- 1Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2China National Clinical Research Center for Neurological Diseases, Beijing, China
- 3Cure & Sure Biotech Co., LTD, Shenzhen, China
Surgical resection remains a first-line therapy for glioblastoma multiforme (GBM). Increased extent of resection (EOR) of noncontrast-enhancing regions in T2-weighted MRI images (T2-EOR) provides a survival benefit for GBM patients receiving standard radio/chemotherapy. However, whether it also improves immunotherapeutic outcomes remains unclear. We calculated the T2-EOR by comparing the preoperative and postoperative MRI T2 hyperintensity outside the enhancing tumour and correlated the T2-EOR with immunological and clinical outcomes from our published early-phase trial of heat shock protein peptide complex-96 (HSPPC-96) vaccination in treating a cohort of 19 patients with newly diagnosed GBMs (NCT02122822). Patients with higher T2-EOR exhibited shorter progression-free survival (PFS) (HR 11.29, p=0.002) and overall survival (OS) (HR 6.5, p=0.003) times than patients with lower T2-EOR. T2-EOR was negatively correlated with the levels of tumour specific immune response (TSIR) post-vaccination (R=-0.725, p<0.001) and absolute TSIR increase from pre- to post-vaccination (R=-0.679, p=0.001). Multivariate Cox regression models revealed that higher T2-EOR represented an independent risk factor for PFS (HR 19.85, p=0.0068) and OS (HR 21.24, p=0.0185) in this patient cohort. Taken together, increased T2-EOR deteriorated immunotherapeutic outcomes by suppressing TSIR, suggesting the potential of T2-EOR as an early biomarker for predicting the immunotherapeutic efficacy of HSPPC-96 vaccination.
Introduction
Glioblastoma multiforme (GBM), the most common and deadliest primary malignant brain tumour in adults, presents a great challenge for neurooncologists and neurosurgeons, as the outcomes of GBM patients have not greatly improved over the past two decades (1). The median survival for GBM is still limited to 15-20 months after standard-of-care treatment, which includes surgical resection, radiotherapy, temozolomide-based chemotherapy (2) and the recently developed tumour treating fields therapy (3). Among these therapies, surgical resection remains a primary treatment option for GBM. The extent of resection (EOR) greatly impacts clinical outcomes, with gross total resection of contrast-enhancing regions in GBMs significantly extending progression-free survival (PFS) and overall survival (OS) times (4, 5) compared with subtotal resection. Meanwhile, the rapid progress in novel intraoperative assistive technologies (6–8) has facilitated the transition from gross total resection to supratotal resection (SpTR), which expanded the EOR into noncontrast-enhancing regions outside the contrast-enhancing regions (9, 10). The noncontrast-enhancing region usually contains a small number of tumour cells, rendering it a region susceptible to tumour recurrence (11, 12). Therefore, recent clinical findings have supported the view that SpTR improves PFS and OS in a fraction of GBM patients receiving standard-of-care radiotherapy and chemotherapy (9, 13).
The last decade has witnessed unprecedented progress in cancer immunotherapies, which have already been approved as primary treatment options for a variety of malignancies (14–16). In glioma, immunotherapies, including immune checkpoint blockade, dendritic cell vaccines, peptide vaccines as well as chimeric antigens receptor-T cell therapy, have also generated encouraging early-clinical results in a fraction of patients, thus bringing hope for GBM patients (17–22). Heat shock protein peptide complex-96 (HSPPC-96) is a tumor-derived protein complex that comprises heat shock protein glycoprotein 96 kDa (gp96) and its binding tumor antigen derived peptides (23, 24). HSPPC-96 can be taken up directly by antigen-presenting cells (dendritic cells) and then trigger specific antitumor responses (25, 26), rendering it available as a cancer-treatment vaccine (27–29). Several studies have demonstrated its preliminary effectiveness in treating recurrent (17) and newly diagnosed GBMs (19). We previously conducted a single-arm, phase I clinical study that enrolled a total of 20 patients to evaluate its safety and efficacy in treating newly-diagnosed GBMs (NCT02122822) (19). We observed a median PFS of 11.0 months and a median OS of 31.4 months in the 19 patients with complete follow-up information. Meanwhile, we uncovered that the level of tumour-specific immune response (TSIR) post-vaccination were closely associated with the survival time (19). The median OS time for patients with high TSIR post-vaccination was >40.5 months as compared with 14.6 months for patients with low TSIR post-vaccination (Hazard ratio, 0.25; 95% CI, 0.071–0.90; P = 0.034) (19). We further revealed the presence of specific intratumoral T cell receptor clones were capable of predicting a durable response to the immunotherapy (30). However, whether SpTR improves outcomes in immunotherapies such as HSPPC-96 vaccination is still unclear.
In this study, we reviewed the clinical and immunological data, as well as the preoperative and postoperative (48-72 hours after surgery) MRI images, of patients in the HSPPC-96 vaccinated cohort (19). We calculated the EOR of noncontrast-enhancing regions in T2-weighted MRI images (T2-EOR) by comparing preoperative and postoperative T2-weighted MRI images and then correlated the T2-EOR with the TSIR levels as well as clinical outcomes to explore the impact of SpTR on the effectiveness of GBM immunotherapy.
Materials and Methods
Patients
We previously conducted a single-arm phase I clinical trial to determine the safety and efficacy of HSPPC-96 vaccination, combined with standard adjuvant radiotherapy and chemotherapy, in treating newly diagnosed GBM patients (19). A total of 20 patients with histologically confirmed GBMs were enrolled and received a total of six doses of the vaccine. The start of vaccination was concurrent with the beginning of adjuvant temozolomide chemotherapy. Vaccines were administered via subcutaneous injection in 25-μg doses every week for 6 weeks. Cyclophosphamide (400 mg) was given through intravenous injection before each HSPPC-96 vaccination (19). The primary endpoint of this study was PFS at 6 months and the frequency of adverse events. The secondary endpoints included OS and PFS at the end of the study and TSIR during the vaccinations. The clinical trial was approved by the ethics committee of Beijing Tiantan Hospital (JS2012-001-03) and was registered at ClinicalTrials.gov (NCT02122822) and http://www.chictr.org.cn/enindex.aspx (ChiCTR-ONC-13003309). Written informed consent was obtained from all participants. All the included patients underwent gross total resection of contrast-enhancing regions in the tumour and had a Karnofsky performance status (KPS) scale (range 0-100%) score of ≥ 70% before vaccination (19). Finally, 19 patients with available follow-up information for clinical efficacy analysis were included in the current study.
