- 1Family Cancer Assessment Clinic, Huntsman Cancer Institute, Salt Lake City, UT, United States
- 2Department of Internal Medicine, Huntsman Cancer Institute, Salt Lake City, UT, United States
The largest proportion of hereditary melanoma cases are due to pathogenic variants (PVs) in the CDKN2A/p16 gene, which account for 20%-40% of familial melanomas and confer up to a 30%-70% lifetime risk for melanoma in individuals with these variants. In addition, PVs in the CDKN2A gene also increase risk for pancreatic cancer (~5–24% lifetime risk). Individuals with PVs in the CDKN2A gene also tend to have an earlier onset of cancer. Despite these known risks, uptake of germline testing has been limited in the past, largely due to perceptions of limited benefit for patients. Prevention recommendations have been developed for individuals with CDKN2A PVs as well the providers who care for them. On the patient level, behavioral modifications regarding melanoma prevention such as wearing sunscreen, limiting prolonged sun exposure and practicing general sun safety can help reduce risks. Germline testing can provide motivation for some individuals to adhere to these lifestyle changes. On the provider level, pancreatic cancer surveillance for individuals with CDKN2A PVs has been increasingly endorsed by expert consensus, although the efficacy of these surveillance methods remains under study. This review summarizes the updated surveillance guidelines for individuals with CDKN2A PVs and explores the impact of genetic counseling and testing in influencing behavioral changes in these individuals.
Background
Most melanomas are sporadic; however, between 7-15% of melanomas occur in those with a family history of the cancer (1, 2). Many factors are involved in increasing an individual’s risk for melanoma, most of which influence a family as a whole. While sun exposure experiences, skin pigmentation, and geographic location have been well-characterized as risk factors (3–8), more recently, genetics have been a topic of interest in the melanoma world. Germline pathogenic variants (PVs) in a number of genes predispose to melanoma (9–12), but the largest proportion of familial melanoma cases (20%-40%) are due to PVs in the gene CDKN2A (cyclin-dependent kinase inhibitor 2A) (2, 12).
CDKN2A functions as a tumor suppressor gene, and somatic CDKN2A PVs are commonly found in both sporadic and hereditary melanomas (1). This gene is located on chromosome 9p21.3 and has two main transcripts (isoforms 1 and 4). Isoform 1 encodes the protein p16 (INK4a), while isoform 4 encodes the protein p14 (ARF). Germline PVs in the CDKN2A gene more commonly affect protein p16 than p14 and typically affect function of the G1/S checkpoint in the cell cycle by inhibiting cyclin-dependent kinases CDK4 and CDK6. This inhibition allows for uncontrolled cellular proliferation, which has many downstream carcinogenic affects (13). For simplicity, we will be using CDKN2A to refer to the PVs that occur in the p16 isoform as these are more common and better described than PVs in the p14 isoform.
Familial Atypical Multiple Mole Melanoma Syndrome
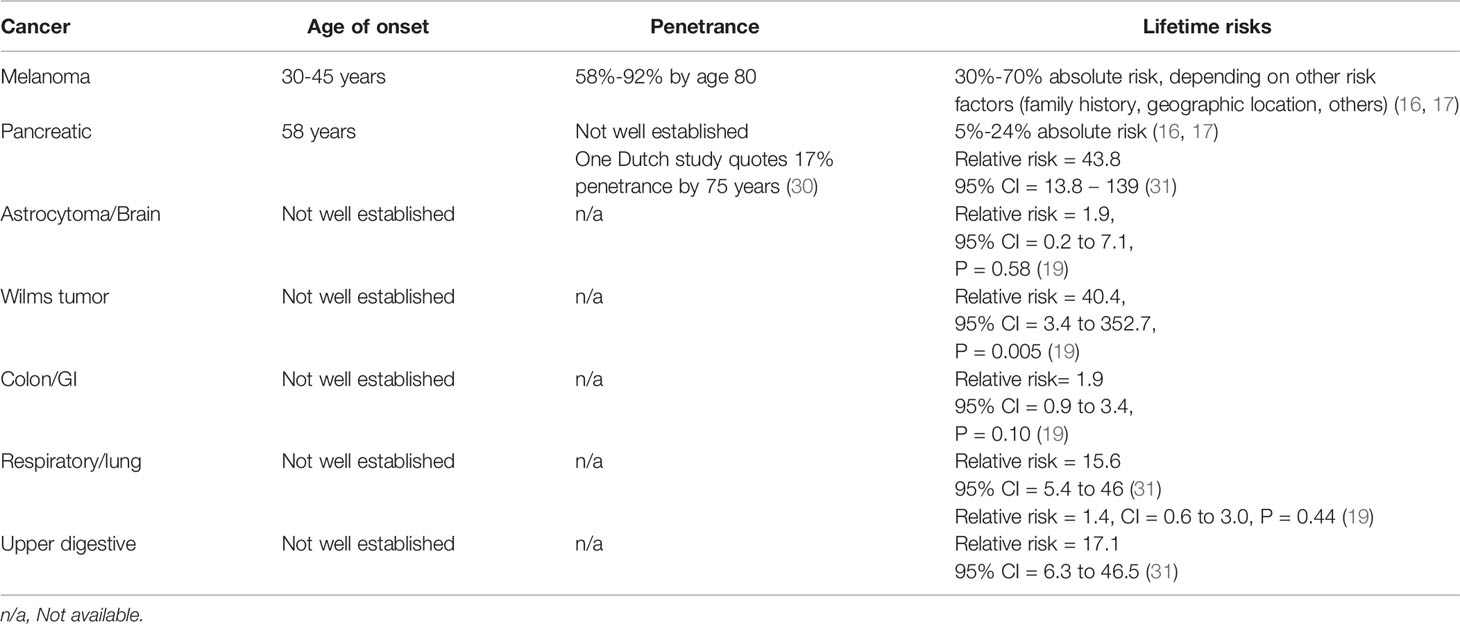
Germline PVs in the CDKN2A gene are consistent with the condition called familial atypical multiple mole melanoma syndrome (FAMMM) (13). FAMMM is an autosomal dominant condition characterized by a large number of melanocytic nevi (often >50), up to 65-fold increased risk for cutaneous melanoma, and a 13-47-fold increased risk for pancreatic cancer (13–15). This translates to a 30%-70% lifetime risk for melanoma and a 5%-24% lifetime risk for pancreatic cancer (16, 17). In addition, other cancers have been observed in carriers, although actionable guidelines for increased surveillance for these cancers are not available at this time (18, 19).The penetrance rate for melanoma in individuals with CDKN2A PVs is estimated at 58-92% by age 80 (13, 20, 21). This variance in penetration may be related to location and associated sun exposure, although studies are conflicting on this point (22, 23). Variants in MC1R, often but not always associated with a red hair phenotype, can act as a modifier gene for CDKN2A mutation carriers, as well as their known effect as an independent low-penetrance susceptibility gene for melanoma (24). Histopathologic characteristics and somatic mutations of melanomas in individuals with CDKN2A PVs are similar to those with sporadic melanoma (13, 14, 25–28). Of note, several CDKN2A patients have been reported with melanomas with coexisting BRAF and NRAS mutations, which is uncommon in sporadic melanomas (28). Higher melanoma mortality rates have been described in CDKN2A families than in wild-type melanoma families (10); however other studies have found no difference in survival rates between CDKN2A carriers and non-carriers (29). (see Table 1)
Clinical characteristics of FAMMM include a large number of atypical melanocytic nevi; however, multiple nevi, while characteristic, are not diagnostic of FAMMM (1, 32). Multiple and/or dysplastic nevi are not restricted to inherited syndromes and are considered a strong risk factor for both sporadic melanoma and melanoma in CDKN2A carriers (33–36). Atypical nevi may transform into malignant melanoma, but melanomas in FAMMM patients often also develop on normal skin (13, 33, 37, 38).
Melanoma diagnosis in FAMMM cohorts typically occurs over a decade earlier than that in sporadic melanoma cases. Sporadic melanoma is typically diagnosed between the ages of 53-61, whereas individuals with a CDKN2A PV are often diagnosed between ages 30-45 (1, 39–41). The youngest reported cases of melanoma in FAMMM families have been seen at age 13 (42, 43). Additionally, individuals with CDKN2A PVs have an increased probability of multiple primary melanomas: one study reported a 23% incidence of second melanoma primary diagnosed within 5 years of the first, representing a 10-fold increase over that of melanoma patients without CDKN2A PVs (39). In a genotype-phenotype correlation study, multiple primary melanomas were the most predictive factor for presence of a CDKN2A mutation (44). Given the earlier presentation of melanoma in this population and potential for multiple primary lesions, increased and intensive surveillance for cutaneous melanomas is routinely recommended with screening starting at a young age (13, 45).
Melanoma Surveillance
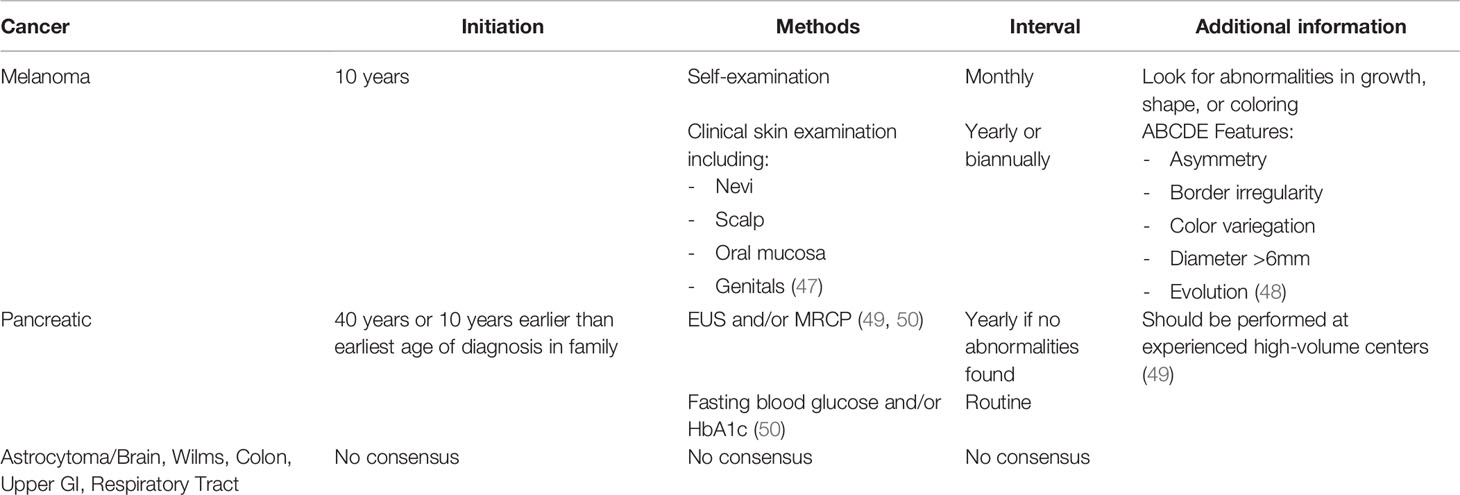
Many groups have provided recommendations for high-risk families with CDKN2A PVs, which include increased frequency of clinical skin examinations beginning in childhood (12, 46, 47). Suggested surveillance include clinical skin examinations yearly or biannually starting from age 10 with monthly self-examination of the skin beginning in childhood (47). When identified, suspicious moles should be biopsied and removed (47). Lifestyle modifications have been recommended for individuals with CDKN2A PVs that include limiting exposure to the sun and to ultraviolet radiation. Protective clothing should be worn when exposure is unavoidable (47). (see Table 2)
Full body skin examinations should include the scalp, oral mucosa, and genitals, as significant variability has been reported regarding location of melanomas (41). A healthcare provider should examine nevi for features of melanoma every 6 to 12 months. The patient should look for abnormalities in growth, shape or coloring through self-examinations of the skin. The ABCDE features (Asymmetry, Border irregularity, Color variegation, Diameter >6mm, Evolution) of melanoma should be screened for in these patients (13, 48).
