- 1University of Pittsburgh Medical Center Hillman Cancer Center, University of Pittsburgh Medical Center (UPMC), School of Medicine, Pittsburgh, PA, United States
- 2Medical Pfizer Inc, New York, NY, United States
- 3Department of Breast Medical Oncology The University of Texas MD Anderson Cancer Center, Houston, TX, United States
Background: Cyclin-dependent kinase 4/6 inhibitors are a standard treatment for patients with hormone receptor−positive (HR+)/human epidermal growth factor receptor 2−negative (HER2−) metastatic breast cancer (MBC). However, real-world data on effectiveness in patients with liver or lung metastatic disease is limited. This study compared outcomes of palbociclib plus letrozole versus letrozole alone in patients with HR+/HER2− MBC with lung or liver metastasis treated in routine clinical practice in the United States.
Methods: This retrospective analysis used Flatiron Health’s database of electronic health records. Women with HR+/HER2− MBC and liver or lung metastasis received first-line palbociclib plus letrozole or letrozole alone between February 2015 and February 2019. Real-world progression-free survival (rwPFS) was defined as time from start of treatment to death or disease progression. Stabilized inverse probability treatment weighting (sIPTW) was used to balance baseline demographic and clinical characteristics between palbociclib plus letrozole versus letrozole cohorts. Cox proportional-hazards models were used to estimate the effectiveness of palbociclib plus letrozole versus letrozole alone in rwPFS and overall survival (OS).
Results: The study included 353 patients with lung metastasis, 123 with liver metastasis, and 75 with both. After sIPTW, palbociclib plus letrozole versus letrozole alone was significantlly associated with prolonged rwPFS (hazard ratio (HR), 0.56) and OS (HR, 0.58) (both p<0.001) in all patients. Palbociclib plus letrozole compared with letrozole alone demonstrated a median rwPFS of 16.5 versus 10.5 months, respectively (adjusted HR, 0.52; P<0.001), a median OS of not reached versus 40.3 months (adjusted HR, 0.60; P<0.01) in patients with lung metastasis, and median OS of 30.1 versus 16.8 months (adjusted HR, 0.56; P<0.03 in patients with liver metastasis. In patients with liver metastasis, palbociclib plus letrozole had a median rwPFS of 10.7 months versus 8.0 months in the letrozole alone cohort (adjusted HR, 0.70; P=0.12).
Conclusions: In this real-world population, palbociclib in combination with letrozole is associated with improved outcomes compared with letrozole alone for patients with HR+/HER2− MBC and liver or lung metastasis in the first-line setting. The findings support first-line palbociclib in combination with an aromatase inhibitor as standard of care for HR+/HER2− MBC regardless of visceral disease.
Clinical Trial Registration: NCT04176354.
Introduction
Breast cancer is the most common malignancy in women, accounting for more than 43,000 deaths in the United States in 2021 (1). Approximately 6% of patients with breast cancer are initially diagnosed with metastatic disease and, of patients who are diagnosed with early-stage disease, about 30% will go on to develop metastatic breast cancer (MBC). Despite improvements in treatment, MBC has a poor prognosis and an overall 5-year survival rate of only 27% for patients in the United States (2).
Hormone receptor-positive (HR+) breast cancer, which accounts for approximately 70% of all breast cancers, most commonly metastasizes to the bone, lung, and liver, with <10% metastasizing to the brain and other tissues (3, 4). An analysis of more than 9500 patients with HR+/human epidermal growth factor receptor 2−negative (HER2−) MBC found that patients with brain and liver metastasis had significantly lower overall survival (OS) compared with those with lung metastasis, whereas patients with bone metastasis exhibited the most favorable OS (3).
Endocrine-based therapy (ET) is recommended as a first-line treatment for patients with HR+ advanced breast cancer (5). A recent meta-analysis of patients with HR+/HER2− advanced breast cancer receiving ET (either an aromatase inhibitor, fulvestrant, or tamoxifen) found that progression-free survival (PFS) and OS were significantly longer in patients with non-visceral versus visceral metastatic disease (6). Furthermore, patients with liver metastasis had a significantly shorter PFS and OS than patients with non-liver visceral metastasis. Similarly, in a single-center study of patients with HR+/HER2− MBC receiving fulvestrant, PFS was nearly the same in patients with non-visceral metastasis and lung metastasis (without liver metastasis) whereas those with liver metastasis had significantly worse PFS (7). These findings demonstrate the heterogeneity in MBC with respect to response to therapy and highlight the difficulty in treating patients with liver metastasis.
Cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) in combination with ET have become the standard of care for HR+/HER2− MBC (8). The safety and efficacy of palbociclib, the first-in-class CDK4/6i inhibitor, in combination with letrozole as a first-line treatment for patients with estrogen receptor–positive/HER2− MBC were demonstrated in the phase 2 PALOMA-1 trial and subsequently validated in the phase 3 PALOMA-2 trial (9, 10). A subgroup analysis of patients in PALOMA-2 demonstrated that palbociclib in combination with letrozole provided a significant PFS benefit compared with letrozole in combination with placebo in those with visceral and non-visceral disease (10). A recent retrospective analysis of a real-world population of patients with HR+/HER2− MBC with visceral crisis found that patients receiving CDK4/6i had a 5-month improvement in OS compared with those receiving chemotherapy, consistent with CDK4/6i providing benefit in patients with MBC with visceral disease (11).
Observational real-world studies complement findings from clinical trials, providing important evidence demonstrating a therapy’s efficacy in populations that are more heterogeneous than those in clinical trials (12). An understanding of real-world treatment practices and how they affect the efficacy of new therapies can also help guide clinicians on optimal drug use and indications (13). In this study, we evaluated the efficacy of palbociclib in combination with letrozole as a first-line treatment in patients with HR+/HER2− MBC with lung or liver metastasis in a real-world setting.
Materials and Methods
Database
This was a retrospective analysis of electronic health records (EHRs) from the Flatiron Health’s analytic longitudinal database. The Flatiron database undergoes rigorous data curation and abstraction and is regarded as one of the industry’s foremost oncology databases (14). It includes de-identified structured and unstructured EHRs from more than 280 cancer clinics, including approximately 800 sites of care, and represents 2.4 million patients with cancer actively being treated in the United States. This database has been validated and has been widely used for multiple real-world studies in cancer, including breast cancer (15–17).
