- 1Department of Pediatrics, University of San Francisco, San Francisco, CA, United States
- 2Department of Neurology, Neurosurgery and Pediatrics, University of San Francisco, San Francisco, CA, United States
- 3Division of Neurology, Department of Pediatrics, Baylor College of Medicine, Houston, TX, United States
Surgery, chemotherapy and radiation have been the mainstay of pediatric brain tumor treatment over the past decades. Recently, new treatment modalities have emerged for the management of pediatric brain tumors. These therapies range from novel radiotherapy techniques and targeted immunotherapies to checkpoint inhibitors and T cell transfer therapies. These treatments are currently investigated with the goal of improving survival and decreasing morbidity. However, compared to traditional therapies, these novel modalities are not as well elucidated and similarly has the potential to cause significant short and long-term sequelae, impacting quality of life. Treatment complications are commonly mediated through direct drug toxicity or vascular, infectious, or autoimmune mechanisms, ranging from immune effector cell associated neurotoxicity syndrome with CART-cells to neuropathy with checkpoint inhibitors. Addressing treatment-induced complications is the focus of new trials, specifically improving neurocognitive outcomes. The aim of this review is to explore the pathophysiology underlying treatment related neurologic side effects, highlight associated complications, and describe the future direction of brain tumor protocols. Increasing awareness of these neurologic complications from novel therapies underscores the need for quality-of-life metrics and considerations in clinical trials to decrease associated treatment-induced morbidity.
Introduction
Advances in therapeutic modalities, stemming from broader understanding of biologic pathways of cancer, ranging from targeted molecular treatments to viral-based therapies, have demonstrated and continue to show great potential for improving pediatric neuro-oncologic outcomes. As survivorship for pediatric brain tumor patients continues to improve, the corresponding increase in morbidity from treatment-associated complications are a growing and significant concern. Patients who undergo craniospinal radiation are at a significant risk of secondary malignancies, especially those with an underlying defect in tumor suppressor genes, such as neurofibromatosis type 1 and 2 and Li Fraumeni syndrome. Compared to their healthy peers and survivors of non-central nervous system (CNS) cancers, survivors of brain tumors experience the lowest physical, psychological, and social health outcomes (1–3). With these developing concerns, ongoing trials aim to address the management of therapy-induced complications. To further understand the landscape of brain tumor therapies, we reviewed traditional and novel treatment modalities of CNS tumors, explore their underlying molecular mechanism of action, highlight associated complications, and outline the future direction of brain tumor treatment protocols.
Traditional Therapies
Chemotherapy
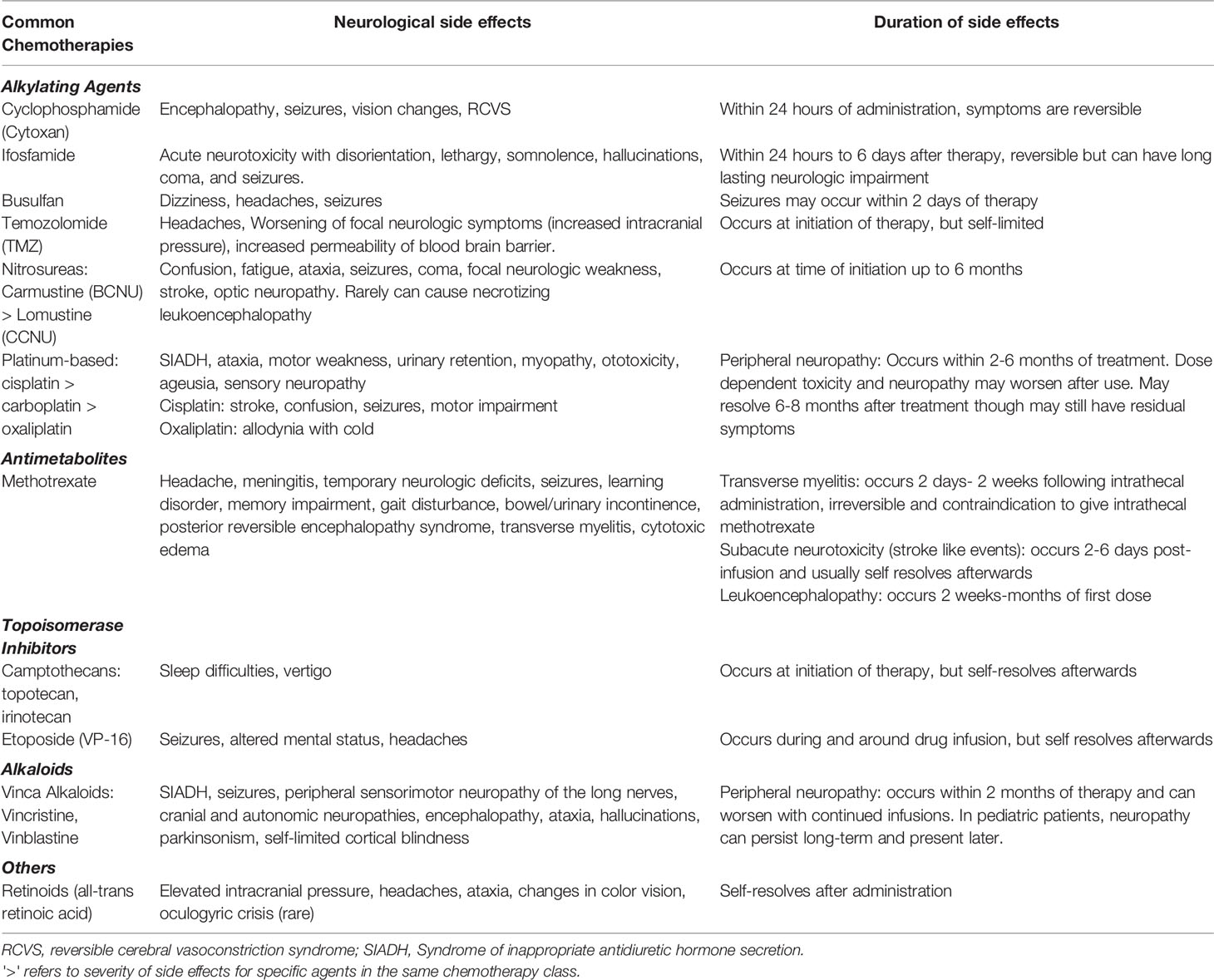
Chemotherapy, in addition to surgery and radiation therapy have been the mainstay of pediatric brain tumor treatment over the past decades. In addition to profound pancytopenia, chemotherapies can cause neurologic deficits, including encephalopathy, ataxia and motor weakness (4). The most used chemotherapeutic agents for brain tumors include vincristine, vinblastine, carboplatin, cyclophosphamide, cisplatin, ifosfamide, and etoposide, of which the latter two are most associated with encephalopathy (incidence of 10-40%) (4–7). Vincristine and vinblastine can rarely cause syndrome of inappropriate antidiuretic hormone secretion (SIADH) which can subsequently lead to seizures. Neuropathy from vinka alkaloids is a more common adverse effect (16-78%) (5, 8). Cisplatin and carboplatin are ototoxic drugs, which can cause permanent high-frequenting hearing loss (42-71%) and consequently severe learning and developmental impairments in young children (5, 9–14). Among patients, who received cisplatin and carboplatin therapy for brain tumors, 4 patients with glioma (57%), 15 with medulloblastoma (88%) and 3 with primitive neuroectodermal tumor (50%) had ototoxicity. However, sodium thiosulfate has demonstrated otoprotective effects with lower rates of hearing loss for children with hepatoblastoma, who underwent cisplatin treatment in a randomized phase III trial (15). In this study of 109 children, patients who received sodium thiosulfate had a 48% lower incidence of hearing loss (RR= 0.52; 95% CI, 0.33 to 0.81; P = 0.002) compared to those who did not receive sodium thiosulfate, with no impact on overall survival (15). A similar phase III trial (NCT00716976) investigating the use of sodium thiosulfate for cisplatin-associated hearing loss in brain tumors, specifically medulloblastoma, neuroblastoma and germ cell tumors has recently been completed with results pending.
Chemotherapeutic agents, such as vincristine, cyclophosphamide, ifosfamide, etoposide, gemcitabine, and cisplatin can result in blood pressure elevations, which may cause posterior reversible encephalopathy syndrome (1-10%), presenting with headaches, seizures, vomiting visual changes, and altered mental status (16, 17). Generally, management of these chemotherapy associated complications is supportive care. Intolerable neurologic side effects can often be managed by dose reduction or modifying the treatment regimen (18). Table 1 summarizes neurologic side effects of commonly used chemotherapeutic agents for the treatment of brain tumors.
Radiation Therapy (RT)
Of the RT modalities, fractionated RT is commonly used in pediatric brain tumors, because it delivers a lower dose per fraction, while increasing the probability of interrupting tumor cells during times of cell division and radiosensitivity, sparing normal tissue (19, 20). Radiation dose and volume correlate with functional outcomes, especially in the pediatric population. The extent of RT (focal vs. craniospinal) depends on the brain tumor pathology with certain brain tumors, such as medulloblastoma, receiving craniospinal RT due to the higher risk of CNS metastasis.
RT can cause both short and long-term neurotoxicity. While short-term neurotoxicity presents as pseudoprogression with worsening of acute neurologic symptoms, somnolence syndrome, radiation necrosis, long-term toxicity can include neurocognitive impairment, vasculopathy, and secondary malignancies like high grade gliomas and meningiomas (21). Proton therapy, a newer radiotherapeutic modality has been successful in improving cognitive outcomes but has not shown benefit with any other RT-associated complications.
Cognitive Dysfunction
Decline in cognitive ability, including attention, memory, and information processing have profound influences on pediatric patients’ long-term health-related quality of life as it impairs job and education prospects, as well as hinders social interactions (22, 23). Children are most vulnerable to RT-associated cognitive complications with the greatest risk during infancy to early childhood. Not surprisingly, radiation dose and field and photon therapy have the most impact on neurocognitive outcomes with pediatric patients treated with craniospinal irradiation associated with the poorest cognitive outcomes compared to those receiving focal radiation only (24). The prevalence of cognitive dysfunction ranges from 40-100% for pediatric brain tumor survivors (25–27). In respect to time course, cognitive complications may occur shortly after RT but also persist years after treatment. Within the first 3-12 weeks, post radiation somnolence syndrome can occur, which presents with fatigue, nausea, vomiting fever, dysphagia, and ataxia. Though post radiation somnolence syndrome can last several weeks, they are typically self-limiting (28). RT-cognitive dysfunction occurs frequently after treatment, is associated with younger age and can be very challenging to manage (29). Pediatric patients who are treated with RT should undergo yearly neuropsychologic assessments to ensure normal development and additional educational and school support as needed.
Metformin has the potential to improve cognitive recovery in pediatric patients post-RT (30). Metformin acts on the neural stem cells in the subventricular zone and the dentate gyrus, restoring neurogenesis after RT and in turn has been associated with improvement in working memory performance in mice models (30). In a randomized placebo controlled early phase clinical trial with pediatric patients, metformin enhanced auditory-verbal recall and working memory, both associated with neurogenesis in the dentate gyrus of the hippocampus (31, 32). Side effects from metformin were safe and tolerable, including mild gastrointestinal distress, and diarrhea, consistent with its known adverse effects. Overall, metformin shows promise as a safe therapy for RT-induced cognitive dysfunction, but larger clinical trials are required before widespread adoption (30).
Due to negative impacts of quality of life from neurocognitive decline, several clinical trials have sought to proactively address and improve neurocognitive outcomes in pediatric patients post- RT through cognitive training or stimulant medications. The Children’s Oncology Group (COG) ACCL10P1 (NCT01503086) employs a computerized cognitive training program for children, who recently completed cranial RT, in efforts to prevent memory and attention dysfunction. In this study, pediatric patients engage with interactive computerized educational modules, targeting verbal working memory and visual-special skills 3-5 times a week for several weeks. COG’s ACCL0922 (NCT01381718) investigates modafinil’s role in improving attention, executive function and fatigue compared to placebo in pediatric patients, who previously underwent treatment for their primary brain tumor. Most recently, COG is conducting a Phase 3 randomized, placebo-controlled trial evaluating memantine for neurocognitive protection in children receiving cranial radiation therapy for their primary CNS tumors, ACCL2031 (NCT04939597). Though the results of these studies are pending, these trials refocus efforts towards enhancing not only survival, but also improving quality of life.
