
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 18 May 2022
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.837408
This article is part of the Research Topic Women in Gastrointestinal Cancers, volume II: 2022 View all 10 articles
Background: Being “positive” has been one of the most frustrating words anyone could hear since the end of 2019. This word had been overused globally due to the high infectious nature of SARS-CoV-2. All citizens are at risk of being infected with SARS-CoV-2, but a red warning sign has been directed towards cancer and immune-compromised patients in particular. These groups of patients are not only more prone to catch the virus but also more predisposed to its deadly consequences, something that urged the research community to seek other effective and safe solutions that could be used as a protective measurement for cancer and autoimmune patients during the pandemic.
Aim: The authors aimed to turn the spotlight on specific herbal remedies that showed potential anticancer activity, immuno-modulatory roles, and promising anti-SARS-CoV-2 actions.
Methodology: To attain the purpose of the review, the research was conducted at the States National Library of Medicine (PubMed). To search databases, the descriptors used were as follows: “COVID-19”/”SARS-CoV-2”, “Herbal Drugs”, “Autoimmune diseases”, “Rheumatoid Arthritis”, “Asthma”, “Multiple Sclerosis”, “Systemic Lupus Erythematosus” “Nutraceuticals”, “Matcha”, “EGCG”, “Quercetin”, “Cancer”, and key molecular pathways.
Results: This manuscript reviewed most of the herbal drugs that showed a triple action concerning anticancer, immunomodulation, and anti-SARS-CoV-2 activities. Special attention was directed towards “matcha” as a novel potential protective and therapeutic agent for cancer and immunocompromised patients during the SARS-CoV-2 pandemic.
Conclusion: This review sheds light on the pivotal role of “matcha” as a tri-acting herbal tea having a potent antitumorigenic effect, immunomodulatory role, and proven anti-SARS-CoV-2 activity, thus providing a powerful shield for high-risk patients such as cancer and autoimmune patients during the pandemic.
In October 2007, a warning letter was issued but no one responded (1). The warning letter was issued by Cheng and his colleagues mentioning that “Horseshoe bats resemble a large reservoir for SARS-CoV-like and the possibility of its reemergence with another novel virus should be taken into consideration because it is a time bomb” (1). The warning letter became a reality 12 years later in December 2019; the city of Wuhan in China experienced the emergence of a novel coronavirus that was initially called “Wuhan pneumonia” (2). It was further classified by the WHO on March 11, 2020 as the 5th documented pandemic since the 1918 Spanish flu pandemic (H1N1) (3).
SARS-CoV-2 has been the main cause of death in 2020 and 2021, accounting for more than 5 million deaths (4). Upon stratification of the mortality lists and the morbidity rates around the globe, several observations have been observed (5). Cancer and autoimmune patients such as those with asthma (6), rheumatoid arthritis (RA) (7), multiple sclerosis (MS) (8), and systemic lupus erythematosus (SLE) (7) were reported to be among high-risk patients during the pandemic (9).
In the case of cancer patients, their chemotherapy-induced immune-compromised status puts them at a higher risk to be easily infected by the virus, and at the same time, such patients should receive their treatment protocols to avoid complications from their oncological diseases (10, 11). Several reports from China (12–14), United States (15), and Italy (16–18) confirmed that cancer patients are at very high risk of developing severe complications upon SARS-CoV-2 infection. Among cancer patients, those with lung cancer are the least fortunate as it was reported that the highest incidence of comorbidity with SARS-CoV-2 was in lung cancer patients (19). Consequently, such patients experience severe symptoms of SARS-CoV-2 that may require intensive care admission and mechanical ventilation, or could result in loss of life (11). This issue encouraged oncological societies such as the European Association for Medical Oncology (ESMO) (20), the American Society of Clinical Oncology (ASCO), the National Comprehensive Cancer Network (NCCN), and many others to provide new guidelines for cancer patients’ treatment protocols and diagnostic tests during the pandemic (20). The main ideology behind the new guidelines is to calculate the benefit:risk ratio and categorize cancer patients into high, medium, and low priority based on Ontario Heath Cancer Care as previously reviewed in (21).
The same goes for patients suffering from autoimmune disorders where their immune-compromised status puts them at a higher risk of infection by the virus and developing more severe symptoms (22). In addition, their treatment protocols are mainly dependent on immunomodulatory disease-modifying therapies (DMTs) including glucocorticoids and immunosuppressants that are mainly prescribed to mitigate the immune attacks towards their normal body organs (22). For instance, a study focused on MS patients highlights that younger MS patients with lower socioeconomic status are at a higher risk of exposure to an unfavorable course of SARS-CoV-2 infection (23). In the case of SLE patients, it was first predicted that hydroxyl-chloroquine in their treatment protocol might provide a type of protection from COVID-19 complications (24). Yet, preliminary results from the clinics showed total opposite morbidity and mortality rates (25, 26). Mathian’s group reported that SLE patients also showed a high incidence of severe and even fatal cases of infection, confirming that, despite the co-treatment of SLE patients with antimalarial drugs, a high risk of unfavorable infection course has still been witnessed among SLE patients (25). Also, a more coherent study that included 417 SLE patients showed that the morbidity rates are moderately higher in the case of SLE patients (7).
Therefore, it is highly recommended that rheumatologists and oncologists encourage their patients to continue their ongoing treatment to avoid dangerous flare-ups of their autoimmune diseases or complications of their oncological diseases. It is imperative for those patients to have a nutritional plan that shields them from SARS-CoV-2 infection and at the same time improves their autoimmune status in the case of autoimmune patients and/or provide antitumor actions in the case of cancer patients.
In this review, we will show a glimpse of all the therapeutical trials that were carried out during the last couple of years to decrease the socioeconomic burden of such a pandemic. Yet, several failed attempts were witnessed starting from repurposing of conventional drugs, discovery of new medications that might take years of validation, to several vaccination approaches that go in parallel with the high viral mutational capacity (27, 28). However, less attention was given to the ideal remedy—”herbal drugs”—that might be the ultimate route to treat such deadly disease.
In this review, the authors will try to emphasize the significance of herbal drugs that should not be less than that of vaccines and antivirals during the pandemic. Herbal drugs have an edge regarding high-risk patients (cancer and autoimmune patients) in that they might play a dual/triple role in alleviating the primary disease and act as a protective shield during the pandemic.
Upon focusing on the herbal products with their immense roles starting from being antioxidants and holding anti-inflammatory and antiviral activities, we had a closer look at “matcha”, which we expect to have a great impact in the upcoming years because of its potent immune-modulatory capabilities and its recent validated activity against SARS-CoV-2 (29, 30). Nonetheless, matcha was also reported to hold a lot of promise for cancer (31, 32) and autoimmune patients (33, 34). Yet, in this review, the authors shed light on the research gap concerning the molecular mechanism of actions underlying matcha as a potent immunomodulatory, anticancer, and antiviral activity.
In this review, the authors screened literature covering the therapeutic effects of “matcha” as a SARS-CoV-2 antiviral herbal drug; this review also focused on the anticancer activity and immunomodulatory role of “matcha”. To attain the purpose of the review, research was conducted at the States National Library of Medicine (PubMed). For the search in databases, the descriptors used were “COVID-19”/“SARS-CoV-2”, “Herbal Drugs”, “Autoimmune diseases”, “Rheumatoid Arthritis”, “Asthma”, “Multiple Sclerosis”, “Systemic Lupus Erythematosus” “Nutraceuticals”, “Matcha”, “Green tea”, “EGCG”, “Quercetin”, “Cancer”, and key molecular pathways. Research papers, books, and published data were reviewed for their relevance to the aim of the review and summarized. Criteria for inclusion were complete, relevant publication, available online, in English, published between 1997 and 2022, and with detailed information about participants, methods, and analyses. Data collection was performed, and data abstracted were in the form of descriptive information, covering the type of samples used, techniques, and findings or effects reported. Bias was limited through the evaluation of the studies through their internal validity rather than the conclusion.
SARS-CoV-2 has a spherical shape with a positive single-strand RNA composed of approximately 30,000 nucleotides and enclosed inside a capsid (35). The genome encodes four structural proteins and many non-structural proteins (nsp) as previously reviewed (36, 37). The structural proteins are Spike (S) protein, Envelope (E) protein, Membrane (M) protein, and Nucleocapsid (N) protein. Inside the capsid, there is a nuclear capsid or the N protein, which is bound to the positive single-stranded RNA and coating it as demonstrated in Figure 1. The SARS-CoV-2 life cycle is briefly described in Figure 2, since it has been extensively discussed and reviewed in previous reviews (35, 38).
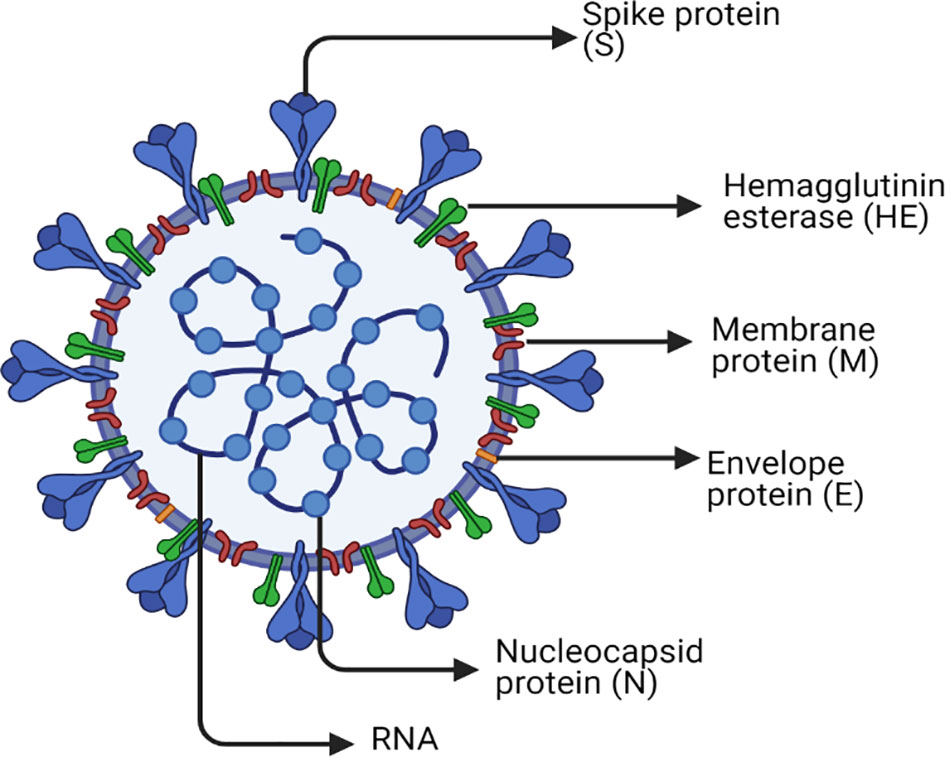
Figure 1 SARS-CoV-2 structure. The figure represents a graphical representation of the viral structural proteins spike (S), envelope (E), and membrane (M), which are embedded in the lipid surface. The positive single-stranded RNA is bound to the nucleocapsid protein (N) in the core of the capsid. Each one of these proteins plays a crucial role in the replication life cycle of the virus. The spike protein (S) is the master that supports the attachment and entry of host cell via fusion. The nucleocapsid protein (N) is the one used in transcription, which is included in the replication cycle. The membrane protein (M) that is most abundant on the viral surface drives the viral assembly. Furthermore, the envelope protein (E) has an indispensable role in assembly, host cell membrane permeability, and interactions between the host and virus. Another surface protein is Hemagglutinin esterase dimer (HE) that is found to play a role in cell entry and its infection without having a role in the replication process itself. Finally, the lipid envelope encircles the approximately 30,000 nucleotides, which is the genome of the virus encoding its four structural and many nonstructural proteins (nsp).
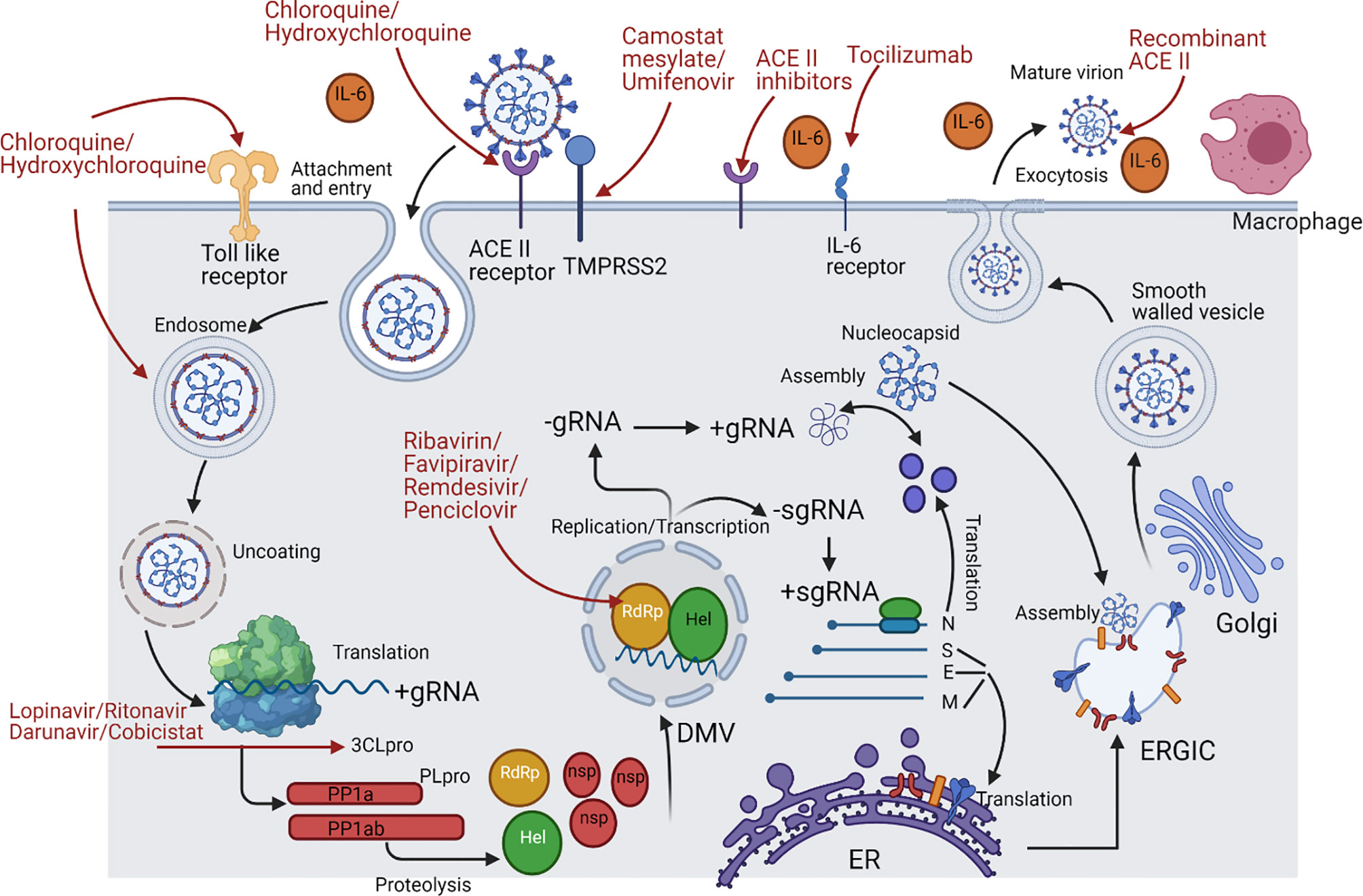
Figure 2 SARS-CoV-2 life cycle and the repurposed drugs targeting specific stages throughout its life cycle. This figure represents a schematic description for the SARS-Cov-2 life cycle with the repurposed drugs targeting specific stages in it. ACE2 receptor on the lung cells is targeted by the RBD of the S1 region in the viral spike protein; however, this binding could be targeted by chloroquine and hydroxychloroquine, recombinant ACEII receptor, or ACEII inhibitors. After the attachment, transmembrane protease serine 2 (TMPRSS2) of the host cell makes a proteolytic cleavage between S1 and S2 subunits, thus separating RBD from the fusion domains, yet this step could be targeted by camostat mesylate and Umifenovir. Consequently, a major step is taken, which is the exposure of fusion peptide domain, enabling the virus to fuse with the cell and pave its way by endocytosis then enclosed in an acidified endosome. Proteasomes then act on the nucleocapsid protein (N), uncoating it and releasing the genetic material freely in the cytoplasm, but this can be inhibited by chloroquine and hydroxychloroquine due to rendering alkaline endosomal PH. Once the positive strand becomes free, translation of the open reading frame 1a/b and production of polyproteins pp1a and pp1ab takes place. The polyproteins undergo cleavage by the viral proteases Papain like protease (PLpro) and Chemotrypsin like protease (3C like protease or 3CLpro or Mpro). Lopinavir/Ritonavir and Darunavir/Cobicistat are the ones used to inhibit 3CLpro. On the other hand, the transcription process start since the Replication/Transcription complex (RTC) was translated, and at this point, there are many nucleoside analogue drugs that were repurposed for inhibiting RNA-dependent RNA polymerase (RdRp) such as Ribavirin, Favipiravir, Remdesivir, and Penciclovir. The RTC will supervise the formation of double membrane vesicle structures (DMV) in the cytoplasm to shield the transcription process. The positive strand is used as a template for making the negative strand, which is then transcribed to make more positive strands. Moreover, subgenomic mRNAs are produced by discontinuous transcription for the sake of being translated to form the 4 viral structural proteins. Once N protein is finished, it combines with a new positive strand for the nucleocapsid to be done. However, S, E, and M proteins proceed to the endoplasmic reticulum (ER) and then to the Golgi apparatus. Last but not least, both the nucleocapsid and structural proteins will be assembled at the ER-Golgi intermediate compartment (ERGIC) to the viral envelope followed by exocytosis of mature virions through smooth-walled vesicles. Many immune components can be released during the whole process such as IL-6, leading to a cytokine storm, so the monoclonal antibody Tocilizumab is used as well as the Toll-like receptor (TLR) inhibitors chloroquine and hydroxychloroquine.
The current pandemic has urged the public health systems and pharmaceutical companies to develop new antiviral drugs and vaccines against SARS-CoV-2 after being the leading cause of death recently. In an attempt to find effective treatment for COVID-19 patients, enormous efforts were exerted in handling the pandemic. Several approaches were considered such as repurposing of FDA-approved drugs where the doctors were permitted to carry out such clinical trials using a combination of these drugs due to the urgent need to reduce cost, time, and risk of the drug development processes, but this was accompanied by several side effects and limitations as shown in Table 1. Thus, not all the repurposed drugs have been approved to be used in ameliorating this pandemic, and some of them were suspended by WHO such as chloroquine, hydroxychloroquine, remdesivir, and lopinavir/ritonavir (74). It is also important to note that all clinical trials highlighted in Table 1 do not include any of the high-risk patients like cancer and autoimmune patients, who are the main concern of this review.
Throwing light on the currently available vaccines’ effectiveness, it was reported that only 30.7% protection was acquired against the new variants of concern “delta” when compared to the “alpha” variant of the virus, which has provided 48.7% protection from a single dose of either BNT162b2 or ChAdOx1 nCoV-19 vaccines (75). However, two doses from these vaccines give a 93.7% protection against alpha and 88% protection against delta for BNT162b2, while for ChAdOx1 nCoV-19, it has an efficacy of 74.5% against alpha vs. 67% for delta (76). For the Pfizer/BioNTech vaccine efficiency, it has 88% protection against the alpha variant, and this percentage has significantly decreased against delta (76). Nonetheless, it was reported that certain mutations were identified in the most recent “omicron” variant that led to higher transmission ability, higher infectivity and binding affinity to ACE2 receptors, and increase in the failure of neutralizing antibodies and immune defense (77). Therefore, relying only on the significance of vaccinations to rescue us from such virulent variants is not a wise solution, especially since it has been well documented and experienced that the vaccines developed against the wild SARS-CoV-2 have lower efficiency rates against the mutated variants (78). Collectively, it has to be recognized that at this stage, vaccine development is important, but still the nourishment of our immune systems has a greater weight in fighting this ongoing pandemic.
After shedding light onto the evolution of new variants of SARS-CoV-2, it is essential to recall the long-lasting Influenza A virus as a live example, which can be compared in parallel with SARS-CoV-2 nowadays. The Influenza A virus causes one of the annual epidemics; even so, it continues to represent a significant threat to global public health due to its very high mutation rates and its ability to cross-transmit between species (79). The same scenario applies where Influenza A rapid evolution resulted in the loss of optimal efficacy for vaccines and antiviral drugs, to which the virus became resistant and thus complete eradication was not achieved (79). As a result, the scientific communities were compelled to use natural therapies and herbal products to boost the immune system as an alternative plan, which showed great success. Some of these herbal products include licorice roots, pomegranate, guava tea, vitamin C supplements, and zinc supplements (80).
Focusing on cancer patients and patients with autoimmune diseases, there are several studies that showed impaired antibody responses following dual COVID-19 vaccination in patients with chronic lymphocytic leukemia (81) and lung cancer (82). Furthermore, it was proved that humoral protection against the delta variant is markedly impaired among chronic lymphocytic leukemia patients, indicating the urgent need for further optimization of immune protection in this patient cohort (81). Yet, not enough data were reported about the status humoral protection for patients with autoimmune diseases.
Applying the concepts of ancient people about natural remedies in defending against colds and flu ensures that natural products were always side by side with any respiratory viral infection (83, 84). By now, most of the population had experienced the impaired protection of the currently available drugs against SARS-CoV-2 and vaccines due to the high rate of naturally occurring mutations. Consequently, a noteworthy concept is that we need an immune-modulatory and broad-spectrum antiviral agent with diverse mechanisms of action that can be readily used for the prevention of future pandemics. In this review, the authors will focus on candidates from herbal medicines exerting their immunomodulatory and antiviral effects especially for immune-compromised COVID-19 patients, and a special focus on the Japanese green tea “matcha” will be addressed. It is also worth mentioning that several reviews had shed light onto the potent role of natural compounds in the prevention of and/or as an adjunct treatment for COVID-19 (85–88). Yet, this review focuses on the tri-acting natural compounds that possess anticancer, immunomodulatory, and anti-SARS-CoV-2 activities, which were proposed as protective shields for cancer and autoimmune patients in particular during the pandemic.
In this section, the authors will focus on candidates from the herbal medicine field that have been suggested to be used during the pandemic. During the last couple of years, a huge number of herbal medicines have been suggested as anti-SARS-CoV-2 agents, for example, purple coneflower, the bark of cinchona trees, Java turmeric, ashwagandha leaves, ginger, turmeric, garlic, flaxseed, tick berry leaves, oregano, elderberry, green tea, orange, and citrus peel as previously reviewed in (89, 90).
This review focuses on natural compounds that possess a triple action including anticancer, immunomodulatory, and anti-SARS-CoV-2 activities as listed below and as summarized in Table 2. The inclusion criteria include natural compounds that possess the 3 activities, with a known mechanism of action and molecular targets, and entered clinical trials in the case of anti-SARS-CoV-2 herbal drugs. A detailed list of natural compounds that entered clinical trials as anti-SARS-CoV-2 agents is shown in Table 3. The exclusion criteria used in this review include natural compounds that possess only one of the above-mentioned actions, and/or unknown mechanism of action.
Shufeng Jiedu capsule (SFJDC) is an oral Chinese herbal medicine prepared from many different plants as rhizome and root of Polygonum cuspidatum, root of Isatis indigotica Fort, dried roots of Phragmites communis, and many others (53, 152). SFJDC was proven to have antibacterial, antiviral, anti-inflammatory, and antitumor effects (153). The capsule preparations are often used to cure Influenza, the thing that made these preparations to be suggested for investigating it against COVID-19 (53). Yet, it is worth mentioning that SFJDC is contraindicated in patients with known serious hypersensitivity to the product itself or any component of the dosage form.
In a study held to discover the effects of combining SFJDC with doxorubicin to treat hepatocellular carcinoma cells, results showed higher incidence of apoptosis along with more inhibition in cancer migration and invasion, indicating that SFJDC could be a potential complementary anticancer medication (153).
The anti-inflammatory action of SFJDC was studied using mouse models infected with HCoV-229E, and the study indicated the ability of SFJDC to decrease IL-6, IL-10, TNF-α, and IFN-γ in lungs. This has created the hypothesis about the ability of herbal medicines to attenuate the cytokine storm caused by COVID-19 (154). These effects could be explained by the following mechanisms in which SFJDC was found to be acting with them: the PI3K-Akt signaling pathway was attenuated and the NF-κB-mediated transcription of pro-inflammatory cytokines was inhibited as well (155, 156).
