- 1Department of Urology, Kaohsiung Municipal Ta-Tung Hospital, Kaohsiung, Taiwan
- 2Department of Urology, School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
- 3Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
- 4Department of Urology, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan
- 5Department of Urology, China Medical University, Taichung, Taiwan
- 6School of Medicine, China Medical University, Taichung, Taiwan
- 7Department of Urology, China Medical University Hospital, Taichung, Taiwan
- 8Division of Urology, Department of Surgery, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan
- 9Institute of Biomedical Engineering, National Taiwan University, Taipei, Taiwan
- 10Department of Urology, National Taiwan University Hospital, College of Medicine, National Taiwan University, Taipei, Taiwan
- 11Division of Urology, Department of Surgery, Taichung Veterans General Hospital, Taichung, Taiwan
- 12Institute of Medicine, Chung Shan Medical University, Taichung, Taiwan
- 13Department of Applied Chemistry, National Chi Nan University, Nantou, Taiwan
- 14Department of Senior Citizen Service Management, National Taichung University of Science and Technology, Taichung, Taiwan
- 15Division of Urology, Department of Surgery, Taipei Tzu Chi Hospital, The Buddhist Medical Foundation, New Taipei City, Taiwan
- 16School of Medicine, Buddhist Tzu Chi University, Hualien, Taiwan
- 17Department of Urology, MacKay Memorial Hospital, Taipei, Taiwan
- 18Mackay Medical College, New Taipei City, Taiwan
- 19Institute of Biomedical Informatics, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 20Department of Urology, Ditmanson Medical Foundation Chiayi Christian Hospital, Chiayi, Taiwan
- 21Department of Health and Nutrition Biotechnology, Asian University, Taichung, Taiwan
- 22Department of Urology, Hualien Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation and Tzu Chi University, Hualien, Taiwan
- 23Division of Urology, Department of Surgery, Far Eastern Memorial Hospital, New Taipei City, Taiwan
- 24Department of Healthcare Information & Management, Ming Chuan University, Taoyuan, Taiwan
- 25Division of Urology, Department of Surgery, Taipei City Hospital renai branch, Taipei, Taiwan
- 26Department of Urology, School of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan
- 27Department of Urology, Taiwan Adventist Hospital, Taipei, Taiwan
- 28Division of Urology, Department of Surgery, Cardinal Tien Hospital, New Taipei City, Taiwan
- 29Department of Life Science, College of Science, National Taiwan Normal University, Taipei, Taiwan
- 30Department of Urology, School of Medicine, College of Medicine, Taipei Medical University, Taipei, Taiwan
- 31Department of Urology, Taipei Medical University Hospital, Taipei Medical University, Taipei, Taiwan
- 32Division of Urology, Department of Surgery, Chang Gung Memorial Hospital, Chiayi, Taiwan
- 33Chang Gung University of Science and Technology, Chiayi, Taiwan