Enzyme-Linked Immunospot Assay
TSIR was evaluated by the number of stimulated peripheral blood mononuclear cells (PBMCs) in response to autologous tumour lysate in an IFN-γ release ELISPOT assay, as previously reported (19). In the assay, PMA (Millipore Sigma) was included as a positive control, and wells without added tumour lysates or PMA were used as negative controls. IL-2 (Millipore Sigma) was also added to each well (50 IU/ml). A total of 3 × 105 PBMCs were added to each well. Spots were counted using an ELISPOT reader (Cellular Technology Ltd.). The number of PBMCs responding to tumour lysate, as measured by IFN-γ release, was calculated by subtracting the background number of IFN-γ spots in the negative control well.
Imaging Studies
Preoperative MRI images were obtained within 14 days before surgery, and postoperative MRI images were obtained within 3 days after surgery. The noncontrast-enhancing regions were represented as T2-hyperintense areas outside the contrast-enhancing lesions in T1-weighted images, termed T2-nonenhancing regions. After the T2-nonenhancing regions were manually outlined, the volume was automatically calculated with Neusoft PACS (version 5.5, Neusoft, China) software, which has been shown to be reproducible and accurate for tumour volume estimation in previous studies (9, 31).
Statistical Analysis
Categorical data were compared using Fisher’s exact test. Continuous data were compared using analysis of variance (ANOVA) or Wilcoxon nonparametric tests. Pearson correlation tests were applied to determine the association between T2-EOR and TSIR indicators. PFS and OS were estimated by the Kaplan–Meier method, and the difference between groups was assessed by the log-rank test. Multivariate Cox regression models were fitted to identify independent risk factors for survival. Statistical significance was set as a 2-tailed p value of less than 0.05. All statistics were analysed using R (version 4.1.2) statistical software.
Result
Baseline Characteristics and T2-EOR Evaluation
Recent clinical studies have shown the benefits of SpTR of noncontrast-enhancing regions, not just gross total resection of enhancing lesions, for survival outcomes in GBM patients (9, 10, 13). The noncontrast-enhancing regions were represented as T2-hyperintense areas outside the contrast-enhancing lesions and were termed T2-nonenhancing regions (Figure 1). Since the survival benefit of SpTR was achieved in the context of patients receiving standard-of-care radiotherapy and chemotherapy after surgery, we then wondered whether SpTR could improve the immunotherapeutic outcomes. To answer this question, we reviewed the clinical and immunological data, as well as the preoperative and postoperative (48-72 hours after surgery) MRI