
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Oncol. , 30 March 2022
Sec. Molecular and Cellular Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.874554
This article is part of the Research Topic Vascular Co-option and Beyond for Cancer Biology View all 8 articles
Non-angiogenic tumors grow in the absence of angiogenesis by two main mechanisms: cancer cells infiltrating and occupying the normal tissues to exploit pre-existing vessels (vascular co-option); the cancer cells themselves forms channels able to provide blood flow (the so called vasculogenic mimicry). In the original work on vascular co-option initiated by Francesco Pezzella, the non-angiogenic cancer cells were described as “exploiting” pre-existing vessels. Vascular co-option has been described in primary and secondary (metastatic) sites. Vascular co-option is defined as a process in which tumor cells interact with and exploit the pre-existing vasculature of the normal tissue in which they grow. As part of this process, cancer cells first migrate toward vessels of the primary tumor, or extravasate at a metastatic site and rest along the ab-luminal vascular surface. The second hallmark of vascular co-option is the interaction of cancer cells with the ab-luminal vascular surface. The first evidence for this was provided in a rat C6 glioblastoma model, showing that the initial tumor growth phase was not always avascular as these initial tumors can be vascularized by pre-existing vessels. The aim of this review article is to analyze together with vascular co-option, other alternative mode of vascularization occurring in glioblastoma multiforme (GBM), including vasculogenic mimicry, angiotropism and trans-differentiation of glioblastoma stem cells.
Three types of angiogenesis have been described in tumor growth: sprouting angiogenesis (1), intussusceptive microvascular growth (IMG) (2), and glomeruloid vascular proliferation (3) (Figure 1). Sprouting angiogenesis in tumor growth include the following stages: The basement membrane is locally degraded on the side of the dilated peritumoral postcapillary venule situated closed to the angiogenic stimulus; Interendothelial contacts are weakened and endothelial cells migrate into the connective tissue; A solid cord of endothelial cells form; Lumen formation occurs proximal to the migrating front, contiguous tubular sprouts anastomose to form functionally capillary loops, parallel with the synthesis of the new basement membrane and the recruitment of pericytes (1).
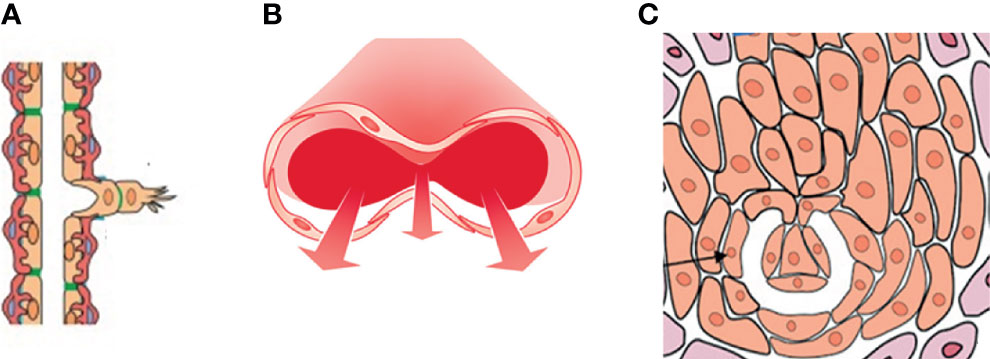
Figure 1 A drawing showing the three types of angiogenesis have been described in tumor growth: (A) sprouting angiogenesis, (B) intussusceptive microvascular growth (IMG), and (C) glomeruloid vascular proliferation. Sprouting angiogenesis involves formation and outgrowth of sprouts; IMG involves the formation of new vasculature where a pre-existing vessel splits in two; in glomeruloid vascular proliferation small glomeruloid bodies, so-called for their morphological resemblance with the renal glomeruli, are recognizable.
In IMG, the vascular network expands by insertion of newly formed columns of interstitial tissue structures (tissue pillars) into the vascular lumen. IMG proceeds through these steps: Protrusion of opposing capillary walls into the lumen and the creation of a contact zone between facing endothelial cells; Reorganization of their intercellular junctions and central perforation of the endothelial bilayer; Formation of an interstitial pillar core by invading supporting cells (myofibroblasts, pericytes) and deposition of matrix, such pillars ranging in diameter from 1 to 2.5 μm; Enlargement in thickness of the pillars without additional qualitative alteration (2). IMG occurs in different tumors, including colon and mammary carcinomas, melanoma, B-cell non-Hodgkin’s lymphoma and glioma (4).
A switch from sprouting to IMG might represent an adaptive response to treatment with various antitumor and anti-angiogenic compounds to restore the hemodynamic and structural properties of the vasculature enhancing tumor drug delivery and sensitivity to treatments (5).
In glomeruloid vascular described in glioblastoma (6), small glomeruloid bodies, so-called for their morphological resemblance with the renal glomeruli, are recognizable (Figure 2). Glomeruloid bodies are made up by small vessels lined by hyperplastic endothelial cells surrounded by a discontinuous layer of pericytes. Two types of glomeruloid bodies might exist (6). The first, formed by an “active” mechanism would be the one in which angiogenesis occurs and the glomeruloid vessels are newly formed, possibly because of the action of vascular endothelial growth factor (VEGF) (3). The second type or “passive” is one in which no new vessels are formed but pre-existing capillaries are coiled and folded by metastatic cells which extravasate and then adhere to the abluminal surface of the capillaries and pulling them into a glomeruloid shape (6).
