- Department of Oncology, Shengjing Hospital of China Medical University, Shenyang, China
Purpose: Epidermal growth factor receptor (EGFR) T790M-negative/unknown advanced non-small cell lung cancer (NSCLC) patients lack subsequent approved targeted therapies. This meta-analysis aimed to assess the efficacy of osimertinib in advanced NSCLC patients with different T790M status after resistance to prior first- or second-generation EGFR-tyrosine kinase inhibitors (EGFR-TKIs) and to predict the subgroups that may benefit beside T790M-positive disease.
Methods: PubMed, Embase, Web of Science, and Cochrane Library databases were searched for relevant trials. Meeting abstracts were also reviewed to identify appropriate studies. Studies evaluating the efficacy and/or survival outcomes of osimertinib in patients with different T790M status (positive, negative, or unknown) after resistance to prior first- or second-generation EGFR-TKIs were enrolled, and data were pooled to assess hazard ratios (HRs) or relative risk ratios (RRs) in terms of overall survival (OS), progression-free survival (PFS), and objective response rate (ORR).
Results: A total of 1,313 EGFR-mutated NSCLC patients from 10 retrospective and one prospective studies treated with osimertinib after resistance to first- or second-generation EGFR-TKIs were included. In overall groups, T790M-positive patients showed an improved OS (HR=0.574, p=0.015), PFS (HR = 0.476, p = 0.017), and ORR (RR = 2.025, p = 0.000) compared with T790M-negative patients. In the brain metastases subgroup, no significant difference in OS was observed between T790M-positive and T790M-negative patients (HR = 0.75, p = 0.449) or between T790M-positive and T790M-unknown patients (HR = 0.90, p = 0.673). In the plasma genotyping subgroup, PFS was similar between T790M-positive and T790M-negative patients (HR = 1.033, p = 0.959).
Conclusion: Patients with progressive brain metastases on first- or second-generation EGFR-TKIs can benefit from subsequent osimertinib therapy regardless of T790M status. Patients with plasma T790M-negative status and lack of tissue genotyping should be allowed to receive osimertinib treatment.
Introduction
Lung cancer is the leading cause of cancer-related mortality, and the most common type is non-small-cell lung cancer (NSCLC), accounting for 85% (1). Because of the high incidence rate and poor prognosis of advanced NSCLC, effective treatment strategies are urgently needed. Activating mutations in the tyrosine kinase domain of epidermal growth factor receptor (EGFR) as one of the significant drivers are mainly found in NSCLC patients; these mutations have motivated the emergency of targeted therapy, which has notably improved the survival of NSCLC patients. For treatment-naive advanced NSCLC patients with EGFR-sensitizing mutations, first-line EGFR-TKIs including first-generation gefitinib, erlotinib, and icotinib, second-generation afatinib and dacomitinib, and third-generation osimertinib and almonertinib have replaced traditional platinum-based chemotherapy as the current therapeutic standard, with a progression-free survival (PFS) range of 9–19.3 months (2–8). Although osimertinib, an irreversible third-generation EGFR-TKI, has been recommended by the National Comprehensive Cancer Network guidelines as a preferred first-line treatment for patients with EGFR-sensitizing mutation advanced NSCLC, first- or second-generation EGFR-TKIs are still an important first-line choice in some parts of the world due to cost and lower overall survival (OS) benefit of osimertinib in subgroups of the Asian population or patients with the 21L858R point mutation compared to first-generation EGFR-TKIs gefitinib and erlotinib observed in the FLAURA study (7).
The most common acquired resistance mechanism to first- or second-generation EGFR-TKIs is a threonine-to-methionine substitution at amino acid position 790 in exon 20 (i.e., T790M mutation), accounting for 49%–73% of the cases of resistance (9–11). Patients with acquired T790M will benefit from subsequent treatment with osimertinib that selectively targets both EGFR-sensitizing mutations and the T790M mutation (12, 13). However, only 50%–60% of resistant patients can undergo tissue rebiopsy to test for T790M (14–16). Plasma circulating tumor DNA (ctDNA), a type of liquid biopsy, is often used as an alternative for genotyping. However, it only exhibits 30%–70% sensitivity for detection of T790M compared with tissue genotyping using next-generation sequencing (NGS) or polymerase chain reaction (PCR)-based detection (17–19). As a result, <30% (14%–27.2%) of patients after resistance to prior EGFR-TKIs can be subsequently treated with osimertinib, and some patients who would likely benefit from osimertinib will go untreated due to a lack of detection or false-negative report of T790M by ctDNA detection (10, 20).
Osimertinib has also been shown to exhibit clinically significant activity for some T790M-negative patients after resistance to first- or second-generation EGFR-TKIs, especially in patients with brain metastasis (BM) (21, 22). Therefore, this meta-analysis aimed to assess the efficacy of osimertinib in advanced NSCLC patients with different T790M status after resistance to prior first- or second-generation EGFR-TKI treatment and to predict the subgroups that may benefit.
Methods
Search Strategy
PubMed, Embase, Web of Science, and Cochrane Library databases were searched using the following search terms: (“non-small cell lung cancer” OR “NSCLC”) AND (“osimertinib” OR “AZD9291” OR “third-generation EGFR-TKI”) AND ((“EGFR” AND “mutation”) OR (“epidermal growth factor receptor” AND “mutation”)) to find relevant articles. In addition, abstracts from the American Society of Clinical Oncology (ASCO), European Society of Medical Oncology (ESMO), and World Conference on Lung Cancer were reviewed. Finally, the reference lists of the eligible articles were manually checked to ensure all relevant literature was retrieved. The search end date was October 26, 2021. The article search was performed separately by two investigators.
Eligibility Criteria
Studies that met the following criteria were included (1): advanced EGFR-mutant NSCLC patients treated with third-generation EGFR-TKIs after resistance to first- or second-generation EGFR-TKIs (2); evaluation of the efficacy and/or survival outcomes of different T790M statuses (positive, negative, or unknown); and (3) outcomes including at least one of the following endpoints, namely, overall survival (OS), PFS, ORR, and duration of response (DOR). The selection of articles was separately performed by two investigators based on a common set of criteria. Differences in opinion were settled through discussion.
Data Extraction
The extractable data included authors, year of publication, number of patients, gene detection information (T790M positive, negative, or unknown) after resistance to prior-generation EGFR-TKIs, BM status, genotyping sample types, OS, PFS, and hazard ratios (HRs) with 95% confidence interval (CI) for OS and/or PFS, ORR, DOR. Data extraction was performed separately by two investigators.
Statistical Analysis
All statistical analyses were performed with STATA 14.0 (StataCorp, College Station, TX, USA). The primary endpoints were OS and PFS, and the secondary endpoints were ORR and DOR. The effects of all outcomes were presented with HRs or relative risk ratios (RRs), 95% CIs, and p-values. Subgroup analyses were performed on BM and genotyping samples. HRs and 95% CIs were estimated using the procedures described by Tierney et al. if not reported in a study (23). Kaplan–Meier curve data were recovered via Engauge Digitizer version 11.1. This process was repeated two times to reduce variability. The I2 statistic was applied to evaluate heterogeneity. The random effect models were chosen if I2 was >50% or the p-value was <0.05, implying obvious heterogeneity; otherwise, fixed-effects models were applied. Two‐sided p < 0.05 was considered statistically significant.
