- 1Division of Population Sciences, Comprehensive Cancer Center, The Ohio State University, Columbus, OH, United States
- 2Division of Epidemiology, College of Public Health, The Ohio State University, Columbus, OH, United States
- 3Arthur G. James Cancer Hospital and Richard J. Solove Research Institute, Columbus, OH, United States
- 4Division of Cancer Prevention and Control, Department of Internal Medicine, College of Medicine, The Ohio State University, Columbus, OH, United States
Objective: The oral-cervical human papillomavirus (HPV) infection/cancer relationship is not well established. Oral-cervical HPV studies were reviewed to assess dual-site occurrence, HPV type concordance, and study quality/deficiencies.
Methods: PubMed, EMBASE, Ovid Medline, and Web of Science were searched between 1/1/1990 and 8/10/2021 for studies investigating HPV infections/cancers and type concordance between the oral cavity/oropharynx and cervix. Dual-site and concordant HPV infection rates were summarized as percentages; cancer diagnoses studies were summarized using standardized incidence ratios (SIR). The Quality Assessment Tool for Quantitative Studies (QATQS) evaluated study methodology.
Results: One hundred fourteen papers were identified. Most were cross-sectional (n=79, 69%), involved synchronous dual-site HPV testing (n=80, 70%), did not report HPV type concordance (n=62, 54%), and achieved moderate methodological QATQS ratings (n=81, 71%). The overall dual-site infection rate averaged 16%; the HPV type concordance rate averaged 41%, among those dually-infected women. Most HPV-related cancer diagnoses studies reported increased secondary cancer risk, with SIRs generally ranging from 1.4 to 29.4 for secondary cervical cancer after primary oral cancer and from 1.4 to 6.3 for secondary oral cancer after primary cervical cancer.
Conclusion/Impact: Oral-cervical HPV infections/cancers remain understudied. Future research should use stronger methodologies and HPV concordance analyses to better understand oral-cervical HPV epidemiology.
Introduction
Human papillomavirus (HPV) is the most prevalent sexually transmitted infection (1). The virus exists in 200+ types—some more high risk (i.e., potentially malignant) than others (1). Various HPV types can infect the cervix, vagina, vulva, penis, anus, and/or oropharyngeal region, increasing the risk for the development of warts and/or cancers (1). Globally, about 630,000 incident cancers are HPV-related with most occurring in the oropharynx and cervix (1, 2). Oral HPV infections and cancer biology remain less understood than cervical HPV (3–7). Cervical HPV infection is clearly acquired through vaginal intercourse, whereas acquisition of oral HPV, potentially during orogenital sex, remains uncertain, especially in women (3, 4, 8, 9). Therefore, women are disproportionately burdened with the disease, amassing 90% of all HPV-related cancers (1).
HPV can be attributed to more than 70% of oropharyngeal cancers in the United States (US) (8, 10). In 2020, there were 98,412 new oropharyngeal cancer cases worldwide (11). High-risk HPV types (e.g., HPV16) account for a substantial proportion of oral HPV cases (3). HPV tends to infect the back of the oral cavity from the base of the tongue through the esophagus, including the oropharynx and tonsils (3, 9). However, there is no routine screening for oral HPV infection and methods are less-refined for oral HPV cancer detection, resulting in later stage diagnoses and more aggressive cancer treatments (3).
Approximately 604,127 women were diagnosed with cervical cancer worldwide in 2020 (11). Essentially all cervical cancers are HPV-related (10). HPV types 16 and 31/18/33 are the first and second most common type groupings routinely identified in advanced cervical infections and cancers, respectively (5). Slow disease progression and effective screening methods, including Papanicolaou (Pap) tests, allow for opportunities to detect and treat cervical abnormalities to reduce the risk for cancer development (12).
Results from studies of dual-site oral-cervical HPV infections/cancers are inconsistent. Investigating HPV status in both oral and cervical sites in women can aid in determining how HPV is transmitted (e.g., orogenital interaction, autoinoculation, unrelated events) (4). For example, oral-cervical HPV type concordance (i.e., same HPV type(s) in both sites) would suggest a transfer of infection across sites. Whereas HPV type discordance would suggest the infections were separate. Clarity in the oral-cervical HPV+ association could improve prevention, screening, and/or treatment approaches for both diseases, ultimately reducing HPV-related cancer rates overall.
Current systematic reviews on the topic of oral HPV infections and cervical cancers have only studied the infections independently of one another. This prohibits a complete assessment of HPV type concordance between the anatomical sites. The one meta-analysis that investigated oral and cervical HPV infections estimated an HPV concordance rate of 27% (4). However, the study was limited in publication years, databases, search terms, and oral HPV data collection methodologies, including just 10 studies, and without any quality assessment.
To date, there is no published comprehensive systematic review incorporating a quality assessment of the literature that examines the potential for both oral and cervical HPV infections in women. This systematic review aims to fill significant gaps in the HPV literature regarding oral and cervical dual-site and concordance rates of HPV. In summary, there is no consensus on whether oral and cervical HPV-related infections and/or cancers are more likely to be related or unrelated events. This systematic review aims to critically assess studies with participants who have at least one HPV-related oral and/or cervical infection/cancer diagnosis, comparing any HPV types across the two biological sites, to determine if there is a higher probability that any HPV types at the two sites had concordance.
Materials and Methods
Literature Search Strategy
A review of the literature was conducted in PubMed, EMBASE, Ovid Medline, and Web of Science databases using variations to the search terms oropharynx and cervix and human papillomavirus and infection or cancer. Searches were restricted to peer-reviewed papers published from January 1, 1990 to August 10, 2021. For example in PubMed, the following terms were used:
These search strategies were reproduced in each of the other three databases (Supplementary Table). Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines were used in this systematic review with respect to design and reporting.
Eligibility Criteria
Inclusion Criteria
Studies were eligible for inclusion if they: involved human subjects; investigated both person-specific anatomical sites of oral cavity/oropharynx and cervix for HPV synchronously (i.e., evaluated simultaneously) or asynchronously (i.e., evaluated at different times); and were full-text papers of original research written in English. Partner studies with both men and women were included if any HPV data pertaining to women could be independently differentiated from any HPV data presented on men. Studies including participants with a positive oral HPV test or any HPV-related oral cancer (i.e., non-tobacco/alcohol-related oral cancers) were included. Oral sites could range from the oral cavity to the esophagus (both potentially HPV-associated), including the oropharyngeal region with the base of the tongue and the tonsils (both HPV-related), as long as the original study authors had justified the sites to be at least possibly oral HPV-related (3, 9). All cervical abnormalities/cancers were assumed to be HPV-related since 95–99% of cervical cancer cases involve HPV (13).
Exclusion Criteria
Studies were excluded if they were not relevant to within-person HPV evaluation of both oral and cervical infections (e.g., both sites but in different people, wrong biological site or cancer or population), involved only HPV infections in the oral cavity/oropharynx or cervix, not original research (e.g., reviews, abstracts, letters, commentaries, meetings, protocols), or were case reports or series (i.e., N <10).
Data Collection, Categories, and Analyses
Data Extraction
Duplicate citations from the four databases were reviewed and removed. The remaining citations were divided equally, reviewed separately, and then summarized with data extraction by three study authors (KHJ, CBH, XZ). Any questions regarding inclusion were resolved by consensus among the three authors listed above.
Assessment of Risk of Bias and Quality of Studies
As described by Thomas et al., the Quality Assessment Tool for Quantitative Studies (QATQS) from the Effective Public Health Practice Project criteria was utilized to determine the quality of each included study (14). The assessment tool evaluates: 1) selection bias, 2) study design, 3) confounder adjustment, 4) blinding, 5) data collection methods, and 6) withdrawals and dropouts (14). All topics were evaluated for studies included in this systematic review, excluding blinding since all studies were observational in nature and no intervention or randomized control trial methods were considered for HPV evaluation in the oropharynx/oral cavity sites and/or cervix. Included papers were divided such that two authors (KHJ, CBH, or XZ) reviewed and scored the QATQS for each study independently. Each topic area evaluated received a rating of strong, moderate, or weak quality, dependent on topic-specific criteria. Studies attaining only moderate and/or strong quality topic ratings were classified as “strong”; studies with one weak quality topic rating were classified as “moderate” while studies with two or more weak quality topic ratings were classified as “weak” (14). The primary paper evaluator (KHJ, CBH, or XZ) compared the two-author ratings for inconsistencies. Discrepancies were discussed amongst authors and a consensus was reached.
Outcomes
Concurrent infections were defined as any HPV infection(s) occurring in both the oral cavity/oropharynx and cervix simultaneously due to synchronous site testing. If HPV infection was absent at either or both sites, then any infection was not considered concurrent. “Dual-site infections” were defined as any HPV infections occurring in both the oral cavity/oropharynx and cervix at different times due to asynchronous (i.e., non-simultaneous) testing of the two sites. Concordant infections were identified in women who shared at least one HPV type across oral and cervical sites at any time (synchronously or asynchronously) (Table 1).
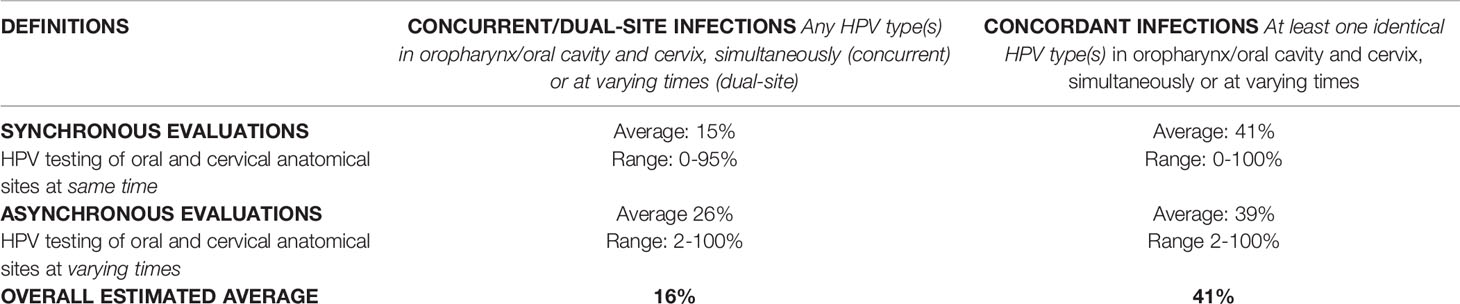
Table 1 Definitions and summary statistics for a 1990-2021 systematic review of oral-cervical human papillomavirus (HPV) infection/cancer rates in women.
Studies investigating oral-cervical cancer diagnoses from registry data were also considered. We included studies that examined the occurrence of cervical cancer after a primary diagnosis of HPV-related oral cancer and occurrence of HPV-related oral cancer after a primary diagnosis of cervical cancer. Infections were also included here, if documented accordingly in the registries/databases.
Categories
Eligible studies were divided into three categories based on the timing of HPV evaluation at both sites (i.e., synchronously, asynchronously, or cancer diagnoses). Synchronous HPV evaluation studies actively collected oral and cervical samples and tested them both for HPV DNA at the same visit (with one study testing oral samples within three weeks of cervical samples). Asynchronous HPV evaluation studies either HPV-tested the oral and cervical sites at separate visits or one anatomical site was previously diagnosed with a HPV-related cancer and the other anatomical site was actively tested for HPV infection during the study. Cancer diagnoses only studies relied on data from cancer registries or medical records to determine prior primary and secondary cancer diagnoses of the oral cavity/oropharyngeal region and cervix.
Statistical Analyses
When individual synchronous and asynchronous studies presented sufficient results, we summarized concurrent/dual-site infection data as percentages of women with any oral-cervical HPV infections at any time. HPV type concordance data was summarized as percentages of women with the same oral-cervical HPV type(s) at any time. Overall concurrent/dual-site and concordant oral-cervical HPV infection rates were determined by averaging respective individual study percentages (Table 1). For cancer diagnoses studies, we summarized the overall rates of secondary cervical and/or oral cancers (number of cases per 10,000 women) and reported the standardized incidence ratios (SIR) to indicate whether the age-adjusted observed cancer cases were higher than expected for individual study populations. Results were not pooled across studies but stated as ranges.