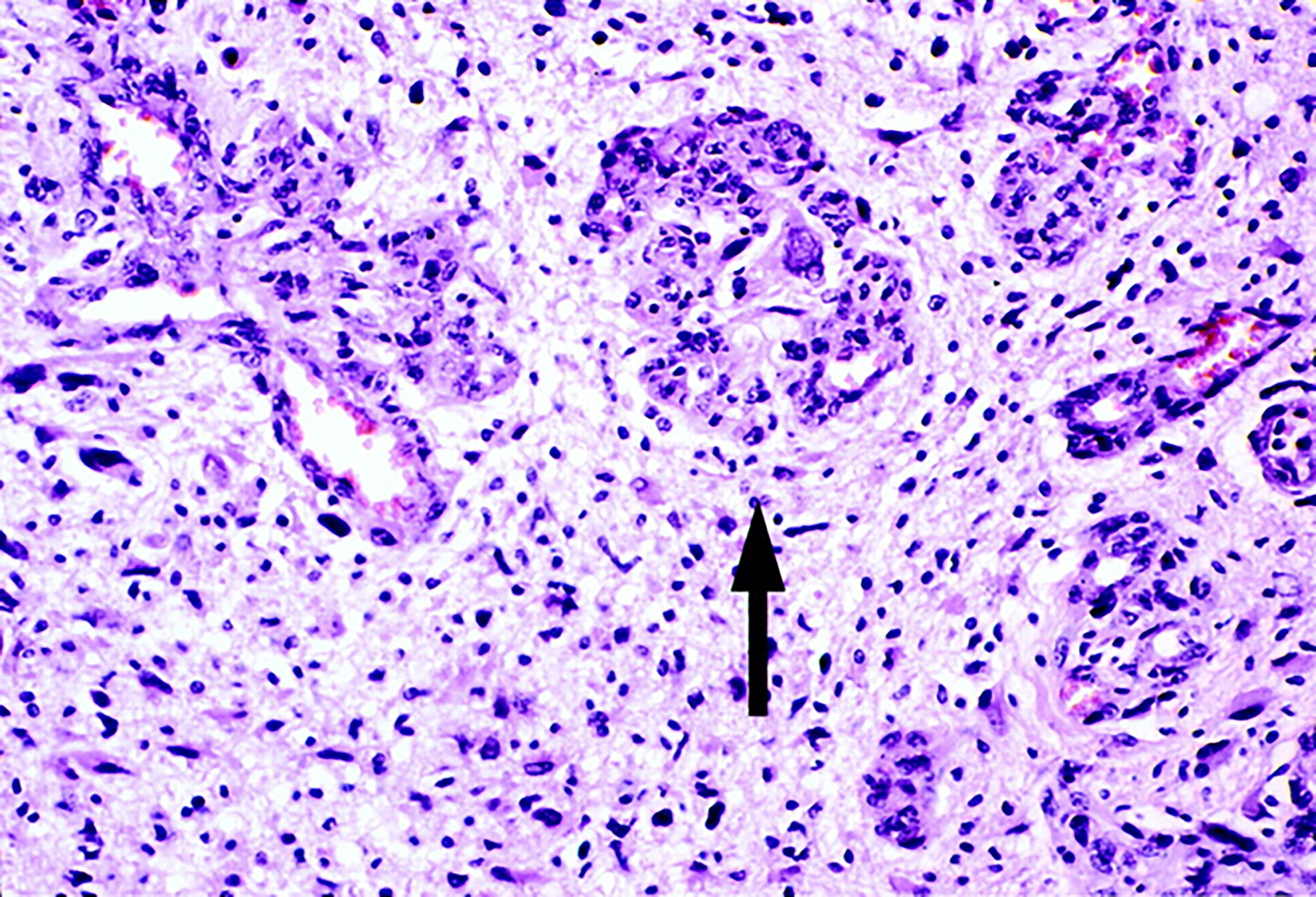
Figure 2 Glomeruloid vascular proliferation in a human glioblastoma multiforme bioptic specimen (arrow). Newly sprouted vessels arranged in tufted aggregates resemble renal glomeruli. Adjacent vessels demonstrate other morphological forms of microvascular hyperplasia in glioblastoma. Blue toluidine staining. Original magnification: x 25 (Reproduced from 7).
Tumors can also grow without inducing angiogenesis, as occurs in vessel co-option or vascular co-option (8), vasculogenic mimicry and angiotropism (9). In the original work on vascular co-option initiated by Francesco Pezzella, the non-angiogenic cancer cells were described as “exploiting” pre-existing vessels (10). Vascular co-option, described in primary and secondary (metastatic) sites, is defined as a process in which tumor cells interact with and exploit the pre-existing vasculature of the normal tissue in which they grow. In vessel co-option, tumors utilize alternative mechanisms besides angiogenesis to obtain nutrients for growth through local tumor invasion and proliferation along co-opted vessels. Cancer cells migrate along the pre-existing vessels and infiltrate tissues between co-opted vessels (8).
Vessel co-option was initially described in gliomas and lung metastasis (11–13). The first event observed following co-option was an increase in the levels of angiopoietin-2 (Ang-2) in the pre-existing vessels surrounded by tumor cells (11), without increase of VEGF expression, leading to vascular regression by detachment of the endothelium from the basement membrane. Ang-2 binds to its receptor Tie-2 inducing dissociation of the mural cells from endothelial cells (11). Moreover, Angiopoietin-2 (Ang-2) increases the secretion of matrix metalloproteinase-2 (MMP-2) favoring human glioma cells invasive capacity (14).
In vasculogenic mimicry, first described in uveal melanoma (15), tumor cells form vessel-like networks. In this condition, tumor cells reverse to an embryonic-like phenotype and mimic endothelial cells. Vasculogenic mimicry can serve as a marker for tumor metastasis, a poor prognosis, worse survival, and the highest risk of cancer recurrence.
Angiotropism (the pericytic-like location of tumor cells) is a microscopic marker of migration of tumor cells along the abluminal vascular surface (9). Glioma cells follow ab-luminal surface of blood vessels (16) and migrate considerable distances without employing intravascular dissemination (17).
Glioblastoma multiforme (glioblastoma IDH-wild type) is the most aggressive brain tumor with high recurrence and mortality rate. To further limit the molecular heterogeneity of tumors subsumed as ‘glioblastoma’, the upcoming 2021 World Health Organization (WHO) classification of primary brain tumors will introduce a definition of glioblastoma based on typical histological features and the absence of IDH mutations (18). IDH mutations characterize a subpopulation of glioblastomas and indicate a better prognosis (18). The vasculature of IDH mutated glioblastomas differs from that of IDH wild-type GBM, including a lower frequency of vascular abnormalities in IDH mutated glioblastomas (19).
