- 1Zhongshan People’s Hospital, Guangdong Medical University, Zhongshan, China
- 2Department of Hepatic Surgery Center, Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China
Background: Large hepatocellular carcinoma (LHCC) is highly malignant and prone to recurrence, leading to a poor long-term prognosis for patients. There is an urgent need for measures to intervene in postoperative recurrence. Preoperative Transcatheter Arterial Embolization (TACE) is an effective treatment. However, there is a lack of reliable preoperative indicators to guide the application of preoperative TACE. We, therefore, investigated whether the preoperative status of circulating tumor cells (CTCs) could be used to guide preoperative TACE for HCC treatment.
Methods: This study recruited 361 HCC patients and compared recurrence-free survival (RFS) and overall survival (OS) in patients treated with TACE prior to surgery and those not treated with TACE. Patients were divided into CTC-positive group and CTC-negative group according to CTC status, and the effect of preoperative TACE on RFS and OS was compared in each subgroup.
Results: In CTC-positive patients, preoperative TACE reduces early recurrence and improves long-term survival. However, HCC patients did not benefit from preoperative TACE for the overall population and CTC-negative patients.
Conclusions: Preoperative CTC testing is a reliable indicator of whether HCC patients received TACE preoperatively. CTC positivity was associated with early tumor recurrence, and preoperative TACE could reduce early recurrence and long-term prognosis in CTC-positive patients.
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common malignancy globally and the third leading cause of cancer deaths (1). For early-stage HCC, partial hepatectomy prolongs disease-free survival (RFS) and overall survival (OS) in HCC patients (2, 3). However, for HCC patients with large hepatocellular carcinoma (> 5 cm), the tumor is highly malignant and prone to recurrence after surgery, with the vast majority of patients eventually dying due to tumor recurrence (4). This creates an urgent need for appropriate treatment measures to control postoperative recurrence of HCC (5).
Previous studies have reported that preoperative transcatheter arterial embolization (TACE) plays an essential role in improving RFS and OS in patients with HCC (6–15). It has also been suggested that preoperative TACE does not improve RFS and OS in HCC patients (16–25). Therefore, many scholars have speculated that preoperative TACE may only benefit certain particular types of HCC groups, especially those with a higher degree of malignancy (26). However, there is no relevant definition of this special type; there is a lack of relevant and reliable preoperative information to distinguish which HCC patients may benefit from preoperative TACE.
A new study from Zhongshan Hospital reports that positive pre-surgical circulating tumor cells (CTCs) are associated with early postoperative recurrence (27). In addition, it can guide whether postoperative adjuvant TACE should be performed to avoid unnecessary postoperative TACE and achieve precise treatment (28). In the same study, we also noted that in the HCC population with positive preoperative CTC testing, postoperative TACE reduced early postoperative recurrence and facilitated the survival of HCC patients (28). We, therefore, envisaged whether preoperative CTC could guide preoperative adjuvant TACE in HCC patients? Our research team carried out a correlation retrospective study based on this.
Materials and Methods
Patient Population
This study recruited HCC patients who underwent preoperative CTC testing at the Department of General Surgery I of Zhongshan People’s Hospital from January 2010 to December 2017, with the following inclusion criteria: (1) patients diagnosed with HCC by postoperative pathology, (2) no treatment other than TACE, (3) radical tumor resection (R0) that is, negative macroscopic and microscopic tumor resection margins, (4) complete serological and imaging data, (5) tumor diameter not less than 5 cm, and (6) single tumor. Exclusion criteria were (1) patients younger than 18 years of age, (2) presence of vascular tumor thrombus and distant metastasis, (3) patients with Child-Pugh grade C liver function, (4) no severe vital organ dysfunction, (5) patients who underwent palliative resection, and (6) loss of postoperative follow-up data. The Ethics Committee of Zhongshan People’s Hospital has approved the retrospective study, all patients have signed an informed consent form.
Data Collection
All patients underwent abdominal enhanced CT or MRI, chest CT or X-ray scan. Laboratory tests include blood routine, liver and kidney function, coagulation function, hepatitis B surface antigen, hepatitis C antibody, AFP, and other examinations. The basic data of the included study population, such as gender, age, hepatitis B surface antigen, hepatitis C antibody, AFP level, alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl aminotransferase (GGT), alkaline phosphatase (ALP), creatinine (Cr), albumin (Alb), total bilirubin (TIBL), direct bilirubin (DIBL), international normalized ratio (INR), platelet count, presence of cirrhosis, Child-Pugh grade, presence of microvascular infiltration (MVI), maximum tumor diameter, pathological classification, extent of liver resection, type of liver resection and other data were collected. Minor liver resection was defined as resection of fewer than three Couinaud liver segments, while major liver resection was defined as resection of three or more liver segments. Non-anatomical liver resection included a limited resection or wedge resection; anatomical resections were defined by the Brisbane 2000 system. The continuous variables are transformed into binary variables, and the cut-off value is the upper and lower lines of the recognized normal value.
Preoperative TACE
Considering that this was a retrospective study, the decision to use TACE prior to surgery was left to the discretion of the treating surgeon and the patient at that time. The patient was placed supine, locally disinfected, draped, and given local anesthetized. The puncture site was chosen to be 2 cm below the inguinal ligament, and the catheter sheath was placed into the femoral artery using the Seldinger technique. Firstly, the DSA technique helps with abdominal trunk and standard hepatic artery angiography to determine the tumor’s location, size, and condition of the tumor. Once the tumor is understood, the catheter sheath is continued deeper into the left or right hepatic artery or the vessel that feeds the tumor, 5-fluorouracil (500 mg/m2) or oxaliplatin (100 mg/m2) was injected into the proper hepatic artery, and embolization was performed using different embolization materials. Patients were asked to return to the hospital 4-6 weeks after embolization for follow-up serology, including blood routine, liver and kidney function, coagulation function, AFP, and imaging included abdominal enhanced CT or MRI, chest X-ray scan, etc. All of the above procedures were performed by highly qualified attending physicians who received relevant interventional medicine.
Isolation and Identification of CTC
The Cyttel method is used to detect CTCs, and its main principles include the negative immunomagnetic particle assay and immunofluorescence in situ hybridization (im-FISH). Jiangsu Lyle Biomedical Technology Co manufactures the kit. For patients with preoperative TACE, samples were obtained within three days before TACE, while for patients without preoperative TACE, the sample extraction must also be completed within three days before surgery. Generally, we draw 5ml peripheral blood, and process the samples strictly according to the manufacturer’s instructions. Firstly, the samples was treated with negative immunomagnetic powder method to remove leukocytes from the peripheral blood, and isolate rare cells in the blood, and finally obtain CTCs. Then, the im-FISH technique was used to fix and dehydrate the samples, then hybridization with chromosome centromeres 1 and 8, followed by sealing with 4-diamidine-2-phenylindole (DAPI) staining solution, and then observation and counting under a fluorescence microscope (29–32). It defined CTC count ≥1 as CTC-positive (32).
Follow-Up
Each follow-up visit for all patients include AFP, routine blood tests, liver and kidney function tests, coagulation function tests. Enhanced CT or MRI of the abdomen, chest CT, and the bone scan will be performed if tumor residue and signs of tumor recurrence are suspected. The first postoperative follow-up visit would performed one month after the operation. The follow-up frequency was once every 2-3 months within six months after the operation, once every 3-4 months within 6-24 months after the operation, and once every 4-6 months after 24 months after the operation. After a recurrence of HCC, treatment options are chosen according to the recurrence and the patient’s general condition. Treatment options include surgical re-resection, radiofrequency ablation (RFA), percutaneous ethanol injection (PEI), TACE, taking targeted drugs, immune drug therapy, and even liver transplantation. OS was defined as the date of surgery until patient death or last follow-up, and RFS was defined as the date of surgery until patient signs of recurrence or last follow-up. Recurrence was classified as early recurrence and late recurrence using a cut-off value of 24 months.
Statistical Analysis
Continuous variables were expressed as median ± square difference (Median ± SD), and categorical variables were expressed as number (n) or percentage (%) of patients. The t-test or Mann-Whitney test was used to compare two groups of continuous variables, and the χ2 or Fisher’s exact test was used to compare two groups of categorical variables. The survival curves of OS and RFS of the patients were plotted using the Kaplan-Meier method, and the OS and RFS of the patients in the preoperative TACE group and the two groups without preoperative TACE were compared using the log-rank. We also used the Landmark analysis method to analyze the results of assessing early recurrence (recurrence 24 months after surgery) and late recurrence. Univariate and multivariate Cox regression models were used to analyze the independent risk factors of each factor on patients’ RFS and OS. All statistics and graphs for this study were completed in R (version 3.62). P values < 0.05 were considered statistically significant.
