
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 15 July 2022
Sec. Breast Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.876487
This article is part of the Research Topic Quantitative Imaging and Artificial Intelligence in Breast Tumor Diagnosis View all 27 articles
Objective: To investigate the Contrast-enhanced ultrasound (CEUS) imaging characteristics of granulomatous lobular mastitis (GLM) and the value of differentiating GLM from breast cancer.
Materials and methods: The study included 30 women with GLM (mean age 36.7 ± 5 years [SD]) and 58 women with breast cancer (mean age 48. ± 8 years [SD]) who were scheduled for ultrasound-guided tissue biopsy. All patients were evaluated with conventional US and CEUS prior to the biopsy. In both groups, the parameters of the quantitative and qualitative analysis of the CEUS were recorded and compared. The receiver-operating-characteristics curves (ROC) were created. Sensitivity, specificity, cut-off, and area under the curve (AUC) values were calculated.
Results: TTP values in GLM were statistically higher than in breast cancer (mean, 27.63 ± 7.29 vs. 20.10 ± 6.11), but WIS values were lower (mean, 0.16 ± 0.05 vs. 0.28 ± 0.17). Rich vascularity was discovered in 54.45% of breast cancer patients, but only 30.00% of GLM patients had rich vascularity. The AUC for the ROC test was 0.791 and 0.807, respectively. The optimal cut-off value for TTP was 24.5s, and the WIS cut-off value was 0.185dB/s, yielding 73.33% sensitivity, 84.48% specificity, and 86.21% sensitivity, 70% specificity respectively in the diagnosis of GLM. The lesion scores reduced from 4 to 3 with the addition of CEUS for the patients with GLM. However, the scores did not change for the patients with breast cancer.
Conclusion: CEUS could help distinguish GLM from breast cancer by detecting higher TTP and WIS values, potentially influencing clinical decision-making for additional biopsies.
Granulomatous lobular mastitis (GLM) is a rare, benign chronic inflammatory disease with unclear etiology that is frequently mistaken as a malignant process both clinically and radiographically (1) Imaging features suggestive of GLM remain nonspecific and are not always present in all patients. As a result, accurate diagnosis necessitates pathological examination (2, 3). Unnecessary biopsies or surgical excision can result in chronic fistulas and breast deformities. During the evaluation, the patient may experience a great deal of anxiety.
Ultrasonography (US) is frequently used to assess lesions using Breast Imaging Reporting and Data System (BI-RADS), because it has a high sensitivity in diagnosing breast lesions (4). BI-RADS 3 lesions are likely to be benign and should be monitored; BI-RADS 4 and 5 lesions are suspected malignant and must be pathologically confirmed. Contrast-enhanced ultrasound (CEUS) imaging with microbubble contrast agents has significantly improved microcirculation visualization and allowed researchers to overcome the limitations of traditional B-mode US techniques. Previous studies have shown that combining CEUS with conventional US could improve diagnostic performance in breast lesions. The kinetic parameters of tumor tissue in CEUS can also be quantified by generating the time-intensity (T/I) curve with specialized software. Several studies have shown that CEUS can help distinguish between benign and malignant breast lesions (5–7). Because the current imaging modalities are not sufficient to establish a definitive diagnosis of GLM in most patients. Prior studies of breast cancer reveal increased amounts of microvessels in cancerous lesions.Based on this data, CEUS may provide additional information for distinguishing GLM from breast cancer lesions.
The purpose of this study was to investigate CEUS parameters of GLM and breast cancer and analyze their values in distinguishing GLM from breast cancer.
The ethics committee of Jiangsu University Affiliated People’s Hospital approved this study (K-20190175-W), and written informed consent for breast CEUS examination was obtained prior to enrolling a patient in our study. Between September 2019 and November 2021, consecutive patients were screened for breast cancer at Jiangsu University Affiliated People’s Hospital’s Department of Breast Surgery. According to the BI-RADS classification scheme, the selection criteria were represented by US findings classified as BI-RADS category 3–5. All those patients contraindicated for CEUS were excluded from the study. After grayscale and contrast-enhanced ultrasound, a core needle biopsy (CNB) or vacuum-assisted biopsy (VAB) of BI-RADS 4 and 5 lesions was performed. Only the most suspicious lesion fulfilling the selection criteria was evaluated when a patient had multiple lesions. GLM diagnosis criteria was based on the management of granulomatous lobular mastitis: an international multidisciplinary consensus (2021 edition) (8). The histopathologic results of biopsy served as the diagnosis gold standard in this study. All patients had standard mammography and magnetic resonance imaging (MRI) (according to age).
All examinations were performed with a Philips EPI Q5 color Doppler ultrasound equipped with a high-frequency linear array probe (using a 12-5 MHZ and 9-3 MHZ linear-array transducer) and dedicated contrast pulse sequences. To reduce contrast agent destruction, low mechanical index values (MI=0.08) were used. The contrast medium employed was SonoVue (Bracco Imaging, Milan, Italy).
