
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 21 April 2022
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.852583
This article is part of the Research TopicEmerging Therapeutic Targets, Potential Diagnostic or Prognostic markers for Colorectal CancerView all 28 articles
 Stefano Mariani1†
Stefano Mariani1† Marco Puzzoni1†
Marco Puzzoni1† Riccardo Giampieri2
Riccardo Giampieri2 Pina Ziranu1
Pina Ziranu1 Valeria Pusceddu1
Valeria Pusceddu1 Clelia Donisi1
Clelia Donisi1 Mara Persano1
Mara Persano1 Giovanna Pinna1
Giovanna Pinna1 Erika Cimbro1
Erika Cimbro1 Alissa Parrino1
Alissa Parrino1 Dario Spanu1
Dario Spanu1 Andrea Pretta1
Andrea Pretta1 Eleonora Lai1
Eleonora Lai1 Nicole Liscia1
Nicole Liscia1 Alessio Lupi2
Alessio Lupi2 Enrica Giglio2
Enrica Giglio2 Grazia Palomba3
Grazia Palomba3 Milena Casula3
Milena Casula3 Marina Pisano3
Marina Pisano3 Giuseppe Palmieri3
Giuseppe Palmieri3 Mario Scartozzi1*
Mario Scartozzi1*Background: Rechallenge with EGFR inhibitors represents a promising strategy for patients with RAS wild type (WT) colorectal cancer (CRC) but definitive selection criteria are lacking. Recently, the RAS WT status on circulating tumor DNA (ct-DNA) emerged as a potential watershed for this strategy. Our study explored the liquid biopsy-driven cetuximab rechallenge in a RAS and BRAF WT selected population.
Methods: CRC patients with RAS and BRAF WT both on tumor tissue and on ct-DNA at baseline receiving rechallenge with cetuximab were eligible for our analysis. Ct-DNA was analyzed for RAS-BRAF mutations with pyro-sequencing and nucleotide sequencing assays. Real-time PCR and droplet digital PCR were performed to confirm the RAS-BRAF mutational status.
Results: A total of 26 patients were included in our analysis. In the global population, RR was 25.0%, median overall survival (mOS) was 5.0 months, and median progression-free survival (mPFS) was 3.5 months. Previous response to anti-EGFR was associated with improved mPFS (5.0 vs. 2.0 months, HR: 0.26, p = 0.048); anti-EGFR free interval > 14 months and anti-EGFR free interval > 16 months were associated with improved mPFS (respectively 7.0 vs. 3.0 months, HR: 0.27, p = 0.013 and not reached vs. 3.0 months, HR: 0.20, p = 0.002) and with improved mOS (respectively 13.0 vs. 5.0 months, HR: 0.27, p = 0.013 and 13.0 vs. 5.0 months, HR: 0.20, p = 0.002). Previous lines >2 were correlated with improved mPFS (4.0 vs. 1.0 month, HR: 0.05, p = 0.041) and with improved mOS (7.0 vs. 1.0 month, HR: 0.045, p = 0.034). In a multiple logistic regression model, only the anti-EGFR free interval was confirmed to be a significant predictor for mOS and mPFS.
Conclusions: Liquid biopsy-driven cetuximab rechallenge was confirmed to be effective. The clinical outcome was consistent with available results from phase II studies. In addition to the molecular selection through the analysis of ct-DNA for RAS, the long anti-EGFR free interval is confirmed as a prospective selection criterion for this therapeutic option.
The anti-epidermal growth factor receptor (EGFR) antibodies (i.e., cetuximab and panitumumab) have a key role in the treatment of metastatic colorectal cancer. The resistance to anti-EGFR antibodies represents a challenging issue in this context. In particular the genetic heterogeneity of colorectal cancer along with the dynamic nature of tumor biology are at the basis of secondary resistance to EGFR blockade (1). The mechanism underlying the secondary resistance to EGFR blockade, although not yet fully understood, seems to be linked to the emergence of EGFR downstream pathway mutation including RAS mutations (2) and EGFR mutations (3). The K- and N-RAS mutations, in this respect, are the most important mediators of secondary resistance to EGFR blockade, and they can be detected by circulating cell-free tumor DNA analysis (2). The RAS and EGFR mutant alleles emerging at the time of disease progression to EGFR blockade have been shown to decline in blood during EGFR blockade suspension (4, 5). This biological phenomenon can be predicted by an exponential decay model (4, 5).
