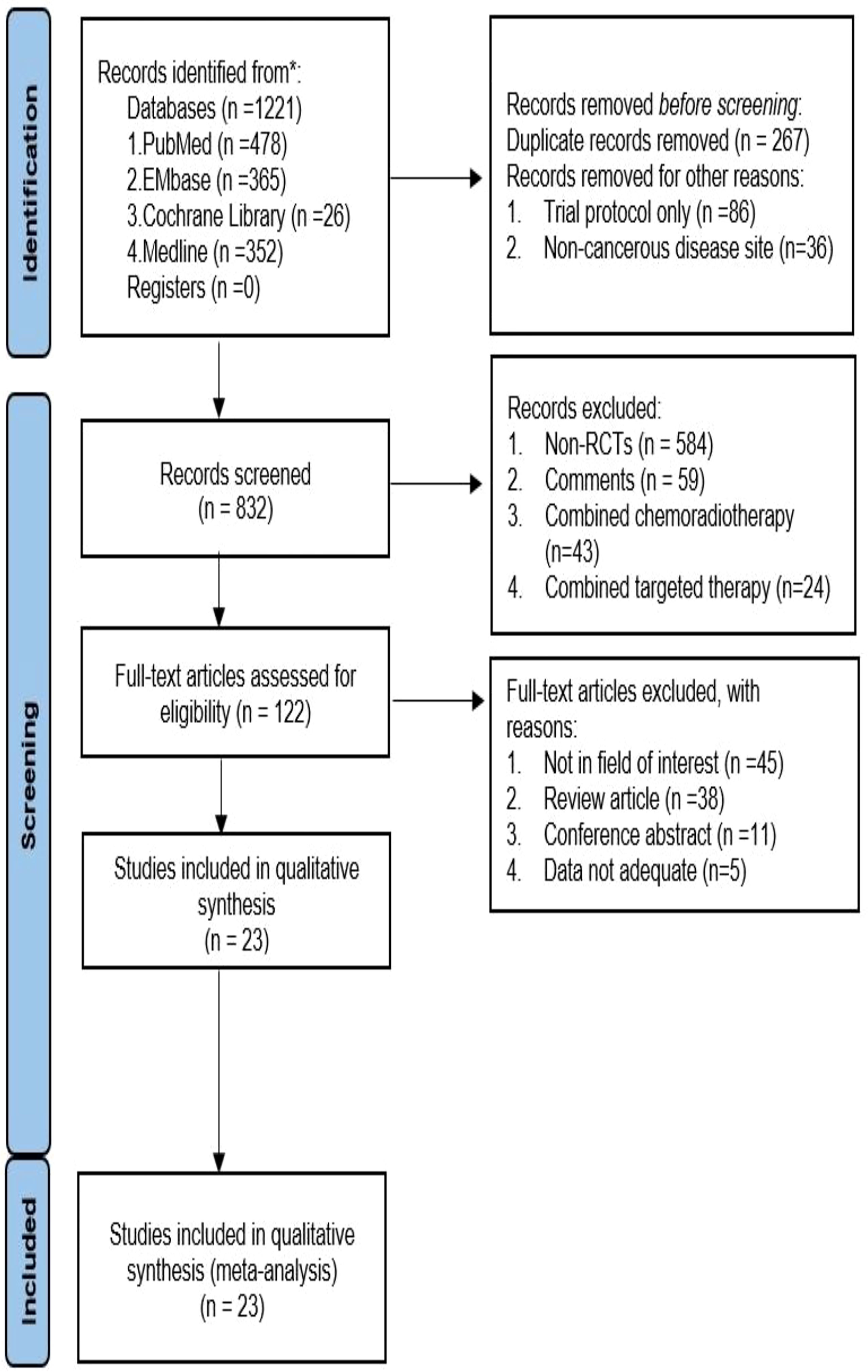
We initially retrieved 1221 studies following the set retrieval strategy. Duplicate records were subsequently eliminated, leaving 832 studies after excluding trial protocol and non-cancerous disease site. Another 710 studies were excluded after reading the title and abstract, Including 584 non-randomized controlled studies(Non-RCTs), 59 were Comments, 43 were Combined chemoradiotherapy, 24 were Combined targeted therapy. Full-text reading of the 122 studies led to the elimination of 99 articles. Amongst the 99 studies, 45 were not in the field of interest, 38 were review articles, 11 were conference abstracts, and 5 had insufficient data. The remaining 23 studies were included for meta-analysis (14–36), which included 32 single arms (see Figure 1 and Table 1) and a total of 3970 patients with malignant tumors. The tumor types included malignant melanoma, non-small cell lung cancer, advanced renal cell carcinoma, malignant pleural mesothelioma, malignant sarcoma, esophageal gastric junction cancer, colorectal cancer, malignant glioma, and urothelial, ovarian, and hepatocellular