images, of an early-phase trial that evaluated the safety and preliminary efficacy of HSPPC-96 vaccination in a cohort of 19 patients with newly diagnosed GBMs (19). The baseline epidemiological and clinical characteristics, including sex, age at diagnosis, KPS scale score (range 0-100%), MGMT promoter methylation status, IDH 1/2 mutations and TERT promoter mutations, were reported in our previous study (Supplementary Table 1) (19).
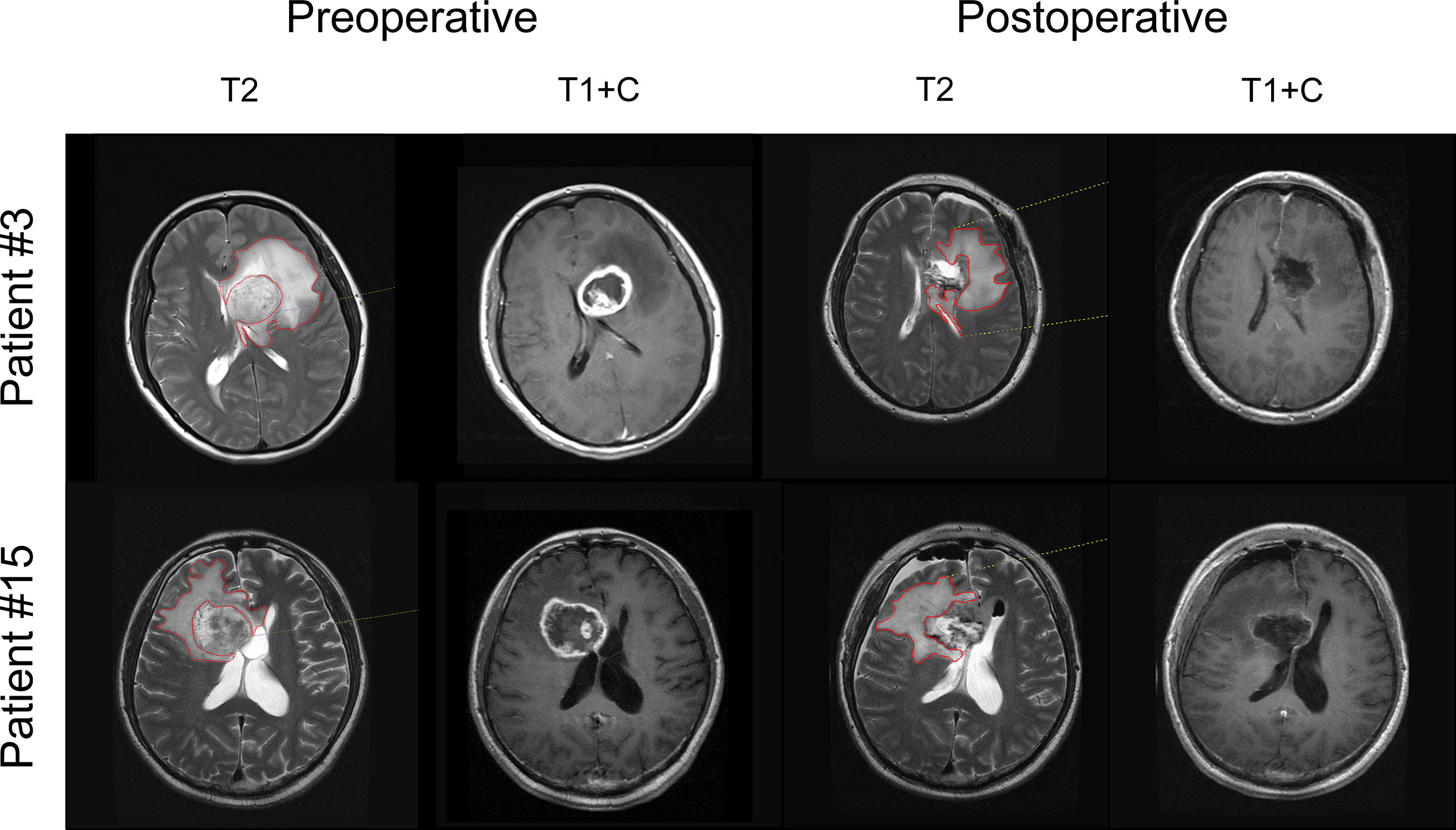
Figure 1 T2-EOR determination on preoperative and postoperative MRI images. The noncontrast-enhancing regions were represented as T2-weighted hyperintense areas outside the T1-weighted contrast-enhancing lesions and were termed T2-nonenhancing regions. T2-nonenhancing regions were manually outlined in red at each level, and the volume was automatically calculated by Neusoft PACS software. T2-EOR was defined as the preoperative-to-postoperative change in T2-nonenhancing region volume divided by the preoperative T2-nonenhancing region volume.
Gross total resection of contrast-enhancing tumour areas was achieved in all patients (Supplementary Table 1). However, the median preoperative and postoperative volumes of T2-nonenhancing region were 75.69 cm3 (range 40.57-153.25 cm3) and 45.11 cm3 (range 24.60-154.15 cm3), respectively, resulting in a 40.1% median T2-EOR (range -12.7%-67.8%), as shown in Supplementary Table 1. Based on the T2-EOR, we divided the 19 included patients into high (T2-EOR ≥ median) and low (T2-EOR < median) T2-EOR groups. We compared all the baseline features between the two groups but did not observe any significant differences (Supplementary Table 2).
Patients With Increased T2-EOR Exhibited Poorer Prognosis
The median PFS was 11.0 months, and the median OS was 31.4 months in this vaccinated cohort (30). Surprisingly, in contrast to the standard-of-care treatment, increased T2-EOR deteriorated the immunotherapeutic outcomes, as shown by significantly shortened PFS (hazard ratio (HR) 11.29, p=0.002) and OS (HR 6.5, p=0.003) times in the high T2-EOR group as compared with the low T2-EOR group (Figure 2). This finding indicates that T2-EOR is negatively correlated with patient survival time and reflects a possibly divergent effect of SpTR on the outcomes of GBM patients receiving distinct therapeutic modalities.
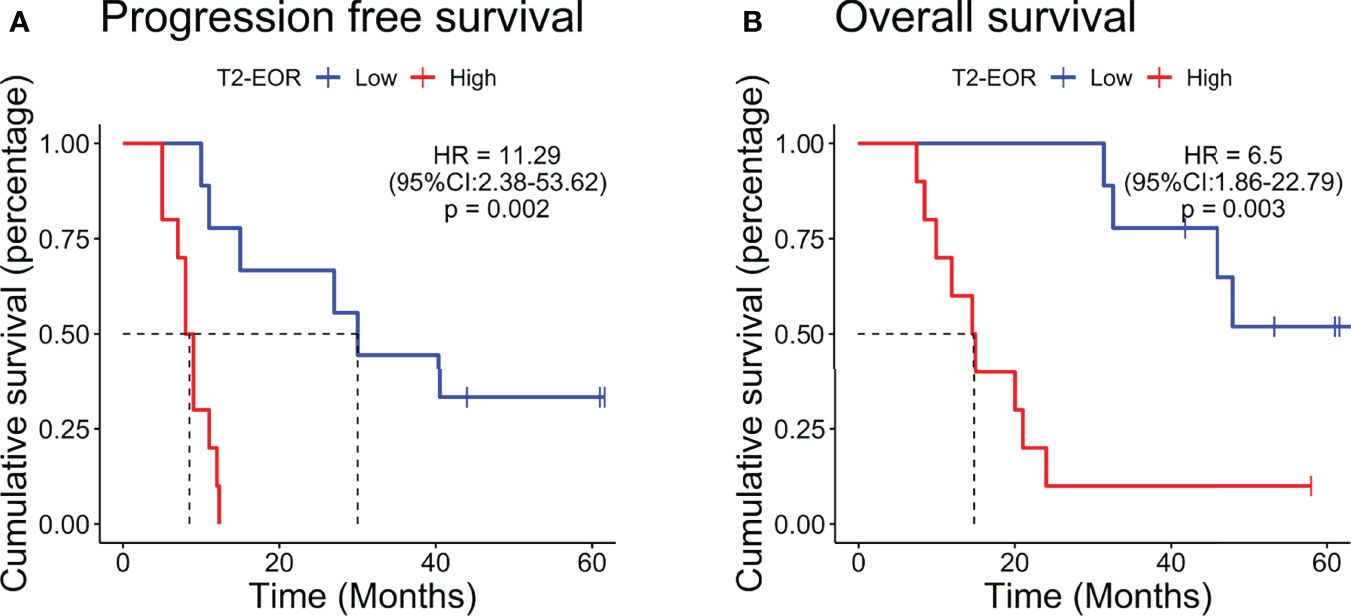
Figure 2 Patients with increased T2-EOR exhibited poorer prognosis. Kaplan–Meier estimates of (A) progression-free survival and (B) overall survival in 19 GBM patients, which were divided into the high (T2-EOR ≥ median) and low (T2-EOR < median) T2-EOR groups. The log-rank test was applied to estimate the difference. Vertical lines indicate time points at which patients were censored.