Results
A total of 8768 papers were identified through PubMed, EMBASE, Ovid Medline, and Web of Science databases after removing duplicates (Figure 1). Titles, abstracts, and full-text papers were screened, 8654 did not meet the eligibility criteria and subsequently were removed. Specifically, 1842 (21%) studies were not topic relevant, 3071 (35%) studies evaluated HPV only in the oropharynx/oral cavity [2289 (26%) studies] or cervix [782 (9%) studies], 5 (0.06%) studies did not relate oral cancers to HPV status, 3412 (39%) studies were not original research, and 324 (4%) studies were case reports or series. A total of 114 papers were included.
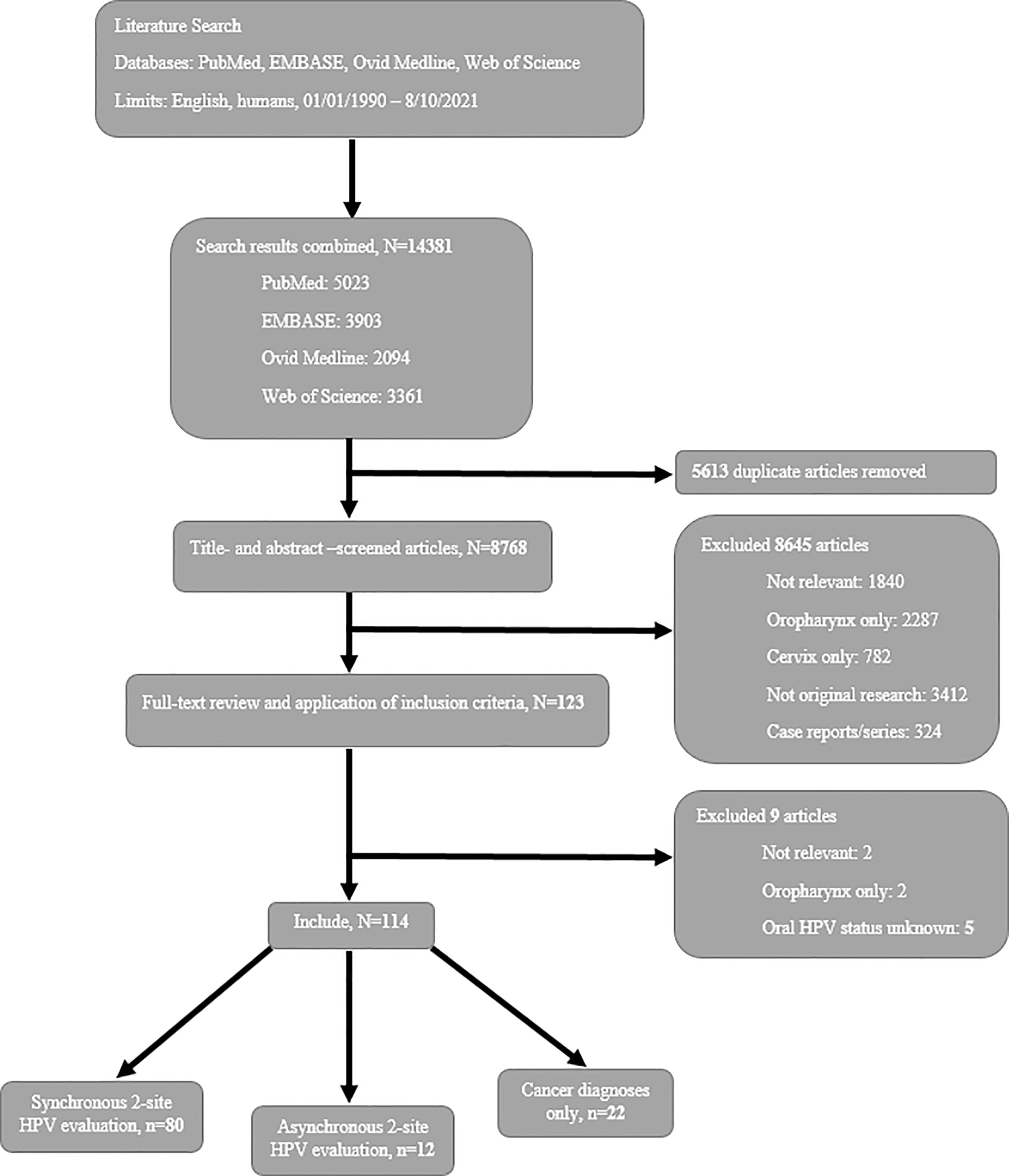
Figure 1 Flow diagram of study selection for a systematic review of oral-cervical HPV infection/cancer epidemiology literature.
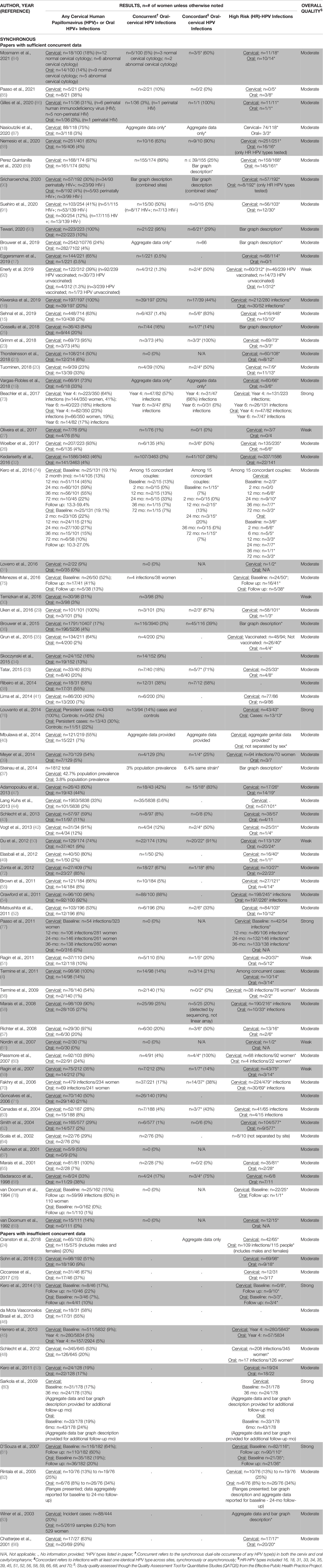
Most studies (n=80, 70%) (4, 15–93) evaluated oral and cervical sites with synchronous HPV testing; the remaining studies were divided between asynchronous evaluations (n=12, 11%) (94–105) and cancer diagnoses only (n=22, 19%) (106–127). Combining synchronous (4, 15–93) and asynchronous (94–105) study data, the overall estimate of oral and cervical dual-site HPV infections was 16% and the overall estimate of oral-cervical HPV type concordance among dually-infected women was 41% (Table 1). Among cancer diagnoses only studies (106–127), the incidence of a secondary cervical cancer diagnosis ranged from as few as 4.5/10,000 to as many as 192.5/10,000 women; the incidence of a secondary oral HPV-related cancer ranged from 1.0 to 45.8 per 10,000 women.
Synchronous Oral-Cervical HPV Testing (n=80)
Eighty studies synchronously evaluated HPV-related infections at both the oral and cervical sites (4, 15–93) (Table 2A). Cervical samples were collected by a variety of measures with most studies using swabs or a combination of methods; oral samples were collected mainly by rinses or swabs. HPV DNA detection was most often determined through polymerase chain reaction (PCR) (n=60, 75%) (4, 16–20, 24–29, 33–35, 38, 39, 42–49, 52–56, 61–65, 67–76, 78–89, 91–93).
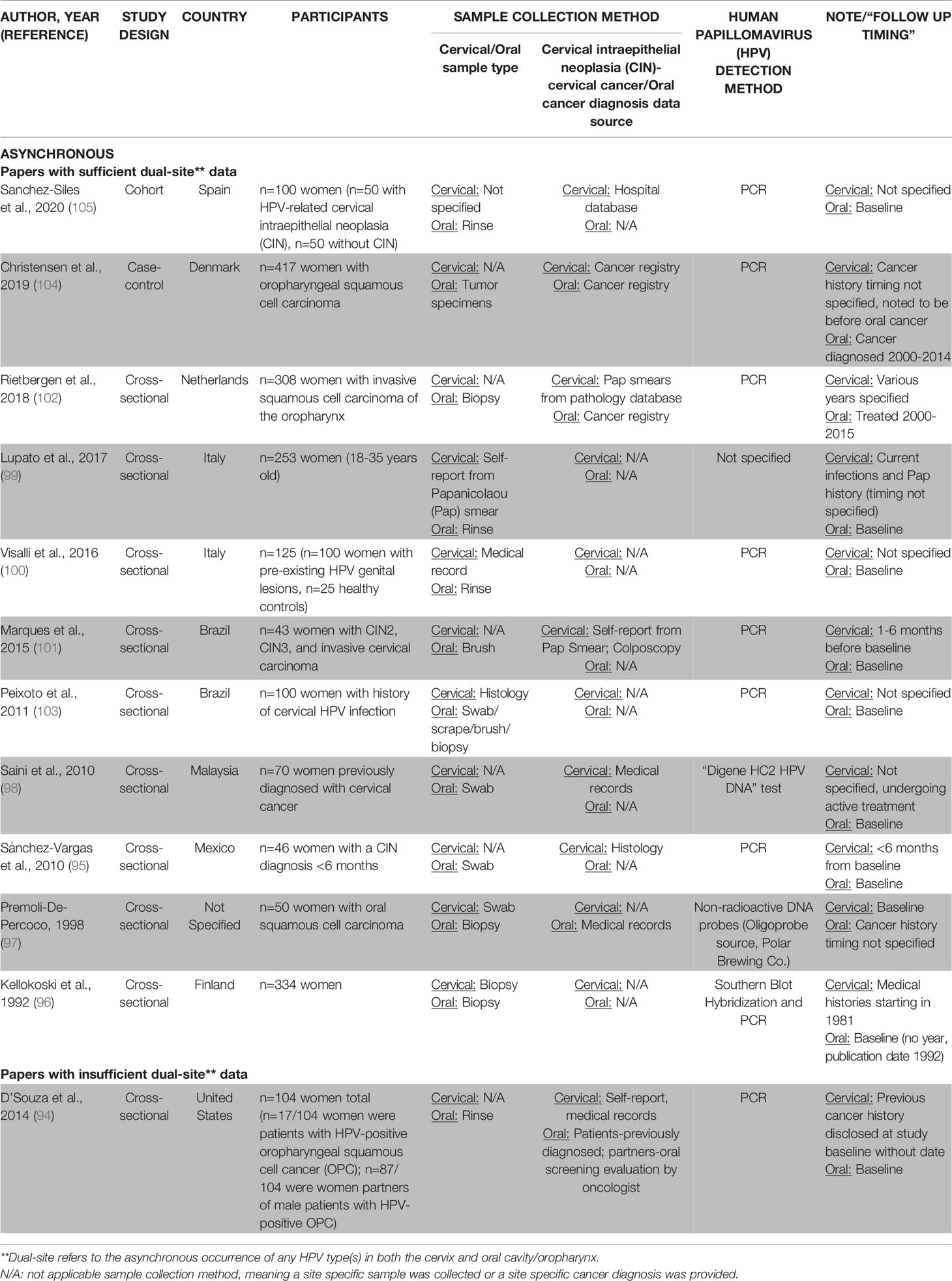
Table 2B Description of methodology used in asynchronous oral-cervical HPV evaluation papers (n=12).
