
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 05 April 2022
Sec. Head and Neck Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.854755
This article is part of the Research Topic Improving Quality of Life in Patients with Differentiated Thyroid Cancer View all 20 articles
 Antonio Matrone1†
Antonio Matrone1† Luigi De Napoli2†
Luigi De Napoli2† Liborio Torregrossa3
Liborio Torregrossa3 Aleksandr Aghababyan2
Aleksandr Aghababyan2 Piermarco Papini2
Piermarco Papini2 Carlo Enrico Ambrosini2
Carlo Enrico Ambrosini2 Rosa Cervelli4
Rosa Cervelli4 Clara Ugolini3
Clara Ugolini3 Fulvio Basolo3
Fulvio Basolo3 Eleonora Molinaro1
Eleonora Molinaro1 Rossella Elisei1*
Rossella Elisei1* Gabriele Materazzi2
Gabriele Materazzi2Background: Large thyroid masses, particularly if rapidly growing, are often characterized by compression and infiltration of the vital structures of the neck. Therefore, an early and precise diagnosis, not only of malignancy but also of histotype, is mandatory to set up the right therapy. The aim of this study was to evaluate the diagnostic performance of fine needle aspiration cytology (FNAC) and core needle biopsy (CNB) in this setting.
Patients and Methods: We prospectively evaluated 95 patients with large and rapidly growing thyroid masses admitted to the University Hospital of Pisa between April 2014 and January 2020. All patients were submitted to FNAC and CNB in the same session. The ability of both procedures to diagnose the malignancy of the lesions, particularly the histotype, and to obtain sufficient material to perform molecular analysis was evaluated.
Results: FNAC obtained adequate tumor sample to reach a diagnosis in 76 of 95 (80%) patients, while a higher percentage was obtained with CNB (92/95, 96.8%). FNAC was able to identify the malignancy of the lesion in 74 of 95 (77.9%) cases, but only in 16 of 74 (21.6%) cases was it able to define the histotype. CNB was able to define the malignancy of the lesion in all but three cases (92/95, 96.8%), and in all specimens, the histotype was identified. Moreover, in all cases, the material extracted from CNB was optimal to perform molecular analysis. No surgery-related complications were experienced with both procedures.
Conclusions: CNB is a rapid and safe procedure with higher performance compared to FNAC in identifying the histotype of large and rapidly growing thyroid masses. Moreover, adequate material can be obtained to characterize the molecular profile for the treatment of potentially lethal cancers. In the era of precision medicine, CNB should be introduced in routine clinical practice as a key procedure for an early diagnosis and therapy of these diseases.
Large thyroid masses, particularly if rapidly growing, are often life-threatening events because of the mechanical compression and/or infiltration of the vital structures of the neck (1–3). The most common is anaplastic thyroid carcinoma (ATC), which often appears with hoarseness, cervical pain, dysphagia, dyspnea, and stridor (4, 5). Moreover, beyond local compression, ATC is characterized by high rates of distant metastases and rapidly fatal clinical outcomes (6), and most patients die within 6 months from the diagnosis (1, 7). Conversely, large thyroid masses other than ATC are not necessarily life threatening and can be successfully treated with specific therapies. A clear identification of the histology of these masses is essential to select the most appropriate therapeutic approach (8–10).
All ATC cases are defined as stage IV and primary tumor considered T4 by the American Joint Committee on Cancer (11). According to the American Thyroid Association (12), the therapeutic options include radical surgery, if possible, external radiotherapy, and/or chemotherapy, which should be combined to maximize the disease control (3, 13, 14).
Since ATC is highly aggressive, a rapid diagnosis and treatment should be mandatory. Moreover, since other thyroid masses can have similar clinical presentation, but different outcomes, differentiating ATC from thyroid lymphoma (TL), poorly differentiated thyroid cancer (PDTC), and thyroid gland metastases (TGM) could improve the therapeutic approach and survival (8).
Currently, fine needle aspiration cytology (FNAC) is the most common diagnostic procedure (3), but it has shown several limitations particularly in the identification of ATC or TL (15–17). Therefore, patients with a suspicion of ATC or TL frequently require a surgical conventional biopsy, with more time elapsed to achieve the correct diagnosis (18). Moreover, in the case of surgical conventional biopsy, particularly in ATC, the surgical wound does not quickly heal, thus leading to a further delay in beginning potential treatments.
Core needle biopsy (CNB) represents a minimally invasive, safe, and accurate procedure providing a histological sample, which retains not only its cytologic appearance but also the tissue architecture. CNB has been suggested as a complementary method to FNAC, mainly to overcome possible inconclusive diagnosis (19). Accordingly, FNAC can play an important diagnostic role in the initial evaluation of ATC, but CNB may be necessary for definitive diagnosis and to perform molecular analysis (3). To our knowledge, no studies comparing the sensitivity of FNAC and CNB, on the same patient, in the diagnostic accuracy for detecting the malignancy of large thyroid masses and discriminating the histology have been performed.
The aim of this prospective study was to compare the diagnostic performance of FNAC and CNB in a large series of patients, thus exploring the possibility that CNB could be the first and main diagnostic tool in the presence of a large and rapidly growing thyroid mass.
We prospectively collected the data of 95 consecutive patients with large thyroid masses admitted to either the Endocrine Unit or the Endocrine Surgery Unit of the University Hospital of Pisa between April 2014 and January 2020.
The study was conducted according to the guidelines of the Declaration of Helsinki, approved by the local Ethical Committee (CEAVNO, Comitato Etico Area Vasta Nord Ovest). For the policy of the University Hospital, all patients signed an informed consent both for the performance of invasive procedures and for the use of their data for scientific purposes.
All patients were submitted to routine blood evaluation and coagulation tests, and the medical history and hemorrhagic risk were carefully evaluated. When receiving antiplatelet or anticoagulation therapy, the diagnostic procedures were still performed, without withdrawal. Only warfarin was discontinued up to 3 days before the procedure. No antibiotics and/or analgesics were used after the procedures.
For each patient, a total body spiral computed tomography (CT) scan with intravenous contrast medium, particularly focused on the neck–mediastinum, was performed. Bronchoscopy and esophagoscopy were performed, if needed.
As per protocol, all patients were submitted to FNAC and CNB in the same time session.
An Aloka ProSound Alpha-5sv ultrasound system with a 7.5- to 12-MHz linear transducer (Hitachi Aloka Medical, LTD., Tokyo, Japan) was used for the neck ultrasound (neck US) examination. Neck US assessment is necessary to evaluate the composition and vascularity of the lesion in order to avoid necrotic spaces and vascular bundle and to select the most appropriate area of the mass for tissue sampling. For both procedures, a trans-isthmic or a lateral approach was performed according to each specific case.
