
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 01 April 2022
Sec. Hematologic Malignancies
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.846044
This article is part of the Research Topic Risk-Stratification in Myelodysplastic Syndromes View all 6 articles
 Qiuni Chen1,2†
Qiuni Chen1,2† Yue Chen1,2†
Yue Chen1,2† Yijing Zhang1,2
Yijing Zhang1,2 Lijuan Zhang1,2
Lijuan Zhang1,2 Kankan Chen1,2
Kankan Chen1,2 Zhengmei He1,2
Zhengmei He1,2 Chunling Wang1,2*
Chunling Wang1,2* Liang Yu1,2*
Liang Yu1,2*Background: Myelodysplastic syndromes (MDSs) are a very heterogeneous group of myeloid disorders with high prevalence and risk of developing acute myeloid leukemia. The more accurate risk stratification can provide a better guidance of treatment. The platelet–large cell ratio (P-LCR) is a parameter reported in complete blood cell count tests, and was associated with many diseases, but its role in MDS is not clear.
Purpose: This study aims to explore the impact of the P-LCR on the prognosis of patients with MDS, which is of great significance for clinical treatment.
Methods: In the retrospective study, 122 newly diagnosed MDS patients were enrolled. We used the bioinformatics tool X-tile to define a P-LCR threshold of 36.7% to predict prognosis. Patients were divided into P-LCRlow and P-LCRhigh groups, and their characteristics were compared between the two groups.
Results: Results show that the P-LCRlow was associated with worse overall survival (OS) than the P-LCRhigh patients (median OS, 18.53 months versus 25.77 months, p=0.0057), but there were no statistical differences in progression-free survival (PFS) between the two groups (p=0.2001). The results of univariate and multivariate Cox proportional hazard analyses adjusted for gender, bone marrow blast level, platelet count, and International Prognostic Scoring System scores showed that the P-LCR was useful in the evaluation of PFS [hazard ratio (HR) 0.212, 95%CI 0.064–0.702, p=0.011] and OS of MDS (HR 0.464, 95%CI 0.284–0.757, p=0.002).
Conclusion: This study is the first report showing that the P-LCR would be a simple and immediately available biomarker for predicting the prognosis of MDS.
Myelodysplastic syndromes (MDS) are one of the most prevalent hematological malignancies originating from hematopoietic stem/progenitor cells. It is a very heterogeneous group of myeloid disorders characterized by somatic mutations in hematopoietic stem cells, leading to ineffective hematopoiesis, bone marrow dysplasia, and an increased risk of transformation to acute leukemia (1).
The prevalence of MDS increases with age. The choice and timing of therapy depend on the risk stratification. The International Prognostic Scoring System (IPSS) (2) and revised International Prognostic Scoring System (IPSS-R) (3) are the most commonly used prognostic models. In general, all these scoring systems include the analyses of peripheral cytopenias, percentage of blasts in the bone marrow, and cytogenetic characteristics. Patients with MDS are generally divided into two different broad subgroups according to two scoring systems: lower- and higher-risk groups. Patients with a lower risk group by IPSS are those with low and intermediate-1(INT-1) diseases and very low, low, and some subsets of intermediate risk by the IPSS-R. Patients with a higher-risk disease are those with intermediate-2 (INT-2) and high risk by the IPSS and some subsets of intermediate-, high-, and very high-risk disease by the IPSS-R. Although the IPSS and IPSS-R are very important and serve as part of the main eligibility criteria for past and ongoing registration in clinical trials, many limitations remain.
With the deepening of biomarker research, various types of indicators, including genetics, blood parameters, and even nutritional indexes, had been found to be related to the biological characteristics and even the prognosis of MDS (4–6). Therefore, the concise indexes may help to more accurately evaluate the prognosis of MDS.
The platelet large–cell ratio (P-LCR) is an index representing the percentage of platelets larger than 12 fl in the circulating pool (7), generated by the automatic blood cell counter, thus probably identifying those platelets that are metabolically and enzymatically more active than small platelets, and it is easily available at an affordable cost. The original description of this parameter is from 1981 (7). It is determined based on flow cytometry and automated blood cell analysis techniques. The P-LCR was the best tool to assess megakaryocyte activity and a good monitoring tool for platelet activity. Evidence has indicated that the P-LCR contributes to the diagnosis of certain diseases (8–10). For instance, the P-LCR was proven higher in patients with chronic myeloid leukemia than patients with reactive thrombocytosis, essential thrombocythemia, or polycythemia vera (11). In clinical practice, abnormal platelet parameters are relatively common in MDS patients at initial diagnosis, but its impact on the prognosis remains unclear.