With a median survival of 14-18 months and 5-year survival rates of less than 5%, the prognosis of GBM patients is very poor (20). The standard treatment for GBM patients is maximal tumor resection followed by adjuvant radiotherapy and adjuvant chemotherapy using alkylating agent temozolomide (the “stupp protocol”, 21).
One of the most significant features of GBM is the hypervascularity and there is a significant correlation between the degree of angiogenesis and prognosis (22). VEGF is highly expressed in GBM and is correlated with the grade of malignancy and prognosis (23, 24). Other angiogenic cytokines, including hepatocyte growth factor (HGF), fibroblast growth factor-2 (FGF-2), platelet derived growth factor (PDGF), Angs, and interleukin-8 (IL-8) are also up-regulated in GBM (24–27). In GBM, tumor-associated macrophages (TAMs) crosstalk with Treg cells to release pro-angiogenic and immune-suppressive VEGF (28).
GBM vessels are characterized by structural and functional abnormalities, including altered association between endothelial cells and pericytes, leading to chronic hyperpermeability, vessel leakage, poor vessel perfusion and delivery of nutrients (29). All these morphological characteristics contribute to hypoxia, interstitial fluid pressure and enhanced susceptibility to metastatic invasion (30). Furthermore, hypoxia-mediated up-regulation of pro-angiogenic factors secretion by inflammatory and tumor cells, enhance vascular abnormalities.
Different types of neovascularization occur in GBM, including vasculogenesis, angiogenesis, IMG (Figure 3), vascular co-option, vasculogenic mimicry, and trans-differentiation of glioblastoma stem-like cells (GSCs) in endothelial cell-like cells (31, 32). When GSCs were cultured ex vivo under endothelial favorable conditions, they expressed typical endothelial markers, such as CD31, von Willebrand factor (vWF), and Tie-2 (32, 33). Endothelial cells promote the GSC phenotype in the perivascular niche through direct cell–cell interactions by activating the Notch pathway in GSCs through the expression of Notch ligands and release of nitric oxide (34–37). Moreover, GSCs can secrete diffusible factors such as VEGF, which recruit tumor blood vessels to the niche (38, 39). Other modalities of interactions between tumor cells and endothelial cells in GMB include microRNA-containing extracellular vesicles, gap junctions and non-coding RNAs (40–43).
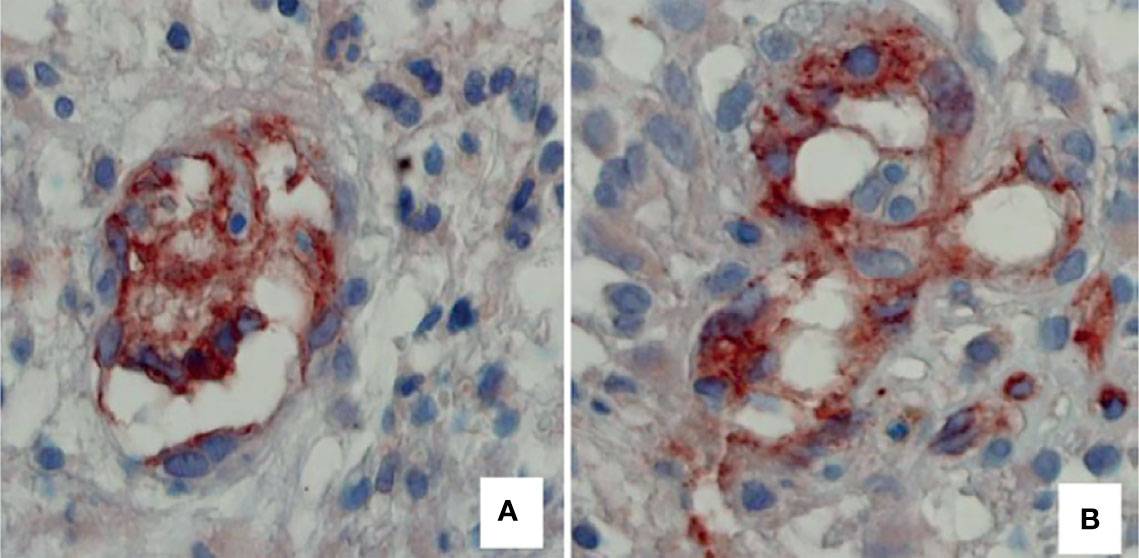
Figure 3 Two examples of tumor vessels, respectively, with a low and high number of connections of intraluminal tissue folds with the opposite vascular wall, expression of intussusceptive microvascular growth in II malignancy grade tumor specimen (A), compared with IV malignancy grade (B). Blood vessels have been identified by immunohistochemical reaction with an anti-CD31 antibody. Original magnification: x 60 (Reproduced from 4).
C6 rat glioma cells co-opted brain vessels at early stages soon after their orthotopic injection (11). After serial transplantation of human derived GBM cells, early passaged tumor cells co-opted the brain vasculature, while at later passaged, angiogenesis occurs. Spheroids from human glioma patient tumors co-opt the host vasculature, showing an aggressive infiltrative growth pattern (44).
In GBM, tumor cells displace astrocytic endfeet from endothelial cells, leading to abnormal blood-brain barrier (BBB) permeability and loss of astrocytic-mediated glio-vascular coupling (17, 45–47). Caspani et al. (46) studied interactions occurring between GBM cells and pericytes associated with brain blood vessels and demonstrated that GBM cells produced cytoplasmic expansions denominated flectopodia which adhere to pericytes, forming hybrid cells.
Orthotopic injection of GSCs in immunocompromised mice generated large anaplastic tumor xenografts, showing a vessel wall formed by endothelial cells derived from GSCs (33). GSCs support vascular function by generating pericytes in a process enhanced by hypoxia (48). Endothelial cells induce GSCs features in differentiated GBM cells through FGF-2 (49), and tumor-derived endothelial cells share the same somatic mutations as GBM cells, suggesting that tumor endothelial cells derive from GMB cells (31).
