- 1School of Medicine, Western Sydney University, Campbelltown, NSW, Australia
- 2Medical Oncology, Ingham Institute of Applied Medical Research, Liverpool, NSW, Australia
- 3Centre of Circulating Tumour Cell Diagnostics & Research, Ingham Institute of Applied Medical Research, Liverpool, NSW, Australia
- 4South West Sydney Clinical School, University of New South Wales, Liverpool Hospital, Liverpool, NSW, Australia
- 5Ingham Institute of Applied Medical Research, Liverpool, NSW, Australia
- 6School of Medicine, University of New South Wales, Kensington, NSW, Australia
- 7Medical Oncology, Liverpool Hospital, Liverpool, NSW, Australia
In advanced prostate cancer, access to recent diagnostic tissue samples is restricted and this affects the analysis of the association of evolving biomarkers such as AR-V7 with metastatic castrate resistance. Liquid biopsies are emerging as alternative analytes. To clarify clinical value of AR-V7 detection from liquid biopsies, here we performed a meta-analysis on the prognostic and predictive value of androgen receptor variant 7 (AR-V7) detected from liquid biopsy for patients with prostate cancer (PC), three databases, the Embase, Medline, and Scopus were searched up to September 2021. A total of 37 studies were included. The effects of liquid biopsy AR-V7 status on overall survival (OS), radiographic progression-free survival (PFS), and prostate-specific antigen (PSA)-PFS were calculated with RevMan 5.3 software. AR-V7 positivity detected in liquid biopsy significantly associates with worse OS, PFS, and PSA-PFS (P <0.00001). A subgroup analysis of patients treated with androgen receptor signaling inhibitors (ARSi such as abiraterone and enzalutamide) showed a significant association of AR-V7 positivity with poorer OS, PFS, and PSA-PFS. A statistically significant association with OS was also found in taxane-treated patients (P = 0.04), but not for PFS (P = 0.21) or PSA-PFS (P = 0.93). For AR-V7 positive patients, taxane treatment has better OS outcomes than ARSi (P = 0.01). Study quality, publication bias and sensitivity analysis were integrated in the assessment. Our data show that liquid biopsy AR-V7 is a clinically useful biomarker that is associated with poor outcomes of ARSi-treated castrate resistant PC (CRPC) patients and thus has the potential to guide patient management and also to stratify patients for clinical trials. More studies on chemotherapy-treated patients are warranted.
Systematic Review Registration: PROSPERO, CRD42021239353.
Introduction
Prostate cancer (PC) is one of the most common male cancers. The androgen receptor (AR) pathway is critical in maintaining normal prostate tissue homeostasis, cancer development and progression (1). Therapies for PC include surgery and radiation for localized or early-stage cancer, while for advanced or metastatic PC, androgen deprivation therapy (ADT), with or without chemotherapy is the standard of care. However, patients eventually develop castration resistant PC (CRPC). Recent incorporation of novel androgen receptor signaling inhibitors (ARSi, e.g., enzalutamide (Enz), abiraterone (Abi)) and taxane-based chemotherapy have improved outcomes of CRPC patients over the past two decades (2).
Biomarkers detected in liquid biopsy (such as circulating tumor cells and cell-free tumor DNA) demonstrate good concordance with biomarkers detected in conventional tissue biopsy, especially for metastatic CRPC (3). Liquid biopsy is emerging as a reliable source of biological data for biomarker discovery, especially in advanced PC when tissue biopsy is often not obtainable or can be used longitudinally to monitor tumor evolution and changes in biomarker characteristics. In CRPC, one of most promising prognostic markers is the constitutively active AR splice variant 7 (AR-V7). AR-V7 lacks the ligand binding domain and substitutes for functional AR even in the absence of the ligand testosterone, and differentially regulates AR-dependent gene expression (4). Thus far, current literature suggests that expression or nuclear subcellular location of AR-V7 is associated with overall survival (OS) and progression free survival (PFS) when found in tissue biopsy (5) or liquid biopsy [whole blood (6, 7), circulating tumor cells (8), and exosomes (9, 10)]. However, the study cohorts are variable in patient numbers and stages and also treatment options; the clinical relevance of AR-V7, especially liquid biopsy detectable AR-V7, is still not clear or widely accepted and need further investigation.
To clarify the clinical utility of AR-V7 detection from liquid biopsies, we undertook a comprehensive systematic review and meta-analysis to evaluate the available data from the clinical studies published up to September 2021. Prognostic and predictive value of liquid biopsy derived AR-V7 data in PC patients were evaluated from 37 studies that met the inclusion criteria.
Methods
Study Design and Literature Searches
This study was conducted according to preferred reporting items for systematic reviews and meta-analysis (PRISMA) (11). The protocol has been registered on PROSPERO (CRD42021239353). Detailed literature searches up to September 10, 2021 in the Embase, PubMed, and Scopus databases were conducted thoroughly to check the prognostic role of AR-V7 in PC. The used search terms were (~Androgen Receptor Variant 7) OR (~ARV7) OR (~AR3) AND (~”prostate cancer”). The searched study citations were imported to EndNote (version X9) for duplicate checking and title and/or abstract screening and then uploaded to the online systematic review research tool Rayyan (https://www.rayyan.ai/) for independent systematic review according to selection criteria. Two independent, blinded observers (TK and YM) reviewed all candidate articles. Any discrepancies in the article selections were resolved by discussion.
Selection Criteria
Pre-set exclusion criteria of this study were: (1) publication type: review articles, letters, comments, questionnaires, conference papers, corrections, reply to editor, case reports, book chapters, abstracts only, research highlights, summaries; (2) non-human studies (animal or cell line study); (3) non-prostate cancer; (4) AR-V7 data are not derived from human; (5) survival data not related to AR-V7 or with insufficient data to calculate the hazard ratios (HRs) and their 95% CIs, or the Kaplan–Meier (K–M) curve unable to calculate HRs and 95% CI parameters. Finally, studies were only included when they met the following criteria: (1) AR-V7 assayed in liquid biopsies (whole blood, circulating tumor cells, PBMC, plasma, exosome); (2) A reported relationship between AR-V7 and prognostic/predictive indicators, namely, OS, PFS, and PSA-PFS; (3) patient cohorts with n >25, and (4) English language only.
Data Extraction and Quality Assessment
This study focuses on the prognostic value of AR-V7 detected from liquid biopsy and its predictive value for ARSi and chemotherapy. According to a pre-designed table, the items of data extraction included the last name of the first author, publication year, study country, number of patients included, age of patient, sample resource (processing method) and AR-V7 detection method, type of therapies, endpoints of oncological outcomes, HRs and 95% CIs (from univariate or multivariate Cox analysis), follow-up durations and definitions of OS, PFS, and PSA-PFS (Supplementary Table 1). When HRs and 95% CIs were not presented in the study, an Engauge Digitizer (version 12.1) was used to digitalize the K–M survival curve to re-calculate HRs and 95% CI as described previously (12). Data was extracted by two authors (TK and YM) independently and any inconsistencies were resolved by discussion. Notably, when several publications were retrieved reporting the same trial or patient cohort or from same author(s), study question and data from this publication were discussed by two authors (TK and YM) and uniqueness of the included data was ensured.
