
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Oncol. , 13 September 2022
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.843116
This article is part of the Research Topic Optimizing Outcomes and Addressing Adversities of Immunotherapy in Lung Cancer View all 17 articles
 Xin Li1†
Xin Li1† Chunqiu Xia1†
Chunqiu Xia1† Minghui Liu1†
Minghui Liu1† Jinghao Liu1
Jinghao Liu1 Ming Dong1
Ming Dong1 Honglin Zhao1
Honglin Zhao1 Song Xu1
Song Xu1 Dan Wang2
Dan Wang2 Sen Wei1
Sen Wei1 Zuoqing Song1
Zuoqing Song1 Gang Chen1
Gang Chen1 Hongyu Liu3
Hongyu Liu3 Jun Chen1,3,4*
Jun Chen1,3,4*Neoadjuvant immunochemotherapy has attracted much attention as a treatment for locally advanced non-small-cell lung cancer. However, there is scarce evidence of the safety and efficacy of camrelizumab as neoadjuvant in lung cancer. Here, we present three patients who were diagnosed with IIIA squamous non-small-cell lung cancer from September to December in 2020 and received two cycles of neoadjuvant camrelizumab plus nab-paclitaxel and nedaplatin, followed by surgical resection. All three patients had a reduction in the tumor size on CT image and not delayed planned surgery. We did not observe grade 3 or 4 adverse events. Two of the three patients achieved a major pathological response (MPR), including one complete tumor regression of the primary lung tumor. Multiplex fluorescent immunohistochemistry revealed that CD8+ T cells, FoxP3+ regulatory T cells, and PD-L1 expression on immune cells in the surgical specimen were much higher than in the pretreatment biopsy sample in patients with MPR. This was not observed in the patient without MPR. Camrelizumab plus chemotherapy could potentially be a neoadjuvant regimen for resectable IIIA squamous non-small-cell lung cancer, with a high MPR proportion, and did not compromise surgical procedure. Our findings should be validated in a future randomized clinical trial.
Neoadjuvant therapy is one of the many approaches to locally advanced non-small cell lung cancer (NSCLC) (1). However, traditional platinum-based chemotherapy either before or after resection provides only 5% higher of overall survival (OS) than surgery alone for treating patients with stage IB–IIIA NSCLC (2–4). Recently, checkpoint inhibitors targeting PD-1 and PD-L1 have revolutionized the treatment paradigm for several cancers. On the basis of previous success, several studies have focused on the utility of immune checkpoint inhibitors as neoadjuvant therapy for treating patients with surgically resectable NSCLC (5–7). Camrelizumab, a humanized monoclonal antibody against PD-1, has proved to be effective and safe in multiple tumor types including advanced NSCLC in phase 1, 2, and 3 studies (8–11). However, there has been no report regarding the efficacy and safety of the combination of camrelizumab with chemotherapy as a neoadjuvant treatment in patients with resectable lung cancer to date. Hence, we evaluated the safety and feasibility of the use of neoadjuvant camrelizumab in a small group of patients with resectable stage IIIA squamous lung cancer.
This single-group study was developed by the author’s medical center. Three patients who were diagnosed with stage IIIA squamous lung cancer between September and December in 2020 were evaluated to undergo lobectomy surgery. Patients received the following drugs intravenously: camrelizumab (200 mg) on day 1; nab-paclitaxel (100 mg/m²) on days 1, 8, and 15; and nedaplatin (80 mg/m²) on day 1 of every 21 days for 4–6 cycles. Surgery was performed after the first two treatment cycles with an interval of 3–6 weeks (12). All the patients underwent baseline tumor assessment, including pretreatment pathological diagnosis by means of bronchoscopy or percutaneous core needle lung biopsy, contrast-enhanced CT of chest and abdomen, single photon emission computed tomography (SPECT) of bone, and magnetic resonance imaging (MRI) of brain; chest CT was repeated within 1 week before surgery. The changes in tumor size were judged according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Major pathological response (MPR) was defined as the presence of 10% or less residues of cancer cells in the primary tumor surgical specimen (13).
