
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 14 April 2022
Sec. Skin Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.873771
Background: Atypical fibroxanthoma (AFX) and pleomorphic dermal sarcoma (PDS) are increasingly common sarcomas of the skin with a genetic UV signature. Immunosuppression is a known risk factor for developing other UV-induced skin cancers such as cutaneous squamous cell carcinoma (cSCC), basal cell carcinoma (BCC), and Merkel cell carcinoma with increased mortality. In case reports or small case series of AFX/PDS patients, immunosuppression has been hypothesized as a risk factor for the development of distant metastases. The aim of the present study was to analyze immunosuppression as a risk factor for AFX/PDS in a large patient cohort.
Methods: A cohort of 164 patients with AFX/PDS (47 AFX and 117 PDS) was collected between 2003 and 2021 and analyzed for clinicopathological data with a special focus on immunosuppression.
Results: Of all patients, 29.9% had any kind of immunosuppression; 6.4% of the AFX and 12.0% of the PDS patients had underlying hemato-oncological diseases. Patients with immunosuppression due to an underlying hemato-oncological disease had a significantly increased risk of progressing to (p = 0.010) and developing distant organ metastases (p = 0.000).
Conclusions: Immunosuppression seems to be a risk factor for developing AFX/PDS with worse clinical outcomes. Therefore, immunosuppression, especially underlying hemato-oncological diseases, should be considered in the treatment and follow-up care of patients with AFX/PDS.
Atypical fibroxanthoma (AFX) and pleomorphic dermal sarcoma (PDS) are defined as rare neoplasms of the skin. Although accurate incidence data do not exist, the incidence is increasing due to demographic changes. Therefore, up to now, they represent the most common sarcomas of the skin (1, 2).
Given the similarities in clinical presentation, histology, and (epi)genetics, AFX and PDS are now considered to be a spectrum of one entity (3–6). They typically occur in chronically light-exposed sites with a non-specific clinical presentation in the form of ulcerated or polypoid tumors (7, 8). Histopathologically, both tumors are very poorly differentiated (“dedifferentiated”) atypical malignant skin neoplasms requiring the exclusion of other poorly differentiated skin tumors such as “dedifferentiated” cutaneous squamous cell carcinoma (cSCC), malignant melanomas, vascular tumors, and other sarcomas, as well as reticulohistiocytomas and atypical fibrous histiocytomas (1, 2, 8–11). Tumor cell morphology includes a variable spectrum of atypical spindle-shaped and epithelioid cells with pleomorphic, vesicular, or hyperchromatic nuclei, as well as atypical multinucleated giant cells and often atypical mitoses, which in the case of AFX remain confined to the dermis and in the case of PDS encompass significant portions of subcutaneous adipose tissue or other deeper structures.
AFX and PDS harbor a UV-induced genetic mutation signature with a very high mutational burden, which is even higher than that of other UV-induced tumors such as cSCC and malignant melanomas (1). The most common genetic alterations include TP53 loss of function mutations, followed by alterations in CDKN2A/B gene (CDKN2A/B mutations in 68%, deletions in 71%, and both in 46%) (1, 5, 12).
The local recurrence rate of AFX is less than 5% after complete excision with a lower recurrence rate of patients operated on with microscopically controlled surgery in contrast to surgery with a wide clinical safety margin (13). In PDS, local recurrences have been described in 5%–28% of cases (7, 9, 14, 15), usually occurring within the first 2 years after primary excision. However, a safety margin of 2 cm was associated with a lower local recurrence risk (7). Metastasis rates in PDS range from 8.8% to 20% with an increased risk in very thick primary tumors (7, 9, 14). Metastases are mainly observed in the skin and regional lymph nodes; organ distant metastases are diagnosed in 4% to 10%, most frequently in the lungs. Metastatic cases of AFX published in the literature on the elderly mostly represent PDS according to the current definition (infiltration of subcutis) or were even diagnosed without the use of immunohistochemical markers and are therefore not reliably attributable to AFX/PDS (15–21).
In addition to UV light as a proven etiopathogenetic factor for the development of AFX/PDS, immunosuppression has been recurrently propagated as a risk factor for the development of distant metastases. Nevertheless, in the majority of case reports or case series of metastasized AFX/PDS, the immune status of the patients remains unclear (9, 16, 20, 21).
