- 1Medical School, National and Kapodistrian University of Athens, Athens, Greece
- 2Department of Surgery, Division of Hepatobiliary Surgery and Liver Transplantation, Vanderbilt University Medical Center, Nashville, TN, United States
- 3Department of Surgery, Duke University Medical Center, Durham, NC, United States
Irreversible electroporation (IRE) is a local ablative technique used in conjunction with chemotherapy to treat locally advanced pancreatic cancer (LAPC). The combination of IRE and chemotherapy has showed increased overall survival when compared to chemotherapy alone, pointing towards a possible facilitating effect of IRE on chemotherapeutic drug action and delivery. This review aims to present current chemotherapeutic regimens for LAPC and their co-implementation with IRE, with an emphasis on possible molecular augmentative mechanisms of drug delivery and action. Moreover, the potentiating mechanism of IRE on immunotherapy, M1 oncolytic virus and dendritic cell (DC)-based treatments is briefly explored. Investigating the synergistic effect of IRE on currently established treatment regimens as well as newer ones, may present exciting new possibilities for future studies seeking to improve current LAPC treatment algorithms.
Introduction
By 2030, pancreatic cancer is expected to be the second most common cause of malignancy-related mortality in the United States (1). Although surgical resection is the mainstay treatment with curative intent, only up to 30% of pancreatic cancer cases are amenable to resection (2, 3). Hence, according to the extent of vascular involvement and tumor resectability, non-metastatic disease is classified as resectable, borderline resectable, and locally advanced pancreatic cancer (LAPC) (4), with LAPC having a variable definition depending on the different consensus guidelines (Table 1) (5–11). The standard of care for resectable pancreatic cancer is resection followed by adjuvant chemotherapy, and for borderline pancreatic cancer is neoadjuvant therapy (12). A recent meta-analysis of randomized clinical trials also demonstrated that neoadjuvant gemcitabine-based chemo(radio)therapy – without adjuvant FOLFIRINOX (5-fluorouracil/leucovorin plus oxaliplatin and irinotecan) – led to improved overall survival in patients with borderline resectable pancreatic cancer, yet its role in resectable cases warrants further exploration (4). The introduction of more effective chemotherapy regimens, such as FOLFIRINOX and gemcitabine plus albumin-bound paclitaxel, within the past 10 years, has also been important for patients with LAPC as both the resectability and survival of LAPC after neoadjuvant therapy have increased (13–20). Therefore, either FOLFIRINOX or gemcitabine plus albumin-bound paclitaxel chemotherapy is currently the first-line treatment for patients with LAPC, and response is monitored using biochemical (e.g., carbohydrate antigen 19-9), radiographic [e.g., Response Evaluation Criteria in Solid Tumors (RECIST) criteria on computed tomography scan], and metabolic response (e.g., positron emission tomography scan) criteria (21).
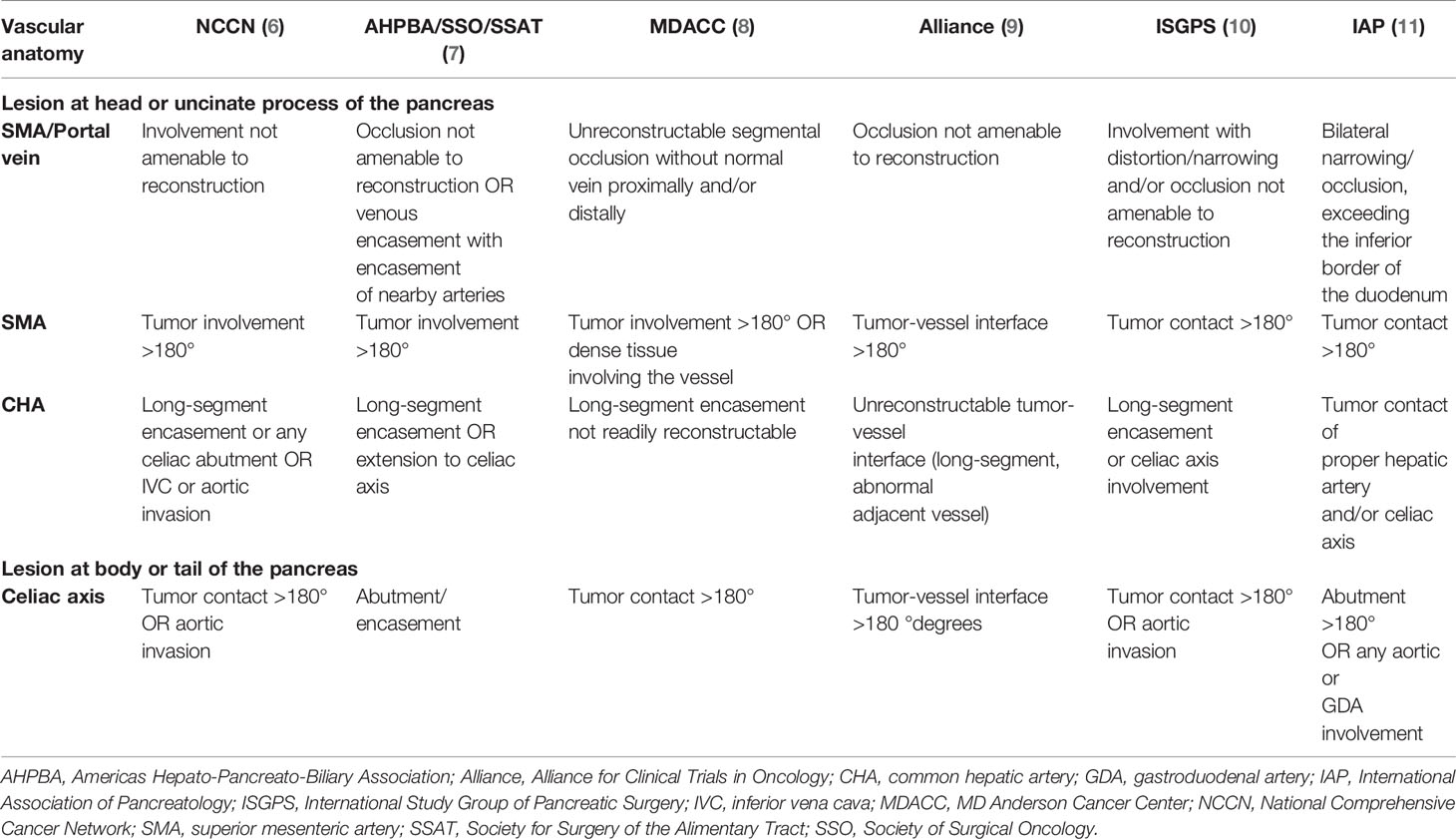
Table 1 Locally advanced pancreatic cancer definition according to different guidelines (5).
Admittedly, the management of patients with LAPC unresponsive to neoadjuvant therapy is an area of great debate among the pancreatic surgery community. Of note, the current level of evidence precludes us from deducing meaningful conclusions on whether surgery can yield a survival benefit over a non-surgical approach, especially since these pancreatic resections involve major vessel resections and reconstructions commonly accompanied by great morbidity (22–29). Instead, irreversible electroporation (IRE), a form of nonthermal injury initially used in 2009 for LAPC (30, 31), has been more frequently utilized as a consolidative therapy for LAPC with favorable outcomes. Whereas other locally ablative techniques, such as radiofrequency ablation, have been employed in treating simple-shaped, mass-forming LAPC, IRE is known to target tumors with intricate formations, even in case of major blood vessel encasement (32). In addition, IRE has been proposed to have a complementary role to other modalities, such as chemotherapy, newer immunotherapies, or electrogene therapy. The aim of this review is to discuss current chemotherapy options for LAPC patients and elaborate on the facilitative effect of IRE on intra-tumoral delivery and action of chemotherapy, immunotherapy, newer electrogene and dendritic cell (DC)-based ex vivo techniques.
