- 1Department of Obstetrics and Gynecology, Zhongnan Hospital of Wuhan University, Wuhan, China
- 2Department of Obstetrics and Gynecology, The Central Hospital of Wuhan, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background: Unilateral oophorectomy has the benefits of preserving the ovarian function of fertility and hormone secretion, but the precise inclusion criteria for candidates for this procedure remain controversial. This study aimed to compare the prevalence and therapeutic efficiency of unilateral oophorectomy in women with ovarian cancer who underwent bilateral oophorectomy; moreover, it aimed to identify the appropriate candidates for unilateral oophorectomy.
Methods: Female patients diagnosed with stage I-III ovarian cancer between 2000 and 2017 were retrospectively identified from the Surveillance, Epidemiology, and End Results program database. Overall survival (OS) and disease-specific survival (DSS) after unilateral or bilateral (salpingo-) oophorectomy were estimated. Cumulative mortality rates (CMRs) for non-cancer comorbidities were also estimated.
Results: A total of 28,480 women with ovarian cancer were included in this study, of whom 11,517 died during the study period. Of the patients, 7.5% and 48.0% underwent unilateral and bilateral oophorectomy, respectively. Overall, for stage-Ia tumors, unilateral oophorectomy was associated with remarkably better OS and DSS than bilateral oophorectomy (OS: p < 0.001; DSS: p = 0.01). For stage-Ib and stage-Ic ovarian tumor, there was no significant difference between the OS and DSS of patients treated by unilateral oophorectomy and those treated by bilateral oophorectomy. For stage-II and stage-III ovarian cancer, unilateral oophorectomy was associated with remarkably worse OS and DSS than bilateral oophorectomy. Among the reproductive-age women younger than 50 years, the OS and DSS of patients with stage-I tumors receiving unilateral oophorectomy were comparable to those receiving bilateral oophorectomy, even for high-grade stage-Ic tumors (all p > 0.05). For those aged 50 years and older, OS and DSS of patients with stage-I tumor receiving unilateral oophorectomy were significantly worse than those receiving bilateral oophorectomy, even for low-grade stage-Ia ovarian tumor (OS: p < 0.001; DSS: p = 0.02).
Conclusion: Unilateral oophorectomy exhibited excellent oncological superiority and was equivalent to bilateral oophorectomy for stage-I ovarian tumors among women of reproductive age. For women of reproductive age, the criteria of unilateral oophorectomy can be appropriately broadened to high-grade stage-Ic diseases because of the better performance of unilateral oophorectomy in this population.
Introduction
Ovarian cancer is the sixth leading cause of global cancer deaths, accounting for over 294,000 new cancer cases and 198,000 new cancer deaths worldwide in 2019 (1). Ovarian cancer ranks fifth in cancer deaths among American women, accounting for more deaths than any other female reproductive system cancer. A woman’s risk of developing ovarian cancer during her lifetime is about 1 in 78, and her lifetime chance of dying from ovarian cancer is approximately 1 in 108 (2). Although the 5-year relative-survival rate of localized ovarian cancer can reach 93%, only 16% of the cases have the opportunity to be diagnosed at an early stage (3). Of the tumors, 57% were accompanied by distant metastases at the time of cancer diagnosis, implying a particularly unfavorable prognosis with a survival rate of only 30% (3, 4). As a consequence of the low early detection, as well as the relatively high malignant potential, the overall 5-year relative survival rate generally ranges between 30% and 40% across the globe (5). Strikingly, only very modest increases have been achieved (2%–4%) since 1995 (5). Therefore, despite its significance to public health, the etiology of this lethal disease is not completely understood.
The standard treatment for ovarian cancer includes upfront surgery to accurately diagnose and stage the disease and perform maximal cytoreduction, followed by taxanes and platinum–based combination chemotherapy in most patients (6). Traditionally, surgical staging of ovarian cancer has included exploratory laparotomy with peritoneal washings, hysterectomy, salpingo-oophorectomy, omentectomy, multiple peritoneal biopsies, and potential pelvic and para-aortic lymphadenectomy.
When preservation of fertility is desired and the disease seems confined to a single ovary, preservation of the uterus and contralateral ovary is often possible (6). Compared with bilateral oophorectomy, unilateral oophorectomy can preserve the other side of the ovary to maintain the ovarian function of fertility and hormone secretion. The major concerns of unilateral oophorectomy focused on the potential risks of residual tumor, tumor recurrence, and a newly occurring tumor on the other side of the ovary (7, 8). Therefore, it is important to determine which proportion of patients are suitable and/or have the opportunity to receive unilateral oophorectomy and preserve the other side of the ovary.
This study aimed to characterize the prevalence and outcomes of unilateral oophorectomy in women with ovarian cancer and compare it with bilateral oophorectomy to distinguish the appropriate proportion of patients with ovarian cancer to be treated with unilateral oophorectomy. The results will guide researchers and clinicians in determining the optimal therapy for patients with ovarian cancer.
Materials and Methods
Data Sources and Study Population
This retrospective cohort study used data from the Surveillance, Epidemiology, and End Results (SEER) program. The SEER database is a population-based cancer registry covering nearly 30% of the US population and collecting cancer demographics, incidence, survival, and treatment data. The SEER*Stat software version 8.3.8 was used for the analysis (9). This study was performed according to the STROCSS guidelines (Strengthening the reporting of cohort, cross-sectional and case-control studies in surgery) (10).
Female patients diagnosed with the first primary malignant ovarian cancer (site codes: C56.9) between 2000 and 2017 were extracted from the SEER 18 database (2020 submission) (11). Only patients with unilateral-originated ovarian cancer were included because patients with bilateral origin might have lost their opportunity to undergo unilateral oophorectomy. Patients diagnosed only through autopsy or death certificates were excluded. We further excluded patients without complete follow-up information, including follow-up duration and age at diagnosis. To accurately evaluate the effects of surgical operations, we further excluded patients with advanced-stage cancer or those with a cancer of unknown stage (Figure S1).
Since it is a publicly available database, access to the SEER data required a signed research data agreement form. The Institutional Review Board of Zhongnan Hospital of Wuhan University waived the institutional review board approval for the data obtained from the SEER database, as the study did not directly involve human subjects, and all data were anonymized. The requirement for informed consent was waived.
Definition of Variables
All patients were followed between the time of the first primary diagnosis of ovarian cancer and the time of their death, exiting the study alive, or the end of the study (December 31, 2017). Among the patients included in this study, we evaluated the following variables: age at diagnosis, race, year of diagnosis, cancer stage, American Joint Committee on Cancer Staging (AJCC) N stage, AJCC T stage, surgical therapy, cause of death, histological types, urban/rural residency at diagnosis, median household income, follow-up time, and vital status at the end of follow-up.
As the SEER database records the survival duration in months, and a month was the shortest time interval available for analysis, survival durations shorter than 1 month were recorded as 0 months in the SEER program. Age at cancer diagnosis was divided into 4 groups for comparison: “15-39 years,” “40-59 years,” “60-79 years,” and “80+ years”. Patients aged 15-49 years were selected for specific analyses, as this proportion of women had a greater desire for fertility preservation.
For ovarian cancer, the SEER program derived TNM values of the stage from the International Federation of Gynecology and Obstetrics (FIGO) stage. Thus, FIGO information of this study was inferred from TNM-stage values. TNM-stage values were extracted from AJCC 3rd stage codes for patients diagnosed between 2000 and 2003, AJCC 6th stage codes for patients diagnosed between 2004 and 2009, AJCC 7th stage codes for patients diagnosed between 2010 and 2015, and SEER combined stage for patients diagnosed in 2016 and 2017 (12). We excluded the patients with stage IV ovarian cancer.
The SEER program provided detailed site-specific surgical information for the included patients (13–15). Surgical operations for ovarian cancer were divided into two major groups: unilateral and bilateral oophorectomy. To avoid confusion, local excision/destruction and unknown surgical operations were excluded (surgery codes: 17 and 90-99). Unilateral oophorectomy was defined as total removal of the tumor or (single) ovary, and unilateral (salpingo-) oophorectomy with or without hysterectomy (surgery codes: 25-28 and 35-37). Bilateral oophorectomy includes bilateral (salpingo-) oophorectomy with or without hysterectomy, cytoreductive surgery, and pelvic exenteration (surgery codes: 60-74). Surgical operations with unknown laterality were excluded for accuracy (surgery codes: 55-57 and 80) (13).