Patients
Patients included in the analysis were female and ≥18 years old, were diagnosed with HR+/HER2− MBC with liver or lung involvement, and initiated palbociclib plus letrozole or letrozole alone in the first-line setting between February 2015 and February 2019. The study data cutoff was May 31, 2019, to allow a potential minimum follow-up of 3 months from the date of palbociclib plus letrozole or letrozole initiation (ie, index date). Patients were excluded if they had previously been treated with a CDK4/6i, an aromatase inhibitor, tamoxifen, raloxifene, toremifene, or fulvestrant for MBC or with a CDK4/6i as part of a clinical trial. Only patients whose first structured activity was ≤90 days after their MBC diagnosis date were included to exclude patients who might have been treated for their MBC. Patients were followed up from the start of treatment with palbociclib plus letrozole or letrozole alone to death, the last visit, or study end, whichever came first. This retrospective deidentified database analysis was exempt from institutional review board approval and included a waiver of informed consent.
Outcomes
Real-world PFS (rwPFS) was defined as the time from the start of treatment to death or disease progression, whichever came first, as described previously (18). Disease progression was determined by the recorded assessment of the treating clinician based on radiology, laboratory evidence, pathology, or clinical evaluation. Patients who did not die or experience disease progression were censored at the date of initiation of the next line of therapy (for patients with ≥2 lines of therapy) or their last visit during the study period (for patients with only 1 line of therapy). OS was defined as the length of time from the start of the first line of therapy to the date of death due to any cause, as previously described (18). The date of death was determined by Flatiron based on a recent mortality dataset generated by combining multiple data sources and benchmarked against the National Death Index. If a patient did not die during the study period, they were censored at the end of study.
Statistical Analyses
Descriptive statistics were used for demographic and clinical characteristics. Follow-up duration was defined as the time from the start of treatment with palbociclib plus letrozole or letrozole alone to death, the last visit, or study end, whichever came first.
Stabilized inverse probability treatment weighting (sIPTW) and propensity score matching (PSM) methods were used to balance baseline demographic and clinical characteristics between comparison cohorts (palbociclib plus letrozole vs letrozole alone), as previously described (18). PSM was conducted as a sensitivity analysis to assess the robustness of the sIPTW results. Propensity scores were generated using a multivariable binomial logistic regression model with age group, number of metastatic sites, practice type, race, disease stage, Eastern Cooperative Oncology Group (ECOG) performance status, and cancer type (de novo vs recurrent) as covariates (19). Matches were made using 1:1 nearest neighbor matching without replacement.
The Kaplan-Meier method was used to estimate medians and 95% CIs and for landmark analyses for rwPFS and OS. To compare rwPFS and OS between treatment groups, univariate and multivariate Cox proportional hazards models with a robust sandwich estimator were used for patients with lung and liver metastasis, respectively. In multivariate analyses, models included age group, number of metastatic sites, practice type, race, disease stage, ECOG performance status, and cancer type (de novo vs recurrent).
Chi-squared test was performed to compare disease progression during the second-line treatment in patients who were initially treated with palbociclib plus letrozole versus letrozole alone.
Results
Patients
A total of 551 eligible patients, 353 (64.1%) with lung metastasis, 123 (22.3%) with liver metastasis, and 75 (13.6%) with both lung and liver metastases, were included in the analysis. Of these patients, 330 (59.9%) initiated first-line therapy with palbociclib plus letrozole and 221 (40.1%) with letrozole alone. The median (interquartile range) follow-up was 22.6 (17.3) and 22.1 (21.5) months for patients receiving palbociclib plus letrozole and letrozole, respectively. Demographic and clinical characteristics are shown in Table 1. Across both treatment groups, approximately 95% of patients were from a community setting and about 4.0% from an academic setting. Compared with the letrozole group, patients in the palbociclib plus letrozole group tended to be younger, were less likely to be Black, and were more likely to have a better ECOG performance status and ≥3 metastatic sites. A small percentage of patients, 4.2% in the palbociclib plus letrozole group and 6.3% in the letrozole group, also had brain metastases. Patient characteristics were generally balanced after sIPTW and propensity score matching (Table 1).
rwPFS and OS in Patients With Lung or Liver Metastasis
Evaluation of landmark rwPFS demonstrated that the percentage of patients with rwPFS at 6 months was higher in the palbociclib plus letrozole group (76.2%) versus the letrozole group (63.2%), and this benefit was maintained at 24 months (38.5% vs 22.4%).