Current Chemotherapy Options for Locally Advanced Pancreatic Cancer
In the era of genome-wide association studies and next generation sequencing, chemotherapy is tailored to address individual tumors down to the level of single-nucleotide-polymorphisms (33). It has been made possible to estimate qualities, such as tumor burden and resistance to chemotherapeutic regimens, by recognizing key mutations in the cancer cell genome. Nowadays, personalized medicine has evolved into a tool of paramount importance in choosing the appropriate chemotherapeutic regimen against LAPC.
The randomized phase III PRODIGE trial evaluated the use of FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer in patients with good performance status and demonstrated significant improvements in survival using FOLFIRINOX (13). Based on these results, FOLFIRINOX is included as a preferred, category 1 recommendation for first-line therapy for patients with metastatic pancreatic cancer and good performance status [i.e., Eastern Cooperative Oncology Group (ECOG) performance status 0-1]. By extrapolation, this regimen is considered a category 2A recommendation for LAPC patients in the National Comprehensive Cancer Network (NCCN) guidelines (12). However, there are certain concerns regarding FOLFIRINOX-induced toxicity, as in the PRODIGE trial, the FOLFIRINOX group had significantly higher rates of neutropenia, diarrhea, thrombocytopenia, and sensory neuropathy and decreased quality of life compared to the gemcitabine group (13). Therefore, prospective data showed that a modified FOLFIRINOX regimen with a 25% reduced initial bolus dose of 5-fluorouracil and irinotecan can mitigate chemotherapy-induced toxicities without a negative effect on survival; as such, the modified FOLFIRINOX regimen is also included as a preferred treatment option in the NCCN guidelines (12, 34).
For metastatic or LAPC, gemcitabine has shown a modest clinical and survival benefit over bolus 5-fluorouracil (35). Therefore, the NCCN guidelines recommend gemcitabine monotherapy as a category 1 front-line option for good performance status metastatic and LAPC patients and as a reasonable category 1 first- or second-line option for symptomatic poor performance status patients (12). Gemcitabine monotherapy is also a category 1 therapy for adjuvant treatment after resection as the phase III CONKO-001 trial showed a survival benefit using adjuvant gemcitabine over observation after macroscopically complete resection in patients without prior chemotherapy or radiation (36, 37). Additionally, the randomized phase III MPACT trial compared gemcitabine plus albumin-bound paclitaxel versus gemcitabine alone in patients with metastatic pancreatic cancer and no prior chemotherapy and demonstrated improved response and survival in the gemcitabine plus albumin-bound paclitaxel group (14, 38). Therefore, gemcitabine plus albumin-bound paclitaxel is listed in the NCCN guidelines as a preferred category 1 recommendation for good performance status (i.e., ECOG performance status 0-2) metastatic pancreatic cancer patients, and by extrapolation as a category 2A recommendation for LAPC patients as well (12).
Current Experience With IRE
IRE initially showed promise when applied to animal models (39, 40). Moreover, in human patients, since 2009, Nanoknife (Angiodynamics, Latham, NY, USA) has been used as a Food and Drug Administration-approved IRE delivery system. Currently, IRE has proven to be a safe procedure with a clear benefit in overall survival of LAPC patients when combined with chemotherapy. Recently, experience from a cohort of 40 patients undergoing IRE in Greece from 2015 to 2019 showed a median overall survival of 24.2 months with few major grade III complications (two out of 40 patients developing pancreatic fistula). Importantly, 33 out of 40 patients had undergone FOLFIRINOX or nab-paclitaxel neoadjuvant therapy prior to IRE, and after repeat imaging showed no disease progression, they underwent local ablation through electroporation (41).
Martin et al. (30) initially commented on the safety of IRE in treating LAPC in 2012. They recruited 27 patients with grade III pancreatic adenocarcinoma who had previously received various chemotherapy regimens. Preoperatively, 24 patients had reported 100% performance status. IRE delivery had a 100% technical success. Median stay in the hospital following IRE was 9 days. The cost of the procedure, however, was high (2,000$ per probe). Importantly, the role of extensive surgeon experience using IRE (50 cases at minimum using NanoKnife), or similar ablative techniques, in pancreas or other organs (i.e., liver, kidney), was emphasized, based on predictive learning curves (30).
More recently, in a systematic review by Moris et al. (42) gathering results up to August 2018, IRE was found to be technically feasible and with few side-effects in a total of 498 patients. Open, laparoscopic, and percutaneous approaches were compared. However, results regarding the survival benefit of laparoscopic IRE vs open therapies were ambiguous. Additionally, with open-approach IRE, 35.8% of patients experienced postoperative morbidity, with 21.5% being major incidents (Clavien-Dindo grade ≥III) (42). On the other hand, laparoscopic IRE was associated with lower morbidity (24.3% overall morbidity and 13.3% Clavien-Dindo grade ≥III) (42). The overall reported mortality following IRE was 2% (42). However, open IRE is most commonly performed, as it allows for more accurate placement of the probes and gives the surgeon the opportunity to evaluate whether a LAPC may be resectable, despite previous imaging studies suggesting otherwise (43, 44).
Lafranceschina et al. (45) summarized the outcomes of 691 patients with unresectable LAPC previously treated with chemotherapy who underwent IRE. The CROSSFIRE trial, gathering 138 patients from the Netherlands, and the AHPBA trial, with 500 patients from the USA, Japan, Taiwan, and the UK, as well as smaller studies from China, France, and Canada, were combined. Median patient age was 63 years, and tumor size ranged between 2.8 to 4.5 cm. Ideally, tumor size between 3-4 cm showed best results following IRE. The overall morbidity rate was 30.5% and complications included pancreatic fistula, pancreatitis, thrombosis, and pseudoaneurysm formation, while mortality following IRE was 3.4%. Interestingly, overall survival was between 10-27 months following IRE compared to 6-11.5 months in patients treated with chemotherapy and/or radiation without IRE.
In 2020, Ruarus et al. (46) published a phase II study with a total of 50 patients. Despite an increased survival in patients undergoing IRE compared to chemotherapy, there was no proof of synergy between the two. Ten patients had recurrent LAPC. Overall survival was 17 months following diagnosis (10 months following IRE application), and 16 months in the recurrence group. Only 22 patients had received induction chemotherapy with FOLFIRINOX. Of the 50 patients, there was one recorded death related to IRE. Of note, there was no difference in survival in patients who had received FOLFIRINOX induction vs those with gemcitabine or no chemotherapy. Therefore, IRE was the main determinant of increased survival in those patients. The aim of 12-14 months survival following only conventional chemotherapy was exceeded by patients participating in the study.
A poorly addressed topic in the literature is that of IRE-related morbidity and mortality. In a review of the literature, Charalambous et al. in 2020 included a total of 460 patients across 9 studies (47). They concluded that overall mortality was 3.4% (amongst the causes were peritonitis, hepatojejunostomy site ulceration and bleeding, bile duct necrosis, enterocutaneous fistula, gastroduodenal artery bleeding and multisystem organ failure) while major (Clavien-Dindo class III or higher) morbidity was 10.2% (Table 2). Overall morbidity was 29.4% at 90 days following open IRE. An open approach seems to be associated with higher morbidity. Nevertheless, it is the most commonly administered method, and, according to the authors, is associated with increased overall survival versus laparoscopic or percutaneous approaches. Sugumar et al. in 2021 conducted a systematic review of the literature including a total of 2,768 patients (53). They reported 12-month major complication and mortality rates of 18% and 2.65%, respectively, following a combination of multimodal therapy and IRE. One-year overall and progression-free survival rates were 55% and 12%, respectively (53). Importantly, the authors concluded that multimodal LAPC therapy with IRE had a similar overall mortality rate to multimodal therapy without IRE. Therefore, they recommended the use of IRE only as an experimental treatment modality in current clinical practice (53).