The main constituents of SFJDC are quercetin, wogonin, and polydatin, indicating their ability to bind to Mpro of SARS-CoV-2 by means of molecular docking studies (157). There are clinical data as well for the addition of SFJDC with the standard antiviral therapy, indicating the high probability of SFJDC to shorten the duration of COVID-19 symptoms in mild to moderate cases (157). Another study was conducted at Bozhou People’s hospital where the effect of combining SFJDC with arbidol hydrochloride was studied in comparison to the arbidol hydrochloride alone (158). The results revealed clinical improvements in the combined group compared to the other one (152, 159).
Zingiber officinale or ginger belongs to the family Zingiberaceae, it is an extremely beneficial herbal medicine used in many aspects. It originated in Southeast Asia but nowadays used worldwide as a food spice (160). Ginger rhizome is used for pain, nausea, and vomiting (161). A very wide range of active constituents are available and divided into two groups: volatile and non-volatile. The volatile group is definitely responsible for the odor and taste of ginger such as sesquiterpene and monoterpenoid hydrocarbons. However, gingerols, shogaols, parasols, and zingerone are the non-volatile constituents (162). Yet, it is worth mentioning that the usage of ginger might be accompanied by several side effects such as abdominal discomfort, diarrhea, heartburn, increased bleeding tendency, and mouth or throat irritation.
Ginger’s active constituents 6-gingerol and 6-shogaol are the main anticancer agents. Ginger has a broad spectrum anticancer activity against an array of solid malignancies such as gastric, pancreatic, colorectal, and liver cancers as shown in Table 2 and as previously reviewed in (91). The anticancer activity of ginger is accredited to its aptitude to repress several signaling pathways simultaneously such as the PI3K/AKT/mTOR pathway, the JAK/STAT pathway, the NF-κB pathway, COX-2 signaling, and caspase molecules (91).
Ginger is now considered a perfect choice for COVID-19 patients as it has analgesic, anti-inflammatory, antiviral, and immunomodulatory effects that can have a great role in the prevention of lung damage and respiratory disorders as listed in Table 2. Mechanistically, this analgesic effect is achieved by inhibiting prostaglandin (PG) production through cyclooxygenase (COX) and lipoxygenase (LOX) pathways. This is also achieved by its antioxidant activity where inhibition of the transcription factor Nf-ĸB occurs. It also acts as an agonist of vanilloid nociceptor, which represses the pain sensation (163). Considering the anti-inflammatory effect, several pathways are involved, but we will focus only on the effect of 6-gingerol, which inhibits the production of pro-inflammatory cytokines from LPS-stimulated macrophages as shown in Table 2 (164). In the case of immune-compromised patients such as patients with rheumatoid arthritis, its manifestations are proved to be decreased by ginger as it increases the transcription factor forkhead box protein 3 (FoxP3) gene expression and decreases retinoic acid receptor-related orphan receptor γt (RORγt) and T-box expressed in T-cell (T-bet) gene expression (165).
As illustrated earlier, one of the drug targeting mechanisms for SARS-CoV-2 is a papain-like protease (PL pro) that cleaves viral polyproteins that are very important for viral replication and survival. It was recently reported that ginger has the potential to act as a PL pro inhibitor for SARS-CoV-2, expressing its antiviral effect (89). Nonetheless, ginger has proven to relieve symptoms associated with COVID-19 infection such as chest pain. Ginger has proven to reduce chest pain and induce relaxation in airway smooth muscle, hindering airway resistance and inflammation as shown in Table 3 (166).
Curcuma longa or turmeric is a widely known herbal medicine; its main active constituent is the polyphenolic compound curcumin. It belongs to the family Zingiberaceae and used as a food spice, same as ginger. In Asian countries, it is used as a supplement and medicine to treat many diseases such as diabetes mellitus, cardiovascular diseases, obesity, neurodegenerative diseases, inflammatory bowel disease, allergy or asthma, and psoriasis. As mentioned above, turmeric extract is known for its polyphenol curcumin, constituting up to 77%; it contains other active constituents such as demethoxy-curcumin and bis-demethoxy-curcumin (167, 168). Turmeric can be used as an antiviral, antioxidant, anti-inflammatory, and anticancer agent. It is also important to note that turmeric does not usually cause severe side effects. Some users experience mild side effects such as abdominal discomfort, nausea, diarrhea, and dizziness.
Turmeric is one of the well-investigated anticancer nutraceuticals. It was named the golden spice, whose use was passed on from the kitchen to the clinic (169). Curcumin shows an anti-neoplastic activity against solid malignancies such as breast, liver, colorectal, and prostate cancers, and several types of leukemias and lymphomas as shown in Table 2 and previously reviewed in (170–172).
Turmeric is ranked as one of the most common immunomodulatory herbal drugs as curcumin shows strong antioxidant and anti-inflammatory effects (173). Mechanistically, its anti-inflammatory effects are prominent through the inhibition of the pro-inflammatory molecules: toll-like receptor (TLR-4), phosphatidylinositol-3 kinase (PI3K), and nuclear factor-kappa B (NF-κB). Turmeric also has the potential to repress the production of an array of pro-inflammatory cytokines such as IL-6, tumor necrosis factor-alpha (TNF-α), and interleukin 1 beta (IL-1β) (174, 175).
Concerning the SARS-CoV-2 antiviral activity of turmeric, one of the proposed mechanisms of action is acting as a PL pro inhibitor, same as ginger (89). Yet, in the case of turmeric (curcumin), this is not the only known antiviral mechanism; it is also known to act as an ACE II inhibitor. As previously illustrated, the virus enters the host by its S protein binding to the ACE II receptor. ACE II expression is detected in nasal epithelial cells, alveolar epithelial type II cells (AEC type II) of lungs, and the luminal surface of intestinal epithelial cells. Consequently, it stops viral entry and invasion in these cells (176–178).
Allium sativum or garlic is one of the world’s oldest cultivated plants and has developed a well-established reputation across many cultures for embodying promising therapeutic benefits (179). More specifically, garlic is famed for its immunomodulatory role. Garlic contains a wide range of active constituents such as allicin, alliin, ajoenes, vinyldithiins, and diallyl sulfide. S-allyl-cysteine, S-ally-mercapto cysteine, and N-acetylcysteine resemble organosulfur examples, but concerning the flavonoidal constituents, quercetin is the main active constituent. The sulfur-containing phytochemicals are mainly responsible for its immunomodulatory, anti-inflammatory, anticancer, antitumor, antidiabetic, anti-atherosclerotic, and cardioprotective features (180, 181). Similar to other herbal drugs mentioned above, some mild side effects could accompany the usage of garlic such as unpleasant mouth or body odor, nausea, vomiting, and diarrhea.
The anticancer properties of garlic have been well-documented in several types of neoplastic conditions such as breast, nasopharyngeal, oral, esophageal, and gastric carcinomas, which were previously reviewed in (179). Digging deeper to understand the molecular mechanism of garlic as an anticancer agent, this could be directly related to the sulfur-containing active constituents as they provide a source of hydrogen sulfide (182). In particular, our research group has recently shown the vital role of hydrogen sulfide in cancer progression in different contexts (183, 184).
As previously mentioned, the immunomodulatory role of garlic extracts has a well-documented property that is mainly related to the sulfur-containing active constituents as well (180, 181). There are extensive mechanisms for the immunomodulatory and anti-inflammatory actions of garlic, specifically alliin, which effectively suppresses the expression of several proinflammatory cytokines such as interleukin 6 (IL-6) and mature plasma cell 1 (MCP-1) (110). In the case of asthmatic patients, garlic has also a proven anti-asthmatic property through repressing IL-4, IL-5, and IL-13 secretion (185). Moreover, the S-allyl cysteine constituent of garlic has also proven to ameliorate MS-related pathology and relieve the associated symptoms through altering tumor necrosis-α level in the MS-mouse model (186). However, concerning SLE patients, no information was reported concerning the impact of garlic on the pathogenesis of the disease or its associated symptoms. Collectively, garlic has been proven to have several features that could provide a protective shield for high-risk autoimmune patients in the current pandemic.
Garlic has shown potential antiviral activity against a myriad array of viruses. Its antiviral activity against SARS-CoV-2 has been estimated. It was reported to act as a chymotrypsin-like protease (3CLpro) inhibitor, resulting in hindering viral attachment to host cells. Such antiviral activity has been acknowledged to the alliin and quercetin constituents in the garlic (187). In the shadow of SARS-CoV-2-associated high risk of blood clots and increase in D-dimer levels that are directly proportional to mortality rate, it is important to decrease other risk factors for blood clots such as lipids, triglycerides, and cholesterol levels in high-risk patients in particular. In such context, black garlic extracts were proven to have an anti-atherosclerotic action, meaning to decrease the blood levels of total lipids, triglycerides, and cholesterol as they lower sterol regulatory element-binding protein 1 (SREBP-1C) mRNA expression causing downregulation of lipid and cholesterol metabolism (188).
Linum usitatissimum or flaxseed has been known for its potential anticancer and anti-angiogenic properties against several solid and non-solid malignancies. Nonetheless, it has also been known for its promising immunomodulatory role and recent anti-SARS-CoV-2 activity. This has been associated with its high abundance of lignans and Omega 3. The most common lignin is secoisolariciresinol diglucoside (SDG).
Literature supports the anticancer activity of flaxseed oil and other isolated compounds from flaxseed both in vitro and in vivo (189–191). Ezzat et al. have recently validated the anticancer activity of lignin-rich fraction from flaxseed against breast cancer cell lines and mice bearing tumors as well. It was reported that lignin-rich flaxseed fractions markedly repressed vascular endothelial growth factor (VEGF) and 1-α, metalloproteinases harnessing breast cancer metastasis in vitro and in vivo (191). Moreover, it was reported to activate the caspase-3-dependent apoptosis as a mechanism of its antiproliferative activity (190, 191).
For a very long time, PUFA has been known to treat metabolic, cardiac, inflammatory, and autoimmune diseases and reduce the risk of cancers (192). Generally, omega 3 PUFA has a great immunomodulatory effect in cases of acute pneumonia and acute respiratory distress syndrome (ARDS) by reducing reactive oxygen species and pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, and IL-8 (193, 194).
Since flaxseed’s immunomodulatory role and its inhibitory impact on several cytokines that are reported to be dominant players in the cytokine storm manifested by SARS-CoV-2 patients were validated, the effect of flaxseed on SARS-CoV-2 patients was evaluated. It was found that omega 3 reduces lung inflammation caused by the SARS-CoV-2 infection by decreasing IL-6 production, extracellular signal-regulated kinases 1 and 2, COX-2 activation, and the nuclear translocation of NF-κB (144).
Citrus fruits such as Citrus sinensis (sweet orange) are the most widely used functional food during the pandemic. This was definitely because of its highly relevant active constituents in combating SARS-CoV-2. Citrus fruits are rich in vitamin C, carotenoids, and flavanones (195). Nonetheless, even the hesperidin flavone is found in the peel and the white part (albedo) of citrus fruits (196). Hesperidin has manifold properties such as antiviral, antimicrobial, antioxidant, antitumor, antihypertensive, and immunostimulant activities (197).
Our research group has recently focused on the anticancer activity of hesperidin and its glycoside hesperetin, where we and others showed that hesperidin has potent anticancer properties against several hallmarks of breast cancer such as cellular viability, proliferation, colony-forming ability, migration, and invasion in vitro (198–200). Moreover, it was found to have a direct impact on the tumor microenvironment at the tumor-immune synapse through altering ICAM-1 and ULBP2 in MDA-MB-231 breast cancer cell lines.
Hesperidin has been recently reported to have a direct post immunomodulatory role on autoimmune patients. It was reported that hesperidin can reduce the neuroinflammation episodes experienced by MS victims as well as ameliorates the immunological outcome in an MS-mouse model (118). In a more comprehensive study, it was reported that hesperidin alleviates several neurological disorders including MS through its anti-inflammatory and potent antioxidant activities (201). It is also important to note that hesperidin was also found to have anti-arthritogenic effects in an experimental model of RA (202).
More than one mechanism was proposed for the anti-SARS-CoV-2 activity of hesperidin; as explained before, SARS-CoV-2 is internalized by binding of the spike glycoprotein of the virus with ACE2 receptors. Hesperidin superimposes the ACE2-receptor-bidomain (RBD) complex, so it binds to the virus spike protein (197). Also, it was suggested that it binds “3Clpro” or “Mpro”, preventing the processing of viral proteins pp1a and pp1ab into functional proteins in the host cells (145). Furthermore, it is considered a powerful antioxidant as it is powerful against superoxide and hydroxyl radicals that cause oxidative stress, and it can help control specific phases of the life cycle of SARS-CoV-2 and finally prevent cell death (197, 203–205). However, the main antioxidant effect of orange peel goes back to vitamin C content. It was suggested that increasing vitamin C daily intake during the COVID-19 pandemic is a useful protective measure as it stimulates antiviral immune responses and reduces the lungs’ inflammatory status (144, 206, 207).
Echinacea purpurea or the purple coneflower is a well-known herb highly recommended for respiratory infectious diseases in Europe as it is already present in different forms such as extracts, tinctures, teas, and sprays, and at different dosages as well (208). The purple coneflower contains many bioactive compounds such as chicoric acid and caffeic acids, alkylamides, and polysaccharides (209). These active constituents were proven to have antiviral effects against enveloped viruses such as human coronavirus (209). Its supplements are widely recommended by naturopathic doctors for their immune support function (210). Moreover, it is well known for its various immunomodulatory, antioxidant, anti-inflammatory, and antibacterial properties (211, 212).
The anticancer mechanism is still not clear, but a study showed that chicoric acid has the ability to induce apoptosis in colon cancer cells and to decrease the telomerase activity in HCT-116 cells (213). Another study done on human pancreatic cancer cells and colon cancer cells indicated the ability of the root extract to induce DNA fragmentation and increase the activity of caspase 3/7 in a dose- and time-dependent manner, thus inducing apoptosis (214).
Extracts of E. purpurea, both aqueous and alcoholic, regulate the immune cells in both adaptive and innate systems (215–217). It works by improving CD4+ and CD8+ T lymphocyte and cytokine levels in blood. These cytokines include the three interleukins IL-6, IL-10, and IL-17 (218). To inhibit inflammation, it suppresses interleukins IL-2, IL-6, and tumor necrosis factor (TNF-α) (219).
E. purpurea seems to augment its antiviral response by influencing PRRs on the innate immune cells, pathogen-associated molecular pattern PAMPs on the virus (220). Such interaction triggers phagocytosis and initiation of other antiviral responses by the immune system (221). It was also noted that E. purpurea has an effective antiviral role against rhinoviruses (222), influenza virus (223), RSV (208), herpes virus (208), adenoviruses (208), and coronaviruses (224, 225).
Another potential candidate is Java turmeric, also known as Curcuma zanthorrhiza. It is a highly promising candidate as its major active constituent is xanthorrhizol (accounts for 44.5%) (226). Java turmeric is widely used in Southeast Asian countries and belongs to Zingiberaceae and Curcuma genus (90). This plant is usually used as an important food additive to enhance flavors (227), but as a treatment component, it is well approved for some diseases and can be used as supplements (226–229). This plant has a myriad of functions, namely, it has antimicrobial, antioxidant, antihyperglycemic, antihypertensive, antiplatelet, anticancer, and nephroprotective effects, and it can be used as a supplement in SLE (230–233). These characteristics make it a potential adjuvant therapy for COVID-19 patients and a preventive measure for high-risk patients, especially SLE patients.
The anticancer activity of Java turmeric can be due to the induction of the TP53-dependent mitochondrial pathway and thus induction of apoptosis (234–236). It can also induce caspase activation, which will lead to enzymatic proteolysis of DNA and cytoplasmic proteins leading to cell death (227). A study done on HCT166 colon cancer showed that xanthorrhizol leads to higher expression of NAG-1 and increases the activity of its promoter (237). NAG-1 (non-steroidal anti-inflammatory drug-activated gene 1) is a pro-apoptotic and is a member of (TGF-β) (237). Regulation of the MAPK pathway is another function for xanthorrhizol; it increases ROS levels intracellularly and enhances phosphorylation of p38 and JNK in SCC-15 oral squamous cell carcinoma (238).
It was proven that it inhibits the production of inflammatory cytokines from adipose tissue by downregulation of inflammatory cytokine genes and inhibits the expression of TNF-α as well (90). For SLE patients with hypovitamin D levels, a study showed that xanthorrhizol can lower the serum level of IL-6 and increase the serum level of TGF-β (229). Another study released the same results when done on hippocampal neurons and primary culture microglia, and this inhibition of inflammation could be due to inhibition of nitric oxide synthase (iNOS), and consequently, lower levels of nitric oxide (NO) are produced (235, 239, 240). Collectively, it may play an immunosuppressant role (90).
Xanthorrhizol was shown to have a potent antiviral activity against SARS-CoV-2 variants such as GH clade strain and delta strain, so it can be a promising antiviral plant against COVID-19 (241).
Withania somnifera is widely known for its antiviral, immunomodulatory, anti-inflammatory, anti-stress, antihypertensive, and antidiabetic effects, and many clinical trials were made to study its safety profile in humans (which eventually confirmed its safe use in humans) (242, 243). Moreover, there are scientific proofs for the ability of W. somnifera to maintain immune homeostasis in states of infection and inflammation (244, 245). The main active constituent in this plant is called Withanolides, which is a group of C28 steroidal lactone triterpinoids, including withaferin A; withanolide A, B, and D; withanoside IV and V; withasomniferin A; withanone; sitoondoside IX and X; and 12-deoxywithastramonolide. Furthermore, there are other active constituents such as catechin, naringenin, and syringic acid p-coumarin. This combination of such significant components endows W. somnifera superior protective capability (245, 246).
Withaferin A is considered to be the principal component in W. somnifera; it works by inhibition of β-tubulin and consequently stops the proliferation of cells (247); it also inhibits tumor proteasomal chymotrypsin (248). It was also proven that withaferin A inhibits the cancer chaperon Hsp90 as it stabilizes the signaling proteins (249). Moreover, Notch1, which mediates the survival of colon cancer cells, is inhibited as well by withaferin (250).
Collectively, withaferin A and withanone promote ROS signaling, so they induce cancer killing by oxidative stress along with other pathways (251, 252). Finally, a study was carried out in a mouse model that concluded that W. somnifera alcoholic extracts inhibit tumor proliferation and growth and increase life span (253).
Withanolide A encourages B- and T-cell proliferation with improvements in TH1 response as well (254–258). In mice, W. somnifera extracts led to higher counts of leukocytes and platelets (259, 260), and in chicks, the count of CD4+ and CD8+ also increased when compared to normal levels (261, 262). W. somnifera extracts were found by a study to be an immunostimulant when administered with anupana as vehicle, and the results revealed the activation of T cells and NK cells after 4 days only with BID consumption (263).
W. somnifera can impede the viral replication cycle. Withanone destabilizes the complex between the ACE2 receptor (host) and spike protein (virus) (264), and in addition, withaferin A and withanone are responsible for blocking Mpro and TMPRSS2 enzymes, which could interfere with the entry of the virus (142, 264, 265). Withacoagin and withanolide B have the ability to block the spike protein and also the RdRp with a high affinity (266). It was reported that they prevent virus entry to the host through inhibition of the trans-membrane protease serine 2 (TMPRSS2)/ACE II complex, thus hindering SARS-CoV-2 entrance to host cells (142).
Tea is from the plant Camellia sinensis, which is a highly consumed beverage worldwide, with approximately 2.5 million tons produced each year. The difference between green and black tea is in the manufacturing process as green tea, once harvested, is steamed to prevent fermentation, while black tea is left as it is, causing the dimerization of catechins to theaflavins (267, 268). Although the composition of tea can change according to the climate, leaves, season, etc., the main constituent in it is considered to be polyphenols (269). Tea is not just a normal beverage, as research has turned a spotlight on it to study its various effects whether in vivo or in vitro (269). The studies revealed that polyphenols present in the tea can have a role in several diseases including cancer, diabetes, and cardiovascular diseases (269).
The polyphenols present in black tea are mainly theaflavins and thearubigins (269). Derivatives of theaflavins are theaflavin (TF1), theaflavin-3-gallate (TF2A), theaflavin-3’-gallate (TF2B), and theaflavin-3,3’-digallate (TF3) (270). Investigations of their biological properties found a myriad of benefits including antiviral, anti-inflammatory, antioxidant, antitumor, and antibacterial activities (271–273).
Black tea shows a potential in the treatment of many types of cancer such as breast, prostate, lung, ovarian, cervical, and liver (274). In vitro studies for breast cancer showed 40% smaller tumor size for the intervention group when compared to controls (275). For prostatic cancer, significant inhibition for the androgen receptor promoter region along with inhibition of androgen receptor expression was noticed (276). Moreover, prostatic adenocarcinoma cell viability is inhibited in a dose-dependent fashion with TF1, TF2a, TF2b, and TF3 (277). A myriad of studies have proven the antiproliferative activity of theaflavins and the inhibition of survival and migration ability of cancer cells (274). There are studies that reveal the proapoptotic potential of theaflavins by observing higher levels of Bax (apoptotic protein) and lower levels of Bcl-2 (antiapoptotic protein) (274). Furthermore, P53 levels are increased by theaflavins and reduction in the levels of phosphorylated Akt, phosphorylated mTOR, and c-Myc occurs (274). Generally, theaflavins show a potential for cancer treatment and prevention (274).
Theaflavins were proven to have the potential for inhibition of not only lipopolysaccharide (LPS)-induced intracellular adhesion molecule (ICAM)-1 but also the expression of the vascular cell adhesion molecule (VCAM)-1 by blocking pathways of NF-kB and c-Jun N-terminal kinase (JNK); this in turn will shut down the neutrophils since ICAM-1 and VCAM-1 are expressed on the endothelial cell surfaces (278–280). Theaflavins also have the capability to inhibit ROS and neutrophil elastase enzyme (the one that increases the permeability of alveolar epithelium) in a promising way (280–282).
The antiviral activity of black tea comes from TF1, TF2a, TF2b, and TF3, which were proven to have high affinity for 3CLpro and inhibit it (270). Theaflavins also showed a potential for inhibiting RNA-dependent RNA polymerase RdRp (283) and RBD in the spike at locations near the contact between ACE2 and spike protein (270). The roles of theaflavins go beyond treatment since TF3 was found to be able to bind to the ACE2 receptor, thus preventing spike RBD from attaching (284), leading to prophylaxis effects (285).
The main polyphenolics in green tea are quercetin and catechins, which include epigallocatechin-3-gallate (EGCG) (the most predominant one), epigallocatechin, epicatechin-3-gallate, epicatechin, gallocatechins, and gallocatechin gallate (269, 286). The main investigated biological effects were anti-inflammatory, antibacterial, antioxidant, antiproliferative, and antitumor (267), as shown in Figure 3 and briefly described below. The main prominent effect for EGCG is being a potent antiviral more than the chemically synthesized drugs (270).
