- Centre for Colorectal Disease, St Vincent's University Hospital, Dublin, Ireland
Background: Impairment of bowel, urogenital and fertility-related function in patients treated for rectal cancer is common. While the rate of rectal cancer in the young (<50 years) is rising, there is little data on functional outcomes in this group.
Methods: The REACCT international collaborative database was reviewed and data on eligible patients analysed. Inclusion criteria comprised patients with a histologically confirmed rectal cancer, <50 years of age at time of diagnosis and with documented follow-up including functional outcomes.
Results: A total of 1428 (n=1428) patients met the eligibility criteria and were included in the final analysis. Metastatic disease was present at diagnosis in 13%. Of these, 40% received neoadjuvant therapy and 50% adjuvant chemotherapy. The incidence of post-operative major morbidity was 10%. A defunctioning stoma was placed for 621 patients (43%); 534 of these proceeded to elective restoration of bowel continuity. The median follow-up time was 42 months. Of this cohort, a total of 415 (29%) reported persistent impairment of functional outcomes, the most frequent of which was bowel dysfunction (16%), followed by bladder dysfunction (7%), sexual dysfunction (4.5%) and infertility (1%).
Conclusion: A substantial proportion of patients with early-onset rectal cancer who undergo surgery report persistent impairment of functional status. Patients should be involved in the discussion regarding their treatment options and potential impact on quality of life. Functional outcomes should be routinely recorded as part of follow up alongside oncological parameters.
Introduction
While globally colorectal cancer incidence rates are overall predominantly stable or declining, a slew of data suggests that in Westernised countries incidence in the younger population (<50 years) is markedly increasing (1–8). Although there is no agreed definition of ‘young-onset’ colorectal cancer, most literature includes those diagnosed at ≤ 50 years of age (9–11). A proportion of these are due to inherited colorectal cancer syndromes (e.g. Lynch syndrome, familial adenomatous polyposis etc.); however, the majority are sporadic (9). Colorectal cancer in the young demonstrates some distinct features comparative to the disease which occurs in the older age group. The vast majority of the increase in incidence is accounted for by left-sided tumors, with little-no increase in right-sided neoplasms (9, 10, 12). Compared to the older age group, a greater proportion of young rectal cancers present symptomatically, rather than being detected incidentally or by screening. Despite this, the interval from onset of symptoms to diagnosis is on average six months greater for the young cohort than in older adults (13, 14). As such these patients tend to present with a more advanced stage at diagnosis, with 71% diagnosed at stage III or IV (15, 16). In addition, high risk features such as signet-ring mucinous histology and poor differentiation are more frequently seen. However, these factors do not confer a worse prognosis, with stage-adjusted cancer-specific survival similar or better than older patients (10, 17–19). This may reflect more aggressive treatment strategies. Young patients with rectal cancer are more likely than older patients to undergo systemic therapy and complex surgical interventions for equivalent stage disease (19–21).
Functional impairment after treatment for rectal cancer is unfortunately common. With respect to low anterior resection syndrome (LARS), the reported incidence of major symptomatology after curative restorative anterior resection varies from 37 to up to 90% of patients (22–26). The presence of a stoma, whether permanent or temporary, has negative effects on body image – which may persist even after restoration of bowel continuity – and may delay the ability of young patients to return to work (27). Urogenital symptoms are less frequently discussed by healthcare providers but are also common (28, 29).
A diagnosis of rectal cancer in the young carries several special considerations. The increased frequency of adverse pathological features warrants aggressive management strategies to ensure optimal oncological outcomes. Nevertheless, long-term health-related quality of life (HRQOL) is also of critical importance. Particular consideration should be given to preservation of a functional sphincter complex, avoidance of a permanent stoma and autonomic nerve sparing. Other special considerations include fertility and the potential impact of pelvic radiotherapy and surgery, especially in female patients of child-bearing age. The importance of measuring functional outcomes after treatment for rectal cancer in addition to post-operative morbidity and oncological outcomes is increasingly recognized and reported. There is a paucity of data examining functional impairment after rectal cancer treatment in young-onset patients (30). This study evaluated gastrointestinal, genitourinary and fertility concerns in a large cohort of young-onset rectal cancer patients.
Methods
Study Participants
An international multicentre observational study to assess the functional outcomes of patients diagnosed with early age onset rectal cancer was performed. Inclusion criteria were adults aged between 18 and 49 years with a histologically confirmed diagnosis of rectal cancer, with known post-operative bowel, bladder or sexual functional status.
Data Collection and Analysis
Patients who fulfilled the inclusion criteria of the study were selected from the REACCT Collaborative database. All participating institutions are tertiary referral units with specialist expertise in CRC. Ethical approval was sought at an individual institutional level. Collected data included baseline patient demographics, clinical, stage, surgical, and treatment data, and functional outcomes. A microscopically clear resection (R0) was defined by a tumor-free resection margin of at least 1 mm. Bowel dysfunction was defined as frequency, urgency, clustering or incontinence. Bladder dysfunction was defined as voiding difficulties or need for catheterization. Comprehensive investigation was required for a diagnosis of infertility. Basic descriptive statistics were calculated.