Results
Characteristics of Patients With HCC
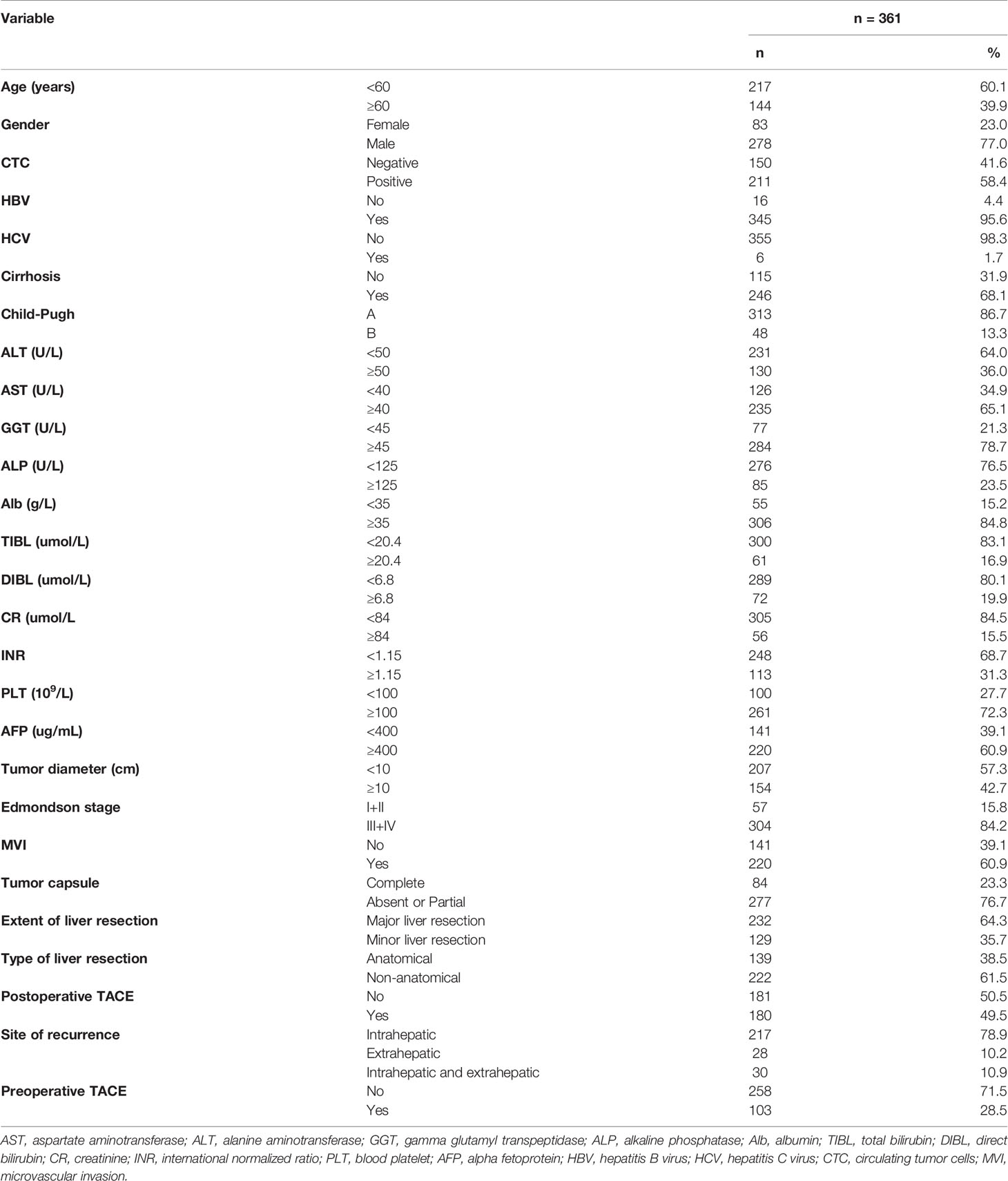
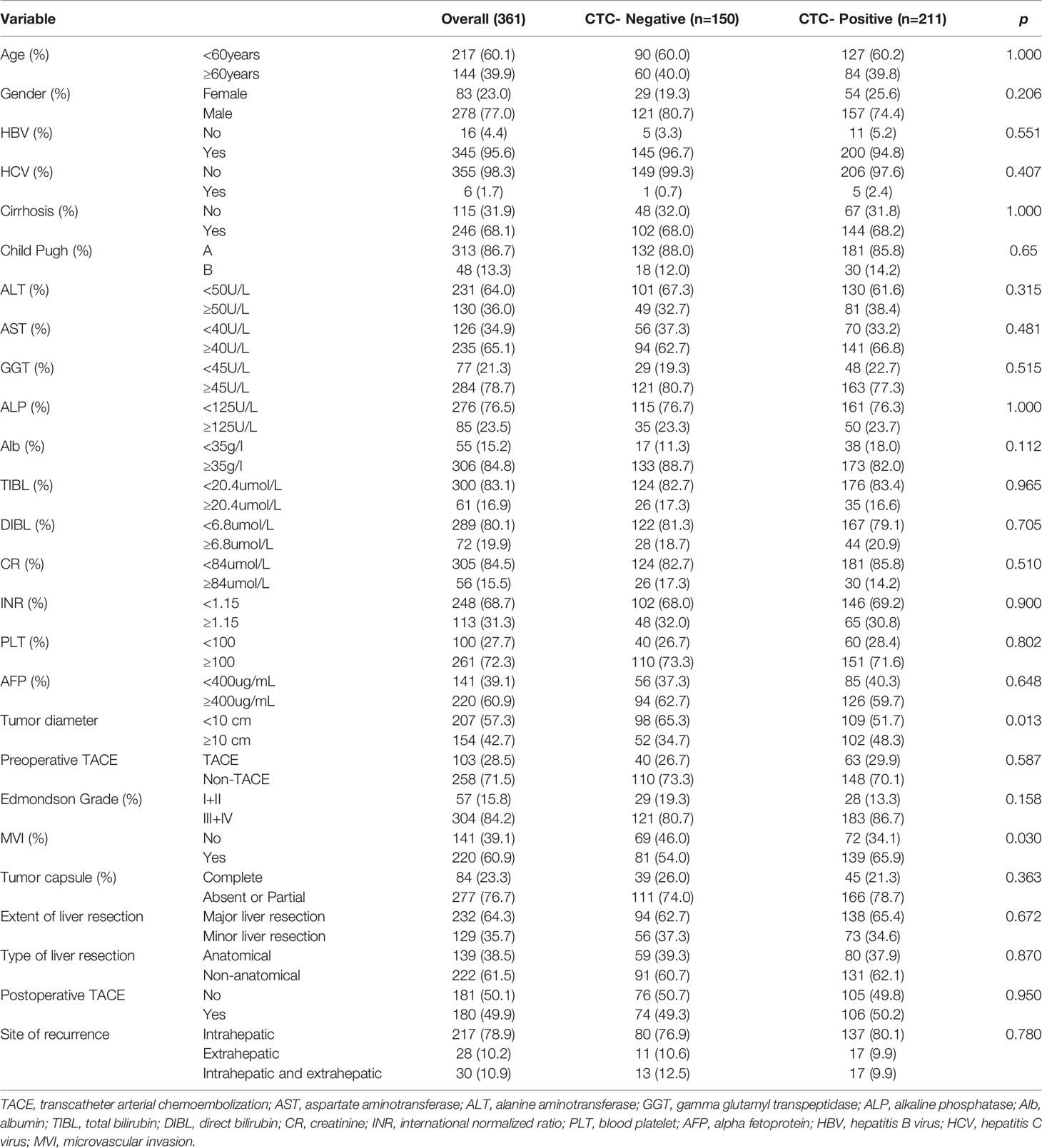
Baseline characteristics of the total population of HCC are listed in Table 1. The population was divided into positive and negative subgroups based on the preoperative CTCs count. The clinical baseline of each subgroup is shown in Table 2. In this study, a total of 361 patients with HCC were enrolled in this study, including 211 patients of CTC-positive (58.4%) and 103 patients of preoperative TACE (28.5%). The median follow-up time of the CTC-positive group was 38.0 months, while the median follow-up time of the CTC-negative group was 44.5 months. The median follow-up time of HCC patients with preoperative TACE was 41.0 months, while patients without TACE were 36.5 months. During follow-up, 134 patients died, and 275 patients developed tumor recurrence. In the CTC-positive and CTC-negative subgroups, the clinicopathological variables were similar, comparable and not statistically significant between patients who underwent preoperative TACE and those who did not (P > 0.05; Table 2). In the overall population, RFS and OS were similar of patients with and without preoperative TACE; Preoperative TACE did not improve the prognosis of HCC (P > 0.05; Figures 1A, B).
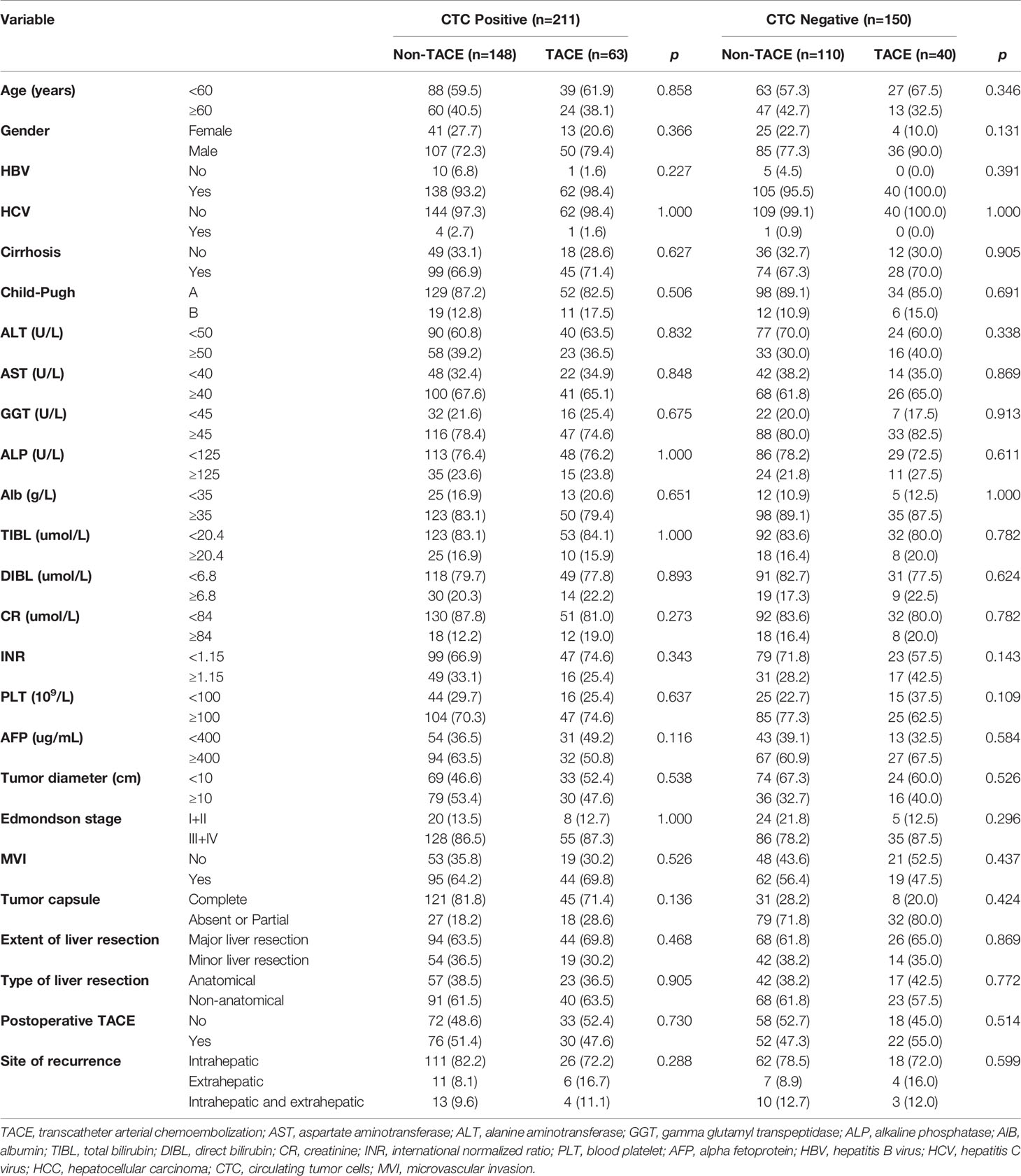
Table 2 Comparison of clinicopathological variables between preoperative TACE and control group in HCC patients with CTC-positive and CTC-negative groups.
