
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 08 March 2022
Sec. Surgical Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.838103
This article is part of the Research Topic Neuroendocrine Tumours of the Gastrointestinal Tract, Liver and Pancreas: Current management and treatment strategies View all 11 articles
Background: Liver metastases (LMs) are common in advanced pancreatic neuroendocrine tumor (PNET) patients. Currently, the benefit of primary tumor resection (PTR) in the setting of PNET patients with liver metastases is still controversial in several guidelines.
Methods: Data were extracted from the Surveillance, Epidemiology and End Results (SEER) database to evaluate this issue. The main index of interest in our study was overall survival time.
Results: Information on 536 PNET patients with liver metastases from the SEER database was identified. A total of 214 patients (PTR group) received primary tumor resection, and more than half of them (132 patients) had synchronous LM resection. The other 322 PNET patients (non-PTR group) with liver metastases did not receive primary tumor resection. A significant survival benefit was gained from PTR when compared with non-PTR patients, both in OS (72.93 ± 2.7 vs. 36.80 ± 2.22 months) and 3- or 5-year survival rates (75.1% vs. 28.9% and 67.9% vs. 22.3%, respectively). No difference was found between PTR alone and PTR with synchronous LM resection. From univariate and multivariate analyses, younger age (<65 years) and good or moderate tumor differentiation may be more important when considering primary tumor resection. However, we found that all grades of tumor differentiation could result in a better overall survival time after primary tumor resection.
Conclusion: Our study suggested that primary tumor resection in pancreatic neuroendocrine patients with liver metastases could result in a longer survival time. Primary tumor resection with synchronous liver metastasis resection was not related to a better survival benefit. This treatment strategy may routinely be taken into consideration in these patients.
Pancreatic neuroendocrine tumors (PNETs) are a heterogeneous group of neoplasms representing approximately 1% of all pancreatic cancers by incidence and 10% of pancreatic cancers by prevalence (1). Surgical resection remains the primary and potentially curative treatment approach for PNETs. However, most patients have metastatic disease at diagnosis that often occurs first in the liver, and approximately 28-77% of patients develop liver metastases (LM) in their lifetime (2, 3). Management of pancreatic neuroendocrine tumor liver metastases (PNELMs) may depend on whether the liver disease is resectable.
For patients with limited liver metastases, surgical resection of both the primary tumor and hepatic disease in a staged or synchronous fashion is recommended. The role and benefit of primary site resection (PTR) in patients with unresectable liver metastases are still controversial. A recent systematic review and meta-analysis showed that palliative resection of primary PNETs in patients with unresectable metastatic liver disease can increase overall survival time (OS), but there was a bias toward patients with better performance status, less advanced disease, or a tumor located in the body or tail of the pancreas (4). Similar findings were demonstrated in another meta-analysis, but the limitations of the included studies do not allow firm conclusions (5). Until now, there has been no adequate robust evidence for whether a primary tumor should be resected in the presence of unresectable liver metastases. Moreover, additional pancreatic resection morbidity, the relatively indolent behavior, and the lower symptomatic presentation of nonfunctional PNETs should be taken into consideration.
Therefore, we designed this study to investigate whether primary tumor resection has a survival benefit in patients with pancreatic neuroendocrine tumors with liver metastases, even if the liver metastases are unresectable.
We used SEER*Stat software version 8.3.8 to retrieve the data for our study from the Surveillance, Epidemiology, and End Research (SEER) database (SEER Research Data, 18 Registries, Nov 2019 Sub 2000–2017). The primary sites for tumors of the pancreas were based on the column of site and morphology, which was labeled C25.0 to C25.9. The patients were enrolled according to the International Classification of Disease for Oncology, third edition (ICD-O-3) histology/behavior codes: pancreatic endocrine tumor, malignant (8150/3), insulinoma, malignant (8152/3), glucagonoma, malignant (8153/3), vipoma, malignant (8155/3), somatostatinoma, malignant (8156/3), enterochromaffin-like cell tumor, malignant (8242/3), goblet cell carcinoid (8243/3), neuroendocrine carcinoma, NOS (8246/3), and atypical carcinoid tumor (8249/3). Patient demographics included sex, age at diagnosis, year of diagnosis, grade, tumor size, surgery for primary site (derived from column RX Summ—Surg Prim Site (1998+)), surgery for distant sites (derived from column RX Summ—Surg Oth Reg/Dis (2003+)), survival months, vital status and SEER cause-specific death classification.