carcinomas. According to the number of reports, we analyzed 19 common adverse events, namely diet, pruritus, diarrhea, rash, nausea, hyperthyroidism, hyperthyroidism, discredited appetite, pyrexia, headache, maculopapular rash, pneumonitis, adrenal insufficiency, colitis, vomiting, and increased aspartate aminotransferase (AST), increased alanine aminotransferase (ALT), increased amylase, and increased lipase.
Incidence of any adverse events and risk ratio of grade 3 or higher adverse events
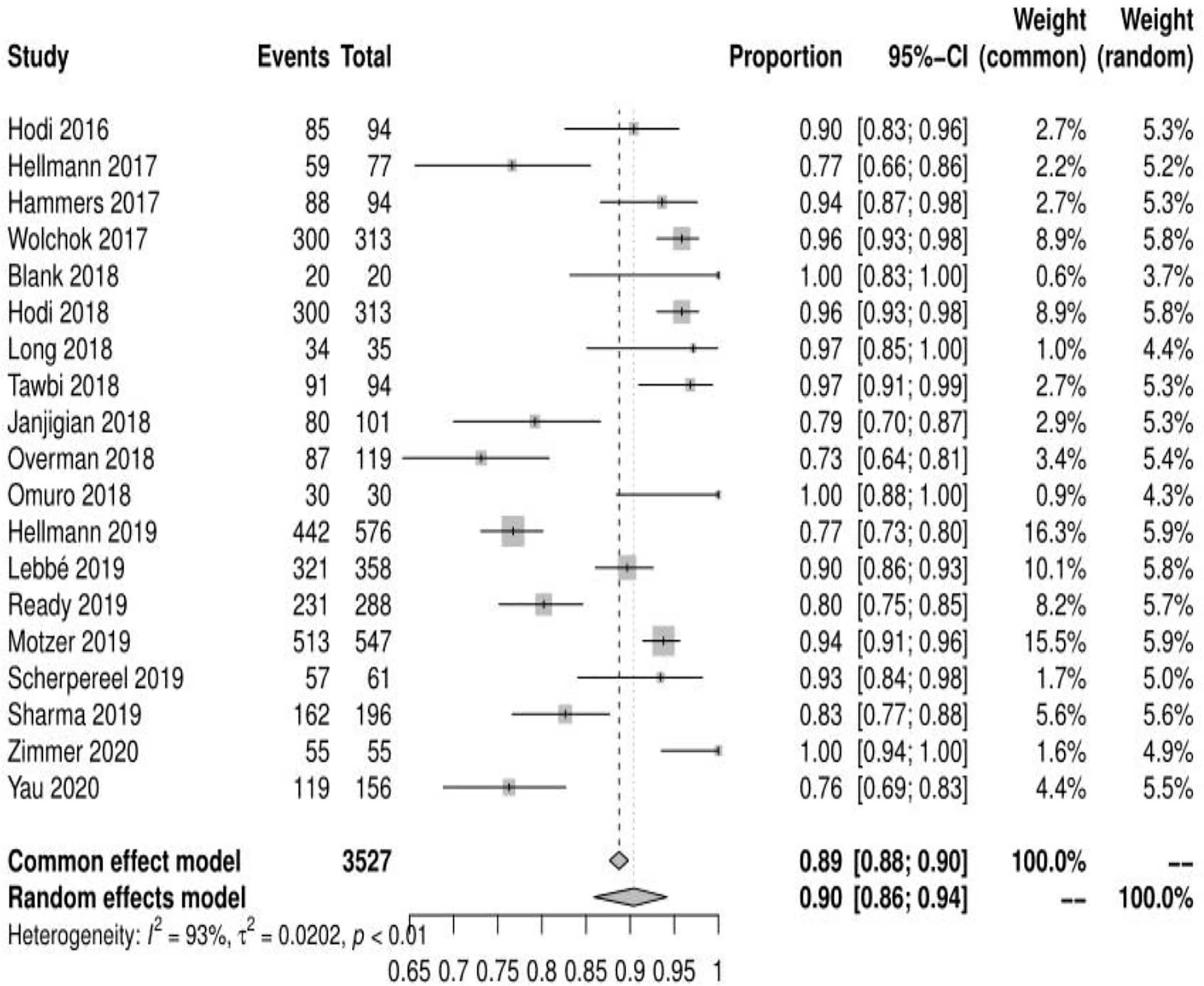
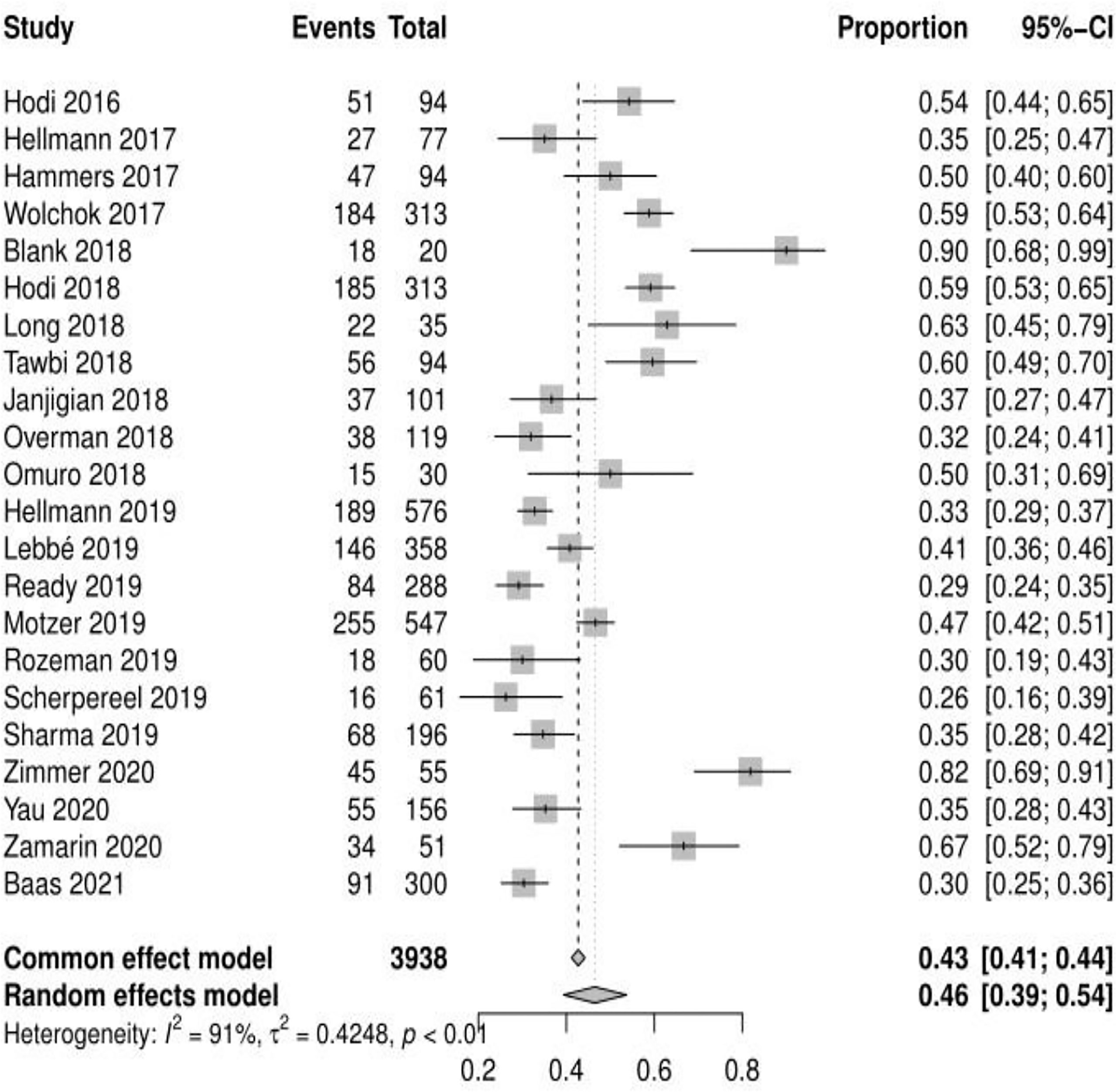
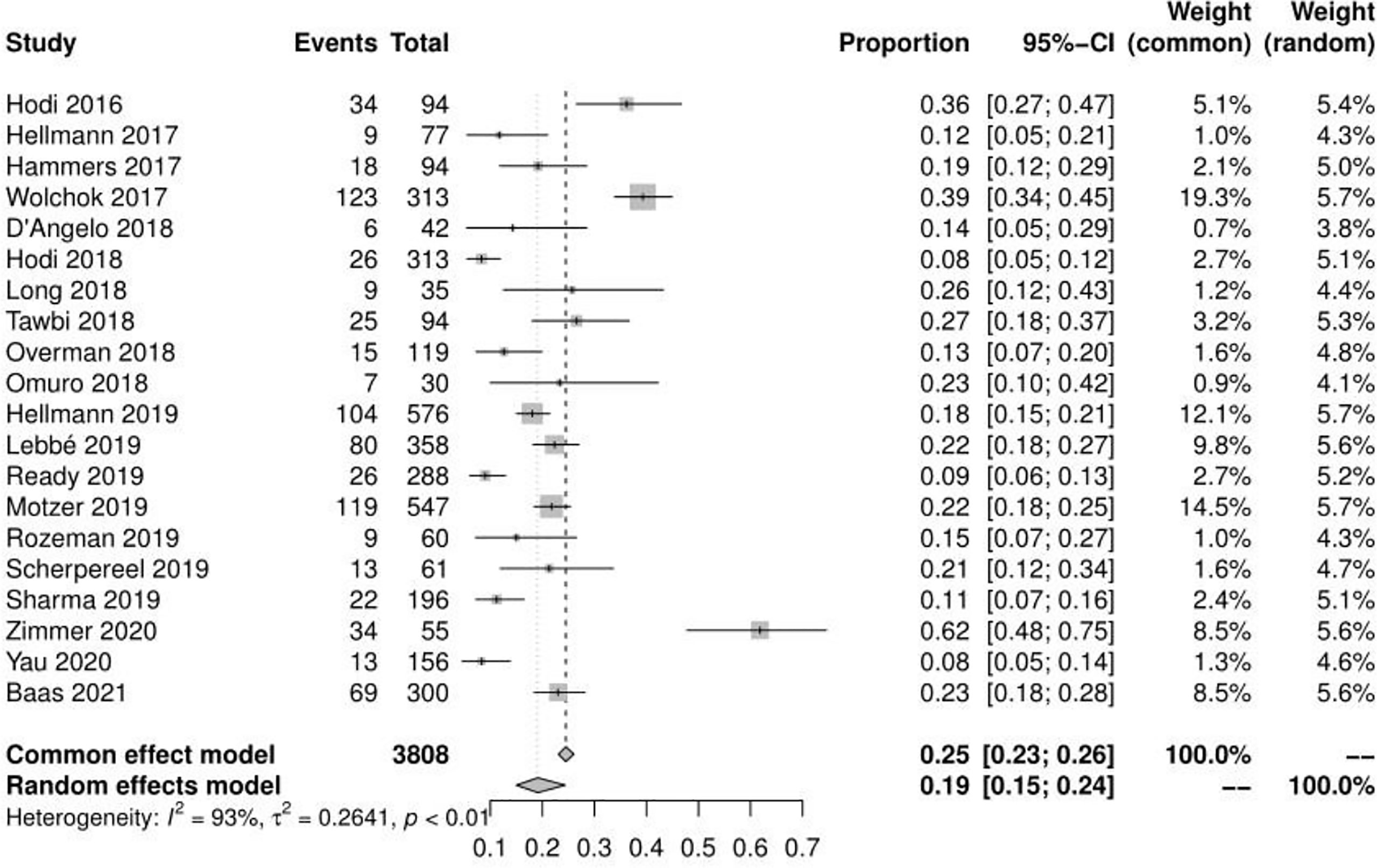
Of the 23 studies analyzed, 19 reported adverse events of any grade, the mean incidence of any adverse events was 90.42% (95% CI, 85.91% ~ 94.18%, I2 = 93%) (see Figure 2). 22 reported adverse events of grade ≥ 3, and the mean incidence of grade 3 or higher adverse events was 46.46% (95% CI, 39.37% ~ 53.69%, I2 = 91%) (see Figure 3). Subgroup analysis revealed that the mean incidence of any adverse event and that of a grade ≥ 3 adverse event were 94.53% (95% CI, 91.18% ~ 97.21%, I2 = 71%) and 55.29% (95% CI, 46.73% ~ 