
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Oncol. , 25 August 2022
Sec. Gynecological Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.841547
This article is part of the Research Topic Women in Gynecological Oncology: 2021 View all 34 articles
 Qimin Wang1,2†
Qimin Wang1,2† Yingying He1,3†
Yingying He1,3† Fang Long1,4,5†
Fang Long1,4,5† Chaoran Li4,6†
Chaoran Li4,6† Zhuowei Shen1,4†
Zhuowei Shen1,4† Dongxing Guo1,4,5†
Dongxing Guo1,4,5† Duoji Zhaxi1
Duoji Zhaxi1 Lamu Bumu5
Lamu Bumu5 Zhengyu Hua7
Zhengyu Hua7 Zhigang Sun7
Zhigang Sun7 Nan Jiang7
Nan Jiang7 Xu Han4
Xu Han4 Jing Li4
Jing Li4 Keqing Yan2
Keqing Yan2 Siqi Bai4
Siqi Bai4 Muhan Tao4
Muhan Tao4 Xiaoguang Xu1,8*
Xiaoguang Xu1,8* Zhen Xiao1,4,5*
Zhen Xiao1,4,5*Background: Cervical cancer has become a worldwide concern owing to its high incidence and mortality rates. To date, high-altitude areas of Tibet have not benefited from any large-scale cervical cancer screening programs. Therefore, we initiated a screening program to investigate the prevalence of human papilloma virus (HPV) and HPV genotype distribution to reveal cervical cancer and its precursor which lead to morbidity among women in the city of Nagqu in northern Tib3et.
Methods: A total of 25,173 women were recruited to undergo HPV genotype tests between June and December 2019. Women infected with HPV 16 and/or 18 underwent colposcopy and histological examination. Women with other high-risk HPV type (hr-HPV) underwent cytological tests to determine whether to conduct further colposcopy and histological examination for diagnosis. HPV prevalence was calculated in the total population and further stratified according to various parameters, such as age group, area location (altitude level), and single or mixed infection status. The HPV genotype distribution was also investigated accordingly. Cervical lesions revealed by further colposcopic findings were also analyzed; high-grade and malignant lesion morbidities were calculated in total and in each county. Most data were collected and analyzed using descriptive and consistency check statistical methods, and a risk factor investigation for HPV infection was performed using logistic regression models.
Results: The total HPV infection rate among women in Nagqu was 13.42%. Of the 25,173 women in the study, 999 (3.97%) were HPV 16/18 positive, 2,379 (9.45%) were other hr-HPV-positive, and 21,795 (86.58%) were HPV-negative. The five most common HPV genotypes, accounting for more than 60% of all HPV infections in Nagqu people, were HPV 16, 58, 31, 18, and 52. Tibetan women younger than 20 years and older than 60 years were the two age groups with the highest rates of HPV infection, 26.7% and 19.8%, respectively. Among the HPV-positive women, 2,656 (78.33%) were infected with a single strain and 732 (21.67%) were infected with multiple strains (more than two genotypes). HPV prevalence increased in high-altitude areas (positive rate highest in Nyima with an altitude of 5,000 m, 23.9%) and decreased in relatively low-altitude areas (positive rate lowest in Lhari with an altitude of 4,000 m, 6.6%). Multiple analyses showed that age, parity, age at first delivery, and altitude of residence were independent factors facilitating HPV infection in Tibetan women. High-grade and malignant cervical lesions revealed by histological findings were different among living locations, with the highest rates in Xainza, Baingoin, and Nyainrong, these being 2.019%, 1.820%, and 1.116%, respectively, among women in these areas.
Conclusion: Our survey provides an overall perspective on HPV genotype infection and cervical lesions in women in northern Tibet. The data not only provide useful information for the treatment of cervical lesions but also has great value in terms of the primary and secondary prevention measures that can be taken for women living in these regions.
Clinical Trial Registration: www.chictr.org.cn, indentifier ChiCTR2000035061.
Cervical cancer is the third most common cancer worldwide and the fourth most common cancer among Chinese women. Reportedly, there are 34,000 and 275,000 cervical cancer-related deaths in China and globally, respectively (1). Human papillomavirus (HPV) has been confirmed as a pathogenic cause of cervical invasive cancer and its precancerous lesions, and cervical cancer is now regarded as a preventable disease (2).
Several cervical screening programs have been developed and applied to populations in various countries to identify precursors of cervical cancer. Multiple strategies are available for cervical cancer screening. The cheapest and simplest method of visual inspection with acetic acid has a high false-positive rate, requires a large number of trained clinicians, and is time-consuming, all of which make it difficult to access in Tibet despite its relative simplicity and low cost. The pap smear or cytology test has been used for several decades (3, 4) ; however, it has low sensitivity and specificity. Primary cytology with HPV triage has been widely used in women older than 30 years (testing HPV together with cytology), and it has high sensitivity and specificity (5–7). However, these tests are expensive and require abundant medical resources. Recently, another screening method solely for HPV testing (as the primary screening test) has been developed following cytology triage (8, 9). This method is extensively used in European countries, and it has an enhanced negative predictive value (10, 11). This screening method allows cervical cancer screening safety intervals to be extended from 3 to 5 years (12).
In most parts of China, for cervical cancer screening, we adopt the principle of “Opportunistic screening.” That is, when a woman comes to the hospital for various reasons, doctors suggest additional cervical cancer screening. Usually, combined cytology and HPV tests are used in most Chinese medical affiliations, and patients can afford the expense by themselves (13, 14). However, this strategy has not yet been widely used in Tibet. This is because Tibet has a relatively small population, large territory, and limited medical resources (15). Moreover, the majority of women in Tibet rarely visit hospitals voluntarily, except in emergencies, and they are uninclined to pay for, even affordable, screening programs. Therefore, government-financed HPV primary screening programs for all the population at right ages (16) may be the best choice for women in Tibet.
This is the first large-scale cervical cancer screening program conducted in the city of Nagqu, northern Tibet. This study aimed to investigate the prevalence and distribution of HPV genotypes and cervical lesions in Nagqu women. This is the first regional epidemiological study to elucidate the incidence of cervical cancer and its precursor which leads to morbidity in Nagqu.