Behavioral Changes in CDKN2A Carriers
Predictive genetic testing has been shown to increase the uptake of cancer screening and prevention (51–53). In the past, concerns were raised about offering predictive DNA analysis for families suspicious of harboring a CDKN2A PV outside of defined research protocols. The concern was that the likelihood of finding a PV was low and the efficacy of melanoma prevention was lacking (54). In general meta-analysis of the benefits of predictive genetic testing for disease prevention in cohorts of multiple complex hereditary conditions showed mixed results (52, 55). In studies of CDNK2A carriers specifically, positive outcomes were reported one year post-counseling, including fewer reported sunburns and lower daily ultraviolet radiation dose compared to baseline analysis (53). Another study found that two years following genetic counseling, unaffected CDKN2A carriers reported improvements in following the recommendation to undergo annual total body skin examinations and increased thoroughness in their monthly skin self-examinations (51). Genetic counseling is recommended by the NCCN for familial melanoma following the “rule of three,” including three family members with melanoma/pancreatic cancer/astrocytoma on the same side of the family or an individual with three malignant melanomas or associated tumors (49).
Genetic testing to identify hereditary cancer risks has the potential for preventative surveillance and medical management options if a genetic predisposition is identified in an individual. While some hereditary cancer syndromes have surveillance guidelines that require routine medical follow-ups and options for additional imaging or surgery to reduce risks, hereditary predisposition to melanoma can be complicated by its multifactorial nature. The greater onus of prevention may fall on the individual. Behavioral modifications such as wearing sunscreen, limiting prolonged sun exposure and practicing general sun safety can allow some individuals to feel a greater sense of control in their medical management, but can also create limitations for other individuals who may not feel adequately prepared to make such behavioral modifications (56, 57).
Given the risk for melanoma is impacted by environmental factors such as UV exposure, a deeper understanding of the behavioral changes among individuals with a CDKN2A PV is important in tailoring medical management and targeting surveillance efforts. Several studies have indicated that identification of carrier status with a CDKN2A pathogenic variant can have cognitive and behavioral impacts beyond family history-based risk assessment alone (53, 58, 59). For example, one study showed that two years after undergoing genetic testing, CDKN2A carriers without a personal history of melanoma were found to have a 30% increase in adherence to total body skin examination (TBSEs) (p=0.032, one tailed) (60). This adherence was comparable to family members with melanoma who tested positive for the PV (p= 0.635).
While receiving a test result indicating a CDKN2A PV has been showing to have dramatic effects on behavior, not all patients undergoing testing will receive this result. Testing negative for a familial CDKN2A has not been found to have negative effects such as promoting increased UV exposure. At-risk relatives from melanoma-prone families without a known genetic etiology and for whom genetic testing was not available, also have been shown to benefit from genetic counseling about melanoma risk. Following genetic counseling they also exhibited significantly decreased UV exposure, though not as quickly or to the extent as CDKN2A carriers (53).
CDKN2A testing and test reporting in these studies was conducted in the setting of pre- and post-test genetic counseling. Pairing genetic testing with appropriate genetic counseling will maximize the benefit of this information for patients. Studies have shown that relatives at risk for CDKN2A PVs exhibit high levels of interest in genetic testing, similar to levels of interest in families with other hereditary cancer syndromes.
Predictive Genetic Testing for Minors
Offering genetic testing to children is recommended only for conditions in which early intervention is available and the potential benefit of testing at that age outweighs the potential psychological harms (13). While the melanoma risk associated with CDKN2A PVs typically present in adulthood, screening recommendations initiate at age 10. Additionally, childhood is a time of significant UV exposure, and testing earlier in life may present an opportunity to minimize exposures that would contribute to melanoma risk later in life.
Genetic testing and counseling for CDKN2A has been shown to improve photoprotective behaviors among children (ages 10-15y), decrease sunburns by over 50% (p>.05), and increase adherence to sun-protective behaviors (55.6% vs. 88.9%, p = 0.04) one year after genetic counseling and testing (61). The decrease in sunburns and adherence to sun protection was reported equally by both carriers and non-carriers (p > 0.05) highlighting the importance of pre-test genetic counseling in improving awareness regarding sun-protective behaviors (61). There was no perceived increase noted in anxiety or depression among minors who underwent genetic testing for CDKN2A. Counseling and testing of children may heighten parents’ awareness of the need for sun protection in childhood.