- 34Department of Medicine, College of Medicine, Chang Gung University, Taoyuan, Taiwan
- 35Department of Urology, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan
- 36Taipei Medical University Research Center of Urology and Kidney (TMU-RCUK), Taipei Medical University, Taipei, Taiwan
- 37Division of Urology, Department of Surgery, Chang Gung Memorial Hospital, Taoyuan, Taiwan
- 38Graduate Institute of Clinical Medical Science, College of Medicine, Chang Gung University, Taoyuan, Taiwan
- 39School of Medicine, College of Medicine, Chang Gung University, Taoyuan, Taiwan
- 40School of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
- 41Cohort Research Center, Kaohsiung Medical University, Kaohsiung, Taiwan
Purpose: We aimed to evaluate the impact of preoperative local symptoms on prognosis after radical nephroureterectomy in patients with upper tract urothelial carcinoma (UTUC).
Methods: This retrospective study consisted of 2,662 UTUC patients treated at 15 institutions in Taiwan from 1988 to 2019. Clinicopathological data were retrospectively collected for analysis by the Taiwan UTUC Collaboration Group. The Kaplan-Meier method was used to calculate overall survival (OS), cancer-specific survival (CSS), disease-free survival (DFS), and bladder recurrence-free survival (BRFS). The prognostic value of preoperative local symptoms in OS, CSS, DFS, and BRFS was investigated using Cox proportional hazards models.
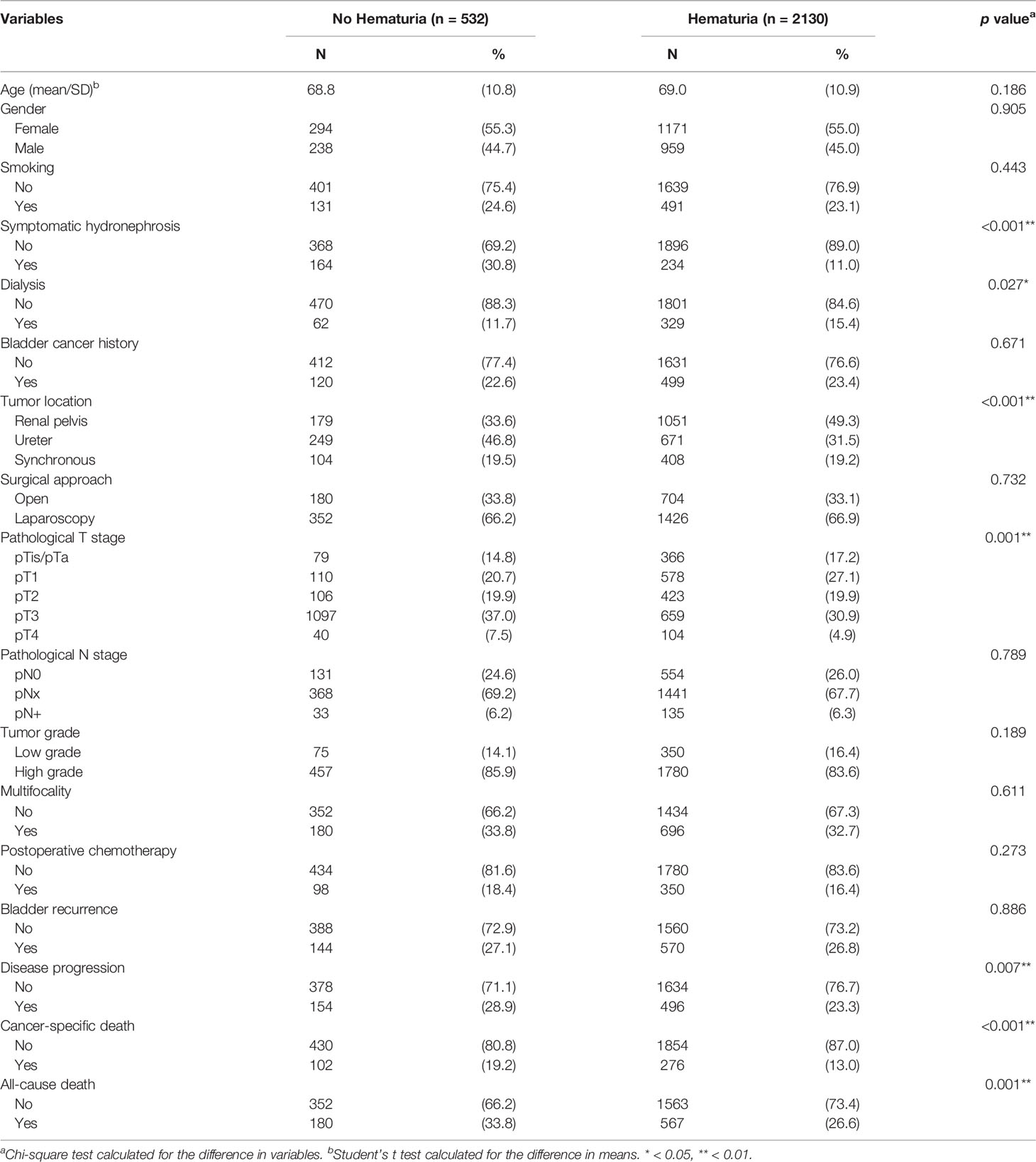
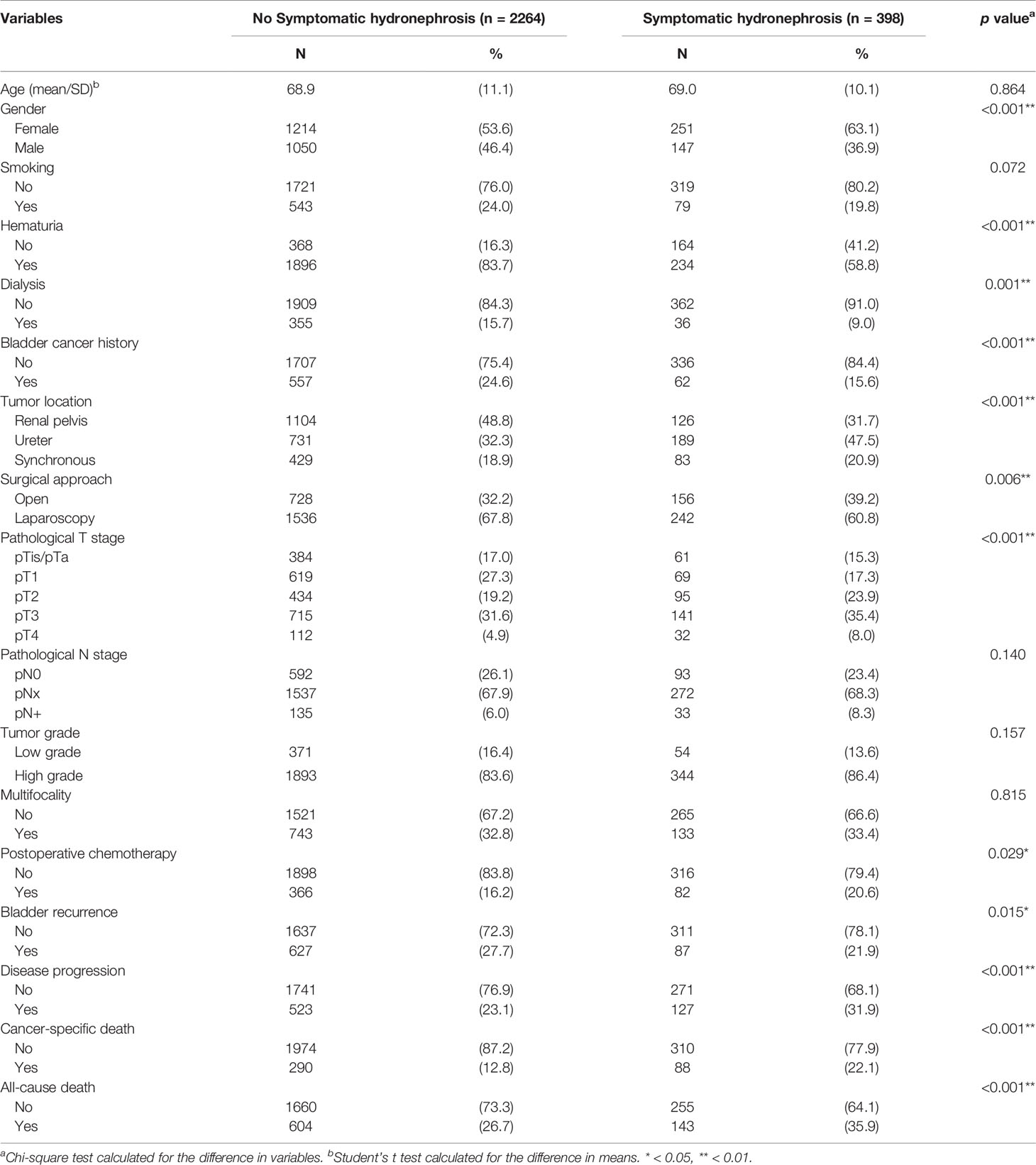
Results: The median follow-up was 36.6 months. Among 2,662 patients, 2,130 (80.0%) presented with hematuria and 398 (15.0%) had symptomatic hydronephrosis at diagnosis. Hematuria was associated with less symptomatic hydronephrosis (p <0.001), more dialysis status (p = 0.027), renal pelvic tumors (p <0.001), and early pathological tumor stage (p = 0.001). Symptomatic hydronephrosis was associated with female patients (p <0.001), less dialysis status (p = 0.001), less bladder cancer history (p <0.001), ureteral tumors (p <0.001), open surgery (p = 0.006), advanced pathological tumor stage (p <0.001), and postoperative chemotherapy (p = 0.029). Kaplan-Meier analysis showed that patients with hematuria or without symptomatic hydronephrosis had significantly higher rates of OS, CSS, and DFS (all p <0.001). Multivariate analysis confirmed that presence of hematuria was independently associated with better OS (HR 0.789, 95% CI 0.661–0.942) and CSS (HR 0.772, 95% CI 0.607–0.980), while symptomatic hydronephrosis was a significant prognostic factor for poorer OS (HR 1.387, 95% CI 1.142–1.683), CSS (HR 1.587, 95% CI 1.229–2.050), and DFS (HR 1.378, 95% CI 1.122–1.693).
Conclusions: Preoperative local symptoms were significantly associated with oncological outcomes, whereas symptomatic hydronephrosis and hematuria had opposite prognostic effects. Preoperative symptoms may provide additional information on risk stratification and perioperative treatment selection for patients with UTUC.