The rechallenge strategy with anti-EGFR might then represent a promising therapeutic weapon aiming to overcome secondary resistance to anti-EGFR in the light of the knowledge about the pulsatile behavior of RAS clones under EGFR blockade pressure. The basic idea supporting the concept of rechallenge with anti-EGFR is the possibility to successfully treat patients previously exposed and resistant to such drugs after an anti-EGFR interval in which tumor cells may have gone back to prevalent RAS wild-type (WT) status after developing a prevalent RAS mutant status due to anti-EGFR pressure.
Santini et al. (6) firstly investigated the activity of rechallenge with a cetuximab-based therapy in metastatic colorectal cancer patients achieving encouraging results in terms of clinical outcome. Response rate (RR) was 53.8% and median progression-free survival (mPFS) was 6.6 months.
Subsequently, Cremolini et al. (7) prospectively assessed the activity of cetuximab plus irinotecan as third-line treatment for patients with strict clinical criteria (i.e., prior first-line irinotecan and cetuximab-based regimen with at least partial response, progression-free survival of at least 6 months with first-line therapy, progression within 4 weeks after last dose of cetuximab, and prior second-line oxaliplatin and bevacizumab-based treatment). In the global population, RR was 21%, median PFS was 3.4 months, and median overall survival (OS) was 9.8 months. Particularly noteworthy was the finding of improved mPFS for patients with RAS wild-type ct-DNA (4.0 vs. 1.9 months, p = 0.03).
Similarly, Masuishi et al. (8) conducted a phase II trial of irinotecan-cetuximab rechallenge as third line confirming the clinical activity of this treatment strategy (3-month PFS rate was 44.1%, mPFS was 2.4 months, and mOS was 8.2 months). The long anti-EGFR free interval (>372 days) was related to an improved outcome in terms of RR, OS, and PFS.
Results from CHRONOS, a multicenter phase II trial of liquid biopsy-driven anti-EGFR rechallenge with panitumumab (9), have been presented at the ASCO 2021 annual meeting. Molecular inclusion criteria for enrolment in this study were very restrictive (patients showing a >50% drop in RAS mutational load compared to baseline were considered molecularly eligible). The study met the primary endpoint; overall response rate (ORR) was 30%, mPFS was 16 weeks, and, interestingly, response occurred independently of number of prior treatments and sidedness. Furthermore, the presence of resistance conferring mutations and responses were independent of the time since last anti-EGFR.
However, selection criteria allowing clinicians to reliably select patients most likely to benefit from such a treatment approach are yet to be defined. This is of particular clinical relevance when we consider that metastatic colorectal cancer patients who are potential candidates for a rechallenge with anti-EGFR may also be eligible, at the same time, for other therapeutic options with higher level of evidence (10, 11). In this scenario, liquid biopsy for RAS and BRAF mutational status should be considered a valuable criteria for patient selection (7, 9), as well as the hypothesis of delaying the introduction of rechallenge with anti-EGFR in order to both expose the patient to treatment options with phase III evidence (10, 11) of activity and take advantage of a longer anti-EGFR free interval.
Further studies are urgently needed to better understand the prognostic and predictive factors along with the better timeline for rechallenge strategy. The present study explored the role of liquid biopsy-driven rechallenge strategy with EGFR blockade in molecularly and clinically selected metastatic colorectal cancer patients.
