- 1School of Public Health, Fudan University, Shanghai, China
- 2Children’s Hospital, Fudan University, Shanghai, China
- 3Department of Cancer Prevention, Fudan University Shanghai Cancer Center, Shanghai, China
- 4Cancer Prevention and Control, Shanghai Municipal Center for Disease Control and Prevention, Shanghai, China
Background: Routine vaccination of infants for protecting against hepatitis B virus (HBV) infection and its serious consequences, including hepatocellular cancer (HCC), has been carried out in Shanghai, China, since 1986. We therefore have examined the trend of HBV infection and HCC incidences before and after HBV vaccination over decades to assess the potential influences of the Shanghai HBV vaccination program.
Methods: Data on incidences of HBV infection and HCC were collected from the Shanghai Cancer Registry and the Shanghai HBV vaccination follow-up study. Joint-point regression and the Bayesian age-period-cohort statistical analysis methods were used.
Results: The incidences of HBV infection dramatically declined from 23.09 and 1.13 per 100,000 for males and females in 2000 to 3.24 (-85.97%) and 0.22 (-80.53%) per 100,000 in 2014, respectively. Sero-epidemiological data from the sampling surveys during 20 years of follow-up showed that less than 1% of people undergoing HBV vaccination have a positive serum HBsAg. Consistently, the annual adjusted standardization rates (ASR) of HCC steadily fell from 33.38 and 11.65 per 100,000 for males and females in 1973 to 17.34 (-49.2%) and 5.60 (-51.9%) per 100,000 in 2014, respectively. The annual percentage change in overall HCC incidences is about -2%. HCC incidences in males at younger age groups (age <50 years old), particularly in those with age <34 groups, showed an accelerating decrease over time, whereas HCC incidences significantly declined in the female population across all age groups except for those under 19 years of age. The results supported that the universal HBV vaccination in newborns is easy to implement with high coverages and is effective for preventing both HBV infection and HCC in populations.
Introduction
Primary liver cancer including hepatocellular carcinoma (HCC, 75%–85%) and intrahepatic cholangiocarcinoma (10%–15%), as well as other rare types, is the sixth most diagnosed malignancy and the third most common cause of cancer death in the world (1). According to the International Agency for Research on Cancer (IARC) report, approximately 906,000 new cases of primary liver cancer were diagnosed worldwide in 2020, about half of them in China (1). HCC is a fatal disease; less than 5% of HCC cases survive over a year after their initial diagnosis (2). The mortality and incidence of HCC are very similar, presenting with an annual fatality ratio of 0.97 (2). Therefore, primary prevention of HCC is a clear priority to effectively reduce the disease burden.
Up to 80% of all cases of HCC worldwide have HBV and/or HCV infection. Adults who have had a chronic HBV infection since childhood develop HCC at a rate of 5% per decade, which is 100–300 times the rate among uninfected people (3, 4). Therefore, HBV is one of the most known human carcinogens only second to tobacco smoking (3, 4). Because HBV most commonly spreads from mothers to infants or to those in their early childhood and the substantial burden of HBV-related diseases developed from HBV infections in childhood, universal vaccination in infants and children has been a most effective way to prevent HBV infection and reduce the prevalence of HBsAg and incidence of HCC (5).
The first HBV vaccine was blood-derived and approved in the United States in 1981 (6). Due to the concern about the possibility of introducing viral DNA into the vaccine, the safer recombinant HBV vaccine that contains the non-infectious surface protein of HBV was then generated in the yeast Saccharomyces cerevisiae to replace the blood-derived HBV vaccine in 1986 in the United States (7). In 1991, the Advisory Committee on Immunization Practices (ACIP) in the United States recommended universal childhood vaccination for eliminating HBV transmission in the United States (8). Now, many countries have implemented a routine HBV vaccination program in infants against chronic HBV infection and HCC (5, 9–11). Notably in Taiwan, China, with high rates of HBV infection, a universal HBV vaccination program has been launched in the region since 1984, which resulted in a significant reduction of HBV infection and the incidence of childhood HCC (5). Shanghai is one of the first cities in mainland China that started the hepatitis B immunization program in infants born to HBsAg-positive mothers in 1986 (12). A universal infant immunization program then extended to the whole Shanghai city in 1989 (13). Here we analyzed the impact of hepatitis B immunization in infants on the incidences of HBV infection and HCC after three decades of the vaccination in Shanghai. Our results show that the incidence of HBV infection in the Shanghai population decreased by more than 80% and age-standardized rates (ASRs) of HCC by around 50%. There is also an accelerating decrease in the incidence of HCC at the young male population in Shanghai.
Methods
Sources of Data
The Shanghai Cancer Registry was established by the Shanghai Tumor Hospital in the early 1960s and became a population-based registry in 1972. Since 1973, the incident cases of cancers and their mortality data have covered approximately all the permanent residents in Shanghai city. In 2002, a computer-based data collection system was established and used for the Shanghai Cancer Registry by the Shanghai Municipal Center for Disease Control and Prevention (CDC). The quality of submitted data for each local registry was examined by the registration officers at the Shanghai Municipal CDC according to the published guidelines by the International Agency for Research on Cancer (IARC) and the Chinese National Cancer Registry Center (14, 15). The ICD-9 coding system was used before 2002. Between 2002 and 2008, ICD-10 and ICD-O-2 were used. ICD-O-3 has been implemented since 2008. Cancer diagnosis data are received by regional CDCs from local hospitals and community centers. Mortality data are based on death certificates, which include information regarding primary cancer site, metastasis, follow-up, and other cancer-related clinical, pathological, and treatment information.