A total of 25,173 married women aged 18–65 years in Nagqu City, Tibet Autonomous Region of China, who were available at home and who consented to undergo screening between June and December 2019 were recruited in this study. HPV genotype testing was the first step for all these participants. Women with positive results were divided into two groups. The first group included those with HPV 16 and/or HPV 18 infections, and the second group included high-risk HPV (hr-HPV) infections with genotypes other than 16 and 18. Women with HPV 16 and/or 18 infection in the first group underwent colposcopy and histological examination at the People’s Hospital of Nagqu. Women in the second group with other types of hr-HPV infection underwent cytology testing initially. If cytology results showed ASCUS (atypical squamous cells, undetermined significance) or more in these women, they were requested to undergo further colposcopy and histological examination for diagnosis. The flow diagram (Figure 1) summarizes this process.
All HPV samplings were performed by trained general practitioners at every primary hospital in all parts of Nagqu City. HPV samples were analyzed using a BGI 16-type HPV genotyping sequencing assay (BGI, Shenzhen, China). HPV testing was performed using an HPV genotyping sequencing system. This assay could detect 16 hr-HPV genotypes (16, 18, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, and 73), as described here in detail (17, 18). Briefly, this assay aimed to target and amplify DNA of the HPV L1 gene. MiSeq or personal genome machine (PGM) sequencing strategies were used for testing. HPV16/18-positive samples were subjected to histological examination while cytology tests were also maintained for these participants for study purposes; hr-HPVs other than 16/18 infected women were referred for cytology analysis with liquid-based cytology (LBC) (Thin Prep, Hologic, Boxborough, Massachusetts, USA).
Women who required colposcopy examinations were referred to a single expert gynecologist at the People’s Hospital of Nagqu, Tibet. All examinations were conducted according to routine procedures described previously (19). Hematoxylin and eosin (H&E) staining was performed for histological diagnosis.
SPSS 21.0 (version 21.0; SPSS, Inc. Chicago, IL, USA) was used for statistical analysis. All participants were divided by age group and county to calculate the prevalence of the overall HPV genotype, HPV 16/18, and other hr-HPV genotypes. Additionally, a stratifying strategy was used to analyze the cytology and histology results. To compare differences in HPV infection prevalence and cervical lesion rates among various locations and age groups, the chi-square test was used accordingly. Also, a logistic regression model was used to investigate factors influencing HPV infection. The p value was considered significant when p < 0.05.
The total HPV infection rate among women in Nagqu was 13.42%. Of the 25,173 women in the study, 999 (3.97%) were HPV 16/18 positive, 2,379 (9.45%) were other hr-HPV HPV positive, and 21,795 (86.58%) were HPV negative (Figure 2A and Table 1).
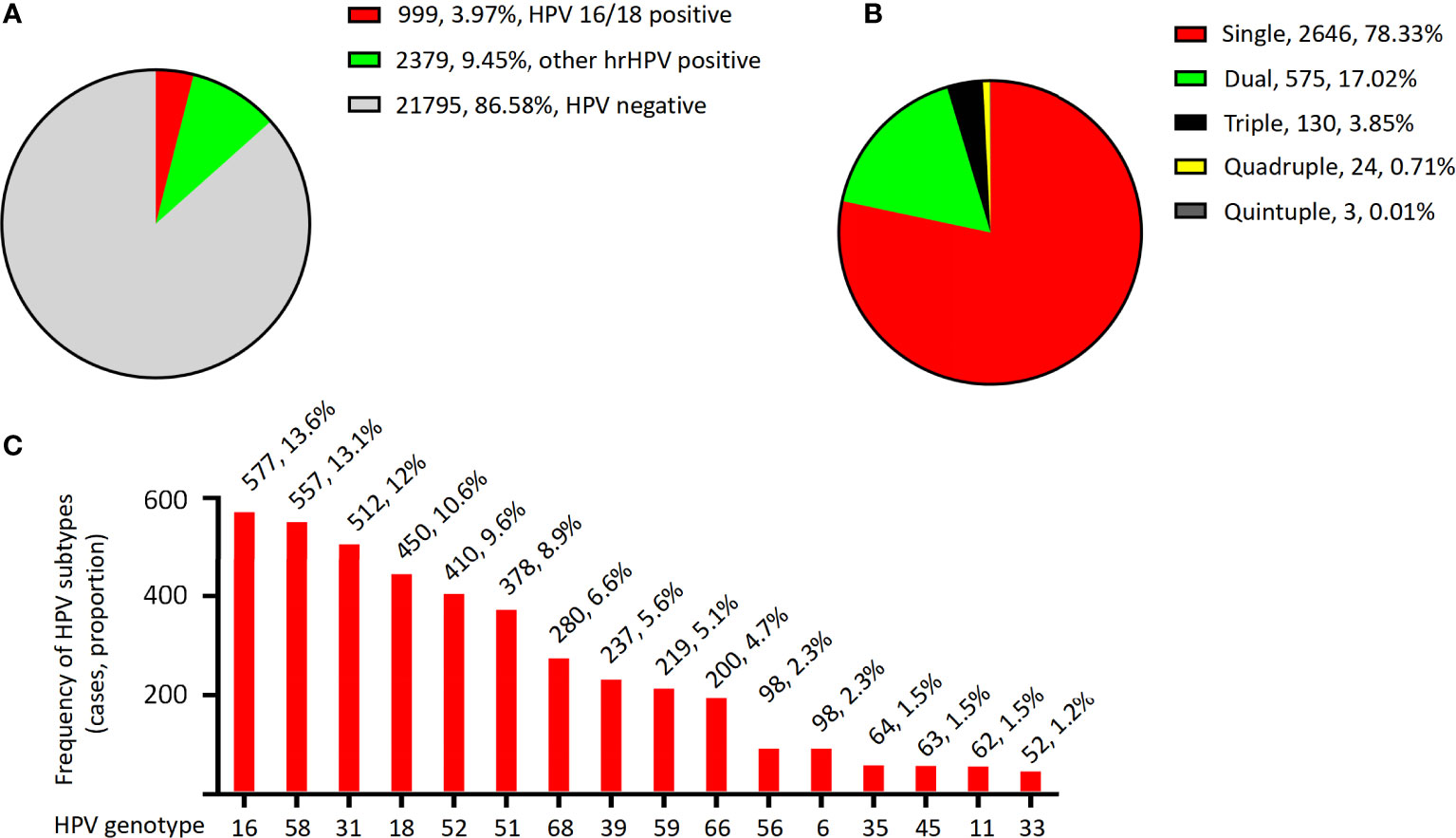
Figure 2 The HPV infection distribution. (A) Distribution of HPV genotypes in the women of Nagqu. (B) Distribution of multiple and simple HPV infection in the women of Nagqu. (C) Overall prevalence distribution of each HPV genotype.