63.86%, I2 = 85%) in the N1–I3 subgroup (see Supplementary Material Figure S1, S2), respectively, and 84.91% (95% CI, 80.02% ~ 90.10%, I2 = 90%) and 36.72% (95% CI, 30.51% ~ 43.39%, I2 = 81%) in the N3–I1 subgroup (see Supplementary Material Figure S3, S4).
Incidence of Treatment-Related Deaths and Treatment−Related Adverse Event Leading to Discontinuation
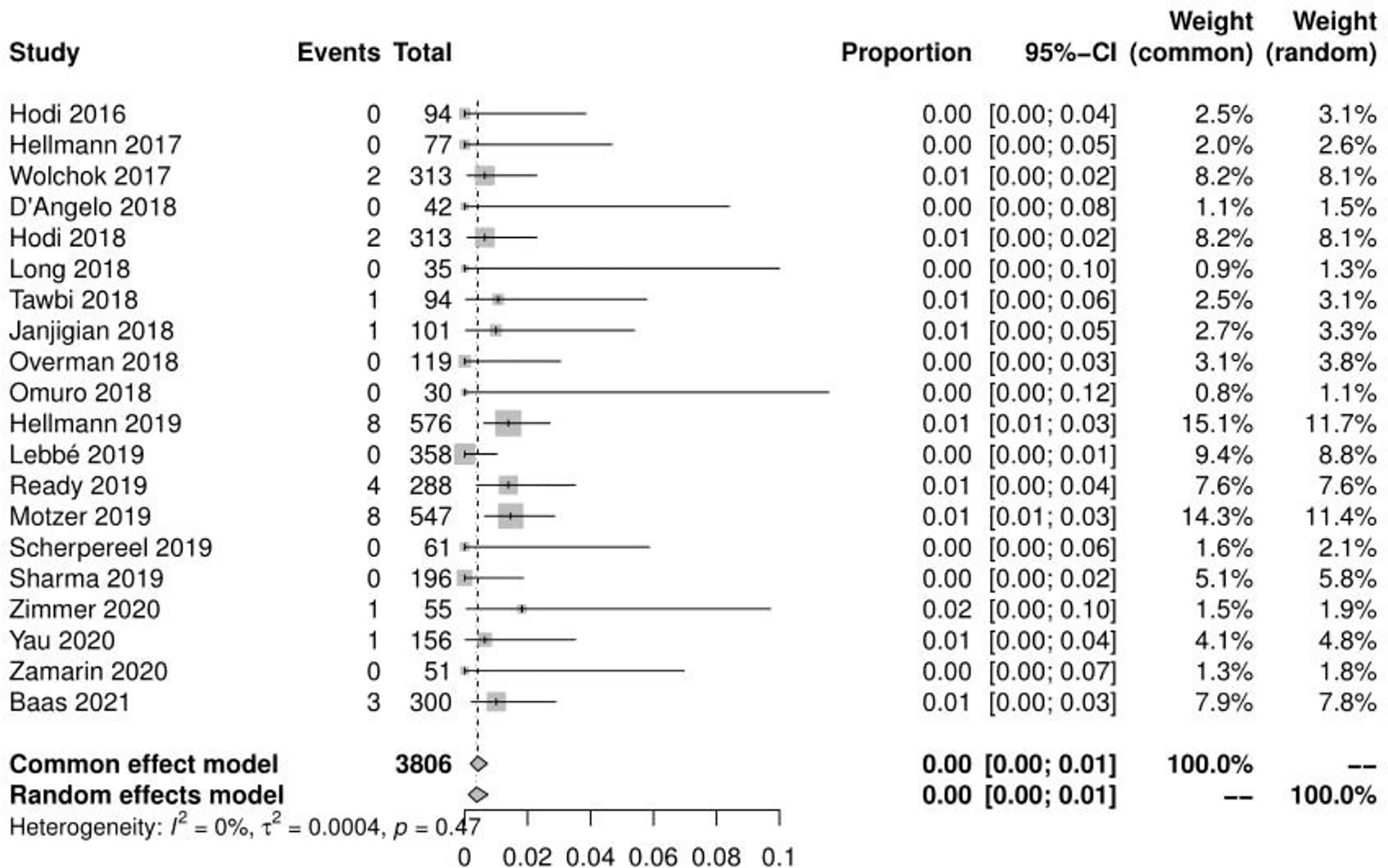
Of the 23 studies, 20 reported a total of 31 treatment-related deaths, with a mean incidence of 0.42%(95% CI, 0.18% ~ 0.72%, I2 = 0%) (see Figure 4). 20 reported the number of adverse events leading to discontinuation, with a mean incidence of 19.11% (95% CI, 14.99% ~ 24.38%, I2 = 93%) (see Figure 5). The mean incidence of treatment-related death and that of a treatment-related adverse event leading to discontinuation were 0.06% (95% CI, 0.00% ~ 0.44%, I2 = 0%) and 27.51% (95% CI, 21.45% ~ 35.29%, I2 = 83%) in the N1–I3 subgroup(see Supplementary Material Figure S5-S6), respectively, and 0.43% (95% CI, 0.14% ~ 0.83%, I2 = 0%) and 14.65% (95% CI, 11.54% ~ 18.04%, I2 = 75%) in the N3–I1 subgroup(see Supplementary Material Figure S7-S8).
