- 1Department of Epidemiology, Center for Global Health, School of Public Health, Nanjing Medical University, Nanjing, China
- 2Department of Thoracic Surgery, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China
- 3Department of Thoracic Surgery, Jiangsu Key Laboratory of Molecular and Translational Cancer Research, Jiangsu Cancer Hospital, Jiangsu Institute of Cancer Research, The Affiliated Cancer Hospital of Nanjing Medical University, Nanjing, China
- 4Jiangsu Key Lab of Cancer Biomarkers, Prevention and Treatment, Collaborative Innovation Center for Cancer Personalized Medicine, Nanjing Medical University, Nanjing, China
- 5Department of Cancer Prevention, The Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), Hangzhou, China
- 6Institute of Basic Medicine and Cancer (IBMC), Chinese Academy of Sciences, Hangzhou, China
- 7Research Units of Cohort Study on Cardiovascular Diseases and Cancers, Chinese Academy of Medical Sciences, Beijing, China
Background: It remains undetermined whether neuroticism affects the risk of lung cancer. Therefore, we performed complementary observational and Mendelian randomization (MR) analyses to investigate the association between neuroticism and lung cancer risk.
Methods: We included 364,451 UK Biobank participants free of cancer at baseline. Neuroticism was ascertained using the 12-item of Eysenck Personality Inventory Neuroticism Scale. Multivariable Cox regression models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). Two-sample MR analysis was carried out with summary genetic data from UK Biobank (374,323 individuals) and International Lung Cancer Consortium (29,266 lung cancer cases and 56,450 controls). Furthermore, we calculated a polygenic risk score of lung cancer, and examined the joint-effect and interaction between neuroticism and genetic susceptibility on lung cancer risk.
Results: During a median follow-up of 7.13 years, 1573 lung cancer cases were documented. After adjusting for smoking and other confounders, higher neuroticism was associated with an increased risk of lung cancer (HR per 1 SD=1.07, 95% CI: 1.02-1.12). Consistently, MR analysis suggested a causal effect of neuroticism on lung cancer risk (OR IVW=1.10, 95% CI: 1.03-1.17). Compared to individuals with low neuroticism and low PRS, those with both high neuroticism and high PRS had the greatest risk of lung cancer (HR=1.82, 95%CI: 1.51-2.20). Furthermore, there was a positive additive but no multiplicative interaction between neuroticism and genetic risk.
Conclusions: Our findings suggest that neuroticism is associated with an elevated risk of incident lung cancer, which is strengthened by the genetic susceptibility to lung cancer. Further studies are necessary to elucidate underlying mechanisms.
Introduction
Neuroticism is a personality trait that reflects relative stability to experience negative emotions. Neuroticism is one of the major dimensions of the Five-Factor Model of personality (1), and it is also one of the most studied psychological dispositions because of its relevance ranging from normal to abnormal emotional functioning (2). Individuals with higher neuroticism would be more likely to be worried, anxious and emotionally unstable (3). There is growing evidence that neuroticism is associated with a wide range of adverse health outcomes, including the occurrence of mental disorders (4), diabetes (5), cardiovascular disease (CVD) (6), cancer (7, 8), and mortality (9). The underlying mechanisms may be directly or indirectly related to endocrine, inflammation, and harmful behaviors (e.g., cigarette smoking and alcohol abuse) (10, 11).
Evidence linking regarding neuroticism to lung cancer risk, however, is scarce and inconsistent. For instance, several retrospective case-control studies have suggested that a low degree of neuroticism was significantly associated with an increased risk of lung cancer (7, 12), while prospective studies found either null (13–17) or positive associations (18) between higher neuroticism and lung cancer risk. Notably, most of the previous studies were limited by the lack of strict adjustment for confounding variables (e.g., smoking and alcohol use), a short follow-up period or small numbers of lung cancer cases. Thus, the exact association between neuroticism and lung cancer still needs to be determined in well-designed prospective studies with large samples.
Moreover, in contrast to observational studies, which are susceptible to reverse causation and confounding bias, Mendelian randomization (MR) analysis is an established approach to assess the causal effect of an exposure on an outcome by using genetic variants as a proxy for the exposure (19). Genetic variants are randomly assigned at gametogenesis, independent of environmental factors and unaffected by disease processes, thus can minimize the influence of residual confounding and reverse causation (20). This approach has been successfully applied to verify different etiological associations (21–24), such as low education is a causal risk factor in the development of lung cancer.