In GBM, vasculogenic mimicry is characterized by the activation of epithelial-mesenchymal transition (EMT)-related proteins, such as Twist1 (50), up-regulation of IL-6 expression in glioma cells (51), and trans-differentiation of GSCs into mural cells (52).
Resistance to anti-angiogenic treatment can be intrinsic, when it is observed at the beginning of the treatment, or acquired, i.e., that it affects the relapsing disease after an initial response to therapy (53).
Resistance to VEGF pathway inhibitors involves different mechanisms, including normalization of tumor blood vessels, alternative mechanisms of vessel formation, hypoxia, recruitment of inflammatory cells and immature myeloid cells (53). The most accepted hypothesis for acquired resistance to anti-angiogenic therapies is based on the induction or up-regulation of other pro-angiogenic factor pathways, including IL-8, FGF-2, PDGF and Angs (53). PDGF-BB can induce GBM formation when overexpressed with the RCAS system (54).
Non-angiogenic growth is an important mechanism of acquired resistance to anti-angiogenic therapy. Tumor cells might evade anti-VEGF therapies using existing vasculature and increasing the fraction of co-opted vessels (55). Vascular co-option has been proposed to be a mechanism of resistance to anti-VEGF therapies (56–58). In GBM, the aberrant vasculature favor increasing resistance and limitations to the efficacy of conventional therapies.
Anti-VEGF antibody treatment increased the fraction of co-opted vasculature in human glioblastoma cells injected into nude rat striatum (59). Treatment of GBM with a monoclonal antibody against VEGF receptor-2 (VEGFR-2) induces co-option of quiescent cerebral vessels (60). Modified GBM-resident endothelial cells express lower levels of VEGFR and this might ultimately dampen the efficacy of anti-VEGF therapies (61). Vascular co-option has been observed in GBM after anti-angiogenic therapy with cediranib (62).
Intravital imaging identified ephrin-B2 on endothelial cells and GSCs as an important regulator of vessel co-option and B11, a single-chain variable fragment directed against ephrin-B2 efficiently blocked cooption and tumor growth (13, 63). Chemotherapy and/or radiation therapeutic might increase GSC subpopulation and emerging tumor-derived endothelial cells. For instance, irradiated GSCs express Tie2, migrate towards VEGF, and form tubes on Matrigel in vitro (64). Moreover, temozolomide combined or not with bevacizumab, potentiates tumor-derived endothelial cell incorporation in vessels from xenograft models (65). In this context, GSC trans differentiation contributes to both resistance to anti-angiogenic therapies and re-vascularization following chemotherapy and/or radiation.
Bevacizumab obtained clinical approval by the US Food and Drug Administration for the treatment of GBM at progression after standard chemoradiotherapy. Bevacizumab inhibits angiogenesis and tumor growth in pre-clinical models of GBM (59, 66–68), and in combination with radiotherapy and chemotherapy with temozolomide was associated with a significant improvement of progression free survival (PFS), but only a modest improvement of overall survival (OS) (69–71). However, bevacizumab in combination with temozolomide or lomustine, respectively, did not prolong OS in patients with newly diagnosed or recurrent GBM in phase III clinical trials (71–73).
Several tyrosine kinase inhibitors, which inhibit PDGF receptor (PDGFR) and transforming growth factor beta (TGFβ), were ineffective in clinical trials (74–76). Chemotherapeutic stress after temozolomide treatment increase HIF response in recurrent GBM, leading to trans-differentiation of GSCs to endothelial cells, promoting vasculogenic mimicry (77).
Immune check-points inhibitors might induce an improved immune response against the co-opting cancer cells and might synergize with anti-angiogenic therapies (78). Immune check-points inhibitors have been successfully used in GBM mouse models (79–83), while immunotherapy is not working in human glioblastomas (84)”.
Blockade of VEGF, Ang-2, and PD-1 increased the survival of GBM-bearing mice in comparison to anti-VEGF and anti-Ang-2 alone (85). Targeting endothelial PAK4 promoted GBM vessel normalization, which in turn improved engineered chimeric antigen receptor T cells (CAR-T) infiltration and extended mouse survival (86).
DR conceived and wrote the manuscript; FP revised the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported in part by HORIZON EUROPE, GRANT CODE S08 (INTERGLIO) funded by the University of Bari Aldo Moro, Bari, Italy.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ausprunk DH, Folkman J. Migration and Proliferation of Endothelial Cells in Preformed and Newly Formed Blood Vessels During Tumor Angiogenesis. Microvasc Res (1977) 14:53–65. doi: 10.1016/0026-2862(77)90141-8
2. Ribatti D, Djonov V. Intussusceptive Microvascular Growth in Tumors. Cancer Lett (2012) 316:126–31. doi: 10.1016/j.canlet.2011.10.040
3. Sundberg C, Nagy JA, Brown LF, Feng D, Eckelhoefer IA, Manseau AM, et al. Glomeruloid Microvascular Proliferation Follows Adenoviral Vascular Permeability Factor/Vascular Endothelial Growth Factor-164 Gene Delivery. Am J Pathol (2001) 158:1145–60. doi: 10.1016/S0002-9440(10)64062-X
4. Nico B, Crivellato E, Guidolin D, Annese T, Longo V, Finato N, et al. Intussusceptive Microvascular Growth in Human Glioma. Clin Exp Med (2010) 10:93–8. doi: 10.1007/s10238-009-0076-7
5. Semela D, Piguet AC, Kolev M, Schmitter K, Hlushechuk R, Djonov V, et al. Vascular Remodeling and Antitumoral Effects of mTOR Inhibition in a Rat Model of Hepatocellular Carcinoma. J Hepatol (2007) 46:840–8. doi: 10.1016/j.jhep.2006.11.021
6. Dome B, Tımár J, Paku S. A Novel Concept of Glomeruloid Body Formation in Experimental Cerebral Metastases. J Neuropathol Exp Neurol (2003) 62:655–61. doi: 10.1093/jnen/62.6.655
7. Brat DJ, Van Meir EG. Glomeruoloid Microvascular Proliferation Orchestrated by VPF/VEGF. Am J Pathol (2001) 158:789–96. doi: 10.1016/S0002-9440(10)64025-4
8. Kuczynski EA, Vermeulen PB, Pezzella F, Kerbel RS, Reynolds AR. Vessel Co-Option in Cancer. Nat Rev Clin Oncol (2019) 16:469–83. doi: 10.1038/s41571-019-0181-9
9. Lugassy C, Zadran S, Bentolila LA, Wadehra M, Prakash R, Carmichael ST, et al. Angiotropism, Pericytic Mimicry and Extravascular Migratory Metastasis in Melanoma; an Alternative to Intravascular Cancer Dissemination. Cancer Microenviron (2014) 7:139–52. doi: 10.1007/s12307-014-0156-4
10. Pezzella F, Pastorino U, Tagliabue E, Andreola S, Sozzi G, Gasparini G, et al. Non-Small-Cell Lung Carcinoma Tumor Growth Without Morphological Evidence of Neoangiogenesis. Am J Pathol (1997) 151:1417–23.
