
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 20 April 2022
Sec. Neuro-Oncology and Neurosurgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.849109
Objective: We aimed to explore the application and prospects of the subperineural resection technique for tumor separation and removal under the perineurium during surgery for vestibular schwannomas (VSs).
Methods: This study retrospectively analyzed 124 patients with VSs who underwent surgery via a retrosigmoid approach from July 2015 to October 2020 in the Department of Neurosurgery, Tangdu Hospital, Air Force Military Medical University. The data will be discussed with regard to the following aspects: clinical features, surgical strategies, tumor resection extent, facial–acoustic function preservation, and postoperative complications.
Results: Gross total resection (GTR) of the tumor was achieved in 104 patients, with a GTR rate of 83.9%, and subtotal resection (STR) of the tumor was achieved in 20 patients. There was no significant difference in facial and acoustic nerve functional preservation between GTR and STR, as well as in tumor resection between solid and cystic tumors. The retention rate reached 97.6% in terms of complete anatomical facial nerve preservation. Facial nerve function was assessed using the House–Brackmann (HB) grading score. Consequently, HB grades of I–II, III–IV, and V–VI were determined for 96 (77.4%), 25 (20.2%), and 3 (2.4%) cases, respectively, 1 week postoperatively and accounted for 110 cases (88.7%), 13 cases (10.5%), and 1 case (0.8%), respectively, at 6 months. Fifteen of 35 (42.9%) patients with serviceable hearing before the operation still had serviceable hearing at 6 months postoperatively. There were 5 cases of cerebellar or brainstem bleeding after the operation, and one patient died. Multivariate logistic regression analysis showed that older age (≥60 years, p = 0.011), large tumor (>3 cm, p = 0.004), and cystic tumor (p = 0.046) were independent risk factors associated with the extent of adhesion between the tumor and the brainstem and facial–acoustic nerve.
Conclusion: We successfully applied the subperineural resection technique to a large series of patients with VSs and achieved satisfactory results. Accurate identification of the perineurium and subperineural resection of the tumor can effectively reduce the disturbance of the facial–acoustic nerve during the operation and provide an intuitive basis for judging the tumor boundary. The subperineural resection technique may be conducive to improving the rate of total tumor resection and facial–acoustic nerve functional preservation in the surgical treatment of VSs.
With the development of microneurosurgical techniques, the application of various advanced instruments, and intraoperative electrical nerve monitoring (1, 2), the gross total resection (GTR) rate and the postoperative preservation of facial nerve function in vestibular schwannomas (VSs) have significantly improved in recent years. Meanwhile, hearing retention is increasingly becoming the goal of surgical treatment (3, 4). According to previously reported results, in patients with small tumors (<1.5 cm in diameter), 40%–70% have serviceable hearing postoperatively and less than 10% of these have permanent facial weakness (5–7). However, for patients with tumors larger than 2.5 cm in diameter, the rate of serviceable hearing preservation after surgery is less than 5%, and the risk of partial or permanent facial paralysis is about 50% in those with total resection of large tumors (8–10). The complexity of the structure increases with tumor volume, which may cause difficulties for neurosurgeons to identify and protect cranial nerves (CNs) VII/VIII and the brainstem. Surgical techniques based on a better understanding of the membranous characteristics of VSs are necessary for the achievement of satisfactory results.
Sasaki et al. (11) firstly demonstrated the perineurium structure around VSs based on histological observations and introduced the subperineural dissection technique in surgery. Satisfactory resection rates and facial nerve function preservation were achieved in subsequent reports of the application of this technique in VS treatment (12, 13). However, the complicated membranous structure around VSs makes understanding and duplicating the subperineural separation technique difficult. Moreover, there is no report on the effects of the application of this technology in a large series of cases so far. In this study, we applied the subperineural resection technique in the surgical treatment of 124 patients with VSs. We proposed an easily accessible model of membranous structure around VSs and introduced the nuances and pearls of the subperineural technique based on this model in protecting related cranial nerves and the brainstem. Furthermore, we retrospectively analyzed the surgical effects and complications of this technique and presented our experiences.
A total of 124 patients with VSs recruited from July 2015 to October 2020 underwent microsurgical treatment in the Department of Neurosurgery, Tangdu Hospital, Air Force Military Medical University. Among these patients, 48 were men and 76 were women. The age range of the patients was 21–75 years, with an average age of 48.5 ± 12.9 years (Table 1). VSs were confirmed pathologically in all patients. Patients diagnosed with neurofibromatosis type II and who previously received surgical treatment were excluded.
Preoperative hearing assessment included pure tone hearing, speech discrimination score, and acoustic brainstem response. The following House–Brackmann (HB) grading system was used to evaluate facial nerve function (14): grades I–II, good function; grades III–IV, moderate decline; and grades V–VI, severe decline. Facial nerve function was assessed on the first day and at 1 week, 3 months, 6 months, and 1 year postoperatively. Magnetic resonance imaging (MRI) plain and enhanced scans were performed in all patients before the operation and at 3 days, 3 months, 6 months, and 1 year postoperatively, and then annually at follow-up visits. Samii classification was utilized to assess tumor size and compression on the surrounding cerebellum and brainstem (15). Thin-layer CT scan of the petrous bone was performed to observe the size of the internal auditory canal and the location of the labyrinth; moreover, the presence of a high jugular bulb was detected.
For the facial nerve, the recording electrodes were placed on the ipsilateral orbicularis oculi and orbicularis oris. For the trigeminal nerve, the electrode was placed on the ipsilateral masticatory muscles. For the accessory nerve, the electrode was placed on the ipsilateral trapezius muscle or sternocleidomastoid muscle. For the auditory nerve, a positive electrode on the top of the head and negative electrodes on both sides of the auricular lobule were able to monitor brainstem auditory-evoked potentials. The stimulation electrode was located in the internal auditory canal.
Lumbar cistern drainage was performed routinely before surgery. A craniotomy was conducted with a suboccipital retrosigmoid approach in the lateral prone position intraoperatively (16). The transverse sinuses and the intersection of transverse and sigmoid were located on the basis of MRI. The bone flap was designed according to tumor size. Cerebrospinal fluid (60–80 ml) was released after bone removal. When the cerebellum collapses naturally, the arachnoid fold (the first and second layers of the arachnoid) (Figures 1A, 2B) can be discerned with separation along the cerebellopontine angle (CPA) cistern. The deep tumor and its surface “capsule” (the third layer of the arachnoid membrane plus the perineurium anterior to point L, or the perineurium only posterior to point L) (Figures 1A, 2B) can be seen when the arachnoid fold is pulled apart. Electrical stimulation on the surface of the tumor was used to detect the facial nerve. In the position where the facial nerve is not detected, the “capsule” of the tumor was separated from the surface of the tumor using a hook microdissector under the perineural layer (Figures 1B, 2B), and the tumor was removed according to the order: center, brainstem end (Figures 1C, 2B), caudal end, and rostral end (Figures 1D, 2D). As shown in Figure 2, even the “capsule” of the tumor consists of different layers of membranous structure at different points individually, and the perineurium is always the innermost and only layer that fully covers the tumor. Identifying the interface between the tumor and the perineurium and keeping resection of the tumor under the perineurium (subperineural technique) are vital to guarantee that the brainstem is intact and CN VII and the cochlear nerve are out of the tumor “capsule” (Figures 1E, F). A blunt or sharp separation technique was applied according to the degree of adhesion of the tumor to the perineurium.