The same sonologist, with 20 years of experience with breast US, performed all US and CEUS examinations. When a breast lesion was discovered, its location, maximum diameter, 2-D characteristics, and color Doppler characteristics were all recorded. Shape, margin, orientation, inner echo, posterior echo, and calcification were all 2-D characteristics. A dual display of grayscale and contrast-enhanced images was used to allow simultaneous visualization to keep the probe position constant during the examination. The plane with the most significant lesion diameter was chosen as the reference scan. In addition to keeping the transducer in a stable position throughout the scan, the target area was compressed as little as possible. The contrast reagent suspension used consisted of 59 mg of SonoVue powder mixed with 5 mL of saline and was administered via a 20-gauge cannula into the antecubital vein. Following a bolus injection of 4.8 mL of contrast agent via the intravenous cannula, a saline injection of 5–10 mL was administered. A two-minute dynamic image image was recorded and saved on a hard disk as raw data for later analysis.
Two other investigators who had not performed the conventional US and CEUS examinations and were blinded to surgical, histopathologic, and other imaging findings independently analyzed the US imaging data. The findings of the conventional ultrasound examination were evaluated using a standardized BI -RADS™ (Breast Imaging Reporting and Data System) for breast ultrasound. The diagnostic criteria for CEUS, according to a previously published study (5).
A dedicated sonographic quantification software (Qontrast, Bracco, Milan, Italy) based on signal intensity pixel by pixel over time was used to generate color-coded maps of the studied lesion’s perfusion parameters. The enhancement patterns were evaluated as qualitative parameters, while the time-intensity curve was analyzed quantitatively (9). The qualitative variables were classified as follows: The degree of enhancement of lesions in comparison to surrounding tissue, the type of vascularization (peripheral or central), the homogeneity of perfusion (homogeneous vs. heterogeneous), and the degree of vascularization (peripheral or central) (weak or absent vs. intermediate vs. rich). The time-intensity curve’s quantitative parameters were determined. The region of interest (ROI) was placed