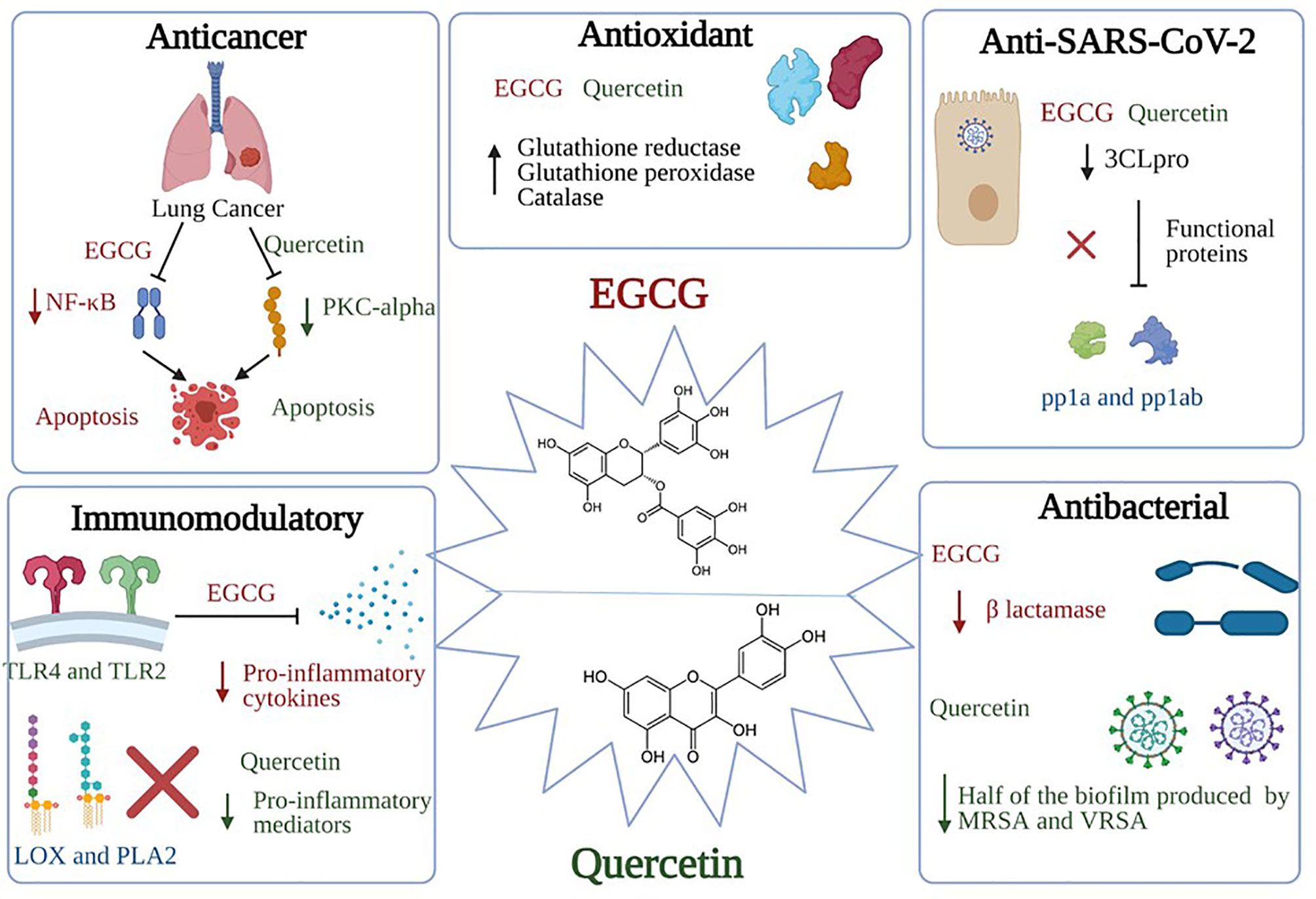
Figure 3 Significant pharmacological activities of EGCG and quercetin. Green tea, with its main two active constituents EGCG and quercetin, contributes to a wide range of medicinal activities such as antioxidant, immunomodulatory, anticancer, antiviral, and antibacterial actions. EGCG anticancer activity is produced by suppressing the NF-κB signaling in A549 and H1299 cells; activation of apoptotic cascades are also initiated, resulting in a marked hindering of cellular proliferation. On the other hand, quercetin inhibits the protein kinase C (PKC-α), a survival signaling protein, repressing several cancer hallmarks. Concerning the immunomodulatory actions, EGCG induces TLR4 and TLR2 expression levels, thus depleting the mitogen-activated protein kinase (MAPK) pathway and repression of pro-inflammatory cytokines release. As for the quercetin, it inhibits pro-inflammatory mediators such as Lipoxygenase and Phospholipase A2. Besides, both constituents share the same antiviral mechanism against SARS-CoV-2, which is binding to “3Clpro” or “Mpro”, preventing the processing of viral proteins pp1a and pp1ab into functional proteins in the host cells. Bona fida, the antioxidant effects of both EGCG and quercetin are mediated through scavenging and neutralizing the free radicals and boosting the enzymes that are responsible for detoxification such as glutathione reductase, glutathione peroxidase, and catalase. Finally, quercetin showed potential antibacterial activity through inhibiting half of the biofilm production by methicillin-resistant S. aureus (MRSA) and vancomycin-resistant S. aureus (VRSA). Similarly, EGCG showed antibacterial activities through inhibiting the B lactamase production and neutralizing the released toxins.
Regarding the scope of the review, their antiviral activity and their potential as anti-SARS-CoV-2 nutraceuticals will be the main focus especially since the anticancer activity and immunomodulatory role of EGCG and quercetin have been validated and previously reviewed (287, 288). This part will discuss some of the proposed mechanisms of EGCG (the main catechin) as an anticancer and immunomodulatory constituent of green tea as an introduction for its significance, then the spotlight on it, as well as on quercetin, will be turned on again in the matcha part.
Apoptosis is the needed end result in the treatment of any cancer, so highlight was thrown on EGCG’s ability to induce apoptosis. Studies showed its ability to induce apoptosis by generating ROS and activating caspase-3 and caspase-9. Consequently, this leads to cycle arrest at the G1 phase (289, 290). NF-kB, which has a major role in apoptosis inhibition in cancer (291), was inhibited by EGCG in breast cancer, lung cancer, and human non-squamous cell carcinoma (292, 293). Activator protein-1 (AP-1), which induces proliferation, is also downregulated by EGCG (294). Actually, EGCG was proven by several studies to inhibit VEGF production through inhibition of STAT-3 and NF-kB in breast and human non-squamous cell carcinoma (292). Another study indicated the efficacy of EGCG on the inhibition of the IGF/IGF-1R axis (295, 296). Even in epigenetics, EGCG inhibits the activation of DNA methyltransferase, leading to restoration of silenced tumor suppressor genes as an end result; these genes include retinoic acid receptor- β (RARβ), p16INK4a, and O6-methylguanine-DNA methyltransferase (297).
In an aim to investigate the effects of EGCG on cytokine level modulation, a study was done on activated human primary T cells to see the effect on atherogenesis (298). This study found that EGCG has successfully decreased the level of interleukins IL-2 and IL-4, INF-γ, and TNF-α. EGCG also decreased the level of phosphorylated c-Jun N-terminal (p-JNK) and extracellular signal-regulated kinase (p-ERK), and this could explain the mechanism used by EGCG to exert its anti-inflammatory effects (298). In addition, EGCG seems to have a role in symptoms reduction and pathology improvement in autoimmune diseases (299). Inhibition effects of EGCG on CD4+ T-cell expansion in response to stimulation was observed (299). The differentiation of naïve CD4+ T cells and that of Th1 and Th17 was also affected (299). This obstructed differentiation of Th1 and Th17 can be due to downregulation of transcription factors by EGCG, for instance, STAT1 and T-bet for Th1, while STAT3 and RORγt for Th17 (299). A study on multiple sclerosis in an animal model showed that EGCG weakened the disease severity in a dose-dependent manner and suppressed the proliferation of T cells along with reducing pro-inflammatory cytokine production (299). Besides, EGCG anti-inflammatory effects were proven as well in inflammatory arthritis disease (299).
EGCG has the ability to downregulate MAPK and NF-kb signaling pathways leading to the inhibition of pro-inflammatory cytokines as a result (300, 301). EGCG can weaken the transmigration of neutrophils through vascular endothelial cells (281) and decrease the neutrophil elastase enzyme, which increases the permeability of alveolar epithelium (302). As mentioned in black tea, EGCG in green tea can also inhibit LPS-induced ICAM-1 as well as the expression of the VCAM-1 by blocking pathways of NF-kB and c-Jun N-terminal kinase (JNK); this in turn will shut down the neutrophils since ICAM-1 and VCAM-1 are expressed on the endothelial cell surfaces (278–280). Last but not least, EGCG can scavenge for ROS and neutrophil elastase enzyme (the one that increases the permeability of alveolar epithelium) in a promising way (280–282), making it a strong immunomodulatory agent that can help fight infections that consequently will have an impact on controlling COVID-19.
EGCG was shown to possess antiviral activity against many viruses such as porcine reproductive and respiratory syndrome virus (PRRSV), hepatitis C virus (HCV), ZIKA virus, chikungunya virus, influenza virus, and HIV-1 (303–307). Consequently, this inspired researchers to evaluate its antiviral potential against SARS-CoV-2. Initially, EGCG and quercetin were reported to be among the most effective inhibitors for 3CLpro as presented in Figure 4 (148). EGCG was proven by molecular docking studies to be the most potent inhibitor for 3CLpro among all the nature-based phytochemicals (308). Then, it was reported that EGCG inhibits many structural proteins such as the HR2 domain, the post-fusion core of the S2 subunit, S protein, the RBD-ACE2 complex, and NSP15 endoribonuclease as shown in Figure 4 (270). Also, another mechanism of action mediated by EGCG was the inhibition of the complex formation between glucose-regulated protein-78 (GRP-78) and the virus (309), as shown in Figure 4. GRP78 is a chaperone protein that is normally expressed in the lumen of the endoplasmic reticulum. Under cell stress conditions, overexpression of this protein occurs and is then translocated to the plasma membrane where SARS-CoV-2 interacts with it by the S protein, and subsequently, virus entry happens (310). Another molecular docking study was made on the binding affinity to the viral structural protein finding that EGCG has the highest affinity among the other substances that are included in the study. This study underlined a very important discovery: the affinity of EGCG to inhibition was higher than that of the well-known drugs used during the pandemic, remdesivir and chloroquine, suggesting a better antiviral activity for EGCG (270, 311).
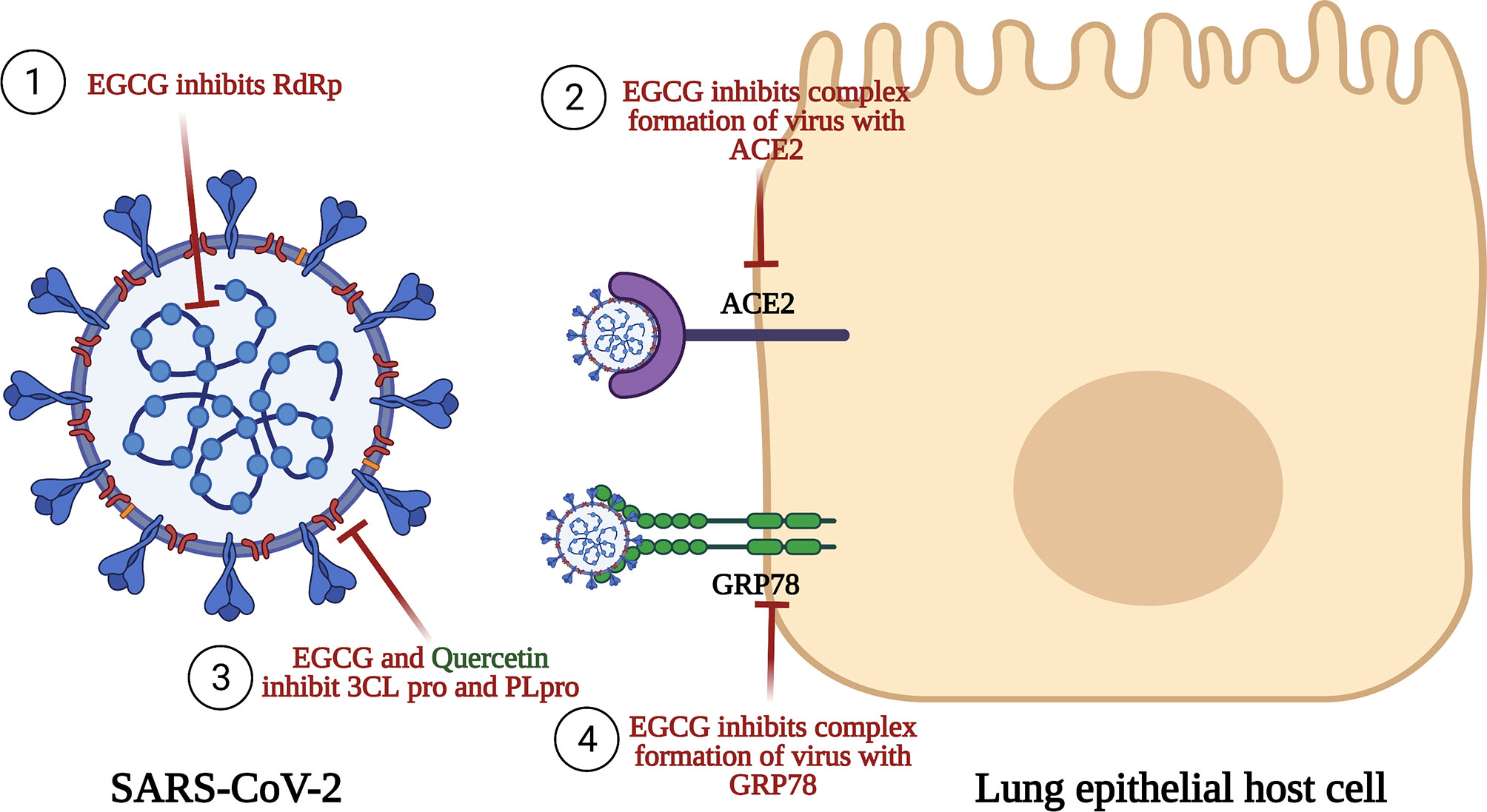
Figure 4 Anti-SARS-CoV-2 activities of EGCG and quercetin. This figure illustrates the anti-SARS-CoV-2 for both EGCG and quercetin. EGCG inhibits RNA-dependent RNA polymerase (RdRp), an enzyme having an important role n replication and transcription of the virus. EGCG also inhibits the binding of the S1 region of the viral spike protein to the ACE2 receptor on the lung host cells. EGCG and quercetin prevent the processing of polyproteins pp1a and pp1ab by Papain-like protease (PLpro) and Chemotrypsin-like protease (3C like protease or 3CLpro or Mpro). Finally, EGCG inhibits complex formation of the virus with GRP78 receptor and thus inhibits viral entry.
Collectively, among all the natural active constituents isolated from phytochemical plants, EGCG and quercetin showed an exceptionally potent antiviral activity harnessing the SARS-CoV-2 life cycle through a myriad of mechanisms as summarized in Figure 4. Accordingly, our next step was to screen for herbal drugs that were reported to contain the highest phenolic contents of EGCG and quercetin. Undoubtedly, the choice was matcha, especially since it has been recently reported that several types of green tea could effectively block infection due to SARS-CoV-2 and its new variants by mainly abrogating the spike binding to the ACE2 receptor (29, 30).
Matcha powder is a herbal drug that was reported to contain at least three times higher EGCG content than green tea, providing an economic and beneficial beverage for SARS-CoV-2-infected patients and a preventive measure for high-risk patients such as cancer and autoimmune patients (312). Nowadays, matcha tea powder is widely known and used for its abundant health benefits and its exceptional quality. Matcha is the powdered form of green tea that originated in Japan (313). The high nutritional benefits of matcha come from the presence of many powerful active constituents as listed in Table 4 below. The main forms of catechins and the most active ones found in higher amounts are (-) epigallocatechin 3-gallate (EGCG), caffeine, quercetin, phenolic acids, rutin, vitamin C, chlorophyll, and theanine (313). Catechins are present in four types: (-) epicatechin (EC), (-) epicatechin 3-gallate, (-) epigallocatechin (EGC), and (-) epigallocatechin 3-gallate (EGCG) (344, 345).
Catechins have an indisputable role as antioxidants by scavenging and neutralizing the free radicals and boosting the enzymes that are responsible for detoxification such as glutathione reductase, glutathione peroxidase, and catalase (346). It is also worth mentioning that the cellular redox homeostasis can be well maintained by the intake of catechins more often in the human diet (316). As previously mentioned, EGCG has a powerful antiviral effect. Compared to vitamin C, flavonoids, and glutathione, it has been proved that catechins have a higher antioxidant potential (316).
This antioxidant effect contributes to one of the anti-carcinogenic mechanisms of EGCG, as it was previously mentioned that the catechins can quench the reactive oxygen species at any stage and consequently halt the malignant transformation process (347). Other studies have shown that the EGCG has several anticancer activities as shown in Figure 5. EGCG exhibits antitumorigenic properties in lung cancer. It suppresses the NF-κB signaling in A549 and H1299 cells; this leads to the inhibition of cell proliferation and induces apoptosis as shown in Figure 5 (348). EGCG suppresses breast cancer progression through the tight binding of EGCG to signal transduction activator proteins of transcription 1 (STAT1) by its three hydroxyl groups of the B ring and one hydroxyl group of the D ring; this bond leads to the blockage of the phosphorylation of STAT1 by Janus Kinase 2 (JAK2) and inhibition of its carcinogenic effects since STAT1 in cancer cases can act as an oncogenic protein. It was worth noting that EGCG promotes Fas/CD95-mediated apoptosis in the neck and head squamous carcinoma by inhibiting JAK/STAT3 (317, 349).
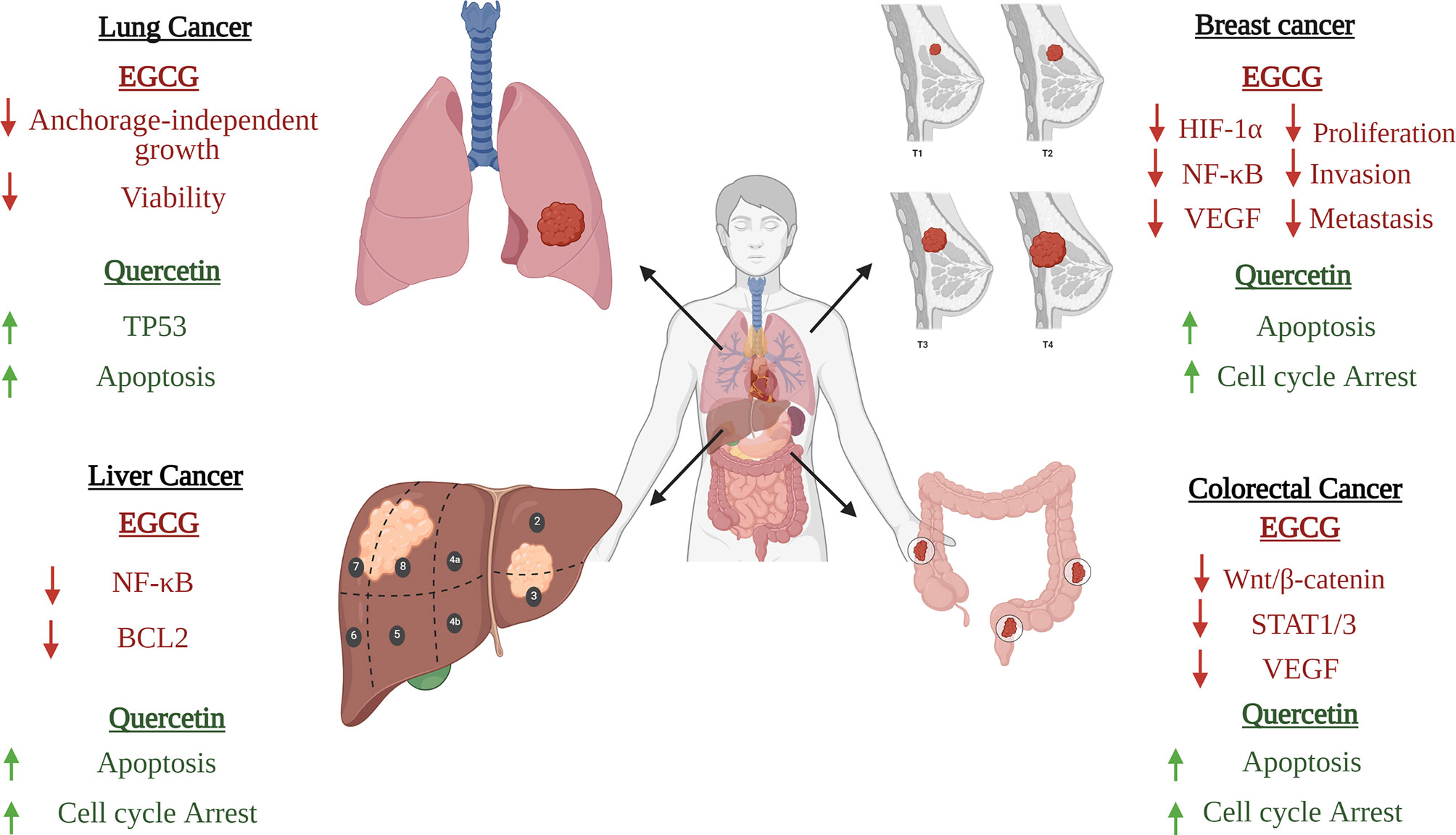
Figure 5 Anticancer activities of EGCG and quercetin. Epigallocatechin-3-gallate (EGCG) and quercetin modulate several canonical oncological pathways such as Wnt/β-catenin and JAK/STAT pathways. Also, they modulate the expression of several anti-apoptotic proteins such as BCL2, tumor suppressor proteins such as TP53, and oncogenic drivers such as VEGF. Both active ingredients have proven to be effective in halting the malignant transformation process in several types of cancers such as breast, lung, liver, and colorectal cancers.
As per the current treatment protocol for SARS-CoV-2, several antibiotics are prescribed especially in high-risk patients to avoid the complications of secondary bacterial infections, an act that would result in an antibiotic resistance catastrophe post-pandemic as described by the WHO in November 2020. As presented in Figure 3, EGCG has promising antibacterial activity while saving the world from the antibiotic resistance dilemma that would threaten our lives enormously. EGCG has shown bactericidal activity against staphylococci. Moreover, EGCG also shows an antibiofilm activity when co-administered with other antibiotics; it gives a powerful synergistic action. EGCG also can inhibit beta-lactamase production and neutralize the released toxins. The negatively charged property of EGCG makes it more effective against Gram-positive bacteria (318).
Nutrition immunity is a new concept that has been revolutionized during the pandemic (350). Massive attack on the respiratory epithelium (host cells for SARS-CoV-2) can lead to acute respiratory distress syndrome (ARDS), characterized by the uncontrolled release of pro-inflammatory cytokines leading to damaging the host cells, a vicious process termed as cytokine storm (350). It is important to note that the cytokine storm is also a common feature in the case of chemotherapy-treated cancer patients (351). Therefore, it is a crucially important measure to protect such high-risk patients during the pandemic. SARS-CoV-2-infected patients have a reduction in IFN-α and IFN-β levels, thus increasing the chance for the virus to invade and take over the immune system (319, 352). It is noteworthy that EGCG has a dominant immunomodulatory role by inducing TLR4 and TLR2 expression levels. Such induction occurs as a result of the repression of the mitogen-activated protein kinase (MAPK) and the pro-inflammatory cytokines as presented in Figure 3 (300, 301). EGCG also modulates the immune system through inhibition of the RIG-I (acts as a RIG-I inhibitor), thus protecting the infected patient from the cytokine storm and its notorious consequences (353).
As presented in Table 4, one of the main matcha constituents is quercetin. Quercetin possesses an array of pharmacological activities such as neuroprotection, antioxidant, and antineoplastic activities. It also has a vital role in diabetes mellitus patients, where it inhibits glucose absorption and thus it regulates carbohydrate metabolism, thus regulating the insulin secretion and sensitivity to tissues (320, 321, 354).
Our research group has recently highlighted the potential anticancer activity of quercetin and its derivatives in liver and breast cancers (355, 356). Studies show that if quercetin was ingested on a daily basis, it was found to decrease the risk of cancer incidence (326). Quercetin was also reported to retain its antitumorigenic properties against several types of leukemias, melanoma, lung, colorectal, and ovarian cancers (325). In vivo studies also supported the promising anticancer properties of quercetin in several animal models (324). Molecularly, quercetin inhibits protein kinase C signaling protein, resulting in the activation of apoptotic death signals and cell cycle arrest as shown in Figure 5 (329). It also has powerful induction effects on TP53, Fas/FADD, caspases, and suppression of vital anti-apoptotic proteins (330, 331, 357). From an immune-oncological point of view, long-term intake of quercetin was proved to improve natural killer cells’ cytotoxic activity, neutrophil chemotaxis, and lymphocyte proliferation (323, 358). In addition, quercetin induces T helper cells to produce TH1-derived interferon-gamma (IFN-γ) and downregulates TH2-derived IL-4 (327, 359). Altogether, it is quite evident that quercetin possesses potential intrinsic anticancer activity together with activating the innate and adaptive immune arms to halt oncological progression in several malignant contexts.
Similar to EGCG, quercetin has shown potent bactericidal properties against an array of bacteria, such as Enterococcus faecalis and Listeria monocytogenes. Both are resistant to several antibiotics and have a detrimental ability to produce biofilms on an artificial device such as stents. Quercetin was reported to effectively inhibit 95% of biofilm formation, also by stopping several glycolytic enzymes such as 2,3-bisphosphoglycerate-dependent phosphoglycerate mutase (GpmA) and ATP-dependent phosphofructokinase (PfkA) in L. monocytogenes. Quercetin also represses the secretion of the bacterial adhesion molecules that have a vital role in L. monocytogenes (foodborne illness bacteria) infection. Thus, the incorporation of quercetin as a food additive to minimize the adhesion, proliferation, and biofilm growth of the bacteria is a safe and economic idea (328). It is also important to note that quercetin was reported to inhibit half of the biofilm production by methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant S. aureus (VRSA), thus shedding light on its powerful antibacterial activity (328).