The importance of the immune system in human skin cancer has been long recognized based primarily upon the increased incidence of skin cancers in organ transplant recipients (OTRs), patients with hemato-oncological diseases, and mechanisms of UV light-mediated immunomodulation.
The present study investigated in a large cohort of patients whether immunosuppression is a risk factor for AFX/PDS in general as well as for advanced-stage disease.
AFX and PDS were selected from the Clinic for Dermatology and Venereology and the archive of Dermatopathology of the University Hospital between 2003 and 2021. AFX and PDS were diagnosed based on the histopathologic criteria described by Fletcher: invasion of the subcutaneous fat or other deeper structures, necrosis, and lymphovascular or perineural invasion were used to distinguish PDS from AFX (22). As part of the diagnostic procedure performed at the time of diagnosis, immunohistochemical staining of at least one cytokeratin (such as p40, pan-cytokeratin, or CK5/6), melanocytic (such as SOX10, S100, melan-A, and HMB45), and vascular markers (such as ERG, CD31, CD34, and podoplanin) as well as desmin had to be performed to exclude potential differential diagnoses such as malignant melanoma, sarcomatoid SCC, leiomyosarcoma, and vascular malignancies.
Mitoses were counted per 10 high-power fields (HPF) and scored according to the FNCLCC Grading System: score 1, 0–9 mitoses per 10 HPF; score 2, 10–19 mitoses per 10 HPF; and score 3, ≥20 mitoses per 10 HPF (23).
Clinicopathological data were retrospectively collected from the medical records of the patients. All tumors had been completely excised. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected by the approval of the institution’s human research review committee of the University of Cologne, Germany (registration no. 15-307).
Statistical analyses were performed using the statistical analysis software package IBM SPSS, version 27.0 (Chicago, IL, USA). Pearson’s chi-squared test, t-tests, and univariate hazard ratios were calculated with 95% CIs by use of the Cox proportional hazards model. The significance level was determined at p < 0.05. The Kaplan–Meier curves have been generated for progression-free and overall survival. The significance for the survival curves has been determined using the log-rank test.
A total of 164 patients (47 with AFX and 117 with PDS) were included in our study with a mean follow-up of 25 months (range 0–156 months). The majority of patients were male (89% versus 11%) with a mean age of 79 years at initial diagnosis. The most frequent tumor location was the scalp followed by the trunk and extremities. None of the AFX recurred in contrast to 30 of 117 PDS (25.6%) after complete excision of the primary tumor (see Table 1).
Overall, 49 of 164 patients (29.9%) were immunosuppressed; 6.4% of the AFX and 12.0% of the PDS patients had underlying hemato-oncological diseases (see Table 1).
Moreover, these immunosuppressed patients with an underlying hemato-oncological disease had a significantly increased risk of progressing to (p = 0.010) and developing distant organ metastases (p = 0.000). In contrast, patients with underlying hemato-oncological diseases were neither more likely to have other solid tumors nor to be on drug immunosuppression. Moreover, the mitotic count of the tumors was similar in patients with underlying hemato-oncological diseases and all other patients (Table 2).