The quality of cancer report in each year from 1973 to 2013 in Shanghai was evaluated by 1) death certificate only, DCO%, 2) histological verification, HV%, and 3) the mortality-to-incidence ratio, M/I%, according to the published method by IARC (15). The data were presented as shown in Supplementary Table 1 and Supplementary Figure 1.
Shanghai Hepatitis B Vaccination Program
Three different types of hepatitis B vaccines have been used since 1986 in Shanghai. The first hepatitis B vaccine was derived from the plasma of patients with chronical HBV infection and produced by the Shanghai Biological Manufacture. The second one, named as the Recombivax hepatitis B vaccine, was a purified hepatitis B virus surface antigen (HBsAg) that was expressed by Saccharomyces cerevisiae (i.e., common baker’s yeast). The third one is a heat-inactivated and whole recombinant Hansenula polymorpha yeast expressing HBsAg.
The plasma-derived Hepatitis B vaccine was first tested in the field in 7,470 infants born in 1986 at Huangpu district (formally named as the Nanshi district), Shanghai. These hepatitis B-vaccinated infants were then followed up for the changes of HBV immunity, including HBsAg and anti-HBs. In addition, a cohort of infants born at a neighboring district Changning, Shanghai, without hepatitis B vaccination in the same year was used as the baseline control group in this study. In 1989, the plasma-derived hepatitis B vaccine inoculation was expanded to infants from 12 districts and counties in Shanghai. In 1995, a uniformed vaccine schedule and record with the first dose to be administered within 24 h of birth and subsequent doses at ages of 1 and 6 month(s) were developed for all infants in Shanghai city and a total of 128,000 subjects were inoculated with the plasma-derived hepatitis B vaccine with a full-course inoculation rate of over 99%.
In 1997, a yeast-derived recombinant hepatitis B vaccine was imported into China to replace the plasma-derived hepatitis B vaccine and a total of 81, 661 neonates were inoculated within 24 h of birth. In 2001, a total of 158,239 subjects, including neonates, children at ages of 13 and 17, and university freshmen in Shanghai, were inoculated with the hepatitis B vaccine. Since 2002, the hepatitis B vaccination program has become a part of the National Immunization Program of China. Since then, around 200,000 or more neonates each year in Shanghai have been inoculated with recombinant hepatitis B vaccine with a full-course inoculation rate of 99% or more.
Hepatitis B Vaccination Follow-Up Study in Huangpu and Changning Districts, Shanghai
Blood samples had been collected from the hepatitis B-vaccinated infants at Huangpu district, Shanghai, and the hepatitis B-unvaccinated infants (baseline control group) at Changning district once every 2 years for a total of 28 years through a sampling approach. Serum HBsAg, anti-HBs, and anti-HBc levels were determined using enzyme-linked immunosorbent assay (ELISA) kits from Abbott Laboratories (Abbott Park, IL, USA), according to the manufacturer’s protocol. The anti-HBs cutoff value was ≥10.0 mIU/ml, and the anti-HBc sample value/cutoff value (S/CO) was ≥1.00.
Statistical Analysis
The incidence of HCC among the population in each birth cohort was calculated by dividing the number of HCC cases by the total number of person years observed when the subjects were including the total population within Shanghai city. The standardized incidence rates in different diagnostic years ranging from 1973 to 2012 were adjusted by the 1960 world standard population. The trend of HCC incidences before (1973 to 2006) and after (1973 to 2012) hepatitis B vaccination was analyzed by joint-point regression analysis, as described by Kim et al. (16).
The Bayesian Age-Period-Cohort approach was used to analyze changes in HCC incidences in Shanghai by gender, age group, time period, and birth cohort and to project the incidence and mortality rates of HCC in Shanghai from years 2013 to 2020 based on the demographic shifts. The Poisson linear model was applied to estimate the annual percentage change (EAPC). These analyses were conducted with EAPC (17, 18) in Epi software package and INLA (19, 20) procedure in R software package (Version 3.3.3).