Introduction
Urothelial carcinoma (UC) is a malignant tumor of the lining of the urinary tract. While the majority of UCs arise in the bladder, upper tract UC (UTUC) is less common (1, 2). Unlike bladder UC, almost 60% of UTUCs are invasive at diagnosis (3–5). The standard treatment for invasive UTUC is radical nephroureterectomy (RNU) with bladder cuff excision, but the cancer often recurs after surgical intervention (2, 3). Therefore, it is important to select candidates who require adjunctive therapy in the perioperative period. Identifying useful prognostic markers for UTUC is one way to aid patient selection. Previous studies have demonstrated the prognostic significance of pathological features such as tumor stage and lymphovascular invasion (2). However, these characteristics are only obtained after RNU and cannot be incorporated into preoperative assessment. Preoperative prognostic factors are more conducive to make up for the inadequacy of clinical staging of UTUC.
UTUC is usually initially diagnosed by examination after seeking medical attention for clinical symptoms. The most common symptom of UTUC is hematuria, which occurs in approximately 70%–80% of patients (2, 6). Flank pain is the second most common symptom (20%), usually caused by obstruction of urine flow, and is closely related to hydronephrosis (7–9). Other systemic symptoms, including weight loss, general malaise, fatigue, and cachexia, may be associated with worse prognosis and should be treated promptly (2, 8, 9). Although local symptoms are common, their impact on survival outcomes in patients with UTUC remains questionable (7–14). This study aimed to evaluate the value of preoperative local symptoms on the prognosis of UTUC after nephroureterectomy.
Materials and Methods
Patient Collection and Tumor Specimens
The Taiwan UTUC Collaboration Group collected 4,813 patients from 15 institutions in Taiwan from 1988 to 2019. A total of 2,662 patients were included in this study after we excluded patients who underwent surgery other than RNU or who had incomplete medical records. This study was supervised by the review board of our institution (KMUHIRB-E(I)-20180214). RNU was performed with either an open or laparoscopic approach. The open approach used one or two incisions, such as a midline incision or a flank plus Gibson incision, while the laparoscopic approach employed a camera port to minimize trauma caused by the incision through a transperitoneal or retroperitoneal access. Lymph node dissection was performed when lymph node involvement was suspected on preoperative imaging or when lymphadenopathy was found during surgery. Intravesical chemotherapy was not routinely performed after RNU, except in patients with bladder recurrence during follow-up. Various clinicopathological data were included for analysis, including age, gender, smoking, local symptoms, dialysis, bladder cancer history, tumor location, surgical approach, pathological features (pathological T stage, pathological N stage, tumor grade, multifocality), and postoperative chemotherapy.
All tumor specimens were reviewed by genitourinary pathologists at each medical center, and the criteria for pathological characteristics were uniform. Tumor stage was defined according to the 2010 American Joint Committee Cancer TNM (Tumor, Lymph Node, Metastasis) system (15), while tumor grade was based on the 2004 World Health Organization/International Society of Urologic Pathology Consensus Classification (16).
Preoperative Symptom Assessment
Patients with hematuria may or may not have visible red urine. The presence of hematuria was determined by urinalysis prior to the diagnosis of UTUC. Patients were considered to have hematuria if urinalysis revealed more than 3 red blood cells per high-power field on two consecutive microscopic evaluations. We used renal ultrasonography, computed tomography, magnetic resonance imaging, or intravenous pyelography to detect hydronephrosis. Consistent with previous studies (10, 17), any degree of dilatation of the renal collecting system was defined as hydronephrosis. If the pelvicalyceal dilatation was caused by noncancerous condition such as urolithiasis and benign ureteral stricture, it was not considered hydronephrosis. Symptomatic hydronephrosis was defined as moderate to severe flank pain on the same side of the hydronephrosis (10). Patients with nonspecific lumbago or flank pain contralateral to UTUC were not considered symptomatic hydronephrosis. Patients were divided into two groups according to the presence of hematuria or symptomatic hydronephrosis to assess the prognostic value of local symptoms.
Follow-Up
Typically, patients were followed up every 3 months for the first 2 years after RNU. Follow-up visits were performed every 6 months from years 3 to 4, and annually after year 5 if there was no disease recurrence. Workup included a thorough history taking, physical examination, urine cytology, blood tests, chest X-ray, cystoscopy, and abdominal computed tomography. Disease progression was defined as distant metastasis or cancer development in the tumor bed or regional lymph nodes. Bladder recurrence was considered an independent entity for survival analysis. Cancer-specific and overall mortality was determined by reviewing death certificates and medical records.
Statistical Analysis
Differences in categorical parameters between the presence and absence of each local symptom were assessed by Pearson’s chi-square test, and continuous variables were compared by Student’s t test. The Kaplan-Meier method was used to evaluate the effect of local symptoms on overall survival (OS), cancer-specific survival (CSS), disease-free survival (DFS), and bladder recurrence-free survival (BRFS). Survival curves were compared by log-rank test, and survival time from surgery date to each endpoint (i.e., all-cause death, cancer-specific mortality, disease progression, bladder recurrence) or last visit was calculated. In addition, we used Cox proportional hazards models to assess the effect of each variable on oncological outcomes. The effects of all variables on each survival rate were examined in univariate analysis, and statistically significant variables were adjusted to evaluate their prognostic value in multivariate analysis. We used SPSS 26.0 (SPSS Inc., Chicago, IL, USA) for all analyses and p <0.05 was defined as statistically significant.
Results
Clinicopathological Data and Preoperative Symptoms
The median and mean follow-up was 36.6 and 47.7 months, respectively. This study consisted of 2,662 UTUC patients, including 1,465 (55.0%) women and 1,197 (45.0%) men. Demographic and clinicopathological characteristics were compared according to the presence of hematuria (Table 1) and symptomatic hydronephrosis (Table 2). Hematuria occurred in 2,130 (80.0%) patients. Table 1 shows that patients with hematuria were associated with less symptomatic hydronephrosis (p <0.001), more dialysis status (p = 0.027), renal pelvic tumors (p <0.001), early pathological T stage (p = 0.001), less disease progression (p = 0.007), fewer cancer-specific deaths (p <0.001), and fewer all-cause deaths (p = 0.001). Several parameters were found to be not significantly different between the two groups, including age (p = 0.186), gender (p = 0.905), smoking (p = 0.443), bladder cancer history (p = 0.671), surgical approach (p = 0.732), pathological N stage (p = 0.789), tumor grade (p = 0.189), multifocality (p = 0.611), postoperative chemotherapy (p = 0.273), and bladder recurrence (p = 0.886).
In Table 2, 398 (15.0%) patients had symptomatic hydronephrosis at the initial presentation. They were associated with female patients (p <0.001), less hematuria (p <0.001), less dialysis status (p = 0.001), less bladder cancer history (p <0.001), ureteral tumors (p <0.001), open surgical approach (p = 0.006), advanced pathological T stage (p <0.001), postoperative chemotherapy (p = 0.029), less bladder recurrence (p = 0.015), more disease progression (p <0.001), more cancer-specific mortality (p <0.001), and more all-cause mortality (p <0.001). No differences in age (p = 0.864), smoking (p = 0.072), pathological N stage (p = 0.140), tumor grade (p = 0.157), and multifocality (p = 0.815) were observed between the two groups.