In this study, we performed a retrospective review to evaluate whether the P-LCR is associated with the prognosis of MDS.
One hundred and sixty-four patients with newly diagnosed MDS between March 2010 and January 2021 were reviewed in the Huai’an No.1 People’s Hospital. MDS was defined according to the World Health Organization (WHO) 2008 and 2016 classification for MDS. The study population was selected according to the following criteria and followed up to April 2021.The study was approved by the Institutional Review Committee of Huai’an No.1 People’s Hospital and implemented in conformity with the Declaration of Helsinki. All the patients were anonymous. Informed consent was waived because of the retrospective design of the data collection.
The inclusion criteria are as follows: a) diagnosed with MDS according to the 2008 and 2016 WHO definitions; b) complete blood samples were obtained at diagnosis and before any interventions; and c) detailed clinical data were available.
The exclusion criteria are as follows: a) age < 18 years; b) platelet transfusion before the measurement of the P-LCR; and c) active bleeding symptoms.
Whole-blood samples collected into tubes were used for the measurement of the P-LCR. The baseline P-LCR level at diagnosis was defined as the value that was obtained on the nearest day before the diagnosis. The P-LCR was measured using XN-9000 (Sysmex, Kobe, Japan). The reference range for the P-LCR in our institution is 13.0%–43.0%. We used the bioinformatics tool X-tile to define a P-LCR threshold of 36.7% to predict prognosis (12). Subjects were classified as P-LCR low (<36.7%; N = 74) or P-LCR high (≥36.7%; N = 48) cohorts.
Data analyses were performed with the Statistical Package (SPSS 26.0 Inc., Chicago, IL, United States) and Graphpad Prism 6 (Graphpad Software, California, United States). The differences of categorical variables between groups were made by using the Mann–Whitney U-test or chi-squared test. Kaplan–Meier analysis was used to assess the associations of the P-LCR with progression-free survival (PFS) and overall survival (OS). PFS, the primary end point, was defined as the duration from the first treatment to the progression of MDS, death of any cause, or end of clinical follow-up. OS, the secondary end point, was defined as the duration from the first treatment to all-cause death or the end of follow-up. Kaplan–Meier survival curves were performed to compare the prognosis between the P-LCRlow and P-LCRhigh groups by the log rank test. The X-tile software (Version 3.6.1; Yale University, New Haven, CT, United States) was conducted to evaluate the optimal cut-off P-LCR. Univariate and multivariate Cox proportional hazards models were performed to identify significant prognostic predictors. The hazard ratio (HR) and 95% confidence interval (CI) were calculated. The significant variables with p <0.1 defined in univariate survival analyses (by the log rank test) were included for the multivariate analyses to validate the prognostic value of the P-LCR. A p-value less than 0.05 (2-tailed) indicated a statistical significance.
A total of 122 newly diagnosed MDS patients were included in our cohort. The median follow-up time was 48.27 months (range, 21.50–59.83 months). A total of 85 patients died. The demographics of patients are summarized in Table 1. The median age was 64.48 (26–88) years, and 84 (68.9%) were men. Subjects were classified as P-LCRlow (<36.7%; N = 74) or P-LCRhigh (≥36.7%; N = 48) cohorts. The distribution of characteristics such as age, gender, WHO subtype, Hb level, bone marrow blast%, WBC count, ANC count, PLT count, IPSS subgroups, and treatment were not different between the two groups. Patients with IPSS low-risk disease presented with a lower P-LCR compared to INT-1 (p=0.011), INT-2 (p=0.004), and high risk (p=0.021) patients (Figure 1).
Kaplan–Meier survival curves were performed to compare the prognosis between P-LCRlow and P-LCRhigh groups by the log rank test. The data showed that P-LCRlow was associated with worse OS compared to P-LCRhigh patients (median OS, 18.53 months versus 25.77 months, p=0.0057, Figure 2A). A similar tendency was observed in PFS but with no statistical difference (p=0.2001, Figure 2B). Subgroup analyses were done in lower risk groups (including low risk and INT-1) and higher risk groups (including INT-2 and high risk). Patients with lower risk were expected to have better prognosis. The analyses of relationship between the P-LCR and prognosis in lower risk patients indicated that there was no statistical significance (p=0.0980 and p=0.0587; Figure 3). The association was also explored in higher risk groups. The results show that patients in higher risk groups with low P-LCR have shorter OS compared to those with high P-LCR, respectively (p=0.0171, Figure 4). There was no similar observation in PFS analysis (p=0.0771, Figure 4).