Herein, we performed complementary observational and Mendelian randomization (MR) analyses to assess the association between neuroticism and lung cancer risk based on the UK Biobank resource. In addition, because previous studies, including ours (25, 26), suggest that the genetic factors may modify the associations between behavioral/environmental risk factors and lung cancer risk; therefore, we further assessed the potential joint and interactive effect between neuroticism and genetic susceptibility represented by a polygenic risk score (PRS) on lung cancer risk.
Subjects and Methods
Study Design and Participants
The UK Biobank is a large population-based prospective cohort study, with the study design and data acquisition process described in detail previously (27). Briefly, over 500,000 people aged 40-69, were enrolled between 2006 and 2010 via 22 health assessment centers across England, Wales and Scotland. Social demographics, lifestyle, health-related information are obtained through touch-screen questionnaires and physical measurements. Blood, saliva, and urine were also collected from each participant. The UK Biobank has full ethical approval from the North West Multi-center Research Ethics Committee. All participants provided written informed consent at recruitment.
In the current study, we excluded participants with prevalent cancer at recruitment (n=46,533) and missing data on neuroticism (n=91,523), leaving 364,451 participants in the observational analysis. In addition, only 300,465 individuals of European descent were available for the genetic analysis (Supplementary Figure S1).
Exposure and Covariate Ascertainment
In the baseline survey, neuroticism was assessed using the 12-item neuroticism subscale from the Eysenck Personality Inventory Neuroticism Scale (EPIN‐R) (28). Responses to each item were either “Yes” or “No”, which were summed to produce a total score that varied from 0 to 12. A higher score indicated greater neuroticism. Other covariate data were collected at baseline using standard protocols, including socioeconomic characteristics (age, sex, ethnic background and education level), health-related factor (family history of lung cancer), and lifestyle factors (smoking, alcohol intake, body mass index and physical activity).
Outcome Ascertainment
Incident cases of lung cancer within the UK Biobank cohort were identified through linkage to national cancer registries in England, Wales and Scotland. Participants were followed up to date of diagnosis of lung cancer, date of withdrawal from the study, date of death or loss follow-up (referring to March 31, 2016, for England and Wales and October 31, 2015, for Scotland), whichever came first. Incident lung cancer was identified using the International Classification of Diseases, Tenth Revision codes of C33 and C34.
Mendelian Randomization Analyses
We conducted two-sample Mendelian randomization (MR) analyses to assess the causal association between neuroticism and lung cancer risk. Instrumental variables for neuroticism were selected from a Genome-wide association study (GWAS) of 374,323 individuals of European ancestry in the UK Biobank (GWAS ID: ukb-b-4630), available in the IEU GWAS database (https://gwas.mrcieu.ac.uk/) (29). Among the genome-wide-significant SNPs (P<5×10-8) identified in neuroticism GWAS, we obtained 116 independent SNPs after exclusion of correlated SNPs based on a linkage disequilibrium level of r2 <0.01 (Supplementary Table S1). Moreover, SNPs with intermediate allele frequencies (MAF >0.42) were considered to be strand-ambiguous and removed from the analysis. Consequently, the instruments for neuroticism explained 1.26% (F-statistic 40.63). Besides, summary-level data for lung cancer was obtained from the International Lung Cancer Consortium (totalling 29,266 lung cancer cases and 56,450 controls; dbGAP: phs000876) (30).
The primary method in MR analyses was the inverse-variance weighted (IVW) (31), followed by sensitivity analyses using the maximum likelihood (ML) methods (32), weighted median (WM) (33), MR-Egger regression (34) and MR Robust Adjusted Profile Score (RAPS) (35). We further applied two diagnostic tests, including the MR Egger intercept test of significant deviation from the null (36), and Cochran’s Q-statistic to assess heterogeneity. Moreover, to rule out possible pleiotropic effects, we examined the genetic instruments in the Phenoscanner GWAS database (http://www.phenoscanner.medschl.cam.ac.uk/) to assess previously reported associations (P <5 × 10−8) with confounders (smoking or BMI), and then assessed the effects after manual filtering the related SNPs from the MR analyses. Finally, we performed reverse-direction MR analyses to estimate the effect of lung cancer (exposure) on neuroticism. The MR analysis was performed using the TwoSampleMR R package.
