- 1Department of Otolaryngology-Head and Neck Surgery, Nanchong Central Hospital, The Second Clinical Medical College, North Sichuan Medical College, Nanchong, China
- 2Department of Pathogen Biology, School of Basic Medical Sciences & Forensic Medicine, North Sichuan Medical College, Nanchong, China
Objective: Several studies were conducted to explore the clinical significance of cyclooxygenase-2 (COX-2) overexpression in laryngeal cancer. However, the associations between COX-2 overexpression and clinicopathological characteristics of laryngeal cancer patients remained unclear. Here, we performed a meta-analysis to eva-TY -40luate the role of COX-2 overexpression in the risk, clinical progression, and progno\sis of laryngeal cancer.
Methods: The eligible literature was obtained from PubMed, Embase, Web of Science, and China National Knowledge Infrastructure (CNKI) databases. Odds ratio (OR), risk ratio (RR), and 95% confidence interval (CI) were calculated to assess the strength of the associations, and I2 statistics were used to evaluate heterogeneity among studies. Publication bias was detected with Begg’s test and Egger’s test.
Results: A total of 47 eligible articles were included for the meta-analysis after screening. COX-2 expression levels in the laryngeal cancer patients were significantly higher than those in the normal controls (OR = 11.62, 95% CI: 6.96–19.40, P < 0.05). The pooled results also showed that there were significant correlations between COX-2 overexpression and clinicopathological characteristics (tumor stage, OR = 3.26, 95% CI: 2.13–4.98, P < 0.05; lymph node metastasis, in Asians, OR = 2.35, 95% CI: 1.53–3.60, P < 0.05; recurrence, OR = 10.71, 95% CI: 3.54–32.38, P < 0.05; T stage, in Asians, OR = 2.52, 95% CI: 1.66–3.83, P < 0.05). In addition, significant correlations between COX-2 overexpression and overall survival of laryngeal cancer were found both in Asians and in Caucasians (total, HR = 1.73, 95% CI: 1.23–2.24, P < 0.05; survival in Asians, HR = 2.59, 95% CI: 1.27–3.92, P < 0.05; survival in Caucasians, HR = 1.59, 95% CI: 1.03–2.14, P < 0.05).
Conclusions: The meta-analysis results suggested that COX-2 overexpression was significantly associated with the increased risk, worse clinicopathological progression, and poorer prognosis of laryngeal cancer.
Introduction
Laryngeal cancer is the second most common cancer of the upper respiratory tract, and it includes approximately 30% of all head and neck cancers (1). It was estimated that 13,000 new laryngeal cancer patients were diagnosed in the USA yearly, while approximately 25,300 new cases of laryngeal cancer were reported annually (2, 3). Although laryngectomy, radiotherapy, and chemotherapy were used to treat laryngeal cancer, the 5-year rate was still less than 30% (4). The selection of therapeutic methods depended on the tumor’s stage and sensitivity to chemoradiotherapy. In general, the larynx was sacrificed to obtain a better prognosis in the treatment of laryngeal cancer, which often affected physiological function and psychological health (1). To date, there were no effective biomarkers or tools to predict the progression and prognosis of laryngeal cancer, and almost 40% of cases were diagnosed as advanced stage tumors (5, 6). Therefore, studies were urgently conducted to discover effective biomarkers for the progression of laryngeal cancer and to elucidate the molecular mechanism of laryngeal carcinoma.
Cyclooxygenase-2 (COX-2) is one of the isoforms of cyclooxygenase, a membrane-bound and rate-limiting enzyme (7). COX-2 could catalyze the generation of prostaglandin E2 (PGE2), which is responsible for the normal physiological functions of human bodies (8). In general, COX-2 presents a lower expression level in normal tissues, and a higher expression of COX-2 is often found in many tumor tissues such as gastric cancer, breast cancer, endometrial cancer, and liver cancer (9–12). Considering the significant expression differences between normal tissues and cancer tissues, COX-2 might be a potential biomarker for the early diagnosis of tumors. The positive rate of COX-2 in lung cancer tissue was much higher than that in normal tissues (13). Furthermore, chemotherapy, radiotherapy, and proinflammatory cytokines promoted the expression of COX-2; thus, COX-2 might be related to the clinical progression of cancers (8). It has been reported that elevated COX-2 levels predicted poorer survival of non-small cell lung cancer (14), and higher COX-2 expression was significantly associated with histological type, lymph node metastasis, and venous invasion of liver cancer (15). Recently, many studies have been conducted to explore the correlation between the clinical progression of laryngeal cancer and COX-2 expression. However, the results were not consistent and convincing due to the sample size, source of the patients, and detection methods of COX-2 expression. Therefore, we carried out this meta-analysis to clarify the role of COX-2 overexpression in the risk, progression, and prognosis of laryngeal cancer.
Methods
Literature Search Strategy and Selection
PubMed, Embase, Web of Science, and CNKI were retrieved to search relevant literature in December 2021 with the following search terms: “COX-2”, “COX2”, “cyclooxygenase-2”, “expression”, “laryngeal cancer”, “laryngeal carcinoma”, and “prognosis”. Finally, relevant studies involving the associations of COX-2 expression with the risk, clinicopathological characteristics, and prognosis of laryngeal cancer were included.
Inclusion and Exclusion Criteria
The inclusion criteria were as follows: 1) studies involving the associations of COX-2 expression with the risk, clinical characteristics, and prognosis of laryngeal cancer; 2) studies applying immunohistochemistry (IHC) to assess the expression of COX-2 in laryngeal tissue; 3) studies including enough data on the risk, clinical characteristics, and prognosis of laryngeal cancer to estimate OR, HR, and 95% CI; and 4) studies dividing COX-2 expression levels into positive and negative categories. The exclusion criteria included the following: 1) duplicate studies, letters, and reviews; 2) detection methods of COX-2 expression were not IHC; 3) studies conducted in cell lines and animal models; and 4) studies that did not offer enough data to calculate OR, HR, and 95% CI.
Data Extraction and Quality Assessment
The following data were extracted from the eligible studies: “first authors’ name”, “publication year”, “patients’ country”, “cancer type”, “number of positive and negative cases of COX-2 in the case group and control group”, “detection method of COX-2 expression”, “cutoff value for COX-2 expression levels”, “overall survival curve for extracting HR and 95% CI”, and “HR and 95% CI data for overall survival of laryngeal cancer”. Two reviewers independently extracted the data from the included studies, and the two researchers discussed and resolved any discrepancies. To evaluate the methodological quality of eligible studies, the Newcastle-Ottawa Quality Assessment Scale (NOQAS) was used, and the included literature was scored from 0 to 9 (16).
Statistical Analysis
Pooled OR and 95% CI were calculated and used to evaluate the associations of COX-2 overexpression with risk and clinical characteristics of laryngeal cancer. In comparison, HR and 95% CI were applied to assess the role of COX-2 overexpression in the overall survival of laryngeal cancer. Cochran’s Q statistic and I2 tests were used to assess the statistical heterogeneity among studies (17, 18). A random-effects model was used when the I2 value was >50% or P <0.05; when the I2 value was <50% or P >0.05, a fixed-effects model was applied (19). Begg’s and Egger’s tests were conducted to estimate publication bias (20, 21). Sensitivity analyses were also performed to observe the effects of individual studies on the overall results. If HR and 95% CI for laryngeal cancer survival were not directly provided, survival data would be extracted from the survival curve with Engauge Digitizer 11.1 software (22). All P-values were two-sided and P <0.05 was statistically significant. STATA 12.0 software (Stata Corporation, College Station, TX, USA) was used to carry out all the statistical analyses.