We conducted a multicenter retrospective analysis in patients with molecularly selected colorectal cancer who underwent rechallenge with anti-EGFR antibodies. The aim of the study was to evaluate the correlation of several putative predictive/prognostic factors for rechallenge strategy with clinical outcome. Patients were included in our analysis if they met the following criteria: histologically confirmed RAS and BRAF wild-type colorectal cancer, first-line anti-EGFR-based therapy with documentation of progression to first-line therapy within 4 weeks after the last administration of anti-EGFR, rechallenge with anti-EGFR antibody (e.g., irinotecan + cetuximab or cetuximab monotherapy), and measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. To be eligible, patients must also have circulating tumor DNA (ct-DNA) RAS-BRAF WT profile at the rechallenge baseline. The ct-DNA was analyzed at the rechallenge baseline for RAS-BRAF mutations using pyro-sequencing (PyroMark Q24 MDx Workstation) and nucleotide sequencing (Genetic Analyzer ABI3130) assays. Real-time PCR (Idylla) and droplet digital PCR (QX200 System) were performed to confirm the RAS-BRAF mutation status. The limit of detection for RAS BRAF mutations was 5%. Tumor response was evaluated by clinicians’ assessment according to the Response Evaluation Criteria in Solid Tumors (RECIST v1.1). Several clinical variables including previous response to anti-EGFR containing therapy, anti-EGFR free interval, and previous lines for metastatic disease > 2 were evaluated in relation to outcome in terms of RR, mPFS, and mOS. Statistical analysis was performed with the MedCalc Statistical Software version 14.10.2 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2014). For study purposes, the optimal anti-EGFR free interval threshold was defined according to receiver operating characteristic (ROC) curve analysis. The association between categorical variables was estimated by Fisher exact test for categorical binomial variables. Survival probability over time was estimated by the Kaplan–Meier method. Significant differences in the probability of survival between the strata were evaluated by log-rank test. Multiple logistic regression was used to assess the role of significant variables in the univariate analysis. The study was performed in accordance with the protocol, which was approved by the AOU Cagliari Ethical committee (approval number: 3.32 n.14 28/04/21) along with all experimental procedures. Written informed consent was obtained for all patients enrolled into the analysis, and methods were carried out in accordance with the Declaration of Helsinki.
A total of 26 patients with RAS-BRAF wild-type metastatic colorectal cancer receiving rechallenge with cetuximab between July 2018 and February 2021 were included in our analysis. All patients had RAS-BRAF wild-type status on ct-DNA at baseline. Overall clinical and pathological characteristics are summarized in Table 1. The median follow-up duration was 6.0 months (95% CI for the median 4.1 to 11.3). The most common treatment-related adverse events of any grade were skin rash (81%), diarrhea (35%), neutropenia (19%), and fatigue (12%). The most common grade 3 adverse event was neutropenia (8%). No grade 4 adverse events occurred.
Long anti-EGFR free interval, previous response to anti-EGFR therapy, and previous lines >2 for metastatic disease were associated with clinical outcome in the univariate analysis. In particular, the previous response to anti-EGFR was associated with improved mPFS (5.0 vs. 2.0 months, HR: 0.26, p = 0.048); long anti-EGFR free interval > 14 months and long anti-EGFR free interval > 16 months were correlated with improved mPFS (respectively 7.0 vs. 3.0 months, HR: 0.27, p = 0.013 and not reached vs. 3.0 months, HR: 0.20, p = 0.002) and with mOS (respectively 13.0 vs. 5.0 months, HR: 0.27, p = 0.013 and 13.0 vs. 5.0 months, HR: 0.20, p = 0.002). Previous lines >2 for metastatic disease were correlated with improved mPFS (4.0 vs. 1.0, HR: 0.05, p = 0.041) and mOS (7.0 vs. 1.0 months, HR: 0.045 p = 0.034). The optimal anti-EGFR free interval threshold identified for predicting prognosis was 14 months (Figure 1), whereas the optimal anti-EGFR free interval threshold identified for predicting response was 16 months (Figure 2). Prior responders with longer anti-EGFR free interval experienced the best clinical outcome in terms of RR, mPFS, and mOS (Table 2) to rechallenge strategy in our clinical series (Figure 3).