The adapted Newcastle–Ottawa Scale (NOS) scales for cohort study (13) were used to evaluate the quality of enrolled studies, which embraced three aspects, namely, patient selection, comparability, and assessment of outcome with a total score of 9. In addition, the quality of statistical evaluation was assessed to give a maximal score of 1 as described in Supplementary Table 2; a score of 7 or more is considered as high-quality and a score of 6 or less is considered as low quality.
Statistical Analysis
Pooled HR and 95% CI were used to evaluate the prognostic and predictive value of AR-V7 presence or high expression (in some studies, authors set a threshold to discriminate high or low expression level) on the patient survival parameters (OS, PFS, PFA-PFS) in Review Manager 5.3 software (RevMan v.5.3, Denmark). The Cochran Q and I2 statistical methods were applied to evaluate the heterogeneity among included studies and a random effects model was used for data consolidation. If the heterogeneity was very high, only a descriptive score was given. Further subgroup analysis based on patient treatment was also conducted. The inverted funnel plots with Egger’s test were used to analyze potential publication bias with R software. A sensitivity analysis was carried out to assess the influence of each individual study on the pooled results by sequentially excluding each study. A two-tailed p-value <0.05 was regarded as statistically significant.
Results
Search Results, Study and Patient Characteristics
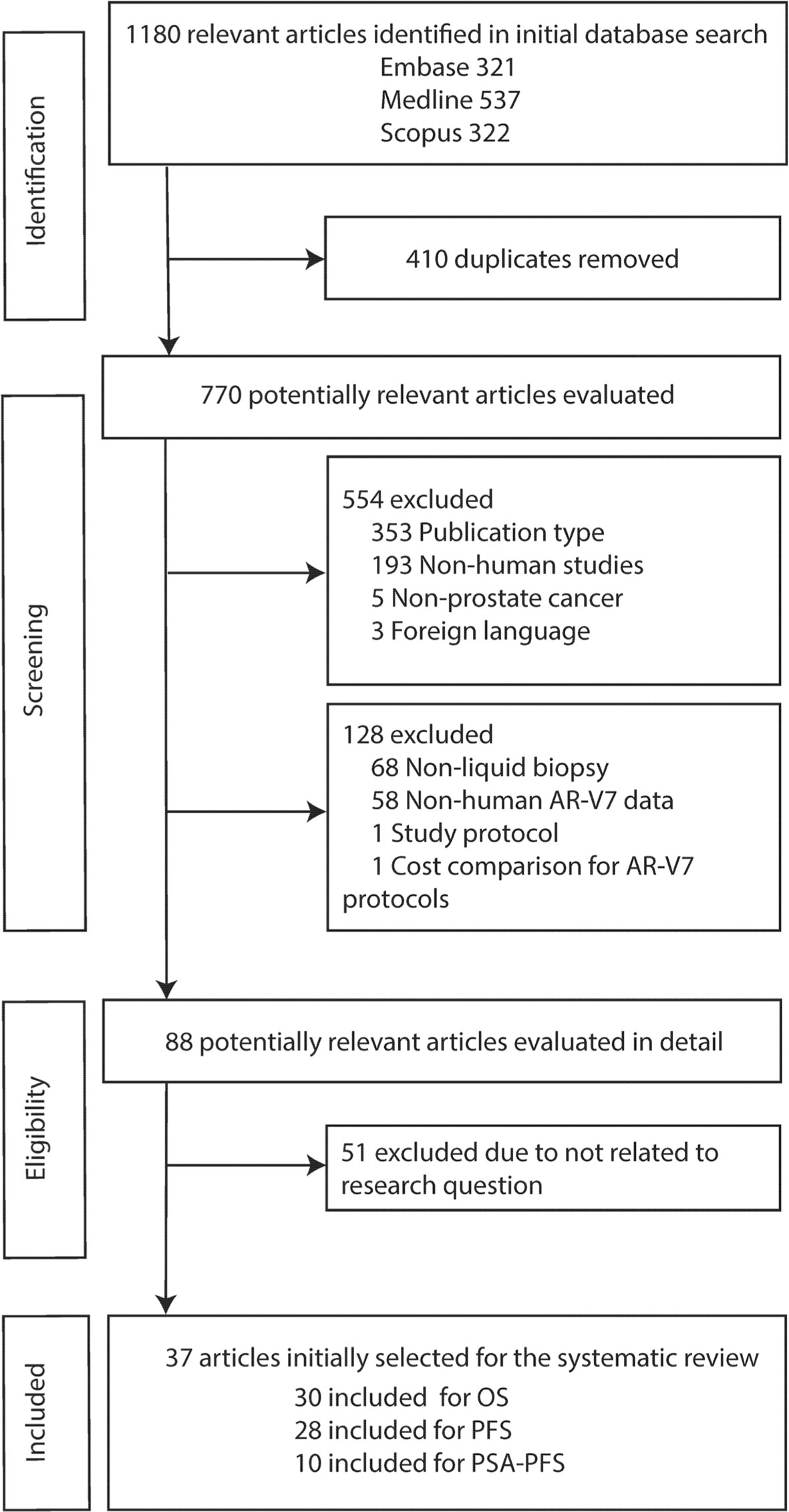
The flowchart outlining the results of the literature search and application of the strategic inclusion and exclusion criteria is presented in Figure 1. A total of 1,180 relevant articles were identified in initial database searches (Embase: 321, Medline: 537, Scopus: 322). After screening research title and abstract to remove duplicates (n = 410) and excluding the non-relevant studies based on publication type (n = 353), non-human studies (n = 193), non-prostate cancer (n = 5) and foreign language (n = 3) followed by a review of full text for eligibility, 37 articles were identified based on inclusion criteria ‘human data’, ‘AR-V7’, ‘liquid biopsy’, and ‘survival’. Although we initially only searched quite a broad terminology ‘prostate cancer’, all 37 studies investigated CRPC (n = 4) or metastatic CRPC (mCRPC) (n = 33) as defined in the reports (Supplementary Table 1). Baseline characteristics of all eligible articles are listed in Table 1. All articles were published from 2014 to 2021 and included studies from Europe (46%), America and Canada (46%), and Asia-Pacific (8%). Liquid biopsy AR-V7 was detected from CTC (n = 28), PBMC (n = 2), whole blood (n = 4) or exosomes (n = 3). The patient cohort size ranged from 26 to 202 and the median or mean patient age ranged from 56 to 78. CTC enrichment methods included (modified) AdnaTest ® (Qiagen) (n = 13), Oncoquick® (Greiner Bio-One GmbH) (n = 1), red blood cell (RBC) lysis (n = 3), and immunomagnetic beads-based methods (such as CellSearch® or IsoFlux®, dynabeads) (n = 9). The method of AR-V7 detection was primarily by PCR (quantitative PCR and droplet digital PCR, 92%). Endpoint of patient outcomes include OS (n = 30), PFS (n = 28) and PSA-PFS (n = 10) (Table 1).