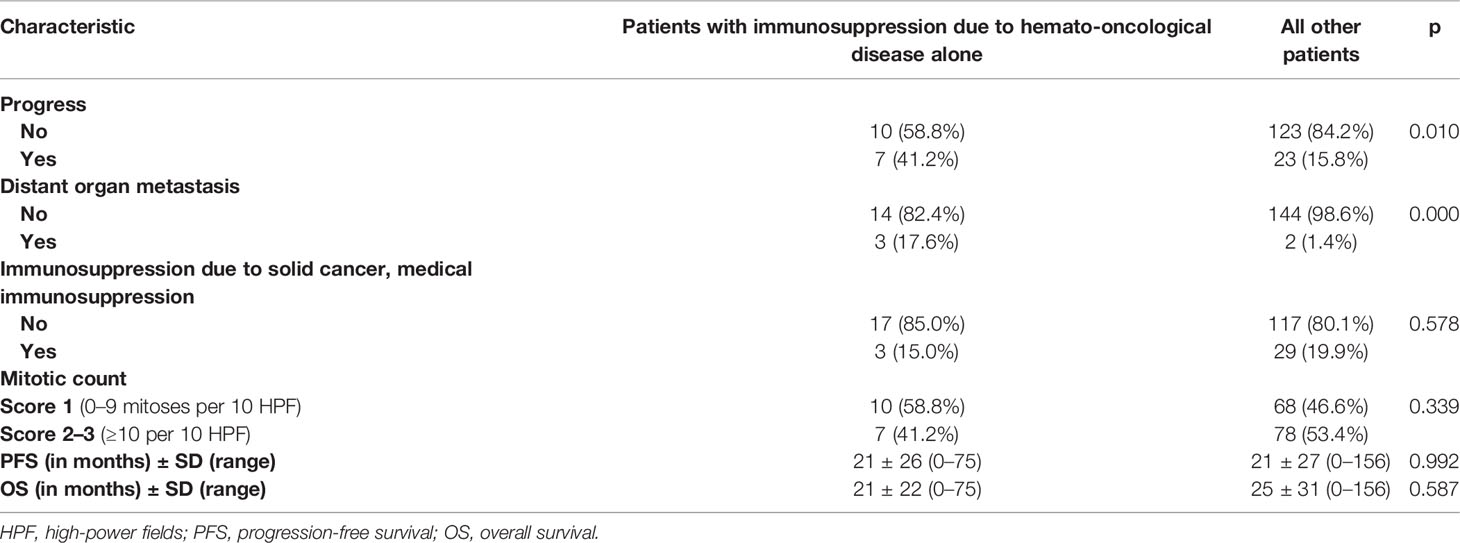
Table 2 Correlation of immunosuppression due to a hemato-oncological disease and progress or distant organ metastasis.
Progression-free survival and overall survival were similar in immunosuppressed patients with hemato-oncological diseases as compared to all other patients (see Table 2).
This is probably due to the fact that both progression-free survival and overall survival were not achieved in the majority of cases and that many of these elderly patients were lost to follow-up.
Although the exact prevalence of immunosuppression in Germany is unknown, it can be assumed that it is increasing due to greater life expectancy in general and among immunosuppressed adults because of improvements in its medical management as well as new indications for immunosuppressive treatments (24, 25). In a study from the United States, an immunosuppression rate of 2.7% was estimated by self-reports of the adult population.
Up to now, no systematic investigation of immunosuppression as a risk factor for AFX/PDS has been performed. There are only case reports or small case series reporting on immunosuppression in the setting of metastasized AFX/PDS (2, 14–19).
Looking at the most common UV-induced skin cancers, basal cell carcinoma (BCC) and cSCC, the distribution of immunosuppressed versus immunocompetent patients has not been systematically documented. However, a large series in OTRs estimated a 65- to 250-fold increased incidence of cSCC and a 10-fold increased incidence of BCC in renal transplant recipients compared to immunocompetent persons. Moreover, the 3-year disease-specific survival is 56%, with the metastasis rate at 7%, much higher in these patients compared to 0.25% in the general population (26, 27). A first invasive cSCC, often at the base of a field cancerization in chronically UV-damaged skin, typically represents an indicator lesion of an at least 10-fold increased risk for the development of further cSCC in often increasingly shorter time intervals (28). Generally, the cutaneous cancer incidence correlates with the degree and duration of immunosuppression (29).
Regarding other types of immunosuppressed patients, it has been reported that patients with chronic lymphocytic leukemia (CLL) have an 8-fold increased risk for cSCC and BCC with 7 to 14 times increased risk to develop recurrences and/or metastases (30). Hematopoietic cell transplantation recipients have shown a modest risk of skin cancer (31). Age, chronic lymphocytic leukemia, clinically photodamaged skin, and history of cSCC have been defined as independent risk factors for developing cSCC in these patients (32). Interestingly, an azathioprine-specific genetic signature 32 could be detected in both well and poorly differentiated cSCC. Although no therapeutic approach emerges from this study, azathioprine should be avoided in high-risk cSCC patients (33). The risk of Merkel cell carcinoma is also significantly increased in patients with different types of immunosuppression including autoimmune diseases, neoplastic comorbidities, immunosuppression due to hemato-lymphoid disorders such as chronic lymphocytic leukemia/small lymphocytic lymphoma, and immune-modulating drugs or after solid organ transplantation. Moreover, immunosuppression significantly correlated with disease progression in these patients (34–37).