Causes of death of patients with ovarian cancer were classified into two major groups: death from cancer (i.e., a second primary cancer) and death from non-cancer comorbidities (i.e., deaths from any medical cause other than cancer). Causes of death were defined by the SEER cause-specific death classification variable from death certificates (15–17). Non-cancer causes were categorized into 26 major groups. These groups were further divided into seven broad categories: infectious diseases, cardiovascular diseases (CVD), respiratory diseases, gastrointestinal and liver diseases, renal diseases, external injuries, and other non-cancer causes.
Statistical Analysis
We estimated the characteristics of the patients with ovarian cancer. Trends in surgical operations were characterized by age at diagnosis and year of diagnosis. Moreover, we analyzed the overall survival (OS) and disease-specific survival (DSS) of patients using the Kaplan-Meier method. The OS rate was defined as the percentage of survivors (all causes of death) after follow-up. The DSS rate was defined as the percentage of patients who have not died from ovarian cancer (rather than from other causes) in a defined period of time (18). Cox regression models were used to assess the significance of differences in the OS and DSS analyses. The cumulative mortality rate (CMR) was estimated for non-cancer comorbidities (15).
All analyses were performed using SEER*Stat software version 8.3.8 (9) and R 3.6.3 (19). Tests were two-tailed, with a p-value of less than 0.05 considered statistically significant.
Results
Baseline Characteristics
In this population-based study involving 28,480 women with stage I-III ovarian cancer, 11,517 (40.4%) deaths were recorded, with a median follow-up time of 4.1 years (range: 0–17.9 years) (Figure S1 and Table 1). Most of the patients were aged 40–79 years (83.3%) and were white (82.6%). Of the cancers, 46.8% were stage-I tumors. Serous ovarian cancer accounted for the majority of tumors (51.9%), followed by endometrioid carcinoma (20.7%) (Table 1).
Of the patients, 95.5% (N = 27,197) had undergone surgical operations, among whom 7.9%, 50.3%, and 41.8% underwent unilateral oophorectomy, bilateral oophorectomy, and other surgical procedures, respectively (Table S1). Patients who underwent unilateral oophorectomy were younger. Most patients who underwent unilateral oophorectomy (70.9%) were younger than 60. Of the patients aged 15–39 years, 33.7% and 22.7% underwent unilateral oophorectomy and bilateral oophorectomy, respectively (Table 1). Moreover, there was a decreasing trend in the unilateral oophorectomy rate by age at cancer diagnosis (Figure 1A). Similarly, the unilateral oophorectomy rate decreased by FIGO stage (Figure 1B), especially for younger patients (Figure 1C). Most tumors treated by unilateral oophorectomy were in the Ia stage (53.1%), followed by Ic stage (19.4%). The Hispanic population had a higher unilateral oophorectomy rate (11.3%) than the non-Hispanic population (7.0%) (Table 1).
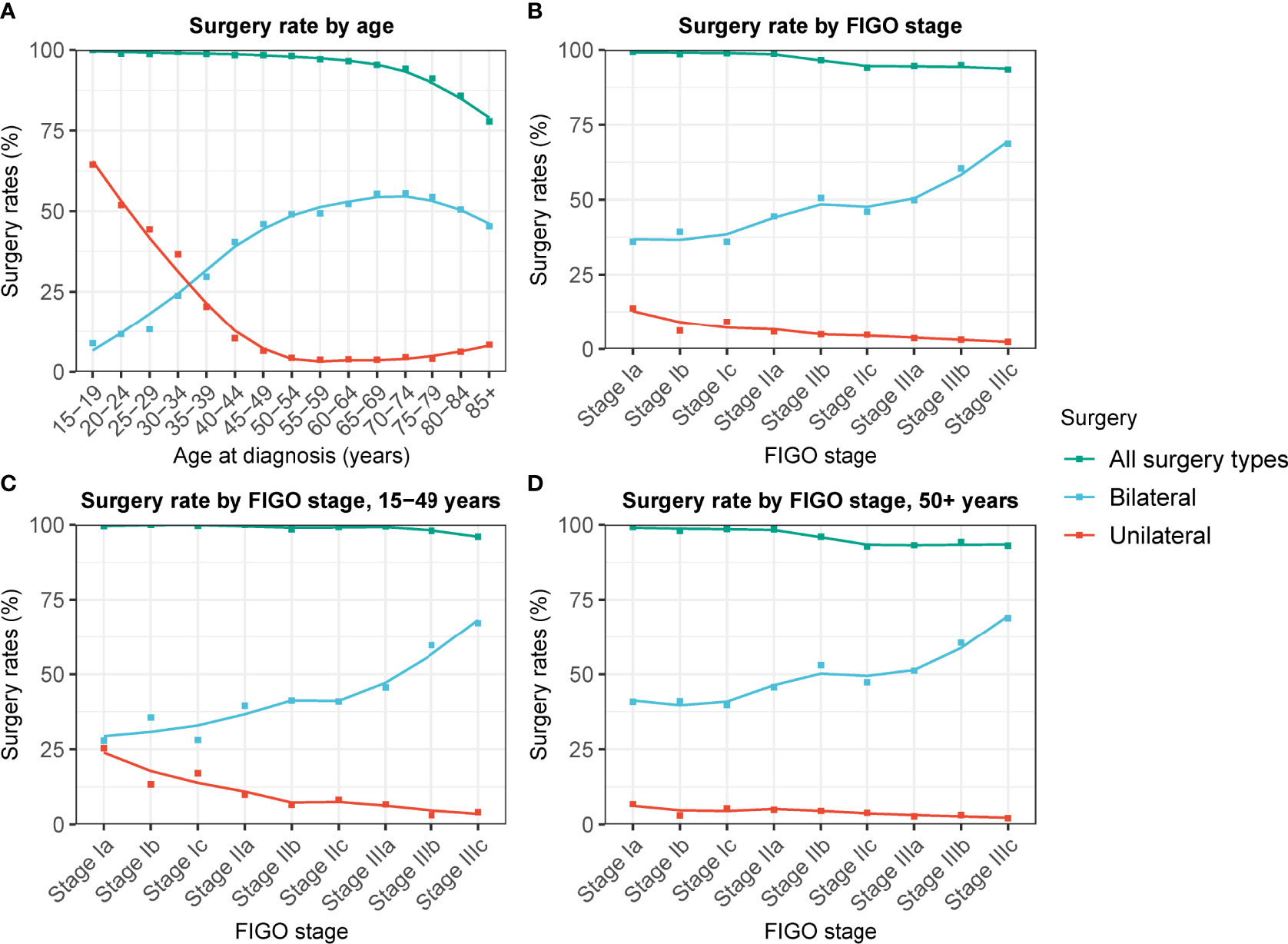
Figure 1 Changes in unilateral and bilateral oophorectomy rate of patients with ovarian cancer by FIGO stage and age at cancer diagnosis. (A) Changes in unilateral and bilateral oophorectomy rate of patients with ovarian cancer of all stage by age at cancer diagnosis. (B) Changes in unilateral and bilateral oophorectomy rate of patients with ovarian cancer by FIGO stage. (C) Changes in unilateral and bilateral oophorectomy rate of patients aged 15-49 years with ovarian cancer by FIGO stage. (D) Changes in unilateral and bilateral oophorectomy rate of patients aged 50+ years with ovarian cancer by FIGO stage.
Survival Analysis of Surgical Interventions for Patients With Ovarian Cancer
The OS and DSS of patients who had undergone surgery were significantly better than those of patients who did not (all p < 0.001) (Figure 2 and Figure S2). The prognostic superiority of surgical operation could be observed in ovarian cancer at all stages (Figures 2B, C and Figure S2).