11. Holash J, Maisonpierre PC, Compton D, Boland P, Alexander CR, Zagzag D, et al. Vessel Cooption, Regression, and Growth in Tumors Mediated by Angiopoietins and VEGF. Science (1999) 284:1994–8. doi: 10.1126/science.284.5422.1994
12. Cai Y, Wu J, Li Z, Long Q. Mathematical Modelling of a Brain Tumour Initiation and Early Development: A Coupled Model of Glioblastoma Growth, Pre-Existing Vessel Co-Option, Angiogenesis and Blood Perfusion. PloS One (2016) 11:e0150296. doi: 10.1371/journal.pone.0150296
13. Krusche B, Ottone C, Clements MP, Johnstone ER, Goetsch K, Lieven H, et al. EphrinB2 Drives Perivascular Invasion and Proliferation of Glioblastoma Stem-Like Cells. eLife (2016) 5:e14845. doi: 10.7554/eLife.14845
14. Hu B, Guo P, Fang Q, Tao HQ, Wang D, Nagane M, et al. Angiopoietin-2 Induces Human Glioma Invasion Through the Activation of Matrix Metalloprotease-2. Proc Natl Acad Sci USA (2003) 100:8904–9. doi: 10.1073/pnas.1533394100
15. Maniotis AJ, Folberg R, Hess A, Seftor EA, Gardner LM, Pe'er J, et al. Vascular Channel Formation by Human Melanoma Cells In Vivo and In Vitro: Vasculogenic Mimicry. Am J Pathol (1999) 155:739–52. doi: 10.1016/S0002-9440(10)65173-5
16. Gritsensko P, Leenders W, Friedl P. Recapitulating In Vivo-Like Plasticity of Glioma Cell Invasion Along Blood Vessels and in Astrocyte-Rich Stroma. Histochem Cell Biol (2017) 148:395–406. doi: 10.1007/s00418-017-1604-2
17. Lugassy C, Vernon SE, Busam K, Engbring JA, Welch DR, Poulos EG, et al. Pericytic-Like Angiotropism of Glioma and Melanoma Cells. Am J Dermatopathol (2002) 24:473–8. doi: 10.1097/00000372-200212000-00003
18. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro Oncol (2021) 23:1231–51. doi: 10.1093/neuonc/noab106
19. Lai A, Kharbanda S, Pope WB, Tran A, Solis OE, Peale F, et al. Evidence for Sequenced Molecular Evolution of IDH1 Mutant Glioblastoma From a Distinct Cell of Origin. J Clin Oncol (2011) 29:4482–90. doi: 10.1200/JCO.2010.33.8715
20. Wen PY, Kesari S. Malignant Gliomas in Adults. N Engl J Med (2008) 359:492–507. doi: 10.1056/NEJMra0708126
21. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy Plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N Engl J Med (2005) 352:987–96. doi: 10.1056/NEJMoa043330
22. Norden AD, Drappatz J, Wen PY. Antiangiogenic Therapies for High-Grade Glioma. Nat Rev Neurol (2009) 5:619. doi: 10.1038/nrneurol.2009.159
23. Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meiret EG. Hypoxia and the Hypoxia-Inducible-Factor Pathway in Glioma Growth and Angiogenesis. Neuro Oncol (2005) 7:134–54. doi: 10.1215/S1152851704001115
24. Schmidt NO, Westphal M, Hagel C, Ergün S, Stavrou D, Eliot M, et al. Levels of Vascular Endothelial Growth Factor, Hepatocyte Growth Factor-Scatter Factor and Basic Fibroblast Growth Factor in Human Gliomas and Their Relation to Angiogenesis. Int J Cancer (1999) 84:10–8. doi: 10.1002/(SICI)1097-0215(19990219)84:1<10::AID-IJC3>3.0.CO;2-L
25. Shih AH, Holland EC. Platelet-Derived Growth Factor (PDGF) and Glial Tumorigenesis. Cancer Lett (2006) 232:139–47. doi: 10.1016/j.canlet.2005.02.002
26. Reiss Y, Machein MR, Plate KH. The Role of Angiopoietins During Angiogenesis in Gliomas. Brain Pathol (2005) 15:311–7. doi: 10.1111/j.1750-3639.2005.tb00116.x
27. Brat DJ, Bellail AC, Van Meir EG. The Role of Interleukin-8 and Its Receptors in Gliomagenesis and Tumoral Angiogenesis. Neuro Oncol (2005) 7:122–33. doi: 10.1215/S1152851704001061
28. Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, et al. Tumour Hypoxia Promotes Tolerance and Angiogenesis via CCL28 and T(reg) Cells. Nature (2011) 475:226–30. doi: 10.1038/nature10169
29. Plate KH, Mennel HD. Vascular Morphology and Angiogenesis in Glial Tumors. Exp Toxicol Pathol (1995) 47:89–94. doi: 10.1016/S0940-2993(11)80292-7
30. Barlow KD, Sanders AM, Soker S, Ergun S, Metheny-Barlow LJ. Pericytes on the Tumor Vasculature: Jekyll or Hyde? Cancer Microenviron (2013) 6:1–17. doi: 10.1007/s12307-012-0102-2
31. Wang R, Chadalavada K, Wilshire J, Kowalik U, Hovinga KE, Geber A, et al. Glioblastoma Stem-Like Cells Give Rise to Tumour Endothelium. Nature (2010) 468:829–33. doi: 10.1038/nature09624
32. Soda Y, Marumoto T, Friedmann-Morvinski D, Soda M, Liu F, Michiue H, et al. Transdifferentiation of Glioblastoma Cells Into Vascular Endothelial Cells. Proc Natl Acad Sci USA (2011) 108:4274–80. doi: 10.1073/pnas.1016030108
33. Ricci-Vitiani L, Pallini R, Biffoni B, Todaro M, Invernici G, Cenci T, et al. Tumour Vascularization via Endothelial Differentiation of Glioblastoma Stem-Like Cells. Nature (2010) 468:824–8. doi: 10.1038/nature09557