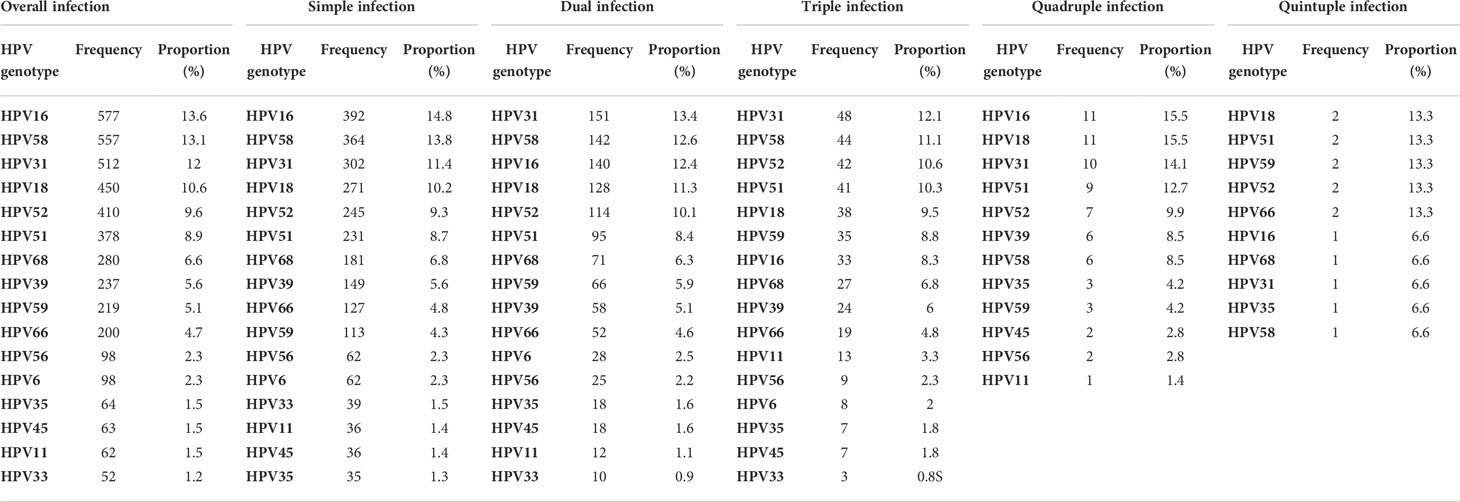
Table 1 Prevalence of HPV genotypes in all patients and prevalence of HPV genotypes in simple- and multiple-HPV-infection patients.
For the HPV-positive patients, the genotyping results showed that 2,646 (78.33%) were singly infected, 575 (17.02%) were doubly infected, 130 (3.85%) were triple infected, 24 (0.71%) were quadruply infected, and only three patients (0.01%) were quintuply infected (Figure 2B and Table 1).
The overall prevalence of each HPV genotype in the HPV-positive patients is shown in Figure 2C; Table 1. The most frequently detected HPV genotype was HPV 16, which was detected in 577 women (13.6%). The relatively less common genotypes were HPV 58 (557, 13.1%), HPV 31 (512, 12%), HPV 18 (450, 10.6%), HPV 52 (410, 9,6%), HPV 51 (378, 8.9%), HPV 68 (280, 6.6%), HPV 39 (237, 5.6%), HPV 59 (219, 5.1%), HPV 66 (200, 4.7%), HPV 56 (98, 2.3%), HPV 6 (98, 2.3%), HPV 35 (64, 1.5%), HPV 45 (63, 1.5%), HPV 11 (62, 1.5%), and HPV 33 (52, 1.2%). In patients with single and multiple HPV infections, the HPV prevalence was slightly different from the total prevalence, as summarized in Table 1. For example, in single- and dual-infection groups, the four most common genotypes were HPV 16, 58, 31, and 18; however, in patients infected with three HPV genotypes, the four most common infection genotypes were HPV 31, 58, 52, and 51.
Based on the age groups, as shown in Table 2, women with a younger age (less than 30 years) and older age (>50 years) had higher total HPV infection rates of 15.9% and 18.4%, respectively, p < 0.01. Women with a median age of (31–50 years) had a lower positive rate (12.6%), and the difference was statistically significant.
As the city of Nagqu has an area of 370,000 km2, and its 11 counties have different altitudes and different modes of production and life, we calculated the HPV infection rate in women according to each of the 11 areas. Generally, women living in eastern areas with lower altitudes had lower HPV infection rates than those living in western areas with higher altitudes (Figure 3A and Table 3). HPV infection rates in women were lowest in Lhari, Bagen, and Biru counties at 6.6%, 7%, and 9.2%, respectively. The highest infection rate was noted in the ultra-high-altitude areas of Amdo (18.6%), Nyima (23.9%), and Shuanghu (20.3%). When we combined the 11 counties into three regions according to altitude level (region A, altitude level <4,000 m; region B, altitude level within 4,000–5,000 m, region C altitude level >5000 m), we found that regions with lower altitudes had the lowest HPV-positive rate of 9.8%, while the rates in regions B and C were 13.2% and 20.9%, respectively (p = 0.001). Correlation analysis also indicated a significant correlation between altitude and HPV infection rates, p = 0.001, gamma = -0.251 (Figure 3B).