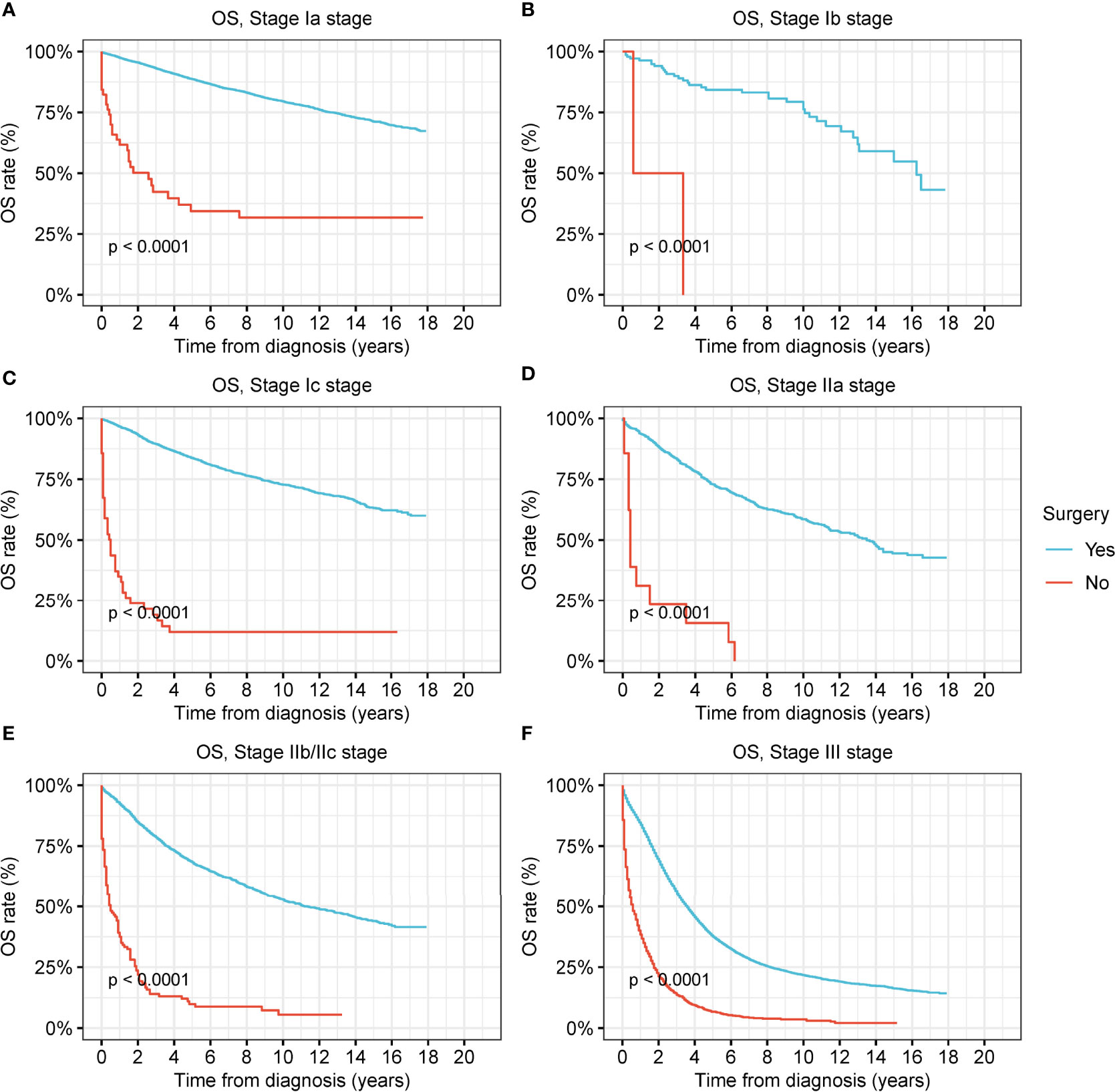
Figure 2 Overall survival (OS) of patients with ovarian cancer by surgery. (A) OS of patients with stage-Ia ovarian cancer by surgery. (B) OS of patients with stage-Ib ovarian cancer by surgery. (C) OS of patients with stage-Ic ovarian cancer by surgery. (D) OS of patients with stage-IIa ovarian cancer by surgery. (E) OS of patients with stage-IIb/IIc ovarian cancer by surgery. (F) OS of patients with stage-III ovarian cancer by surgery.
To examine the therapeutic effects of unilateral oophorectomy, we performed survival analyses according to the type of surgical intervention (Figure 3 and Figure S3). In stage-Ia tumor, unilateral oophorectomy was associated with remarkably better OS and DSS compared with bilateral oophorectomy, with a 5-year OS rate of 89.9% for unilateral oophorectomy and 87.9% for bilateral oophorectomy (OS: p < 0.001; DSS: p = 0.01) (Figure 3A and Figure S3A). For stage-Ib and stage-Ic ovarian tumor, there was no significant difference between the OS and DSS of patients treated by unilateral oophorectomy and those of patients treated by bilateral oophorectomy (stage Ib: OS: p = 0.6; DSS: p = 0.8; stage Ic: OS: p = 0.06; DSS: p = 0.2) (Figures 3B, C and Figures S3B, C). For stage-IIa tumors, the OS and DSS after unilateral oophorectomy were significantly worse than those after bilateral oophorectomy (5-year OS: 50.3% vs. 72.0%, p < 0.001; 5-year DSS: 61.6% vs. 72.0%, p < 0.001) (Figure 3D and Figure S3D). For stage-IIb/IIc tumors, there was no significant difference between the OS and DSS of patients treated by unilateral oophorectomy and those of patients treated by bilateral oophorectomy (OS: p = 0.6; DSS: p = 0.6) (Figure 3E and Figure S3E). For stage-III tumors, the OS and DSS after unilateral oophorectomy were significantly worse than those after bilateral oophorectomy (OS: p < 0.001; DSS: p < 0.001) (Figure 3F and Figure S3F).
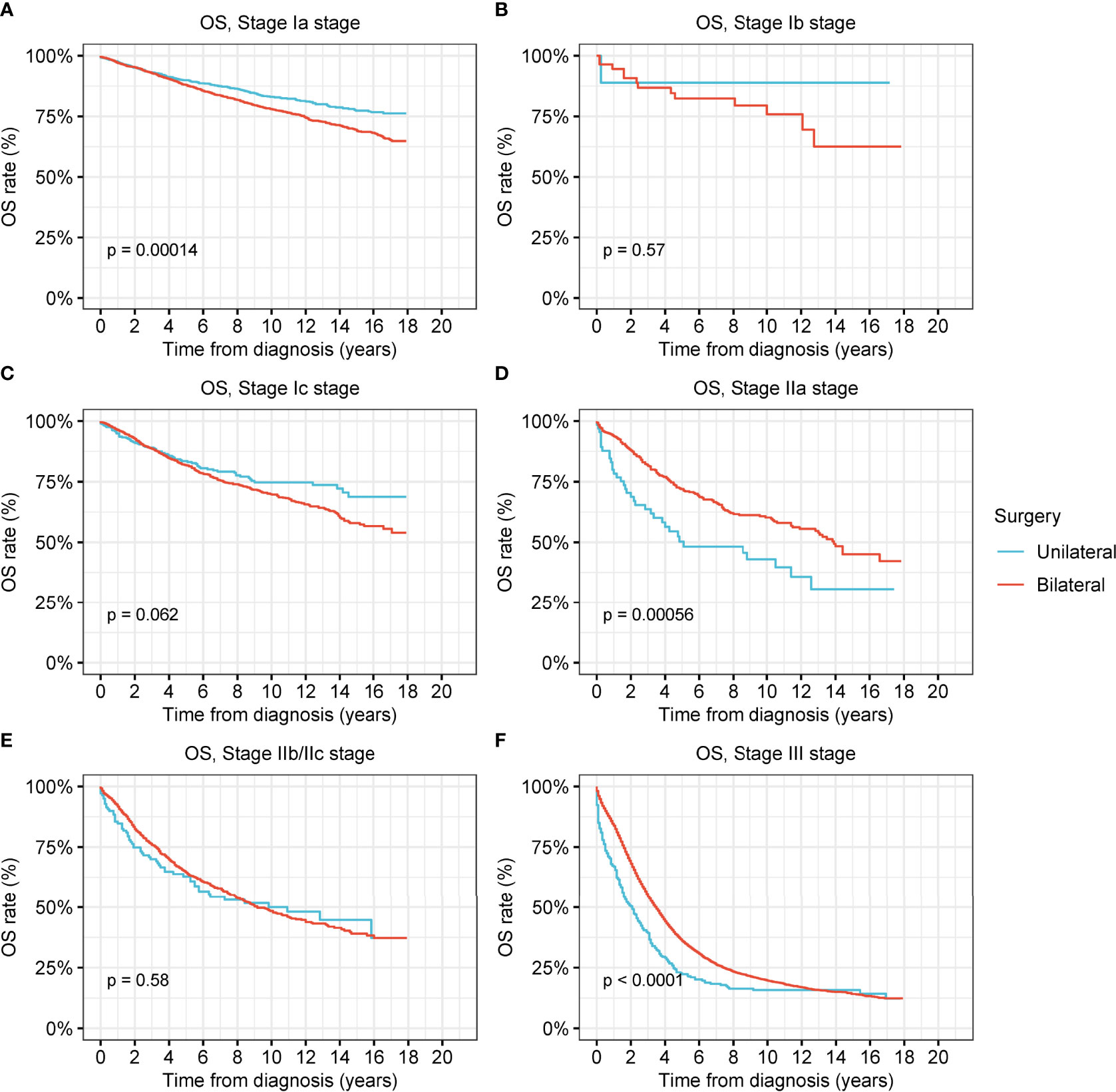
Figure 3 Overall survival (OS) of patients with ovarian cancer by cancer stage and different types of surgical operation. (A) OS of patients with stage-Ia ovarian cancer by different types of surgical operation. (B) OS of patients with stage-Ib ovarian cancer by different types of surgical operation. (C) OS of patients with stage-Ic ovarian cancer by different types of surgical operation. (D) OS of patients with stage-IIa ovarian cancer by different types of surgical operation. (E) OS of patients with stage-IIb/IIc ovarian cancer by different types of surgical operation. (F) OS of patients with stage-III ovarian cancer by different types of surgical operation.