Results
A total of 1,428 patients were included in the study. The median (range) age was 42 (18–49) years and 816 (57%) were male. Median (range) BMI was 22 (13–50). The majority (86%) had non-metastatic disease. Baseline demographics are summarised in Table 1.
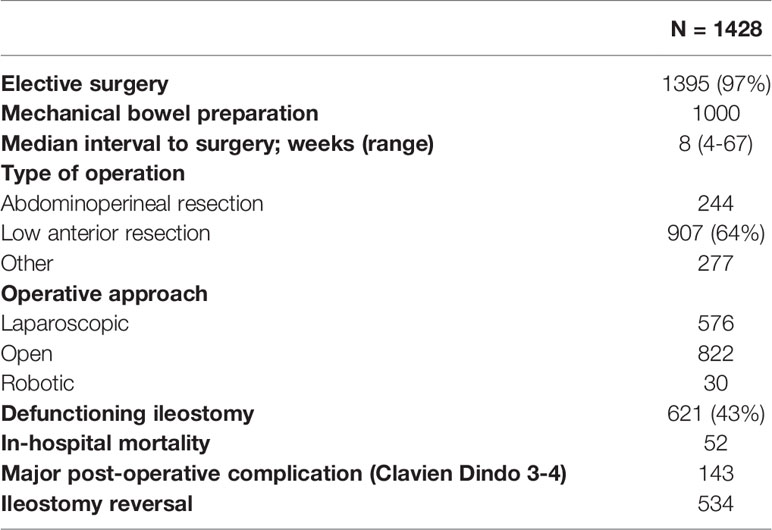
All patients underwent surgical intervention with 97% (n=1395) having an elective procedure. Neoadjuvant therapy was administered to 39% patients. The median (range) interval to surgery was 8 (4-67) weeks. The most commonly performed operation was a low anterior resection (LAR) whilst 17% underwent an APR and 19% had another procedure such as local excision (LE). Defunctioning stomas were placed in two-thirds of those undergoing LAR. Adjuvant chemotherapy was given to 51% (n=724). Operative details are summarized in Table 2.
The rate of major post-operative morbidity within 30 days was 9.6%, with ileus the most commonly reported post-operative morbidity. The median follow-up was 42 months (range 1-180). The most common functional impairment related to the gastrointestinal tract. Symptoms of bowel dysfunction such as frequency, urgency, clustering or incontinence were present in 17% (n=235). Urinary dysfunction (e.g. voiding difficulties or the need for intermittent self-catheterization) was reported by 7% (n=100) whilst 6% (n=65) experienced symptoms of sexual dysfunction. Only fifteen patients reported fertility-related problems.
Discussion
In general, some degree of functional impairment post treatment for rectal cancer is extremely common. With respect to bowel dysfunction, as outlined in the Introduction, rates of up to 90% have been reported. These symptoms may persist well beyond the initial post-operative phase, with as many as 71-74% patients reporting some degree of faecal incontinence and rectal evacuation difficulties at 15 years post-surgery (25, 31, 32). The prevalence of major LARS at long-term follow up is high, with data from a recent meta-analysis reporting its frequency at 41% (33, 34). The need for a defunctioning stoma may also be associated with post-operative LARS (35, 36). Furthermore, a longer interval from stoma formation to restoration of bowel continuity may be associated with worse final bowel function, particularly with respect to urgency and continence (35, 37). In the present study of functional outcomes in young patients with rectal cancer, 29% reported bowel, bladder and/or sexual dysfunction.
Impairment of bladder and sexual function in patients with rectal cancer are also common, although these are less frequently reported on than bowel dysfunction, especially in female patients (38, 39). These effects are primarily due to division of the pelvic autonomic nerves during surgery, although some symptoms may also be a sequela of pelvic radiotherapy. Aside from impact on bowel function, presence of a stoma is associated with worse sexual function, negative body image and a delay in return to work (27, 40). Rates of urinary dysfunction post treatment range from 58-77% of patients reporting urgency, 46% voiding difficulty and 20-63% urinary incontinence (39, 41). Rectal cancer patients also report more sexual dysfunction symptoms and body image issues comparative to those with more proximal neoplasms. Up to 54% of male rectal cancer survivors report erectile dysfunction in contrast to 25% of those with colonic cancer (42). In women, dyspareunia occurs in 26-53% and vaginal dryness in up to 75% (39, 43). Incidence of sexual dysfunction in both males and females appears to be associated those undergoing with APR and who received NCRT (38, 39, 44, 45).
The data above pertain to patients of all ages. Despite the rapid acceleration in rectal cancer incidence in the younger population, there is scant literature specifically examining post-operative functional outcomes in young-onset rectal cancer patients. Nevertheless, this cohort has specific needs and considerations. Data from the QoLiRECT study suggests that younger patients are disproportionately affected by LARS (34). Aside from impact on bowel function, presence of a stoma is associated with worse sexual function, negative body image and a delay in return to work (27, 40). For premenopausal female patients the effect of treatment on fertility must also be borne in mind, primarily due to pelvic radiotherapy but also potentially from adhesions post pelvic surgery and the effect of chemotherapeutic drugs such as oxaliplatin (46–49).