Vascular Complications
There are both short and long-term vascular complications that arise following RT therapy. While cerebral microbleeds can be detected as early as eight months after RT, cavernous malformations and atherosclerosis can present decades later (33, 34). Cerebral microbleeds are RT induced small vessel vasculopathies that are associated with poorer executive function, working memory and cognitive decline in pediatric patients (35–37). Patients who are treated at a younger age, with higher RT doses, and a larger treatment volume are at greater risk of developing cerebral microbleeds (38–40).
Though the mechanism of these RT-associated vascular complications remains unclear, it is thought that radiation causes hyperplasia to the endothelium and fibrinoid necrosis of the vascular walls, resulting in vessel stenosis (41–43). The resulting impaired flow and increased venous pressure can lead to migraines, Moyamoya syndrome (3%), cavernoma formation (50%) and ischemic stroke (1-12%) (43–51). Post-radiation arteriopathy increases the risk of strokes due to stenosis of the distal internal carotid arteries and proximal cerebral arteries with significant neurovascular risk in pediatric patients who receive RT adjacent to the circle of Willis (4). The Childhood Cancer Survivor Study (CCSS) revealed that pediatric brain tumor survivors, who completed therapy 5 years or longer, had a significantly elevated risk of late-occurring stroke (RR = 29; 95% CI, 13.8 to 60.6; P <.0001) compared to the sibling control group (52). The cranial RT dose was directly correlated with late-onset stroke with doses greater than 30 gray (Gy) associated with an increased risk, and the highest risk of stroke noted in RT doses above 50 Gy (52, 53). In the CCSS group, for patients who received greater than 50 Gy, the risk of stroke continued to rise throughout adulthood with the cumulative incidence of 1.1% at 10 years post diagnosis and increasing more than tenfold to 12% at 30 years (53). This increased risk over time may be due to accelerated intracranial atherosclerosis following RT as atherosclerotic risk factors of hypertension and diabetes both separately and in combination were significant predictors of stroke in the CCSS cohort (53). Due to this progressive stroke risk and modifiable risk factors, the Children’s Oncology Group recommends serial monitoring for strokes at clinic visits, routine screening for hypertension, blood glucose and lipid panel every two years in children with an elevated BMI, and yearly imaging with MRI and MRA as indicated for possible intervention, such as aspirin and surgical revascularization.
Other vascular malformations, such as cerebral cavernous malformations can occur on average 10 years post-RT and present with seizures or hemorrhage. Although there is a relatively low incidence of cavernomas 10 years post-RT, around 3-4%, patients with medulloblastoma are at a significantly higher risk (34, 43, 54). Surgical intervention may be required if there are concerns for rupture and hemorrhage (45).
Though not clearly a vascular complication, stroke-like migraine attacks (SMART) syndrome is a late-onset sequalae of radiation, which can occur 1-40 years post-RT (49). SMART syndrome presents with symptoms of repetitive migraine headaches, aphasia, persistent seizures, and other focal neurologic deficits, spanning days to weeks (49). Symptoms typically self-resolve for more than 80% of patients, but some may experience permanent neurologic deficits (55). Though the underlying etiology of SMART syndrome is not clearly defined, it is postulated that hypoperfusion and cerebrovascular changes observed on imaging may be contributing factors (56, 57). Treatment involves symptom management.
Radiation Necrosis
RT is thought to induce radiation necrosis via two mechanisms: (1) vascular injury from upregulation of pro-inflammatory markers [Vascular endothelial growth factor (VEGF), Intercellular Adhesion Molecule 1 (ICAM-1), and Tumor Necrosis Factor alpha (TNF-alpha)] and activation of sphingomyelinase and ceramide, which results in endothelial cell death, (2) reactive astrogliosis, creating pro-inflammatory environment and demyelination (58). Typically, radiation necrosis is observed within the first two years of RT but can occur as early as 6 months. However, early tumor recurrence can be often radiographically indistinguishable from radiation necrosis in high grade tumors.
While asymptomatic radiation necrosis can be managed with close observation, patients with symptomatic radiation necrosis causing focal neurologic deficits, mass effect and elevated intracranial pressure, should be initiated on corticosteroids as first-line therapy (59, 60). For patients requiring longer term treatment, bevacizumab (an anti-vascular endothelial growth factor (VEGF) monoclonal antibody) has demonstrated robust clinical and radiographic response in multicenter prospective trials, avoiding steroid-treatment toxicity (61, 62). Bevacizumab has also shown significant improvement in neurocognitive outcomes, including attention, language, concentration, memory, visuospatial orientation, and calculation at two months compared to patients treated with corticosteroids only (63). However, as an anti-angiogenesis agent, bevacizumab decreases wound healing and predisposes patients to hemorrhage and thrombosis. Aside from bevacizumab, hyperbaric oxygen therapy has also been explored but the benefit remains controversial (64). Despite proton therapy’s ability to deliver radiation more precisely, it has not been demonstrated to reduce the risk of radiation necrosis. For patients with severe symptoms and/or treatment-refractory radiation necrosis, including persistent neurologic deficits and mass effect, surgical intervention could be considered (65).
Novel Therapies
The increasing need for targeted therapies is multifactorial and includes the need to improve outcomes where chemotherapy and radiation have failed. Concurrently, advances in molecular and gene profiling of brain tumors have enhanced our ability to diagnose, risk stratify and identify key aberrant genetic pathways, paving the way for new tailored modalities (62). However, breakthrough for novel therapies has been limited to low grade glioma and a small number of specific high-grade gliomas, namely mismatch repair-deficient glioblastoma (GBM). These new therapies can be categorized into two main approaches: (1) targeting gene mutations and dysregulated pathways involved in cellular growth differentiation, and apoptosis, such as with Mitogen-activated protein kinase kinase (MEK) and B-Raf proto-oncogene (BRAF) inhibitors, and small molecule inhibitors, and (2) upregulating immunosurveillance and immune system activation, such as through checkpoint inhibitors, chimeric antigen receptor T-cells (CAR T-cells), vaccine therapy and virotherapies.
These new modalities face a myriad of extrinsic, intrinsic and tumor-associated limitations (66, 67). Extrinsic factors consist of physical and biological barriers preventing trafficking of therapies into the intracranial space, such as the blood brain barrier and tumor microenvironment (65). For immunotherapies like CAR T-cell and virotherapies, intrinsic limitations such as limited targetable tumor antigens, insufficient proliferation or expansion and lack of durable response can also hinder clinical efficacy (63). Brain tumors, especially gliomas, can circumvent the binding and targeting of these novel therapies by upregulating expression of inhibitory ligands and cytokines, and downregulating targeted tumor antigens, preventing recognition by CAR T-cells, viral vectors, and avoiding check point inhibition (68, 69).
Targeted Therapy
Mitogen-Activated Protein Kinase Kinase (MEK) Inhibitors
Aberrant mitogen-activated protein kinase (MAPK) pathway signaling is the most prevalent genetic abnormality underlying low grade gliomas in pediatric patients (70–72). In phase II clinical trials of pediatric patients, MEK inhibitors have demonstrated efficacy in pretreated, non- neurofibromatosis type 1 (NF1), recurrent optic pathway and hypothalamic low-grade gliomas, as well as BRAF-mutated or NF1- associated low grade gliomas (73, 74). MEK inhibitors can be considered as an alternative treatment modality for children with multiple lesions or have underlying mutations in BRAF and NF1 as they can avoid the paradoxical MAPK pathway activation observed when low-grade gliomas with aberrant BRAF-KIAA1549 mutations are treated with BRAF inhibitors (75). Phase 3 COG trials are currently underway to directly compare standard chemotherapy versus MEK inhibitors in NF1 associated and sporadic low-grade gliomas in the upfront as well as recurrent setting. From phase I/II trials of pediatric patients, MEK inhibitors have tolerable neurotoxicity with the most common neurologic symptom being headache (30%) and myopathy (73, 74, 76). Cobimetinib was more frequently associated with myopathy compared to other MEK inhibitors (77). Dose reduction and/or discontinuation resolved all acute and severe toxicities. These acute complications are similar to what is observed in the adult population. However, the long-term effects of continued MEK inhibition are not fully elucidated in the pediatric population. In vivo models of juvenile rats have demonstrated decreased bone length and delayed sexual maturation with prolonged treatment with MEK inhibitors (78). However, these changes were dose-dependent decreases in body weight, food consumption and long bone growth and consequently, the decrease of bone growth were likely related to overall growth and not due to a bone specific effect of MEK inhibition.
B-Raf Proto-Oncogene (BRAF) Inhibitors
The BRAF protein is a serine/threonine kinase protein, which modulates activity downstream of the Ras-Raf-MEK extracellular signal-regulated kinase (ERK) and MAPK pathway (79). Both signaling pathways play integral roles in regulating cellular differentiation, growth, development, and apoptosis. The most common mutation in the BRAF gene involves a missense mutation in the activation segment on exon 15, codon 600, resulting in a valine to glutamic acid substitution (V600E) (79, 80). This subsequent BRAF-V600E mutation is most commonly observed in gangliogliomas, papillary craniopharyngiomas, pleomorphic xanthoastrocytomas, and epithelioid glioblastomas (81). In patients with pilocytic astrocytomas, fusion of KIAA1549-BRAF gene is more often noted (81).
For brain tumors that harbor the BRAF V600E mutation, direct inhibition of this oncogenic kinase and upstream MAPK proteins can provide targeted therapy as demonstrated in a phase I/II study (82). BRAF V600E inhibitors are well tolerated with no significant neurotoxicities (83). Non-CNS related complications included pyrexia, fatigue, rash, arthralgias, arterial hypertension, decrease in left ventricular ejection fracture, and QT prolongation (83, 84). These side effects are directly correlated to the drug dosage and improved with alternation/reduction of medication. Like MEK inhibitors, the acute complications from BRAF inhibition are similar between adult and pediatric population. Long-term, the use of BRAF V600E inhibitors can lead to squamous cell carcinoma and keratoacanthoma, which may be attributed to paradoxical activation of MAP kinase in cells lacking the BRAF mutation (83).
Though BRAF V600E monotherapy can be effective for a period of time, tumors typically progress in a matter of months (85). To prevent tumor progression, concurrent inhibition of both BRAF and MEK has demonstrated efficacy in pre-clinical models. A recent phase II trial for high- and low-grade gliomas has illustrated a clinical response with combination dabrafenib, a BRAF inhibitor, and trametinib, a MEK inhibitor (86, 87). In this trial, common neurological complications included fatigue, and headache, consistent with prior side effects seen with monotherapy. However, there is an increased risk of cardiovascular adverse events, including pulmonary embolisms, arterial hypertension and left ventricular ejection fracture with dual BRAF and MEK inhibitor therapy compared to BRAF monotherapy (84). As with MEK inhibitors, cessation and dose reduction of these drugs decreased toxicity, but also decreased efficacy.
Small Molecule Inhibitors
Small molecule inhibitors target the tumor microenvironment, as well as tumor-dependent pathways involved with cell growth, invasion and angiogenesis. Because of their small size, they can readily pass through the blood brain barrier to reach CNS tumors. There are several small molecule inhibitors currently studied in clinical trials, including inhibitors of vascular endothelial growth factor (VEGF), cyclin dependent kinase (CDK) 4/6, and histone deacetylase pathways, as well as Onc 201, a dopamine receptor D2 (DRD2) antagonist.