Risk Ratio of Grade 1 and 2 Adverse Events
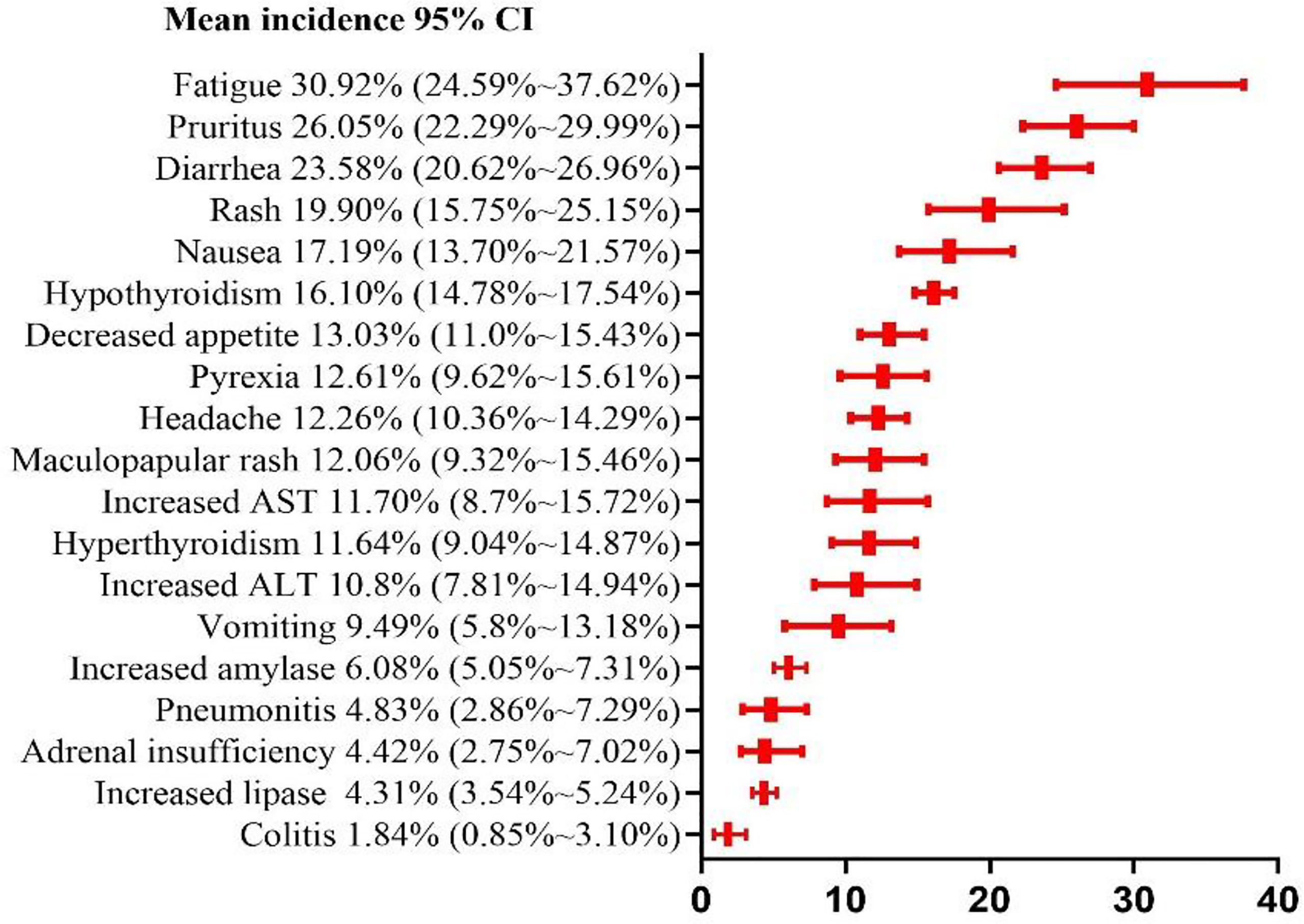
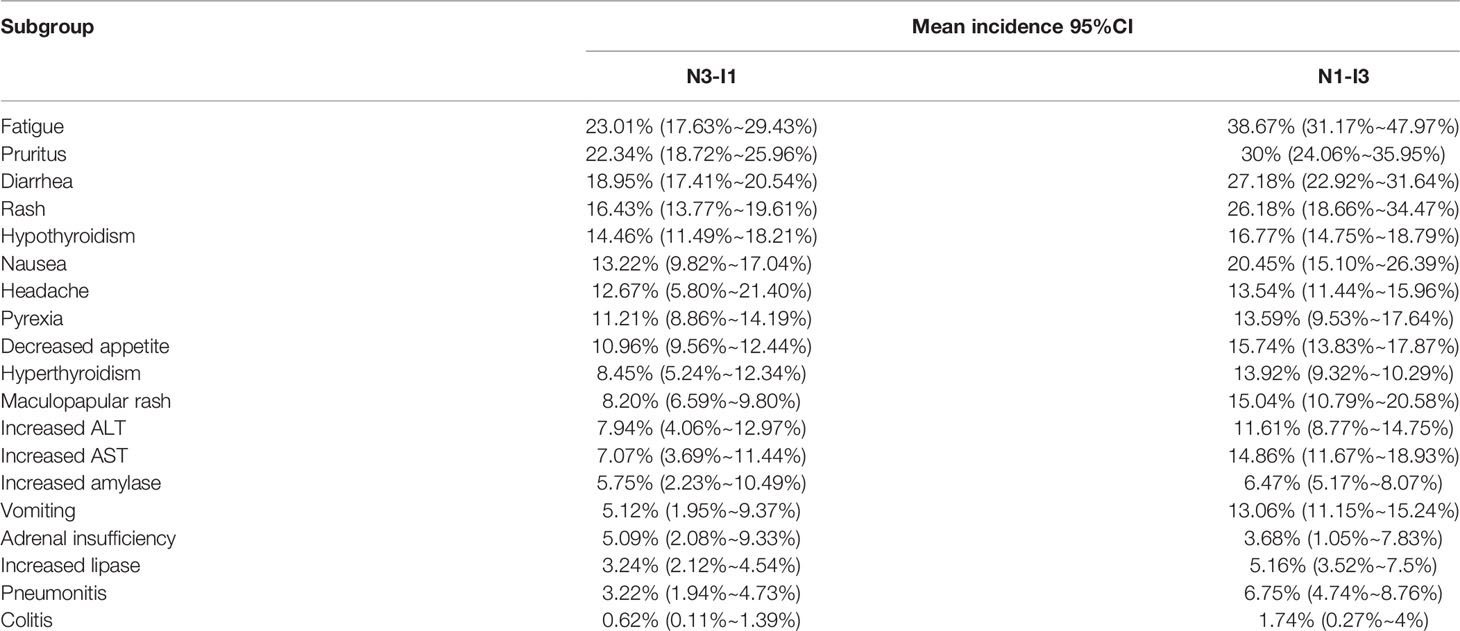
Among the 19 common adverse events analyzed, the risk of grade 1–2 adverse events was > 10%. The top 5 risks were fatigue(30.92%, 95% CI: 24.59% ~ 37.62%, I2 = 93%), pruritus (26.05%, 95% CI: 22.29% ~ 29.99%, I2 = 82%), diarrhea(23.58%, 95% CI: 20.62% ~ 26.96%, I2 = 88%), rash(19.90%, 95% CI: 15.75% ~ 25.15%, I2 = 88%), nausea (17.19%, 95% CI: 13.7% ~ 21.57%, I2 = 86%), the risks of other common adverse events are presented in Figure 6. Fatigue, pruritus, diarrhea, and rash were also among the top 5 risks in the N1–I3 and N3–I1 subgroups, which additionally included nausea and hypothyroidism, respectively. The risks of other common adverse events in each subgroup are presented in Table 2.