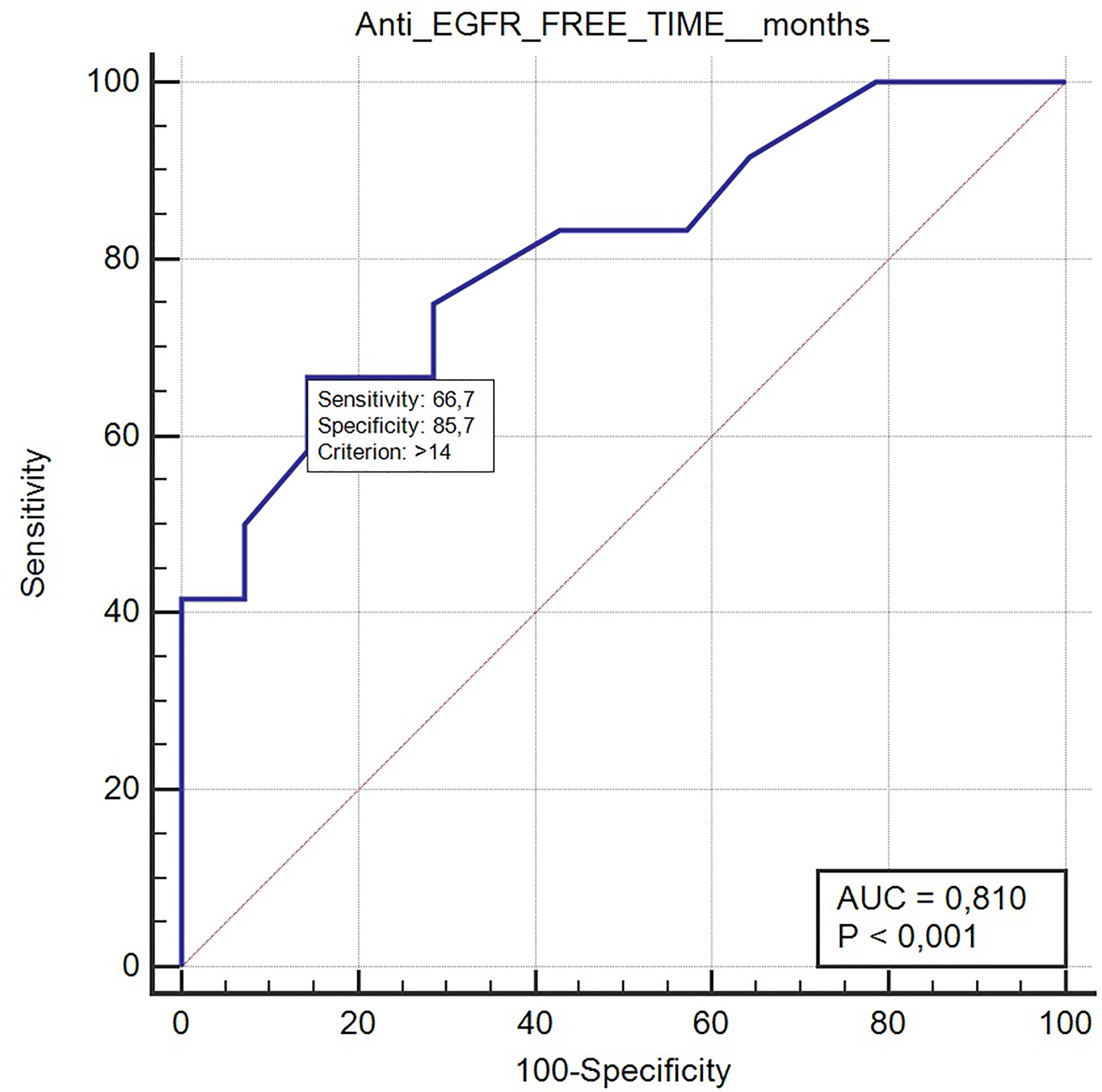
Figure 1 ROC curve of anti-EGFR free-time predictive of prognosis with rechallenge treatment (AUC 0.81, p < 0.001).
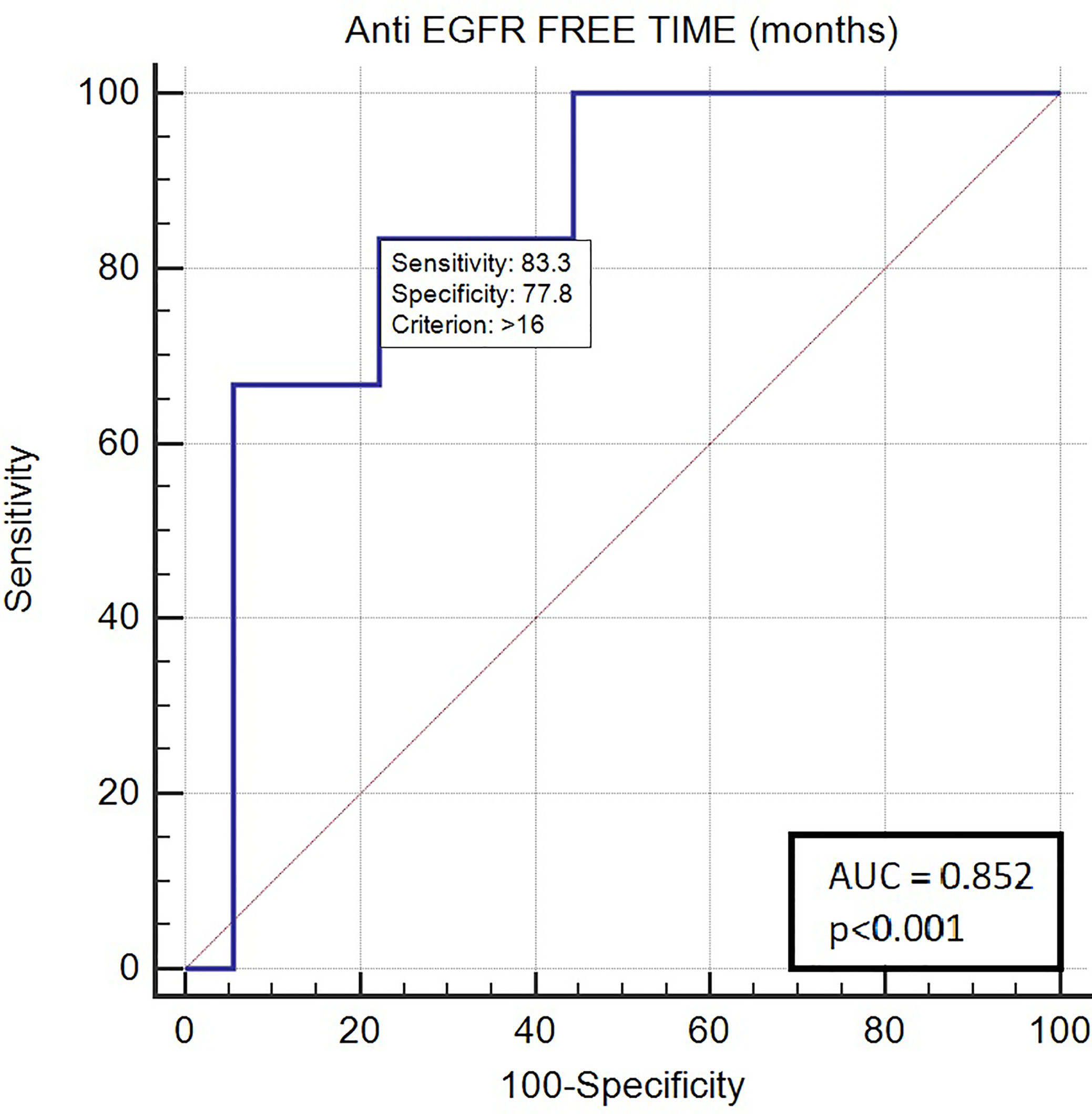
Figure 2 ROC curve of anti-EGFR free-time predictive of response with rechallenge treatment (AUC 0.852; p < 0.001).
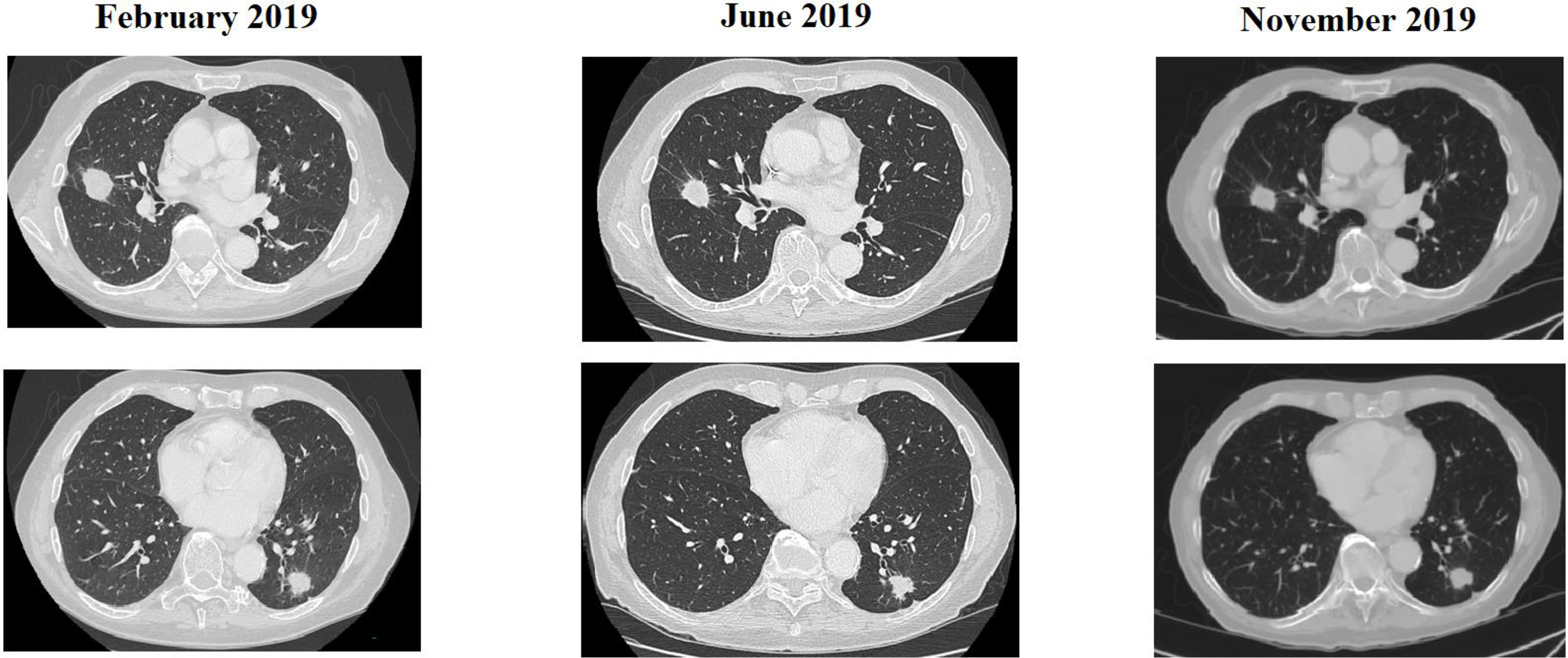
Figure 3 CT scan of a patient treated with rechallenge strategy after >2 prior lines of treatment and an anti-EGFR free interval > 16 months.
In a multiple logistic regression model, the anti-EGFR free interval >16 months as well as the anti-EGFR free interval >14, among the variables (i.e., long anti-EGFR free interval, previous response to anti-EGFR treatment, and previous lines >2 for metastatic disease), maintained their independent role for OS (p = 0.033 and p = 0.028, respectively). Likewise, the anti-EGFR free interval >16 months maintained the independent role for PFS (p = 0.020) among the significant variables in the univariate analysis (i.e., long