in the area of most significant enhancement, and its size was set to the default value of 3 (mean, 6.9 ± 0.3 mm2; range, 5.6-7.5 mm2). The quantitative parameters were classified as follows: TTP (time to peak, s), PI (peak intensity, dB), WIS (wash in slope, dB/s), AUC (area under curve, dB x s). The integral value of the curve is associated with total blood volume and the sum of the area wash-in and area wash-out (Figure 1). Predefined motion compensation and background set were also applied to obtain these parameters. Motion compensation is an automatic function that detects slight movements in concordance with movements of ROI and eliminates their influence. Application examples are given (Figure 2).

Figure 1 Flow chart with patients in the study. BC: breast cancer, granulomatous lobular mastitis : GLM; CEUS: contrast-enhanced ultrasound; CNB: Core needle biopsy; VAB: vcauum-assisted biopsy.
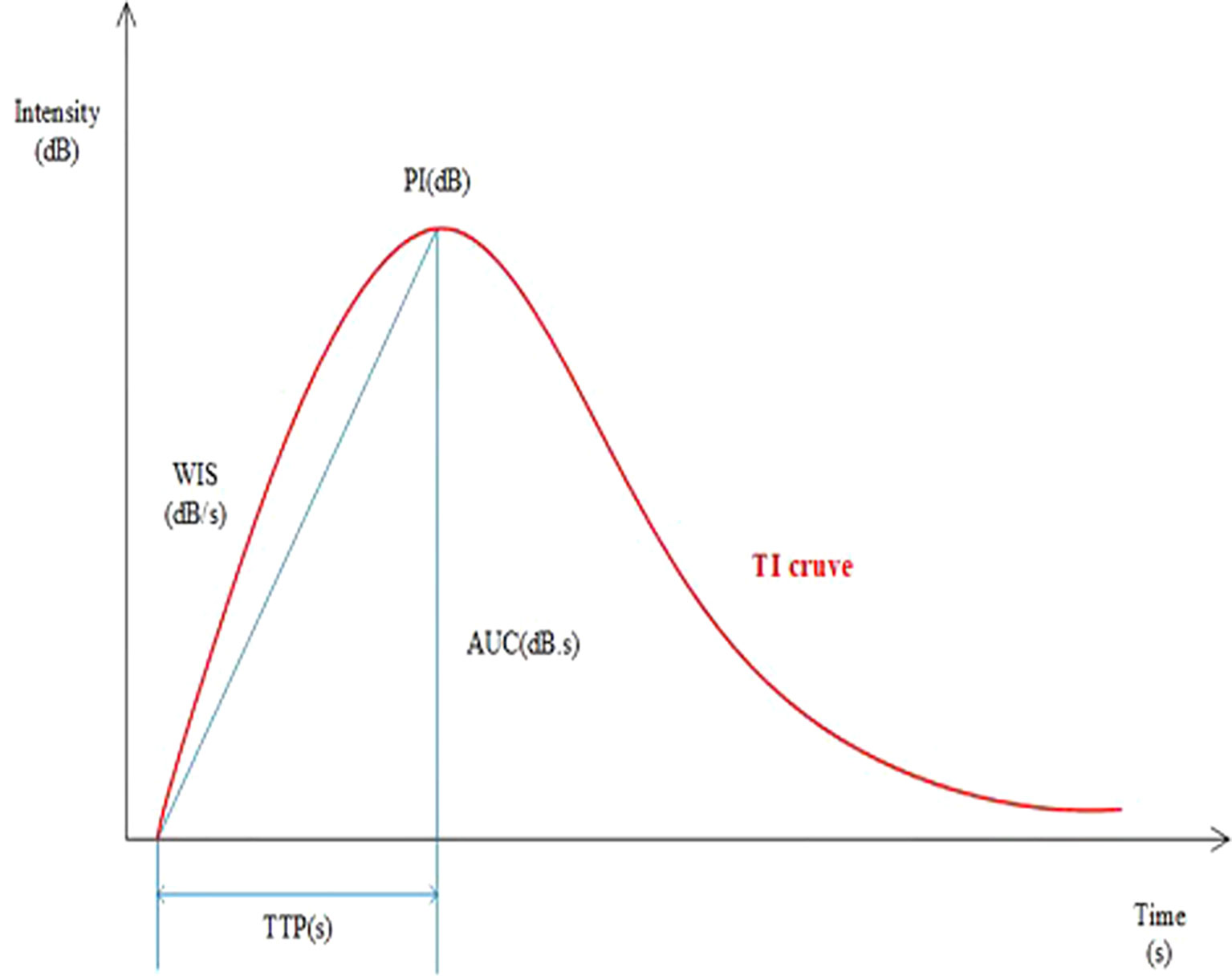
Figure 2 Representation of the time-intensity curve including parameters: TI (time intensity curves), PI (peakintensity), WIS (wash in slope), AUC (area under curve), TTP (time to peak).
For the statistical analysis, GraphPad Prism 7 was used. The student’s t-test and ANOVA were used to examine the differences between independent groups. The Mann-Whitney U test was used to compare differences between two independent groups. Fisher’s exact test was used to compare categorical (qualitative) parameters summarized using absolute and relative frequencies. The p< 0.05 level was considered statistically significant.
A total of 130 patients who underwent image-guided biopsy were enrolled in this study. 2 patients were contraindicated for CEUS, and 40 patients diagnosed with the benign non-inflammatory disease (fibroadenoma, for example) were excluded. Fifty-eight patients with breast cancer and 30 patients with GLM were included in the analysis of the quantitative CEUS parameters (Figure 3).
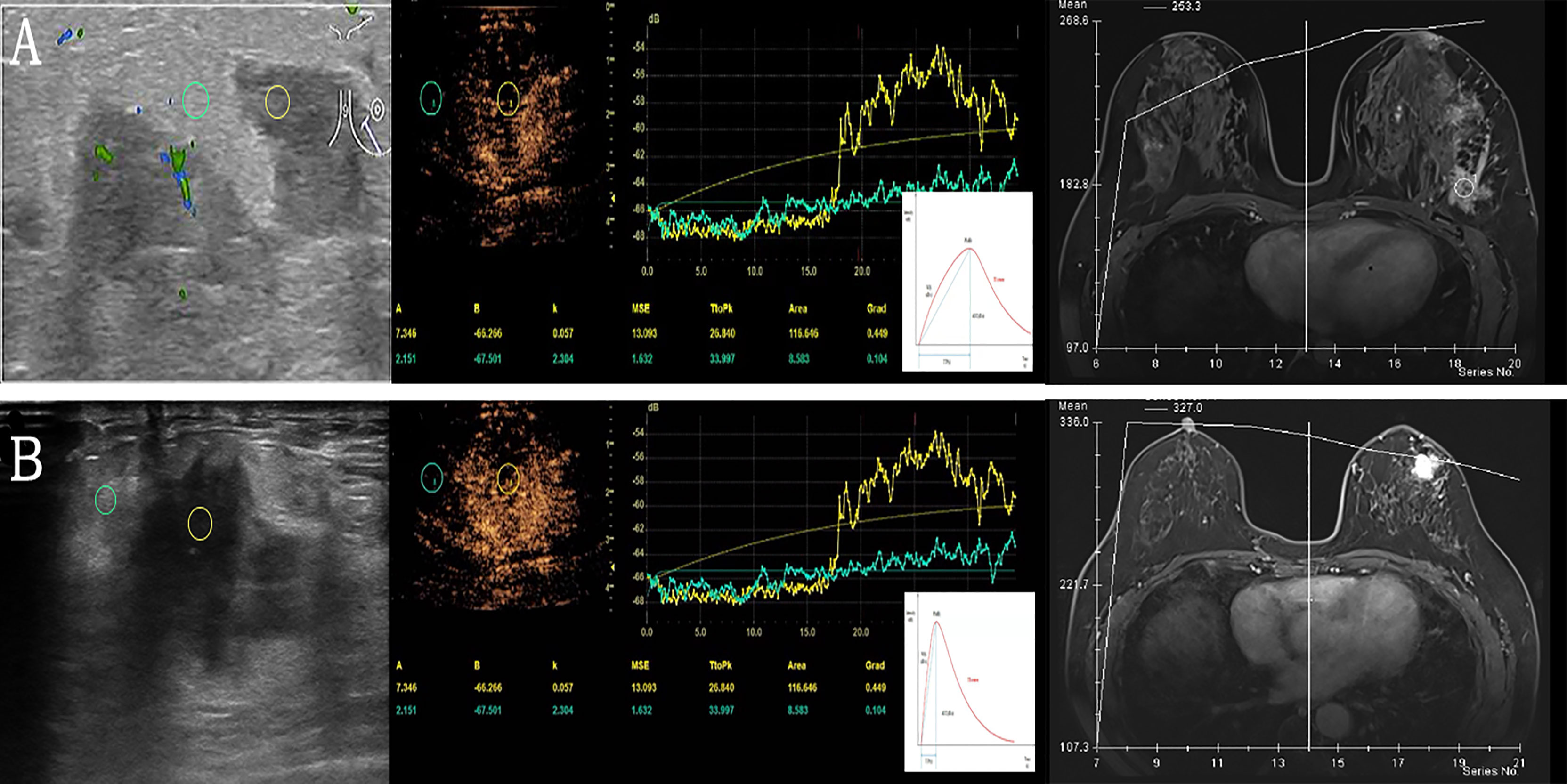
Figure 3 Examples of quantitative data acquisition using ROI. (A) Granulomatous lobular mastitis in a 28-year-old female. Moderate enhancement of the lesion (yellow ROI) compared to minimal enhancement in surrounding breast tissue (green ROI). Gradual enhancement and a gradual wash out of contrast agent (corresponding TIC below) versus TIC of DEC-MRI. (B) Invasive ductal carcinoma in a 61-year-old