T2-EOR Was Negatively Correlated With TSIR Levels
We next explored the mechanism underlying the link between T2-EOR and therapeutic outcomes. We hypothesized that T2-EOR would be negatively associated with TSIR, measured by an interferon-γ release ELISPOT assay on PBMCs, which has been shown to be closely associated with survival time (19). To test this hypothesis, we correlated T2-EOR with the levels of four indexes regarding TSIR: TSIR pre-vaccination (Figure 3A), TSIR post-vaccination (Figure 3B), absolute change in TSIR from pre- to post-vaccination (Figure 3C) and fold change in TSIR from pre- to post-vaccination (Figure 3D). We observed that the levels of post-vaccination TSIR (R=-0.725, p<0.001) and absolute TSIR change (R=-0.679, p=0.001) were negatively correlated with T2-EOR, whereas correlations between T2-EOR and pre-vaccination TSIR or fold change of TSIR did not reach a significant level. Accordingly, the high T2-EOR group exhibited reduced levels of post-vaccination TSIR and absolute TSIR change compared with the low group (Figures 3B, C). Figure 3E shows a distinct difference in post-vaccination TSIR levels between the two groups. Our previous study revealed high post-vaccination TSIR as a favourable prognosticator for patients’ PFS and OS (Supplementary Figures 1A, B) (30), and we also observed a similar trend in survival analysis for absolute TSIR change (Supplementary Figures 1C, D). Therefore, these findings indicate that the link between high T2-EOR and poorer outcomes could be attributed to its inverse correlation with the TSIR levels.
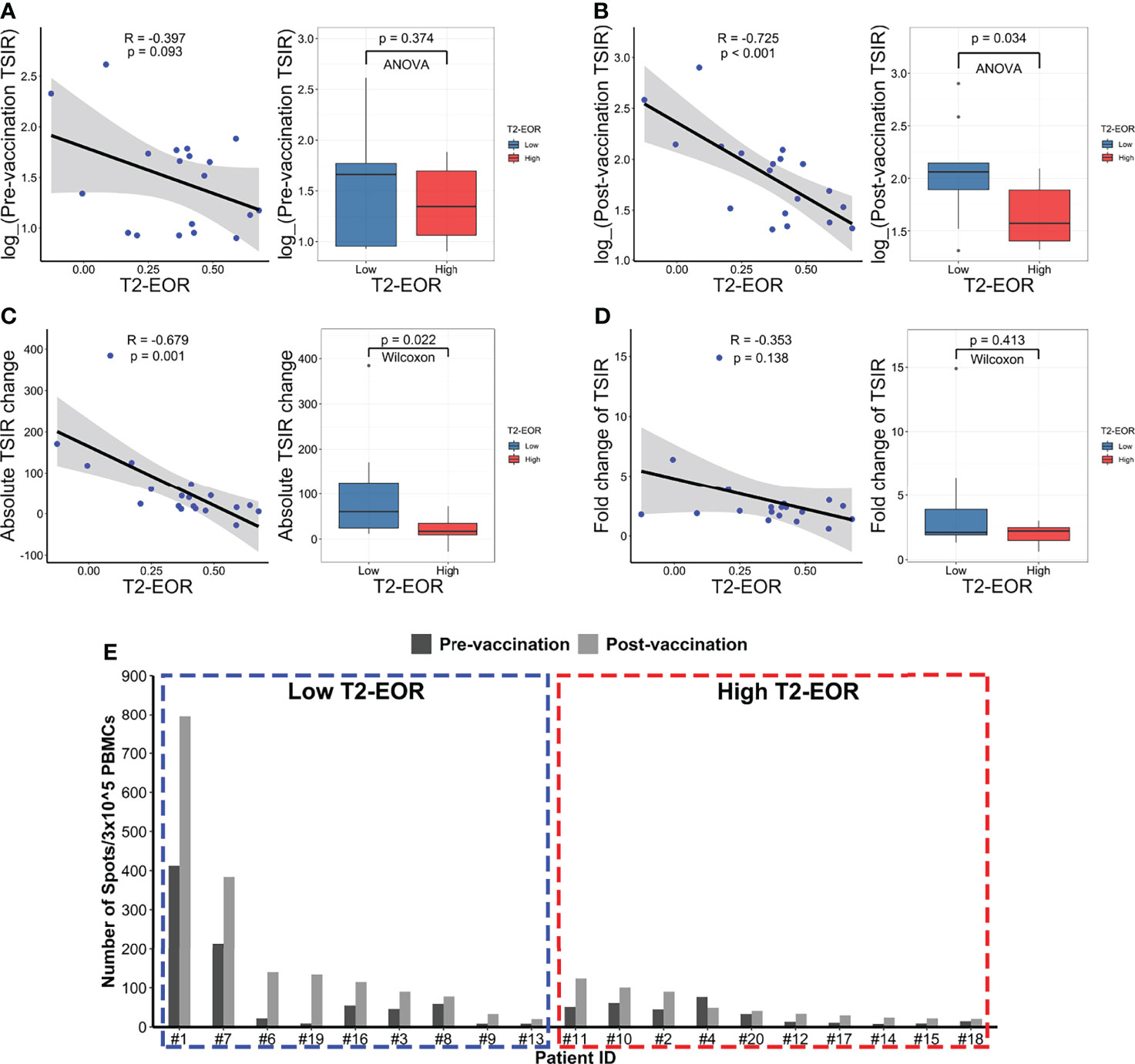
Figure 3 T2-EOR was negatively correlated with the levels of the tumour-specific immune response. Pearson correlation of T2-EOR with (A) pre-vaccination TSIR, (B) post-vaccination TSIR, (C) absolute change in TSIR from pre- to post-vaccination and (D) fold change in TSIR from pre- to post-vaccination. The differences in these TSIR indexes were also determined between the high and low T2-EOR groups using analysis of variance (ANOVA) or Wilcoxon nonparametric tests. (E) The levels of pre- and post-vaccination TSIR in each patient in this cohort are displayed as the high and low T2-EOR groups, respectively.