Risk Ratio of Grade 3 or Higher Adverse Events
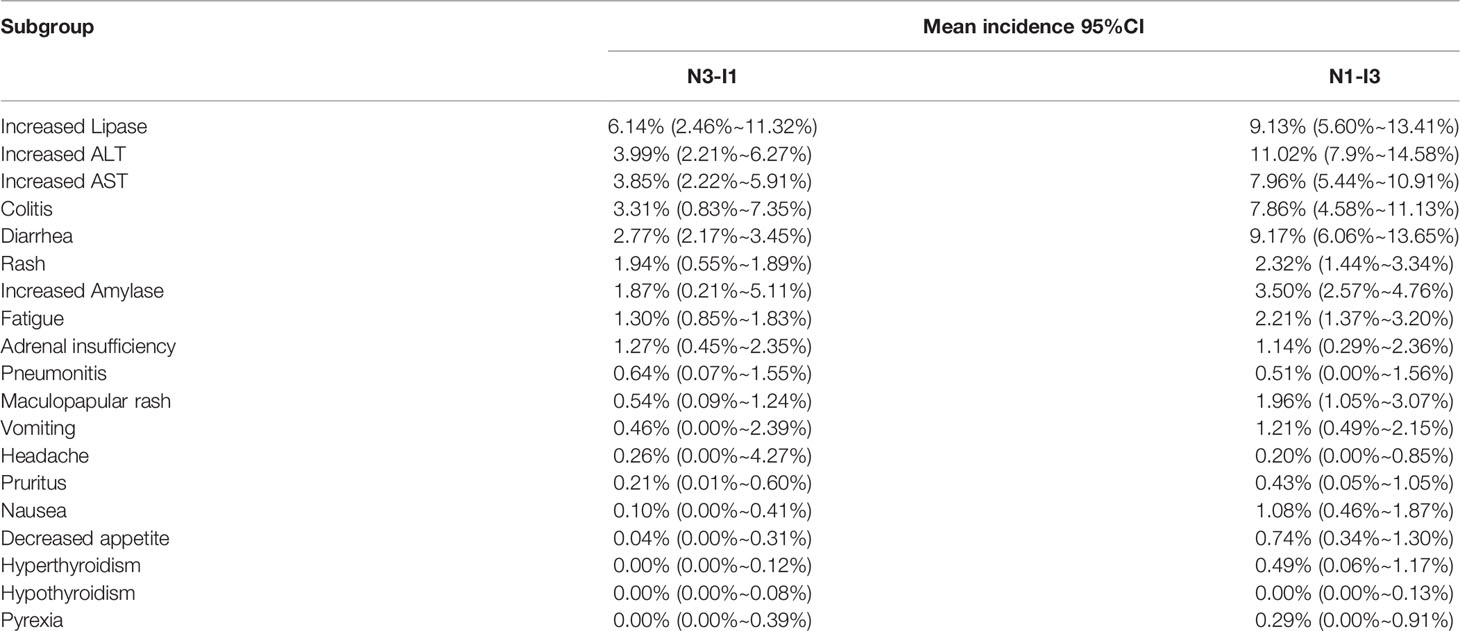
Among the 19 common adverse events, the risk of a grade ≥ 3 adverse event was > 5%. The top 5 risks were increased ALT (8.12%, 95% CI: 5.90% ~ 10.65%, I2 = 73%), increased lipase (7.62%, 95% CI: 4.88% ~ 10.89%, I2 = 77%), colitis (6.39%, 95% CI: 3.98% ~ 10.25%, I2 = 80%), increased AST (6.30%, 95% CI: 4.61% ~ 8.22%, I2 = 68%), diarrhea (5.72%, 95% CI: 3.50% ~ 8.44%, I2 = 83%), the risks of other common adverse events are presented in Figure 7. Sub-group analysis yielded the same adverse events as the top 5 risks in both N1–I3 and N3–I1 subgroups. The risks of other common adverse events are presented in Figure 7 and Table 3.
Subgroup Analysis of the Incidence of Adverse Events Based on Cancer Type
Based on the risk of any adverse events (Supplementary Material Figure S9), melanoma had the highest risk (95.87%, 95% CI: 92.93% ~ 98.12%, I2 = 72.4%), while colorectal cancer had the lowest risk (73.11%, 95% CI: 64.75% ~ 80.73%, I2 = 0%). Similarly, based on the risk of grade 3 and higher adverse events (Supplementary Material Figure S10), melanoma had the highest risk (58.13%, 95% CI: 47.67% ~ 70.88%, I2 = 92.3%), while pleural mesothelioma had the lowest risk (26.23%, 95% CI: 17.22% ~ 39.95%, I2 = 0%). Melanoma also had the highest risk (25.83%, 95% CI: 16.79) % ~ 39.75%, I2 = 94.2%) based on risk of any adverse event leading to discontinuation (Supplementary Material Figure S11), while glioblastoma had the lowest risk (23.33%, 95% CI: 12.20% ~ 44.64%, I2 = 0%). In contrast, glioblastoma had the highest risk (1.64%, 95% CI: 0.10% ~ 25.62%, I2 = 0%) based on the risk of treatment-related deaths (Supplementary Material Figure S12), while urothelial carcinoma had the lowest risk (0.25%, 95% CI: 0.02% ~ 4.05%, I2 = 0%).
Discussion
ICIs are monoclonal antibodies against regulatory immune checkpoint factors that inhibit T cell activation. These antibodies promote immune-mediated tumor-cell clearance by enhancing T cell-mediated anti-tumor immunity. At present, their targets mainly include CTLA-4 and PD-1/PD-L1, CTLA-4 regulates the activation of T cells by preventing the generation of T cell inhibitory signals (37, 38). let’s first talk about the five common adverse events, and then describe whether the risk of grade ≥ 3 is > 5%, so as to promote the further proliferation of T cells, thereby achieving the anti-tumor effect of PD-1. PD-L1 inhibits the signal transduction by blocking the interaction between T cells and antigen-presenting cells, promotes the proliferation of activated T cells, and then kills tumor cells (39, 40). However, activation of the immune system also impairs the immune homeostasis in non-tumor tissues, resulting in a series of adverse reactions, mainly involving the skin, gastrointestinal tract, liver, lung, and endocrine glands (41, 42). In recent years, combinatorial immunotherapy has gradually become a research hotspot, and its antitumor effectiveness has been confirmed by multiple studies. However, combination of drugs seems to increase the risk of adverse events. The results of the meta-analyses by Yang et al. and Chen et al (43, 44). have shown that the antitumor effect of nivolumab and ipilimumab co-treatment was better than that of nivolumab or ipilimumab alone. The results of the meta-analysis by Xing et al. have shown that the risk of adverse events related to nivolumab and ipilimumab co-treatment was higher than that of the single-drug use (45). To the best of our knowledge, the study presented here is the largest and most comprehensive meta-analysis to evaluate the common adverse events of nivolumab and ipilimumab combination. The study by Xing et al. analyzed fewer studies than this study and did not include subgroup analysis of the initial medication regimen.