Results
Characteristics of the Included Studies
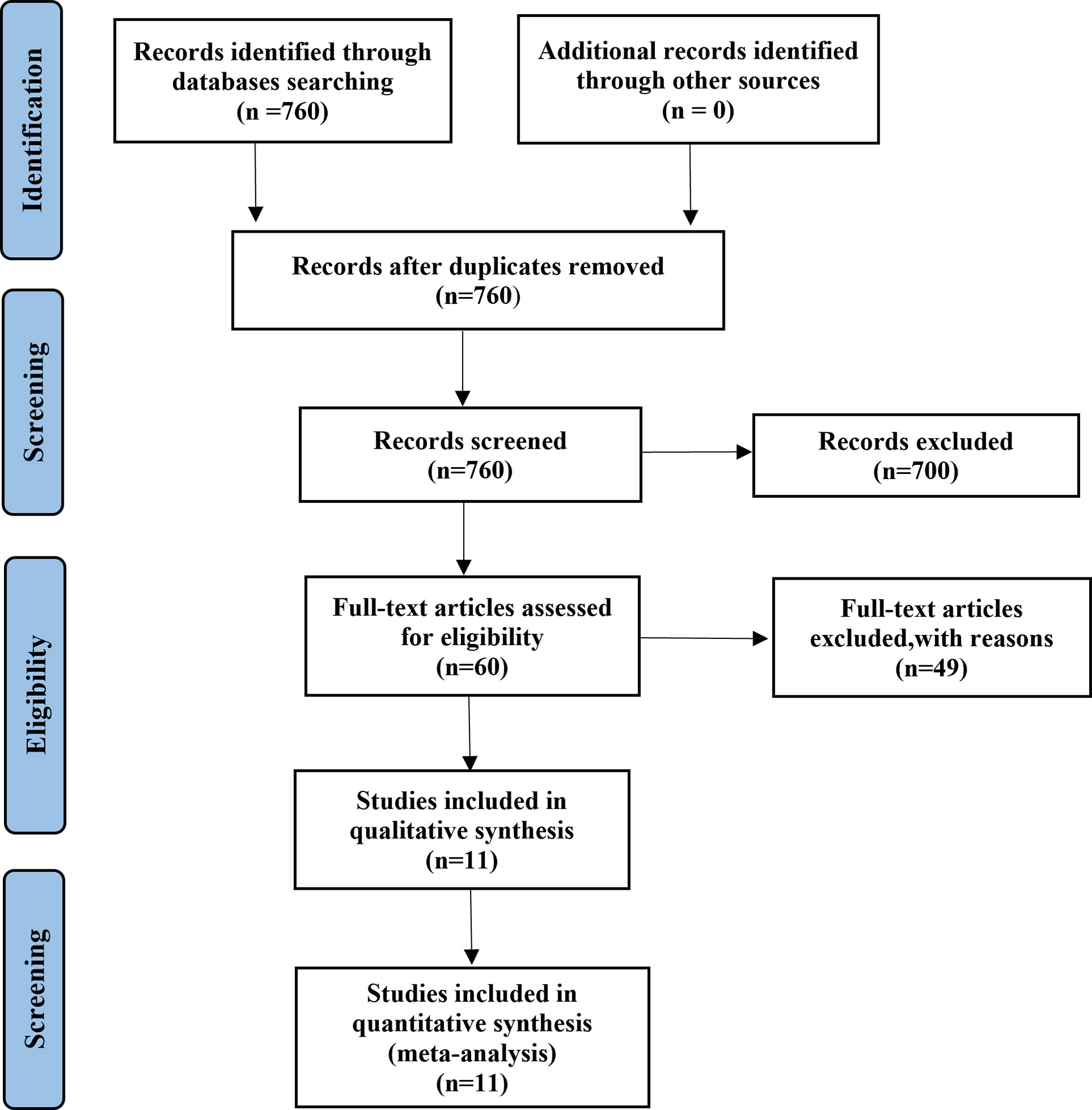
A total of 1,313 EGFR-mutated NSCLC patients from 10 retrospective and one prospective study (18, 21, 22, 24–31) treated with osimertinib after resistance to first- or second-generation EGFR-TKIs were included in the meta-analysis (Table 1). A Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) flow diagram of the retrieval process is presented in Figure 1. Eight studies compared outcomes between T790M-positive and T790M-negative patients, one compared outcomes between T790M-positive and T790M-unknown patients, and two provided survival outcomes of BM subgroups among T790M-positive, T790M-negative, and T790M-unknown patients. The percentages of T790M-positive, T790M-negative, and T790M-unknown patients were 65.80%, 26.70%, and 7.50%, respectively (Figure 2).
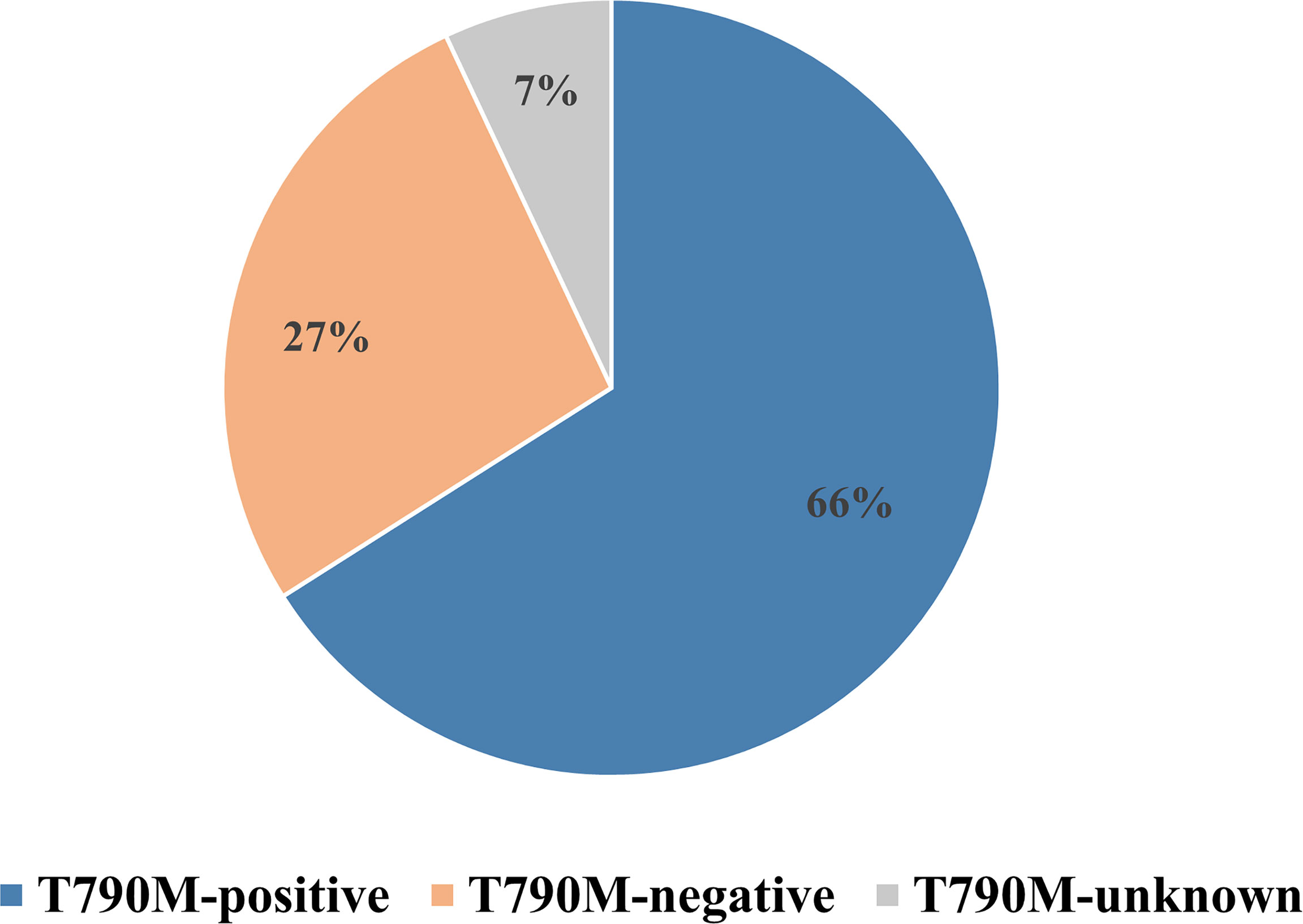
Figure 2 Percentages of T790M-positive, T790M-negative, and T790M-unknown patients in enrolled studies.