A Lightspeed 16 RT, Lightspeed 64 VCT, and a Discovery HD 750 CT scan (GE Medical Systems, Waukesha, WI, USA) was used in patients scanned in our institution. Images of the total body CT scan were utilized for the evaluation of the tumor dimension; the presence of necrosis and/or calcifications; esophageal, tracheal, or laryngeal invasion; vascular involvement; and lymph node and/or distant metastases.
Local anesthesia with 1% lidocaine was applied just before performing both FNAC and CNB, and a 2- to 3-mm skin incision was performed using no. 11 surgical blade, specifically for the aim of this study.
US-guided FNAC was performed with a 21-gauge needle using a 10- or 20-ml syringe with standard aspiration technique. During FNA, four to eight needle passes were performed during one single puncture in the analyzed nodule. The appropriateness of the material obtained by FNA was evaluated on site by the endocrinologist or the surgeon who performed the procedure and was then used to prepare smears, which were examined by pathologists after staining with hematoxylin and eosin.
Cytological results were classified based on the Italian Consensus (20). Accordingly, the samples were defined as non-diagnostic if they were “inadequate”, when biased by smearing and/or fixing and/or staining artifacts or by obscuring blood, or “non-representative”, when the number of epithelial cells collected from the mass was insufficient for a definitive diagnosis. After FNA, we immediately performed CNB using the same skin incision.
CNB was performed using a 16-gauge disposable double-component spring-activated needle. The needle was positioned above the mass, in the same point of the previous FNAC, and was pushed to shoot the cutting cannula. The entire procedure was US guided. The biopsy needles were about 100 mm long. In all cases, we used a 2.0-cm excursion needle. Usually, two or three core samples were picked up from the same skin incision and fixed with 10% formaldehyde solution. After biopsy, the skin incision was dabbed and disinfected, but not sutured. Immediately after CNB, a manual compression of the neck was applied by the patient, and all patients were observed in hospital for the following 20–30 minutes.
Both procedures, FNAC and CNB, were conducted in Fowler’s position to avoid respiratory failure.
FNAC and CNB were analyzed by three different pathologists (LT, CU, and FB) in a double-blinded protocol. Immunohistochemical analyses were performed on each tissue sample obtained by CNB using the VENTANA BenchMark immunostaining system (Ventana Medical Systems, Tucson, AZ, USA). From formalin-fixed and paraffin-embedded specimens, tissue sections (3–5 mm) were deparaffinized and processed. Different panels of immunostaining were performed according to the morphological aspect on tissue section.
Since a greater amount of tissue was obtained by CNB, we decided to perform immunohistochemical and molecular analyses only on CNB samples.
Molecular analysis of samples was not an aim of this study and was not performed systematically. However, we collected the available data found in the pathological report for a descriptive analysis. From 2018, genetic analysis for potential actionable mutations, such as BRAF V600E first and then RET/PTC and NTRK rearrangements, has been performed (21–24). Moreover, in some cases, other oncogenes, especially those beneficial for a differential diagnosis, were analyzed. Good quality DNA and RNA extracted from CNB were obtained and were used to perform molecular analysis. We then performed real-time PCR to analyze codons 600 and 601 of the BRAF gene (EasyPGX® ready THYROID), RET/PTC 1–3, NTRK 1–3, and PAX8/PPAR gamma rearrangements (EasyPGX® ready NTRK and THYROID Fusion). Analysis of the TERT gene promoter hotspot mutations C228T and C250T and the TP53 gene mutations in exons 4–9 was performed on specific request, not in all cases, using direct Sanger sequencing. The MassARRAY system (Sequenom, San Diego, CA, USA) was utilized for the evaluation of exons 18–21 of the EGFR gene, exon 20 of the HER-2 gene, and exons 9 and 20 of the PIK3CA gene. Fluorescence in situ hybridization (FISH) analysis was conducted for ROS1 rearrangement (Vysis 6q22 ROS1 Break Apart FISH Probe Kit), MYC translocation (Vysis MYC Break Apart FISH Probe Kit), and BCL2 translocation (Vysis BCL2 Break Apart FISH Probe Kit (all from Abbott Laboratories, Chicago, IL, USA).
Data are presented as median and interquartile range (IQR) or as frequency (percentage). Diagnostic accuracy was evaluated for both procedures. The χ2 test was used to assess differences between the categorical variables in both procedures. A p-value <0.05 was considered statistically significant. Statistical analysis was performed with SPSS 21.0 software (IBM Corp., Armonk, NY, USA).
At the time of enrollment, patients had a median age of 70 years (IQR = 58–79 years, range = 28–91 years), and 55 out of 95 (57.9%) were women (Table 1).

Table 1 Epidemiological features of the study group (n = 95) and CT scan features of the 76 patients (76/95, 80%) with large thyroid masses who had a CT scan in our department.
In 19 out of 95 (20%) cases, CT scan was not performed in our hospital, and images were evaluated at the time of procedures by the medical team involved in the treatment of the patients. Conversely, 76 of 95 (80%) patients had the CT scan done in our hospital, and images were reviewed by a dedicated radiologist (RC); the radiological features of the neck mass of these patients are reported in Table 1.
The median estimated volume of the thyroid gland, including the surrounding parenchyma not involved in the neoplasia, was 114.5 ml (IQR = 48.25–232.5), while that of the malignant lesion was 106.5 ml (IQR = 40.5–210.75). In most cases, the CT scan showed the invasion of some structures of the neck (73.7%), and about half of them revealed the presence of intratumoral macro- or microcalcifications (50%) and necrosis (63.2%). Lymph node (80.3%) and distant (55.3%) metastases were already present at diagnosis.
The correlation of the results of CT scan with those of FNAC, CNB, and specific immunohistochemical staining for ATC, PDTC, TL, and TGM of lung adenocarcinoma is shown in Figure 1.