It should be acknowledged that there are many barriers to sustainable and life-long behavioral changes and these can be challenging for individuals and families. According to Wu et al., peer influence can be an important factor impacting engagement in sun protective behaviors among children (56). Family modeling and communication, such as parents modeling preventative behaviors, can allow for improved engagement in sun protective behaviors among children. Interventions targeting education for broader populations regarding sun protection and dispelling of myths related to UV exposure/sun safety, such as the perception of reduced UVR exposure in winter, may be beneficial in addressing gaps in education and awareness among the general population.
Pancreatic Cancer Surveillance
Pancreatic ductal adenocarcinoma (PDAC) is seen in association with FAMMM as the second most frequent cancer diagnosis in these kindreds (14, 15). Pancreatic cancer is often diagnosed at later stages, which is associated with poorer prognoses (62, 63). Less than ¼ of patients are candidates for potentially curative surgical resection at the time of diagnosis (64), therefore early detection is extremely important in improving survival outcomes (50).
While effective screening and prevention measures for melanoma exist, the efficacy of pancreatic cancer surveillance has not been as well established (65). It is also unknown how individuals with a CDKN2A PV may make behavioral changes regarding their pancreatic cancer risk, given there is greater individual control over melanoma prevention than pancreatic cancer prevention, at least at this time. In one study disclosing the return of research results, 85.7% (n = 12) of CDKN2A carriers indicated that they planned to have their pancreas checked in the next six months. However, not all carriers who intended to be screened for pancreatic cancer did so within six months. Those with positive CDKN2A results were more likely to communicate these results to their healthcare teams than non-carriers (66).
PDAC Surveillance Guidelines
Pancreatic cancer screening guidelines have evolved over the years. Most recently, the International Cancer of the Pancreas Screening (CAPS) Consortium and the National Comprehensive Cancer Network (NCCN) have established consensus guidelines for surveillance of high-risk individuals (49, 50). Current CAPS and NCCN recommendations support pancreatic cancer screening for individuals with CDKN2A PVs, regardless of their family history.
For CDKN2A PV carriers, these guidelines recommend initiation of surveillance 10 years earlier than the earliest age of pancreatic cancer diagnosis in the family, or at age 40, whichever is earlier. The NCCN guidelines recommend that individuals considered to be at high risk for pancreatic cancer pursue these screenings at experienced high-volume centers after having in-depth discussions about the benefits and limitations of these screenings with their healthcare providers (49).
Surveillance methods include annual imaging with endoscopic ultrasound (EUS) and/or MRI/Magnetic resonance cholangiopancreatography (MRCP) per both CAPS and NCCN recommendations. CAPS guidelines recommend routine testing for late onset diabetes with fasting blood glucose and/or HbA1c, adding that high-risk individuals with new-onset diabetes should prompt additional screening (50). One year interval surveillance was recommended for those without abnormalities on imaging (49, 50). However, the CAPS Consortium did not reach a consensus on how to alternate the two screening modalities (50).
The Dutch Familial Pancreatic Cancer surveillance study performed a prospective study aimed at determining the long-term yield of PDAC surveillance in high-risk individuals between the years 2006-2019 (63). PDACs found through surveillance in the high-risk group were more likely to be resectable than sporadic PDACs diagnosed on the basis of development of symptoms. Of the 96 participants with CDKN2A PVs in this study, 7 were found to have PDAC through surveillance. EUS was found to be a superior imaging tool at detecting PDAC lesions with a solid component when compared to MRI/MRCP, while MRI/MRCP was found to be more sensitive at identifying small (sub-cm) cystic lesions. The diagnostic yield of PDAC was beneficial in high-risk patients, including those with CDKN2A PVs, but timely identification of disease in these patients still remains challenging. Individuals included in the study were highly adherent to scheduled procedures, which suggests that those with PDAC susceptibility PVs are ideally suited for increased surveillance (63).
Other studies have shown that PDAC surveillance of CDKN2A PV carriers is beneficial in detecting PDACs at a more resectable stage (66). Prospective screening data from three European centers were collected. Of those individuals who participated in surveillance programs diagnosed with PDAC, the resection rate was found to be 75% with a 5-year survival rate of 24% (compared historically to 13-21.2% with a 5-year survival rate of 4-7% for sporadic PDAC) (62, 66).
A second study following patients enrolled in the Cancer of the Pancreas Screening cohort also found strong evidence supporting the use of pancreatic surveillance in high-risk individuals (62, 67). This study found the majority of PDACs detected during screening to be resectable (90%) with a significantly increased 3-year survival outcome (85%). These two studies highlight the potential benefit of PDAC surveillance in high-risk cohorts and were used to justify the update to the CAPS guidelines (50).
Conclusions
Historically germline genetic testing for cancer susceptibility was encouraged for genes with established clinical utility (68, 69). For many years, CDKN2A genetic testing has been felt to limit uptake on this basis. In recent years, developments in the behavioral science literature as well as the pancreatic surveillance literature have altered the risk-benefit ratio in CDKN2A testing. Behavioral literature has demonstrated increased sun-protective behaviors and surveillance, not just for individuals who were positive for CDKN2A PVs but also for those who underwent genetic evaluation for this condition. Additionally, pancreatic surveillance has been effective in identifying asymptomatic pancreatic cancers in this population and may be effective in down staging this disease. For this reason, expert consensus has recommended pancreatic cancer surveillance for all individuals with CDKN2A PVs (49, 50). Based on data emerging in these two areas, re-evaluation of the clinical utility of germline CDKN2A testing is appropriate.
Author Contributions
AK, KP, WK, and JJ contributed to conception and design of the manuscript. AK and KP wrote the first draft of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Author Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors extend gratitude to Katy Nottingham, for editorial assistance. This publication utilized the Genetic Counseling Shared Resource at Huntsman Cancer Institute at the University of Utah and was supported by the National Cancer Institute of the National Institutes of Health under Award Number P30CA042014.
