
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 28 February 2022
Sec. Radiation Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.837577
Introduction: The cooperative group experience of thoracic sterotactic body radiation therapy (SBRT) in medically inoperable patients with early stage non-small cell lung cancer (NSCLC) historically utilized corticosteroid premedication. Patterns of care have been mixed as to whether premedication adds benefit in terms of improved lung toxicity and treatment tolerance.
Methods: Patients treated for NSCLC from 2014 to 2017 with definitive thoracic SBRT (BED10≥100) at a single institution, in a prospectively collected database were evaluated. Pretreatment clinicopathologic characteristics, including Eastern Cooperative Oncology Group (ECOG) performance status, PFT parameters of FEV1, and diffusing capacity for carbon monoxide (DLCO) were collected. Treatment and dosimetric characteristics were collected, and patients were scored as to whether dexamethasone was prescribed and utilized with each fraction. Toxicity was graded on multiple domains including lung as during and 30 days after completion of treatment using Common Terminology Criteria for Adverse Events Version 4. Univariate analysis was performed with Fisher’s exact test for categorical variables and two-tailed Student’s t-test for continuous variables. Multivariate analysis was performed with Cox proportional hazards model to adjust for age, pretreatment DLCO, ECOG, tumor size, central versus peripheral location, and biological effective dose.
Results: A total of 86 patients treated with thoracic SBRT with 54–60 Gy in 3–8 fractions met inclusion criteria, with the majority (70%) receiving 5 fractions. Of these patients, 45 (52%) received 4 mg dexamethasone premedication prior to each fraction of SBRT and 41 (48%) were treated without dexamethasone premedication. Overall acute lung toxicity was low in both groups. Between the two groups of patients, 5/45 (11%) developed grade 2 or higher lung toxicity including hospital admission in the dexamethasone premedication arm vs. 2/41 (5%) without premedication (p = 0.4370, Fisher’s exact test). Freedom from acute SBRT lung toxicity was no different between dexamethasone premedication arm and no premedication (Log rank, p = 0.45). On multivariate Cox proportional hazard modeling adjusting for age, ECOG, tumor size, central vs. peripheral location, pretreatment DLCO, and BED, there was no difference in freedom from acute lung toxicity without dexamethasone premedication (HR: 0.305; 95% CI: 0.033, 2.792; p = 0.293).
Conclusions: In this retrospective analysis, pretreatment steroid prophylaxis with dexamethasone confers a similar acute toxicity profile and no added clinical benefit to treatment without pretreatment steroid prophylaxis.
Sterotactic body radiation therapy (SBRT) has evolved as the standard of care for medically inoperable early stage non-small cell lung cancer (NSCLC) and is being increasingly employed to ablate oligometastatic sites of disease progression to the lungs (1, 2). Early historical experiences with SBRT of 1–3 fractions generally employing higher doses of 18–30 Gy per fraction had consistently utilized corticosteroid premedication prior to each session of radiation (3–5). The use of oral and inhaled corticosteroids to prevent and/or treat acute and posttreatment radiation-induced lung injury is well established in clinical practice of conventionally fractionated thoracic radiation (6, 7). Early protocols of thoracic SBRT espoused that the expected acute inflammatory effects of large dose per fraction to lung parenchyma could be potentially abated by the use of steroid prophylaxis (4). Corticosteroid premedication, 15–60 min prior to each fraction of radiation, was mandated in the first phase II cooperative group trial (Radiation Therapy Oncology Group (RTOG) 0236) and has influenced the pattern of practice among treating radiation oncologists (5). Some rationale for this approach can be extrapolated from the available randomized evidence showing the benefit of corticosteroid prophylaxis administration in mitigating the acute inflammatory effects of radiation and thus preventing “pain-flare” phenomenon when utilizing single-fraction stereotactic radiation therapy for metastatic spinal metastases (8–10). However, whether the use of corticosteroid prophylaxis results in clinically meaningful improvements in the prevention of acute lung injury and therefore clinical toxicity has not been analyzed or reported on before in the literature. Although prophylactic treatment with corticosteroids may be a safeguard to prevent acute lung injury, mechanistic immune interactions of corticosterioid use may implicate efficacy of the programmed cell death 1 inhibitor (PD-1) inhibitors, which are being increasingly integrated with stereotactic ablative forms of radiation in all stages of NSCLC. We aimed to evaluate whether corticosteroid prophylaxis influenced the development of acute lung toxicity in patients receiving thoracic SBRT in our multicenter research database.