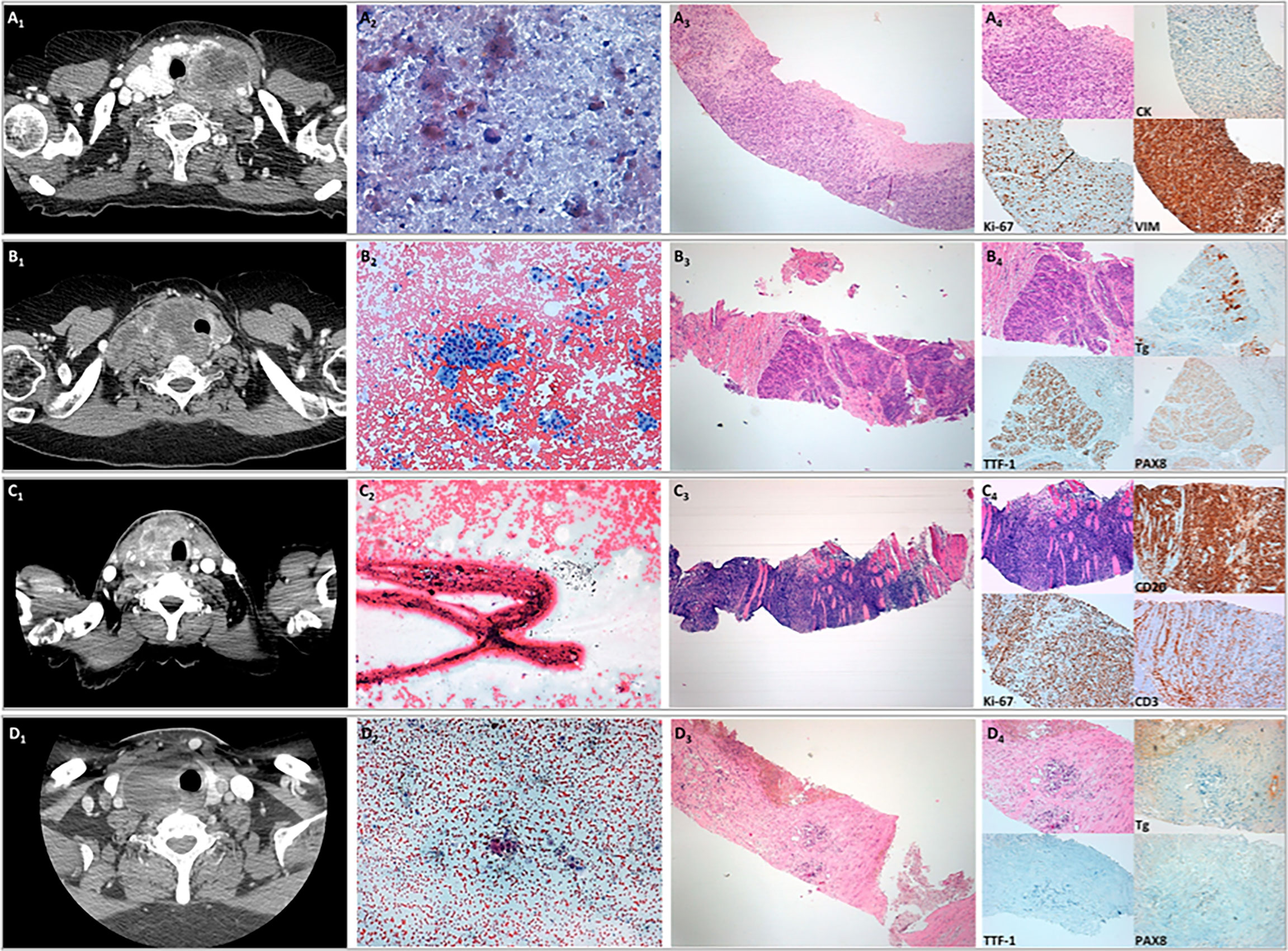
Figure 1 Correlation of the results of CT scan with those of fine needle aspiration cytology (FNAC), core needle biopsy (CNB), and specific immunohistochemical staining for anaplastic thyroid carcinoma (ATC), poorly differentiated thyroid cancer (PDTC), thyroid lymphoma (TL), and thyroid gland metastases (TGM) of lung adenocarcinoma. (A) Representative cytological and histological images of a case of ATC. (A1) CT scan with i.v contrast imaging. (A2) FNAC sample showing a few isolated atypical cells in a necrotic background (original magnification, ×40; Papanicolaou staining). (A3) CNB provided a tissue fragment composed of malignant undifferentiated neoplasia (original magnifications, ×4 and ×10; H&E staining). (A4) Immunohistochemical staining showing neoplastic cells with a high proliferative index, immunoreactivity for vimentin, and patchy weak immunopositivity for cytokeratins. (B) Representative cytological and histological images of a case of poorly differentiated thyroid carcinoma. (B1) CT scan with i.v contrast imaging. (B2) FNAC sample showing numerous groups of follicular cells with moderate nuclear atypia (original magnification, ×10; Papanicolaou staining). (B3) CNB showing neoplastic cells arranged in solid and trabecular architecture (original magnifications, ×4 and ×10; H&E staining). (B4) Immunohistochemical staining showing focal weak immunoreactivity for thyroglobulin and diffuse immunoreactivity for TTF-1 and PAX8. (C) Representative cytological and histological images of a case of TL. (C1) CT scan with i.v. contrast imaging. (C2) FNAC sample not diagnostic for the presence of extensive crush artifacts (original magnification, ×20; Papanicolaou staining). (C3) CNB provided a fragment of tissue composed of muscular tissue with intense lymphoid infiltration (original magnifications, ×4 and ×10; H&E staining). (C4) Immunohistochemical staining showing that neoplastic cells were CD20 positive and CD3 negative with high proliferative indices compatible with B-cell lymphoma. (D) Representative cytological and histological images of a case of carcinoma of extra-thyroid origin. (D1) CT scan with i.v contrast imaging. (D2) FNAC sample showing a few groups of epithelial cells with marked nuclear atypia (original magnifications, ×10 and ×40 in the insert; Papanicolaou staining). (D3) CNB showing a few clusters of neoplastic cells interspersed in fibrotic stroma (original magnifications, ×4 and ×10; H&E staining). (D4) Immunohistochemical staining showing the absence of immunoreactivity for thyroglobulin, TTF-1, and PAX8, suggesting an extra-thyroid origin.
We firstly analyzed the ability of both FNA and CNB to provide adequate tumor samples to reach a diagnosis. Enough and adequate material to perform the cytological analysis was obtained in 76 out of 95 (80%) FNA specimens. A significantly higher percentage of good quality tissue, defined as a minimum 20% of tumor content in the specimens, was obtained with CNB (92/95, 96.8%; p < 0.01). Using this material, immunohistochemistry was performed in 89 of 95 (93.7%) cases, which was useful to the pathologists in clarifying the diagnosis in 88 of 89 (98.9%) cases. The main immunohistochemical markers evaluated according to the histotype revealed by CNB are reported in Table 2. The molecular analysis performed in 17 cases is reported in Table 3.

Table 2 Panel of the main immunohistochemical markers evaluated in our series of rapidly growing thyroid masses according to histology diagnosed by CNB (n = 89).
A comparison of the cytological results of FNAC and the histological results of CNB is reported in Table 4. FNAC was able to identify a malignant lesion in 74 of 95 (77.9%) cases: in 12 out of 74 (16.2%), it provided suspicion for malignancy (TIR 4); in 54 of 74 (73%), it was definitively positive for malignancy (TIR 5); and in 8 of 74 (10.8%) cases, it was suggestive for TL. In 19 (20%) cases, FNAC was not diagnostic (TIR 1), likely due to the presence of necrotic material and inflammatory cells not clearly identifiable during the neck US; in two cases (2.1%), an indeterminate cytology was obtained (TIR 3).
Conversely, CNB was able to diagnose the malignant lesion in all but three cases (92/95, 96.8%). As expected, in all diagnostic specimens, CNB was able to define the histotype of the malignancy, while FNAC did it in 16 of 74 (21.6%) cases (p < 0.01) (Figure 2).