In our cohort of 164 AFX/PDS patients, around one-third of patients had any kind of immunosuppression, the majority of them due to a hemato-oncological disease, which is significantly more than one would expect in the general population or population of other skin cancers such as cSCC or BCC.
Furthermore, these patients with an underlying hemato-oncological disease progressed significantly more often and developed distant organ metastases significantly more often. These details should be considered in the treatment and follow-up care of these patients.
The raw data supporting the conclusions of this article will be made available by the author, without undue reservation.
The author confirms being the sole contributor of this work and has approved it for publication.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Klein S, Quaas A, Noh KW, Cartolano M, Abedpour N, Mauch C, et al. Integrative Analysis of Pleomorphic Dermal Sarcomas Reveals Fibroblastic Differentiation and Susceptibility to Immunotherapy. Clin Cancer Res (2020) 26(21):5638–45. doi: 10.1158/1078-0432.CCR-20-1899
2. Helbig D, Ziemer M, Dippel E, Erdmann M, Hillen U, Leiter U, et al. S1-Guideline Atypical Fibroxanthoma (AFX) and Pleomorphic Dermal Sarcoma (PDS). J Dtsch Dermatol Ges (2022) 20(2):235–43. doi: 10.1111/ddg.14700
3. Griewank KG, Wiesner T, Murali R, Pischler C, Muller H, Koelsche C, et al. Atypical Fibroxanthoma and Pleomorphic Dermal Sarcoma Harbor Frequent NOTCH1/2 and FAT1 Mutations and Similar DNA Copy Number Alteration Profiles. Mod Pathol (2018) 31(3):418–28. doi: 10.1038/modpathol.2017.146
4. Helbig D, Quaas A, Mauch C, Merkelbach-Bruse S, Buttner R, Emberger M, et al. Copy Number Variations in Atypical Fibroxanthomas and Pleomorphic Dermal Sarcomas. Oncotarget (2017) 8(65):109457–67. doi: 10.18632/oncotarget.22691
5. Helbig D, Ihle MA, Putz K, Tantcheva-Poor I, Mauch C, Buttner R, et al. Oncogene and Therapeutic Target Analyses in Atypical Fibroxanthomas and Pleomorphic Dermal Sarcomas. Oncotarget (2016) 7(16):21763–74. doi: 10.18632/oncotarget.7845
6. Koelsche C, Stichel D, Griewank KG, Schrimpf D, Reuss DE, Bewerunge-Hudler M, et al. Genome-Wide Methylation Profiling and Copy Number Analysis in Atypical Fibroxanthomas and Pleomorphic Dermal Sarcomas Indicate a Similar Molecular Phenotype. Clin Sarcoma Res (2019) 9:2. doi: 10.1186/s13569-019-0113-6
7. Persa OD, Loquai C, Wobser M, Baltaci M, Dengler S, Kreuter A, et al. Extended Surgical Safety Margins and Ulceration are Associated With an Improved Prognosis in Pleomorphic Dermal Sarcomas. J Eur Acad Dermatol Venereol (2019) 33(8):1577–80. doi: 10.1111/jdv.15493
8. Calonje E, Wadden C, Wilson-Jones E, Fletcher CD. Spindle-Cell Non-Pleomorphic Atypical Fibroxanthoma: Analysis of a Series and Delineation of a Distinctive Variant. Histopathology (1993) 22(3):247–54. doi: 10.1111/j.1365-2559.1993.tb00114.x
9. Tardio JC, Pinedo F, Aramburu JA, Suarez-Massa D, Pampin A, Requena L, et al. Pleomorphic Dermal Sarcoma: A More Aggressive Neoplasm Than Previously Estimated. J Cutan Pathol (2016) 43(2):101–12. doi: 10.1111/cup.12603
10. Choy B, Hyjek E, Montag AG, Pytel P, Haydon R, Luu HH, et al. High Prevalence of MiTF Staining in Undifferentiated Pleomorphic Sarcoma: Caution in the Use of Melanocytic Markers in Sarcoma. Histopathology (2017) 70(5):734–45. doi: 10.1111/his.13139