To our knowledge, this is the first large-scale series reporting the incidence of bowel, urogenital and fertility-related dysfunction in a cohort of young-onset rectal cancer patients. Although not approaching the rates reported in some of the literature described above, nevertheless a substantial minority of patients reported impairment of at least one functional domain. In line with the existing body of literature, bowel dysfunction was the most prevalent functional impairment, followed by bladder and sexual dysfunction. There are several acknowledged contributing factors which increase the likelihood of functional impairment post-operatively. These include tumor height – thus influencing the surgical procedure undertaken and feasibility of sphincter preservation -, administration of NCRT, TME versus organ sparing approaches, and occurrence of post-operative morbidity, in particular anastomotic leak.
Many of the factors described above which affect functional impairment post-treatment are non-modifiable. Therefore, thorough pre-operative counselling on the potential outcomes to ensure informed consent and engaging in a shared decision-making process with these patients on the risks and benefits of individual treatment options is critical. This process may be assisted by utilization of aids such as the pre-operative LARS score (POLARS) tool, a nomogram which inputs clinico-pathological characteristics including patient age, tumor height, administration of NCRT, planned total or partial mesorectal excision and plan for a defunctioning stoma in order to predict the likelihood of post-operative bowel dysfunction (50). Models also exist to predict the likelihood of permanent stoma at 2 years post-operatively and may similarly be used to assist in providing informed consent and facilitate shared decision-making between patients and healthcare providers (51). Patients of reproductive age should be specifically counselled on and referred for fertility preservation options prior to commencement of treatment (20, 52, 53).
Consideration should be given to organ or sphincter-preserving and nerve-sparing approaches where feasible without threatening oncological safety. Data from the International Watch and Wait Database suggests that a strategy of non-operative management in young-onset patients with a cCR is not associated with worse oncological outcomes (54). For low-risk early rectal cancers, organ-sparing local excision in the form of transanal endoscopic microsurgery (TEM) or transanal minimally invasive surgery (TAMIS) with or without NCRT may be adequate and avoid the functional morbidity associated with total mesorectal excision (55–58). In patients with locally advanced rectal cancer, total neoadjuvant treatment (TNT) in the form of radiotherapy with either induction or consolidation chemotherapy appears to result in increased rates of pCR, reduced toxicity comparative to adjuvant chemotherapy and improved survival (59–62). For patients requiring TME, there is some evidence in the literature to suggest that utilization of a robotic-assisted approach over laparoscopic may facilitate sparing of the pelvic autonomic nerves and thus reduce the incidence of post-operative bladder and sexual dysfunction (63–67). However, further data will be required to definitively confirm this. In this series, only 2% of procedures were undertaken robotically, although this proportion is likely to increase over time with the burgeoning uptake of the technology.
The decision whether to place a defunctioning stoma should be carefully weighed against the negative impacts on sexual function, ability to return to work and body image (20, 27, 40, 42). Early recognition and prompt treatment of anastomotic leak is important to minimize adverse effects on long-term bowel function (68–70). Restoration of bowel continuity where a diverting stoma has been placed should be a priority, as a long interval to reversal is associated with dysfunction (37).
In the immediate post-operative period, the major consideration should be early recognition and prompt treatment of morbidity, specifically anastomotic leak, to minimize the recognized adverse effect of this complication on long-term bowel function (68–70). After the immediate post-operative period, restoration of bowel continuity where a diverting stoma has been placed should be a priority, as a longer interval to reversal of a defunctioning stoma is associated with a higher incidence of long-term faecal incontinence (37). At long-term follow-up, functional outcomes should be routinely recorded alongside oncological parameters.
