
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 23 May 2022
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.855909
Background: Microwave ablation (MWA) for hepatocellular carcinomas (HCCs) in the elderly has been the subject of new research in recent years. However, there are currently no strong lines of evidence for the prognosis following MWA treatment for HCC in the elderly. Therefore, we conducted a systematic review to assess the safety and feasibility of MWA for HCC in elderly patients.
Methods: Up until August 15, 2021, a comprehensive literature search was undertaken in PubMed, Scopus, CENTRAL (Cochrane Central Register of Controlled Trials), and Google Scholar databases for all published articles. Observational studies reporting the safety and feasibility of MWA for HCC in elderly patients were included. The Newcastle–Ottawa Scale (NOS) was used to measure the quality assessment.
Results: Our review, composed of 7 observational studies, including a total of 7,683 HCC patients, looked at the safety and feasibility of MWA for HCC in the elderly. Current lines of evidence on the risks and outcomes of MWA of HCC treatments in elderly patients are discussed.
Conclusions: According to our findings, elderly patients, even those with a high comorbidity index, benefited from MWA of HCC similar to younger patients. More clinical data are needed to determine selection criteria for elderly HCC patients to increase the possibility of receiving MWA as a potential lifesaving option. As such, further studies evaluating the outcomes of MWA for HCC treatment modalities in elderly patients are warranted.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42021273091.
Hepatocellular carcinoma (HCC), the most prevalent primary liver cancer, is the world’s fifth most common cancer and the third leading cause of cancer-related death (1). As life expectancy has increased, the number of older people with HCC has also increased (2). It is widely acknowledged that aging is a risk factor for HCC development (3). Recent studies from the United States, the United Kingdom, and Japan have found a significant age-related rise in the development of HCC in those over the age of 75 (4). In the elderly aged >71 years, it has been found that liver weight (5) and portal blood flow velocity decrease (6), resulting in a reduced liver repair capability compared to younger people. As a result, elderly individuals with liver cancer can expect a worse prognosis after treatment. As human life expectancy increases, a number of studies have advised that the minimum age for elderly groups should be 75 years (7, 8). The majority of studies have found that age distribution at HCC diagnosis has shifted over time, and that those 65 and older with HCC had fewer effective treatments and worse prognoses than younger adults (2, 9, 10).
Management of malignant diseases in elderly individuals is becoming a prominent concern worldwide as the population ages due to improved treatment and healthcare (11). For the majority of older persons, surgery or liver transplantation is difficult (12). As a result, novel therapeutic techniques for the treatment of HCC, such as local radical ablation, targeted chemotherapeutic drugs, and radiation therapy, continue to be researched and developed (13). Hepatic resection (HR) is generally considered as the first-line treatment for HCC patients (10). Studies have shown the feasibility and safety of HR for elderly patients with HCC. The indication of HR is limited because of comorbidity or a poor general status of elderly patients (10). Radiofrequency ablation (RFA), trans-arterial chemoembolization (TACE), and microwave ablation (MWA) have received recognition as alternative treatment strategies as local ablation therapy for HCC treatment (14–17). Among these local ablation therapies, only the efficacy and safety of RFA have been reported in elderly patients with HCC (14, 18–21).
Both RFA and MWA rely on thermal injury, but MWA uses an electromagnetic field as opposed to electrical current used in RFA. Unlike MWA, the effect of RFA is partially limited by the heat-sink effect and increased impedance of the ablated tissue (22). Compared with RFA, MWA attains a more predictable ablation zone, permits simultaneous treatment of multiple lesions, and achieves larger coagulation volumes in a shorter procedural time (23).
Compared to RFA, MWA has a few advantages. First, the heat-sink effect, which occurs when thermal energy from the target lesion is distributed due to blood flow in nearby vessels, is a significant disadvantage of RFA (22). Second, the time required for MWA ablation is smaller than that required for RFA. Third, MWA has the ability to deliver higher temperatures in the ablation zone (24). MWA’s two characteristics result in a more predictable ablation zone (25–27). MWA zones are uniform in shape and size and are not impacted by convective heat loss (24, 28). Because of these benefits, MWA has become a popular therapeutic option for hepatic malignancies. Recently, emerging studies have evaluated the efficacy of MWA of HCC in the elderly with conflicting findings (9, 29–34). Therefore, we conducted this systematic review to assess the safety and feasibility of MWA for HCC in elderly patients.
This study is a systematic review for the critical assessment and evaluation of all published literatures investigating MWA in the elderly population with HCC.
Ethical clearance for this manuscript was not required because it was a systematic review performed by using prevailing published data.
This meta-analysis was conducted in accordance with standard guidelines using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement35 and has a PROSPERO number CRD42021273091.
All studies (controlled or uncontrolled) reporting outcomes of MWA for elderly patients with HCC.
(a) Duplicate studies, case series, case reports, systematic reviews, conference abstracts, preprints, and editorials; (b) studies that do not describe relevant outcomes; and (c) full texts are unavailable.
This systematic review was performed following the guidelines of the PRISMA (35) and Cochrane (36). An electronic search of PubMed, Scopus, CENTRAL (Cochrane Central Register of Controlled Trials), and Google Scholar databases was performed for English language papers published up to August 15, 2021. Searches were performed using keywords including “microwave”, OR “microwave ablation”, AND “Elderly”, AND “liver transplantation”, AND “Hepatocellular Carcinoma”, OR “HCC”. Reference lists of the identified studies and relevant reviews on the subject were also scanned for additional studies.