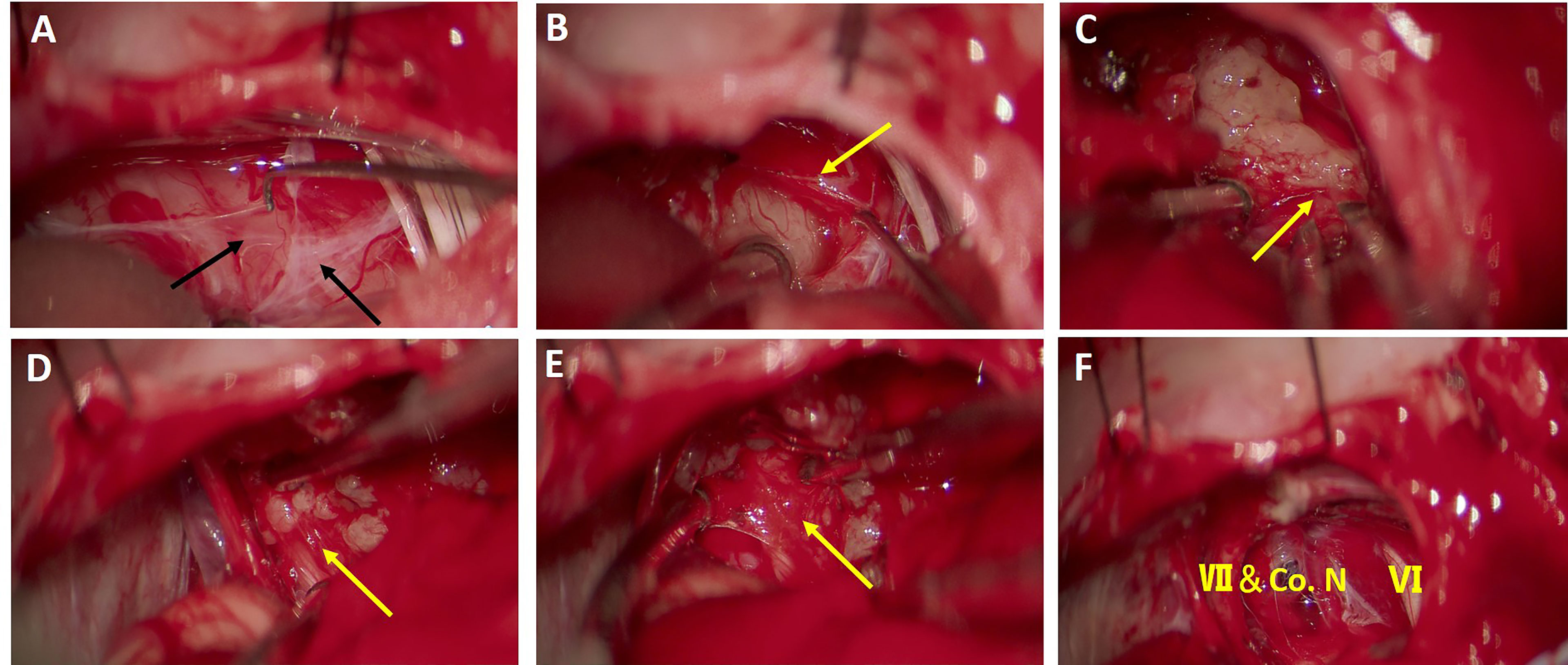
Figure 1 Surgical procedure of tumor separation and removal under the perineurium. (A) An arachnoid fold consisting of the first and second layers of the arachnoid membrane can be identified on the superficial surface of the tumor. (B) Under the arachnoid fold, the tumor capsule was identified and reflected under the perineural plane to create a distinguished interface for resection of the tumor. (C) The tumor was separated from the surface of the brainstem, with the perineurium remaining on the brainstem. (D) Rostrally, facial nerve was preserved under the perineurium and the tumor was dissected from the perineurium. (E) The perineurium above the facial nerve and cochlear nerve was kept intact after tumor removal. (F) Cerebellopontine angle (CPA) area after tumor removal. The brainstem, facial nerve (VII), cochlea nerve (Co. N), and abducens nerve (VI) were preserved well after subperineural tumor resection. Black arrows indicate arachnoid membrane and yellow arrows indicate the perineurium.