The class of VEGF inhibitors prevent angiogenesis (88). VEGF modulates vasculature formation and permeability, crucial for tumor infiltration, growth, and metastasis (89). Several VEGF inhibitors can also block platelet derived growth factor receptors, another angiogenesis pathway and c-kit signaling, involved with driving CNS tumor growth (90–92). In phase I/II trials for pediatric patients with brain tumors, who underwent VEGF inhibitor therapy, the following neurologic side effects were reported: intracranial hemorrhage (8-10%), lower extremity pain (8%), dizziness (6%), headache (5-15%), muscle weakness (5%), myalgia (4%), altered consciousness (4%), dysphagia (2-11%), reversible posterior leukoencephalopathy syndrome (2%), seizures (2%), hydrocephalus (2%), peripheral motor neuropathy (2%), blurry vision (2%), leukoencephalopathy (2%), papilledema (2%), and fecal incontinence (2%) (93–96). Of note, because neovascularization is critical for physiologic skeletal develop, these children also had notable growth plate abnormalities (9%), which may severely impact growth, adult height, and skeletal maturation (97). Overall, when compared to children with solid tumors who underwent treatment with VEGF inhibitors, there were significantly fewer neurologic side effects, with the most common being pain (10%), suggesting that these neurologic adverse events may be intrinsic to CNS tumors and related to disease progression (90, 91, 98).
CDK are vital regulators of cell cycle progression with CDK4 and CDK6 as integral components (99). The overexpression of CDK4 and 6 are linked to tumor invasion and tumorigenesis in several cancer phenotypes (100). CDK 4/6 inhibitors prevent downstream phosphorylation of retinoblastoma, arresting cell division at the G1 check point. From phase I/II trials for pediatric patients with progressive brain tumors, CDK 4/6 inhibitors were well tolerated with neurotoxicities including: ataxia (24%), dizziness (23%), seizure (21%), headache (19-31%), gait disturbance (14%), and muscle weakness (14%) (101, 102). Of note, seizures were only seen in the heavily pre-treated patients, defined as those who underwent more than 4 prior interventions, such as RT and/or myeloablative chemotherapy/biologics and/or myeloablative chemotherapy with bone marrow stem cell rescue (101).
Histone deacetylase inhibitors are epigenetic regulators that block histone deacetyltransferase (HDAC) activity (103). HDAC condense chromatin by removing acetyl groups from histones, which can repress gene transcription and activity, enabling overexpression of genes involved with proliferation, while downregulating genes involved with differentiation and tumor regulation. HDAC inhibitors regulate gene expression not only through blockade of HDAC, but also by regulating aberrant chromatin structures, which can alter cell migration, differentiation, growth, and angiogenesis (104, 105). In phase I/II trials with HDAC inhibitors for all tumors types, notable neurologic side effects include: lower extremity myopathy (33%), hydrocephalus (17%), pyramidal tract syndrome (17%), seizures (17%), ataxia (17%), Bell’s palsy (17%), muscle weakness (17%), nystagmus (17%), blurry vision (17%), dysgeusia (17-28%), dizziness (15%), headache (6-33%) and neuropathy (6%) (106–110). There were no improvements in either progression or overall survival in these early phase trials.
Onc201 is a small molecule in the imipridone class, which can bypass the BBB, and acts through several mechanisms for anti-tumor effect. Originally, Onc201 was discovered as a downstream regulator of the TNF-related apoptosis-inducing ligand (TRAIL) gene transcription pathway (111). Because immune cells express TRAIL receptors on their surface, Onc201 can selectively induce apoptosis, activating the body’s anti-tumor immunosurveillance without damaging normal cells. Subsequent work has identified underlying molecular targets, including selective competitive and non-competitive antagonism of DRD2 and allosteric activation of caseinolytic protease P (ClpP), both of which are overexpressed in multiple malignancies and can serve as specific anti-tumor therapeutic targets (112–115).
Phase I/II trials with Onc201 have demonstrated tolerable neurotoxicity, with no patients requiring dose adjustment or drug discontinuation for adverse effects (116, 117). In a Phase II recurrent glioblastoma trial, the most common side effect likely attributable to weekly Onc201 was dizziness (5%) (116). While other neurological adverse events were also reported in the trial, including headache (50%), memory impairment (20%), seizure (20%), and paresthesia (15%), these symptoms were attributed to disease progression and not Onc201. Additional potential neurologic complications reported include tinnitus, dysgeusia, altered mental status and gait instability (116–118). Because of the limited data of adverse side effects, and unique mechanism of action, several open trials have explored the potential for synergistic benefit of Onc201 in conjunction with RT, chemotherapy, checkpoint inhibitors and targeted agents to potentiate therapy response and target separate mechanisms of resistance (118). Thus far, pre-clinical in vivo data has yielded promising responses though clinical trial results have yet to be reported (111, 119).
Immunotherapy
Check Point Inhibitors
Recently, check point inhibitors have gained traction in pediatric trials given their efficacy across different cancers. However, they have shown limited efficacy in brain tumors, except in hypermutant glioblastoma with mismatch repair deficiency (120). Ordinarily, T-cells interact with antigen presenting cell’s major histocompatibility complex receptors through programmed death 1 (PD-1) and its associated programed cell death ligand 1/2 (PD-L1, PDL-2), as well as cytotoxic T lymphocyte antigen 4 (CTLA-4) receptors to modulate self-immunity and immune tolerance. PD-1 inhibits T cell activity through interaction with PD-L1 and PD-L2 (121). Tumor cells can evade immunodetection by up-regulating immune checkpoint expression of these negative regulators PD-1 and CTLA-4 and in effect desensitize and exhaust T-cells from recognizing the tumor (122). By blocking the tumor’s PD-L1/PDL-2 binding sites to the T-cell, these checkpoint inhibitors allow for persistent T-cell activation, immunosurveillance and recognition of the tumor (123–125). However, these uninhibited T-cells also have capacity to target normal tissue and result in adverse autoimmune-associated complications (125).
The timing of checkpoint inhibitors may influence the anti-tumor immune response for brain tumor patients. In a randomized multi-institution clinical trial, patients who received both neoadjuvant and adjuvant pembrolizumab (an anti-PD-1 monoclonal antibody) after surgical resection showed improved overall and progression free survival compared to those who received adjuvant therapy alone (126). This clinical response can be attributed to neoadjuvant inhibition of PD-1, resulting in upregulation of interferon response by tumor infiltrating lymphocytes in the tumor microenvironment (126). In turn, inhibition of tumor cell proliferation and cell cycle function occurs. Patients who received only adjuvant PD-1 blockade did not demonstrate similar T-cell upregulation, interferon gene expression, or inhibition of tumor cell proliferation (126).
Though the specific mechanism underlying autoimmune complications from check point inhibitors are unclear, it is thought to be mediated through aberrant T-regulatory cell function from up-regulation of Th-1 and Th-17 activity, and in turn cytokine production, such as IL-6 and IL-17 (124, 125, 127). In addition, enhanced autoantibody production following PD-1 and CTLA-4 inhibition can contribute to cross-reactivity of antigens found on both tumors and normal nervous system tissue, resulting in non-specific targeting (125). This enables these antibodies to bind and impair muscles, nerves, and neuromuscular junctions. Because of this molecular mimicry and widespread involvement of the nervous system, checkpoint inhibitor therapy can induce a significant autoimmune response, which can include: fatigue, headache, inflammation of the pituitary gland, CNS vasculitis, aseptic meningitis, myositis, retinopathy, posterior reversible encephalopathy, auto-immune mediated disorders (myasthenia gravis, cerebellar degeneration, enteric neuropathy, polyradiculopathy, vascular neuropathy, autoimmune encephalitis, progression of demyelinating diseases) (4, 128).
Current management of neurologic complications from check point inhibitors depends on the severity of neurologic deficits, associated patient comorbidities, and risks/benefits of initiating immunotherapy. Though there are no current guidelines, corticosteroids, IVIG, and plasmapheresis are considered first line management strategies. If these initial therapies are unsuccessful, immunosuppressants, such as: rituximab, mycophenolate, cyclophosphamide, and methotrexate can be used (125, 129). For refractory cases, proteasome inhibitors (bortezomib), IL-17 blockers and tacrolimus have also been trialed (130). The decision to discontinuing check point inhibitors in the setting of treatable auto-immune related neurologic complications is controversial. Resumption of checkpoint therapy is dependent on various factors including life expectancy, cancer progression, severity of initial immune-associated neurologic events, and the oncologist’s own comfort with management (125).
Chimeric Antigen Receptor T-Cells (CAR T-Cell)
The initial success with CD19 targeted CAR T-cells for CNS leukemia garnered attention for the potential for immunotherapy for CNS tumors (131). Recent trials have targeted common antigens against epidermal growth factor receptor variant III (EGFRvIII), GD2 and HER2 found in pediatric CNS tumors (132, 133). However, one of the unique difficulties with immunotherapy for CNS lesions, involves managing the potential tumor inflammation associated neurotoxicity, which can be fatal (134).
Three sequelae of CAR T cell treatment include: cytokine release syndrome (CRS), immune effector cell-associated neurotoxicity syndrome (ICANS) or CAR T cell neurotoxicity, and recently tumor inflammation associated neurotoxicity (TIAN), which is specifically observed in CNS tumors. CRS typically occurs the first week of therapy and initially presents with fever, but can develop with symptoms of tachycardia, hypotension and multi-organ dysregulation, including liver failure and disseminated intravascular coagulation (135). Depending on the treatment approach, symptoms can resolve within 2 weeks (136). CRS is mediated through macrophage activation and subsequent cytokine release by monocytes, macrophages and CAR T-cells (137).
Compared to CRS, ICANS occurs 2-11 days after therapy, presenting with tremor, lethargy, cognitive, motor and speech disturbances and in severe cases focal neurologic deficits, such as seizures, cerebral edema, increased intracranial pressure, severe ataxia, and loss of consciousness. Unfortunately, patients may experience chronic neurotoxicity, lasting over several months (138). ICANS can occur independently of CRS; however, more severe CRS symptoms, as well as higher serum CAR T-cells, and elevated CSF protein, leukocytes, interferon-gamma, IL-6 and IL-10, are correlated with the presence of neurotoxicity (138, 139). Though the exact mechanism of these neurologic complications is poorly understood, in vivo models show pronounced meningeal inflammation on histopathology in mice with ICANs compared to those without neurotoxicity (140).
Uniquely observed in brain tumors, TIAN occurs due to intratumoral inflammation (141). As a result of tumor inflammation, patients can experience worsening neurologic deficits and concerns for increased ICP, hydrocephalus and herniation. There remains a paucity of literature and experience with TIAN, but in one single case, management was centered on CSF diversion for elevated intracranial pressure (141).
Generally, the treatment for CRS involves supportive measures as these adverse events are self-limited but can be lethal. Initiation of treatment will depend on the grade/severity of CRS. Since these complications are likely mediated through the release of cytokines, including IL-6, IL-1, and GM-CSF, tocilizumab, an IL-6 inhibitor can be used to treat severe CRS without affecting the immune response (136, 142). Unlike for CRS, tocilizumab has no effect on ICANS, due to difficulty passing the BBB. In a similar mechanism, anakinra, an IL-1 receptor antagonist, has demonstrated efficacy in murine models with reversing neurotoxicity, not observed with IL-6 blockade, as well as, in a case series treating high grade ICANs, and achieving clinical response in half of patients (143, 144). However, further data is required before anakinra can be adopted for ICANS therapy. Steroids can be used for ICANS though it can decrease the efficacy of CAR T-cell therapy (145). In practice, steroids are reserved for severe CRS refractory to tocilizumab treatment and for patients presenting with ICANS.
Vaccine Therapies
Several phase I/II clinical trials have demonstrated increased overall survival with active immunotherapy using autologous dendritic cell (DC) vaccines to target glioblastoma and high-grade gliomas (146–153). As antigen presenting cells, DC can activate and maintain primary immune responses when paired with tumor associated antigens. After exposure to tumor antigens, DC phagocytize and express them as cell surface receptors for CD4+ and CD8+ lymphocyte activation. Similar to CAR T-cells, which require autologous T-cell collection, DC are harvested from the patient, cultured and pulsed with tumor associated antigens before being transfused back to the patient to promote antigen-specific activation of T-cells (154).
In addition to DC vaccines, RNA vaccines have emerged as a promising therapy due to their lower cost, rapid development, and high potency. RNA vaccines can activate both the humoral and cell-mediated immune response as it can deliver different tumor specific antigens and can encode entire tumor antigens, activating a more robust T-cell response (155). Because nucleic acid vaccines also lack viral or protein-derived products during manufacturing, these RNA based therapies are well tolerated with lower cross reactivity (156). One emerging trial from the Pacific Pediatric Neuro-oncology Consortium trial 20 (PNOC020) utilizes an RNA-lipid particle vaccine to treat high grade gliomas in children and adults.