Our multicenter prospectively collected institutional database of lung SBRT approved by the institutional review and privacy boards (IRB Number G2017-0242) was analyzed. Patients treated for early stages I–II NSCLC from 2014 to 2017 with definitive intent thoracic SBRT (BED10≥100) were included. Pretreatment baseline clinicopathologic characteristics expected to influence the development of acute lung toxicity were collected, including ECOG performance status, PFT parameters of FEV1, diffusing capacity for carbon monoxide (DLCO), tumor size, T stage, lobe of lung involved, and RTOG-defined central vs. peripheral locations. Treatment and dosimetric characteristics of dose, dose per fraction, prescription isodose line, and delivery schedule were also collected. Patients were scored as to whether corticosteroid prophylaxis was prescribed and utilized prior to each fraction. We recorded medication utilized, timing, and medication dose, and administration was confirmed through the electronic medical record (EMR).
Toxicity was graded on multiple domains including pulmonary domain using the Common Terminology Criteria for Adverse Events Version 4. Patients were scored as to whether they develop acute lung toxicity which was defined as grade 2 or higher pneumonitis or admission during or within 30 days of treatment completion. Hospitalization for acute exacerbation of COPD during or within 30 days after completion of treatment was also considered in the analysis. SBRT technique included simulation with helical 4-dimensional (4D) computed tomography (CT) scan utilizing respiratory imaging and synchronization via Real-Time Position Management System (Varian Oncology, Palo Alto, CA, USA). Patient immobilization and abdominal compression utilized the Civco Pro-Lok body system (CIVCO Medical Solutions, Orange City, IA, USA). An internal target volume (ITV) was delineated incorporating maximum inspiratory, maximum expiratory, as well as maximum intensity projection of the CT data. Uniform 5-mm planning target margin (PTV) was utilized around the ITV. Respiratory-gated treatment was employed if tumors demonstrated motion of greater than 1 cm. Static intensity-modulated radiation therapy and volumetric modulated arc therapy were employed for treatment delivery. All patients received daily volumetric image guidance with cone-beam CT, matching to the ITV.
Univariate analysis was performed with Fisher's exact test for categorical variables and two-tailed Student's t-test for continuous variables. Multivariate analysis was performed with Cox proportional hazards model to adjust for age, pretreatment DLCO, ECOG, tumor size, central versus peripheral location, and biological effective dose.
A total of 86 patients met the inclusion criteria for analysis. The median follow-up for the included cohort was 18.6 months.
Most patients received thoracic SBRT with 54–60 Gy in 3–8 fractions, with the majority (70%) receiving 5 fractions. Forty-five patients (52%) were treated with 4 mg dexamethasone premedication 30 min prior to each fraction of SBRT, and forty-one patients (48%) were treated without any dexamethasone premedication. Compliance with MD instructions for dexamethasone premedication was verified in the EMR.
Table 1 summarizes baseline treatment, patient, and clinicoradiographic characteristics for the entire cohort and by pretreatment with dexamethasone.
Overall acute lung toxicity was low in both groups, with seven patients (8.1%) experiencing grade 2 or greater acute pneumonitis or admitted to a hospital during or 30 days postcompletion of SBRT (see Figure 1).
Between the two groups, 5/45 (11%) patients developed grade 2 or higher lung toxicity including hospital admission in the dexamethasone premedication arm vs. 2/41 (5%) patients without premedication (p = 0.4370, Fisher’s exact test).
Freedom from acute SBRT lung toxicity was 91.1% in the dexamethasone-premedicated group vs. 97.6% in the group receiving SBRT without premedication (Log rank, p = 0.45) (see Figure 2).