female with significant enhancement of the lesion (yellow ROI) compared to slight enhancement in surrounding breast tissue (green ROI), the lesion is ill-defined. After rapid enhancement of the tumor, early wash-out can be observed (corresponding TIC below) versus TIC of DEC-MRI. ROI, region of interest; TIC, time intensity curves; DEC-MRI, dynamic contrast enhancement magnetic resonance imaging.
Basic patient and lesion characteristics are summarized in Table 1. Patients with GLM were significantly younger (mean 36.7 ± 5 years vs. 48 ± 8 years) and had larger lesions (40.67 ± 8.38 vs. 29.02 ± 6.05 mm) (p< 0.01). There was no statistically significant difference in US-BI-RADS scores between breast cancer patients and GLM patients (p = 0.19). When only conventional US was used to evaluate patients with GLM, the scores were predominantly determined to be four according to the BI-RADS classification, similar to breast cancer, preventing differentiation. With the addition of CEUS for patients with GLM, the lesion scores decreased from 4 to 3. The scores for patients with breast cancer, on the other hand, remained unchanged.
The parameters of the quantitative and qualitative analysis of the CEUS of GLM and breast cancer are summarized in Table 2. GLM had statistically higher TTP values (on average by 7 s) and lower WIS values (on average by 0.12 dB/s) than breast cancer. A statistically significant difference in the degree of enhancement was observed when rich vascularity was detected in 54.45% of breast cancer but only 30.00% of benign lesions. There was no discernible difference in the nature of the blood supply in the surrounding tissue of GLM and breast cancer.
For GLM patients, the mean TTP and WIS distributions for the BI-RADS categories are shown. There was a significant difference between BI-RADS 3 and 4 lesions (p< 0.05, 95% CI). There was a negative correlation between BI-RADS scores and TTP (p< 0.01) (Table 3). According to ROC curve analysis, the best cut-off value of TTP for distinguishing between GLM and breast cancer was 24.5s, yielding sensitivity and specificity of 73.33% and 84.48%, respectively, for the diagnosis of GLM. The cut-off value of WIS was 0.185dB/s, at which the sensitivity and specificity for diagnosing GLM were 86.21% and 70%. ROC results revealed an area under the curve values (TTP-AUC: 0.791, WSI-AUC: 0.807) (Figure 4).