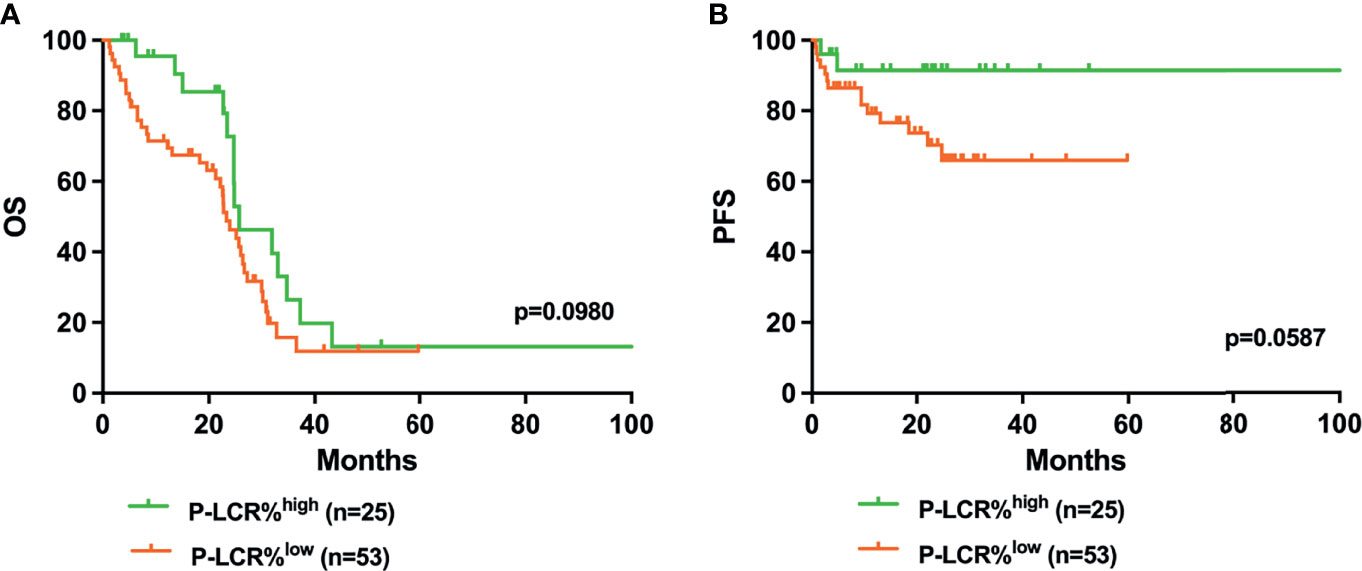
Figure 3 Higher P-LCR was not associated with better OS (A) and better PFS (B) in lower risk MDS groups (lower risk MDS group was defined as IPSS=low risk+INT-1).
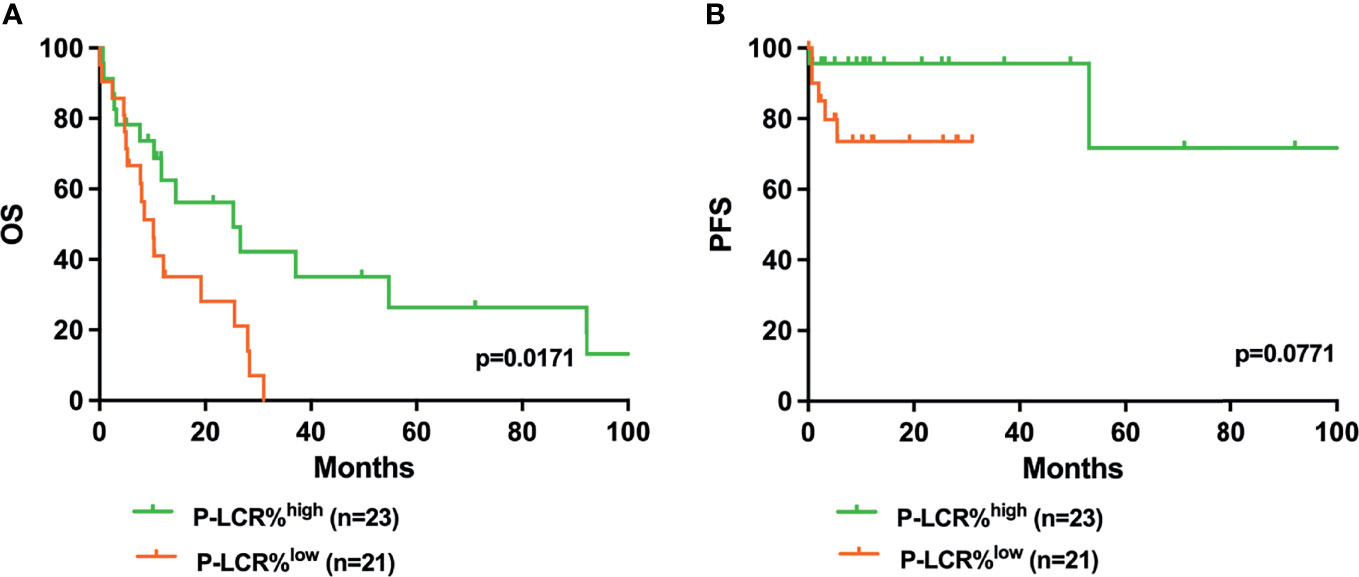
Figure 4 Higher P-LCR was associated with better OS (A) but not with better PFS (B) in higher risk MDS groups (higher risk MDS group was defined as IPSS=INT-2+high risk).