Results
Search Results and Study Characteristics
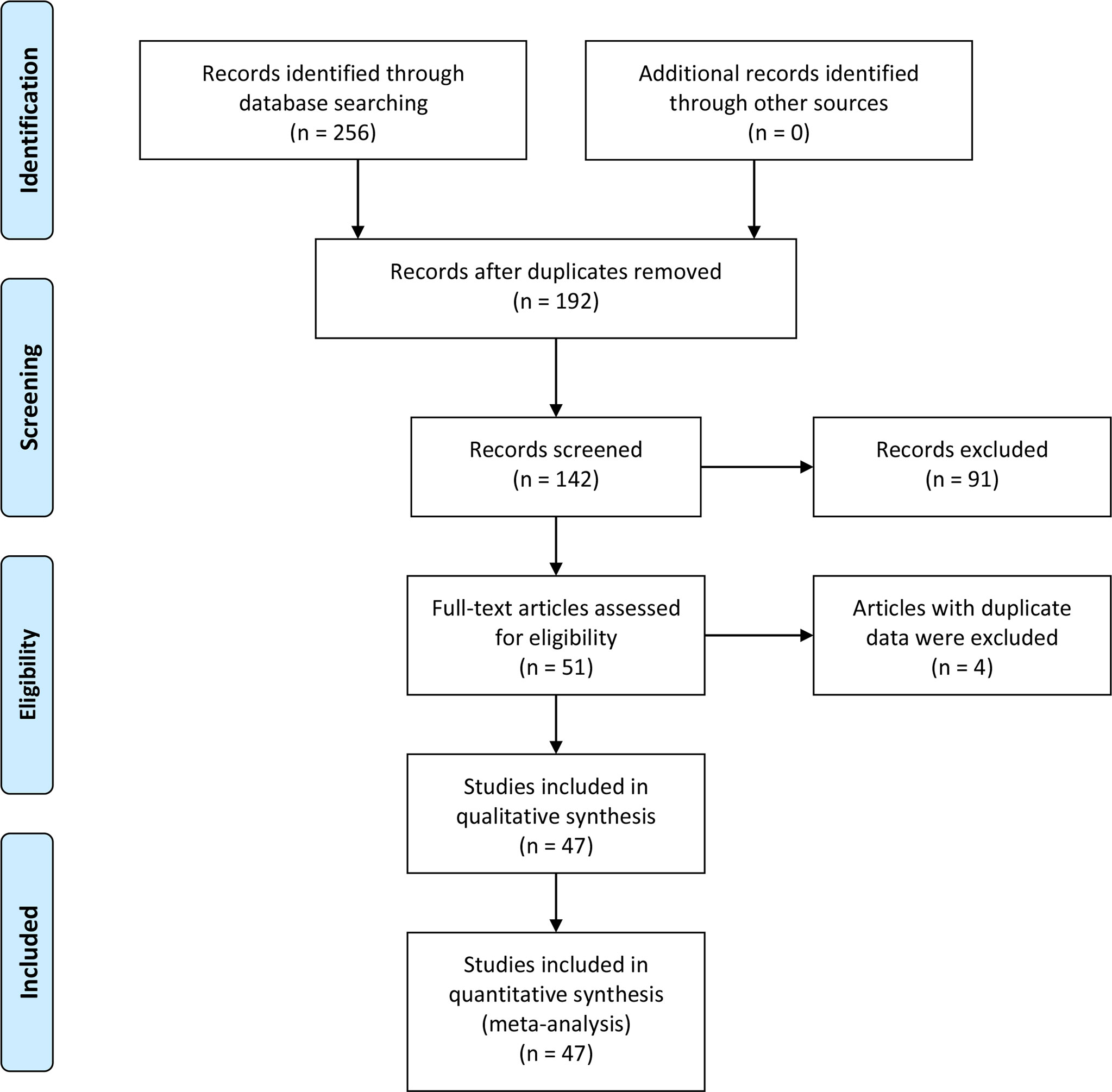
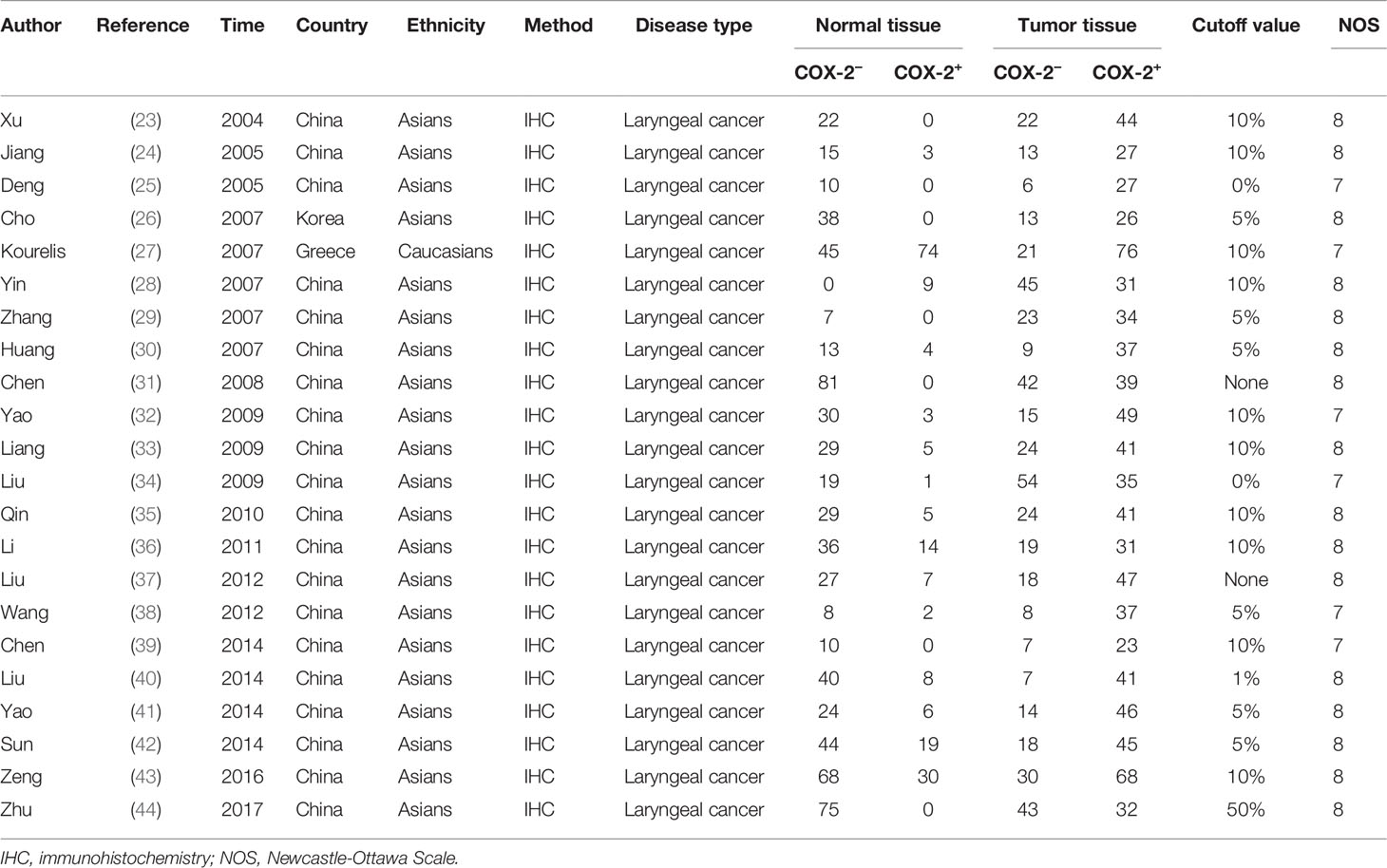
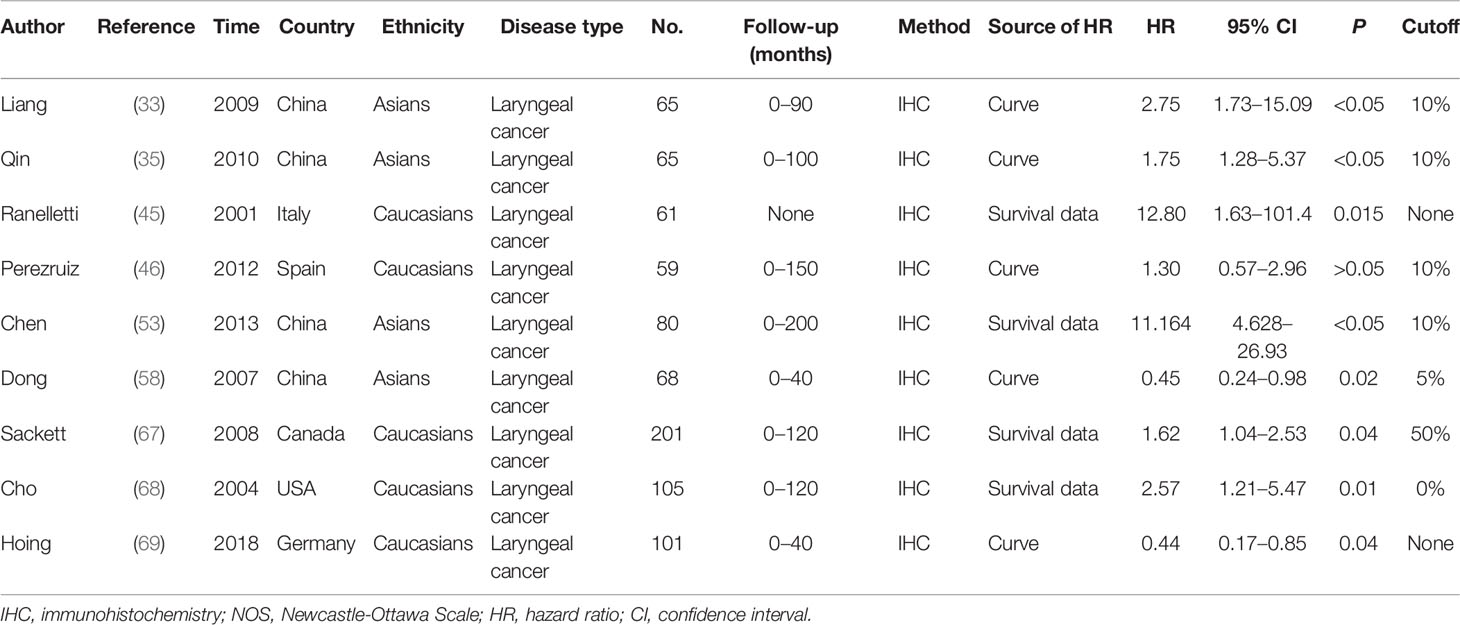
Two hundred fifty-six studies were acquired from an initial retrieval. Then, titles, abstracts, and full text were read in detail. Finally, according to the inclusion and exclusion criteria, 47 articles with 860 normal controls and 1,352 laryngeal cancer patients were identified (23–69). Twelve studies were conducted in Caucasians, and 35 reports were performed in Asians. In these included studies, 22 studies observed the correlation between COX-2 expression and risk of laryngeal cancer, and 46 reports involved the associations of COX-2 expression with clinical characteristics of laryngeal cancer. In addition, nine pieces of literature that explored the role of COX-2 in the overall survival of laryngeal cancer were included. The survival curve was extracted from five articles used to extract the HR and 95% CI, while HR and 95% CI data were extracted directly from the other four pieces of literature. The quality scores of eligible studies were all >6, which indicated the high quality of the included studies (Figure 1 and Tables 1, 2).
Meta-Analysis Results