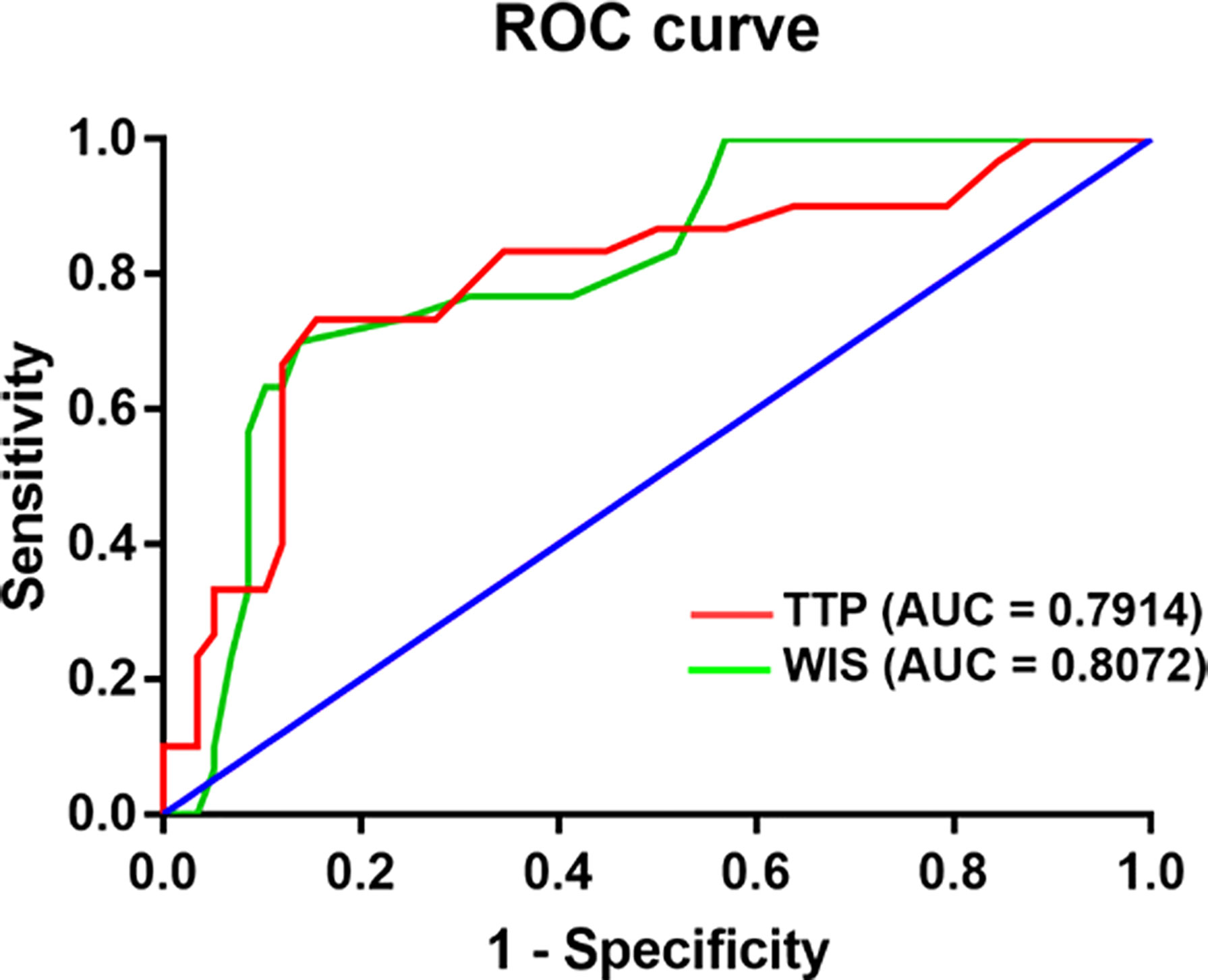
Figure 4 Diagram shows Comparison receiver operating characteristic curve of CEUS between granulomatous lobular mastitis and breast cancer.
This study aimed to compare the CEUS characteristics of GLM to those of breast cancer. To interpret the CEUS results, we used quantitative and qualitative examination parameters that provide a measurable value for the lesions. In our study, GLM had statistically higher TTP parameters (sensitivity 73.33%, specificity 84.48%) and lower WIS values (sensitivity 86.21%, specificity 70%) than breast cancer. The CEUS adds valuable information to that obtained through the US. Our findings indicated that this approach could help to avoid unnecessary biopsies.
GLM is a rare chronic inflammatory, benign breast disease. It primarily affects women of childbearing age, and its etiology is unknown. It is difficult to diagnose because it can mimic breast cancer clinically and radiologically (1). For the initial assessment of this rare entity, ultrasound and mammography are commonly used imaging modalities. However, the imaging findings on ultrasound and mammography are often inconclusive, making it difficult to distinguish this disease from malignancy using these traditional imaging techniques (2, 10). Dynamic contrast enhancement Magnetic resonance imaging (DCE-MRI) is a powerful tool for detecting breast disease. DCE-MRI parameters of breast cancer were found to be related to the expression of histopathological factors (11, 12). GLM characteristics detected by MRI commonly manifest as heterogeneous enhancing masses, segmental non-mass enhancement (NME), or focal non-massive lesions. The most common MRI finding in GLM patients is NME, which is characterized by heterogeneous and clustered ring enhancement patterns (13, 14). However, access to MRI may be limited, and exams are relatively expensive. Furthermore, patients who are contraindicated for MRI or who cannot tolerate MRI are not candidates for these exams. Our study only performed mammography on patients over 40 years of age who had non-diagnostic images. Four patients with GLM for DCE-MRI, and two of the images show a ring-shaped pattern of non-massive enhancement around the lesion.