Polygenic Risk Score Calculation
The procedure for genotyping, imputation and quality control of the SNPs in the UK Biobank has been described elsewhere (37). In the present study, we created a polygenic risk score for lung cancer using 18 SNPs based on the largest available lung cancer GWAS of European descent (Supplementary Table S2) (30). Each SNP was recoded as 0, 1, or 2 according to the number of risk alleles; and then multiplied by its respective effect size (β-coefficient) of lung cancer to calculate the PRS: PRS =β1 × SNP1 + β2 × SNP2 +…+ βn × SNPn (38). The genetic risk was categorized into low (lowest tertile), intermediate (second tertile), and high (highest tertile) based on distributions of PRS in non-cases (38, 39).
Other Statistical Analysis
Cox proportional hazards model was used to estimate the hazard ratio (HR) and corresponding 95% confidence interval (CI) for the association between neuroticism and incident lung cancer. The proportional hazards assumption was tested using Schoenfeld residuals. Neuroticism was modeled on the continuous (per 1-standard deviation [SD] increment) or quintile scale. Model 1 was adjusted for age at recruitment (continuous), sex, ethnic background (white, non-white), education (college or university degree, no degree) and family history of lung cancer (no, yes). In the multivariable model 2, we additionally adjusted for lifestyle factors, including smoking status (never, former, current <15, current ≥15 cigarettes/day, current amount unknown), alcohol intake frequency (daily or almost daily, three or four times a week, once or twice a week, once to three times a month, special occasions, never), BMI (kg/m2, <25, 25-29.9, ≥30), and physical activity (MET-h/week, <10, 10-50, ≥50). In genetic analyses, we further adjusted for the first ten genetic principal components and genotyping array batch. Missing data on covariates were coded as a missing indicator for categorical variables and with sex-specific median values for continuous variables. Statistical tests for trends were assessed by treating the variables continuously.
To assess the joint associations, we further classified participants into six groups according to PRS (low, intermediate, high) and neuroticism score (low, high), and estimated hazard ratios of incident lung cancer in different groups compared with those with low PRS and low neuroticism. To quantify the multiplicative interaction between neuroticism and genetic risk of lung cancer, we added an interaction term in the Cox proportional hazards regression models. Additive interaction was evaluated by relative excess risk due to interaction (RERI) and attributable proportion because of the interaction (AP), and its 95% confidence interval (CI) was calculated by drawing 1000 bootstrap samples from the estimation dataset (40). If there was additive interaction, the 95% CIs of the RERI and AP would not include 0.
In addition, to examine whether the associations between neuroticism and lung cancer risk differed by subgroups, stratified analyses were conducted by age at recruitment, sex, ethnic background, education, family history of lung cancer, smoking status, alcohol intake frequency, BMI, physical activity and histological subtypes. The heterogeneity of these stratified estimates was evaluated using the χ2 -based Cochrane’s Q test. Two sensitivity analyses were performed to assess the robustness of the findings: (1) excluding participants with less than one year of follow-up; and (2) using the Fine and Gray competing risk model (41) to account for potential bias due to the competing risks of death.
All above statistical analyses were performed with R (version 3.61) and P < 0.05 (two-sided) was considered statistically significant.