11. Helbig D, Mauch C, Buettner R, Quaas A. Immunohistochemical Expression of Melanocytic and Myofibroblastic Markers and Their Molecular Correlation in Atypical Fibroxanthomas and Pleomorphic Dermal Sarcomas. J Cutan Pathol (2018) 45(12):880–5. doi: 10.1111/cup.13346
12. Lai K, Harwood CA, Purdie KJ, Proby CM, Leigh IM, Ravi N, et al. Genomic Analysis of Atypical Fibroxanthoma. PloS One (2017) 12(11):e0188272. doi: 10.1371/journal.pone.0188272
13. Bitel A, Schonlebe J, Kronert C, Wollina U. Atypical Fibroxanthoma: An Analysis of 105 Tumors. Dermatol Ther (2020) 33(6):e13962. doi: 10.1111/dth.13962
14. Miller K, Goodlad JR, Brenn T. Pleomorphic Dermal Sarcoma: Adverse Histologic Features Predict Aggressive Behavior and Allow Distinction From Atypical Fibroxanthoma. Am J Surg Pathol (2012) 36(9):1317–26. doi: 10.1097/PAS.0b013e31825359e1
15. Wang WL, Torres-Cabala C, Curry JL, Ivan D, McLemore M, Tetzlaff M, et al. Metastatic Atypical Fibroxanthoma: A Series of 11 Cases Including With Minimal and No Subcutaneous Involvement. Am J Dermatopathol (2015) 37(6):455–61. doi: 10.1097/DAD.0000000000000237
16. Helwig EB, May D. Atypical Fibroxanthoma of the Skin With Metastasis. Cancer (1986) 57(2):368–76. doi: 10.1002/1097-0142(19860115)57:2<368::AID-CNCR2820570230>3.0.CO;2-N
17. Cooper JZ, Newman SR, Scott GA, Brown MD. Metastasizing Atypical Fibroxanthoma (Cutaneous Malignant Histiocytoma): Report of Five Cases. Dermatol Surg (2005) 31(2):221–5. doi: 10.1097/00042728-200502000-00019
18. Kargi E, Gungor E, Verdi M, Kuiacogiu S, Erdogan B, Alli N, et al. Atypical Fibroxanthoma and Metastasis to the Lung. Plast Reconstr Surg (2003) 111(5):1760–2. doi: 10.1097/00006534-200304150-00032
19. Armstrong S, Dwyer P, Bettington A, Strutton G. Brain and Lung Metastasis Secondary to Metastatic Atypical Fibroxanthoma: A Rare Australian Case. Australas J Dermatol (2017) 58(2):150–1. doi: 10.1111/ajd.12513
20. Davis JL, Randle HW, Zalla MJ, Roenigk RK, Brodland DG. A comparison of Mohs micrographic surgery and wide excision for the treatment of atypical fibroxanthoma. Dermatol Surg (1997) 23(2):105–10. doi: 10.1111/j.1524-4725.1997.tb00670.x
21. Davidson JS, Demsey D. Atypical Fibroxanthoma: Clinicopathologic Determinants for Recurrence and Implications for Surgical Management. J Surg Oncol (2012) 105(6):559–62. doi: 10.1002/jso.22128
22. McCalmont TH. Correction and Clarification Regarding AFX and Pleomorphic Dermal Sarcoma. J Cutan Pathol (2012) 39(1):8. doi: 10.1111/j.1600-0560.2011.01851.x
23. Trojani M, Contesso G, Coindre JM, Rouesse J, Bui NB, de Mascarel A, et al. Soft-Tissue Sarcomas of Adults; Study of Pathological Prognostic Variables and Definition of a Histopathological Grading System. Int J Cancer (1984) 33(1):37–42. doi: 10.1002/ijc.2910330108
24. Harpaz R, Dahl RM, Dooling KL. Prevalence of Immunosuppression Among US Adults, 2013. JAMA (2016) 316(23):2547–8. doi: 10.1001/jama.2016.16477
25. Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. Closing the Gap: Increases in Life Expectancy Among Treated HIV-Positive Individuals in the United States and Canada. PloS One (2013) 8(12):e81355. doi: 10.1371/journal.pone.0081355
26. Lindelof B, Sigurgeirsson B, Gabel H, Stern RS. Incidence of Skin Cancer in 5356 Patients Following Organ Transplantation. Br J Dermatol (2000) 143(3):513–9. doi: 10.1111/j.1365-2133.2000.03703.x