Kaplan-Meier Analysis of OS, CSS, DFS, and BRFS Based on Hematuria or Symptomatic Hydronephrosis
During follow-up, all-cause death, cancer-specific mortality, disease progression, and bladder recurrence occurred in 747 (28.1%), 378 (14.2%), 650 (24.4%), and 714 (26.8%) patients, respectively. As shown in Tables 1 and 2, the absence of hematuria or the presence of symptomatic hydronephrosis was significantly associated with more crude events in OS, CSS, and DFS (all p <0.01). In Kaplan-Meier analysis, patients with hematuria had significantly better OS, CSS, and DFS than those without hematuria (Figures 1A–C, respectively; all p <0.001). In contrast, patients with symptomatic hydronephrosis had significantly lower OS, CSS, and DFS than cases without symptomatic hydronephrosis (Figures 2A–C, respectively; all p <0.001). For BRFS, there was no significant difference according to hematuria (Figure 1D; p = 0.385) or symptomatic hydronephrosis (Figure 2D; p = 0.050).
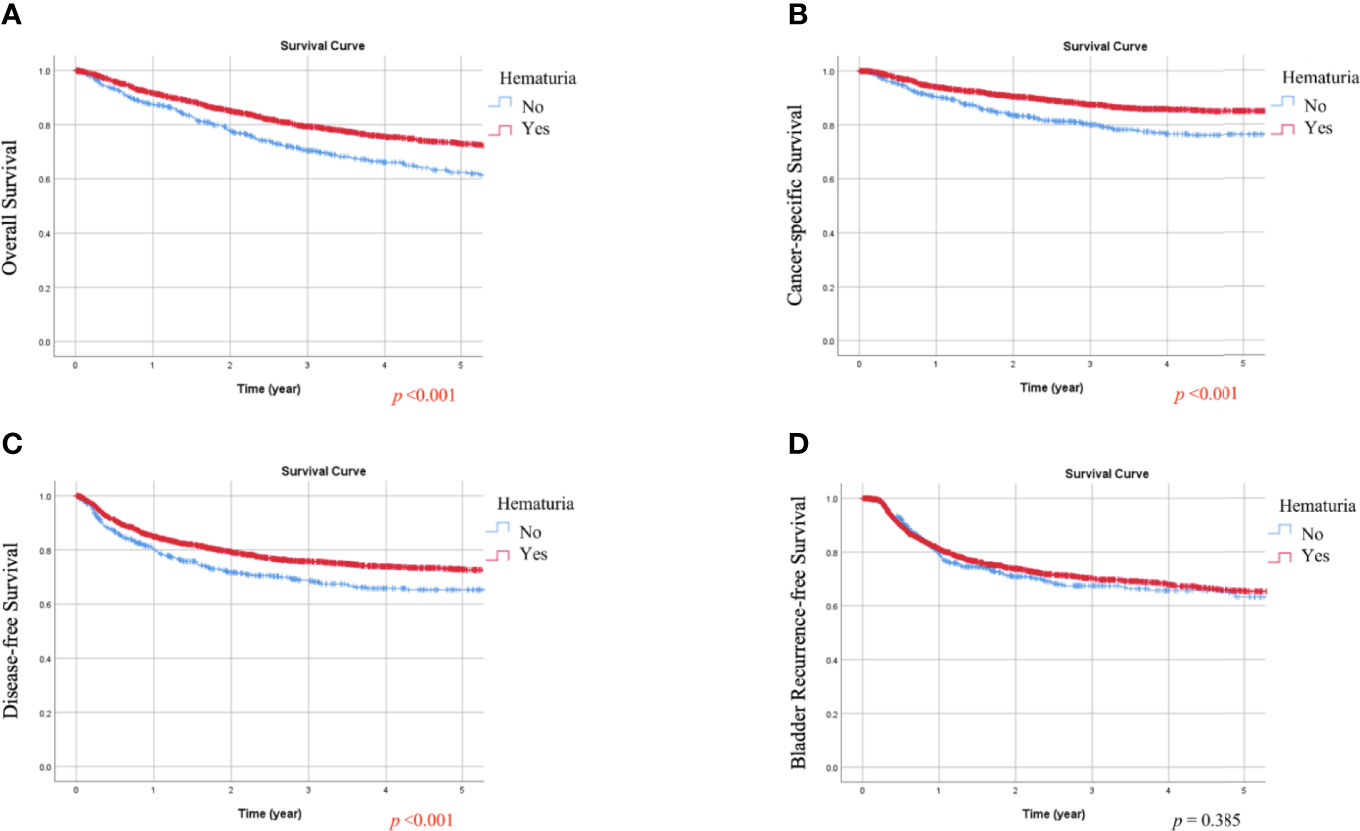
Figure 1 Kaplan-Meier estimates of overall survival (A), cancer-specific survival (B), disease-free survival (C), and bladder recurrence-free survival (D) according to hematuria.
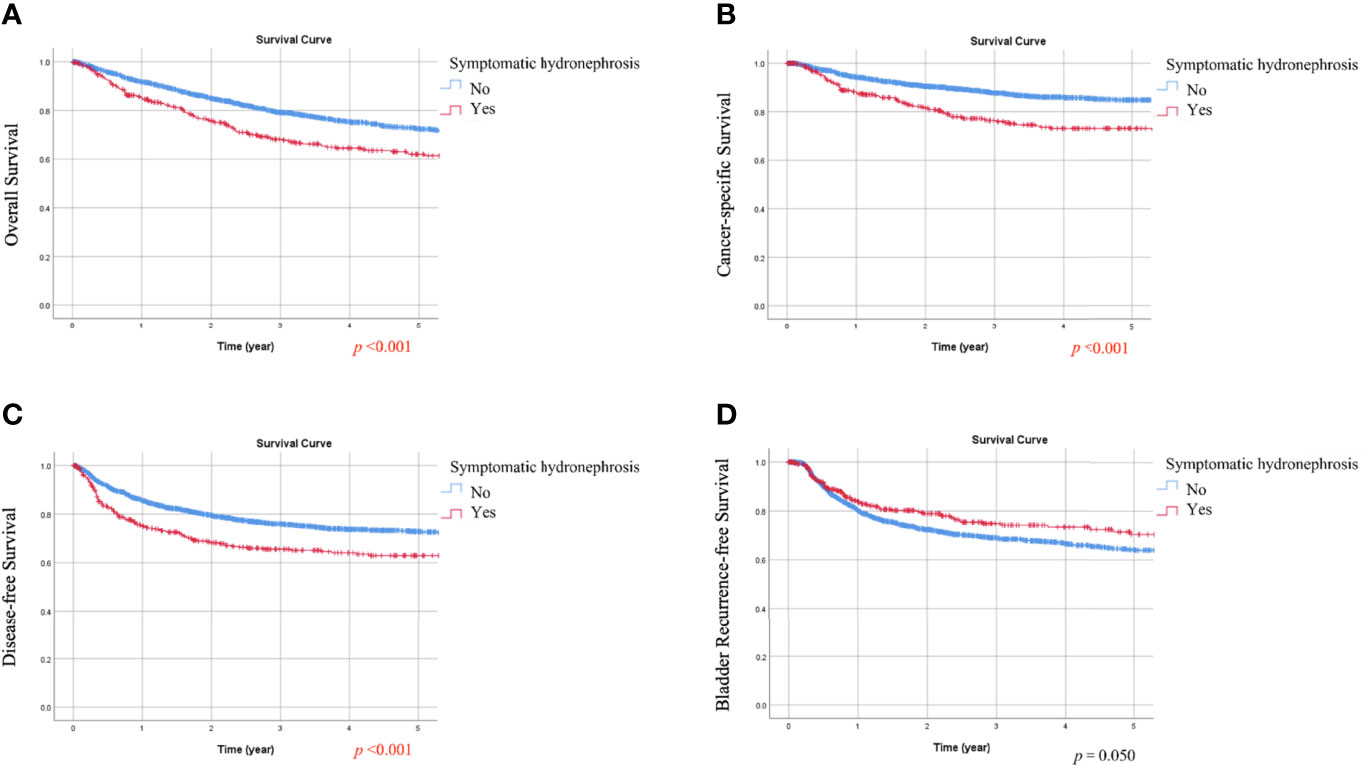
Figure 2 Kaplan-Meier estimates of the overall survival (A), cancer-specific survival (B), disease-free survival (C), and bladder recurrence-free survival (D) according to symptomatic hydronephrosis.