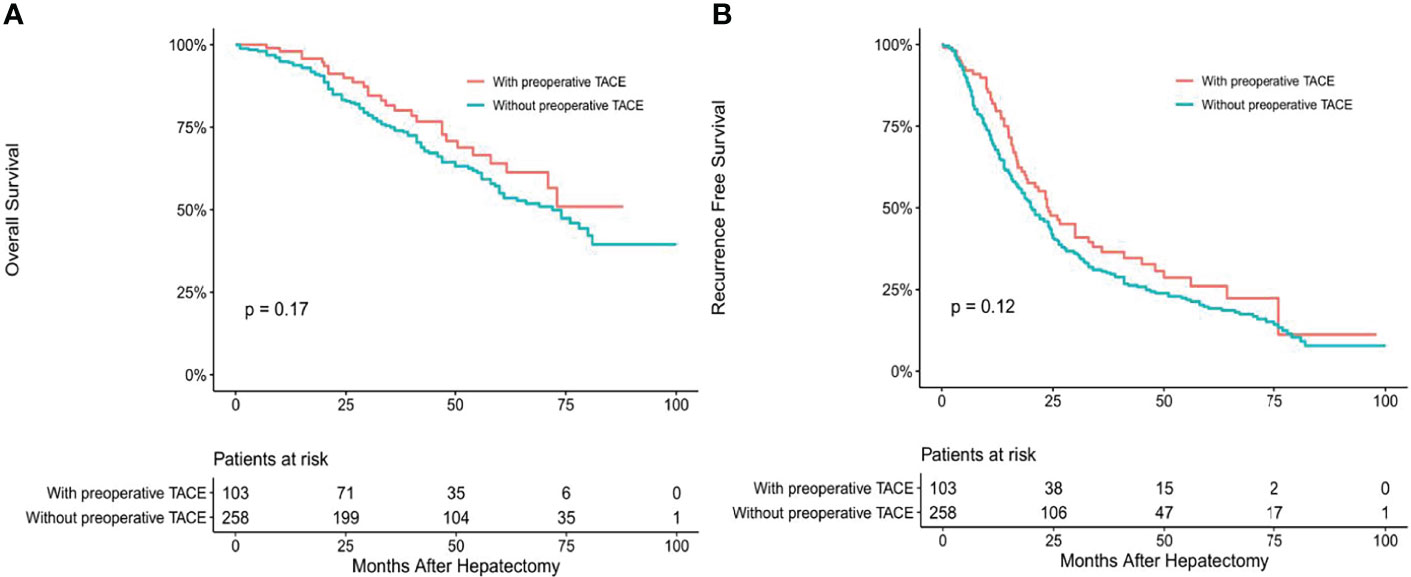
Figure 1 Overall (A) and recurrence-free (B) survival curves of overall HCC patients with or without preoperative TACE.
CTCs Status Affects OS and RFS of HCC Patients
Using survival curves drawn by the Kaplan Meier method, we found that OS (median 39 months vs. 47 months, P < 0.05, Supplementary Figure 1A) and RFS (median 17.0 months vs. 24 months, P < 0.05 Supplementary Figure 1B) in CTC-positive group were worse than those in CTC-negative group. We also analyzed the effect of CTCs status on postoperative recurrence patterns using the landmark method. Using a 24-month cut-off, postoperative recurrence was divided into an early recurrence and late recurrence. We found that CTC positive was associated with postoperative early recurrence (P < 0.05; Supplementary Figure 2) but not with late recurrence (P > 0.05; Supplementary Figure 2).
The Clinical Efficacy of Preoperative TACE Was Evaluated in Subgroups of CTC-Positive and CTC-Negative Groups
To determine whether CTCs status affects the clinical efficacy of TACE, we stratified patients’ CTCs status of and compared the OS and RFS between patients with and without preoperative TACE at different CTCs status. In the CTC-positive group, preoperative TACE prolonged OS and RFS in HCC patients; the difference was statistically significant (P < 0.05; Figures 2A, B). In CTC-negative group, preoperative TACE could not improve RFS and OS, and the difference was not statistically significant (P > 0.05; Figures 3A, B).
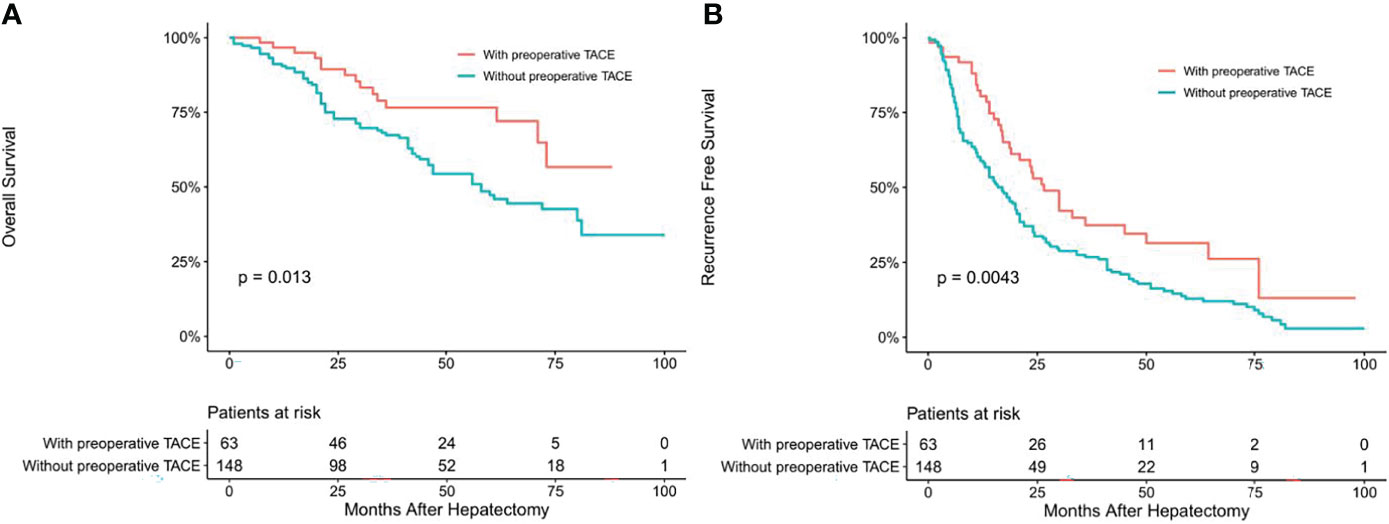
Figure 2 Overall (A) and recurrence-free (B) survival curves of HCC patients in CTC-positive patients with or without preoperative TACE.
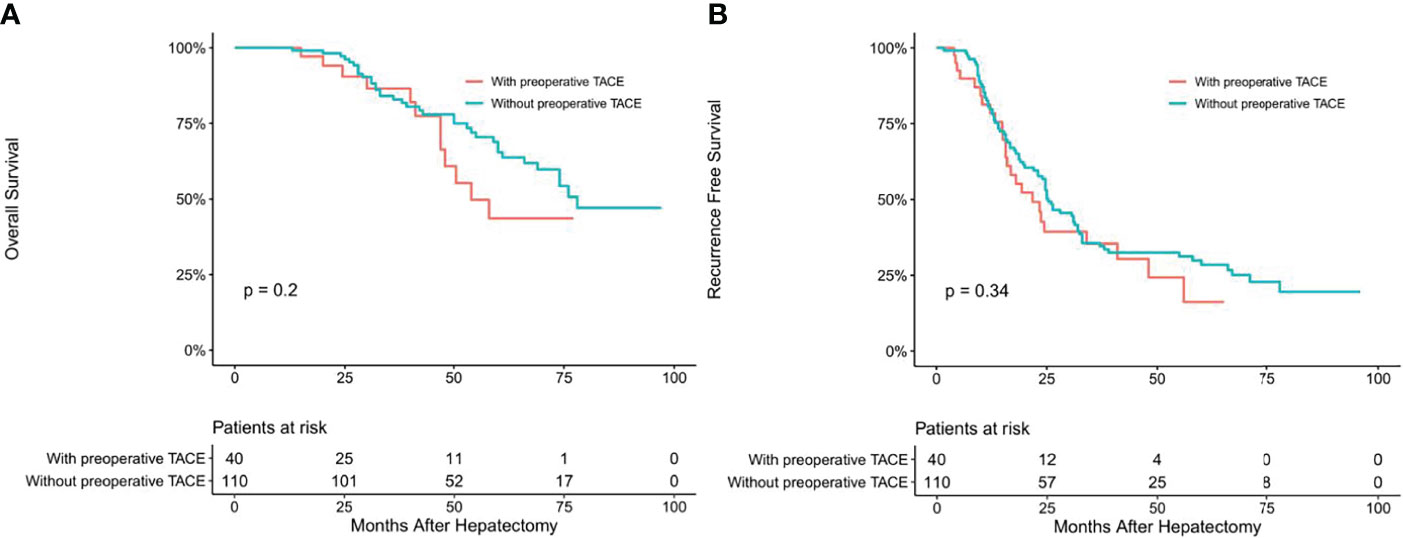
Figure 3 Overall (A) and recurrence-free (B) survival curves of HCC patients in CTC-negative patients with or without preoperative TACE.