Several Multiple irregular hypoechoic masses with multiple tubular extensions are a symptom of GLM (15). Shear wave elastography (SWE) values were significantly higher in breast cancer patients than in GLM patients (16, 17). This study’s most common ultrasound findings were increased skin thickness and irregular heterogeneous hypoechoic masses with tubular extension, which is consistent with previous research. These findings mainly were BI-RADS 4-5 and necessitated a biopsy for a definitive diagnosis (18).
CEUS is currently a widely used diagnostic method for assessing microvascular architecture in real-time. CEUS has an advantage over power Doppler, which has been widely used to assess the vascularity of liver and other organ masses (19). Ultrasound contrast agents (UCA) are gas-filled microbubbles (3–10 m diameter) supported by a flexible shell, such as phospholipids or albumin, employed in CEUS. These microbubbles operate as resonant entities in an ultrasonic field, generating nonlinear scattered signals that distinguish blood flow from surrounding tissue (20). In an ultrasound examination, SonoVue is used to enhance the vascular signals. As a result, the breast’s detection, morphology, and flow of microvessels are improved (21, 22). Previous research has found that CEUS has a higher diagnostic performance than the conventional US in distinguishing benign from malignant breast lesions (23). The use of contrast-enhanced ultrasound in conjunction with blood cell analysis improved the diagnostic accuracy of plasma cell mastitis (24). Min Tang demonstrated that CEUS has high diagnostic accuracy in distinguishing benign inflammation from the malignant peripheral pulmonary disease (25).
In the present study, according to the BI-RADS classification, US scores for GLM in this study were predominantly 4. In patients with GLM, the addition of CEUS reduced the score from 4 to 3. The scores, however, did not change in patients with breast cancer. We hypothesize that CEUS will be an effective tool in evaluating GLM with unclear findings on conventional ultrasound to differentiate between categories 3 and 4. As a result, CEUS may reduce the number of tissue core needle biopsies of GLM. In comparison to MRI, CEUS is a relatively simple, quick, and inexpensive method suited to becoming part of the diagnostic algorithm of breast examination prior to biopsy.
In our study, we used the TI curves to look at differences in vascular perfusion kinetics. When compared to breast cancer, GLM was associated with significantly higher TTP values and significantly lower WIS values. These findings can be explained by the earlier and faster onset of breast cancer enhancement. Several other studies that focused solely on CEUS characteristics of confirmed breast cancer found earlier peak enhancement (analogous to TTP and WIS parameters) and faster microbubble elimination in more aggressive forms of cancer associated with poor prognosis (7, 26, 27). In our research, evaluating other qualitative (vascularization type, perfusion homogeneity) and quantitative parameters (PI, AUC) did not significantly improve the ability to distinguish between GLM and breast cancer. Thus, quantitative CEUS analysis provides an objective and reproducible assessment of lesion vascularization, whereas some quantitative parameters between GLM and breast cancer still overlap. One possible explanation for this is that some hypervascular GLM lesions, particularly at the chronic inflammation stage, mimic malignant tumors because their enhancement dynamics are similar to those of carcinomas.
Sonovue was the most widely used UCA in previous studies, but its mechanical properties limit its use with high-frequency linear array probes for breast scanning and its capacity for long-term imaging. In our study, Sonovue imaging could only last 4 minutes, limiting access to high-quality imaging parameters compared to the more stable Optison or Sonazoid (28). External perfusion software, such as VueBox (Bracco, Italy), with integrated motion correction, allows for a more detailed evaluation of micro vascularization in terms of wash-in and wash-out kinetics because cine loops for up to 2min can be evaluated and more parameters are determined (29). As a result, new UCA in conjunction with analysis software may improve CEUS diagnostic performance.
There are several limitations to our study. First, neither inter nor intra-observer variability was assessed. Second, statistical power may have been compromised because this was a single-center clinical study with a small number of patients. This study was not intended to replace conventional US for the diagnosis of GLM and breast cancer but rather to describe how a complementary method, based on the microcirculation of the tissues examined, can provide valuable additional information to that obtained using the US.
According to the findings of this study, CEUS has a favorable diagnostic performance with a higher TTP and a lower WIS value in distinguishing GLM from breast cancer. Applying this method in clinical practice can influence clinical decision-making for further biopsies.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics Committee of Jiangsu University Affiliated People’s Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
LY, AA, and QZ contributed to the conception and design of the study. XX organized the database. LP performed the statistical analysis. LY wrote the first draft of the manuscript. TW, AA, and XQ wrote sections of the manuscript. AA and XQ revised the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported Research Project of Jiangsu Maternal and Child Health Association (Grant No. FYX202004) and Zhenjiang Social Development Guidance Project (Grant NO. FZ2018035).