Cox Proportional Hazards Models for OS, CSS, DFS, and BRFS
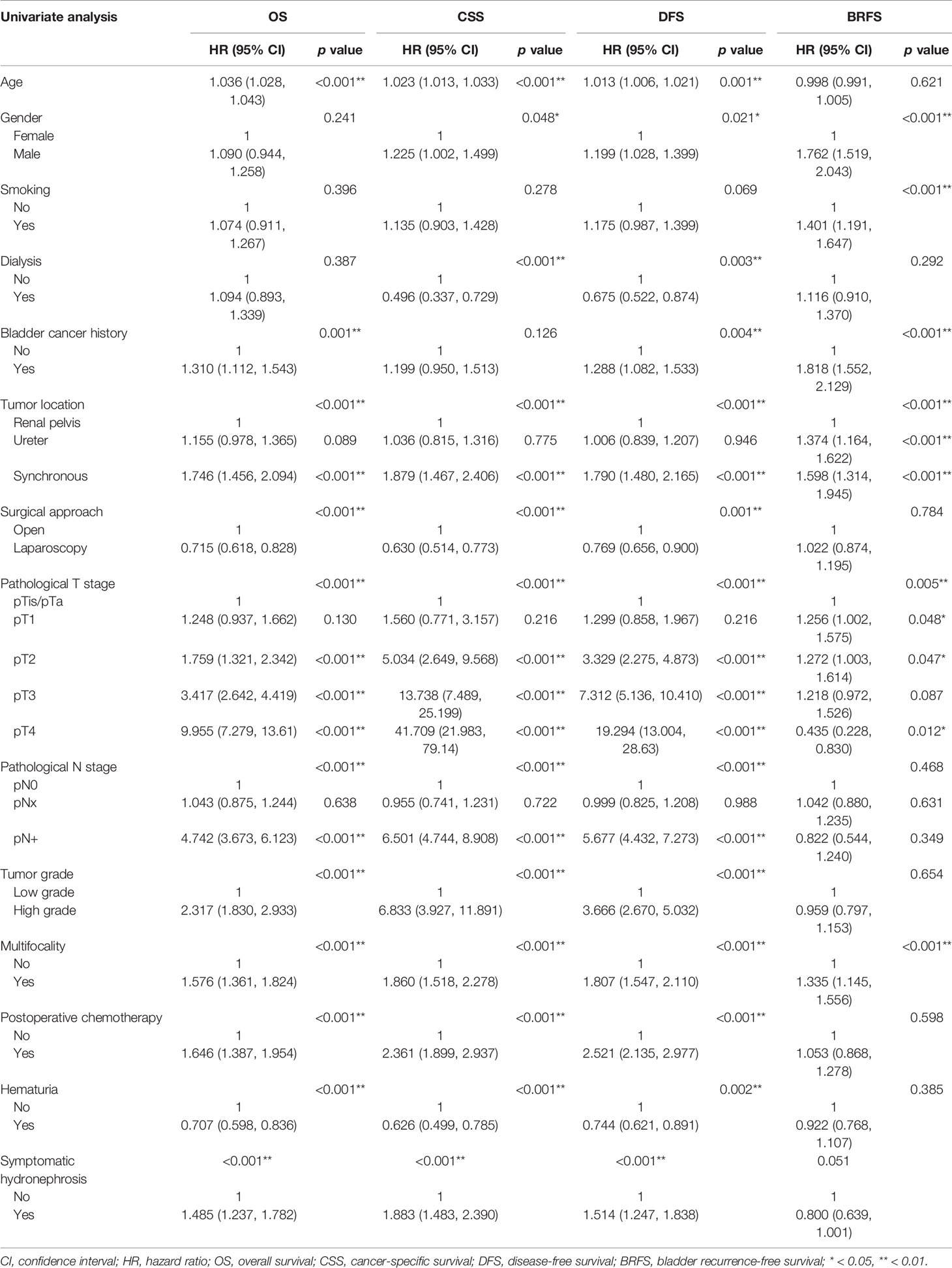
In univariate analysis (Table 3), multiple variables were associated with poorer OS, CSS, and DFS, including advanced age, synchronous renal pelvic and ureteral tumors, open surgical approach, advanced pathological T stage, lymph node metastasis, high tumor grade, multifocality, postoperative chemotherapy, absence of hematuria, and symptomatic hydronephrosis. As for bladder recurrence, gender, smoking, bladder cancer history, tumor location, pathological T stage, and multifocality were significantly associated with BRFS.
After adjusting for significant variables in multivariate analysis (Table 4), bladder cancer history was associated with worse OS and DFS. Statistically significant factors for worse OS and CSS were open surgical approach and absence of hematuria. Variables independently associated with worse OS, CSS, and DFS were advanced age, advanced pathological T stage, lymph node metastasis, high tumor grade, and symptomatic hydronephrosis. In multivariate analysis of BRFS, gender, tumor location, bladder cancer history, and pathological T stage were significantly associated with bladder recurrence.
In summary, the presence of hematuria was associated with better OS and CSS in univariate analysis (HR 0.707, 95% CI 0.598–0.836 and HR 0.626, 95% CI 0.499–0.785) and multivariate analysis (HR 0.789, 95% CI 0.661–0.942 and HR 0.772, 95% CI 0.607–0.980). On the other hand, symptomatic hydronephrosis was a significant prognostic factor for worse OS, CSS, and DFS in univariate analysis (HR 1.485, 95% CI 1.237–1.782, HR 1.883, 95% CI 1.483–2.390, and HR 1.514, 95% CI 1.247–1.838, respectively) and multivariate analysis (HR 1.387, 95% CI 1.142–1.683, HR 1.587, 95% CI 1.229–2.050, and HR 1.378, 95% CI 1.122–1.693, respectively).