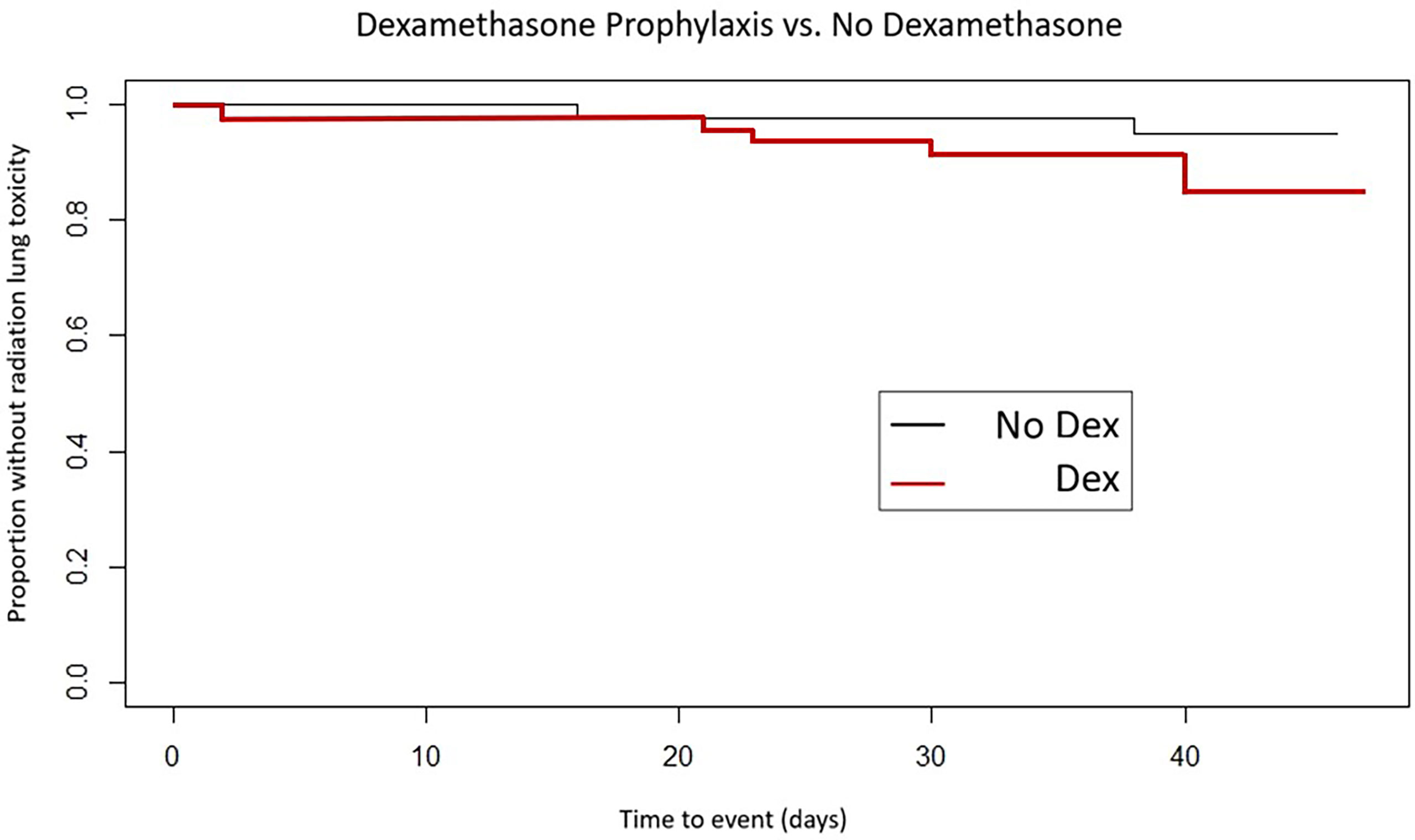
Figure 2 Time to development of acute lung toxicity by dexamethasone premedication vs. no premedication.