In conclusion, future studies focusing on patient-reported as well as oncological outcomes in young rectal cancer patients will result in improved characterization of functional outcomes in this group. Enhanced functional outcomes via organ preservation, sphincter preservation and other approaches will have consequent positive effects on quality of life.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Research Ethics Committee, St Vincent’s University Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
REACCT Collaborative
Members of the REACCT Collaborative are:
Lauren V. O’Connell, Alexandra M. Zaborowski, Ahmed Abdile. Michel Adamina, Felix Aigner, Laura d’Allens, Caterina Allmer, Andrea Álvarez, Rocio Anula, Mihailo Andric, Sam Atallah Simon Bach, Miklosh Bala, Marie Barussaud, Augustinas Bausys, Andrew Beggs, Felipe Bellolio, Melissa-Rose Bennett, Vicki Bevan, Sebastiano Biondo, Gabriele Bislenghi, Marc Bludau, Carl Brown, Christiane Bruns, Daniel D. Buchanan, Pamela Buchwald, Jacobus W.A. Burger, Nikita Burlov, Michela Campanelli, Maylis Capdepont, Michele Carvello, Hwee-Hoon Chew, Dimitri Christoforidis, David Clark, Marta Climent, Rowan Collinson, Kyle G. Cologne, Tomas Contreras, Roland Croner, Ian R. Daniels, Giovanni Dapri, Justin Davies, Paolo Delrio, Quentin Denost, Michael Deutsch, Andre Dias, André D’Hoore, Evgeniy Drozdov, Daniel Duek, Malcolm Dunlop, Adam Dziki, Aleksandra Edmundson, Sergey Efetov, Alaa El-Hussuna, Brodie Elliott, Sameh Emile, Eloy Espin-Basany, Martyn Evans, Seraina Faes, Omar Faiz, Nuno Figueiredo, Fergal Fleming, Caterina Foppa, George Fowler, Matteo Frasson, Tim Forgan, Frank Frizelle, Shamil Gadaev, Jose Gellona, Tamara Glyn, Barisic Goran, Emma Greenwood, Marianne G. Guren, Stephanie Guillon, Ida Gutlic, Dieter Hahnloser, Heather Hampel, Ann Hanly, Hirotoshi Hasegawa, Lene Hjerrild Iversen, Andrew Hill, James Hill, Jiri Hoch, Roel Hompes, Luis Hurtado, Fabiano Iaquinandi, Ugne Imbrasaite, Rumana Islam, Mehrenah D Jafari, Andrea Jiménez Salido, Marta Jiménez-Toscano, Yukihide Kanemitsu, Aleksei Karachun, Ahmer A. Karimuddin, Deborah S. Keller, Justin Kelly, Rory Kennelly, Gleb Khrykov, Petr Kocian, Cherry Koh, Neils Kok, Katrina A. Knight, Joep Knol, Christos Kontovounisios, Hartwig Korner, Zoran Krivokapic, Irmgard Kronberger, Hidde Maarten Kroon, Marius Kryzauskas, Said Kural, Miranda Kusters, Zaher Lakkis, Timur Lankov, David Larson, György Lázár, Kai-Yin Lee, Suk Hwan Lee, Jérémie H. Lefèvre, Anna Lepisto, Christopher Lieu, Lynette Loi, Craig Lynch, Helene Maillou-Martinaud, Annalisa Maroli, Sean T. Martin, Anna Martling, Klaus E. Matzel, Julio Mayol, Frank McDermott, Guillaume Meurette, Monica Millan, Martin Mitteregger, Andrei Moiseenko, John RT. Monson, Stefan Morarasu, Konosuke Moritani, Gabriela Möslein, Martino Munini, Caio Nahas, Sergio Nahas, Ionut Negoi, Anastasia Novikova, Misael Ocares, Koji Okabayashi, Alexandra Olkina, Luis Oñate-Ocaña, Jaime Otero, Cihan Ozen, Ugo Pace, Guilherme Pagin São Julião, Lidiia Panaiotti, Yves Panis, Demetris Papamichael, Swati Patel, Juan Carlos Patrón Uriburu, Sze-Lin Peng, Miguel Pera, Rodrigo O. Perez, Alexei Petrov, Frank Pfeffer, Terry P. Phang, Tomas Poskus, Heather Pringle, David Proud, Ivana Raguz, Nuno Rama, Shahnawaz Rasheed, Manoj J. Raval, Daniela Rega, Christoph Reissfelder, Juan Carlos Reyes Meneses, Frederic Ris, Stefan Riss, Homero Rodriguez-Zentner, Campbell S Roxburgh, Avanish Saklani, Tarik Sammour, Deborah Saraste, Martin Schneider, Ryo Seishima, Aleksandar Sekulic, Toni Seppala, Kieran Sheahan, Alexandra Shlomina, Guiseppe Sica, Tongplaew Singnomklao, Leandro Siragusa, Neil Smart, Alejandro Solis-Peña, Antonino Spinelli, Roxane D. Staiger, Michael J. Stamos, Scott Steele, Ker-Kan Tan, Pieter J Tanis, Paris Tekkis, Biniam Teklay, Sabrina Tengku, Petr Tsarkov, Matthias Turina, Alexis Ulrich, Bruna B. Vailati, Meike van Harten, Cornelis Verhoef, Satish Warrier, Steven Wexner, Benjamin A. Weinberg, Cameron Wells, Albert Wolthuis, Evangelos Xynos, Nancy You, Alexander Zakharenko, Justino Zeballos, Youzhi Zhou, Des C. Winter.