The pooled results suggested that COX-2 overexpression was significantly associated with the risk of laryngeal cancer (COX-2 positive in laryngeal cancer vs. COX-2 positive in normal control: 64.87% vs. 22.09%; OR = 11.62, 95% CI: 6.96–19.40, P < 0.05). However, a small heterogeneity was found in the analysis for the risk of laryngeal cancer (I2 = 69.1%, P < 0.001), in which the type of antibodies, experimental methods of IHC, and cutoff values for evaluating COX-2 expression might lead to the heterogeneity. In addition, we found that COX-2 overexpression was significantly associated with the tumor stage and T stage of laryngeal cancer (tumor stage, OR = 3.26, 95% CI: 2.13–4.98, P < 0.05; recurrence, OR = 10.71, 95% CI: 3.54–32.38, P < 0.05). Subgroup analysis suggested that there was a significant association of COX-2 overexpression with the lymph node metastasis and T stage of laryngeal cancer (lymph node metastasis, total, OR = 2.11, 95% CI: 1.38–3.23, P < 0.05, in Asians, OR = 2.35, 95% CI: 1.53–3.60, P < 0.05; T stage, total, OR = 2.18, 95% CI: 1.49–3.20, P < 0.05, in Asians, OR = 2.52, 95% CI: 1.66–3.83, P < 0.05), and heterogeneity among studies was significantly decreased in the subgroup analysis based on ethnicity. Finally, we estimated the prognostic value of COX-2 overexpression in laryngeal cancer, and significant correlations between COX-2 overexpression and poor prognosis of laryngeal cancer were found both in Asians and Caucasians (total, HR = 1.73, 95% CI: 1.23–2.24; survival in Asians, HR = 2.59, 95% CI: 1.27–3.92, P < 0.05; survival in Caucasians, HR = 1.59, 95% CI: 1.03–2.14, P < 0.05) (Figure 2 and Table 3).