On multivariate Cox proportional hazard modeling adjusting for age, ECOG performance status, tumor size, central vs. peripheral locations, pretreatment DLCO, dose per fraction, and biologically equivalent dose, there was no difference in freedom from acute lung toxicity with dexamethasone premedication (HR: 0.305; 95% CI: 0.033, 2.792; p = 0.293) (see Table 2).
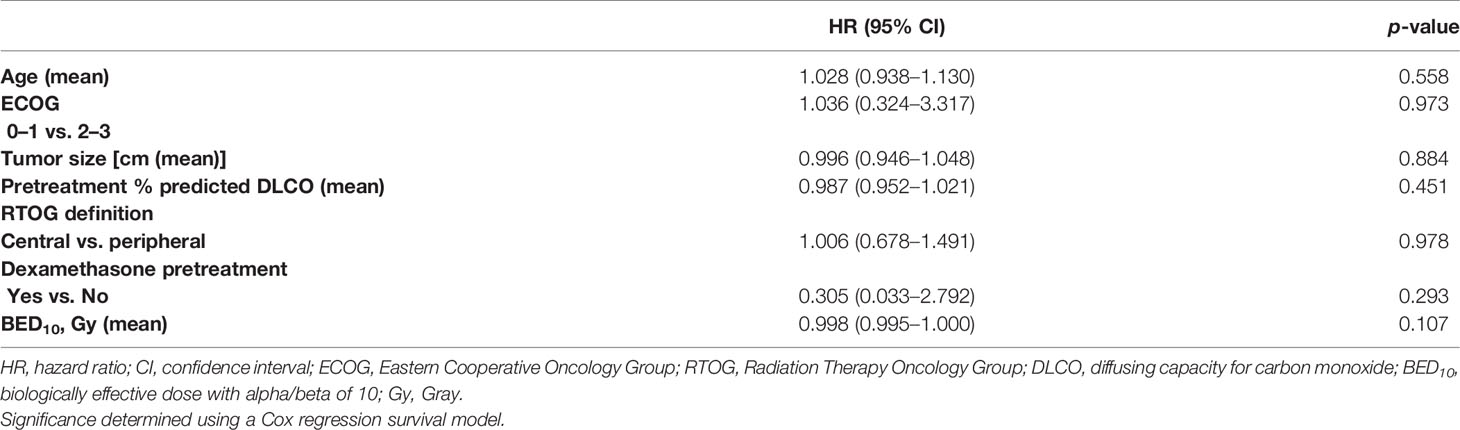
Table 2 Freedom from development of acute lung toxicity controlling for other variables in multivariate model.
Dexamethasone premedication did not appear to prevent against or mitigate acute lung toxicity or hospital admission during or in the subacute post-SBRT setting. To our knowledge, this represents the first report analyzing the influence of corticosteroid premedication in the development of acute lung injury in patients treated with thoracic SBRT.
Corticosteroid premedication has been utilized in large-dose per fraction stereotactic ablative approaches to the lung and abdominal sites since the advent of SBRT in the 1990s namely due to the bioplausibility of deterring acute inflammatory response in radiated tissue. The Karolinska Hospital experience first described stereotactic high-dose per fraction radiotherapy to the body and noted that acute high-grade toxicity could be mitigated with steroid premedication when treating tumors in the lung and liver (3, 4).
Clinical practice has been informed since then predominantly by its required use in the seminal RTOG 0236 phase II experience, but actual premedication use has been varied and largely based on institutional practice patterns. In addition, as lung SBRT cooperative group protocols have evolved, corticosteroid premedication has been increasingly left out or made optional at the discretion of the treating physician (11–20) (see Table 3).

Table 3 Incorporation of corticosteroid premedication evolution among clinical experience and cooperative group trials of thoracic SBRT.