Author Contributions
LO’C: drafting of manuscript. DW: conceptualization of study, editing of manuscript and confirmation of final version for submission. AMZ: drafting and editing of manuscript, approval of final version for submission. All other authors - data collection and submission, statistical analysis. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen Y-J, Ciombor KK, et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2018) 16(7):874–901. doi: 10.6004/jnccn.2018.0061
2. Keum N, Giovannucci E. Global Burden of Colorectal Cancer: Emerging Trends, Risk Factors and Prevention Strategies. Nat Rev Gastroenterol Hepatol (2019) 16(12):713–32. doi: 10.1038/s41575-019-0189-8
3. Araghi M, Soerjomataram I, Bardot A, Ferlay J, Cabasag CJ, Morrison DS, et al. Changes in Colorectal Cancer Incidence in Seven High-Income Countries: A Population-Based Study. Lancet Gastroenterol Hepatol (2019) 4(7):511–8. doi: 10.1016/S2468-1253(19)30147-5
4. Siegel RL, Jemal A, Ward EM. Increase in Incidence of Colorectal Cancer Among Young Men and Women in the United States. Cancer Epidemiol Biomarkers Prev (2009) 18(6):1695–8. doi: 10.1158/1055-9965.EPI-09-0186
5. Bailey CE, Hu C-Y, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM, et al. Increasing Disparities in the Age-Related Incidences of Colon and Rectal Cancers in the United States, 1975-2010. JAMA Surg (2015) 150(1):17–22. doi: 10.1001/jamasurg.2014.1756
6. Vuik FE, Nieuwenburg SA, Bardou M, Lansdorp-Vogelaar I, Dinis-Ribeiro M, Bento MJ, et al. Increasing Incidence of Colorectal Cancer in Young Adults in Europe Over the Last 25 Years. Gut (2019) 68(10):1820–6. doi: 10.1136/gutjnl-2018-317592
7. Petersson J, Bock D, Martling A, Smedby KE, Angenete E, Saraste D. Increasing Incidence of Colorectal Cancer Among the Younger Population in Sweden. BJS Open (2020) 4(4):645–58. doi: 10.1002/bjs5.50279
8. Chambers AC, Dixon SW, White P, Williams AC, Thomas MG, Messenger DE. Demographic Trends in the Incidence of Young-Onset Colorectal Cancer: A Population-Based Study. Br J Surg (2020) 107(5):595–605. doi: 10.1002/bjs.11486
9. Mauri G, Sartore-Bianchi A, Russo A-G, Marsoni S, Bardelli A, Siena S. Early-Onset Colorectal Cancer in Young Individuals. Mol Oncol (2019) 13(2):109–31. doi: 10.1002/1878-0261.12417
10. Patel SG, Ahnen DJ. Colorectal Cancer in the Young. Curr Gastroenterol Rep (2018) 20(4):15. doi: 10.1007/s11894-018-0618-9
11. REACCT Collaborative, Zaborowski AM, Abdile A, Adamina M, Aigner F, d’Allens L, et al. Characteristics of Early-Onset vs Late-Onset Colorectal Cancer: A Review. JAMA Surg (2021) 156(9):865–74. doi: 10.1001/jamasurg.2021.2380
12. Kasi PM, Shahjehan F, Cochuyt JJ, Li Z, Colibaseanu DT, Merchea A. Rising Proportion of Young Individuals With Rectal and Colon Cancer. Clin Colorectal Cancer (2019) 18(1):e87–95. doi: 10.1016/j.clcc.2018.10.002
13. Scott RB, Rangel LE, Osler TM, Hyman NH. Rectal Cancer in Patients Under the Age of 50 Years: The Delayed Diagnosis. Am J Surg (2016) 211(6):1014–8. doi: 10.1016/j.amjsurg.2015.08.031
14. Mitchell E, Macdonald S, Campbell NC, Weller D, Macleod U. Influences on Pre-Hospital Delay in the Diagnosis of Colorectal Cancer: A Systematic Review. Br J Cancer (2008) 98(1):60–70. doi: 10.1038/sj.bjc.6604096
15. Meester RGS, Mannalithara A, Lansdorp-Vogelaar I, Ladabaum U. Trends in Incidence and Stage at Diagnosis of Colorectal Cancer in Adults Aged 40 Through 49 Years, 1975-2015. JAMA (2019) 321(19):1933–4. doi: 10.1001/jama.2019.3076
16. Yarden RI, Newcomer KL, Never Too Young Advisory Board, CancerAlliance C. Abstract 3347: Young Onset Colorectal Cancer Patients Are Diagnosed With Advanced Disease After Multiple Misdiagnoses. Sci Health Policy (2019) 79 (3_Supplement):3347. doi: 10.1158/1538-7445.am2019-3347
17. Abdelsattar ZM, Wong SL, Regenbogen SE, Jomaa DM, Hardiman KM, Hendren S. Colorectal Cancer Outcomes and Treatment Patterns in Patients Too Young for Average-Risk Screening. Cancer (2016) 122(6):929–34. doi: 10.1002/cncr.29716
18. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. CA Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
19. Saraste D, Järås J, Martling A. Population-Based Analysis of Outcomes With Early-Age Colorectal Cancer. Br J Surg (2020) 107(3):301–9. doi: 10.1002/bjs.11333
20. Siegel RL, Jakubowski CD, Fedewa SA, Davis A, Azad NS. Colorectal Cancer in the Young: Epidemiology, Prevention, Management. Am Soc Clin Oncol Educ Book (2020) 40:1–14. doi: 10.1200/EDBK_279901