Univariate analyses were performed to investigate the prognostic factors affecting disease progression and death (Table 2). The baseline P-LCR level (HR 0.578, 95%CI 0.363–0.919, p=0.020), bone marrow blast level (HR 1.083, 95%CI 1.042–1.126, p<0.001), platelet count (HR 0.997, 95%CI 0.994-0.999, p=0.018), and IPSS scores (HR 1.530, 95%CI 1.160-2.018, p=0.003) were potential risk factors for poor OS. The P-LCR was also prognostic for PFS (HR 0.263, 95%CI 0.087-0.794, p=0.018).
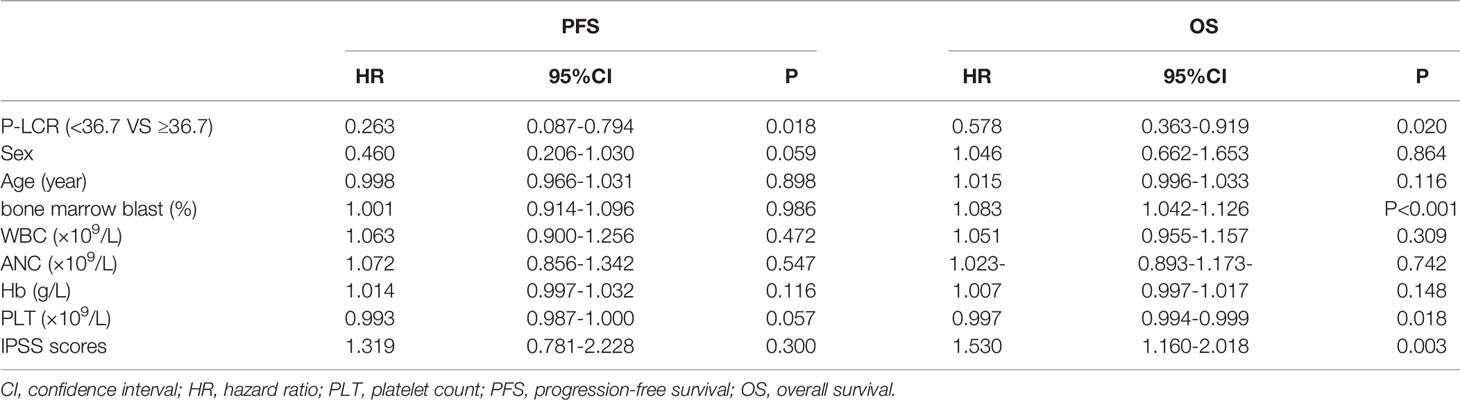
Table 2 Univariate analyses for PFS and OS in 122 MDS patients in relation to prognostic parameters.
All variables with P < 0.1 in univariate analyses were included in multivariate analyses. The P-LCR, gender, bone marrow blast, PLT count and IPSS scores were the prognostic-related risk factors. We performed multivariate Cox regression analyses with the clinical variables based on the risk factors above. The results showed that the P-LCR at diagnosis in patients with MDS is an independent predictor for PFS in the total cohort in multivariate analyses (HR 0.212, 95%CI 0.064-0.701, p=0.011, Table 3) after the adjustment with gender, age, blasts, and PLT count. The P-LCR level (HR 0.464, 95%CI 0.284-0.757, p=0.002) was a potential prognostic factor for OS in multivariate analyses are shown in Table 3.