Comparison Between T790M-Positive and T790M-Negative Patients
Overall Group
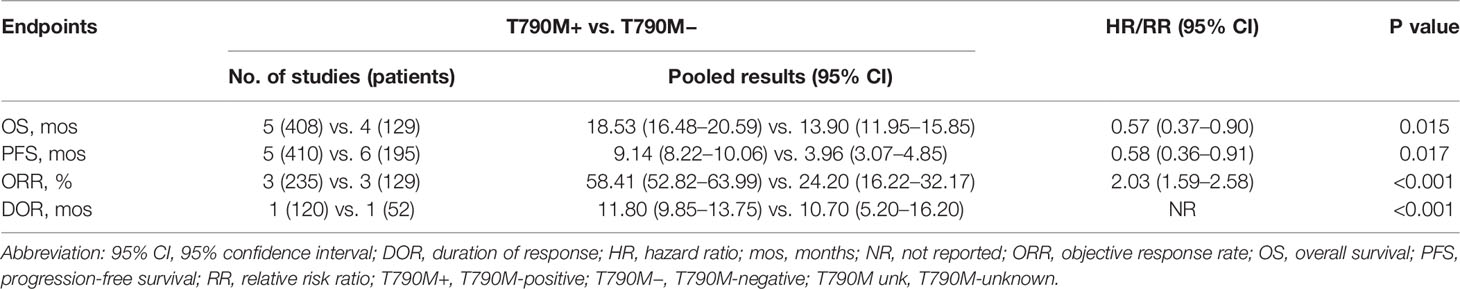
As shown in Table 2, overall OS in osimertinib-treated patients was 18.53 months (95% CI, 16.48–20.59) vs. 13.90 months (95% CI, 11.95–15.85) in T790M-positive and T790M-negative groups, respectively, with an HR of 0.57 (95% CI, 0.37–0.90; p = 0.015) (Figure 3A). Overall PFS for T790M-positive vs. T790M-negative groups was 9.14 months (95% CI, 8.22–10.06) vs. 3.96 months (95% CI, 3.07–4.85), with an HR of 0.58 (95% CI, 0.36–0.91; p = 0.017) (Figure 3B). Overall ORR for T790M-positive vs. T790M-negative groups was 58.41% (95% CI, 52.82–63.99) vs. 24.20% (95% CI, 16.22–32.17), with an RR of 2.03 (95% CI, 1.59–2.58, p < 0.001).
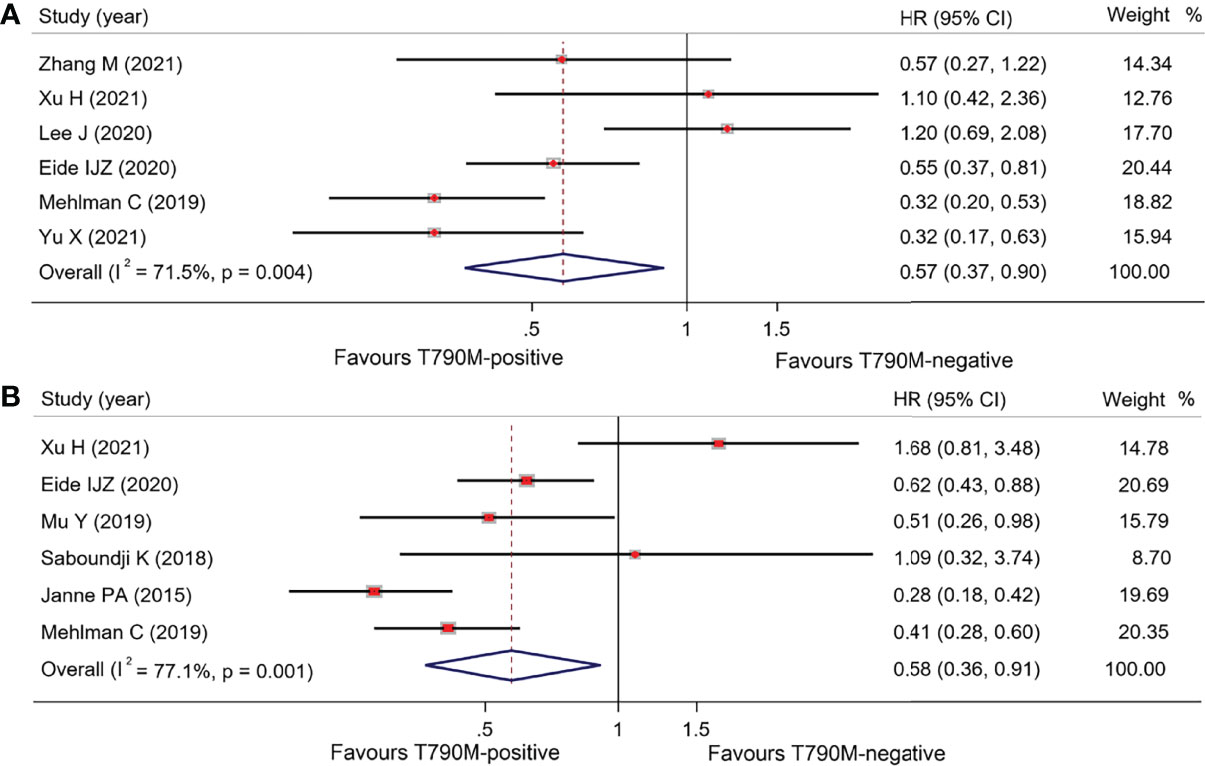
Figure 3 Comparison between T790M-positive and T790M-negative patients of overall group (A) and forest plot of OS (B), and forest plot of PFS.
Subgroup of Plasma Detection
PFS was not different between plasma detection T790M-positive and T790M-negative subgroups: 9.09 months (95% CI, 8.16–10.02) vs. 9.84 months (95% CI, 8.00–11.69), respectively, with an HR of 1.02 (95% CI, 0.44–2.35) (p = 0.959). ORRs in T790M-positive and T790M-negative subgroups were 63% (95% CI, 55.50–70.50) and 46% (95% CI, 36–56), respectively, with an RR of 1.36 (95% CI, 1.07–1.73; p < 0.001). Tissue genotyping outcomes were extracted from one study with PFS of 9.70 vs. 3.40 months, respectively, in T790M-positive and T790M-negative patients (HR, 0.36; 95% CI, 0.26–0.49) (18). Results are summarized in Table 3.
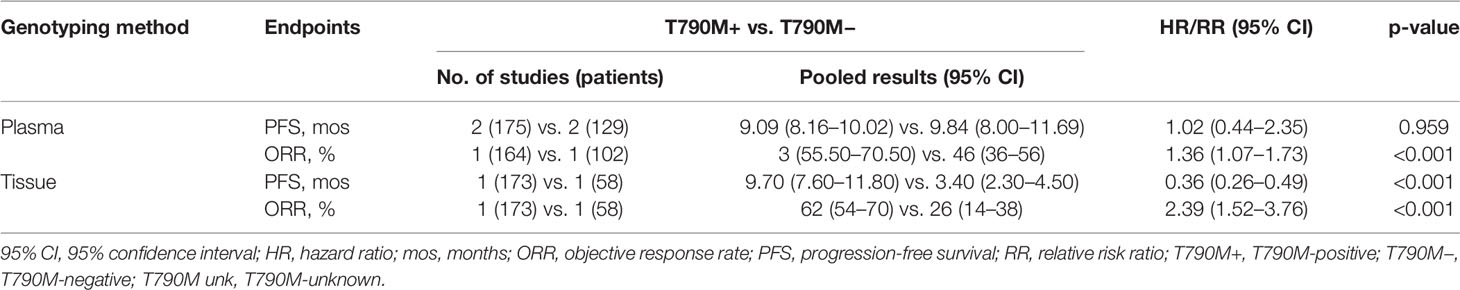
Table 3 Pooled results of survival and response rate the T790M-positive and T790M-negative groups with different genotyping samples.
Comparison Among BM Patients With Different T790M Mutation Status
T790M-Positive vs. T790M-Negative Groups
Pooled results of the subgroup with regard to BM demonstrated that there was no significant difference in OS between the T790-positive and T790-negative groups. OS in T790M-positive and T790M-negative patients was 16.28 months (95% CI, 13.62–18.94) and 17.50 months (95% CI, 14.61–20.39), respectively, with an HR of 0.75 (95% CI, 0.36–1.58; p = 0.449) (Figure 4A). PFS data were only available in one trial: 8.80 vs. 10.80 months, respectively, in T790M-positive and T790M-negative patients (26). These results are summarized in Table 4.