Two authors (JZ and HZ) independently extracted the following information from each included study: first author name, country, ethnicity, year of publication, duration of the study, number of patients, treatment methods, study design, duration of the study, group investigated, sample size, number of male/female patients, mean age, cutoff age for elderly definition, tumor size, number of single or multiple tumors, Model For End-Stage Liver Disease (MELD) score, Child–Pugh score, hospital stay duration, objectives, endpoints, and conclusions. Additional information on technical efficacy, local tumor progression, frequency of complications, quality of life, and duration of hospital stay was also extracted from the available included studies. At each stage, publications were examined twice, with conflicts addressed by consensus or adjudication by a third reviewer (DZ).
Assessment of the quality of the included studies was conducted by using the Newcastle–Ottawa Scale (NOS) (37). The NOS comprises the following three aspects: selection of study subjects (4 points), comparability of study subjects (2 points), and exposure or outcomes (3 points). The total score ranges from 0 to 9, and those with a score ≥ 6 were considered as high-quality studies. Two authors independently rated the study’s quality. Any discrepancies in the quality scores were resolved by consensus among the authors.
Only descriptive analysis of results was performed.
The initial search generated 339 records, as shown in Figure 1. A total of 127 records were checked after duplicates were removed. After carefully reading the titles and abstracts, 29 articles were selected for further eligibility. Finally, after evaluating full texts, 22 articles were removed due to insufficient data or overlapping data, leaving the current systematic review with 7 total studies.
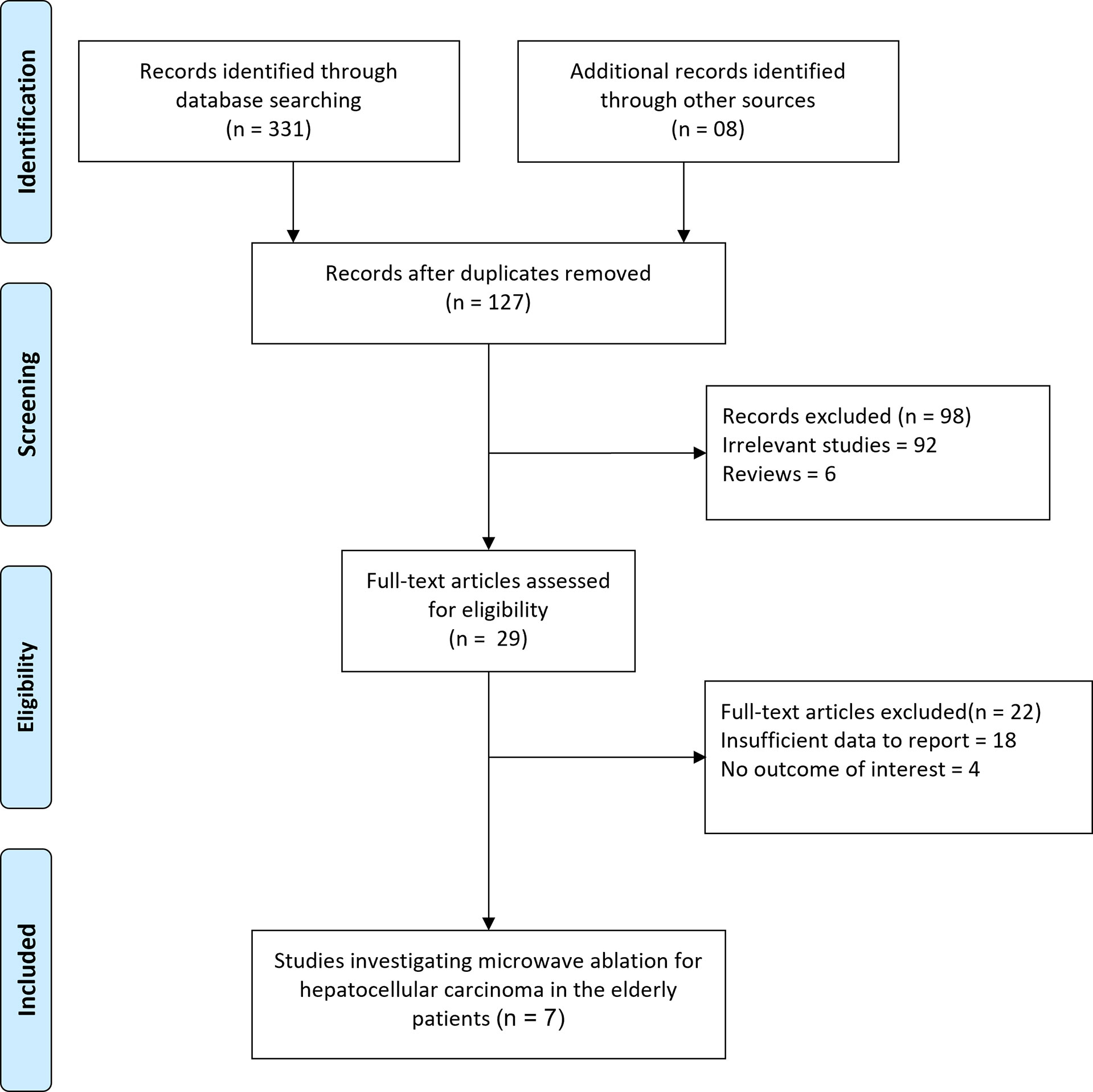
Figure 1 Flow diagram for the selection of studies and specific reasons for exclusion from the present meta-analysis.
Seven observational studies (9, 29–34) involving 7,683 HCC patients were included in our systematic review. All included studies were retrospective cohort studies and the published year ranged between 2018 and 2020 with sample sizes of HCC patients ranging from 30 to 2,389. Baseline and clinical characteristics for the included studies are shown in Tables 1 and 2. Three studies were conducted in Caucasian patients, while 4 studies were conducted in Asian patients. The median age ranged from 71.4 years to 82.3 years old and median tumor size ranged from 2.4 to 3.2 cm.