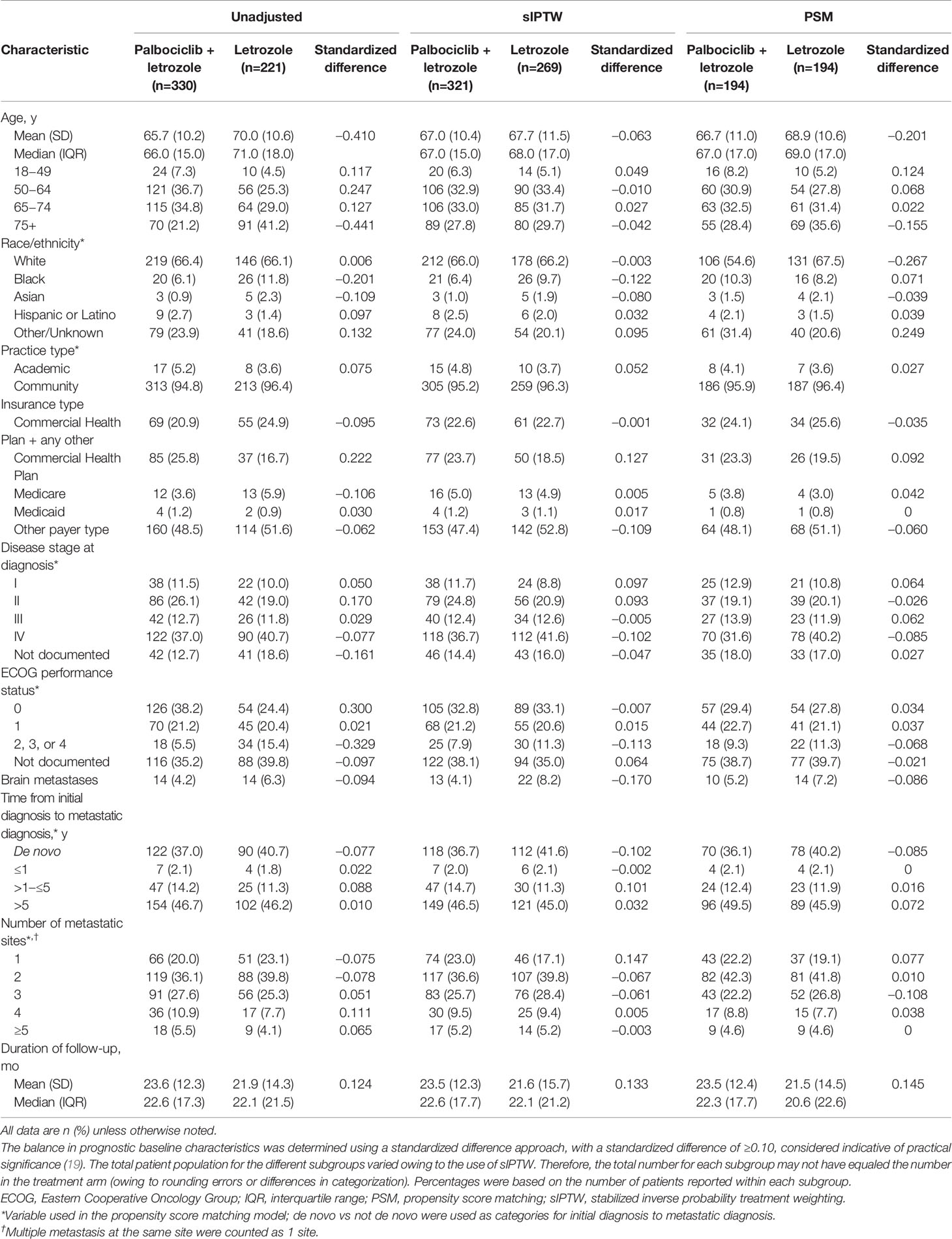
In the unadjusted analysis of patients with visceral metastasis, median rwPFS was significantly longer among those in the palbociclib plus letrozole group versus the letrozole group (15.4 [95% CI, 12.5–19.5] months vs 10.2 [95% CI, 8.0–11.7] months; hazard ratio, 0.60 [95% CI, 0.49–0.74]; P<0.001). In the sIPTW-adjusted analysis of patients with visceral metastasis, median rwPFS was significantly longer among those in the palbociclib plus letrozole group versus the letrozole group (16.1 [95% CI, 13.0–20.2] months vs 9.6 [95% CI, 7.2–11.0] months; hazard ratio, 0.56 [95% CI, 0.45–0.69]; P<0.001; Figure 1A). In a sensitivity analysis using the PSM method, median rwPFS was also significantly longer among those in the palbociclib plus letrozole group versus the letrozole group (15.7 [95% CI, 12.7–20.2] months vs 9.5 [95% CI, 6.7–10.8] months; hazard ratio, 0.57 [95% CI, 0.44–0.72]; P<0.001; Figure 1B)
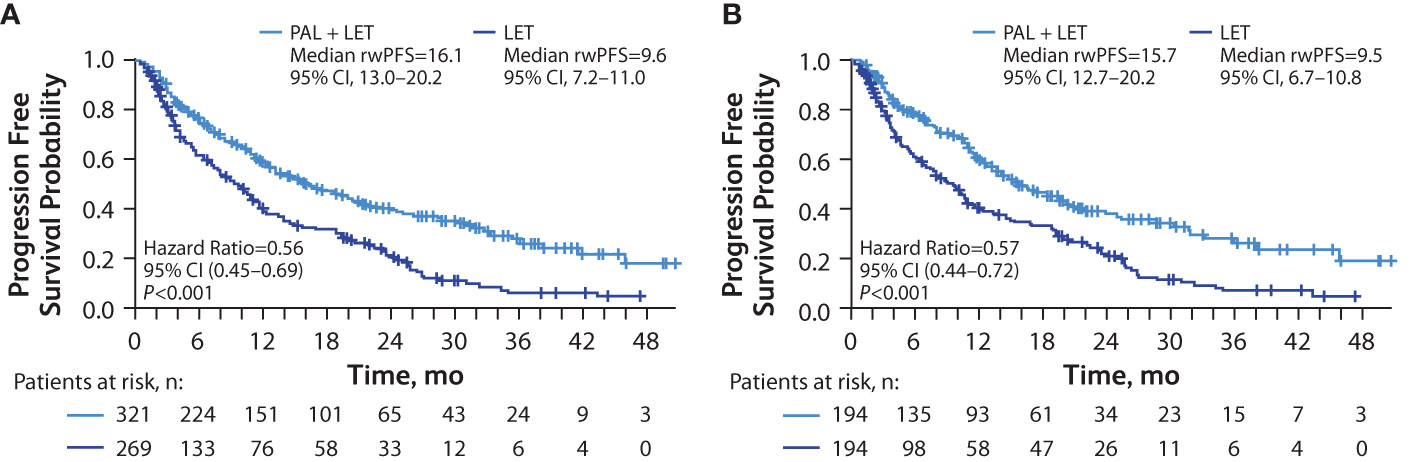
Figure 1 Kaplan-Meier curves of real-world progression-free survival in patients with visceral (lung and/or liver) metastasis. sIPTW analysis (A) and PSM analysis (B); number of patients at risk are shown. LET, letrozole; PAL, palbociclib; PSM, propensity score matching; rwPFS, real-world progression-free survival; sIPTW, stabilized inverse probability treatment weighting.