Thirty studies including 976 AR-V7 positive (or high level, as defined by authors) and 2,056 AR-V7 negative (or low level) patients were used for OS comparison, while 28 studies including 697 AR-V7 positive and 1,553 AR-V7 negative patients were used for PFS analysis and 10 studies including 216 AR-V7 positive and 425 AR-V7 negative patients for PSA-PFS analysis. Most patients in the cohort of studies were treated with ARSi (either enzalutamide, abiraterone, or not specified) or taxane-based chemotherapy. Some reports included miscellaneous treatments [such as Bipolar Androgen-based therapy (32)]. Overall AR-V7 positive patients had significantly worse OS (HR 3.36, 95% CI 2.56–4.41, P <0.00001), PFS (HR 2.96, 95% CI 2.20–3.98, P <0.00001) and PSA-PFS (HR 4.34, 95% CI 2.15–8.76, P <0.00001) than AR-V7 negative patients. Due to significant study heterogeneity (I2 ≥80%), random effects model was applied to calculate HR value and 95% CI for all survival parameters.
Predictive Value of AR-V7 for ARSi-Treatment
AR-V7 positive patients treated with ARSi (enzalutamide or abiraterone) had significant poorer OS (HR 4.34, 95% CI 3.00–6.28, P <0.00001), PFS (HR 2.89, 95% CI 2.15–3.87, P <0.00001) and PSA-PFS (HR 4.69, 95% CI 2.50–8.82, P <0.0001) compared with AR-V7 negative patients (Figures 2–4). When analyzed based on specific treatment, compared to negative patients, AR-V7 positive patients also had significant worse OS (Enz: HR 2.93, 95% CI 1.71–5.01, P <0.0001; Abi: HR 6.59, 95% CI 2.18–19.94, P = 0.0008, respectively) (Figure 2), PFS (Enz: HR 4.38, 95% CI 2.44–7.84, P <0.0001; Abi: HR 6.88, 95% CI 1.99–23.73, P = 0.002, respectively) (Figure 3) and PSA-PFS (Enz: HR 7.40, 95% CI 2.66–20.60, one study, P = 0.0008; Abi: HR 11.39, 95% CI 4.53–28.67, two studies, P <0.00001, respectively) (Figure 4).
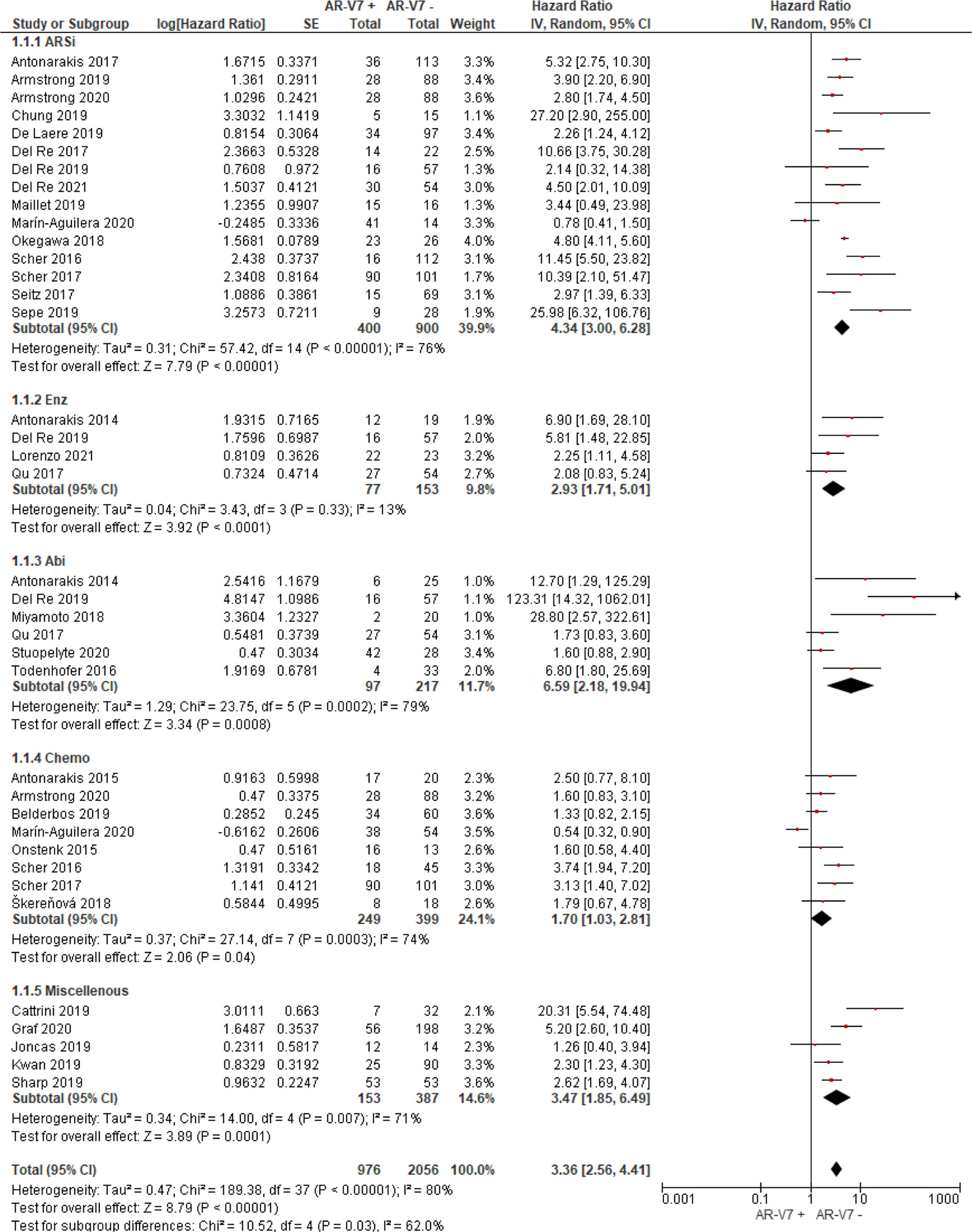
Figure 2 Forest plot of hazard ratios (HRs) for association of liquid biopsy AR-V7 status with overall survival (OS) in all included studies. Pooled HRs were calculated using random effect model. AR-V7, androgen receptor splice variant 7; CI, confidence interval and bars indicate 95% CIs. Subgroup analysis (ARSi, enzalutamide or abiraterone; Enz, enzalutamide; Abi, abiraterone; Chemo, taxane based chemotherapy; Miscellaneous, treatments that do not belong to above treatments or not clearly defined) were assessed.