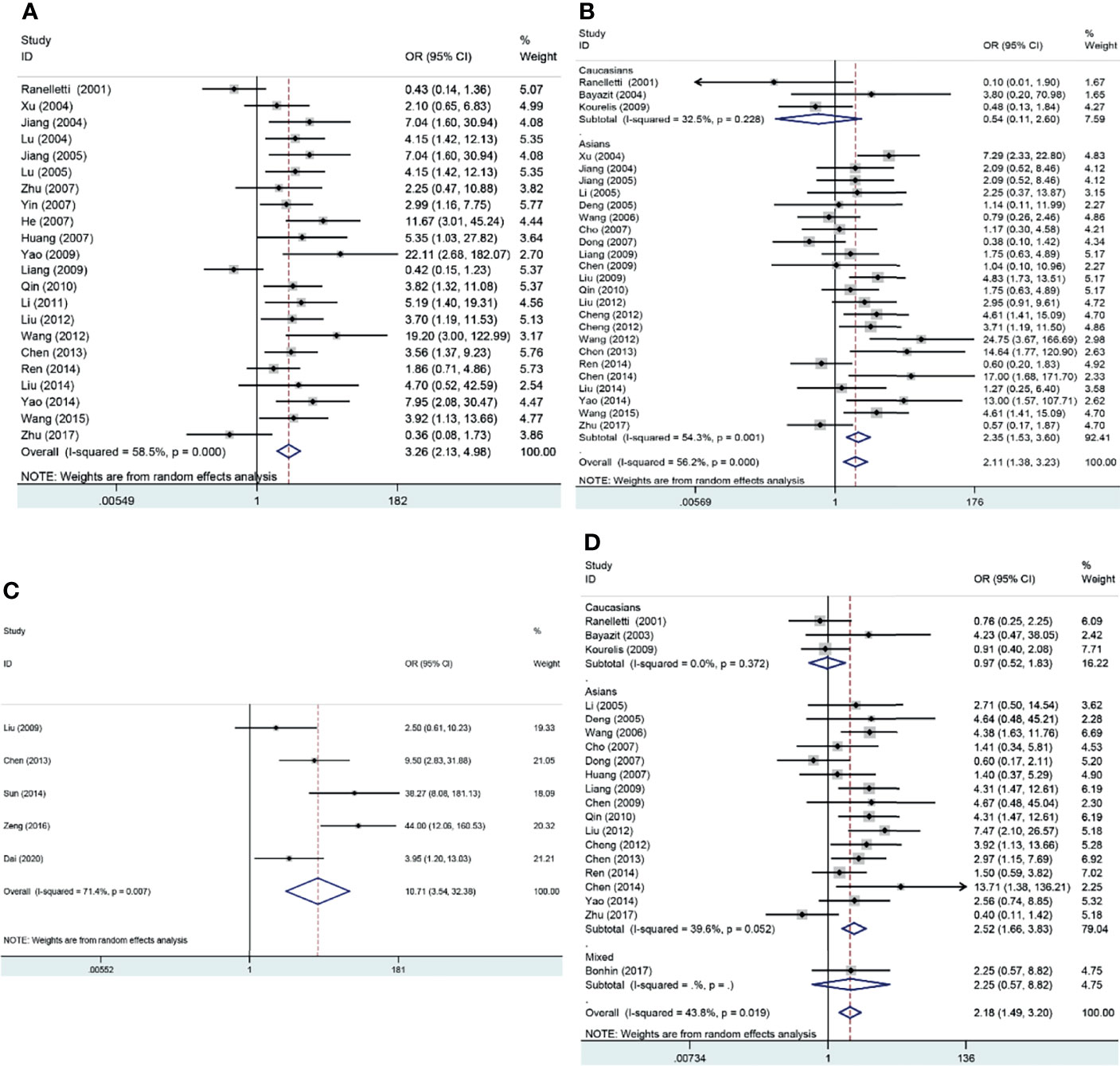
Figure 2 Forest plots of the associations of COX-2 overexpression with clinical features of laryngeal cancer. (A) Tumor stage for laryngeal cancer. (B) Lymph node metastasis for laryngeal cancer. (C) Recurrence for laryngeal cancer. (D) T stage for laryngeal cancer. OR, odds ratio; CI, confidence interval.
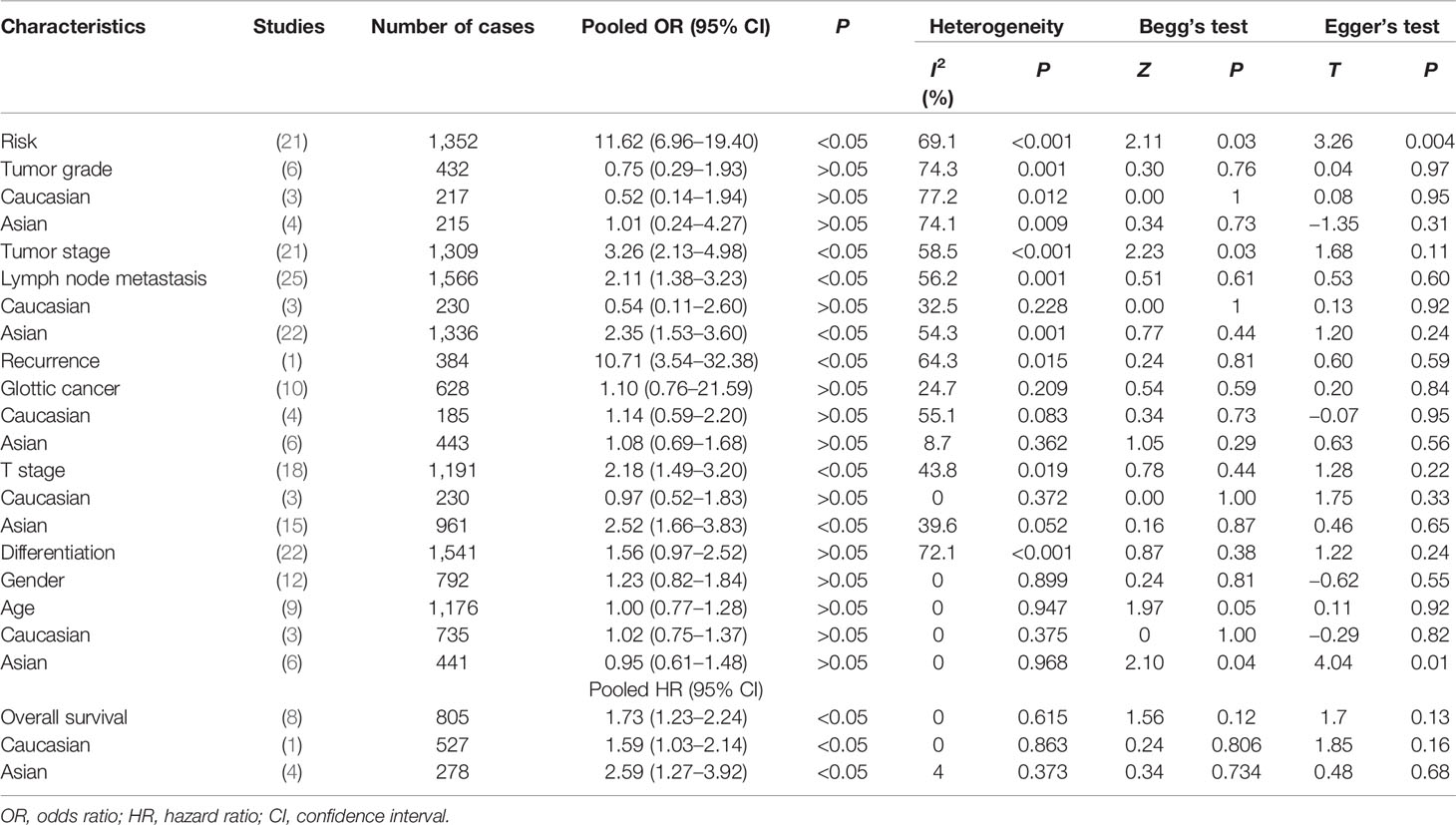
Table 3 Meta-analysis results for COX-2 overexpression in risk, clinical features, and overall survival of laryngeal cancer patients.
Publication Bias and Sensitivity Analysis
Although a small heterogeneity was found, sensitivity analysis did not identify any studies that significantly affected the overall statistical results. According to Begg’s test and Egger’s test, publication bias was found in the analysis for the risk and tumor stage of laryngeal cancer. The studies were mainly conducted in the Chinese population. Moreover, we speculated that antibody differences, experimental methods of IHC, evaluation methods of COX-2 expression, and cutoff values for COX-2 expression might contribute to the slight publication bias (Figures 3, 4).