The selection criteria were as follows: (1) patients who had one primary cancer only and pancreatic NETs was the first; and (2) patients who had liver metastasis only at the time of diagnosis without other known sites of metastasis. The exclusion criteria were as follows: (1) incomplete follow-up information; (2) unknown cause of death or death attributed to causes other than this cancer; and (3) unknown characteristics.
Statistical analysis was performed using IBM SPSS Statistics 21.0. Patients’ baseline characteristics, tumor characteristics, and treatments were compared by the Mann–Whitney U test or Pearson chi-squared test. Data are presented as percentages or mean values. We used Kaplan–Meier curves to analyze the overall survival time (OS), and the differences between groups were compared by the log-rank test. Univariate and multivariate analyses were performed using Cox proportional hazards models. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. A p-value less than 0.05 was defined as statistically significant.
A total of 536 patients were included based on our inclusion criteria (Figure 1). All of these patients were diagnosed with pancreatic neuroendocrine tumors and had liver metastasis at diagnosis. The baseline characteristics are summarized in Table 1. There were 323 men and 213 women in this population, and the median age at diagnosis was 58 years. The number of patients with pancreatic head tumors (171 patients, 31.9%) was similar to that at the pancreatic tail (190 patients, 35.4%). The mean tumor size was 54.44 ± 31.57 mm, and 59.9% of tumors were more than 4 cm. All patients were pathologically diagnosed after resection surgery of biopsy, and the most common pathological type was neuroendocrine carcinoma, comprising 54.5% of these populations. Moreover, five tumors were functional PNETs, including two patients with insulinoma, two patients with gastrinoma, and one with glucagonoma. Based on the degree of differentiation, tumors were divided into four grades (grade I: well differentiated; grade II: moderately differentiated, grade III: poorly differentiated, grade IV: undifferentiated). Approximately 76.3% of patients were well and moderately differentiated. All patients had liver metastases at diagnosis without other known sites (such as lung, brain, bone) of metastases.
A total of 39.9% of patients (214 of 536 patients) received primary tumor resection, except for 8 patients who were recommended for surgery but not performed. The rest of the patients were not recommended for surgery. Surgical procedures included partial pancreatectomy (consisting of partial pancreatectomy and local excision of tumors), pancreaticoduodenectomy (with or without distal/partial gastrectomy), and total pancreatectomy. The median follow-up