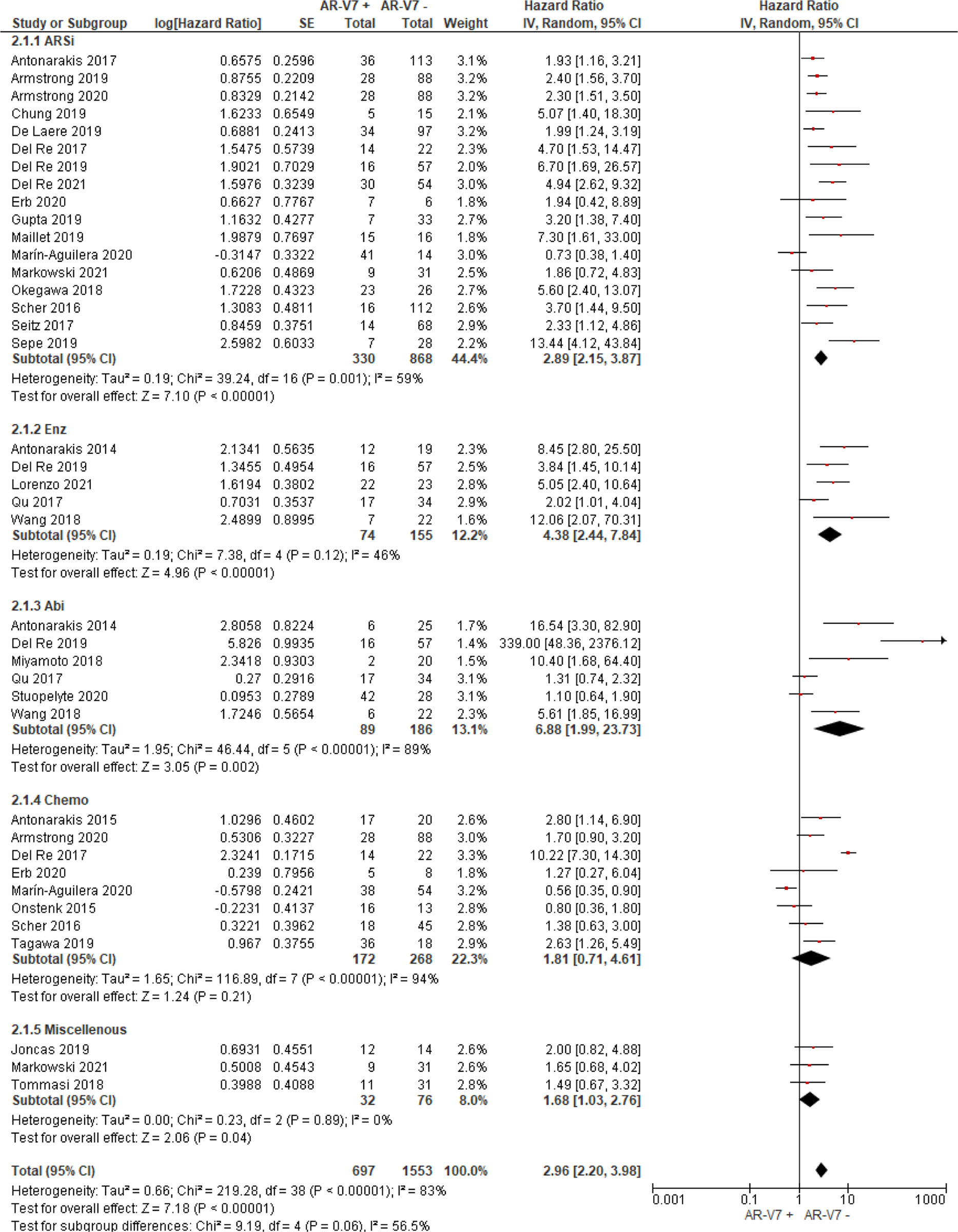
Figure 3 Forest plot of hazard ratios (HRs) for association of liquid biopsy AR-V7 status with PFS in all studies. Pooled HRs were calculated using random effect model. AR-V7: androgen receptor splice variant 7. CI, confidence interval and bars indicate 95% CIs. Subgroup analysis (ARSi, enzalutamide or abiraterone; Enz, enzalutamide; Abi, abiraterone; Chemo, taxane based chemotherapy; Miscellaneous, treatments that do not belong to above treatments or not clearly defined) were assessed.
Figure 4 Forest plot of hazard ratios (HRs) for association of liquid biopsy AR-V7 status with PSA-PFS in all studies. Pooled HRs were calculated using random effect model. AR-V7, androgen receptor splice variant 7; CI, confidence interval and bars indicate 95% CIs. Subgroup analysis (ARSi, enzalutamide or abiraterone; Enz, enzalutamide; Abi, abiraterone; Chemo, taxane based chemotherapy; Miscellaneous, treatments that do not belong to above treatments or not clearly defined) were assessed.
Chemotherapy-Treated Patients and Outcome Association With AR-V7
In the subgroup analysis of the patients treated with taxane-based chemotherapy, the association of AR-V7 positivity with worse OS was observed (HR 1.70, 95% CI 1.03–2.81, P = 0.04) (Figure 2), but no conclusive association between AR-V7 positive status and worse PFS and PSA-PFS were apparent, likely due to inadequate power (PFS: HR 1.81, 95% CI 0.71–4.61, P = 0.21, Figure 3; PSA-PFS: HR 0.93, 95% CI 0.21–4.22, P = 0.93, Figure 4). It is to be emphasised that data is only derived from two studies and a total of 129 patients (Figure 4).
AR-V7 Effect on Non-Defined (Miscellaneous) Treatments
For the studies in which the authors did not clarify treatments and were unable to be classified as either ARSi or taxane chemotherapy, AR-V7 presence is associated with worse OS (HR 3.47, 95% CI 1.85–6.49, P = 0.0001, 5 studies) and PFS (3 studies, HR 1.68, 95% CI 1.03–2.76, P = 0.04) (Figures 2, 3).
ARSi vs. Chemotherapy in AR-V7 Positive or Negative Patients
Four studies compared treatment response in AR-V7 positive or negative patients. Taxane treatment is linked to superior OS (HR 0.54, 95% CI 0.34–0.87, P = 0.01) in patients positive for AR-V7, compared to ARSi (Figure 5A). In contrast, for AR-V7 negative patients, OS in taxane or ARSi treated patients is not significantly different (HR 1.17, 95% CI 0.71–1.92, P = 0.54) (Figure 5B).