Discussion
Studies have shown that systemic symptoms such as weight loss, general malaise, and fatigue are associated with poor prognosis in UTUC (2, 8, 9). However, the prognostic significance of local symptoms directly attributable to the tumor, such as hematuria and symptomatic hydronephrosis, have been poorly studied. This is the largest multicenter study investigating the prognostic value of preoperative local symptoms in UTUC, showing that hematuria and symptomatic hydronephrosis independently lead to better and worse survival, respectively. These findings suggest that hematuria and symptomatic hydronephrosis are not only helpful in disease detection, but also have prognostic value.
Although hematuria is the most common symptom of UC, few studies have investigated the prognostic role of hematuria. Ramirez et al. showed that the severity of hematuria was associated with more advanced pathological stages of bladder UC (18). However, inherent anatomical differences between the bladder and upper urinary tract may prevent extrapolation of this result to UTUC (4). Bladder UC is almost impossible to block the urinary tract before hematuria occurs. In contrast, UTUC is prone to urinary obstruction due to the small diameter of the upper urinary tract and may not present with hematuria. In the absence of hematuria, accurate diagnosis may be delayed, leading to tumor upstaging. As our results showed, the pathological tumor stage was significantly higher in patients without hematuria than in patients with hematuria (p = 0.001).
Previous studies have not established the protective effect of hematuria on UTUC (10–13, 19). Of these studies, some of them showed that hematuria was associated with better prognosis in patients with UTUC (12, 13), but not others (10, 11, 19). In the present study, the significance of hematuria in OS and CSS remained after adjustment for various clinicopathological variables. With three times the number of patients compared to the largest previous study (11), we believe that patients presenting with hematuria have better survival rates. Notably, the results were very similar if only gross hematuria was defined as hematuria (Supplementary Table).
Another key finding of the study was that symptomatic hydronephrosis predicted worse outcomes. Similar to the results of hematuria, not all previous studies showed that flank pain (11–13) or hydronephrosis (20–24) was unfavorable for the prognosis of UTUC. In current guidelines and in a recent meta-analysis, hydronephrosis is considered a high-risk feature (2, 25), but its prognostic significance is greatly reduced after multivariate adjustment (20, 21). Although high-grade hydronephrosis may be a better indicator of poor prognosis (22–24), interobserver variability and how severe hydronephrosis is significant are problematic. Symptomatic hydronephrosis was described as co-occurring flank pain and hydronephrosis, which was clearly defined and confirmed as an important prognostic factor for UTUC in a previous study (10). Notably, neither flank pain nor hydronephrosis alone was significant in multivariate analysis. Our results also supported the prognostic value of symptomatic hydronephrosis in UTUC.
Some previous studies grouped all local symptoms as a whole to assess their impact on prognosis. For example, in patients with renal cell carcinoma (RCC), those with flank pain, hematuria, and palpable tumors had a worse prognosis than those without these symptoms (26–28). Raman et al. used the same criteria, but local symptoms failed to predict outcomes in patients with UTUC (8). Although the etiology of flank pain can differ between RCC and UTUC (local mass effect and urinary tract obstruction, respectively), pain is probably related to tumor aggressiveness. On the contrary, hematuria generally represents the invasion of advanced RCC into the collecting system, but is a warning symptom for early diagnosis and prompt treatment of UTUC. In addition, Ataus et al. showed that in UTUC, patients with flank pain had lower survival than those with hematuria (13). Taken together, we believe that dissecting local symptoms in detail is important to obtain additional prognostic information.
Zhao et al. found that UTUC patients with hydronephrosis should have a shorter waiting time for surgery (13), otherwise the increased intraluminal pressure may lead to wall thinning and a greater chance of peripheral invasion of tumor cells (29). Since symptomatic hydronephrosis was a more reliable predictor of cancer invasiveness than hydronephrosis alone (10), we supposed that these patients may require more timely treatment to avoid upstaging. Another potential clinical application is for monitoring treatment efficacy. In the study by Miyake et al., down-grading of hydronephrosis after neoadjuvant chemotherapy was associated with favorable oncological outcomes (30). Likewise, relief of flank pain could reflect a favorable response to therapy and a proxy for downstaging.
This study has some limitations. First, this is a retrospective study. Second, we were unable to determine the exact extent of the patient’s local symptoms. Third, several important factors, such as surgical margins, histological variants, and tumor necrosis, were not included in the analysis because information on many patients was not available. We performed a sensitivity analysis in a limited subgroup and found similar results. To provide more information to those who may be wondering, we analyzed the effect of positive surgical margins on survival at different pT stages. As shown in the Supplementary Figure, positive surgical margins appeared to have the greatest impact on pT3 disease. Fourth, data collection was performed by collaborating with multiple medical centers, so surgical specimens were reviewed by different genitourinary pathologists and operated by different surgeons. To our knowledge, there is no consensus on the impact of local symptoms on the prognosis of UTUC. We have included most of the recognized clinicopathological variables in our comprehensive survival analysis, and this is the largest multicenter study to date evaluating the effect of preoperative symptoms on UTUC outcomes. We demonstrate that preoperative local symptoms are important prognostic factors, and our promising results support further prospective studies for validation.