From recent trials, there have been limited neurotoxicities following DC or RNA vaccine administration, with reports of increased seizures and radiographic pseudoprogression in a small subset. In adult patients, who underwent DC vaccine therapy for their high-grade gliomas, a small cohort of patients had increased seizure frequency (5-10%) (157–159). In pediatric patients with recently diagnosed high grade glioma or diffuse midline glioma, after administration of the peptide vaccine with glioma-associated antigen epitopes, radiographic evidence of pseudoprogression (19%) was noted, with corresponding symptoms of ataxia, cranial neuropathies and hyperventilation (153). Dexamethasone improved neurologic symptoms from pseudoprogression. The most common complications were typical for routine vaccines, including fatigue, myalgia, headaches, fevers and localized rashes at the injection site (150, 160–162). The incidence of these complications was not significantly different between the treatment and control group. Lymphopenia was the most serious adverse event for patients with high grade gliomas, who received the vaccinations (149). In all trials, there were no deaths were attributable to the DC or peptide vaccination.
Aside from the complications of the vaccine, the optimal injection method has not been clearly delineated, as many different routes have been described, including subcutaneous, intraperitoneal, intranodal, and intrathecal (160). Overall, vaccine therapies are an emerging class of therapeutics that may be efficacious against gliomas with tolerable adverse effects.
Virotherapy
Like vaccine therapy, virotherapy elicits an inflammatory response and induces tumoricidal effects to overcome glioma’s immunosuppressive microenvironment, but through viral vectors, such as herpes, coxsackie, parvovirus, poliovirus, cytomegalovirus, and adenovirus (163–165). Currently two classes of virotherapies have been used: (1) replication-competent oncolytic viruses, and (2) gene therapy viral vectors, both which require direct injection into the tumor or resection cavity.
Oncolytic viruses are genetically modified to specifically recognize and infect tumor cells, while avoiding normal cells. Following replication within the cancer cell, these oncolytic viruses can induce apoptosis, triggering the release of additional viral progeny and tumor antigen, which activates a robust innate and adaptive immune response (166, 167). Conversely, gene therapy viral vectors induce immune response by inserting desired genomic information into the tumor cell’s transcription and translation mechanism and in turn manufacture new proteins, such as toxic byproducts or cytokines to trigger an anti-tumor response (168). With new advances in viral vectors, current virotherapies now encompass components of both oncolytic and gene-therapy viral properties to enhance therapeutic effects, such as with DNX-2440 and Toca 511. DNX-2440 was designed as an oncolytic virus, but can also transduce X40L, a gene which activates immune response in tumors to augment the anti-tumor response (14). Toca 511, a gene therapy virus with the ability to replicate, can continue to proliferate and infect tumor cells even after the initial treatment (169).
From initial studies for the treatment of recurrent high-grade gliomas, virotherapy has been well tolerated though this in part depends on the specific viral vector (170). In one study of 61 adult glioblastoma patients with non-pathogenic polio virus-rhinovirus vaccine through intra-tumoral infusion, most patients experienced at minimum one neurologic side effect though over 66% were grade 1 (171). These adverse events included: headache (52%), hemiparesis (50%), seizures (45%), dysphagia (28%), cognitive changes (25%), visual field deficits (19%), paresthesia (13%), gait abnormalities (10%), dystonia (2%), and facial weakness (2%) (171). There were three severe adverse events, which ranged from grade 4 cerebral edema (2%) to grade 5 intracranial hemorrhage (2%) and grade 5 seizure (2%).
Toxicities from other early clinical trials have been primarily associated with viral infection and replication, including fever, lymphocyte depletion and malaise (168). These systemic responses were mild and self-limited. The most serious complications noted in those trials was also encephalopathy and cerebral edema though again patient sample size is limited (172). Although there are theoretical concerns of off-receptor viral targeting or uncontrolled viral replication, studies have failed to demonstrate any evidence of neurovirulence. For virotherapy-induced neurotoxicity, bevacizumab was used during these trials to manage tumor associated edema. Other symptoms of viral malaise and fever were managed with supportive care (171).
Future of CNS Therapies to Reduce Morbidity and Mortality
With the implementation of new treatment modalities for brain tumors, therapy-associated morbidity should also be considered. To address therapy complications, we need to transition to create protocols that both enhance survival and decrease morbidity yet maintain efficacy. The goals of reducing chemo- and radiation neurotoxicity have been accomplished through de-escalation of therapy and revision of protocols for brain tumors with good prognosis, as well as targeted drug therapies. For example, patients with medulloblastoma, WNT subtype, have a more favorable prognosis and are therefore being treated, in the setting of clinical trials, with de-escalation of therapy, including reduction of the radiation and chemotherapy doses. In similar efforts, protocols for germinomas, which are generally radio-sensitive and with low mortality, have been modified to incorporate neoadjuvant chemotherapy followed by a reduced RT dose to alleviate long-term radiation-associated neurotoxicity (173). In children with low grade gliomas, treatment with targeted therapies have higher response rates than chemotherapy. However, there are concerns for significant toxicity. Phase III clinical trials are underway comparing chemotherapy versus targeted therapy, which should provide additional information on response and toxicity (NCT04166409).
In addition to de-escalation of therapy or using targeted therapy, other trials are investigating neuroprotective medications. For example, COG clinical trials are exploring sodium thiosulfate for otoprotection in patients with medulloblastoma and memantine for preventing cognitive dysfunction in children receiving cranial radiation. Ultimately, the goal of brain tumor treatment is transitioning towards increasing long term survivorship without impacting patients’ quality of life.
Conclusion
As new promising therapies emerge for pediatric patients with brain tumors, long-term morbidity must also be considered and factored into clinical trial design. The ultimate goal for future treatment should be to provide targeted, effective, and safe therapy to improve the lives of all children with brain tumors.
Author Contributions
TN and FM wrote and revised the manuscript. SM revised and contributed through discussion to the manuscript. All authors have read and approved the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Macartney G, Harrison MB, VanDenKerkhof E, Stacey D, McCarthy P. Quality of Life and Symptoms in Pediatric Brain Tumor Survivors: A Systematic Review. J Pediatr Oncol Nursing: Off J Assoc Pediatr Oncol Nurses (2014) 31(2):65–77. doi: 10.1177/1043454213520191
2. Zeltzer LK, Recklitis C, Buchbinder D, Zebrack B, Casillas J, Tsao JC, et al. Psychological Status in Childhood Cancer Survivors: A Report From the Childhood Cancer Survivor Study. J Clin Oncol (2009) 27(14):2396–404. doi: 10.1200/JCO.2008.21.1433
3. Aarsen FK, Paquier PF, Reddingius RE, Streng IC, Arts WF, Evera-Preesman M, et al. Functional Outcome After Low-Grade Astrocytoma Treatment in Childhood. Cancer (2006) 106(2):396–402. doi: 10.1002/cncr.21612
4. Armstrong C, Sun LR. Neurological Complications of Pediatric Cancer. Cancer Metastasis Rev (2020) 39(1):3–23. doi: 10.1007/s10555-020-09847-0
5. Allen JC. Complications of Chemotherapy in Patients With Brain and Spinal Cord Tumors. Pediatr Neurosurg (1991) 17(4):218–24. doi: 10.1159/000120601
6. Ajithkumar T, Parkinson C, Shamshad F, Murray P. Ifosfamide Encephalopathy. Clin Oncol (R Coll Radiol) (2007) 19(2):108–14. doi: 10.1016/j.clon.2006.11.003
7. Ames B, Lewis LD, Chaffee S, Kim J, Morse R. Ifosfamide-Induced Encephalopathy and Movement Disorder. Pediatr Blood Cancer (2010) 54(4):624–6. doi: 10.1002/pbc.22361
8. Al-Mahayri ZN, AlAhmad MM, Ali BR. Long-Term Effects of Pediatric Acute Lymphoblastic Leukemia Chemotherapy: Can Recent Findings Inform Old Strategies? Front Oncol (2021) 11:710163. doi: 10.3389/fonc.2021.710163
9. Knight KRG, Kraemer DF, Neuwelt EA. Ototoxicity in Children Receiving Platinum Chemotherapy: Underestimating a Commonly Occurring Toxicity That May Influence Academic and Social Development. J Clin Oncol (2005) 23(34):8588–96. doi: 10.1200/JCO.2004.00.5355
10. Clemens E, de Vries AC, Pluijm SF, am Zehnhoff-Dinnesen A, Tissing WJ, Loonen JJ, et al. Determinants of Ototoxicity in 451 Platinum-Treated Dutch Survivors of Childhood Cancer: A DCOG Late-Effects Study. Eur J Cancer (2016) 69:77–85. doi: 10.1016/j.ejca.2016.09.023
11. Knight KR, Kraemer DF, Winter C, Neuwelt EA. Early Changes in Auditory Function as a Result of Platinum Chemotherapy: Use of Extended High-Frequency Audiometry and Evoked Distortion Product Otoacoustic Emissions. J Clin Oncol (2007) 25(10):1190–5. doi: 10.1200/JCO.2006.07.9723
12. Stöhr W, Langer T, Kremers A, Bielack S, Lamprecht-Dinnesen A, Frey E, et al. Cisplatin-Induced Ototoxicity in Osteosarcoma Patients: A Report From the Late Effects Surveillance System. Cancer Invest (2005) 23(3):201–7. doi: 10.1081/CNV-200055951