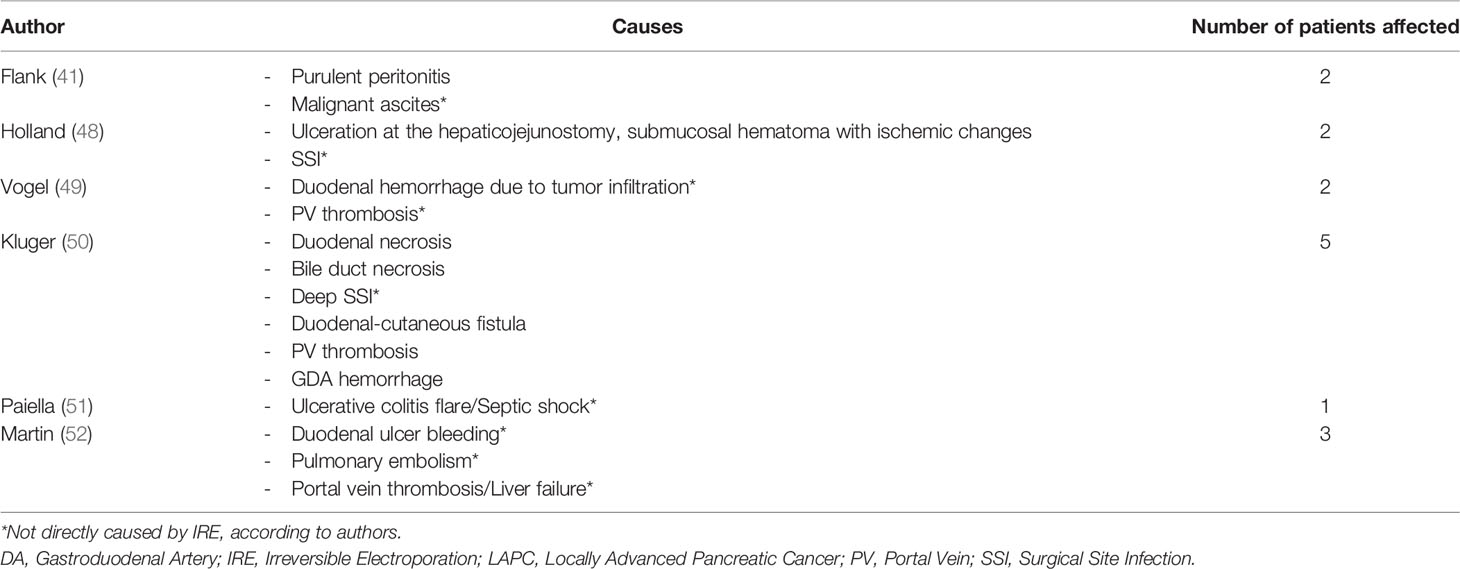
Table 2 Mortality causes (in patients treated with IRE for LAPC) (45).
IRE Amplification of LAPC-Targeted Drug Effect and Delivery
Aside from direct apoptotic and possible chemotherapy-augmenting effects, IRE has been postulated to have a local immunologic impact on the tumor bed, which provides an opportunity for immunotherapeutic regimens to take effect (54). Hence, there are many ways IRE can affect tumor cells, either directly by promoting apoptosis, or indirectly, through facilitation of other drug mechanisms (Figure 1).
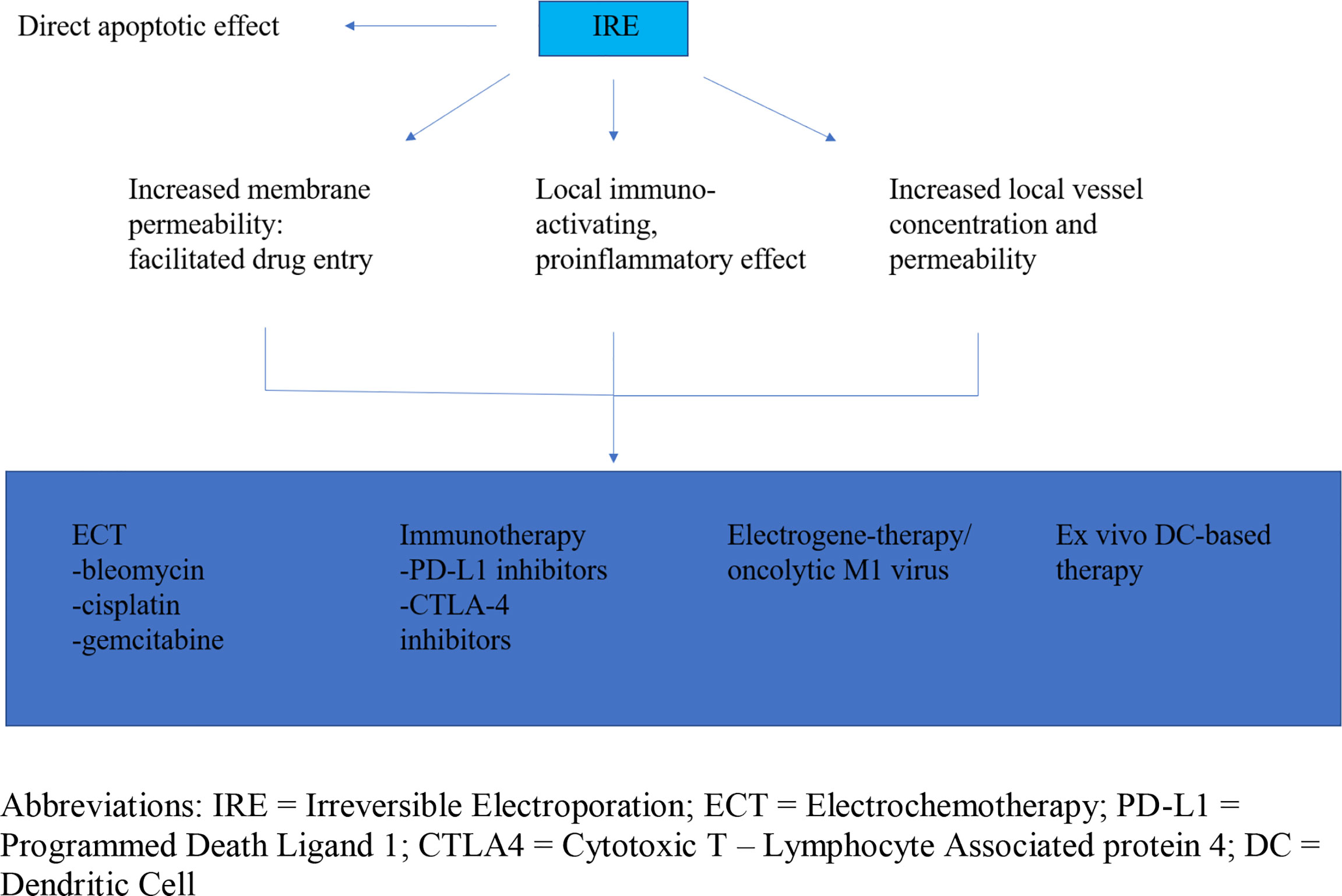
Figure 1 Irreversible electroporation anti-tumor effect and facilitation of drug mechanism and delivery. IRE, Irreversible Electroporation; ECT, Electrochemotherapy; PD-L1, Programmed Death Ligand 1; CTLA4, Cytotoxic T – Lymphocyte Associated protein 4; DC, Dendritic Cell.