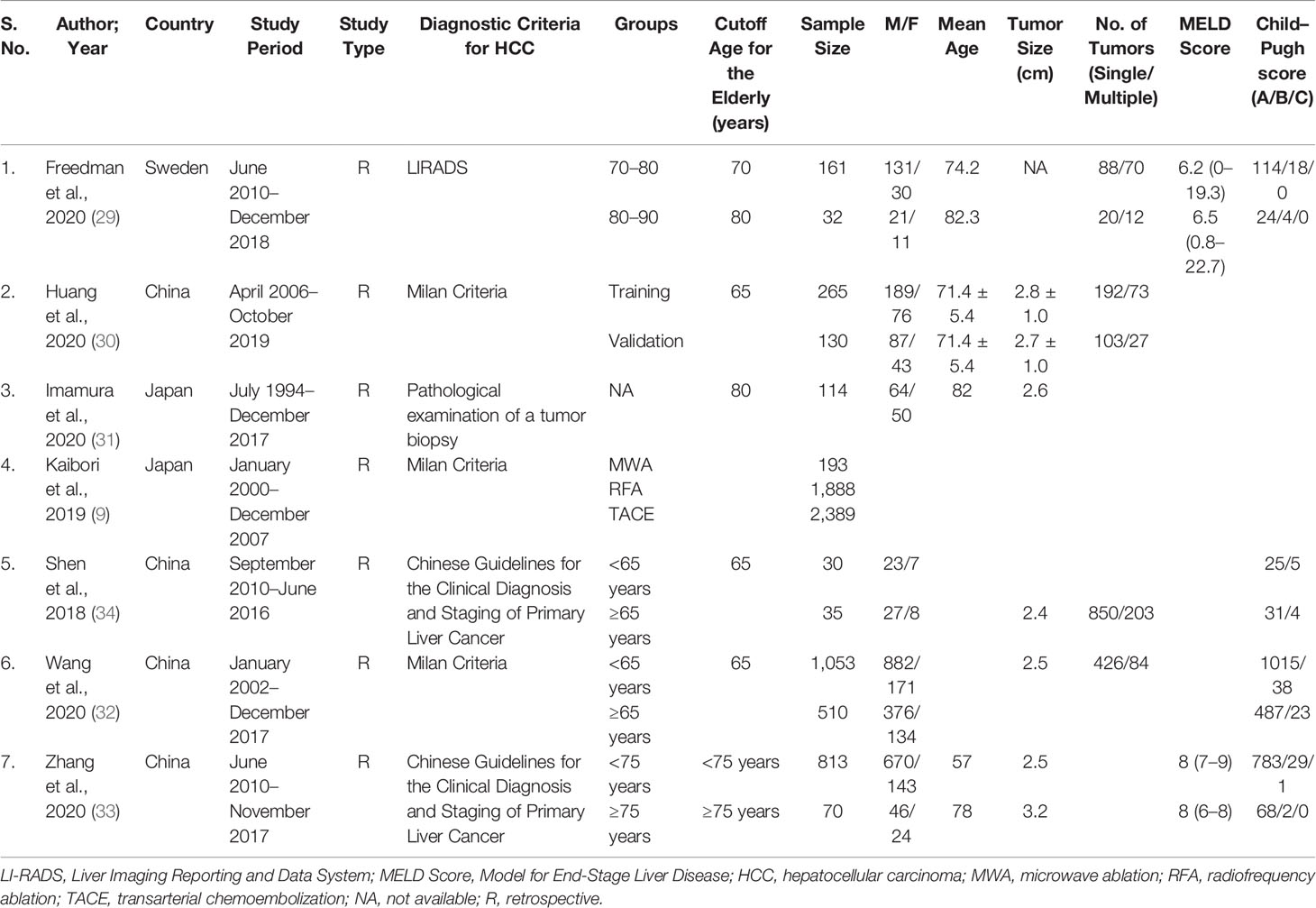
Table 1 Baseline characteristics of included studies in the systematic review investigating microwave ablation for hepatocellular carcinoma in elderly patients.