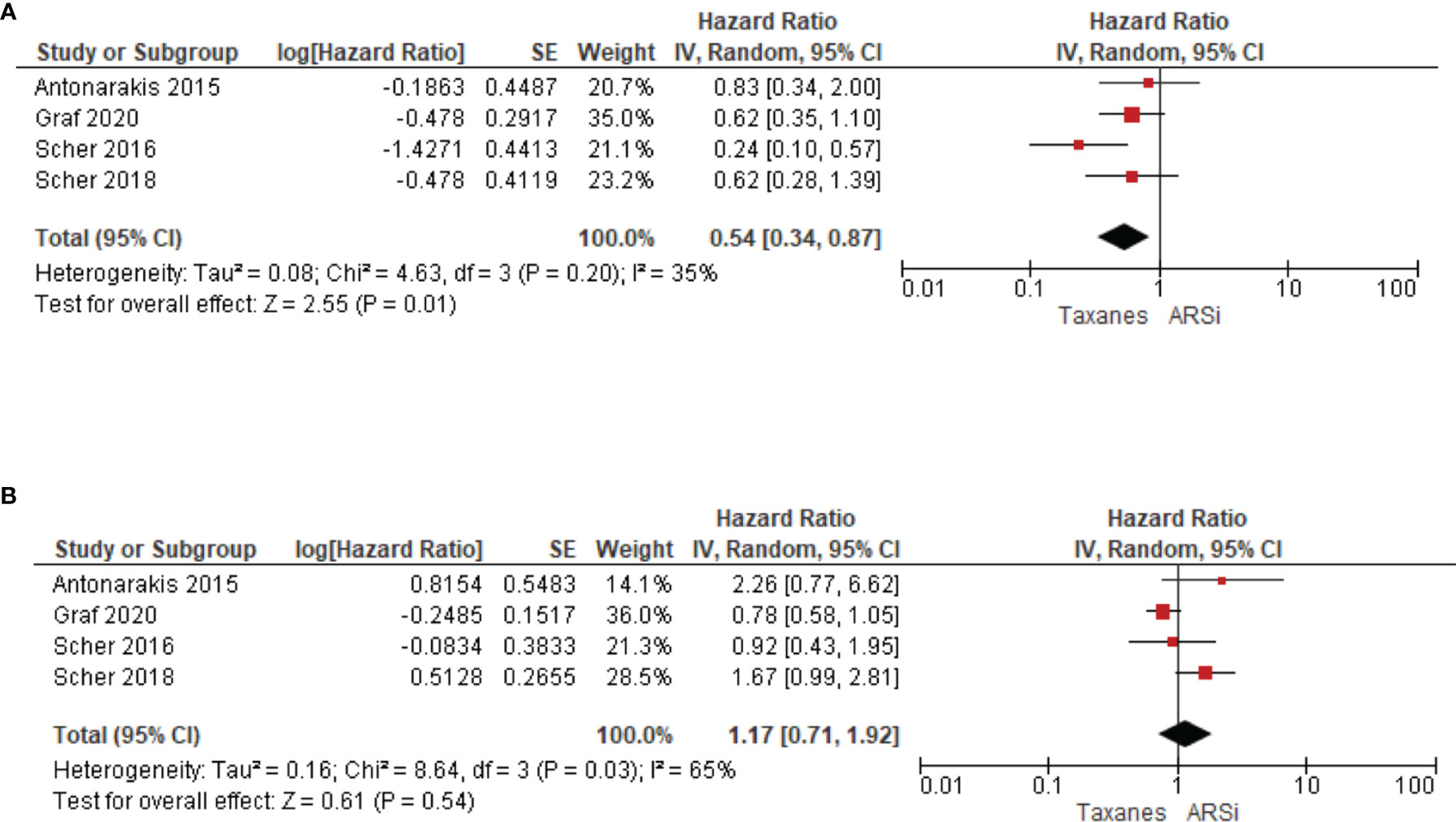
Figure 5 Forest plots for association of liquid biopsy AR-V7 status with OS in (A) AR-V7 positive (ARSi vs. Chemotherapy) and (B) AR-V7 negative patients (ARSi vs. Chemotherapy). Pooled HRs were calculated using random effect model. AR-V7, androgen receptor splice variant 7; CI, confidence interval and bars indicate 95% CIs.
Quality Assessment, Publication Bias and Sensitivity Analysis
Thirty five articles were assessed as high-quality studies while 2 were deemed low quality studies (Table 1 and Supplementary Table 2). Overall, the average quality of studies is 8.5. Publication biases were evaluated for subgroups with more than 10 publications; no publication bias was observed for OS (Egger’s test P = 0.9925, 15 publications, Supplementary Figure 1A) whereas publication bias was observed for PFS (Egger’s test P = 0.0411, 17 publications, Supplementary Figure 1B) in ARSi-treated subgroups. Sensitivity analyses were performed on the subgroups of more than 6 studies and the results were relatively stable except for overall survival in chemotherapy-treated group, where missing data in one study (31) had a significant effect on data outcome (Supplementary Table 3).
Discussion
AR splice variants have been proposed as a cause of resistance to ARSi and taxane-based chemotherapy (46). AR-V7, the most-studied AR splice variant, is emerging as a clinically relevant biomarker in CRPC, with a detection incidence ranging between 20 and 60%, depending on biopsy source, detection methods, and disease stage. Given that tumor tissue of advanced PC is rarely available and archival tissue may not reflect the biology of the current tumor stage, liquid biopsies, mainly blood, are becoming attractive resources for AR-V7 and other biomarker evaluation. Technical advances, different detection methods for AR-V7 from liquid biopsies are now available, including modified AdnaTest ProstateCancer, and droplet digital PCR of CTCs enriched by various CTC isolation platforms (see Table 1). We recently confirmed CTC-based AR-V7 testing is more reliable than exosomal RNA and cell free tumor RNA in plasma (47). Accumulating reports on the association of AR-V7 detectability in liquid biopsy with therapy response and patient survival have prompted us to perform this systematic review and meta-analysis on the prognostic and predictive utility of liquid biopsy-based AR-V7 identification. Our data show that liquid biopsy detectable AR-V7 significantly associates with poor outcomes to ARSi treatment as shown for OS, PFS, PSA-PFS (P <0.001). This strongly supports the notion that AR-V7 detection from CRPC patient liquid biopsies has prognostic and predictive power. This observation is highly clinically relevant and could affect how clinicians make treatment decisions for patients with (metastatic) CRPC and when to transition patients to taxane-based chemotherapy.
While on taxane-based treatment, the association of AR-V7 presence with poorer outcome is still significant (P = 0.04) for OS data and lack adequate power for PFS (P = 0.21) or PSA-PFS (P = 0.93). However, there are relatively fewer publications in this subgroup, so these conclusions are based on weaker datasets compared to the ARSi treated subgroup; for instance, the omitting one publication changes the P-value and AR-V7 impact on OS would no longer be significant (Supplementary Table 3). Our data agree with a recent report that AR-V7 may contribute to taxane resistance by circumventing taxane-induced inhibitory effects both in vitro (cell lines) and in vivo (PC tissue) (43, 48). On the other hand, we cannot exclude the possibility that AR-V7 expression was induced in CRPC patients who had received ARSi prior to chemotherapy, and that its effect on OS has not been completely washed out by taxanes. We note that four studies suggest that chemotherapy would be a better option compared to ARSi (HR 0.54, P = 0.01) in AR-V7 positive CRPC, suggesting that AR-V7 determination is important in chemotherapy-treated patients. More studies in this subgroup are warranted.