In conclusion, symptomatic hydronephrosis was an independent prognostic factor for worse disease outcomes, while the presence of hematuria was associated with better survival. Preoperative local symptoms could be a novel variable to risk stratify patients with UTUC and help physicians make better treatment decisions.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The studies involving human participants were reviewed and approved by Kaohsiung Medical University Hospital [KMUHIRB-E(I)-20180214]. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
H-CY and W-JW conceived the project. All authors collected the data. H-CY analyzed the results. T-WL and H-CY drafted the manuscript. H-CY and W-JW edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Kaohsiung Municipal Ta-Tung Hospital (kmtth-110-015) and supported partially by the Ministry of Science and Technology (MOST 109-2314-B-037-095), Ministry of Health and Welfare (MOHW111-TDU-B-212-134006), Kaohsiung Medical University Hospital (KMUH-DK-111007C), Kaohsiung Medical University Regenerative Medicine and Cell Therapy Research Center (KMU-TC109A02), and Kaohsiung Medical University Center for Liquid Biopsy and Cohort Research (KMU-TC109B05).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Dr. Yu-Tsai Li for statistical assistance and all members of the Taiwan Upper Tract Urothelial Carcinoma Collaboration Group. All members of the Taiwan Upper Tract Urothelial Carcinoma Collaboration Group: Allen W. Chiu, Bing-Juin Chiang, Chao- Hsiang Chang, Chao-Yuan Huang, Cheng-Huang Shen, Cheng- Kuang Yang, Cheng-Ling Lee, Chen-Hsun Ho, Che-Wei Chang, Chia-Chang Wu, Chieh-Chun Liao, Chien-Hui Ou, Chih-Chen Hsu, Chih-Chin Yu, Chih-Hung Lin, Chih-Ming Lu, Chih-Yin Yeh, Ching-Chia Li, Chi-Ping Huang, Chi-Rei Yang, Chi-Wen Lo, Chuan-Shu Chen, Chung-Hsin Chen, Chung-You Tsai, Chung-Yu Lin, Chun-Hou Liao, Chun-Kai Hsu, Fang-Yu Ku, Hann-Chorng Kuo, Han-Yu Weng, Hao-Han Chang, Hong-Chiang Chang, Hsiao-Jen Chung, Hsin-Chih Yeh, Hsu-Che Huang, Ian-Seng Cheong, I-Hsuan Alan Chen, Jen-Kai Fang, Jen-Shu Tseng, Jen- Tai Lin, Jian-Hua Hong, Jih-Sheng Chen, Jungle Chi-Hsiang Wu, Kai-Jie Yu, Keng-Kok Tan, Kuan-Hsun Huang, Kun-Lin Hsieh, Lian-Ching Yu, Lun-Hsiang Yuan, Hao-Lun Luo, Marcelo Chen, Min-Hsin Yang, Pai-Yu Cheng, Po-Hung Lin, Richard Chen-Yu Wu, See-Tong Pang, Shin-Hong Chen, Shin-Mei Wong, Shiu-Dong Chung, Shi-Wei Huang, Shuo-Meng Wang, Shu-Yu Wu, Steven Kuan-Hua Huang, Ta-Yao Tai, Thomas Y. Hsueh, Ting-En Tai, Victor Chia-Hsiang Lin, Wei-Chieh Chen, Wei-Ming Li, Wei-Yu Lin, Wen-Hsin Tseng, Wen-Jeng Wu, Wun-Rong Lin, Yao-Chou Tsai, Yen-Chuan Ou, Yeong-Chin Jou, Yeong-Shiau Pu, Yi-Chia Lin, Yi-Hsuan Wu, Yi-Huei Chang, Yi-sheng Lin, Yi-Sheng Tai, Yu- Khun Lee, Yuan-Hong Jiang, Yu-Che Hsieh, Yu-Chi Chen, Yu- Ching Wen, Yung-Tai Chen, Zhe-Rui Yang.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.872849/full#supplementary-material
Supplementary Figure | Kaplan-Meier analyses of overall survival, cancer-specific survival, disease-free survival, and bladder recurrence-free survival based on positive surgical margins and stratified for different pT stages.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2022. CA Cancer J Clin (2022) 72:7–33. doi: 10.3322/caac.21708
2. Rouprêt M, Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur Urol (2021) 79:62–79. doi: 10.1016/j.eururo.2020.05.042
3. Leow JJ, Chong KT, Chang SL, Bellmunt J. Upper Tract Urothelial Carcinoma: A Different Disease Entity in Terms of Management. ESMO Open (2016) 1:e000126. doi: 10.1136/esmoopen-2016-000126
4. Green DA, Rink M, Xylinas E, Matin SF, Stenzl A, Roupret M, et al. Urothelial Carcinoma of the Bladder and the Upper Tract: Disparate Twins. J Urol (2013) 189:1214–21. doi: 10.1016/j.juro.2012.05.079
5. Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, Kikuchi E, et al. Outcomes of Radical Nephroureterectomy: A Series From the Upper Tract Urothelial Carcinoma Collaboration. Cancer (2009) 115:1224–33. doi: 10.1002/cncr.24135
7. Ito Y, Kikuchi E, Tanaka N, Miyajima A, Mikami S, Jinzaki M, et al. Preoperative Hydronephrosis Grade Independently Predicts Worse Pathological Outcomes in Patients Undergoing Nephroureterectomy for Upper Tract Urothelial Carcinoma. J Urol (2011) 185:1621–26. doi: 10.1016/j.juro.2010.12.035
8. Raman JD, Shariat SF, Karakiewicz PI, Lotan Y, Sagalowsky AI, Roscigno M, et al. Does Preoperative Symptom Classification Impact Prognosis in Patients With Clinically Localized Upper-Tract Urothelial Carcinoma Managed by Radical Nephroureterectomy? Urol Oncol (2011) 29:716–23. doi: 10.1016/j.urolonc.2009.11.007
9. Inman BA, Tran VT, Fradet Y, Lacombe L. Carcinoma of the Upper Urinary Tract: Predictors of Survival and Competing Causes of Mortality. Cancer (2009) 115:2853–62. doi: 10.1002/cncr.24339
10. Yeh HC, Jan HC, Wu WJ, Li CC, Li WM, Ke HL, et al. Concurrent Preoperative Presence of Hydronephrosis and Flank Pain Independently Predicts Worse Outcome of Upper Tract Urothelial Carcinoma. PLoS One (2015) 10:e0139624. doi: 10.1371/journal.pone.0139624
11. Fang D, Gong YQ, Singla N, Yang KL, Xiong GY, Zhang L, et al. The Significance of the Initial Symptom in Chinese Patients With Upper Tract Urothelial Carcinoma: Regular Health Examination Is Still Underutilized. Kaohsiung J Med Sci (2018) 34:511–21. doi: 10.1016/j.kjms.2018.01.003
12. Feng C, Wang L, Ding G, Ding Q, Zhou Z, Jiang H, et al. Predictive Value of Clinicopathological Markers for the Metachronous Bladder Cancer and Prognosis of Upper Tract Urothelial Carcinoma. Sci Rep (2014) 4:4015. doi: 10.1038/srep04015
13. Ataus S, Onal B, Tunc B, Erozenci A, Cekmen A, Kural AR, et al. Factors Affecting the Survival of Patients Treated by Standard Nephroureterectomy for Transitional Cell Carcinoma of the Upper Urinary Tract. Int Urol Nephrol (2006) 38:9–13. doi: 10.1007/s11255-005-3151-3
14. Zhao F, Qi N, Zhang C, Xue N, Li S, Zhou R, et al. Impact of Surgical Wait Time on Survival in Patients With Upper Urinary Tract Urothelial Carcinoma With Hydronephrosis. Front Oncol (2021) 11:698594. doi: 10.3389/fonc.2021.698594
15. Eble JN, Sauter G, Epstein JI, Sesterhenn IA. ‘Tumours of the Urinary System’. In: Beltran AL, editor. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Lyon, France: IARC Press (2004). p. 90.
16. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, et al. AJCC Cancer Staging Manual. 7th Edition. Chicago: Springer (2010). p. 491.