Quercetin possesses an immunomodulatory role through repressing platelet aggregation, lipid peroxidation, inhibition of pro-inflammatory mediators such as lipoxygenase and phospholipase A2, and the expression levels of MHG class II and co-stimulatory molecules. Digging deeper, it was found that attenuation of several canonical and non-canonical immunomodulatory pathways such as arachidonic acid metabolism, the associated leukotriene/prostaglandins, and mTOR signaling pathways are the molecular mechanisms by which quercetin possesses its immunomodulatory role in several contexts (360, 361).
Caffeine is one of the constituents of matcha; it has a strong antioxidant activity where it acts by neutralizing the ROS and it induces the antioxidant enzyme activities and also increases the glutathione levels, thus reducing oxidative stress. Besides the antioxidant effect, caffeine also has an anti-inflammatory activity where it reduces the secretion of pro-inflammatory cytokines. It was found that the caffeine content in matcha is greater than that in green tea, thus making matcha tea more effective (333, 334, 339).
Phenolic acids are found at their maximum levels in matcha tea. They are well known to have powerful antioxidant and anti-inflammatory effects as well as hypoglycemic and neuroprotective effects. Moreover, regulation of several metabolic disorders is controlled by some of the phenolic acids by regulating carbohydrates and lipid metabolisms (333, 338–341).
Rutin is a polyphenolic compound. Among all the kinds of tea available in the market, matcha contains very high amounts of rutin. It has several benefits such as antioxidant, antidiabetic, and anti-inflammatory effects (332, 342, 343, 362).
Chlorophyll is responsible for the bright green color of matcha. It was reported that it has powerful anti-inflammatory and antioxidant effects (314, 315). Nevertheless, the amino acid theanine provides the taste of matcha, which is distinctive and non-bitter. Also, it was found that the presence of caffeine with theanine improves efficiency rather than using them separately (335–337). Last but not least, the presence of vitamin C is also important and possesses several beneficial effects since it cannot be synthesized within the human body. It is considered a strong exogenous antioxidant, and it must be supplied via nutritional intake as it reinforces the immune system (363).
COVID-19 has made the world face a war with a different meaning this time—a war in which the entire world population are warriors, whose main slogan was “Stay Safe”, a war whose weapons consist of open-ended practical trials that take place in research labs to find a solution to finally end the war. On one hand, the hospitals were at full capacity, and the demand for oxygen supplies was increasing. On the other hand, this virus impacted the entire globe with deleterious effects economically. The mess was escalating.
The trials for combating this virus were numerous, and carried out with different aims, whether for drug repurposing or trying to develop new antiviral agents. Vaccine development was also one of the main goals of researchers, yet many other researchers have shed light on the use of herbal medicine. In silico, in vivo, and in vitro studies were conducted all with only one aim, which is to find a solution to solve this mess. In fact, none of the studies could underestimate the significance of the others, and all can work in harmony with each other or help each other to reach the main curative goal at the end.
Herbal medicine was one of the major routes that were investigated throughout this pandemic by many researchers as plants have been proven to be a miracle drug throughout the generations for combating many diseases, which also gave many researchers hope for defeating COVID-19. Nature has never failed to protect us, that is why the first routine that was followed since the start of this pandemic is to eat fruits and vegetables because of their potential to strengthen our immune system and act as a preventive measure. For this reason, it was not surprising that many review articles, clinical trials, and molecular docking studies investigated the antiviral potential of many plants against SARS-CoV-2, and many of them showed a strong potential to improve the pandemic situation as previously reviewed in (364–366).
However, there was another dark side to the story, and this darkness relies mainly on the ones who were suffering every minute whether for fear of catching the virus because they know how weak their bodies are to defeat this enemy or for the difficulties they would face to follow their treatment plans in the hospitals or clinics during this pandemic. These sufferers are mainly the cancer patients and immune-compromised patients such as those with SLE, RA, and MS who have higher mortality rates and exacerbated conditions upon exposure to the virus (367) compared to other normal individuals. Although vaccines seemed to be a proper solution, there are still limitations that should be taken into consideration as regards the efficacy of the vaccine for this type of patients as well as the possible interactions between both the vaccine and their treatments or the disease condition (367). Moreover, these patients needed to be tracked routinely for any signs of unexpected adverse events or if they are on active cancer therapy, so the relation between the timing of the vaccine and its safety and efficacy with the treatments and immune deficiency should be evaluated (368). Because of this, it was logical to think of herbal medicines as an option for these people due to their potential to defend against a myriad of viruses and strengthen the immune system, and certain herbs could have a role in attacking cancer as well when compared to vaccines or synthetic drugs.
In this review, we focused on the significant role of herbal medicines in helping cancer and immune-compromised patients. A spotlight was thrown on many plants such as ginger, turmeric, garlic, flaxseed, citrus fruits, Echinacea purpurea, Java turmeric, ashwagandha, and black tea. All of the plants highlighted in this review have proven their efficacy as anticancer, immunomodulatory, and antiviral agents; many of them already show an anti-COVID-19 potential. The combination of these three actions suggests herbal medicines as a good option for these patients. Yet, it is worth mentioning that most of the herbal products’ actions mentioned in this review are dose-dependent effects. For instance, it should be noted that garlic is an anticancer agent in several oncological contexts, but it is a source of organosulfur compounds, which are hydrogen sulfide donors (182). Hydrogen sulfide is a well-known biphasic gasotransmitter molecule that, at low concentrations, plays an oncogenic role while having an anticancer activity at higher concentration (183, 184).
One of the herbal plants that were discussed in this review was green tea, and while focusing on its constituents, which were mainly EGCG and quercetin, they were found to have very potent multiple mechanisms for defending against different cancer types, acting as immunomodulatory, anti-inflammatory, and antiviral agents specifically towards COVID-19. Matcha was able to obtain these protective properties in the highest possible amount.
Matcha is a Japanese green tea in which nowadays seems to be trendy in certain populations for its claimed ability to boost health and immunity, also it has been used recently in some of the cosmetic products for its ability to participate in a healthy skin conditions. Digging deeper in the Matcha constituents, we found that it contains EGCG and quercetin, the proven ones for their efficacy, in much concentrated amounts than in the normal green tea along with other constituents that were discussed as well in this review and as shown in Figure 6. This can explain the potential for that herbal tea in specific to be an indispensable way for cancer and immunocompromised individuals to protect themselves against COVID-19 along with alleviating their health states.
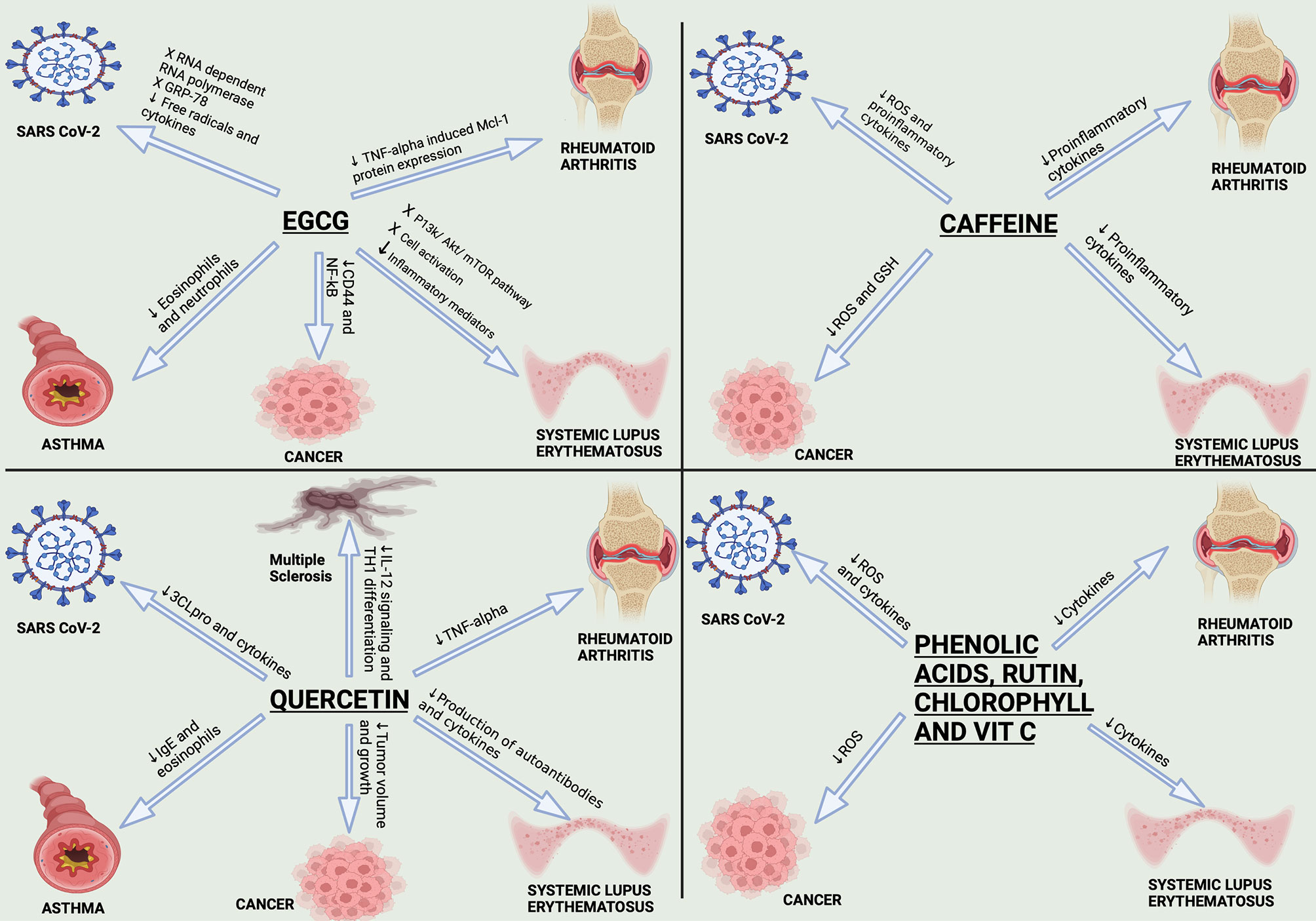
Figure 6 Matcha beneficial effects in protecting cancer and autoimmune patients from SARS-CoV-2 infection. This figure highlights the dynamic constituents of matcha and its beneficial effects in preventing SARS-CoV-2 infection and also ameliorating SARS-CoV-2-positive patients. A special focus on cancer and autoimmune patients is presented. Patients who have caught SARS-CoV-2 were found to have a decrease in IFNs; thus, EGCG stimulates the expression of both TLR4 and TLR2, and this helps in reducing the pro-inflammatory cytokines and the cytokine storm. Since the viral nucleic acid activates the RIG-1 that increases IFN-1, EGCG showed to act as a RIG-1 inhibitor. In addition, EGCG has an antioxidant effect by neutralizing the free radicals and boosting the detoxification enzymes. Moreover, quercetin is a potent immunomodulatory in SARS-CoV-2, as it has many functions such as antiviral activity, platelet aggregation inhibition, and inhibition of proinflammatory mediators like Lipoxygenase (LOX) and Phospholipase A2. Rutin has an antioxidant and anti-inflammatory activity; thus, it is useful in the management of COVID-19. The chlorophyll content in matcha has proven to be beneficial due to its anti-inflammatory and antioxidant effect, which assists to overcome the cytokine storm. Besides, caffeine has a potential anti-inflammatory effect by decreasing proinflammatory cytokines, and its antioxidant effect is due to reduction of ROS and increase of glutathione. In addition, Vitamin C greatly stimulates antiviral immune responses and reduces the lungs’ inflammatory state. It is essential to highlight that the intake of matcha tea not only will manage COVID-19 symptoms but also can prevent the virus itself from infecting humans.
This review suggests matcha as one of the potential options that should be highlighted during this period as it offers a great amount of potential to battling the current pandemic.
Since herbal medicines have always been able to tackle many health issues throughout the past pandemics and act as prophylaxis against a myriad of diseases, this encourages us to have a wider look in generalizing herbal medicines to the entire population. As reviewed, it has been documented that with the emergence of each new mutation, the efficacy of vaccines and drugs becomes negatively affected. Such information should encourage global health organizations to tackle this issue in a different way. More in-depth studies on herbal medicines need to be conducted using more clinical trials. In vivo and in vitro studies should be carried out for much more medicinal plants as well as in silico and molecular docking studies to further study and discover new effects for secondary metabolites. Such studies and clinical trials should also include the more susceptible populations such as cancer patients, immune disease patients, and children since they are not included in the current studies. Because of this, WHO will be encouraged to advise doctors about prescribing these herbal medicines along with other synthetic drugs if needed, and the media will also play a role as they will start to encourage people to use more herbal medicines and to make them aware of such medicines’ benefits. Consequently, this can lead to the discovery of more new plants and the investigation of new research areas by pharmaceutical companies in order to meet the market need. This can be a plan for any upcoming SARS-CoV-2 outbreak or for any new pandemic for either normal or ill people, and this plan will most probably succeed. One of the herbal medicines that is highlighted by this review for future use is matcha.
Such a wide range of therapeutic potentials ofwfi 2 “matcha” constituents whether as an immunomodulatory agent or as an anti-SARS-CoV-2 agent might be the reason why “matcha” would acquire a high market share in the upcoming years, especially since SARS-CoV-2 might behave like seasonal flu (after having more than eight waves to date). Nonetheless, we should not ignore the fact that more coronaviruses might appear at any time, since bats act as a reservoir or a storage tank for them. Collectively, this might highlight the potential of “matcha” to be the stone that could hit 3 birds (cancer, autoimmune disease, and SARS-CoV-2).
Although “matcha” seems to be very promising, there are a lot of challenges that may hinder its usage. For instance, “matcha” might not be accessible for many people due to its high price especially since we are focusing on cancer and autoimmune disease patients who already have very high expenses for their medications. Therefore, it is recommended to be produced in larger amounts for the sake of reducing its price, and this could be done by pharmaceutical companies. One of the major disadvantages that might discourage people to use “matcha” is its bad taste; however, this could be masked by the addition of flavors during the production phase, which again sheds light on the importance of pharmaceutical companies in “matcha” production. Therefore, this review elucidates the importance of having a cup of “matcha” to reinforce and strengthen the immune system in cancer and autoimmune disease patients who have a higher risk of catching SARS-CoV-2. Yet, this also can be generalized for everyone as it is powerful enough to prevent and protect them from catching the virus.
In conclusion, this review stresses the fact that the probability of the current pandemic to continue for a long time and the probability of developing future pandemics are extremely high, especially after the emergence of several VOCs. In this review, the authors highlight the great potential held by herbal medicine especially for high-risk patients such as cancer and autoimmune patients. Also, the authors shed light onto our new norm and how herbal products are considered risk-free solutions. In this review, it was set clear that after the SARS-CoV-2 pandemic experience, it should be noted that the development of new drugs and effective vaccines will not always be the easiest option. This review presents the current herbal medicines that could be used in preventing and fighting COVID-19, which happen to have three roles: as an immunomodulatory and anticancer agent, aside from displaying anti-SARS-CoV-2 activities. A special spotlight was turned on for the Japanese green tea “matcha”. The authors elucidating the promising use of matcha as a prophylactic agent during the SARS-CoV-2 pandemic can have a significant impact on the socioeconomic and health status in general and on cancer and autoimmune patients in particular. This was mainly based on their major constituents: EGCG and quercetin and their well-reported anticancer activity, immunomodulatory effects, and their recent anti-SARS-CoV-2 activity. Yet, more detailed studies about the usage of “matcha” among cancer and autoimmune patients have to be conducted in the future.
CK, MK, and MT contributed to drafting the original draft of the manuscript and data collection (literature reviewing) and sketching the figures. The conception of the work, critical revision of the article, and data interpretation were performed by the principal investigator of the work, RY. The final version of the manuscript was approved and revised by all the authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Cheng VC, Lau SK, Woo PC, Yuen KY. Severe Acute Respiratory Syndrome Coronavirus as an Agent of Emerging and Reemerging Infection. Clin Microbiol Rev (2007) 20(4):660–94. doi: 10.1128/CMR.00023-07
2. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical Features of Patients Infected With 2019 Novel Coronavirus in Wuhan, China. Lancet (2020) 395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5
3. Coronaviridae Study Group of the International Committee on Taxonomy of, V. The Species Severe Acute Respiratory Syndrome-Related Coronavirus, Classifying 2019-Ncov and Naming It SARS-CoV-2. Nat Microbiol (2020) 5(4):536–44. doi: 10.1038/s41564-020-0695-z
4. Woolf SH, Chapman DA, Lee JH. COVID-19 as the Leading Cause of Death in the United States. JAMA (2021) 325(2):123–4. doi: 10.1001/jama.2020.24865
5. Koh HK, Geller AC, VanderWeele TJ. Deaths From COVID-19. JAMA (2021) 325(2):133–4. doi: 10.1001/jama.2020.25381
6. Liu S, Zhi Y, Ying S. COVID-19 and Asthma, Reflection During the Pandemic. Clin Rev Allergy Immunol (2020) 59(1):78–88. doi: 10.1007/s12016-020-08797-3
7. Ramirez GA, Gerosa M, Beretta L, Bellocchi C, Argolini LM, Moroni L, et al. COVID-19 in Systemic Lupus Erythematosus, Data From a Survey on 417 Patients. Semin Arthritis Rheum (2020) 50(5):1150–7. doi: 10.1016/j.semarthrit.2020.06.012
8. Bsteh G, Bitschnau C, Hegen H, Auer M, Di Pauli F, Rommer P, et al. Multiple Sclerosis and COVID-19, How Many Are at Risk? Eur J Neurol (2020) 28(10):3369–74. doi: 10.1111/ene.14555
9. Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and Clinical Outcomes of COVID-19 in Patients With Autoimmune Diseases, a Systematic Review and Meta-Analysis. Ann Rheum Dis (2021) 80(3):384–91. doi: 10.1136/annrheumdis-2020-218946
10. Cortiula F, Pettke A, Bartoletti M, Puglisi F, Helleday T. Managing COVID-19 in the Oncology Clinic and Avoiding the Distraction Effect. Ann Oncol (2020) 31(5):553–5. doi: 10.1016/j.annonc.2020.03.286
11. Rogado J, Pangua C, Serrano-Montero G, Obispo B, Marino AM, Perez-Perez M, et al. Covid-19 and Lung Cancer, A Greater Fatality Rate? Lung Cancer (2020) 146:19–22. doi: 10.1016/j.lungcan.2020.05.034
12. Tian J, Yuan X, Xiao J, Zhong Q, Yang C, Liu B, et al. Clinical Characteristics and Risk Factors Associated With COVID-19 Disease Severity in Patients With Cancer in Wuhan, China, a Multicentre, Retrospective, Cohort Study. Lancet Oncol (2020) 21(7):893–903. doi: 10.1016/S1470-2045(20)30309-0
13. Yang K, Sheng Y, Huang C, Jin Y, Xiong N, Jiang K, et al. Clinical Characteristics, Outcomes, and Risk Factors for Mortality in Patients With Cancer and COVID-19 in Hubei, China, a Multicentre, Retrospective, Cohort Study. Lancet Oncol (2020) 21(7):904–13. doi: 10.1016/S1470-2045(20)30310-7
14. Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, et al. Clinical Characteristics of COVID-19-Infected Cancer Patients, a Retrospective Case Study in Three Hospitals Within Wuhan, China. Ann Oncol (2020) 31(7):894–901. doi: 10.1016/j.annonc.2020.03.296
15. Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients With Cancer Appear More Vulnerable to SARS-CoV-2, A Multicenter Study During the COVID-19 Outbreak. Cancer Discov (2020) 10(6):783–91. doi: 10.1158/2159-8290.CD-20-0422
16. Cavanna L, Citterio C, Toscani I, Franco C, Magnacavallo A, Caprioli S, et al. Cancer Patients With COVID-19, a Retrospective Study of 51 Patients in the District of Piacenza, Northern Italy. Future Sci OA (2020) 7(1):FSO645. doi: 10.2144/fsoa-2020-0157
17. Di Lorenzo G, Buonerba L, Ingenito C, Crocetto F, Buonerba C, Libroia A, et al. Clinical Characteristics of Metastatic Prostate Cancer Patients Infected With COVID-19 in South Italy. Oncology (2020) 98(10):743–7. doi: 10.1159/000509434
18. Pietrantonio F, Garassino MC. Caring for Patients With Cancer During the COVID-19 Outbreak in Italy. JAMA Oncol (2020) 6(6):821–2. doi: 10.1001/jamaoncol.2020.1426
19. Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 Transmission in Patients With Cancer at a Tertiary Care Hospital in Wuhan, China. JAMA Oncol (2020) 6(7):1108–10. doi: 10.1001/jamaoncol.2020.0980
20. Passaro A, Addeo A, Von Garnier C, Blackhall F, Planchard D, Felip E, et al. ESMO Management and Treatment Adapted Recommendations in the COVID-19 Era, Lung Cancer. ESMO Open (2020) 5(Suppl 3). doi: 10.1136/esmoopen-2020-000820
21. Gosain R, Abdou Y, Singh A, Rana N, Puzanov I, Ernstoff MS. COVID-19 and Cancer, a Comprehensive Review. Curr Oncol Rep (2020) 22(5):53. doi: 10.1007/s11912-020-00934-7
22. Askanase AD, Khalili L, Buyon JP. Thoughts on COVID-19 and Autoimmune Diseases. Lupus Sci Med (2020) 7(1):e000396. doi: 10.1136/lupus-2020-000396
23. Willis MD, Robertson NP. Multiple Sclerosis and the Risk of Infection, Considerations in the Threat of the Novel Coronavirus, COVID-19/SARS-CoV-2. J Neurol (2020) 267(5):1567–9. doi: 10.1007/s00415-020-09822-3
24. Fernandez-Ruiz R, Paredes JL, Niewold TB. COVID-19 in Patients With Systemic Lupus Erythematosus, Lessons Learned From the Inflammatory Disease. Transl Res (2021) 232:13–36. doi: 10.1016/j.trsl.2020.12.007
25. Mathian A, Mahevas M, Rohmer J, Roumier M, Cohen-Aubart F, Amador-Borrero B, et al. Clinical Course of Coronavirus Disease 2019 (COVID-19) in a Series of 17 Patients With Systemic Lupus Erythematosus Under Long-Term Treatment With Hydroxychloroquine. Ann Rheum Dis (2020) 79(6):837–9. doi: 10.1136/annrheumdis-2020-218795
26. Favalli EG, Gerosa M, Murgo A, Caporali R. Are Patients With Systemic Lupus Erythematosus at Increased Risk for COVID-19? Ann Rheum Dis (2021) 80(2):e25. doi: 10.1136/annrheumdis-2020-217787
27. Lefebvre M, Vignier N, Pitard B, Botelho-Nevers E, Wyplosz B, Cohen R, et al. COVID-19 Vaccines, Frequently Asked Questions and Updated Answers. Infect Dis Now (2021) 51(4):319–33. doi: 10.1016/j.idnow.2021.02.007
28. Kaptein SJF, Jacobs S, Langendries L, Seldeslachts L, Ter Horst S, Liesenborghs L, et al. Favipiravir at High Doses has Potent Antiviral Activity in SARS-CoV-2-Infected Hamsters, Whereas Hydroxychloroquine Lacks Activity. Proc Natl Acad Sci USA (2020) 117(43):26955–65. doi: 10.1073/pnas.2014441117
29. Liu J, Bodnar BH, Meng F, Khan AI, Wang X, Saribas S, et al. Epigallocatechin Gallate From Green Tea Effectively Blocks Infection of SARS-CoV-2 and New Variants by Inhibiting Spike Binding to ACE2 Receptor. Cell Biosci (2021) 11(1):168. doi: 10.1186/s13578-021-00680-8
30. Henss L, Auste A, Schürmann C, Schmidt C, von Rhein C, Mühlebach MD, et al. The Green Tea Catechin Epigallocatechin Gallate Inhibits SARS-CoV-2 Infection. J Gen Virol (2021) 102(4):001574. doi: 10.1099/jgv.0.001574
31. Bonuccelli G, Sotgia F, Lisanti MP. Matcha Green Tea (MGT) Inhibits the Propagation of Cancer Stem Cells (CSCs), by Targeting Mitochondrial Metabolism, Glycolysis and Multiple Cell Signalling Pathways. Aging (Albany NY) (2018) 10(8):1867–83. doi: 10.18632/aging.101483
32. Keckstein S, Tilgener C, Jeschke U, Hofmann S, Vilsmaier T, Kaltofen T, et al. Effects of Matcha Tea Extract on Cell Viability and Peroxisome Proliferator-Activated Receptor γ Expression on T47D Breast Cancer Cells. Arch Gynecol Obstet (2022). doi: 10.1007/s00404-021-06381-4
33. Westerlind H, Palmqvist I, Saevarsdottir S, Alfredsson L, Klareskog L, Di Giuseppe D. Is Tea Consumption Associated With Reduction of Risk of Rheumatoid Arthritis? A Swedish case-control study. Arthritis Res Ther (2021) 23(1):209. doi: 10.1186/s13075-021-02583-y