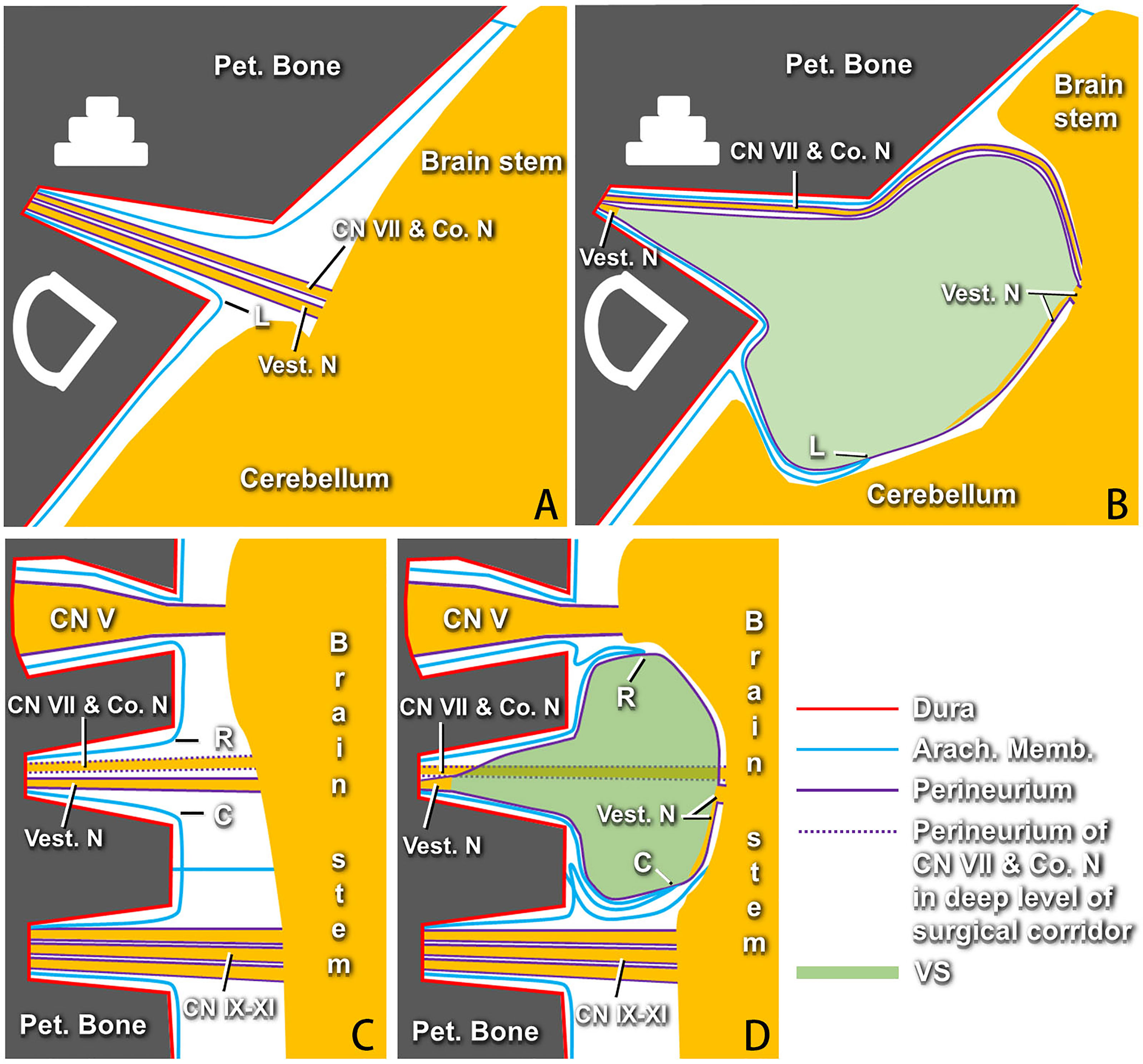
Figure 2 Models of membranous structures around vestibular schwannomas (VSs) proposed by the authors according to the surgical findings (right side). (A) Axial section showing the facial nerve, cochlear nerve, and vestibular nerves, which are covered with perineurium, running from the brainstem into the internal acoustic canal (IAC) fundus under the arachnid membrane in the normal situation. Point “L” indicates the arachnoid membrane at the lateral point of the IAC porus. (B) Axial section showing the arachnoid membrane at point “L” being adhered and stretched by the tumor to the cerebellopontine angle (CPA) with the progress of VSs, which causes “three arachnoid layers” on the lateral surface of the perineurium-covered tumor anterior to the dislocated point “L.” The site of point “L” pulled by the tumor to the CPA is different for each case. There remain some degenerated vestibular nerve fibers that can be identified between the perineurium and the tumor, as well as the IAC fundus. (C) Coronal section showing the normal profile of membranous structures at the rostral and caudal sides of cranial nerves (CNs) VII and VIII. Points “R” and “C” represent the arachnoid membrane at the rostral and caudal points of the IAC porus, respectively. (D) Membranous structures between the VSs and CN V or CN IX–XI varying greatly at different sites due to the different dislocations of points “R” and “C.” The innermost layer of the perineurium can be identified constantly around the tumor. Pet., petrous; Co. N, cochlear nerve; Vest. N, vestibular nerve; Arach., arachnoid; Memb., membrane; VSs, vestibular schwannomas.
After removing the CPA tumor, individualized drilling of the lateral wall of the internal acoustic canal (IAC) was performed. Over-drilling was avoided by continuous checking in order to protect hearing structures until the outermost pole of the tumor can just be directly seen with opening of the dura. The superficial layers of the arachnoid membrane and the perineurium on the IAC tumor surface were opened (Figure 2D). After debulking, the tumor in the IAC was also separated bluntly or sharply from the perineurium and dissected backward from the fundus to the porus of the IAC. When the tumor was resected completely, bone wax and muscle were applied to close the opened lateral wall of the IAC, the layers were sutured, and the incision was closed after hemostasis.
Mild adhesion: the tumor can be easily detached from the perineurium with a “peeling-off” technique.
Moderate adhesion: the tumor cannot be detached directly from the perineurium. A microdissector is required to create an interface between the tumor and the perineurium for further subperineural resection.
Severe adhesion: even with manipulation using the microdissector, the tumor cannot be separated from the perineurium. A sharp dissection technique is required in this situation.
SPSS 23.0 was used for statistical analysis. The measurement data were expressed as x ± s with a t-test, and the enumeration data were expressed as n (percent) with a chi-squared test or Fisher’s exact test. Univariate analysis was performed to assess influencing factors associated with the extent of tumor adhesion. Multivariate logistic regression was then performed to identify the independent predictors found significant in the univariate analysis. A p-value <0.05 was considered as statistically significant.
The extent of tumor resection was distinguished by postoperative MRI performed 3 months after surgery (Figure 3). Among the 124 patients with VSs, 104 had GTR, with a GTR rate of 83.9%, and 20 underwent STR, with an STR rate of 16.1%. There was no significant difference in facial and acoustic nerve functional preservation and in the configuration of the facial nerve in relation to the tumor between GTR and STR (Table 2). No significant difference was observed in the tumor resection between solid and cystic tumors (Table 2).