21. Zaborowski AM, Murphy B, Creavin B, Rogers AC, Kennelly R, Hanly A, et al. Clinicopathological Features and Oncological Outcomes of Patients With Young-Onset Rectal Cancer. Br J Surg (2020) 107(5):606–12. doi: 10.1002/bjs.11526
22. Croese AD, Zubair ON, Lonie J, Trollope AF, Vangaveti VN, Mushaya C, et al. Prevalence of Low Anterior Resection Syndrome at a Regional Australian Centre. ANZ J Surg (2018) 88(12):E813–7. doi: 10.1111/ans.14749
23. Hughes DL, Cornish J, Morris C, LARRIS Trial Management Group. Functional Outcome Following Rectal Surgery-Predisposing Factors for Low Anterior Resection Syndrome. Int J Colorectal Dis (2017) 32(5):691–7. doi: 10.1007/s00384-017-2765-0
24. Battersby NJ, Juul T, Christensen P, Janjua AZ, Branagan G, Emmertsen KJ, et al. Predicting the Risk of Bowel-Related Quality-Of-Life Impairment After Restorative Resection for Rectal Cancer: A Multicenter Cross-Sectional Study. Dis Colon Rectum (2016) 59(4):270–80. doi: 10.1097/DCR.0000000000000552
25. Pieniowski EHA, Palmer GJ, Juul T, Lagergren P, Johar A, Emmertsen KJ, et al. Low Anterior Resection Syndrome and Quality of Life After Sphincter-Sparing Rectal Cancer Surgery: A Long-Term Longitudinal Follow-Up. Dis Colon Rectum (2019) 62(1):14–20. doi: 10.1097/DCR.0000000000001228
26. Sun W, Dou R, Chen J, Lai S, Zhang C, Ruan L, et al. Impact of Long-Course Neoadjuvant Radiation on Postoperative Low Anterior Resection Syndrome and Quality of Life in Rectal Cancer: Post Hoc Analysis of a Randomized Controlled Trial. Ann Surg Oncol (2019) 26(3):746–55. doi: 10.1245/s10434-018-07096-8
27. den Bakker CM, Anema JR, Huirne JAF, Twisk J, Bonjer HJ, Schaafsma FG. Predicting Return to Work Among Patients With Colorectal Cancer. Br J Surg (2020) 107(1):140–8. doi: 10.1002/bjs.11313
28. Celentano V, Cohen R, Warusavitarne J, Faiz O, Chand M. Sexual Dysfunction Following Rectal Cancer Surgery. Int J Colorectal Dis (2017) 32(11):1523–30. doi: 10.1007/s00384-017-2826-4
29. Nocera F, Angehrn F, von Flüe M, Steinemann DC. Optimising Functional Outcomes in Rectal Cancer Surgery. Langenbecks Arch Surg (2021) 406(2):233–50. doi: 10.1007/s00423-020-01937-5
30. Denost Q. The Challenge Posed by Young-Onset Rectal Cancer. Br J Surg (2020) 107(5):481–3. doi: 10.1002/bjs.11591
31. Oya M, Komatsu J, Takase Y, Nakamura T, Ishikawa H. Comparison of Defecatory Function After Colonic J-Pouch Anastomosis and Straight Anastomosis for Stapled Low Anterior Resection: Results of a Prospective Randomized Trial. Surg Today (2002) 32(2):104–10. doi: 10.1007/s005950200001
32. van Duijvendijk P, Slors JFM, Taat CW, van Tets WF, van Tienhoven G, Obertop H, et al. Prospective Evaluation of Anorectal Function After Total Mesorectal Excision for Rectal Carcinoma With or Without Preoperative Radiotherapy. Am J Gastroenterol (2002) 97(9):2282–9. doi: 10.1111/j.1572-0241.2002.05782.x
33. Croese AD, Lonie JM, Trollope AF, Vangaveti VN, Ho Y-H. A Meta-Analysis of the Prevalence of Low Anterior Resection Syndrome and Systematic Review of Risk Factors. Int J Surg (2018) 56:234–41. doi: 10.1016/j.ijsu.2018.06.031
34. Sandberg S, Asplund D, Bisgaard T, Bock D, González E, Karlsson L, et al. Low Anterior Resection Syndrome in a Scandinavian Population of Patients With Rectal Cancer: A Longitudinal Follow-Up Within the QoLiRECT Study. Colorectal Dis (2020) 22(10):1367–78. doi: 10.1111/codi.15095
35. Vogel I, Reeves N, Tanis PJ, Bemelman WA, Torkington J, Hompes R, et al. Impact of a Defunctioning Ileostomy and Time to Stoma Closure on Bowel Function After Low Anterior Resection for Rectal Cancer: A Systematic Review and Meta-Analysis. Tech Coloproctol (2021) 25(7):751–60. doi: 10.1007/s10151-021-02436-5
36. Keane C, Sharma P, Yuan L, Bissett I, O’Grady G. Impact of Temporary Ileostomy on Long-Term Quality of Life and Bowel Function: A Systematic Review and Meta-Analysis. ANZ J Surg (2020) 90(5):687–92. doi: 10.1111/ans.15552
37. Keane C, Park J, Öberg S, Wedin A, Bock D, O’Grady G, et al. Functional Outcomes From a Randomized Trial of Early Closure of Temporary Ileostomy After Rectal Excision for Cancer. Br J Surg (2019) 106(5):645–52. doi: 10.1002/bjs.11092
38. Kasparek MS, Hassan I, Cima RR, Larson DR, Gullerud RE, Wolff BG. Long-Term Quality of Life and Sexual and Urinary Function After Abdominoperineal Resection for Distal Rectal Cancer. Dis Colon Rectum (2012) 55(2):147–54. doi: 10.1097/DCR.0b013e31823d2606