Three other meta-analyses on AR-V7 prognostication (13, 49, 50) have been published recently, but given the common inaccessibility of current tissue biopsies, our meta-analysis exclusively focuses on liquid biopsies and includes the most up-to-date studies. Further, we not only include all studies with author self-reported HR and 95% CI, but also calculate HR and 95% CI with established methods (12) for some papers with insufficient and incomplete statistical reporting. Nevertheless, our systematic review has limitations. We only examined OS, PFS, and PSA-PFS, and did not assess other treatment outcomes such as PSA response. Discrepancies in the definition of PSA response (e.g., extent of PSA fall in a specific timeframe) exist across studies and given our selection criteria, papers were excluded if they only reported PSA response without survival data. Secondly, statistical power was limited by the numbers of studies available and small sample sizes in some of the subgroups analysed. Thirdly, included study designs differed greatly in biological material investigated (type of liquid biopsy and content such as CTCs or exosomes). For some studies, patients were enrolled from a single centre, potentially leading to publication bias and selection bias. Also, no randomized study has ever directly compared the predictive value of AR-V7 in patients treated with chemotherapy vs. ARSi. Therefore, the results are indirect with potential bias. Lastly, the variability of techniques used to determine AR-V7 positivity, namely, qRT-PCR and ddPCR of mRNA derived from CTC, whole blood, exosome, could result in differing conclusions. The cut-off value is essential in defining and interpretation of AR-V7 positivity, due to the continuous nature of this variable; more work is required to answer the question of whether the degree of AR-V7 presence is important. Last but not least, other CTC AR detection methods have been adopted such as RNA-seq and immunostaining. Despite the variety of methodologies, we found that liquid biopsy detectable AR-V7 correlates with disease outcomes (Supplementary Figure 2).
In conclusion, ARSi and taxane-based chemotherapy are approved treatment options for CPRC patients and are used globally. Use of emerging methodologies, such as liquid biopsy-determined AR-V7, to optimize utility of a known predictive biomarker could help to guide the optimal treatment sequencing pathway for each patient in a personalised manner and is therefore of clinical importance. Standardization of liquid biopsy AR-V7 detection would underpin utility in clinical practice. Avoiding ineffective therapies or early switching to more effective approaches should ensure better outcomes for patients. However, further studies on chemotherapy-treated patient cohort and direct comparison of chemotherapy vs. ARSi are warranted.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
Project development, methodology, data collection and analysis: TK and YM. Conceptualization: YM and TK. Project development: TB, KS, PDS and WC. Statistics: JD, TK and YM. Manuscript writing, editing, and reviewing: all authors. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
TK received an Ingham Institute/Narellan Rotary Club Men’s health grant 2018 and a WSU School of Medicine Androgen Receptor Research scholarship.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.868031/full#supplementary-material
Supplementary Table 2 | Quality assessment of included studies based on adapted NOS scales.
Supplementary Table 3 | Sensitivity analysis of subgroups with more than 6 studies.
Supplementary Figure 1 | Inverted funnel plot to evaluate potential publication bias in OS (A) and PFS (B) of ARSi treated patients.
Supplementary Figure 2 | Forest plot of hazard ratios (HRs) for association of liquid biopsy AR-V7 status with OS (A), PFS (B), PSA-PFS (C) in all studies. Subgroup analysis were performed based on AR-V7 detection technique type. Pooled HRs were calculated using random effect model. AR-V7: androgen receptor splice variant 7. CI: confidence interval and bars indicate 95% CIs. PCR, polymerase chain reaction; qRT-PCR, quantitative real time PCR; ddPCR, droplet digital PCR; IF, immunofluorescence; IHC, immunohistochemistry; FC, flow cytometry; RNA-seq, RNA-sequencing.
References
1. Lonergan PE, Tindall DJ. Androgen Receptor Signaling in Prostate Cancer Development and Progression. J Carcinog (2011) 10:20–. doi: 10.4103/1477-3163.83937
2. Cattrini CA-O, España R, Mennitto A, Bersanelli MA-O, Castro EA-O, Olmos D, et al. Optimal Sequencing and Predictive Biomarkers in Patients With Advanced Prostate Cancer. Cancers (2021) 13:4522. doi: 10.3390/cancers13184522
3. Wyatt AW, Annala M, Aggarwal R, Beja K, Feng F, Youngren J, et al. Concordance of Circulating Tumor DNA and Matched Metastatic Tissue Biopsy in Prostate Cancer. J Natl Cancer Inst (2017) 109:djx118. doi: 10.1093/jnci/djx118
4. Shafi AA, Putluri V, Arnold JM, Tsouko E, Maity S, Roberts JM, et al. Differential Regulation of Metabolic Pathways by Androgen Receptor (AR) and its Constitutively Active Splice Variant, AR-V7, in Prostate Cancer Cells. Oncotarget (2015) 6:31997–2012. doi: 10.18632/oncotarget.5585
5. Sharp A, Coleman I, Yuan W, Sprenger C, Dolling D, Rodrigues DN, et al. Androgen Receptor Splice Variant-7 Expression Emerges With Castration Resistance in Prostate Cancer. J Clin Invest (2019) 129:192–208. doi: 10.1172/JCI122819
6. Stuopelyte K, Sabaliauskaite R, Bakavicius A, Haflidadóttir BS, Visakorpi T, Väänänen RM, et al. Analysis of AR-FL and AR-V1 in Whole Blood of Patients With Castration Resistant Prostate Cancer as a Tool for Predicting Response to Abiraterone Acetate. J Urol (2020) 204(1):71–8. doi: 10.1097/JU.0000000000000803
7. Todenhöfer T, Azad A, Stewart C, Gao J, Eigl BJ, Gleave ME, et al. AR-V7 Transcripts in Whole Blood RNA of Patients With Metastatic Castration Resistant Prostate Cancer Correlate With Response to Abiraterone Acetate. J Urol (2016) 197(1):135–42. doi: 10.1016/j.juro.2016.06.094
8. Sharp A, Welti JC, Lambros MBK, Dolling D, Rodrigues DN, Pope L, et al. Clinical Utility of Circulating Tumour Cell Androgen Receptor Splice Variant-7 Status in Metastatic Castration-Resistant Prostate Cancer. Eur Urol (2019) 76(5):676–85. doi: 10.1016/j.eururo.2019.04.006