Electrochemotherapy (ECT) is the co-implementation of IRE and chemotherapy in treating LAPC (55). IRE penetrates cell membranes rendering cancer cells susceptible to the effects of chemotherapeutic drugs with otherwise little ability to infiltrate the cell. Current ECT regimens include bleomycin and cisplatin. With regards to pancreatic cancer, few small studies so far have exhibited promising results with minimal side effects (56–58). Most recently, a randomized control trial by Izzo et al. evaluated the effectiveness of ECT with subsequent chemotherapy (FOLFOXIRI) versus only chemotherapy in inoperable LAPC and highlighted the importance of multiple insertions with variable geometry ensuring a more complete coverage of the tumoral lesion as this can lead to improvements in both local disease control and overall survival (59). However, one drawback of ECT is its limited effect on distant metastases, despite its good local suppressive effects (60). Recently, IRE-facilitated intra-tumoral transfer of gemcitabine, an established chemotherapeutic regimen for metastatic pancreatic cancer and patients with poor functional status, has also demonstrated promising results (61). Finally, another study beginning in 2021 aims to compare overall survival and progression-free survival rates of ECT (bleomycin) to IRE and calcium electroporation (i.e., the influx of calcium in electro-porated cells resulting in cell death) in pancreatic cancer patients with poor prognosis in Poland (62). It remains to be seen whether those innovative approaches to dealing with inoperable disease will provide a viable alternative for LAPC patients.
Newer immunotherapeutic regimens, such as immune checkpoint inhibitors against cytotoxic T-lymphocyte-associated protein 4 and programmed cell death protein-1, did not show favorable results when used alone for LAPC (63). Notably, IRE is known to have an immunologic effect, affecting the tumor milieu in such a way that it tips the scale from local immunosuppression to inflammation and tumor cell immune recognition and destruction (64). Namely, 14 days after IRE application, there was notable helper and memory T cell number proliferation, whilst Tregs were shown to be decreased. The number of macrophages rose as well, while natural killer (NK) cells did not show a significant increase (65–67). Therefore, it would be clinically interesting to examine the interaction of IRE with current immunotherapy regimens during this short window of immunologic opportunity.
Electrogene-therapy is the transfer of therapeutic genes inside tumor cells by means of electroporation (60). A similar principle is used in M1 virus insertion into pancreatic cancer cells. M1 is an anti-tumor RNA virus whose protein product stimulates cancer cell apoptosis. M1 oncolytic virus-treated mice with LAPC showed increased survival after treatment (68). Following IRE, pores are created on the cell surface, allowing M1 to enter without requiring a specialized viral transporter. Furthermore, the local application of IRE enhances vessel permeability and increases local vessel concentration within the tumor bed, thereby achieving larger concentrations of M1 virus. And as IRE transforms the immune-suppressed tumor microenvironment into an immuno-active, proinflammatory one, T cell activation against M1 oncolytic virus-infected cells is facilitated even further.
DCs are antigen-presenting cells of the immune system with a role in stimulating immune response against foreign antigens. DCs presenting certain antigens on their surface are taken from patients and cultured ex vivo. They are then re-introduced into the patient, exerting an anti-tumor effect by alerting the host immune system. Local tumor immunosuppression, however, has an inhibitory effect on the action of DCs on the tumor bed (69, 70). Once again, the local immune-activating effect of IRE allows downstream activated T and B cells to penetrate cancer cells, therefore facilitating the effect of injected DCs (71).
Application of IRE in Conjunction With Chemotherapy
Before commencing with either FOLFIRINOX or gemcitabine and subsequent IRE, triphasic computed tomography scan with pancreatic protocol (0.7 mm cuts) and three-dimensional reconstruction is performed to appropriately stage the tumor (72). The presence or absence of superior mesenteric artery or vein or celiac artery encasement, distant metastases or peritoneal spread on imaging will guide the choice of chemotherapy and determine whether an operative approach is possible (5–11). Moreover, diagnostic laparoscopy is performed with paracolic and pelvic washing to detect smaller distant tumor foci that would again signify inoperable disease. Importantly, IRE is a locally ablative technique with no effect on distant metastases. After induction chemotherapy, and if no metastases are detected on repeat imaging, and pancreatic tumor axial diameter is < 4.0 cm, IRE can be performed 2-4 weeks after the last dose of chemotherapy, per RECIST criteria (Figure 2) (73).
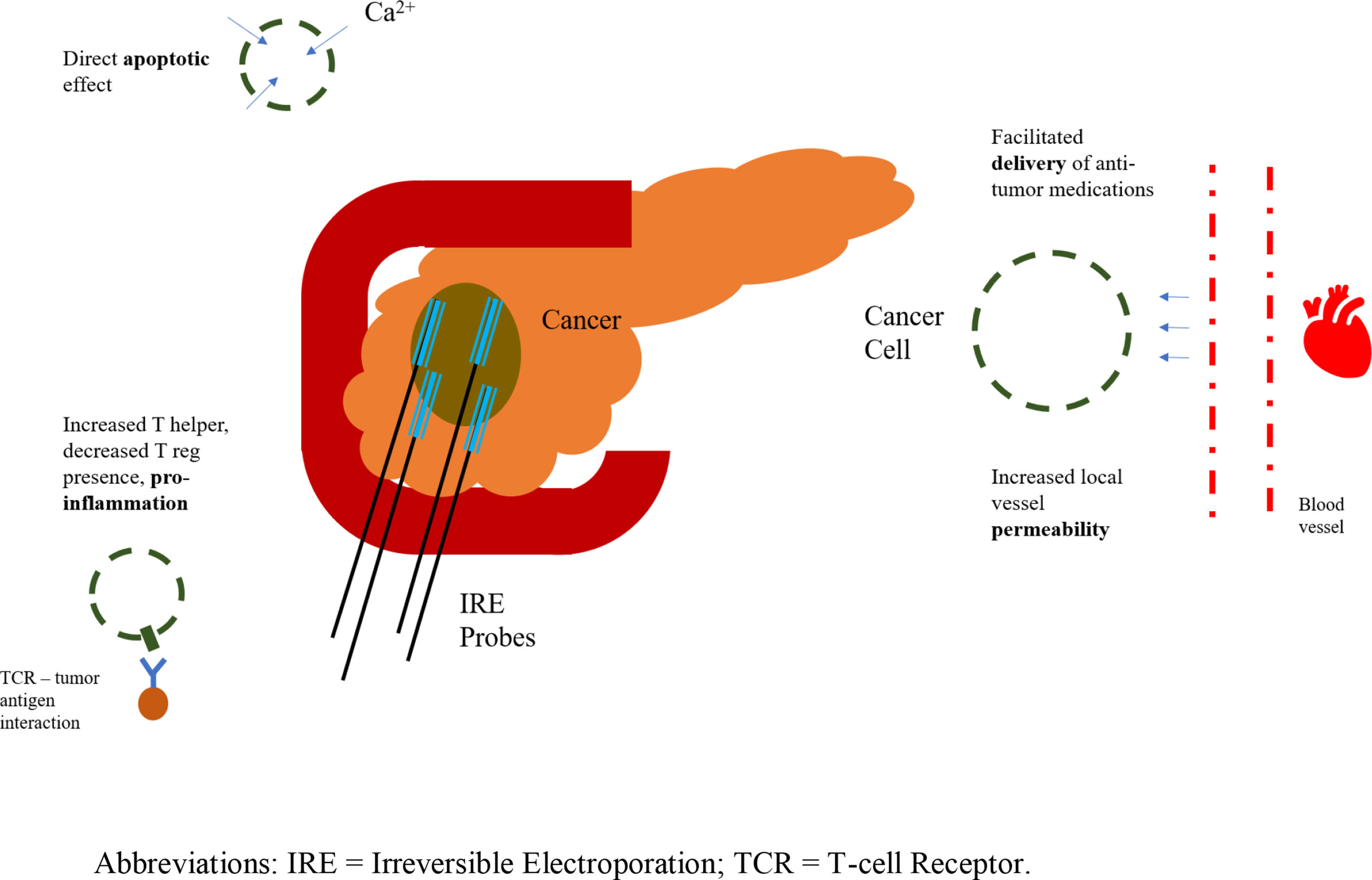
Figure 2 Irreversible electroporation application; representation of probes and anti-tumor effects. IRE, Irreversible Electroporation; TCR, T-cell Receptor.