39. Bregendahl S, Emmertsen KJ, Lindegaard JC, Laurberg S. Urinary and Sexual Dysfunction in Women After Resection With and Without Preoperative Radiotherapy for Rectal Cancer: A Population-Based Cross-Sectional Study. Colorectal Dis (2015) 17(1):26–37. doi: 10.1111/codi.12758
40. Reese JB, Finan PH, Haythornthwaite JA, Kadan M, Regan KR, Herman JM, et al. Gastrointestinal Ostomies and Sexual Outcomes: A Comparison of Colorectal Cancer Patients by Ostomy Status. Support Care Cancer (2014) 22(2):461–8. doi: 10.1007/s00520-013-1998-x
41. Karlsson L, Bock D, Asplund D, Ohlsson B, Rosenberg J, Angenete E. Urinary Dysfunction in Patients With Rectal Cancer: A Prospective Cohort Study. Colorectal Dis (2020) 22(1):18–28. doi: 10.1111/codi.14784
42. Reese JB, Handorf E, Haythornthwaite JA. Sexual Quality of Life, Body Image Distress, and Psychosocial Outcomes in Colorectal Cancer: A Longitudinal Study. Support Care Cancer (2018) 26(10):3431–40. doi: 10.1007/s00520-018-4204-3
43. Sörensson M, Asplund D, Matthiessen P, Rosenberg J, Hallgren T, Rosander C, et al. Self-Reported Sexual Dysfunction in Patients With Rectal Cancer. Colorectal Dis (2020) 22(5):500–12. doi: 10.1111/codi.14907
44. Lange MM, van de Velde CJH. Urinary and Sexual Dysfunction After Rectal Cancer Treatment. Nat Rev Urol (2011) 8(1):51–7. doi: 10.1038/nrurol.2010.206
45. Marijnen CAM, van de Velde CJH, Putter H, van den Brink M, Maas CP, Martijn H, et al. Impact of Short-Term Preoperative Radiotherapy on Health-Related Quality of Life and Sexual Functioning in Primary Rectal Cancer: Report of a Multicenter Randomized Trial. J Clin Oncol (2005) 23(9):1847–58. doi: 10.1200/JCO.2005.05.256
46. Maltaris T, Seufert R, Fischl F, Schaffrath M, Pollow K, Koelbl H, et al. The Effect of Cancer Treatment on Female Fertility and Strategies for Preserving Fertility. Eur J Obstet Gynecol Reprod Biol (2007) 130(2):148–55. doi: 10.1016/j.ejogrb.2006.08.006
47. Marhhom E, Cohen I. Fertility Preservation Options for Women With Malignancies. Obstet Gynecol Surv (2007) 62(1):58–72. doi: 10.1097/01.ogx.0000251029.93792.5d
48. Oresland T, Palmblad S, Ellström M, Berndtsson I, Crona N, Hultén L. Gynaecological and Sexual Function Related to Anatomical Changes in the Female Pelvis After Restorative Proctocolectomy. Int J Colorectal Dis (1994) 9(2):77–81. doi: 10.1007/BF00699417
49. Segelman J, Buchli C, Svanström Röjvall A, Matthiessen P, Arver S, Bottai M, et al. Effect of Radiotherapy for Rectal Cancer on Ovarian Androgen Production. Br J Surg (2019) 106(3):267–75. doi: 10.1002/bjs.10980
50. Battersby NJ, Bouliotis G, Emmertsen KJ, Juul T, Glynne-Jones R, Branagan G, et al. Development and External Validation of a Nomogram and Online Tool to Predict Bowel Dysfunction Following Restorative Rectal Cancer Resection: The POLARS Score. Gut (2018) 67(4):688–96. doi: 10.1136/gutjnl-2016-312695
51. Back E, Häggström J, Holmgren K, Haapamäki MM, Matthiessen P, Rutegård J, et al. Permanent Stoma Rates After Anterior Resection for Rectal Cancer: Risk Prediction Scoring Using Preoperative Variables. Br J Surg (2021) 108(11):1388–95. doi: 10.1093/bjs/znab260
52. Spanos CP, Mamopoulos A, Tsapas A, Syrakos T, Kiskinis D. Female Fertility and Colorectal Cancer. Int J Colorectal Dis (2008) 23(8):735–43. doi: 10.1007/s00384-008-0483-3
53. O’Neill MT, Ni Dhonnchu T, Brannigan AE. Topic Update: Effects of Colorectal Cancer Treatments on Female Fertility and Potential Methods for Fertility Preservation. Dis Colon Rectum (2011) 54(3):363–9. doi: 10.1007/DCR.0b013e31820240b3
54. Bahadoer RR, Peeters KCMJ, Beets GL, Figueiredo NL, Bastiaannet E, Vahrmeijer A, et al. Watch and Wait After a Clinical Complete Response in Rectal Cancer Patients Younger Than 50 Years. Br J Surg (2021) 109(1):114–120. doi: 10.1093/bjs/znab372
55. Melnitchouk N, Fields AC, Lu P, Scully RE, Powell AC, Maldonado L, et al. Local Versus Radical Excision of Early Distal Rectal Cancers: A National Cancer Database Analysis. Ann Surg Oncol (2020) 27(7):2169–76. doi: 10.1245/s10434-019-08155-4
56. Halverson AL, Morris AM, Cleary RK, Chang GJ. For Patients With Early Rectal Cancer, Does Local Excision Have an Impact on Recurrence, Survival, and Quality of Life Relative to Radical Resection? Ann Surg Oncol (2019) 26(8):2497–506. doi: 10.1245/s10434-019-07328-5