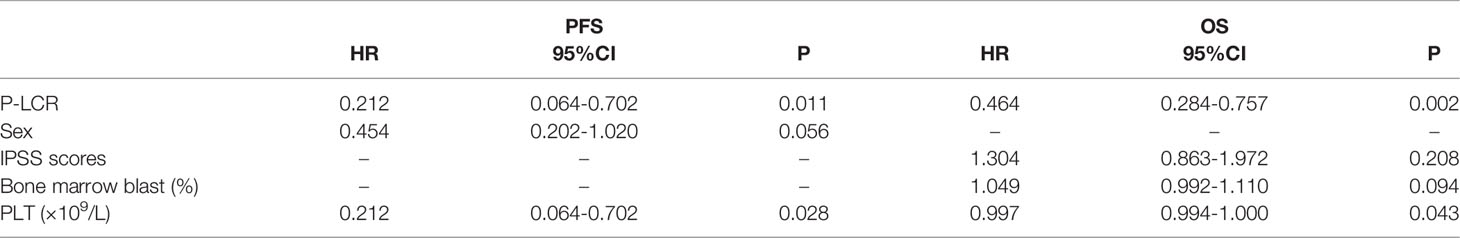
Table 3 Multivariate analyses for PFS and OS in 122 MDS patients in relation to prognostic parameters.
The present study showed that the P-LCR level at diagnosis was associated with the prognosis in patients with MDS. As far as we are aware, this study is the first report showing the P-LCR as an independent prognostic variable in MDS. We found that the patients whose P-LCR level was less than 36.7% at diagnosis experienced shorter overall survival compared to patients with a high P-LCR. There seems to be a similar tendency in the relationship between the P-LCR and PFS. PFS in the P-LCRlow group seemed to be shorter compared to the P-LCRhigh group, although it was not statistically significant in the presented data. Subgroup analyses indicated that the P-LCR has a higher prognostic value in patients with higher risk.
Univariate and multivariate analyses were also performed to investigate the prognostic factors affecting disease progression and death. Results indicated that the P-LCR is a prognostic parameter in patients with MDS. Although the mechanism of action is still unclear, there is a consensus that the P-LCR is a potential biomarker, which can be quickly and accurately detected in peripheral blood test. Moreover, the baseline platelet count seemingly has a prognostic impact in MDS. While various studies have evaluated the prognostic significance of thrombocytosis in cancers, such as colon cancer, ovarian cancer, and hepatocellular cancer (13–15), no significance for platelet count in MDS has been reported. Gender seems to influence the prognosis of MDS patients; the same conclusion was also found in previous studies (16, 17). Some possible explanations for that might include 1) the increased comorbidities upon diagnosis in male patients, which limited preferred choices and the aggressiveness of treatment options (18, 19), while large cohort-based studies confirmed that MDS patients with the above comorbidities had significantly greater risk of death than those without comorbidities (20); 2) male MDS patients might be associated with molecular abnormalities such as faster methylome aging and shorter telomeres (21), both of which often correlate with shorter survival (22).
The present data suggested that the elevated P-LCR level at diagnosis was associated with better prognosis in MDS patients, especially for those with higher risk disease. We speculate that the reasons may be that, firstly, more large platelets are involved in the neoplastic consumed, thereby speeding up the progression of the tumor (23); secondly, the degree of platelet activation affects various effector factors, such as Vascular Endothelial Growth Factor (VEGF), Epidermal Growth Factor (EGF), transforming growth factor-beta (TGFβ), Platelet-derived growth factor (PDGF), and Interleukin-6 (IL-6), that impact vascular maturation in the tumor microenvironment and mediate the invasion of cancer cells (24), which are associated with survival in MDS. Third, large platelet cells might be associated with greater platelet–tumor complex formation; therefore, patients with a high P-LCR could gain more benefit from antiplatelet drugs than could patients with low P-LCR levels (25).
Platelets play a critical role in the development and progression of different cancers by promoting cancer cell proliferation, survival, angiogenesis, and metastasis (26, 27). There have been several studies revealing that platelet indices are associated with prognosis in patients with various diseases. Thrombocytosis was related to poor prognosis in several cancers, such as ovarian, gastric, lung, breast, hepatocellular, and bladder cancer (28–33). Higher platelet distribution width (PDW) was correlated with unfavorable prognosis in ovarian, and breast cancer (34, 35). Moreover, the mean platelet volume (MPV) level was evaluated for association with the development of diabetes mellitus (36) and outcomes of cancer patients (37, 38). However, the impact of the P-LCR in MDS is unclear.