27. Harwood CA, Mesher D, McGregor JM, Mitchell L, Leedham-Green M, Raftery M, et al. A Surveillance Model for Skin Cancer in Organ Transplant Recipients: A 22-Year Prospective Study in an Ethnically Diverse Population. Am J Transpl (2013) 13(1):119–29. doi: 10.1111/j.1600-6143.2012.04292.x
28. Jiyad Z, O'Rourke P, Soyer HP, Green AC. Actinic Keratosis-Related Signs Predictive of Squamous Cell Carcinoma in Renal Transplant Recipients: A Nested Case-Control Study. Br J Dermatol (2017) 176(4):965–70. doi: 10.1111/bjd.15019
29. Jensen P, Hansen S, Moller B, Leivestad T, Pfeffer P, Geiran O, et al. Skin Cancer in Kidney and Heart Transplant Recipients and Different Long-Term Immunosuppressive Therapy Regimens. J Am Acad Dermatol (1999) 40Pt 1):177–86. doi: 10.1016/S0190-9622(99)70185-4
30. Adami J, Frisch M, Yuen J, Glimelius B, Melbye M. Evidence of an Association Between non-Hodgkin's Lymphoma and Skin Cancer. BMJ (1995) 310(6993):1491–5. doi: 10.1136/bmj.310.6993.1491
31. Curtis RE, Metayer C, Rizzo JD, Socie G, Sobocinski KA, Flowers ME, et al. Impact of Chronic GVHD Therapy on the Development of Squamous-Cell Cancers After Hematopoietic Stem-Cell Transplantation: An International Case-Control Study. Blood (2005) 105(10):3802–11. doi: 10.1182/blood-2004-09-3411
32. Scott JF, Brough KR, Grigoryan KV, Muzic JG, Kim GY, Conic RRZ, et al. Risk Factors for Keratinocyte Carcinoma in Recipients of Allogeneic Hematopoietic Cell Transplants. JAMA Dermatol (2020) 156(6):631–9. doi: 10.1001/jamadermatol.2020.0559
33. Inman GJ, Wang J, Nagano A, Alexandrov LB, Purdie KJ, Taylor RG, et al. The Genomic Landscape of Cutaneous SCC Reveals Drivers and a Novel Azathioprine Associated Mutational Signature. Nat Commun (2018) 9(1):3667. doi: 10.1038/s41467-018-06027-1
34. Rastrelli M, Del Fiore P, Russo I, Tartaglia J, Dal Monico A, Cappellesso R, et al. Merkel Cell Carcinoma: Evaluation of the Clinico-Pathological Characteristics, Treatment Strategies and Prognostic Factors in a Monocentric Retrospective Series (N=143). Front Oncol (2021) 11:737842. doi: 10.3389/fonc.2021.737842
35. Sargen MR, Cahoon EK, Yu KJ, Madeleine MM, Zeng Y, Rees JR, et al. Spectrum of Nonkeratinocyte Skin Cancer Risk Among Solid Organ Transplant Recipients in the US. JAMA Dermatol (2022). doi: 10.1001/jamadermatol.2022.0036
36. Katerji R, Yigit N, Lozeau D, Liu Y, Tam W, Crane GM. Merkel Cell Carcinoma in the Setting of Hematologic Disease is Associated With Unique Features and Potential Pitfalls. Ann Diagn Pathol (2022) 56:151868. doi: 10.1016/j.anndiagpath.2021.151868
Keywords: atypical fibroxanthoma (AFX), pleomorphic dermal sarcoma (PDS), immunosuppression, hemato-oncological disease, metastases
Citation: Helbig D (2022) Hemato-Oncological Diseases as Risk Factor for Recurrence or Metastasis of Pleomorphic Dermal Sarcoma. Front. Oncol. 12:873771. doi: 10.3389/fonc.2022.873771
Received: 11 February 2022; Accepted: 21 March 2022;
Published: 14 April 2022.
Edited by:
Gil Bar-Sela, Ha’Emek Medical Center, IsraelReviewed by:
Roni Pircha Dodiuk-Gad, Technion Israel Institute of Technology, IsraelCopyright © 2022 Helbig. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Doris Helbig, RG9yaXMuaGVsYmlnQHVrLWtvZWxuLmRl; orcid.org/0000-0002-5841-4631
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.