time was 43 months (1–95 months). The mean OS of all patients was 53.54 ± 2.03 months. Significant differences existed between PTR patients and non-PTR patients, and the OS of these two groups was 72.93 ± 2.70 and 36.80 ± 2.22 months, respectively (p = 0.000). The 3- and 5-year survival rates of PTR patients were 75.1% and 67.9%, respectively, while the same indexes of non-PTR patients were 28.9% and 22.3%, respectively. Additionally, we found no significant difference in the PTR group with or without LM resection. (PTR patients with LM resection vs. without LM resection: 71.48 ± 3.46 vs. 73.47 ± 4.01, p = 0.528) (Figure 2).
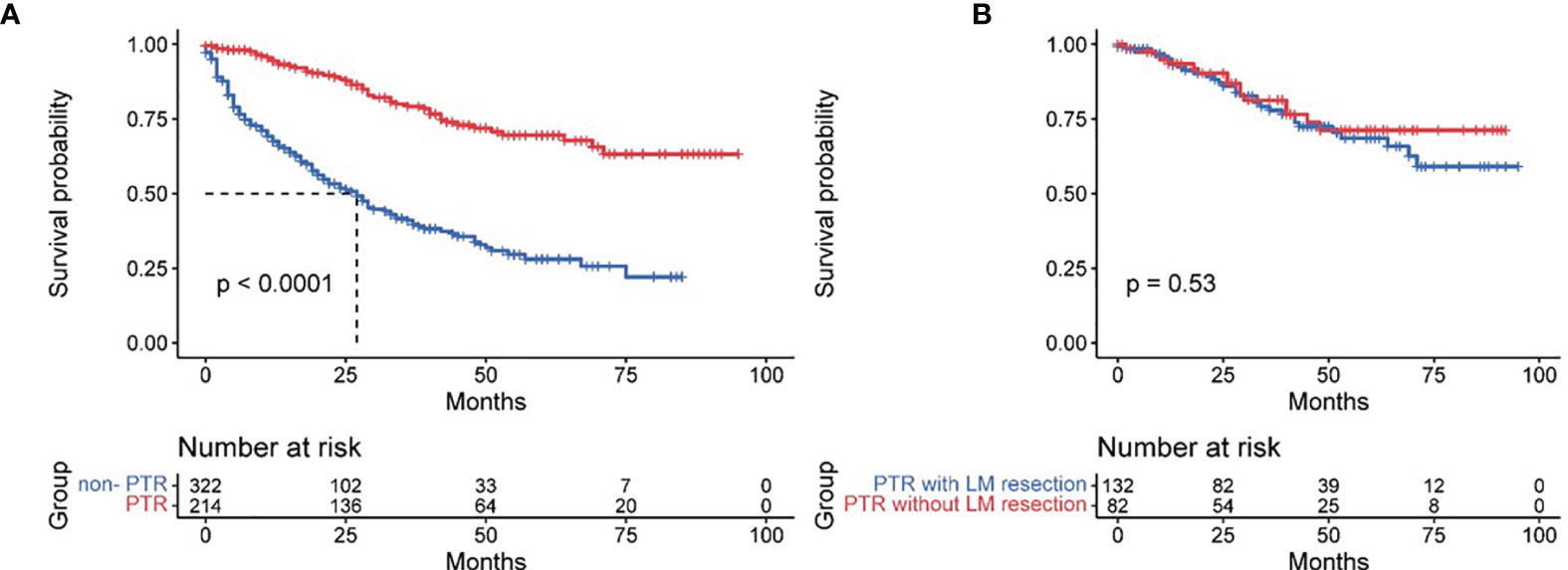
Figure 2 Effect of primary tumor resection (A) and with/without liver metastases resection (B) in pancreatic neuroendocrine tumor patients with liver metastases.
Based on tumor differentiation, all four grade groups showed that PTR significantly improved survival time (Table 2), especially in the grade III group (poor differentiation). The OS of the PTR patients was nearly 5-fold that of the non-PTR patients (64.58 ± 7.90 vs. 12.95 ± 2.53). In PTR patients, worse tumor differentiation was associated with decreased OS. The same results were shown when dividing patients based on tumor size into three groups (tumor size ≤2 cm, 2 cm < tumor size ≤4 cm, tumor size >4 cm). All 14 patients who received primary tumor