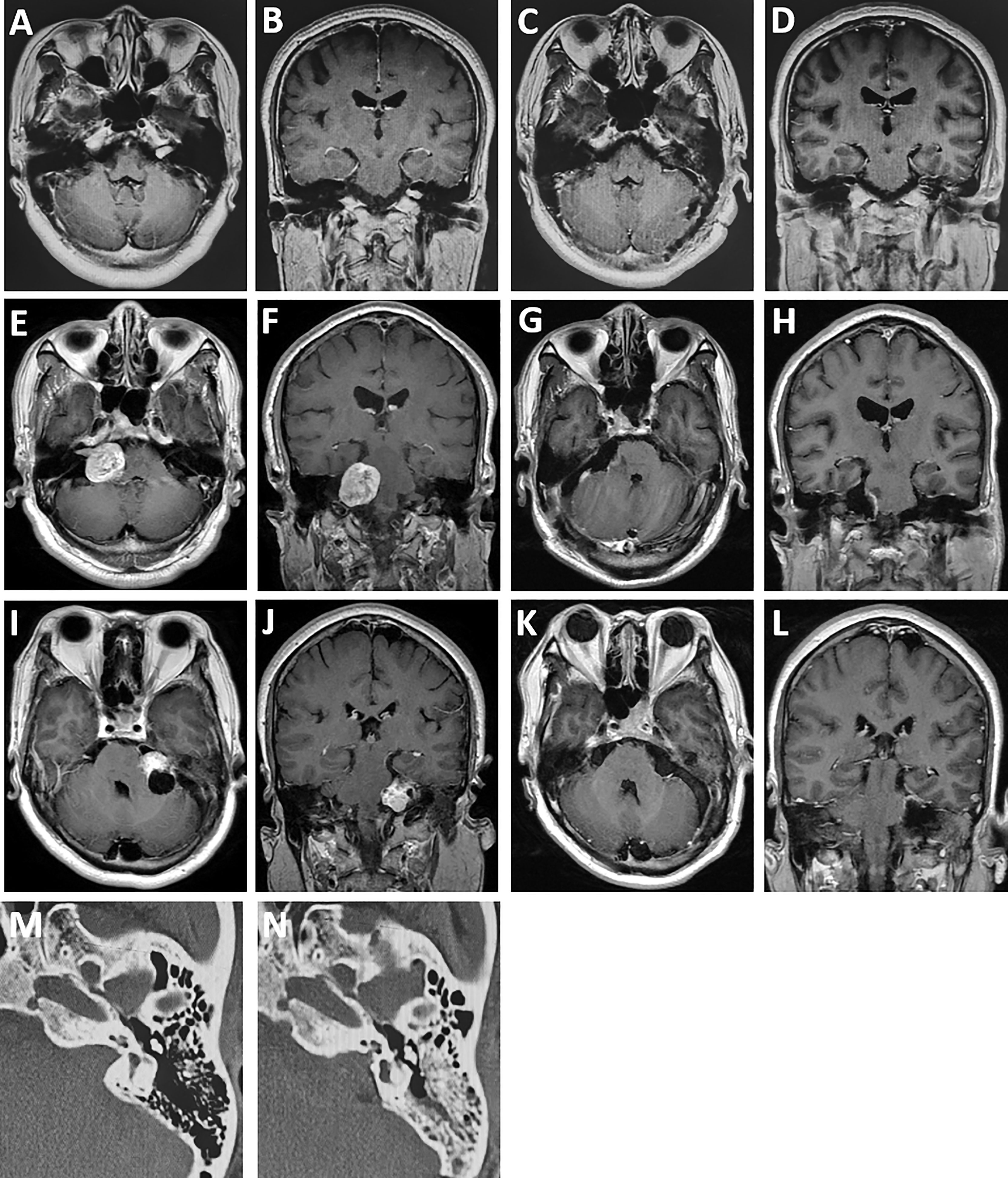
Figure 3 Comparison of preoperative and postoperative MRI. (A, B) A patient with vestibular schwannoma in the internal auditory canal with effective hearing preoperatively. (C, D) MRI at 6-month follow-up revealing total tumor resection and hearing preservation obtained in the patient. (E, F) A patient with solid vestibular schwannoma in the cerebellopontine angle. (G, H) Baseline MRI at 6-month follow-up revealing gross total resection of the tumor and good facial nerve functional preservation (House–Brackmann grade I) after surgery. (I, J) A patient with predominantly cystic vestibular schwannoma with hearing loss before surgery. (K, L) MRI at 6-month follow-up showing no tumor recurrence. (M, N) Comparison of the bone window CT scans of the internal auditory canal before and after grinding.
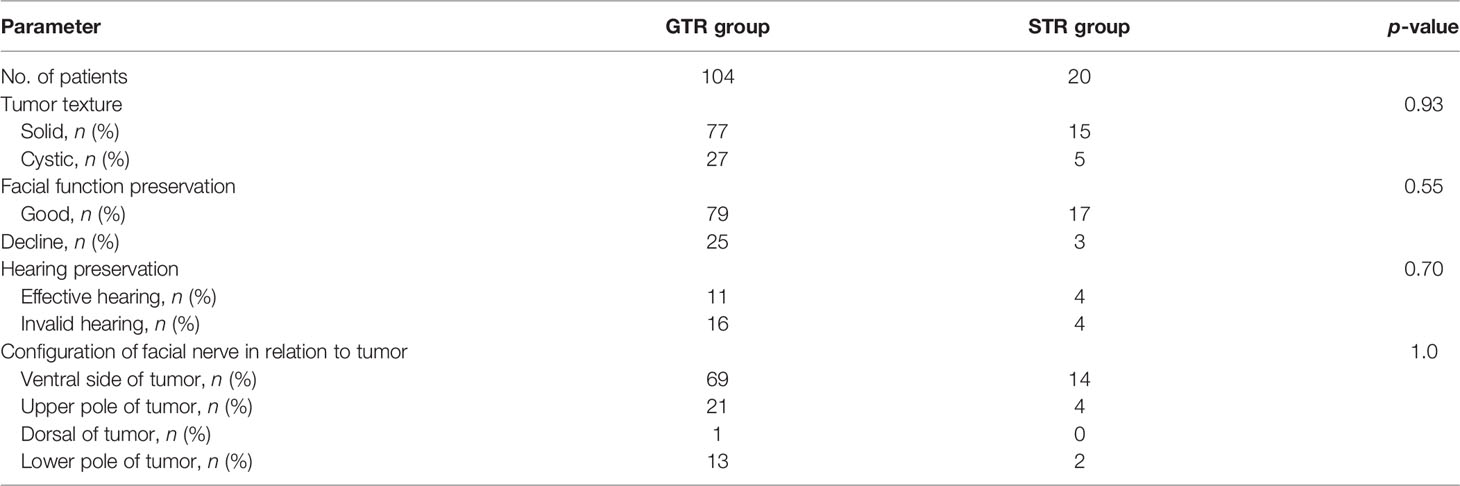
Table 2 Analysis of categorical variables between the gross total resection (GTR) group and subtotal resection (STR) group.
The facial nerve was completely anatomically preserved after the operation in 121 cases (97.6%); the facial nerve was not completely retained in the other 3 because of tumor erosion or adhesion. The HB scores for facial nerve function 1 week after the operation were as follows: 96 cases (77.4%) were of grades I–II (good function), 25 cases (20.2%) of grades III–IV (moderate decline), and 3 cases (2.4%) of grades V–VI (severe decline). The scores at 6 months after operation were as follows: 110 cases (88.7%) with grades I–II, 13 cases (10.5%) with grades III–IV, and 1 case (0.8%) with grade V–VI.
Among 35 patients with serviceable hearing before the operation, 17 had normal hearing and 18 patients had a hearing decline. At 6 months post-operation, of the 17 patients with normal hearing preoperatively, 3 (17.6%) still had normal hearing postoperatively, 5 (29.4%) had a hearing decline, and 9 (52.9%) had hearing loss. Of the 18 patients with a hearing decline, 7 still had serviceable hearing and the other 8 had hearing loss. Thus, 15 out of 35 (42.9%) patients still had serviceable hearing 6 months after the operation.