Univariate and multivariate Cox regression analysis also showed that in CTC-positive group, non-preoperative TACE was an independent risk factor for OS (hazard ratio [HR]= 2.330, 95% confidence interval [CI], 1.318-4.120, P <0.05; Table 3) and RFS(hazard ratio [HR]= 2.332, 95% confidence interval [CI], 1.584-3.432, P <0.05; Table 4) of HCC patients, while in CTC-negative group, preoperative TACE had no effect on OS (hazard ratio [HR]= 0.655, 95% confidence interval [CI], 0.338-1.267, P >0.05; Table 5)and RFS (hazard ratio [HR]= 0.805, 95% confidence interval [CI], 0.511-1.269,P >0.05; Table 6) of HCC patients.
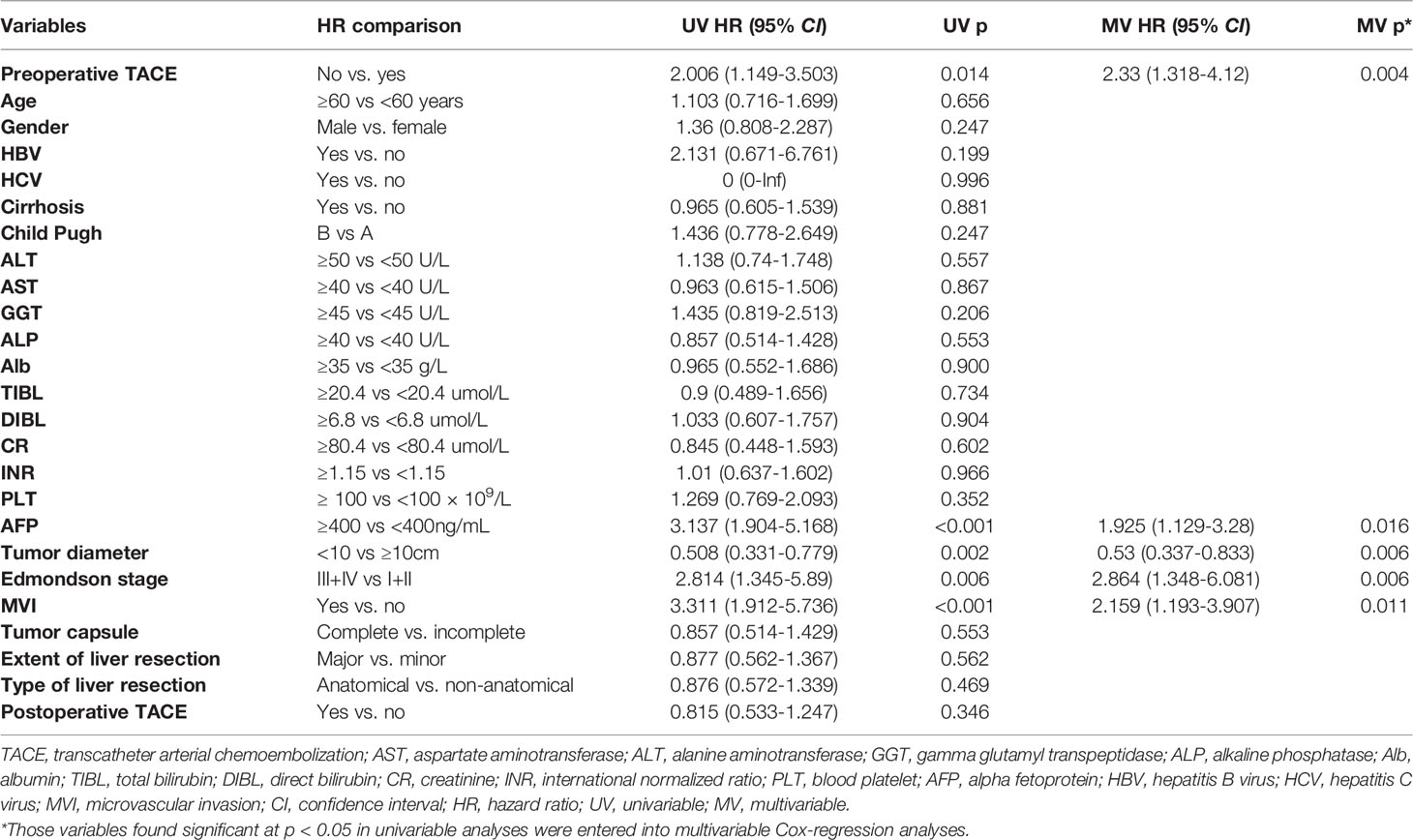
Table 3 Univariate and multivariate Cox regression analyses were used to identify independent risk factors for overall survival in CTC-positive patients.
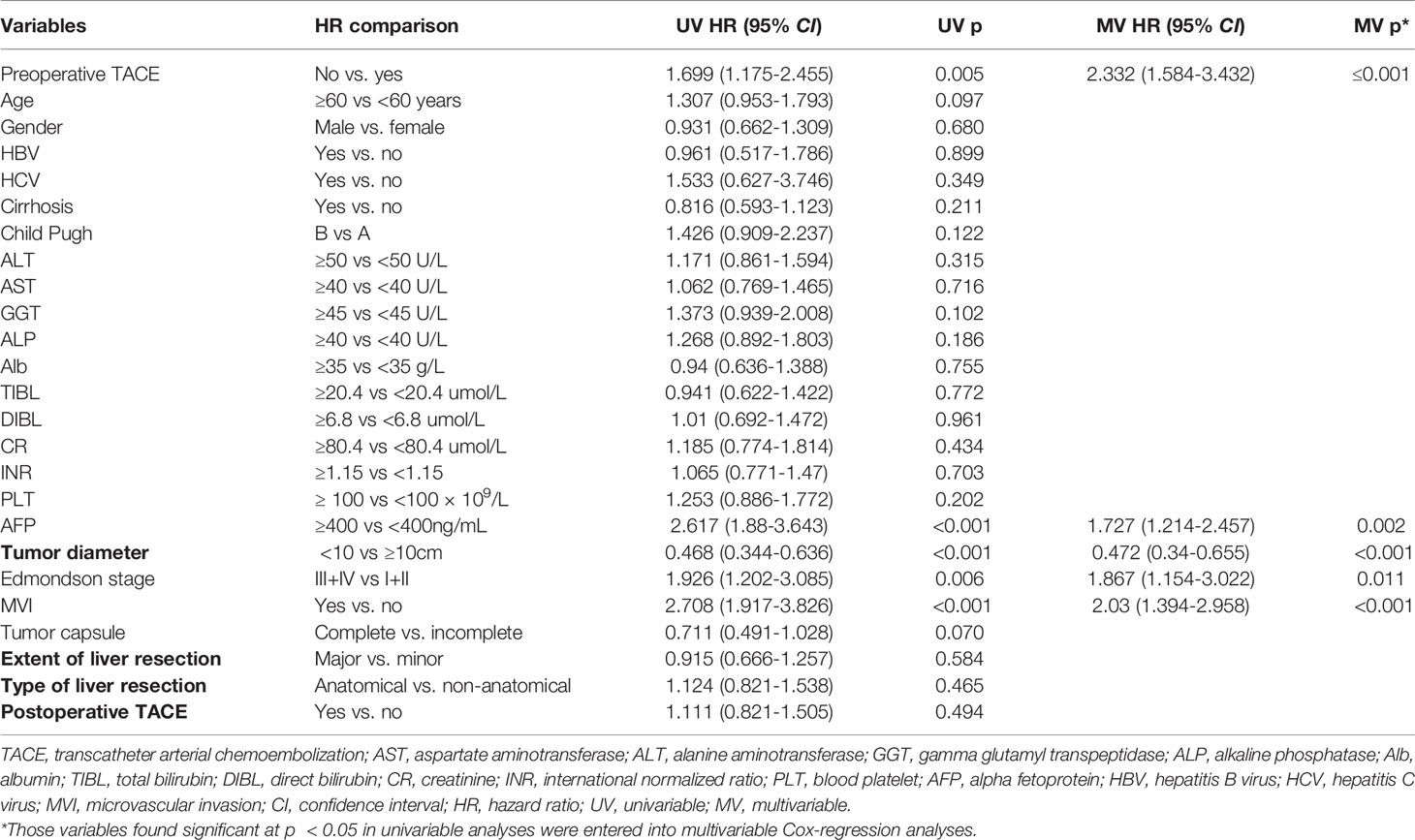
Table 4 Univariate and multivariate Cox regression analyses were used to identify independent risk factors for recurrence free survival in CTC positive patients.