Results
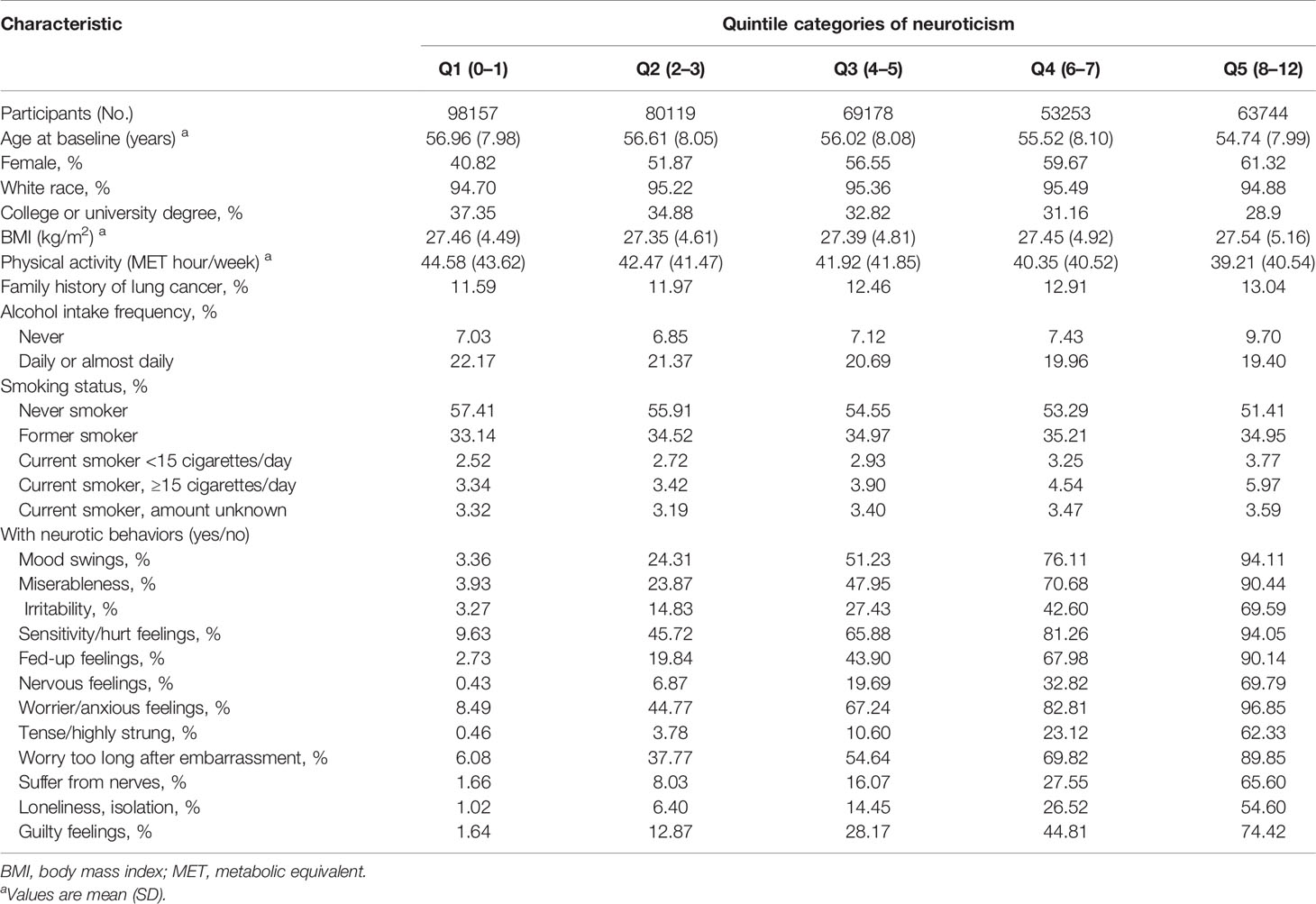
Of the 364,451 participants included in the analysis, 1573 developed incident lung cancer during a median follow-up period of 7.13 years. The baseline characteristics of the participants are shown in Table 1. Compared with those in the lowest quintile of neuroticism, participants in the highest quintile were younger, less educated, less physical activity, and more likely to be smokers and have higher BMI.
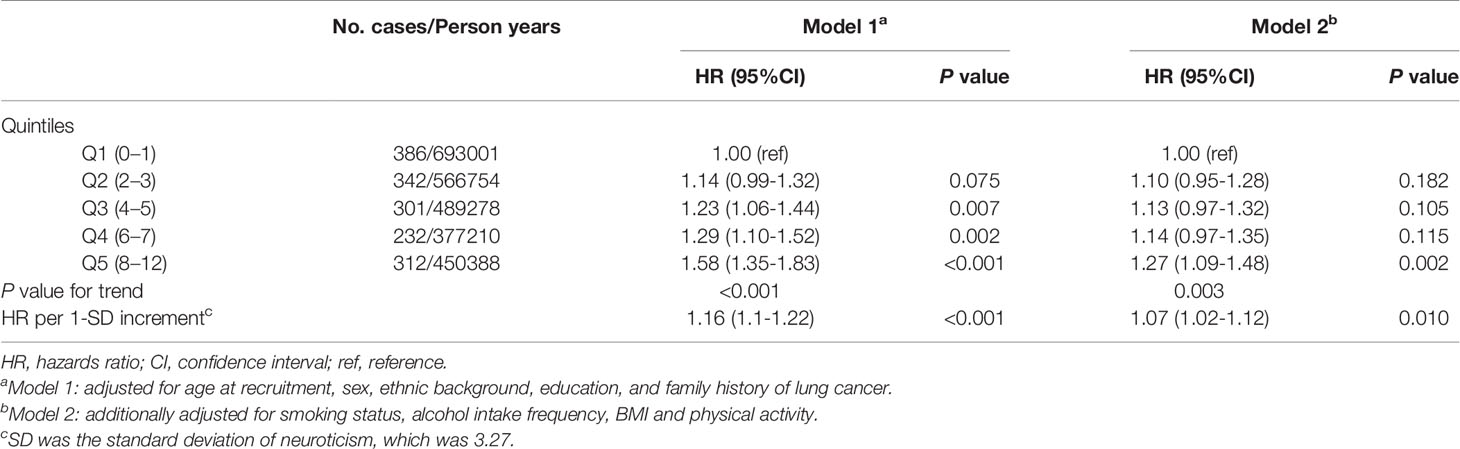
The association between neuroticism and risk of lung cancer is shown in Table 2. Neuroticism was positively associated with lung cancer risk in Model 1 (HR Q5 vs. Q1=1.58, 95% CI: 1.35-1.83), although the estimates were substantially attenuated in model additional adjustment for lifestyle factors (smoking status, alcohol intake, BMI and physical activity) (Model 2: HR Q5 vs. Q1=1.27, 95% CI: 1.09-1.48). Per 1-SD increment in neuroticism was associated with a 7% higher lung cancer risk (Model 2: HR=1.07; 95% CI: 1.02-1.12). In sensitivity analyses, the associations were basically unchanged after excluding individuals with less than one year of follow-up (Supplementary Table S3) and using Fine-Gray models treating the death as a competing risk (Supplementary Table S4).