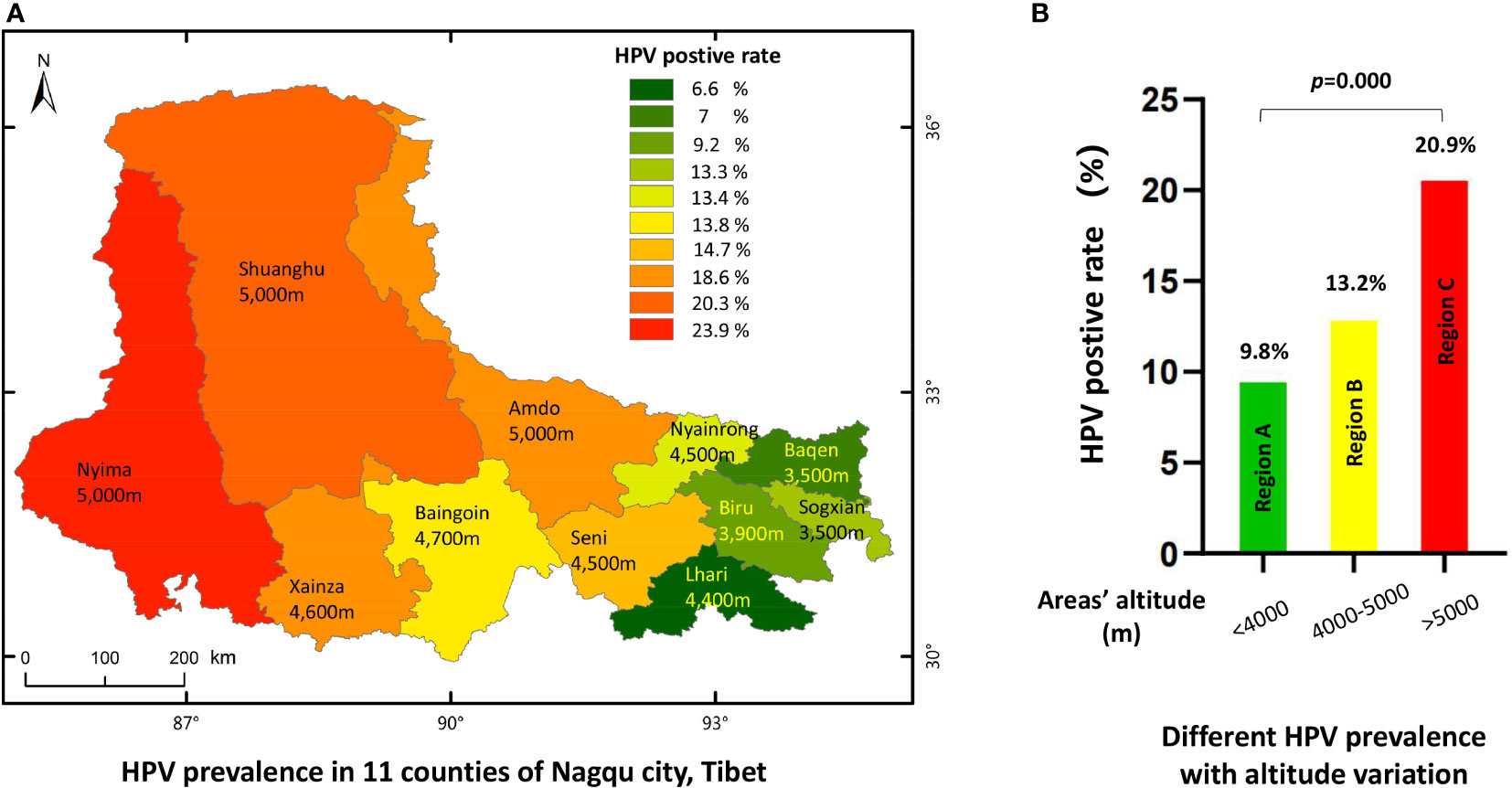
Figure 3 The HPV prevalence in 11 countries of Nagqu. (A) HPV prevalence in 11 counties of Nagqu City, Tibet. (B) Different HPV prevalence with altitude variation.
According to the above findings, it can be mentioned that HPV infection was associated with factors such as women’s age, living locations, and altitude. Our analysis also showed that women with HPV infection had less children and younger age in first delivery. Therefore, we introduced logistic regression to perform multiple-factor analysis to identify the independent factors facilitating HPV infection in Nagqu women. Statistics indicated that age group, living location altitude, and parity were the independent factors influencing HPV infection (Table 4). The P-value was 0.031 for those aged between 31 and 35 years and 0.001 for those aged over 50 years. The P-values for altitude and yield were 0.001.
The most common finding was low-grade squamous intraepithelial lesion (LSIL), and 739 women (78.8%) were diagnosed with HPV 16/18. Meanwhile, among other hr-HPV-positive women, only 53.5% (1206 individuals) were diagnosed with LSIL. Additionally, in cytology tests of HPV 16/18 and other hr-HPV-positive patients, the diagnosis of atypical squamous cells of unknown significance (ASCUS), atypical squamous cells cannot exclude HSIL (ASCH), high-grade squamous intraepithelial lesion (HSIL), and cancer was very rare. The most dominant difference was the proportion of Negative for Intra-epithelial Lesions or malignancies (NILM). In the 16/18-positive patients, only 3.1% (29 women) were diagnosed with NILM, whereas in other hr-HPV-positive patients, nearly 40% were diagnosed with NILM. The results are summarized in Table 5.
A total of 2,315 women underwent histological examinations, as shown in Table 5. As shown in Table 5, of HPV 16/18-positive patients, 70.2%, 18.5%, 8.9%, and 2.3% of patients had LSIL, NILM, HSIL, and invasive cancers, respectively. In other hr-HPV-positive patients, the diagnosis of NILM was slightly higher (31.6%), LSIL patients were slightly less than HPV 16/18 patients, with a proportion of 58.2%, HSIL was diagnosed in 9.6% of HPV-positive patients, and cervical cancer was found in only 0.6% of HPV-positive women. The histological differences between the two HPV groups were statistically significant (p = 0.001).
As there were different HPV infection rates in different counties, we next investigated the distribution of HSIL and cervical cancer lesions in each of the 1 different counties. The analysis indicated that, consistent with the HPV infection profiles, patients with HSIL and cancer lesions seemed to have a similar distribution (Figure 4A and Table 6). For HPV-positive women, lesions of HSIL and invasive cancer were most prevalent in some high-altitude areas such as Xainza (2.019%), Baingoin (1.820%), and Nyainrong (1.116%), while the incidence was lower in lower-altitude areas such as Sogxian (0.744%) and Baqen (0.826%). After stratifying the lesion rates by the three altitude levels, the HSIL or cancer incidence also presented a significant positive correlation with altitude, as shown in Figure 4B, p = 0.001.