Some potential predictors including the preoperative hearing level, origin of the tumor, tumor texture, maximum diameter of the tumor, fundal fluid, and adhesion were analyzed. The results demonstrated that there was better hearing preservation with fundal fluid on preoperative MRI than without fundal fluid. The hearing preservation rate (4/5, 80%) with maximum tumor diameter ≤2 cm was higher than that (11/30, 37%) with maximum tumor diameter >2cm, although there was no significant statistical difference (Table 3).
All patients with GTR of VSs had not received radiotherapy. Thirteen of the 20 patients undergoing STR of lesions received stereotactic radiosurgery 1 month after surgery. Contrast-enhanced T1- and T2-weighted (T2W) MRI, as well as contrast-enhanced CT scans, was used to plan the radiosurgery. A prescription dose of 12 Gy was delivered to the planning target volume for the tumor.
We observed that the extent of adhesion of the tumor to the brainstem and facial–acoustic nerve during surgery was the important risk factor that affected the total resection rate of tumor and the preservation rate of facial and acoustic nerve function; therefore, candidate variables including sex, age, maximum diameter of tumor, tumor texture, Ki-67, peritumoral edema, Samii classification, and tumor blood supply were analyzed. The outcomes revealed that age (p = 0.0046), maximum diameter of tumor (p = 0.0023), tumor texture (p = 0.033), and peritumoral edema (p = 0.045) significantly affected the extent of tumor adhesion (Table 4). When age, maximum diameter of tumor, tumor texture, and peritumoral edema were entered into the multivariate logistic regression analysis, older age (≥60 years, p = 0.011), large tumor (>3 cm, p = 0.004), and cystic tumor (p = 0.046) were independent risk factors for the extent of tumor adhesion (Table 5).
Five cases of cerebellar or brainstem hemorrhage occurred in the operation area, of which 1 case died. Two patients underwent sustained craniotomy and decompressive craniectomy, and only mild neurological dysfunction remained postoperatively. Two patients were cured and discharged with conservative treatment. Nine patients with postoperative high fever complicated with intracranial infection were cured when they were treated with lumbar cistern drainage of the cerebrospinal fluid and anti-infection. Cerebrospinal fluid leakage occurred in 4 patients, of which 1 case was otorrhea and was cured conservatively. The remaining 3 cases developed incision leakage due to improper dura mater suture, but the leak disappeared when repaired. Cerebellar ataxia appeared postoperatively in 8 patients, but all returned to normal 3–6 months later. Symptoms of posterior cranial nerve injury emerged in 4 patients, and abducent nerve injury emerged in 2 patients postoperatively, which manifested as attenuation of the cough reflex and dysphagia and ocular movement disorder.
The follow-up period ranged from 11 to 70 months, with an average of 34.6 ± 17.4 months. During the follow-up, two patients receiving STR but no radiotherapy underwent reoperation due to tumor recurrence. The recurrence times were 28 and 41 months, respectively. The other patients had no recurrence in the follow-up period.
Conservative treatment, stereotactic radiotherapy, and surgery were employed in the treatment of VSs. For patients with large VSs and obvious clinical symptoms, surgery is still the only option to alleviate the clinical symptoms and cure the tumors (17). The difficulty in obtaining GTR and functional preservation of the facial and auditory nerves increases with the increase of tumor volume.
At present, studies have confirmed that most VSs are subarachnoid, whereas those located outside the arachnoid region occur quite rarely. Kohno et al. (18) confirmed that 86 of 118 patients (73%) were subarachnoid, 2 (2%) cases were extra-arachnoid, and the remaining 30 cases (25%) were relatively large tumors that were difficult to determine. Lescanne et al. (19, 20) also reported that the arachnoid membrane of the CPA cistern continued from the brainstem to the bottom of the internal carotid artery (ICA), and the facial–acoustic nerve complex had no interval and was located under the arachnoid, indicating that the VS was a non-extraarachnoid tumor. It was suggested, by means of optical microscopy and immunohistochemical staining, that the membranous structure covering the tumor surface was arachnoid (18, 21, 22). Ohata et al. (22) further elucidated the rearrangement of the adjacent arachnoid membrane with the tumor growth, which caused different capsule layers of the arachnoid membrane at different sites on VSs, and advocated that the tumor should be dissected and resected along the subarachnoid plane. Furthermore, Sasaki et al. (11) demonstrated that there is a thin layer of connective tissue membrane composed of the perineurium and that some degenerated nerve fibers under it lie on the surface of the tumor. Subsequently, the subperineural VS resection technique was introduced and demonstrated to obtain good surgical results (12, 13). However, it is still difficult to identify the perineurium accurately and to get to the right dissection plane due to the “rearrangement of adjacent arachnoid membrane” out of the perineurium, which may cause this technique to be less repeatable. Based on our surgical experience on 124 VS cases, we combined the “arachnoid membrane rearrangement theory” and the “subperineural resection technique” and proposed a more comprehensive descriptive model of the membranous structure around VSs (Figures 2A–D). Better understanding of the membranous structure around VSs based on this model will be greatly helpful for neurosurgeons to identify the accurate subperineural dissecting plane.
In the present study, the subperineural resection technique was demonstrated to effectively reduce the disturbance of the facial–acoustic nerve during operation and to provide an intuitive basis for judging the tumor boundary. It is important to try to avoid breaking through the perineural layer during the entire process of tumor resection. If the perineurium has been accidentally pierced and enters the extra-perineural space as a result, it is necessary to try to reenter the subperineural plane. Esquia-Medina et al. (23) also found that the degree of tumor adhesion, which is mainly evaluated by the nerve displacement, was an important predictive factor associated with facial function. In this study, we proposed a manipulation-based classification in depicting the extent of tumor adhesion to the perineurium and found that older patients and large and cystic tumors were closely associated with severe tumor adhesion, which may significantly affect total tumor removal and nerve function preservation when using this technique. When the tumor has a clear boundary with the perineurium above the brainstem and the facial and cochlear nerves, the tumor could be separated and removed under the perineurium to achieve GTR. However, in the instance of severe adhesion of the tumor to the perineurium (usually at the site near the ICA porus), the function of the facial–acoustic nerve might be damaged if the tumor and the perineurium were detached roughly from the nerves. Therefore, when the interface between the tumor and the perineurium could not be separated using the microdissector, neurosurgeons must use a microscissor to perform sharp dissection in order to create an interface at the mixed level of nerve fibers and tumor cells. In this way, although a small part of the tumor tissue may remain on the perineurium above nerves, the possibility of preserving the function of the facial–auditory nerve will be significantly improved (11, 24, 25).