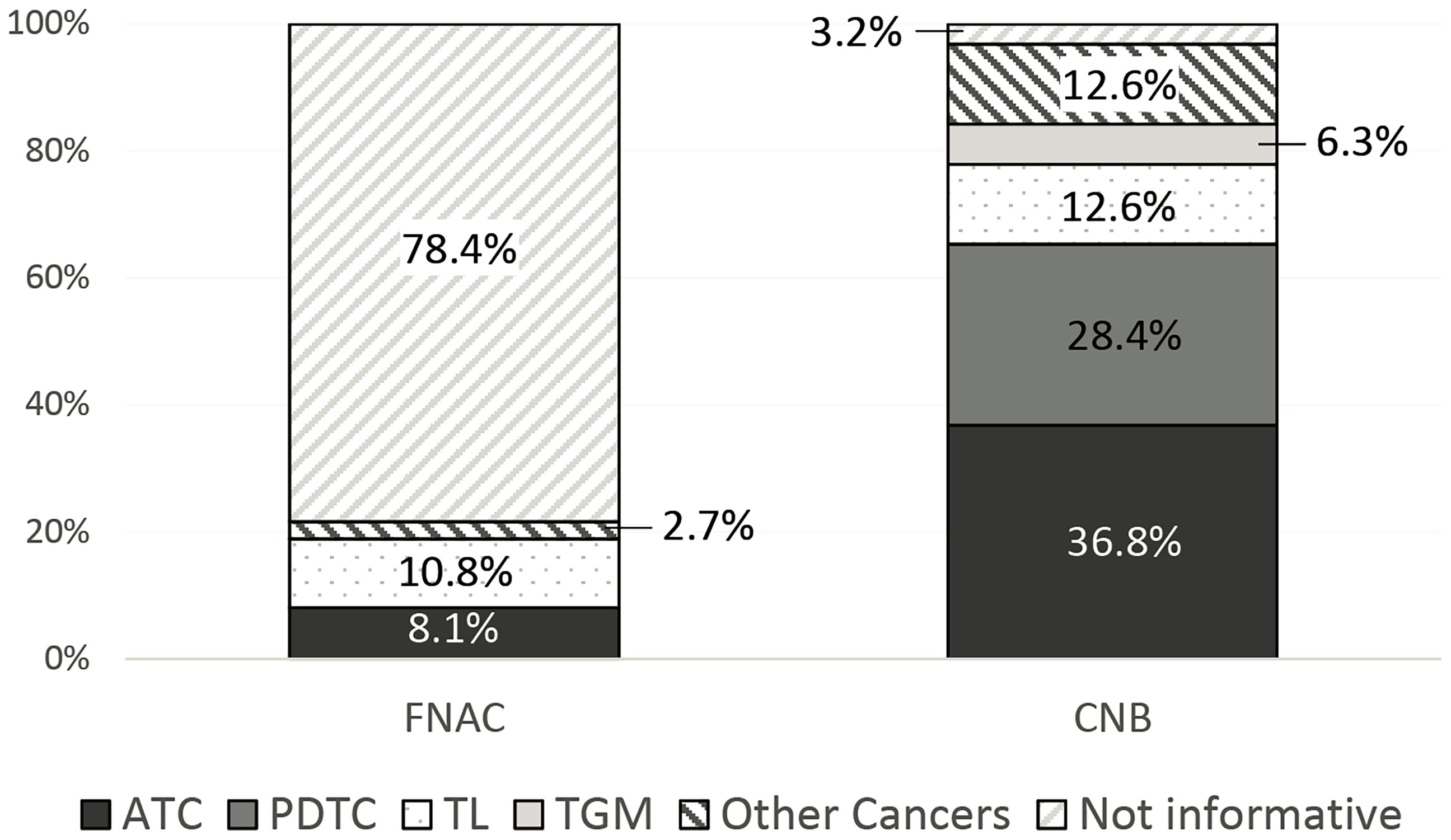
Figure 2 Diagnostic ability of fine needle aspiration cytology (FNAC) and core needle biopsy (CNB) in identifying tumor histotype.
ATC was diagnosed overall in 35 out of 95 (36.8%) cases by CNB. Conversely, FNAC was suspicious for or diagnostic of malignancy in most of the ATCs identified by CNB (29/35, 82.9%), but the specific diagnosis of ATC was only made in six of them (17.1%).
Similarly, FNAC was suspicious for or diagnostic of malignancy in 23 of 27 (85.2%) PDTCs identified by CNB, but the specific diagnosis of PDTC could not be established in any of them based on the cytological specimen.
Of the 13 cases of TL, 12 were correctly identified by CNB (92.3%). On the other hand, FNAC provided the correct diagnosis of TL in seven cases (53.8%), and another one (7.7%) was classified as thyroid malignancy (TIR 5), but it was not diagnostic in the other five (38.4%).
Six cases in the whole series had TGM (6.3%), and CNB showed that they were metastases from the kidney, colon, lung (two cases), and breast (two cases). Conversely, FNAC was inconclusive (TIR 1) in three cases, suspicious for malignancy in one case, and positive for malignancy in two cases, but in all of them, FNAC did not perform a correct histological diagnosis, only predicted it correctly.
Moreover, several other rare neck cancers (12/95, 12.6%) were diagnosed in our series (Table 4). In 9 out of 12 cases, FNAC was able to identify the malignancy of the lesion and, in two of them, also suggested the presence of squamous cell carcinoma. Conversely, in all cases of rare neck cancers, CNB correctly identified both the malignancy of the lesion and the histotype.
In 24 of 95 (25.2%) patients, surgery was performed because there was no preoperative evidence of cervical bundle and massive esophageal and/or tracheal infiltration, as assessed by CT scan, bronchoscopy, and esophagoscopy. In this subgroup, we compared the histology and the results of both CNB and FNAC.
As shown in Table 5, in 20 out of 24 (83.3%) cases, the CNB results were concordant with those of histology, while in 3 of 24 (12.5%) cases, CNB showed a PDTC, but the histology demonstrated the presence of ATC. In only one case of those treated with surgery was the material obtained by CNB inadequate to reach a specific histological diagnosis.