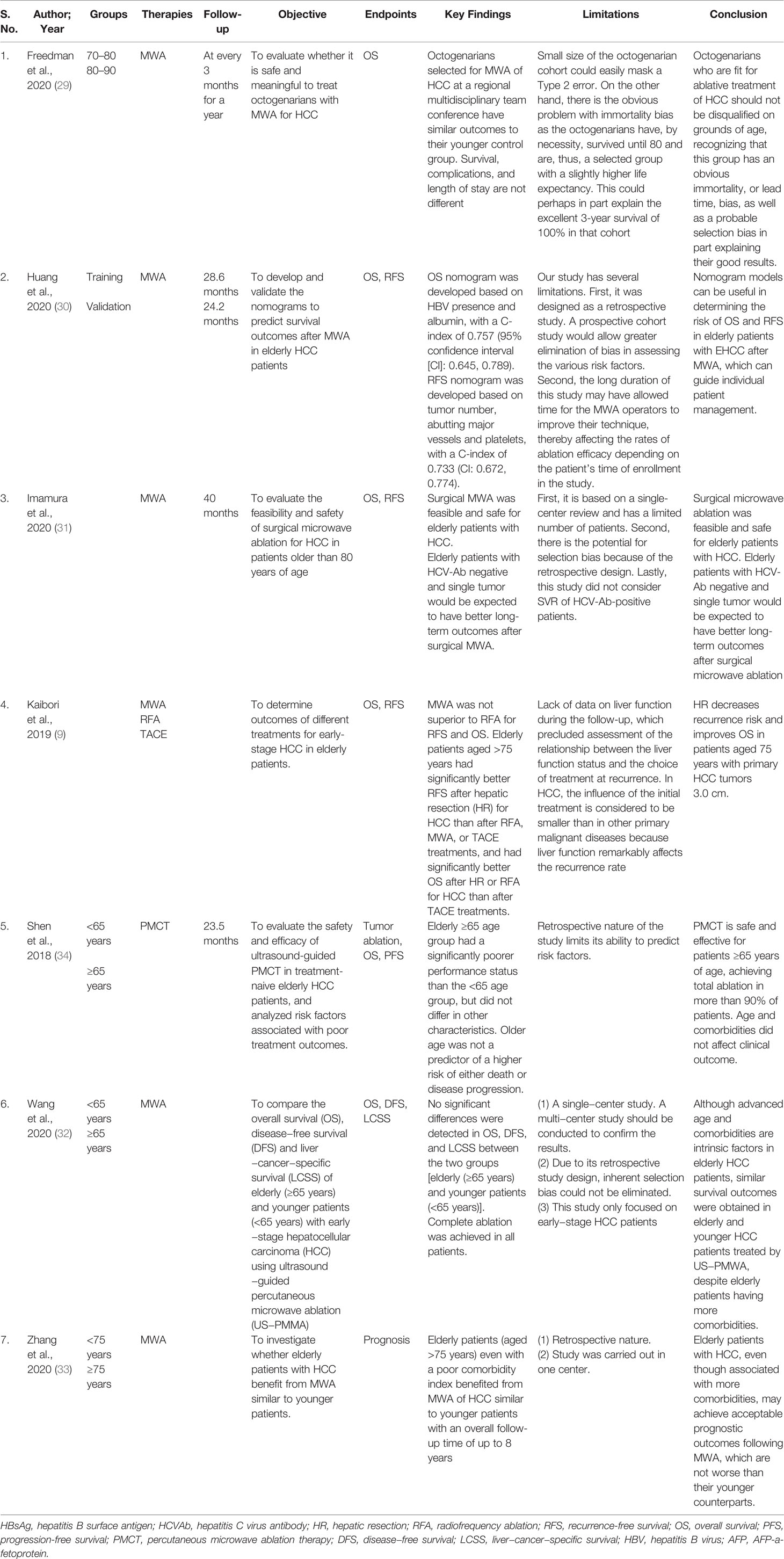
Table 2 Descriptive summary of findings for the included studies investigating microwave ablation for hepatocellular carcinoma in elderly patients.
Three studies reported Milan criteria (9, 30, 32), one study used LICRADS (29), one used pathological examination of a tumor biopsy (31), and two studies used Chinese guidelines for the clinical diagnosis and staging of primary liver cancer (33, 34) as the diagnostic criteria for defining HCC. Only two studies (29, 33) reported MELD Score data and four studies (29, 32–34) reported Child–Pugh score data. However, data to determine outcomes of different treatments (MWA/RFA and TACE) for early-stage HCC in elderly patients were only reported by a single study (9). Majority of the studies included were of good quality, with a NOS of six or higher (Table 3).
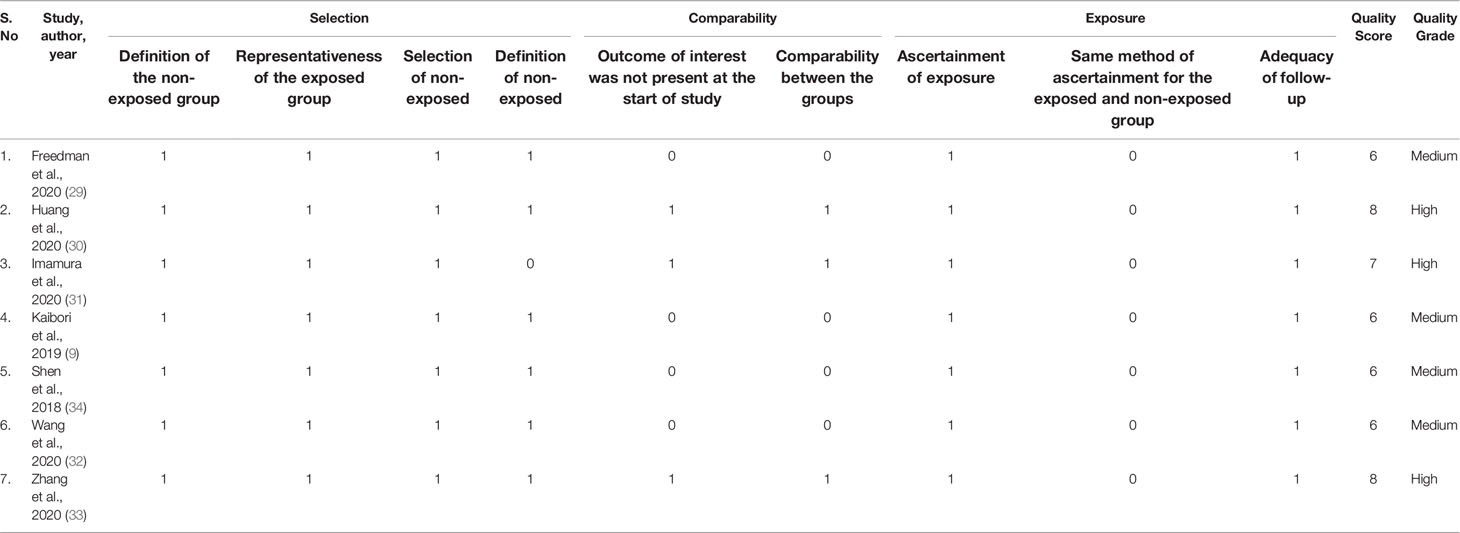
Table 3 Quality assessment of the included studies in the systematic review using the Newcastle–Ottawa Scale.