In addition to the membrane resection technique, which improves the functional preservation of the facial auditory nerve, intraoperative neurophysiological monitoring also plays an important role in anatomical recognition, intraoperative functional preservation, and postoperative prediction (26, 27). Neff et al. (28) pointed out that if the stimulus threshold was ≤0.05 mA or the response amplitude was ≥240 μV, then there is a 98% chance for predicting postoperative facial auditory nerve function as HB grade I or II. Boublata et al. (29) reported on 151 cases of VSs with diameters of 31–60 mm under neurophysiological monitoring and demonstrated that the GTR rate was 82.8%, the anatomical retention rate of facial nerves was 98.7%, and that patients with HB grades of I and II at 2 years postoperatively accounted for 82%. Although the facial–acoustic nerve cannot be seen directly with neurophysiological monitoring, the relative distance between the nerve and the probe can be inferred by adjusting the current required to stimulate the muscle. This suggests that the nerve is highly exposed close to the probe when the evoked potential is obtained with a current of <0.2 mA for stimulation. In contrast, it indicated that a larger tissue or bone barrier existed between the probe and the nerve with stimulation under a current >0.5 mA (30). The retention of auditory nerve function was still not comparable to that of facial nerve function despite the combination of brainstem auditory-evoked potentials (BAEPs), electrocochleograms (ECochGs), and cochlear nerve action potentials (CNAPs) for vestibulocochlear nerve monitoring. More importantly, the larger the tumor, the higher the probability of postoperative hearing loss; for this reason, further clinical research and other electrophysiological monitoring techniques are needed to improve hearing retention (30).
Boublata et al. (29) reported on 126 patients (82.8%) who had GTR tumors, 20 patients (13.9%) who had subtotal tumors, and 124 patients (82%) who had facial nerve function of HB grades I–II 2 years after surgery. The study of Sughrue et al. (31) included 74% of patients who underwent GTR, 11.5% underwent near-total resection, and 14.5% underwent SRT. In a retrospective study of the efficacy of the facial nerve-sparing approach, functional preservation of the facial nerve with HB grade I or II was achieved in 97% after surgery (32). In our group of 124 patients with VSs, the GTR rate was 83.9%, and patients with HB grades I–II postoperatively accounted for 77.4% at 1 week and 88.7% at 6 months. These data indicated that the subperineural technique is effective in obtaining total tumor resection and in preserving facial nerve function.
Although satisfactory results were reported for the resection of total tumor and preservation of facial nerve function, preservation of hearing was still unsatisfactory, especially for large VSs. Zhu et al. (33) reported a postoperative rate of serviceable hearing preservation of 60.9% (67/110) in 110 small VSs (≤1.5cm). Wanibuchi et al. (4) also determined a hearing preservation rate of 53.7% in 592 consecutive patients with VSs (>2 cm in diameter). Surgical tips were introduced as vital to preserving hearing, including bloodless microdissection, subperineural or subcapsular dissection, real-time neurophysiological monitoring, sharp resection of the facial–acoustic nerve, and avoidance of mechanical and heat damage to the brainstem, the nerves, and the internal auditory artery (4, 33). In this study, the hearing preservation rate at 6 months was 43% (15/35). The prognostic factors were analyzed for postoperative hearing preservation, and it indicated that the presence of fundal fluid was a positive prognostic factor. Several documents also stated that the presence of fundal fluid on T2W MRI was predictive of postoperative hearing preservation (33–35). A number of studies confirmed a higher postoperative rate of hearing preservation in tumors originating from the superior vestibular nerve than in those originating from the inferior vestibular nerve, as well as in small tumors than in large tumors (33, 35). However, in our study, we did not find hearing preservation to be related to the tumor origin and size. This may be due to the limited sample of patients with serviceable hearing before operation and most of the recruited patients with preoperative serviceable hearing having large tumors, which may have resulted in poor hearing preservation rate.
The effect of stereotactic radiotherapy in VSs is more and more accepted. For small and medium VSs (≤2.8 cm), gamma knife surgery (GKS) showed good results in terms of tumor control and functional preservation of the facial and acoustic nerves (36). Larger VSs adhere more tightly to the brainstem and facial–acoustic nerve, and the risk of brainstem injury or facial paralysis may be higher after surgery. The treatment strategy combining STR with GKS has been usually selected for larger VSs, also achieving good facial–acoustic nerve functional preservation (32, 37). The classification criterion for the degree of tumor adhesion proposed in this study provides a good reference for neurosurgeons in planning surgical strategy. GTR is relatively easy to achieve in the mild and moderate adhesion group. We strongly recommend that STR combined with postoperative GKS strategy should be adopted when operating on tumors firmly adhered to the perineurium in order to maintain good life quality of patients.
The most serious complications after microsurgery of VSs were intraoperative brainstem injury and postoperative bleeding in the operation area, which were consistent with previous reports (38–40). Bleeding occurred in 5 cases. Of these patients, 1 died due to respiratory and cardiac arrest the next day postoperatively. Craniotomy and hematoma evacuation occurred in 2 cases, and conservative treatment was given in the other 2 cases; eventually, all patients were cured and discharged. Adhesion of the tumor to the brainstem and cerebellum leads to microdamage to the small blood vessels at the junction of tumor and brain tissue, which is the main cause of bleeding (40). Due to the complexity of the surgical procedures, the operation generally lasts longer, especially for larger VSs, and the risks of postoperative fever and intracranial infection could be greater. We carried out procedures such as preoperative lumbar cistern to facilitate intraoperative cerebellar collapse in order to extend the operating space and postoperative continuous drainage of the cerebrospinal fluid until it tested normal in laboratory analysis, which significantly reduced the duration of fever or infection in patients. Another common complication is cerebrospinal fluid otorrhea or incision leakage (5, 41). The incidence of leakage was 3.2% in these cases, which was not very high compared to that in previous literature (40, 41). It is recommended that once mastoid pneumatization is opened, bone wax should be used to seal it tightly, and sometimes it is necessary to cover the muscles on the mastoid for fixation. For the dura mater, a 4–0 absorbable suture was used to close the dural gap, and autologous fascia was responsible for repairing larger dura defects. The procedures were finished by covering the artificial dura, fixing with tissue colloid, and using an elastic bandage for incision compression.
The membranous structure around VSs is complicated and is divergent for each case. The perineurium provides a safe and constant dissecting plane for neurosurgeons to remove the tumor. Fine outcomes have been achieved regarding the GTR rate and the facial–acoustic nerve functional preservation rate with the use of the subperineural technique under neuroelectrophysiological monitoring. The decision to perform radical GTR or STR combined with GKS can be made according to the degree of tumor adherence.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
QY and ZT: chief surgeon, writing—review and editing. WYX, WC, and ZY: writing—original draft preparation. WP, WY, and XYF: data collection and analysis. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (no. 81971153) and Tangdu Hospital New Technology Plan (nos. XJSXYW2021120, XJSXYW202126).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
MRI, magnetic resonance imaging; CPA, cerebellopontine angle; IAC, internal auditory canal; GTR, gross total resection; STR, subtotal resection; HB, House Brackmann; GKS, gamma knife surgery; VSs, vestibular schwannomas.