anti-EGFR free interval, previous response to anti-EGFR treatment, and previous lines >2 for metastatic disease). None of them were statistically correlated to RR at multivariate analysis.
The rechallenge strategy with anti-EGFR antibodies can boast a plausible biological rationale along with promising results both from retrospective (6, 12, 13) and from phase II studies (7–9). However, prognostic and predictive factors, both molecular and clinical, along with the most effective treatment’s timeline are not yet fully defined. The response to previous anti-EGFR blockade and the anti-EGFR free interval are among the most studied prognostic and predictive factors for clinical-based rechallenge strategy. The genetic selection through ct-DNA analysis for RAS status, however, represents the most important tool in guiding patient selection, and the liquid biopsy-driven rechallenge with anti-EGFR showed improved outcome compared to clinical-based rechallenge strategy (9). Though the design of phase II trials investigating the rechallenge with anti-EGFR suggests a role in a third-line setting, a number of previous lines >2 (12) and longer anti-EGFR free interval (7, 8) were associated with improved clinical outcome. This suggests a possible usage in later lines (i.e., >3). Moreover, in clinical practice, rechallenge with anti-EGFR blockade is often used in later lines, whereas in third- and fourth-line settings, regorafenib and trifluridine-tipiracil are the preferred options on the basis of the phase III clinical trial data.
In the present study, we explored the rechallenge strategy with cetuximab-based therapy in clinically and molecularly selected, heavily pretreated (i.e., 92.3% received rechallenge with anti-EGFR in fourth- or fifth-line therapy) colorectal cancer patients. Notably, the response rate and the mPFS reported in the heavily pretreated population (i.e., RR: 25.0%, mPFS: 3.5 months) (Table 1) is worth mentioning and consistent with already available literature data from phase II studies.