resection with a tumor size less than 2 cm survived at the end of follow-up. Different surgical procedures also led to different outcomes. Patients who received partial pancreatectomy had better OS than the other two groups, which may be related to higher tumor differentiation, smaller tumor size, and lower additional mortality associated with the surgical procedure (Figures 3–5).
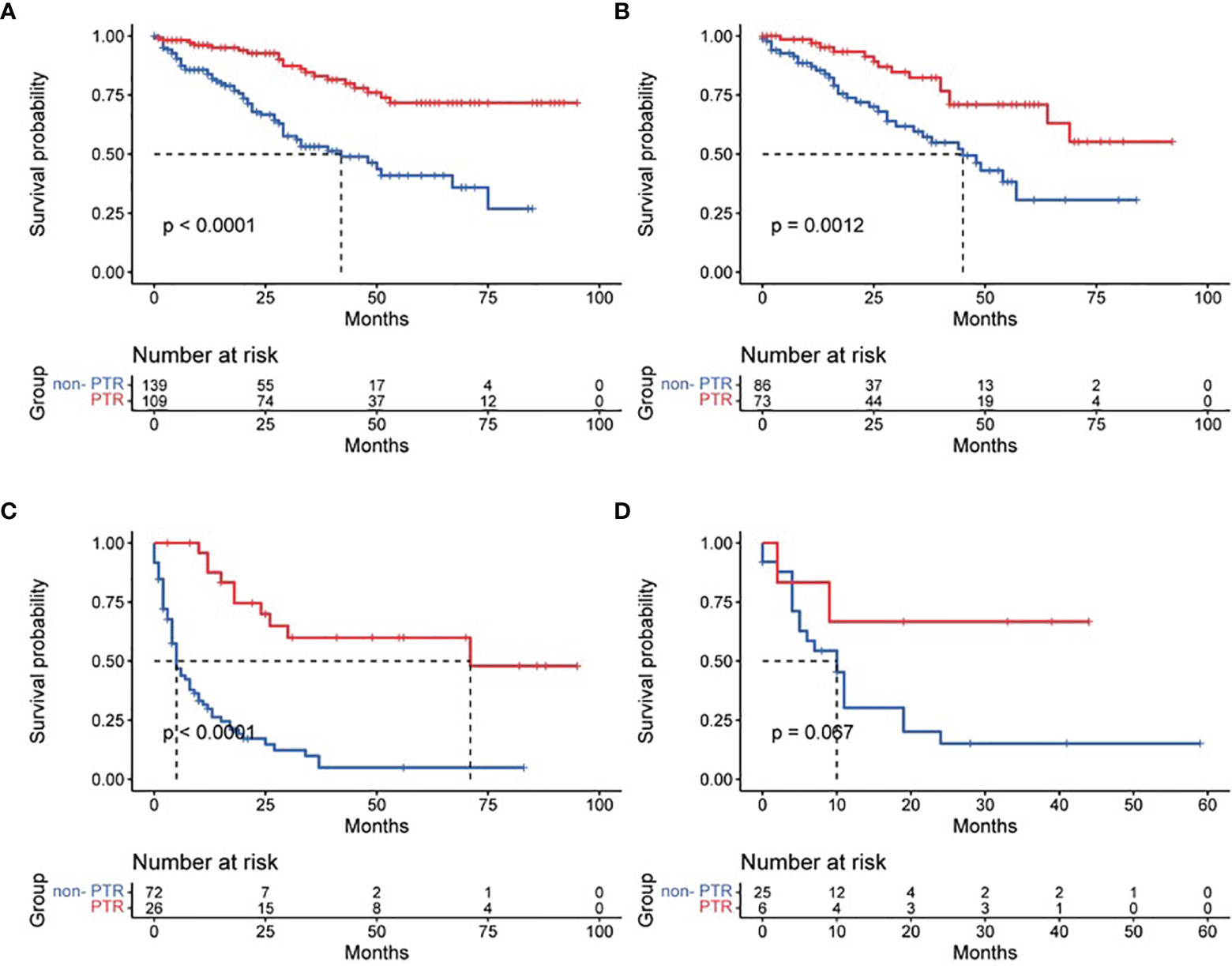
Figure 3 Effect of primary tumor resection in patients with different tumor differentiation: (A) well-differentiated patients; (B) moderately differentiated patients; (C) poorly differentiated patients; and (D) undifferentiated patients.
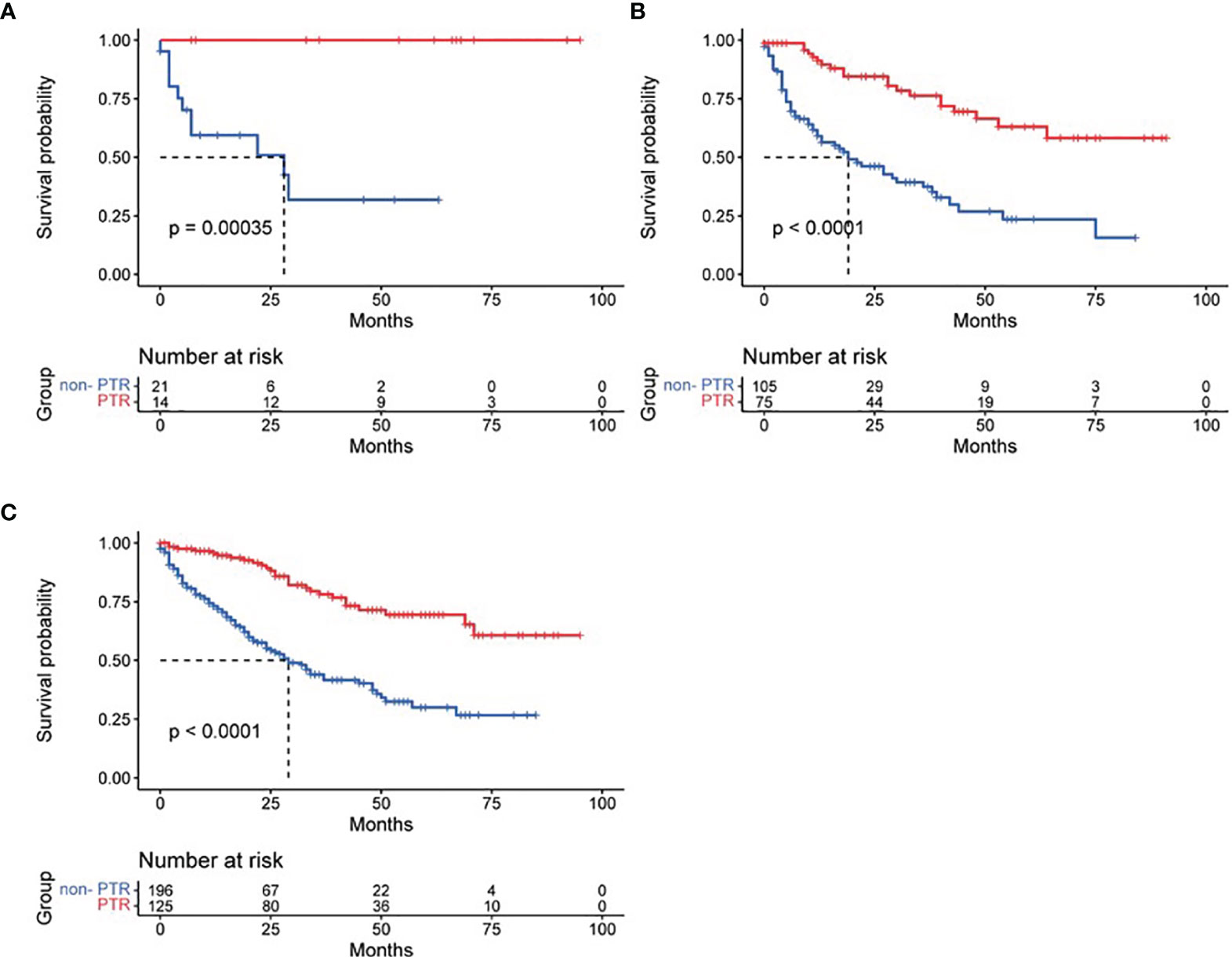
Figure 4 Effect of primary tumor resection in patients with different tumor sizes: (A) tumor size ≤2 cm; (B) tumor size 2–4 cm; (C) tumor size >4 cm.
From univariate and multivariate analyses, we found that age over 65 years (HR: 1.493, 95% CI: 1.137–1.962), poorly differentiated or undifferentiated tumors (HR: 4.102, 95% CI: 2.942-5.721; HR: 3.338, 95% CI: 2.043–5.455, respectively) and primary tumor resection (HR: 3.771, 95% CI: 2.702–5.263) were independent risk factors related to overall survival time (Table 3).
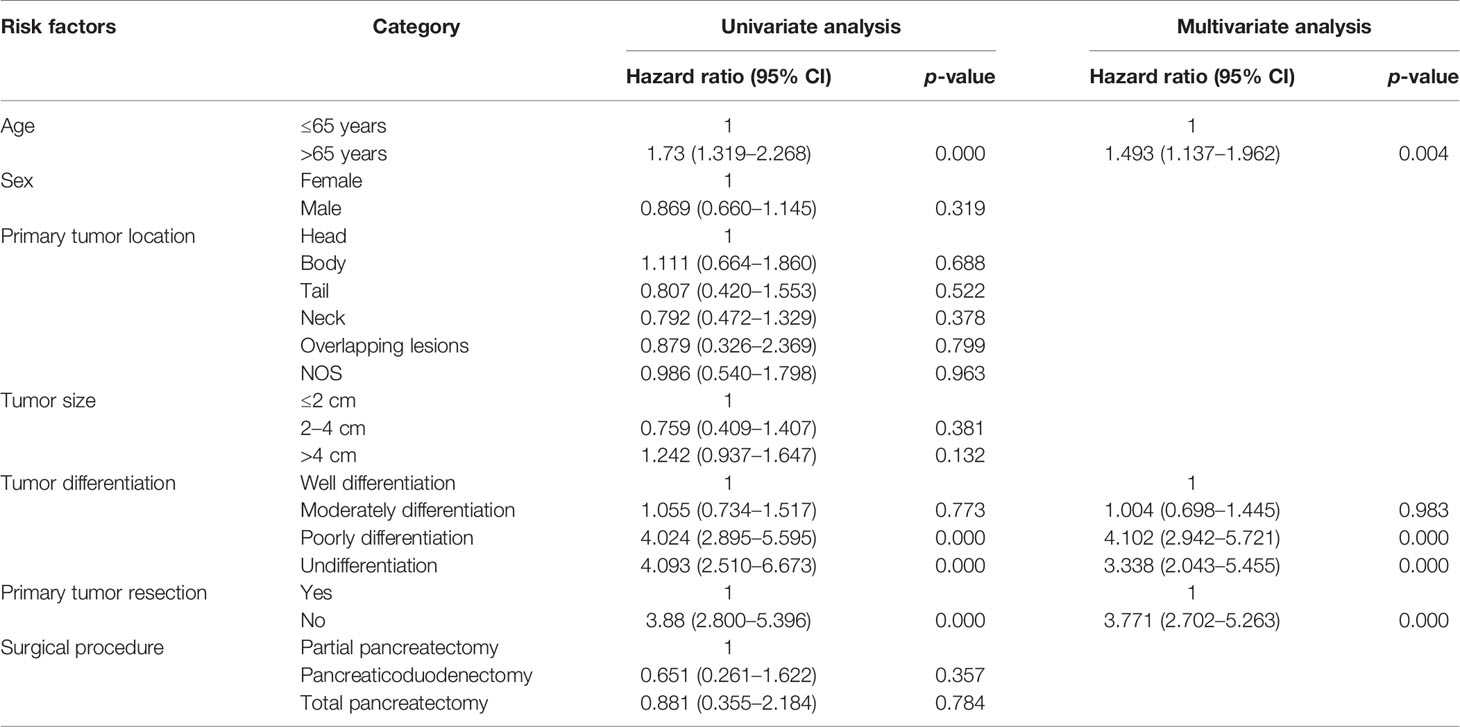
Table 3 Possible variables at univariate and multivariate analyses in pancreatic neuroendocrine patients with liver metastases.