For low-grade and high-grade stage-I ovarian tumors, there was no significant difference between the OS and DSS of patients treated by unilateral oophorectomy and those of patients treated by bilateral oophorectomy (Figures 4A–D and Figures S4B, C). The OS of patients with low-grade and high-grade stage-IIa ovarian tumors undergoing unilateral oophorectomy were worse than those of patients undergoing bilateral oophorectomy (low-grade: p = 0.03; high-grade: p < 0.001) (Figures 4E, F). The DSS of patients with high-grade stage-IIa ovarian tumor undergoing unilateral oophorectomy were worse than those of patients undergoing bilateral oophorectomy (high-grade: p < 0.001) (Figure S4F).
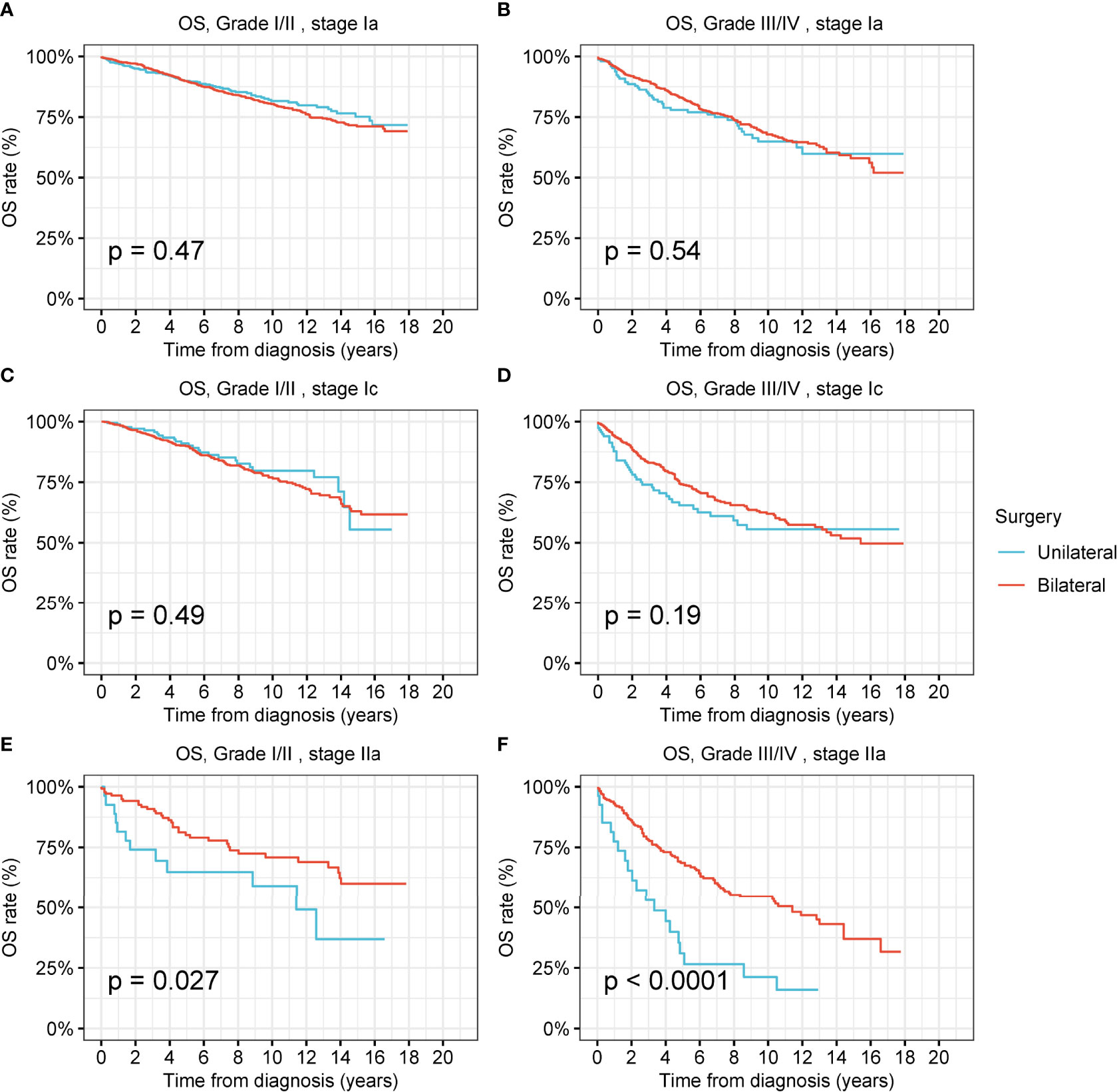
Figure 4 Overall survival (OS) of patients with ovarian cancer by cancer stage, cancer grade and different types of surgical operation. (A) OS of patients with low-grade stage-Ia ovarian cancer by different types of surgical operation. (B) OS of patients with high-grade stage-Ia ovarian cancer by different types of surgical operation. (C) OS of patients with low-grade stage-Ic ovarian cancer by different types of surgical operation. (D) OS of patients with high-grade stage-Ic ovarian cancer by different types of surgical operation. (E) OS of patients with low-grade stage-IIa ovarian cancer by different types of surgical operation. (F) OS of patients with high-grade stage-IIa ovarian cancer by different types of surgical operation.
We further analyzed the survival after unilateral oophorectomy or bilateral oophorectomy in patients with ovarian cancers of different histology (Figures S5–S8). We found that, except for high-grade stage-Ic serous ovarian cancer (Due to the inadequate cases with stage-Ib tumors, stage-Ib tumors were not included for further analyses hereafter), unilateral oophorectomy was comparable to bilateral oophorectomy in low-grade and high-grade stage-I ovarian cancer of any histology, including serous, mucinous, endometrioid, and clear cell carcinoma (Figures S5–S8). For high-grade stage-Ic serous ovarian cancer, the OS after unilateral oophorectomy was significantly worse than that after bilateral oophorectomy (p = 0.03) (Figure S5D).
Survival Analysis of Surgical Interventions by Stage and Age at Cancer Diagnosis
Female patients of reproductive age had a greater desire to preserve fertility; thus, we investigated the prevalence and therapeutic effects of unilateral oophorectomy in this peculiar population (Figure 5 and Figure S9). Among the reproductive-age women younger than 50 years, 22.8% received unilateral oophorectomy, and 27.9% underwent bilateral oophorectomy. We found that OS and DSS of patients with low-grade and high-grade stage-I receiving unilateral oophorectomy were comparable to those of patients receiving bilateral oophorectomy (all p > 0.05) (Figure 6 and Figure S9). For patients aged 15–59 years with high-grade stage-Ic ovarian tumor, the OS and DSS of patients receiving unilateral oophorectomy were similar to those of patients receiving bilateral oophorectomy (OS: p = 1; DSS: p = 0.7) (Figure 5F and Figure S9F).