References
1. Toussi A, Mans N, Welborn J, Kiuru M. Germline Mutations Predisposing to Melanoma. J Cutaneous Pathol (2020) 47(7):606–16. doi: 10.1111/cup.13689
2. Soura E, Eliades PJ, Shannon K, Stratigos AJ, Tsao H. Hereditary Melanoma: Update on Syndromes and Management: Genetics of Familial Atypical Multiple Mole Melanoma Syndrome. J Am Acad Dermatol (2016) 74(3):395–407quiz 8–10. doi: 10.1016/j.jaad.2015.08.038
3. Arnold M, de Vries E, Whiteman DC, Jemal A, Bray F, Parkin DM, et al. Global Burden of Cutaneous Melanoma Attributable to Ultraviolet Radiation in 2012. Int J Cancer (2018) 143(6):1305–14. doi: 10.1002/ijc.31527
4. Eide MJ, Weinstock MA. Association of UV Index, Latitude, and Melanoma Incidence in Nonwhite Populations–US Surveillance, Epidemiology, and End Results (SEER) Program, 1992 to 2001. Arch Dermatol (2005) 141(4):477–81. doi: 10.1001/archderm.141.4.477
5. Elwood JM, Jopson J. Melanoma and Sun Exposure: An Overview of Published Studies. Int J Cancer (1997) 73(2):198–203. doi: 10.1002/(SICI)1097-0215(19971009)73:2<198::AID-IJC6>3.0.CO;2-R
6. Oliveria SA, Saraiya M, Geller AC, Heneghan MK, Jorgensen C. Sun Exposure and Risk of Melanoma. Arch Dis Child (2006) 91(2):131–8. doi: 10.1136/adc.2005.086918
7. Qureshi AA, Laden F, Colditz GA, Hunter DJ. Geographic Variation and Risk of Skin Cancer in US Women. Differences Between Melanoma, Squamous Cell Carcinoma, and Basal Cell Carcinoma. Arch Intern Med (2008) 168(5):501–7. doi: 10.1001/archinte.168.5.501
8. Scherer D, Kumar R. Genetics of Pigmentation in Skin Cancer–A Review. Mutat Res (2010) 705(2):141–53. doi: 10.1016/j.mrrev.2010.06.002
9. Aoude LG, Heitzer E, Johansson P, Gartside M, Wadt K, Pritchard AL, et al. POLE Mutations in Families Predisposed to Cutaneous Melanoma. Fam Cancer (2015) 14(4):621–8. doi: 10.1007/s10689-015-9826-8
10. Pissa M, Helkkula T, Appelqvist F, Silander G, Borg ÅVerifytat, Pettersson J, et al. CDKN2A Genetic Testing in Melanoma-Prone Families in Sweden in the Years 2015–2020: Implications for Novel National Recommendations. Acta Oncol (2021) 60(7):888–96. doi: 10.1080/0284186X.2021.1914346
11. Robles-Espinoza CD, Harland M, Ramsay AJ, Aoude LG, Quesada V, Ding Z, et al. POT1 Loss-of-Function Variants Predispose to Familial Melanoma. Nat Genet (2014) 46(5):478–81. doi: 10.1038/ng.2947
12. Rossi M, Pellegrini C, Cardelli L, Ciciarelli V, Di Nardo L, Fargnoli MC. Familial Melanoma: Diagnostic and Management Implications. Dermatol Pract Concept (2019) 9(1):10–6. doi: 10.5826/dpc.0901a03
13. Eckerle Mize D, Bishop M, Resse E, Sluzevich J. Familial Atypical Multiple Mole Melanoma Syndrome. In: Riegert-Johnson DL, Boardman LA, Hefferon T, Roberts M, editors. Cancer Syndromes. Bethesda (MD: National Center for Biotechnology Information (US) Copyright © 2009-, Douglas L Riegert-Johnson (2009).