Different study protocols were used in all the included seven studies as mentioned briefly in Table 4 for the type of approach, type of MWA device, type of MW needle, ablation per lesion, average ablation time, and average ablation energy. Only five studies reported the type of MWA device used, namely, MTC-3C (Nanjing, China) (30), Microtaze generator (Alfresa Pharma, Osaka, Japan) (32), FORSEA MTC3C microwave tumor therapy system (Nanjing Qinghai) (35), and KY−2000 (Kangyou Medical, Nanjing, China) [KY-2,00,02,450 MHz (32) and KY-21,00,915 MHz (33)]. The high degree of heterogeneity for the data availability in all the included studies restricted us to perform any analysis in order to reach any conclusive point.
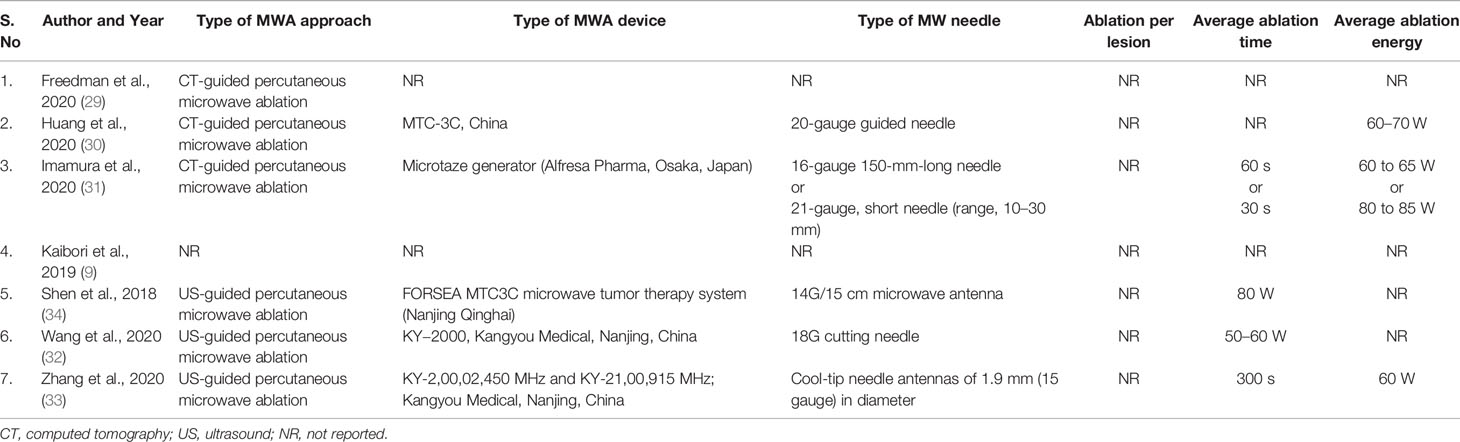
Table 4 Detailed MWA characteristics of included studies in the systematic review investigating microwave ablation for hepatocellular carcinoma in elderly patients.
Kaibori et al. (9) observed that patients over 75 years old with primary HCC with a tumor size of less than 3.0 cm had a lower risk of hepatic resection and had an improved OS. Another study by Shen et al. (34) showed that the elderly (age ≥65 years) group had a considerably poorer performance status than the younger (age <65 years) group, while tumor size and partial ablation were found to be predictors of disease progression. Zhang et al. (33) also found that the size of tumors was a significant predictive variable for OS in a Cox analysis. The tumor size increases with age and may be one of the main reasons for the poorer immune system in elderly patients. Moreover, the probability of liver cancer was higher in those with HCV infection but lower in those with HBV infection as age increased (33).
Kaibori et al. (9) suggested that MWA was not superior to RFA for OS. The 5-year OS rates in each group were HR: 59.5%, RFA: 53.2%, MWA: 40.2%, and TACE: 29.2%, and differed significantly among the 4 groups (9). OS was significantly better after HR or RFA for HCC than after TACE treatments in elderly patients aged >75 years. HR: 39.6%, RFA: 34.5%, MWA: 23.8%, and TACE: 19.3%, with significant differences between the four groups. Freedman et al. (29) in their retrospective study comparing first MWA therapy for HCC in septuagenarians (n = 161) versus octogenarians (n = 32) showed no difference in OS between the two groups, with a median survival time of 3.9 years for patients between 70 and 80 years of age and 4.3 years for octogenarians (p = 0.416). The older group had an average age of 82 and a median survival of 4.3 years, whereas the younger cohort had an average age of 74 and a median survival of 3.9 years.
Another finding by Wang et al. (32) showed no significant differences in OS between two groups [elderly (more than 65 years) and younger patients (less than 65 years)]. HCV infection, comorbidities, cirrhosis, larger tumors, poor liver functional status, more ablation points, longer ablation time, longer hospital stays, and greater hospitalization expenditures were all more common in elderly individuals. Albumin, r-glutamyl transpeptidase (rGT), and ablation session were found to be significant predictors for OS.