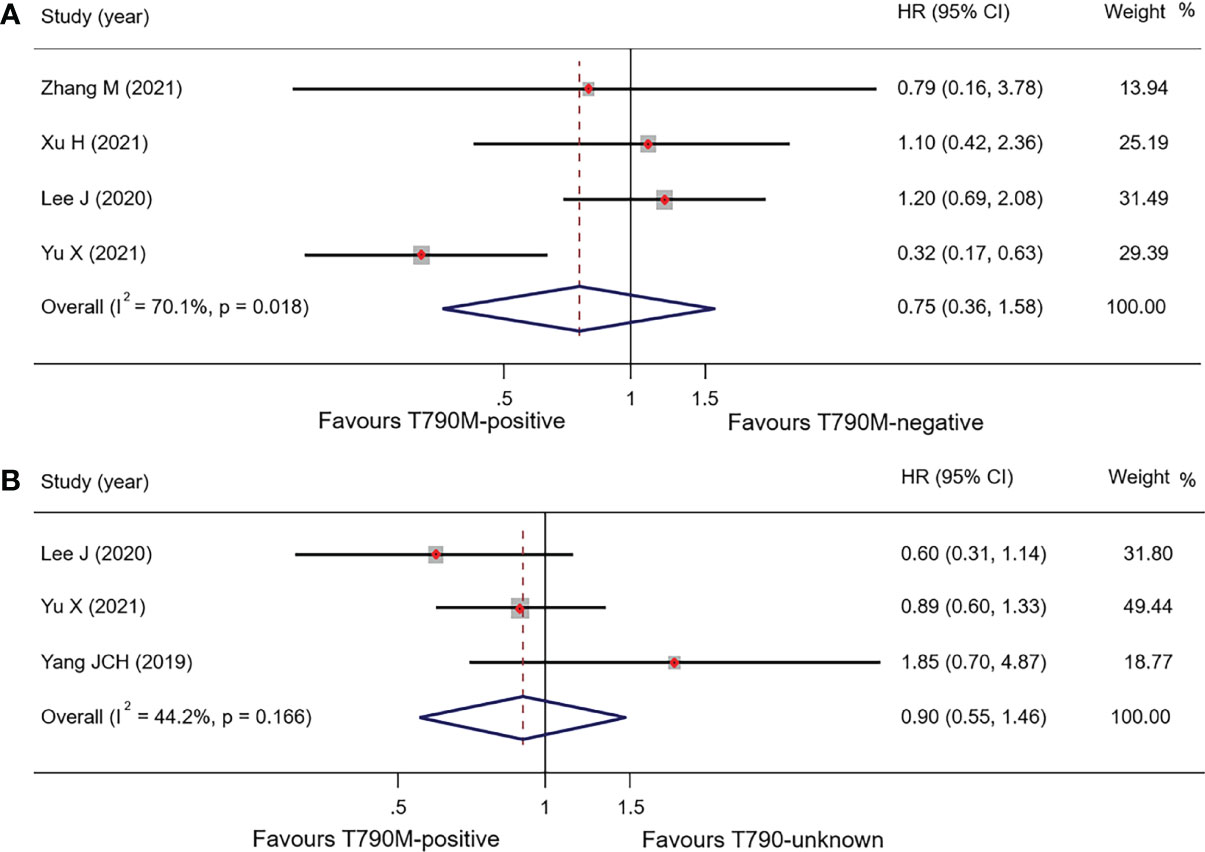
Figure 4 Comparison of BM patients based on T790M mutation status of OS (A), forest plot of comparison of T790M-positive and T790M-negative (B), and forest plot of comparison of T790M-positive and T790M-unknown groups.

Table 4 Pooled results of survival and response rate for the different T790M statuses with brain metastases.
T790M-Positive vs. T790M-Unknown Groups
Three studies reported OS in BM patients with T790M-positive and T790-unknown statuses. Pooled OS results in T790M-positive and T790-unknown groups were 20.78 and 22.98 months, respectively (these were calculated using a weighted average of single study medians because of insufficient data of the 95% CI values) (32), with an HR of 0.90 (95% CI, 0.55–1.47; p = 0.673) (Table 4; Figure 4B).
T790M-Positive vs. T790M-Negative vs. T790M-Unknown Groups
A direct comparison of BM patients with the three T790M statuses was also performed in two studies. OS was 22.59, 21.17, and 24.86 months in T790M-positive, T790M-negative, and T790M-unknown groups, respectively; these were calculated using a weighted average of single study medians because of insufficient data of the 95% CI values (32) (Table 4).
Discussion
Patients with advanced NSCLC harboring a secondary EGFR T790M mutation following treatment with first- or second-generation EGFR-TKIs can benefit from subsequent treatment with osimertinib. However, other patients exhibiting resistance with T790M-negative/T790M-unknown statuses lack subsequent approved targeted therapies, and the efficacy of osimertinib in these patients remains unclear. Therefore, it is necessary to explore other subgroups of patients who may benefit from osimertinib treatment to expand its scope of application. Our meta-analysis showed that patients with plasma T790M-negative status or BM patients with T790M-negative or T790M-unknown statuses had similar efficacy to that of T790M-positive patients when treated with osimertinib, suggesting that patients with BM progression with first- or second-generation EGFR-TKIs can benefit from subsequent osimertinib therapy regardless of T790M status, and patients with plasma T790M test-negative status and lack of tissue rebiopsy and genotyping should be allowed to receive osimertinib treatment, especially in the absence of later standard treatment.
Studies have shown that osimertinib can overcome the resistance of acquired T790M mutation, with median PFS of 9.9–12.3 months and ORR of 60–71% (31, 33, 34). A randomized phase III trial, AURA 3, showed that compared with chemotherapy, osimertinib can significantly improve ORR (71% vs. 31%) and PFS (10.1 vs. 4.4 months) in patients with acquired T790M (35). These encouraging results led to the approval of osimertinib as a subsequent treatment for advanced NSCLC patients who developed resistance to prior EGFR-TKIs and acquired a T790M resistance mutation. However, studies have shown that osimertinib also appears to be effective in T790M-negative resistant patients. A study that enrolled 62 T790M-negative patients receiving osimertinib reported a PFS of 2.8 months and an ORR of 21% (31). In a prospective TREM study, 52 EGFR-TKI-resistant patients with T790M-negative status who received osimertinib treatment showed PFS, OS, and ORR of 5.1 months, 13.4 months, and 28%, respectively (22). Furthermore, some retrospective studies have reported that osimertinib had an ORR of 21%–40% and OS of 14–27 months in prior EGFR-TKI-resistant T790M-negative patients (25, 28, 29). This efficacy is similar to the previously reported efficacy of chemotherapy after EGFR-TKI failure. Two studies (AURA3 and IMPRESS) reported PFS of 4.4–5.3 months and ORR of 31.0%–39.5% in patients treated with chemotherapy after resistance to first- or second-generation EGFR-TKIs (35, 36). In our study, the pooled results of osimertinib-treated T790M-negative patients showed similar PFS (3.96 months) and ORR (24.20%) to previous chemotherapy results, indicating that osimertinib may be clinically significant for some patients with a T790M-negative status, although results were not as significant as with T790M-positive patients. However, it is clear that it will be necessary to identify subgroups of these patients that will truly benefit from treatment with osimertinib.