1. Carlson ML, Link MJ. Vestibular Schwannomas. N Engl J Med (2021) 384(14):1335–48. doi: 10.1056/NEJMra2020394
2. Macielak RJ, Wallerius KP, Lawlor SK, Lohse CM, Marinelli JP, Neff BA, et al. Defining Clinically Significant Tumor Size in Vestibular Schwannoma to Inform Timing of Microsurgery During Wait-and-Scan Management: Moving Beyond Minimum Detectable Growth. J Neurosurg (2021) 136(3): 1–9. doi: 10.3171/2021.4.JNS21465
3. Matsushima K, Kohno M, Nakajima N. Hearing Preservation in Vestibular Schwannoma Surgery via Retrosigmoid Transmeatal Approach. Acta Neurochir (Wien) (2019) 161(11):2265–9. doi: 10.1007/s00701-019-04034-9
4. Wanibuchi M, Fukushima T, Friedman AH, Watanabe K, Akiyama Y, Mikami T, et al. Hearing Preservation Surgery for Vestibular Schwannomas via the Retrosigmoid Transmeatal Approach: Surgical Tips. Neurosurg Rev (2014) 37(3):431–44; discussion 44. doi: 10.1007/s10143-014-0543-9
5. Ansari SF, Terry C, Cohen-Gadol AA. Surgery for Vestibular Schwannomas: A Systematic Review of Complications by Approach. Neurosurg Focus (2012) 33(3):E14. doi: 10.3171/2012.6.FOCUS12163
6. Carlson ML, Vivas EX, McCracken DJ, Sweeney AD, Neff BA, Shepard NT, et al. Congress of Neurological Surgeons Systematic Review and Evidence-Based Guidelines on Hearing Preservation Outcomes in Patients With Sporadic Vestibular Schwannomas. Neurosurgery (2018) 82(2):E35–E9. doi: 10.1093/neuros/nyx511
7. Preet K, Ong V, Sheppard JP, Udawatta M, Duong C, Romiyo P, et al. Postoperative Hearing Preservation in Patients Undergoing Retrosigmoid Craniotomy for Resection of Vestibular Schwannomas: A Systematic Review of 2034 Patients. Neurosurgery (2020) 86(3):332–42. doi: 10.1093/neuros/nyz147
8. Grinblat G, Dandinarasaiah M, Braverman I, Taibah A, Lisma DG, Sanna M. “Large and Giant Vestibular Schwannomas: Overall Outcomes and the Factors Influencing Facial Nerve Function”. Neurosurg Rev (2021) 44(4):2119–31. doi: 10.1007/s10143-020-01380-6
9. Gurgel RK, Dogru S, Amdur RL, Monfared A. Facial Nerve Outcomes After Surgery for Large Vestibular Schwannomas: Do Surgical Approach and Extent of Resection Matter? Neurosurg Focus (2012) 33(3):E16. doi: 10.3171/2012.7.FOCUS12199
10. Wallerius KP, Macielak RJ, Lawlor SK, Lohse CM, Neff BA, Van Gompel JJ, et al. Hearing Preservation Microsurgery in Vestibular Schwannomas: Worth Attempting in "Larger" Tumors? Laryngoscope (2021) 00:1–8. doi: 10.1002/lary.29968
11. Sasaki T, Shono T, Hashiguchi K, Yoshida F, Suzuki SO. Histological Considerations of the Cleavage Plane for Preservation of Facial and Cochlear Nerve Functions in Vestibular Schwannoma Surgery. J Neurosurg (2009) 110(4):648–55. doi: 10.3171/2008.4.17514
12. Giammattei L, Passeri T, Padovan S, Froelich S. Vestibular Schwannoma: Care for Soft Tissues and Subperineural Dissection: How I do it. Acta Neurochir (Wien) (2021) 163(8):2247–51. doi: 10.1007/s00701-021-04801-7
13. Tayebi Meybodi A JR, Liu JK. Retrosigmoid Approach for Giant Cystic Vestibular Schwannoma: Subperineural Dissection Technique for Facial Nerve Preservation. Neurosurg Focus (2021) 5:1–3. doi: 10.3171/2021.7.FOCVID21128
14. Lanman TH, Brackmann DE, Hitselberger WE, Subin B. Report of 190 Consecutive Cases of Large Acoustic Tumors (Vestibular Schwannoma) Removed via the Translabyrinthine Approach. J Neurosurg (1999) 90(4):617–23. doi: 10.3171/jns.1999.90.4.0617
15. Wang X, Long W, Liu D, Yuan J, Xiao Q, Liu Q. Optimal Surgical Approaches and Treatment Outcomes in Patients With Jugular Foramen Schwannomas: A Single Institution Series of 31 Cases and a Literature Review. Neurosurg Rev (2020) 43(5):1339–50. doi: 10.1007/s10143-019-01165-6
16. Graffeo CS, Peris-Celda M, Perry A, Carlstrom LP, Driscoll CLW, Link MJ. Anatomical Step-By-Step Dissection of Complex Skull Base Approaches for Trainees: Surgical Anatomy of the Retrosigmoid Approach. J Neurol Surg B Skull Base (2021) 82(3):321–32. doi: 10.1055/s-0039-1700513
17. Zhang S, Liu W, Hui X, You C. Surgical Treatment of Giant Vestibular Schwannomas: Facial Nerve Outcome and Tumor Control. World Neurosurg (2016) 94:137–44. doi: 10.1016/j.wneu.2016.06.119
18. Kohno M, Sato H, Sora S, Miwa H, Yokoyama M. Is an Acoustic Neuroma an Epiarachnoid or Subarachnoid Tumor? Neurosurgery (2011) 68(4):1006–16; discussion 16-7. doi: 10.1227/NEU.0b013e318208f37f
19. Lescanne E, Velut S, Lefrancq T, Destrieux C. The Internal Acoustic Meatus and its Meningeal Layers: A Microanatomical Study. J Neurosurg (2002) 97(5):1191–7. doi: 10.3171/jns.2002.97.5.1191
20. Lescanne E, Francois P, Velut S. Cerebellopontine Cistern: Microanatomy Applied to Vestibular Schwannomas. Prog Neurol Surg (2008) 21:43–53. doi: 10.1159/000156580
21. Kuo TC, Jackler RK, Wong K, Blevins NH, Pitts LH. Are Acoustic Neuromas Encapsulated Tumors? Otolaryngol Head Neck Surg (1997) 117(6):606–9. doi: 10.1016/S0194-5998(97)70040-8
22. Ohata K, Tsuyuguchi N, Morino M, Takami T, Goto T, Hakuba A, et al. A Hypothesis of Epiarachnoidal Growth of Vestibular Schwannoma at the Cerebello-Pontine Angle: Surgical Importance. J Postgrad Med (2002) 48(4):253–8; discussion 8-9.