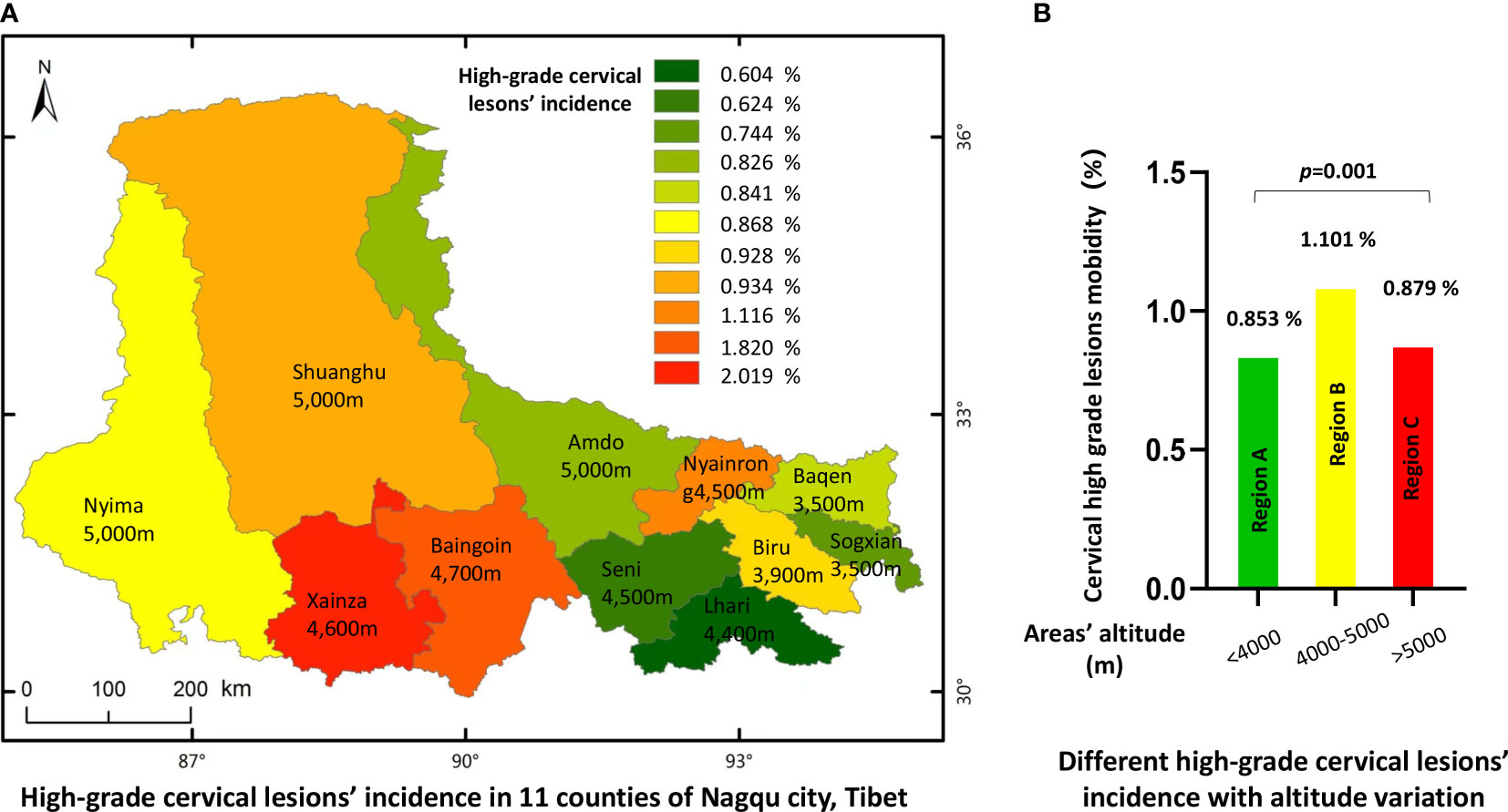
Figure 4 Incidence of high grade cervical lesions in 11 countries of Nagqu. (A) High-grade cervical lesions' incidence in 11 counties of Nagqu city, Tibet. (B) Different high-grade cervical lesions' incidence with altitude variation.
To the best of our knowledge, this is the first large-scale population-based survey in Tibet to investigate the prevalence of HPV infection and related cervical lesions in women. This study revealed that the overall HPV infection rate in the women of Nagqu was 13.42%, consistent with recent studies in other high-altitude areas such as Nyingchi, Tibet, 12.81% (20), and Xining, Qinghai, 16.72% (21), while it was slightly higher compared with the investigation conducted about 10 years ago, in which the HPV positivity rate was 9.82% (22). In previous studies, HPV prevalence varied greatly among areas in China and worldwide. Within China, different districts have different HPV infection rates, such as Shanxi (14.8%), Shenzhen (11.3%), Shenyang (17%), Zhejiang (13.3%), and Shandong (18.1% (23–27). According to reports on global levels, HPV-positive rates range from 5% to 10% (28–31). Our data also showed that cervical lesion rates fluctuated significantly among women in different counties of Nagqu Tibet, ranging from 0.605% to 2.019% (Table 6).
Intriguingly, there seemed to be a positive correlation between HPV prevalence rate/cervical high-grade lesion incidence and altitude. The higher the altitude, the higher the prevalence of HPV in women. We searched for related studies performed in high-altitude areas all over the world and found that HPV prevalence was high (38.2%) in high-altitude areas of Ecuador, a Latin American country with a largely high altitude, although it was a hospital-based rather than a community-based investigation (32). As mentioned earlier, in China, HPV infection rates at different altitudes in Shanxi were 14.8%, 11.3%, 17%, 13.3%, and 18.1%, respectively. Shanxi, Shenyang, and Shandong are in northern China, whereas Shenzhen, Zhejiang, and Tibet are in the south.
Therefore, we assumed that possible reasons may be attributed to the following two elements: hypoxia from high altitude and cold weather because of extreme climate, both of which facilitate HPV transmission.
Although a few studies have suggested a higher risk of HPV prevalence and cervical cancer in women living in high-altitude areas, there is solid evidence that many types of cancer are more common in high-altitude-living people, such as gastric cancer and breast cancer. The mechanism, which has been comprehensively reviewed (33), is complex and controversial.
Researchers have also revealed an association between cold weather and a high risk of many cancers, such as breast, prostate, and colon cancer. The reasons may be related to evolutionary adaption, high concentrations of certain air pollutants, and low levels of serum vitamin D, although any single factor cannot explain this epidemiological phenomenon (34).
Another possible reason we suspected, although with no scientific data, may be attributed to the social and living styles of Tibetan women, especially in pastoral areas. From our examinations and the subsequent interviews, women in pastoral areas in northern Tibet had very poor sanitation, and many of them did not clean their anogenital areas with tissue or other materials after defecation. In addition, in many places, polyandry and premature sex are common in society. Compared with Han women, Tibetan women have a lower probability of using prenatal care or receiving maternal and child healthcare (35), and Tibetan women have lower knowledge and acceptance of cervical cancer screening (2). All of these behaviors are believed to facilitate HPV transmission.
In our study, we found that the main form of HPV infection was a single infection, while multiple HPV infections were less common, and the results were consistent with those of previous studies. The five most common HPV genotypes in Nagqu people were HPV 16, 58, 31, 18, and 52, accounting for approximately 60% of all infections. This result differed slightly from those of previous studies on the top five HPV genotypes in China (36). Fortunately, all five genotypes were within the nine-valent HPV vaccine spectrum. Thus, there should be an HPV vaccine program initiated in northern Tibet, and our data will provide great value in this effort. The manner of infection in Tibet was approximately the same as that encountered in previous studies in other areas of China (37).