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hovanessian Larsen LJ, Peyvandi B, Klipfel N, Grant E, Iyengar G. Granulomatous Lobular Mastitis: Imaging, Diagnosis, and Treatment. AJR Am J Roentgenol (2009) 193(2):574–81. doi: 10.2214/AJR.08.1528
2. Yin Y, Liu X, Meng Q, Han X, Zhang H, Lv Y. Idiopathic Granulomatous Mastitis: Etiology, Clinical Manifestation, Diagnosis and Treatment. J Invest Surg (2021) 35(3):709–20. doi: 10.1080/08941939.2021.1894516
3. Steuer AB, Stern MJ, Cobos G, Castilla C, Joseph K-A, Pomeranz MK, et al. Clinical Characteristics and Medical Management of Idiopathic Granulomatous Mastitis. JAMA Dermatol (2020) 156(4):460–4. doi: 10.1001/jamadermatol.2019.4516
4. Mendelson EB, Böhm-Vélez M, Berg WA, et al. ACR BI-RADS® Ultrasound. In: ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA, American College of Radiology (2013).
5. Du J, Wang L, Wan C-F, Hua J, Fang H, Chen J, et al. Differentiating Benign From Malignant Solid Breast Lesions: Combined Utility of Conventional Ultrasound and Contrast-Enhanced Ultrasound in Comparison With Magnetic Resonance Imaging. Eur J Radiol (2012) 81(12):3890–9. doi: 10.1016/j.ejrad.2012.09.004
6. Zhang Q, Agyekum EA, Zhu L, Yan L, Zhang L, Wang X, et al. Clinical Value of Three Combined Ultrasonography Modalities in Predicting the Risk of Metastasis to Axillary Lymph Nodes in Breast Invasive Ductal Carcinoma. Front Oncol (2021) 11:715097(3867). doi: 10.3389/fonc.2021.715097
7. Janu E, Krikavova L, Little J, Dvorak K, Brancikova D, Jandakova E, et al. Prospective Evaluation of Contrast-Enhanced Ultrasound of Breast BI-RADS 3-5 Lesions. BMC Med Imaging (2020) 20(1):66. doi: 10.1186/s12880-020-00467-2
8. Yuan Q-Q, Xiao S-X, Farouk O, Du Y-T, Sheybani F, Tan QT, et al. Management of Granulomatous Lobular Mastitis: An International Multidisciplinary Consensus (2021 Edition). Mil Med Res (2022) 9(1):20. doi: 10.1186/s40779-022-00380-5
9. Wan C, Du J, Fang H, Li F, Wang L. Evaluation of Breast Lesions by Contrast Enhanced Ultrasound: Qualitative and Quantitative Analysis. Eur J Radiol (2012) 81(4):e444–50. doi: 10.1016/j.ejrad.2011.03.094
10. Yin L, Agyekum EA, Zhang Q, Wu T, Qian X. Gynecomastia With Rare Granulomatous Lobular Mastitis: A Case Report and Literature Review. J Int Med Res (2022) 50(1):3000605221075815. doi: 10.1177/03000605221075815
11. Tuan Linh L, Minh Duc N, Minh Duc N, Tra My T-T, Viet Bang L, Cong Tien N, et al. Correlations Between Apparent Diffusion Coefficient Values and Histopathologic Factors in Breast Cancer. Clin Ter (2021) 172(3):218–24. doi: 10.7417/CT.2021.2318
12. Tuan Linh L, Minh Duc N, Tra My T-T, Viet Bang L, Minh Thong P. Correlations Between Dynamic Contrast-Enhanced Magnetic Resonance Imaging Parameters and Histopathologic Factors in Breast Cancer. Clin Ter (2021) 172(5):453–60. doi: 10.7417/CT.2021.2358
13. Zhao Q, Xie T, Fu C, Chen L, Bai Q, Grimm R, et al. Differentiation Between Idiopathic Granulomatous Mastitis and Invasive Breast Carcinoma, Both Presenting With non-Mass Enhancement Without Rim-Enhanced Masses: The Value of Whole-Lesion Histogram and Texture Analysis Using Apparent Diffusion Coefficient. Eur J Radiol (2020) 123:108782. doi: 10.1016/j.ejrad.2019.108782
14. Chu AN, Seiler SJ, Hayes JC, Wooldridge R, Porembka JH. Magnetic Resonance Imaging Characteristics of Granulomatous Mastitis. Clin Imaging (2017) 43:199–201. doi: 10.1016/j.clinimag.2017.03.012
15. Yildiz S, Aralasmak A, Kadioglu H, Toprak H, Yetis H, Gucin Z, et al. Radiologic Findings of Idiopathic Granulomatous Mastitis. Med Ultrason (2015) 17(1):39–44. doi: 10.11152/mu.2013.2066.171.rfm
16. Arslan S, Öncü F, Eryılmaz MA, Durmaz MS, Altunkeser A, Ünlü Y. Advantages of B-Mode Ultrasound Combined With Strain Elastography in Differentiation of Idiopathic Granulomatous Mastitis From Malignant Breast Lesions. Turk J Med Sci (2018) 48(1):16–23. doi: 10.3906/sag-1708-34