23. Esquia-Medina GN, Grayeli AB, Ferrary E, Tubach F, Bernat I, Zhang Z, et al. Do Facial Nerve Displacement Pattern and Tumor Adhesion Influence the Facial Nerve Outcome in Vestibular Schwannoma Surgery? Otol Neurotol (2009) 30(3):392–7. doi: 10.1097/MAO.0b013e3181967874
24. Vellutini EA, Beer-Furlan A, Brock RS, Gomes MQ, Stamm A, Cruz OL. The Extracisternal Approach in Vestibular Schwannoma Surgery and Facial Nerve Preservation. Arq Neuropsiquiatr (2014) 72(12):925–30. doi: 10.1590/0004-282X20140152
25. Tomio R, Yoshida K, Kohno M, Kamamoto D, Mikami S. The Outermost "Dura-Like Membrane" of Vestibular Schwannoma. Surg Neurol Int (2016) 7:71. doi: 10.4103/2152-7806.185008
26. Youssef AS, Downes AE. Intraoperative Neurophysiological Monitoring in Vestibular Schwannoma Surgery: Advances and Clinical Implications. Neurosurg Focus (2009) 27(4):E9. doi: 10.3171/2009.8.FOCUS09144
27. Matsushima K, Kohno M, Sakamoto H, Ichimasu N, Nakajima N. Intraoperative Continuous Neuromonitoring for Vestibular Schwannoma Surgery: Real-Time, Quantitative, and Functional Evaluation. World Neurosurg (2021) 158:189. doi: 10.1016/j.wneu.2021.11.102.
28. Neff BA, Ting J, Dickinson SL, Welling DB. Facial Nerve Monitoring Parameters as a Predictor of Postoperative Facial Nerve Outcomes After Vestibular Schwannoma Resection. Otol Neurotol (2005) 26(4):728–32. doi: 10.1097/01.mao.0000178137.81729.35
29. Boublata L, Belahreche M, Ouchtati R, Shabhay Z, Boutiah L, Kabache M, et al. Facial Nerve Function and Quality of Resection in Large and Giant Vestibular Schwannomas Surgery Operated By Retrosigmoid Transmeatal Approach in Semi-Sitting Position With Intraoperative Facial Nerve Monitoring. World Neurosurg (2017) 103:231–40. doi: 10.1016/j.wneu.2017.02.053
30. Oh T, Nagasawa DT, Fong BM, Trang A, Gopen Q, Parsa AT, et al. Intraoperative Neuromonitoring Techniques in the Surgical Management of Acoustic Neuromas. Neurosurg Focus (2012) 33(3):E6. doi: 10.3171/2012.6.FOCUS12194
31. Sughrue ME, Kaur R, Rutkowski MJ, Kane AJ, Kaur G, Yang I, et al. Extent of Resection and the Long-Term Durability of Vestibular Schwannoma Surgery. J Neurosurg (2011) 114(5):1218–23. doi: 10.3171/2010.11.JNS10257
32. Haque R, Wojtasiewicz TJ, Gigante PR, Attiah MA, Huang B, Isaacson SR, et al. Efficacy of Facial Nerve-Sparing Approach in Patients With Vestibular Schwannomas. J Neurosurg (2011) 115(5):917–23. doi: 10.3171/2011.7.JNS101921
33. Zhu W, Chen H, Jia H, Chai Y, Yang J, Wang Z, et al. Long-Term Hearing Preservation Outcomes for Small Vestibular Schwannomas: Retrosigmoid Removal Versus Observation. Otol Neurotol (2018) 39(2):e158–e65. doi: 10.1097/MAO.0000000000001684
34. Tamura M, Carron R, Yomo S, Arkha Y, Muraciolle X, Porcheron D, et al. Hearing Preservation After Gamma Knife Radiosurgery for Vestibular Schwannomas Presenting With High-Level Hearing. Neurosurgery (2009) 64(2):289–96; discussion 96. doi: 10.1227/01.NEU.0000338256.87936.7C
35. Goddard JC, Schwartz MS, Friedman RA. Fundal Fluid as a Predictor of Hearing Preservation in the Middle Cranial Fossa Approach for Vestibular Schwannoma. Otol Neurotol (2010) 31(7):1128–34. doi: 10.1097/MAO.0b013e3181e8fc3f
36. Golfinos JG, Hill TC, Rokosh R, Choudhry O, Shinseki M, Mansouri A, et al. A Matched Cohort Comparison of Clinical Outcomes Following Microsurgical Resection or Stereotactic Radiosurgery for Patients With Small- and Medium-Sized Vestibular Schwannomas. J Neurosurg (2016) 125(6):1472–82. doi: 10.3171/2015.12.JNS151857
37. Gupta A, Turel MK, Moorthy RK, Joseph V, Rajshekhar V. Treatment Outcomes and Follow-Up Compliance After Less Than Total and Total Resection of Vestibular Schwannomas in 294 Patients. Neurol India (2020) 68(6):1351–60. doi: 10.4103/0028-3886.304069
38. Huang X, Xu J, Xu M, Chen M, Ji K, Ren J, et al. Functional Outcome and Complications After the Microsurgical Removal of Giant Vestibular Schwannomas via the Retrosigmoid Approach: A Retrospective Review of 16-Year Experience in a Single Hospital. BMC Neurol (2017) 17(1):18. doi: 10.1186/s12883-017-0805-6
39. Samii M, Giordano M, Metwali H, Almarzooq O, Samii A, Gerganov VM. Prognostic Significance of Peritumoral Edema in Patients With Vestibular Schwannomas. Neurosurgery (2015) 77(1):81–5; discussion 5-6. doi: 10.1227/NEU.0000000000000748
40. Betka J, Zverina E, Balogova Z, Profant O, Skrivan J, Kraus J, et al. Complications of Microsurgery of Vestibular Schwannoma. BioMed Res Int (2014) 2014:315952. doi: 10.1155/2014/315952
Keywords: subperineural resection technique, facial–acoustic nerve function, gross total resection, neurophysiological monitoring, vestibular schwannomas, microsurgery, extent of tumor adhesion
Citation: Wu Y, Wei C, Wang P, Zhang Y, Wu Y, Xue Y, Zhao T and Qu Y (2022) Application of Subperineural Resection Technique in Vestibular Schwannomas: Surgical Efficacy and Outcomes in 124 patients. Front. Oncol. 12:849109. doi: 10.3389/fonc.2022.849109
Received: 05 January 2022; Accepted: 14 March 2022;
Published: 20 April 2022.
Edited by:
Rachel Grossman, Tel Aviv Sourasky Medical Center, IsraelReviewed by:
Mario Zuccarello, University of Cincinnati, United StatesCopyright © 2022 Wu, Wei, Wang, Zhang, Wu, Xue, Zhao and Qu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianzhi Zhao, emhhb3RpYW56aGkxOTgxQDE2My5jb20=; Yan Qu, eWFucXUwMTIzQGZtbXUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.