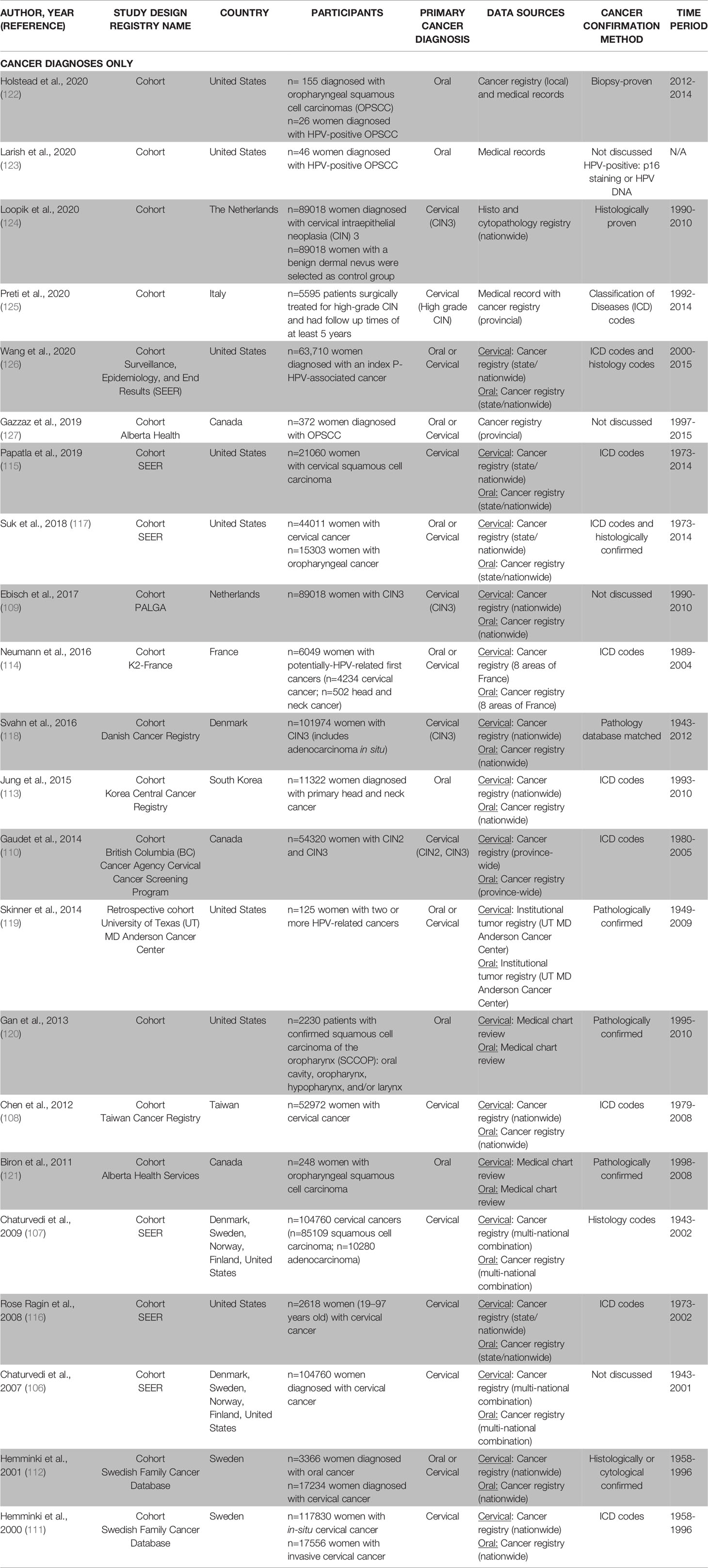
Table 2C Description of methodology used in oral-cervical human papillomavirus (HPV)-related cancer diagnoses only papers (n=22).
Overall rates of cervical HPV+ and oral HPV+ cases varied by study (Table 3A). Almost all studies found higher rates of cervical HPV+ than oral HPV+ (n=76/80, 95%) (4, 15–19, 21–67, 69–79, 81–84, 86–93). On average, 53% of women were HPV+ in the cervix; an average of 15% of women were HPV+ in the oral cavity/oropharyngeal region. Most papers included high-risk HPV type results from DNA genotyping (n=74/80, 93%) (4, 15–29, 31–33, 35–37, 39–45, 47–66, 68–70, 72–93) with 82% (n=61/74) (4, 15–26, 29, 31, 33, 35–37, 40, 42, 44, 45, 47–52, 54–62, 65, 66, 69, 70, 72, 74–79, 81, 83–93) reporting exact HPV types observed.
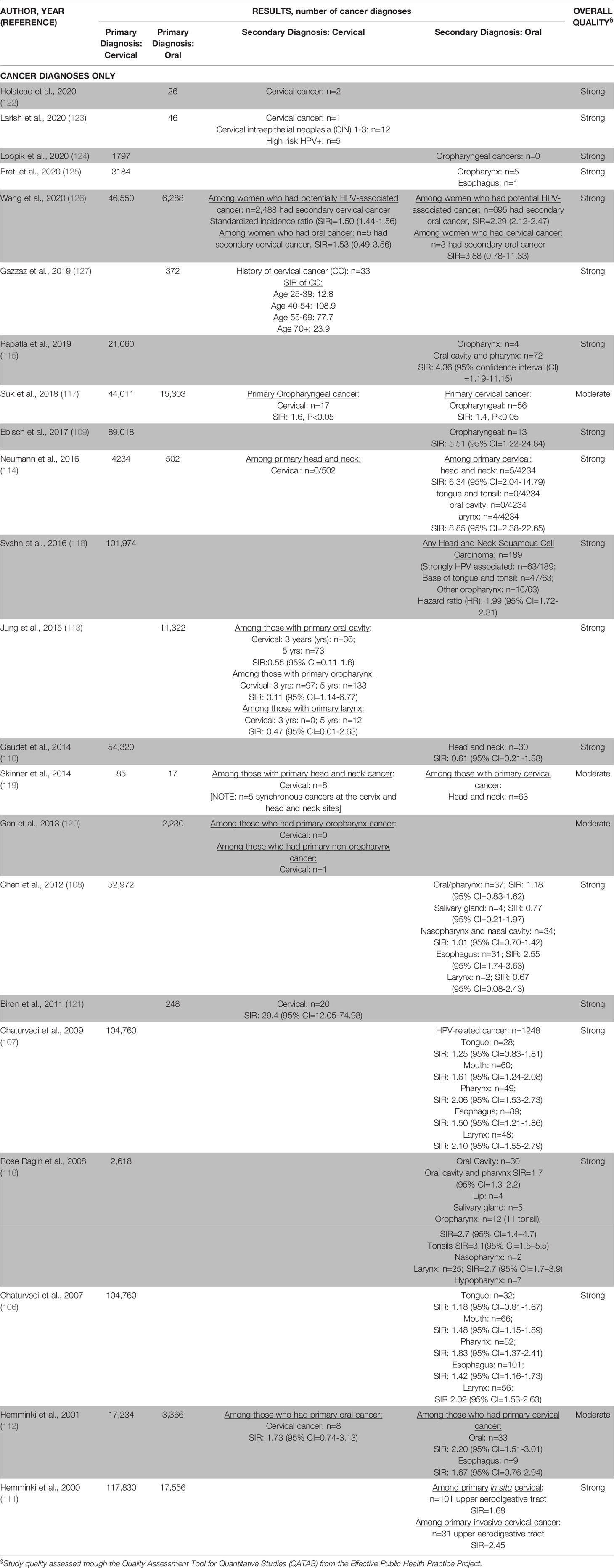
Table 3C Results of the oral-cervical human papillomavirus (HPV)-related cancer diagnoses only papers (n=22).
Eighty-three percent of all synchronous testing studies (n=67/80, Table 3A) (4, 15–21, 23, 25–27, 29–44, 47, 49–52, 54–65, 67–78, 84–93) provided some form of data on concurrent oral-cervical HPV+ cases. Concurrent oral and cervical HPV infection rates could be calculated for most, but not all, of these studies (n=59/67, 88%) (4, 15–17, 20, 21, 23, 25–27, 29–39, 41–44, 47, 49–52, 54–65, 67–72, 76–78, 84–89, 91–93). The calculated concurrent rates ranged from 0% to 95%, depending on the study. On average, 15% of women had HPV infections occurring concurrently in both sites. Most rates of concurrent oral and cervical HPV infections were ≤10% (n=39/59, 66%) (15, 17, 20, 21, 23, 26, 27, 29–32, 34–37, 39, 41, 43, 44, 49, 51, 52, 55, 56, 59–65, 67, 69, 77, 78, 84–86, 92). Only four studies (7%) (54, 72, 89, 93) had concurrent oral and cervical HPV infection rates over 65%.
Among the 67 studies identifying concurrent oral and cervical HPV+ cases, 70% (n=47/67) (4, 15, 16, 18–20, 23, 25–27, 29, 32, 33, 36–40, 42, 43, 47, 50–52, 56–60, 62, 63, 65, 68, 70, 72–74, 84–93) determined concordance in oral-cervical HPV types (Table 3A). For studies reporting overall rates (n=40/47, 85%) (4, 15, 16, 20, 23, 25–27, 29, 32, 33, 36–39, 42, 43, 47, 50–52, 56–60, 62, 63, 65, 68, 70, 72, 84–86, 88, 89, 91–93), concordance in oral and cervical HPV infection types ranged from 0% to 100%, with an average of 41% of the women having infections of the same type in both sites. More than half of the studies had oral-cervical HPV type concordance rates of <50% (n=23/40, 58%) (4, 16, 25, 27, 32, 36, 37, 39, 43, 51, 52, 56, 58, 59, 62, 63, 65, 70, 72, 85, 89, 91, 93), yet seven studies reported concordance rates of >80% (15, 23, 47, 50, 60, 86, 88).
Asynchronous Oral-Cervical HPV Testing (n=12)
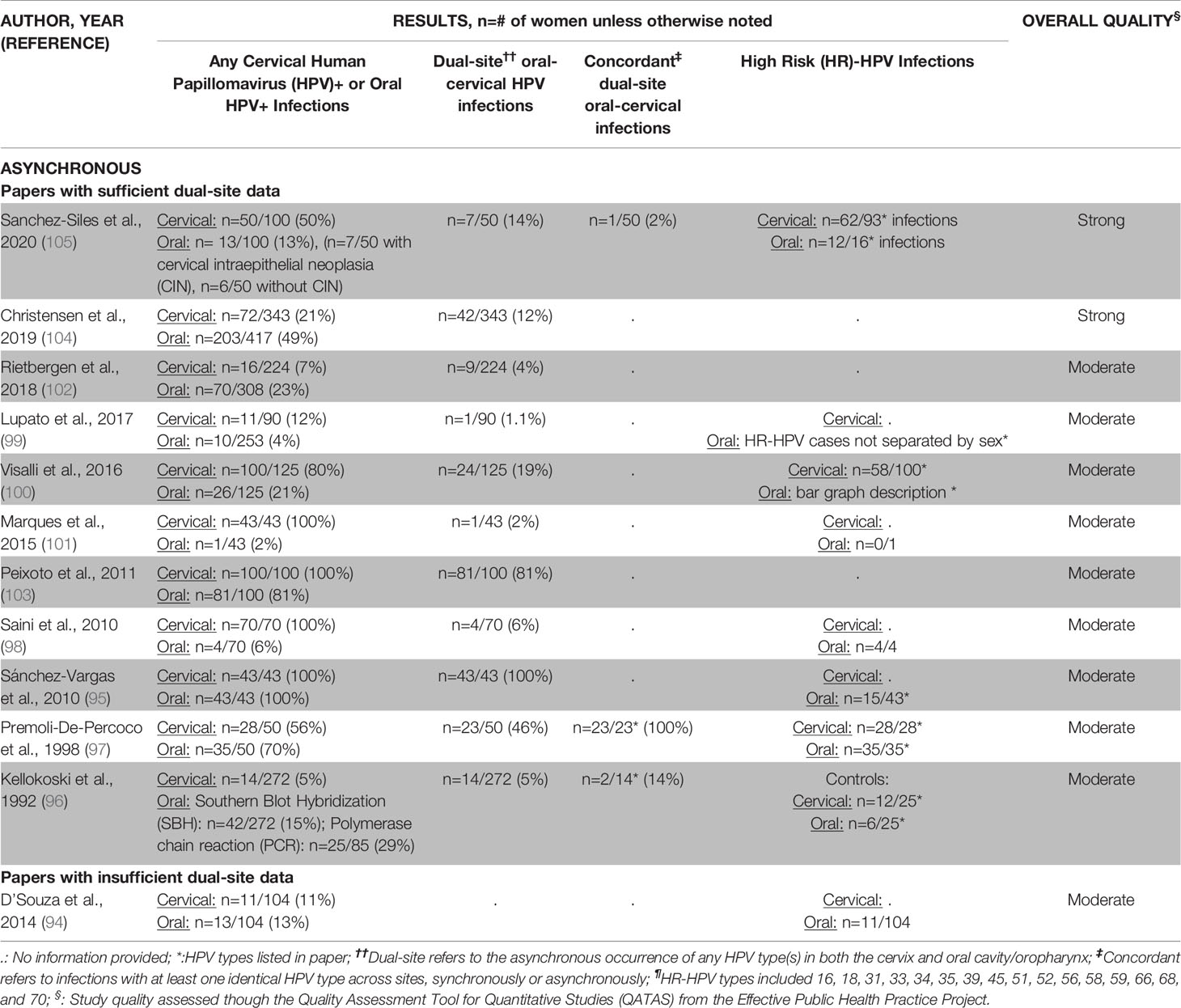
Twelve studies evaluated HPV-related infections of the oral cavity/oropharynx and cervix asynchronously (94–105) (Table 2B). Most studies sampled women with cervical infections for oral HPV (n=7/12, 58%) (95, 96, 98, 100, 101, 103, 105). Cervical HPV data collection usually relied on medical records (94–96, 98, 100–105) while at least some oral samples were actively evaluated for HPV during the study (94–105). Oral HPV sampling methodology used a buccal (brush) sample (95, 98, 101, 103), biopsied lesions (96, 97, 102, 104), or a gargle/rinse sample (94, 99, 100, 105). Cervical and oral HPV DNA was often detected by PCR (94–96, 100–105).