The percentage of patients with OS at 12 months was higher in the palbociclib plus letrozole group (89.4%) versus the letrozole group (75.0%), and this benefit was maintained at 36 months (59.7% vs 44.5%).
Among the patients with visceral metastasis, the median OS was significantly longer in the palbociclib plus letrozole group versus the letrozole group in the unadjusted analysis (not reached [NR; 95% CI, 38.3–NR] months vs 29.4 [95% CI, 25.8–40.8] months; hazard ratio, 0.56 [95% CI, 0.43–0.74]; P<0.001) and in the sIPTW-adjusted analysis (NR [95% CI, 38.3–NR] months vs 32.4 [95% CI, 26.0–40.8] months; hazard ratio, 0.58 [95% CI, 0.43–0.77]; P<0.001; Figure 2A). In a sensitivity analysis using the PSM method, median OS was also significantly longer among those in the palbociclib plus letrozole group versus the letrozole group (NR [95% CI, 42.7–NR] months vs 29.1 [95% CI, 25.3–40.8] months; hazard ratio, 0.53 [95% CI, 0.39–0.74]; P<0.001; Figure 2B)
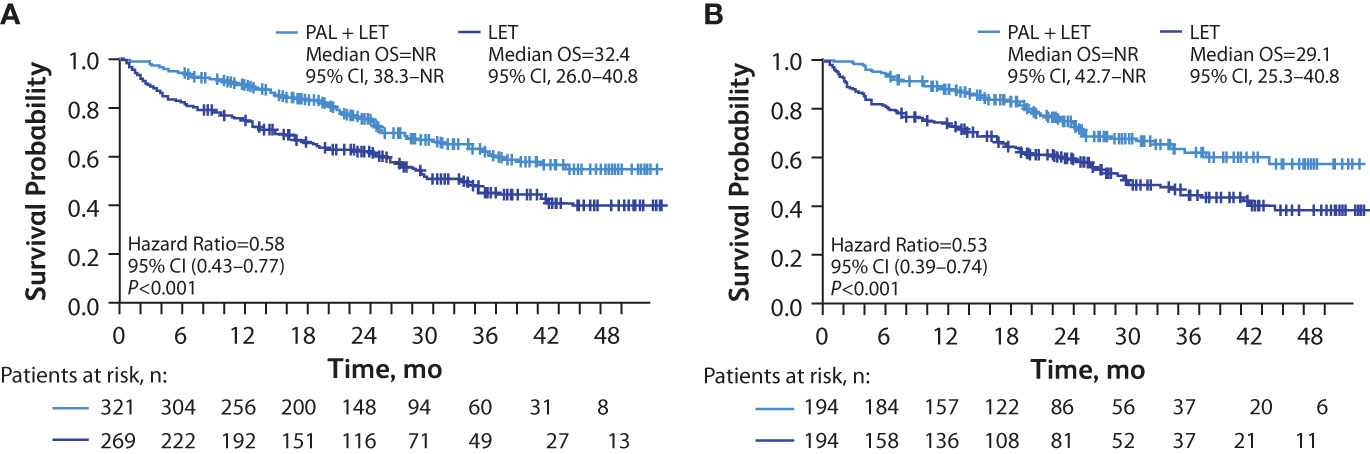
Figure 2 Kaplan-Meier curves of overall survival in patients with visceral (lung and/or liver) metastasis. sIPTW analysis (A) and PSM analysis (B); number of patients at risk are shown. LET, letrozole; NR, not reached; OS, overall survival; PAL, palbociclib; PSM, propensity score matching; sIPTW, stabilized inverse probability treatment weighting.
Outcomes in Patients With Lung Metastasis
In patients with lung metastasis, median rwPFS was significantly longer among those in the palbociclib plus letrozole group versus the letrozole group in unadjusted analysis (16.5 [95% CI, 14.0–21.9] months vs 10.5 [95% CI, 8.0–12.3] months; hazard ratio, 0.58 [95% CI, 0.45–0.74]; P<0.001) and remained significantly longer among those in the palbociclib plus letrozole group versus the letrozole group after adjusting for baseline covariates hazard ratio, 0.52 [95% CI, 0.40–0.69]; P<0.001; Figure 3A). Median OS was also significantly longer among those in the palbociclib plus letrozole group versus the letrozole group in unadjusted analysis (NR [95% CI, NR–NR] vs 40.3 [95% CI, 29.0–NR] months; hazard ratio, 0.58 [95% CI, 0.41–0.82]; P<0.01 and in the multivariate analysis hazard ratio, 0.60 [95% CI, 0.41–0.88]; P<0.01; Figure 3B).
Figure 3 Kaplan-Meier curves of real-world progression-free survival and overall survival in patients with lung metastasis. Analysis of rwPFS (A) and OS (B) adjusted for demographic and clinical variables; number of patients at risk are shown. LET, letrozole; NR, not reached; OS, overall survival; PAL, palbociclib; rwPFS, real-world progression-free survival. *Hazard ratios were estimated by multivariate Cox regression models adjusted for baseline demographic and clinical variables.
Outcomes in Patients With Liver Metastasis
In patients with liver metastasis, median rwPFS was significantly longer among those in the palbociclib plus letrozole group versus the letrozole group in unadjusted analysis (10.7 [95% CI, 7.9–12.7] months vs 8.0 [95% CI, 4.5–10.7] months; hazard ratio, 0.71 [95% CI, 0.51–0.99]; P=0.04). After adjusting for baseline covariates, the median rwPFS remained longer among those in the palbociclib plus letrozole group versus the letrozole group hazard ratio, 0.70 [95% CI, 0.44–1.10]; P=0.12; Figure 4A), although the analysis did not reach statistical significance.