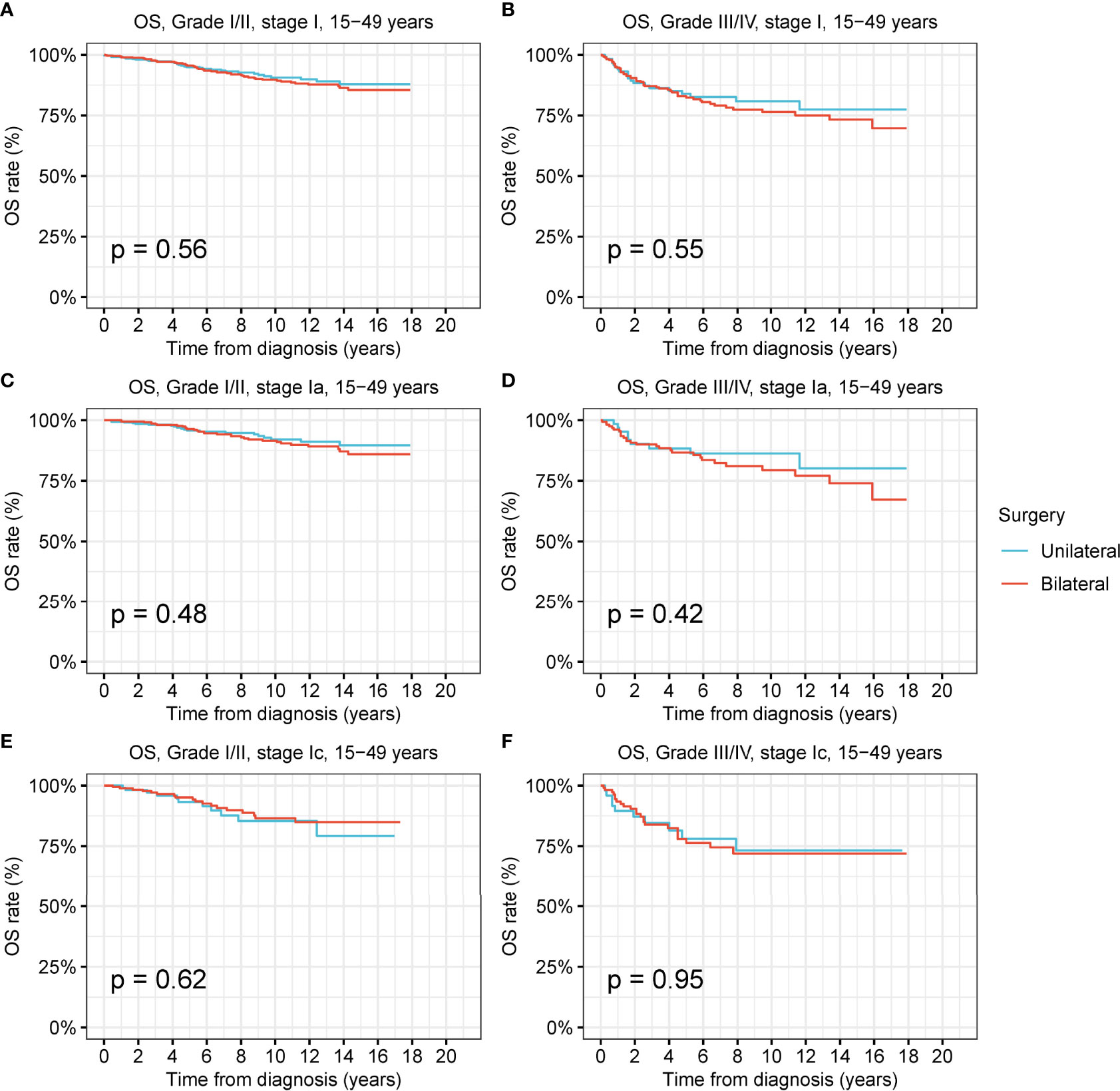
Figure 5 Overall survival (OS) of patients of productive age (15-50 years) with ovarian cancer by cancer stage, cancer grade and different types of surgical operation. (A) OS of patients of productive age with low-grade stage-I ovarian cancer by different types of surgical operation. (B) OS of patients of productive age with high-grade stage-I ovarian cancer by different types of surgical operation. (C) OS of patients of productive age with low-grade stage-Ia ovarian cancer by different types of surgical operation. (D) OS of patients of productive age with high-grade stage-Ia ovarian cancer by different types of surgical operation. (E) OS of patients of productive age with low-grade stage-Ic ovarian cancer by different types of surgical operation. (F) OS of patients of productive age with high-grade stage-Ic ovarian cancer by different types of surgical operation.
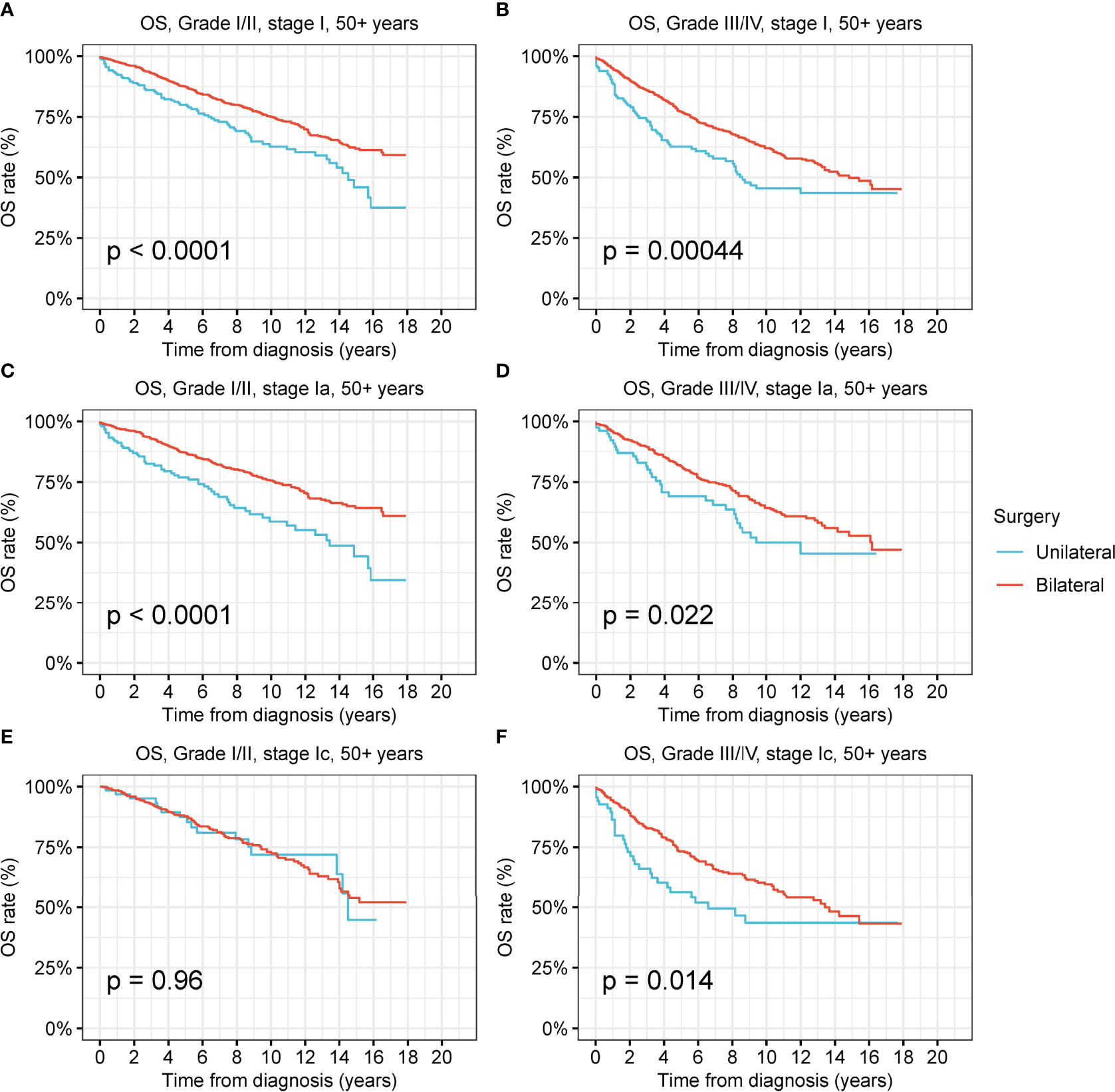
Figure 6 Overall survival (OS) of patients aged 50+ years with ovarian cancer by cancer stage, cancer grade and different types of surgical operation. (A) OS of patients aged 50+ years with low-grade stage-I ovarian cancer by different types of surgical operation. (B) OS of patients aged 50+ years with high-grade stage-I ovarian cancer by different types of surgical operation. (C) OS of patients aged 50+ years with low-grade stage-Ia ovarian cancer by different types of surgical operation. (D) OS of patients aged 50+ years with high-grade stage-Ia ovarian cancer by different types of surgical operation. (E) OS of patients aged 50+ years with low-grade stage-Ic ovarian cancer by different types of surgical operation. (F) OS of patients aged 50+ years with high-grade stage-Ic ovarian cancer by different types of surgical operation.