Half of the asynchronous studies (n=6/12, 50%) showed that more women were HPV+ in the cervix than in the oral cavity/oropharynx (98–101, 103, 105) while essentially the other half (n=5/12, 42%) found the opposite (94, 96, 97, 102, 104). Most asynchronous studies (n=9/12, 75%) provided some data regarding the high-risk HPV types (94–101, 105) (Table 3B), tending to only specify when high-risk oral HPV was found (n=5/9, 56%) (94, 95, 98, 99, 101). Due to HPV assessments occurring at different times, studies rarely (n=4/9, 44%) reported both the specific high-risk oral and cervical HPV types found at the person level (96, 97, 100, 105).
Most asynchronous studies (n=11/12, 92%) differentiated between women with and without dual-site oral and cervical HPV infections at any time (95–105) (Table 3B). One woman (2%) to as many as all (100%) women asynchronously tested positive for HPV in both the oral cavity/oropharynx and cervix. The overall dual-site oral and cervical HPV+ infection rate estimate was 26% (95–105). On average, when women had (pre)existing cervical infections (95, 98, 100, 101, 103, 105), almost twice as many were dually-infected with HPV in the oral cavity/oropharynx (avg.: 37%, range: 2-100%) as compared to women with (pre)existing oral HPV infections who were also cervical HPV+ (avg.: 21%, range: 4-46%) (97, 102, 104). Women without a known, prior oral or cervical HPV infection were not as likely to be dually HPV infected at both sites, with rates ranging from 1.1-5% (96, 99).
Among studies where women were known to be dually-infected with oral and cervical HPV, 27% (n=3/11) measured concordance in HPV types across both sites at any time (96, 97, 105). On average, 39% of asynchronous oral and cervical infections within women had an HPV type in common (96, 97, 105) (Table 3B). Women who had an HPV+ oral cancer and a cervical HPV infection present had the greatest concordance in oral-cervical HPV types (100%) (96, 97, 105). Rates of concordant oral-cervical HPV types were lower in studies where not all women had prior HPV-related infections (2-14%) (96, 97, 105).
Cancer Diagnoses Only (Primary Oral/Cervical, Secondary Cervical/Oral, n=22)
Twenty-two retrospective studies focused on the diagnosis of a secondary cervical or oral cancer after a primary cancer diagnosis of oral or cervical cancer (106–127) (Table 2C). Although we specifically included studies focused on HPV-related oral cancers, the sites of oral cancers varied across studies (e.g., some studies included oropharynx, oral cavity and pharynx, some only included oropharyngeal, and some vaguely defined HPV-related head and neck sites). Five studies examined the risk of a secondary cervical cancer after a primary diagnosis of oral cancer (113, 120–123). Half of the studies (n=11/22, 50%) examined the risk of a secondary oral cancer diagnosis after a primary diagnosis of a cervical cancer (n=6) (106–108, 111, 115, 116) or a cervical intraepithelial neoplasia (CIN) (n=5) (109, 110, 118, 124, 125). Six studies investigated the risk of a secondary cervical and/or oral cancer after a primary diagnosis of an oral and/or cervical cancer (112, 114, 117, 119, 126, 127). Most studies utilized data from country or state level cancer registries to monitor disease surveillance (n=15/22, 68%) (106–118, 124, 126); three studies conducted medical chart reviews (120, 121, 123); four studies collected at least some data from institutional or provincial tumor registries (119, 122, 125, 127).
Among women with a primary diagnosis of oral cancer, the number of secondary cervical cancers was lowest among medical record-based studies (122, 123), followed by provincial registries (121, 127), and highest among national studies (112, 113, 117, 120, 126). National studies reported that the incidence of a secondary cervical cancer ranged from 4.5-192.5 per 10,000 women (112, 113, 117, 120, 126) (Table 3C). The observed cases of a secondary cervical cancer were higher than expected in five studies with the SIR generally ranging from 1.4-29.4 (113, 117, 120, 121, 127). Interestingly, Gan et al. found that the SIR of a secondary cervical cancer was smaller among women diagnosed with HPV-related oral cancers (SIR range: 3.3-4.0) compared to women diagnosed with non-HPV-related oral cancers (SIR range: 8.3-12.8) (120). Two studies did not observe any differences between the numbers of observed and expected cases of a secondary cervical cancer among women who had a primary oral cancer (112, 126).
Among women with a primary cervical cancer, one provincial-level registry found very few cases of secondary oral cancers (125). Nationally, studies that reported the incidence of a secondary oral/head and neck cancer ranged from 1.0-45.8 per 10,000 women (106–108, 110–112, 114–117, 126); one study had an incidence of zero for secondary oropharyngeal cancers (124) (Table 3C). The incidence rates varied due to differences in included oral cancer sites across studies. The observed cases of a secondary oral cancer were higher than expected in almost all national studies, including primary CIN3 cases, with the SIR ranging from 1.4-6.3 (106–109, 111, 112, 114–118, 126).
Quality Assessment
For the quality assessment of the 114 included papers based on the QATQS tool, 26 studies (23%) were classified as strong (73, 76, 77, 79–81, 104–111, 113–116, 118, 121–127), 81 studies (71%) were moderate (4, 15–26, 28, 29, 31–49, 52–58, 60, 62–72, 74, 75, 78, 82–91, 93–103, 112, 117, 119, 120), and 7 (6%) were weak (27, 30, 50, 51, 59, 61, 92) (Tables 3A–C). The most common component rated as weak was study design (n=79, 69%) (4, 15–72, 84–103); only a few studies used a case-control design (n=2, 2%) (76, 104) or cohort design (n=33, 29%) (73–75, 77–83, 105–127) with the majority being cross-sectional designs (n=79, 69%) (4, 15–72, 84–103). In addition, few studies randomly selected participants for inclusion from a comprehensive list of the target population (n=26, 23%) (18, 21, 24, 32, 34, 36, 37, 44, 45, 83, 92, 104, 108, 109, 111–113, 115–119, 121, 124, 126, 127). This contributed to most studies being classified as ‘moderate’ for selection bias (n=99, 87%) (4, 15–26, 28–33, 35–43, 46–49, 51–58, 60–77, 79–82, 84–91, 93–103, 105–107, 109–116, 118–123, 125). For data collection within synchronous and asynchronous HPV testing studies, some studies did not specify an HPV infection sample collection method, so the validity and reliability were unknown or they relied on self-reported HPV infections (n=10/92, 11%) (4, 33, 40, 55, 63, 64, 73, 94, 99, 101). For the last criteria, withdrawals and dropouts, few cohort studies described the number of and/or reasons for participants being lost-to-follow-up (n=8/33, 24%) (73, 74, 78–81, 83, 108).
Discussion
After an expansive search of four databases for studies of dual-site oral and cervical HPV infections/cancers, we included 114 papers that evaluated the sites synchronously (n=80) (4, 15–93), asynchronously (n=12) (94–105), or by cancer diagnoses only (n=22) (106–127). This systematic review enhances the previous meta-analysis (4) by including more publication years, comprehensive search terms, databases, general oral HPV testing approaches, and formal study quality assessments of included studies using QATQS. We found that studies evaluating both oral and cervical HPV infections had cervical HPV+ rates that were higher than oral HPV+ rates.
The reporting of dual-site oral and cervical HPV infection rates was wide-ranging. On average, 15% of infections occurred concurrently in the oral cavity/oropharynx and cervix. Among concurrent oral-cervical HPV+ cases, HPV types were concordant across the two sites in an average of 41% of women. Asynchronous dual-site (oral-cervical) HPV infection rates also varied, spanning from 1.1% to 100%, with an average of 26% of study populations testing positive for both oral and cervical HPV at different times. Oral-cervical HPV type concordance was either very low (2%) or high (100%) for these asynchronously tested and dually-infected women, producing an average concordance rate of 39%. Combining synchronous and asynchronous oral-cervical HPV testing data, it was estimated that 16% of women were dually infected and 41% of the dually infected women had at least one concordant HPV type across sites. Most cancer diagnoses only studies reported an increased risk for a secondary cervical and/or oral cancer, resulting in incidence spanning 1.0-192.5/10,000 women. Regardless of timing, most studies were cross-sectional (n=79, 69%) (4, 15–72, 84–103) and therefore achieved an overall moderate rating with QATQS scoring criteria (n=81, 71%) (4, 15–26, 28, 29, 31–49, 52–58, 60, 62–72, 74, 75, 78, 82–91, 93–103, 112, 117, 119, 120).
Oral HPV infection can be especially difficult to detect which may explain the lower oral-cervical HPV type concordance rates or lack of significant findings in the reviewed studies. Saliva continuously rinses the mouth so it may aid in regional virus clearance, making oral HPV more transient than HPV infections at other sites. Most people clear oral HPV infections, often in as little as a few months, which means it can easily be missed (3). HPV detection in the oral cavity is not indicative of oropharyngeal cancer either. The virus tends to inhabit the oropharynx (e.g., tonsils), so if only buccal samples are being tested, HPV may go undetected. Rinsing or gargling within the oral cavity may only partially capture any HPV inhabiting the oropharyngeal region (3, 9, 99, 128). HPV testing materials were originally designed for cervical HPV; although repeatedly shown to be capable of HPV detection at other sites, materials might not be as effective at identifying oral HPV (94, 129). Many existing oral cancer diagnostic tests are questionable, lacking standard diagnostic protocols. New diagnostic approaches are evolving but are not yet validated (3). With the increase in oral HPV cancers, oral HPV sampling and testing methodology should improve over time.
Other reasons for non-significant findings within studies might be site-independent or biological in nature. Virus detection methods (e.g., assay, technique) vary in sensitivity levels and are often HPV type specific, so the chosen HPV test may not be able to detect the HPV type present, suggesting no infection (98–100, 103, 129–131). Poor or inappropriate sample collections at either site might also hinder a positive HPV result (98). HPV-infected, but otherwise healthy people can test negative for the virus and/or may develop HPV type-specific immunity at other uninfected mucosal sites (96, 98, 132). It is also biologically plausible for a cervical HPV+ woman to not be oral HPV+ given that cervical-oral HPV transmission between heterogeneous partners is common, but oral-oral HPV transmission is infrequent (94).
The current systematic review expands upon the narrowly-focused topic-related meta-analysis of 2010 (4) with the inclusion of additional oral-cervical HPV studies and their quality assessments. We identified a significant gap in the oral-cervical HPV literature with HPV type concordance between sites being understudied, highlighting the need for better HPV data collection and reporting efforts. HPV type was frequently missing. Synchronous studies usually provided HPV type data for one site and only HPV+/- status for the other site, despite data for both sites being collected concurrently. Asynchronous studies recruiting women with (pre)existing HPV conditions tended to only report basic HPV status for the secondary anatomical site. Cancer diagnoses only or registry-based studies did not collect any HPV type information. When HPV types were reported, data tended to be presented at aggregate levels with either totals or broad categories by anatomical site and/or HPV type (e.g., HPV+/- status only, HPV16/not HPV16, oncogenic/not oncogenic, groups of multiple HPV types).