34. Saleh F, Raghupathy R, Asfar S, Oteifa M, Al-Saleh N. Analysis of the Effect of the Active Compound of Green Tea (EGCG) on the Proliferation of Peripheral Blood Mononuclear Cells. BMC Complement Altern Med (2014) 14:322. doi: 10.1186/1472-6882-14-322
35. Kirtipal N, Bharadwaj S, Kang SG. From SARS to SARS-CoV-2, Insights on Structure, Pathogenicity and Immunity Aspects of Pandemic Human Coronaviruses. Infect Genet Evol (2020) 85:104502. doi: 10.1016/j.meegid.2020.104502
36. Wang MY, Zhao R, Gao LJ, Gao XF, Wang DP, Cao JM. SARS-CoV-2: Structure, Biology, and Structure-Based Therapeutics Development. Front Cell Infect Microbiol (2020) 10:587269. doi: 10.3389/fcimb.2020.587269
37. Kadam SB, Sukhramani GS, Bishnoi P, Pable AA, Barvkar VT. SARS-CoV-2, the Pandemic Coronavirus, Molecular and Structural Insights. J Basic Microbiol (2021) 61(3):180–202. doi: 10.1002/jobm.202000537
38. Harrison AG, Lin T, Wang P. Mechanisms of SARS-CoV-2 Transmission and Pathogenesis. Trends Immunol (2020) 41(12):1100–15. doi: 10.1016/j.it.2020.10.004
39. Elhusseiny KM, Abd-Elhay FA, Kamel MG. Possible Therapeutic Agents for COVID-19, a Comprehensive Review. Expert Rev Anti Infect Ther (2020) 18(10):1005–20. doi: 10.1080/14787210.2020.1782742
40. Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and Chloroquine Effectively Inhibit the Recently Emerged Novel Coronavirus (2019-Ncov) In Vitro. Cell Res (2020) 30(3):269–71. doi: 10.1038/s41422-020-0282-0
41. Ferron F, Subissi L, Silveira De Morais AT, Le NTT, Sevajol M, Gluais L, et al. Structural and Molecular Basis of Mismatch Correction and Ribavirin Excision From Coronavirus RNA. Proc Natl Acad Sci USA (2018) 115(2):E162–71. doi: 10.1073/pnas.1718806115
42. Omrani AS, Saad MM, Baig K, Bahloul A, Abdul-Matin M, Alaidaroos AY, et al. Ribavirin and Interferon Alfa-2a for Severe Middle East Respiratory Syndrome Coronavirus Infection, a Retrospective Cohort Study. Lancet Infect Dis (2014) 14(11):1090–5. doi: 10.1016/S1473-3099(14)70920-X
43. Prakash A, Singh H, Kaur H, Semwal A, Sarma P, Bhattacharyya A, et al. Systematic Review and Meta-Analysis of Effectiveness and Safety of Favipiravir in the Management of Novel Coronavirus (COVID-19) Patients. Indian J Pharmacol (2020) 52(5):414–21. doi: 10.4103/ijp.ijp_998_20
44. Pilkington V, Pepperrell T, Hill A. A Review of the Safety of Favipiravir - a Potential Treatment in the COVID-19 Pandemic? J Virus Erad (2020) 6(2):45–51. doi: 10.1016/S2055-6640(20)30016-9
45. Lou Y, Liu L, Yao H, Hu X, Su J, Xu K, et al. Clinical Outcomes and Plasma Concentrations of Baloxavir Marboxil and Favipiravir in COVID-19 Patients, An Exploratory Randomized, Controlled Trial. Eur J Pharm Sci (2021) 157:105631. doi: 10.1016/j.ejps.2020.105631
46. Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med (2020) 383(19):1813–26. doi: 10.1056/NEJMc2022236
47. Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, et al. Remdesivir for 5 or 10 Days in Patients With Severe Covid-19. N Engl J Med (2020) 383(19):1827–37. doi: 10.1056/NEJMoa2015301
48. Mulangu S, Dodd LE, Davey RT Jr., Tshiani Mbaya O, Proschan M, Mukadi D, et al. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N Engl J Med (2019) 381(24):2293–303. doi: 10.1056/NEJMoa1910993
49. Jin Y, Yang H, Ji W, Wu W, Chen S, Zhang W, et al. Virology, Epidemiology, Pathogenesis, and Control of COVID-19. Viruses (2020) 12(4):372. doi: 10.3390/v12040372
50. Friess H, Kleeff J, Isenmann R, Malfertheiner P, Buchler MW. Adaptation of the Human Pancreas to Inhibition of Luminal Proteolytic Activity. Gastroenterology (1998) 115(2):388–96. doi: 10.1016/S0016-5085(98)70205-7
51. Chakraborty C, Sharma AR, Bhattacharya M, Agoramoorthy G, Lee SS. The Drug Repurposing for COVID-19 Clinical Trials Provide Very Effective Therapeutic Combinations, Lessons Learned From Major Clinical Studies. Front Pharmacol (2021) 12:704205. doi: 10.3389/fphar.2021.704205
52. Triant VA, Siedner MJ. Darunavir and Cardiovascular Risk, Evaluating the Data to Inform Clinical Care. J Infect Dis (2020) 221(4):498–500. doi: 10.1093/infdis/jiz482
53. Chen X, Yin YH, Zhang MY, Liu JY, Li R, Qu YQ. Investigating the Mechanism of ShuFeng JieDu Capsule for the Treatment of Novel Coronavirus Pneumonia (COVID-19) Based on Network Pharmacology. Int J Med Sci (2020) 17(16):2511–30. doi: 10.7150/ijms.46378
54. Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized With Severe Covid-19. N Engl J Med (2020) 382(19):1787–99. doi: 10.1056/NEJMc2008043
55. Saleh M, Gabriels J, Chang D, Fishbein J, Qiu M, Mountantonakis SE, et al. Safely Administering Potential QTc Prolonging Therapy Across a Large Health Care System in the COVID-19 Era. Circ Arrhythm Electrophysiol (2020) 13(11):e008937. doi: 10.1161/CIRCEP.120.008937
56. Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, et al. Risk of QT Interval Prolongation Associated With Use of Hydroxychloroquine With or Without Concomitant Azithromycin Among Hospitalized Patients Testing Positive for Coronavirus Disease 2019 (COVID-19). JAMA Cardiol (2020) 5(9):1036–41. doi: 10.1001/jamacardio.2020.1834
57. Chorin E, Wadhwani L, Magnani S, Stefano C, Chinitz LA, Jankelson L, et al. QT Interval Prolongation and Torsade De Pointes in Patients With COVID-19 Treated With Hydroxychloroquine/Azithromycin. Heart Rhythm (2020) 17(9):1425–33. doi: 10.1016/j.hrthm.2020.05.014
58. Touret F, de Lamballerie X. Of Chloroquine and COVID-19. Antiviral Res (2020) 177:104762. doi: 10.1016/j.antiviral.2020.104762
59. Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, et al. Hydroxychloroquine With or Without Azithromycin in Mild-To-Moderate Covid-19. N Engl J Med (2020) 383(21):2041–52. doi: 10.1056/NEJMx200021
60. Pepperrell T, Pilkington V, Owen A, Wang J, Hill AM. Review of Safety and Minimum Pricing of Nitazoxanide for Potential Treatment of COVID-19. J Virus Erad (2020) 6(2):52–60. doi: 10.1016/S2055-6640(20)30017-0
61. Nojomi M, Yassin Z, Keyvani H, Makiani MJ, Roham M, Laali A, et al. Effect of Arbidol (Umifenovir) on COVID-19, a Randomized Controlled Trial. BMC Infect Dis (2020) 20(1):954. doi: 10.1186/s12879-020-05698-w
62. Estacio RO, Jeffers BW, Hiatt WR, Biggerstaff SL, Gifford N, Schrier RW. The Effect of Nisoldipine as Compared With Enalapril on Cardiovascular Outcomes in Patients With Non-Insulin-Dependent Diabetes and Hypertension. N Engl J Med (1998) 338(10):645–52. doi: 10.1056/NEJM199803053381003
63. Aghili R, Honardoost M, Khamseh ME. COVID-19, Case Fatality and ACE2 Inhibitors Treatment Concerns in Patients With Comorbidities. Med J Islam Repub Iran (2020) 34:147. doi: 10.47176/mjiri.34.147
64. Lopes RD, Macedo AVS, de Barros ESPGM, Moll-Bernardes RJ, Dos Santos TM, Mazza L, et al. Effect of Discontinuing vs Continuing Angiotensin-Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers on Days Alive and Out of the Hospital in Patients Admitted With COVID-19, A Randomized Clinical Trial. JAMA (2021) 325(3):254–64. doi: 10.1001/jama.2020.25864
65. Abd El-Aziz TM, Al-Sabi A, Stockand JD. Human Recombinant Soluble ACE2 (Hrsace2) Shows Promise for Treating Severe COVID-19. Signal Transduct Target Ther (2020) 5(1):258. doi: 10.1038/s41392-020-00374-6
66. Ragia G, Manolopoulos VG. Inhibition of SARS-CoV-2 Entry Through the ACE2/TMPRSS2 Pathway, a Promising Approach for Uncovering Early COVID-19 Drug Therapies. Eur J Clin Pharmacol (2020) 76(12):1623–30. doi: 10.1007/s00228-020-02963-4
67. Casadevall A, Joyner MJ, Pirofski LA. A Randomized Trial of Convalescent Plasma for COVID-19-Potentially Hopeful Signals. JAMA (2020) 324(5):455–7. doi: 10.1001/jama.2020.10218
68. Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-Threatening COVID-19, A Randomized Clinical Trial. JAMA (2020) 324(5):460–70. doi: 10.1001/jama.2020.12607
69. van Griensven J, Edwards T, de Lamballerie X, Semple MG, Gallian P, Baize S, et al. Evaluation of Convalescent Plasma for Ebola Virus Disease in Guinea. N Engl J Med (2016) 374(1):33–42. doi: 10.1056/NEJMoa1511812
70. Dulipsingh L, Ibrahim D, Schaefer EJ, Crowell R, Diffenderfer MR, Williams K, et al. SARS-CoV-2 Serology and Virology Trends in Donors and Recipients of Convalescent Plasma. Transfus Apher Sci (2020) 59(6):102922. doi: 10.1016/j.transci.2020.102922
71. Singh TU, Parida S, Lingaraju MC, Kesavan M, Kumar D, Singh RK. Drug Repurposing Approach to Fight COVID-19. Pharmacol Rep (2020) 72(6):1479–508. doi: 10.1007/s43440-020-00155-6
72. Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, et al. Tocilizumab for the Treatment of Severe COVID-19 Pneumonia With Hyperinflammatory Syndrome and Acute Respiratory Failure, A Single Center Study of 100 Patients in Brescia, Italy. Autoimmun Rev (2020) 19(7):102568. doi: 10.1016/j.autrev.2020.102568
73. Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of Tocilizumab in Patients Hospitalized With Covid-19. N Engl J Med (2020) 383(24):2333–44. doi: 10.1056/NEJMoa2028836
74. Alam S, Kamal TB, Sarker MMR, Zhou JR, Rahman SMA, Mohamed IN, et al. Therapeutic Effectiveness and Safety of Repurposing Drugs for the Treatment of COVID-19, Position Standing in 2021. Front Pharmacol (2021) 12:659577. doi: 10.3389/fphar.2021.659577
75. Shiehzadegan S, Alaghemand N, Fox M, Venketaraman V. Analysis of the Delta Variant B.1.617.2 COVID-19. Clin Pract (2021) 11(4):778–84. doi: 10.3390/clinpract11040093
76. Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 Vaccines Against the B.1.617.2 (Delta) Variant. N Engl J Med (2021) 385(7):585–94. doi: 10.1056/NEJMoa2108891
77. He X, Hong W, Pan X, Lu G, Wei X. SARS-CoV-2 Omicron Variant: Characteristics and Prevention. MedComm (2020) (2021) 2(4):838–45. doi: 10.1002/mco2.110
78. Pilishvili T, Gierke R, Fleming-Dutra KE, Farrar JL, Mohr NM, Talan DA, et al. Effectiveness of mRNA Covid-19 Vaccine Among U.S. Health Care Personnel N Engl J Med (2021) 385(25):e90.
79. Lyons DM, Lauring AS. Mutation and Epistasis in Influenza Virus Evolution. Viruses (2018) 10(8):407. doi: 10.3390/v10080407
80. Mousa HA. Prevention and Treatment of Influenza, Influenza-Like Illness, and Common Cold by Herbal, Complementary, and Natural Therapies. J Evid Based Complement Altern Med (2017) 22(1):166–74. doi: 10.1177/2156587216641831
81. Parry H, McIlroy G, Bruton R, Damery S, Tyson G, Logan N, et al. Impaired Neutralisation of SARS-CoV-2 Delta Variant in Vaccinated Patients With B Cell Chronic Lymphocytic Leukaemia. J Hematol Oncol (2022) 15(1):3. doi: 10.1186/s13045-021-01219-7
82. Valanparambil R, Carlisle J, Linderman S, Akthar A, Millett RL, Lai L, et al. Antibody Response to SARS-CoV-2 mRNA Vaccine in Lung Cancer Patients, Reactivity to Vaccine Antigen and Variants of Concern. medRxiv (2022). doi: 10.1101/2022.01.03.22268599
83. Mirzaie A, Halaji M, Dehkordi FS, Ranjbar R, Noorbazargan H. A Narrative Literature Review on Traditional Medicine Options for Treatment of Corona Virus Disease 2019 (COVID-19). Complement Ther Clin Pract (2020) 40:101214. doi: 10.1016/j.ctcp.2020.101214
84. Ang L, Lee HW, Kim A, Lee JA, Zhang J, Lee MS. Herbal Medicine for Treatment of Children Diagnosed With COVID-19, A Review of Guidelines. Complement Ther Clin Pract (2020) 39:101174. doi: 10.1016/j.ctcp.2020.101174
85. Kabir MT, Uddin MS, Hossain MF, Abdulhakim JA, Alam MA, Ashraf GM, et al. nCOVID-19 Pandemic, From Molecular Pathogenesis to Potential Investigational Therapeutics. Front Cell Dev Biol (2020) 8. doi: 10.3389/fcell.2020.00616
86. Hossain MF, Hasana S, Mamun AA, Uddin MS, Wahed MII, Sarker S, et al. COVID-19 Outbreak, Pathogenesis, Current Therapies, and Potentials for Future Management. Front Pharmacol (2020) 11. doi: 10.3389/fphar.2020.563478
87. Kumar R, Srivastava JK, Singh R, Siddiqui MH, Mansouri RA, Abdulhakim JA, et al. Available Compounds With Therapeutic Potential Against COVID-19, Antimicrobial Therapies, Supportive Care, and Probable Vaccines. Front Pharmacol (2020) 11. doi: 10.3389/fphar.2020.582025
88. Masoud AT, Zaazouee MS, Elsayed SM, Ragab KM, Kamal EM, Alnasser YT, et al. KAP-COVIDGLOBAL, a Multinational Survey of the Levels and Determinants of Public Knowledge, Attitudes and Practices Towards COVID-19. BMJ Open (2021) 11(2):e043971. doi: 10.1136/bmjopen-2020-043971
89. Huang J, Tao G, Liu J, Cai J, Huang Z, Chen JX. Current Prevention of COVID-19, Natural Products and Herbal Medicine. Front Pharmacol (2020) 11:588508. doi: 10.3389/fphar.2020.588508
90. Nugraha RV, Ridwansyah H, Ghozali M, Khairani AF, Atik N. Traditional Herbal Medicine Candidates as Complementary Treatments for COVID-19, A Review of Their Mechanisms, Pros and Cons. Evid Based Complement Alternat Med (2020) 2020:2560645. doi: 10.1155/2020/2560645
91. Prasad S, Tyagi AK. Ginger and Its Constituents, Role in Prevention and Treatment of Gastrointestinal Cancer. Gastroenterol Res Pract (2015) 2015:142979. doi: 10.1155/2015/142979
92. Mojaverrostami S, Bojnordi MN, Ghasemi-Kasman M, Ebrahimzadeh MA, Hamidabadi HG. A Review of Herbal Therapy in Multiple Sclerosis. Adv Pharm Bull (2018) 8(4):575–90. doi: 10.15171/apb.2018.066
93. Ali BH, Blunden G, Tanira MO, Nemmar A. Some Phytochemical, Pharmacological and Toxicological Properties of Ginger (Zingiber Officinale Roscoe), a Review of Recent Research. Food Chem Toxicol (2008) 46(2):409–20. doi: 10.1016/j.fct.2007.09.085
94. Ho SC, Chang KS, Lin CC. Anti-Neuroinflammatory Capacity of Fresh Ginger Is Attributed Mainly to 10-Gingerol. Food Chem (2013) 141(3):3183–91. doi: 10.1016/j.foodchem.2013.06.010
95. Haridas M, Sasidhar V, Nath P, Abhithaj J, Sabu A, Rammanohar P. Compounds of Citrus Medica and Zingiber Officinale for COVID-19 Inhibition, in Silico Evidence for Cues From Ayurveda. Futur J Pharm Sci (2021) 7(1):13. doi: 10.1186/s43094-020-00171-6
96. Giordano A, Tommonaro G. Curcumin and Cancer. Nutrients (2019) 11(10):2376. doi: 10.3390/nu11102376
97. Zheng Z, Sun Y, Liu Z, Zhang M, Li C, Cai H. The Effect of Curcumin and Its Nanoformulation on Adjuvant-Induced Arthritis in Rats. Drug Des Devel Ther (2015) 9:4931–42. doi: 10.2147/DDDT.S90147
98. Xue M, McKelvey K, Shen K, Minhas N, March L, Park SY, et al. Endogenous MMP-9 and Not MMP-2 Promotes Rheumatoid Synovial Fibroblast Survival, Inflammation and Cartilage Degradation. Rheumatol (Oxford) (2014) 53(12):2270–9. doi: 10.1093/rheumatology/keu254
99. Aggarwal BB, Gupta SC, Sung B. Curcumin, an Orally Bioavailable Blocker of TNF and Other Pro-Inflammatory Biomarkers. Br J Pharmacol (2013) 169(8):1672–92. doi: 10.1111/bph.12131
100. Shakibaei M, John T, Schulze-Tanzil G, Lehmann I, Mobasheri A, et al. Suppression of NF-KappaB Activation by Curcumin Leads to Inhibition of Expression of Cyclo-Oxygenase-2 and Matrix Metalloproteinase-9 in Human Articular Chondrocytes, Implications for the Treatment of Osteoarthritis. Biochem Pharmacol (2007) 73(9):1434–45. doi: 10.1016/j.bcp.2007.01.005
101. Natarajan C, Bright JJ. Curcumin Inhibits Experimental Allergic Encephalomyelitis by Blocking IL-12 Signaling Through Janus Kinase-STAT Pathway in T Lymphocytes. J Immunol (2002) 168(12):6506–13. doi: 10.4049/jimmunol.168.12.6506
102. Moore BA, Aznavoorian S, Engler JA, Windsor LJ. Induction of Collagenase-3 (MMP-13) in Rheumatoid Arthritis Synovial Fibroblasts. Biochim Biophys Acta (2000) 1502(2):307–18. doi: 10.1016/S0925-4439(00)00056-9
103. Jin H, Qiao F, Wang Y, Xu Y, Shang Y. Curcumin Inhibits Cell Proliferation and Induces Apoptosis of Human Non-Small Cell Lung Cancer Cells Through the Upregulation of miR-192-5p and Suppression of PI3K/Akt Signaling Pathway. Oncol Rep (2015) 34(5):2782–9. doi: 10.3892/or.2015.4258
104. Babaei F, Nassiri-Asl M, Hosseinzadeh H. Curcumin (a Constituent of Turmeric), New Treatment Option Against COVID-19. Food Sci Nutr (2020) 8(10):5215–27. doi: 10.1002/fsn3.1858
105. Zhou X, Qian H, Zhang D, Zeng L. Garlic Intake and the Risk of Colorectal Cancer, A Meta-Analysis. Med (Baltimore) (2020) 99(1):e18575. doi: 10.1097/MD.0000000000018575
106. Tsubura A, Lai YC, Kuwata M, Uehara N, Yoshizawa K. Anticancer Effects of Garlic and Garlic-Derived Compounds for Breast Cancer Control. Anticancer Agents Med Chem (2011) 11(3):249–53. doi: 10.2174/187152011795347441
107. Lamm DL, Riggs DR. The Potential Application of Allium Sativum (Garlic) for the Treatment of Bladder Cancer. Urol Clin North Am (2000) 27(1):157–62, xi. doi: 10.1016/S0094-0143(05)70243-3
108. Ryu HW, Lee SU, Lee S, Song HH, Son TH, Kim YU, et al. 3-Methoxy-Catalposide Inhibits Inflammatory Effects in Lipopolysaccharide-Stimulated RAW264.7 Macrophages. Cytokine (2017) 91:57–64. doi: 10.1016/j.cyto.2016.12.006
109. Arreola R, Quintero-Fabian S, Lopez-Roa RI, Flores-Gutierrez EO, Reyes-Grajeda JP, Carrera-Quintanar L, et al. Immunomodulation and Anti-Inflammatory Effects of Garlic Compounds. J Immunol Res (2015) 2015: 401630. doi: 10.1155/2015/401630
110. Quintero-Fabian S, Ortuno-Sahagun D, Vazquez-Carrera M, Lopez-Roa RI. Alliin, a Garlic (Allium Sativum) Compound, Prevents LPS-Induced Inflammation in 3T3-L1 Adipocytes. Mediators Inflamm (2013) 2013:381815. doi: 10.1155/2013/381815
111. Lei XY, Yao SQ, Zu XY, Huang ZX, Liu LJ, Zhong M, et al. Apoptosis Induced by Diallyl Disulfide in Human Breast Cancer Cell Line MCF-7. Acta Pharmacol Sin (2008) 29(10):1233–9. doi: 10.1111/j.1745-7254.2008.00851.x
112. Altonsy MO, Habib TN, Andrews SC. Diallyl Disulfide-Induced Apoptosis in a Breast-Cancer Cell Line (MCF-7) may be Caused by Inhibition of Histone Deacetylation. Nutr Cancer (2012) 64(8):1251–60. doi: 10.1080/01635581.2012.721156
113. Shekh S, Reddy KKA, Gowd KH. In Silico Allicin Induced S-Thioallylation of SARS-CoV-2 Main Protease. J Sulfur Chem (2021) 42(1):109–20. doi: 10.1080/17415993.2020.1817457
114. Tannous S, Haykal T, Dhaini J, Hodroj MH, Rizk S. The Anti-Cancer Effect of Flaxseed Lignan Derivatives on Different Acute Myeloid Leukemia Cancer Cells. BioMed Pharmacother (2020) 132:110884. doi: 10.1016/j.biopha.2020.110884
115. Constantin MM, Nita IE, Olteanu R, Constantin T, Bucur S, Matei C, et al. Significance and Impact of Dietary Factors on Systemic Lupus Erythematosus Pathogenesis. Exp Ther Med (2019) 17(2):1085–90. doi: 10.3892/etm.2018.6986
116. Hathaway D, Pandav K, Patel M, Riva-Moscoso A, Singh BM, Patel A, et al. Omega 3 Fatty Acids and COVID-19, A Comprehensive Review. Infect Chemother (2020) 52(4):478–95. doi: 10.3947/ic.2020.52.4.478
117. Cheng FJ, Huynh TK, Yang CS, Hu DW, Shen YC, Tu CY, et al. Hesperidin Is a Potential Inhibitor Against SARS-CoV-2 Infection. Nutrients (2021) 13(8):2800. doi: 10.3390/nu13082800
118. Haghmorad D, Mahmoudi MB, Salehipour Z, Jalayer Z, Momtazi Brojeni AA, Rastin M, et al. Hesperidin Ameliorates Immunological Outcome and Reduces Neuroinflammation in the Mouse Model of Multiple Sclerosis. J Neuroimmunol (2017) 302:23–33. doi: 10.1016/j.jneuroim.2016.11.009
119. Wei D, Ci X, Chu X, Wei M, Hua S, Deng X, et al. Hesperidin Suppresses Ovalbumin-Induced Airway Inflammation in a Mouse Allergic Asthma Model. Inflammation (2012) 35(1):114–21. doi: 10.1007/s10753-011-9295-7
120. Abdallah RM, Elkhouly AM, Soliman RA, El Mechawy N, El Sebaei A, Motaal AA, et al. Hindering the Synchronization Between miR-486-5p and H19 lncRNA by Hesperetin Halts Breast Cancer Aggressiveness Through Tuning ICAM-1. Anticancer Agents Med Chem (2022) 22(3):586–95. doi: 10.2174/1871520621666210419093652
121. Pan H, Wang F, Rankin GO, Rojanasakul Y, Tu Y, Chen YC. Inhibitory Effect of Black Tea Pigments, Theaflavin3/3’-Gallate Against Cisplatin-Resistant Ovarian Cancer Cells by Inducing Apoptosis and G1 Cell Cycle Arrest. Int J Oncol (2017) 51(5):1508–20. doi: 10.3892/ijo.2017.4145
122. Way TD, Lee HH, Kao MC, Lin JK. Black Tea Polyphenol Theaflavins Inhibit Aromatase Activity and Attenuate Tamoxifen Resistance in HER2/neu-Transfected Human Breast Cancer Cells Through Tyrosine Kinase Suppression. Eur J Cancer (2004) 40(14):2165–74. doi: 10.1016/j.ejca.2004.06.018
123. Gao Y, Rankin GO, Tu Y, Chen YC. Theaflavin-3, 3’-Digallate Decreases Human Ovarian Carcinoma OVCAR-3 Cell-Induced Angiogenesis via Akt and Notch-1 Pathways, Not via MAPK Pathways. Int J Oncol (2016) 48(1):281–92. doi: 10.3892/ijo.2015.3257
124. Liu W, Li J. Theaflavin-3, 3’-Digallate Attenuates Rheumatoid Inflammation in Mice Through the Nuclear Factor-kappaB and MAPK Pathways. Arch Immunol Ther Exp (Warsz) (2019) 67(3):153–60. doi: 10.1007/s00005-019-00536-7
125. Banerjee A, Kanwar M, Maiti S. Theaflavin-3’-O-Gallate a Black-Tea Constituent Blocked SARS CoV-2 RNA Dependant RNA Polymerase Active-Site With Better Docking Results Than Remdesivir. Drug Res (Stuttg) (2021) 71(8):462–72. doi: 10.1055/a-1467-5828
126. Almatroodi SA, Almatroudi A, Khan AA, Alhumaydhi FA, Alsahli MA, Rahmani AH. Potential Therapeutic Targets of Epigallocatechin Gallate (EGCG), the Most Abundant Catechin in Green Tea, and Its Role in the Therapy of Various Types of Cancer. Molecules (2020) 25(14):3146. doi: 10.3390/molecules25143146
127. Rauf A, Imran M, Khan IA, Ur-Rehman M, Gilani SA, Mehmood Z, et al. Anticancer Potential of Quercetin, A Comprehensive Review. Phytother Res (2018) 32(11):2109–30. doi: 10.1002/ptr.6155
128. Li QS, Wang YQ, Liang YR, Lu JL. The Anti-Allergic Potential of Tea, a Review of Its Components, Mechanisms and Risks. Food Funct (2021) 12(1):57–69. doi: 10.1039/D0FO02091E
129. Maurya AK, Vinayak M. Anticarcinogenic Action of Quercetin by Downregulation of Phosphatidylinositol 3-Kinase (PI3K) and Protein Kinase C (PKC) via Induction of P53 in Hepatocellular Carcinoma (HepG2) Cell Line. Mol Biol Rep (2015) 42(9):1419–29. doi: 10.1007/s11033-015-3921-7
130. Guan X, Gao M, Xu H, Zhang C, Liu H, Lv L, et al. Quercetin-Loaded Poly (Lactic-Co-Glycolic Acid)-D-Alpha-Tocopheryl Polyethylene Glycol 1000 Succinate Nanoparticles for the Targeted Treatment of Liver Cancer. Drug Delivery (2016) 23(9):3307–18. doi: 10.1080/10717544.2016.1176087
131. Ghosh R, Chakraborty A, Biswas A, Chowdhuri S. Evaluation of Green Tea Polyphenols as Novel Corona Virus (SARS CoV-2) Main Protease (Mpro) Inhibitors - an in Silico Docking and Molecular Dynamics Simulation Study. J Biomol Struct Dyn (2021) 39(12):4362–74. doi: 10.1080/07391102.2020.1779818
132. Derosa G, Maffioli P, D’Angelo A, Di Pierro F. A Role for Quercetin in Coronavirus Disease 2019 (COVID-19). Phytother Res (2021) 35(3):1230–6. doi: 10.1002/ptr.6887
133. Brinker AM, Ma J, Lipsky PE, Raskin I. Medicinal Chemistry and Pharmacology of Genus Tripterygium (Celastraceae). Phytochemistry (2007) 68(6):732–66. doi: 10.1016/j.phytochem.2006.11.029
134. Kiviharju TM, Lecane PS, Sellers RG, Peehl DM. Antiproliferative and Proapoptotic Activities of Triptolide (PG490), a Natural Product Entering Clinical Trials, on Primary Cultures of Human Prostatic Epithelial Cells. Clin Cancer Res (2002) 8(8):2666–74.