As indicated in other studies, our survey also demonstrated that the age-stratified HPV genotype prevalence has a U-shaped pattern. That is, the infection rate was highest in both the youngest and oldest groups and lowest in the median group (38). This was consistent with previous studies in China that showed a bimodal distribution with age (39). Moreover, the prevalence of HPV genotypes differed according to age group. In the group of women younger than 30 years, the top four HPV genotypes were HPV 16, 31, 58, and 52; in those aged 31–50 years, the top four HPV genotypes were HPV 16, 58, 52, and 18. In women aged >50 years, the top four frequently infected HPV genotypes were HPV 31, 58, 16, and 18. Even when we stratified the women into simple and mixed infection groups, this pattern remained consistent. HPV infection is closely related to women’s age, and women who are sexually active and postmenopausal women have a higher probability of infection with HPV (37, 40, 41), which is also applicable in the Nagqu area. This may be related to factors such as age at first sexual encounter, number of sexual partners, frequency of sexual intercourse, and sexual hygiene for sexually active women (42). The immune capacity of women decreases with age, especially in pre- and postmenopausal women, and the ability to eliminate past and new infections weakens (43). Furthermore, education, economic status, and awareness of cervical cancer and cervical cancer screening are perhaps also the influencing factors (42), although we did not explore this in detail in this research. Therefore, it is necessary to strengthen health knowledge education, improve women’s awareness of self-health care, and conduct regular HPV screening to prevent cervical cancer. Our research may thus be helpful for the prevention of cervical cancer in women in Nagqu.
Although the incidence of HSIL and cervical cancer was nearly the same between the HPV 16/18 infection group and the other hr-HPV infection group, the LSIL frequencies were significantly different (44). In the HPV 16/18 group, LSIL was detected in approximately 70.2% of patients, whereas in the other hr-HPV infection group, LSIL only accounted for 58.2% of women. This phenomenon was also observed in other similar research (45–48). According to our data, HPV16/18 positivity results in different LSIL rates in Tibet compared to non-16/18, which may suggest different management strategies for the two types of infection. Previous studies have shown that persistent HPV infections, such as HPV 16, 18, and 31, are closely related to the occurrence of cervical cancer (49). The occurrence of low-risk HPV is closely related to the occurrence of skin venereal diseases such as condyloma acuminatum (50), suggesting that close attention should be paid to hr-HPV infection in the prevention and treatment of cervical cancer.
As mentioned above, the HPV infection rate correlated with altitude; a similar phenomenon was also observed in the histological results (Figure 4 and Table 6). Some studies have indicated that severe lesions might be attributed to the high prevalence of HPV 16/18 infections instead of the overall HPV infection rate (51), which was also verified in our survey. The top three counties with a high incidence of cervical lesions were Xainza (2.019%), Baingoin (1.820%), and Nyainrong (1.116%), and HPV 16/18 infection rates were also higher in 11 counties (5.6%, 3.8%, and 3.1%, respectively).
The strength of this study is highlighted by the fact that it showed a higher prevalence of HPV infection in women residing at higher altitudes in Tibet. Further analysis indicated that HPV infection and cervical lesion incidence might have a certain correlation with location altitude and it might also vary in different age groups. This information not only provides useful information for the treatment of cervical lesions but also has great value in primary and secondary cervical cancer prevention measures for women residing in high-altitude areas.
The weakness of our survey included missing some important information of the women participating in the study such as the number of sex partner, smoking or alcohol drinking habits, and economic status, so that statistical analyses could not be performed to investigate more risk factors facilitating HPV infection and cervical precancer pathogenesis. Further studies are needed not only for more rigorous and comprehensive investigations but also for long-term surveillance of HPV-positive Tibetan women.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Ethics Committee of Nagqu people’s Hospital. The patients/participants provided their written informed consent to participate in this study.
QW, YH, FL, CL, ZS, and DG contributed equally to this work and shared first authorship. DZ, LB, ZH, ZS, NJ, XH, JL, KY, SB, and MT helped in writing this article together. ZX and XX are the authors of this article. All authors have made significant contributions to this article. All authors agree to be responsible for the content of this article and have submitted the manuscript.
National Natural Science Foundation of Liaoning, China, 2019-BS-073 and Public health scientific research project of Futian District, Shenzhen hospital of Guangzhou University of Chinese Medicine, NO. FTWS2019104. Interregion collaborative innovation science and technology program, QYXTZX-NQ2022-03.