High T2-EOR Was an Independent Predictor Associated With Shortened PFS and OS Times
We fitted two Cox regression models, including age, sex, KPS score, T2-EOR and post-vaccination TSIR, to predict PFS and OS in this vaccinated cohort. As a result, both T2-EOR and post-vaccination TSIR emerged as significant independent prognosticators for PFS and OS (Figures 4A, B). High T2-EOR was significantly associated with shortened PFS (HR 19.85, p=0.0086) and OS (HR 21.24, p=0.0185) times. Compared with post-vaccination TSIR, T2-EOR can be measured prior to vaccination. Thus, T2-EOR is a potential biomarker that not only predicts immunotherapeutic outcomes but also facilitates the early recognition of patients who are more likely to benefit from the immunotherapy of HSPPC-96 vaccination.
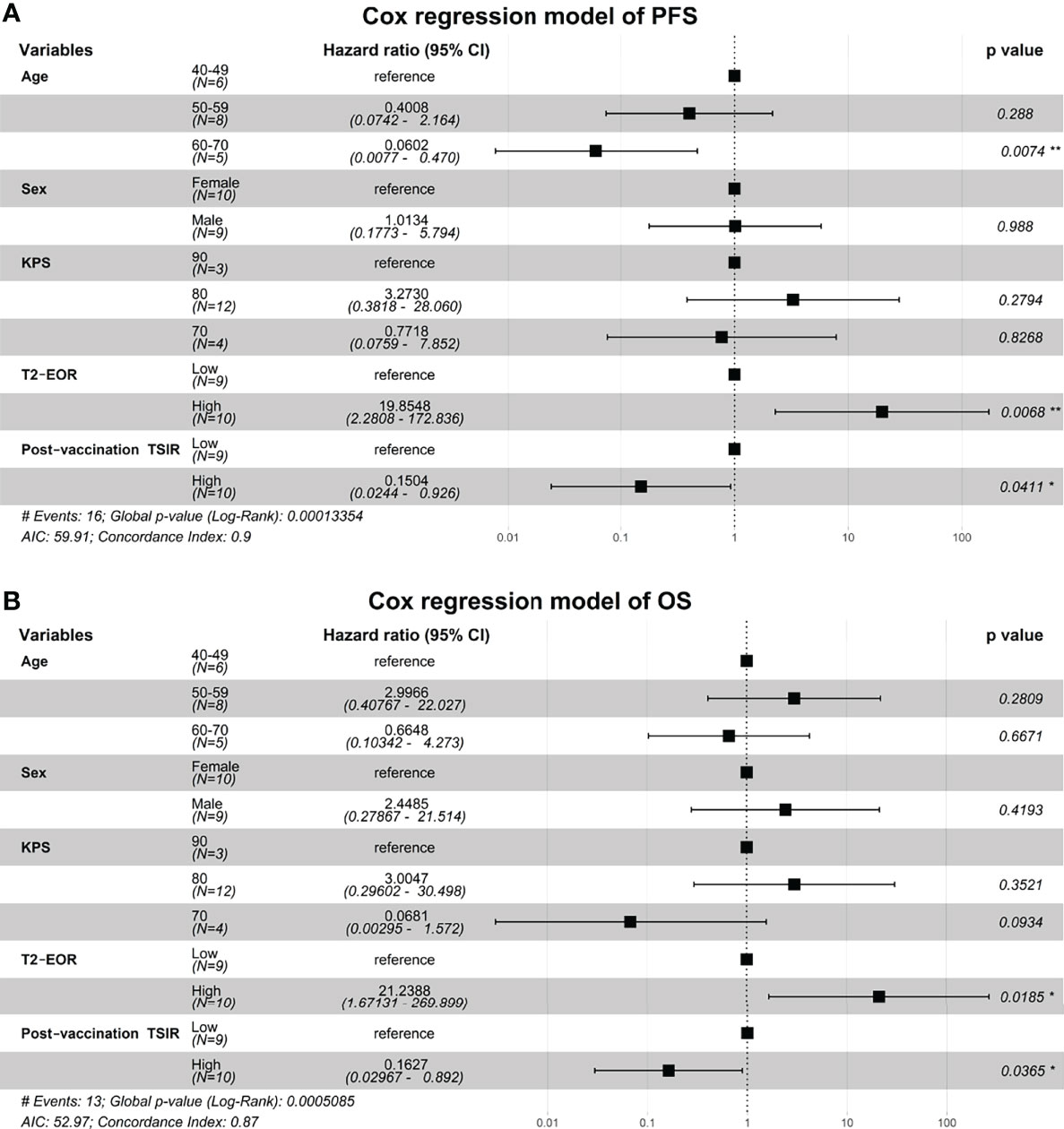
Figure 4 High T2-EOR was an independent predictor associated with shortened PFS and OS times. Two Cox regression models were fitted to identify independent prognosticators for (A) progression-free survival and (B) overall survival. *, P < 0.05. **, P < 0.01.