Current National Comprehensive Cancer Network (NCCN) guidelines for neuroendocrine tumors of the pancreas support resection of the primary site and metastases if complete resection is possible, and both staged and synchronous resection are recommended (6). However, the role of primary tumor resection for PNET patients with unresectable liver metastases is still controversial. For small intestinal neuroendocrine tumors (SI-NETs), palliative PTR may prevent or solve complications such as bowel obstruction or intestinal ischemia associated with primary tumors. Thus, primary tumor resection of intestinal NETs is strongly recommended even in the presence of liver or lymph node metastases (7). In contrast, a systematic review meta-analysis of midgut neuroendocrine tumor patients with unresectable metastatic liver disease suggested that PTR had a significant role in improving OS with a low perioperative risk of mortality (8). In the setting of unresectable PNELM, neither the European Neuroendocrine Tumor Society (ENETS) nor the North American Neuroendocrine Tumor Society (NANETS) guidelines recommend routine palliative primary resection (9, 10).
Our findings show that primary tumor resection in pancreatic neuroendocrine tumor patients with liver metastases is significantly associated with improved survival time. Furthermore, we evaluated potential risk factors related to OS. We found that age less than 65 years and well-differentiated or moderately differentiated tumor grade were associated with prolonged survival. Younger age and well-differentiated tumors may be important selected factors when considering primary tumor resection. Younger patients may have a better physical status to tolerate more aggressive treatment and fewer comorbidities. Citterio reported improved survival times in primary tumors resected from well-differentiated pancreatic NETs (median survival times were 138 and 37 months, respectively) (11). Furthermore, according to our findings, all differentiation grades had significantly better OS after PTR, especially in patients with poorly differentiated tumors, and PTR increased survival by nearly 5-fold.
Other factors, such as sex, tumor location, tumor size, and surgical procedures, were not significantly independent factors. Both the ENATS and NANETS guidelines proposed tumor location as a surgical selection factor. For nonfunctional PNETs, primary tumors located in the head of the pancreas are related to higher odds of specific symptoms, such as jaundice or duodenal occlusion, and these complications could be solved by endoscopic or surgical bypasses (9). In addition, distal pancreatectomy has lower morbidity than pancreaticoduodenectomy (Whipple procedure). Thus, primary lesions located in the body or tail may be more favorable for resection and derive better quality of life and outcomes (9, 10). A previous study supported the positive survival benefit of PTR in PNET patients of the body and tail with unresectable liver metastases when compared with non-PTR individuals (median survival time: 111 vs. 52 months) (12). We evaluated whether different surgical procedures had different outcomes, and the results showed that partial pancreatectomy and pancreaticoduodenectomy had similar OS. Moreover, univariate analysis also showed that both tumor location and surgical procedure were not significant independent risk factors. Tumor location and surgical procedures may not be limiting conditions in deciding whether to perform primary tumor resection with liver metastases.