Science Foundation of Liaoning Provincial Department of Education LZ2019044.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.841547/full#supplementary-material
1. Youlin ZYQ. Epidemiological status and prevention of cervical cancer. Chin J Matern Child Clin Med (Electron Ed) (2015) 11:02.1–6. doi: 10.3877/cma.j.issn.1673-5250.2015.02.001
2. Wu E, Tiggelaar SM, Jiang T, Zhao H, Wu R, Wu R, et al. Cervical cancer prevention-related knowledge and attitudes among female undergraduate students from different ethnic groups within china, a survey-based study. Women Health (2018) 58:661–84. doi: 10.1080/03630242.2017.1333076
3. Nayar R, Wilbur DC. The Bethesda system for reporting cervical cytology: A historical perspective. Acta Cytol (2017) 61:359–72. doi: 10.1159/000477556
4. Solomon D, Davey D, Kurman R, Moriarty A, O'Connor D, Prey M, et al. The 2001 bethesda system: Terminology for reporting results of cervical cytology. JAMA (2002) 287:2114–9. doi: 10.1001/jama.287.16.2114
5. Mariani L, Igidbashian S, Sandri MT, Vici P, Landoni F. The clinical implementation of primary hpv screening. Int J Gynaecol Obstet (2017) 136:266–71. doi: 10.1002/ijgo.12065
6. Ogilvie GS, van Niekerk D, Krajden M, Smith LW, Cook D, Gondara L, et al. Effect of screening with primary cervical HPV testing vs cytology testing on high-grade cervical intraepithelial neoplasia at 48 months: The HPV FOCAL randomized clinical trial. JAMA (2018) 320:1.43–52. doi: 10.1001/jama.2018.7464
7. Machalek DA, Roberts JM, Garland SM, Thurloe J, Richards A, Chambers I, et al. Routine cervical screening by primary hpv testing: Early findings in the renewed national cervical screening program. Med J Aust (2019) 211:113–9. doi: 10.5694/mja2.50223
8. Goodman A. Hpv testing as a screen for cervical cancer. BMJ (2015) 350:h2372. doi: 10.1136/bmj.h2372
9. Agorastos T, Chatzistamatiou K, Katsamagkas T, Koliopoulos G, Daponte A, Constantinidis T, et al. Primary screening for cervical cancer based on high-risk human papillomavirus (hpv) detection and hpv 16 and hpv 18 genotyping, in comparison to cytology. PloS One (2015) 10:e0119755. doi: 10.1371/journal.pone.0119755
10. Sahlgren H, Elfström KM, Lamin H, Carlsten-Thor A, Eklund C, Dillner J, et al. Colposcopic and histopathologic evaluation of women with hpv persistence exiting an organized screening program. Am J Obstet Gynecol. Carlsten-Thor Eklund (2020) 222:253.e1–8. doi: 10.1016/j.ajog.2019.09.039
11. Bergengren L, Lillsunde-Larsson G, Helenius G, Karlsson MG. Hpv-based screening for cervical cancer among women 55-59 years of age. PloS One (2019) 14:e0217108. doi: 10.1371/journal.pone.0217108
12. Silver MI, Rositch AF, Phelan-Emrick DF, Gravitt PE. Uptake of hpv testing and extended cervical cancer screening intervals following cytology alone and pap/hpv cotesting in women aged 30–65 years. Cancer Causes Control (2018) 29:43–50. doi: 10.1007/s10552-017-0976-x
13. Wang X, Wu S, Li Y. Risks for cervical abnormalities in women with non-16/18 high-risk human papillomavirus infections in south shanghai, China. J Med Virol (2021) 93 93:6355–61. doi: 10.1002/jmv.27185
14. Dehui ZJW. Application of liquid-based thin layer cytometry (lct) in cervical cancer screening at high altitude. Matern Child Health Care China (2008) 23:3628–9. doi: 10.01-4411(2008)25-3628-02
15. Foggin PM, Dorje D, Xuri W, Marc Foggin J, Torrance J. Assessment of the health status and risk factors of kham tibetan pastoralists in the alpine grasslands of the tibetan plateau. Soc Sci Med (2006) 63:2512–32. doi: 10.1016/j.socscimed.2006.06.018
16. Zhong S, Ou Y, Zhang F, Lin Z, Huang R, Nong A, et al. Prevalence trends and risk factors associated with HIV, syphilis, and hepatitis c virus among pregnant women in southwest China, 2009-2018. AIDS Res Ther (2022) 19:31. doi: 10.1186/s12981-022-00450-7
17. Gao G, Wang J, Kasperbauer JL, Tombers NM, Teng F, Gou H, et al. Whole genome sequencing reveals complexity in both hpv sequences present and hpv integrations in hpv-positive oropharyngeal squamous cell carcinomas. BMC Cancer (2019) 19:352. doi: 10.1186/s12885-019-5536-1
18. Yi JZX, Xu J, Liu T, Liu T. Development and validation of a new genotyping assay based on next-generation sequencing. Am J Clin Pathol (2014) 141:796–804. doi: 10.1309/AJCP9P2KJSXEKCJB
20. Jianqi LXL, Zhen L, Jundong W, De Jicuomu YQ. Qu xiwangmu analysis of cervical high-risk human papillomavirus infection status and subtypes of women in rural areas of linzhi city, tibet. J Pract Med (2022) 38:4.502–506. doi: 10.3969/j.issn.1006⁃5725.2022.04.021
21. Cairen Zhuoma ZJ. Wei human papillomavirus infection in 26,622 women in qinghai. Chin J Fam Plan (2013) 21:12.812–816. doi: 10.3969/j.issn.1004-8189.2013.12
22. Qiong SKJ, Hui L, Rong ZX, Fang HH, Jinhua L. Investigation and related factors of cervical human papillomavirus infection in women in tibet autonomous region. Chin Women J Obstet (2009) 12:898–902. doi: 10.3760/cmaJ.issn.0529-567x2009.12005
23. Bergengren L, Lillsunde-Larsson G, Helenius G, Karlsson MG. HPV-based screening for cervical cancer among women 55-59 years of age. PloS One (2019) 14:e0217108. doi: 10.1371/journal.pone.0217108
24. Tang Y, Zheng L, Yang S, Li B, Su H, Zhang LP. Epidemiology and genotype distribution of human papillomavirus (hpv) in southwest china: A cross-sectional five years study in non-vaccinated women. Virol J (2017) 14:84. doi: 10.1186/s12985-017-0751-3
25. Yang L, He Z, Huang XY, Liu HN, Tao JY. Prevalence of human papillomavirus and the correlation of HPV infection with cervical disease in weihai, China. Eur J Gynaecol Oncol (2015) 36:73–7. doi: 10.12892/ejgo2581.2015
26. Mai Q, Yang X, Cheng H, Wu G, Wu Z. Prevalence and genotype distribution of human papillomavirus among women with cervical lesions in shenzhen city, China. Hum Vaccin Immunother (2021) 17:965–71. doi: 10.1080/21645515.2020.1805993
27. Luo LP, He P, Liu QT, Jiang YH, Zhang YN, Li QZ, et al. Prevalence and genotype distribution of hpv infection among 214,715 women from southern china, 2012-2018: Baseline measures prior to mass hpv vaccination. BMC Infect Dis (2021) 21:328. doi: 10.1186/s12879-021-06019-5