For those aged 50 years and older, the OS of patients with low-grade and high-grade stage-I receiving unilateral oophorectomy was significantly worse than that of patients receiving bilateral oophorectomy (low-grade: p < 0.001; high-grade: p < 0.001) (Figures 6A, B). The DSS of patients aged 50 years and older with low-grade stage-I receiving unilateral oophorectomy were similar to those of patients receiving bilateral oophorectomy (p = 0.2) (Figure S10A). The DSS of patients aged 50 years and older with high-grade stage-I receiving unilateral oophorectomy were worse than that of patients receiving bilateral oophorectomy (p < 0.001) (Figure S10B). For patients aged 50 years and older with low-grade stage-Ia ovarian tumor, the OS and DSS of patients receiving unilateral oophorectomy were significantly worse than those of patients receiving bilateral oophorectomy (OS: p < 0.001; DSS: p = 0.01) (Figure 6C and Figure S10C).
Comorbidity Analysis of Patients With Ovarian Cancer Treated by Different Surgical Interventions
A comorbidity analysis was carried out on the causes of death for the patients with ovarian cancer (Figure 7, Figure S11, and Figure S12). In stage-I ovarian cancer, the CMR of cancer-related deaths was significantly lower in patients who underwent unilateral oophorectomy than in those who underwent bilateral oophorectomy (p < 0.001) (Figure 7A). CVDs were also remarkably decreased in patients who underwent unilateral oophorectomy (5-year CMR: unilateral oophorectomy, 1.5%; bilateral oophorectomy, 1.7%; p = 0.04) (Figure 7C). For stage-II and stage-III tumors, there were no significant differences between the CMR of unilateral and bilateral oophorectomy for both cancer-related deaths and non-cancer comorbidities (Figure S11 and Figure S12).
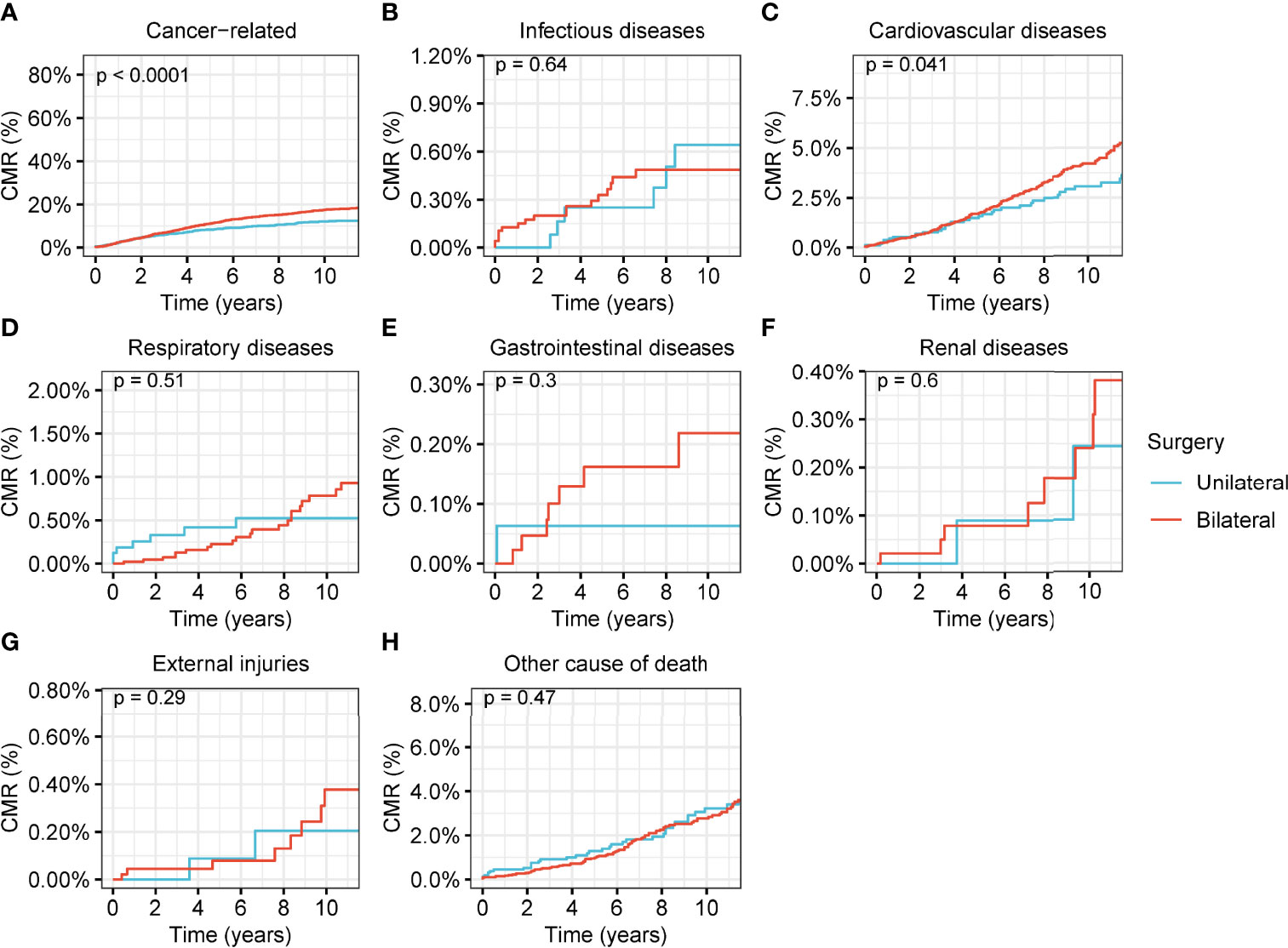
Figure 7 Cumulative mortality rate (CMR) among women of productive age with stage-I ovarian cancer by different types of surgical operation. (A) CMR from cancer-related deaths among women of productive age with stage-I ovarian cancer by different types of surgical operation. (B) CMR from infectious diseases among women of productive age with stage-I ovarian cancer by different types of surgical operation. (C) CMR from cardiovascular diseases among women of productive age with stage-I ovarian cancer by different types of surgical operation. (D) CMR from respiratory diseases among women of productive age with stage-I ovarian cancer by different types of surgical operation. (E) CMR from gastrointestinal diseases among women of productive age with stage-I ovarian cancer by different types of surgical operation. (F) CMR from renal diseases among women of productive age with stage-I ovarian cancer by different types of surgical operation. (G) CMR from external injuries among women of productive age with stage-I ovarian cancer by different types of surgical operation. (H) CMR from other non-cancer causes among women of productive age with stage-I ovarian cancer by different types of surgical operation.
Discussion
In this population-based study involving more than 28,000 women with ovarian cancer, we compared the prevalence and therapeutic efficacy of unilateral oophorectomy with bilateral oophorectomy. We found that unilateral oophorectomy exhibited excellent oncological superiority and was equivalent to bilateral oophorectomy for stage-I ovarian tumors among women of productive age; this equivalence to bilateral oophorectomy remained true for high-grade stage-Ic ovarian tumors. For patients aged 50 years and older, the performance of unilateral oophorectomy was worse than that of bilateral oophorectomy, even for low-grade stage-Ia ovarian tumors. These results indicated that unilateral oophorectomy was valuable for stage-I ovarian tumors among women of productive age.
Unilateral oophorectomy has the advantages of preserving fertility and part or full function of the ovary, while fertility is completely destroyed after bilateral oophorectomy. Fertility preservation is an important component of cervical cancer survivors’ overall quality of life (20). Fertility-preserving procedures in cases of borderline ovarian tumors are now well-established because this type of lesion is often diagnosed in young women whose fertility issues are primordial (21). The status of fertility-preserving procedures in malignant ovarian cancer remains controversial. Data on the conservative management of ovarian cancer are still limited; however, the oncologic safety of fertility-sparing procedures in early ovarian cancer has been confirmed (22–24). Researchers also proposed that high-risk disease should not be considered a contraindication to conservative surgery (23, 25). This procedure is mainly limited to women with IA grade 1 disease who wish to preserve their fertility. For some investigators, fertility-sparing procedures were found to be safe in women with more advanced-stage disease until stage IC (26). Our results further confirmed the potential candidates for this procedure. We found that age is an important factor in selecting potential candidates, as unilateral oophorectomy is valuable for stage-I ovarian tumors among women of productive age, even for high-grade stage-Ic diseases. In contrast, the performance of unilateral oophorectomy is demonstrated to be greatly weakened by age. For patients aged 50 years or older, the long-term survival after unilateral oophorectomy is worse than that after bilateral oophorectomy, even for the tumors with the lowest risk, namely, the low-grade stage-Ia tumors. Therefore, we recommend the inclusion criteria of unilateral oophorectomy be extended to high-grade stage-Ic diseases; in contrast, for those aged 50 years and older without fertility desire, unilateral oophorectomy is not recommended, and bilateral oophorectomy should be adopted as the first choice.