Similar positive associations were observed in the stratified analyses according to age at recruitment, sex, ethnic background, education, family history of lung cancer, smoking status, alcohol intake frequency, BMI, physical activity and histological subtypes (all Pheterogeneity > 0.05) (Supplementary Table S5). Examination of individual neuroticism items showed that mood swings (HR=1.19, 95% CI: 1.07-1.31), miserableness (HR=1.16, 95%CI: 1.05-1.28), irritability (HR=1.13, 95% CI: 1.01-1.26), and fed up feelings (HR=1.34, 95% CI: 1.21-1.48) were positively associated with lung cancer risk (Supplementary Table S6).
In MR analysis, we obtained 107 SNPs for neuroticism after excluding palindromic SNPs or unavailable SNPs in lung cancer GWAS dataset. A genetically predicted neuroticism was also associated with an increased risk of lung cancer (OR IVW=1.10, 95% CI: 1.03-1.17), and the association was stable in sensitivity analyses using other MR methods (Figure 1). The MR Egger intercept tests yielded no indication of directional horizontal pleiotropy (Pintercept=0.147), but Cochran’s Q test suggested significant heterogeneity among estimates obtained from individual SNPs (P=1.69×10-6). Furthermore, we identified 9 genetic instruments that were also associated with smoking or BMI in the PhenoScanner database, but similar associations were observed after removing these variants (Supplementary Table S7). Additionally, we performed a reverse-direction MR analysis, showing that the genetically instrumented lung cancer was not associated with neuroticism (Supplementary Table S8).