BM progression is a unique disease progression pattern with insufficient response to anti-tumor drugs and poor prognosis because of the active blood–brain barrier (BBB); it accounts for approximately 40% of prior generation EGFR-TKI-resistant metastasis sites (37, 38). In our study, there was no significant OS difference between BM patients with and without T790M, and between those with T790M-positive and T790M-unknown statuses. Furthermore, no significant OS difference was observed in a direct comparison of T790M-positive, T790M-negative, and T790M-unknown patients. These outcomes are generally consistent with the following clinical studies. A retrospective analysis of studies within the AURA series (AURA extension, AURA2, AURA17, and AURA3) exhibited a CNS ORR of 54%–70%, a median CNS PFS of 11.1–11.7 months, and an OS of 18.8 months in T790M-positive patients (33, 34, 39, 40), while some studies also exhibited a CNS PFS of 10.8 months and an OS of 17.2–27 months in T790M-negative patients (24–26). The BLOOM study demonstrated a PFS of 12.3 months and an ORR of 38% in the T790M-unselected population (21). Accordingly, it is worthwhile to discuss whether osimertinib should be used in all patients with progressive BM regardless of T790M status. One of the reasons for the promising efficacy of osimertinib in the CNS may depend on its adequate BBB-penetrating capabilities. The APOLLO and BLOOM studies showed superior BBB penetrations of osimertinib of 31.7% and 16%, respectively (21, 41). However, the BBB penetrations of prior generation EGFR-TKIs were all <6%, with erlotinib at 2.8%–5.1%, gefitinib at 1%–3%, and afatinib at 0.7% (42–45). The insufficient concentration of TKIs in cerebrospinal fluid (CSF), which is less likely to permanently control the dissemination of tumor cells, is crucial in BM after resistance to prior generation EGFR TKIs, apart from the mechanism-induced acquired resistance. Another intriguing circumstance is the mismatching of the T790M mutation detection rate between plasma- or tissue-based genotyping and CSF-based genotyping. A study directly comparing paired plasma and CSF samples in lung adenocarcinoma patients with BM confirmed the lower prevalence of T790M mutation in CSF (3/23) than in plasma (9/23) (46). This result is consistent with other studies reporting a 13%–16% T790M mutation detection rate in CSF, which is significantly lower than the T790M mutation detection rate in plasma of 41%–45% (47, 48). However, one study of 45 EGFR-TKI-treated NSCLC patients with leptomeningeal metastases reported a higher detection rate of the T790M mutation (30.4% vs. 21.7%) and gene copy number variations (CNVs) such as MET (47.8% vs. 0) in CSF than in the plasma, indicating that genetic profiles in CSF may be different from those in plasma, and T790M status in the plasma or primary tumor cannot fully represent the mutation status in CSF (49). In addition, low exposure to first- or second-generation EGFR-TKIs in CSF may also result in “occult” T790M clones within CSF, i.e., low T790M mutation abundance, which may lead to false-negative test results for the T790M mutation. This may be another reason why some patients with BM progression benefit from osimertinib (50). Recently, a study classifying patients into T790M-positive and T790M-negative cohorts based on detection in CSF showed promising efficacy of osimertinib in the T790M-negative cohort with a median intracranial PFS of 7.0 months (51). Thus, plasma and CSF may be complementary for EGFR-TKI resistance patients with BM progression. However, CSF genotyping-based analyses were not included in this meta-analysis for several reasons. First, these data are from retrospective studies with small sample sizes, leading to various biases, such as low statistical power and inflated effect size estimation. Second, because the absolute amount of tumor-derived cell-free DNA in CSF is very low, the method of detecting mutations in CSF is important to the test results. However, techniques used in CSF detection are under exploration with no definitive conclusion. Therefore, osimertinib may be the better choice for patients with BM progression after prior first- or second-generation EGFR-TKIs, regardless of the T790M status.
Tissue genotyping is currently the standard detection approach due to its sensitivity, but is an invasive procedure that may pose danger or cause treatment delays and is often not feasible. For patients inaccessible to tissue biopsy, liquid biopsy, such as plasma genotyping, may be a non-invasive alternative. In the real world, however, approximately 50% of drug-resistant patients underwent tissue rebiopsy, and 20%–50% patients underwent liquid biopsy (20, 52). Previous studies also showed approximately 70% consistency between liquid biopsy- and tissue rebiopsy-based genetic tests in detecting T790M (18, 19). In our meta-analysis, PFS in plasma T790M-positive and T790M-negative patients was 9.09 vs. 9.84 months. PFS provided by one study in tissue T790M-positive vs. tissue T790M-negative patients was 9.7 vs. 3.4 months. There were dramatic differences observed between tissue and plasma genotyping, indicating that there exist sensitivity differences between these methods. The Cobas EGFR Mutation Test v2 for the analysis of T790M in plasma was approved by the US Food and Drug Administration in 2016 because the detection of L858R point mutation and exon 19 deletions in plasma samples with this test method was highly consistent with that in tissue samples (53). Although plasma genotyping has been widely applied in clinical practice, its sensitivity has not been estimated by well-designed, large-scale prospective randomized trials. In terms of the T790M mutation, Arcila et al. had assessed the credibility of plasma genotyping before the emergence of osimertinib (17). Of 64 patients who were confirmed to harbor the T790M mutation with tissue genotyping, 45 were T790M positive with plasma genotyping, including 11 patients who were positive in the second testing, and the overall sensitivity of plasma genotyping was 70%. In the analysis of AURA extension and AURA studies, the sensitivity was 61% and only 51% in the AURA3 study (33, 34, 39). Furthermore, a cross-comparison study of Cobas, Therascreen, ddPCR, and BEAMing provided sensitivities of 41%, 29%, 71%, and 71%, respectively (53). Plasma genotyping has a relatively high positive predictive value, which can avoid biopsies for most patients, but a large proportion of patients with false-negative T790M mutation may miss the chance of osimertinib treatment. For EGFR T790M-negative patients after prior EGFR-TKI therapy, platinum-doublet chemotherapy is considered the standard treatment with a PFS of 4.5–5.4 months and an ORR of 24–30.9% (54, 55). Data on tissue T790M-negative patients treated by osimertinib after failure of prior generation EGFR-TKI treatment are limited; the only study included in this meta-analysis provided a PFS of 3.4 months (95% CI, 2.3–4.5 months) and an ORR of 26% (95% CI, 14–38%) (18). Therefore, osimertinib appears to have similar efficacy compared to chemotherapy but with more manageable toxicity. As a result, for patients in whom tissue genotyping is ultimately unavailable and are plasma T790M-negative, osimertinib is a moderately recommended subsequent line treatment, and for patients who are tissue T790M-negative, osimertinib may also be a choice given that more than a quarter of patients have a response; at the very least, it has certain advantages over chemotherapy.
There are several limitations to this meta-analysis. First, the number of studies and patients included in this pooled analysis is limited. The major reason is that there are few studies assessing the efficacy of osimertinib in advanced NSCLC patients with T790M-negative or T790M-unknown statuses. Second, the included studies are almost all retrospective, with only one prospective study, so selection bias and public bias are difficult to avoid. Third, we failed to further analyze the different detection methods used in the target population after resistance to prior generation EGFR-TKIs, which may have affected the end results. Therefore, larger-scale clinical studies are needed to confirm the efficacy of osimertinib in advanced NSCLC patients with different T790M statuses following resistance to prior generation EGFR-TKIs.
Conclusion
Many studies have shown that when off-target (non-EGFR) pathway resistance mechanisms occur, such as MET/HER2 amplification, BRAF mutation, or RET rearrangement, continuously blocking the EGFR pathway with osimertinib in combination with drugs targeting these off-target activating pathway is a promising treatment strategy regardless of the type of EGFR-TKI treatment previously received. Thus, inhibition of the EGFR pathway is important regardless of the cause of EGFR-TKI resistance. This meta-analysis showed that osimertinib has an encouraging efficacy for plasma T790M-negative patients and progressive BM patients regardless of T790M status after resistance to prior generation EGFR-TKIs. Thus, based on the results of this meta-analysis and given the lack of approved effective targeted therapy, we strongly recommend that patients with progressive BM receive osimertinib treatment, even if the T790M test is negative; we moderately recommend osimertinib as a subsequent treatment for advanced NSCLC patients whose tissue rebiopsy is unavailable (T790M-unknown) and plasma T790M test is negative. Finally, for patients who tested negative for T790M by tissue rebiopsy, we only give a low-level recommendation (Figure 5).