All three patients received the two planned cycles and underwent complete tumor resection. The clinical characteristics of these patients are presented in Table 1. No grade 3 or 4 adverse events or treatment-related deaths occurred. Neoadjuvant camrelizumab and chemotherapy were not associated with any toxic effects, which was not previously reported. There were no treatment-related surgeries that were postponed or canceled. The intervals between the administration of the second dose of camrelizumab and surgery were 32, 39, and 41 days, respectively. Of the three patients, two received the six planned treatment cycles; one patient (patient B) was diagnosed with Alzheimer’s disease while on study and terminated the therapy after one postoperative treatment cycle. The median follow-up was 10 months (10, 11, and 12 months, respectively). None of the patients died or experienced disease recurrence during the follow-up.
All three patients were operated by video thoracoscopy approach and had an R0 surgical resection. The durations of the surgery were 90, 115, and 130 min, respectively. No significant hemorrhaging occurred during surgery. The length of postoperative hospital stay was 3, 4, and 5 days, respectively, without stay in the intensive care unit (ICU). No postoperative complications, such as pneumothorax, hemoptysis, chylothorax, and pneumonia, occurred.
Two of the three patients (patient A and patient B) achieved MPR, including one complete tumor regression of the primary lung tumor but had residual lymph-node metastases (patient A). One patient (patient C) had an incomplete pathological response to the neoadjuvant treatment. Reduction in the tumor size was noted in all patients’ CT images after two cycles of treatment (Figure 1).