13. Coradini PP, Cigana L, Selistre SG, Rosito LS, Brunetto AL. Ototoxicity From Cisplatin Therapy in Childhood Cancer. J Pediatr Hematol Oncol (2007) 29(6):355–60. doi: 10.1097/MPH.0b013e318059c220
14. Patel DM, Foreman PM, Nabors LB, Riley KO, Gillespie GY, Markert JM. Design of a Phase I Clinical Trial to Evaluate M032, a Genetically Engineered HSV-1 Expressing IL-12, in Patients With Recurrent/Progressive Glioblastoma Multiforme, Anaplastic Astrocytoma, or Gliosarcoma. Hum Gene Ther Clin Dev (2016) 27(2):69–78. doi: 10.1089/humc.2016.031
15. Brock PR, Maibach R, Childs M, Rajput K, Roebuck D, Sullivan MJ, et al. Sodium Thiosulfate for Protection From Cisplatin-Induced Hearing Loss. New Engl J Med (2018) 378(25):2376–85. doi: 10.1056/NEJMoa1801109
16. Tam CS, Galanos J, Seymour JF, Pitman AG, Stark RJ, Prince HM. Reversible Posterior Leukoencephalopathy Syndrome Complicating Cytotoxic Chemotherapy for Hematologic Malignancies. Am J Hematol (2004) 77(1):72–6. doi: 10.1002/ajh.20147
17. de Laat P, Te Winkel ML, Devos AS, Catsman-Berrevoets CE, Pieters R, van den Heuvel-Eibrink MM. Posterior Reversible Encephalopathy Syndrome in Childhood Cancer. Ann Oncol (2011) 22(2):472–8. doi: 10.1093/annonc/mdq382
18. Neil EC, Hanmantgad S, Khakoo Y. Neurological Complications of Pediatric Cancer. J Child Neurol (2016) 31(12):1412–20. doi: 10.1177/0883073815620673
19. Pawlik TM, Keyomarsi K. Role of Cell Cycle in Mediating Sensitivity to Radiotherapy. Int J Radiat Oncol Biol Phys (2004) 59(4):928–42. doi: 10.1016/j.ijrobp.2004.03.005
20. Nguyen T, Duong C, Sheppard JP, Lee SJ, Kishan AU, Lee P, et al. Hypo-Fractionated Stereotactic Radiotherapy of Five Fractions With Linear Accelerator for Vestibular Schwannomas: A Systematic Review and Meta-Analysis. Clin Neurol Neurosurg (2018) 166:116–23. doi: 10.1016/j.clineuro.2018.01.005
21. Bowers DC, Verbruggen LC, Kremer LCM, Hudson MM, Skinner R, Constine LS, et al. Surveillance for Subsequent Neoplasms of the CNS for Childhood, Adolescent, and Young Adult Cancer Survivors: A Systematic Review and Recommendations From the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol (2021) 22(5):e196–206. doi: 10.1016/S1470-2045(20)30688-4
22. Schulte F, Kunin-Batson AS, Olson-Bullis BA, Banerjee P, Hocking MC, Janzen L, et al. Social Attainment in Survivors of Pediatric Central Nervous System Tumors: A Systematic Review and Meta-Analysis From the Children’s Oncology Group. J Cancer Surviv (2019) 13(6):921–31. doi: 10.1007/s11764-019-00808-3
23. Ventura LM, Grieco JA, Evans CL, Kuhlthau KA, MacDonald SM, Tarbell NJ, et al. Executive Functioning, Academic Skills, and Quality of Life in Pediatric Patients With Brain Tumors Post-Proton Radiation Therapy. J Neurooncol (2018) 137(1):119–26. doi: 10.1007/s11060-017-2703-6
24. Yahya N, Manan HA. Neurocognitive Impairment Following Proton Therapy for Paediatric Brain Tumour: A Systematic Review of Post-Therapy Assessments. Support Care Cancer (2021) 29(6):3035–47. doi: 10.1007/s00520-020-05808-z
25. Duffner PK. Risk Factors for Cognitive Decline in Children Treated for Brain Tumors. Eur J Paediatr Neurol (2010) 14(2):106–15. doi: 10.1016/j.ejpn.2009.10.005
26. Glauser TA, Packer RJ. Cognitive Deficits in Long-Term Survivors of Childhood Brain Tumors. Childs Nerv Syst (1991) 7(1):2–12. doi: 10.1007/BF00263824
27. Aarsen FK, Paquier PF, Arts WF, Van Veelen ML, Michiels E, Lequin M, et al. Cognitive Deficits and Predictors 3 Years After Diagnosis of a Pilocytic Astrocytoma in Childhood. J Clin Oncol (2009) 27(21):3526–32. doi: 10.1200/JCO.2008.19.6303
28. Aspesberro F, Milewski LS, Brogan TV. Acute Central Nervous System Complications in Pediatric Hematopoietic Stem Cell Patients. J Pediatr Intensive Care (2014) 3(3):169–81. doi: 10.3233/PIC-14100
29. Sun LR, Cooper S. Neurological Complications of the Treatment of Pediatric Neoplastic Disorders. Pediatr Neurol (2018) 85:33–42. doi: 10.1016/j.pediatrneurol.2018.05.011
30. Ayoub R, Ruddy RM, Cox E, Oyefiade A, Derkach D, Laughlin S, et al. Assessment of Cognitive and Neural Recovery in Survivors of Pediatric Brain Tumors in a Pilot Clinical Trial Using Metformin. Nat Med (2020) 26(8):1285–94. doi: 10.1038/s41591-020-0985-2
31. Zammit AR, Ezzati A, Zimmerman ME, Lipton RB, Lipton ML, Katz MJ. Roles of Hippocampal Subfields in Verbal and Visual Episodic Memory. Behav Brain Res (2017) 317:157–62. doi: 10.1016/j.bbr.2016.09.038
32. Zheng F, Cui D, Zhang L, Zhang S, Zhao Y, Liu X, et al. The Volume of Hippocampal Subfields in Relation to Decline of Memory Recall Across the Adult Lifespan. Front Aging Neurosci (2018) 10:320. doi: 10.3389/fnagi.2018.00320
33. Lupo JM, Molinaro AM, Essock-Burns E, Butowski N, Chang SM, Cha S, et al. The Effects of Anti-Angiogenic Therapy on the Formation of Radiation-Induced Microbleeds in Normal Brain Tissue of Patients With Glioma. Neuro Oncol (2016) 18(1):87–95. doi: 10.1093/neuonc/nov128
34. Gastelum E, Sear K, Hills N, Roddy E, Randazzo D, Chettout N, et al. Rates and Characteristics of Radiographically Detected Intracerebral Cavernous Malformations After Cranial Radiation Therapy in Pediatric Cancer Patients. J Child Neurol (2015) 30(7):842–9. doi: 10.1177/0883073814544364
35. Cole FM, Yates P. Intracerebral Microaneurysms and Small Cerebrovascular Lesions. Brain (1967) 90(4):759–68. doi: 10.1093/brain/90.4.759
36. Roddy E, Sear K, Felton E, Tamrazi B, Gauvain K, Torkildson J, et al. Presence of Cerebral Microbleeds is Associated With Worse Executive Function in Pediatric Brain Tumor Survivors. Neuro Oncol (2016) 18(11):1548–58. doi: 10.1093/neuonc/now163
37. Morrison MA, Payabvash S, Chen Y, Avadiappan S, Shah M, Zou X, et al. A User-Guided Tool for Semi-Automated Cerebral Microbleed Detection and Volume Segmentation: Evaluating Vascular Injury and Data Labelling for Machine Learning. NeuroImage Clin (2018) 20:498–505. doi: 10.1016/j.nicl.2018.08.002
38. Wahl M, Anwar M, Hess CP, Chang SM, Lupo JM. Relationship Between Radiation Dose and Microbleed Formation in Patients With Malignant Glioma. Radiat Oncol (2017) 12(1):126. doi: 10.1186/s13014-017-0861-5
39. Avadiappan S, Morrison MA, Jakary A, Felton E, Stoller S, Hess CP, et al. Relationship Between 7t MR-Angiography Features of Vascular Injury and Cognitive Decline in Young Brain Tumor Patients Treated With Radiation Therapy. J Neurooncol (2021) 153(1):143–52. doi: 10.1007/s11060-021-03753-3
40. Morrison MA, Hess CP, Clarke JL, Butowski N, Chang SM, Molinaro AM, et al. Risk Factors of Radiotherapy-Induced Cerebral Microbleeds and Serial Analysis of Their Size Compared With White Matter Changes: A 7t MRI Study in 113 Adult Patients With Brain Tumors. J Magn Reson Imaging (2019) 50(3):868–77. doi: 10.1002/jmri.26651
41. Porter PJ, Willinsky RA, Harper W, Wallace MC. Cerebral Cavernous Malformations: Natural History and Prognosis After Clinical Deterioration With or Without Hemorrhage. J Neurosurg (1997) 87(2):190–7. doi: 10.3171/jns.1997.87.2.0190
42. Topper R, Jurgens E, Reul J, Thron A. Clinical Significance of Intracranial Developmental Venous Anomalies. J Neurol Neurosurg Psychiatry (1999) 67(2):234–8. doi: 10.1136/jnnp.67.2.234
43. Lew SM, Morgan JN, Psaty E, Lefton DR, Allen JC, Abbott R. Cumulative Incidence of Radiation-Induced Cavernomas in Long-Term Survivors of Medulloblastoma. J Neurosurg (2006) 104(2 Suppl):103–7. doi: 10.3171/ped.2006.104.2.6
44. Scala M, Fiaschi P, Cama A, Consales A, Piatelli G, Giannelli F, et al. Radiation-Induced Moyamoya Syndrome in Children With Brain Tumors: Case Series and Literature Review. World Neurosurg (2020) 135:118–29. doi: 10.1016/j.wneu.2019.11.155
45. Kralik SF, Watson GA, Shih CS, Ho CY, Finke W, Buchsbaum J. Radiation-Induced Large Vessel Cerebral Vasculopathy in Pediatric Patients With Brain Tumors Treated With Proton Radiation Therapy. Int J Radiat Oncol Biol Phys (2017) 99(4):817–24. doi: 10.1016/j.ijrobp.2017.07.009
46. Wu YH, Chang FC, Liang ML, Chen HH, Wong TT, Yen SH, et al. Incidence and Long-Term Outcome of Postradiotherapy Moyamoya Syndrome in Pediatric Patients With Primary Brain Tumors: A Single Institute Experience in Taiwan. Cancer Med (2016) 5(8):2155–60. doi: 10.1002/cam4.785
47. Armstrong AE, Gillan E, DiMario FJ Jr. SMART Syndrome (Stroke-Like Migraine Attacks After Radiation Therapy) in Adult and Pediatric Patients. J Child Neurol (2014) 29(3):336–41. doi: 10.1177/0883073812474843
48. Jia W, Saito R, Kanamori M, Iwabuchi N, Iwasaki M, Tominaga T. SMART (Stroke-Like Migraine Attacks After Radiation Therapy) Syndrome Responded to Steroid Pulse Therapy: Report of a Case and Review of the Literature. eNeurologicalSci (2018) 12:1–4. doi: 10.1016/j.ensci.2018.05.003
49. Dominguez M, Malani R. Stroke-Like Migraine Attacks After Radiation Therapy (SMART) Syndrome: A Comprehensive Review. Curr Pain Headache Rep (2021) 25(5):33. doi: 10.1007/s11916-021-00946-3
50. Hall MD, Bradley JA, Rotondo RL, Hanel R, Shah C, Morris CG, et al. Risk of Radiation Vasculopathy and Stroke in Pediatric Patients Treated With Proton Therapy for Brain and Skull Base Tumors. Int J Radiat Oncol Biol Phys (2018) 101(4):854–9. doi: 10.1016/j.ijrobp.2018.03.027
51. Passos J, Nzwalo H, Marques J, Azevedo A, Netto E, Nunes S, et al. Late Cerebrovascular Complications After Radiotherapy for Childhood Primary Central Nervous System Tumors. Pediatr Neurol (2015) 53(3):211–5. doi: 10.1016/j.pediatrneurol.2015.05.015
52. Bowers DC, Liu Y, Leisenring W, McNeil E, Stovall M, Gurney JG, et al. Late-Occurring Stroke Among Long-Term Survivors of Childhood Leukemia and Brain Tumors: A Report From the Childhood Cancer Survivor Study. J Clin Oncol (2006) 24(33):5277–82. doi: 10.1200/JCO.2006.07.2884