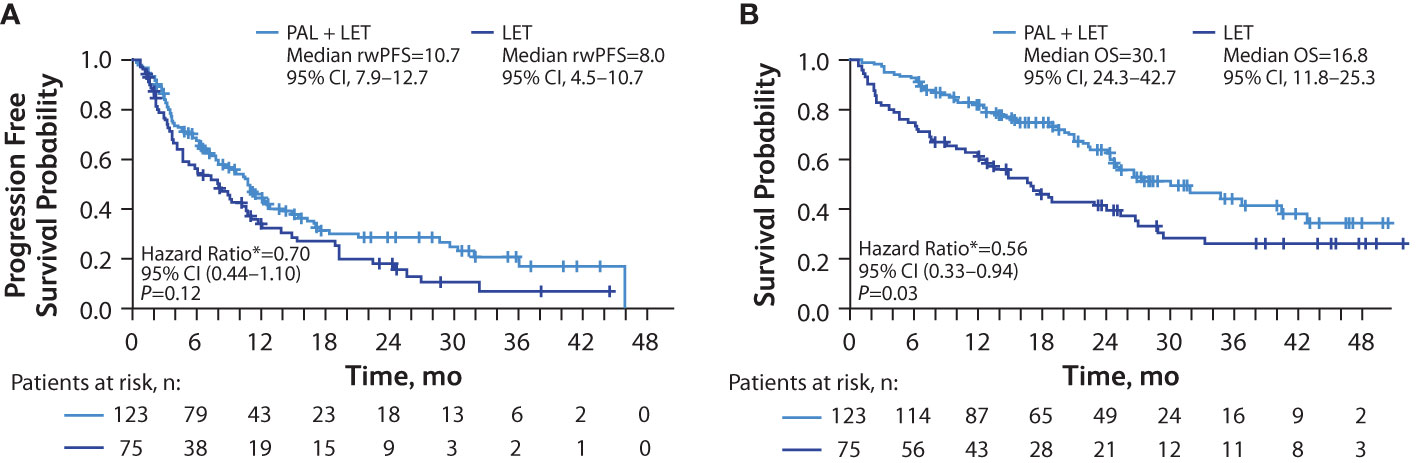
Figure 4 Kaplan-Meier curves of real-world progression-free survival and overall survival in patients with liver metastasis. Analysis of rwPFS (A) and OS (B) adjusted for demographic and clinical variables; number of patients at risk are shown. LET, letrozole; OS, overall survival; PAL, palbociclib; rwPFS, real-world progression-free survival. *Hazard ratios were estimated by multivariate Cox regression models adjusted for baseline demographic and clinical variables.
Median OS was significantly longer among those in the palbociclib plus letrozole group versus the letrozole group in the unadjusted analysis (30.1 [95% CI, 24.3–42.7] months vs 16.8 [95% CI, 11.8–25.3] months; hazard ratio, 0.55 [95% CI, 0.37–0.81]; P<0.01 and remained significantly longer among those in the palbociclib plus letrozole group versus the letrozole group in the multivariate analysis hazard ratio, 0.56 [95% CI, 0.33–0.94]; P=0.03; Figure 4B).
Subsequent Second-Line Anticancer Treatments and Disease Progression During the Second-Line Treatment
Table 2 shows treatments following first-line treatment and disease progression among patients receiving second-line treatment. Among patients with lung metastasis, 51.4% in the palbociclib plus letrozole group and 60.8% in the letrozole group had second line treatment. Of the patients in the palbociclib plus letrozole group, 36.4% and 28.0% received a CDK4/6i and chemotherapy, respectively, as a second-line treatment. Of the patients in the letrozole group, 60.6% and 13.5% received a CDK4/6i and chemotherapy, respectively, as a second-line treatment. Among the patients with lung metastasis that were being treated in the second line, there was no significant difference in the proportion of patients that experienced disease progression between the palbociclib plus letrozole group and the letrozole group (52.3% vs 45.2%, χ2 = 1.17; P=0.28).
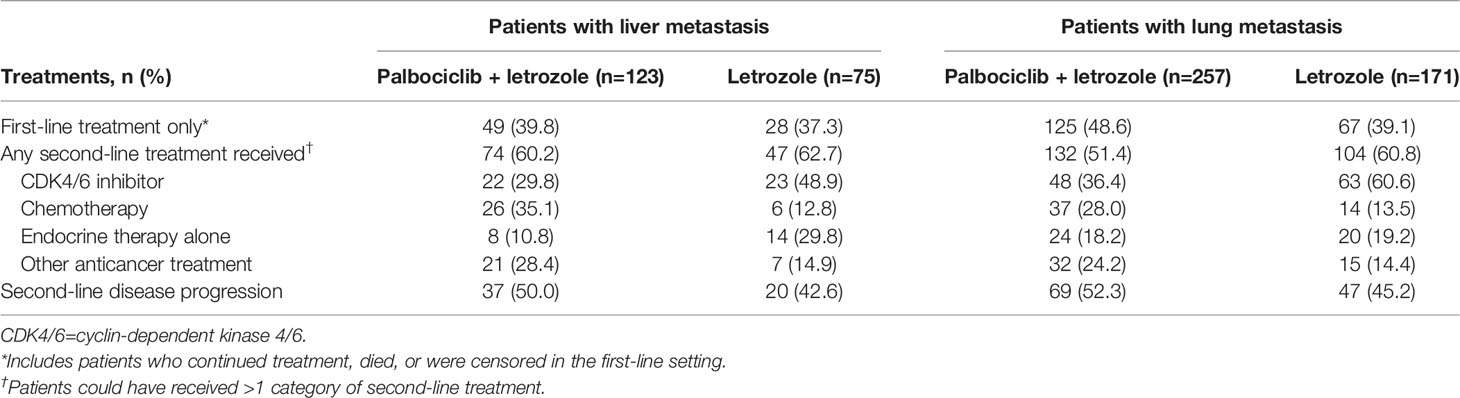
Table 2 Subsequent second-line anticancer treatments and disease progression in the second-line treatment.
Among patients with liver metastasis, 60.2% in the palbociclib plus letrozole group and 62.7% in the letrozole group had second-line treatment. Of the patients in the palbociclib plus letrozole group, 29.8% and 35.1% received a CDK4/6i and chemotherapy, respectively, as a second-line treatment. Of the patients in the letrozole group, 48.9% and 12.8% received a CDK4/6i and chemotherapy, respectively, as a second line treatment. Among the patients with liver metastasis that were being treated in the second line, there was no significant difference in disease progression between patients in the palbociclib plus letrozole group and the letrozole group (50.0% vs 42.6%, χ2 = 0.64; P=0.42).