34. Zhu TS, Costello MA, Talsma CE, Flack CG, Crowley JG, Hamm LL, et al. Endothelial Cells Create a Stem Cell Niche in Glioblastoma by Providing NOTCH Ligands That Nurture Self-Renewal of Cancer Stem-Like Cells. Cancer Res (2011) 71:6061–72. doi: 10.1158/0008-5472.CAN-10-4269
35. Hovinga KE, Shimizu F, Wang R, Panagiotakos G, Van Der Heijden M, Moayedpardazi H, et al. Inhibition of Notch Signaling in Glioblastoma Targets Cancer Stem Cells via an Endothelial Cell Intermediate. Stem Cells (2010) 28:1019–29. doi: 10.1002/stem.429
36. Charles N, Ozawa T, Squatrito M, Bleau AM, Brennan CW, Hambardzumyan D, et al. Perivascular Nitric Oxide Activates Notch Signaling and Promotes Stem-Like Character in PDGF-Induced Glioma Cells. Cell Stem Cell (2010) 6:141–52. doi: 10.1016/j.stem.2010.01.001
37. Eyler CE, Wu Q, Yan K, MacSwords JM, Chandler-Militello D, Misuraca KL, et al. Glioma Stem Cell Proliferation and Tumor Growth Are Promoted by Nitric Oxide Synthase 2. Cell (2011) 146:53–66. doi: 10.1016/j.cell.2011.06.006
38. Gilbertson RJ, Rich JN. Making a Tumour’s Bed: Glioblastoma Stem Cells and the Vascular Niche. Nat Rev Cancer (2007) 7:733–6. doi: 10.1038/nrc2246
39. Kumar S, Bar−Lev L, Sharife H, Grunewald M, Mogilevsky M, Licht T, et al. Identification of Vascular Cues Contributing to Cancer Cell Stemness and Function. Angiogenesis (2022) 25. doi: 10.1007/s10456-022-09830-z
40. Würdinger T, Tannous BA, Saydam O, Skog J, Grau S, Soutschek J, et al. miR-296 Regulates Growth Factor Receptor Overexpression in Angiogenic Endothelial Cells. Cancer Cell (2008) 14:382–93. doi: 10.1016/j.ccr.2008.10.005
41. Thuringer D, Boucher J, Jego G, Pernet N, Cronier L, Hammann A, et al. Transfer of Functional microRNAs Between Glioblastoma and Microvascular Endothelial Cells Through Gap Junctions. Oncotarget (2016) 7:73925–34. doi: 10.18632/oncotarget.12136
42. Lucero R, Zappulli V, Sammarco A, Murillo OD, See Cheah P, Srinivasan S, et al. Glioma-Derived miRNA-Containing Extracellular Vesicles Induce Angiogenesis by Reprogramming Brain Endothelial Cells. Cell Rep (2020) 30:2065–74. doi: 10.1016/j.celrep.2020.01.073
43. Li D, Zhang Z, Xia C, Niu C, Zhou W. Non-Coding RNAs in Glioma Microenvironment and Angiogenesis. Front Mol Neurosci (2021) 14:763610. doi: 10.3389/fnmol.2021.763610
44. Huszthy PC, Daphu I, Niclou SP, Stieber D, Nigro JM, Sakariassen PØ, et al. In Vivo Models of Primary Brain Tumors: Pitfalls and Perspectives. Neuro Oncol (2012) 14:979–93. doi: 10.1093/neuonc/nos135
45. Watkins S, Robel S, Kimbrough IF, Robert SM, Ellis-Davies G, Sontheimer H. Disruption of Astrocyte-Vascular Coupling and the Blood-Brain Barrier by Invading Glioma Cells. Nat Commun (2014) 5:4196. doi: 10.1038/ncomms5196
46. Caspani EM, Crossley PH, Redondo-Garcia C, Martinez S. Glioblastoma: A Pathogenetic Crosstalk Between Tumor Cells and Pericytes. PLoS ONE (2014) 9:e101402. doi: 10.1371/journal.pone.0101402
47. Nagano N, Sasaki H, Aoyagi M, Hirakawa K. Invasion of Experimental Rat Brain Tumor: Early Morphological Changes Following Microinjection of C6 Glioma Cells. Acta Neuropathol (1993) 86:117–25. doi: 10.1007/BF00334878
48. Cheng L, Huang Z, Zhou W, Wu Q, Donnola S, Liu JK, et al. Glioblastoma Stem Cells Generate Vascular Pericytes to Support Vessel Function and Tumor Growth. Cell (2013) 153:139–52. doi: 10.1016/j.cell.2013.02.021
49. Fessler E, Borovski T, Medema JP. Endothelial Cells Induce Cancer Stem Cell Features in Differentiated Glioblastoma Cells via bFGF. Mol Cancer (2015) 14:157. doi: 10.1186/s12943-015-0420-3
50. Cao W, Xu C, Li X, Yang X. Twist1 Promotes Astrocytoma Development by Stimulating Vasculogenic Mimicry. Oncol Lett (2019) 18:846–55. doi: 10.3892/ol.2019.10380
51. Zhang L, Xu Y, Sun J, Chen W, Zhao L, Ma C, et al. M2-Like Tumor-Associated Macrophages Drive Vasculogenic Mimicry Through Amplification of IL-6 Expression in Glioma Cells. Oncotarget (2017) 8:819–32. doi: 10.18632/oncotarget.13661
52. Scully S, Francescone R, Faibish M, Bentley B, Taylor SL, Oh D, et al. Transdifferentiation of Glioblastoma Stem-Like Cells Into Mural Cells Drives Vasculogenic Mimicry in Glioblastomas. J Neurosci (2012) 32:12950–60. doi: 10.1523/JNEUROSCI.2017-12.2012
53. Ribatti D. Anti-Angiogenic Cancer Therapy: Development of Resistance. In: Marmé D, editor. Tumor Angiogenesis. A Key Target for Cancer Therapy. Switzerland: Springer Nature (2019). p. 313–23.