The platelet–large cell ratio (P-LCR) is an indicator of circulating larger platelets (>12 fl) and the best tool to assess megakaryocyte activity (24). A large platelet is somehow a representative marker of immature platelets; thus, the lower P-LCR observed in MDS patients in comparison to the respectively high group suggests increased platelet maturity. These findings are also similar to those of Renate Asare et al. (39), whose results suggested that there are significantly (p<0.05) lower levels of P-LCR in children with Burkitt lymphoma than the controls. The mechanisms for the phenomenon remain uncertain. Psaila et al. (40) found that patients with AML/MDS had smaller platelets and lower in vivo platelet activation and ex vivo platelet reactivity than patients with immune thrombocytopenia. All these studies prompt that the flow cytometric analyses of platelet function and cell parameter analyzers could establish the expression of the platelet indices and reflect the megakaryocyte activity, so as to judge the marrow environment indirectly. The P-LCR has good prognostic evaluation efficiency and could also reflect the changes of the general states of MDS from different aspects. As an easily obtained index, it may help to more accurately evaluate the prognosis of MDS.
This is the first documentation on the prognostic value of P-LCR in patients with MDS with long-term follow-up. However, our data are preliminary; further prospective analyses and the mechanism studies are necessary.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Institutional Review Committee of Huai’an No.1 People’s Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
LY designed the study. QC and YC wrote the manuscript. YZ collected data. LZ and KC were responsible for the tables. ZH was responsible for the figures. CW and LY modified the manuscript.
This work was funded by Science and Technology Fund of Huaian City [grant # HAB202020] and Commission of Health of Jiangsu Province [grant # 2019082].
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.846044/full#supplementary-material
1. Saygin C, Godley LA. Genetics of Myelodysplastic Syndromes. Cancers (Basel) (2021) 13(14):3380. doi: 10.3390/cancers13143380
2. Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International Scoring System for Evaluating Prognosis in Myelodysplastic Syndromes. Blood (1997) 89(6):2079–88. doi: 10.1182/blood.V89.6.2079
3. Greenberg PL, Tuechler H, Schanz J, Sanz G, Garcia-Manero G, Solé F, et al. Revised International Prognostic Scoring System for Myelodysplastic Syndromes. Blood (2012) 120(12):2454–65. doi: 10.1182/blood-2012-03-420489
5. Shi Z, Li B, Huang H, Qin T, Xu Z, Zhang H, et al. Prognostic Impact of Red Blood Cell Distribution Width in Myelodysplastic Syndromes. Br J Haematol (2019) 186(2):352–5. doi: 10.1111/bjh.15830
6. Teras LR, Patel AV, Carter BD, Rees-Punia E, McCullough ML, Gapstur SM. Anthropometric Factors and Risk of Myeloid Leukaemias and Myelodysplastic Syndromes: A Prospective Study and Meta-Analysis. Br J Haematol (2019) 186(2):243–54. doi: 10.1111/bjh.15904
7. Bessman JD, Williams LJ, Gilmer PR Jr. Mean Platelet Volume. The Inverse Relation of Platelet Size and Count in Normal Subjects, and an Artifact of Other Particles. Am J Clin Pathol (1981) 76(3):289–93. doi: 10.1093/ajcp/76.3.289
8. Turk U, Tengiz I, Ozpelit E, Celebiler A, Pekel N, Ozyurtlu F, et al. The Relationship Between Platelet Indices and Clinical Features of Coronary Artery Disease. Kardiol Pol (2013) 71(11):1129–34. doi: 10.5603/KP.2013.0293
9. Yan K, Ding B, Huang J, Dai Y, Xiong S, Zhai Z. Normal Platelet Counts Mask Abnormal Thrombopoiesis in Patients With Chronic Myeloid Leukemia. Oncol Lett (2015) 10(4):2390–4. doi: 10.3892/ol.2015.3502
10. Wysokiński A, Szczepocka E. Platelet Parameters (PLT, MPV, P-LCR) in Patients With Schizophrenia, Unipolar Depression and Bipolar Disorder. Psychiatry Res (2016) 237:238–45. doi: 10.1016/j.psychres.2016.01.034
11. Kabutomori O, Kanakura Y, Iwatani Y. Increase in Platelet-Large Cell Ratio in Chronic Myeloid Leukemia. Leuk Res (2001) 25(10):873. doi: 10.1016/S0145-2126(01)00017-0
12. Camp RL, Dolled-Filhart M, Rimm DL. X-Tile: A New Bio-Informatics Tool for Biomarker Assessment and Outcome-Based Cut-Point Optimization. Clin Cancer Res (2004) 10(21):7252–9. doi: 10.1158/1078-0432.CCR-04-0713