Age-Period-Cohort model for incidences of HCC
Results
The Trend of HCC and Different Types of Viral Hepatitis Incidences From 1973 to 2014 in Shanghai, China
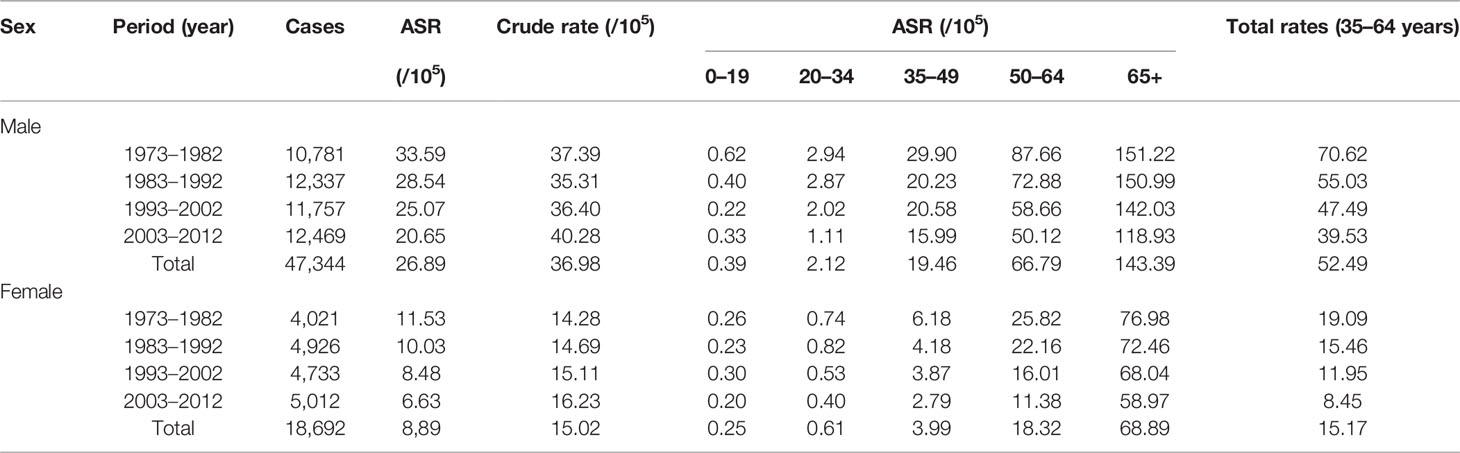
Figure 1A and Supplementary Table 2 show a continuously decreasing trend of HCC incidences from 1973 to 2014 in Shanghai City, China. The age-standardized rates (ASRs) of HCC were 33.38 in males and 11.65 in females per 100,000 in 1973 compared to 17.34 in males and 5.60 in females in 2014, respectively. The incidences of male and female HCC decreased by 49.2% and 51.9% from 1973 to 2014, respectively.
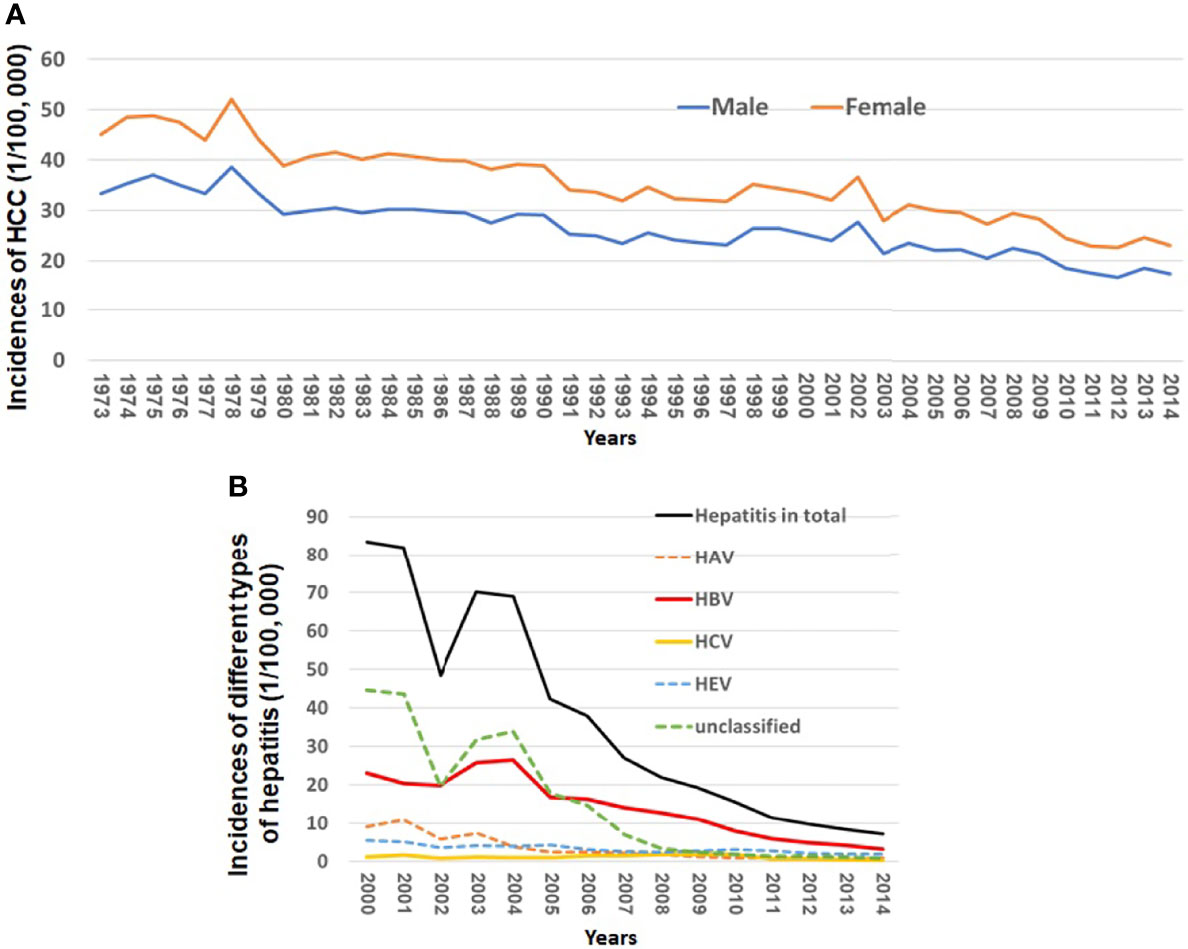
Figure 1 Incidences of HCC and different types of viral hepatitis from 1973 to 2014 in Shanghai City, China. (A) Incidences of HCC from 1973 to 2014 in Shanghai City and (B) incidences of HAV, HBV, HCV, HEV, and unclassified hepatitis from 2000 to 2014 in Shanghai City.