Discussion
Surgical resection remains a standard-of-care treatment for GBMs (32). Gross total resection of contrast-enhancing areas in GBMs has been widely reported to significantly extend PFS and OS in GBM patients receiving radiotherapy and temozolomide-based chemotherapy (33–36). Given the infiltrative growth nature of GBM, peritumoural oedema, shown as T2-nonenhancing region, also contains a small number of tumour cells, thus making it a region susceptible to tumour recurrence (11, 12). Therefore, SpTR, which leads to increased T2-EOR by minimizing T2-nonenhancing region, is theoretically associated with improved PFS and OS compared with gross total resection of contrast-enhancing areas. Recently, mounting evidence has supported the benefit of SpTR for the outcomes of glioma patients, with increased T2-EOR resulting in longer PFS and OS times (5, 9, 13, 35). However, the impact of T2-EOR on immunotherapeutic efficacy has not been elucidated. In this study, we reviewed the preoperative and postoperative MRI images of GBM patients who received HSPPC-96 vaccination in our previous clinical trial (NCT02122822). We revealed an inverse correlation of T2-EOR with vaccination-related antitumour immune response, represented as post-vaccination TSIR (Figure 3B) and absolute TSIR change (Figure 3C). Meanwhile, high T2-EOR was independently correlated with poorer clinical outcomes (Figures 2, 4). Taken together, our results indicate that increased T2-EOR or SpTR has a detrimental impact on the immunotherapeutic efficacy of HSPPC-96 vaccination.
We proposed two possible mechanisms to explain the link of SpTR to worse immunotherapeutic efficacy. The first is that SpTR can reduce the residual tumour burdens, which are required to generate an effective immune response in immunotherapies such as CAR-T therapies (37), HSPPC-96 vaccination (27) and rindopepimut (a peptide vaccine against EGFR-vIII-expressing GBMs). Patients with a higher tumour burden at the time of CAR-T cell infusion exhibited better CAR-T cell persistence and proliferation (37). The HSPPC-96 vaccination trial on melanoma (27) revealed that the immunotherapy provided a significant benefit in patients with residual disease. The rindopepimut trial showed a possible long-term survival benefit with rindopepimut for GBM patients with significant residual disease (≥2 cm2 of residual enhancing tumour on post-chemoradiation imaging) compared with those with minimal residual disease (< 2 cm2 of residual enhancing tumour) (38). They suggested that residual disease is associated with greater antigen expression, which would lead to the enhanced anti-tumour immunity required for a therapeutic effect (38). In this study, we also observed a direct positive correlation between the T2-nonenhancing region residual volumes and TSIR levels (Supplementary Figures 2A, B), although this correlation did not have a significant impact on outcomes (Supplementary Figures 2C, D), likely due to the limited sample size of the study. Therefore, a larger sample-size trial with a prospective design is needed to further confirm this possible mechanism.
Another possible mechanism is that T2-EOR also represents a change in T2-nonenhancing region before and after surgery and thus could be an indicator reflecting the intensity of the immune response to surgery that would predict immunotherapeutic outcomes. In this scenario, T2-nonenhancing region, representing peritumoural oedema, is positively correlated with the immune response to surgical stress (31, 39). An increased T2-EOR reflects less change in postoperative T2-nonenhancing region than preoperative T2-nonenhancing region, indicating a less intensive immune response to surgeries and thus reflecting a lower native capacity to trigger the antitumour immunity required for immunotherapeutic effectiveness. However, to our knowledge, there is no direct evidence supporting this hypothesis, thus requiring further animal studies as well as early clinical trials to test it.
Again, given the limited sample size and retrospective design of this study, the current findings should be further confirmed in another larger and carefully designed cohort, although we observed a statistically significant association of T2-EOR with immunotherapeutic efficacy.
Conclusion
In conclusion, to our knowledge, our study first revealed a significant association of SpTR or increased T2-EOR with worse immunotherapeutic outcomes in GBM patients, suggesting the potential of T2-EOR as an early biomarker for predicting the immunotherapeutic efficacy of HSPPC-96 vaccination.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
Conceptualization, YZ and NJ. Methodology, XC. Software, XC. Formal analysis YZ and XC. Resources, NJ. Data curation, XC and YW. Writing—original draft preparation, XC. Writing—review and editing, YZ and NJ. Visualization, XC and CL. Supervision, YZ and NJ. Project administration, YZ and NJ. Funding acquisition, XH, HG, YZ and NJ. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (81702451, 81930048), Capital characteristic Clinical Application Project (Z181100001718196), Capital Health Research and Development of Special Grant (2022-2-2047).
Conflict of Interest
XH is a co-founder and shareholder of Cure & Sure Biotech Co., LTD, Shenzhen, China, 100070; HG is a shareholder of Cure & Sure Biotech Co., LTD, Shenzhen, China, 100070; Cure & Sure Biotech Co., LTD, Shenzhen, China, 100070 developed the HSPCC-96 vaccine and provided financial support to the vaccine trial (NCT02122822), on which the current study is based.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.877190/full#supplementary-material.