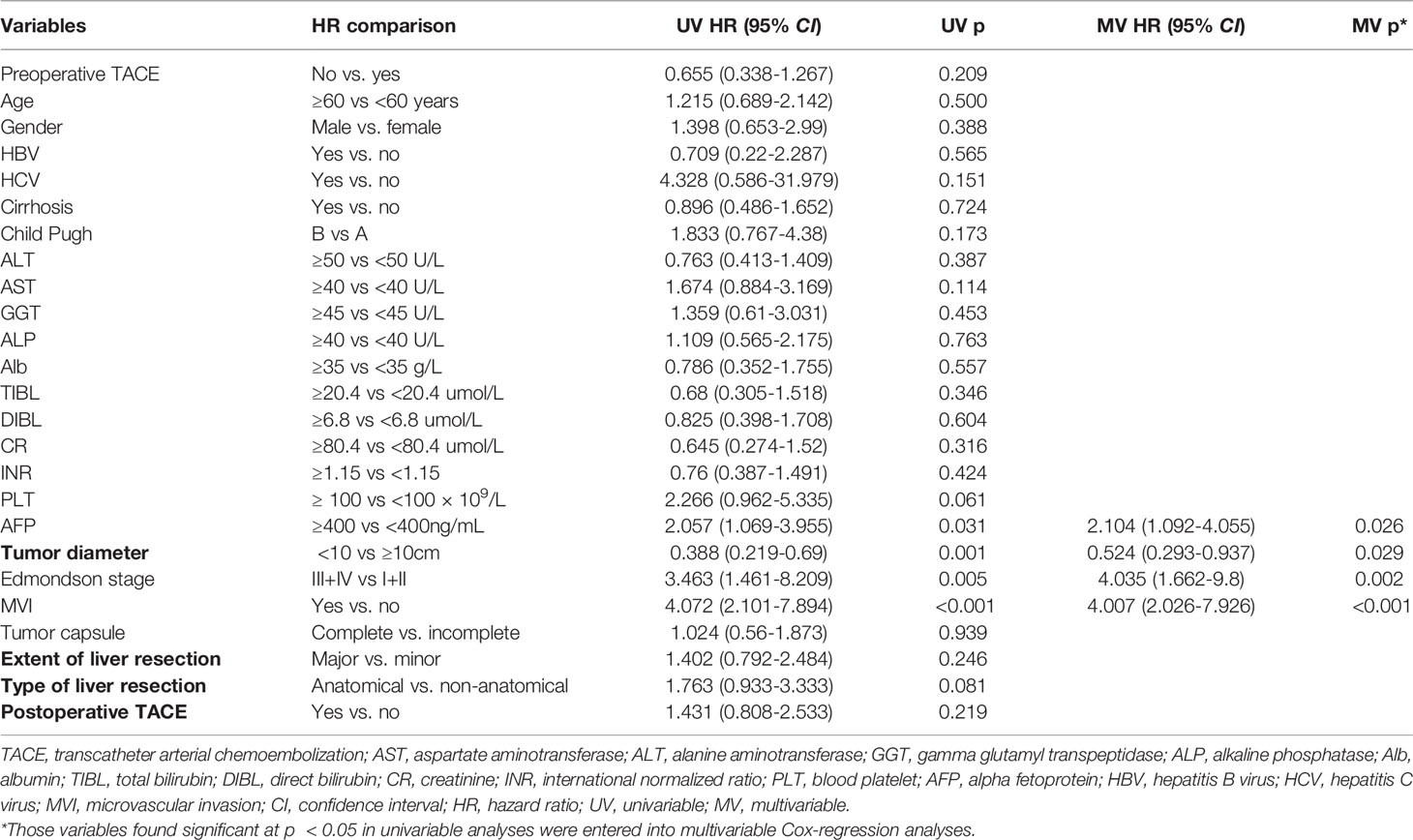
Table 5 Univariate and multivariate Cox regression analyses were used to identify independent risk factors for overall survival in CTC-negative patients.
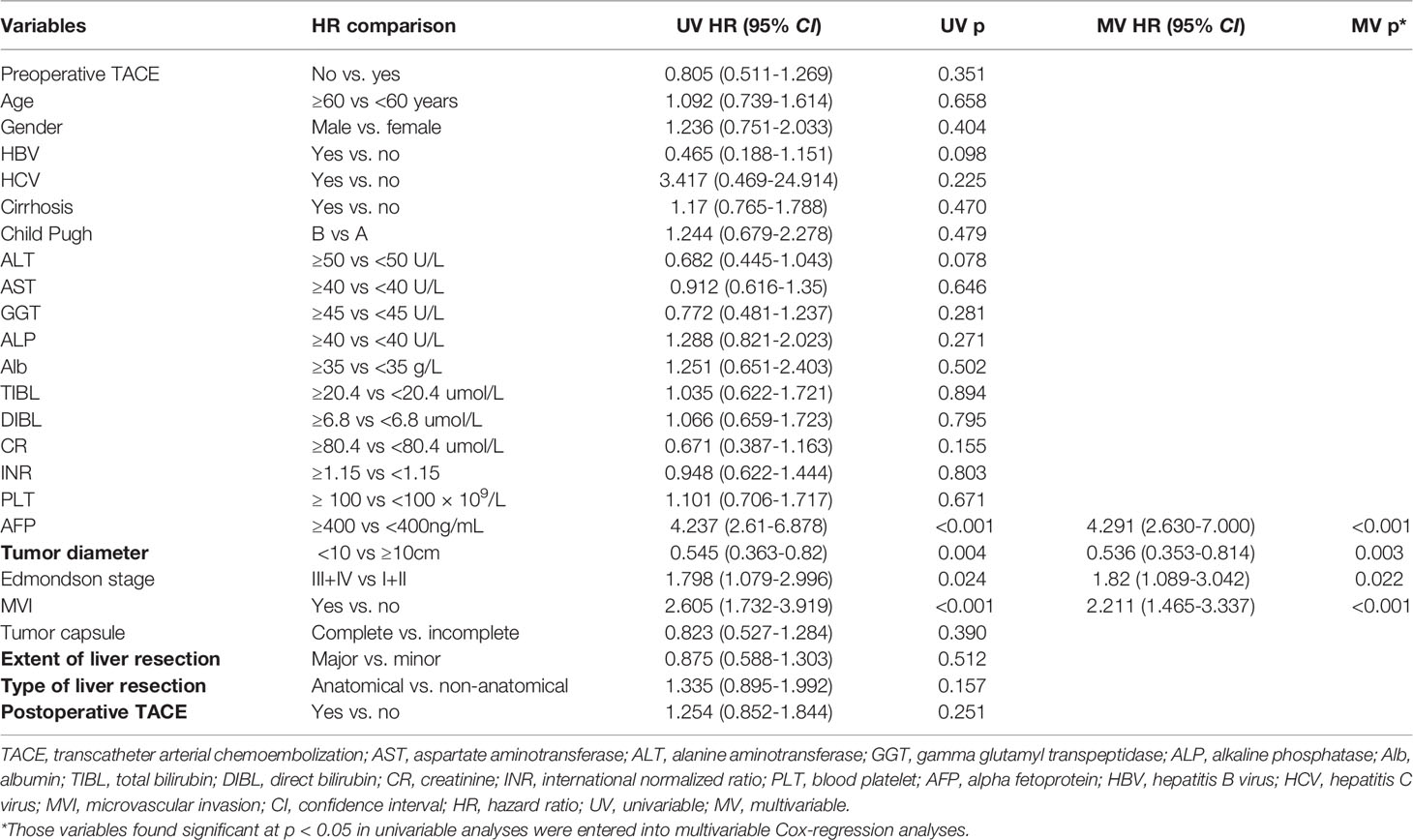
Table 6 Univariate and multivariate Cox regression analyses were used to identify independent risk factors for recurrence free survival in CTC negative patients.
Preoperative Adjuvant TACE Can Reduce the Early Recurrence of CTC-Positive Patients
Using landmark analysis and taking 24 months as the cutoff value, we found that preoperative TACE could reduce the early recurrence of patients in CTC-positive group (P < 0.05, Figure 4A), but could not improve the late recurrence rate of patients (P > 0.05, Figure 4A). In the CTC-negative group, preoperative TACE could not improve the early and late recurrence (P > 0.05, Figure 4B).
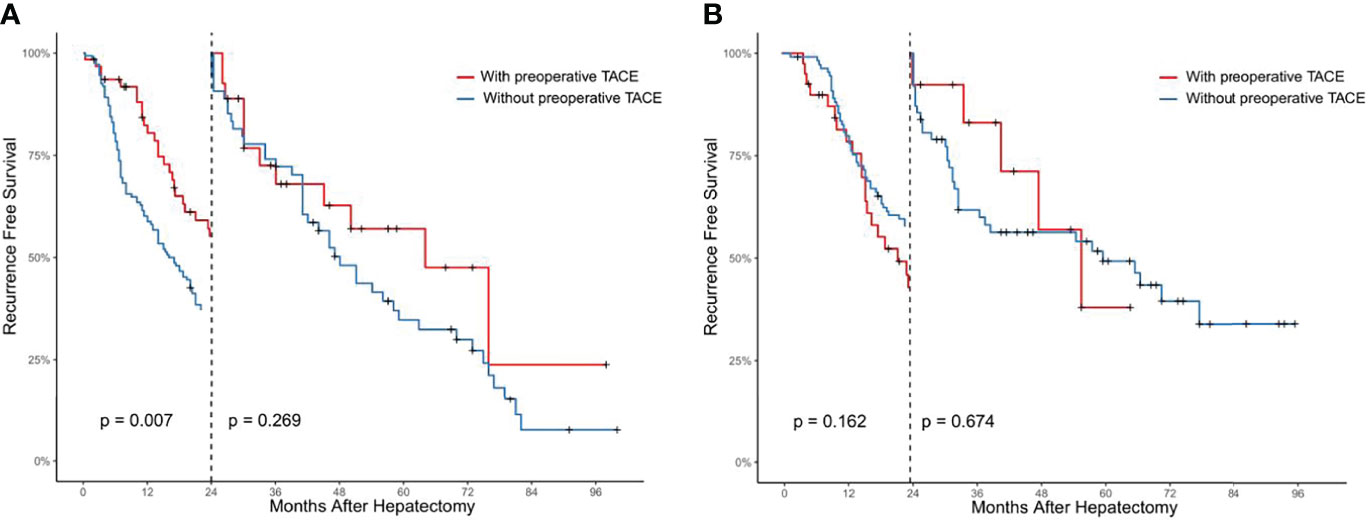
Figure 4 Analysis of the effect of preoperative TACE on early and late postoperative recurrence in CTC positive (A) and CTC negative (B) HCC patients by landmark method.