Chemotherapy in LAPC aims to shrink the existing tumor to make negative-margin surgical resection feasible. Neoadjuvant chemotherapy has also decreased the need for vascular reconstruction, a particularly challenging aspect of surgical resection for LAPC (74). In the past, vascular reconstruction was carried out at highly specialized centers but was not a technique widely available (75, 76). However, advances in surgical technique combined with newer chemotherapeutic regimens have rendered vascular resection and subsequent reconstruction in LAPC a common practice (77). Currently, patients with LAPC being treated with chemotherapy can either remain stable or, rarely, have their disease downgraded (78). In a subset of patients, unfortunately, LAPC can progress and metastasize during neoadjuvant chemotherapy. Should LAPC remain stable or be downgraded to borderline resectable disease, surgical excision becomes an option (75). Locally ablative techniques, such as IRE, can also be implemented towards that goal. Indeed, Sadot et al. in 2015 reported that in a total of 101 patients with LAPC and median follow-up of 12 months, following 6 cycles of FOLFIRINOX, one third were able to undergo local resection (25). Suker et al. in 2016 conducted a systematic review showing that close to 30 percent of patients receiving FOLFIRINOX were able to have their LAPC resected by the end of the treatment (91 of 325 patients) (79). However, if metastases occur, the tumor is inoperable and there is no utility in performing IRE, since it has no effect on distant foci of disease. Sadot et al. reported that 23% of their patients developed distant metastatic foci by the end of the neoadjuvant treatment (25). Hence, the benefit of possible synergism between chemotherapy and IRE must be weighed against the risk of disease progression during initial neoadjuvant chemotherapy.
An important consideration regarding current chemotherapy regimens (i.e., FOLFIRINOX, gemcitabine) is that trials to date have primarily included patients with metastatic disease, not LAPC (13, 14). While chemotherapy can be used in both metastatic and LAPC, IRE is only applicable in the latter. LAPC-specific randomized clinical trials that compare FOLFIRINOX to gemcitabine are yet to be undertaken.
The mechanism by which IRE facilitates the delivery and action of chemotherapeutic drugs is complex. Chemotherapy, however, even without IRE, has an established benefit in treating pancreatic cancer patients. While studies have shown mixed results, the application of neoadjuvant chemotherapy with subsequent IRE has previously proven superior to chemotherapy alone in extending overall survival in patients with LAPC (80). This possible synergism points to an potentiating effect of IRE on existing chemotherapeutic regimen mechanism of action. Understanding the way IRE facilitates the delivery and action of various immunotherapeutic regimens, electrogene modalities and DC based treatments is key in incorporating IRE in the current standard treatment algorithm for LAPC, including chemotherapy and possibly surgical resection.
Conclusion
IRE has a direct pro-apoptotic effect on LAPC cells by increasing membrane permeability and disrupting cancer cell homeostasis. However, it also seems to facilitate the delivery and action of chemotherapeutic regimens (bleomycin, cisplatin, and gemcitabine). Indeed, adjunctive chemotherapy followed by IRE has shown superior overall survival over chemotherapy alone. IRE, however, also appears to augment the effect of immunotherapy, M1 oncovirus- and DC-based therapies for LAPC. Further research is needed to examine the potentiating effects of IRE on anti-LAPC drug delivery and action. Current examination of potential facilitating mechanisms points to a key role of IRE in future LAPC treatment algorithms.
Author Contributions
AG, IZ, and DM conceived and designed the study, acquired, analyzed, and interpreted the data, drafted, and critically revised the manuscript, and approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
IRE, Irreversible Electroporation; LAPC, Locally Advanced Pancreatic Cancer, ECT, Electrochemotherapy, DC, Dendritic Cell.
References
1. Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting Cancer Incidence and Deaths to 2030: The Unexpected Burden of Thyroid, Liver, and Pancreas Cancers in the United States. Cancer Res (2014) 74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155
2. Huang L, Jansen L, Balavarca Y, Molina-Montes E, Babaei M, van der Geest L, et al. Resection of Pancreatic Cancer in Europe and USA: An International Large-Scale Study Highlighting Large Variations. Gut (2019) 68:130–9. doi: 10.1136/gutjnl-2017-314828
3. Loveday BPT, Zilbert N, Serrano PE, Tomiyama K, Tremblay A, Fox AM, et al. Neoadjuvant Therapy and Major Arterial Resection for Potentially Reconstructable Arterial Involvement by Stage 3 Adenocarcinoma of the Pancreas. HPB (2019) 21:643–52. doi: 10.1016/j.hpb.2018.10.004
4. van Dam JL, Janssen QP, Besselink MG, Homs MYV, van Santvoort HC, van Tienhoven G, et al. Neoadjuvant Therapy or Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: A Meta-Analysis of Randomised Controlled Trials. Eur J Cancer (Oxford England: 1990) (2021). doi: 10.1016/j.ejca.2021.10.023
5. Fromer MW, Hawthorne J, Philips P, Egger ME, Scoggins CR, McMasters KM, et al. An Improved Staging System for Locally Advanced Pancreatic Cancer: A Critical Need in the Multidisciplinary Era. Ann Surg Oncol (2021) 28:6201–10. doi: 10.1245/s10434-021-10174-z
6. Tempero MA, Malafa MP, Behrman SW, Benson AB 3rd, Casper ES, Chiorean EG, et al. Pancreatic Adenocarcinoma, Version 2.2014: Featured Updates to the NCCN Guidelines. J Natl Compr Cancer Network: JNCCN (2014) 12:1083–93. doi: 10.6004/jnccn.2014.0106
7. Vauthey J-N, Dixon E. AHPBA/SSO/SSAT Consensus Conference on Resectable and Borderline Resectable Pancreatic Cancer: Rationale and Overview of the Conference. Ann Surg Oncol (2009) 16:1725–6. doi: 10.1245/s10434-009-0409-5
8. Varadhachary GR, Tamm EP, Abbruzzese JL, Xiong HQ, Crane CH, Wang H, et al. Borderline Resectable Pancreatic Cancer: Definitions, Management, and Role of Preoperative Therapy. Ann Surg Oncol (2006) 13:1035–46. doi: 10.1245/ASO.2006.08.011