The major limitations of this procedure are the underlying risks of residual tumor, tumor recurrence, and possible newly occurring carcinoma in the remaining ovarian tissue. To address these concerns, postoperative chemotherapy, radiotherapy, or molecular targeted therapy should be employed for the high-risk population (27). Precise diagnosis and stage of the disease before the surgery are vital to guarantee the tumor clearance of surgery (28). Minimally invasive surgery, if necessary, is a viable approach to accurately diagnose and stage the tumor (29–31). Routine screening and active follow-up should be performed after this procedure to avoid future rumor recurrence or newly developed tumors.
Our results revealed that unilateral oophorectomy can decrease the long-term mortality risk of CVD; this might be a consequence of the stable hormone levels generated from the preservation of part or full ovarian function, while bilateral oophorectomy will lead to a sudden disruption in the secretion of sex hormones, mainly estrogen. In addition to its powerful roles in regulating the development and homeostasis of reproductive tissues, estrogen provides critical signaling and trophic support to a range of tissues throughout the body and across the lifespan through the activation of estrogen receptors, ERα (encoded by ESR1), ERβ (encoded by ESR2), and G-protein-coupled estrogen receptor (GPER; also known as GPR30) (32–35). Estrogens act in target tissues through estrogen receptors and G protein-coupled ERα to reduce CVD risk (33). Premenopausal women are protected from CVD relative to age-matched men (36, 37), and low levels of estrogens (i.e., hypo-estrogenemia) in young women (18–40 years) increase CVD risk (38). Moreover, early menopause (before 40 years of age) (39) is associated with accelerated atherosclerosis, a 2.6-fold increase in CVD risk (40), and an increased risk of CVD-related mortality (41, 42). These studies supported the role of estrogens in determining CVD risk. Therefore, after bilateral oophorectomy, the destruction of ovarian function results in the demand for hormone replacement therapy (HRT), while unilateral oophorectomy, which maintains part or whole of ovarian function, does not need HRT. Furthermore, HRT may be difficult and even dangerous for some women. Endogenous estrogen from the remaining ovary after unilateral oophorectomy eliminates these difficulties and dangers.
This study had several limitations. First, given the study’s descriptive and retrospective design, we could not prospectively assess the effects of surgical interventions in patients with ovarian cancer and could not draw causal inferences. Second, we could not assess the patients’ physical conditions, comorbidities, and other health factors. Given the high incidence of comorbidities, cognitive impairment, frailty, functional losses, social isolation, and other factors in this population, it is important to assess these variables when proposing treatment decisions; however, the SEER program did not provide this information. Third, we could not investigate the influence of other therapies, such as radiotherapy or chemotherapy. The SEER program only provided detailed information on surgical operations.
Notwithstanding these limitations, this study may contribute to the surgical interventions and cancer surveillance literature for ovarian cancer. The strength of this study is that the data were derived from a high-quality, population-based, real-world cancer registry. Real-world data reflect the realistic effects of different interventions in the real scenario of cancer treatment, which may avoid the limitations of clinical trials. The implications of this study are important for the development of ovarian cancer.
Conclusions
In conclusion, unilateral oophorectomy exhibited excellent oncological superiority and was equivalent to bilateral oophorectomy for stage-I ovarian tumors among women of productive age. For women of reproductive age, the criteria for unilateral oophorectomy can be appropriately broadened to high-grade stage-Ic diseases because of the comparable performance of unilateral oophorectomy in this population. For those aged 50 years and older without fertility desire, unilateral oophorectomy is not recommended, and bilateral oophorectomy should be adopted as the first choice. Moreover, unilateral oophorectomy can reduce mortality and CVD risk in women. As unilateral oophorectomy has the advantage of preserving fertility and the hormone secretion function of the ovary, guidance on selecting appropriate candidates should be developed.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: http://www.seer.cancer.gov.
Author Contributions
WZ and KW designed the study. JX, YL, and GF performed the research. ZZ, KW, and WZ developed the typescript. All of the authors approved the typescript and agree to submit for publication.
Funding
This study was supported by National Key Research and Development Program of China (2021YFC2009105) and National Natural Science Foundation of China (82002760).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.866443/full#supplementary-material
References
1. Global Burden of Disease 2019 Cancer Collaboration, Kocarnik JM, Compton K, Dean FE, Weijia F, Brian LG, et al. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol (2021) 8:420–44. doi: 10.1001/jamaoncol.2021.6987
2. American Cancer Society. Key Statistics for Ovarian Cancer . Available at: https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html (Accessed January 1, 2022).
3. The SEER program. Cancer Stat Facts: Ovarian Cancer . Available at: https://seer.cancer.gov/statfacts/html/ovary.html (Accessed January 1, 2022).
4. Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA eds. SEER Cancer Statistics Review, 1975-2018. Bethesda, MD: National Cancer Institute (2021). Available at: https://seer.cancer.gov/csr/1975_2018/.
5. Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. Global Surveillance of Cancer Survival 1995-2009: Analysis of Individual Data for 25,676,887 Patients From 279 Population-Based Registries in 67 Countries (CONCORD-2). Lancet (2015) 385(9972):977–1010. doi: 10.1016/S0140-6736(14)62038-9
6. Nezhat FR, Pejovic T, Finger TN, Khalil SS. Role of Minimally Invasive Surgery in Ovarian Cancer. J Minim Invasive Gynecol (2013) 20(6):754–65. doi: 10.1016/j.jmig.2013.04.027
7. Dewilde K, Moerman P, Leunen K, Amant F, Neven P, Vergote I. Staging With Unilateral Salpingo-Oophorectomy and Expert Pathological Review Result in No Recurrences in a Series of 81 Intestinal-Type Mucinous Borderline Ovarian Tumors. Gynecol Obstet Invest (2018) 83(1):65–9. doi: 10.1159/000478929
8. Tsai HW, Ko CC, Yeh CC, Chen YJ, Twu NF, Chao KC, et al. Unilateral Salpingo-Oophorectomy as Fertility-Sparing Surgery for Borderline Ovarian Tumors. J Chin Med Assoc (2011) 74(6):250–4. doi: 10.1016/j.jcma.2011.04.003
9. Surveillance Research Program. National Cancer Institute SEER*Stat Software Version 8.3.8 . Available at: www.seer.cancer.gov/seerstat.
10. Mathew G, Agha R, Group S. STROCSS 2021: Strengthening the Reporting of Cohort, Cross-Sectional and Case-Control Studies in Surgery. Int J Surg (2021) 96:106165. doi: 10.1016/j.ijsu.2021.106165
11. Surveillance, Epidemiology, and End Results (SEER) Program. SEER*Stat Database. In: Mortality - All COD, Aggregated With State, Total U.S. (1969-2017) <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, Released December 2019. Underlying Mortality Data Provided by NCHS. Bethesda, MD: National Cancer Institute. Available at: www.cdc.gov/nchs. Bethesda, MD National Cancer Institute
12. The SEER program. SEER Combined/AJCC Cancer Staging . Available at: https://seer.cancer.gov/seerstat/variables/seer/ajcc-stage/ (Accessed April 1, 2020).
13. The SEER program. Surgery Codes of Ovary . Available at: https://seer.cancer.gov/manuals/2022/AppendixC/Surgery_Codes_Ovary_2022.pdf (Accessed January 1, 2022).
14. Yang P, Zheng Y, Zhang L, Hou X. Endoscopic Excision for Proximal Colonic Cancer: A Challenge in Transanal Endoscopic Microsurgery - Letter to the Editor. Int J Surg (2021) 92:106035. doi: 10.1016/j.ijsu.2021.106035
15. Zheng Y, Yang P, Chen J, Yu K, Ye Y, Zhang L, et al. Endoscopic Excision as a Viable Alternative to Major Resection for Early Duodenal Cancers: A Population-Based Cohort Study. Int J Surg 106644 (2022) 101:106644. doi: 10.1016/j.ijsu.2022.106644
16. Anderson C, Lund JL, Weaver MA, Wood WA, Olshan AF, Nichols HB. Noncancer Mortality Among Adolescents and Young Adults With Cancer. Cancer (2019) 125(12):2107–14. doi: 10.1002/cncr.32063
17. Zheng Y, Ye Y, Chen L, Ma Z, Liu L, Cheng G, et al. Prevalence and Outcomes of Focal Ablation Versus Prostatectomy for Elderly Patients With Prostate Cancer: A Population-Based Study. J Natl Cancer Center (2021) 2:25–32. doi: 10.1016/j.jncc.2021.11.005
18. Gschwend JE, Dahm P, Fair WR. Disease Specific Survival as Endpoint of Outcome for Bladder Cancer Patients Following Radical Cystectomy. Eur Urol (2002) 41(4):440–8. doi: 10.1016/S0302-2838(02)00060-X
19. R Core Team. R: A Language and Environment for Statistical Computing (2019). Vienna, Austria: R Foundation for Statistical Computing. Available at: https://www.R-project.org/ (Accessed December 12, 2019).