Figure 1 CT images of lesions before and after two cycles of neoadjuvant camrelizumab plus chemotherapy. After two doses of camrelizumab plus chemotherapy, the lesions of three patients were significantly reduced.
Hematoxylin–eosin staining (HE staining) revealed that residual non-viable tumor and lymph nodes of patients with MPR composed of extensive necrosis and a large amount of inflammatory cell infiltration, foamy histiocytes, and multinucleated giant cells can be seen locally. These features were not evident in areas distant from the tumor bed, which was consistent with immunological response, but this response was barely noticeable in the patient without MPR (Figure 2). To further explore these cases, multiplex immunofluorescence analysis which contained PD-1-positive, PD-L1-positive, CD8+ T cells, CD68+ macrophages, and FoxP3+ regulatory T cells was performed in both pretreatment and surgical specimens (Figure 3). In two patients with MPR, CD8+ T cell, FoxP3+ regulatory T cell, and PD-L1 expression on immune cells in the surgical specimen was much higher than in the pretreatment biopsy sample, whereas this immunoreactive intensity was faint in the patient without MPR. Table S1 shows the changes of tumor cells and immune cells in lesions before and after neoadjuvant treatment.
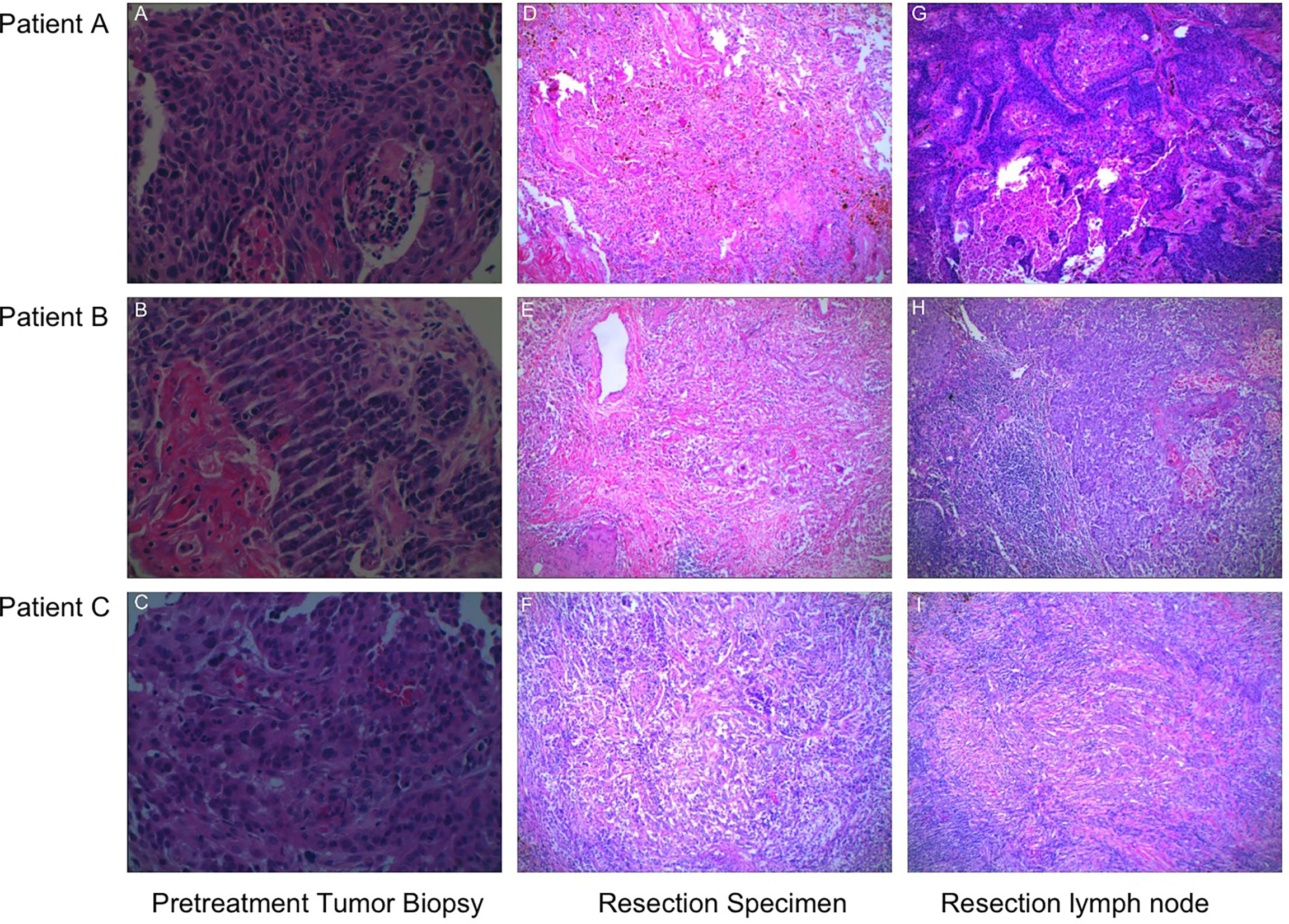
Figure 2 The representative sections of tumor specimens from three patients before and after the administration of camrelizumab plus chemotherapy. (A–C) Pretreatment tumor biopsy, HE staining (×400). (D–E) The resection specimens were infiltrated by lymphocytes and macrophages, and there were over 90% tumor tissue regression, HE staining (×100). (F) Over 50% residual tumor cells were present, and a little fibrous tissue can be observed, HE staining (×100). The presence of necrosis, fibrosis, and macrophages was observed in the metastatic lymph nodes of patients with MPR (G, H), whereas this founding was not observed in the patient without MPR (I), HE staining (×100).