Additional problems with vague data reporting were observed, irrespective of whether or not studies involved synchronous or asynchronous oral and cervical HPV testing or cancer diagnoses only. Many studies provided a general summary statement regarding the oral-cervical HPV relationship across sites with the corresponding statistics (e.g., odds ratios, (Cohen) kappa statistics, p-values). Enumeration of sub-sites of oral cancers made it difficult to calculate incidence consistently among cancer diagnoses only papers. Information on HPV type was represented as ranges or in bar graphs, which made it difficult to extract specific values and interpret results. Still others did not stratify oral HPV results by sex so cervical HPV data could not be cross-compared with respective oral samples in females.
Without specific HPV type information presented at the person-level for both the oral cavity/oropharynx and cervix, concurrent/dual-site versus concordant infections could not be elucidated. Additionally, not all dual-site HPV+ studies, especially asynchronous and cancer diagnoses only papers, discussed the potential for concurrent infections which made it difficult to determine if the identified oral and cervical HPV infections could be related. Few studies listed participant data individually, making it unclear if a participant had the same infection in both sites. In turn, oral-cervical HPV type concordance data was missing or could not be deduced for more than half of the papers (n=62/114, 54%) (17–19, 22, 24, 28, 30, 34, 35, 40, 41, 44–46, 48, 49, 53–55, 64, 66, 71, 75, 76, 79–83, 87, 90, 94, 95, 98–104, 106–127).
Lack of HPV type details also made it difficult to describe the oral-cervical HPV infection epidemiology more generally. About 10% of synchronous and asynchronous studies quantified the number of HPV infections (vs. HPV+ women) to account for multiple infections in women, which is an important detail to note, but complicated the estimation of the disease burden. More than 10% of synchronous and asynchronous studies did not discuss if any detected HPV types were high-risk. The interpretation of cancer diagnoses only papers could not be compared collectively with synchronous and asynchronous papers due to different effect estimates being used (i.e., SIR). Additionally, almost one-third of cancer diagnoses only papers (n=7/22, 32%) were missing SIR values (118–120, 122–125).
The current systematic review also had its limitations. Unpublished works and conference abstracts were excluded, potentially missing some information, however, we evaluated many peer-reviewed publications with broad search terms. Only papers written and published in English were included so there could be a lack of generalizability to international research. However, 94 international studies were captured with our search criteria (or 82.5% of all papers included in this systematic review were conducted outside the US). The inability to decipher the vagueness in oral cancer types (i.e., HPV vs. tobacco/alcohol related) and/or a lack of differentiation between HPV infection sites (e.g., oral-cervical data combined within multi-site results) potentially prevented some topic-relevant papers from being included in the current review. Regardless, studies had to justify oral cancers as potentially HPV-related to be included. Using strict review criteria, the current review included studies focused on HPV-related oral and cervical infections/cancers only, minimizing misclassification bias concerns. The systematic review also relied on literature-reported “oral HPV-related cancer” terminology to portray results. Inconsistent use of varying terms across publications impeded the summarization of results across studies. The standardized QATQS tool could not be fully utilized due to the topic-related nature of this systematic review relying only on observational studies.
To better understand the epidemiology of oral HPV transmission moving forward, data collection efforts need to be improved to include standardized reporting of HPV type data. Individual-level, site-specific HPV type data should be reported for every sample evaluated, especially when investigators are already using HPV DNA tests that provide such detailed information. Cancer diagnoses studies/registries should include a repository of HPV-evaluated bio-specimens such that site-specific HPV types can be identified. Broad categories, aggregated data, summary statistics, and analyses without stratum-specific results only provide an overview of a potential association of HPV infections/cancers across sites without being able to hone in on possible transmission routes which can only be divulged if HPV types are compared.
Detailed documentation of the timing of HPV site-specific sampling and evaluations are also needed to aid in determining concurrent HPV infections or the likelihood of an association between dual/multi-site HPV infections. Generation of a special access database to pool this person-level, site-specific HPV infection/cancer data would facilitate the additional analyses needed to understand the epidemiology of HPV transmission between sites. Better understanding site-specific HPV infection/cancer biology, including transmission routes, can further aid in preventing and minimizing future disease burdens.
In conclusion, few reviewed studies utilized strong epidemiological methodologies to determine HPV type concordance in dual-site oral and cervical infections. The results from this systematic review are inconclusive given the heterogeneity of included studies with wide-ranging oral-cervical HPV infection/cancer rates. Cervical HPV+ infection/cancer diagnoses tended to be more prevalent in women than oral HPV+ infections/cancers were. Given that these dual-site infection rates can vary significantly by female population and no oral HPV+ cancer screening approach exists, oral HPV+ cancer incidence may continue to increase unchecked. Additional studies identifying specific HPV infection types, both concurrently and over time, at multiple biological sites (especially oral and cervical, but also vaginal, vulval, penial, and anal) within women and men are needed to better understand how HPV is transmitted and determine any relationships between potentially HPV-related cancer sites. Pooling of these individual-level study results into a special access HPV database could facilitate future research investigations. From there, risk factors and populations with potentially increased oral and/or cervical HPV cancer risks could more easily be identified and incorporated into future public health prevention and control efforts, locally and globally, to reduce the HPV-related cancer burden in men and women.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
All authors agree to be accountable for the content of the work. KJ: validation, formal analysis, investigation, data curation, writing-original draft, writing-review and editing, and project administration. CB: validation, formal analysis, investigation, data curation, writing-original draft, writing-review and editing, and visualization. XZ: validation, formal analysis, investigation, data curation, writing-original draft, and writing-review and editing. EP: conceptualization, methodology, resources, writing-review and editing, and supervision. All authors contributed to the article and approved the submitted version.
Funding
KJ (P01 CA229143-S1) and XZ (F99CA253745-01) both have National Cancer Institute (NCI) funding through the National Institutes of Health (NIH). CB is funded through The Ohio State University Comprehensive Cancer Center.
Conflict of Interest
EP receives grant funding through the university from Pfizer and Merck Foundation.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.848628/full#supplementary-material
References
1. Szymonowicz KA, Chen J. Biological and Clinical Aspects of HPV-Related Cancers. Cancer Biol Med (2020) 17:864–78. doi: 10.20892/j.issn.2095-3941.2020.0370
2. de Martel C, Plummer M, Vignat J, Franceschi S. Worldwide Burden of Cancer Attributable to HPV by Site, Country and HPV Type. Int J Cancer (2017) 141:664–70. doi: 10.1002/ijc.30716
3. Candotto V, Lauritano D, Nardone M, Baggi L, Arcuri C, Gatto R, et al. HPV Infection in the Oral Cavity: Epidemiology, Clinical Manifestations and Relationship With Oral Cancer. Oral Implantol (Rome) (2017) 10:209–20. doi: 10.11138/orl/2017.10.3.209
4. Termine N, Giovannelli L, Matranga D, Caleca MP, Bellavia C, Perino A, et al. Oral Human Papillomavirus Infection in Women With Cervical HPV Infection: New Data From an Italian Cohort and a Metanalysis of the Literature. Oral Oncol (2011) 47:244–50. doi: 10.1016/j.oraloncology.2011.02.011
5. Bonde JH, Sandri MT, Gary DS, Andrews JC. Clinical Utility of Human Papillomavirus Genotyping in Cervical Cancer Screening: A Systematic Review. J Low Genit Tract Dis (2020) 24:1–13. doi: 10.1097/lgt.0000000000000494
6. Shrestha AD, Neupane D, Vedsted P, Kallestrup P. Cervical Cancer Prevalence, Incidence and Mortality in Low and Middle Income Countries: A Systematic Review. Asian Pac J Cancer Prev (2018) 19:319–24. doi: 10.22034/apjcp.2018.19.2.319
7. Wheeler BS, Rositch AF, Poole C, Taylor SM, Smith JS. Patterns of Incident Genital Human Papillomavirus Infection in Women: A Literature Review and Meta-Analysis. Int J STD AIDS (2019) 30:1246–56. doi: 10.1177/0956462418824441
8. Berman TA, Schiller JT. Human Papillomavirus in Cervical Cancer and Oropharyngeal Cancer: One Cause, Two Diseases. Cancer (2017) 123:2219–29. doi: 10.1002/cncr.30588
9. Elrefaey S, Massaro MA, Chiocca S, Chiesa F, Ansarin M. HPV in Oropharyngeal Cancer: The Basics to Know in Clinical Practice. Acta Otorhinolaryngol Ital (2014) 34:299–309.
10. Bruni L, Albero G, Serrano B, Mena M, Gomez D, Munoz J, et al. ICO/IARC Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in the World (Accessed April 22, 2020). Summary Report 17 June 2019.