From the perspective of patient consultation, several results of this meta-analysis are crucial. Our results showed that approximately 9 of the 10 patients treated with nivolumab alongside ipilimumab had at least one adverse event, and 5 of the 10 patients had at least one grade ≥ 3 adverse event. Among them, fatigue was the most common mild adverse event (30.92%), and increased ALT level was the most common grade ≥ 3 adverse event (8.12%). Patients should also be informed that pruritus, diarrhea, and rash are also common adverse events but are remotely likely to manifest as serious complications. The fatality rate of any of these adverse events was very low (0.5%). The risk of drug withdrawal due to an adverse event was estimated at 42%. Approximately 1 of the 5 patients discontinued the treatment because of an adverse event.
In this meta-analysis, fast, headache, decreased appetite, and pyrexia were found to be subjective symptoms. Of the remaining adverse events, diarrhea, nausea, vomiting, colitis, and increased AST, ALT, amylase, and lipase levels are related to the digestive system; rash, pruritus, and maculopapular rash are skin-related; hyperthyroidism and adult insufficiency are endocrine-related; and pneumonitis is mainly related to the respiratory system. Regarding the grade 1–2 adverse events, 13 had risks of > 10%, and 15 had > 5%. Fatigue (30.92%) had the highest risk, which was higher than the risk of adverse events reported in PD-1 (18.7%) and PD-L1 (26%) meta-analysis studies by Wang et al (42). Grade 1–2 adverse events often do not have serious consequences for patients but increase patient discomfort and weaken the eagerness of the patient for the treatment. Some grade 1–2 adverse events often develop into grade ≥ 3 adverse events, such as colitis and pneumonitis, if not managed timely. We found that 5 grade ≥ 3 events had risks of > 5%, and 12 had > 1%. Among such events, increased ALT (8.12%) level was the most common. Increased ALT and AST levels are symptoms of hepatitis; increased lipase and amylase levels are symptoms of pancreatitis; hyperthyroidism and hyperthyroidism are symptoms of thyroiditis; diarrhea is a symptom of colitis. If autoimmune diseases are not identified early, they often cause severe health problems and can even be fatal. Pneumonia is the most common cause of treatment-related deaths in patients treated with immunosuppressants, and we estimated the incidence at 1.5% and 11%. In addition, our results show that the types of adverse events in the digestive system and their risks are significantly higher than those in other systems. Therefore, it is necessary to monitor the digestive system of the patients under treatment for such events to prevent development of severe problems in the digestive system.
In our study, we sub-grouped the patients according to their initial dosing regimen, namely N1–I3 and N3–I1 subgroups, and performed subgroup analysis. The N3–I1 subgroup had higher risks of adverse events of any grade, grade 1–2, and grade ≥ 3 (both with and without classification) than the N1–I3 subgroup, consistent with the results of the meta-analysis by Xu et al (46). The risk of adverse events of any grade was not classified, and nearly 10% (94.53% vs. 84.51%) of the N3–I1 subgroup had a higher risk than the N1–I3 subgroup, whereas nearly 20% (55.29% vs. 36.72%) of the N3–I1 subgroup had a higher risk of adverse events of grade ≥ 3 than the N1–I3 subgroup. Regarding the risk of classified grade 1 and grade 2 common adverse events, the most common risk in both N1–I3 and N3–I1 subgroups was fatigue (38.67% and 23.01%, respectively), whereas, regarding the classified grade ≥ 3 common adverse events, the most common risks in the N1–I3 and N3–I1 subgroups were increased ALT (11.02%) and lipase (6.14%) levels, respectively. Therefore, initial medication schemes have a certain impact on the occurrence of adverse events. When deciding on the medication scheme, treatment effectiveness and cost should also be considered in addition to treatment safety. We should be more cautious about the impact of different initial medication regimens of N3-I1 and N1-I3 on adverse events, because we did not consider the impact of treatment period and sequence.
Our preliminary analysis of the incidence of adverse events in different types of tumors further revealed that melanoma had a higher overall risk of adverse events. However, the differences between different cancer types were not significant. A meta-analysis of PD-1 and PD-L1 by Wang et al. revealed a similar average incidence of adverse events across various cancer types (42). However, this conclusion could not be fully explained in our study, possibly because of the small sample size of some tumor types. Some studies postulate that there are certain differences in the risk of adverse events for different types of tumors (47, 48). Based on the dose subgroup analysis for different cancer types, choosing the best drug regimen can prevent the occurrence of some adverse events to a certain extent. Taking timely intervention measures to the occurrence of common adverse events can further reduce the occurrence of serious adverse events and deaths.
Nonetheless, this study was limited by several factors, some with high heterogeneity (I2> 90%). We did not conduct subgroup analysis by cancer type for different types of adverse events. We also did not further analyze race, age, gender, and smoking history, amongst other demographic and clinical factors, which may have led to deviations in the analysis results. The length of treatment cycles may also have impacted the results despite performing subgroup analyses based on different initial doses. The different follow-up times for each study may have also biased the results.
In conclusion, this study estimated the risks of common adverse events in the co-treatment of malignant-tumor patients with ipilimumab and nivolumab and identified the impacts of different initial administration schemes on the risks of such events. Accordingly, this study provides an important reference for the toxicity of co-treatment with ipilimumab and nivolumab.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
XZ, FG, and ZL conceived the study, had full access to all the data in the study, and take responsibility for the integrity of the data and the accuracy of the data analysis. XZ and FG designed the search strategy and discussed with JY, HF, and QX, BG, QY, and KJ performed study selection, data extraction and synthesis. XZ and FG drafted and led on the writing of the manuscript. All the other authors participated in the analysis and interpretation of the data, revised the manuscript critically for important intellectual content and re-drafted some of its section. All the authors read and approved the final version of the manuscript, and agreed to be accountable for all aspects of the work to ensure its accuracy and integrity.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.877434/full#supplementary-material
Supplementary Figure 1 | Forest plot of any adverse events in N1-I3 subgroup.
Supplementary Figure 2 | Forest plot of grade 3 or higher adverse events in N1-I3 subgroup.
Supplementary Figure 3 | Forest plot of any adverse events in N3-I1 subgroup.
Supplementary Figure 4 | Forest plot of grade 3 or higher adverse events in N3-I1 subgroup.
Supplementary Figure 5 | Forest plot of treatment-related deaths in N1-I3 subgroup.
Supplementary Figure 6 | Forest plot of adverse events leading to discontinuation in N1-I3 subgroup.
Supplementary Figure 7 | Forest plot of treatment-related deaths in N3-I1 subgroup.
Supplementary Figure 8 | Forest plot of adverse events leading to discontinuation in N3-I1 subgroup.
Supplementary Figure 9 | Forest plot of any adverse events in different cancers.
Supplementary Figure 10 | Forest plot of grade 3 and higher adverse events in different cancers.
Supplementary Figure 11 | Forest plot of any adverse event leading to discontinuation in different cancers.
Supplementary Figure 12 | Forest plot of treatment-related deaths in different cancers.