Our study has several strengths. We were able to assess a relatively uniformly treated group of patients with similar disease and treatment characteristics in large part because our database spans an integrated healthcare system with 3 different treatment sites which employed different dexamethasone premedication based on prespecified site-specific SBRT protocol in terms of corticosteroid use and not based on patient selection. This allowed for a relatively uniform set of patients treated with similar SBRT technique, and steroid agent and dose were also uniform among the premedicated cohort. Nevertheless, the retrospective nature of the design does predispose to possible patient selection bias influencing the results. Multivariate analysis controlling for possible confounders, continued to show no added benefit to dexamethasone premedication. In addition, given the sample size of the study, it remains possible that small effect sizes from the influence of dexamethasone may not be adequately evaluated, and results should be confirmed in larger datasets.
Numerically, the steroid-premedicated arm had higher rates of hospitalization and acute lung toxicity. Although this was not statistically significant between groups, and may be largely influenced by selection bias, it is important to consider that dexamethasone use is not without inherent patient risks.
The side effect profile of corticosteroid use including effects on immunosuppression, hyperglycemia, electrolyte abnormalities, sleep disturbance, and gastric ulcer disease need to be carefully considered in light of our findings. The initial report of RTOG 0236, for example, did report one event of grade 4 hypocalcemia, largely felt to be related to steroid prophylaxis (5).
In addition, the emergence and increased integration of ablative radiotherapy with immune checkpoint inhibitors in NSCLC and oligometastatic progression to the lung, requires further careful consideration and judicious use of corticosteroid prophylaxis. Several reports have pointed to decreased efficacy of PDL1 blockade in patients receiving baseline corticosteroids, mechanistically related to a blunting of peripheral bursts of CD8-positive T cells needed in response to PDL1 blockade (21, 22). Adjuvant PDL1 blockage has shown significant survival benefits in more advanced lung cancer and is now being tested in earlier stages of NSCLC treated with SBRT (23, 24). Hence, our findings suggest significant information on this clinical scenario in patients planned to initiate PDL1 inhibition post-SBRT, showing that no significant safety signals were noted when employing high-dose per fraction lung SBRT without steroid prophylaxis. Further investigations tailoring radiation dose and fractionation schedule, for example, in the case of centrally located lesions, to minimize acute toxicity and optimize immunotherapy synergy may represent future avenues of investigation instead of relying on steroid premedication (25).
In this retrospective analysis, pretreatment steroid prophylaxis with dexamethasone confers a similar acute toxicity profile to treatment without steroid prophylaxis. The limitations of the study, including its retrospective nature, are that pre- and posttreatment pulmonary function tests and patient-reported symptomatic outcomes were not routinely and rigourously assessed. Further follow-up is needed, and the impact of steroid premedication on clinical (local control and survival) outcomes should be determined. These results should also be validated in independent datasets and prospectively with a patient-reported outcome instrument.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Geisinger Cancer Institute IRB. The patients/participants provided their written informed consent to participate in this study.
Conceptualization: FA and AM. Data curation: FA. Formal analysis: FA and PS. Investigation: FA, PS, and AM. Methodology: FA, PS, and AM. Resources: FA. Supervision: AM. Validation: FA, PS, and AM. Writing—original draft preparation: FA. Writing—review and editing: FA, PS, and AM. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Timmerman RD, Herman J, Cho LC. Emergence of Stereotactic Body Radiation Therapy and its Impact on Current and Future Clinical Practice. J Clin Oncol (2014) 32(26):2847–54. doi: 10.1200/JCO.2014.55.4675
2. Palma DA, Salama JK, Lo SS, Senan S, Treasure T, Govindan R, et al. The Oligometastatic State - Separating Truth From Wishful Thinking. Nat Rev Clin Oncol (2014) 11(9):549–57. doi: 10.1038/nrclinonc.2014.96
3. Blomgren H, Lax I, Naslund I, Svanstrom R. Stereotactic High Dose Fractionation Radiation Therapy of Extracranial Tumors Using an Accelerator. Clinical Experience of the First Thirty-One Patients. Acta Oncol (1995) 34:861–70. doi: 10.3109/02841869509127197
4. Blomgren H, Lax I, Goranson H, Kraepelian T, Nilsson B, Naslund I, et al. Radiosurgery for Tumors in the Body: Clinical Experience Using a New Method. J Radiosurg (1998) 1(1):63–74. doi: 10.1023/B:JORA.0000010880.40483.c4
5. Timmerman R, Paulus R, Galvin J, Michalski J, Straube W, Bradley J, et al. Stereotactic Body Radiation Therapy for Inoperable Early Stage Lung Cancer. JAMA (2010) 303(11):1070–6. doi: 10.1001/jama.2010.261
6. Bledsoe T, Nath S, Decker R. Radiation Pneumonitis. Clin Chest Med (2017) 38(2):201. doi: 10.1016/j.ccm.2016.12.004
7. Camus P. Interstitial Lung Disease From Drugs, Biologics, and Radiation. In: Schwartz MI, King TE Jr, editors. Interstitial Lung Disease, 5th. Shelton, CT: People's Medical Publishing House-USA (2011). p. 637.