14. Goldstein AM, Fraser MC, Struewing JP, Hussussian CJ, Ranade K, Zametkin DP, et al. Increased Risk of Pancreatic Cancer in Melanoma-Prone Kindreds With P16 INK4 Mutations. N Engl J Med (1995) 333(15):970–5. doi: 10.1056/NEJM199510123331504
15. Habbe N, Langer P, Sina-Frey M, Bartsch D. Familial Pancreatic Cancer Syndromes. Endocrinol Metab Clinics North Am (2006) 35:417–30, xi. doi: 10.1016/j.ecl.2006.02.016
16. Hruban RH, Canto MI, Goggins M, Schulick R, Klein AP. Update on Familial Pancreatic Cancer. Adv Surg (2010) 44:293–311. doi: 10.1016/j.yasu.2010.05.011
17. Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley JW, Kamel I, et al. International Cancer of the Pancreas Screening (CAPS) Consortium Summit on the Management of Patients With Increased Risk for Familial Pancreatic Cancer. Gut (2013) 62(3):339–47. doi: 10.1136/gutjnl-2012-303108
18. Middlebrooks CD, Stacey ML, Li Q, Snyder C, Shaw TG, Richardson-Nelson T, et al. Analysis of the CDKN2A Gene in FAMMM Syndrome Families Reveals Early Age of Onset for Additional Syndromic Cancers. Cancer Res (2019) 79(11):2992–3000. doi: 10.1158/0008-5472.CAN-18-1580
19. Mukherjee B, Delancey JO, Raskin L, Everett J, Jeter J, Begg CB, et al. Risk of Non-Melanoma Cancers in First-Degree Relatives of CDKN2A Mutation Carriers. J Natl Cancer Inst (2012) 104(12):953–6. doi: 10.1093/jnci/djs221
20. Parker JF, Florell SR, Alexander A, DiSario JA, Shami PJ, Leachman SA. Pancreatic Carcinoma Surveillance in Patients With Familial Melanoma. Arch Dermatol (2003) 139(8):1019–25. doi: 10.1001/archderm.139.8.1019
21. Rulyak SJ, Lowenfels AB, Maisonneuve P, Brentnall TA. Risk Factors for the Development of Pancreatic Cancer in Familial Pancreatic Cancer Kindreds. Gastroenterology (2003) 124(5):1292–9. doi: 10.1016/S0016-5085(03)00272-5
22. Bishop DT, Demenais F, Goldstein AM, Bergman W, Bishop JN, Bressac-de Paillerets B, et al. Geographical Variation in the Penetrance of CDKN2A Mutations for Melanoma. J Natl Cancer Inst (2002) 94(12):894–903. doi: 10.1093/jnci/94.12.894
23. Cust AE, Harland M, Makalic E, Schmidt D, Dowty JG, Aitken JF, et al. Melanoma Risk for CDKN2A Mutation Carriers Who Are Relatives of Population-Based Case Carriers in Australia and the UK. J Med Genet (2011) 48(4):266–72. doi: 10.1136/jmg.2010.086538
24. Santillan AA, Cherpelis BS, Glass LF, Sondak VK. Management of Familial Melanoma and Nonmelanoma Skin Cancer Syndromes. Surg Oncol Clin N Am (2009) 18(1):73–98, viii. doi: 10.1016/j.soc.2008.08.003
25. Ford D, Bliss JM, Swerdlow AJ, Armstrong BK, Franceschi S, Green A, et al. Risk of Cutaneous Melanoma Associated With a Family History of the Disease. Int J Cancer (1995) 62(4):377–81. doi: 10.1002/ijc.2910620403
26. Eskandarpour M, Hashemi J, Kanter L, Ringborg U, Platz A, Hansson J. Frequency of UV-Inducible NRAS Mutations in Melanomas of Patients With Germline CDKN2A Mutations. JNCI: J Natl Cancer Institute (2003) 95(11):790–8. doi: 10.1093/jnci/95.11.790
27. Zebary A, Omholt K, van Doorn R, Ghiorzo P, Harbst K, Hertzman Johansson C, et al. Somatic BRAF and NRAS Mutations in Familial Melanomas With Known Germline CDKN2A Status: A Genomel Study. J Invest Dermatol (2014) 134(1):287–90. doi: 10.1038/jid.2013.270
28. Jovanovic B, Egyhazi S, Eskandarpour M, Ghiorzo P, Palmer JM, Bianchi Scarrà G, et al. Coexisting NRAS and BRAF Mutations in Primary Familial Melanomas With Specific CDKN2A Germline Alterations. J Invest Dermatol (2010) 130(2):618–20. doi: 10.1038/jid.2009.287
29. Dalmasso B, Pastorino L, Ciccarese G, Andreotti V, Grillo F, Mastracci L, et al. CDKN2A Germline Mutations are Not Associated With Poor Survival in an Italian Cohort of Melanoma Patients. J Am Acad Dermatol (2019) 80(5):1263–71. doi: 10.1016/j.jaad.2018.07.060
30. Vasen H, Ibrahim I, Ponce CG, Slater EP, Matthäi E, Carrato A, et al. Benefit of Surveillance for Pancreatic Cancer in High-Risk Individuals: Outcome of Long-Term Prospective Follow-Up Studies From Three European Expert Centers. J Clin Oncol (2016) 34(17):2010–9. doi: 10.1200/JCO.2015.64.0730
31. Helgadottir H, Höiom V, Jönsson G, Tuominen R, JIngvar C, Borg A, et al. High Risk of Tobacco-Related Cancers in CDKN2A Mutation-Positive Melanoma Families. J Med Genet (2014) 51(8):545–52. doi: 10.1136/jmedgenet-2014-102320