The Clinicopathological Baseline of CTC-Positive Group and CTC-Negative Group Were Compared
The comparison of clinicopathological variables between the CTC-positive group and CTC-negative group is shown in Table 7; the proportion of patients with tumor diameter ≥10cm (48.3.1% vs. 34.7%, P < 0.05; Table 7) and positive rate of MVI (65.9% vs. 54.0%, P < 0.05; Table 7) in CTC-positive group was higher than that in CTC-negative group. At the same time, other clinicopathological indicators such as age, sex, HBV, cirrhosis, Child-Pugh, Edmondson stage, and AFP≥400ng/ml were not significantly different(P > 0.05; Table 7).
Comparison of Perioperative Complications Between Patients With Preoperative TACE and Those Without Preoperative TACE
We compared the effects of preoperative TACE on perioperative complications and mortality. We found that preoperative TACE did not increase perioperative mortality, liver failure, bile leakage, ascites, wound infection, and other complications compared to patients without preoperative TACE (P > 0.05; Table 8).
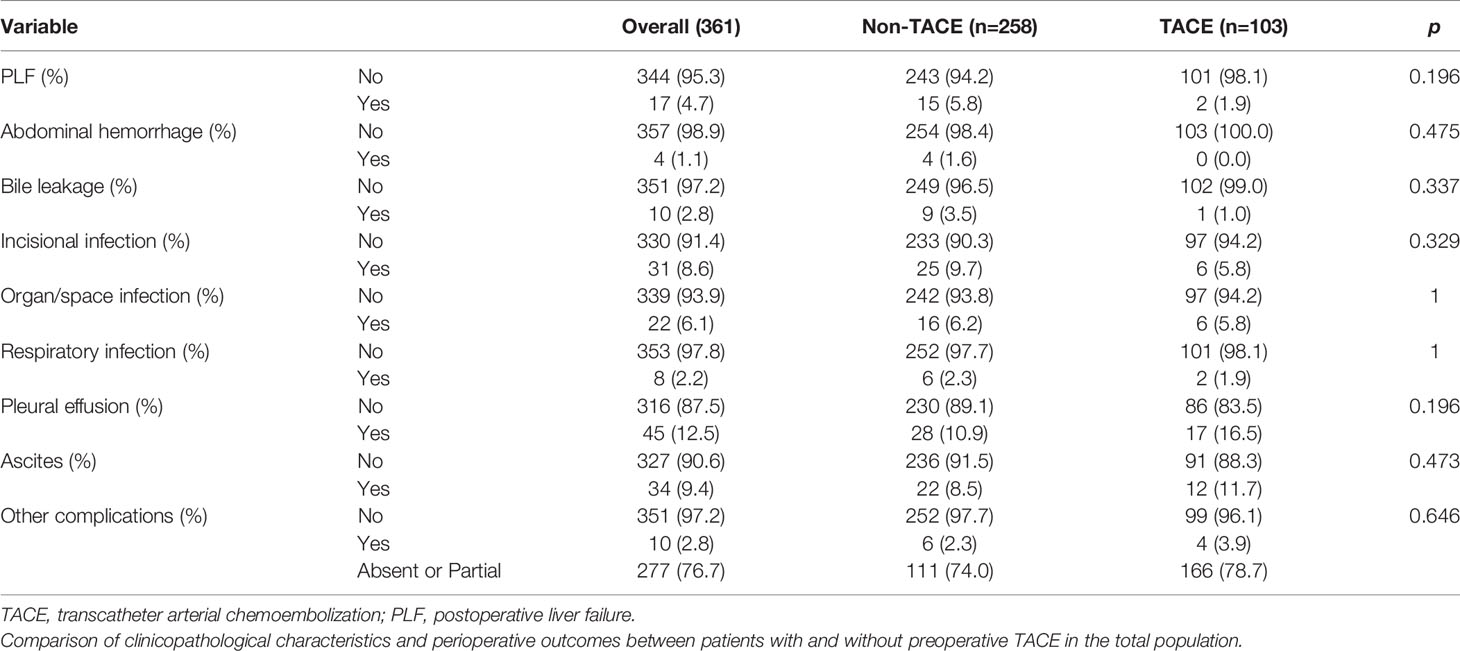
Table 8 Comparison of perioperative complications between patients with preoperative TACE and those without preoperative TACE.
Discussion
TACE has been one of the most effective and safest local treatment for patients with unresectable HCC (8). Its use of embolic material to occlude the main blood vessels that will supply the tumor leads to ischaemic necrosis of the tumor and the chemotherapeutic drugs that can effectively kill the tumor tissue or tumor cells. In the last 20 years, many scholars have also applied TACE in the preoperative adjuvant treatment of large HCC (6–25, 33–36). The main objectives of preoperative TACE were: (1) induce tumor volume shrinkage and convert unresectable HCC into resectable HCC; (2) reduce postoperative tumor recurrence and improve long-term patient survival; (3) improve the detection of occult lesions not detected by preoperative imaging beyond TACE (6, 37). However, the effectiveness of preoperative TACE has been controversial (6–25, 33–36), and some scholars believe that preoperative TACE may only be benefit for certain specific groups of HCC, such as patients with large tumor diameters, multiple nodes and invasive HCC (8, 14, 26). However, there is no consensus on which type of HCC patients can benefit from TACE, so there is an urgent need to explore a reliable preoperative indicator to guide preoperative TACE.
CTCs are malignant tumor cells that invade into the peripheral blood via epithelial-mesenchymal (EMT) form, which reflect the tumor’s aggressiveness and is often used for prognostic monitoring in breast, colorectal, and prostate cancers (29, 30, 38, 39). CTCs testing is considered to be a reliable means of early screening for cancer, postoperative recurrence, or metastasis monitoring in HCC patients (40). There are various methods on the market to detect circulating tumor cells, among them the Cyttel method (30–32, 41, 42) and CellSearch™ are the most common (27, 28, 43). The CellSearch™ system assay uses the traditional EpCAM-dependent enrichment method to identify CTCs (41, 44), which has certain limitations. The most important point is that not all peripheral blood CTCs of HCC patients express EpCAM, only 30-40% of HCC cells express EpCAM (45). This results in the low sensitivity of the CellSearch™ system to detect CTCs (44). To overcome this problem, we used a negative immunomagnetic particle method to detect CTCs to improve the assay’s sensitivity. Our retrospective study found that patients with positive CTCs had shorter RFS and OS than those with negative CTCs, and the landmark analysis also found that CTCs status was associated with early postoperative recurrence (P < 0.05), possibly by causing early recurrence leading to patient death. These findings are consistent with recent studies (46–50).
In addition, we divided the overall population into CTC-positive group and negative-group based on CTCs status and explored whether patients in each group would benefit from preoperative TACE. This study suggested that preoperative TACE may prolong survival prognosis by reducing RFS in the CTC-positive population. At the same time, we also showed that non-preoperative TACE was a risk factor for RFS and OS in HCC patients by univariate and multivariate Cox regression analyses. However, in the CTC-negative group, preoperative TACE was not found to reduce postoperative recurrence and improve survival prognosis, and univariate and multivariate Cox regression analyses also showed that preoperative TACE did not improve long-term prognosis by reducing early recurrence in HCC patients but not affecting late recurrence.
Many studies have suggested that early postoperative recurrence of HCC may be associated with occult micrometastases remaining in the liver (26, 50, 51), and many factors influence the patient’s early postoperative tumor recurrence, including CTCs status, tumor diameter, tumor number, microvascular invasion, incomplete tumor envelope and satellite nodules (26, 50, 51). In this study, we found that patients with positive CTCs had a relatively larger tumor diameter (P < 0.05) and a higher positive rate of MVI (P < 0.05), so we hypothesized that the proportion of patients with occult metastases was higher in the CTC-positive group (52). As surgical resection alone does not remove residual occult foci, preoperative TACE can theoretically remove it. This also explains why preoperative TACE reduce early recurrence in CTC-positive patients and prolongs survival prognosis of patient (26, 28, 52). Second, consider that the vast majority of early recurrence are intrahepatic recurrence. According to the “seed” and “soil” theory of HCC recurrence and metastasis after surgery, preoperative TACE causes changes in the tumor microenvironment of hepatocellular carcinoma. Preoperative TACE may act as a herbicide, making it difficult for CTCs (seeds) to grow in the residual liver (soil) (52). Therefore, preoperative CTC testing is relevant to guide preoperative TACE treatment. In the comprehensive management of hepatocellular carcinoma, clinicians need to pay more attention to the clinical value of preoperative CTC testing. For CTC-positive patients, preoperative TACE is necessary to reduce early postoperative recurrence and prolong OS. However, for patients with CTC-negative, preoperative TACE may not be necessary.