8. Chow E, Meyer RM, Ding K, Nabid A, Chabot P, Wong P, et al. Dexamethasone in the Prophylaxis of Radiation-Induced Pain Flare After Palliative Radiotherapy for Bone Metastases: A Double-Blind, Randomised Placebo-Controlled, Phase 3 Trial. Lancet Oncol (2015) 16(15):1463–72. doi: 10.1016/S1470-2045(15)00199-0
9. Niglas M, Raman S, Rodin D, Detsky J, DeAngelis C, Soliman H, et al. Should Dexamethasone be Standard in the Prophylaxis of Pain Flare After Palliative Radiotherapy for Bone Metastases?-a Debate. Ann Palliat Med (2018) 7(2):279–83. doi: 10.21037/apm.2017.04.08
10. Westhoff PG, de Graeff A, Geerling JI, Reyners AK, van der Linden YM. Dexamethasone for the Prevention of a Pain Flare After Palliative Radiotherapy for Painful Bone Metastases: A Multicenter Double-Blind Placebo-Controlled Randomized Trial. BMC Cancer (2014) 14:347. doi: 10.1186/1471-2407-14-347
11. Nagata Y, Hiraoka M, Shibata T, Onishi H, Kokubo M, Karasawa K, et al. Prospective Trial of Stereotactic Body Radiation Therapy for Both Operable and Inoperable T1N0M0 Non-Small Cell Lung Cancer: Japan Clinical Oncology Group Study Jcog0403. Int J Radiat Oncol Biol Phys (2015) 93(5):989–96. doi: 10.1016/j.ijrobp.2015.07.2278
12. Timmerman RD, Paulus R, Pass HI, Gore EM, Edelman MJ, Galvin J, et al. Stereotactic Body Radiation Therapy for Operable Early-Stage Lung Cancer: Findings From the NRG Oncology RTOG 0618 Trial. JAMA Oncol (2018) 4(9):1263–6. doi: 10.1001/jamaoncol.2018.1251
13. Chang JY, Senan S, Paul MA, Mehran RJ, Louie AV, Balter P, et al. Stereotactic Ablative Radiotherapy Versus Lobectomy for Operable Stage I Non-Small-Cell Lung Cancer: A Pooled Analysis of Two Randomised Trials. Lancet Oncol (2015) 16(6):630–7. doi: 10.1016/S1470-2045(15)70168-3
14. Bezjak A, Bradley J, Gaspar L. Seamless Phase I/II Study of Stereotactic Lung Radiotherapy (SBRT) for Early Stage, Centrally Located, Non-Small Cell Lung Cancer (NSCLC) in Medically Inoperable Patients (2009). Radiation Therapy Oncology Group (RTOG) 0813. Available at: https://clinicaltrials.gov/ct2/show/NCT00750269 (Accessed 4.1.2019).