11. IARC. Cancer Today: Cancer Fact Sheets (2020). Available at: https://gco.iarc.fr/ (Accessed January 19, 2021).
12. Sawaya GF, Smith-McCune K, Kuppermann M. Cervical Cancer Screening: More Choices in 2019. JAMA (2019) 321:2018–9. doi: 10.1001/jama.2019.4595
13. Burd EM. Human Papillomavirus and Cervical Cancer. Clin Microbiol Rev (2003) 16:1–17. doi: 10.1128/cmr.16.1.1-17.2003
14. Thomas BH. A Process for Systematically Reviewing the Literature: Providing the Research Evidence for Public Health Nursing Interventions. Worldviews Evidence-Based Nurs (2004) 1:176–84. doi: 10.1111/j.1524-475X.2004.04006.x
15. Sehnal B, Zikan M, Nipcova M, Dusek L, Cibula D, Slama J. The Association Among Cervical, Anal, and Oral HPV Infections in High-Risk and Low-Risk Women. Eur J Obstet Gynecol Reprod Biol (2019) 4:1–5. doi: 10.1016/j.eurox.2019.100061
16. Kiwerska K, Jozefiak A, Markowska J, Kedzia W, Jackowska J, Wierzbicka M. Oral-Genital Human Papillomavirus Infection in Polish Couples: Frequent Detection of HPV 42. BMC Infect Dis (2019) 19:122. doi: 10.1186/s12879-018-3645-0
17. Eggersmann TK, Sharaf K, Baumeister P, Thaler C, Dannecker CJ, Jeschke U, et al. Prevalence of Oral HPV Infection in Cervical HPV Positive Women and Their Sexual Partners. Arch Gynecol Obstet (2019) 299:1659–65. doi: 10.1007/s00404-019-05135-7
18. Brouwer AF, Eisenberg MC, Carey TE, Meza R. Multisite HPV Infections in the United States (NHANES 2003–2014): An Overview and Synthesis. Prev Med (2019) 123:288–98. doi: 10.1016/j.ypmed.2019.03.040
19. Vargas-Robles D, Magris M, Morales N, de Koning MNC, Rodriguez I, Nieves T, et al. High Rate of Infection by Only Oncogenic Human Papillomavirus in Amerindians. mSphere (2018) 3:1–13. doi: 10.1128/mSphere.00176-18
20. Tuominen H, Rautava S, Syrjanen S, Collado MC, Rautava J. HPV Infection and Bacterial Microbiota in the Placenta, Uterine Cervix and Oral Mucosa. Sci Rep (2018) 8:11. doi: 10.1038/s41598-018-27980-3
21. Thorsteinsson K, Storgaard M, Katzenstein TL, Ladelund S, Ronsholt FF, Johansen IS, et al. Prevalence of Cervical, Oral, and Anal Human Papillomavirus Infection in Women Living With HIV in Denmark - The SHADE Cohort Study. J Clin Virol (2018) 105:64–71. doi: 10.1016/j.jcv.2018.05.010
22. Sohn AH, Kerr SJ, Hansudewechakul R, Gatechompol S, Chokephaibulkit K, Dang HLD, et al. Risk Factors for Human Papillomavirus Infection and Abnormal Cervical Cytology Among Perinatally Human Immunodeficiency Virus-Infected and Uninfected Asian Youth. Clin Infect Dis (2018) 67:606–13. doi: 10.1093/cid/ciy144
23. Grimm D, Woelber L, Prieske K, Schmalfeldt B, Kurti S, Busch CJ, et al. Comparison of PapilloCheck and Linear Array to Detect and Differentiate Human Papillomaviruses in Cervical and Tonsillar Smears From Females With Cervical Intraepithelial Lesions. Eur J Microbiol Immunol (2018) 8:107–11. doi: 10.1556/1886.2018.00018
24. Cranston RD, Cespedes MS, Paczuski P, Yang M, Coombs RW, Dragavon J, et al. High Baseline Anal Human Papillomavirus and Abnormal Anal Cytology in a Phase 3 Trial of the Quadrivalent Human Papillomavirus Vaccine in Human Immunodeficiency Virus-Infected Individuals Older Than 26 Years: ACTG 5298. Sex Transm Dis (2018) 45:266–71. doi: 10.1097/olq.0000000000000745
25. Cossellu G, Fedele L, Badaoui B, Angiero F, Farronato G, Monti E, et al. Prevalence and Concordance of Oral and Genital HPV in Women Positive for Cervical HPV Infection and in Their Sexual Stable Partners: An Italian Screening Study. PloS One (2018) 13:e0205574. doi: 10.1371/journal.pone.0205574
26. Woelber L, Breuer J, Meyer T, Vettorazzi E, Prieske K, Bohlmann I, et al. Oral Human Papillomavirus in Women With High-Grade Cervical Intraepithelial Neoplasia. J Low Genit Tract Dis (2017) 21:177–83. doi: 10.1097/lgt.0000000000000313
27. Oliveira LH, Santos LS, Silva CO, Augusto EF, Neves FP. Papillomavirus Infections in the Oral and Genital Mucosa of Asymptomatic Women. Braz J Infect Dis (2017) 21:88–91. doi: 10.1016/j.bjid.2016.08.015
28. Ciccarese G, Herzum A, Rebora A, Drago F. Prevalence of Genital, Oral, and Anal HPV Infection Among STI Patients in Italy. J Med Virol (2017) 89:1121–4. doi: 10.1002/jmv.24746
29. Uken RB, Brummer O, von Schubert-Bayer C, Brodegger T, Teudt IU. Oral HPV Prevalence in Women Positive for Cervical HPV Infection and Their Sexual Partners: A German Screening Study. Eur Arch Otorhinolaryngol (2016) 273:1933–42. doi: 10.1007/s00405-016-3953-1
30. Temizkan O, Aydogan B, Sanverdi I, Sakiz D, Arici B, Karaca K, et al. Prevalence of Abnormal Oral Cytology and Impact of Sexual Behavior in Women With Abnormal Cervical Cytology. Clin Exp Obstet Gynecol (2016) 43:388–92. doi: 10.12891/ceog2158.2016
31. Loverro G, Di Naro E, Caringella AM, De Robertis AL, Loconsole D, Chironna M. Prevalence of Human Papillomavirus Infection in a Clinic Sample of Transsexuals in Italy. Sex Transm Infect (2016) 92:67–9. doi: 10.1136/sextrans-2014-051987
32. Kedarisetty S, Orosco RK, Hecht AS, Chang DC, Weissbrod PA, Coffey CS. Concordant Oral and Vaginal Human Papillomavirus Infection in the United States. JAMA Otolaryngology– Head Neck Surg (2016) 142:457–65. doi: 10.1001/jamaoto.2016.0064
33. Tatar TZ, Kis A, Szabo E, Czompa L, Boda R, Tar I, et al. Prevalence of Human Papillomaviruses in the Healthy Oral Mucosa of Women With High-Grade Squamous Intra-Epithelial Lesion and of Their Partners as Compared to Healthy Controls. J Oral Pathol Med (2015) 44:722–7. doi: 10.1111/jop.12302
34. Skoczynski M, Gozdzicka-Jozefiak A, Kwasniewska A. The Prevalence of Human Papillomavirus Between the Neonates and Their Mothers. BioMed Res Int (2015) 2015:126417. doi: 10.1155/2015/126417
35. Grun N, Ahrlund-Richter A, Franzen J, Mirzaie L, Marions L, Ramqvist T, et al. Oral Human Papillomavirus (HPV) Prevalence in Youth and Cervical HPV Prevalence in Women Attending a Youth Clinic in Sweden, a Follow Up-Study 2013-2014 After Gradual Introduction of Public HPV Vaccination. Infect Dis (Lond) (2015) 47:57–61. doi: 10.3109/00365548.2014.964764
36. Brouwer AF, Eisenberg MC, Carey TE, Meza R. Trends in HPV Cervical and Seroprevalence and Associations Between Oral and Genital Infection and Serum Antibodies in NHANES 2003-2012. BMC Infect Dis (2015) 15:575. doi: 10.1186/s12879-015-1314-0
37. Steinau M, Hariri S, Gillison ML, Broutian TR, Dunne EF, Tong ZY, et al. Prevalence of Cervical and Oral Human Papillomavirus Infections Among US Women. J Infect Dis (2014) 209:1739–43. doi: 10.1093/infdis/jit799
38. Ribeiro CMB, Ferrer I, de Farias ABS, Fonseca D, Silva IHM, Gueiros LAM, et al. Oral and Genital HPV Genotypic Concordance Between Sexual Partners. Clin Oral Investig (2014) 18:261–8. doi: 10.1007/s00784-013-0959-6
39. Meyer MF, Huebbers CU, Siefer OG, Vent J, Engbert I, Eslick GD, et al. Prevalence and Risk Factors for Oral Human Papillomavirus Infection in 129 Women Screened for Cervical HPV Infection. Oral Oncol (2014) 50:27–31. doi: 10.1016/j.oraloncology.2013.10.009
40. Mbulawa ZZ, Johnson LF, Marais DJ, Coetzee D, Williamson AL. Risk Factors for Oral Human Papillomavirus in Heterosexual Couples in an African Setting. J Infect (2014) 68:185–9. doi: 10.1016/j.jinf.2013.10.012
41. Lima MD, Braz-Silva PH, Pereira SM, Riera C, Coelho AC, Gallottini M. Oral and Cervical HPV Infection in HIV-Positive and HIV-Negative Women Attending a Sexual Health Clinic in Sao Paulo, Brazil. Int J Gynaecol Obstet (2014) 126:33–6. doi: 10.1016/j.ijgo.2014.01.017
42. Vogt SL, Gravitt PE, Martinson NA, Hoffmann J, D'Souza G. Concordant Oral-Genital HPV Infection in South Africa Couples: Evidence for Transmission. Front Oncol (2013) 3(303):1–7. doi: 10.3389/fonc.2013.00303
43. Schlecht NF, Rojas M, Lorde-Rollins E, Nucci-Sack A, Strickler HD, Burk RD, et al. Burden of Cervical, Anal, and Oral HPV in an Inner-City Pre-Vaccine Adolescent Population. J Urban Health (2013) 90:141–6. doi: 10.1007/s11524-012-9756-9
44. Lang Kuhs KA, Gonzalez P, Struijk L, Castro F, Hildesheim A, van Doorn LJ, et al. Prevalence of and Risk Factors for Oral Human Papillomavirus Among Young Women in Costa Rica. J Infect Dis (2013) 208:1643–52. doi: 10.1093/infdis/jit369
45. Herrero R, Quint W, Hildesheim A, Gonzalez P, Struijk L, Katki HA, et al. Reduced Prevalence of Oral Human Papillomavirus (HPV) 4 Years After Bivalent HPV Vaccination in a Randomized Clinical Trial in Costa Rica. PloS One (2013) 8:e68329. doi: 10.1371/journal.pone.0068329
46. da Mota Vasconcelos Brasil C, Ribeiro CMB, Leão JC. Oral and Genital Human Herpesvirus 8 and Human Papillomavirus in Heterosexual Partners. J Oral Pathol Med (2013) 42:61–5. doi: 10.1111/j.1600-0714.2012.01184.x
47. Adamopoulou M, Vairaktaris E, Nkenke E, Avgoustidis D, Karakitsos P, Sioulas V, et al. Prevalence of Human Papillomavirus in Saliva and Cervix of Sexually Active Women. Gynecol Oncol (2013) 129:395–400. doi: 10.1016/j.ygyno.2013.02.015
48. Schlecht NF, Burk RD, Nucci-Sack A, Shankar V, Peake K, Lorde-Rollins E, et al. Cervical, Anal and Oral HPV in an Adolescent Inner-City Health Clinic Providing Free Vaccinations. PloS One (2012) 7:e37419. doi: 10.1371/journal.pone.0037419
49. Elasbali AM, El Din AHS, Abdallah RAH, Ahmed HG. Cervical and Oral Screening for HR-HPV Types 16 and 18 Among Sudanese Women Cervical Lesions. Infect Agents Cancer (2012) 7:6. doi: 10.1186/1750-9378-7-17
50. Du J, Nordfors C, Ahrlund-Richter A, Sobkowiak M, Romanitan M, Nasman A, et al. Prevalence of Oral Human Papillomavirus Infection Among Youth, Sweden. Emerg Infect Dis (2012) 18:1468–71. doi: 10.3201/eid1809.111731
51. Ragin C, Edwards R, Larkins-Pettigrew M, Taioli E, Eckstein S, Thurman N, et al. Oral HPV Infection and Sexuality: A Cross-Sectional Study in Women. Int J Mol Sci (2011) 12:3928–40. doi: 10.3390/ijms12063928
52. Matsushita K, Sasagawa T, Miyashita M, Ishizaki A, Morishita A, Hosaka N, et al. Oral and Cervical Human Papillomavirus Infection Among Female Sex Workers in Japan. Jpn J Infect Dis (2011) 64:34–9.
53. Kero K, Rautava J, Syrjanen K, Grenman S, Syrjanen S. Human Papillomavirus Genotypes in Male Genitalia and Their Concordance Among Pregnant Spouses Participating in the Finnish Family HPV Study. J Sex Med (2011) 8:2522–31. doi: 10.1111/j.1743-6109.2011.02378.x
54. Crawford R, Grignon AL, Kitson S, Winder DM, Ball SL, Vaughan K, et al. High Prevalence of HPV in Non-Cervical Sites of Women With Abnormal Cervical Cytology. BMC Cancer (2011) 11:473. doi: 10.1186/1471-2407-11-473
55. Brown B, Blas MM, Cabral A, Carcamo C, Gravitt PE, Halsey N. Oral Sex Practices, Oral Human Papillomavirus and Correlations Between Oral and Cervical Human Papillomavirus Prevalence Among Female Sex Workers in Lima, Peru. Int J STD AIDS (2011) 22:655–8. doi: 10.1258/ijsa.2011.010541
56. Termine N, Giovannelli L, Matranga D, Perino A, Panzarella V, Ammatuna P, et al. Low Rate of Oral Human Papillomavirus (HPV) Infection in Women Screened for Cervical HPV Infection in Southern Italy: A Cross-Sectional Study of 140 Immunocompetent Subjects. J Med Virol (2009) 81:1438–43. doi: 10.1002/jmv.21509
57. Richter KL, van Rensburg EJ, van Heerden WF, Boy SC. Human Papilloma Virus Types in the Oral and Cervical Mucosa of HIV-Positive South African Women Prior to Antiretroviral Therapy. J Oral Pathol Med (2008) 37:555–9. doi: 10.1111/j.1600-0714.2008.00670.x
58. Marais DJ, Passmore JA, Denny L, Sampson C, Allan BR, Williamson AL. Cervical and Oral Human Papillomavirus Types in HIV-1 Positive and Negative Women With Cervical Disease in South Africa. J Med Virol (2008) 80:953–9. doi: 10.1002/jmv.21166
59. Ragin CC, Wheeler VW, Wilson JB, Bunker CH, Gollin SM, Patrick AL, et al. Distinct Distribution of HPV Types Among Cancer-Free Afro-Caribbean Women From Tobago. Biomarkers (2007) 12:510–22. doi: 10.1080/13547500701340384
60. Passmore JA, Marais DJ, Sampson C, Allan B, Parker N, Milner M, et al. Cervicovaginal, Oral, and Serum IgG and IgA Responses to Human Papillomavirus Type 16 in Women With Cervical Intraepithelial Neoplasia. J Med Virol (2007) 79:1375–80. doi: 10.1002/jmv.20901
61. Nordin P, Hansson BG, Hansson C, Blohme I, Larko O, Andersson K. Human Papilloma Virus in Skin, Mouth and Uterine Cervix in Female Renal Transplant Recipients With or Without a History of Cutaneous Squamous Cell Carcinoma. Acta Derm Venereol (2007) 87:219–22. doi: 10.2340/00015555-0235
62. Smith EM, Ritchie JM, Yankowitz J, Wang D, Turek LP, Haugen TH. HPV Prevalence and Concordance in the Cervix and Oral Cavity of Pregnant Women. Infect Dis Obstet Gynecol (2004) 12:45–56. doi: 10.1080/10647440400009896
63. Canadas MP, Bosch FX, Junquera ML, Ejarque M, Font R, Ordonez E, et al. Concordance of Prevalence of Human Papillomavirus DNA in Anogenital and Oral Infections in a High-Risk Population. J Clin Microbiol (2004) 42:1330–2. doi: 10.1128/jcm.42.3.1330-1332.2004
64. Scala M, Bonelli G, Gipponi M, Margarino G, Muzza A. Cryosurgery Plus Adjuvant Systemic Alpha2-Interferon for HPV-Associated Lesions. Anticancer Res (2002) 22:1171–6.