References
1. Alexander BM, Cloughesy TF. Adult Glioblastoma. J Clin Oncol (2017) 35(21):2402–9. doi: 10.1200/JCO2017730119
2. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy Plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med (2005) 352(10):987–96. doi: 10.1056/NEJMoa043330
3. Stupp R, Taillibert S, Kanner A, Read W, Steinberg D, Lhermitte B, et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide Vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA (2017) 318(23):2306–16. doi: 10.1001/jama201718718
4. Juan W, Yin-Sheng C, Xiao-Bing J, Fu-Hua L, Zheng-He C, Jian W, et al. The Mediating Role of Extent of Resection in the Relationship Between the Tumor Characteristics and Survival Outcome of Glioma. J Cancer (2019) 10(14):3232–8. doi: 10.7150/jca30159
5. D'Amico RS, Englander ZK, Canoll P. Bruce JN Extent of Resection in Glioma-A Review of the Cutting Edge. World Neurosurg (2017) 103:538–49. doi: 10.1016/jwneu201704041
6. Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen H-J. Fluorescence-Guided Surgery With 5-Aminolevulinic Acid for Resection of Malignant Glioma: A Randomised Controlled Multicentre Phase Iii Trial. Lancet Oncol (2006) 7(5):392–401. doi: 10.1016/s1470-2045(06)70665-9
7. Moiraghi A, Roux A, Peeters S, Pelletier JB, Baroud M, Trancart B, et al. Feasibility, Safety and Impact on Overall Survival of Awake Resection for Newly Diagnosed Supratentorial Idh-Wildtype Glioblastomas in Adults. Cancers (Basel) (2021) 13(12):2932–53. doi: 10.3390/cancers13122911
8. Senft C, Bink A, Franz K, Vatter H, Gasser T. Seifert V Intraoperative Mri Guidance and Extent of Resection in Glioma Surgery: A Randomised, Controlled Trial. Lancet Oncol (2011) 12(11):997–1003. doi: 10.1016/s1470-2045(11)70196-6
9. Li YM, Suki D, Hess K, Sawaya R. The Influence of Maximum Safe Resection of Glioblastoma on Survival in 1229 Patients: Can We Do Better Than Gross-Total Resection? J Neurosurg (2016) 124(4):977–88. doi: 10.3171/20155JNS142087
10. de Leeuw CN, Vogelbaum MA. Supratotal Resection in Glioma: A Systematic Review. Neuro Oncol (2019) 21(2):179–88. doi: 10.1093/neuonc/noy166
11. Choi Y, Ahn KJ, Nam Y, Jang J, Shin NY, Choi HS, et al. Analysis of Peritumoral Hyperintensity on Pre-Operative T2-Weighted Mr Images in Glioblastoma: Additive Prognostic Value of Minkowski Functionals. PloS One (2019) 14(5):e0217785. doi: 10.1371/journalpone0217785
12. Lemee JM, Clavreul A, Menei P. Intratumoral Heterogeneity in Glioblastoma: Don't Forget the Peritumoral Brain Zone. Neuro Oncol (2015) 17(10):1322–32. doi: 10.1093/neuonc/nov119
13. Jackson C, Choi J, Khalafallah AM, Price C, Bettegowda C, Lim M, et al. A Systematic Review and Meta-Analysis of Supratotal Versus Gross Total Resection for Glioblastoma. J Neurooncol (2020) 148(3):419–31. doi: 10.1007/s11060-020-03556-y
14. Rizvi NA, Mazieres J, Planchard D, Stinchcombe TE, Dy GK, Antonia SJ, et al. Activity and Safety of Nivolumab, an Anti-Pd-1 Immune Checkpoint Inhibitor, for Patients With Advanced, Refractory Squamous Non-Small-Cell Lung Cancer (Checkmate 063): A Phase 2, Single-Arm Trial. Lancet Oncol (2015) 16(3):257–65. doi: 10.1016/S1470-2045(15)70054-9
15. Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, et al. Axicabtagene Ciloleucel Car T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med (2017) 377(26):2531–44. doi: 10.1056/NEJMoa1707447
16. Carlino MS, Larkin J, Long GV. Immune Checkpoint Inhibitors in Melanoma. Lancet (2021) 398(10304):1002–14. doi: 10.1016/S0140-6736(21)01206-X
17. Crane CA, Han SJ, Ahn B, Oehlke J, Kivett V, Fedoroff A, et al. Individual Patient-Specific Immunity Against High-Grade Glioma After Vaccination With Autologous Tumor Derived Peptides Bound to the 96 Kd Chaperone Protein. Clin Cancer Res (2013) 19(1):205–14. doi: 10.1158/1078-0432CCR-11-3358
18. Batich KA, Reap EA, Archer GE, Sanchez-Perez L, Nair SK, Schmittling RJ, et al. Long-Term Survival in Glioblastoma With Cytomegalovirus Pp65-Targeted Vaccination. Clin Cancer Res (2017) 23(8):1898–909. doi: 10.1158/1078-0432CCR-16-2057
19. Ji N, Zhang Y, Liu Y, Xie J, Wang Y, Hao S, et al. Heat Shock Protein Peptide Complex-96 Vaccination for Newly Diagnosed Glioblastoma: A Phase I, Single-Arm Trial. JCI Insight (2018) 3(10):e99145–53. doi: 10.1172/jciinsight99145
20. Wen PY, Reardon DA, Armstrong TS, Phuphanich S, Aiken RD, Landolfi JC, et al. A Randomized Double-Blind Placebo-Controlled Phase Ii Trial of Dendritic Cell Vaccine Ict-107 in Newly Diagnosed Patients With Glioblastoma. Clin Cancer Res (2019) 25(19):5799–807. doi: 10.1158/1078-0432CCR-19-0261
21. O'Rourke DM, Nasrallah MP, Desai A, Melenhorst JJ, Mansfield K, Morrissette JJD, et al. A Single Dose of Peripherally Infused Egfrviii-Directed Car T Cells Mediates Antigen Loss and Induces Adaptive Resistance in Patients With Recurrent Glioblastoma. Sci Transl Med (2017) 9(399):eaaa0984–98. doi: 10.1126/scitranslmedaaa0984
22. Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, et al. Regression of Glioblastoma After Chimeric Antigen Receptor T-Cell Therapy. N Engl J Med (2016) 375(26):2561–9. doi: 10.1056/NEJMoa1610497
23. Caudill MM, Li Z. Hsppc-96: A Personalised Cancer Vaccine. Expert Opin Biol Ther (2001) 1(3):539–47. doi: 10.1517/1471259813539