32. Bishop JA, Wachsmuth RC, Harland M, Bataille V, Pinney E, Mac KP, et al. Genotype/Phenotype and Penetrance Studies in Melanoma Families With Germline CDKN2A Mutations. J Invest Dermatol (2000) 114(1):28–33. doi: 10.1046/j.1523-1747.2000.00823.x
33. Carey WP Jr., Thompson CJ, Synnestvedt M, Dt G, Halpern A, Schultz D, et al. Dysplastic Nevi as a Melanoma Risk Factor in Patients With Familial Melanoma. Cancer (1994) 74(12):3118–25. doi: 10.1002/1097-0142(19941215)74:12<3118::AID-CNCR2820741210>3.0.CO;2-7
34. Swerdlow AJ, English J, MacKie RM, O’Doherty CJ, Hunter JA, Clark J, et al. Benign Melanocytic Naevi as a Risk Factor for Malignant Melanoma. (Clin Res Ed) (1986) 292(6535):1555–9. doi: 10.1136/bmj.292.6535.1555
35. Halpern AC, Guerry Dt, Elder DE, Clark WH Jr., Synnestvedt M, Norman S, et al. Dysplastic Nevi as Risk Markers of Sporadic (Nonfamilial) Melanoma. A Case-Control Study Arch Dermatol (1991) 127(7):995–9. doi: 10.1001/archderm.127.7.995
36. Bataille V, Bishop JA, Sasieni P, Swerdlow AJ, Pinney E, Griffiths K, et al. Risk of Cutaneous Melanoma in Relation to the Numbers, Types and Sites of Naevi: A Case-Control Study. Br J Cancer (1996) 73(12):1605–11. doi: 10.1038/bjc.1996.302
37. Czajkowski R, Placek W, Drewa G, Czajkowska A, Uchańska G. FAMMM Syndrome: Pathogenesis and Management. Dermatol Surg (2004) 30(2 Pt 2):291–6. doi: 10.1111/j.1524-4725.2004.30088.x
38. Kelly JW, Yeatman JM, Regalia C, Mason G, Henham AP. A High Incidence of Melanoma Found in Patients With Multiple Dysplastic Naevi by Photographic Surveillance. Med J Aust (1997) 167(4):191–4. doi: 10.5694/j.1326-5377.1997.tb138843.x
39. van der Rhee JI, Krijnen P, Gruis NA, de Snoo FA, Vasen HFA, Putter H, et al. Clinical and Histologic Characteristics of Malignant Melanoma in Families With a Germline Mutation in CDKN2A. J Am Acad Dermatol (2011) 65(2):281–8. doi: 10.1016/j.jaad.2010.06.044
40. Goldstein AM, Chan M, Harland M, Gillanders EM, Hayward NK, Avril MF, et al. High-Risk Melanoma Susceptibility Genes and Pancreatic Cancer, Neural System Tumors, and Uveal Melanoma Across Genomel. Cancer Res (2006) 66(20):9818–28. doi: 10.1158/0008-5472.CAN-06-0494
41. Måsbäck A, Olsson H, Westerdahl J, Sandberg T, Borg A, Jonsson N, et al. Clinical and Histopathological Features of Malignant Melanoma in Germline CDKN2A Mutation Families. Melanoma Res (2002) 12(6):549–57. doi: 10.1097/00008390-200212000-00004
42. Lynch HT, Brand RE, Hogg D, Deters CA, Fusaro RM, Lynch JF, et al. Phenotypic Variation in Eight Extended CDKN2A Germline Mutation Familial Atypical Multiple Mole Melanoma-Pancreatic Carcinoma-Prone Families: The Familial Atypical Mole Melanoma-Pancreatic Carcinoma Syndrome. Cancer (2002) 94(1):84–96. doi: 10.1002/cncr.10159
43. Gironi LC, Colombo E, Farinelli P, Giorgione R, Bozzola C, Ogliara P, et al. Germline CDKN2A Mutations in Childhood Melanoma: A Case of Melanoma-Pancreatic Cancer Syndrome. Int J Dermatol (2015) 54(12):e553–5. doi: 10.1111/ijd.12933
44. Pedace L, De Simone P, Castori M, et al. Clinical Features Predicting Identification of CDKN2A Mutations in Italian Patients With Familial Cutaneous Melanoma. Cancer Epidemiol (2011) 35(6):e116–20. doi: 10.1016/j.canep.2011.07.007
45. Goldstein AM, Struewing JP, Chidambaram A, Fraser MC, Tucker MA. Genotype-Phenotype Relationships in U.S. Melanoma-Prone Families With CDKN2A and CDK4 Mutations. J Natl Cancer Inst (2000) 92(12):1006–10. doi: 10.1093/jnci/92.12.1006
46. Niendorf KB, Tsao H. Cutaneous Melanoma: Family Screening and Genetic Testing. Dermatol Ther (2006) 19(1):1–8. doi: 10.1111/j.1529-8019.2005.00050.x
47. CDKN2A Mutations: Cancer Risk and Management Recommendations 2020 (2020). UT Southwestern Harold C: Simmons Comprehensive Cancer Center. Available at: https://s3-us-west-2.amazonaws.com/utsw-patientcare-web-production/documents/Familial_Atypical_Mole_Melanoma_CDKN2A.pdf (Accessed November 30, 2021).
48. Abbasi NR, Shaw HM, Rigel DS, Friedman RJ, McCarthy WH, Osman I, et al. Early Diagnosis of Cutaneous Melanoma: Revisiting the ABCD Criteria. Jama (2004) 292(22):2771–6. doi: 10.1001/jama.292.22.2771
49. National Comprehensive Cancer Network. Genetic/Familial High-Risk Assessment: Breast, Ovarian and Pancreatic (Version 1.2022) . Available at: https://www.nccn.org/guidelines/guidelines-detail?category=2&id=1503 (Accessed December 10, 2021).