9. Katz MHG, Marsh R, Herman JM, Shi Q, Collison E, Venook AP, et al. Borderline Resectable Pancreatic Cancer: Need for Standardization and Methods for Optimal Clinical Trial Design. Ann Surg Oncol (2013) 20:2787–95. doi: 10.1245/s10434-013-2886-9
10. Bockhorn M, Uzunoglu FG, Adham M, Imrie C, Milicevic M, Sandberg AA, et al. Borderline Resectable Pancreatic Cancer: A Consensus Statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery (2014) 155:977–88. doi: 10.1016/j.surg.2014.02.001
11. Isaji S, Mizuno S, Windsor JA, Bassi C, Fernández-Del Castillo C, Hackert T, et al. International Consensus on Definition and Criteria of Borderline Resectable Pancreatic Ductal Adenocarcinoma 2017. Pancreatology (2018) 18:2–11. doi: 10.1016/j.pan.2017.11.011
12. Tempero MA, Malafa MP, Al-Hawary M, Behrman SW, Benson AB, Cardin DB, et al Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2021) 19(4):439–57. doi: 10.6004/jnccn.2021.0017
13. Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX Versus Gemcitabine for Metastatic Pancreatic Cancer. N Engl J Med (2011) 364:1817–25. doi: 10.1056/NEJMoa1011923
14. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased Survival in Pancreatic Cancer With Nab-Paclitaxel Plus Gemcitabine. N Engl J Med (2013) 369:1691–703. doi: 10.1056/NEJMoa1304369
15. Satoi S, Yamaue H, Kato K, Takahashi S, Hirono S, Takeda S, et al. Role of Adjuvant Surgery for Patients With Initially Unresectable Pancreatic Cancer With a Long-Term Favorable Response to Non-Surgical Anti-Cancer Treatments: Results of a Project Study for Pancreatic Surgery by the Japanese Society of Hepato-Biliary-Pancreatic Surgery. J Hepato Biliary Pancreatic Sci (2013) 20:590–600. doi: 10.1007/s00534-013-0616-0
16. Hackert T, Sachsenmaier M, Hinz U, Schneider L, Michalski CW, Springfeld C, et al. Locally Advanced Pancreatic Cancer: Neoadjuvant Therapy With Folfirinox Results in Resectability in 60% of the Patients. Ann Surg (2016) 264:457–63. doi: 10.1097/SLA.0000000000001850
17. Gemenetzis G, Groot VP, Blair AB, Laheru DA, Zheng L, Narang AK, et al. Survival in Locally Advanced Pancreatic Cancer After Neoadjuvant Therapy and Surgical Resection. Ann Surg (2019) 270:340–7. doi: 10.1097/SLA.0000000000002753
18. Michelakos T, Pergolini I, Castillo CF-D, Honselmann KC, Cai L, Deshpande V, et al. Predictors of Resectability and Survival in Patients With Borderline and Locally Advanced Pancreatic Cancer Who Underwent Neoadjuvant Treatment With FOLFIRINOX. Ann Surg (2019) 269:733–40. doi: 10.1097/SLA.0000000000002600
19. Tanaka M, Heckler M, Mihaljevic AL, Sun H, Klaiber U, Heger U, et al. CT Response of Primary Tumor and CA19-9 Predict Resectability of Metastasized Pancreatic Cancer After FOLFIRINOX. Eur J Surg Oncol (2019) 45:1453–9. doi: 10.1016/j.ejso.2019.03.039
20. Rangelova E, Wefer A, Persson S, Valente R, Tanaka K, Orsini N, et al. Surgery Improves Survival After Neoadjuvant Therapy for Borderline and Locally Advanced Pancreatic Cancer: A Single Institution Experience. Ann Surg (2021) 273:579–86. doi: 10.1097/SLA.0000000000003301
21. Wu YHA, Oba A, Lin R, Watanabe S, Meguid C, Schulick RD, et al. Selecting Surgical Candidates With Locally Advanced Pancreatic Cancer: A Review for Modern Pancreatology. J Gastrointest Oncol (2021) 12:2475–83. doi: 10.21037/jgo-21-119
22. Datta J, Wilson GC, D’Angelica MI, Katz MHG, Maithel SK, Merchant NB, et al. A Call for Caution in Overinterpreting Exceptional Outcomes After Radical Surgery for Pancreatic Cancer: Let the Data Speak. Ann Surg (2021) 274:e82–4. doi: 10.1097/SLA.0000000000004471
23. Tee MC, Krajewski AC, Groeschl RT, Farnell MB, Nagorney DM, Kendrick ML, et al. Indications and Perioperative Outcomes for Pancreatectomy With Arterial Resection. J Am Coll Surgeons (2018) 227:255–69. doi: 10.1016/j.jamcollsurg.2018.05.001
24. Hammel P, Huguet F, van Laethem J-L, Goldstein D, Glimelius B, Artru P, et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA (2016) 315:1844–53. doi: 10.1001/jama.2016.4324
25. Sadot E, Doussot A, O’Reilly EM, Lowery MA, Goodman KA, Do RKG, et al. FOLFIRINOX Induction Therapy for Stage 3 Pancreatic Adenocarcinoma. Ann Surg Oncol (2015) 22:3512–21. doi: 10.1245/s10434-015-4647-4
26. Reni M, Zanon S, Balzano G, Nobile S, Pircher CC, Chiaravalli M, et al. Selecting Patients for Resection After Primary Chemotherapy for Non-Metastatic Pancreatic Adenocarcinoma. Ann Oncol (2017) 28:2786–92. doi: 10.1093/annonc/mdx495
27. Bachellier P, Addeo P, Faitot F, Nappo G, Dufour P. Pancreatectomy With Arterial Resection for Pancreatic Adenocarcinoma: How Can It Be Done Safely and With Which Outcomes?: A Single Institution’s Experience With 118 Patients. Ann Surg (2020) 271:932–40. doi: 10.1097/SLA.0000000000003010
28. Klaiber U, Schnaidt ES, Hinz U, Gaida MM, Heger U, Hank T, et al. Prognostic Factors of Survival After Neoadjuvant Treatment and Resection for Initially Unresectable Pancreatic Cancer. Ann Surg (2021) 273:154–62. doi: 10.1097/SLA.0000000000003270
29. Nimptsch U, Krautz C, Weber GF, Mansky T, Grützmann R. Nationwide In-Hospital Mortality Following Pancreatic Surgery in Germany Is Higher Than Anticipated. Ann Surg (2016) 264:1082–90. doi: 10.1097/SLA.0000000000001693
30. Martin RCG 2nd, McFarland K, Ellis S, Velanovich V. Irreversible Electroporation Therapy in the Management of Locally Advanced Pancreatic Adenocarcinoma. J Am Coll Surgeons (2012) 215:361–9. doi: 10.1016/j.jamcollsurg.2012.05.021
31. Martin RCG 2nd, McFarland K, Ellis S, Velanovich V. Irreversible Electroporation in Locally Advanced Pancreatic Cancer: Potential Improved Overall Survival. Ann Surg Oncol (2013) 20 Suppl 3:S443–9. doi: 10.1245/s10434-012-2736-1
32. Paiella S, de Pastena M, D’Onofrio M, Crinò SF, Pan TL, de Robertis R, et al. Palliative Therapy in Pancreatic Cancer-Interventional Treatment With Radiofrequency Ablation/Irreversible Electroporation. Trans Gastroenterol Hepatol (2018) 3:80. doi: 10.21037/tgh.2018.10.05
33. Morganti S, Tarantino P, Ferraro E, D’Amico P, Duso BA, Curigliano G. Next Generation Sequencing (NGS): A Revolutionary Technology in Pharmacogenomics and Personalized Medicine in Cancer. Adv Exp Med Biol. (2019) 1168:9–30. doi: 10.1007/978-3-030-24100-1_2