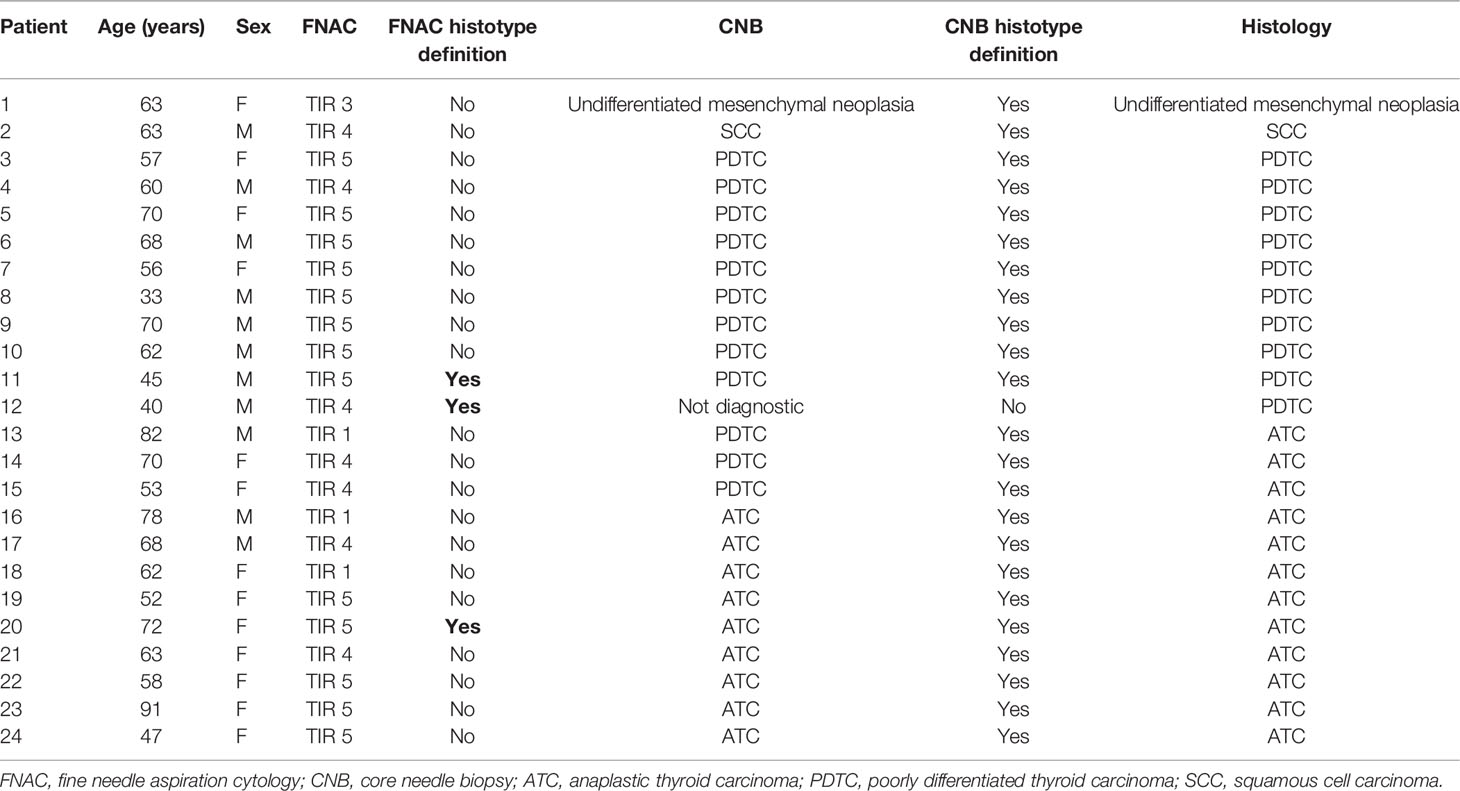
Table 5 Diagnostic performance of FNAC and CNB compared with histology in patients treated with surgery (n = 24).
Regarding FNAC, the cytological material was inadequate to reach a diagnosis in three cases (TIR 1), in one case showed an indeterminate lesion (TIR 3), and in 20 out of 24 (83.3%) cases was suggestive or suspicious for malignancy not further characterized. Overall, the ability of FNAC to define the histotype concordantly with histology was observed in only three cases (12.5%) (Table 5).
In our series, no complications related to FNAC and CNB were experienced during and after the procedures.
Large thyroid masses, particularly if rapidly growing, represent a clinical challenge as they are often related to life-threatening events. In order to quickly reach a diagnosis and carry out appropriate treatments, several procedures have been proposed (3, 25). Surgical conventional biopsy achieves diagnosis in almost all cases, but it has several limitations. It may increase the morbidity and mortality, especially in the elderly, is time-consuming, is invasive, may cause tissue damage, and often requires hospital admission, general anesthesia, and potential transfer to the intensive care unit. Therefore, due to the surgical risk, this approach is not be suitable for all patients (26). Conversely, FNAC is usually the first type of biopsy chosen because it is more immediate, is performed without general anesthesia, and can allow the diagnosis of malignancy in >60% of ATC cases (27, 28). However, cytology, particularly in rare and high-grade malignant neoplasms, does not often give information about the histotype of the tumor, which is, indeed, necessary for the planning of further therapeutic procedures (29). Because of the presence of necrotic material and inflammatory cells in aspirates, we also had 20% of non-diagnostic cases, a higher prevalence when compared to the non-diagnostic FNAC commonly observed when thyroid nodules were submitted to the procedure in our center (30). This percentage of non-diagnostic cases could potentially be reduced by using cell block specimens on the FNA material. However, this is not a routine procedure, the rate of non-diagnostic results could be slightly reduced (31), and, although commonly applied in our center in lung and liver lesions, it is not performed on thyroid nodules.
Several types of neoplasia were diagnosed in our series. Indeed, beyond ATC and PDTC, the most frequently diagnosed neoplasia on CNB (36.8% and 28.4%, respectively), TL (13.7%), TGM (6.3%), and other rare neck cancers (12.6%), such as angiosarcoma, mesenchymal neoplasia, squamous cell carcinoma, and plasmacytoma, were also diagnosed. Similar results were reported by Choi et al. (32), who performed FNAC and CNB in a series of 52 thyroid nodules clinically suspicious for TGM. Unlike in our study, FNAC and CNB were not performed in all cases; nevertheless, their study demonstrated the higher sensitivity of CNB vs. FNAC in the diagnosis of TGM.
It was clearly demonstrated that 18.9% (18/95) of these masses, although localized in the thyroid, were indeed either metastases from other types of tumors or quite rare tumors. However, none of them was identified by FNAC, but were clearly recognized by CNB, which is a true biopsy and allows the pathologist to perform immunohistochemistry, which can better characterize the origin of the tumor (33).
The role of CNB compared to FNAC has already been explored in the diagnosis of thyroid nodules, particularly in cytologically indeterminate lesions. Na et al. (34) evaluated 161 patients with indeterminate cytology and confirmed the higher sensitivity of CNB compared to FNAC in detecting malignancy. However, in our experience, the CNB procedure required that the nodule dimension be ≥3 cm, thus representing a limitation in its routine application. The higher sensitivity of CNB compared to FNAC in detecting malignancy was also confirmed in other human tumors (35–38).