Previous response to anti-EGFR, long anti-EGFR free interval, and previous lines >2 were correlated to better clinical outcome in our clinical series. In particular, previous responders with longer anti-EGFR free interval (i.e., >16 months) achieved the best outcome from rechallenge with anti-EGFR. PFS and OS were significantly longer in patients with long anti-EGFR free interval, consistent with results from phase II trials of clinical-driven rechallenge with anti-EGFR (7, 8). A number of previous lines >2 were associated with improved outcome (i.e., in terms of PFS and OS) according to results from previous retrospective analysis (i.e., improved outcome in terms of RR but no longer PFS and OS) (12). Similarly, the association of previous response to anti-EGFR with improved PFS is consistent with previous retrospective studies (13). Nevertheless, only long anti-EGFR free interval was significantly correlated to better outcome at multivariate analysis.
All this, in the light of the exponential decay kinetics of RAS mutant alleles after EGFR blockade suspension (5), suggests that the choice of the optimal timing of rechallenge strategy represents a key factor for this treatment approach and also that several factors others than RAS and BRAF mutations might be involved in secondary resistance to EGFR blockade (e.g., EGFR mutations).
Nevertheless, the results of the CHRONOS study (9) are partially contrary to our findings and to results from clinical-based rechallenge studies. In the CHRONOS study, the response occurred independently from the number of prior treatments, and the presence of resistance conferring mutations and responses was independent of time since last anti-EGFR. The very restrictive molecular inclusion criteria of this trial could possibly justify these findings, underlying the idea that the molecular selection is more effective in interpreting the dynamic nature of tumor biology compared to clinical factors.
Our study has some limitations; in particular, the small sample size along with the retrospective nature of the study deeply influenced the interpretations of our findings. This analysis, therefore, must be considered exploratory and caution is needed in data interpretation.
Taking into account the current data regarding secondary resistance to EGFR blockade and the biological rationale for rechallenge strategy, the results of the present study are impressive and promising for future development. This especially applies in the form in which the results underlying the hypothesis of delaying the introduction of rechallenge with anti-EGFR would maintain efficacy in later lines. The liquid biopsy-driven anti-EGFR rechallenge is confirmed to be viable in clinical practice, and it should be considered the main tool for selected patients. Previous response to anti-EGFR and EGFR free interval might just represent a surrogate factor substituting in fact for acquired gene alteration status. Nevertheless, given the difficulties in applying the molecular methods from interventional liquid biopsy-driven anti-EGFR rechallenge studies, these clinical predictive factors maintain their value and a combination of the two approaches is also possible in the interest of greater effectiveness.
The up-to-date results from prospective liquid biopsy-driven rechallenge strategy trials (9, 14) are expected to give new insight into this challenging issue.
The raw data supporting the conclusions of this article will be made available by the authors upon request.
The studies involving human participants were reviewed and approved by AOU Cagliari Ethical committee (approval number: 3.32 n.14 28/04/21). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Author GiuP reports advisory role for BMS, MSD, Roche, Pierre-Fabre, Novartis, and Incyte.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.852583/full#supplementary-material
1. Puzzoni M, Ziranu P, Demurtas L, Lai E, Mariani S, Liscia N, et al. Why Precision Medicine Should Be Applied Across the Continuum of Care for Metastatic Colorectal Cancer Patients. Future Oncol (2020) 16(2):4337–9. doi: 10.2217/fon-2019-0624