Discussion
Lung and liver are common sites of metastasis in HR+/HER2− MBC (4). Visceral involvement at these sites has been associated with poorer prognosis, and metastatic liver disease has been shown to be particularly difficult to treat (3, 6). Previous subgroup analysis of patients in the PALOMA-2 trial showed that first-line palbociclib in combination with letrozole provided a PFS benefit compared with letrozole alone in patients with visceral and nonvisceral disease (10). However, real-world data on the efficacy of CDK4/6i in patients with lung and liver metastasis are scarce. This study demonstrates in a real-world population that first-line palbociclib in combination with letrozole provided a significant benefit in rwPFS and OS compared with letrozole alone in patients with lung or liver metastasis after adjusting for important demographic and clinicopathologic factors.
Subgroup exploratory analyses in clinical trials have shown that a CDK4/6i prolongs PFS in patients with HR+/HER2− advanced breast cancer and visceral metastasis. The MONALEESA-7 and MONALEESA-2 trials of pre/perimenopausal and postmenopausal women, respectively, with HR+/HER2− advanced breast cancer and visceral metastasis have shown that ribociclib in combination with ET provided a significant PFS benefit compared with placebo in combination with ET (20, 21). A pooled analysis of 7 phase 3 trials in patients with HR+/HER2− advanced breast cancer receiving first-line CDK4/6i (N=1111) demonstrated that CDK4/6i plus ET provided greater PFS benefit compared with placebo plus ET in patients with visceral metastasis, with a hazard ratio similar to those in the broader intended-use population (22). In our real-world population of patients with visceral metastasis (lung and/or liver), first-line palbociclib in combination with letrozole significantly prolonged rwPFS compared with letrozole alone. When analysis was restricted to patients with lung metastasis, palbociclib in combination with letrozole also significantly prolonged rwPFS compared with letrozole alone.
Previous studies have also provided evidence that treatment with CDK4/6i prolongs OS in patients with visceral metastasis. A subgroup analysis of patients with pre/perimenopausal HR+/HER2− advanced breast cancer with liver or lung metastasis who enrolled in the MONALEESA-7 trial demonstrated that ribociclib in combination with ET provided an OS benefit compared with placebo plus ET, although the results did not reach statistical significance (23). A large meta-analysis of HR+/HER2− MBC patients from the MONALEESA-3 and -7, MONARCH 2 and PALOMA 3 trials (N=1390) demonstrated that CDK4/6i in combination with ET (first or laterr lines), compared with ET alone, significantly prolonged OS in subgroups of patients with and without visceral involvement (24). A recent retrospective analysis of a real-world population of patients with HR+/HER2− MBC evaluated OS in patients with visceral crisis at diagnosis (N=336) (11). Patients receiving CDK4/6i had a 5-month improvement in OS compared with those receiving chemotherapy (11). In this real-world study, first-line palbociclib in combination with letrozole also significantly prolonged OS in patients with visceral metastasis (lung and/or liver) and patients with lung metastasis compared with letrozole alone.
Compared with patients with HR+ MBC and visceral non-liver metastasis or lung metastasis, patients with liver metastasis have been shown to respond poorly to treatment with ET, underscoring the more aggressive course of disease in patients with liver involvement (6, 7). A recent subgroup analysis of patients with HR+/HER2− MBC and liver metastasis enrolled in the MONARCH trials demonstrated that abemaciclib in combination with ET as first-line treatment was shown to provide a substantial benefit over ET alone, characterized by significantly prolonged PFS (25). In our population of patients with liver metastasis, palbociclib in combination with letrozole was associated with a significant benefit over letrozole alone in rwPFS. After adjusting for covariates, the benefit of palbociclib in combination with letrozole over letrozole alone remained (hazard ratio = 0.70, palbociclib plus letrozole vs letrozole), although the results did not reach statistical significance. The small sample size of patients with liver metastases (n=198 [palbociclib + letrozole, n=123; letrozole alone, n=75]) makes this analysis difficult to interpret. However, in the OS analysis of patients with liver metastasis, the addition of palbociclib to letrozole significantly lengthened OS compared with letrozole alone, even after adjustment for covariates.
Our findings in this real-world population are consistent with findings from clinical trials, and demonstrate that palbociclib in combination with ET as first line treatment is effective in patients with more difficult to treat lung and liver metastatic disease. Recently, it was demonstrated that in a real-world population of HR+/HER2− advanced breast cancer patients receiving CDK4/6i plus ET in the first line, the proliferative index marker Ki67 was significantly inversely correlated with PFS, suggesting that this may be a marker of CDK4/6i resistance (26). In addition, a recent gene expression analysis of tumors from patients in two neoadjuvant trials of CDK4/6i plus ET found that tumors from patients that exhibited intrinsic resistance to CDK4/6i were highly enriched in interferon-related signatures (27). Such findings suggest that future clinical studies evaluating the Ki67 index and interferon signaling as biomarkers of CDK4/6i resistance are warranted.
By the study cutoff date, 40%-50% of patients remained on first-line treatment. Among patients who received second-line treatment, CDK4/6is were more commonly used in the letrozole alone cohort than in the palbociclib plus letrozole cohort. However, no significant difference in disease progression during second-line treatment was observed between patients who were initially treated with palbociclib plus letrozole versus letrozole alone. Further research with large sample sizes is warranted to demonstrate the effects of subsequent treatments following first-line CDK4/6i treatment on disease progression and OS.
Although the large size and geographic distribution of the Flatiron database is a strength of this study, there are inherent limitations to retrospective analysis of real-world data. The quality of information extracted from the EHR depends on the quality of information entered by the clinician, and there is a potential for missing or incomplete data.