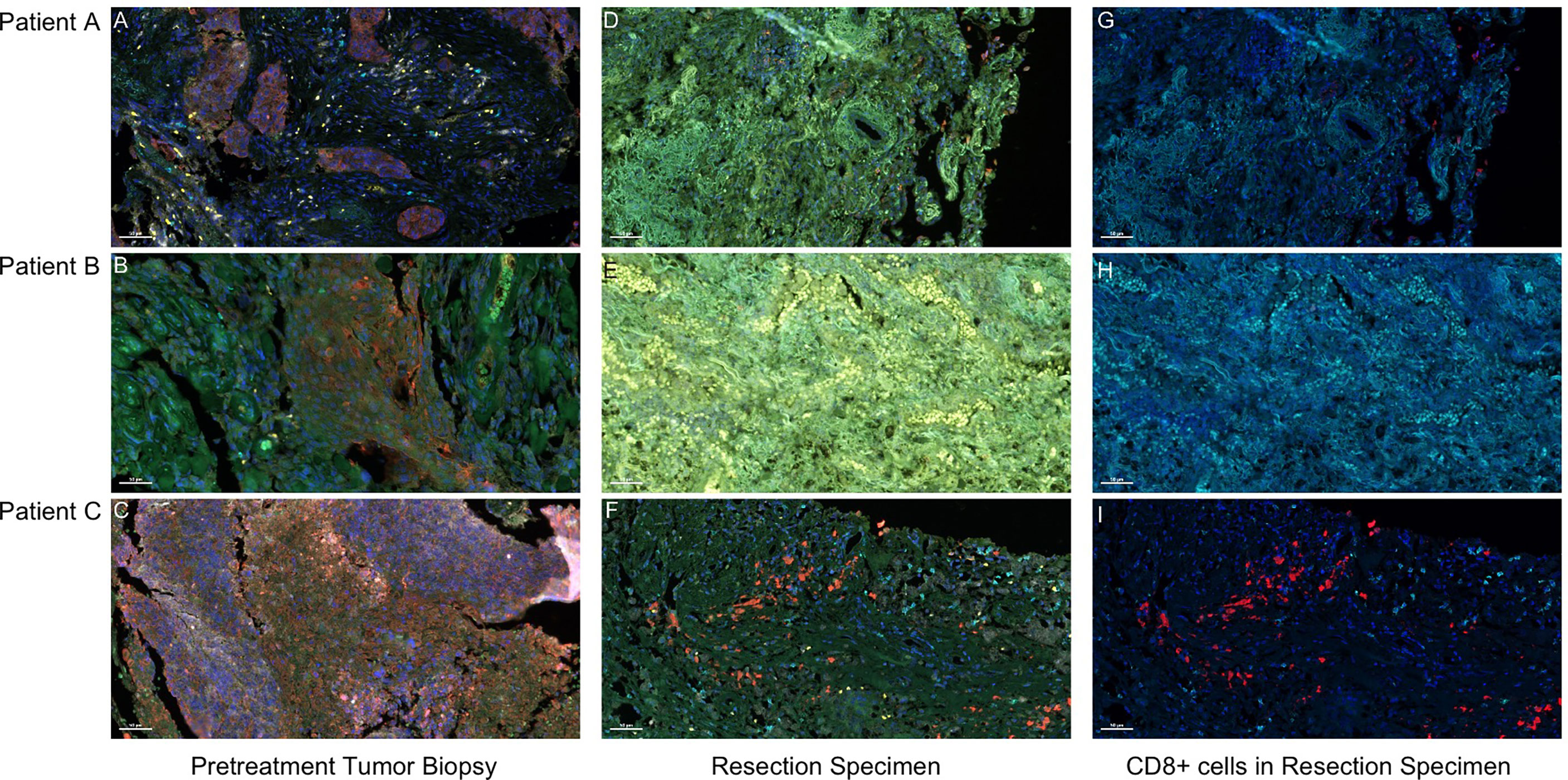
Figure 3 Presence of CD 68+ macrophages, CD8+ T cells, and FoxP3+ Treg cells in pretreatment and surgical specimen detected by multiplex fluorescent immunohistochemistry. Visible structures include cytokeratin-positive tumor cells (red), PD-1+ cells (orange), PD-L1+ cells (green), CD68+ macrophages (white), CD8+ T cells (cyan), and FoxP3+ regulatory T cells (yellow). (A–C) Pretreatment tumor biopsy tissues. After two doses of camrelizumab plus chemotherapy, the surgical specimens of patients A and B who achieved MPR contained an influx of CD8+ T cells (G, H), and the presence of macrophages, Treg cells, and PD-1 and PD-L1+ immune cells were more common in the tumor area (D, E). By contrast, in patient C who had no MPR, the tumor immune response was not obvious, and the amount of PD-1 and PD-L1+ immune cells was reduced after treatment (F, I). Scale bar, 50 μm.
In our study, we observed that neoadjuvant administration of two cycles of camrelizumab plus nab-paclitaxel and nedaplatin in patients with stage IIIA squamous NSCLC was not associated with additional adverse events than in previous studies (8–11). There was no delay of the planned surgery in all three patients. In addition, a radiological reduction in the tumor size was noted in all patients, which made the surgery easier to perform. Among them, two patients had MPR. The adverse events in this study were grade 1 or 2 and consistent with those of the individual drugs. The reactive cutaneous capillary endothelial proliferation that was most commonly reported related to camrelizumab was not observed in our study, which might be contributed to the combination with chemotherapy (14, 15).