Virus infection-related hepatitis can be divided into at least five types, namely, hepatitis A, B, C, and E and unclassified hepatitis. The incident cases of the viral infection related to hepatitis in Shanghai have been reported to the Shanghai Municipal CDC since 2000. The incidences of such viral infection in total decreased by 91.48% from 83.41 per 100,000 in 2000 to 7.11 per 100,000 in 2014 (Figure 1B). Among all types of hepatitis, the chronic infection of HBV or HCV plays a major role in development of HCC. Figure 1B and Supplementary Table 3 show that the incidences of new HBV and HCV infection declined by 85.96% from 23.0 per 100,000 in 2000 to 3.24 in 2014 per 100,000 and by 80.53% from 1.13 per 100,000 to 0.22 per 100,000 in 2014, respectively.
The Protective Effect of HBV Vaccine Against HBV Infection in the HBV-Vaccinated 1986 Birth Cohort in Huangpu District Compared to the Unvaccinated 1984 Baseline Birth Cohort in Changning District, Shanghai
The vaccine-induced immunity against HBV infection in the population was evaluated by three serum biomarkers, namely, HBsAg and anti-HBs and anti-HBc antibodies, from subjects who were selected randomly or non-randomly from the name lists of HBV-vaccinated infants (based on the consents or availability of the study subjects) in the population and followed up for 20 years since their HBV vaccination. Table 1 shows that the positive rates of HBsAg were less than 1% ranging from 0.19% to 0.98% and that the positive rates of anti-HBs antibody and anti-HBc antibody were from 20. 65% to 89. 01%, and from 0. 96% to 3. 40%, respectively, during the past 20 years after the HBV vaccination in the population.
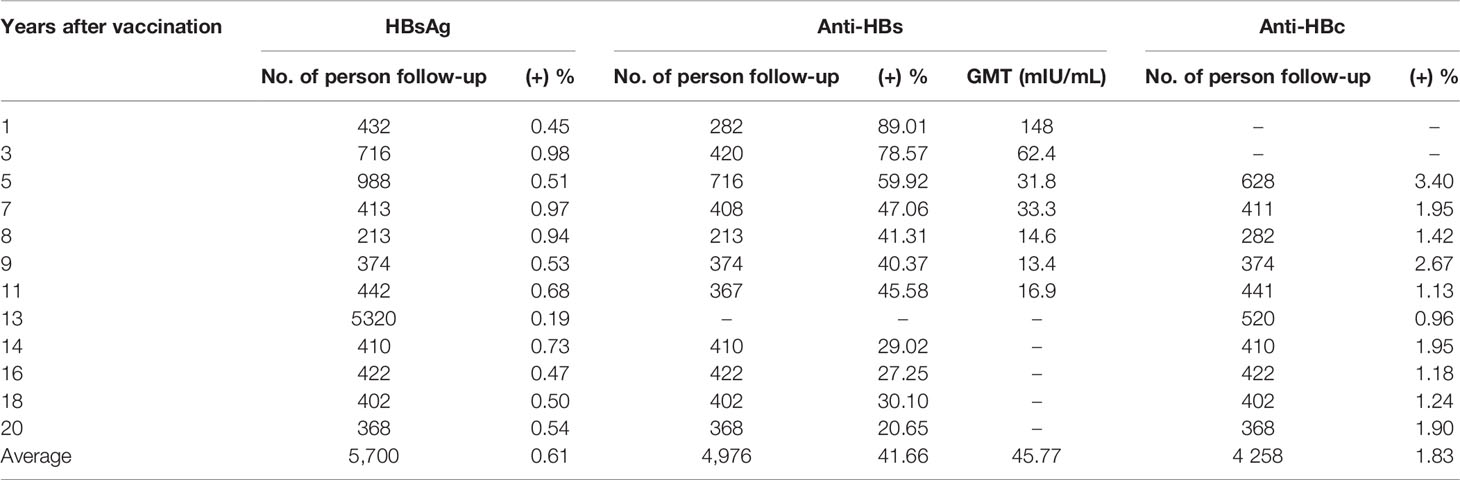
Table 1 Positive rates of HBV immunity-related biomarkers in randomly selected groups each year in the follow-up cohort of 7470 HBV-vaccinated infants in Shanghai (12).
In addition, infants born in a neighboring district Changning, Shanghai City, in 1984 served as a baseline control birth cohort. Figure 2 shows that the HBsAg-positive rates in different ages in the 1986 birth cohort at Huangpu district were all below 0.8%, whereas the HBsAg-positive rates in the unvaccinated population in Changning district exhibited an age-dependent increase from 2.47% for those age less than 2 years old to 14.21% for those age equal or more than 21 years old. This result supports a significant protective effect of HBV vaccination against HBV infection in the 1986 birth cohort at Huangpu district.