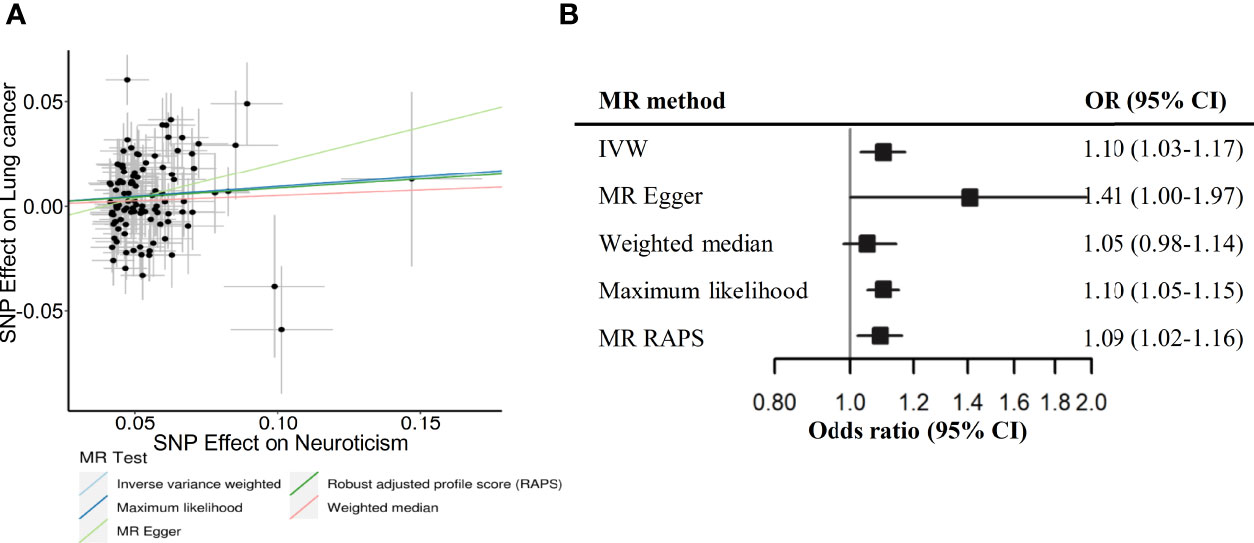
Figure 1 Scatterplot (A) and forest plot (B) depicting Mendelian randomization (MR) results for neuroticism and risk of lung cancer. NSNPs, number of SNP instruments used in the MR analysis; OR, odds ratio; CI, confidence interval; IVW, Inverse variance weighted; RAPS, Robust adjusted profile score - Huber loss function.
The PRS of lung cancer was significantly associated with an increased risk of incident lung cancer (Supplementary Table S9 and Supplementary Figures S2A, B). When combing neuroticism and genetic risk, we observed the joint effect of neuroticism and PRS the risk of incident lung cancer showed a dose-response manner (Ptrend < 0.001, Figure 2). Specifically, compared with participants with low PRS and low neuroticism, those with high PRS and high neuroticism had the highest risk of incident lung cancer (HR=1.82, 95%CI: 1.51-2.20). Meanwhile, there was a positive additive interaction between neuroticism and PRS but no multiplicative interaction (Supplementary Table S10). In addition, among participants with a high PRS, high neuroticism was associated with a 21% increased risk of lung cancer (HR High vs. Low=1.21, 95%CI: 1.02-1.44) than those with low neuroticism (Supplementary Table S11), indicating that even in the context of high genetic risk, low neuroticism implied a reduced risk of lung cancer.
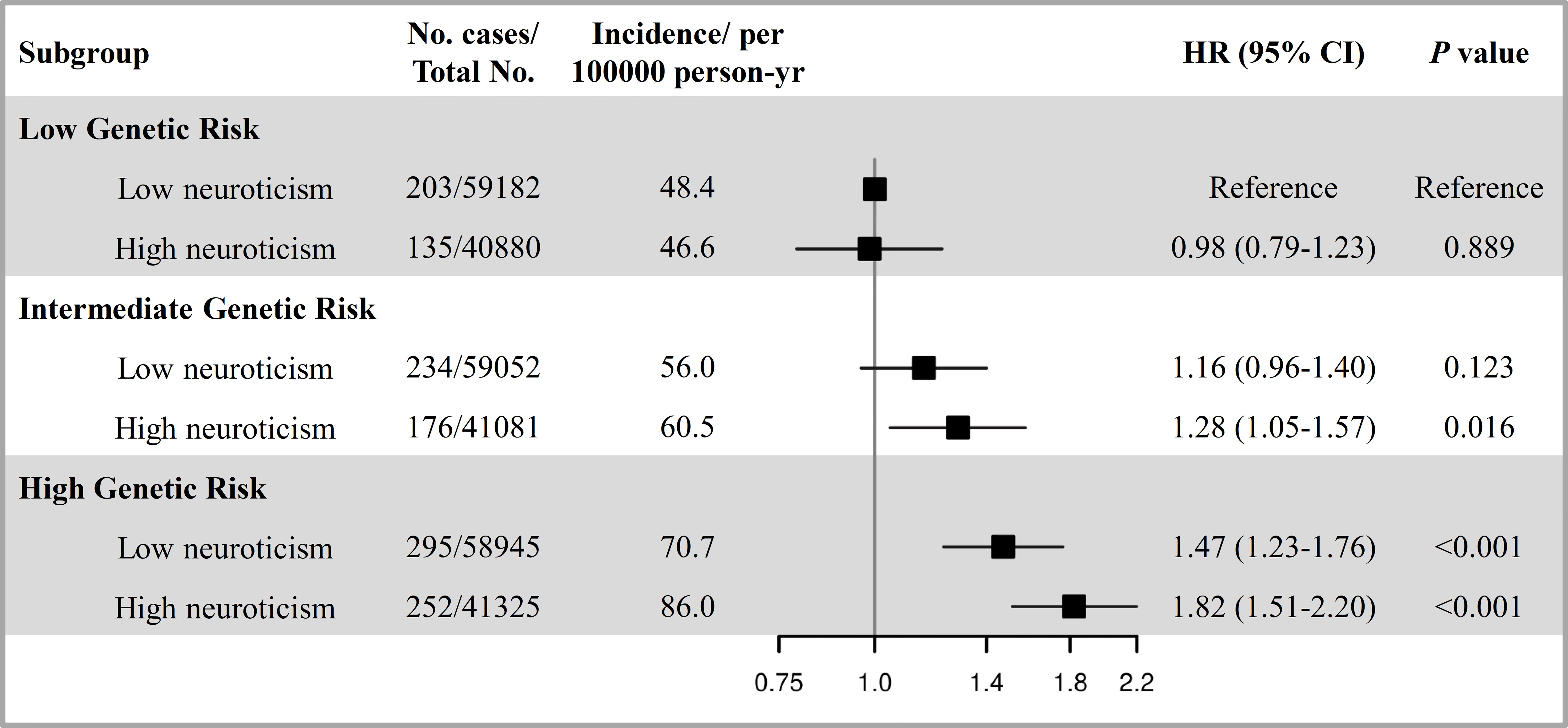
Figure 2 The joint effect and additive interaction of neuroticism and genetic risk with the risk of incident lung cancer. The genetic risk was categorized into low (lowest tertile), intermediate (second tertile), and high (highest tertile). The neuroticism was defined as low and high according to median level. HRs and 95% CIs were estimated using Cox proportional-hazard models with adjustment for age at recruitment, sex, ethnic background, education, family history of lung cancer, smoking status, alcohol intake frequency, BMI, physical activity, the first ten principal components of ancestry and genotyping batch.
Discussion