Limited to traditional cytotoxic chemotherapy, advanced squamous NSCLC was associated with shorter survival than non-squamous NSCLC (16). Recently, several studies have shown that anti-PD-1 or anti-PD-L1 plus chemotherapy could improve progression-free survival and overall survival compared with chemotherapy alone in advanced squamous NSCLC (17, 18). Furthermore, MPR was more frequently observed in squamous cell carcinoma than in adenocarcinoma in neoadjuvant immunotherapy (5), which correlated with overall survival (13). The CheckMate 816 study, a phase 3 trial of neoadjuvant anti-PD-1 immunotherapy (nivolumab) plus chemotherapy versus chemotherapy alone in resectable NSCLC, reported that neoadjuvant nivolumab plus chemotherapy did not delay surgery and achieved a remarkable higher of pathologic complete response (pCR) than chemotherapy alone (24% vs. 2.2%). The study also showed that, compared with chemotherapy, the improvement of nivolumab plus chemotherapy on pCR was consistent in key subgroups, including disease stage (IB/II [26.2% vs. 4.8%]; ≥IIIA [23.0% vs. 0.9%]), PD-L1 tumor proportion score (<1% [16.7% vs. 2.6%]; ≥1% [32.6% vs. 2.2%]), and tumor mutational burden (low [22.4% vs. 1.9%]); high [30.8% vs. 2.7%]) (19). In terms of safety, neoadjuvant therapy with nivolumab combined with chemotherapy did not increase postoperative complications. The CheckMate 816 study reported that 11% of the patients encountered grade 3–4 surgery-relate adverse events in the nivolumab plus chemotherapy group, compared with 15% in the chemotherapy group (19). The heterogeneous ethnicities may lead to different responses to therapies. In previous studies for advanced NSCLC, the effect of camrelizumab was not inferior to nivolumab or pembrolizumab in Chinese populations (10, 11). Therefore, camrelizumab might be more suitable and economical for Chinese patients with NSCLC (10, 11). We chose nab-paclitaxel to avoid the need for steroid. Because of the concerns about serious side effects and effectiveness, patients in our study received two cycles of camrelizumab plus nab-paclitaxel and nedaplatin followed by surgical removal of the tumor, which was less than in most studies. Our results demonstrated that this shorter cycle was also very effective.
Compared to adjuvant therapy, neoadjuvant immunotherapy may improve efficacy by reducing metastasis or recurrence in early-stage NSCLC (20). PD-1 blocking enhances T-cell-mediated antitumor activity not only directly killing tumor cells but also increasing tumor antigen-specific T-cell priming (6, 21). The activated tumor-specific T cells circulate in the body to eradicate micrometastatic tumor deposits that might otherwise drive postsurgical relapse (20). Platinum-based chemotherapy could induce immunogenic tumor elimination by increasing antigen presentation, following T-cell priming (22). In our study, more infiltration of CD8+ T cells and of PD-L1 on immune cells was observed in patients with MPR after PD-1 blockade, which was consistent with an adaptive PD-L1 upregulation mechanism (23, 24). It was unexpected that patient A with complete tumor regression of the primary lung tumor had a 1.1% tumor cell PD-L1 expression in the pretreatment specimen, which was the lowest among the three patients. However, the high expression of his PD-1 on immune cells was the highest after treatment, which may contribute to the treatment response. Furthermore, the CD8+ T cells of his surgical specimen were lower than those of another patient with MPR, but his FoxP3+ regulatory T cells were also lower, which may increase immune response. By contrast, in patient C who was without MPR, the presence of PD-1 and PD-L1+ immune cells was reduced after treatment (Table S1). The limitations of our study include the small patient numbers, the absence of a randomized control group, and the short postoperative follow-up period. Larger studies are needed to correlate the pathological response resulting from neoadjuvant therapy with overall survival.
In conclusion, all three patients in this study had a radiographic response, and two of them reached a major pathological response. There were no new safety signals identified. Neoadjuvant camrelizumab plus chemotherapy could potentially be a therapeutic option for patients with stage IIIA squamous NSCLC, which requires confirmation in future prospective multicenter randomized studies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This study was reviewed and approved by Review Board of Tianjin Medical University General Hospital. The patients/participants provided their written informed consent to participate in this study.
XL, CX, ML, HL, and JC wrote the manuscript. XL, CX, ML, JL, MD, HZ, SX, SW, DW, ZS, and GC took care of the patients, collected, and analyzed the data. JC and HL supervised the research. All authors contributed to the article and approved the submitted version.