54. Hambardzumyan D, Amankulor NM, Helmy KY, Becher OJ, Holland EC. Modeling Adult Glioma Using RCSA/t-Va Technology. Transl Oncol (2009) 2:89–95. doi: 10.1593/tlo.09100
55. Zhang Y, Wang S, Dudley AC. Models and Molecular Mechanisms of Blood Vessel Co-Option by Cancer Cells. Angiogenesis (2020) 23:17–25. doi: 10.1007/s10456-019-09684-y
56. Bridgeman VL, Vermeulen PB, Foo S, Bilecz A, Daley F, Kostaras E, et al. Vessel Co-Option Is Common in Human Lung Metastases and Mediates Resistance to Anti-Angiogenic Therapy in Preclinical Lung Metastasis Models. J Pathol (2017) 241:362–74. doi: 10.1002/path.4845
57. Frentzas S, Simoneau E, Bridgeman VL, Vermeulen PB, Foo S, Kostaras E, et al. Vessel Co-Option Mediates Resistance to Anti-Angiogenic Therapy in Liver Metastasis. Nat Med (2016) 22:1294–302. doi: 10.1038/nm.4197
58. Kuczynski EA, Yin M, Bar-Zion A, Lee CR, Butz H, Man S, et al. Co-Option of Liver Vessels and Not Sprouting Angiogenesis Drives Acquired Sorafenib Resistance in Hepatocellular Carcinoma. J Natl Cancer Inst (2016) 108:djw 030. doi: 10.1093/jnci/djw030
59. Rubenstein JL, Kim J, Ozawa T, Zhang M, Westphal M, Deen D, et al. Anti-VEGF Antibody Treatment of Glioblastoma Prolongs Survival But Results in Increased Vascular Co-Option. Neoplasia (2000) 2:306–14. doi: 10.1038/sj.neo.7900102
60. Kunkel P, Ulbricht U, Bohlen P, Brockmann MA, Fillbrandt R, Stavrou D, et al. Inhibition of Glioma Angiogenesis and Growth In Vivo by Systemic Treatment With a Monoclonal Antibody Against Vascular Endothelial Growth Factor Receptor-2. Cancer Res (2001) 61:6624–8.
61. Liu T, Ma W, Xu H, Huang M, Zhang D, He Z, et al. PDGF-Mediated Mesenchymal Transformation Renders Endothelial Resistance to Anti-VEGF Treatment in Glioblastoma. Nat Commun (2018) 9:3439. doi: 10.1038/s41467-018-05982-z
62. Di Tomaso E, Snuderl M, Kamoun WS, Duda DG, Auluck PA, Fazlollahi L, et al. Glioblastoma Recurrence After Cediranib Therapy in Patients: Lack of ‘Rebound’ Revascularization as a Mode of Escape. Cancer Res (2003) 71:19–28. doi: 10.1158/0008-5472.CAN-10-2602
63. Abengozar MA, de Frutos S, Ferreiro S, Soriano J, Perez-Martinez M, Olmeda D, et al. Blocking Ephrinb2 With Highly Specific Antibodies Inhibits Angiogenesis, Lymphangiogenesis, and Tumor Growth. Blood (2012) 119:4565–76. doi: 10.1182/blood-2011-09-380006
64. Deshors P, Toulas C, Arnauduc F, Malric L, Siegfried A, Nicaise Y, et al. Ionizing Radiation Induces Endothelial Transdifferentiation of Glioblastoma Stem-Like Cells Through the Tie2 Signaling Pathway. Cell Death Dis (2019) 10:816. doi: 10.1038/s41419-019-2055-6
65. Xue W, Du X, Wu H, Liu H, Xie T, Tong H, et al. Aberrant Glioblastoma Neovascularization Patterns and Their Correlation With DCE-MRI-Derived Parameters Following Temozolomide and Bevacizumab Treatment. Sci Rep (2017) 7:13894. doi: 10.1038/s41598-017-14341-9
66. Kim KJ, Li B, Winer J, Armanini M, Gillett N, Phillips HS, et al. Inhibition of Vascular Endothelial Growth Factor-Induced Angiogenesis Suppresses Tumour Growth In Vivo. Nature (1993) 362:841–4. doi: 10.1038/362841a0
67. Jahnke K, Muldoon LL, Varallyay CG, Lewin SJ, Kraemer DF, Neuwelt EA. Bevacizumab and Carboplatin Increase Survival and Asymptomatic Tumor Volume in a Glioma Model. Neuro Oncol (2009) 11:142–50. doi: 10.1215/15228517-2008-077
68. Lee CG, Heijn M, di Tomaso E, Griffon-Etienne G, Ancukiewicz M, Koike C, et al. Anti-Vascular Endothelial Growth Factor Treatment Augments Tumor Radiation Response Under Normoxic or Hypoxic Conditions. Cancer Res (2000) 60:5565–70.