In this large-scale prospective study, we observed that higher neuroticism was associated with an increased risk of incident lung cancer. Consistently, the MR analysis demonstrated that a positive causal association of neuroticism with lung cancer. Further, when examining the joint effects of neuroticism and genetic risk on lung cancer risk, we demonstrated that the greatest relative increase of risk was among those with high neuroticism and high genetic risk, and there was a positive additive interaction.
Previous studies of neuroticism and lung cancer have led to inconsistent results, and most of these studies found null or weak positive associations that did not reach the statistical threshold, which may partly be due to study designs and small sample sizes. To our knowledge, this is the largest study to date to examine the association between neuroticism and lung cancer incidence (including 1573 cases), thereby having greater statistical power to detect existing associations. In line with our study, a population-based cohort study comprised 59,548 Swedish and Finnish participants also showed a positive association between neuroticism and lung cancer (18). To exclude the interference of confounding factors, we further carried out MR analyses, which confirmed a causal link between neuroticism and lung cancer. Meanwhile, our MR results were robust to numerous sensitivity analyses for confounding, horizontal pleiotropy, and reverse causality. These findings together suggested that neuroticism may be one etiological factor for lung cancer.
Several underlying mechanisms may mediate the association between neuroticism and lung cancer. Biologically, neuroticism may lead to dysregulation of the immune and endocrine systems (11) and an increase in chronic inflammation (42). Higher neuroticism has been reported to be associated with the atypical response of natural killer cells to stress (43), blunted cortisol response to stress (44) and higher IL6, CRP, and WBC counts (45, 46). Besides, stress may also enhance carcinogenesis through changes in DNA repair and/or apoptosis (47). Moreover, individuals with higher neuroticism tend to live less healthy lifestyles, including cigarette smoking (48), alcohol consumption (49), obesity (50) and physical inactivity (51), which may lead to an increased risk of lung cancer. However, the exact underlying mechanisms linking neuroticism to lung cancer still need to be elucidated by further research.