57. Jones HJS, Al-Najami I, Cunningham C. Quality of Life After Rectal-Preserving Treatment of Rectal Cancer. Eur J Surg Oncol (2020) 46(11):2050–6. doi: 10.1016/j.ejso.2020.07.018
58. Rullier E, Vendrely V, Asselineau J, Rouanet P, Tuech J-J, Valverde A, et al. Organ Preservation With Chemoradiotherapy Plus Local Excision for Rectal Cancer: 5-Year Results of the GRECCAR 2 Randomised Trial. Lancet Gastroenterol Hepatol (2020) 5(5):465–74. doi: 10.1016/S2468-1253(19)30410-8
59. Prabhakaran S, Yang TWW, Johnson N, Bell S, Chin M, Simpson P, et al. Latest Evidence on the Management of Early-Stage and Locally Advanced Rectal Cancer: A Narrative Review. ANZ J Surg (2022) 92(3):365–372. doi: 10.1111/ans.17429
60. Conroy T, Bosset J-F, Etienne P-L, Rio E, François É, Mesgouez-Nebout N, et al. Neoadjuvant Chemotherapy With FOLFIRINOX and Preoperative Chemoradiotherapy for Patients With Locally Advanced Rectal Cancer (UNICANCER-PRODIGE 23): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2021) 22(5):702–15. doi: 10.1016/S1470-2045(21)00079-6
61. Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM-K, et al. Short-Course Radiotherapy Followed by Chemotherapy Before Total Mesorectal Excision (TME) Versus Preoperative Chemoradiotherapy, TME, and Optional Adjuvant Chemotherapy in Locally Advanced Rectal Cancer (RAPIDO): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2021) 22(1):29–42. doi: 10.1016/S1470-2045(20)30555-6
62. Zaborowski A, Stakelum A, Winter DC. Systematic Review of Outcomes After Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. Br J Surg (2019) 106(8):979–87. doi: 10.1002/bjs.11171
63. Kim JY, Kim N-K, Lee KY, Hur H, Min BS, Kim JH. A Comparative Study of Voiding and Sexual Function After Total Mesorectal Excision With Autonomic Nerve Preservation for Rectal Cancer: Laparoscopic Versus Robotic Surgery. Ann Surg Oncol (2012) 19(8):2485–93. doi: 10.1245/s10434-012-2262-1
64. Broholm M, Pommergaard H-C, Gögenür I. Possible Benefits of Robot-Assisted Rectal Cancer Surgery Regarding Urological and Sexual Dysfunction: A Systematic Review and Meta-Analysis. Colorectal Dis (2015) 17(5):375–81. doi: 10.1111/codi.12872
65. Wee IJY, Kuo L-J, Ngu JC-Y. Urological and Sexual Function After Robotic and Laparoscopic Surgery for Rectal Cancer: A Systematic Review, Meta-Analysis and Meta-Regression. Int J Med Robot (2021) 17(1):1–8. doi: 10.1002/rcs.2164
66. Wang G, Wang Z, Jiang Z, Liu J, Zhao J, Li J. Male Urinary and Sexual Function After Robotic Pelvic Autonomic Nerve-Preserving Surgery for Rectal Cancer. Int J Med Robot (2017) 13(1). doi: 10.1002/rcs.1725
67. Kim HJ, Choi G-S, Park JS, Park SY, Yang CS, Lee HJ. The Impact of Robotic Surgery on Quality of Life, Urinary and Sexual Function Following Total Mesorectal Excision for Rectal Cancer: A Propensity Score-Matched Analysis With Laparoscopic Surgery. Colorectal Dis (2018) 20(5):O103–13. doi: 10.1111/codi.14051
68. Nesbakken A, Nygaard K, Lunde OC. Outcome and Late Functional Results After Anastomotic Leakage Following Mesorectal Excision for Rectal Cancer. Br J Surg (2001) 88(3):400–4. doi: 10.1046/j.1365-2168.2001.01719.x
69. Hain E, Manceau G, Maggiori L, Mongin C, Prost À la Denise J, Panis Y. Bowel Dysfunction After Anastomotic Leakage in Laparoscopic Sphincter-Saving Operative Intervention for Rectal Cancer: A Case-Matched Study in 46 Patients Using the Low Anterior Resection Score. Surgery (2017) 161(4):1028–39. doi: 10.1016/j.surg.2016.09.037
70. Mongin C, Maggiori L, Agostini J, Ferron M, Panis Y. Does Anastomotic Leakage Impair Functional Results and Quality of Life After Laparoscopic Sphincter-Saving Total Mesorectal Excision for Rectal Cancer? A Case-Matched Study. Int J Colorectal Dis (2014) 29(4):459–67. doi: 10.1007/s00384-014-1833-y
Keywords: functional outcome, young rectal cancer, patient reported outcome (PROM), rectal cancer, early onset rectal cancer
Citation: REACCT Collaborative (2022) Post-Operative Functional Outcomes in Early Age Onset Rectal Cancer. Front. Oncol. 12:868359. doi: 10.3389/fonc.2022.868359
Received: 02 February 2022; Accepted: 18 March 2022;
Published: 30 May 2022.
Edited by:
Cesare Ruffolo, University Hospital of Padua, ItalyReviewed by:
Giulio Aniello Santoro, Ospedale di Treviso, ItalyMichele Manigrasso, University of Naples Federico II, Italy
Copyright © 2022 REACCT Collaborative. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lauren V. O’Connell, bGF1cmVub2Nvbm5lbGxAcmNzaS5pZQ==
 REACCT Collaborative
REACCT Collaborative