9. Del Re M, Conteduca V, Crucitta S, Gurioli G, Casadei C, Restante G, et al. Androgen Receptor Gain in Circulating Free DNA and Splicing Variant 7 in Exosomes Predict Clinical Outcome in CRPC Patients Treated With Abiraterone and Enzalutamide. Prostate Cancer Prostatic Dis (2021) 24(2):524–31. doi: 10.1038/s41391-020-00309-w
10. Del Re M, Crucitta S, Sbrana A, Rofi E, Paolieri F, Gianfilippo G, et al. AR-V7 and AR-FL Expression Is Associated With Clinical Outcome: A Translational Study in Patients With Castrate Resistant Prostate Cancer. BJU Int (2019) 124:693–700. doi: 10.1111/bju.14792
11. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PloS Med (2009) 6(7):e1000100. doi: 10.1136/bmj.b2700
12. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical Methods for Incorporating Summary Time-to-Event Data Into Meta-Analysis. Trials (2007) 8:16–. doi: 10.1186/1745-6215-8-16
13. Wang J, Zhang Y, Wei C, Gao X, Yuan P, Gan J, et al. Prognostic Value of Androgen Receptor Splice Variant 7 in the Treatment of Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Front Oncol (2020) 10:562504. doi: 10.3389/fonc.2020.562504
14. Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Nakazawa M, et al. Androgen Receptor Splice Variant 7 and Efficacy of Taxane Chemotherapy in Patients With Metastatic Castration-Resistant Prostate Cancer. JAMA Oncol (2015) 1(5):582–91. doi: 10.1001/jamaoncol.2015.1341
15. Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Zhu Y, et al. Clinical Significance of Androgen Receptor Splice Variant-7 Mrna Detection in Circulating Tumor Cells of Men With Metastatic Castration-Resistant Prostate Cancer Treated With First- and Second-Line Abiraterone and Enzalutamide. J Clin Oncol (2017) 35(19):2149–56. doi: 10.1200/JCO.2016.70.1961
16. Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, et al. AR-V7 and Resistance to Enzalutamide and Abiraterone in Prostate Cancer. New Engl J Med (2014) 371(11):1028–38. doi: 10.1056/NEJMoa1315815
17. Armstrong AJ, Halabi S, Luo J, Nanus DM, Giannakakou P, Szmulewitz RZ, et al. Prospective Multicenter Validation of Androgen Receptor Splice Variant 7 and Hormone Therapy Resistance in High-Risk Castration-Resistant Prostate Cancer: The PROPHECY Study. J Clin Oncol (2019) 37(13):1120–9. doi: 10.1200/JCO.18.01731
18. Armstrong AJ, Luo J, Nanus DM, Giannakakou P, Szmulewitz RZ, Danila DC, et al. Prospective Multicenter Study of Circulating Tumor Cell AR-V7 and Taxane Versus Hormonal Treatment Outcomes in Metastatic Castration-Resistant Prostate Cancer. JCO Precis Oncol (2020) 4:1285–301. doi: 10.1200/PO.20.00200
19. Belderbos BPS, Sieuwerts AM, Hoop EO, Mostert B, Kraan J, Hamberg P, et al. Associations Between AR-V7 Status in Circulating Tumour Cells, Circulating Tumour Cell Count and Survival in Men With Metastatic Castration-Resistant Prostate Cancer. Eur J Cancer (2019) 121:48–54. doi: 10.1016/j.ejca.2019.08.005
20. Cattrini C, Rubagotti A, Zinoli L, Cerbone L, Zanardi E, Capaia M, et al. Role of Circulating Tumor Cells (CTC), Androgen Receptor Full Length (AR-FL) and Androgen Receptor Splice Variant 7 (AR-V7) in a Prospective Cohort of Castration-Resistant Metastatic Prostate Cancer Patients. Cancers (2019) 11(9):1365. doi: 10.3390/cancers11091365
21. Chung JS, Wang Y, Henderson J, Singhal U, Qiao Y, Zaslavsky AB, et al. Circulating Tumor Cell–Based Molecular Classifier for Predicting Resistance to Abiraterone and Enzalutamide in Metastatic Castration-Resistant Prostate Cancer. Neoplasia (United States) (2019) 21(8):802–9. doi: 10.1016/j.neo.2019.06.002
22. De Laere B, Oeyen S, Mayrhofer M, Whitington T, van Dam PJ, Van Oyen P, et al. TP53 Outperforms Other Androgen Receptor Biomarkers to Predict Abiraterone or Enzalutamide Outcome in Metastatic Castration-Resistant Prostate Cancer. Clin Cancer Res (2019) 25(6):1766–73. doi: 10.1158/1078-0432.CCR-18-1943
23. Del Re M, Biasco E, Crucitta S, Derosa L, Rofi E, Orlandini C, et al. The Detection of Androgen Receptor Splice Variant 7 in Plasma-Derived Exosomal RNA Strongly Predicts Resistance to Hormonal Therapy in Metastatic Prostate Cancer Patients. Eur Urol (2017) 71(4):680–7. doi: 10.1016/j.eururo.2016.08.012
24. Erb HHH, Sparwasser P, Diehl T, Hemmerlein-Thomas M, Tsaur I, Jüngel E, et al. AR-V7 Protein Expression in Circulating Tumour Cells is Not Predictive of Treatment Response in Mcrpc. Urologia Internationalis (2020) 104(3):253–62. doi: 10.1159/000504416
25. Graf RP, Hullings M, Barnett ES, Carbone E, Dittamore R, Scher HI. Clinical Utility of the Nuclear-Localized AR-V7 Biomarker in Circulating Tumor Cells in Improving Physician Treatment Choice in Castration-Resistant Prostate Cancer. Eur Urol (2020) 77(2):170–7. doi: 10.1016/j.eururo.2019.08.020
26. Gupta S, Hovelson DH, Kemeny G, Halabi S, Foo WC, Anand M, et al. Discordant and Heterogeneous Clinically Relevant Genomic Alterations in Circulating Tumor Cells vs Plasma DNA From Men With Metastatic Castration Resistant Prostate Cancer. Genes Chromosomes Cancer (2019) 59(4):225–39. doi: 10.1002/gcc.22824
27. Joncas FH, Lucien F, Rouleau M, Morin F, Leong HS, Pouliot F, et al. Plasma Extracellular Vesicles as Phenotypic Biomarkers in Prostate Cancer Patients. Prostate (2019) 79(15):1767–76. doi: 10.1002/pros.23901
28. Kwan EM, Fettke H, Docanto MM, To SQ, Bukczynska P, Mant A, et al. Prognostic Utility of a Whole-Blood Androgen Receptor-Based Gene Signature in Metastatic Castration-Resistant Prostate Cancer. Eur Urol Focus (2021) 7:63–70. doi: 10.1016/j.euf.2019.04.020
29. Di Lorenzo G, Zappavigna S, Crocetto F, Giuliano M, Ribera D, Morra R, et al. Assessment of Total, PTEN(-), and AR-V7(+) Circulating Tumor Cell Count by Flow Cytometry in Patients With Metastatic Castration-Resistant Prostate Cancer Receiving Enzalutamide. Clin Genitourin Cancer (2021) 19:e286–98. doi: 10.1016/j.clgc.2021.03.021
30. Maillet D, Allioli N, Peron J, Plesa A, Decaussin-Petrucci M, Tartas S, et al. Improved Androgen Receptor Splice Variant 7 Detection Using a Highly Sensitive Assay to Predict Resistance to Abiraterone or Enzalutamide in Metastatic Prostate Cancer Patients. Eur Urol Oncol (2021) 4:609–17. doi: 10.1016/j.euo.2019.08.010