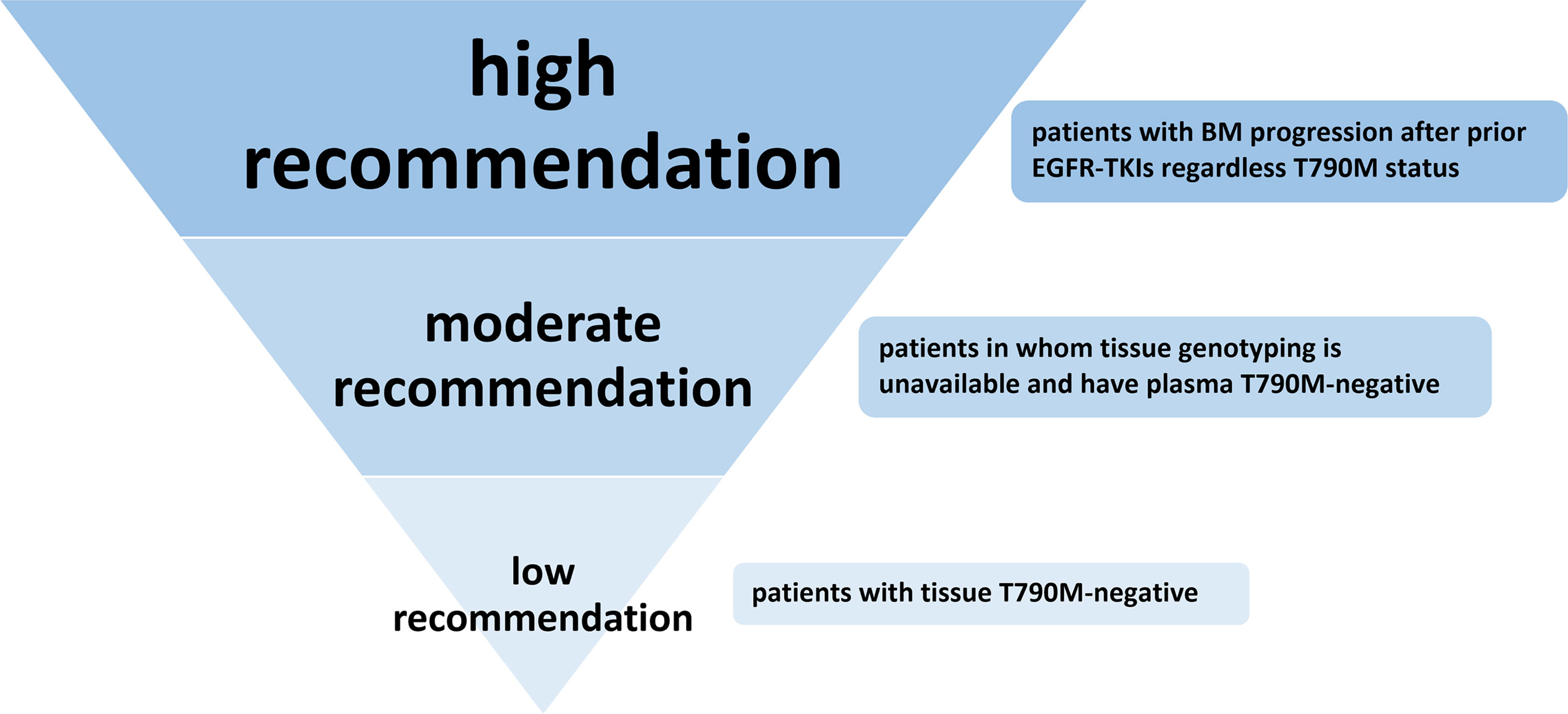
Figure 5 Recommendation level of osimertinib treatment for NSCLC patients with T790M-negative or T790M-unknown status after resistance to first- or second-generation EGFR-TKIs.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contribution
X-FY: writing of the original draft. SJ: data extraction and collection. R-LG: data extraction and collection. LS: software. Z-XW: software. S-LZ: formal analysis. L-TH: table editing. C-BH: conceptualization, methodology, and supervision. J-TM: conceptualization, methodology, manuscript review, and revision. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the 345 Talent Project of Shengjing Hospital and the Liaoning Province Key Research and Development Plan Projects (No. 2020JH2/10300149).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. CA Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
2. Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, et al. Gefitinib or Carboplatin-Paclitaxel in Pulmonary Adenocarcinoma. N Engl J Med (2009) 361(10):947–57. doi: 10.1056/NEJMoa0810699
3. Zhou CC, Wu YL, Chen GY, Feng JF, Liu XQ, Wang CL, et al. Erlotinib Versus Chemotherapy as First-Line Treatment for Patients With Advanced EGFR Mutation-Positive non-Small-Cell Lung Cancer (OPTIMAL, CTONG-0802): A Multicentre, Open-Label, Randomised, Phase 3 Study. Lancet Oncol (2011) 12(8):735–42. doi: 10.1016/s1470-2045(11)70184-x
4. Yang JCH, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, et al. Afatinib Versus Cisplatin-Based Chemotherapy for EGFR Mutation-Positive Lung Adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of Overall Survival Data From Two Randomised, Phase 3 Trials. Lancet Oncol (2015) 16(2):141–51. doi: 10.1016/s1470-2045(14)71173-8
5. Wu YL, Cheng Y, Zhou XD, Lee KH, Nakagawa K, Niho S, et al. Dacomitinib Versus Gefitinib as First-Line Treatment for Patients With EGFR-Mutation-Positive non-Small-Cell Lung Cancer (ARCHER 1050): A Randomised, Open-Label, Phase 3 Trial. Lancet Oncol (2017) 18(11):1454–66. doi: 10.1016/s1470-2045(17)30608-3
6. Shi YK, Wang L, Han BH, Li W, Yu P, Liu YP, et al. First-Line Icotinib Versus Cisplatin/Pemetrexed Plus Pemetrexed Maintenance Therapy for Patients With Advanced EGFR Mutation-Positive Lung Adenocarcinoma (CONVINCE): A Phase 3, Open-Label, Randomized Study. Ann Oncol (2017) 28(10):2443–50. doi: 10.1093/annonc/mdx359
7. Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, et al. Overall Survival With Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med (2020) 382(1):41–50. doi: 10.1056/NEJMoa1913662
8. Lu S, Dong XR, Jian H, Chen JH, Chen GY, Sun YP, et al. Randomized Phase III Trial of Aumolertinib (HS-10296, Au) Versus Gefitinib (G) as First-Line Treatment of Patients With Locally Advanced or Metastatic Non-Small Cell Lung Cancer (NSCLC) and EGFR Exon 19 Del or L858R Mutations (EGFRm). J Clin Oncol (2021) 39(15):4. doi: 10.1200/JCO.2021.39.15_suppl.9013
9. Pereira I, Gaspar C, Pina M, Azevedo I, Rodrigues A. Real-World T790M Mutation Frequency and Impact of Rebiopsy in Patients With EGFR-Mutated Advanced Non-Small Cell Lung Cancer. Cureus (2020) 12(12):e12128. doi: 10.7759/cureus.12128
10. Komiya K, Nakashima C, Nakamura T, Hirakawa H, Abe T, Ogusu S, et al. Current Status and Problems of T790M Detection, a Molecular Biomarker of Acquired Resistance to EGFR Tyrosine Kinase Inhibitors, With Liquid Biopsy and Re-Biopsy. Anticancer Res (2018) 38(6):3559–66. doi: 10.21873/anticanres.12628
11. Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, et al. Analysis of Tumor Specimens at the Time of Acquired Resistance to EGFR-TKI Therapy in 155 Patients With EGFR-Mutant Lung Cancers. Clin Cancer Res (2013) 19(8):2240–7. doi: 10.1158/1078-0432.Ccr-12-2246
12. Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, et al. AZD9291, an Irreversible EGFR TKI, Overcomes T790M-Mediated Resistance to EGFR Inhibitors in Lung Cancer. Cancer Discov (2014) 4(9):1046–61. doi: 10.1158/2159-8290.Cd-14-0337
13. Papadimitrakopoulou VA, Mok TS, Han JY, Ahn MJ, Delmonte A, Ramalingam SS, et al. Osimertinib Versus Platinum-Pemetrexed for Patients With EGFR T790M Advanced NSCLC and Progression on a Prior EGFR-Tyrosine Kinase Inhibitor: AURA3 Overall Survival Analysis. Ann Oncol (2020) 31(11):1536–44. doi: 10.1016/j.annonc.2020.08.2100
14. Gyawali B, West HJ. Plasma vs Tissue Next-Generation Sequencing in Non-Small Cell Lung Cancer-Either, Both, or Neither? JAMA Oncol (2019) 5(2):148–9. doi: 10.1001/jamaoncol.2018.4304
15. Oellerich M, Schütz E, Beck J, Kanzow P, Plowman PN, Weiss GJ, et al. Using Circulating Cell-Free DNA to Monitor Personalized Cancer Therapy. Crit Rev Clin Lab Sci (2017) 54(3):205–18. doi: 10.1080/10408363.2017.1299683
16. Zugazagoitia J, Ramos I, Trigo JM, Palka M, Gomez-Rueda A, Jantus-Lewintre E, et al. Clinical Utility of Plasma-Based Digital Next-Generation Sequencing in Patients With Advance-Stage Lung Adenocarcinomas With Insufficient Tumor Samples for Tissue Genotyping. Ann Oncologym (2019) 30(2):290–6. doi: 10.1093/annonc/mdy512
17. Arcila ME, Oxnard GR, Nafa K, Riely GJ, Solomon SB, Zakowski MF, et al. Rebiopsy of Lung Cancer Patients With Acquired Resistance to EGFR Inhibitors and Enhanced Detection of the T790M Mutation Using a Locked Nucleic Acid-Based Assay. Clin Cancer Res (2011) 17(5):1169–80. doi: 10.1158/1078-0432.CCR-10-2277
18. Oxnard GR, Thress KS, Alden RS, Lawrance R, Paweletz CP, Cantarini M, et al. Association Between Plasma Genotyping and Outcomes of Treatment With Osimertinib (AZD9291) in Advanced Non-Small-Cell Lung Cancer. J Clin Oncol (2016) 34(28):3375–82. doi: 10.1200/jco.2016.66.7162