References
1. Miller KD, Ortiz AP, Pinheiro PS, Bandi P, Minihan A, Fuchs HE, et al. Cancer Statistics for the US Hispanic/Latino Population, 2021. CA Cancer J Clin (2021) 71:466–87. doi: 10.3322/caac.21695
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin (2021) 71:7–33. doi: 10.3322/caac.21654
3. Hegde PS, Chen DS. Top 10 Challenges in Cancer Immunotherapy. Immunity (2020) 52:17–35. doi: 10.1016/j.immuni.2019.12.011
4. Nakajima EC, Lipson EJ, Brahmer JR. Challenge of Rechallenge: When to Resume Immunotherapy Following an Immune-Related Adverse Event. J Clin Oncol (2019) 37:2714–8. doi: 10.1200/JCO.19.01623
5. Owonikoko TK, Park K, Govindan R, Ready N, Reck M, Peters S, et al. Nivolumab and Ipilimumab as Maintenance Therapy in Extensive-Disease Small-Cell Lung Cancer: CheckMate 451. J Clin Oncol (2021) 39:1349–59. doi: 10.1200/JCO.20.02212
6. Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, et al. First-Line Nivolumab Plus Ipilimumab Combined With Two Cycles of Chemotherapy in Patients With Non-Small-Cell Lung Cancer (CheckMate 9LA): An International, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2021) 22:198–211. doi: 10.1016/S1470-2045(20)30641-0
7. Bhoori S, Mazzaferro V. Combined Immunotherapy and VEGF-Antagonist in Hepatocellular Carcinoma: A Step Forward. Lancet Oncol (2020) 21:740–1. doi: 10.1016/S1470-2045(20)30211-4
8. Mayor S. Combined Immunotherapy Improves Survival in Metastatic Melanoma. BMJ (2015) 350:h2993. doi: 10.1136/bmj.h2993
9. Di Federico A, Rizzo A, Ricci AD, Frega G, Palloni A, Tavolari S, et al. Nivolumab: An Investigational Agent for the Treatment of Biliary Tract Cancer. Expert Opin Investig Drugs (2021) 30:325–32. doi: 10.1080/13543784.2021.1863946
10. Baxi S, Yang A, Gennarelli RL, Khan N, Wang Z, Boyce L, et al. Immune-Related Adverse Events for Anti-PD-1 and Anti-PD-L1 Drugs: Systematic Review and Meta-Analysis. Bmj (2018) 360:k793. doi: 10.1136/bmj.k793
11. De Velasco G, Je Y, Bossé D, Ott PA, Moreira RB, et al. Comprehensive Meta-Analysis of Key Immune-Related Adverse Events From CTLA-4 and PD-1/PD-L1 Inhibitors in Cancer Patients. Cancer Immunol Res (2017) 5:312–8. doi: 10.1158/2326-6066.CIR-16-0237
12. Yan BY, Wasilewski G, Lacouture ME, Barker CA. Incidence of Dermatologic Adverse Events in Patients With Cancer Treated With Concurrent Immune Checkpoint Inhibitors and Radiation Therapy: A Systematic Review and Meta-Analysis. J Am Acad Dermatol (2021) 84:871–5. doi: 10.1016/j.jaad.2020.10.071
13. Shim S, Kim S-J. Intervention Meta-Analysis: Application and Practice Using R Software. Epidemiol Health (2019) 41:e2019008. doi: 10.4178/epih.e2019008
14. Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, et al. First-Line Nivolumab Plus Ipilimumab in Unresectable Malignant Pleural Mesothelioma (CheckMate 743): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet (2021) 397:375–86. doi: 10.1016/S0140-6736(20)32714-8
15. Blank CU, Rozeman EA, Fanchi LF, Sikorska K, van deWiel B, Kvistborg P, et al. Neoadjuvant Versus Adjuvant Ipilimumab Plus Nivolumab in Macroscopic Stage III Melanoma. Nat Med (2018) 24:1655–61. doi: 10.1038/s41591-018-0198-0
16. D'Angelo SP, Mahoney MR, Van Tine BA, Atkins J, Milhem MM, Jahagirdar BN, et al. Nivolumab With or Without Ipilimumab Treatment for Metastatic Sarcoma (Alliance A091401): Two Open-Label, Non-Comparative, Randomised, Phase 2 Trials. Lancet Oncol (2018) 19:416–26. doi: 10.1016/S1470-2045(18)30006-8
17. Hammers HJ, Plimack ER, Infante JR, Rini BI, McDermott DF, Lewis LD, et al. Safety and Efficacy of Nivolumab in Combination With Ipilimumab in Metastatic Renal Cell Carcinoma: The CheckMate 016 Study. J Clin Oncol (2017) 35:3851–8. doi: 10.1200/JCO.2016.72.1985
18. Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, et al. Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med (2019) 381:2020–31. doi: 10.1056/NEJMoa1910231
19. Hellmann MD, Rizvi NA, Goldman JW, Gettinger SN, Borghaei H, Brahmer JR, et al. Nivolumab Plus Ipilimumab as First-Line Treatment for Advanced non-Small-Cell Lung Cancer (CheckMate 012): Results of an Open-Label, Phase 1, Multicohort Study. Lancet Oncol (2017) 18:31–41. doi: 10.1016/S1470-2045(16)30624-6
20. Hodi FS, Chesney J, Pavlick AC, Robert C, Grossmann KF, McDermott DF, et al. Combined Nivolumab and Ipilimumab Versus Ipilimumab Alone in Patients With Advanced Melanoma: 2-Year Overall Survival Outcomes in a Multicentre, Randomised, Controlled, Phase 2 Trial. Lancet Oncol (2016) 17:1558–68. doi: 10.1016/S1470-2045(16)30366-7
21. Hodi FS, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Cowey CL, et al. Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab Alone in Advanced Melanoma (CheckMate 067): 4-Year Outcomes of a Multicentre, Randomised, Phase 3 Trial. Lancet Oncol (2018) 19:1480–92. doi: 10.1016/S1470-2045(18)30700-9
22. Janjigian YY, Bendell J, Calvo E, Kim JW, Ascierto PA, Sharma P, et al. CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer. J Clin Oncol (2018) 36:2836–44. doi: 10.1200/JCO.2017.76.6212
23. Lebbé C, Meyer N, Mortier L, Marquez-Rodas I, Robert C, Rutkowski P, et al. Evaluation of Two Dosing Regimens for Nivolumab in Combination With Ipilimumab in Patients With Advanced Melanoma: Results From the Phase IIIb/IV CheckMate 511 Trial. J Clin Oncol (2019) 37:867–75. doi: 10.1200/JCO.18.01998
24. Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, et al. Combination Nivolumab and Ipilimumab or Nivolumab Alone in Melanoma Brain Metastases: A Multicentre Randomised Phase 2 Study. Lancet Oncol (2018) 19:672–81. doi: 10.1016/S1470-2045(18)30139-6
25. Motzer RJ, Rini BI, McDermott DF, Arén Frontera O, Hammers HJ, Carducci MA, et al. Nivolumab Plus Ipilimumab Versus Sunitinib in First-Line Treatment for Advanced Renal Cell Carcinoma: Extended Follow-Up of Efficacy and Safety Results From a Randomised, Controlled, Phase 3 Trial. Lancet Oncol (2019) 20:1370–85. doi: 10.1016/S1470-2045(19)30413-9
26. Omuro A, Vlahovic G, Lim M, Sahebjam S, Baehring J, Cloughesy T, et al. Nivolumab With or Without Ipilimumab in Patients With Recurrent Glioblastoma: Results From Exploratory Phase I Cohorts of CheckMate 143. Neuro Oncol (2018) 20:674–86. doi: 10.1093/neuonc/nox208
27. Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M, et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J Clin Oncol (2018) 36:773–9. doi: 10.1200/JCO.2017.76.9901
28. Ready N, Hellmann MD, Awad MM, Otterson GA, Gutierrez M, Gainor JF, et al. First-Line Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer (CheckMate 568): Outcomes by Programmed Death Ligand 1 and Tumor Mutational Burden as Biomarkers. J Clin Oncol (2019) 37:992–1000. doi: 10.1200/JCO.18.01042
29. Rozeman EA, Menzies AM, van Akkooi ACJ, Adhikari C, Bierman C, van deWiel BA, et al. Identification of the Optimal Combination Dosing Schedule of Neoadjuvant Ipilimumab Plus Nivolumab in Macroscopic Stage III Melanoma (OpACIN-Neo): A Multicentre, Phase 2, Randomised, Controlled Trial. Lancet Oncol (2019) 20:948–60. doi: 10.1016/S1470-2045(19)30151-2
30. Scherpereel A, Mazieres J, Greillier L, Lantuejoul S, Dô P, Bylicki O, et al. Nivolumab or Nivolumab Plus Ipilimumab in Patients With Relapsed Malignant Pleural Mesothelioma (IFCT-1501 MAPS2): A Multicentre, Open-Label, Randomised, non-Comparative, Phase 2 Trial. Lancet Oncol (2019) 20:239–53. doi: 10.1016/S1470-2045(18)30765-4
31. Sharma P, Siefker-Radtke A, de Braud F, Calvo E, Bono P, et al. Nivolumab Alone and With Ipilimumab in Previously Treated Metastatic Urothelial Carcinoma: CheckMate 032 Nivolumab 1 Mg/Kg Plus Ipilimumab 3 Mg/Kg Expansion Cohort Results. J Clin Oncol (2019) 37:1608–16. doi: 10.1200/JCO.19.00538
32. Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi FS, Moschos SJ, et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N Engl J Med (2018) 379:722–30. doi: 10.1056/NEJMoa1805453
33. Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall Survival With Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med (2017) 377:1345–56. doi: 10.1056/NEJMoa1709684
34. Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol (2020) 6:e204564. doi: 10.1001/jamaoncol.2020.4564
35. Zamarin D, Burger RA, Sill MW, Powell DJ Jr, Lankes HA, Feldman MD, et al. Randomized Phase II Trial of Nivolumab Versus Nivolumab and Ipilimumab for Recurrent or Persistent Ovarian Cancer: An NRG Oncology Study. J Clin Oncol (2020) 38:1814–23. doi: 10.1200/JCO.19.02059
36. Zimmer L, Livingstone E, Hassel JC, Fluck M, Eigentler T, Loquai C, et al. Adjuvant Nivolumab Plus Ipilimumab or Nivolumab Monotherapy Versus Placebo in Patients With Resected Stage IV Melanoma With No Evidence of Disease (IMMUNED): A Randomised, Double-Blind, Placebo-Controlled, Phase 2 Trial. Lancet (2020) 395:1558–68. doi: 10.1016/S0140-6736(20)30417-7
37. Barrios DM, Do MH, Phillips GS, Postow MA, Akaike T, Nghiem P, et al. Immune Checkpoint Inhibitors to Treat Cutaneous Malignancies. J Am Acad Dermatol (2020) 83:1239–53. doi: 10.1016/j.jaad.2020.03.131
38. de Miguel M, Calvo E. Clinical Challenges of Immune Checkpoint Inhibitors. Cancer Cell (2020) 38:326–33. doi: 10.1016/j.ccell.2020.07.004
39. Dall'Olio FG, Marabelle A, Caramella C, Garcia C, Aldea M, Chaput N, et al. Tumour Burden and Efficacy of Immune-Checkpoint Inhibitors. Nat Rev Clin Oncol (2021) 19:75–90. doi: 10.1038/s41571-021-00564-3
40. Doroshow DB, Bhalla S, Beasley MB, Sholl LM, Kerr KM, Gnjatic S, et al. PD-L1 as a Biomarker of Response to Immune-Checkpoint Inhibitors. Nat Rev Clin Oncol (2021) 18:345–62. doi: 10.1038/s41571-021-00473-5
41. Nadelmann ER, Yeh JE, Chen ST. Management of Cutaneous Immune-Related Adverse Events in Patients With Cancer Treated With Immune Checkpoint Inhibitors: A Systematic Review. JAMA Oncol (2021) 8:130–8. doi: 10.1001/jamaoncol.2021.4318
42. Wang Y, Zhou S, Yang F, Qi X, Wang X, Guan X, et al. Treatment-Related Adverse Events of PD-1 and PD-L1 Inhibitors in Clinical Trials: A Systematic Review and Meta-Analysis. JAMA Oncol (2019) 5:1008–19. doi: 10.1001/jamaoncol.2019.0393
43. Chen J, Li S, Yao Q, Du N, Fu X, Lou Y, et al. The Efficacy and Safety of Combined Immune Checkpoint Inhibitors (Nivolumab Plus Ipilimumab): A Systematic Review and Meta-Analysis. World J Surg Oncol (2020) 18:150. doi: 10.1186/s12957-020-01933-5
44. Yang Y, Jin G, Pang Y, Huang Y, Wang W, Zhang H, et al. Comparative Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Advanced Cancer: A Systematic Review and Meta-Analysis. Front Pharmacol (2020) 11:40. doi: 10.3389/fphar.2020.00040
45. Xing P, Zhang F, Wang G, Xu Y, Li C, Wang S, et al. Incidence Rates of Immune-Related Adverse Events and Their Correlation With Response in Advanced Solid Tumours Treated With NIVO or NIVO+IPI: A Systematic Review and Meta-Analysis. J Immunother Cancer (2019) 7:341. doi: 10.1186/s40425-019-0779-6
46. Xu H, Tan P, Ai J, Zhang S, Zheng X, Liao X, et al. Antitumor Activity and Treatment-Related Toxicity Associated With Nivolumab Plus Ipilimumab in Advanced Malignancies: A Systematic Review and Meta-Analysis. Front Pharmacol (2019) 10:1300. doi: 10.3389/fphar.2019.01300
47. Wang F, Yang S, Palmer N, Fox K, Kohane IS, Liao KP, et al. Real-World Data Analyses Unveiled the Immune-Related Adverse Effects of Immune Checkpoint Inhibitors Across Cancer Types. NPJ Precis Oncol (2021) 5(1):82. doi: 10.1038/s41698-021-00223-x
Keywords: immune checkpoint inhibitors (ICI), ipilimumab, adverse events, nivolumab, meta-analysis
Citation: Zhao X, Gao F, Yang J, Fan H, Xie Q, Jiang K, Gong J, Gao B, Yang Q and Lei Z (2022) Risk of Adverse Events in Cancer Patients Receiving Nivolumab With Ipilimumab: A Meta-Analysis. Front. Oncol. 12:877434. doi: 10.3389/fonc.2022.877434
Received: 16 February 2022; Accepted: 25 May 2022;
Published: 23 June 2022.
Edited by:
Xian Zeng, Fudan University, ChinaReviewed by:
Alessandro Morabito, G. Pascale National Cancer Institute Foundation (IRCCS), ItalyXi Lou, University of Alabama at Birmingham, United States
Zeguo Sun, Icahn School of Medicine at Mount Sinai, United States
Copyright © 2022 Zhao, Gao, Yang, Fan, Xie, Jiang, Gong, Gao, Yang and Lei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zehua Lei, bGVpdHNlaHVhQDEyNi5jb20=
 Xin Zhao
Xin Zhao Fengwei Gao1
Fengwei Gao1 Qian Yang
Qian Yang