13. Shen Y, Xu H, Guan Z, Lv M, Qian T, Wu Y. Effect of Rho GTPase Activating Protein 9 Combined With Preoperative Ratio of Platelet Distribution Width to Platelet Count on Prognosis of Patients With Serous Ovarian Cancer. Transl Cancer Res (2021) 10(10):4440–53. doi: 10.21037/tcr-21-1946
14. Papila Kundaktepe B, Papila C. The Clinical Significance of Preoperative Plasma Fibrinogen Levels and Platelet Counts in Resectable Colon Cancer. World J Surg Oncol (2021) 19(1):69. doi: 10.1186/s12957-021-02180-y
15. Pang Q, Liu S, Wang L, Pan H, Wang C, Zhou L, et al. The Significance of Platelet-Albumin-Bilirubin (PALBI) Grade in Hepatocellular Carcinoma Patients Stratified According to Platelet Count. Cancer Manag Res (2020) 12:12811–22. doi: 10.2147/CMAR.S277013
16. Wang F, Ni J, Wu L, Wang Y, He B, Yu D. Gender Disparity in the Survival of Patients With Primary Myelodysplastic Syndrome. J Cancer (2019) 10(5):1325–32. doi: 10.7150/jca.28220
17. Nösslinger T, Tüchler H, Germing U, Sperr W, Krieger O, Haase D, et al. Prognostic Impact of Age and Gender in 897 Untreated Patients With Primary Myelodysplastic Syndromes. Ann Oncol (2010) 21(1):120–5. doi: 10.1093/annonc/mdp264
18. Bammer C, Sperr WR, Kemmler G, Wimazal F, Nösslinger T, Schönmetzler A, et al. Clustering of Comorbidities is Related to Age and Sex and Impacts Clinical Outcome in Myelodysplastic Syndromes. J Geriatr Oncol (2014) 5(3):299–306. doi: 10.1016/j.jgo.2014.02.002
19. Zipperer E, Tanha N, Strupp C, Kündgen A, Nachtkamp K, Neukirchen J, et al. The Myelodysplastic Syndrome-Comorbidity Index Provides Additional Prognostic Information on Patients Stratified According to the Revised International Prognostic Scoring System. Haematologica (2014) 99(3):e31–2. doi: 10.3324/haematol.2013.101055
20. Wang R, Gross CP, Halene S, Ma X. Comorbidities and Survival in a Large Cohort of Patients With Newly Diagnosed Myelodysplastic Syndromes. Leuk Res (2009) 33(12):1594–8. doi: 10.1016/j.leukres.2009.02.005
21. Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda G, et al. Genome-Wide Methylation Profiles Reveal Quantitative Views of Human Aging Rates. Mol Cell (2013) 49(2):359–67. doi: 10.1016/j.molcel.2012.10.016
22. Jemielity S, Kimura M, Parker KM, Parker J, Cao X, Aviv A, et al. Short Telomeres in Short-Lived Males: What are the Molecular and Evolutionary Causes. Aging Cell (2007) 6(2):225–33. doi: 10.1111/j.1474-9726.2007.00279.x
23. Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean Platelet Volume: A Link Between Thrombosis and Inflammation. Curr Pharm Des (2011) 17(1):47–58. doi: 10.2174/138161211795049804
24. Pujol-Moix N, Vázquez-Santiago M, Morera A, Ziyatdinov A, Remacha A, Nomdedeu J, et al. Genetic Determinants of Platelet Large-Cell Ratio, Immature Platelet Fraction, and Other Platelet-Related Phenotypes. Thromb Res (2015) 136(2):361–6. doi: 10.1016/j.thromres.2015.06.016
25. Ishibashi Y, Tsujimoto H, Kouzu K, Itazaki Y, Tsuchiya S, Fujishima S, et al. Impact of Antiplatelet and Anticoagulant Therapies on Platelet-Related Prognostic Markers in Patients With Esophageal Cancer. In Vivo (2020) 34(4):1941–9. doi: 10.21873/invivo.11991
26. Cho MS, Bottsford-Miller J, Vasquez HG, Stone R, Zand B, Kroll MH, et al. Platelets Increase the Proliferation of Ovarian Cancer Cells. Blood (2012) 120(24):4869–72. doi: 10.1182/blood-2012-06-438598
27. Wu B, Ye Y, Xie S, Li Y, Sun X, Lv M, et al. Megakaryocytes Mediate Hyperglycemia-Induced Tumor Metastasis. Cancer Res (2021) 81(21):5506–22. doi: 10.1158/0008-5472.CAN-21-1180
28. Hufnagel DH, Cozzi GD, Crispens MA, Beeghly-Fadiel A. Platelets, Thrombocytosis, and Ovarian Cancer Prognosis: Surveying the Landscape of the Literature. Int J Mol Sci (2020) 21(21):8169. doi: 10.3390/ijms21218169
29. Wang YH, Kang JK, Zhi YF, Zhang Y, Wang Y, Zhou Q, et al. The Pretreatment Thrombocytosis as One of Prognostic Factors for Gastric Cancer: A Systematic Review and Meta-Analysis. Int J Surg (2018) 53:304–11. doi: 10.1016/j.ijsu.2018.03.084
30. Ma Y, Li G, Yu M, Sun X, Nian J, Gao Y, et al. Prognostic Significance of Thrombocytosis in Lung Cancer: A Systematic Review and Meta-Analysis. Platelets (2021) 32(7):919–27. doi: 10.1080/09537104.2020.1810653
31. Garmi N, Nasrallah S, Baram Y, Katz A, Koren A, First M, et al. Platelets and Breast Cancer. Isr Med Assoc J (2020) 22(10):613–7.