31. Marín-Aguilera M, Jiménez N, Reig Ò, Montalbo R, Verma AK, Castellano G, et al. Androgen Receptor and its Splicing Variant 7 Expression in Peripheral Blood Mononuclear Cells and in Circulating Tumor Cells in Metastatic Castration-Resistant Prostate Cancer. Cells (2020) 9(1):203. doi: 10.3390/cells9010203
32. Markowski MC, Wang H, Sullivan R, Rifkind I, Sinibaldi V, Schweizer MT, et al. A Multicohort Open-Label Phase II Trial of Bipolar Androgen Therapy in Men With Metastatic Castration-Resistant Prostate Cancer (RESTORE): A Comparison of Post-Abiraterone Versus Post-Enzalutamide Cohorts. Eur Urol (2021) 79:692–9. doi: 10.1016/j.eururo.2020.06.042
33. Miyamoto DT, Lee RJ, Kalinich M, LiCausi JA, Zheng Y, Chen T, et al. An RNA-Based Digital Circulating Tumor Cell Signature is Predictive of Drug Response and Early Dissemination in Prostate Cancer. Cancer Discovery (2018) 8(3):288–303. doi: 10.1158/2159-8290.CD-16-1406
34. Okegawa T, Ninomiya N, Masuda K, Nakamura Y, Tambo M, Nutahara K. AR-V7 in Circulating Tumor Cells Cluster as a Predictive Biomarker of Abiraterone Acetate and Enzalutamide Treatment in Castration-Resistant Prostate Cancer Patients. Prostate (2018) 78(8):576–82. doi: 10.1002/pros.23501
35. Onstenk W, Sieuwerts AM, Kraan J, Van M, Nieuweboer AJM, Mathijssen RHJ, et al. Efficacy of Cabazitaxel in Castration-Resistant Prostate Cancer is Independent of the Presence of AR-V7 in Circulating Tumor Cells. Eur Urol (2015) 68(6):939–45. doi: 10.1016/j.eururo.2015.07.007
36. Qu F, Xie W, Nakabayashi M, Zhang H, Jeong SH, Wang X, et al. Association of AR-V7 and Prostate-Specific Antigen RNA Levels in Blood With Efficacy of Abiraterone Acetate and Enzalutamide Treatment in Men With Prostate Cancer. Clin Cancer Res (2017) 23(3):726–34. doi: 10.1158/1078-0432.CCR-16-1070
37. Scher HI, Graf RP, Schreiber NA, Jayaram A, Winquist E, McLaughlin B, et al. Assessment of the Validity of Nuclear-Localized Androgen Receptor Splice Variant 7 in Circulating Tumor Cells as a Predictive Biomarker for Castration-Resistant Prostate Cancer. JAMA Oncol (2018) 4(9):1179–86. doi: 10.1001/jamaoncol.2018.1621
38. Scher HI, Graf RP, Schreiber NA, McLaughlin B, Lu D, Louw J, et al. Nuclear-Specific AR-V7 Protein Localization is Necessary to Guide Treatment Selection in Metastatic Castration-Resistant Prostate Cancer. Eur Urol (2017) 71(6):874–82. doi: 10.1016/j.eururo.2016.11.024
39. Scher HI, Lu D, Schreiber NA, Louw J, Graf RP, Vargas HA, et al. Association of AR-V7 on Circulating Tumor Cells as a Treatment-Specific Biomarker With Outcomes and Survival in Castration-Resistant Prostate Cancer. JAMA Oncol (2016) 2(11):1441–9. doi: 10.1001/jamaoncol.2016.1828
40. Seitz AK, Thoene S, Bietenbeck A, Nawroth R, Tauber R, Thalgott M, et al. AR-V7 in Peripheral Whole Blood of Patients With Castration-Resistant Prostate Cancer: Association With Treatment-Specific Outcome Under Abiraterone and Enzalutamide. Eur Urol (2017) 72(5):828–34. doi: 10.1016/j.eururo.2017.07.024
41. Sepe P, Verzoni E, Miodini P, Claps M, Ratta R, Martinetti A, et al. Could Circulating Tumor Cells and ARV7 Detection Improve Clinical Decisions in Metastatic Castration-Resistant Prostate Cancer? The Istituto Nazionale Dei Tumori (INT) Experience. Cancers (2019) 11(7):980. doi: 10.3390/cancers11070980
42. Škereňová M, Mikulová V, Čapoun O, Švec D, Kološtová K, Soukup V, et al. Gene Expression Analysis of Immunomagnetically Enriched Circulating Tumor Cell Fraction in Castration-Resistant Prostate Cancer. Mol Diagnosis Ther (2018) 22(3):381–90. doi: 10.1007/s40291-018-0333-0
43. Tagawa ST, Antonarakis ES, Gjyrezi A, Galletti G, Kim S, Worroll D, et al. Expression of AR-V7 and Arv(567es) in Circulating Tumor Cells Correlates With Outcomes to Taxane Therapy in Men With Metastatic Prostate Cancer Treated in TAXYNERGY. Clin Cancer Res (2019) 25(6):1880–8. doi: 10.1158/1078-0432.CCR-18-0320
44. Tommasi S, Pilato B, Carella C, Lasorella A, Danza K, Vallini I, et al. Standardization of CTC AR-V7 PCR Assay and Evaluation of its Role in Castration Resistant Prostate Cancer Progression. Prostate (2018) 79(1):54–61. doi: 10.1002/pros.23710
45. Wang S, Yang S, Nan C, Wang Y, He Y, Mu H. Expression of Androgen Receptor Variant 7 (AR-V7) in Circulated Tumor Cells and Correlation With Drug Resistance of Prostate Cancer Cells. Med Sci Monitor (2018) 24:7051–6. doi: 10.12659/MSM.909669
46. Galletti G, Leach BI, Lam L, Tagawa ST. Mechanisms of Resistance to Systemic Therapy in Metastatic Castration-Resistant Prostate Cancer. Cancer Treat Rev (2017) 57:16–27. doi: 10.1016/j.ctrv.2017.04.008
47. Nimir M, Ma Y, Jeffreys SA, Opperman T, Young F, Khan T, et al. Detection of AR-V7 in Liquid Biopsies of Castrate Resistant Prostate Cancer Patients: A Comparison of AR-V7 Analysis in Circulating Tumor Cells, Circulating Tumor RNA and Exosomes. Cells (2019) 8(7):688. doi: 10.3390/cells8070688
48. Zhang G, Liu X, Li J, Ledet E, Alvarez X, Qi Y, et al. Androgen Receptor Splice Variants Circumvent AR Blockade by Microtubule-Targeting Agents. Oncotarget (2015) 6(27):23358–71. doi: 10.18632/oncotarget.4396
49. Wang Z, Shen H, Ma N, Li Q, Mao Y, Wang C, et al. The Prognostic Value of Androgen Receptor Splice Variant 7 in Castration-Resistant Prostate Cancer Treated With Novel Hormonal Therapy or Chemotherapy: A Systematic Review and Meta-Analysis. Front Oncol (2020) 10:572590. doi: 10.3389/fonc.2020.572590
Keywords: prostate cancer, AR-V7, liquid biopsy, prognosis, meta-analysis
Citation: Khan T, Becker TM, Scott KF, Descallar J, de Souza P, Chua W and Ma Y (2022) Prognostic and Predictive Value of Liquid Biopsy-Derived Androgen Receptor Variant 7 (AR-V7) in Prostate Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 12:868031. doi: 10.3389/fonc.2022.868031
Received: 02 February 2022; Accepted: 21 February 2022;
Published: 18 March 2022.
Edited by:
Riccardo Tellini, Careggi University Hospital, ItalyReviewed by:
Hideki Maeda, Meiji Pharmaceutical University, JapanAasems Jacob, Pikeville Medical Center, United States
Haoran Liu, Stanford University, United States
Carlo Cattrini, Azienda Ospedaliero Universitaria Maggiore della Carità, Italy
Copyright © 2022 Khan, Becker, Scott, Descallar, de Souza, Chua and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yafeng Ma, eWFmZW5nLm1hQHVuc3cuZWR1LmF1
 Tanzila Khan1,2,3
Tanzila Khan1,2,3 Kieran F. Scott
Kieran F. Scott Yafeng Ma
Yafeng Ma