Peptide receptor radionuclide therapy (PRRT) in somatostatin-positive NETs is a replacement treatment strategy in patients who are not suitable for radical resection, and PRRT could result in disease stabilization, partial remission, or reduction of tumor mass (13). A lower tumor burden and smaller lesions may allow a high dose of concentration and a higher chance of tumor response (14); thus, palliative or debulking surgery may increase the response to PRRT. Based on this hypothesis, a previous study in the setting of G1-G2 PNETs with diffuse liver metastases suggested that PTR prior to PRRT results in better progression-free survival (PFS) (70 vs. 30 months, p = 0.02) and OS (112 vs. 65 months, p = 0.011) (15). Another recent study also found that PTR before PRRT provides a significant survival benefit in patients with stage IV neuroendocrine neoplasms, and both PFS and OS improved (134 vs. 67 months, p < 0.001 and 18 vs. 14 months, p = 0.012, respectively) (16). These results provide us with a novel strategy for the combination of primary tumor resection and PRRT for advanced pancreatic neuroendocrine tumors with distant metastases.
Due to the relatively low incidence and heterogeneity of pancreatic neuroendocrine tumors, it is difficult to design randomized trials to provide strong evidence for standard treatment strategies. Our study also had some limitations due to its retrospective nature and selection bias. First, the tumor differentiation grade from the SEER database is different from the current guidelines, which are based on mitoses in a high power field and the Ki-67 index. Second, we do not have information about adjuvant therapies and postoperative therapies, which may influence the survival analysis in all patients. Third, the tumor burden of liver metastases (tumor location and number of lesions) may be a confounding variable. Fourth, the SEER database did not include tumor margin information.
Although several limitations exist in our study, we still suggest the significant role of primary tumor resection in pancreatic neuroendocrine tumors with liver metastases for improving survival time. Although all patients who receive resectable primary tumors may be potentially beneficial, younger patients and well- or moderately differentiated primary PNETs should be preferentially considered.
Primary tumor resection is associated with longer survival in pancreatic neuroendocrine tumor patients with liver metastases, but additional synchronous liver metastasis resection was not related to better overall survival time. The combination of primary tumor resection and other treatment strategies (e.g., peptide receptor radionuclide therapy) may result in a better outcome.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
N-WK and X-BL designed the study. Y-HC and C-LT acquired the data. Z-YW analyzed and interpreted the data. YM wrote the paper. N-WK critically revised the manuscript for important intellectual content. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This research was supported by the Sichuan Province Science and Technology Planning Project (2020YFS0262), West China Hospital Clinical Research Incubation Project (21HXFH058), and the 1.3.5 Project for Disciplines of Excellence-Clinical Research Incubation Project (ZY2017302 and ZYJC21037), West China Hospital, Sichuan University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
LM, liver metastases; PNETs, pancreatic neuroendocrine tumors; PTR, primary tumor resection; SEER, Surveillance, Epidemiology and End Results; PNELM, pancreatic neuroendocrine tumors liver metastases; OS, overall survival; NCCN, National Comprehensive Cancer Network; SI-NETs, small intestinal neuroendocrine tumors; ENETS, European Neuroendocrine Tumor Society; NANETS, North American Neuroendocrine Tumor Society; PRRT, peptide receptor radionuclide therapy; PFS, progression-free survival.
1. Yao JC, Eisner MP, Leary C, Dagohoy C, Phan A, Rashid A, et al. Population-Based Study of Islet Cell Carcinoma. Ann Surg Oncol (2007) 14:3492–500. doi: 10.1245/s10434-007-9566-6
2. Pavel M, Baudin E, Couvelard A, Krenning E, Öberget K, Steinmüller T, et al. ENETS Consensus Guidelines for the Management of Patients With Liver and Other Distant Metastases From Neuroendocrine Neoplasms of Foregut, Midgut, Hindgut, and Unknown Primary. Neuroendocrinology (2012) 95:157–76. doi: 10.1159/000335597
3. Yao JC, Hassan M, Phan A, Dagohoy C, Leary C, Mares JE, et al. One Hundred Years After “Carcinoid”: Epidemiology of and Prognostic Factors for Neuroendocrine Tumors in 35,825 Cases in the United States. J Clin Oncol (2008) 26:3063–72. doi: 10.1200/JCO.2007.15.4377
4. Zhou B, Zhan C, Ding Y, Yan S, Zheng S. Role of Palliative Resection of the Primary Pancreatic Neuroendocrine Tumor in Patients With Unresectable Metastatic Liver Disease: A Systematic Review and Meta-Analysis. Onco Targets Ther (2018) 11:975–82. doi: 10.2147/OTT.S158171
5. Partelli S, Cirocchi R, Rancoita PMV, Muffatti F, Andreasi V, Crippa S, et al. A Systematic Review and Meta-Analysis on the Role of Palliative Primary Resection for Pancreatic Neuroendocrine Neoplasm With Liver Metastases. HPB (Oxford) (2018) 20:197–203. doi: 10.1016/j.hpb.2017.10.014
6. NCCN Clinical Practice Guidelines in Oncology-Neuroendocrine and Adrenal Tumors (Version 2.2020)[EB/OL]. Available at: http://www.nccn.org.