Huang et al. (30) developed and validated nomograms to predict survival outcomes after MWA in 265 early-stage HCC (EHCC) patients showed that older patients with EHCC who had MWA had satisfactory OS rates, with a 10-year rate of 32.8%. Multiple tumors, abutting major vessels, and low platelet levels were related with significant recurrence rates following MWA; HCV or other etiologies, high AFP levels, and low albumin levels were associated with a low OS rate. They concluded that that OS in patients over 75 years old was equivalent to that in individuals 65 to 75 years of age.
Imamura et al. (31) also found that surgical MWA can be performed safely and effectively in older patients with primary HCC, with a 5-year OS rate of 49.2%. HCV-Ab positivity and multiple tumors were found to be independent predictive variables for OS in their multivariate analysis. Zhang et al. (33) also confirmed that elderly patients (age >75 years), even with a poor comorbidity index, benefited from MWA of HCC similar to younger patients with an overall follow-up time of up to 8 years. After matching, there were no significant differences in the rates of complete ablation and major complications, as well as OS and PFS, between those aged >75 years and those aged <75 years. The findings of Shen et al. (34) also suggested that older age was not associated with an increased risk of mortality or disease progression. Multiple tumors, hypertension, and lower postoperative ALT levels were found to be predictors of death. Their data imply that there is no link between age and clinical success following HCC treatment with percutaneous microwave ablation therapy (PMCT) (34).
The prevalence of HCC in elderly people is expected to continue to increase in the near future (3, 38). Minimally invasive therapy is often recommended in elderly patients considering their reduced tolerance to surgery and the presence of comorbidities. In recent years, interest in MWA has increased due to its potential physical advantages, which have been facilitated by modern high-powered devices (39). Microwaves may provide more direct heating than other energies, making MWA more effective in organs with high blood perfusion or near vascular heat sinks than other thermo-ablative modalities. A previous systematic review and meta-analysis conducted by Glassberg et al. (40) indicated that MWA is safe and effective as RFA for the treatment of HCC or liver metastases and MWA is significantly associated with lowering the rates of local tumor progression as compared to RFA. MWA obtains a larger area of tumor necrosis compared with RFA. At present, MWA with a water-cooling cycle can obtain a larger ablation boundary and avoid the effect of tissue electrical conduction, and tissue carbonization prevents the effect of its energy diffusion (41, 42).
To our knowledge, this is the first systematic review to qualitatively show that elderly patients, despite having a high comorbidity index, benefited from MWA of HCC in a similar manner to younger patients. It should be noted that all included clinical studies are retrospective (9, 29–34). This increases the risk of clinical consequences being under-reported or misreported. Furthermore, in all investigations that included MWA, no severe problems were reported. Even with a high comorbidity index, elderly patients benefited from MWA of HCC in a similar manner to younger patients with a longer overall follow-up time (9, 29–34). Although advanced age and comorbidities are fundamental variables in older HCC patients, senior and younger HCC patients treated by ultrasound percutaneous MWA had similar survival outcomes, despite elderly patients having greater comorbidities (32). However, most of the included studies in our review confirmed that MWA can be performed safely and effectively in older patients with primary HCC with a similar overall survival to younger subjects.
Shen et al. (34) confirm that older age was not associated with an increased risk of mortality or disease progression. Multiple tumors, hypertension, and lower postoperative ALT levels were found to be predictors of death, while tumor size and partial ablation were found to be predictors of disease progression. Huang et al. (30) developed a clinicopathological-based nomogram having the consistent ability to predict survival outcomes in elderly with HCC and showed that multiple tumors, abutting major vessels, and low platelet levels were related with significant recurrence rates following MWA; HCV or other etiologies, high AFP levels, and low albumin levels were associated with a low OS rate. Zhang et al. also suggested that the size of tumors and Child–Pugh grade, rather than age or the Charlson comorbidity index, were found to be significant predictive variables for OS in a Cox analysis (33). Therefore, summarizing lines of evidence suggests that age and comorbidities may not have an effect on MWA in older HCC patients, which could assist in broadening the criteria for MWA in clinical practice.
There were some limitations in our systematic review: (1) only limited number of studies were published investigating the impact of MWA in the elderly; (2) all the included studies (n = 7) were of retrospective nature, which may lead to recall bias for the observed findings; (3) we could not perform a meta-analysis due to the availability of heterogenous data in all the included studies; (4) findings must be interpreted with caution as the definition for elderly age varied in the included studies; (5) different types of available MWA machines may have varying efficacies and were not reported; (6) tumor numbers (single/multiple) varied in the elderly and only few articles reported tumor size; and, lastly, (7) all included studies were conducted over different time periods and an increase in the experience of operators may affect results.
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
WL and WH conceived and designed the study. JZ, HZ, DZ, and HY collected the data and performed the analysis. GS was involved in the writing of the manuscript and is responsible for the integrity of the study. All authors contributed to the article and approved the submitted version.