32. Liu PH, Hsu CY, Su CW, Huang YH, Hou MC, Rich NE, et al. Thrombocytosis is Associated With Worse Survival in Patients With Hepatocellular Carcinoma. Liver Int (2020) 40(10):2522–34. doi: 10.1111/liv.14560
33. Jokisch JF, Grimm T, Buchner A, Kretschmer A, Weinhold P, Stief CG, et al. Preoperative Thrombocytosis in Patients Undergoing Radical Cystectomy for Urothelial Cancer of the Bladder: An Independent Prognostic Parameter for an Impaired Oncological Outcome. Urol Int (2020) 104(1-2):36–41. doi: 10.1159/000500729
34. Qin L, Li JY, Huang WJ, Zhang ML, Wang RT, Shen W. Higher Platelet Distribution Width is Associated With Unfavorable Prognosis in Ovarian Cancer. Cancer biomark (2020) 28(3):365–70. doi: 10.3233/CBM-191190
35. Huang Y, Cui MM, Huang YX, Fu S, Zhang X, Guo H, et al. Preoperative Platelet Distribution Width Predicts Breast Cancer Survival. Cancer biomark (2018) 23(2):205–11. doi: 10.3233/CBM-181267
36. Citirik M, Beyazyildiz E, Simsek M, Beyazyildiz O, Haznedaroglu IC. MPV may Reflect Subcinical Platelet Activation in Diabetic Patients With and Without Diabetic Retinopathy. Eye (Lond) (2015) 29(3):376–9. doi: 10.1038/eye.2014.298
37. Deng Q, Long Q, Liu Y, Yang Z, Du Y, Chen X. Prognostic Value of Preoperative Peripheral Blood Mean Platelet Volume/Platelet Count Ratio (MPV/PC) in Patients With Resectable Cervical Cancer. BMC Cancer (2021) 21(1):1282. doi: 10.1186/s12885-021-09016-8
38. Ning Y, Yang H, Qin S, Cao BR, Zhong ZX, He CS, et al. Prognostic Value of Preoperative Mean Platelet Volume and a Predictive Nomogram in Oral Squamous Cell Carcinoma Patients Based on Real-World Data. Cancer Manag Res (2021) 13:8495–509. doi: 10.2147/CMAR.S323117
39. Asare R, Opoku-Okrah C, Danquah KO, Opare-Sem O, Addai-Mensah O, Gyamfi D, et al. Expression of Platelet Parameters and Platelet Membrane Glycoproteins in Childhood Burkitt Lymphoma. Leuk Res (2019) 84:106189. doi: 10.1016/j.leukres.2019.106189
40. Psaila B, Bussel JB, Frelinger AL, Babula B, Linden MD, Li Y, et al. Differences in Platelet Function in Patients With Acute Myeloid Leukemia and Myelodysplasia Compared to Equally Thrombocytopenic Patients With Immune Thrombocytopenia. J Thromb Haemost (2011) 9(11):2302–10. doi: 10.1111/j.1538-7836.2011.04506.x
Keywords: myelodysplastic syndromes (MDS), platelet–large cell ratio (P-LCR), survival, prognosis, biomarker
Citation: Chen Q, Chen Y, Zhang Y, Zhang L, Chen K, He Z, Wang C and Yu L (2022) Prognostic Impact of Platelet-Large Cell Ratio In Myelodysplastic Syndromes. Front. Oncol. 12:846044. doi: 10.3389/fonc.2022.846044
Received: 30 December 2021; Accepted: 08 March 2022;
Published: 01 April 2022.
Edited by:
Lorenzo Falchi, Memorial Sloan Kettering Cancer Center, United StatesReviewed by:
Ibrahim C. Haznedaroglu, Hacettepe University Hospital, TurkeyCopyright © 2022 Chen, Chen, Zhang, Zhang, Chen, He, Wang and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunling Wang, d2NsNjUwNkAxNjMuY29t; Liang Yu, eXVsaWFuZ2hhQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.