28. Mutombo AB, Benoy I, Tozin R, Bogers J, Van Geertruyden JP, Jacquemyn Y. Prevalence and distribution of human papillomavirus genotypes among women in Kinshasa. J Glob Oncol (2019) 5:1–9. doi: 10.1200/JGO.19.00110
29. Yang J, Wang W, Wang Z, Wang Z, Wang Y, Wang J, et al. Prevalence, genotype distribution and risk factors of cervical hpv infection in yangqu, china: A population-based survey of 10086 women. Hum Vaccin Immunother (2020) 16:1645–52. doi: 10.1080/21645515.2019.1689743
30. Sankaranarayanan R, Nene BM, Shastri SS, Jayant K, Muwonge R, Budukh AM, et al. HPV screening for cervical cancer in rural India. N Engl J Med (2009) 360:1385–94. doi: 10.1056/NEJMoa0808516
31. Comerlato J, Kops NL, Bessel M, Horvath JD, Fernandes BV, Villa LL, et al. Sex differences in the prevalence and determinants of hpv-related external genital lesions in young adults: A national cross-sectional survey in brazil. BMC Infect Dis (2020) 20:683. doi: 10.1186/s12879-020-05376-x
32. Vega Crespo B, Neira VA, Ortíz Segarra J, Rengel RM, López D, Orellana MP, et al. Role of self-sampling for cervical cancer screening: Diagnostic test properties of three tests for the diagnosis of hpv in rural communities of cuenca, Ecuador. Int J Environ Res Pub Health (2022) 19:4619. doi: 10.3390/ijerph19084619
33. Calderón-Gerstein WS, Torres-Samaniego G. High altitude and cancer: An old controversy. Respir Physiol Neurobiol (2021) 289:103655. doi: 10.1016/j.resp.2021.103655
34. Voskarides K. The “Cancer-cold” hypothesis and possible extensions for the Nordic populations. Scand J Pub Health (2019) 47(5):477–481. doi: 10.1177/1403494819831905
35. Huang Y, Shallcross D, Pi L, Tian F, Pan J, Ronsmans C. Ethnicity and maternal and child health outcomes and service coverage in western china: A systematic review and meta-analysis. Lancet Glob Health (2018) 6:e39–56. doi: 10.1016/S2214-109X(17)30445-X
36. Zhu B, Liu Y, Zuo T, Cui X, Li M, Zhang J, et al. The prevalence, trends, and geographical distribution of human papillomavirus infection in china: The pooled analysis of 1.7 million women. Cancer Med (2019) 8:5373–85. doi: 10.1002/cam4.2017
37. Feng D, Wei S, Chen J, Yu Z, Lhamo Y, Wang H, et al. Human papillomavirus prevalence and genotype distribution landscapes in shannan city, Tibet Tibetan autonomous region, China. Virol J (2022) 19:46. doi: 10.1186/s12985-022-01775-5
38. Evans AC, Adamson CS, Papillo JL, St John TL, Leiman G, Cooper K. Distribution of human papillomavirus types in ThinPrep papanicolaou tests classified according to the Bethesda 2001 terminology and correlations with patient age and biopsy outcomes. Cancer (2006) 106:1054–64. doi: 10.1002/cncr.21664
39. Yuqian HSZFZ, Wen C, Youlin Q. Multicenter cross-sectional study on cervical human papillomavirus infection and type distribution in Chinese women. Chin J Epidemiol (2015) 36:12.1351–1356. doi: 10.3760/cma.j.issn.0254-6450.2015.12.006
40. Guo C, Du H, Qu X, Duan X, Li J, Li R, et al. Prevalence of human papillomavirus among chinese han and mongols minority women in inner mongolia, china: Reflected by self-collected samples in chimust. Front Public Health (2022) 10:840879. doi: 10.3389/fpubh.2022.840879
41. Wei L, Ma L, Qin L, Huang Z. The prevalence and genotype distribution of human papillomavirus among women in guangxi, southern China. Infect Agent Cancer (2022) 17:19. doi: 10.1186/s13027-022-00431-5
42. Zhixiang LFC, Shujuan W. Analysis of current situation and high risk factors of cervical hpv infection in 680 women. Ningxia Med J (2022) 42:6.545–547. doi: 10.13621/j.1001-5949.2020.060.545
43. Carozzi FM, Ocello C, Burroni E, Faust H, Zappa M, Paci E, et al. Effectiveness of HPV vaccination in women reaching screening age in Italy. J Clin Virol (2016) 84:74–81. doi: 10.1016/j.jcv.2016.09.011
44. Wei H, Wang N, Zhang Y, Zhang J, Wang S, Zhang S. Distribution of various types of low-risk human papillomavirus according to cervical cytology and histology in northern Chinese women. Int J Gynaecol Obstet (2014) 126:28–32. doi: 10.1016/j.ijgo.2014.01.020
45. Lufang DHD, Aimin X, Chun W, Xia H, Meifang Z. Study on the relationship between type specific hr-hpv subtype viral load reflected by ct value detected by cobas 4800 hpv and cervical lesions. Chin J Obstet Gynecol (2019) 07:458–63. doi: 10.3760/cma.j.issn.0529⁃567x.2019.07.005
46. Gallegos-Bolaños J, Rivera-Domínguez JA, Presno-Bernal JM, Cervantes-Villagrana RD. High prevalence of co-infection between human papillomavirus (hpv) 51 and 52 in Mexican population. BMC Cancer (2017) 17:531. doi: 10.1186/s12885-017-3519-7
47. Tao X, Zhang H, Zhang H, Xiao J, Li J, Zhou X, et al. Follow-up with histopathology and HPV testing on LSIL cytology in china's largest academic woman's hospital. Cancer Cytopathol (2019) 127:258–66. doi: 10.1002/cncy.22119
48. Wu J, Li X, Liu X, Gao Z. Human papillomavirus genotype prevalence in the women of shanghai, china and its association with the severity of cervical neoplasia. Int J Clin Exp Pathol (2018) 11:4614–21.
49. Dunne EF, Markowitz LE. Genital human papillomavirus infection. Clin Infect Dis (2006) 43:624–9. doi: 10.1086/505982
50. Seong SH, Jeong SM, Jung JH, Kwon DI, Jang JY, Kim JH, et al. Ms. human papillomavirus genotype distribution of genital keratotic lesions. Australas J Dermatol (2022). doi: 10.1111/ajd.13867
Keywords: cervical cancer, HPV, cervical cancer screening, genotype, disease distribution
Citation: Wang Q, He Y, Long F, Li C, Shen Z, Guo D, Zhaxi D, Bumu L, Hua Z, Sun Z, Jiang N, Han X, Li J, Yan K, Bai S, Tao M, Xu X and Xiao Z (2022) Cervical cancer screening in high-altitude areas in China: A large cross-section study of 25,173 women in northern Tibet. Front. Oncol. 12:841547. doi: 10.3389/fonc.2022.841547
Received: 22 December 2021; Accepted: 13 July 2022;
Published: 25 August 2022.
Edited by:
Debabrata Barmon, Bhubaneswar Borooah Cancer Institute, IndiaReviewed by:
Timothy Abiola Olusesan Oluwasola, University of Ibadan, NigeriaCopyright © 2022 Wang, He, Long, Li, Shen, Guo, Zhaxi, Bumu, Hua, Sun, Jiang, Han, Li, Yan, Bai, Tao, Xu and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen Xiao, c2VyaW91c2RvY0AxNjMuY29t; Xiaoguang Xu, ZHJ4Z3h1QDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.