In addition to analyzing the impact of preoperative TACE on the prognosis of HCC patients, we also evaluated the impact of perioperative complications of the subsequent surgery with preoperative TACE. The results found that preoperative TACE did not increase the complications such as liver failure, postoperative ascites, and associated postoperative infections (P > 0.05). Some papers reported that the effect of preoperative TACE on surgery was rare if the interval between preoperative TACE and surgery was more than four weeks (8). To be precise, the median time from preoperative TACE to surgical resection at our affiliated medical centre is 4.5 weeks (range 3-6 weeks). Secondly, liver resection is only performed by an experienced team of surgeons. The above results may minimise the impact of preoperative TACE in the perioperative period.
Our research has limitations. Firstly, this study is a single-center retrospective study with few cases. Therefore, in the follow-up study, we will conduct a multi-center, large sample prospective study with multiple medical centers to further demonstrate the value of CTC testing as a guide for preoperative TACE. Secondly, most of the population we include were infected with HBV, whereas most HCC patients in western countries are caused by factors such as HCV or alcohol. The result may not be suitable for Western populations.
In conclusion, this study suggests for the first to propose that preoperative CTC testing is a guide to predicting the efficacy of preoperative TACE for HCC. For patients with positive preoperative CTCs, preoperative TACE may be a reliable means to prevent early recurrence and improve patients’ postoperative prognosis.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Zhongshan Hospital Affiliated to Guangdong Medical University, Tongji Hospital of Tongji Medical College of Huazhong University of Science and Technology, Huangshi Central Hospital of Edong Healthcare Group, Hubei Polytechnic University, Xiaogan Central Hospital, General Hospital of Central Theater, Qinghai University Affiliated Hospital, and Renmin Hospital of Wuhan University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
QZ wrote the paper. WH, QZ, and AM provided the data. FX analysed the data. XF, JC, and WZ reviewed and edited the manuscript. All authors read and approved the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.839597/full#supplementary-material
Supplementary Figure 1 | Comparison of overall survival (A) and recurrence free survival (B) between CTC-positive and CTC-negative patients. Analysis of the effect of CTC status on early and late postoperative recurrence in overall HCC patients by landmark method.
Supplementary Figure 2 | Analysis of the effect of CTC status on early and late postoperative recurrence in overall HCC patients by landmark method.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Agrawal S, Belghiti J. Oncologic Resection for Malignant Tumors of the Liver. Ann Surg (2011) 253(4):656–65. doi: 10.1097/SLA.0b013e3181fc08ca
3. Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of Hepatocellular Cancer After Resection: Patterns, Treatments, and Prognosis. Ann Surg (2015) 261(5):947–55. doi: 10.1097/SLA.0000000000000710
4. Poon RT, Fan ST, Wong J. Selection Criteria for Hepatic Resection in Patients With Large Hepatocellular Carcinoma Larger Than 10 Cm in Diameter. J Am Coll Surg (2002) 194(5):592–602. doi: 10.1016/S1072-7515(02)01163-8
5. Yang Y, Lin K, Liu L, Qian Y, Yang Y, Yuan S, et al. Impact of Preoperative TACE on Incidences of Microvascular Invasion and Long-Term Post-Hepatectomy Survival in Hepatocellular Carcinoma Patients: A Propensity Score Matching Analysis. Cancer Med (2021) 10(6):2100–11. doi: 10.1002/cam4.3814
6. Chen XP, Hu DY, Zhang ZW, Zhang BX, Chen YF, Zhang WG, et al. Role of Mesohepatectomy With or Without Transcatheter Arterial Chemoembolization for Large Centrally Located Hepatocellular Carcinoma. Dig Surg (2007) 24(3):208–13. doi: 10.1159/000102901
7. Gerunda GE, Neri D, Merenda R, Barbazza F, Zangrandi F, Meduri F, et al. Role of Transarterial Chemoembolization Before Liver Resection for Hepatocarcinoma. Liver Transpl (2000) 6(5):619–26. doi: 10.1053/jlts.2000.8312
8. Li C, Wang MD, Lu L, Wu H, Yu JJ, Zhang WG, et al. Preoperative Transcatheter Arterial Chemoembolization for Surgical Resection of Huge Hepatocellular Carcinoma (≥ 10 Cm): A Multicenter Propensity Matching Analysis. Hepatol Int (2019) 13):736–47. doi: 10.1007/s12072-019-09981-0
9. Liao M, Zhu Z, Wang H, Huang J. Adjuvant Transarterial Chemoembolization for Patients After Curative Resection of Hepatocellular Carcinoma: A Meta-Analysis. Scand J Gastroenterol (2017) 52(6-7):624–34. doi: 10.1080/00365521.2017.1292365
10. Lu CD, Peng SY, Jiang XC, Chiba Y, Tanigawa N. Preoperative Transcatheter Arterial Chemoembolization and Prognosis of Patients With Hepatocellular Carcinomas: Retrospective Analysis of 120 Cases. World J Surg (1999) 23(3):293–300. doi: 10.1007/PL00013185
11. Majno PE, Adam R, Bismuth H, Castaing D, Ariche A, Krissat J, et al. Influence of Preoperative Transarterial Lipiodol Chemoembolization on Resection and Transplantation for Hepatocellular Carcinoma in Patients With Cirrhosis. Ann Surg (1997) 226(6):688–701. doi: 10.1097/00000658-199712000-00006
12. Nishikawa H, Arimoto A, Wakasa T, Kita R, Kimura T, Osaki Y. Effect of Transcatheter Arterial Chemoembolization Prior to Surgical Resection for Hepatocellular Carcinoma. Int J Oncol (2013) 42(1):151–60. doi: 10.3892/ijo.2012.1711
13. Ochiai T, Sonoyama T, Hironaka T, Yamagishi H. Hepatectomy With Chemoembolization for Treatment of Hepatocellular Carcinoma. Hepatogastroenterology (2003) 50(51):750–5.
14. Yamashita Y, Takeishi K, Tsuijita E, Yoshiya S, Morita K, Kayashima H, et al. Beneficial Effects of Preoperative Lipiodolization for Resectable Large Hepatocellular Carcinoma (≥5 Cm in Diameter). J Surg Oncol (2012) 106:498–503. doi: 10.1002/jso.23098
15. Zhang Z, Liu Q, He J, Yang J, Yang G, Wu M. The Effect of Preoperative Transcatheter Hepatic Arterial Chemoembolization on Disease-Free Survival After Hepatectomy for Hepatocellular Carcinoma. Cancer (2000) 89(12):2606–12. doi: 10.1002/1097-0142(20001215)89:12<2606::AID-CNCR13>3.0.CO;2-T
16. Choi GH, Kim DH, Kang CM, Kim KS, Choi JS, Lee WJ, et al. Is Preoperative Transarterial Chemoembolization Needed for a Resectable Hepatocellular Carcinoma? World J Surg (2007) 31(12):2370–7. doi: 10.1007/s00268-007-9245-6
17. Ha TY, Hwang S, Lee YJ, Kim KH, Ko GY, Ii Gwon D, et al. Absence of Benefit of Transcatheter Arterial Chemoembolization (TACE) in Patients With Resectable Solitary Hepatocellular Carcinoma. World J Surg (2016) 40(5):1200–10. doi: 10.1007/s00268-015-3373-1
18. Jianyong L, Jinjing Z, Lunan Y, Jingqiang Z, Wentao W, Yong Z, et al. Preoperative Adjuvant Transarterial Chemoembolization Cannot Improve the Long Term Outcome of Radical Therapies for Hepatocellular Carcinoma. Sci Rep (2017) 7:41624. doi: 10.1038/srep41624
19. Kim IS, Lim YS, Lee HC, Suh DJ, Lee YJ, Lee SG. Pre-Operative Transarterial Chemoembolization for Resectable Hepatocellular Carcinoma Adversely Affects Post-Operative Patient Outcome. Aliment Pharmacol Ther (2008) 27(4):338–45. doi: 10.1111/j.1365-2036.2007.03580.x
20. Lee KT, Lu YW, Wang SN, Chen HY, Chuang SC, Chang WT, et al. The Effect of Preoperative Transarterial Chemoembolization of Resectable Hepatocellular Carcinoma on Clinical and Economic Outcomes. J Surg Oncol (2009) 99(6):343–50. doi: 10.1002/jso.21248
21. Paye F, Jagot P, Vilgrain V, Farges O, Borie D, Belghiti J. Preoperative Chemoembolization of Hepatocellular Carcinoma: A Comparative Study. Arch Surg (1998) 133(7):767–72. doi: 10.1001/archsurg.133.7.767
22. Sasaki A, Iwashita Y, Shibata K, Ohta M, Kitano S, Mori M. Preoperative Transcatheter Arterial Chemoembolization Reduces Long-Term Survival Rate After Hepatic Resection for Resectable Hepatocellular Carcinoma. Eur J Surg Oncol (2006) 32(7):773–9. doi: 10.1016/j.ejso.2006.04.002
23. Shi HY, Wang SN, Wang SC, Chuang SC, Chen CM, Lee KT. Preoperative Transarterial Chemoembolization and Resection for Hepatocellular Carcinoma: A Nationwide Taiwan Database Analysis of Long-Term Outcome Predictors. J Surg Oncol (2014) 109(5):487–93. doi: 10.1002/jso.23521