34. Stein SM, James ES, Deng Y, Cong X, Kortmansky JS, Li J, et al. Final Analysis of a Phase II Study of Modified FOLFIRINOX in Locally Advanced and Metastatic Pancreatic Cancer. Br J Cancer (2016) 114:737–43. doi: 10.1038/bjc.2016.45
35. Burris HA 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, et al. Improvements in Survival and Clinical Benefit With Gemcitabine as First-Line Therapy for Patients With Advanced Pancreas Cancer: A Randomized Trial. J Clin Oncol (1997) 15:2403–13. doi: 10.1200/JCO.1997.15.6.2403
36. Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant Chemotherapy With Gemcitabine vs Observation in Patients Undergoing Curative-Intent Resection of Pancreatic Cancer: A Randomized Controlled Trial. JAMA (2007) 297:267–77. doi: 10.1001/jama.297.3.267
37. Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, et al. Adjuvant Chemotherapy With Gemcitabine and Long-Term Outcomes Among Patients With Resected Pancreatic Cancer: The CONKO-001 Randomized Trial. JAMA (2013) 310:1473–81. doi: 10.1001/jama.2013.279201
38. Chiorean EG, Von Hoff DD, Reni M. CA19-9 Decrease at 8 Weeks as a Predictor of Overall Survival in a Randomized Phase III Trial (MPACT) of Weekly Nab-Paclitaxel Plus Gemcitabine Versus Gemcitabine Alone in Patients With Metastatic Pancreatic Cancer. Ann Oncol (2016) 27:654–60. doi: 10.1093/annonc/mdw006
39. Rubinsky B, Onik G, Mikus P. Irreversible Electroporation: A New Ablation Modality — Clinical Implications. Technol Cancer Res Treat (2007) 6:37–48. doi: 10.1177/153303460700600106
40. Charpentier KP, Wolf F, Noble L, Winn B, Resnick M, Dupuy DE. Irreversible Electroporation of the Pancreas in Swine: A Pilot Study. HPB (2010) 12:348–51. doi: 10.1111/j.1477-2574.2010.00174.x
41. Oikonomou D, Karamouzis MV, Moris D, Dimitrokallis N, Papamichael D, Kountourakis P, et al. Irreversible Electroporation (IRE) Combined With Chemotherapy Increases Survival in Locally Advanced Pancreatic Cancer (LAPC). Am J Clin Oncol (2021) 44:325–30. doi: 10.1097/COC.0000000000000826
42. Moris D, Machairas N, Tsilimigras DI, Prodromidou A, Ejaz A, Weiss M, et al. Systematic Review of Surgical and Percutaneous Irreversible Electroporation in the Treatment of Locally Advanced Pancreatic Cancer. Ann Surg Oncol (2019) 26:1657–68. doi: 10.1245/s10434-019-07261-7
43. Katz MHG, Fleming JB, Bhosale P, Varadhachary G, Lee JE, Wolff R, et al. Response of Borderline Resectable Pancreatic Cancer to Neoadjuvant Therapy Is Not Reflected by Radiographic Indicators. Cancer (2012) 118:5749–56. doi: 10.1002/cncr.27636
44. Dholakia AS, Hacker-Prietz A, Wild AT, Raman SP, Wood LD, Huang P, et al. Resection of Borderline Resectable Pancreatic Cancer After Neoadjuvant Chemoradiation Does Not Depend on Improved Radiographic Appearance of Tumor–Vessel Relationships. J Radiat Oncol (2013) 2:413–25. doi: 10.1007/s13566-013-0115-6
45. Lafranceschina S, Brunetti O, Delvecchio A, Conticchio M, Ammendola M, Currò G, et al. Systematic Review of Irreversible Electroporation Role in Management of Locally Advanced Pancreatic Cancer. Cancers (2019) 11:1718. doi: 10.3390/cancers11111718
46. Ruarus AH, Vroomen LGPH, Geboers B, van Veldhuisen E, Puijk RS, Nieuwenhuizen S, et al. Percutaneous Irreversible Electroporation in Locally Advanced and Recurrent Pancreatic Cancer (PANFIRE-2): A Multicenter, Prospective, Single-Arm, Phase II Study. Radiology (2020) 294:212–20. doi: 10.1148/radiol.2019191109
47. Charalambous P, Moris D, Karachaliou G-S, Papalampros A, Dimitrokallis N, Tsilimigras DI, et al. The Efficacy and Safety of the Open Approach Irreversible Electroporation in the Treatment of Pancreatic Cancer: A Systematic Review. Eur J Surg Oncol (2020) 46:1565–72. doi: 10.1016/j.ejso.2020.05.017
48. Holland MM, Bhutiani N, Kruse EJ, Weiss MJ, Christein JD, White RR, et al. A Prospective, Multi-Institution Assessment of Irreversible Electroporation for Treatment of Locally Advanced Pancreatic Adenocarcinoma: Initial Outcomes From the AHPBA Pancreatic Registry. HPB (2019) 21:1024–31. doi: 10.1016/j.hpb.2018.12.004
49. Vogel JA, Rombouts SJ, de Rooij T, van Delden OM, Dijkgraaf MG, van Gulik TM, et al. Induction Chemotherapy Followed by Resection or Irreversible Electroporation in Locally Advanced Pancreatic Cancer (IMPALA): A Prospective Cohort Study. Ann Surg Oncol (2017) 24:2734–43. doi: 10.1245/s10434-017-5900-9
50. Kluger MD, Epelboym I, Schrope BA, Mahendraraj K, Hecht EM, Susman J, et al. Single-Institution Experience With Irreversible Electroporation for T4 Pancreatic Cancer: First 50 Patients. Ann Surg Oncol (2016) 23:1736–43. doi: 10.1245/s10434-015-5034-x
51. Paiella S, Butturini G, Frigerio I, Salvia R, Armatura G, Bacchion M, et al. Safety and Feasibility of Irreversible Electroporation (IRE) in Patients With Locally Advanced Pancreatic Cancer: Results of a Prospective Study. Digest Surg (2015) 32:90–7. doi: 10.1159/000375323
52. Martin RCG, Kwon D, Chalikonda S, Sellers M, Kotz E, Scoggins C, et al. Treatment of 200 Locally Advanced (Stage III) Pancreatic Adenocarcinoma Patients With Irreversible Electroporation. Ann Surg (2015) 262:486–94. doi: 10.1097/SLA.0000000000001441
53. Sugumar K, Hurtado A, Naik I, Hue JJ, Rothermel LD, Ammori JB, et al. Multimodal Therapy With or Without Irreversible Electroporation for Unresectable Locally Advanced Pancreatic Adenocarcinoma: A Systematic Review and Meta-Analysis. HPB (2021) 24(5):586–95. doi: 10.1016/j.hpb.2021.12.014
54. Tian G, Guan J, Chu Y, Zhao Q, Jiang T. Immunomodulatory Effect of Irreversible Electroporation Alone and Its Cooperating With Immunotherapy in Pancreatic Cancer. Front Oncol (2021) 11:712042. doi: 10.3389/fonc.2021.712042
55. Geboers B, Scheffer HJ, Graybill PM, Ruarus AH, Nieuwenhuizen S, Puijk RS, et al. High-Voltage Electrical Pulses in Oncology: Irreversible Electroporation, Electrochemotherapy, Gene Electrotransfer, Electrofusion, and Electroimmunotherapy. Radiology (2020) 295:254–72. doi: 10.1148/radiol.2020192190
56. Granata V, Fusco R, Piccirillo M, Palaia R, Petrillo A, Lastoria S, et al. Electrochemotherapy in Locally Advanced Pancreatic Cancer: Preliminary Results. Int J Surg (2015) 18:230–6. doi: 10.1016/j.ijsu.2015.04.055