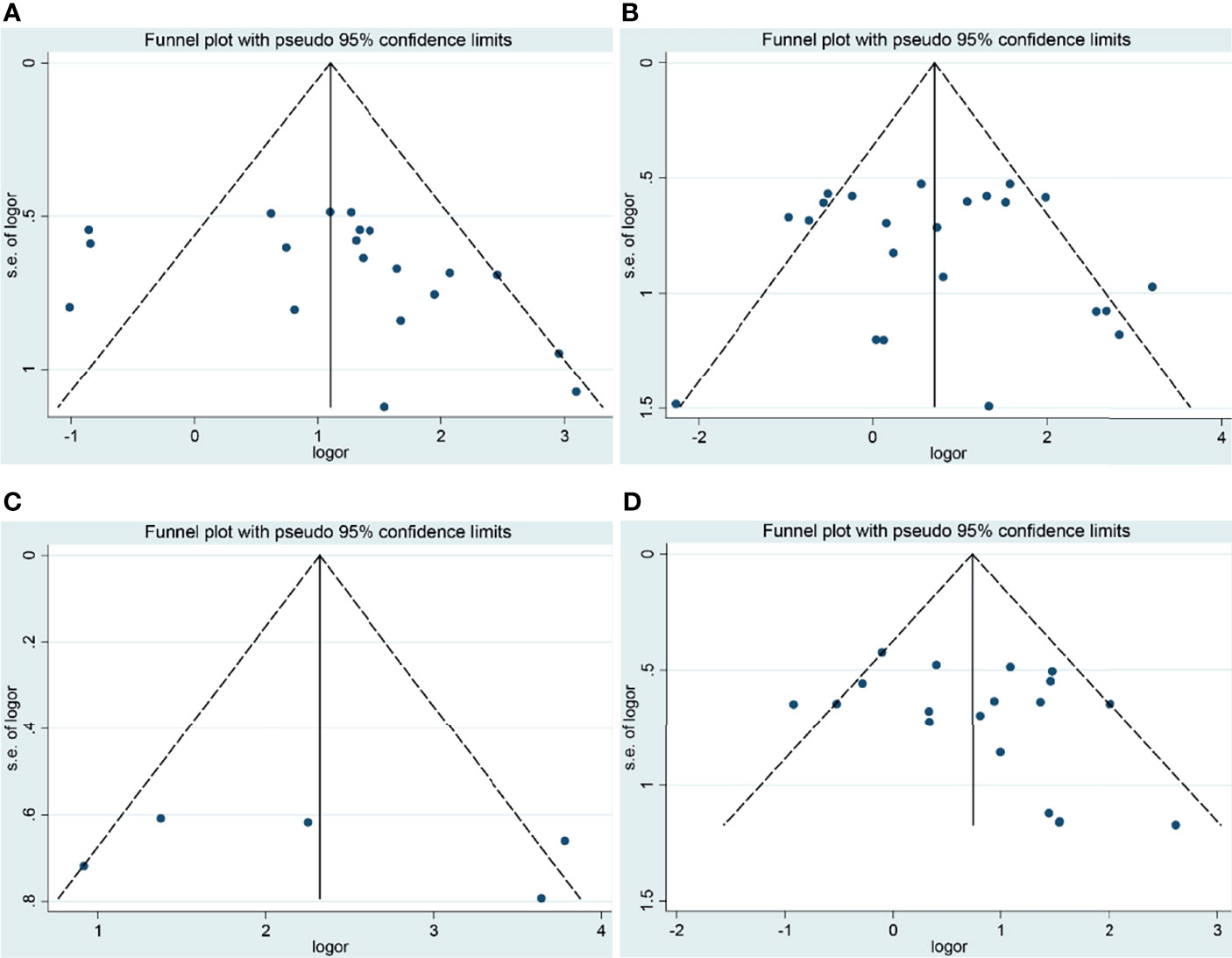
Figure 3 Funnel plots of the association of COX-2 expression with clinical features of laryngeal cancer. (A) Tumor stage for laryngeal cancer. (B) Lymph node metastasis for laryngeal cancer. (C) Recurrence for laryngeal cancer. (D) T stage for laryngeal cancer. OR, odds ratio; CI, confidence interval.
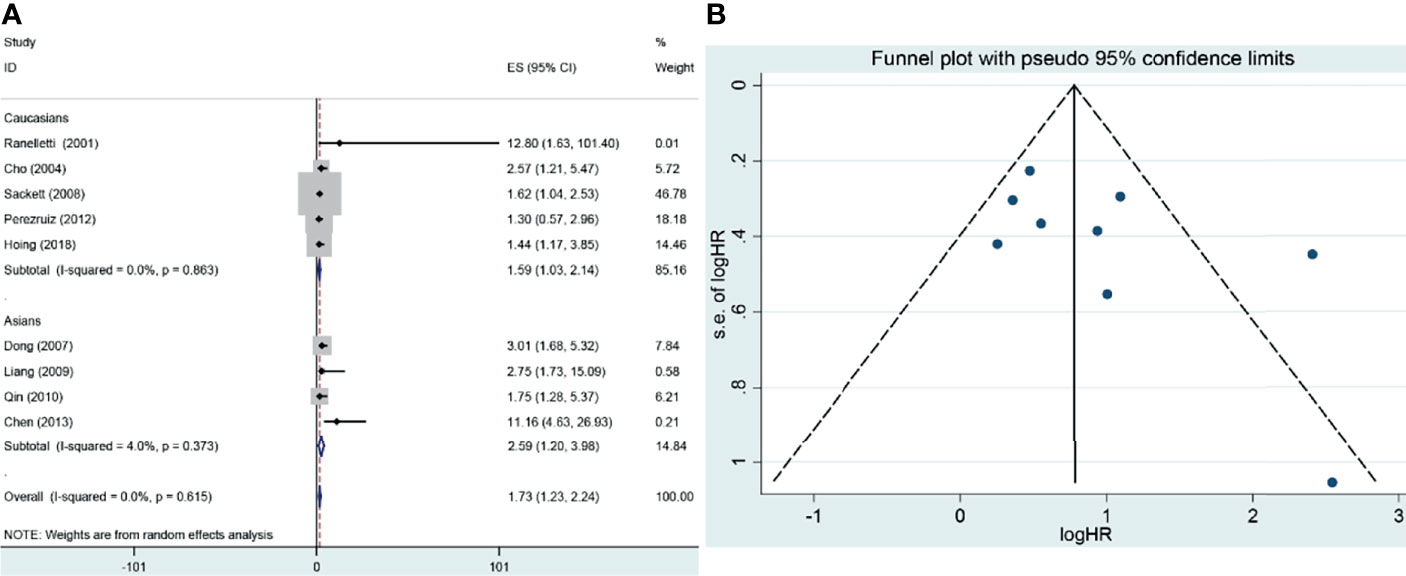
Figure 4 Forest plot and funnel plot of the association between COX-2 expression and overall survival of laryngeal cancer. (A) Forest plot for laryngeal cancer. (B) Funnel plot for laryngeal cancer. HR, hazard ratio; CI, confidence interval.
Discussion
The main risk factors for laryngeal cancer were tobacco use, alcohol consumption, and human papillomavirus infection. However, recent studies have found numerous genetic alterations in laryngeal cancer, which suggested that genetics also was involved in the occurrence of laryngeal cancer. In a genome-wide association study involving 993 laryngeal squamous cell carcinoma patients and 1,995 controls, researchers found the three most significant SNPs—rs174549, rs2857595, and rs10492336—which are located in FASD1, AIF1, and TBX5 genes, respectively (70). In addition, a gene expression study conducted in laryngeal cancer tissues and adjacent normal tissues suggested that differentially expressed genes were mainly enriched in cell cycle, DNA replication, metabolic pathways, mucin-type O-glycan biosynthesis, and drug metabolism-cytochrome P450 (71). Therefore, the internal genetic mechanism might be responsible for laryngeal cancer’s occurrence and clinical progression. In addition to the high-throughput studies, some articles were also conducted to detect the expression of biomarkers in scattered tumor tissues. In these biomarkers, COX-2 was involved in inflammation, cellular invasion, angiogenesis, antiapoptotic cellular defenses, and immunological resistance (72).