53. Mueller S, Fullerton HJ, Stratton K, Leisenring W, Weathers RE, Stovall M, et al. Radiation, Atherosclerotic Risk Factors, and Stroke Risk in Survivors of Pediatric Cancer: A Report From the Childhood Cancer Survivor Study. Int J Radiat Oncol Biol Phys (2013) 86(4):649–55. doi: 10.1016/j.ijrobp.2013.03.034
54. Strenger V, Sovinz P, Lackner H, Dornbusch HJ, Lingitz H, Eder HG, et al. Intracerebral Cavernous Hemangioma After Cranial Irradiation in Childhood. Incidence and Risk Factors. Strahlenther Onkol (2008) 184(5):276–80. doi: 10.1007/s00066-008-1817-3
55. Lim SY, Brooke J, Dineen R, O’Donoghue M. Stroke-Like Migraine Attack After Cranial Radiation Therapy: The SMART Syndrome. Pract Neurol (2016) 16(5):406–8. doi: 10.1136/practneurol-2016-001385
56. Fan EP, Heiber G, Gerard EE, Schuele S. Stroke-Like Migraine Attacks After Radiation Therapy: A Misnomer? Epilepsia (2018) 59(1):259–68. doi: 10.1111/epi.13963
57. Wai K, Balabanski A, Chia N, Kleinig T. Reversible Hemispheric Hypoperfusion in Two Cases of SMART Syndrome. J Clin Neurosci (2017) 43:146–8. doi: 10.1016/j.jocn.2017.05.013
58. Vellayappan BA, McGranahan T, Graber J, Taylor L, Venur V, Ellenbogen R, et al. Radiation Necrosis From Stereotactic Radiosurgery-How Do We Mitigate? Curr Treat Options Oncol (2021) 22(7):57. doi: 10.1007/s11864-021-00854-z
59. Wang YX, King AD, Zhou H, Leung SF, Abrigo J, Chan YL, et al. Evolution of Radiation-Induced Brain Injury: MR Imaging-Based Study. Radiology (2010) 254(1):210–8. doi: 10.1148/radiol.09090428
60. Kotsarini C, Griffiths PD, Wilkinson ID, Hoggard N. A Systematic Review of the Literature on the Effects of Dexamethasone on the Brain From In Vivo Human-Based Studies: Implications for Physiological Brain Imaging of Patients With Intracranial Tumors. Neurosurgery (2010) 67(6):1799–815; discussion 815. doi: 10.1227/NEU.0b013e3181fa775b
61. Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, et al. Randomized Double-Blind Placebo-Controlled Trial of Bevacizumab Therapy for Radiation Necrosis of the Central Nervous System. Int J Radiat Oncol Biol Phys (2011) 79(5):1487–95. doi: 10.1016/j.ijrobp.2009.12.061
62. Furuse M, Nonoguchi N, Kuroiwa T, Miyamoto S, Arakawa Y, Shinoda J, et al. A Prospective, Multicentre, Single-Arm Clinical Trial of Bevacizumab for Patients With Surgically Untreatable, Symptomatic Brain Radiation Necrosis(Dagger). Neurooncol Pract (2016) 3(4):272–80. doi: 10.1093/nop/npv064
63. Liao G, Khan M, Zhao Z, Arooj S, Yan M, Li X. Bevacizumab Treatment of Radiation-Induced Brain Necrosis: A Systematic Review. Front Oncol (2021) 11:593449. doi: 10.3389/fonc.2021.593449
64. Leber KA, Eder HG, Kovac H, Anegg U, Pendl G. Treatment of Cerebral Radionecrosis by Hyperbaric Oxygen Therapy. Stereotact Funct Neurosurg (1998) 70 Suppl 1:229–36. doi: 10.1159/000056426
65. McPherson CM, Warnick RE. Results of Contemporary Surgical Management of Radiation Necrosis Using Frameless Stereotaxis and Intraoperative Magnetic Resonance Imaging. J Neurooncol (2004) 68(1):41–7. doi: 10.1023/B:NEON.0000024744.16031.e9
66. Haydar D, Ibanez-Vega J, Krenciute G. T-Cell Immunotherapy for Pediatric High-Grade Gliomas: New Insights to Overcoming Therapeutic Challenges. Front Oncol (2021) 11:718030. doi: 10.3389/fonc.2021.718030
67. El Demerdash N, Kedda J, Ram N, Brem H, Tyler B. Novel Therapeutics for Brain Tumors: Current Practice and Future Prospects. Expert Opin Drug Deliv (2020) 17(1):9–21. doi: 10.1080/17425247.2019.1676227
68. Bloch O, Crane CA, Kaur R, Safaee M, Rutkowski MJ, Parsa AT. Gliomas Promote Immunosuppression Through Induction of B7-H1 Expression in Tumor-Associated Macrophages. Clin Cancer Res (2013) 19(12):3165–75. doi: 10.1158/1078-0432.CCR-12-3314
69. Colombo MP, Piconese S. Regulatory-T-Cell Inhibition Versus Depletion: The Right Choice in Cancer Immunotherapy. Nat Rev Cancer (2007) 7(11):880–7. doi: 10.1038/nrc2250
70. Zhang J, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, et al. Whole-Genome Sequencing Identifies Genetic Alterations in Pediatric Low-Grade Gliomas. Nat Genet (2013) 45(6):602–12. doi: 10.1038/ng.2611
71. Hargrave D. Paediatric High and Low Grade Glioma: The Impact of Tumour Biology on Current and Future Therapy. Br J Neurosurg (2009) 23(4):351–63. doi: 10.1080/02688690903158809
72. Jones DTW, Kieran MW, Bouffet E, Alexandrescu S, Bandopadhayay P, Bornhorst M, et al. Pediatric Low-Grade Gliomas: Next Biologically Driven Steps. Neuro-oncology (2018) 20(2):160–73. doi: 10.1093/neuonc/nox141
73. Fangusaro J, Onar-Thomas A, Young Poussaint T, Wu S, Ligon AH, Lindeman N, et al. Selumetinib in Paediatric Patients With BRAF-Aberrant or Neurofibromatosis Type 1-Associated Recurrent, Refractory, or Progressive Low-Grade Glioma: A Multicentre, Phase 2 Trial. Lancet Oncol (2019) 20(7):1011–22. doi: 10.1016/S1470-2045(19)30277-3
74. Fangusaro J, Onar-Thomas A, Poussaint TY, Wu S, Ligon AH, Lindeman N, et al. A Phase II Trial of Selumetinib in Children With Recurrent Optic Pathway and Hypothalamic Low-Grade Glioma Without NF1: A Pediatric Brain Tumor Consortium Study. Neuro Oncol (2021) 23(10):1777–88. doi: 10.1093/neuonc/noab047
75. Karajannis MA, Legault G, Fisher MJ, Milla SS, Cohen KJ, Wisoff JH, et al. Phase II Study of Sorafenib in Children With Recurrent or Progressive Low-Grade Astrocytomas. Neuro Oncol (2014) 16(10):1408–16. doi: 10.1093/neuonc/nou059
76. Banerjee A, Jakacki RI, Onar-Thomas A, Wu S, Nicolaides T, Young Poussaint T, et al. A Phase I Trial of the MEK Inhibitor Selumetinib (AZD6244) in Pediatric Patients With Recurrent or Refractory Low-Grade Glioma: A Pediatric Brain Tumor Consortium (PBTC) Study. Neuro-oncology (2017) 19(8):1135–44. doi: 10.1093/neuonc/now282
77. Kostine M, Rouxel L, Barnetche T, Veillon R, Martin F, Dutriaux C, et al. Rheumatic Disorders Associated With Immune Checkpoint Inhibitors in Patients With Cancer—Clinical Aspects and Relationship With Tumour Response: A Single-Centre Prospective Cohort Study. Ann Rheum Dis (2018) 77(3):393–8. doi: 10.1136/annrheumdis-2017-212257
78. Ney GM, McKay L, Koschmann C, Mody R, Li Q. The Emerging Role of Ras Pathway Signaling in Pediatric Cancer. Cancer Res (2020) 80(23):5155–63. doi: 10.1158/0008-5472.CAN-20-0916
79. Matallanas D, Birtwistle M, Romano D, Zebisch A, Rauch J, von Kriegsheim A, et al. Raf Family Kinases: Old Dogs Have Learned New Tricks. Genes Cancer (2011) 2(3):232–60. doi: 10.1177/1947601911407323
80. Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF Gene in Human Cancer. Nature (2002) 417(6892):949–54. doi: 10.1038/nature00766
81. Maraka S, Janku F. BRAF Alterations in Primary Brain Tumors. Discovery Med (2018) 26(141):51–60.
82. Hargrave DR, Bouffet E, Tabori U, Broniscer A, Cohen KJ, Hansford JR, et al. Efficacy and Safety of Dabrafenib in Pediatric Patients With BRAF V600 Mutation-Positive Relapsed or Refractory Low-Grade Glioma: Results From a Phase I/IIa Study. Clin Cancer Res: Off J Am Assoc Cancer Res (2019) 25(24):7303–11. doi: 10.1158/1078-0432.CCR-19-2177
83. Flaherty KT, Puzanov I, Kim KB, Ribas A, McArthur GA, Sosman JA, et al. Inhibition of Mutated, Activated BRAF in Metastatic Melanoma. N Engl J Med (2010) 363(9):809–19. doi: 10.1056/NEJMoa1002011
84. Mincu RI, Mahabadi AA, Michel L, Mrotzek SM, Schadendorf D, Rassaf T, et al. Cardiovascular Adverse Events Associated With BRAF and MEK Inhibitors: A Systematic Review and Meta-Analysis. JAMA Netw Open (2019) 2(8):e198890-e. doi: 10.1001/jamanetworkopen.2019.8890
85. Leaver K, Zhang N, Ziskin J. Response of Metastatic Glioma to Vemurafenib. Neurooncol Pract (2016) 3:268–71. doi: 10.1093/nop/npv054
86. Wen PY, Stein A, van den Bent M, De Greve J, Wick A, de Vos F, et al. Dabrafenib Plus Trametinib in Patients With BRAF(V600E)-Mutant Low-Grade and High-Grade Glioma (ROAR): A Multicentre, Open-Label, Single-Arm, Phase 2, Basket Trial. Lancet Oncol (2022) 23(1):53–64. doi: 10.1016/S1470-2045(21)00578-7
87. Zhang J, Yao T, Hashizume R. Combined BRAFV600E and MEK Blockade for BRAFV600E-Mutant Gliomas. J Neurooncol (2016) 131:495–505. doi: 10.1007/s11060-016-2333-4
88. Karkkainen MJ, Petrova TV. Vascular Endothelial Growth Factor Receptors in the Regulation of Angiogenesis and Lymphangiogenesis. Oncogene (2000) 19(49):5598–605. doi: 10.1038/sj.onc.1203855
89. Ferrara N. VEGF as a Therapeutic Target in Cancer. Oncology (2005) 69(Suppl 3):11–6. doi: 10.1159/000088479
90. Geller JI, Fox E, Turpin BK, Goldstein SL, Liu X, Minard CG, et al. A Study of Axitinib, a VEGF Receptor Tyrosine Kinase Inhibitor, in Children and Adolescents With Recurrent or Refractory Solid Tumors: A Children’s Oncology Group Phase 1 and Pilot Consortium Trial (Advl1315). Cancer (2018) 124(23):4548–55. doi: 10.1002/cncr.31725
91. Brown N, McBain C, Nash S, Hopkins K, Sanghera P, Saran F, et al. Multi-Center Randomized Phase II Study Comparing Cediranib Plus Gefitinib With Cediranib Plus Placebo in Subjects With Recurrent/Progressive Glioblastoma. PloS One (2016) 11(5):e0156369. doi: 10.1371/journal.pone.0156369
92. Haberler C, Gelpi E, Marosi C, Rössler K, Birner P, Budka H, et al. Immunohistochemical Analysis of Platelet-Derived Growth Factor Receptor-Alpha, -Beta, C-Kit, C-Abl, and Arg Proteins in Glioblastoma: Possible Implications for Patient Selection for Imatinib Mesylate Therapy. J Neurooncol (2006) 76(2):105–9. doi: 10.1007/s11060-005-4570-9
93. Gururangan S, Fangusaro J, Young Poussaint T, Onar-Thomas A, Gilbertson RJ, Vajapeyam S, et al. Lack of Efficacy of Bevacizumab + Irinotecan in Cases of Pediatric Recurrent Ependymoma–A Pediatric Brain Tumor Consortium Study. Neuro Oncol (2012) 14(11):1404–12. doi: 10.1093/neuonc/nos213
94. Kieran MW, Supko JG, Wallace D, Fruscio R, Poussaint TY, Phillips P, et al. Phase I Study of SU5416, a Small Molecule Inhibitor of the Vascular Endothelial Growth Factor Receptor (VEGFR) in Refractory Pediatric Central Nervous System Tumors. Pediatr Blood Cancer (2009) 52(2):169–76. doi: 10.1002/pbc.21873