57. Rudno-Rudzińska J, Kielan W, Guziński M, Płochocki M, Antończyk A, Kulbacka J. New Therapeutic Strategy: Personalization of Pancreatic Cancer Treatment-Irreversible Electroporation (IRE), Electrochemotherapy (ECT) and Calcium Electroporation (CaEP) – A Pilot Preclinical Study. Surg Oncol (2021) 38:101634. doi: 10.1016/j.suronc.2021.101634
58. Girelli R, Prejanò S, Cataldo I, Corbo V, Martini L, Scarpa A, et al. Feasibility and Safety of Electrochemotherapy (ECT) in the Pancreas: A Pre-Clinical Investigation. Radiol Oncol (2015) 49:147–54. doi: 10.1515/raon-2015-0013
59. Izzo F, Granata V, Fusco R, D’Alessio V, Petrillo A, Lastoria S, et al. Clinical Phase I/II Study: Local Disease Control and Survival in Locally Advanced Pancreatic Cancer Treated With Electrochemotherapy. J Clin Med (2021) 10:1305. doi: 10.3390/jcm10061305
60. Calvet CY, Mir LM. The Promising Alliance of Anti-Cancer Electrochemotherapy With Immunotherapy. Cancer Metastasis Rev (2016) 35:165–77. doi: 10.1007/s10555-016-9615-3
61. Bhutiani N, Agle S, Li Y, Li S, Martin RCG. Irreversible Electroporation Enhances Delivery of Gemcitabine to Pancreatic Adenocarcinoma. J Surg Oncol (2016) 114:181–6. doi: 10.1002/jso.24288
62. Rudno-Rudzińska J, Kielan W, Guziński M, Kulbacka J. Effects of Calcium Electroporation, Electrochemotherapy, and Irreversible Electroporation on Quality of Life and Progression-Free Survival in Patients With Pancreatic Cancer: IREC Clinical Study. Adv Clin Exp Med (2021) 30:765–70. doi: 10.17219/acem/139917
63. Henriksen A, Dyhl-Polk A, Chen I, Nielsen D. Checkpoint Inhibitors in Pancreatic Cancer. Cancer Treat Rev (2019) 78:17–30. doi: 10.1016/j.ctrv.2019.06.005
64. Zhao J, Wen X, Tian L, Li T, Xu C, Wen X, et al. Irreversible Electroporation Reverses Resistance to Immune Checkpoint Blockade in Pancreatic Cancer. Nat Commun (2019) 10:899. doi: 10.1038/s41467-019-08782-1
65. Zhao J, Chen S, Zhu L, Zhang L, Liu J, Xu D, et al. Antitumor Effect and Immune Response of Nanosecond Pulsed Electric Fields in Pancreatic Cancer. Front Oncol (2021) 10:621092. doi: 10.3389/fonc.2020.621092
66. He C, Huang X, Zhang Y, Lin X, Li S. T-Cell Activation and Immune Memory Enhancement Induced by Irreversible Electroporation in Pancreatic Cancer. Clin Trans Med (2020) 10(2):e39. doi: 10.1002/ctm2.39
67. Scheffer HJ, Stam AGM, Geboers B, Vroomen LGPH, Ruarus A, de Bruijn B, et al. Irreversible Electroporation of Locally Advanced Pancreatic Cancer Transiently Alleviates Immune Suppression and Creates a Window for Antitumor T Cell Activation. OncoImmunology (2019) 8:1652532. doi: 10.1080/2162402X.2019.1652532
68. Sun S, Liu Y, He C, Hu W, Liu W, Huang X, et al. Combining NanoKnife With M1 Oncolytic Virus Enhances Anticancer Activity in Pancreatic Cancer. Cancer Lett (2021) 502:9–24. doi: 10.1016/j.canlet.2020.12.018
69. Palucka K, Banchereau J. Cancer Immunotherapy via Dendritic Cells. Nat Rev Cancer (2012) 12:265–77. doi: 10.1038/nrc3258
70. Mehrotra S, Britten CD, Chin S, Garrett-Mayer E, Cloud CA, Li M, et al. Vaccination With Poly(IC:LC) and Peptide-Pulsed Autologous Dendritic Cells in Patients With Pancreatic Cancer. J Hematol Oncol (2017) 10:82. doi: 10.1186/s13045-017-0459-2
71. Yang J, Eresen A, Shangguan J, Ma Q, Yaghmai V, Zhang Z. Irreversible Electroporation Ablation Overcomes Tumor-Associated Immunosuppression to Improve the Efficacy of DC Vaccination in a Mice Model of Pancreatic Cancer. OncoImmunology (2021) 10:1875638. doi: 10.1080/2162402X.2021.1875638
72. Martin RCG 2nd. Irreversible Electroporation of Stage 3 Locally Advanced Pancreatic Cancer: Optimal Technique and Outcomes. J Vis Surg (2015) 2015:2221–965. doi: 10.3978/j.issn.2221-2965.2015.05.02
73. Flak RV, Fisker RV, Bruun NH, Stender MT, Thorlacius-Ussing O, Petersen LJ. Usefulness of Imaging Response Assessment After Irreversible Electroporation of Localized Pancreatic Cancer—Results From a Prospective Cohort. Cancers (2021) 13:2862. doi: 10.3390/cancers13122862
74. al Efishat M, Wolfgang CL, Weiss MJ. Stage III Pancreatic Cancer and the Role of Irreversible Electroporation. BMJ (2015) 350:h521–1. doi: 10.1136/bmj.h521
75. Tseng J, Raut C, Lee J, Pisters P, Vauthey J, Abdalla E, et al. Pancreaticoduodenectomy With Vascular Resection: Margin Status and Survival Duration. J Gastrointest Surg (2004) 8:935–50. doi: 10.1016/j.gassur.2004.09.046
76. Zhou Y, Zhang Z, Liu Y, Li B, Xu D. Pancreatectomy Combined With Superior Mesenteric Vein–Portal Vein Resection for Pancreatic Cancer: A Meta-Analysis. World J Surg (2012) 36:884–91. doi: 10.1007/s00268-012-1461-z
77. Bacalbasa N, Balescu I, Barbu I, Stiru O, Savu C, Pop L, et al. Vascular Resections in Association With Pancreatic Resections for Locally Advanced Pancreatic Cancer. In Vivo (Athens Greece) (2022) 36:1001–6. doi: 10.21873/invivo.12793
78. Balaban EP, Mangu PB, Khorana AA, Shah MA, Mukherjee S, Crane CH, et al. Locally Advanced, Unresectable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol (2016) 34:2654–68. doi: 10.1200/JCO.2016.67.5561
79. Suker M, Beumer BR, Sadot E, Marthey L, Faris JE, Mellon EA, et al. FOLFIRINOX for Locally Advanced Pancreatic Cancer: A Systematic Review and Patient-Level Meta-Analysis. Lancet Oncol (2016) 17:801–10. doi: 10.1016/S1470-2045(16)00172-8
Keywords: irreversible electroporation, locally advanced pancreatic cancer, chemotherapy, electrochemotherapy, electrogene, immunotherapy
Citation: Gyftopoulos A, Ziogas IA, Barbas AS and Moris D (2022) The Synergistic Role of Irreversible Electroporation and Chemotherapy for Locally Advanced Pancreatic Cancer. Front. Oncol. 12:843769. doi: 10.3389/fonc.2022.843769
Received: 05 January 2022; Accepted: 26 April 2022;
Published: 25 May 2022.
Edited by:
Shengping Li, Sun Yat-sen University, ChinaReviewed by:
Stefano Francesco Crinò, University of Verona, ItalyAndrea Laurenzi, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Italy
Copyright © 2022 Gyftopoulos, Ziogas, Barbas and Moris. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dimitrios Moris, ZGltaXRyaW9zLm1vcmlzQGR1a2UuZWR1; orcid.org/0000-0002-5276-0699
 Argyrios Gyftopoulos
Argyrios Gyftopoulos Ioannis A. Ziogas
Ioannis A. Ziogas Andrew S. Barbas
Andrew S. Barbas Dimitrios Moris
Dimitrios Moris