50. Goggins M, Overbeek KA, Brand R, Syngal S, Del Chiaro M, Bartsch DK, et al. Management of Patients With Increased Risk for Familial Pancreatic Cancer: Updated Recommendations From the International Cancer of the Pancreas Screening (CAPS) Consortium. Gut (2020) 69(1):7–17. doi: 10.1136/gutjnl-2019-319352corr1
51. Aspinwall LG, Taber JM, Kohlmann W, Leaf SL, Leachman SA. Unaffected Family Members Report Improvements in Daily Routine Sun Protection 2 Years Following Melanoma Genetic Testing. Genet Med (2014) 16(11):846–53. doi: 10.1038/gim.2014.37
52. Hollands GJ, French DP, Griffin SJ, Prevost AT, Sutton S, King S, et al. The Impact of Communicating Genetic Risks of Disease on Risk-Reducing Health Behaviour: Systematic Review With Meta-Analysis. BMJ (2016) 352:i1102. doi: 10.1136/bmj.i1102
53. Stump TK, Aspinwall LG, Drummond DM, Taber JM, Kohlmann W, Champine M, et al. CDKN2A Testing and Genetic Counseling Promote Reductions in Objectively Measured Sun Exposure One Year Later. Genet Med (2020) 22(1):26–34. doi: 10.1038/s41436-019-0608-9
54. Kefford RF, Mann GJ. Is There a Role for Genetic Testing in Patients With Melanoma? Curr Opin Oncol (2003) 15(2):157–61. doi: 10.1097/00001622-200303000-00007
55. Frieser MJ, Wilson S, Vrieze S. Behavioral Impact of Return of Genetic Test Results for Complex Disease: Systematic Review and Meta-Analysis. Health Psychol (2018) 37(12):1134–44. doi: 10.1037/hea0000683
56. Wu YP, Parsons BG, Mooney R, Aspinwall LG, Cloyes K, Hay JL, et al. Barriers and Facilitators to Melanoma Prevention and Control Behaviors Among at-Risk Children. J Community Health (2018) 43(5):993–1001. doi: 10.1007/s10900-018-0516-y
57. Tripp MK, Peterson SK, Prokhorov AV, Shete SS, Lee JE, Gershenwald JE, et al. Correlates of Sun Protection and Sunburn in Children of Melanoma Survivors. Am J Prev Med (2016) 51(3):e77–85. doi: 10.1016/j.amepre.2016.02.032
58. Aspinwall LG, Stump TK, Taber JM, Drummond DM, Kohlmann W, Champine M, et al. Genetic Test Reporting of CDKN2A Provides Informational and Motivational Benefits for Managing Melanoma Risk. Transl Behav Med (2018) 8(1):29–43. doi: 10.1093/tbm/ibx011
59. Aspinwall LG, Leaf SL, Kohlmann W, Dola ER, Leachman SA. Patterns of Photoprotection Following CDKN2A/P16 Genetic Test Reporting and Counseling. J Am Acad Dermatol (2009) 60(5):745–57. doi: 10.1016/j.jaad.2008.12.034
60. Aspinwall LG, Taber JM, Leaf SL, Kohlmann W, Leachman SA. Melanoma Genetic Counseling and Test Reporting Improve Screening Adherence Among Unaffected Carriers 2 Years Later. Cancer Epidemiol Biomarkers Prev (2013) 22(10):1687–97. doi: 10.1158/1055-9965.EPI-13-0422
61. Stump TK, Aspinwall LG, Kohlmann W, Champine M, Hauglid J, Wu YP, et al. Genetic Test Reporting and Counseling for Melanoma Risk in Minors May Improve Sun Protection Without Inducing Distress. J Genet Couns (2018) 27(4):955–67. doi: 10.1007/s10897-017-0185-5
62. Huang L, Jansen L, Balavarca Y, Molina-Montes E, Babaei M, van der Geest L, et al. Resection of Pancreatic Cancer in Europe and USA: An International Large-Scale Study Highlighting Large Variations. Gut (2019) 68(1):130–9. doi: 10.1136/gutjnl-2017-314828
63. Overbeek KA, Levink IJM, Koopmann BDM, Harinck F, Konings ICAW, Ausems MGEM, et al. Long-Term Yield of Pancreatic Cancer Surveillance in High-Risk Individuals. Gut (2021):gutjnl-2020-323611. doi: 10.1136/gutjnl-2020-323611
64. Gudjonsson B. Pancreatic Cancer: 80 Years of Surgery—Percentage and Repetitions. HPB Surg (2016) 2016:6839687. doi: 10.1155/2016/6839687
65. Poruk KE, Firpo MA, Adler DG, Mulvihill SJ. Screening for Pancreatic Cancer: Why, How, and Who? Ann Surg (2013) 257(1):17–26. doi: 10.1097/SLA.0b013e31825ffbfb
66. Leof ER, Zhu X, Rabe KG, McCormick JB, Petersen GM, Radecki Breitkopf C. Pancreatic Cancer and Melanoma Related Perceptions and Behaviors Following Disclosure of CDKN2A Variant Status as a Research Result. Genet Med (2019) 21(11):2468–77. doi: 10.1038/s41436-019-0517-y
67. Robson ME, Bradbury AR, Arun B, Domchek SM, Ford JM, Hampel HL, et al. American Society of Clinical Oncology Policy Statement Update: Genetic and Genomic Testing for Cancer Susceptibility. J Clin Oncol (2015) 33(31):3660–7. doi: 10.1200/JCO.2015.63.0996
68. ACMG Board of Directors. Clinical Utility of Genetic and Genomic Services: A Position Statement of the American College of Medical Genetics and Genomics. Genet Med (2015) 17:505–7. doi: 10.1038/gim.2015.41
Keywords: CDKN2A, melanoma, pancreatic cancer, surveillance, behavior change, hereditary cancer syndromes
Citation: Pauley K, Khan A, Kohlmann W and Jeter J (2022) Considerations for Germline Testing in Melanoma: Updates in Behavioral Change and Pancreatic Surveillance for Carriers of CDKN2A Pathogenic Variants. Front. Oncol. 12:837057. doi: 10.3389/fonc.2022.837057
Received: 16 December 2021; Accepted: 23 February 2022;
Published: 16 March 2022.
Edited by:
Joshua Arbesman, Cleveland Clinic, United StatesReviewed by:
Lorenza Pastorino, University of Genoa, ItalyPauline Funchain, Cleveland Clinic, United States
Copyright © 2022 Pauley, Khan, Kohlmann and Jeter. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joanne Jeter, Sm9hbm5lLkpldGVyQGhjaS51dGFoLmVkdQ==
†These authors have contributed equally to this work and share first authorship
 Kristen Pauley
Kristen Pauley Ambreen Khan
Ambreen Khan Wendy Kohlmann
Wendy Kohlmann Joanne Jeter
Joanne Jeter