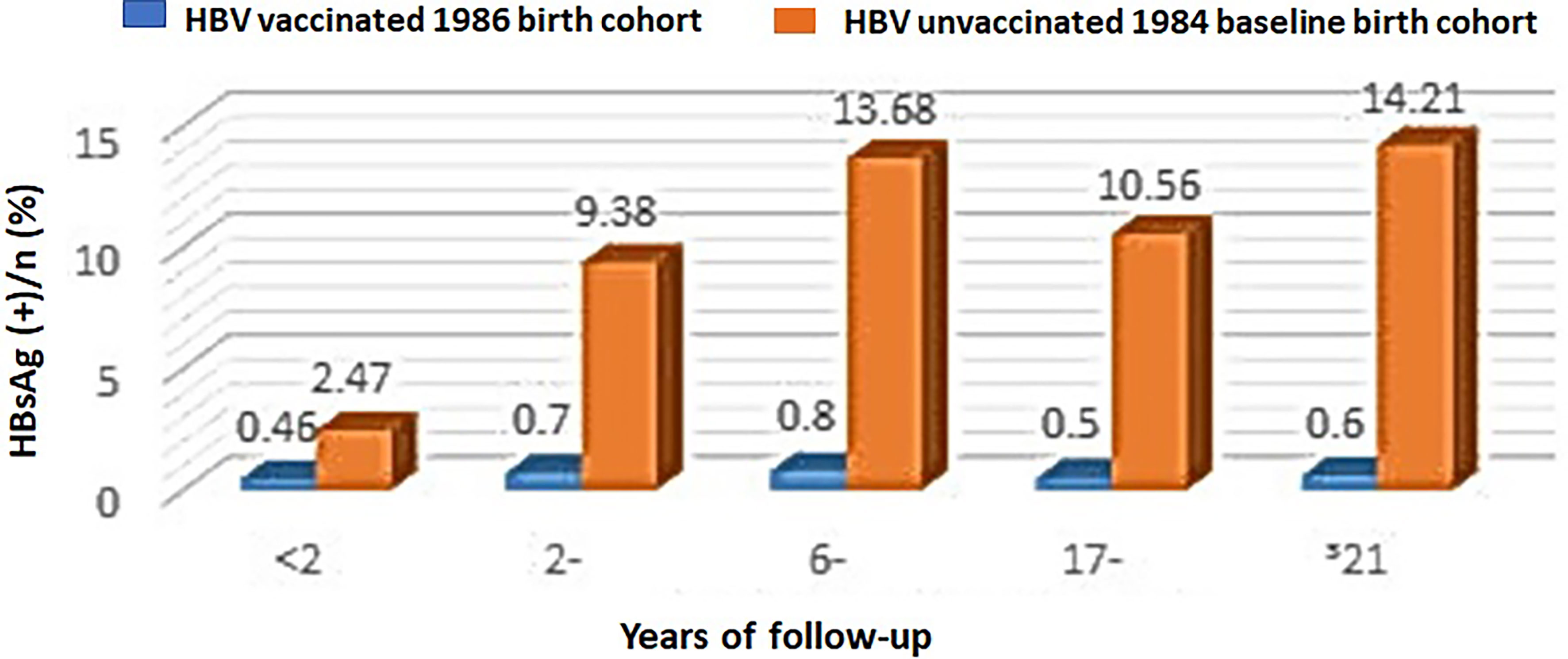
Figure 2 HBsAg-positive rates in the HBV-vaccinated 1986 birth cohort at Huangpu district versus the unvaccinated 1984 birth cohort at Changning district in Shanghai City, China.
The Decreasing Rates of HCC Incidences Before and After HBV Vaccination and in Different Age Groups in Shanghai
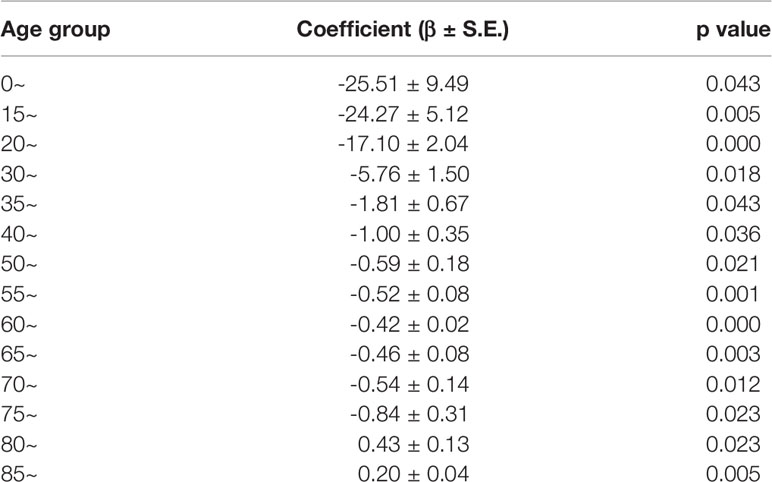
Universal hepatitis B vaccination was extended to all newborns and school students under the age of 12 years in Shanghai to protect against HBV infection and its complications including liver cirrhosis and HCC. We therefore analyzed rates of change in HCC incidences before and after the universal HBV vaccination in Shanghai City by using joint-point regression analysis. Regression coefficient β represents the rate of changes in HCC incidences during the indicated periods shown in Table 2. There is no significant difference in the overall decreasing rates of HCC incidences in all age groups between periods of 1979 to 1999 and 2000 to 2012 (Table 3). However, HCC incidences in the age 0–14, 15–19, and 20–29 groups exhibited an accelerated decrease with the negative two digital regression coefficients. The regression coefficients in age between 30 and 49 and in age of 50 and more are negative for a single digital and less than a single digital, respectively. This result suggested that the HCC incidences dropped significantly in all age groups during the past years in Shanghai, while the same rates in those aged 29 and below had an accelerated decline.
In addition, we have carried out a birth cohort analysis for changes of HCC incidences during the period of 1973–2012. Tables 4A, B show that the incidences of HCC in all age groups decreased over time and the estimated annual percentage changes in the male age group from 20 to 34 and the female age group from age more than 20, respectively, had the most significant decrease.