20. Martinez A, Poilblanc M, Ferron G, De Cuypere M, Jouve E, Querleu D. Fertility-Preserving Surgical Procedures, Techniques. Best Pract Res Clin Obstet Gynaecol (2012) 26(3):407–24. doi: 10.1016/j.bpobgyn.2012.01.009
21. Vasconcelos I, de Sousa Mendes M. Conservative Surgery in Ovarian Borderline Tumours: A Meta-Analysis With Emphasis on Recurrence Risk. Eur J Cancer (2015) 51(5):620–31. doi: 10.1016/j.ejca.2015.01.004
22. Morice P, Leblanc E, Rey A, Baron M, Querleu D, Blanchot J, et al. Gcclcc and Sfog: Conservative Treatment in Epithelial Ovarian Cancer: Results of a Multicentre Study of the GCCLCC (Groupe Des Chirurgiens De Centre De Lutte Contre Le Cancer) and SFOG (Societe Francaise D'oncologie Gynecologique). Hum Reprod (2005) 20(5):1379–85. doi: 10.1093/humrep/deh777
23. Bogani G, Ditto A, Pinelli C, Lopez S, Chiappa V, Raspagliesi F. Ten-Year Follow-Up Study of Long-Term Outcomes After Conservative Surgery for Early-Stage Ovarian Cancer. Int J Gynaecol Obstet (2020) 150(2):169–76. doi: 10.1002/ijgo.13199
24. Ditto A, Martinelli F, Bogani G, Lorusso D, Carcangiu M, Chiappa V, et al. Long-Term Safety of Fertility Sparing Surgery in Early Stage Ovarian Cancer: Comparison to Standard Radical Surgical Procedures. Gynecol Oncol (2015) 138(1):78–82. doi: 10.1016/j.ygyno.2015.05.004
25. Ditto A, Leone Roberti Maggiore U, Bogani G, Martinelli F, Chiappa V, Evangelista MT, et al. Predictive Factors of Recurrence in Patients With Early-Stage Epithelial Ovarian Cancer. Int J Gynaecol Obstet (2019) 145(1):28–33. doi: 10.1002/ijgo.12769
26. Park JY, Kim DY, Suh DS, Kim JH, Kim YM, Kim YT, et al. Outcomes of Fertility-Sparing Surgery for Invasive Epithelial Ovarian Cancer: Oncologic Safety and Reproductive Outcomes. Gynecol Oncol (2008) 110(3):345–53. doi: 10.1016/j.ygyno.2008.04.040
27. Bogani G, Ditto A, Lopez S, Bertolina F, Murgia F, Pinelli C, et al. Adjuvant Chemotherapy vs. Observation in Stage I Clear Cell Ovarian Carcinoma: A Systematic Review and Meta-Analysis. Gynecol Oncol (2020) 157(1):293–8. doi: 10.1016/j.ygyno.2019.12.045
28. Bogani G, Tagliabue E, Ditto A, Signorelli M, Martinelli F, Casarin J, et al. Assessing the Risk of Pelvic and Para-Aortic Nodal Involvement in Apparent Early-Stage Ovarian Cancer: A Predictors- and Nomogram-Based Analyses. Gynecol Oncol (2017) 147(1):61–5. doi: 10.1016/j.ygyno.2017.07.139
29. Bogani G, Borghi C, Leone Roberti Maggiore U, Ditto A, Signorelli M, Martinelli F, et al. Minimally Invasive Surgical Staging in Early-Stage Ovarian Carcinoma: A Systematic Review and Meta-Analysis. J Minim Invasive Gynecol (2017) 24(4):552–62. doi: 10.1016/j.jmig.2017.02.013
30. Ran X, He X, Li Z. Comparison of Laparoscopic and Open Surgery for Women With Early-Stage Epithelial Ovarian Cancer. Front Oncol (2022) 12:879889. doi: 10.3389/fonc.2022.879889
31. Tinelli R, Dellino M, Nappi L, Sorrentino F, D'Alterio MN, Angioni S, et al. Left External Iliac Vein Injury During Laparoscopic Pelvic Lymphadenectomy for Early-Stage Ovarian Cancer: Our Experience and Review of Literature. Front Surg (2022) 9:843641. doi: 10.3389/fsurg.2022.843641
32. Jia M, Dahlman-Wright K, Gustafsson JA. Estrogen Receptor Alpha and Beta in Health and Disease. Best Pract Res Clin Endocrinol Metab (2015) 29(4):557–68. doi: 10.1016/j.beem.2015.04.008
33. Morselli E, Santos RS, Criollo A, Nelson MD, Palmer BF, Clegg DJ. The Effects of Oestrogens and Their Receptors on Cardiometabolic Health. Nat Rev Endocrinol (2017) 13(6):352–64. doi: 10.1038/nrendo.2017.12
34. Prossnitz ER, Barton M. The G-Protein-Coupled Estrogen Receptor GPER in Health and Disease. Nat Rev Endocrinol (2011) 7(12):715–26. doi: 10.1038/nrendo.2011.122
35. Vrtacnik P, Ostanek B, Mencej-Bedrac S, Marc J. The Many Faces of Estrogen Signaling. Biochem Med (Zagreb) (2014) 24(3):329–42. doi: 10.11613/BM.2014.035
36. Yang XP, Reckelhoff JF. Estrogen, Hormonal Replacement Therapy and Cardiovascular Disease. Curr Opin Nephrol Hypertens (2011) 20(2):133–8. doi: 10.1097/MNH.0b013e3283431921
37. Anand SS, Islam S, Rosengren A, Franzosi MG, Steyn K, Yusufali AH, et al. Risk Factors for Myocardial Infarction in Women and Men: Insights From the INTERHEART Study. Eur Heart J (2008) 29(7):932–40. doi: 10.1093/eurheartj/ehn018
38. Kaplan JR, Manuck SB. Ovarian Dysfunction and the Premenopausal Origins of Coronary Heart Disease. Menopause (2008) 15(4 Pt 1):768–76. doi: 10.1097/gme.0b013e31815eb18e
39. Archer DF. Premature Menopause Increases Cardiovascular Risk. Climacteric (2009) 12 Suppl:1, 26–31. doi: 10.1080/13697130903013452
40. Kannel WB, Wilson PW. Risk Factors That Attenuate the Female Coronary Disease Advantage. Arch Intern Med (1995) 155(1):57–61. doi: 10.1001/archinte.1995.00430010063008
41. Jacobsen BK, Nilssen S, Heuch I, Kvale G. Does Age at Natural Menopause Affect Mortality From Ischemic Heart Disease? J Clin Epidemiol (1997) 50(4):475–9. doi: 10.1016/s0895-4356(96)00425-8
Keywords: ovarian cancer, prevalence, outcomes, population-based study, SEER, unilateral oophorectomy, bilateral oophorectomy
Citation: Xiong J, Zhang Z, Liu Y, Fan G, Wu K and Zhang W (2022) Prevalence and Outcomes of Unilateral Versus Bilateral Oophorectomy in Women With Ovarian Cancer: A Population-Based Study. Front. Oncol. 12:866443. doi: 10.3389/fonc.2022.866443
Received: 31 January 2022; Accepted: 16 June 2022;
Published: 08 July 2022.
Edited by:
Sanja Štifter, Skejby Sygehus, DenmarkReviewed by:
Anna Myriam Perrone, Sant’Orsola-Malpighi Polyclinic, ItalyGiorgio Bogani, National Cancer Institute Foundation (IRCCS), Italy
Copyright © 2022 Xiong, Zhang, Liu, Fan, Wu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Zhang, enc2Njc2QDE2My5jb20=; Kejia Wu, d2R6bjIwMTlAMTYzLmNvbQ==
†These authors share first authorship
 Jiaqiang Xiong
Jiaqiang Xiong Zhuoqun Zhang2†
Zhuoqun Zhang2† Yanyan Liu
Yanyan Liu