7. Niederle B, Pape UF, Costa F, Kelestimur F, Knigge U, Öberg K, et al. ENETS Consensus Guidelines Update for Neuroendocrine Neoplasms of the Jejunum and Ileum. Neuroendocrinology (2016) 103(2):125–38. doi: 10.1159/000443170
8. Tsilimigras DI, Ntanasis-Stathopoulos I, Kostakis ID, Moris D, Schizas D, Cloyd JM, et al. Is Resection of Primary Midgut Neuroendocrine Tumors in Patients With Unresectable Metastatic Liver Disease Justified? A Systematic Review and Meta-Analysis. J Gastrointest Surg (2019) 23:1044–54. doi: 10.1007/s11605-018-04094-9
9. Partelli S, Bartsch DK, Capdevila J, Chen J, Knigge U, Niederle B, et al. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumors: Surgery for Small Intestinal and Pancreatic Neuroendocrine Tumors. Neuroendocrinology (2017) 105(3):255–65. doi: 10.1159/000464292
10. Howe JR, Merchant NB, Conrad C, Keutgen XM, Hallet J, Drebin JA, et al. The North American Neuroendocrine Tumor Society Consensus Paper on the Surgical Management of Pancreatic Neuroendocrine Tumors. Pancreas (2020) 49:1–33. doi: 10.1097/MPA.0000000000001454
11. Citterio D, Pusceddu S, Facciorusso A, Coppa J, Milione M, Buzzoni R, et al. Primary Tumor Resection may Improve Survival in Functional Well-Differentiated Neuroendocrine Tumors Metastatic to the Liver. EJSO (2017) 43:380–7. doi: 10.1016/j.ejso.2016.10.031
12. Bertani E, Fazio N, Radice D, Zardini C, Spinoglio G, Chiappa A, et al. Assessed the Role of Primary Tumor Resection in Patients With Synchronous Unresectable Liver Metastases From Pancreatic Neuroendocrine Tumors of the Body and Tail. A Propensity Score Survival Evaluation. EJSO (2017) 43:372–9. doi: 10.1016/j.ejso.2016.09.011
13. van der Zwan WA, Bodei L, Mueller-Brand J, de Herder WW, Kvols LK, Kwekkeboom DJ, et al. GEPNETs Update: Radionuclide Therapy in Neuroendocrine Tumors. Eur J Endocrinol (2015) 172:R1–8. doi: 10.1530/EJE-14-0488
14. Bertani E, Fazio N, Botteri E, Chiappa A, Falconi M, Grana C, et al. Resection of the Primary Pancreatic Neuroendocrine Tumor in Patients With Unresectable Liver Metastases: Possible Indications for a Multimodal Approach. Surgery (2014) 155:607–14. doi: 10.1016/j.surg.2013.12.024
15. Bertani E, Fazio N, Radice D, Zardini C, Grana C, Bodei L, et al. Resection of the Primary Tumor Followed by Peptide Receptor Radionuclide Therapy as Upfront Strategy for the Treatment of G1–G2 Pancreatic Neuroendocrine Tumors With Unresectable Liver Metastases. Ann Surg Oncol (2016) 23:S981–9. doi: 10.1245/s10434-016-5550-3
Keywords: primary tumor resection, pancreatic neuroendocrine tumors, liver metastases, tumor differentiation, overall survival (OS)
Citation: Mou Y, Wang Z-Y, Tan C-L, Chen Y-H, Liu X-B and Ke N-W (2022) The Role of Primary Tumor Resection in Patients With Pancreatic Neuroendocrine Tumors With Liver Metastases. Front. Oncol. 12:838103. doi: 10.3389/fonc.2022.838103
Received: 17 December 2021; Accepted: 10 February 2022;
Published: 08 March 2022.
Edited by:
Alejandro Serrablo, Hospital Universitario Miguel Servet, SpainCopyright © 2022 Mou, Wang, Tan, Chen, Liu and Ke. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Neng-Wen Ke, a2VuZW5nd2VuQHNjdS5lZHUuY24=; Xu-Bao Liu, eGJsaXVAbWVkbWFpbC5jb20uY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.