135. Zhu W, Li J, Wu S, Li S, Le L, Su X, et al. Triptolide Cooperates With Cisplatin to Induce Apoptosis in Gemcitabine-Resistant Pancreatic Cancer. Pancreas (2012) 41(7):1029–38. doi: 10.1097/MPA.0b013e31824abdc0
136. Gong Y, Huang X, Wang D, Li M, Liu Z. Triptolide Protects Bone Against Destruction by Targeting RANKL-Mediated ERK/AKT Signalling Pathway in the Collagen-Induced Rheumatoid Arthritis. Biomed Res (2017) 28(9):4111–6.
137. Teixeira A, DaCunha DC, Barros L, Caires HR, Xavier CPR, Ferreira I, et al. Eucalyptus Globulus Labill. Decoction Extract Inhibits the Growth of NCI-H460 Cells by Increasing the P53 Levels and Altering the Cell Cycle Profile. Food Funct (2019) 10(6):3188–97. doi: 10.1039/C8FO02466A
138. Greiner JF, Muller J, Zeuner MT, Hauser S, Seidel T, Klenke C, et al. 1,8-Cineol Inhibits Nuclear Translocation of NF-kappaB P65 and NF-kappaB-Dependent Transcriptional Activity. Biochim Biophys Acta (2013) 1833(12):2866–78. doi: 10.1016/j.bbamcr.2013.07.001
139. Panikar S, Shoba G, Arun M, Sahayarayan JJ, Usha Raja Nanthini A, Chinnathambi A, et al. Essential Oils as an Effective Alternative for the Treatment of COVID-19, Molecular Interaction Analysis of Protease (M(pro)) With Pharmacokinetics and Toxicological Properties. J Infect Public Health (2021) 14(5):601–10. doi: 10.1016/j.jiph.2020.12.037
140. Signer J, Jonsdottir HR, Albrich WC, Strasser M, Züst R, Ryter S, et al. In Vitro Virucidal Activity of Echinaforce®, an Echinacea Purpurea Preparation, Against Coronaviruses, Including Common Cold Coronavirus 229E and SARS-CoV-2. Virol J (2020) 17(1):136. doi: 10.1186/s12985-020-01401-2
141. Shree P, Mishra P, Selvaraj C, Singh SK, Chaube R, Garg N, et al. Targeting COVID-19 (SARS-CoV-2) Main Protease Through Active Phytochemicals of Ayurvedic Medicinal Plants - Withania Somnifera (Ashwagandha), Tinospora Cordifolia (Giloy) and Ocimum Sanctum (Tulsi) - a Molecular Docking Study. J Biomol Struct Dyn (2022) 40(1):190–203. doi: 10.1080/07391102.2020.1810778
142. Kumar V, Dhanjal JK, Bhargava P, Kaul A, Wang J, Zhang H, et al. Withanone and Withaferin-A Are Predicted to Interact With Transmembrane Protease Serine 2 (TMPRSS2) and Block Entry of SARS-CoV-2 Into Cells. J Biomol Struct Dyn (2022) 40(1):1–13. doi: 10.1080/07391102.2020.1775704
143. Rocha FAC, de Assis MR. Curcumin as a Potential Treatment for COVID-19. Phytother Res (2020) 34(9):2085–7. doi: 10.1002/ptr.6745
144. Messina G, Polito R, Monda V, Cipolloni L, Di Nunno N, Di Mizio G, et al. Functional Role of Dietary Intervention to Improve the Outcome of COVID-19, A Hypothesis of Work. Int J Mol Sci (2020) 21(9):3104. doi: 10.3390/ijms21093104
145. Wu C, Liu Y, Yang Y, Zhang P, Zhong W, Wang Y, et al. Analysis of Therapeutic Targets for SARS-CoV-2 and Discovery of Potential Drugs by Computational Methods. Acta Pharm Sin B (2020) 10(5):766–88. doi: 10.1016/j.apsb.2020.02.008
146. Yadav PK, Jaiswal A, Singh RK. In Silico Study on Spice-Derived Antiviral Phytochemicals Against SARS-CoV-2 TMPRSS2 Target. J Biomol Struct Dyn (2021) p:1–11. doi: 10.1080/07391102.2021.1965658
147. Oluyori AP, Olanipekun BE, Adeyemi OS, Egharevba GO, Adegboyega AE, Oladeji OS. Pharmacophore Modelling, MD Simulation and in Silico ADMET Study Reveals Bitter Cola Constituents as Potential Inhibitors of SARS-CoV-2 Main Protease and RNA Dependent-RNA Polymerase. J Biomol Struct Dyn (2022) p:1–16. doi: 10.1080/07391102.2021.2024883
148. Nguyen TT, Woo HJ, Kang HK, Nguyen VD, Kim YM, Kim DW, et al. Flavonoid-Mediated Inhibition of SARS Coronavirus 3C-Like Protease Expressed in Pichia Pastoris. Biotechnol Lett (2012) 34(5):831–8. doi: 10.1007/s10529-011-0845-8
149. Perricone C, Bartoloni E, Gerli R. Colchicine, an Anti-Rheumatic Agent, as a Potential Compound for the Treatment of COVID-19. Reumatologia (2020) 58(5):261–4. doi: 10.5114/reum.2020.100088
150. Kim JW, Ha TK, Cho H, Kim E, Shim SH, Yang JL, et al. Antiviral Escin Derivatives From the Seeds of Aesculus Turbinata Blume (Japanese Horse Chestnut). Bioorg Med Chem Lett (2017) 27(13):3019–25. doi: 10.1016/j.bmcl.2017.05.022
151. Leung JM, Yang CX, Sin DD. COVID-19 and Nicotine as a Mediator of ACE-2. Eur Respir J (2020) 55(6):2001261. doi: 10.1183/13993003.01261-2020
152. Alam S, Sarker MMR, Afrin S, Richi FT, Zhao C, Zhou JR, et al. Traditional Herbal Medicines, Bioactive Metabolites, and Plant Products Against COVID-19, Update on Clinical Trials and Mechanism of Actions. Front Pharmacol (2021) 12:671498. doi: 10.3389/fphar.2021.671498
153. Xia J, Rong L, Sawakami T, Inagaki Y, Song P, Hasegawa K, et al. Shufeng Jiedu Capsule and Its Active Ingredients Induce Apoptosis, Inhibit Migration and Invasion, and Enhances Doxorubicin Therapeutic Efficacy in Hepatocellular Carcinoma. BioMed Pharmacother (2018) 99:921–30. doi: 10.1016/j.biopha.2018.01.163
154. Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, et al. SARS-CoV-2 and Viral Sepsis, Observations and Hypotheses. Lancet (2020) 395(10235):1517–20. doi: 10.1016/S0140-6736(20)30920-X
155. Zhong W-T, Wu Y-C, Xie X-X, Zhou X, Wei M-M, Soromou L-W, et al. Phillyrin Attenuates LPS-Induced Pulmonary Inflammation via Suppression of MAPK and NF-κb Activation in Acute Lung Injury Mice. Fitoterapia (2013) 90:132–9. doi: 10.1016/j.fitote.2013.06.003
156. Zheng X-Y, Yang S-M, Zhang R, Wang S-M, Li G-B, Zhou S-W. Emodin-Induced Autophagy Against Cell Apoptosis Through the PI3K/AKT/mTOR Pathway in Human Hepatocytes. Drug design Dev Ther (2019) 13:3171. doi: 10.2147/DDDT.S204958
157. Xia L, Shi Y, Su J, Friedemann T, Tao Z, Lu Y, et al. Shufeng Jiedu, a Promising Herbal Therapy for Moderate COVID-19:Antiviral and Anti-Inflammatory Properties, Pathways of Bioactive Compounds, and a Clinical Real-World Pragmatic Study. Phytomedicine (2021) 85:153390. doi: 10.1016/j.phymed.2020.153390
158. Xiao Q, Jiang Y, Wu S. Analysis of the Value of Traditional Chinese Medicine Shufeng Jiedu Capsule Combined With Arbidol in the Treatment of Mild New Coronavirus Pneumonia. Emergency (2020) 29:756–8.
159. Qu X-K. Observation on Clinical Effect of Shufeng Jiedu Capsule Combined With Arbidol Hydrochloride Capsule in Treatment of COVID-19. Chin Traditional Herbal Drugs (2020) p:1167–70.
160. Park EJ, Pezzuto JM. Botanicals in Cancer Chemoprevention. Cancer Metastasis Rev (2002) 21(3-4):231–55. doi: 10.1023/A:1021254725842
161. Li H, Liu Y, Luo D, Ma Y, Zhang J, Li M, et al. Ginger for Health Care: An Overview of Systematic Reviews. Complement Ther Med (2019) 45:114–23. doi: 10.1016/j.ctim.2019.06.002
162. Jolad SD, Lantz RC, Solyom AM, Chen GJ, Bates RB, Timmermann BN. Fresh Organically Grown Ginger (Zingiber Officinale), Composition and Effects on LPS-Induced PGE2 Production. Phytochemistry (2004) 65(13):1937–54. doi: 10.1016/j.phytochem.2004.06.008
163. Rondanelli M, Fossari F, Vecchio V, Gasparri C, Peroni G, Spadaccini D, et al. Clinical Trials on Pain Lowering Effect of Ginger, A Narrative Review. Phytother Res (2020) 34(11):2843–56. doi: 10.1002/ptr.6730
164. Tripathi S, Maier KG, Bruch D, Kittur DS. Effect of 6-Gingerol on Pro-Inflammatory Cytokine Production and Costimulatory Molecule Expression in Murine Peritoneal Macrophages. J Surg Res (2007) 138(2):209–13. doi: 10.1016/j.jss.2006.07.051
165. Aryaeian N, Shahram F, Mahmoudi M, Tavakoli H, Yousefi B, Arablou T, et al. The Effect of Ginger Supplementation on Some Immunity and Inflammation Intermediate Genes Expression in Patients With Active Rheumatoid Arthritis. Gene (2019) 698:179–85. doi: 10.1016/j.gene.2019.01.048
166. Cifci A, Tayman C, Yakut HI, Halil H, Cakir E, Cakir U, et al. Ginger (Zingiber Officinale) Prevents Severe Damage to the Lungs Due to Hyperoxia and Inflammation. Turk J Med Sci (2018) 48(4):892–900. doi: 10.3906/sag-1803-223
167. Hewlings SJ, Kalman DS. Curcumin, A Review of Its Effects on Human Health. Foods (2017) 6(10):92. doi: 10.3390/foods6100092
168. Kocaadam B, Sanlier N. Curcumin, an Active Component of Turmeric (Curcuma Longa), and Its Effects on Health. Crit Rev Food Sci Nutr (2017) 57(13):2889–95. doi: 10.1080/10408398.2015.1077195
169. Gupta SC, Sung B, Kim JH, Prasad S, Li S, Aggarwal BB. Multitargeting by Turmeric, the Golden Spice, From Kitchen to Clinic. Mol Nutr Food Res (2013) 57(9):1510–28. doi: 10.1002/mnfr.201100741
170. Nair A, Amalraj A, Jacob J, Kunnumakkara AB, Gopi S. Non-Curcuminoids From Turmeric and Their Potential in Cancer Therapy and Anticancer Drug Delivery Formulations. Biomolecules (2019) 9(1):13. doi: 10.3390/biom9010013
171. Yeung KS, Gubili J, Mao JJ. Herb-Drug Interactions in Cancer Care. Oncol (Williston Park) (2018) 32(10):516–20.
172. Devassy JG, Nwachukwu ID, Jones PJ. Curcumin and Cancer, Barriers to Obtaining a Health Claim. Nutr Rev (2015) 73(3):155–65. doi: 10.1093/nutrit/nuu064
173. He Y, Yue Y, Zheng X, Zhang K, Chen S, Du Z. Curcumin, Inflammation, and Chronic Diseases, How Are They Linked? Molecules (2015) 20(5):9183–213. doi: 10.3390/molecules20059183
174. Catanzaro M, Corsini E, Rosini M, Racchi M, Lanni C. Immunomodulators Inspired by Nature, A Review on Curcumin and Echinacea. Molecules (2018) 23(11):2778. doi: 10.3390/molecules23112778
175. Srivastava RM, Singh S, Dubey SK, Misra K, Khar A. Immunomodulatory and Therapeutic Activity of Curcumin. Int Immunopharmacol (2011) 11(3):331–41. doi: 10.1016/j.intimp.2010.08.014
176. Zhang H, Penninger JM, Li Y, Zhong N, Slutsky AS. Angiotensin-Converting Enzyme 2 (ACE2) as a SARS-CoV-2 Receptor, Molecular Mechanisms and Potential Therapeutic Target. Intensive Care Med (2020) 46(4):586–90. doi: 10.1007/s00134-020-05985-9
177. Pang XF, Zhang LH, Bai F, Wang NP, Garner RE, McKallip RJ, et al. Attenuation of Myocardial Fibrosis With Curcumin Is Mediated by Modulating Expression of Angiotensin II AT1/AT2 Receptors and ACE2 in Rats. Drug Des Devel Ther (2015) 9:6043–54. doi: 10.2147/DDDT.S95333
178. Jia HP, Look DC, Shi L, Hickey M, Pewe L, Netland J, et al. ACE2 Receptor Expression and Severe Acute Respiratory Syndrome Coronavirus Infection Depend on Differentiation of Human Airway Epithelia. J Virol (2005) 79(23):14614–21. doi: 10.1128/JVI.79.23.14614-14621.2005
179. De Greef D, Barton EM, Sandberg EN, Croley CR, Pumarol J, Wong TL, et al. Anticancer Potential of Garlic and Its Bioactive Constituents, A Systematic and Comprehensive Review. Semin Cancer Biol (2021) 73:219–64. doi: 10.1016/j.semcancer.2020.11.020
180. El-Saber Batiha G, Magdy Beshbishy A, Wasef LG, Elewa YHA, Al-Sagan AA, Abd El-Hack ME, et al. Chemical Constituents and Pharmacological Activities of Garlic (Allium Sativum L.), A Review. Nutrients (2020) 12(3):872. doi: 10.3390/nu12030872
181. Percival SS. Aged Garlic Extract Modifies Human Immunity. J Nutr (2016) 146(2):433S–6S. doi: 10.3945/jn.115.210427
182. Yagdi E, Cerella C, Dicato M, Diederich M. Garlic-Derived Natural Polysulfanes as Hydrogen Sulfide Donors, Friend or Foe? Food Chem Toxicol (2016) 95:219–33. doi: 10.1016/j.fct.2016.07.016
183. Youness RA, Gad AZ, Sanber K, Ahn YJ, Lee GJ, Khallaf E, et al. Targeting Hydrogen Sulphide Signaling in Breast Cancer. J Adv Res (2021) 27:177–90. doi: 10.1016/j.jare.2020.07.006
184. Youness RA, Assal RA, Abdel Motaal A, Gad MZ. A Novel Role of sONE/NOS3/NO Signaling Cascade in Mediating Hydrogen Sulphide Bilateral Effects on Triple Negative Breast Cancer Progression. Nitric Oxide (2018) 80:12–23. doi: 10.1016/j.niox.2018.07.004
185. Hsieh CC, Peng WH, Tseng HH, Liang SY, Chen LJ, Tsai JC. The Protective Role of Garlic on Allergen-Induced Airway Inflammation in Mice. Am J Chin Med (2019) 47(5):1099–112. doi: 10.1142/S0192415X19500563
186. Escribano BM, Luque E, Aguilar-Luque M, Feijoo M, Caballero-Villarraso J, Torres LA, et al. Dose-Dependent S-Allyl Cysteine Ameliorates Multiple Sclerosis Disease-Related Pathology by Reducing Oxidative Stress and Biomarkers of Dysbiosis in Experimental Autoimmune Encephalomyelitis. Eur J Pharmacol (2017) 815:266–73. doi: 10.1016/j.ejphar.2017.09.025
187. Liu X, Wang XJ. Potential Inhibitors Against 2019-Ncov Coronavirus M Protease From Clinically Approved Medicines. J Genet Genomics (2020) 47(2):119–21. doi: 10.1016/j.jgg.2020.02.001
188. Ha AW, Ying T, Kim WK. The Effects of Black Garlic (Allium Satvium) Extracts on Lipid Metabolism in Rats Fed a High Fat Diet. Nutr Res Pract (2015) 9(1):30–6. doi: 10.4162/nrp.2015.9.1.30
189. Zou XG, Li J, Sun PL, Fan YW, Yang JY, Deng ZY. Orbitides Isolated From Flaxseed Induce Apoptosis Against SGC-7901 Adenocarcinoma Cells. Int J Food Sci Nutr (2020) 71(8):929–39. doi: 10.1080/09637486.2020.1750573
190. Buckner AL, Buckner CA, Montaut S, Lafrenie RM. Treatment With Flaxseed Oil Induces Apoptosis in Cultured Malignant Cells. Heliyon (2019) 5(8):e02251. doi: 10.1016/j.heliyon.2019.e02251
191. Ezzat SM, Shouman SA, Elkhoely A, Attia YM, Elsesy MS, El Senousy AS, et al. Anticancer Potentiality of Lignan Rich Fraction of Six Flaxseed Cultivars. Sci Rep (2018) 8(1):544. doi: 10.1038/s41598-017-18944-0
192. Jones GJB, Roper RL. The Effects of Diets Enriched in Omega-3 Polyunsaturated Fatty Acids on Systemic Vaccinia Virus Infection. Sci Rep (2017) 7(1):15999. doi: 10.1038/s41598-017-16098-7
193. Garcia de Acilu M, Leal S, Caralt B, Roca O, Sabater J, Masclans JR. The Role of Omega-3 Polyunsaturated Fatty Acids in the Treatment of Patients With Acute Respiratory Distress Syndrome, A Clinical Review. BioMed Res Int (2015) 2015:653750. doi: 10.1155/2015/653750
194. Sharma S, Chhibber S, Mohan H, Sharma S. Dietary Supplementation With Omega-3 Polyunsaturated Fatty Acids Ameliorates Acute Pneumonia Induced by Klebsiella Pneumoniae in BALB/c Mice. Can J Microbiol (2013) 59(7):503–10. doi: 10.1139/cjm-2012-0521
195. Singh B, Singh JP, Kaur A, Singh N. Phenolic Composition, Antioxidant Potential and Health Benefits of Citrus Peel. Food Res Int (2020) 132:109114. doi: 10.1016/j.foodres.2020.109114
196. Nogata Y, Sakamoto K, Shiratsuchi H, Ishii T, Yano M, Ohta H. Flavonoid Composition of Fruit Tissues of Citrus Species. Biosci Biotechnol Biochem (2006) 70(1):178–92. doi: 10.1271/bbb.70.178
197. Bellavite P, Donzelli A. Hesperidin and SARS-CoV-2, New Light on the Healthy Function of Citrus Fruits. Antioxidants (Basel) (2020) 9(8):742. doi: 10.3390/antiox9080742
198. Nurhayati IP, Khumaira A, Ilmawati GPN, Meiyanto E, Hermawan A. Cytotoxic and Antimetastatic Activity of Hesperetin and Doxorubicin Combination Toward Her2 Expressing Breast Cancer Cells. Asian Pac J Cancer Prev (2020) 21(5):1259–67. doi: 10.31557/APJCP.2020.21.5.1259
199. ElKhouly AM, Youness RA, Gad MZ. MicroRNA-486-5p and microRNA-486-3p, Multifaceted Pleiotropic Mediators in Oncological and Non-Oncological Conditions. Noncoding RNA Res (2020) 5(1):11–21. doi: 10.1016/j.ncrna.2020.01.001
200. Palit S, Kar S, Sharma G, Das PK. Hesperetin Induces Apoptosis in Breast Carcinoma by Triggering Accumulation of ROS and Activation of ASK1/JNK Pathway. J Cell Physiol (2015) 230(8):1729–39. doi: 10.1002/jcp.24818
201. Khan A, Ikram M, Hahm JR, Kim MO. Antioxidant and Anti-Inflammatory Effects of Citrus Flavonoid Hesperetin, Special Focus on Neurological Disorders. Antioxidants (Basel) (2020) 9(7):609. doi: 10.3390/antiox9070609
202. Ahmad S, Alam K, Hossain MM, Fatima M, Firdaus F, Zafeer MF, et al. Anti-Arthritogenic and Cardioprotective Action of Hesperidin and Daidzein in Collagen-Induced Rheumatoid Arthritis. Mol Cell Biochem (2016) 423(1-2):115–27. doi: 10.1007/s11010-016-2830-y
203. Wu J. Tackle the Free Radicals Damage in COVID-19. Nitric Oxide (2020) 102:39–41. doi: 10.1016/j.niox.2020.06.002
204. Park HK, Kang SW, Park MS. Hesperidin Ameliorates Hepatic Ischemia-Reperfusion Injury in Sprague-Dawley Rats. Transplant Proc (2019) 51(8):2828–32. doi: 10.1016/j.transproceed.2019.02.059
205. Haggag YA, El-Ashmawy NE, Okasha KM. Is Hesperidin Essential for Prophylaxis and Treatment of COVID-19 Infection? Med Hypotheses (2020) 144:109957. doi: 10.1016/j.mehy.2020.109957
206. Kim H, Jang M, Kim Y, Choi J, Jeon J, Kim J, et al. Red Ginseng and Vitamin C Increase Immune Cell Activity and Decrease Lung Inflammation Induced by Influenza A Virus/H1N1 Infection. J Pharm Pharmacol (2016) 68(3):406–20. doi: 10.1111/jphp.12529
207. Kim Y, Kim H, Bae S, Choi J, Lim SY, Lee N, et al. Vitamin C Is an Essential Factor on the Anti-Viral Immune Responses Through the Production of Interferon-Alpha/Beta at the Initial Stage of Influenza A Virus (H3N2) Infection. Immune Netw (2013) 13(2):70–4. doi: 10.4110/in.2013.13.2.70
208. Sharma M, Anderson SA, Schoop R, Hudson JB. Induction of Multiple Pro-Inflammatory Cytokines by Respiratory Viruses and Reversal by Standardized Echinacea, a Potent Antiviral Herbal Extract. Antiviral Res (2009) 83(2):165–70. doi: 10.1016/j.antiviral.2009.04.009
209. Barnes J, Anderson LA, Gibbons S, Phillipson JD. Echinacea Species (Echinacea Angustifolia (DC.) Hell., Echinacea Pallida (Nutt.) Nutt., Echinacea Purpurea (L.) Moench), a Review of Their Chemistry, Pharmacology and Clinical Properties. J Pharm Pharmacol (2005) 57(8):929–54. doi: 10.1211/0022357056127
210. Aucoin M, Cooley K, Saunders PR, Care J, Anheyer D, Medina DN, et al. The Effect of Echinacea Spp. On the Prevention or Treatment of COVID-19 and Other Respiratory Tract Infections in Humans, A Rapid Review. Adv Integr Med (2020) 7(4):203–17. doi: 10.1016/j.aimed.2020.07.004
211. Melchart D, Linde K, Worku F, Bauer R, Wagner H. Immunomodulation With Echinacea - a Systematic Review of Controlled Clinical Trials. Phytomedicine (1994) 1(3):245–54. doi: 10.1016/S0944-7113(11)80072-3
212. Sharifi-Rad M, Mnayer D, Morais-Braga MFB, Carneiro JNP, Bezerra CF, Coutinho HDM, et al. Echinacea Plants as Antioxidant and Antibacterial Agents, From Traditional Medicine to Biotechnological Applications. Phytother Res (2018) 32(9):1653–63. doi: 10.1002/ptr.6101
213. Tsai YL, Chiu CC, Yi-Fu Chen J, Chan KC, Lin SD. Cytotoxic Effects of Echinacea Purpurea Flower Extracts and Cichoric Acid on Human Colon Cancer Cells Through Induction of Apoptosis. J Ethnopharmacol (2012) 143(3):914–9. doi: 10.1016/j.jep.2012.08.032
214. Chicca A, Adinolfi B, Martinotti E, Fogli S, Breschi MC, Pellati F, et al. Cytotoxic Effects of Echinacea Root Hexanic Extracts on Human Cancer Cell Lines. J Ethnopharmacol (2007) 110(1):148–53. doi: 10.1016/j.jep.2006.09.013
215. Chaves F, Chacon M, Badilla B, Arevalo C. Effect of Echinacea Purpurea (Asteraceae) Aqueous Extract on Antibody Response to Bothrops Asper Venom and Immune Cell Response. Rev Biol Trop (2007) 55(1):113–9. doi: 10.15517/rbt.v55i1.6061
216. Zhai Z, Liu Y, Wu L, Senchina DS, Wurtele ES, Murphy PA, et al. Enhancement of Innate and Adaptive Immune Functions by Multiple Echinacea Species. J Med Food (2007) 10(3):423–34. doi: 10.1089/jmf.2006.257
217. Sadigh-Eteghad S, Khayat-Nuri H, Abadi N, Ghavami S, Golabi M, Shanebandi D. Synergetic Effects of Oral Administration of Levamisole and Echinacea Purpurea on Immune Response in Wistar Rat. Res Vet Sci (2011) 91(1):82–5. doi: 10.1016/j.rvsc.2010.07.027
218. Park S, Lee MS, Jung S, Lee S, Kwon O, Kreuter MH, et al. Echinacea Purpurea Protects Against Restraint Stress-Induced Immunosuppression in BALB/c Mice. J Med Food (2018) 21(3):261–8. doi: 10.1089/jmf.2017.4073
219. Yu D, Yuan Y, Jiang L, Tai Y, Yang X, Hu F, et al. Anti-Inflammatory Effects of Essential Oil in Echinacea Purpurea L. Pak J Pharm Sci (2013) 26(2):403–8.