Regarding large neck masses, in particular ATC, Ha et al. (26) evaluated the diagnostic performance of FNAC and CNB in a series of patients with ATC or TL. However, FNAC was performed in 83.8% and CNB in only 32.2% of the study group. The authors obtained 100% and 90.9% positive predictive values for CNB and FNAC in diagnosing ATC, respectively. In our series, the positive predictive values for ATC diagnosis were 100% and 17.1% and for TL diagnosis were 92.3% and 61.5% for CNB and FNAC, respectively. Moreover, unlike in our study, their data were retrospectively collected, CNB was performed in only a minority of cases, and only in a few patients (8.1%) were CNB and FNAC simultaneously performed.
In our series, in the 24 cases submitted to surgery, the correlation between the CNB and histology results was very high since in only 12% of the cases was it slightly discordant. This could be, at least in part, due to the heterogeneity of the ATC, being either of pure anaplastic origin or deriving from the dedifferentiation of a preexisting PTC (3). In any case, this discordance did not play any role in the management of the patients. In the era of precision medicine, in which treatments are targeted against specific genes and mutations of the tumor, the ability to perform a rapid and correct histological diagnosis cannot be overlooked. Indeed, particularly in ATC cases, in which a definitive cure is unlikely with standard treatments, the molecular signature of the neoplasia could improve the outcome of patients harboring actionable mutations (i.e., BRAF V600E) (24, 39), as well as in a neoadjuvant setting (40). In our series, although the material obtained from CNB was optimal to perform molecular analysis in most cases (96.8%), it was performed in only 17.9%. This quite low frequency of analyzed cases is not unexpected since the knowledge about the impact of molecular analysis on the treatment of patients with ATC is quite recent (41). Molecular analysis could also be performed on cytological material (42); however, in several cases, cytology does not provide correct information on the histotype, limiting the choice of genetic profile to be analyzed. Conversely, when the histology is known, as in most cases of CNB, a specific molecular profile can be studied, being different not only according to different tumors (43) but also in the context of different thyroid tumors (44, 45). Accordingly, in metastatic malignancies for which the primary site is unknown, the key role of CNB compared to FNAC has been clearly demonstrated, both in clarifying the primary site of the tumor and in obtaining sufficient material to perform the molecular analysis (8, 46).
CNB is a safe and well-tolerated procedure associated with a low incidence of complications when performed in expert hands (47). However, several potential complications have been reported, such as hematoma, voice change, infection, edema, vasovagal reaction, hemoptysis, and dysphagia (34, 48, 49). In a large single-center study, in which CNB was performed on 6,687 thyroid nodules, very few major (0.06%) and minor (0.79%) complications were described (50). Also, in other studies, low rates of major complications, such as bleeding and tumor cell seeding, were observed (51).
These findings are in accordance with our experience, although in the different setting of large thyroid masses, we did not experience any complications related to the CNB procedure. However, to minimize complications, CNB should be performed by well-trained physicians, under real-time US guidance and with a good awareness of neck anatomy and potential complications (52).
To our knowledge, this is the first prospective study comparing the diagnostic performance of FNAC and CNB in a large series of rapidly growing thyroid masses. The main limitation of our study was that it was performed in a tertiary referral center for the treatment of thyroid cancer, therefore making our results not completely reproducible in routine clinical practice. However, it is recommended that these rare thyroid masses should be managed in referral centers able to perform these kinds of procedures. Conversely, the strengths of this study included the large number of patients enrolled; the use of neck US to identify the areas suitable for biopsy, avoiding the presence of tissue necrosis and inflammation; and the simultaneous use of both FNAC and CNB on the same patient, at the same time, from the same skin incision.
In conclusion, our study demonstrates that CNB is a safe procedure able to optimize the diagnostic times and to obtain an early histological diagnosis, which is fundamental to starting an early and specific treatment. Moreover, the CNB sample can also be immediately analyzed for its molecular profile, with the great advantage that, if a druggable mutation is revealed, a more specific and active drug can be immediately started. This evidence strongly supports the official introduction of CNB in routine clinical practice for the diagnosis of large and rapidly growing thyroid masses.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Comitato Etico Area Vasta Nord Ovest (CEAVNO). The patients/participants provided written informed consent to participate in this study.
AM, LDN, RE, and GM: conceptualization, methodology and data curation. LDN, AA, PP, CEA, and GM: surgical procedures. RC: imaging revision. LT, CU, and FB: cytological and histological analysis. AM and LDN: formal analysis. AM, LDN, AA, PP, CEA, EM, RE, and GM: investigation. AM, LDN, and RE: writing—original draft preparation. All authors: writing—review and editing. RE and GM: supervision. All authors contributed to the article and have read and approved the submitted version.
The work was supported by the Ministero dell’Istruzione, dell’Università e della Ricerca Italiano (MIUR, investigator grant 2017, project code PRIN 2017YTWKWH).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kebebew E, Greenspan FS, Clark OH, Woeber KA, McMillan A. Anaplastic Thyroid Carcinoma. Treatment Outcome and Prognostic Factors. Cancer (2005) 103(7):1330–5. doi: 10.1002/cncr.20936