2. Diaz LA Jr, Williams RT, Wu J, Kinde I, Hecht JR, Berlin J, et al. The Molecular Evolution of Acquired Resistance to Targeted EGFR Blockade in Colorectal Cancers. Nature (2012) 486(7404):537–40. doi: 10.1038/nature11219
3. Arena S, Bellosillo B, Siravegna G, Martinez A, Canadas I, Lazzari L, et al. Emergence of Multiple EGFR Extracellular Mutations During Cetuximab Treatment in Colorectal Cancer. Clin Cancer Res (2015) 21(9):2157–66. doi: 10.1158/1078-0432.CCR-14-2821
4. Siravegna G, Mussolin B, Buscarino M, Corti G, Cassingena A, Crisafulli G, et al. Clonal Evolution and Resistance to EGFR Blockade in the Blood of Colorectal Cancer Patients. Nat Med (2015) 21(7):795–801. doi: 10.1038/nm0715-827b
5. Parseghian CM, Loree JM, Morris VK, Liu X, Clifton KK, Napolitano S, et al. Anti-EGFR-Resistant Clones Decay Exponentially After Progression: Implications for Anti-EGFR Re-Challenge. Ann Oncol (2019) 30(2):243–9. doi: 10.1093/annonc/mdy509
6. Santini D, Vincenzi B, Addeo R, Carufi C, Masi G, Scartozzi M, et al. Cetuximab Rechallenge In Metastatic Colorectal Cancer Patients: How to Come Away from Acquired Resistance? Ann Oncol (2017) 28(11):2906. doi: 10.1093/annonc/mdr623
7. Cremolini C, Rossini D, Dell'Aquila E, Leonardi S, Conca E, Del Re M, et al. Rechallenge for Patients With RAS and BRAF Wild-Type Metastatic Colorectal Cancer With Acquired Resistance to First-line Cetuximab and Irinotecan: A Phase 2 Single-Arm Clinical Trial. JAMA Oncol (2019) 5(3):343–50. doi: 10.1001/jamaoncol.2018.5080
8. Masuishi T, Tsuji A, Kotaka M, Nakamur M, Kochi M, Takagene A, et al. Phase 2 Study of Irinotecan Plus Cetuximab Rechallenge as Third-Line Treatment in Kras Wild-Type Metastatic Colorectal Cancer: JACCRO CC-08. Br J Cancer (2020) 123(10):1490–5. doi: 10.1038/s41416-020-01042-w
9. Sartore-Bianchi A, Pietrantonio F, Lonardi S, Mussolin B, Rua F, Fenocchio E, et al. Phase II Study of Anti-EGFR Rechallenge Therapy with Panitumumab Driven by Circulating Tumor DNA Molecular Selection in Metastatic Colorectal Cancer: The CHRONOS Trial. J Clin Oncol (2021) 39(15_suppl):3506. doi: 10.1200/JCO.2021.39.15_suppl.3506
10. Mayer RJ, Van Cutsem E, Falcone A, Yoshino T, Garcia’Carbonero R, Muzunuma N, et al. Randomized Trial of TAS-102 for Refractory Metastatic Colorectal Cancer. N Engl J Med (2015) 372(20):1909–19. doi: 10.1056/NEJMoa1414325
11. Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcona A, Ychou M, et al. Regorafenib Monotherapy for Previously Treated Metastatic Colorectal Cancer (CORRECT): An International, Multicentre, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet (2013) 381(9863):303–12. doi: 10.1016/S0140-6736(12)61900-X
12. Rossini D, Germani MM, Pagani F, Pellino A, Dell’Aquila E, Bensi M, et al. Retreatment With Anti-EGFR Antibodies in Metastatic Colorectal Cancer Patients: A Multi-institutional Analysis. Clin Colorectal Cancer (2020) 19(3):191–9. doi: 10.1016/j.clcc.2020.03.009
13. Liu X, George GC, Tsimberidou AM, Naing A, Wheler JJ, Kopetz S, et al. Retreatment with Anti-EGFR Based Therapies in Metastatic Colorectal Cancer: Impact of Intervening Time Interval and Prior Anti-EGFR Response. BMC Cancer (2015) 15:713. doi: doi.org/10.1186/s12885-015-1701-3
Keywords: colorectal (colon) cancer, epidermal growth factor receptor (EGFR), RAS, liquid biopsy, rechallenge, cetuximab
Citation: Mariani S, Puzzoni M, Giampieri R, Ziranu P, Pusceddu V, Donisi C, Persano M, Pinna G, Cimbro E, Parrino A, Spanu D, Pretta A, Lai E, Liscia N, Lupi A, Giglio E, Palomba G, Casula M, Pisano M, Palmieri G and Scartozzi M (2022) Liquid Biopsy-Driven Cetuximab Rechallenge Strategy in Molecularly Selected Metastatic Colorectal Cancer Patients. Front. Oncol. 12:852583. doi: 10.3389/fonc.2022.852583
Received: 11 January 2022; Accepted: 10 March 2022;
Published: 21 April 2022.
Edited by:
Nadia M. Hamdy, Ain Shams University, EgyptReviewed by:
Bruno Vincenzi, Campus Bio-Medico University, ItalyCopyright © 2022 Mariani, Puzzoni, Giampieri, Ziranu, Pusceddu, Donisi, Persano, Pinna, Cimbro, Parrino, Spanu, Pretta, Lai, Liscia, Lupi, Giglio, Palomba, Casula, Pisano, Palmieri and Scartozzi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mario Scartozzi, bWFyaW9zY2FydG96emlAZ21haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.