220. Nagoor Meeran MF, Javed H, Sharma C, Goyal SN, Kumar S, Jha NK, et al. Can Echinacea be a Potential Candidate to Target Immunity, Inflammation, and Infection - The Trinity of Coronavirus Disease 2019. Heliyon (2021) 7(2):e05990. doi: 10.1016/j.heliyon.2021.e05990
221. Allegra A, Di Gioacchino M, Tonacci A, Musolino C, Gangemi S. Immunopathology of SARS-CoV-2 Infection, Immune Cells and Mediators, Prognostic Factors, and Immune-Therapeutic Implications. Int J Mol Sci (2020) 21(13):4782. doi: 10.3390/ijms21134782
222. Turner RB, Bauer R, Woelkart K, Hulsey TC, Gangemi JD. An Evaluation of Echinacea Angustifolia in Experimental Rhinovirus Infections. N Engl J Med (2005) 353(4):341–8. doi: 10.1056/NEJMoa044441
223. Pleschka S, Stein M, Schoop R, Hudson JB. Anti-Viral Properties and Mode of Action of Standardized Echinacea Purpurea Extract Against Highly Pathogenic Avian Influenza Virus (H5N1, H7N7) and Swine-Origin H1N1 (S-OIV). Virol J (2009) 6:197. doi: 10.1186/1743-422X-6-197
224. Signer J, Jonsdottir HR, Albrich WC, Strasser M, Zust R, Ryter S, et al. In Vitro Virucidal Activity of Echinaforce(R), an Echinacea Purpurea Preparation, Against Coronaviruses, Including Common Cold Coronavirus 229E and SARS-CoV-2. Virol J (2020) 17(1):136. doi: 10.1186/s12985-020-01401-2
225. Hudson JB. Applications of the Phytomedicine Echinacea Purpurea (Purple Coneflower) in Infectious Diseases. J BioMed Biotechnol (2012) 2012:769896. doi: 10.1155/2012/769896
226. Kim MB, Kim C, Song Y, Hwang JK. Antihyperglycemic and Anti-Inflammatory Effects of Standardized Curcuma Xanthorrhiza Roxb. Extract and Its Active Compound Xanthorrhizol in High-Fat Diet-Induced Obese Mice. Evid Based Complement Alternat Med (2014) 2014:205915. doi: 10.1155/2014/205915
227. Oon SF, Nallappan M, Tee TT, Shohaimi S, Kassim NK, Sa’ariwijaya MS, et al. Xanthorrhizol, a Review of Its Pharmacological Activities and Anticancer Properties. Cancer Cell Int (2015) 15:100. doi: 10.1186/s12935-015-0255-4
228. Shimizu K, Funamoto M, Sunagawa Y, Shimizu S, Katanasaka Y, Miyazaki Y, et al. Anti-Inflammatory Action of Curcumin and Its Use in the Treatment of Lifestyle-Related Diseases. Eur Cardiol (2019) 14(2):117–22. doi: 10.15420/ecr.2019.17.2
229. Singgih Wahono C, Diah Setyorini C, Kalim H, Nurdiana N, Handono K. Effect of Curcuma Xanthorrhiza Supplementation on Systemic Lupus Erythematosus Patients With Hypovitamin D Which Were Given Vitamin D3 Towards Disease Activity (SLEDAI), IL-6, and TGF-Beta1 Serum. Int J Rheumatol (2017) 2017:7687053. doi: 10.1155/2017/7687053
230. Maldonado C, Barnes CJ, Cornett C, Holmfred E, Hansen SH, Persson C, et al. Phylogeny Predicts the Quantity of Antimalarial Alkaloids Within the Iconic Yellow Cinchona Bark (Rubiaceae, Cinchona Calisaya). Front Plant Sci (2017) 8:391. doi: 10.3389/fpls.2017.00391
231. Abolghasemi E, Moosa-Kazemi SH, Davoudi M, Reisi A, Satvat MT. Comparative Study of Chloroquine and Quinine on Malaria Rodents and Their Effects on the Mouse Testis. Asian Pac J Trop BioMed (2012) 2(4):311–4. doi: 10.1016/S2221-1691(12)60030-6
232. D’Alessandro S, Scaccabarozzi D, Signorini L, Perego F, Ilboudo DP, Ferrante P, et al. The Use of Antimalarial Drugs Against Viral Infection. Microorganisms (2020) 8(1):85. doi: 10.3390/microorganisms8010085
233. Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. Chloroquine Is a Potent Inhibitor of SARS Coronavirus Infection and Spread. Virol J (2005) 2:69. doi: 10.1186/1743-422X-2-69
234. Handayani T, Sakinah S, Nallappan M, Pihie AH, et al. Regulation of P53-, Bcl-2- and Caspase-Dependent Signaling Pathway in Xanthorrhizol-Induced Apoptosis of HepG2 Hepatoma Cells. Anticancer Res (2007) 27(2):965–71.
235. Ismail N, Pihie AH, Nallapan M. Xanthorrhizol Induces Apoptosis via the Up-Regulation of Bax and P53 in HeLa Cells. Anticancer Res (2005) 25(3B):2221–7.
236. Cheah YH, Azimahtol HL, Abdullah NR. Xanthorrhizol Exhibits Antiproliferative Activity on MCF-7 Breast Cancer Cells via Apoptosis Induction. Anticancer Res (2006) 26(6B):4527–34.
237. Kang YJ, Park KK, Chung WY, Hwang JK, Lee SK. Xanthorrhizol, a Natural Sesquiterpenoid, Induces Apoptosis and Growth Arrest in HCT116 Human Colon Cancer Cells. J Pharmacol Sci (2009) 111(3):276–84. doi: 10.1254/jphs.09141FP
238. Kim JY, An JM, Chung WY, Park KK, Hwang JK, Kim DS, et al. Xanthorrhizol Induces Apoptosis Through ROS-Mediated MAPK Activation in Human Oral Squamous Cell Carcinoma Cells and Inhibits DMBA-Induced Oral Carcinogenesis in Hamsters. Phytother Res (2013) 27(4):493–8. doi: 10.1002/ptr.4746
239. Galen EV, Kroes B. Assessment Report on Curcuma Xanthorrhiza Roxb.(C. Xanthorrhiza D. Dietrich), Rhizoma. Eur Medicines Agency (2014) 44(2).
240. Lim CS, Jin DQ, Mok H, Oh SJ, Lee JU, Hwang JK, et al. Antioxidant and Antiinflammatory Activities of Xanthorrhizol in Hippocampal Neurons and Primary Cultured Microglia. J Neurosci Res (2005) 82(6):831–8. doi: 10.1002/jnr.20692
241. Kim M, Cho H, Ahn DG, Jung HG, Seo HY, Kim JS, et al. In Vitro Replication Inhibitory Activity of Xanthorrhizol Against Severe Acute Respiratory Syndrome Coronavirus 2. Biomedicines (2021) 9(11):1725. doi: 10.3390/biomedicines9111725
242. Saggam A, Limgaokar K, Borse S, Chavan-Gautam P, Dixit S, Tillu G, et al. Withania Somnifera (L.) Dunal, Opportunity for Clinical Repurposing in COVID-19 Management. Front Pharmacol (2021) 12:623795. doi: 10.3389/fphar.2021.623795
243. Mishra LC, Singh BB, Dagenais S. Scientific Basis for the Therapeutic Use of Withania Somnifera (Ashwagandha), a Review. Altern Med Rev (2000) 5(4):334–46.
244. Minhas U, Minz R, Bhatnagar A. Prophylactic Effect of Withania Somnifera on Inflammation in a Non-Autoimmune Prone Murine Model of Lupus. Drug Discov Ther (2011) 5(4):195–201. doi: 10.5582/ddt.2011.v5.4.195
245. Teixeira ST, Valadares MC, Goncalves SA, de Melo A, Queiroz ML. Prophylactic Administration of Withania Somnifera Extract Increases Host Resistance in Listeria Monocytogenes Infected Mice. Int Immunopharmacol (2006) 6(10):1535–42. doi: 10.1016/j.intimp.2006.03.016
246. Kalra R, Kaushik N. Withania Somnifera (Linn.) Dunal, a Review of Chemical and Pharmacological Diversity. Phytochem Rev (2017) 16(5):953–87. doi: 10.1007/s11101-017-9504-6
247. Antony ML, Lee J, Hahm ER, Kim SH, Marcus AI, Kumari V, et al. Growth Arrest by the Antitumor Steroidal Lactone Withaferin A in Human Breast Cancer Cells Is Associated With Down-Regulation and Covalent Binding at Cysteine 303 of Beta-Tubulin. J Biol Chem (2014) 289(3):1852–65. doi: 10.1074/jbc.M113.496844
248. Yang H, Shi G, Dou QP. The Tumor Proteasome Is a Primary Target for the Natural Anticancer Compound Withaferin A Isolated From “Indian Winter Cherry”. Mol Pharmacol (2007) 71(2):426–37. doi: 10.1124/mol.106.030015
249. Yu Y, Hamza A, Zhang T, Gu M, Zou P, Newman B, et al. Withaferin A Targets Heat Shock Protein 90 in Pancreatic Cancer Cells. Biochem Pharmacol (2010) 79(4):542–51. doi: 10.1016/j.bcp.2009.09.017
250. Koduru S, Kumar R, Srinivasan S, Evers MB, Damodaran C. Notch-1 Inhibition by Withaferin-A, a Therapeutic Target Against Colon Carcinogenesis. Mol Cancer Ther (2010) 9(1):202–10. doi: 10.1158/1535-7163.MCT-09-0771
251. Widodo N, Priyandoko D, Shah N, Wadhwa R, Kaul SC. Selective Killing of Cancer Cells by Ashwagandha Leaf Extract and Its Component Withanone Involves ROS Signaling. PloS One (2010) 5(10):e13536. doi: 10.1371/journal.pone.0013536
252. Widodo N, Takagi Y, Shrestha BG, Ishii T, Kaul SC, Wadhwa R. Selective Killing of Cancer Cells by Leaf Extract of Ashwagandha, Components, Activity and Pathway Analyses. Cancer Lett (2008) 262(1):37–47. doi: 10.1016/j.canlet.2007.11.037
253. Christina AJ, Joseph DG, Packialakshmi M, Kothai R, Robert SJ, Chidambaranathan N, et al. Anticarcinogenic Activity of Withania Somnifera Dunal Against Dalton’s Ascitic Lymphoma. J Ethnopharmacol (2004) 93(2-3):359–61. doi: 10.1016/j.jep.2004.04.004
254. Khan B, Ahmad SF, Bani S, Kaul A, Suri KA, Satti NK, et al. Augmentation and Proliferation of T Lymphocytes and Th-1 Cytokines by Withania Somnifera in Stressed Mice. Int Immunopharmacol (2006) 6(9):1394–403. doi: 10.1016/j.intimp.2006.04.001
255. Malik F, Singh J, Khajuria A, Suri KA, Satti NK, Singh S, et al. A Standardized Root Extract of Withania Somnifera and Its Major Constituent Withanolide-A Elicit Humoral and Cell-Mediated Immune Responses by Up Regulation of Th1-Dominant Polarization in BALB/c Mice. Life Sci (2007) 80(16):1525–38. doi: 10.1016/j.lfs.2007.01.029
256. Kour K, Pandey A, Suri KA, Satti NK, Gupta KK, Bani S. Restoration of Stress-Induced Altered T Cell Function and Corresponding Cytokines Patterns by Withanolide A. Int Immunopharmacol (2009) 9(10):1137–44. doi: 10.1016/j.intimp.2009.05.011
257. Khan S, Malik F, Suri KA, Singh J. Molecular Insight Into the Immune Up-Regulatory Properties of the Leaf Extract of Ashwagandha and Identification of Th1 Immunostimulatory Chemical Entity. Vaccine (2009) 27(43):6080–7. doi: 10.1016/j.vaccine.2009.07.011
258. Zhao H, Gao Z, Xie S, Han X, Sun Q. Withaferin A Attenuates Ovalbumin Induced Airway Inflammation. injury (2019) 5:6. doi: 10.2741/4737
259. Davis L, Kuttan G. Immunomodulatory Activity of Withania Somnifera. J Ethnopharmacol (2000) 71(1–2):193–200. doi: 10.1016/S0378-8741(99)00206-8
260. Agarwal R, Diwanay S, Patki P, Patwardhan B. Studies on Immunomodulatory Activity of Withania Somnifera (Ashwagandha) Extracts in Experimental Immune Inflammation. J Ethnopharmacol (1999) 67(1):27–35. doi: 10.1016/S0378-8741(99)00065-3
261. Latheef SK, Dhama K, Samad HA, Wani MY, Kumar MA, Palanivelu M, et al. Immunomodulatory and Prophylactic Efficacy of Herbal Extracts Against Experimentally Induced Chicken Infectious Anaemia in Chicks, Assessing the Viral Load and Cell Mediated Immunity. Virusdisease (2017) 28(1):115–20. doi: 10.1007/s13337-016-0355-3
262. Davis L, Kuttan G. Effect of Withania Somnifera on Cell Mediated Immune Responses in Mice. J Exp Clin Cancer Res (2002) 21(4):585–90.
263. Mikolai J, Erlandsen A, Murison A, Brown KA, Gregory WL, Raman-Caplan P, Zwickey HL, et al. In Vivo Effects of Ashwagandha (Withania Somnifera) Extract on the Activation of Lymphocytes. J Altern Complement Med (2009) 15(4):423–30. doi: 10.1089/acm.2008.0215
264. Balkrishna A, Pokhrel S, Singh H, Joshi M, Mulay VP, Haldar S, et al. Withanone From Withania Somnifera Attenuates SARS-CoV-2 RBD and Host ACE2 Interactions to Rescue Spike Protein Induced Pathologies in Humanized Zebrafish Model. Drug Design Dev Ther (2021) 15:1111. doi: 10.2147/DDDT.S292805
265. Sharma S, Deep S. In-Silico Drug Repurposing for Targeting SARS-CoV-2 Main Protease (Mpro). J Biomolecular Struct Dynam (2020) p:1–8. doi: 10.26434/chemrxiv.12210845
266. Borse S, Joshi M, Saggam A, Bhat V, Walia S, Marathe A, et al. Ayurveda Botanicals in COVID-19 Management, An in Silico Multi-Target Approach. PloS One (2021) 16(6):e0248479. doi: 10.1371/journal.pone.0248479
267. Chacko SM, Thambi PT, Kuttan R, Nishigaki I. Beneficial Effects of Green Tea, a Literature Review. Chin Med (2010) 5:13. doi: 10.1186/1749-8546-5-13
268. Cabrera C, Artacho R, Gimenez R. Beneficial Effects of Green Tea–a Review. J Am Coll Nutr (2006) 25(2):79–99. doi: 10.1080/07315724.2006.10719518
269. Khan N, Mukhtar H. Tea Polyphenols in Promotion of Human Health. Nutrients (2018) 11(1):39. doi: 10.3390/nu11010039
270. Mhatre S, Srivastava T, Naik S, Patravale V. Antiviral Activity of Green Tea and Black Tea Polyphenols in Prophylaxis and Treatment of COVID-19, A Review. Phytomedicine (2021) 85:153286. doi: 10.1016/j.phymed.2020.153286
271. Higdon JV, Frei B. Tea Catechins and Polyphenols, Health Effects, Metabolism, and Antioxidant Functions. Crit Rev Food Sci Nutr (2003) 43(1):89–143. doi: 10.1080/10408690390826464
272. Lambert JD, Yang CS. Mechanisms of Cancer Prevention by Tea Constituents. J Nutr (2003) 133(10):3262S–7S. doi: 10.1093/jn/133.10.3262S
273. Yang CS, Landau JM. Effects of Tea Consumption on Nutrition and Health. J Nutr (2000) 130(10):2409–12. doi: 10.1093/jn/130.10.2409
274. O’Neill EJ, Termini D, Albano A, Tsiani E. Anti-Cancer Properties of Theaflavins. Molecules (2021) 26(4):987. doi: 10.3390/molecules26040987
275. Kaur S, Greaves P, Cooke DN, Edwards R, Steward WP, Gescher AJ, et al. Breast Cancer Prevention by Green Tea Catechins and Black Tea Theaflavins in the C3(1) SV40 T,t Antigen Transgenic Mouse Model Is Accompanied by Increased Apoptosis and a Decrease in Oxidative DNA Adducts. J Agric Food Chem (2007) 55(9):3378–85. doi: 10.1021/jf0633342
276. Ren F, Zhang S, Mitchell SH, Butler R, Young CY. Tea Polyphenols Down-Regulate the Expression of the Androgen Receptor in LNCaP Prostate Cancer Cells. Oncogene (2000) 19(15):1924–32. doi: 10.1038/sj.onc.1203511
277. Lee HH, Ho CT, Lin JK. Theaflavin-3,3’-Digallate and Penta-O-Galloyl-Beta-D-Glucose Inhibit Rat Liver Microsomal 5alpha-Reductase Activity and the Expression of Androgen Receptor in LNCaP Prostate Cancer Cells. Carcinogenesis (2004) 25(7):1109–18. doi: 10.1093/carcin/bgh106
278. Chowdhury P, Barooah AK. Tea Bioactive Modulate Innate Immunity, In Perception to COVID-19 Pandemic. Front Immunol (2020) 11:590716. doi: 10.3389/fimmu.2020.590716
279. Song YA, Park YL, Yoon SH, Kim KY, Cho SB, Lee WS, et al. Black Tea Polyphenol Theaflavin Suppresses LPS-Induced ICAM-1 and VCAM-1 Expression via Blockage of NF-kappaB and JNK Activation in Intestinal Epithelial Cells. Inflammation Res (2011) 60(5):493–500. doi: 10.1007/s00011-010-0296-z
280. Li J, Ye L, Wang X, Liu J, Wang Y, Zhou Y, et al. (-)-Epigallocatechin Gallate Inhibits Endotoxin-Induced Expression of Inflammatory Cytokines in Human Cerebral Microvascular Endothelial Cells. J Neuroinflamm (2012) 9:161. doi: 10.1186/1742-2094-9-161
281. Dona M, Dell’Aica I, Calabrese F, Benelli R, Morini M, Albini A, et al. Neutrophil Restraint by Green Tea, Inhibition of Inflammation, Associated Angiogenesis, and Pulmonary Fibrosis. J Immunol (2003) 170(8):4335–41. doi: 10.4049/jimmunol.170.8.4335
282. Sartor L, Pezzato E, Garbisa S. (-)Epigallocatechin-3-Gallate Inhibits Leukocyte Elastase, Potential of the Phyto-Factor in Hindering Inflammation, Emphysema, and Invasion. J Leukoc Biol (2002) 71(1):73–9.