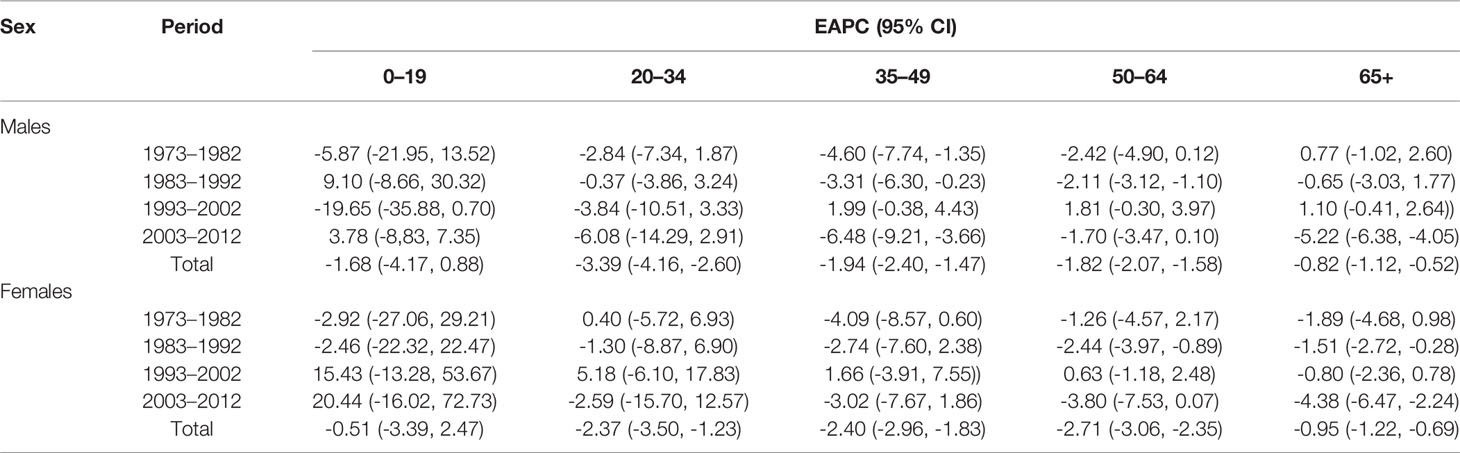
Table 4B The estimated annual percentage changes (EAPC) of liver cancer incidence in Shanghai from 1973 to 2012.
Discussion and Conclusion
Chronic HBV infection has been a major public health issue in China. The majority is infected via mother-to-child transmission in endemic areas (21). The risk of developing chronic infection was also inversely related to the age onset as approximately 90% of individuals who are infected perinatally with HBV are likely to develop chronic infection (21). Therefore, a key strategy for the prevention of HBV chronic infection is universal HBV vaccination in infants beginning at birth (5). To protect people from HBV infection, a plasma-derived inactivated hepatitis B vaccine was first produced in Shanghai, China, in 1983 and 7,470 newborns in Huangpu district, Shanghai City, were inculcated with the vaccine within 24 h and at 1 and 6 month(s) after birth in 1986. The coverage rates of the three-dose hepatitis B vaccine reached to 95% or more. HBsAg-positive rates in the population ranged from 0.19% to 0.98% during the 20 years of follow-up after the vaccination, whereas the HBsAg-positive rates in the unvaccinated population at Changning district, Shanghai, were at higher levels ranging from 2.47% to 14.21%. The HBV vaccination-related seroprevalence of anti-HBs ranged from 89.01% to 20.65% positive rates during the 20 years of follow-up. In some subjects, the protective anti-HBs lasted for at least 20 years and the antibody titrations were more than 10 mIU/ml in the recipients’ serum. Our results clearly indicate that the plasma-derived inactivated hepatitis B vaccine has provided highly protective effects for newborns against HBV infection.
Chronic HBV infection is a major risk factor for HCC. The hepatitis B virus integrates its viral DNA into the host genome and induces chronic necroinflammation and mutations during the infection, which can progress to HCC over a period of decades (22). The lifetime risk of chronic HBV infection for developing HCC was estimated to be between 10% and 25% (4). Therefore, universal hepatitis B vaccination in newborns is an obvious key strategy for primary prevention of HCC. Indeed, Chiang et al. (23) reported a decrease in HCC incidence and mortality by 80% and 92%, respectively, 30 years after the initiation of the whole-Taiwan Hepatitis B Immunization Program in 1984. The Shanghai Hepatitis B Immunization Program started in 1986 with the plasma-derived inactivated hepatitis B vaccine given to newborns in Huangpu district. Coverage was then extended to all infants and preschool and primary schoolchildren using the uniformed vaccine schedule and records during 1989 to 1995. The plasma-derived inactivated hepatitis B vaccine was substituted by recombinant HBV vaccines in 1996, and the vaccine inoculation coverage reached to 96% of all three dosages in infants. Students at ages of 13 and 17 and university freshmen in Shanghai were also inoculated with the HBV vaccine in 1997 with 86% of the population covered. In 2001, more than 6 million infants in each year were inoculated with the recombinant HBV vaccine. The initiation of the Shanghai Hepatitis B Immunization Program was accompanied by a decrease in HCC incidences after 30 years by 49.2% in males and 51.9% in females, respectively. However, the reduction of HCC incidences in Shanghai (by about 50%) is less effective compared to the Taiwan Hepatitis B Immunization Program that is up to an 80% decrease in HCC incidence. The prevalence of HBsAg positivity is similar in the examined populations of these two HBV vaccination programs and less than 1%. This discrepancy suggests that other potential factors, such as food contamination with aflatoxin and algal hepatotoxins in drinking water, may still play an oncogenic role in HCC in Shanghai.