65. Marais DJ, Best JM, Rose RC, Keating P, Soeters R, Denny L, et al. Oral Antibodies to Human Papillomavirus Type 16 in Women With Cervical Neoplasia. J Med Virol (2001) 65:149–54. doi: 10.1002/jmv.2014
66. Chatterjee R, Mukhopadhyay D, Murmu N, Jana S. Prevalence of Human Papillomavirus Infection Among Prostitutes in Calcutta. J Environ Pathol Toxicol Oncol (2001) 20:113–7. doi: 10.1615/JEnvironPatholToxicolOncol.v20.i2.50
67. Aaltonen LM, Auvinen E, Dillner J, Lehtinen M, Paavonen J, Rihkanen H, et al. Poor Antibody Response Against Human Papillomavirus in Adult-Onset Laryngeal Papillomatosis. J Med Microbiol (2001) 50:468–71. doi: 10.1099/0022-1317-50-5-468
68. Badaracco G, Venuti A, Di Lonardo A, Scambia G, Mozzetti S, Benedetti Panici P, et al. Concurrent HPV Infection in Oral and Genital Mucosa. J Oral Pathol Med (1998) 27:130–4. doi: 10.1111/j.1600-0714.1998.tb01928.x
69. van Doornum GJ, Hooykaas C, Juffermans LH, van der Lans SM, van der Linden MM, Coutinho RA, et al. Prevalence of Human Papillomavirus Infections Among Heterosexual Men and Women With Multiple Sexual Partners. J Med Virol (1992) 37:13–21. doi: 10.1002/jmv.1890370104
70. Fakhry C, D'Souza G, Sugar E, Weber K, Goshu E, Minkoff H, et al. Relationship Between Prevalent Oral and Cervical Human Papillomavirus Infections in Human Immunodeficiency Virus-Positive and -Negative Women. J Clin Microbiol (2006) 44:4479–85. doi: 10.1128/JCM.01321-06
71. Goncalves AK, Giraldo P, Barros-Mazon S, Gondo ML, Amaral RL, Jacyntho C. Secretory Immunoglobulin A in Saliva of Women With Oral and Genital HPV Infection. Eur J Obstet Gynecol Reprod Biol (2006) 124:227–31. doi: 10.1016/j.ejogrb.2005.06.028
72. Zonta MA, Monteiro J, Santos G Jr., Pignatari AC. Oral Infection by the Human Papilloma Virus in Women With Cervical Lesions at a Prison in Sao Paulo, Brazil. Braz J Otorhinolaryngol (2012) 78:66–72. doi: 10.1590/S1808-86942012000200011
73. Beachler DC, Lang Kuhs KA, Struijk L, Schussler J, Herrero R, Porras C, et al. The Natural History of Oral Human Papillomavirus in Young Costa Rican Women. Sex Transm Dis (2017) 44:442–9. doi: 10.1097/olq.0000000000000625
74. Kero K, Rautava J, Louvanto K, Syrjanen K, Grenman S, Syrjanen S. Genotype-Specific Concordance of Oral and Genital Human Papillomavirus Infections Among Marital Couples Is Low. Eur J Clin Microbiol Infect Dis (2016) 35:697–704. doi: 10.1007/s10096-016-2589-9
75. Menezes LJ, Poongulali S, Tommasino M, Lin HY, Kumarasamy N, Fisher KJ, et al. Prevalence and Concordance of Human Papillomavirus Infection at Multiple Anatomic Sites Among HIV-Infected Women From Chennai, India. Int J STD AIDS (2016) 27:543–53. doi: 10.1177/0956462415587226
76. Louvanto K, Rautava J, Syrjanen K, Grenman S, Syrjanen S. The Clearance of Oral High-Risk Human Papillomavirus Infection Is Impaired by Long-Term Persistence of Cervical Human Papillomavirus Infection. Clin Microbiol Infect (2014) 20:1167–72. doi: 10.1111/1469-0691.12700
77. Paaso AE, Louvanto K, Syrjanen KJ, Waterboer T, Grenman SE, Pawlita M, et al. Lack of Type-Specific Concordance Between Human Papillomavirus (HPV) Serology and HPV DNA Detection in the Uterine Cervix and Oral Mucosa. J Gen Virol (2011) 92:2034–46. doi: 10.1099/vir.0.032011-0
78. Van Doornum GJ, Prins M, Juffermans LH, Hooykaas C, van den Hoek JA, Coutinho RA, et al. Regional Distribution and Incidence of Human Papillomavirus Infections Among Heterosexual Men and Women With Multiple Sexual Partners: A Prospective Study. Genitourin Med (1994) 70:240–6. doi: 10.1136/sti.70.4.240
79. Kero KM, Rautava J, Syrjanen K, Kortekangas-Savolainen O, Grenman S, Syrjanen S. Stable Marital Relationship Protects Men From Oral and Genital HPV Infections. Eur J Clin Microbiol Infect Dis (2014) 33:1211–21. doi: 10.1007/s10096-014-2061-7
80. Sarkola ME, Grenman SE, Rintala MA, Syrjanen KJ, Syrjanen SM. Effect of Second Pregnancy on Maternal Carriage and Outcome of High-Risk Human Papillomavirus (HPV). Experience From the Prospective Finnish Family HPV Study. Gynecol Obstet Invest (2009) 67:208–16. doi: 10.1159/000209204
81. D'Souza G, Fakhry C, Sugar EA, Seaberg EC, Weber K, Minkoff HL, et al. Six-Month Natural History of Oral Versus Cervical Human Papillomavirus Infection. Int J Cancer (2007) 121:143–50. doi: 10.1002/ijc.22667
82. Rintala MA, Grenman SE, Puranen MH, Isolauri E, Ekblad U, Kero PO, et al. Transmission of High-Risk Human Papillomavirus (HPV) Between Parents and Infant: A Prospective Study of HPV in Families in Finland. J Clin Microbiol (2005) 43:376–81. doi: 10.1128/jcm.43.1.376-381.2005
83. Winer RL, Lee SK, Hughes JP, Adam DE, Kiviat NB, Koutsky LA. Genital Human Papillomavirus Infection: Incidence and Risk Factors in a Cohort of Female University Students. Am J Epidemiol (2003) 157:218–26. doi: 10.1093/aje/kwf180
84. Mosmann JP, Zayas S, Kiguen AX, Venezuela RF, Rosato O, Cuffini CG. Human Papillomavirus and Chlamydia Trachomatis in Oral and Genital Mucosa of Women With Normal and Abnormal Cervical Cytology. BMC Infect Dis (2021) 21:422. doi: 10.1186/s12879-021-06118-3
85. Paaso A, Koskimaa HM, Welters MJP, Kero K, Rautava J, Syrjänen K, et al. Interferon-γ and IL-5 Associated Cell-Mediated Immune Responses to HPV16 E2 and E6 Distinguish Between Persistent Oral HPV16 Infections and Noninfected Mucosa. Clin Exp Dent Res (2021) 7:903–13. doi: 10.1002/cre2.396
86. Gilles C, Buljubasic M, Konopnicki D, Manigart Y, Barlow P, Rozenberg S. Cervical, Anal and Oral Human Papillomavirus (HPV) Infection in Young Women: A Case Control Study Between Women With Perinatally HIV Infection and Women With Non-Perinatally HIV Infection. Eur J Obstet Gynecol Reprod Biol (2020) 244:114–9. doi: 10.1016/j.ejogrb.2019.11.022
87. Nasioutziki M, Chatzistamatiou K, Loufopoulos PD, Vavoulidis E, Tsampazis N, Pratilas GC, et al. Cervical, Anal and Oral HPV Detection and HPV Type Concordance Among Women Referred for Colposcopy. Infect Agent Cancer (2020) 15:22. doi: 10.1186/s13027-020-00287-7
88. Nemesio I, Cury F, Longatto-Filho A, Fregnani JH, Musselwhite L, Vazquez F, et al. Identification of Human Papillomavirus in Oral Rinse Specimens From Women With and Without Cervical Intraepithelial Lesions. Sex Transm Infect (2020) 96:408–10. doi: 10.1136/sextrans-2019-054359
89. Pérez-Quintanilla M, Méndez-Martínez R, Vázquez-Vega S, Espinosa-Romero R, Sotelo-Regil R, Pérez-Montiel MD, et al. High Prevalence of Human Papillomavirus and European Variants of HPV 16 Infecting Concomitantly to Cervix and Oral Cavity in HIV Positive Women. PloS One (2020) 15:e0227900. doi: 10.1371/journal.pone.0227900
90. Sricharoenchai S, Kerr SJ, Gatechompol S, Hansudewechakul R, Dang HLD, Tran DNH, et al. Prevalence of High-Risk Nonavalent Vaccine-Type Human Papillomavirus Infection Among Unvaccinated, Sexually Active Asian Female Adolescents With and Without Perinatally Acquired HIV Infection. Pediatr Infect Dis J (2020) 39:615–9. doi: 10.1097/inf.0000000000002659
91. Suehiro TT, Damke G, Damke E, de Azevedo Ramos PLR, de Andrade Pereira Silva M, Pelloso SM, et al. Cervical and Oral Human Papillomavirus Infection in Women Living With Human Immunodeficiency Virus (HIV) and Matched HIV-Negative Controls in Brazil. Infect Agent Cancer (2020) 15:31. doi: 10.1186/s13027-020-00301-y
92. Enerly E, Flingtorp R, Christiansen IK, Campbell S, Hansen M, Myklebust T, et al. An Observational Study Comparing HPV Prevalence and Type Distribution Between HPV-Vaccinated and -Unvaccinated Girls After Introduction of School-Based HPV Vaccination in Norway. PloS One (2019) 14:e0223612. doi: 10.1371/journal.pone.0223612
93. Tewari P, Banka P, Kernan N, Reynolds S, White C, Pilkington L, et al. Prevalence and Concordance of Oral HPV Infections With Cervical HPV Infections in Women Referred to Colposcopy With Abnormal Cytology. J Oral Pathol Med (2021) 50:692–9. doi: 10.1111/jop.13172
94. D'Souza G, Gross ND, Pai SI, Haddad R, Anderson KS, Rajan S, et al. Oral Human Papillomavirus (HPV) Infection in HPV-Positive Patients With Oropharyngeal Cancer and Their Partners. J Clin Oncol (2014) 32:2408–15. doi: 10.1200/jco.2014.55.1341
95. Sánchez-Vargas LO, Díaz-Hernndez C, Martinez-Martinez A. Detection of Human Papilloma Virus (HPV) in Oral Mucosa of Women With Cervical Lesions and Their Relation to Oral Sex Practices. Infect Agents Cancer (2010) 5(25):1–6. doi: 10.1186/1750-9378-5-25
96. Kellokoski JK, Syrjänen SM, Chang F, Yliskoski M, Syrjänen KJ. Southern Blot Hybridization and PCR in Detection of Oral Human Papillomavirus (HPV) Infections in Women With Genital HPV Infections. J Oral Pathol Med (1992) 21:459–64. doi: 10.1111/j.1600-0714.1992.tb00975.x
97. Premoli-De-Percoco G, Ramirez JL, Galindo I. Correlation Between HPV Types Associated With Oral Squamous Cell Carcinoma and Cervicovaginal Cytology - An in Situ Hybridization Study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod (1998) 86:77–81. doi: 10.1016/s1079-2104(98)90153-6
98. Saini R, Khim TP, Rahman SA, Ismail M, Tang TH. High-Risk Human Papillomavirus in the Oral Cavity of Women With Cervical Cancer, and Their Children. Virol J (2010) 7:131. doi: 10.1186/1743-422x-7-131