24. Srivastava P. Interaction of Heat Shock Proteins With Peptides and Antigen Presenting Cells: Chaperoning of the Innate and Adaptive Immune Responses. Annu Rev Immunol (2002) 20:395–425. doi: 10.1146/annurevimmunol20100301064801
25. Binder RJ. Immunosurveillance of Cancer and the Heat Shock Protein-Cd91 Pathway. Cell Immunol (2019) 343:103814. doi: 10.1016/jcellimm201805007
26. Suto R, Srivastava PK. A Mechanism for the Specific Immunogenicity of Heat Shock Protein-Chaperoned Peptides. Science (1995) 269(5230):1585–8. doi: 10.1126/science7545313
27. Belli F, Testori A, Rivoltini L, Maio M, Andreola G, Sertoli MR, et al. Vaccination of Metastatic Melanoma Patients With Autologous Tumor-Derived Heat Shock Protein Gp96-Peptide Complexes: Clinical and Immunologic Findings. J Clin Oncol (2002) 20(20):4169–80. doi: 10.1200/JCO200209134
28. Testori A, Richards J, Whitman E, Mann GB, Lutzky J, Camacho L, et al. Phase Iii Comparison of Vitespen, an Autologous Tumor-Derived Heat Shock Protein Gp96 Peptide Complex Vaccine, With Physician's Choice of Treatment for Stage Iv Melanoma: The C-100-21 Study Group. J Clin Oncol (2008) 26(6):955–62. doi: 10.1200/JCO2007119941
29. Wood C, Srivastava P, Bukowski R, Lacombe L, Gorelov AI, Gorelov S, et al. An Adjuvant Autologous Therapeutic Vaccine (Hsppc-96. Vitespen) Versus Observation Alone for Patients at High Risk of Recurrence After Nephrectomy for Renal Cell Carcinoma: A Multicentre, Open-Label, Randomised Phase Iii. Trial. Lancet (2008) 372(9633):145–54. doi: 10.1016/S0140-6736(08)60697-2
30. Zhang Y, Mudgal P, Wang L, Wu H, Huang N, Alexander PB, et al. T Cell Receptor Repertoire as a Prognosis Marker for Heat Shock Protein Peptide Complex-96 Vaccine Trial Against Newly Diagnosed Glioblastoma. Oncoimmunology (2020) 9(1):1749476. doi: 10.1080/2162402X20201749476
31. Belhawi SM, Hoefnagels FW, Baaijen JC, Aliaga ES, Reijneveld JC, Heimans JJ, et al. Early Postoperative Mri Overestimates Residual Tumour After Resection of Gliomas With No or Minimal Enhancement. Eur Radiol (2011) 21(7):1526–34. doi: 10.1007/s00330-011-2081-y
32. Sanai N, Berger MS. Surgical Oncology for Gliomas: The State of the Art. Nat Rev Clin Oncol (2018) 15(2):112–25. doi: 10.1038/nrclinonc2017171
33. Kreth FW, Thon N, Simon M, Westphal M, Schackert G, Nikkhah G, et al. Gross Total But Not Incomplete Resection of Glioblastoma Prolongs Survival in the Era of Radiochemotherapy. Ann Oncol (2013) 24(12):3117–23. doi: 10.1093/annonc/mdt388
34. Salvati M, Bruzzaniti P, Relucenti M, Nizzola M, Familiari P, Giugliano M, et al. Retrospective and Randomized Analysis of Influence and Correlation of Clinical and Molecular Prognostic Factors in a Mono-Operative Series of 122 Patients With Glioblastoma Treated With Str or Gtr. Brain Sci (2020) 10(2):91–104. doi: 10.3390/brainsci10020091
35. Molinaro AM, Hervey-Jumper S, Morshed RA, Young J, Han SJ, Chunduru P, et al. Association of Maximal Extent of Resection of Contrast-Enhanced and Non-Contrast-Enhanced Tumor With Survival Within Molecular Subgroups of Patients With Newly Diagnosed Glioblastoma. JAMA Oncol (2020) 6(4):495–503. doi: 10.1001/jamaoncol20196143
36. Lu VM, Goyal A, Graffeo CS, Perry A, Burns TC, Parney IF, et al. Survival Benefit of Maximal Resection for Glioblastoma Reoperation in the Temozolomide Era: A Meta-Analysis. World Neurosurg (2019) 127:31–7. doi: 10.1016/jwneu201903250
37. Brentjens RJ, Davila ML, Riviere I, Park J, Wang X, Cowell LG, et al. Cd19-Targeted T Cells Rapidly Induce Molecular Remissions in Adults With Chemotherapy-Refractory Acute Lymphoblastic Leukemia. Sci Transl Med (2013) 5(177):177ra38. doi: 10.1126/scitranslmed3005930
38. Weller M, Butowski N, Tran DD, Recht LD, Lim M, Hirte H, et al. Rindopepimut With Temozolomide for Patients With Newly Diagnosed, Egfrviii-Expressing Glioblastoma (Act Iv): A Randomised, Double-Blind, International Phase 3 Trial. Lancet Oncol (2017) 18(10):1373–85. doi: 10.1016/s1470-2045(17)30517-x
39. Vogelbaum MA, Jost S, Aghi MK, Heimberger AB, Sampson JH, Wen PY, et al. Application of Novel Response/Progression Measures for Surgically Delivered Therapies for Gliomas: Response Assessment in Neuro-Oncology (Rano) Working Group. Neurosurgery (2012) 70(1):234–43. discussion 43-4. doi: 10.1227/NEU0b013e318223f5a7
Keywords: glioblastoma, extent of resection, immunotherapeutic efficacy, cancer treatment vaccine, HSPPC-96
Citation: Chi X, Wang Y, Li C, Huang X, Gao H, Zhang Y and Ji N (2022) Resection of Noncontrast-Enhancing Regions Deteriorated the Immunotherapeutic Efficacy of HSPPC-96 Vaccination in Treating Glioblastoma. Front. Oncol. 12:877190. doi: 10.3389/fonc.2022.877190
Received: 01 March 2022; Accepted: 19 April 2022;
Published: 18 May 2022.
Edited by:
Jose R. Pineda, University of the Basque Country, SpainReviewed by:
Patricia Garcia Gallastegi, University of the Basque Country, SpainJing Nie, People’s Liberation Army General Hospital, China
Pramod Kumar Srivastava, University of Connecticut, United States
Copyright © 2022 Chi, Wang, Li, Huang, Gao, Zhang and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nan Ji, amluYW5AbWFpbC5jY211LmVkdS5jbg==; Yang Zhang, emhhbmd5YW5nQGJqdHRoLm9yZw==
 Xiaohan Chi1,2
Xiaohan Chi1,2 Yi Wang
Yi Wang Yang Zhang
Yang Zhang Nan Ji
Nan Ji