In the present study, we found that people with elevated COX-2 expression had a higher risk for laryngeal cancer, in which only one study was conducted in Caucasians (27). Thus, the conclusion might be more appropriate to Asians, and more Caucasians should be included in future studies. Moreover, a small heterogeneity was detected in the analysis for the risk of laryngeal cancer, but sensitivity analysis found no significant difference. Moreover, COX-2 expression was significantly associated with the stage and recurrence of laryngeal cancer, in which the included subjects were mostly from China, except for one study that involved participants from Italy. The subgroup analysis found that COX-2 expression had a significant association with lymph node metastasis and T stage of laryngeal cancer in Asians but not in Caucasians. We also conducted a correlation analysis between COX-2 overexpression and tumor grade, tumor type (glottic cancer vs. non-glottic cancer), differentiation, gender, and age (<60 vs. >60). However, no significant associations were found.
To observe the role of COX-2 overexpression in the prognosis of laryngeal cancer, nine studies were included, of which four were from Caucasians and five were from Asians. In the overall analysis, there was a significant association between COX-2 overexpression and overall survival of laryngeal cancer (HR = 1.73, 95% CI: 1.23–2.24, P < 0.05). A significant association was also found in the subgroup analysis based on ethnicity (survival in Asians, HR = 2.59, 95% CI: 1.27–3.92, P < 0.05; survival in Caucasians, HR = 1.59, 95% CI: 1.03–2.14, P < 0.05). In the nine eligible studies, Perezruiz et al. got a negative result, and Hoing et al. obtained an opposite result (46, 69). Sensitivity analysis suggested that these studies did not affect the pooled overall results, and no significant heterogeneity and publication bias were found in the analysis for the overall survival of laryngeal cancer, suggesting that the results were stable.
According to the study results, we speculated that COX-2 overexpression was a clinical biomarker for laryngeal cancer that might affect the clinical progression and prognosis of laryngeal cancer. Although this was the first meta-analysis to assess the association of COX-2 overexpression with laryngeal cancer, some limitations should be addressed. Firstly, the included studies were primarily performed to explore the role of COX-2 expression in the risk, tumor stage, lymph node metastasis, recurrence, differentiation, and gender of laryngeal cancer in Asians. Secondly, the cutoff values of COX-2 expression were not unified, leading to heterogeneity among studies. Thirdly, the overall survival data were extracted from survival curves rather than original variance data, which might lead to the deviation of the final results. Fourthly, the study’s sample size was still too small after performing a subgroup analysis.
In conclusion, COX-2 overexpression was significantly associated with the higher risk and worse prognosis of laryngeal cancer. Moreover, COX-2 overexpression had significant associations with the tumor stage, lymph node metastasis, recurrence, and T stage of laryngeal cancer. Finally, more studies on the correlations of COX-2 overexpression with the risk, clinical characteristics, and prognosis of laryngeal cancer should be performed in the future, especially in Caucasians.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The study was supported by “Nanchong 2020 City-School Science and Technology Strategic Cooperation Special Project (North Sichuan Medical College) (No. 20SXQT0105)”, “Nanchong 2019 City-School Cooperation Scientific Research Special Project (North Sichuan Medical College) (No. 19SXHZ0130)” and “Science Research Project of Sichuan Provincial Department of Education (No. 15ZB0188)”.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann Surg Oncol (2010) 17(6):1471–4. doi: 10.1245/s10434-010-0985-4
2. Steuer CE, El-Deiry M, Parks JR, Higgins KA, Saba NF. An Update on Larynx Cancer. CA Cancer J Clin (2017) 67(1):31–50. doi: 10.3322/caac.21386
3. He YT, Liang D, Li DJ, Shan BE, Zheng RS, Zhang RW, et al. Incidence and Mortality of Laryngeal Cancer in China, 2015. Chin J Cancer Res (2020) 32(1):10–7. doi: 10.21147/j.issn.1000-9604.2020.01.02
4. Rudolph E, Dyckhoff G, Becher H, Dietz A, Ramroth H. Effects of Tumor Stage, Comorbidity and Therapy on Survival of Laryngeal Cancer Patients: A Systematic Review and a Meta-Analysis. Eur Arch Otorhinolaryngol (2011) 268(2):165–79. doi: 10.1007/s00405-010-1395-8
5. Cossu AM, Mosca L, Zappavigna S, Misso G, Bocchetti M, De Micco F, et al. Long non-Coding RNAs as Important Biomarkers in Laryngeal Cancer and Other Head and Neck Tumours. Int J Mol Sci (2019) 20(14):3444. doi: 10.3390/ijms20143444
6. Koontongkaew S. The Tumor Microenvironment Contribution to Development, Growth, Invasion and Metastasis of Head and Neck Squamous Cell Carcinomas. J Cancer (2013) 4(1):66–83. doi: 10.7150/jca.5112
7. Obermoser V, Baecker D, Schuster C, Braun V, Kircher B, Gust R. Chlorinated Cobalt Alkyne Complexes Derived From Acetylsalicylic Acid as New Specific Antitumor Agents. Dalton Transactions (2018) 47(12):4341–51. doi: 10.1039/C7DT04790H
8. Hashemi Goradel N, Najafi M, Salehi E, Farhood B, Mortezaee K. Cyclooxygenase-2 in Cancer: A Review. J Cell Physiol (2019) 234(5):5683–99. doi: 10.1002/jcp.27411
9. Ren JL, Liu J and Sun X. Correlation of COX-2 and MMP-13 Expressions With Gastric Cancer and Their Effects on Prognosis. J BUON (2019) 24(1):187–93.
10. Hugo HJ, Saunders C, Ramsay RG, Thompson EW. New Insights on COX-2 in Chronic Inflammation Driving Breast Cancer Growth and Metastasis. J Mammary Gland Biol Neoplasia (2015) 20(3-4):109–19. doi: 10.1007/s10911-015-9333-4
11. Alves AF, Baldissera VD, Chiela ECF, Cerski CTS, Fontes PRO, Fernandes MDC, et al. Altered Expression of COX-2 and TNF-Alpha in Patients With Hepatocellular Carcinoma. Rev Esp Enferm Dig (2019) 111(5):364–70. doi: 10.17235/reed.2019.5898/2018
12. Li ML, Li MX, Wei YG, Xu H. Prognostic and Clinical Significance of Cyclooxygenase-2 Overexpression in Endometrial Cancer: A Meta-Analysis. Front Oncol (2020) 6(10):1202. doi: 10.3389/fonc.2020.01202
13. Li F, Liu Y, Chen H, Liao D, Shen Y, Xu F, et al. EGFR and COX-2 Protein Expression in non-Small Cell Lung Cancer and the Correlation With Clinical Features. J Exp Clin Cancer Res (2011) 30(1):27. doi: 10.1186/1756-9966-30-27
14. Jiang H, Wang J, Zhao W. Cox-2 in non-Small Cell Lung Cancer: A Meta-Analysis. Clin Chim Acta (2013) 419:26–32. doi: 10.1016/j.cca.2013.01.012
15. Yu JR, Wu YJ, Qin Q, Lu KZ, Yan S, Liu XS, et al. Expression of Cyclooxygenase-2 in Gastric Cancer and its Relation to Liver Metastasis and Long-Term Prognosis. World J Gastroenterol (2005) 11(31):4908–11. doi: 10.3748/wjg.v11.i31.4908
16. Stang A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
17. DerSimonian R. Meta-Analysis in the Design and Monitoring of Clinical Trials. Stat Med (1996) 15(12):1237–1248; discussion 1249–1252. doi: 10.1002/(SICI)1097-0258(19960630)15:12<1237::AID-SIM301>3.0.CO;2-N
18. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring Inconsistency in Meta-Analyses. BMJ (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
19. Bax L, Ikeda N, Fukui N, Yaju Y, Tsuruta H, Moons KG. More Than Numbers: The Power of Graphs in Meta-Analysis. Am J Epidemiol (2009) 169(2):249–55. doi: 10.1093/aje/kwn340
20. Begg CB, Mazumdar M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics (1994) 50(4):1088–101. doi: 10.2307/2533446
21. Egger M, Davey Smith G, Schneider M, Minder C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
22. Parmar MK, Torri V, Stewart L. Extracting Summary Statistics to Perform Meta-Analyses of the Published Literature for Survival Endpoints. Stat Med (1998) 17(24):2815–34. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8
23. Xu WH, Wang J, Zheng ZH. Relationship Between the Expression of COX-2, CD44v6, and Metastasis in Laryngeal Carcinoma. J Oncol (2004) 10(5):327–9.