283. Lung J, Lin YS, Yang YH, Chou YL, Shu LH, Cheng YC, et al. The Potential Chemical Structure of Anti-SARS-CoV-2 RNA-Dependent RNA Polymerase. J Med Virol (2020) 92(6):693–7. doi: 10.1002/jmv.25761
284. Maiti S, Banerjee A. Epigallocatechin Gallate and Theaflavin Gallate Interaction in SARS-CoV-2 Spike-Protein Central Channel With Reference to the Hydroxychloroquine Interaction, Bioinformatics and Molecular Docking Study. Drug Dev Res (2021) 82(1):86–96. doi: 10.1002/ddr.21730
285. Zhang J-J, Shen X, Yan Y-M, Yan W, Cheng Y-X. Discovery of Anti-SARS-CoV-2 Agents From Commercially Available Flavor via Docking Screening. (2020). doi: 10.31219/osf.io/vjch2
287. Aparicio-Soto M, Sanchez-Hidalgo M, Alarcon-de-la-Lastra C. An Update on Diet and Nutritional Factors in Systemic Lupus Erythematosus Management. Nutr Res Rev (2017) 30(1):118–37. doi: 10.1017/S0954422417000026
288. Gan RY, Li HB, Sui ZQ, Corke H. Absorption, Metabolism, Anti-Cancer Effect and Molecular Targets of Epigallocatechin Gallate (EGCG), An Updated Review. Crit Rev Food Sci Nutr (2018) 58(6):924–41. doi: 10.1080/10408398.2016.1231168
289. Shankar S, Ganapathy S, Hingorani SR, Srivastava RK. EGCG Inhibits Growth, Invasion, Angiogenesis and Metastasis of Pancreatic Cancer. Front Biosci (2008) 13:440–52. doi: 10.2741/2691
290. Shankar S, Suthakar G, Srivastava RK. Epigallocatechin-3-Gallate Inhibits Cell Cycle and Induces Apoptosis in Pancreatic Cancer. Front Biosci (2007) 12:5039–51. doi: 10.2741/2446
291. Kaltschmidt B, Greiner JFW, Kadhim HM, Kaltschmidt C. Subunit-Specific Role of NF-KappaB in Cancer. Biomedicines (2018) 6(2):44. doi: 10.3390/biomedicines6020044
292. Masuda M, Suzui M, Lim JT, Deguchi A, Soh JW, Weinstein IB. Epigallocatechin-3-Gallate Decreases VEGF Production in Head and Neck and Breast Carcinoma Cells by Inhibiting EGFR-Related Pathways of Signal Transduction. J Exp Ther Oncol (2002) 2(6):350–9. doi: 10.1046/j.1359-4117.2002.01062.x
293. Fujiki H, Suganuma M, Okabe S, Sueoka N, Komori A, Sueoka E, et al. Cancer Inhibition by Green Tea. Mutat Res/Fundam Mol Mech Mutagenesis (1998) 402(1-2):307–10. doi: 10.1016/S0027-5107(97)00310-2
294. Shirakami Y, Shimizu M. Possible Mechanisms of Green Tea and Its Constituents Against Cancer. Molecules (2018) 23(9):2284. doi: 10.3390/molecules23092284
295. Shimizu M, Deguchi A, Hara Y, Moriwaki H, Weinstein IB. EGCG Inhibits Activation of the Insulin-Like Growth Factor-1 Receptor in Human Colon Cancer Cells. Biochem Biophys Res Commun (2005) 334(3):947–53. doi: 10.1016/j.bbrc.2005.06.182
296. Shimizu M, Shirakami Y, Sakai H, Tatebe H, Nakagawa T, Hara Y, et al. EGCG Inhibits Activation of the Insulin-Like Growth Factor (IGF)/IGF-1 Receptor Axis in Human Hepatocellular Carcinoma Cells. Cancer Lett (2008) 262(1):10–8. doi: 10.1016/j.canlet.2007.11.026
297. Fang M, Chen D, Yang CS. Dietary Polyphenols may Affect DNA Methylation. J Nutr (2007) 137(1 Suppl):223S–8S. doi: 10.1093/jn/137.1.223S
298. Huang SC, Kao YH, Shih SF, Tsai MC, Lin CS, Chen LW, et al. Epigallocatechin-3-Gallate Exhibits Immunomodulatory Effects in Human Primary T Cells. Biochem Biophys Res Commun (2021) 550:70–6. doi: 10.1016/j.bbrc.2021.02.132
299. Wu D, Wang J, Pae M, Meydani SN. Green tea EGCG, T Cells, and T Cell-Mediated Autoimmune Diseases. Mol Aspects Med (2012) 33(1):107–18. doi: 10.1016/j.mam.2011.10.001
300. Byun EH, Omura T, Yamada K, Tachibana H. Green Tea Polyphenol Epigallocatechin-3-Gallate Inhibits TLR2 Signaling Induced by Peptidoglycan Through the Polyphenol Sensing Molecule 67-kDa Laminin Receptor. FEBS Lett (2011) 585(5):814–20. doi: 10.1016/j.febslet.2011.02.010
301. Byun EB, Choi HG, Sung NY, Byun EH. Green Tea Polyphenol Epigallocatechin-3-Gallate Inhibits TLR4 Signaling Through the 67-kDa Laminin Receptor on Lipopolysaccharide-Stimulated Dendritic Cells. Biochem Biophys Res Commun (2012) 426(4):480–5. doi: 10.1016/j.bbrc.2012.08.096
302. Xiaokaiti Y, Wu H, Chen Y, Yang H, Duan J, Li X, et al. EGCG Reverses Human Neutrophil Elastase-Induced Migration in A549 Cells by Directly Binding to HNE and by Regulating Alpha1-AT. Sci Rep (2015) 5:11494. doi: 10.1038/srep11494
303. Mekky RY, El-Ekiaby N, El Sobky SA, Elemam NM, Youness RA, El-Sayed M, et al. Epigallocatechin Gallate (EGCG) and miR-548m Reduce HCV Entry Through Repression of CD81 Receptor in HCV Cell Models. Arch Virol (2019) 164(6):1587–95. doi: 10.1007/s00705-019-04232-x
304. Ge M, Xiao Y, Chen H, Luo F, Du G, Zeng F. Multiple Antiviral Approaches of (-)-Epigallocatechin-3-Gallate (EGCG) Against Porcine Reproductive and Respiratory Syndrome Virus Infection In Vitro. Antiviral Res (2018) 158:52–62. doi: 10.1016/j.antiviral.2018.07.012
305. Lu JW, Hsieh PS, Lin CC, Hu MK, Huang SM, Wang YM, et al. Synergistic Effects of Combination Treatment Using EGCG and Suramin Against the Chikungunya Virus. Biochem Biophys Res Commun (2017) 491(3):595–602. doi: 10.1016/j.bbrc.2017.07.157
306. Yang J, Li L, Tan S, Jin H, Qiu J, Mao Q, et al. A Natural Theaflavins Preparation Inhibits HIV-1 Infection by Targeting the Entry Step, Potential Applications for Preventing HIV-1 Infection. Fitoterapia (2012) 83(2):348–55. doi: 10.1016/j.fitote.2011.11.016
307. Song JM, Lee KH, Seong BL. Antiviral Effect of Catechins in Green Tea on Influenza Virus. Antiviral Res (2005) 68(2):66–74. doi: 10.1016/j.antiviral.2005.06.010
308. Khaerunnisa S, Kurniawan H, Awaluddin R, Suhartati S, Soetjipto S. Potential Inhibitor of COVID-19 Main Protease (Mpro) From Several Medicinal Plant Compounds by Molecular Docking Study. Preprints (2020) 2020:2020030226. doi: 10.20944/preprints202003.0226.v1
309. Ibrahim IM, Abdelmalek DH, Elfiky AA. GRP78, A Cell’s Response to Stress. Life Sci (2019) 226:156–63. doi: 10.1016/j.lfs.2019.04.022
310. Khomari F, Nabi-Afjadi M, Yarahmadi S, Eskandari H, Bahreini E. Effects of Cell Proteostasis Network on the Survival of SARS-CoV-2. Biol Proced Online (2021) 23(1):8. doi: 10.1186/s12575-021-00145-9
311. Khan M, Khan M, Khan Z, Ahamad T, Ansari W. Identification of Dietary Molecules as Therapeutic Agents to Combat COVID-19 Using Molecular Docking Studies. Res Sq (2020). doi: 10.21203/rs.3.rs-19560/v1
312. Weiss DJ, Anderton CR. Determination of Catechins in Matcha Green Tea by Micellar Electrokinetic Chromatography. J Chromatogr A (2003) 1011(1-2):173–80. doi: 10.1016/S0021-9673(03)01133-6
313. Kochman J, Jakubczyk K, Antoniewicz J, Mruk H, Janda K. Health Benefits and Chemical Composition of Matcha Green Tea, A Review. Molecules (2020) 26(1):85. doi: 10.3390/molecules26010085
314. Kang YR, Park J, Jung SK, Chang YH. Synthesis, Characterization, and Functional Properties of Chlorophylls, Pheophytins, and Zn-Pheophytins. Food Chem (2018) 245:943–50. doi: 10.1016/j.foodchem.2017.11.079
315. Suzuki Y, Shioi Y. Identification of Chlorophylls and Carotenoids in Major Teas by High-Performance Liquid Chromatography With Photodiode Array Detection. J Agric Food Chem (2003) 51(18):5307–14. doi: 10.1021/jf030158d
316. Grzesik M, Naparlo K, Bartosz G, Sadowska-Bartosz I. Antioxidant Properties of Catechins: Comparison With Other Antioxidants. Food Chem (2018) 241:480–92. doi: 10.1016/j.foodchem.2017.08.117
317. Menegazzi M, Mariotto S, Dal Bosco M, Darra E, Vaiana N, Shoji K, et al. Direct Interaction of Natural and Synthetic Catechins With Signal Transducer Activator of Transcription 1 Affects Both Its Phosphorylation and Activity. FEBS J (2014) 281(3):724–38. doi: 10.1111/febs.12618
318. Steinmann J, Buer J, Pietschmann T, Steinmann E. Anti-Infective Properties of Epigallocatechin-3-Gallate (EGCG), a Component of Green Tea. Br J Pharmacol (2013) 168(5):1059–73. doi: 10.1111/bph.12009
319. Channappanavar R, Zhao J, Perlman S. T Cell-Mediated Immune Response to Respiratory Coronaviruses. Immunol Res (2014) 59(1-3):118–28. doi: 10.1007/s12026-014-8534-z
320. Babaei F, Mirzababaei M, Nassiri-Asl M. Quercetin in Food, Possible Mechanisms of Its Effect on Memory. J Food Sci (2018) 83(9):2280–7. doi: 10.1111/1750-3841.14317
321. Costa LG, Garrick JM, Roque PJ, Pellacani C. Mechanisms of Neuroprotection by Quercetin, Counteracting Oxidative Stress and More. Oxid Med Cell Longev (2016) 2016:2986796. doi: 10.1155/2016/2986796
322. Senggunprai L, Kukongviriyapan V, Prawan A, Kukongviriyapan U. Quercetin and EGCG Exhibit Chemopreventive Effects in Cholangiocarcinoma Cells via Suppression of JAK/STAT Signaling Pathway. Phytother Res (2014) 28(6):841–8. doi: 10.1002/ptr.5061
323. Alvarez P, Alvarado C, Puerto M, Schlumberger A, Jimenez L, de la Fuente M. Improvement of Leukocyte Functions in Prematurely Aging Mice After Five Weeks of Diet Supplementation With Polyphenol-Rich Cereals. Nutrition (2006) 22(9):913–21. doi: 10.1016/j.nut.2005.12.012
324. Hashemzaei M, Delarami Far A, Yari A, Heravi RE, Tabrizian K, Taghdisi SM, et al. Anticancer and Apoptosisinducing Effects of Quercetin In Vitro and In Vivo. Oncol Rep (2017) 38(2):819–28. doi: 10.3892/or.2017.5766
325. Spagnuolo C, Russo M, Bilotto S, Tedesco I, Laratta B, Russo GL. Dietary Polyphenols in Cancer Prevention, the Example of the Flavonoid Quercetin in Leukemia. Ann N Y Acad Sci (2012) 1259:95–103. doi: 10.1111/j.1749-6632.2012.06599.x
326. Araujo JR, Goncalves P, Martel F. Chemopreventive Effect of Dietary Polyphenols in Colorectal Cancer Cell Lines. Nutr Res (2011) 31(2):77–87. doi: 10.1016/j.nutres.2011.01.006
327. Nair MP, Kandaswami C, Mahajan S, Chadha KC, Chawda R, Nair H, et al. The Flavonoid, Quercetin, Differentially Regulates Th-1 (IFNgamma) and Th-2 (IL4) Cytokine Gene Expression by Normal Peripheral Blood Mononuclear Cells. Biochim Biophys Acta (2002) 1593(1):29–36. doi: 10.1016/S0167-4889(02)00328-2
328. Wang S, Yao J, Zhou B, Yang J, Chaudry MT, Wang M, et al. Bacteriostatic Effect of Quercetin as an Antibiotic Alternative In Vivo and Its Antibacterial Mechanism In Vitro. J Food Prot (2018) 81(1):68–78. doi: 10.4315/0362-028X.JFP-17-214
329. Sahpazidou D, Geromichalos GD, Stagos D, Apostolou A, Haroutounian SA, Tsatsakis AM, et al. Anticarcinogenic Activity of Polyphenolic Extracts From Grape Stems Against Breast, Colon, Renal and Thyroid Cancer Cells. Toxicol Lett (2014) 230(2):218–24. doi: 10.1016/j.toxlet.2014.01.042
330. Vargas AJ, Burd R. Hormesis and Synergy, Pathways and Mechanisms of Quercetin in Cancer Prevention and Management. Nutr Rev (2010) 68(7):418–28. doi: 10.1111/j.1753-4887.2010.00301.x
331. Hsu CL, Yen GC. Phenolic Compounds, Evidence for Inhibitory Effects Against Obesity and Their Underlying Molecular Signaling Mechanisms. Mol Nutr Food Res (2008) 52(1):53–61. doi: 10.1002/mnfr.200700393
332. Jakubczyk K, Kochman J, Kwiatkowska A, Kaldunska J, Dec K, Kawczuga D, et al. Antioxidant Properties and Nutritional Composition of Matcha Green Tea. Foods (2020) 9(4):483. doi: 10.3390/foods9040483
333. Kolackova T, Kolofikova K, Sytarova I, Snopek L, Sumczynski D, Orsavova J. Matcha Tea: Analysis of Nutritional Composition, Phenolics and Antioxidant Activity. Plant Foods Hum Nutr (2020) 75(1):48–53. doi: 10.1007/s11130-019-00777-z
334. Mitani T, Nagano T, Harada K, Yamashita Y, Ashida H. Caffeine-Stimulated Intestinal Epithelial Cells Suppress Lipid Accumulation in Adipocytes. J Nutr Sci Vitaminol (Tokyo) (2017) 63(5):331–8. doi: 10.3177/jnsv.63.331
335. Unno K, Furushima D, Hamamoto S, Iguchi K, Yamada H, Morita A, et al. Stress-Reducing Effect of Cookies Containing Matcha Green Tea, Essential Ratio Among Theanine, Arginine, Caffeine and Epigallocatechin Gallate. Heliyon (2019) 5(5):e01653. doi: 10.1016/j.heliyon.2019.e01653
336. Ku KM, Choi JN, Kim J, Kim JK, Yoo LG, Lee SJ, et al. Metabolomics Analysis Reveals the Compositional Differences of Shade Grown Tea (Camellia Sinensis L.). J Agric Food Chem (2010) 58(1):418–26. doi: 10.1021/jf902929h
337. Kaneko S, Kumazawa K, Masuda H, Henze A, Hofmann T. Molecular and Sensory Studies on the Umami Taste of Japanese Green Tea. J Agric Food Chem (2006) 54(7):2688–94. doi: 10.1021/jf0525232
338. Bialecka-Florjanczyk E, Fabiszewska A, Zieniuk B. Phenolic Acids Derivatives - Biotechnological Methods of Synthesis and Bioactivity. Curr Pharm Biotechnol (2018) 19(14):1098–113. doi: 10.2174/1389201020666181217142051
339. Stefanello N, Spanevello RM, Passamonti S, Porciuncula L, Bonan CD, Olabiyi AA, et al. Coffee, Caffeine, Chlorogenic Acid, and the Purinergic System. Food Chem Toxicol (2019) 123:298–313. doi: 10.1016/j.fct.2018.10.005
340. Naveed M, Hejazi V, Abbas M, Kamboh AA, Khan GJ, Shumzaid M, et al. Chlorogenic Acid (CGA): A Pharmacological Review and Call for Further Research. BioMed Pharmacother (2018) 97:67–74. doi: 10.1016/j.biopha.2017.10.064
341. Weng CJ, Yen GC. Chemopreventive Effects of Dietary Phytochemicals Against Cancer Invasion and Metastasis, Phenolic Acids, Monophenol, Polyphenol, and Their Derivatives. Cancer Treat Rev (2012) 38(1):76–87. doi: 10.1016/j.ctrv.2011.03.001
342. Ghorbani A. Mechanisms of Antidiabetic Effects of Flavonoid Rutin. BioMed Pharmacother (2017) 96:305–12. doi: 10.1016/j.biopha.2017.10.001
343. Hosseinzadeh H, Nassiri-Asl M. Review of the Protective Effects of Rutin on the Metabolic Function as an Important Dietary Flavonoid. J Endocrinol Invest (2014) 37(9):783–8. doi: 10.1007/s40618-014-0096-3
344. Reygaert WC. Green Tea Catechins: Their Use in Treating and Preventing Infectious Diseases. BioMed Res Int (2018) 2018:9105261. doi: 10.1155/2018/9105261
345. Ohishi T, Goto S, Monira P, Isemura M, Nakamura Y. Anti-Inflammatory Action of Green Tea. Antiinflamm Antiallergy Agents Med Chem (2016) 15(2):74–90. doi: 10.2174/1871523015666160915154443
346. Miura Y, Chiba T, Tomita I, Koizumi H, Miura S, Umegaki K, et al. Tea Catechins Prevent the Development of Atherosclerosis in Apoprotein E-Deficient Mice. J Nutr (2001) 131(1):27–32. doi: 10.1093/jn/131.1.27
347. Yang CS, Wang H. Cancer Preventive Activities of Tea Catechins. Molecules (2016) 21(12):1679. doi: 10.3390/molecules21121679
348. Zhang L, Xie J, Gan R, Wu Z, Luo H, Chen X, et al. Synergistic Inhibition of Lung Cancer Cells by EGCG and NF-kappaB Inhibitor BAY11-7082. J Cancer (2019) 10(26):6543–56. doi: 10.7150/jca.34285
349. Lin HY, Hou SC, Chen SC, Kao MC, Yu CC, Funayama S, et al. (-)-Epigallocatechin Gallate Induces Fas/CD95-Mediated Apoptosis Through Inhibiting Constitutive and IL-6-Induced JAK/STAT3 Signaling in Head and Neck Squamous Cell Carcinoma Cells. J Agric Food Chem (2012) 60(10):2480–9. doi: 10.1021/jf204362n
350. Calder PC. Nutrition, Immunity and COVID-19. BMJ Nutr Prev Health (2020) 3(1):74–92. doi: 10.1136/bmjnph-2020-000085
351. Filippou PS, Karagiannis GS. Cytokine Storm During Chemotherapy, a New Companion Diagnostic Emerges? Oncotarget (2020) 11(3):213–5. doi: 10.18632/oncotarget.27442
352. Hadjadj J, Yatim N, Barnabei L, Corneau A, Boussier J, Smith N, et al. Impaired Type I Interferon Activity and Inflammatory Responses in Severe COVID-19 Patients. Science (2020) 369(6504):718–24. doi: 10.1126/science.abc6027
353. Ranjith-Kumar CT, Lai Y, Sarisky RT, Cheng Kao C. Green Tea Catechin, Epigallocatechin Gallate, Suppresses Signaling by the dsRNA Innate Immune Receptor RIG-I. PloS One (2010) 5(9):e12878. doi: 10.1371/journal.pone.0012878
354. Eid HM, Haddad PS. The Antidiabetic Potential of Quercetin, Underlying Mechanisms. Curr Med Chem (2017) 24(4):355–64. doi: 10.2174/0929867323666160909153707
355. Abdel-Latif M, Afifi A, Soliman R, Elkhouly A, Abdelmotaal A, Youness RA. A New Quercetin Glycoside Enhances TNBC Immunological Profile Through TP53/miR-155/MICA/Ulbp2. Ann Oncol (2019) 30:vii7–8. doi: 10.1093/annonc/mdz413.028
356. Ahmed Youness R, Amr Assal R, Mohamed Ezzat S, Zakaria Gad M, Abdel Motaal A. A Methoxylated Quercetin Glycoside Harnesses HCC Tumor Progression in a TP53/miR-15/miR-16 Dependent Manner. Nat Prod Res (2020) 34(10):1475–80. doi: 10.1080/14786419.2018.1509326
357. Kuo PC, Liu HF, Chao JI. Survivin and P53 Modulate Quercetin-Induced Cell Growth Inhibition and Apoptosis in Human Lung Carcinoma Cells. J Biol Chem (2004) 279(53):55875–85. doi: 10.1074/jbc.M407985200
358. Bae JH, Kim JY, Kim MJ, Chang SH, Park YS, Son CH, et al. Quercetin Enhances Susceptibility to NK Cell-Mediated Lysis of Tumor Cells Through Induction of NKG2D Ligands and Suppression of HSP70. J Immunother (2010) 33(4):391–401. doi: 10.1097/CJI.0b013e3181d32f22
359. Ha EJ, Kim KY, Kim CE, Jun DY, Kim YH. Enhancement of Quercetin-Induced Apoptosis by Cotreatment With Autophagy Inhibitor Is Associated With Augmentation of BAK-Dependent Mitochondrial Pathway in Jurkat T Cells. Oxid Med Cell Longev (2019) 2019:7989276. doi: 10.1155/2019/7989276
360. Begum R, Sharma M, Pillai KK, Aeri V, Sheliya MA. Inhibitory Effect of Careya Arborea on Inflammatory Biomarkers in Carrageenan-Induced Inflammation. Pharm Biol (2015) 53(3):437–45. doi: 10.3109/13880209.2014.923005
361. Erden Inal M, Kahraman A. The Protective Effect of Flavonol Quercetin Against Ultraviolet a Induced Oxidative Stress in Rats. Toxicology (2000) 154(1-3):21–9. doi: 10.1016/S0300-483X(00)00268-7
362. Habtemariam S. Rutin as a Natural Therapy for Alzheimer’s Disease, Insights Into Its Mechanisms of Action. Curr Med Chem (2016) 23(9):860–73. doi: 10.2174/0929867323666160217124333
363. Nadi E, Tavakoli F, Zeraati F, Goodarzi MT, Hashemi SH. Effect of Vitamin C Administration on Leukocyte Vitamin C Level and Severity of Bronchial Asthma. Acta Med Iran (2012) 50(4):233–8.
364. Khanna K, Kohli SK, Kaur R, Bhardwaj A, Bhardwaj V, Ohri P, et al. Herbal Immune-Boosters, Substantial Warriors of Pandemic Covid-19 Battle. Phytomedicine (2021) 85:153361. doi: 10.1016/j.phymed.2020.153361
365. Panyod S, Ho CT, Sheen LY. Dietary Therapy and Herbal Medicine for COVID-19 Prevention, A Review and Perspective. J Tradit Complement Med (2020) 10(4):420–7. doi: 10.1016/j.jtcme.2020.05.004
366. Du HZ, Hou XY, Miao YH, Huang BS, Liu DH. Traditional Chinese Medicine: an Effective Treatment for 2019 Novel Coronavirus Pneumonia (NCP). Chin J Nat Med (2020) 18(3):206–10. doi: 10.1016/S1875-5364(20)30022-4
367. Han HJ, Nwagwu C, Anyim O, Ekweremadu C, Kim S, et al. COVID-19 and Cancer, From Basic Mechanisms to Vaccine Development Using Nanotechnology. Int Immunopharmacol (2021) 90:107247. doi: 10.1016/j.intimp.2020.107247
Keywords: SARS-CoV-2, herbal drugs, autoimmune diseases, nutraceuticals, cancer
Citation: Kiriacos CJ, Khedr MR, Tadros M and Youness RA (2022) Prospective Medicinal Plants and Their Phytochemicals Shielding Autoimmune and Cancer Patients Against the SARS-CoV-2 Pandemic: A Special Focus on Matcha. Front. Oncol. 12:837408. doi: 10.3389/fonc.2022.837408
Received: 16 December 2021; Accepted: 21 March 2022;
Published: 18 May 2022.
Edited by:
Nadia M. Hamdy, Ain Shams University, EgyptReviewed by:
Safaet Alam, State University of Bangladesh, BangladeshCopyright © 2022 Kiriacos, Khedr, Tadros and Youness. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rana A. Youness, ci55b3VuZXNzQGhlcnRzLmFjLnVr; cmFuYS55b3VuZXNzMjFAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.