This study was supported by grants from the National Natural Science Foundation of China (82072595, 81773207, and 61973232), Natural Science Foundation of Tianjin (18PTZWHZ00240, 19YFZCSY00040, and 19JCYBJC27000), Shihezi University Oasis Scholars Research Startup Project (LX202002), Special Support Program for the High Tech Leader and Team of Tianjin (TJTZJH-GCCCXCYTD-2-6), Tianjin Municipal Education Commission Natural Science Foundation (2019KJ202), and The Fundamental Research Funds for the Central Universities (3332018180). The funding sources had no role in study design, data collection, and analysis; the decision to publish; or the preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.843116/full#supplementary-material
1. Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman JR, Bharat A, et al. NCCN guidelines insights: Non-small cell lung cancer, version 2.2021. J Natl Compr Canc Netw (2021) 19(3):254–66. doi: 10.6004/jnccn.2021.0013
2. Arriagada R, Bergman B, Dunant A, Le Chevalier T, Pignon JP, Vansteenkiste J. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med (2004) 350(4):351–60. doi: 10.1056/NEJMoa031644
3. Felip E, Rosell R, Maestre JA, Rodríguez-Paniagua JM, Morán T, Astudillo J, et al. Preoperative chemotherapy plus surgery versus surgery plus adjuvant chemotherapy versus surgery alone in early-stage non-small-cell lung cancer. J Clin Oncol (2010) 28(19):3138–45. doi: 10.1200/JCO.2009.27.6204
4. Yendamuri S, Groman A, Miller A, Demmy T, Hennon M, Dexter E, et al. Risk and benefit of neoadjuvant therapy among patients undergoing resection for non-small-cell lung cancer. Eur J Cardiothorac Surg (2018) 53(3):656–63. doi: 10.1093/ejcts/ezx406
5. Shu CA, Gainor JF, Awad MM, Chiuzan C, Grigg CM, Pabani A, et al. Neoadjuvant atezolizumab and chemotherapy in patients with resectable non-small-cell lung cancer: An open-label, multicentre, single-arm, phase 2 trial. Lancet Oncol (2020) 21(6):786–95. doi: 10.1016/S1470-2045(20)30140-6
6. Forde PM, Chaft JE, Smith KN, Anagnostou V, Cottrell TR, Hellmann MD, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med (2018) 378(21):1976–86. doi: 10.1056/NEJMoa1716078
7. Broderick SR, Bott MJ. Neoadjuvant immunotherapy in patients with resectable non-small cell lung cancer. J Thorac Cardiovasc Surg (2019) 158(5):1471–4. doi: 10.1016/j.jtcvs.2019.06.114
8. Fan Y, Zhao J, Wang Q, Huang D, Li X, Chen J, et al. Camrelizumab plus apatinib in extensive-stage SCLC (PASSION): A multicenter, two-stage, phase 2 trial. J Thorac Oncol (2021) 16(2):299–309. doi: 10.1016/j.jtho.2020.10.002
9. Zhou C, Wang Y, Zhao J, Chen G, Liu Z, Gu K, et al. Efficacy and biomarker analysis of camrelizumab in combination with apatinib in patients with advanced nonsquamous NSCLC previously treated with chemotherapy. Clin Cancer Res (2021) 27(5):1296–304. doi: 10.1158/1078-0432.CCR-20-3136
10. Zhou C, Chen G, Huang Y, Zhou J, Lin L, Feng J, et al. Camrelizumab plus carboplatin and pemetrexed versus chemotherapy alone in chemotherapy-naive patients with advanced non-squamous non-small-cell lung cancer (CameL): A randomised, open-label, multicentre, phase 3 trial. Lancet Respir Med (2021) 9(3):305–14. doi: 10.1016/S2213-2600(20)30365-9
11. Wu Y, Huang C, Fan Y, Feng J, Pan H, Jiang L, et al. JCSE01.09 a phase II umbrella study of camrelizumab in different PD-L1 expression cohorts in pre-treated Advanced/Metastatic non-small cell lung cancer. J Thorac Oncol 14(10):S128. doi: 10.1016/j.jtho.2019.08.267
12. Gao SJ, Corso CD, Wang EH, Blasberg JD, Detterbeck FC, Boffa DJ, et al. Timing of surgery after neoadjuvant chemoradiation in locally advanced non-Small cell lung cancer. J Thorac Oncol (2017) 12(2):314–22. doi: 10.1016/j.jtho.2016.09.122