24. Jiang ZH, Pan XL, Luan XY, He Y. Expression of COX-2 in Laryngeal Carcinoma and its Correlation With Expressions of VEGF and MVD. Neoplasm (2005) 25(1):83–6.
25. Deng G, Yang CZ, Chen WY. Expression of COX- 2, Ki- 67 in Laryngeal Squamous Cell Carcinoma. China J Modern Med (2005) 15(17):1–4.
26. Cho NP, Han HS, Soh Y, Lee KY, Son HJ. Cytoplasmic HuR Over-Expression Is Associated With Increased Cyclooxygenase-2 Expression in Laryngeal Squamous Cell Carcinomas. Pathology (2007) 39(6):545–50. doi: 10.1080/00313020701684391
27. Kourelis K, Bonikou GS, Vandoros G, Repanti M, Varakis L, Goumas P. Coordinated Upregulation of COX-2 and NF- κb Is a Steady Feature of Laryngeal Carcinogenesis. Karger (2007) 69:181–9.
28. Yin SH. The Expression and Significance of HIF-1α and COX-2 in Laryngeal Squamous Cell Carcinoma. J Clin Otorhinolaryngol Head Neck Surg (2007) 21(18):820–4.
29. Zhang JH, Dong MM, Huang W. Expression of COX-2, Ki-67, VEGF in Laryngeal Squamous Carcinoma and its Significance. J Henan Med Coll Staff Workers (2007) 19(3):209–12.
30. Huang SH, Wang WW, Chen XH, Wu SE. Expression and Correlation of COX-2 and Ki67 in Laryngeal Carcinoma. Laryngoscope (2007) 23(8):1159–61.
31. Chen YF, Chen FJ, Yang AK, Chen WK. Expression and Clinical Significance of Cyclooxygenase-2 in Tumor of Laryngeal Squamous Cell Carcinoma. Practical Med (2008) 29(1):1–4.
32. Yao HC, Xiao H, Zhao GX, Tang HY, Liu M. COX-2, VEGF-C Expression and Its Relationship With Clinicopathological Parameters in Human Laryngeal Squamous Carcinoma. Prog Modern Biomed (2009) 9(12):2281–3.
33. Liang ZP, Yu L, Chen ZY, Liu WJ, Li WR, Qin G. Expression and Clinical Significance of COX-2 and Survivin in Laryngeal Carcinoma. Chin J Otorhinolaryngol Skull Base Surg (2009) 15(1):1–7.
34. Liu J, Yang WJ, Fu Q, Wu XY. Correlation Between COX-2 Expression and Multidrug Resistance in Laryngeal Cancer. Chinese Otorhinolaryngol (2009) 15(12):1109–11.
35. Qin G, Liang ZP, Yu L, Chen ZY, Liu WJ, Li WR. Expression and Significance of COX-2 and S-100 Positive Dendritic Cell in Laryngeal Carcinoma. J Oncol (2012) 24(3):101–4.
36. Li JY, Xu HL, Xu F. Expression of P16 and Cyclooxygenase-2 in Larynx Carcinoma Tissues and Their Correlation. Chin J Otorhinolaryngol Integ Med (2011) 19(2):72–4.
37. Liu JF, Qin G, Wang LH, Zan MF, Liang CY. The Expression and Clinical Significance of COX-2 and IL-8 in Laryngeal Carcinoma. Med J West China (2012) 24(9):1664–7.
38. Wang BQ, Peng SD. Expression and Relationships Between KAI1 and COX-2 of Laryngeal Squamous Cell Carcinoma. Med J West China (2012) 19(20):1535–8.
39. Chen ZX, Liu YY, Zhang JP, Luo XY, Ye XM, Xiong QB, et al. Expression of β-Catenin, iNOS and COX-2 in Laryngeal Carcinoma and Its Clinical Significance. Chin J Cancer Prevention Treatment (2014) 23(3):1–5.
40. Liu ZB, Lu GP. Expression and Clinical Significance of Osteopontin and COX-2 in Supraglottic Laryngeal Squamous Cell Carcinoma. Jiangsu Med J (2014) 40(9):1045–7.
41. Yao H, You YW, Huang H. Expression Changes and Significance of Beclin1, COX-2 and MVD in Laryngeal Squamous Cell Carcinoma. Shandong Med (2014) 54(23):47–50.
42. Sun YM, Yang JQ, Cao BH, Zhang L, Wang BL, Wu M. Relationship Between Expression of PCNA and COX-2 and Local Recurrence of Tumor in Early Laryngeal Cancer With Negative Surgical Margins. J Clin Exp Pathol (2014) 30(4):408–14.
43. Zeng RH. Expression of P53, P21, PCNA and its Relationship With Recurrence in the Early-Stage Laryngeal Cancer With Negative Surgical Margin. 2016. J Clin Otorhinolaryngol Head Neck Surg (2016) 50(50):349–56.
44. Zhu XC, Qian XY, Gu YJ, Shen XH, Song PP, Li H, et al. Expression of P16 and COX-2 Protein in Laryngeal Squamous Cell Carcinoma and its Clinical Significance and its Clinical Significance. J Clin Otorhinolaryngol Head Neck Surg (2017) 30(10):1336–9.
45. Ranelletti FO, Almandori G, Rocca B, Ferrandina G, Ciabattoni G, Habib A, et al. Prognostic Significance of Cyclooxygenase-2 in Laryngeal Squamous Cell Carcinoma. Chin J Clin Res (2001) 99:343–9.
46. Perezruiz E, Cazorla O, Redondo M, Perez L, Alvarez M, Gallego E, et al. Immunohistochemical Expression of Cyclooxygenase-2 in Patients With Advanced Cancer of the Larynx Who Have Undergone Induction Chemotherapy With the Intention of Preserving Phonation. Clin Trans Oncol (2012) 14:682–8. doi: 10.1007/s12094-012-0859-2
47. Wang ZY, Cheng W, Liang ZM, Liu WQ, Pan ZY and Liang T. The Expression of COX-2 and Relationship With Microvessel Density and Lymphatic Vessel Density in Supraglottic Laryngeal Carcinoma. Chin J Surg Onco (2015) 7(1):27–32.