15. Videtic GM, Paulus R, Singh AK, Chang JY, Parker W, Olivier kR, et al. Long-Term Follow-Up on NRG Oncology RTOG 0915 (NCCTG N0927): A Randomized Phase 2 Study Comparing 2 Stereotactic Body Radiation Therapy Schedules for Medically Inoperable Patients With Stage I Peripheral Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys (2019) 103(5):1077–84. doi: 10.1016/j.ijrobp.2018.11.051
16. Fernando HC, Timmerman R. American College of Surgeons Oncology Group Z4099/Radiation Therapy Oncology Group 1021: A Randomized Study of Sublobar Resection Compared With Stereotactic Body Radiotherapy for High-Risk Stage I Non-Small Cell Lung Cancer. J Thorac Cardiovasc Surg (2012) 144(3):S35–38. doi: 10.1016/j.jtcvs.2012.06.003
17. Palma DA, Haasbeek CJ, Rodrigues GB, Dahele M, Lock M, Yaremko B, et al. Stereotactic Ablative Radiotherapy for Comprehensive Treatment of Oligometastatic Tumors (SABR-COMET): Study Protocol for a Randomized Phase II Trial. BMC Cancer (2012) 12:305. doi: 10.1186/1471-2407-12-305
18. l-Hallaq HA, Chmura S, Salama JK, Winter KA, Robinson CG, Pisansky TM, et al. Rationale of Technical Requirements for NRG-BR001: The First NCI-Sponsored Trial of SBRT for the Treatment of Multiple Metastases. Pract Radiat Oncol (2016) 6(6):e291–8. doi: 10.1016/j.prro.2016.05.004
19. Fernando HC, Timmerman R. A Randomized Phase III Study of Sublobar Resection (SR) Versus Stereotactic Ablative Radiotherapy (SAbR) in High Risk Patients With Stage I Non-Small Cell Lung Cancer (NSCLC) - STABLe-Mates Trial (2015). Available at: https://clinicaltrials.gov/ct2/show/NCT02468024 (Accessed 4.1.2019).
20. Iyengar P, Timmeran R, Gomez D. Maintenance Chemotherapy With or Without Local Consolidative Therapy in Treating Patients With Stage IV Non-Small Cell Lung Cancer (2017). Available at: https://clinicaltrials.gov/ct2/show/NCT03137771 (Accessed 4.1.2019).
21. Arbour KC, Mezquita L, Long N, Rizvi H, Auclin E, Ni A, et al. Impact of Baseline Steroids on Efficacy of Programmed Cell Death-1 and Programmed Death-Ligand 1 Blockade in Patients With Non-Small-Cell Lung Cancer. J Clin Oncol (2018) 36(28):2872–8. doi: 10.1200/JCO.2018.79.0006
22. Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, et al. Defining CD8+ T Cells That Provide the Proliferative Burst After PD-1 Therapy. Nature (2016) 537(7620):417–21. doi: 10.1038/nature19330
23. Antonia S, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, et al. Durvalumab After Chemoradiotherapy in Stage III Non Small Cell Lung Cancer. N Engl J Med (2017) 377:1919–29. doi: 10.1056/NEJMoa1709937
24. Clinicaltrials.gov. National Library of Medicine (US). March 2019- January 2025. Durvalumab vs Placebo Following Stereotactic Body Radiation Therapy in Early Stage Non-Small Cell Lung Cancer Patients: Identifier: Nct03833154. Available at: https://clinicaltrials.gov/ct2/show/NCT03833154 (Accessed 11.5.2019).
Keywords: stereotactic body radiation therapy (SBRT), lung, immunotherapy, dexamethasone, NSCLC
Citation: Alite F, Shaikh PM and Mahadevan A (2022) Influence of Dexamethasone Premedication on Acute Lung Toxicity in Lung SBRT. Front. Oncol. 12:837577. doi: 10.3389/fonc.2022.837577
Received: 16 December 2021; Accepted: 25 January 2022;
Published: 28 February 2022.
Edited by:
Xinglei Shen, University of Kansas Medical Center, United StatesReviewed by:
Francesco Ricchetti, Sacro Cuore Don Calabria Hospital (IRCCS), ItalyCopyright © 2022 Alite, Shaikh and Mahadevan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fiori Alite, ZmFsaXRlQGdlaXNpbmdlci5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.