17. Makal GB, Güvenç İ. The Role of Shear Wave Elastography in Differentiating Idiopathic Granulomatous Mastitis From Breast Cancer. Acad Radiol (2021) 28(3):339–44. doi: 10.1016/j.acra.2020.02.008
18. Xiao X, Dong L, Jiang Q, Guan X, Wu H, Luo B. Incorporating Contrast-Enhanced Ultrasound Into the BI-RADS Scoring System Improves Accuracy in Breast Tumor Diagnosis: A Preliminary Study in China. Ultrasound Med Biol (2016) 42(11):2630–8. doi: 10.1016/j.ultrasmedbio.2016.07.005
19. Dietrich CF, Nolsøe CP, Barr RG, Berzigotti A, Burns PN, Cantisani V, et al. Guidelines and Good Clinical Practice Recommendations for Contrast Enhanced Ultrasound (CEUS) in the Liver - Update 2020 - WFUMB in Cooperation With EFSUMB, AFSUMB, AIUM, and FLAUS. Ultraschall Med (2020) 41(5):562–85. doi: 10.1055/a-1177-0530
20. Mulvana H, Browning RJ, Luan Y, de Jong N, Tang M-X, Eckersley RJ, et al. Characterization of Contrast Agent Microbubbles for Ultrasound Imaging and Therapy Research. IEEE Trans Ultrason Ferroelectr Freq Control (2017) 64(1):232–51. doi: 10.1109/TUFFC.2016.2613991
21. Jung EM, Jung F, Stroszczynski C, Wiesinger I. Quantification of Dynamic Contrast-Enhanced Ultrasound (CEUS) in non-Cystic Breast Lesions Using External Perfusion Software. Sci Rep (2021) 11(1):17677. doi: 10.1038/s41598-021-96137-6
22. Diao X, Zhan J, Chen L, Chen Y, Cao H. Role of Superb Microvascular Imaging in Differentiating Between Malignant and Benign Solid Breast Masses. Clin Breast Cancer (2020) 20(6):e786–93. doi: 10.1016/j.clbc.2020.06.009
23. Li J, Guo L, Yin L, Fang H, Ye W, Zhao B, et al. Can Different Regions of Interest Influence the Diagnosis of Benign and Malignant Breast Lesions Using Quantitative Parameters of Contrast-Enhanced Sonography? Eur J Radiol (2018) 108:1–6. doi: 10.1016/j.ejrad.2018.09.005
24. Zheng Y, Wang L, Han X, Shen L, Ling C, Qian Z, et al. Combining Contrast-Enhanced Ultrasound and Blood Cell Analysis to Improve Diagnostic Accuracy of Plasma Cell Mastitis. Exp Biol Med (Maywood) (2022) 247(2):97–105. doi: 10.1177/15353702211049361
25. Tang M, Xie Q, Wang J, Zhai X, Lin H, Zheng X, et al. Chu Y Et Al: Time Difference of Arrival on Contrast-Enhanced Ultrasound in Distinguishing Benign Inflammation From Malignant Peripheral Pulmonary Lesions. Front Oncol (2020) 10:578884. doi: 10.3389/fonc.2020.578884
26. Szabó BK, Saracco A, Tánczos E, Aspelin P, Leifland K, Wilczek B, et al. Correlation of Contrast-Enhanced Ultrasound Kinetics With Prognostic Factors in Invasive Breast Cancer. Eur Radiol (2013) 23(12):3228–36. doi: 10.1007/s00330-013-2960-5
27. Hu W, Dong Y, Zhang X, Zhang H, Li F, Bai M. The Clinical Value of Arrival-Time Parametric Imaging Using Contrast-Enhanced Ultrasonography in Differentiating Benign and Malignant Breast Lesions. Clin Hemorheol Microcirc (2020) 75(3):369–82. doi: 10.3233/CH-200826
28. Kotopoulis S, Popa M, Mayoral Safont M, Murvold E, Haugse R, Langer A, et al. SonoVue® vs. Sonazoid™ vs. Optison™: Which Bubble Is Best for Low-Intensity Sonoporation of Pancreatic Ductal Adenocarcinoma? Pharmaceutics (2022) 14(1):98. doi: 10.3390/pharmaceutics14010098
Keywords: granulomatous mastitis, breast cancer, quantitative parameters, imaging characteristics, contrasted-enhanced ultrasound
Citation: Yin L, Agyekum EA, Zhang Q, Pan L, Wu T, Xiao X and Qian X-q (2022) Differentiation Between Granulomatous Lobular Mastitis and Breast Cancer Using Quantitative Parameters on Contrast-Enhanced Ultrasound. Front. Oncol. 12:876487. doi: 10.3389/fonc.2022.876487
Received: 15 February 2022; Accepted: 20 June 2022;
Published: 15 July 2022.
Edited by:
Yao Lu, Sun Yat-sen University, ChinaReviewed by:
Atif Ali Hashmi, Liaquat National Medical College, PakistanCopyright © 2022 Yin, Agyekum, Zhang, Pan, Wu, Xiao and Qian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liang Yin, anVzdGluZmx5MjA4MEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.