17. Stimson CJ, Cookson MS, Barocas DA, Clark PE, Humphrey JE, Patel SG, et al. Preoperative Hydronephrosis Predicts Extravesical and Node Positive Disease in Patients Undergoing Cystectomy for Bladder Cancer. J Urol (2010) 183:1732–37. doi: 10.1016/j.juro.2010.01.028
18. Ramirez D, Gupta A, Canter D, Harrow B, Dobbs RW, Kucherov V, et al. Microscopic Haematuria at Time of Diagnosis is Associated With Lower Disease Stage in Patients With Newly Diagnosed Bladder Cancer. BJU Int (2016) 117:783–86. doi: 10.1111/bju.13345
19. Hurel S, Rouprêt M, Seisen T, Comperat E, Phé V, Droupy S, et al. Influence of Preoperative Factors on the Oncologic Outcome for Upper Urinary Tract Urothelial Carcinoma After Radical Nephroureterectomy. World J Urol (2015) 33:335–41. doi: 10.1007/s00345-014-1311-8
20. Sakano S, Matsuyama H, Kamiryo Y, Hayashida S, Yamamoto N, Kaneda Y, et al. Risk Group Stratification Based on Preoperative Factors to Predict Survival After Nephroureterectomy in Patients With Upper Urinary Tract Urothelial Carcinoma. Ann Surg Oncol (2013) 20:4389–96. doi: 10.1245/s10434-013-3259-0
21. Morizane S, Iwamoto H, Masago T, Yao A, Isoyama T, Sejima T, et al. Preoperative Prognostic Factors After Radical Nephroureterectomy in Patients With Upper Urinary Tract Urothelial Carcinoma. Int Urol Nephrol (2013) 45:99–106. doi: 10.1007/s11255-012-0347-1
22. Liang C, Chi R, Huang L, Wang J, Liu H, Xu D, et al. Upper Tract Urothelial Carcinomas Accompanied by Previous or Synchronous Nonmuscle-Invasive Bladder Cancer and Preoperative Hydronephrosis Might Have Worse Oncologic Outcomes After Radical Nephroureterectomy. Clin Genitourin Cancer (2016) 14:e469–77. doi: 10.1016/j.clgc.2016.02.008
23. Luo HL, Kang CH, Chen YT, Chuang YC, Lee WC, Cheng YT, et al. Severity of Hydronephrosis Correlates With Tumour Invasiveness and Urinary Bladder Recurrence of Ureteric Cancer. BJU Int (2013) 112:489–94. doi: 10.1111/bju.12157
24. Hwang I, Jung SI, Nam DH, Hwang EC, Kang TW, Kwon DD, et al. Preoperative Hydronephrosis and Diabetes Mellitus Predict Poor Prognosis in Upper Urinary Tract Urothelial Carcinoma. Can Urol Assoc J (2013) 7:215–20. doi: 10.5489/cuaj.11236
25. Ye T, Yang X, Lv P, Liu H, Ye Z. Prognostic Value of Preoperative Hydronephrosis in Patients Undergoing Radical Nephroureterectomy for Upper Tract Urinary Carcinoma: A Systematic Review and Meta-Analysis. Front Oncol (2020) 10:600511. doi: 10.3389/fonc.2020.600511
26. Schips L, Lipsky K, Zigeuner R, Salfellner M, Winkler S, Langner C, et al. Impact of Tumor-Associated Symptoms on the Prognosis of Patients With Renal Cell Carcinoma: A Single-Center Experience of 683 Patients. Urology (2003) 62:1024–28. doi: 10.1016/s0090-4295(03)00763-5
27. Lee CT, Katz J, Fearn PA, Russo P. Mode of Presentation of Renal Cell Carcinoma Provides Prognostic Information. Urol Oncol (2002) 7:135–40. doi: 10.1016/s1078-1439(01)00185-5
28. Szendroi A, Tabák A, Riesz P, Szucs M, Nyírády P, Majoros A, et al. Clinical Symptoms Related to Renal Cell Carcinoma Are Independent Prognostic Factors for Intraoperative Complications and Overall Survival. Int Urol Nephrol (2009) 41:835–42. doi: 10.1007/s11255-009-9539-8
29. Lee JN, Kwon SY, Choi GS, Kim HT, Kim TH, Kwon TG, et al. Impact of Surgical Wait Time on Oncologic Outcomes in Upper Urinary Tract Urothelial Carcinoma. J Surg Oncol (2014) 110:468–75. doi: 10.1002/jso.23589
30. Miyake M, Marugami N, Fujiwara Y, Komura K, Inamoto T, Azuma H, et al. Down-Grading of Ipsilateral Hydronephrosis by Neoadjuvant Chemotherapy Correlates With Favorable Oncological Outcomes in Patients Undergoing Radical Nephroureterectomy for Ureteral Carcinoma. Diagnostics (Basel) (2019) 10:10. doi: 10.3390/diagnostics10010010
Keywords: hematuria, flank pain, symptomatic hydronephrosis, upper tract urothelial carcinoma (UTUC), radical nephroureterectomy (RNU), prognosis
Citation: Yeh H-C, Chang C-H, Fang J-K, Chen I-HA, Lin J-T, Hong J-H, Huang C-Y, Wang S-S, Chen C-S, Lo C-W, Yu C-C, Tseng J-S, Lin W-R, Jou Y-C, Cheong I-S, Jiang Y-H, Tsai C-Y, Hsueh TY, Chen Y-T, Huang H-C, Tsai Y-C, Lin W-Y, Wu C-C, Lin P-H, Lin T-W and Wu W-J (2022) The Value of Preoperative Local Symptoms in Prognosis of Upper Tract Urothelial Carcinoma After Radical Nephroureterectomy: A Retrospective, Multicenter Cohort Study. Front. Oncol. 12:872849. doi: 10.3389/fonc.2022.872849
Received: 10 February 2022; Accepted: 09 May 2022;
Published: 02 June 2022.
Edited by:
How-Ran Guo, National Cheng Kung University, TaiwanReviewed by:
Fabrizio Di Maida, Careggi University Hospital, ItalyTakumi Takeuchi, Kanto Rosai Hospital, Japan
Copyright © 2022 Yeh, Chang, Fang, Chen, Lin, Hong, Huang, Wang, Chen, Lo, Yu, Tseng, Lin, Jou, Cheong, Jiang, Tsai, Hsueh, Chen, Huang, Tsai, Lin, Wu, Lin, Lin and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Te-Wei Lin, ZGVycmljazgzNTZAeWFob28uY29tLnR3; Wen-Jeng Wu, d2VqZXd1QGttdS5lZHUudHc=
 Hsin-Chih Yeh
Hsin-Chih Yeh Chao-Hsiang Chang
Chao-Hsiang Chang Jen-Kai Fang7
Jen-Kai Fang7 I-Hsuan Alan Chen
I-Hsuan Alan Chen Jen-Tai Lin
Jen-Tai Lin Jian-Hua Hong
Jian-Hua Hong Chao-Yuan Huang
Chao-Yuan Huang Shian-Shiang Wang
Shian-Shiang Wang Chuan-Shu Chen
Chuan-Shu Chen Chi-Wen Lo
Chi-Wen Lo Yung-Tai Chen
Yung-Tai Chen Yao-Chou Tsai
Yao-Chou Tsai Wei-Yu Lin
Wei-Yu Lin Te-Wei Lin
Te-Wei Lin