2. Carcangiu ML, Steeper T, Zampi G, Rosai J. Anaplastic Thyroid Carcinoma. A Study of 70 Cases. Am J Clin Pathol (1985) 83(2):135–58. doi: 10.1093/ajcp/83.2.135
3. Bible KC, Kebebew E, Brierley J, Brito JP, Cabanillas ME, Clark TJ Jr, et al. American Thyroid Association Guidelines for Management of Patients With Anaplastic Thyroid Cancer. Thyroid Off J Am Thyroid Assoc (2021) 31(3):337–86. doi: 10.1089/thy.2020.0944
4. Smallridge RC. Approach to the Patient With Anaplastic Thyroid Carcinoma. J Clin Endocrinol Metab (2012) 97(8):2566–72. doi: 10.1210/jc.2012-1314
5. McIver B, Hay ID, Giuffrida DF, Dvorak CE, Grant CS, Thompson GB, et al. Anaplastic Thyroid Carcinoma: A 50-Year Experience at a Single Institution. Surgery (2001) 130(6):1028–34. doi: 10.1067/msy.2001.118266
6. Masui T, Uemura H, Ota I, Kimura T, Nishikawa D, Yamanaka T, et al. A Study of 17 Cases for the Identification of Prognostic Factors for Anaplastic Thyroid Carcinoma. Mol Clin Oncol (2021) 14(1):1. doi: 10.3892/mco.2020.2163
7. Maniakas A, Dadu R, Busaidy NL, Wang JR, Ferrarotto R, Lu C, et al. Evaluation of Overall Survival in Patients With Anaplastic Thyroid Carcinoma, 2000-2019. JAMA Oncol (2020) 6(9):1397–404. doi: 10.1001/jamaoncol.2020.3362
8. Matrone A, Torregrossa L, Sensi E, Cappellani D, Baronti W, Ciampi R, et al. The Molecular Signature More Than the Site of Localization Defines the Origin of the Malignancy. Front Oncol (2019) 9:1390. doi: 10.3389/fonc.2019.01390
9. Zhu YZ, Li WP, Wang ZY, Yang HF, He QL, Zhu HG, et al. Primary Pulmonary Adenocarcinoma Mimicking Papillary Thyroid Carcinoma. J Cardiothorac Surg (2013) 8:131. doi: 10.1186/1749-8090-8-131
10. Nixon IJ, Coca-Pelaz A, Kaleva AI, Triantafyllou A, Angelos P, Owen RP, et al. Metastasis to the Thyroid Gland: A Critical Review. Ann Surg Oncol (2017) 24(6):1533–9. doi: 10.1245/s10434-016-5683-4
11. Tuttle RM, Haugen B, Perrier ND. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What Changed and Why? Thyroid Off J Am Thyroid Assoc (2017) 27(6):751–6. doi: 10.1089/thy.2017.0102
12. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid Off J Am Thyroid Assoc (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
13. Ain KB. Anaplastic Thyroid Carcinoma: Behavior, Biology, and Therapeutic Approaches. Thyroid Off J Am Thyroid Assoc (1998) 8(8):715–26. doi: 10.1089/thy.1998.8.715
14. Giuffrida D, Gharib H. Anaplastic Thyroid Carcinoma: Current Diagnosis and Treatment. Ann Oncol Off J Eur Soc Med Oncol / ESMO (2000) 11(9):1083–9. doi: 10.1023/A:1008322002520
15. Ljung BM, Drejet A, Chiampi N, Jeffrey J, Goodson WH 3rd, Chew K, et al. Diagnostic Accuracy of Fine-Needle Aspiration Biopsy Is Determined by Physician Training in Sampling Technique. Cancer (2001) 93(4):263–8. doi: 10.1002/cncr.9040
16. Nasuti JF, Gupta PK, Baloch ZW. Diagnostic Value and Cost-Effectiveness of on-Site Evaluation of Fine-Needle Aspiration Specimens: Review of 5,688 Cases. Diagn Cytopathol (2002) 27(1):1–4. doi: 10.1002/dc.10065
17. Baloch ZW, LiVolsi VA. Fine-Needle Aspiration of Thyroid Nodules: Past, Present, and Future. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol (2004) 10(3):234–41. doi: 10.4158/EP.10.3.234
18. Molinaro E, Romei C, Biagini A, Sabini E, Agate L, Mazzeo S, et al. Anaplastic Thyroid Carcinoma: From Clinicopathology to Genetics and Advanced Therapies. Nat Rev Endocrinol (2017) 13(11):644–60. doi: 10.1038/nrendo.2017.76
19. Layfield LJ, Bentz JS, Gopez EV. Immediate on-Site Interpretation of Fine-Needle Aspiration Smears: A Cost and Compensation Analysis. Cancer (2001) 93(5):319–22. doi: 10.1002/cncr.9046
20. Nardi F, Basolo F, Crescenzi A, Fadda G, Frasoldati A, Orlandi F, et al. Italian Consensus for the Classification and Reporting of Thyroid Cytology. J Endocrinol Invest (2014) 37(6):593–9. doi: 10.1007/s40618-014-0062-0
21. Wirth LJ, Sherman E, Robinson B, Solomon B, Kang H, Lorch J, et al. Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N Engl J Med (2020) 383(9):825–35. doi: 10.1056/NEJMoa2005651
22. Subbiah V, Hu MI, Wirth LJ, Schuler M, Mansfield AS, Curigliano G, et al. Pralsetinib for Patients With Advanced or Metastatic RET-Altered Thyroid Cancer (ARROW): A Multi-Cohort, Open-Label, Registrational, Phase 1/2 Study. Lancet Diabetes Endocrinol (2021) 9(8):491–501. doi: 10.1016/S2213-8587(21)00120-0
23. Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med (2018) 378(8):731–9. doi: 10.1056/NEJMoa1714448
24. Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, et al. Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J Clin Oncol Off J Am Soc Clin Oncol (2018) 36(1):7–13. doi: 10.1200/JCO.2017.73.6785
25. Novoa E, Gurtler N, Arnoux A, Kraft M. Role of Ultrasound-Guided Core-Needle Biopsy in the Assessment of Head and Neck Lesions: A Meta-Analysis and Systematic Review of the Literature. Head Neck (2012) 34(10):1497–503. doi: 10.1002/hed.21821
26. Ha EJ, Baek JH, Lee JH, Kim JK, Song DE, Kim WB, et al. Core Needle Biopsy Could Reduce Diagnostic Surgery in Patients With Anaplastic Thyroid Cancer or Thyroid Lymphoma. Eur Radiol (2016) 26(4):1031–6. doi: 10.1007/s00330-015-3921-y
27. Eilers SG, LaPolice P, Mukunyadzi P, Kapur U, Wendel Spiczka A, Shah A, et al. Thyroid Fine-Needle Aspiration Cytology: Performance Data of Neoplastic and Malignant Cases as Identified From 1558 Responses in the ASCP Non-GYN Assessment Program Thyroid Fine-Needle Performance Data. Cancer Cytopathol (2014) 122(10):745–50. doi: 10.1002/cncy.21440
28. Jin M, Jakowski J, Wakely PE Jr. Undifferentiated (Anaplastic) Thyroid Carcinoma and Its Mimics: A Report of 59 Cases. J Am Soc Cytopathol (2016) 5(2):107–15. doi: 10.1016/j.jasc.2015.08.001
29. Shah SS, Faquin WC, Izquierdo R, Khurana KK. FNA of Misclassified Primary Malignant Neoplasms of the Thyroid: Impact on Clinical Management. Cytojournal (2009) 6:1. doi: 10.4103/1742-6413.45191
30. Poma AM, Macerola E, Basolo A, Batini V, Rago T, Santini F, et al. Fine-Needle Aspiration Cytology and Histological Types of Thyroid Cancer in the Elderly: Evaluation of 9070 Patients From a Single Referral Centre. Cancers (Basel) (2021) 13(4):1–10. doi: 10.3390/cancers13040907