24. Si T, Chen Y, Ma D, Gong X, Yang K, Guan R, et al. Preoperative Transarterial Chemoembolization for Resectable Hepatocellular Carcinoma in Asia Area: A Meta-Analysis of Random Controlled Trials. Scand J Gastroenterol (2016) 51(12):1512–9. doi: 10.1080/00365521.2016.1216588
25. Wu CC, Ho YZ, Ho WL, Wu TC, Liu TJ, P’Eng FK. Preoperative Transcatheter Arterial Chemoembolization for Resectable Large Hepatocellular Carcinoma: A Reappraisal. Br J Surg (1995) 82(1):122–6. doi: 10.1002/bjs.1800820141
26. Gao ZH, Bai DS, Jiang GQ, Jin SJ. Review of Preoperative Transarterial Chemoembolization for Resectable Hepatocellular Carcinoma. World J Hepatol (2015) 7(1):40–3. doi: 10.4254/wjh.v7.i1.40
27. Sun YF, Xu Y, Yang XR, Guo W, Zhang X, Qiu SJ, et al. Circulating Stem Cell-Like Epithelial Cell Adhesion Molecule-Positive Tumor Cells Indicate Poor Prognosis of Hepatocellular Carcinoma After Curative Resection. Hepatology (2013) 57(4):1458–68. doi: 10.1002/hep.26151
28. Wang PX, Sun YF, Zhou KQ, Cheng JW, Hu B, Guo W, et al. Circulating Tumor Cells are an Indicator for the Administration of Adjuvant Transarterial Chemoembolization in Hepatocellular Carcinoma: A Single-Center, Retrospective, Propensity-Matched Study. Clin Transl Med (2020) 10(3):e137. doi: 10.1002/ctm2.137
29. Zhang Z, Xiao Y, Zhao J, Chen M, Xu Y, Zhong W, et al. Relationship Between Circulating Tumour Cell Count and Prognosis Following Chemotherapy in Patients With Advanced non-Small-Cell Lung Cancer. Respirology (2016) 21(3):519–25. doi: 10.1111/resp.12696
30. He YZ, He K, Huang RQ, Liu LW, Ye SW, Qian JL, et al. A Clinical Scoring System for Predicting Tumor Recurrence After Percutaneous Radiofrequency Ablation for 3 Cm or Less Hepatocellular Carcinoma. Sci Rep (2021) 11(1):8275. doi: 10.1038/s41598-021-87782-y
31. Chen Z, Lin X, Chen C, Chen Y, Zhao Q, Wu L, et al. Analysis of Preoperative Circulating Tumor Cells for Recurrence in Patients With Hepatocellular Carcinoma After Liver Transplantation. Ann Transl Med (2020) 8(17):1067. doi: 10.21037/atm-20-2751
32. Chen Z, Wang T, Chen C, Hong X, Yu J, Ma Y, et al. Circulating Tumor Cell Is a Clinical Indicator of Pretransplant Radiofrequency Ablation for Patients With Hepatocellular Carcinoma. J Oncol (2021) 2021:7776389. doi: 10.1155/2021/7776389
33. Zhou WP, Lai EC, Li AJ, Fu SY, Zhou JP, Pan ZY, et al. A Prospective, Randomized, Controlled Trial of Preoperative Transarterial Chemoembolization for Resectable Large Hepatocellular Carcinoma. Ann Surg (2009) 249(2):195–202. doi: 10.1097/SLA.0b013e3181961c16
34. Zhong C, Guo RP, Li JQ, Shi M, Wei W, Chen MS, et al. A Randomized Controlled Trial of Hepatectomy With Adjuvant Transcatheter Arterial Chemoembolization Versus Hepatectomy Alone for Stage III A Hepatocellular Carcinoma. J Cancer Res Clin Oncol (2009) 135(10):1437–45. doi: 10.1007/s00432-009-0588-2
35. Kaibori M, Tanigawa N, Kariya S, Ikeda H, Nakahashi Y, Hirohara J, et al. A Prospective Randomized Controlled Trial of Preoperative Whole-Liver Chemolipiodolization for Hepatocellular Carcinoma. Dig Dis Sci (2012) 57(5):1404–12. doi: 10.1007/s10620-012-2029-3
36. Sugo H, Futagawa S, Beppu T, Fukasawa M, Kojima K. Role of Preoperative Transcatheter Arterial Chemoembolization for Resectable Hepatocellular Carcinoma: Relation Between Postoperative Course and the Pattern of Tumor Recurrence. World J Surg (2003) 27(12):1295–9. doi: 10.1007/s00268-003-6817-y
37. Portolani N, Tiberio AM, Bonardelli S, Grazioli L, Matricardi L, Benetti A, et al. Arterial Chemoembolization in Hepatocellular Carcinoma Suitable for Resective Surgery. Hepatogastroenterology (1996) 43(12):1566–74.
38. Bidard FC, Kiavue N, Ychou M, Cabel L, Stern MH, Madic J, et al. Circulating Tumor Cells and Circulating Tumor DNA Detection in Potentially Resectable Metastatic Colorectal Cancer: A Prospective Ancillary Study to the Unicancer Prodige-14 Trial. Cells (2019) 8(6):516. doi: 10.3390/cells8060516
39. Loeian MS, Mehdi Aghaei S, Farhadi F, Rai V, Yang HW, Johnson MD, et al. Liquid Biopsy Using the Nanotube-CTC-Chip: Capture of Invasive CTCs With High Purity Using Preferential Adherence in Breast Cancer Patients. Lab Chip (2019) 19(11):1899–915. doi: 10.1039/C9LC00274J
40. Lin E, Cao T, Nagrath S, King MR. Circulating Tumor Cells: Diagnostic and Therapeutic Applications. Annu Rev BioMed Eng (2018) 20:329–52. doi: 10.1146/annurev-bioeng-062117-120947
41. Gao Y, Zhu Y, Zhang Z, Zhang C, Huang X, Yuan Z. Clinical Significance of Pancreatic Circulating Tumor Cells Using Combined Negative Enrichment and Immunostaining-Fluorescence in Situ Hybridization. J Exp Clin Cancer Res (2016) 35:66. doi: 10.1186/s13046-016-0340-0
42. He YZ, He K, Huang RQ, Wang ZL, Ye SW, Liu LW, et al. Preoperative Evaluation and Prediction of Clinical Scores for Hepatocellular Carcinoma Microvascular Invasion: A Single-Center Retrospective Analysis. Ann Hepatol (2020) 19(6):654–61. doi: 10.1016/j.aohep.2020.07.002
43. Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, et al. Tumor Cells Circulate in the Peripheral Blood of All Major Carcinomas But Not in Healthy Subjects or Patients With Nonmalignant Diseases. Clin Cancer Res (2004) 10(20):6897–904. doi: 10.1158/1078-0432.CCR-04-0378
44. Ahn JC, Teng PC, Chen PJ, Posadas E, Tseng HR, Lu SC, et al. Detection of Circulating Tumor Cells and Their Implications as a Biomarker for Diagnosis, Prognostication, and Therapeutic Monitoring in Hepatocellular Carcinoma. Hepatology (2021) 73(1):422–36. doi: 10.1002/hep.31165
45. Went PT, Lugli A, Meier S, Bundi M, Mirlacher M, Sauter G, et al. Frequent EpCam Protein Expression in Human Carcinomas. Hum Pathol (2004) 35(1):122–8. doi: 10.1016/j.humpath.2003.08.026
46. Hao S, Chen S, Tu C, Huang T. Anterior Approach to Improve the Prognosis in HCC Patients Via Decreasing Dissemination of EpCAM(+) Circulating Tumor Cells. J Gastrointest Surg (2017) 21(7):1112–20. doi: 10.1007/s11605-017-3410-5
47. Zhou KQ, Sun YF, Cheng JW, Du M, Ji Y, Wang PX, et al. Effect of Surgical Margin on Recurrence Based on Preoperative Circulating Tumor Cell Status in Hepatocellular Carcinoma. EBioMedicine (2020) 62:103107. doi: 10.1016/j.ebiom.2020.103107
48. Wang PX, Xu Y, Sun YF, Cheng JW, Zhou KQ, Wu SY, et al. Detection of Circulating Tumour Cells Enables Early Recurrence Prediction in Hepatocellular Carcinoma Patients Undergoing Liver Transplantation. Liver Int (2021) 41(3):562–73. doi: 10.1111/liv.14734
49. Zhou J, Zhang Z, Zhou H, Leng C, Hou B, Zhou C, et al. Preoperative Circulating Tumor Cells to Predict Microvascular Invasion and Dynamical Detection Indicate the Prognosis of Hepatocellular Carcinoma. BMC Cancer (2020) 20(1):1047. doi: 10.1186/s12885-020-07488-8
50. Wang Z, Luo L, Cheng Y, He G, Peng B, Gao Y, et al. Correlation Between Postoperative Early Recurrence of Hepatocellular Carcinoma and Mesenchymal Circulating Tumor Cells in Peripheral Blood. J Gastrointest Surg (2018) 22(4):633–9. doi: 10.1007/s11605-017-3619-3
51. Zhang YM, Zhou ZT, Liu GM. Factors Predicting Early Recurrence After Surgical Resection of Hepatocellular Carcinoma. J Hepatol (2019) 70(3):571–2. doi: 10.1016/j.jhep.2018.10.038
Keywords: preoperative transcatheter arterial embolization, circulating tumor cells, hepatocellular carcinoma, prognosis, TACE
Citation: Zhang Q, Xia F, Mo A, He W, Chen J, Zhang W and Chen W (2022) Guiding Value of Circulating Tumor Cells for Preoperative Transcatheter Arterial Embolization in Solitary Large Hepatocellular Carcinoma: A Single-Center Retrospective Clinical Study. Front. Oncol. 12:839597. doi: 10.3389/fonc.2022.839597
Received: 20 December 2021; Accepted: 15 April 2022;
Published: 18 May 2022.
Edited by:
Wei-lun Tsai, Kaohsiung Veterans General Hospital, TaiwanReviewed by:
Yoshihiro Mise, Juntendo University, JapanAndrea Laurenzi, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Italy
Copyright © 2022 Zhang, Xia, Mo, He, Chen, Zhang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weiqiang Chen, Y3dxMjAxMzhAYWxpeXVuLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Qiao Zhang
Qiao Zhang Feng Xia
Feng Xia Ali Mo
Ali Mo Weiming He1
Weiming He1 Weiqiang Chen
Weiqiang Chen