95. Wetmore C, Daryani VM, Billups CA, Boyett JM, Leary S, Tanos R, et al. Phase II Evaluation of Sunitinib in the Treatment of Recurrent or Refractory High-Grade Glioma or Ependymoma in Children: A Children’s Oncology Group Study Acns1021. Cancer Med (2016) 5(7):1416–24. doi: 10.1002/cam4.713
96. Kieran MW, Chi S, Goldman S, Onar-Thomas A, Poussaint TY, Vajapeyam S, et al. A Phase I Trial and PK Study of Cediranib (AZD2171), an Orally Bioavailable Pan-VEGFR Inhibitor, in Children With Recurrent or Refractory Primary CNS Tumors. Childs Nerv Syst (2015) 31(9):1433–45. doi: 10.1007/s00381-015-2812-5
97. Voss SD, Glade-Bender J, Spunt SL, DuBois SG, Widemann BC, Park JR, et al. Growth Plate Abnormalities in Pediatric Cancer Patients Undergoing Phase 1 Anti-Angiogenic Therapy: A Report From the Children’s Oncology Group Phase I Consortium. Pediatr Blood Cancer (2015) 62(1):45–51. doi: 10.1002/pbc.25229
98. Kim A, Widemann BC, Krailo M, Jayaprakash N, Fox E, Weigel B, et al. Phase 2 Trial of Sorafenib in Children and Young Adults With Refractory Solid Tumors: A Report From the Children’s Oncology Group. Pediatr Blood Cancer (2015) 62(9):1562–6. doi: 10.1002/pbc.25548
99. Sánchez-Martínez C, Gelbert LM, Lallena MJ, de Dios A. Cyclin Dependent Kinase (CDK) Inhibitors as Anticancer Drugs. Bioorg Med Chem Lett (2015) 25(17):3420–35. doi: 10.1016/j.bmcl.2015.05.100
100. Shapiro GI. Cyclin-Dependent Kinase Pathways as Targets for Cancer Treatment. J Clin Oncol (2006) 24(11):1770–83. doi: 10.1200/JCO.2005.03.7689
101. Van Mater D, Gururangan S, Becher O, Campagne O, Leary S, Phillips JJ, et al. A Phase I Trial of the CDK 4/6 Inhibitor Palbociclib in Pediatric Patients With Progressive Brain Tumors: A Pediatric Brain Tumor Consortium Study (PBTC-042). Pediatr Blood Cancer (2021) 68(4):e28879. doi: 10.1002/pbc.28879
102. DeWire M, Fuller C, Hummel TR, Chow LML, Salloum R, de Blank P, et al. A Phase I/II Study of Ribociclib Following Radiation Therapy in Children With Newly Diagnosed Diffuse Intrinsic Pontine Glioma (DIPG). J Neurooncol (2020) 149(3):511–22. doi: 10.1007/s11060-020-03641-2
103. Perla A, Fratini L, Cardoso PS, Nör C, Brunetto AT, Brunetto AL, et al. Histone Deacetylase Inhibitors in Pediatric Brain Cancers: Biological Activities and Therapeutic Potential. Front Cell Dev Biol (2020) 8:546. doi: 10.3389/fcell.2020.00546
104. Bolden JE, Peart MJ, Johnstone RW. Anticancer Activities of Histone Deacetylase Inhibitors. Nat Rev Drug Discov (2006) 5(9):769–84. doi: 10.1038/nrd2133
105. Sanaei M, Kavoosi F. Histone Deacetylases and Histone Deacetylase Inhibitors: Molecular Mechanisms of Action in Various Cancers. Adv Biomed Res (2019) 8(1):63. doi: 10.4103/abr.abr_142_19
106. Wood PJ, Strong R, McArthur GA, Michael M, Algar E, Muscat A, et al. A Phase I Study of Panobinostat in Pediatric Patients With Refractory Solid Tumors, Including CNS Tumors. Cancer Chemother Pharmacol (2018) 82(3):493–503. doi: 10.1007/s00280-018-3634-4
107. Peters KB, Lipp ES, Miller E, Herndon JE 2nd, McSherry F, Desjardins A, et al. Phase I/II Trial of Vorinostat, Bevacizumab, and Daily Temozolomide for Recurrent Malignant Gliomas. J Neurooncol (2018) 137(2):349–56. doi: 10.1007/s11060-017-2724-1
108. Hummel TR, Wagner L, Ahern C, Fouladi M, Reid JM, McGovern RM, et al. A Pediatric Phase 1 Trial of Vorinostat and Temozolomide in Relapsed or Refractory Primary Brain or Spinal Cord Tumors: A Children’s Oncology Group Phase 1 Consortium Study. Pediatr Blood Cancer (2013) 60(9):1452–7. doi: 10.1002/pbc.24541
109. Fouladi M, Park JR, Stewart CF, Gilbertson RJ, Schaiquevich P, Sun J, et al. Pediatric Phase I Trial and Pharmacokinetic Study of Vorinostat: A Children’s Oncology Group Phase I Consortium Report. J Clin Oncol: Off J Am Soc Clin Oncol (2010) 28(22):3623–9. doi: 10.1200/JCO.2009.25.9119
110. Witt O, Milde T, Deubzer HE, Oehme I, Witt R, Kulozik A, et al. Phase I/II Intra-Patient Dose Escalation Study of Vorinostat in Children With Relapsed Solid Tumor, Lymphoma or Leukemia. Klin Padiatr (2012) 224(6):398–403. doi: 10.1055/s-0032-1323692
111. Allen JE, Kline CL, Prabhu VV, Wagner J, Ishizawa J, Madhukar N, et al. Discovery and Clinical Introduction of First-In-Class Imipridone Onc201. Oncotarget (2016) 7(45):74380–92. doi: 10.18632/oncotarget.11814
112. Cole A, Wang Z, Coyaud E, Voisin V, Gronda M, Jitkova Y, et al. Inhibition of the Mitochondrial Protease ClpP as a Therapeutic Strategy for Human Acute Myeloid Leukemia. Cancer Cell (2015) 27(6):864–76. doi: 10.1016/j.ccell.2015.05.004
113. Ishizawa J, Zarabi SF, Davis RE, Halgas O, Nii T, Jitkova Y, et al. Mitochondrial ClpP-Mediated Proteolysis Induces Selective Cancer Cell Lethality. Cancer Cell (2019) 35(5):721–37.e9. doi: 10.1016/j.ccell.2019.03.014
114. Caragher SP, Shireman JM, Huang M, Miska J, Atashi F, Baisiwala S, et al. Activation of Dopamine Receptor 2 Prompts Transcriptomic and Metabolic Plasticity in Glioblastoma. J Neurosci (2019) 39(11):1982–93. doi: 10.1523/JNEUROSCI.1589-18.2018
115. Cheng HW, Liang YH, Kuo YL, Chuu CP, Lin CY, Lee MH, et al. Identification of Thioridazine, an Antipsychotic Drug, as an Antiglioblastoma and Anticancer Stem Cell Agent Using Public Gene Expression Data. Cell Death Dis (2015) 6:e1753. doi: 10.1038/cddis.2015.77
116. Arrillaga-Romany I, Odia Y, Prabhu VV, Tarapore RS, Merdinger K, Stogniew M, et al. Biological Activity of Weekly ONC201 in Adult Recurrent Glioblastoma Patients. Neuro Oncol (2020) 22(1):94–102. doi: 10.1093/neuonc/noz164
117. Arrillaga-Romany I, Chi AS, Allen JE, Oster W, Wen PY, Batchelor TT. A Phase 2 Study of the First Imipridone ONC201, a Selective DRD2 Antagonist for Oncology, Administered Every Three Weeks in Recurrent Glioblastoma. Oncotarget (2017) 8(45):79298–304. doi: 10.18632/oncotarget.17837
118. Prabhu VV, Morrow S, Rahman Kawakibi A, Zhou L, Ralff M, Ray J, et al. ONC201 and Imipridones: Anti-Cancer Compounds With Clinical Efficacy. Neoplasia (2020) 22(12):725–44. doi: 10.1016/j.neo.2020.09.005
119. He L, Bhat K, Ioannidis A, Zhang L, Nguyen NT, Allen JE, et al. Effects of the DRD2/3 Antagonist ONC201 and Radiation in Glioblastoma. Radiother Oncol (2021) 161:140–7. doi: 10.1016/j.radonc.2021.05.027
120. Bouffet E, Larouche V, Campbell BB, Merico D, de Borja R, Aronson M, et al. Immune Checkpoint Inhibition for Hypermutant Glioblastoma Multiforme Resulting From Germline Biallelic Mismatch Repair Deficiency. J Clin Oncol (2016) 34(19):2206–11. doi: 10.1200/JCO.2016.66.6552
121. Kabir TF, Chauhan A, Anthony L, Hildebrandt GC. Immune Checkpoint Inhibitors in Pediatric Solid Tumors: Status in 2018. Ochsner J (2018) 18(4):370–6. doi: 10.31486/toj.18.0055
122. Wherry EJ, Kurachi M. Molecular and Cellular Insights Into T Cell Exhaustion. Nat Rev Immunol (2015) 15(8):486–99. doi: 10.1038/nri3862
123. Hohlfeld R, Dalakas MC. Basic Principles of Immunotherapy for Neurologic Diseases. Semin Neurol (2003) 23(2):121–32. doi: 10.1055/s-2003-41139
124. Yshii LM, Hohlfeld R, Liblau RS. Inflammatory CNS Disease Caused by Immune Checkpoint Inhibitors: Status and Perspectives. Nat Rev Neurol (2017) 13(12):755–63. doi: 10.1038/nrneurol.2017.144
125. Dalakas MC. Neurological Complications of Immune Checkpoint Inhibitors: What Happens When You ‘Take the Brakes Off’ the Immune System. Ther Adv Neurol Disord (2018) 11:1756286418799864. doi: 10.1177/1756286418799864
126. Cloughesy TF, Mochizuki AY, Orpilla JR, Hugo W, Lee AH, Davidson TB, et al. Neoadjuvant Anti-PD-1 Immunotherapy Promotes a Survival Benefit With Intratumoral and Systemic Immune Responses in Recurrent Glioblastoma. Nat Med (2019) 25(3):477–86. doi: 10.1038/s41591-018-0337-7
127. Fellner A, Makranz C, Lotem M, Bokstein F, Taliansky A, Rosenberg S, et al. Neurologic Complications of Immune Checkpoint Inhibitors. J Neurooncol (2018) 137(3):601–9. doi: 10.1007/s11060-018-2752-5
128. Wick W, Hertenstein A, Platten M. Neurological Sequelae of Cancer Immunotherapies and Targeted Therapies. Lancet Oncol (2016) 17(12):e529–e41. doi: 10.1016/S1470-2045(16)30571-X
129. Zekeridou A, Lennon VA. Neurologic Autoimmunity in the Era of Checkpoint Inhibitor Cancer Immunotherapy. Mayo Clin Proc (2019) 94(9):1865–78. doi: 10.1016/j.mayocp.2019.02.003
130. Touat M, Talmasov D, Ricard D, Psimaras D. Neurological Toxicities Associated With Immune-Checkpoint Inhibitors. Curr Opin Neurol (2017) 30(6):659–68. doi: 10.1097/WCO.0000000000000503
131. Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T Cells Expressing CD19 Chimeric Antigen Receptors for Acute Lymphoblastic Leukaemia in Children and Young Adults: A Phase 1 Dose-Escalation Trial. Lancet (2015) 385(9967):517–28. doi: 10.1016/S0140-6736(14)61403-3
132. Paolillo M, Boselli C, Schinelli S. Glioblastoma Under Siege: An Overview of Current Therapeutic Strategies. Brain Sci (2018) 8(1):2–13. doi: 10.3390/brainsci8010015
133. Mount CW, Majzner RG, Sundaresh S, Arnold EP, Kadapakkam M, Haile S, et al. Potent Antitumor Efficacy of Anti-GD2 CAR T Cells in H3-K27M(+) Diffuse Midline Gliomas. Nat Med (2018) 24(5):572–9. doi: 10.1038/s41591-018-0006-x
134. Migliorini D, Dietrich PY, Stupp R, Linette GP, Posey AD Jr., June CH. CAR T-Cell Therapies in Glioblastoma: A First Look. Clin Cancer Res (2018) 24(3):535–40. doi: 10.1158/1078-0432.CCR-17-2871
135. Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ. Toxicity and Management in CAR T-Cell Therapy. Mol Ther Oncol (2016) 3:16011. doi: 10.1038/mto.2016.11
136. Chou CK, Turtle CJ. Assessment and Management of Cytokine Release Syndrome and Neurotoxicity Following CD19 CAR-T Cell Therapy. Expert Opin Biol Ther (2020) 20(6):653–64. doi: 10.1080/14712598.2020.1729735
137. Brudno JN, Kochenderfer JN. Recent Advances in CAR T-Cell Toxicity: Mechanisms, Manifestations and Management. Blood Rev (2019) 34:45–55. doi: 10.1016/j.blre.2018.11.002
138. Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults With B-Cell Lymphoblastic Leukemia. N Engl J Med (2018) 378(5):439–48. doi: 10.1056/NEJMoa1709866
139. Gust J, Finney OC, Li D, Brakke HM, Hicks RM, Futrell RB, et al. Glial Injury in Neurotoxicity After Pediatric CD19-Directed Chimeric Antigen Receptor T Cell Therapy. Ann Neurol (2019) 86(1):42–54. doi: 10.1002/ana.25502
140. Chou CK, Turtle CJ. Insight Into Mechanisms Associated With Cytokine Release Syndrome and Neurotoxicity After CD19 CAR-T Cell Immunotherapy. Bone Marrow Transplant (2019) 54(Suppl 2):780–4. doi: 10.1038/s41409-019-0602-5
141. Majzner R, Ramakrishna S, Mochizuki A, Patel S, Chinnasamy H, Yeom K, et al. EPCT-14. GD2 CAR T-Cells Mediate Clinical Activity And Manageable Toxicity In Children And Young Adults With H3K27M-Mutated Dipg And Spinal Cord DMG. Neuro-Oncology (2021) 23(Supplement_1):i49–50. doi: 10.1093/neuonc/noab090.200
142. Hay KA, Hanafi LA, Li D, Gust J, Liles WC, Wurfel MM, et al. Kinetics and Biomarkers of Severe Cytokine Release Syndrome After CD19 Chimeric Antigen Receptor-Modified T-Cell Therapy. Blood (2017) 130(21):2295–306. doi: 10.1182/blood-2017-06-793141
143. Strati P, Ahmed S, Kebriaei P, Nastoupil LJ, Claussen CM, Watson G, et al. Clinical Efficacy of Anakinra to Mitigate CAR T-Cell Therapy-Associated Toxicity in Large B-Cell Lymphoma. Blood Adv (2020) 4(13):3123–7. doi: 10.1182/bloodadvances.2020002328
144. Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, et al. Monocyte-Derived IL-1 and IL-6 Are Differentially Required for Cytokine-Release Syndrome and Neurotoxicity Due to CAR T Cells. Nat Med (2018) 24(6):739–48. doi: 10.1038/s41591-018-0036-4
145. Brudno JN, Kochenderfer JN. Toxicities of Chimeric Antigen Receptor T Cells: Recognition and Management. Blood (2016) 127(26):3321–30. doi: 10.1182/blood-2016-04-703751
146. Cho DY, Yang WK, Lee HC, Hsu DM, Lin HL, Lin SZ, et al. Adjuvant Immunotherapy With Whole-Cell Lysate Dendritic Cells Vaccine for Glioblastoma Multiforme: A Phase II Clinical Trial. World Neurosurg (2012) 77(5-6):736–44. doi: 10.1016/j.wneu.2011.08.020
147. Yao Y, Luo F, Tang C, Chen D, Qin Z, Hua W, et al. Molecular Subgroups and B7-H4 Expression Levels Predict Responses to Dendritic Cell Vaccines in Glioblastoma: An Exploratory Randomized Phase II Clinical Trial. Cancer Immunol Immunother (2018) 67(11):1777–88. doi: 10.1007/s00262-018-2232-y
148. Wen PY, Reardon DA, Armstrong TS, Phuphanich S, Aiken RD, Landolfi JC, et al. A Randomized Double-Blind Placebo-Controlled Phase II Trial of Dendritic Cell Vaccine ICT-107 in Newly Diagnosed Patients With Glioblastoma. Clin Cancer Res (2019) 25(19):5799–807. doi: 10.1158/1078-0432.CCR-19-0261
149. Chang CN, Huang YC, Yang DM, Kikuta K, Wei KJ, Kubota T, et al. A Phase I/II Clinical Trial Investigating the Adverse and Therapeutic Effects of a Postoperative Autologous Dendritic Cell Tumor Vaccine in Patients With Malignant Glioma. J Clin Neurosci (2011) 18(8):1048–54. doi: 10.1016/j.jocn.2010.11.034
150. Prins RM, Soto H, Konkankit V, Odesa SK, Eskin A, Yong WH, et al. Gene Expression Profile Correlates With T-Cell Infiltration and Relative Survival in Glioblastoma Patients Vaccinated With Dendritic Cell Immunotherapy. Clin Cancer Res (2011) 17(6):1603–15. doi: 10.1158/1078-0432.CCR-10-2563
151. Yamanaka R, Homma J, Yajima N, Tsuchiya N, Sano M, Kobayashi T, et al. Clinical Evaluation of Dendritic Cell Vaccination for Patients With Recurrent Glioma: Results of a Clinical Phase I/II Trial. Clin Cancer Res (2005) 11(11):4160–7. doi: 10.1158/1078-0432.CCR-05-0120
152. Okada H, Kalinski P, Ueda R, Hoji A, Kohanbash G, Donegan TE, et al. Induction of CD8+ T-Cell Responses Against Novel Glioma-Associated Antigen Peptides and Clinical Activity by Vaccinations With {Alpha}-Type 1 Polarized Dendritic Cells and Polyinosinic-Polycytidylic Acid Stabilized by Lysine and Carboxymethylcellulose in Patients With Recurrent Malignant Glioma. J Clin Oncol (2011) 29(3):330–6. doi: 10.1200/JCO.2010.30.7744
153. Pollack IF, Jakacki RI, Butterfield LH, Hamilton RL, Panigrahy A, Potter DM, et al. Antigen-Specific Immune Responses and Clinical Outcome After Vaccination With Glioma-Associated Antigen Peptides and Polyinosinic-Polycytidylic Acid Stabilized by Lysine and Carboxymethylcellulose in Children With Newly Diagnosed Malignant Brainstem and Nonbrainstem Gliomas. J Clin Oncol (2014) 32(19):2050–8. doi: 10.1200/JCO.2013.54.0526
154. Srinivasan VM, Ferguson SD, Lee S, Weathers SP, Kerrigan BCP, Heimberger AB. Tumor Vaccines for Malignant Gliomas. Neurotherapeutics (2017) 14(2):345–57. doi: 10.1007/s13311-017-0522-2
155. Miao L, Zhang Y, Huang L. mRNA Vaccine for Cancer Immunotherapy. Mol Cancer (2021) 20(1):41. doi: 10.1186/s12943-021-01335-5
156. Faghfuri E, Pourfarzi F, Faghfouri AH, Abdoli Shadbad M, Hajiasgharzadeh K, Baradaran B. Recent Developments of RNA-Based Vaccines in Cancer Immunotherapy. Expert Opin Biol Ther (2021) 21(2):201–18. doi: 10.1080/14712598.2020.1815704
157. Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination With Tumor Lysate-Pulsed Dendritic Cells Elicits Antigen-Specific, Cytotoxic T-Cells in Patients With Malignant Glioma. Cancer Res (2004) 64(14):4973–9. doi: 10.1158/0008-5472.CAN-03-3505
158. Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ, et al. Dendritic Cell Vaccination in Glioblastoma Patients Induces Systemic and Intracranial T-Cell Responses Modulated by the Local Central Nervous System Tumor Microenvironment. Clin Cancer Res (2005) 11(15):5515–25. doi: 10.1158/1078-0432.CCR-05-0464
159. De Vleeschouwer S, Fieuws S, Rutkowski S, Van Calenbergh F, Van Loon J, Goffin J, et al. Postoperative Adjuvant Dendritic Cell-Based Immunotherapy in Patients With Relapsed Glioblastoma Multiforme. Clin Cancer Res (2008) 14(10):3098–104. doi: 10.1158/1078-0432.CCR-07-4875
160. Li C, Liu T, Zhou B, Zhou Y, Yu H, Sun Y. Efficacy and Safety Analysis on Dendritic Cell-Based Vaccine-Treated High-Grade Glioma Patients: A Systematic Review and Meta-Analysis. Onco Targets Ther (2018) 11:7277–93. doi: 10.2147/OTT.S177768
161. Lv L, Huang J, Xi H, Zhou X. Efficacy and Safety of Dendritic Cell Vaccines for Patients With Glioblastoma: A Meta-Analysis of Randomized Controlled Trials. Int Immunopharmacol (2020) 83:106336. doi: 10.1016/j.intimp.2020.106336
162. Vik-Mo EO, Nyakas M, Mikkelsen BV, Moe MC, Due-Tonnesen P, Suso EM, et al. Therapeutic Vaccination Against Autologous Cancer Stem Cells With mRNA-Transfected Dendritic Cells in Patients With Glioblastoma. Cancer Immunol Immunother (2013) 62(9):1499–509. doi: 10.1007/s00262-013-1453-3
163. Lawler SE. Cytomegalovirus and Glioblastoma; Controversies and Opportunities. J Neurooncol (2015) 123(3):465–71. doi: 10.1007/s11060-015-1734-0
164. Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental Therapy of Human Glioma by Means of a Genetically Engineered Virus Mutant. Science (1991) 252(5007):854–6. doi: 10.1126/science.1851332
165. Bischoff JR, Kirn DH, Williams A, Heise C, Horn S, Muna M, et al. An Adenovirus Mutant That Replicates Selectively in P53-Deficient Human Tumor Cells. Science (1996) 274(5286):373–6. doi: 10.1126/science.274.5286.373
166. Csatary LK, Bakacs T. Use of Newcastle Disease Virus Vaccine (MTH-68/H) in a Patient With High-Grade Glioblastoma. JAMA (1999) 281(17):1588–9. doi: 10.1001/jama.281.17.1588-a
167. Csatary LK, Gosztonyi G, Szeberenyi J, Fabian Z, Liszka V, Bodey B, et al. MTH-68/H Oncolytic Viral Treatment in Human High-Grade Gliomas. J Neurooncol (2004) 67(1-2):83–93. doi: 10.1023/B:NEON.0000021735.85511.05
168. Wang JL, Scheitler KM, Wenger NM, Elder JB. Viral Therapies for Glioblastoma and High-Grade Gliomas in Adults: A Systematic Review. Neurosurg Focus (2021) 50(2):E2. doi: 10.3171/2020.11.FOCUS20854
169. Cloughesy TF, Landolfi J, Vogelbaum MA, Ostertag D, Elder JB, Bloomfield S, et al. Durable Complete Responses in Some Recurrent High-Grade Glioma Patients Treated With Toca 511 + Toca Fc. Neuro Oncol (2018) 20(10):1383–92. doi: 10.1093/neuonc/noy075
170. Marelli G, Howells A, Lemoine NR, Wang Y. Oncolytic Viral Therapy and the Immune System: A Double-Edged Sword Against Cancer. Front Immunol (2018) 9:866. doi: 10.3389/fimmu.2018.00866
171. Desjardins A, Gromeier M, Herndon JE 2nd, Beaubier N, Bolognesi DP, Friedman AH, et al. Recurrent Glioblastoma Treated With Recombinant Poliovirus. N Engl J Med (2018) 379(2):150–61. doi: 10.1056/NEJMoa1716435
172. Fares J, Ahmed AU, Ulasov IV, Sonabend AM, Miska J, Lee-Chang C, et al. Neural Stem Cell Delivery of an Oncolytic Adenovirus in Newly Diagnosed Malignant Glioma: A First-In-Human, Phase 1, Dose-Escalation Trial. Lancet Oncol (2021) 22(8):1103–14. doi: 10.1016/S1470-2045(21)00245-X
173. Khatua S, Fangusaro J, Dhall G, Boyett J, Wu S, Bartels U. GC-17the Children’s Oncology Group (COG) Current Treatment Approach for Children With Newly Diagnosed Central Nervous System (CNS) Localized Germinoma (ACNS1123 STRATUM 2). Neuro-Oncology (2016) 18(suppl_3):iii45–iii6. doi: 10.1093/neuonc/now072.17
Keywords: neurological complications, brain, cancer, therapeutics, pediatrics
Citation: Nguyen T, Mueller S and Malbari F (2022) Review: Neurological Complications From Therapies for Pediatric Brain Tumors. Front. Oncol. 12:853034. doi: 10.3389/fonc.2022.853034
Received: 12 January 2022; Accepted: 15 March 2022;
Published: 11 April 2022.
Edited by:
Rupert Handgtretinger, University of Tübingen, GermanyReviewed by:
Martin Ebinger, University Children’s Hospital Tübingen, GermanyUte Katharina Bartels, University of Toronto, Canada
Copyright © 2022 Nguyen, Mueller and Malbari. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Thien Nguyen, dGhpZW4ubmd1eWVuM0B1Y3NmLmVkdQ==
 Thien Nguyen
Thien Nguyen Sabine Mueller
Sabine Mueller Fatema Malbari
Fatema Malbari