Unobserved variables cannot be completely addressed through multivariate analysis; however, our analyses did adjust for known clinical confounders that were most likely to affect the outcomes of the study. Sample sizes for subgroup analyses, especially for patients with liver metastasis, are small for robust statistical tests. Also, for OS analysis, the median OS was reached in the letrozole alone group; although significant censoring in the OS analysis indicates the need for subsequent evaluation with longer follow-up. The greater generalizability of the findings may be limited because patients in the Flatiron database may not be reflective of the general population of patients with MBC. Also, rwPFS determination was not based on Response Evaluation Criteria in Solid Tumors (RECIST) criteria and was limited by each clinician’s interpretation and documentation of tumor responses. Furthermore, no causal relationship could be made from the retrpospective database analysis.
Conclusions
This comparative analysis provides evidence that addition of palbociclib to letrozole as first-line therapy significantly improves outcomes for patients with HR+/HER2− MBC with lung or liver metastasis in routine clinical practice. These findings are consistent with subgroup analyses in patients with visceral metastasis from pivotal clinical trials and will better inform clinicians on appropriate therapeutic strategies for patients with poor prognostic characteristics, such as liver metastasis. Further comparative effectiveness research of CDK4/6i combined with ET involving more patients and longer follow-up is warranted in patients with MBC with various visceral metastases.
Data Availability Statement
The data analyzed in this study are subject to the following licenses/restrictions: Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions and exceptions, Pfizer may also provide access to the related individual de-identified participant data. Requests to access these datasets should be directed to https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.
Ethics Statement
The study was retrospective, non-interventional, and used anonymized data provided by Flatiron Health. The Flatiron database is covered under the Health Insurance Portability and Accountability Act of 1996 (HIPAA) through Business Associate Agreements with every provider in the Flatiron network. Ethical approval was not provided for this study on human participants because this study is exempt from institutional review board approval and included a waiver of informed consent. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
Pfizer Inc (NCT04176354).
Conflict of Interest
AB, Consulting fees or honorarium from Pfizer Inc, AstraZeneca, Lilly, Novartis, and Sanofi. XL, BL, and LM, Pfizer employee and Pfizer stockholder. RL, Consulting fees from Pfizer Inc, Eli Lilly, Celcuity, and Novartis, research support from Pfizer Inc, Eli Lilly, Novartis, GlaxoSmithKline, Puma, Zentalis, and Celcuity.
The authors declare that this study received funding from Pfizer Inc. The funder had the following involvement with the study: data acquisition, study design, data analysis, and coauthoring the paper. No authors received funding or payment for coauthoring the paper from the funder.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This study was funded by Pfizer Inc. Editorial support was provided by John Teiber, PhD, of ICON plc (North Wales, PA, USA) and was funded by Pfizer Inc.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin (2021) 71:7–33. doi: 10.3322/caac.21654
2. Redig AJ, McAllister SS. Breast Cancer as a Systemic Disease: A View of Metastasis. J Intern Med (2013) 274:113–26. doi: 10.1111/joim.12084
3. Wang R, Zhu Y, Liu X, Liao X, He J, Niu L. The Clinicopathological Features and Survival Outcomes of Patients With Different Metastatic Sites in Stage IV Breast Cancer. BMC Cancer (2019) 19:1091. doi: 10.1186/s12885-019-6311-z
4. Wu Q, Li J, Zhu S, Wu J, Chen C, Liu Q, et al. Breast Cancer Subtypes Predict the Preferential Site of Distant Metastases: A SEER Based Study. Oncotarget (2017) 8:27990–6. doi: 10.18632/oncotarget.15856
5. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Version 8.2021. National Comprehensive Cancer Network (2021). Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed October 29, 2021.
6. Robertson JFR, Di Leo A, Johnston S, Chia S, Bliss JM, Paridaens RJ, et al. Meta-Analyses of Visceral Versus Non-Visceral Metastatic Hormone Receptor-Positive Breast Cancer Treated by Endocrine Monotherapies. NPJ Breast Cancer (2021) 7:11. doi: 10.1038/s41523-021-00222-y
7. He M, Li JJ, Zuo WJ, Ji L, Jiang YZ, Hu XC, et al. Metastatic Breast Cancer Patients With Lung or Liver Metastases Should Be Distinguished Before Being Treated With Fulvestrant. Cancer Med (2019) 8:6212–20. doi: 10.1002/cam4.2453
8. Ettl J. Management of Adverse Events Due to Cyclin-Dependent Kinase 4/6 Inhibitors. Breast Care (Basel) (2019) 14:86–92. doi: 10.1159/000499534
9. Finn RS, Crown JP, Lang I, Boer K, Bondarenko IM, Kulyk SO, et al. The Cyclin-Dependent Kinase 4/6 Inhibitor Palbociclib in Combination With Letrozole Versus Letrozole Alone as First-Line Treatment of Oestrogen Receptor-Positive, HER2-Negative, Advanced Breast Cancer (PALOMA-1/TRIO-18): A Randomised Phase 2 Study. Lancet Oncol (2015) 16:25–35. doi: 10.1016/S1470-2045(14)71159-3
10. Rugo HS, Finn RS, Dieras V, Ettl J, Lipatov O, Joy AA, et al. Palbociclib Plus Letrozole as First-Line Therapy in Estrogen Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer With Extended Follow-Up. Breast Cancer Res Treat (2019) 174:719–29. doi: 10.1007/s10549-018-05125-4