19. Sacher AG, Paweletz C, Dahlberg SE, Alden RS, O'Connell A, Feeney N, et al. Prospective Validation of Rapid Plasma Genotyping for the Detection of EGFR and KRAS Mutations in Advanced Lung Cancer. JAMA Oncol (2016) 2(8):1014–22. doi: 10.1001/jamaoncol.2016.0173
20. Zhou J, Zhao C, Zhao J, Wang Q, Chu XL, Li JY, et al. Re-Biopsy and Liquid Biopsy for Patients With non-Small Cell Lung Cancer After EGFR-Tyrosine Kinase Inhibitor Failure. Thorac Cancer (2019) 10(4):957–65. doi: 10.1111/1759-7714.13035
21. Yang JCH, Kim SW, Kim DW, Lee JS, Cho BC, Ahn JS, et al. Osimertinib in Patients With Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer and Leptomeningeal Metastases: The BLOOM Study. J Clin Oncol (2020) 38(6):538–47. doi: 10.1200/jco.19.00457
22. Eide IJZ, Helland A, Ekman S, Mellemgaard A, Hansen KH, Cicenas S, et al. Osimertinib in T790M-Positive and -Negative Patients With EGFR-Mutated Advanced non-Small Cell Lung Cancer (the TREM-Study). Lung Cancer (2020) 143:27–35. doi: 10.1016/j.lungcan.2020.03.009
23. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical Methods for Incorporating Summary Time-to-Event Data Into Meta-Analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
24. Zhang ML, Ma WF, Liu HQ, Jiang YS, Qin LZ, Li W, et al. Osimertinib Improves Overall Survival in Patients With Leptomeningeal Metastases Associated With EGFR-Mutated Non-Small-Cell Lung Cancer Regardless of Cerebrospinal Fluid T790M Mutational Status. Evid Based Complement Alternat Med (2021) 2021:6968194. doi: 10.1155/2021/6968194
25. Yu XQ, Sheng JM, Pan GQ, Fan Y. Real-World Utilization of EGFR TKIs and Prognostic Factors for Survival in EGFR-Mutated Non-Small Cell Lung Cancer Patients With Brain Metastases. Int J Cancer (2021) 149(5):1121–8. doi: 10.1002/ijc.33677
26. Xu HY, Chen HQ, Kong JX, Zhang Y, Liu S, Yang GJ, et al. Osimertinib for the Treatment of Epidermal Growth Factor Receptor-Mutated non-Small Cell Lung Cancer Patients With Leptomeningeal Metastases and Different T790M Status. Ann Transl Med (2021) 9(11):937. doi: 10.21037/atm-21-1249
27. Lee J, Choi Y, Han J, Park S, Jung HA, Su JM, et al. Osimertinib Improves Overall Survival in Patients With EGFR-Mutated NSCLC With Leptomeningeal Metastases Regardless of T790M Mutational Status. J Thorac Oncol (2020) 15(11):1758–66. doi: 10.1016/j.jtho.2020.06.018
28. Mu YX, Xing PY, Hao XZ, Wang Y, Li JL. Real-World Data Of Osimertinib In Patients With Pretreated Non-Small Cell Lung Cancer: A Retrospective Study. Cancer Manag Res (2019) 11:9243–51. doi: 10.2147/cmar.S221434
29. Mehlman C, Cadranel J, Rousseau-Bussac G, Lacave R, Pujals A, Girard N, et al. Resistance Mechanisms to Osimertinib in EGFR-Mutated Advanced non-Small-Cell Lung Cancer: A Multicentric Retrospective French Study. Lung Cancer (2019) 137:149–56. doi: 10.1016/j.lungcan.2019.09.019
30. Saboundji K, Auliac JB, Pérol M, François G, Janicot H, Marcq M, et al. Efficacy of Osimertinib in EGFR-Mutated Non-Small Cell Lung Cancer With Leptomeningeal Metastases Pretreated With EGFR-Tyrosine Kinase Inhibitors. Target Oncol (2018) 13(4):501–7. doi: 10.1007/s11523-018-0581-2
31. Jänne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, et al. AZD9291 in EGFR Inhibitor-Resistant non-Small-Cell Lung Cancer. N Engl J Med (2015) 372(18):1689–99. doi: 10.1056/NEJMoa1411817
32. Sun L, Guo YJ, Song J, Wang YR, Zhang SL, Huang LT, et al. Neoadjuvant EGFR-TKI Therapy for EGFR-Mutant NSCLC: A Systematic Review and Pooled Analysis of Five Prospective Clinical Trials. Front Oncol (2020) 10:586596. doi: 10.3389/fonc.2020.586596
33. Yang JC, Ahn MJ, Kim DW, Ramalingam SS, Sequist LV, Su WC, et al. Osimertinib in Pretreated T790M-Positive Advanced Non-Small-Cell Lung Cancer: AURA Study Phase II Extension Component. J Clin Oncol (2017) 35(12):1288–96. doi: 10.1200/jco.2016.70.3223
34. Goss G, Tsai C-M, Shepherd FA, Bazhenova L, Lee JS, Chang G-C, et al. Osimertinib for Pretreated EGFR Thr790Met-Positive Advanced non-Small-Cell Lung Cancer (AURA2): A Multicentre, Open-Label, Single-Arm, Phase 2 Study. Lancet Oncol (2016) 17(12):1643–52. doi: 10.1016/s1470-2045(16)30508-3
35. Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med (2017) 376(7):629–40. doi: 10.1056/NEJMoa1612674
36. Soria JC, Wu YL, Nakagawa K, Kim SW, Yang JJ, Ahn MJ, et al. Gefitinib Plus Chemotherapy Versus Placebo Plus Chemotherapy in EGFR-Mutation-Positive non-Small-Cell Lung Cancer After Progression on First-Line Gefitinib (IMPRESS): A Phase 3 Randomised Trial. Lancet Oncol (2015) 16(8):990–8. doi: 10.1016/s1470-2045(15)00121-7
37. Li LN, Luo SM, Lin H, Yang HT, Chen HJ, Liao ZY, et al. Correlation Between EGFR Mutation Status and the Incidence of Brain Metastases in Patients With non-Small Cell Lung Cancer. J Thorac Dis (2017) 9(8):2510–20. doi: 10.21037/jtd.2017.07.57
38. Mamon HJ, Yeap BY, Jänne PA, Reblando J, Shrager S, Jaklitsch MT, et al. High Risk of Brain Metastases in Surgically Staged IIIA non-Small-Cell Lung Cancer Patients Treated With Surgery, Chemotherapy, and Radiation. J Clin Oncol (2005) 23(7):1530–7. doi: 10.1200/jco.2005.04.123
39. Wu YL, Ahn MJ, Garassino MC, Han JY, Katakami N, Kim HR, et al. CNS Efficacy of Osimertinib in Patients With T790M-Positive Advanced Non-Small-Cell Lung Cancer: Data From a Randomized Phase III Trial (Aura3). J Clin Oncol (2018) 36(26):2702–9. doi: 10.1200/jco.2018.77.9363