31. Vance J, Durbin K, Manglik N, Gilani SM. Diagnostic Utility of Cell Block in Fine Needle Aspiration Cytology of Thyroid Gland. Diagn Cytopathol (2019) 47(12):1245–50. doi: 10.1002/dc.24304
32. Choi SH, Baek JH, Ha EJ, Choi YJ, Song DE, Kim JK, et al. Diagnosis of Metastasis to the Thyroid Gland: Comparison of Core-Needle Biopsy and Fine-Needle Aspiration. Otolaryngol–Head Neck Surg Off J Am Acad Otolaryngol-Head Neck Surg (2016) 154(4):618–25. doi: 10.1177/0194599816629632
33. Higgins SE, Barletta JA. Applications of Immunohistochemistry to Endocrine Pathology. Adv Anat Pathol (2018) 25(6):413–29. doi: 10.1097/PAP.0000000000000209
34. Na DG, Kim JH, Sung JY, Baek JH, Jung KC, Lee H, et al. Core-Needle Biopsy Is More Useful Than Repeat Fine-Needle Aspiration in Thyroid Nodules Read as Nondiagnostic or Atypia of Undetermined Significance by the Bethesda System for Reporting Thyroid Cytopathology. Thyroid Off J Am Thyroid Assoc (2012) 22(5):468–75. doi: 10.1089/thy.2011.0185
35. Cate F, Kapp ME, Arnold SA, Gellert LL, Hameed O, Clark PE, et al. Core Needle Biopsy and Fine Needle Aspiration Alone or in Combination: Diagnostic Accuracy and Impact on Management of Renal Masses. J Urol (2017) 197(6):1396–402. doi: 10.1016/j.juro.2017.01.038
36. Huang Y, Shi J, Chen YY, Li K. Ultrasound-Guided Percutaneous Core Needle Biopsy for the Diagnosis of Pancreatic Disease. Ultrasound Med Biol (2018) 44(6):1145–54. doi: 10.1016/j.ultrasmedbio.2018.02.016
37. Kim HJ, Kim JS. Ultrasound-Guided Core Needle Biopsy in Salivary Glands: A Meta-Analysis. Laryngoscope (2018) 128(1):118–25. doi: 10.1002/lary.26764
38. Wang M, He X, Chang Y, Sun G, Thabane L. A Sensitivity and Specificity Comparison of Fine Needle Aspiration Cytology and Core Needle Biopsy in Evaluation of Suspicious Breast Lesions: A Systematic Review and Meta-Analysis. Breast (2017) 31:157–66. doi: 10.1016/j.breast.2016.11.009
39. Dierks C, Seufert J, Aumann K, Ruf J, Klein C, Kiefer S, et al. The Lenvatinib/Pembrolizumab Combination is an Effective Treatment Option for Anaplastic and Poorly Differentiated Thyroid Carcinoma. Thyroid Off J Am Thyroid Assoc (2021) 31(7):1076–85. doi: 10.1089/thy.2020.0322
40. Wang JR, Zafereo ME, Dadu R, Ferrarotto R, Busaidy NL, Lu C, et al. Complete Surgical Resection Following Neoadjuvant Dabrafenib Plus Trametinib in BRAF(V600E)-Mutated Anaplastic Thyroid Carcinoma. Thyroid Off J Am Thyroid Assoc (2019) 29(8):1036–43. doi: 10.1089/thy.2019.0133
41. Iyer PC, Dadu R, Ferrarotto R, Busaidy NL, Habra MA, Zafereo M, et al. Real-World Experience With Targeted Therapy for the Treatment of Anaplastic Thyroid Carcinoma. Thyroid Off J Am Thyroid Assoc (2018) 28(1):79–87. doi: 10.1089/thy.2017.0285
42. Nishino M. Molecular Cytopathology for Thyroid Nodules: A Review of Methodology and Test Performance. Cancer Cytopathol (2016) 124(1):14–27. doi: 10.1002/cncy.21612
43. Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr, Kinzler KW. Cancer Genome Landscapes. Science (2013) 339(6127):1546–58. doi: 10.1126/science.1235122
44. Landa I, Ibrahimpasic T, Boucai L, Sinha R, Knauf JA, Shah RH, et al. Genomic and Transcriptomic Hallmarks of Poorly Differentiated and Anaplastic Thyroid Cancers. J Clin Invest (2016) 126(3):1052–66. doi: 10.1172/JCI85271
45. Ciampi R, Romei C, Ramone T, Prete A, Tacito A, Cappagli V, et al. Genetic Landscape of Somatic Mutations in a Large Cohort of Sporadic Medullary Thyroid Carcinomas Studied by Next-Generation Targeted Sequencing. iScience (2019) 20:324–36. doi: 10.1016/j.isci.2019.09.030
46. Elsheikh TM, Silverman JF. Fine Needle Aspiration and Core Needle Biopsy of Metastatic Malignancy of Unknown Primary Site. Modern Pathol an Off J U States Can Acad Pathol Inc (2019) 32(Suppl 1):58–70. doi: 10.1038/s41379-018-0149-9
47. Jung CK, Baek JH, Na DG, Oh YL, Yi KH, Kang HC. 2019 Practice Guidelines for Thyroid Core Needle Biopsy: A Report of the Clinical Practice Guidelines Development Committee of the Korean Thyroid Association. J Pathol Transl Med (2020) 54(1):64–86. doi: 10.4132/jptm.2019.12.04
48. Khoo TK, Baker CH, Hallanger-Johnson J, Tom AM, Grant CS, Reading CC, et al. Comparison of Ultrasound-Guided Fine-Needle Aspiration Biopsy With Core-Needle Biopsy in the Evaluation of Thyroid Nodules. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol (2008) 14(4):426–31. doi: 10.4158/EP.14.4.426
49. Screaton NJ, Berman LH, Grant JW. US-Guided Core-Needle Biopsy of the Thyroid Gland. Radiology (2003) 226(3):827–32. doi: 10.1148/radiol.2263012073
50. Ha EJ, Baek JH, Lee JH, Kim JK, Choi YJ, Sung TY, et al. Complications Following US-Guided Core-Needle Biopsy for Thyroid Lesions: A Retrospective Study of 6,169 Consecutive Patients With 6,687 Thyroid Nodules. Eur Radiol (2017) 27(3):1186–94. doi: 10.1007/s00330-016-4461-9
51. Shah KS, Ethunandan M. Tumour Seeding After Fine-Needle Aspiration and Core Biopsy of the Head and Neck–a Systematic Review. Br J Oral Maxillofac Surg (2016) 54(3):260–5. doi: 10.1016/j.bjoms.2016.01.004
Keywords: anaplastic thyroid carcinoma, poorly differentiated thyroid carcinoma, core needle biopsy, fine needle aspiration cytology, thyroid lymphoma
Citation: Matrone A, De Napoli L, Torregrossa L, Aghababyan A, Papini P, Ambrosini CE, Cervelli R, Ugolini C, Basolo F, Molinaro E, Elisei R and Materazzi G (2022) Core Needle Biopsy Can Early and Precisely Identify Large Thyroid Masses. Front. Oncol. 12:854755. doi: 10.3389/fonc.2022.854755
Received: 14 January 2022; Accepted: 10 March 2022;
Published: 05 April 2022.
Edited by:
Giuseppe Mercante, Humanitas University, ItalyReviewed by:
Pirabu Sakthivel, Kovai Medical Center and Hospitals (KMCH), IndiaCopyright © 2022 Matrone, De Napoli, Torregrossa, Aghababyan, Papini, Ambrosini, Cervelli, Ugolini, Basolo, Molinaro, Elisei and Materazzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rossella Elisei, cm9zc2VsbGEuZWxpc2VpQG1lZC51bmlwaS5pdA==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.