In addition, we observed an accelerated decrease in HCC incidences in young adults, in particular for groups of males with an age <34 30 years after the Shanghai Hepatitis B Immunization Program started. This observation is consistent with the reports from Taiwan and many other countries, such as Singapore and Spain, in their implemented HBV vaccination programs in 1980s (24–31). However, our results also showed a similar decrease in HCC incidences in the female population across all the age groups that are more than 19 years old. The results suggest that the HBV vaccination program may have different effects on the reduction in HCC incidence rates between males and females at different ages. Currently, there is very little information or studies for explaining this observation. It has been shown that the incidence rates of female HCC peak consistently by 5 years compared to those of male HCC across different areas of the world regardless of the large variation in their age distributions in different countries or regions (32). It is possible that the other risk factors such as alcohol consumption and tobacco smoking are less common in females and there is a significant difference in sex hormones and hormonal immunity. Such factors may play a role in the differential effects of HBV vaccination on males and females. Further studies are needed to confirm and investigate this phenomenon.
In conclusion, the universal HBV vaccination in newborns is easy to implement with high coverages and is very cost-effective for the prevention of both HBV infection and HCC in populations. However, in HCC-endemic areas, such as Shanghai, more comprehensive prevention measures like improvement of water quality and elimination of aflatoxin-contaminated foods, and drinking less alcohol are also needed to achieve better control of HCC incidences. In addition, the potential difference of HBV vaccination effects on male and female populations is very intriguing and deserves further investigation.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
SY: project design, concept, analysis, funding resource, and manuscript writing. QZ: data collection and resources. YZ: data collection, analysis, and resources. CW: data collection and resources. HR: data collection and resources. XL: data collection and resource. ZL: data collection and resources. YL: data collection and resources. QP: data collection and resources. Y-JZ: data collection and resources. All authors contributed to the article and approved the submitted version.
Conflict of Interest
WS is a member of the CT Advisory Board of Philips Medical Systems.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Dr. Zhu GH supported us with the data of HB vaccine used in Shanghai.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.855945/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Yu SZ, Zheng Y, Wu CX, Zheng YJ. Prevention and Control Hepatocellular Carcinoma in Shanghai During 40 Years. Chin J Epidemiol (2013) 34:637–41. doi: 10.3760/cma.j.issn.0254-6450.2013.06.024
3. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer Statistics in China, 2015. CA Cancer J Clin (2016) 66:115–32. doi: 10.3322/caac.21338
5. Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, et al. Universal Hepatitis B Vaccination in Taiwan and the Incidence of Hepatocellular Carcinoma in Children. Taiwan Childhood Hepatoma Study Group. N Engl J Med (1997) 336:1855–9. doi: 10.1056/NEJM199706263362602
6. Francis DP, Hadler SC, Thompson SE, Maynard JE, Ostrow DG, Altman N, et al. The Prevention of Hepatitis B With Vaccine. Report of the Centers for Disease Control Multi-Center Efficacy Trial Among Homosexual Men. Ann Intern Med (1982) 97:362–6. doi: 10.7326/0003-4819-97-3-362
7. Fisher LM. Special To the New York Times (October 13, 1986). Available at: https://www.nytimes.com/1986/10/13/business/biotechnology-spotlight-now-shines-on-chiron.html (Accessed March 5, 2022).
8. Hepatitis B Virus: A Comprehensive Strategy for Eliminating Transmission in the United States Through Universal Childhood Vaccination. Recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR Recomm Rep (1991) 40:1–25.
9. Peto TJ, Mendy ME, Lowe Y, Webb EL, Whittle HC, Hall AJ. Efficacy and Effectiveness of Infant Vaccination Against Chronic Hepatitis B in the Gambia Hepatitis Intervention Study (1986-90) and in the Nationwide Immunisation Program. BMC Infect Dis (2014) 14:7. doi: 10.1186/1471-2334-14-7
10. Bonanni P, Pesavento G, Boccalini S, Bechini A. Perspectives of Public Health: Present and Foreseen Impact of Vaccination on the Epidemiology of Hepatitis B. J Hepatol (2003) 39:S224–9. doi: 10.1016/S0168-8278(03)00315-5
11. Hadler SC, Fuqiang C, Averhoff F, Taylor T, Fuzhen W, Li L, et al. The Impact of Hepatitis B Vaccine in China and in the China GAVI Project. Vaccine (2013) 31S:J66–72. doi: 10.1016/j.vaccine.2013.03.043
12. Zhang HZ, Wu WS, Su F, Sun CM, Jiang MB, Zhang GH, et al. Valuation on the Immunization Efficacy on the 23 Years Who had Received Plasma-Derived HBV Vaccine as Newborns. Zhonghua Liu Xing Bing Xue Za Zhi (2012) 33:207–9. doi: 10.3760/cma.j.issn.0254-6450.2012.02.018
13. Wang WL, Shu ZJ, Zhou LX, Zhao YR. Clinical Characteristics of Hepatitis B Virus Infection in Middle School Students Born After the Universal Infant Vaccination Program in Shanghai, China. Arch Virol (2012) 157:901–5. doi: 10.1007/s00705-012-1251-9
14. National Central Cancer Registry. Chinese Guideline for Cancer Registration Vol. 4p. Beijing: People’s Medical Publishing House (2016).
15. Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, et al. Cancer Incidence in Five Continents. Volume Ix. Lyon: IARC Sci Publication (2008) p. 1–837.
16. Kim HJ, Luo J, Kim J, Chen HS, Feuer EJ. Clustering of Trend Data Using Joinpoint Regression Models. Stat Med (2014) 33:4087–103. doi: 10.1002/sim.6221
17. Riebler A, Held L. Projecting the Future Burden of Cancer: Bayesian Age-Period-Cohort Analysis With Integrated Nested Laplace Approximations. Biom J (2017) 59:531–49. doi: 10.1002/bimj.201500263
18. Carstensen B. Age-Period-Cohort Models for the Lexis Diagram. Stat Med (2007) 26:3018–45. doi: 10.1002/sim.2764
19. Blangiardo M, Cameletti M, Baio G, Rue H. Spatial and Spatio-Temporal Models With R-INLA. Spat Spatiotemporal Epidemiol (2013) 7:39–55. doi: 10.1016/j.sste.2013.07.003
20. Besag J, Green P, Higdon D, Mengersen K. Bayesian Computation and Stochastic Systems (With Discussion). Stat Sci (1995) 10:3–66. doi: 10.1214/ss/1177010123
21. Le MH, Yeo YH, So S, Gane E, Cheung RC, Nguyen MH. Prevalence of Hepatitis B Vaccination Coverage and Serologic Evidence of Immunity Among US-Born Children and Adolescents From 1999 to 2016. JAMA Netw Open (2020) 3:e2022388. doi: 10.1001/jamanetworkopen.2020.22388
22. McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology (2021) 73(Suppl 1):4–13. doi: 10.1002/hep.31288
23. Chiang CJ, Yang YW, You SL, Lai MS, Chen CJ. Thirty-Year Outcomes of the National Hepatitis B Immunization Program in Taiwan. JAMA (2013) 310:974–6. doi: 10.1001/jama.2013.276701
24. Yuen MF, Hou JL, Chutaputti A. Asia Pacific Working Party on Prevention of Hepatocellular Carcinoma: Hepatocellular Carcinoma in the Asia Pacific Region. J Gastroenterol Hepatol (2009) 24:346–53. doi: 10.1111/j.1440-1746.2009.05784.x
25. Borràs E, Urbiztondo L, Carmona G, Jané M, Barrabeig I, Rosa Sala M, et al. Effectiveness and Impact of the Hepatitis B Vaccination Program in Preadolescents in Catalonia 21 Years After its Introduction. Vaccine (2019) 37:1137–41. doi: 10.1016/j.vaccine.2019.01.024
26. Denis F, Levy-Bruhl D. Mass Vaccination Against Hepatitis B: The French Example. Curr Top Microbiol Immunol (2006) 304:115–29. doi: 10.1007/3-540-36583-4_7
27. Da Villa G. Successful Mass Vaccination Against Hepatitis B Virus in a Hyperendemic Area in Italy. Res Virol (1993) 144:255–8. doi: 10.1016/S0923-2516(06)80036-1
28. Zhou YH, Wu C, Zhuang H. Vaccination Against Hepatitis B: The Chinese Experience. Chin Med J (Engl) (2009) 122:98–102. doi: 10.3760/cma.j.issn.0366-6999.2009.01.018
29. El-Raziky MS, El-Hawary MA, Salama KM, El-Hennawy AM, Helmy HM, Fahmy ME, et al. Patterns of Hepatitis B Infection in Egyptian Children in the Era of Obligatory Hepatitis B Vaccination. Arab J Gastroenterol (2012) 13:1–3. doi: 10.1016/j.ajg.2012.02.003
30. Chub-uppakarn S, Panichart P, Theamboonlers A, Poovorawan Y. Impact of the Hepatitis B Mass Vaccination Program in the Southern Part of Thailand. Southeast Asian J Trop Med Public Health (1998) 29:464–8.
31. Luo Z, Li L, Ruan B. Impact of the Implementation of a Vaccination Strategy on Hepatitis B Virus Infections in China Over a 20-Year Period. Int J Infect Dis (2012) 16:e82–8. doi: 10.1016/j.ijid.2011.10.009
Keywords: hepatitis B virus, mother–infant transmission, hepatitis B vaccination, hepatocellular carcinoma (HCC), prevention
Citation: Yu S, Zhu Q, Zheng Y, Wu C, Ren H, Liu X, Liu Z, Li Y, Pan Q and Zheng Y-J (2022) Accelerating Decreases in the Incidences of Hepatocellular Carcinoma at a Younger Age in Shanghai Are Associated With Hepatitis B Virus Vaccination. Front. Oncol. 12:855945. doi: 10.3389/fonc.2022.855945
Received: 16 January 2022; Accepted: 09 March 2022;
Published: 04 April 2022.
Edited by:
Jianhua Yin, Second Military Medical University, ChinaReviewed by:
Wafaa M. Rashed, Children’s Cancer Hospital, EgyptZhao Li, Peking University People’s Hospital, China
Copyright © 2022 Yu, Zhu, Zheng, Wu, Ren, Liu, Liu, Li, Pan and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shunzhang Yu, c3p5dUBzaG11LmVkdS5jbg==
 Shunzhang Yu1*
Shunzhang Yu1* Ying Zheng
Ying Zheng Xing Liu
Xing Liu Ying-Jie Zheng
Ying-Jie Zheng