99. Lupato V, Holzinger D, Hofler D, Menegaldo A, Giorgi Rossi P, Del Mistro A, et al. Prevalence and Determinants of Oral Human Papillomavirus Infection in 500 Young Adults From Italy. PloS One (2017) 12:e0170091. doi: 10.1371/journal.pone.0170091
100. Visalli G, Curro M, Facciola A, Riso R, Mondello P, Lagana P, et al. Prevalence of Human Papillomavirus in Saliva of Women With HPV Genital Lesions. Infect Agents Cancer (2016) 11:6. doi: 10.1186/s13027-016-0096-3
101. Marques AE, Barra GB, de Resende Oyama CN, Guerra EN. Low Rate of Oropharyngeal Human Papillomavirus Infection of Women With Cervical Lesions and Their Partners: New Data From Brazilian Population. J Oral Pathol Med (2015) 44:453–8. doi: 10.1111/jop.12252
102. Rietbergen MM, van Bokhoven A, Lissenberg-Witte BI, Heideman DAM, Leemans CR, Brakenhoff RH, et al. Epidemiologic Associations of HPV-Positive Oropharyngeal Cancer and (Pre)Cancerous Cervical Lesions. Int J Cancer (2018) 143:283–8. doi: 10.1002/ijc.31315
103. Peixoto AP, Campos GS, Queiroz LB, Sardi SI. Asymptomatic Oral Human Papillomavirus (HPV) Infection in Women With a Histopathologic Diagnosis of Genital HPV. J Oral Sci (2011) 53:451–9. doi: 10.2334/josnusd.53.451
104. Christensen JT, Gronhoj C, Zamani M, Brask J, Kjaer EKR, Lajer H, et al. Association Between Oropharyngeal Cancers With Known HPV and P16 Status and Cervical Intraepithelial Neoplasia: A Danish Population-Based Study. Acta Oncol (2019) 58:267–72. doi: 10.1080/0284186x.2018.1546059
105. Sánchez-Siles M, Remezal-Solano M, López-López AM, Camacho-Alonso F. Prevalence of Human Papillomavirus in the Saliva of Sexually Active Women With Cervical Intraepithelial Neoplasias. Med Oral Patol Oral Cir Bucal (2020) 25:e195–204. doi: 10.4317/medoral.23300
106. Chaturvedi AK, Engels EA, Gilbert ES, Chen BE, Storm H, Lynch CF, et al. Second Cancers Among 104,760 Survivors of Cervical Cancer: Evaluation of Long-Term Risk. J Natl Cancer Inst (2007) 99:1634–43. doi: 10.1093/jnci/djm201
107. Chaturvedi AK, Kleinerman RA, Hildesheim A, Gilbert ES, Storm H, Lynch CF, et al. Second Cancers After Squamous Cell Carcinoma and Adenocarcinoma of the Cervix. J Clin Oncol (2009) 27:967–73. doi: 10.1200/JCO.2008.18.4549
108. Chen CY, Lai CH, Lee KD, Huang SH, Dai YM, Chen MC. Risk of Second Primary Malignancies in Women With Cervical Cancer: A Population-Based Study in Taiwan Over a 30-Year Period. Gynecol Oncol (2012) 127:625–30. doi: 10.1016/j.ygyno.2012.09.004
109. Ebisch RMF, Rutten DWE, IntHout J, Melchers WJG, Massuger LFAG, Bulten J, et al. Long-Lasting Increased Risk of Human Papillomavirus–Related Carcinomas and Premalignancies After Cervical Intraepithelial Neoplasia Grade 3: A Population-Based Cohort Study. J Clin Oncol (2017) 35:2542–50. doi: 10.1200/JCO.2016.71.4543
110. Gaudet M, Hamm J, Aquino-Parsons C. Incidence of Ano-Genital and Head and Neck Malignancies in Women With a Previous Diagnosis of Cervical Intraepithelial Neoplasia. Gynecol Oncol (2014) 134:523–6. doi: 10.1016/j.ygyno.2014.07.088
111. Hemminki K, Dong C, Frisch M. Tonsillar and Other Upper Aerodigestive Tract Cancers Among Cervical Cancer Patients and Their Husbands. Eur J Cancer Prev (2000) 9:433–7. doi: 10.1097/00008469-200012000-00010
112. Hemminki K, Jiang Y, Dong C. Second Primary Cancers After Anogenital, Skin, Oral, Esophageal and Rectal Cancers: Etiological Links? Int J Cancer (2001) 93:294–8. doi: 10.1002/ijc.1319
113. Jung YS, Lim J, Jung KW, Ryu J, Won YJ. Metachronous Second Primary Malignancies After Head and Neck Cancer in a Korean Cohort (1993-2010). PloS One (2015) 10:e0134160. doi: 10.1371/journal.pone.0134160
114. Neumann F, Jegu J, Mougin C, Pretet JL, Guizard AV, Lapotre-Ledoux B, et al. Risk of Second Primary Cancer After a First Potentially-Human Papillomavirus-Related Cancer: A Population-Based Study. Prev Med (2016) 90:52–8. doi: 10.1016/j.ypmed.2016.06.041
115. Papatla K, Halpern MT, Hernandez E, Brown J, Benrubi D, Houck K, et al. Second Primary Anal and Oropharyngeal Cancers in Cervical Cancer Survivors. Am J Obstet Gynecol (2019) 221:478.e1–6. doi: 10.1016/j.ajog.2019.05.025
116. Rose Ragin CC, Taioli E. Second Primary Head and Neck Tumor Risk in Patients With Cervical Cancer–SEER Data Analysis. Head Neck (2008) 30:58–66. doi: 10.1002/hed.20663
117. Suk R, Mahale P, Sonawane K, Sikora AG, Chhatwal J, Schmeler KM, et al. Trends in Risks for Second Primary Cancers Associated With Index Human Papillomavirus-Associated Cancers. JAMA Netw Open (2018) 1:13. doi: 10.1001/jamanetworkopen.2018.1999
118. Svahn MF, Munk C, Jensen SM, von Buchwald C, Frederiksen K, Kjaer SK. Risk of Head-and-Neck Cancer Following a Diagnosis of Severe Cervical Intraepithelial Neoplasia: A Nationwide Population-Based Cohort Study in Denmark. Gynecol Oncol (2016) 142:128–32. doi: 10.1016/j.ygyno.2016.04.023
119. Skinner HD, Sturgis EM, Klopp AH, Ang KK, Rosenthal DI, Garden AS, et al. Clinical Characteristics of Patients With Multiple Potentially Human Papillomavirus-Related Malignancies. Head Neck (2014) 36:819–25. doi: 10.1002/hed.23379
120. Gan SJ, Dahlstrom KR, Peck BW, Caywood W, Li G, Wei Q, et al. Incidence and Pattern of Second Primary Malignancies in Patients With Index Oropharyngeal Cancers Versus Index Nonoropharyngeal Head and Neck Cancers. Cancer (2013) 119:2593–601. doi: 10.1002/cncr.28107
121. Biron VL, Cote DW, Seikaly H. Oropharyngeal Squamous Cell Carcinoma and Human Papillomavirus-Associated Cancers in Women: Epidemiologic Evaluation of Association. J Otolaryngol Head Neck Surg (2011) 40 Suppl 1:S65–9.
122. Holstead R, Rasul R, Golden A, Kamdar D, Ghaly M, Teckie S, et al. Identifying Patterns of Failure and Secondary Primary Malignancies in HPV-Related Oropharyngeal Squamous Cell Carcinomas. Future Oncol (2020) 16:199–207. doi: 10.2217/fon-2019-0673
123. Larish A, Yin L, Glaser G, Moore E, Bakkum-Gamez J, Routman D, et al. Human Papillomavirus-Associated Anogenital Pathology in Females With HPV-Positive Oropharyngeal Squamous Cell Carcinoma. Otolaryngol Head Neck Surg (2021) 164:369–74. doi: 10.1177/0194599820941499
124. Loopik DL, Ebisch RM, IntHout J, Melchers WJ, Massuger LF, Bekkers RL, et al. The Relative Risk of Noncervical High-Risk Human Papillomavirus-Related (Pre)Malignancies After Recurrent Cervical Intraepithelial Neoplasia Grade 3: A Population-Based Study. Int J Cancer (2020) 147:897–900. doi: 10.1002/ijc.32834
125. Preti M, Rosso S, Micheletti L, Libero C, Sobrato I, Giordano L, et al. Risk of HPV-Related Extra-Cervical Cancers in Women Treated for Cervical Intraepithelial Neoplasia. BMC Cancer (2020) 20:972. doi: 10.1186/s12885-020-07452-6
126. Wang M, Sharma A, Osazuwa-Peters N, Simpson MC, Schootman M, Piccirillo JF, et al. Risk of Subsequent Malignant Neoplasms After an Index Potentially-Human Papillomavirus (HPV)-Associated Cancers. Cancer Epidemiol (2020) 64:101649. doi: 10.1016/j.canep.2019.101649
127. Gazzaz MJ, Jeffery C, O'Connell D, Harris J, Seikaly H, Biron V. Association of Human Papillomavirus Related Squamous Cell Carcinomas of the Oropharynx and Cervix. Papillomavirus Res (2019) 8:100188. doi: 10.1016/j.pvr.2019.100188
128. Rogers NL, Cole SA, Lan HC, Crossa A, Demerath EW. New Saliva DNA Collection Method Compared to Buccal Cell Collection Techniques for Epidemiological Studies. Am J Hum Biol (2007) 19:319–26. doi: 10.1002/ajhb.20586
129. Kim KY, Lewis JS Jr., Chen Z. Current Status of Clinical Testing for Human Papillomavirus in Oropharyngeal Squamous Cell Carcinoma. J Pathol Clin Res (2018) 4:213–26. doi: 10.1002/cjp2.111
130. Sasagawa T, Minemoto Y, Basha W, Yamazaki H, Nakamura M, Yoshimoto H, et al. A New PCR-Based Assay Amplifies the E6-E7 Genes of Most Mucosal Human Papillomaviruses (HPV). Virus Res (2000) 67:127–39. doi: 10.1016/s0168-1702(00)00137-4
131. Gravitt PE, Peyton CL, Alessi TQ, Wheeler CM, Coutlée F, Hildesheim A, et al. Improved Amplification of Genital Human Papillomaviruses. J Clin Microbiol (2000) 38:357–61. doi: 10.1128/JCM.38.1.357-361.2000
Keywords: female, (human) papillomavirus (HPV) infection, oropharynx, cervix (uteri), cancer, epidemiology, systematic review
Citation: Jordan KH, Beverly Hery CM, Zhang X and Paskett ED (2022) Low Rates of Dual-Site and Concordant Oral-Cervical Human Papillomavirus Infections and Cancers: A Systematic Review. Front. Oncol. 12:848628. doi: 10.3389/fonc.2022.848628
Received: 04 January 2022; Accepted: 18 February 2022;
Published: 29 March 2022.
Edited by:
Dana Kristjansson, Norwegian Institute of Public Health (NIPH), NorwayReviewed by:
Alfredo Cruz-Gregorio, National Autonomous University of Mexico, MexicoShowket Hussain, ICMR-National Institute of Cancer Prevention and Research, India
Copyright © 2022 Jordan, Beverly Hery, Zhang and Paskett. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kelsey H. Jordan, S2Vsc2V5LkpvcmRhbkBvc3VtYy5lZHU=; Electra D. Paskett, ZWxlY3RyYS5wYXNrZXR0QG9zdW1jLmVkdQ==
†These authors have contributed equally to this work
 Kelsey H. Jordan
Kelsey H. Jordan Chloe M. Beverly Hery
Chloe M. Beverly Hery Xiaochen Zhang
Xiaochen Zhang Electra D. Paskett
Electra D. Paskett