This work was supported by the Medical and Health Science and Technology Plan Project in Zhejiang Province of China (No. 2021427572).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Balogh J, Victor D, Asham EH, Burroughs SG, Boktour M, Saharia A, et al. Hepatocellular Carcinoma: A Review. J Hepatocell Carcinoma (2016) 3:41–53. doi: 10.2147/JHC.S61146
2. Wu F-H, Shen C-H, Luo S-C, Hwang J-I, Chao W-S, Yeh H-Z, et al. Liver Resection for Hepatocellular Carcinoma in Oldest Old Patients. World J Surg Oncol (2019) 17:1. doi: 10.1186/s12957-018-1541-0
3. Nishikawa H, Kimura T, Kita R, Osaki Y. Treatment for Hepatocellular Carcinoma in Elderly Patients: A Literature Review. J Cancer (2013) 4:635–43. doi: 10.7150/jca.7279
4. El-Serag HB, Rudolph KL. Hepatocellular Carcinoma: Epidemiology and Molecular Carcinogenesis. Gastroenterology (2007) 132:2557–76. doi: 10.1053/j.gastro.2007.04.061
6. Zoli M, Iervese T, Abbati S, Bianchi GP, Marchesini G, Pisi E. Portal Blood Velocity and Flow in Aging Man. Gerontology (1989) 35:61–5. doi: 10.1159/000213000
7. Olshansky SJ, Carnes BA, Désesquelles A. Demography. Prospects for Human Longevity. Science (2001) 291:1491–2. doi: 10.1126/science.291.5508.1491
8. Vaupel JW, Carey JR, Christensen K, Johnson TE, Yashin AI, Holm NV, et al. Biodemographic Trajectories of Longevity. Science (1998) 280:855–60. doi: 10.1126/science.280.5365.855
9. Kaibori M, Yoshii K, Hasegawa K, Ogawa A, Kubo S, Tateishi R, et al. Treatment Optimization for Hepatocellular Carcinoma in Elderly Patients in a Japanese Nationwide Cohort. Ann Surg (2019) 270:121–30. doi: 10.1097/SLA.0000000000002751
10. Zarour LR, Billingsley KG, Walker BS, Enestvedt CK, Orloff SL, Maynard E, et al. Hepatic Resection of Solitary HCC in the Elderly: A Unique Disease in a Growing Population. Am J Surg (2019) 217:899–905. doi: 10.1016/j.amjsurg.2019.01.030
11. Yamada S, Shimada M, Miyake H, Utsunomiya T, Morine Y, Imura S, et al. Outcome of Hepatectomy in Super-Elderly Patients With Hepatocellular Carcinoma. Hepatol Res Off J Jpn Soc Hepatol (2012) 42:454–8. doi: 10.1111/j.1872-034X.2011.00952.x
12. Aduen JF, Sujay B, Dickson RC, Heckman MG, Hewitt WR, Stapelfeldt WH, et al. Outcomes After Liver Transplant in Patients Aged 70 Years or Older Compared With Those Younger Than 60 Years. Mayo Clin Proc (2009) 84:973–8. doi: 10.4065/84.11.973
13. Bruix J, Sherman M. Management of Hepatocellular Carcinoma: An Update. Hepatol Baltim Md (2011) 53:1020–2. doi: 10.1002/hep.24199
14. Hiraoka A, Michitaka K, Horiike N, Hidaka S, Uehara T, Ichikawa S, et al. Radiofrequency Ablation Therapy for Hepatocellular Carcinoma in Elderly Patients. J Gastroenterol Hepatol (2010) 25:403–7. doi: 10.1111/j.1440-1746.2009.06037.x
15. Han X, Ni J-Y, Li S-L, Deng H-X, Liang H-M, Xu Y-Y, et al. Radiofrequency Versus Microwave Ablation for Hepatocellular Carcinoma Within the Milan Criteria in Challenging Locations: A Retrospective Controlled Study. Abdom Radiol N Y (2021) 46:3758–71. doi: 10.1007/s00261-021-03105-9
16. Potretzke TA, Ziemlewicz TJ, Hinshaw JL, Lubner MG, Wells SA, Brace CL, et al. Microwave Versus Radiofrequency Ablation Treatment for Hepatocellular Carcinoma: A Comparison of Efficacy at a Single Center. J Vasc Interv Radiol JVIR (2016) 27:631–8. doi: 10.1016/j.jvir.2016.01.136
17. Zhao J, Wu J, He M, Cao M, Lei J, Luo H, et al. Comparison of Transcatheter Arterial Chemoembolization Combined With Radiofrequency Ablation or Microwave Ablation for the Treatment of Unresectable Hepatocellular Carcinoma: A Systemic Review and Meta-Analysis. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group (2020) 37:624–33. doi: 10.1080/02656736.2020.1774667
18. Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, et al. A Randomized Trial Comparing Radiofrequency Ablation and Surgical Resection for HCC Conforming to the Milan Criteria. Ann Surg (2010) 252:903–12. doi: 10.1097/SLA.0b013e3181efc656
19. Doi K, Beppu T, Ishiko T, Chikamoto A, Hayashi H, Imai K, et al. Endoscopic Radiofrequency Ablation in Elderly Patients With Hepatocellular Carcinoma. Anticancer Res (2015) 35:3033–40.
20. Peng Z-W, Liu F-R, Ye S, Xu L, Zhang Y-J, Liang H-H, et al. Radiofrequency Ablation Versus Open Hepatic Resection for Elderly Patients (> 65 Years) With Very Early or Early Hepatocellular Carcinoma. Cancer (2013) 119:3812–20. doi: 10.1002/cncr.28293
21. Takuma Y, Takabatake H, Morimoto Y, Toshikuni N, Yamamoto H. Radiofrequency Ablation for Hepatocellular Carcinoma in Elderly Patients. Nihon Shokakibyo Gakkai Zasshi Jpn J Gastro Enterol (2013) 110:403–11.
22. Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous Microwave Ablation vs Radiofrequency Ablation in the Treatment of Hepatocellular Carcinoma. World J Hepatol (2015) 7:1054–63. doi: 10.4254/wjh.v7.i8.1054
23. Kim C. Understanding the Nuances of Microwave Ablation for More Accurate Post-Treatment Assessment. Future Oncol Lond Engl (2018) 14:1755–64. doi: 10.2217/fon-2017-0736
24. Lubner MG, Brace CL, Hinshaw JL, Lee FT. Microwave Tumor Ablation: Mechanism of Action, Clinical Results, and Devices. J Vasc Interv Radiol JVIR (2010) 21:S192–203. doi: 10.1016/j.jvir.2010.04.007
25. Wright AS, Sampson LA, Warner TF, Mahvi DM, Lee FT. Radiofrequency Versus Microwave Ablation in a Hepatic Porcine Model. Radiology (2005) 236:132–9. doi: 10.1148/radiol.2361031249
26. Qian G-J, Wang N, Shen Q, Sheng YH, Zhao J-Q, Kuang M, et al. Efficacy of Microwave Versus Radiofrequency Ablation for Treatment of Small Hepatocellular Carcinoma: Experimental and Clinical Studies. Eur Radiol (2012) 22:1983–90. doi: 10.1007/s00330-012-2442-1
27. Andreano A, Huang Y, Meloni MF, Lee FT, Brace C. Microwaves Create Larger Ablations Than Radiofrequency When Controlled for Power in Ex Vivo Tissue. Med Phys (2010) 37:2967–73. doi: 10.1118/1.3432569
28. Izzo F, Granata V, Grassi R, Fusco R, Palaia R, Delrio P, et al. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist (2019) 24:e990–e1005. doi: 10.1634/theoncologist.2018-0337
29. Freedman J. Microwave Ablation of Hepatocellular Carcinomas in Octogenarians. Hepatoma Res (2020) 6:10. doi: 10.20517/2394-5079.2019.32
30. Huang Z, Gu Y, Zhang T, Wu S, Wang X, An C, et al. Nomograms to Predict Survival Outcomes After Microwave Ablation in Elderly Patients (>65 Years Old) With Early-Stage Hepatocellular Carcinoma. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group (2020) 37:808–18. doi: 10.1080/02656736.2020.1785556
31. Imamura H, Takami Y, Ryu T, Wada Y, Sasaki S, Ureshino H, et al. Feasibility and Safety of Surgical Microwave Ablation for Hepatocellular Carcinoma in Elderly Patients: A Single Center Analysis in Japan. Sci Rep (2020) 10:14215. doi: 10.1038/s41598-020-71095-7
32. Wang Y, Cheng Z, Yu J, Li X, Hao G, Liu F, et al. US-Guided Percutanous Microwave Ablation for Early-Stage Hepatocellular Carcinoma in Elderly Patients is as Effective as in Younger Patients: A 10-Year Experience. J Cancer Res Ther (2020) 16:292–300. doi: 10.4103/jcrt.JCRT_1021_19
33. Zhang Y-X, Zhang X-H, Yu X-L, Han Z-Y, Yu J, Liu F-Y, et al. Prognosis of Microwave Ablation for Hepatocellular Carcinoma: Does Age Make a Difference? Int J Hyperthermia (2020) 37:688–95. doi: 10.1080/02656736.2020.1778198
34. Shen X, Ma S, Tang X, Wang T, Qi X, Chi J, et al. Clinical Outcome in Elderly Chinese Patients With Primary Hepatocellular Carcinoma Treated With Percutaneous Microwave Coagulation Therapy (PMCT): A Strobe-Compliant Observational Study. Med (Baltimore) (2018) 97:e11618. doi: 10.1097/MD.0000000000011618
35. Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst Rev (2015) 4:1. doi: 10.1186/2046-4053-4-1
36. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated Guidance for Trusted Systematic Reviews: A New Edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev (2019) 10:ED000142. doi: 10.1002/14651858.ED000142
37. Stang A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur J Epidemiol (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
38. Hung AK, Guy J. Hepatocellular Carcinoma in the Elderly: Meta-Analysis and Systematic Literature Review. World J Gastroenterol (2015) 21:12197–210. doi: 10.3748/wjg.v21.i42.12197
39. Facciorusso A, Di Maso M, Muscatiello N. Microwave Ablation Versus Radiofrequency Ablation for the Treatment of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Int J Hyperth Off J Eur Soc Hyperthermic Oncol North Am Hyperth Group (2016) 32:339–44. doi: 10.3109/02656736.2015.1127434
40. Glassberg MB, Ghosh S, Clymer JW, Qadeer RA, Ferko NC, Sadeghirad B, et al. Microwave Ablation Compared With Radiofrequency Ablation for Treatment of Hepatocellular Carcinoma and Liver Metastases: A Systematic Review and Meta-Analysis. OncoTargets Ther (2019) 12:6407–38. doi: 10.2147/OTT.S204340
41. Liu W, Zheng Y, He W, Zou R, Qiu J, Shen J, et al. Microwave vs Radiofrequency Ablation for Hepatocellular Carcinoma Within the Milan Criteria: A Propensity Score Analysis. Aliment Pharmacol Ther (2018) 48:671–81. doi: 10.1111/apt.14929
Keywords: frequency ablation, hepatocellular carcinoma, microwave ablation, elderly, prognosis, review, treatment
Citation: Liang W, Hao W, Shao G, Zheng J, Zeng H, Zhou D and Yao H (2022) Safety and Feasibility of Microwave Ablation for Hepatocellular Carcinomas in the Elderly: A Systematic Review. Front. Oncol. 12:855909. doi: 10.3389/fonc.2022.855909
Received: 16 January 2022; Accepted: 19 April 2022;
Published: 23 May 2022.
Edited by:
Alessio G. Morganti, University of Bologna, ItalyReviewed by:
Marco Massani, ULSS2 Marca Trevigiana, ItalyCopyright © 2022 Liang, Hao, Shao, Zheng, Zeng, Zhou and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoliang Shao, Z3VvbGlhbmdzaGFvMjAxM0AxMjYuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.