69. Lai A, Tran A, Nghiemphu PL, Pope WB, Solis OE, Selch M, et al. Phase II Study of Bevacizumab Plus Temozolomide During and After Radiation Therapy for Patients With Newly Diagnosed Glioblastoma Multiforme. J Clin Oncol (2011) 29:142–8. doi: 10.1200/JCO.2010.30.2729
70. Vredenburgh JJ, Desjardins A, Reardon DA, Peters KB, Herndon JE II, Marcello J, et al. The Addition of Bevacizumab to Standard Radiation Therapy and Temozolomide Followed by Bevacizumab, Temozolomide, and Irinotecan for Newly Diagnosed Glioblastoma. Clin Cancer Res (2011) 17:4199–24. doi: 10.1158/1078-0432.CCR-11-0120
71. Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab Plus Radiotherapy-Temozolomide for Newly Diagnosed Glioblastoma. N Engl J Med (2014) 370:709–22. doi: 10.1056/NEJMoa1308345
72. Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A Randomized Trial of Bevacizumab for Newly Diagnosed Glioblastoma. N Engl J Med (2014) 370:699–708. doi: 10.1056/NEJMoa1308573
73. Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, et al. Lomustine and Bevacizumab Inprogressive Glioblastoma. N Engl J Med (2017) 377:1954–63. doi: 10.1056/NEJMoa1707358
74. Wen PY, Yung WK, Lamborn KR, Dahia PL, Wang Y, Peng B, et al. PhaseI/II Study of Imatinib Mesylate for Recurrent Malignant Gliomas: North American Brain Tumor Consortium Study 99-08. Clin Cancer Res (2006) 12:4899–907. doi: 10.1158/1078-0432.CCR-06-0773
75. Hainsworth JD, Ervin T, Friedman E, Priego V, Murphy PB, Clark BL, et al. Concurrent Radiotherapy and Temozolomide Followed by Temozolomide and Sorafenib in the First-Line Treatment of Patients With Glioblastoma Multiforme. Cancer (2010) 116:3663–9. doi: 10.1002/cncr.25275
76. Neyns B, Sadones J, Chaskis C, Dujardin M, Everaert H, Lv S, et al. Phase II Study of Sunitinib Malate in Patients With Recurrent High-Grade Glioma. J Neurooncol (2011) 103:491–501. doi: 10.1007/s11060-010-0402-7
77. Baisiwala S, Auffinger B, Caragher SP, Shireman JM, Ahsan R, Lee G, et al. Chemotherapeutic Stress Induces Transdifferentiation of Glioblastoma Cells to Endothelial Cells and Promotes Vascular Mimicry. Stem Cells Int (2019) 2019:6107456. doi: 10.1155/2019/6107456
78. Allen E, Jabouille A, Rivera LB, Lodewijckx J, Missiaen R, Steri V, et al. Combined Anti-Angiogenic and Anti-PD-L1 Therapy Stimulates Tumor Immunity Through HEV Formation. Sci Transl Med (2005) 9:eaak9679. doi: 10.1126/scitranslmed.aak9679
79. Huang BY, Zhan YP, Zong WJ, Yu CJ, Li JF, Qu YM, et al. The PD-1/B7-H1 Pathway Modulates the Natural Killer Cells Versus Mouse Glioma Stem Cells. PloS One (2015) 10:e0134715. doi: 10.1371/journal.pone.0134715
80. Zeng J, See AP, Phallen J, Jackson CM, Belcaid Z, Ruzevick J, et al. Anti-PD-1 Blockade and Stereotactic Radiation Produce Long-Term Survival in Mice With Intracranial Gliomas. Int J Radiat Oncol Biol Phys (2013) 86:343–9. doi: 10.1016/j.ijrobp.2012.12.025
81. Harris-Bookman S, Mathios D, Martin AM, Xia Y, Kim E, Xu H, et al. Expression of LAG-3 and Efficacy of Combination Treatment With Anti-LAG-3 and Anti-PD-1 Monoclonal Antibodies in Glioblastoma. Int J Cancer (2018) 143:3201–8. doi: 10.1002/ijc.31661
82. Hung AL, Maxwell R, Theodros D, Belcaid Z, Mathios D, Luksik AS, et al. TIGIT and PD-1 Dual Checkpoint Blockade Enhances Antitumor Immunity and Survival in GBM. Oncoimmunology (2018) 7:e1466769. doi: 10.1080/2162402X.2018.1466769
83. Kim JE, Patel MA, Mangraviti A, Kim ES, Theodros D, Velarde E, et al. Combination Therapy With Anti-PD-1, Anti-TIM-3, and Focal Radiation Results in Regression of Murine Gliomas. Clin Cancer Res (2017) 23:124–36. doi: 10.1158/1078-0432.CCR-15-1535
84. Reardon DA, Brandes AA, Omuro A, Mulholland P, Lim M, Wick A, et al. Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma. JAMA Oncol (2020) 6:1–8. doi: 10.1001/jamaoncol.2020.1024
85. Di Tacchio M, Macas J, Weissenberger J, Sommer K, Bahr O, Steinbach JP, et al. Tumor Vessel Normalization, Immunostimulatory Reprogramming, and Improved Survival in Glioblastoma With Combined Inhibition of PD-1, Angiopoietin-2, and VEGF. Cancer Immunol Res (2019) 7:1910–27. doi: 10.1158/2326-6066.CIR-18-0865
Keywords: angiotropism, glioblastoma, glioblastoma stem cells, vascular co-option, vasculogenic mimicry
Citation: Ribatti D and Pezzella F (2022) Vascular Co-Option and Other Alternative Modalities of Growth of Tumor Vasculature in Glioblastoma. Front. Oncol. 12:874554. doi: 10.3389/fonc.2022.874554
Received: 12 February 2022; Accepted: 04 March 2022;
Published: 30 March 2022.
Edited by:
Monica Fedele, Consiglio Nazionale Delle Ricerche (CNR), ItalyReviewed by:
Aurélie Tchoghandjian, Institut de neurophysiopathologie, FranceCopyright © 2022 Ribatti and Pezzella. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Domenico Ribatti, ZG9tZW5pY28ucmliYXR0aUB1bmliYS5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.