48. Jiang ZH, Li L, Pan XL, Nuan XY. Expression of COX-2 in Laryngeal Carcinoma and Hypopharynx Carcinoma and its Clinical Significance. J Preclin Med Coll Shandong Univ (2004) 18(4):226–8.
49. Liang HT, Gong SS. Expression of Cyclooxygenase-2 and P53 and Their Correlation in Carcinoma of Larynx. J Chin Otorhinolaryngol (China) (2004) 18(7):421–3.
50. Lu HT, Ding J, Gong SS, Wan LJ. Expression of Cyclooxygenase-2 in Laryngeal Squamous Cell Carcinoma and its Relationship With VEGF. J Math Med (2005) 18(4):312–4.
51. Zhu HY, Pan ZH, Xu LM, Mao XL, Chen X. Expressive Activities of COX-2 and Surviving in the Tissues of Various Kinds of Laryngeal Lesions and Their Implications in the Developing Process of Laryngeal Cancer. Chin J Otorhinolaryngol J Integ Med (2007) 15(5):332–84.
52. He Y, Jiang ZH, Song HF. Expression and Significance of COX-2, VEGF and MVD in Laryngeal Carcinoma. Shandong Med (2007) 47(12):42–3.
53. Chen YF, Luo RZ, Li Y, Cui BK, Song M, Yang AK, et al. High Expression Levels of COX-2 and P300 are Associated With Unfavorable Survival in Laryngeal Squamous Cell Carcinoma. Eur Arch Otorhinolaryngol (2013) 270:1009–17. doi: 10.1007/s00405-012-2275-1
54. Ren XT, Jiang J. Expression and Their Clinical Significance of P27, P53, P21, COX-2 and EGFR in Laryngeal Squamous Cell Carcinoma. Chin Arch Otolaryngol Head Neck Surg (2014) 21(7):363–56.
55. Bayazit YA, Buyukberber S, Sari I, Camci C, Ozer E, Sevinc A, et al. Cyclo-Oxygenase 2 Expression in Laryngeal Squamous Cell Carcinoma and Its Clinical Correlates. (2004) 66(2):65–9. doi: 10.1159/000077797
56. Li ZX, Liu GX, Yan MR. Expression of Cyclooxygenase-2 and Metalloproteinase-9 in Laryngeal Carcinoma. J Clin Otolaryngol (2005) 19(12):563–5.
57. Wang JR, Zhang BM, Zhang PF. Expression and Significance of Cyclooxygenase-2 and Vascular Endothelial Growth Factor in Laryngeal Carcinoma. J Fujian Med Univ (2006) 40(2):136–8.
58. Dong P, Li X, Yu Z, Lu G. Expression of Cyclooxygenase-2, Vascular Endothelial Growth Factor and Matrix Metalloproteinase-2 in Patients With Primary Laryngeal Carcinoma: A Tissue Microarray Study. J Laryngol Otology (2007) 121:1177–83. doi: 10.1017/S002221510700031X
59. Kourelis K, Vandoros G, Kourelis T, Papadas T, Goumas P, Bonikou GS. Low COX2 in Tumor and Upregulation in Stroma Mark Laryngeal Squamous Cell Carcinoma Progression. Laryngoscope (2009) 119(9):1723–9. doi: 10.1002/lary.20569
60. Chen WY, Gang C, Qi Y, Yang CZ, Bo L. The Expression of COX-2mrna and Protein in Human Laryngeal Squamous Cell Carcinoma. J Clin Otorhinolaryngol Head Neck Surg (China) (2008) 22(12):532–5.
61. Cheng W, Zhou WG. Clinical Significance and the Relationship Between Vascular Growth Correlation Factor and Microvessel Density, Lymph Vessel Density Expression in Laryngeal Carcinoma. Chin Otolaryngol Head Neck Surg Department (2012) 19(3):117–20.
62. Cheng W, Zhou WG. The Correlations Among the Expression of P53, COX-2, VEGF, and Microvessel Density in Laryngeal Carcinoma. Chin J Othorhinolaryngol Skull Base Surg (2012) 18(2):81–9.
63. Liu ZH, An W, Lin JZ, Li JM, Lin SL, Chen TH. Relationship of Local Recurrence With Expression of Protein Survivin and COX-2 in Laryngeal Carcinoma and its Surgical Margins. Chin J Lab Diagn (2009) 13(7):893–5.
64. Dai WY, Wei XX. Relationship Between Postoperative Recurrence and COX-2 Expression in Patients With Laryngeal Cancer. Exp Lab Med (2020) 38(4):662–5.
65. Bonhin RG, Carvalho GM, Guimaraes AC, Rocha VBC, Chone CT, Crespo AN, et al. Histologic Correlation of VEGF and COX-2 Expression With Tumor Size in Squamous Cell Carcinoma of the Larynx and Hypopharynx. Ear Nose Throat J (2017) 96(4-5):176–82.
66. Xu GQ, Ye YJ, Lu XJ. Expression of COX-2 Protein in Recurrent Laryngeal Cancer After Radiatheraphy. China Med Pharm (2012) 9(11):22–4.
67. Sackett MK, Bairati I, Meyer F, Jobin E, Lussier S, Fortin A, et al. Prognostic Significance of Cyclooxygenase-2 Overexpression in Glottic Cancer. Clin Cancer Res (2008) 14(1):67–73. doi: 10.1158/1078-0432.CCR-07-2028
68. Cho EI, Kowalski DP, Sasaki CT, Haffty BG. Tissue Microarray Analysis Reveals Prognostic Significance of COX-2 Expression for Local Relapse in T1-2N0 Larynx Cancer Treated With Primary Radiation Therapy. Laryngoscope (2004) 114(11):2001–8. doi: 10.1097/01.mlg.0000147936.67379.e7
69. Hong B, Kanaan O, Altenhoff P, Petri R, Thangavelu K, Schluter A, et al. Stromal Versus Tumoral Inflammation Differentially Contribute to Metastasis and Poor Survival in Laryngeal Squamous Cell Carcinoma. Oncotarget (2018) 9(9):8415–26. doi: 10.18632/oncotarget.23865
70. Wei Q, Yu D, Liu M, Wang M, Zhao M, Liu M, et al. Genome-Wide Association Study Identifies Three Susceptibility Loci for Laryngeal Squamous Cell Carcinoma in the Chinese Population. Nat Genet (2014) 46(10):1110–4. doi: 10.1038/ng.3090
71. Wang R, Ma HZ, Lian M, Yang F, Wang H, Feng L, et al. A Preliminary Study on Genome-Wide Expression Profiling of Laryngeal Squamous Cell Carcinoma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi (2014) 49(3):232–5.
Keywords: COX-2, expression, laryngeal cancer, meta-analysis, prognosis
Citation: Du J, Feng J, Luo D and Peng L (2022) Prognostic and Clinical Significance of COX-2 Overexpression in Laryngeal Cancer: A Meta-Analysis. Front. Oncol. 12:854946. doi: 10.3389/fonc.2022.854946
Received: 14 January 2022; Accepted: 14 March 2022;
Published: 22 April 2022.
Edited by:
Boban Erovic, Medical University of Vienna, AustriaReviewed by:
Elena Gershtein, Russian Cancer Research Center NN Blokhin, RussiaNosheen Masood, Fatima Jinnah Women University, Pakistan
Copyright © 2022 Du, Feng, Luo and Peng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijuan Peng, TGlqdWFuX3Blbmc5OUBhbGl5dW4uY29t
 Jingwei Du1
Jingwei Du1 Lijuan Peng
Lijuan Peng