11. Dawood S, Brzozowski K. Efficacy of CDK4/6i in the Visceral Crisis Setting: Result From a Real-World Database. J Clin Oncol (2021) 39:abst 1047. doi: 10.1200/JCO.2021.39.15_suppl.1047
12. Sherman RE, Anderson SA, Dal Pan GJ, Gray GW, Gross T, Hunter NL, et al. Real-World Evidence - What is it and What Can It Tell Us? N Engl J Med (2016) 375:2293–7. doi: 10.1056/NEJMsb1609216
13. Blumenthal GM, Gong Y, Kehl K, Mishra-Kalyani P, Goldberg KB, Khozin S, et al. Analysis of Time-to-Treatment Discontinuation of Targeted Therapy, Immunotherapy, and Chemotherapy in Clinical Trials of Patients With non-Small-Cell Lung Cancer. Ann Oncol (2019) 30:830–8. doi: 10.1093/annonc/mdz060
14. Liu R, Rizzo S, Whipple S, Pal N, Pineda AL, Lu M, et al. Evaluating Eligibility Criteria of Oncology Trials Using Real-World Data and AI. Nature (2021) 592:629–33. doi: 10.1038/s41586-021-03430-5
15. Curtis MD, Griffith SD, Tucker M, Taylor MD, Capra WB, Carrigan G, et al. Development and Validation of a High-Quality Composite Real-World Mortality Endpoint. Health Serv Res (2018) 53:4460–76. doi: 10.1111/1475-6773.12872
16. Singal G, Miller PG, Agarwala V, Li G, Kaushik G, Backenroth D, et al. Association of Patient Characteristics and Tumor Genomics With Clinical Outcomes Among Patients With Non-Small Cell Lung Cancer Using a Clinicogenomic Database. JAMA (2019) 321:1391–9. doi: 10.1001/jama.2019.3241
17. Collins JM, Nordstrom BL, McLaurin KK, Dalvi TB, McCutcheon SC, Bennett JC, et al. A Real-World Evidence Study of CDK4/6 Inhibitor Treatment Patterns and Outcomes in Metastatic Breast Cancer by Germline BRCA Mutation Status. Oncol Ther (2021) 9:575–89. doi: 10.1007/s40487-021-00162-4
18. DeMichele A, Cristofanilli M, Brufsky A, Liu X, Mardekian J, McRoy L, et al. Comparative Effectiveness of First-Line Palbociclib Plus Letrozole Versus Letrozole Alone for HR+/HER2- Metastatic Breast Cancer in US Real-World Clinical Practice. Breast Cancer Res (2021) 23:37. doi: 10.1186/s13058-021-01409-8
19. Austin PC. The Use of Propensity Score Methods With Survival or Time-to-Event Outcomes: Reporting Measures of Effect Similar to Those Used in Randomized Experiments. Stat Med (2014) 33:1242–58. doi: 10.1002/sim.5984
20. Tripathy D, Im SA, Colleoni M, Franke F, Bardia A, Harbeck N, et al. Ribociclib Plus Endocrine Therapy for Premenopausal Women With Hormone-Receptor-Positive, Advanced Breast Cancer (MONALEESA-7): A Randomised Phase 3 Trial. Lancet Oncol (2018) 19:904–15. doi: 10.1016/S1470-2045(18)30292-4
21. Hortobagyi GN. Ribociclib for the First-Line Treatment of Advanced Hormone Receptor-Positive Breast Cancer: A Review of Subgroup Analyses From the MONALEESA-2 Trial. Breast Cancer Res (2018) 20:123. doi: 10.1186/s13058-018-1050-7
22. Gao JJ, Cheng J, Bloomquist E, Sanchez J, Wedam SB, Singh H, et al. CDK4/6 Inhibitor Treatment for Patients With Hormone Receptor-Positive, HER2-Negative, Advanced or Metastatic Breast Cancer: A US Food and Drug Administration Pooled Analysis. Lancet Oncol (2020) 21:250–60. doi: 10.1016/S1470-2045(19)30804-6
23. Im SA, Lu YS, Bardia A, Harbeck N, Colleoni M, Franke F, et al. Overall Survival With Ribociclib Plus Endocrine Therapy in Breast Cancer. N Engl J Med (2019) 381:307–16. doi: 10.1056/NEJMoa1903765
24. Schettini F, Giudici F, Giuliano M, Cristofanilli M, Arpino G, Del Mastro L, et al. Overall Survival of CDK4/6-Inhibitor-Based Treatments in Clinically Relevant Subgroups of Metastatic Breast Cancer: Systematic Review and Meta-Analysis. J Natl Cancer Inst (2020) 112:1089–97. doi: 10.1093/jnci/djaa071
25. Johnston S, O'Shaughnessy J, Martin M, Huober J, Toi M, Sohn J, et al. Abemaciclib as Initial Therapy for Advanced Breast Cancer: MONARCH 3 Updated Results in Prognostic Subgroups. NPJ Breast Cancer (2021) 7:80. doi: 10.1038/s41523-021-00289-7
26. Palleschi M, Maltoni R, Ravaioli S, Vagheggini A, Mannozzi F, Fanini F, et al. Ki67 and PR in Patients Treated With CDK4/6 Inhibitors: A Real-World Experience. Diagnostics (Basel) (2020) 10:573. doi: 10.3390/diagnostics10080573
27. De Angelis C, Fu X, Cataldo ML, Nardone A, Pereira R, Veeraraghavan J, et al. Activation of the IFN Signaling Pathway is Associated With Resistance to CDK4/6 Inhibitors and Immune Checkpoint Activation in ER-Positive Breast Cancer. Clin Cancer Res (2021) 27:4870–82. doi: 10.1158/1078-0432.CCR-19-4191
Keywords: HR+/HER2−, metastatic breast cancer, palbociclib, real-world data, visceral metastasis
Citation: Brufsky A, Liu X, Li B, McRoy L and Layman RM (2022) Real-World Effectiveness of Palbociclib Plus Letrozole vs Letrozole Alone for Metastatic Breast Cancer With Lung or Liver Metastases: Flatiron Database Analysis. Front. Oncol. 12:865292. doi: 10.3389/fonc.2022.865292
Received: 29 January 2022; Accepted: 20 May 2022;
Published: 04 July 2022.
Edited by:
Carmine De Angelis, University of Naples Federico II, ItalyReviewed by:
Michela Palleschi, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyFilippo Montemurro, IRCCS Candiolo Cancer Institute, Italy
Copyright © 2022 Brufsky, Liu, Li, McRoy and Layman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adam Brufsky, YnJ1ZnNreWFtQHVwbWMuZWR1; Xianchen Liu, SmFzb254Yy5MaXVAcGZpemVyLmNvbQ==
†ORCID: Adam Brufsky, orcid.org/0000-0001-8080-7960
Xianchen Liu, orcid.org/0000-0003-0411-1050
 Adam Brufsky
Adam Brufsky Xianchen Liu
Xianchen Liu Benjamin Li2
Benjamin Li2 Rachel M. Layman
Rachel M. Layman