40. Ahn MJ, Chiu CH, Cheng Y, Han JY, Goldberg SB, Greystoke A, et al. Osimertinib for Patients With Leptomeningeal Metastases Associated With EGFR T790M-Positive Advanced NSCLC: The AURA Leptomeningeal Metastases Analysis. J Thorac Oncol (2020) 15(4):637–48. doi: 10.1016/j.jtho.2019.12.113
41. Xing LG, Pan YY, Shi YK, Shu YQ, Feng JF, Li W, et al. Biomarkers of Osimertinib Response in Patients With Refractory, EGFR-T790M-Positive Non-Small Cell Lung Cancer and Central Nervous System Metastases: The APOLLO Study. Clin Cancer Res (2020) 26(23):6168–75. doi: 10.1158/1078-0432.Ccr-20-2081
42. Togashi Y, Masago K, Masuda S, Mizuno T, Fukudo M, Ikemi Y, et al. Cerebrospinal Fluid Concentration of Gefitinib and Erlotinib in Patients With non-Small Cell Lung Cancer. Cancer Chemother Pharmacol (2012) 70(3):399–405. doi: 10.1007/s00280-012-1929-4
43. Togashi Y, Masago K, Fukudo M, Terada T, Fujita S, Irisa K, et al. Cerebrospinal Fluid Concentration of Erlotinib and its Active Metabolite OSI-420 in Patients With Central Nervous System Metastases of Non-Small Cell Lung Cancer. J Thorac Oncol (2010) 5(7):950–5. doi: 10.1097/JTO.0b013e3181e2138b
44. Hoffknecht P, Tufman A, Wehler T, Pelzer T, Wiewrodt R, Schütz M, et al. Efficacy of the Irreversible ErbB Family Blocker Afatinib in Epidermal Growth Factor Receptor (EGFR) Tyrosine Kinase Inhibitor (TKI)-Pretreated non-Small-Cell Lung Cancer Patients With Brain Metastases or Leptomeningeal Disease. J Thorac Oncol (2015) 10(1):156–63. doi: 10.1097/jto.0000000000000380
45. Pareek V, Welch M, Ravera E, Zampolin RL, Sequist LV, Halmos B. Marked Differences in CNS Activity Among EGFR Inhibitors: Case Report and Mini-Review. J Thorac Oncol (2016) 11(11):e135–9. doi: 10.1016/j.jtho.2016.07.010
46. Huang RF, Xu X, Li D, Chen K, Zhan Q, Ge MX, et al. Digital PCR-Based Detection of EGFR Mutations in Paired Plasma and CSF Samples of Lung Adenocarcinoma Patients With Central Nervous System Metastases. Target Oncol (2019) 14(3):343–50. doi: 10.1007/s11523-019-00645-5
47. Hata A, Katakami N, Yoshioka H, Takeshita J, Tanaka K, Masago K, et al. Prognostic Impact of Central Nervous System Metastases After Acquired Resistance to EGFR-TKI: Poorer Prognosis Associated With T790M-Negative Status and Leptomeningeal Metastases. Anticancer Res (2015) 35(2):1025–31.
48. Hata A, Katakami N, Yoshioka H, Takeshita J, Tanaka K, Nanjo S, et al. Rebiopsy of non-Small Cell Lung Cancer Patients With Acquired Resistance to Epidermal Growth Factor Receptor-Tyrosine Kinase Inhibitor: Comparison Between T790M Mutation-Positive and Mutation-Negative Populations. Cancer (2013) 119(24):4325–32. doi: 10.1002/cncr.28364
49. Li YS, Jiang BY, Yang JJ, Zhang XC, Zhang Z, Ye JY, et al. Unique Genetic Profiles From Cerebrospinal Fluid Cell-Free DNA in Leptomeningeal Metastases of EGFR-Mutant non-Small-Cell Lung Cancer: A New Medium of Liquid Biopsy. Ann Oncol Off J Eur Soc Med Oncol (2018) 29(4):945–52. doi: 10.1093/annonc/mdy009
50. Hata A, Katakami N, Yoshioka H, Kaji R, Masago K, Fujita S, et al. Spatiotemporal T790M Heterogeneity in Individual Patients With EGFR-Mutant Non-Small-Cell Lung Cancer After Acquired Resistance to EGFR-TKI. J Thorac Oncol (2015) 10(11):1553–9. doi: 10.1097/jto.0000000000000647
51. Zheng MM, Li YS, Tu HY, Jiang BY, Yang JJ, Zhou Q, et al. Genotyping of Cerebrospinal Fluid Associated With Osimertinib Response and Resistance for Leptomeningeal Metastases in EGFR-Mutated NSCLC. J Thorac Oncol (2021) 16(2):250–8. doi: 10.1016/j.jtho.2020.10.008
52. Wang HP, Zhang L, Si XY, Zhang XT, Wang MZ. Re-Biopsy Status Among Chinese non-Small-Cell Lung Cancer Patients Who Progressed After Icotinib Therapy. Onco Targets Ther (2018) 11:7513–9. doi: 10.2147/ott.S174075
53. Thress KS, Brant R, Carr TH, Dearden S, Jenkins S, Brown H, et al. EGFR Mutation Detection in ctDNA From NSCLC Patient Plasma: A Cross-Platform Comparison of Leading Technologies to Support the Clinical Development of AZD9291. Lung Cancer (2015) 90(3):509–15. doi: 10.1016/j.lungcan.2015.10.004
54. Zhong RB, Xu JL, Lou YQ, Chu TQ, Zhong H, Han BH. Anlotinib or Platinum-Pemetrexed as Second-Line Therapy in EGFR T790M-Negative Lung Cancer. Ann Palliat Med (2020) 9(4):1681–7. doi: 10.21037/apm-20-105
55. Yoshida T, Kuroda H, Oya Y, Shimizu J, Horio Y, Sakao Y, et al. Clinical Outcomes of Platinum-Based Chemotherapy According to T790M Mutation Status in EGFR-Positive non-Small Cell Lung Cancer Patients After Initial EGFR-TKI Failure. Lung Cancer (2017) 109:89–91. doi: 10.1016/j.lungcan.2017.05.001
Keywords: non-small cell lung cancer, epidermal growth factor receptor, osimertinib, T790M mutation, brain metastases
Citation: Yi X-F, Song J, Gao R-L, Sun L, Wu Z-X, Zhang S-L, Huang L-T, Ma J-T and Han C-B (2022) Efficacy of Osimertinib in EGFR-Mutated Advanced Non-small-Cell Lung Cancer With Different T790M Status Following Resistance to Prior EGFR-TKIs: A Systematic Review and Meta-analysis. Front. Oncol. 12:863666. doi: 10.3389/fonc.2022.863666
Received: 30 March 2022; Accepted: 28 April 2022;
Published: 07 June 2022.
Edited by:
Paola Ulivi, Scientific Institute of Romagna for the Study and Treatment of Tumors (IRCCS), ItalyReviewed by:
Qin Wenxing, Shanghai Changzheng Hospital, ChinaMichael Shafique, Moffitt Cancer Center, United States
Copyright © 2022 Yi, Song, Gao, Sun, Wu, Zhang, Huang, Ma and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cheng-Bo Han, aGFuY2hlbmdib0Bzai1ob3NwaXRhbC5vcmc=
 Xiao-Fang Yi
Xiao-Fang Yi Jun Song
Jun Song Ruo-Lin Gao
Ruo-Lin Gao Li Sun
Li Sun Zhi-Xuan Wu
Zhi-Xuan Wu Shu-Ling Zhang
Shu-Ling Zhang Le-Tian Huang
Le-Tian Huang Jie-Tao Ma
Jie-Tao Ma