13. Hellmann MD, Chaft JE, William WN Jr, Rusch V, Pisters KM, Kalhor N, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: Proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol (2014) 15(1):e42–50. doi: 10.1016/S1470-2045(13)70334-6
14. Fang W, Yang Y, Ma Y, Hong S, Lin L, He X, et al. Camrelizumab (SHR-1210) alone or in combination with gemcitabine plus cisplatin for nasopharyngeal carcinoma: Results from two single-arm, phase 1 trials. Lancet Oncol (2018) 19(10):1338–50. doi: 10.1016/S1470-2045(18)30495-9
15. Nie J, Wang C, Liu Y, Yang Q, Mei Q, Dong L, et al. Addition of low-dose decitabine to anti-PD-1 antibody camrelizumab in Relapsed/Refractory classical Hodgkin lymphoma. J Clin Oncol (2019) 37(17):1479–89. doi: 10.1200/JCO.18.02151
16. Blumenthal GM, Bunn PA Jr, Chaft JE, McCoach CE, Perez EA, Scagliotti GV, et al. Current and emergent therapy options for advanced squamous cell lung cancer. J Thorac Oncol (2018) 13(2):165–83. doi: 10.1016/j.jtho.2017.11.111
17. Jotte R, Cappuzzo F, Vynnychenko I, Stroyakovskiy D, Rodríguez-Abreu D, Hussein M, et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): Results from a randomized phase III trial. J Thorac Oncol (2020) 15(8):1351–60. doi: 10.1016/j.jtho.2020.03.028
18. Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gümüş M, Mazières J, et al. Pembrolizumab plus chemotherapy for squamous non-Small-Cell lung cancer. N Engl J Med (2018) 379(21):2040–51. doi: 10.1056/NEJMoa1810865
19. Forde PM, Spicer J, Lu S, Provencio M, Mitsudomi T, Awad MM, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med (2022) 386(21):1973–85. doi: 10.1056/NEJMoa2202170
20. Liu J, Blake SJ, Yong MC, Harjunpää H, Ngiow SF, Takeda K, et al. Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discovery (2016) 6(12):1382–99. doi: 10.1158/2159-8290.CD-16-0577
21. Salmon H, Idoyaga J, Rahman A, Leboeuf M, Remark R, Jordan S, et al. Expansion and activation of CD103(+) dendritic cell progenitors at the tumor site enhances tumor responses to therapeutic PD-L1 and BRAF inhibition. Immunity. (2016) 44(4):924–38. doi: 10.1016/j.immuni.2016.03.012
22. Bezu L, Gomes-de-Silva LC, Dewitte H, Breckpot K, Fucikova J, Spisek R, et al. Combinatorial strategies for the induction of immunogenic cell death. Front Immunol (2015) 6:187. doi: 10.3389/fimmu.2015.00187
23. Huang KC, Chiang SF, Chen WT, Chen TW, Hu CH, Yang PC, et al. Decitabine augments chemotherapy-induced PD-L1 upregulation for PD-L1 blockade in colorectal cancer. Cancers (Basel) (2020) 12(2):462. doi: 10.3390/cancers12020462
Keywords: neoadjuvant immunotherapy, non-small cell lung cancer (NSCLC), camrelizumab, major pathological response (MPR), surgery
Citation: Li X, Xia C, Liu M, Liu J, Dong M, Zhao H, Xu S, Wang D, Wei S, Song Z, Chen G, Liu H and Chen J (2022) Neoadjuvant camrelizumab and chemotherapy in patients with resectable stage IIIA squamous non-small-cell lung cancer: Clinical experience of three cases. Front. Oncol. 12:843116. doi: 10.3389/fonc.2022.843116
Received: 24 December 2021; Accepted: 22 August 2022;
Published: 13 September 2022.
Edited by:
Mohamed Rahouma, New York-Presbyterian, United StatesReviewed by:
Chao H. Huang, University of Kansas Medical Center Research Institute, United StatesCopyright © 2022 Li, Xia, Liu, Liu, Dong, Zhao, Xu, Wang, Wei, Song, Chen, Liu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Chen, aHVudGVyY2oyMDA0QHFxLmNvbQ==
†These authors have contributed equally in this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.