Given that both genetic and behavioral factors may contribute to disease risk collectively, we assessed the joint effect and interaction between neuroticism and genetic factors on lung cancer. Interestingly, we observed an additive interaction between neuroticism and the genetic risk of lung cancer, which revealed that individuals with high genetic risk and high neuroticism synergistically increased the risk of lung cancer, and this form of interaction indicates that there is a biological interaction between risk factors (52). It suggests that individuals with high genetic risk and high neuroticism should pay more attention to their health. Besides, it may be used to guide screening to identify at-risk persons at an early stage.
The current study is the largest and most comprehensive study to investigate the role of neuroticism in the development of lung cancer, we applied two complementary observational and MR analyses, and examined potential joint effects between neuroticism and genetic susceptibility on lung cancer risk, for informing risk stratification and precision preventive strategies. However, several limitations also need to be acknowledged. First, the UK Biobank participants were also more likely to be more educated and healthier, which may not be generalizable to the general UK population due to “healthy volunteer bias” (53). Second, neuroticism was self-reported data at baseline, and therefore, they may have been misclassified. However, the misclassification was more likely to be non-differential and tended to underestimate the magnitude of association. Finally, the generalizability of genetic analyses is limited to individuals of European descent; therefore, the generalization of the results to other populations should be interpreted with caution.
In conclusion, the current study indicated that neuroticism plays a causal role in the development of lung cancer. Moreover, neuroticism and genetic risk jointly contributed to lung cancer incidence. Further studies are needed to confirm our findings in other populations and elucidate the underlying mechanisms.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics Statement
The ethical approval was obtained from North West Multi-centre Research Ethics Committee (REC reference: 11/NW/03820). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
The authors’ responsibilities were as follows—XW, XJ, DH, and HM: conceived and designed the research. XW and MZ: performed the statistical analyses. XZ, XF, MJ, YH, and YW offered statistical support during the study. XW and XJ drafted the manuscript. JX, RY, JD, GJ, LX, LD, and ZH: critically revised the manuscript for important intellectual content. All authors contributed to interpretation of the results, reviewed the manuscript for important intellectual content, and read and approved the final manuscript.
Funding
This work was supported by National Natural Science Foundation of China (81922061, 81803306); Natural Science Foundation of Jiangsu Province (BK20180675); CAMS Innovation Fund for Medical Sciences (2019RU038); and National Science Foundation for Post-doctoral Scientists of China (2018M640466).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.836159/full#supplementary-material
References
1. McCrae RR, John OP. An Introduction to the Five-Factor Model and Its Applications. J Pers (1992) 60(2):175–215. doi: 10.1111/j.1467-6494.1992.tb00970.x
2. Terracciano A, Aschwanden D, Stephan Y, Cerasa A, Passamonti L, Toschi N, et al. Neuroticism and Risk of Parkinson’s Disease: A Meta-Analysis. Mov Disord (2021) 36(8):1863–70. doi: 10.1002/mds.28575
3. Costa PT, Terracciano A, McCrae RR. Gender Differences in Personality Traits Across Cultures: Robust and Surprising Findings. J Pers Soc Psychol (2001) 81(2):322–31. doi: 10.1037/0022-3514.81.2.322
4. Kotov R, Gamez W, Schmidt F, Watson D. Linking “Big” Personality Traits to Anxiety, Depressive, and Substance Use Disorders: A Meta-Analysis. Psychol Bull (2010) 136(5):768–821. doi: 10.1037/a0020327
5. Goodwin RD, Cox BJ, Clara I. Neuroticism and Physical Disorders Among Adults in the Community: Results From the National Comorbidity Survey. J Behav Med (2006) 29(3):229–38. doi: 10.1007/s10865-006-9048-5
6. Almas A, Moller J, Iqbal R, Forsell Y. Effect of Neuroticism on Risk of Cardiovascular Disease in Depressed Persons - A Swedish Population-Based Cohort Study. BMC Cardiovasc Disord (2017) 17(1):185. doi: 10.1186/s12872-017-0604-4
7. Kissen DM, Brown RI, Kissen M. A Further Report on Personality and Psychosocial Factors in Lung Cancer. Ann N Y Acad Sci (1969) 164(2):535–45. doi: 10.1111/j.1749-6632.1969.tb14071.x
8. Morris T, Greer S, Pettingale KW, Watson M. Patterns of Expression of Anger and Their Psychological Correlates in Women With Breast Cancer. J Psychosom Res (1981) 25(2):111–7. doi: 10.1016/0022-3999(81)90098-2
9. Batty GD, McIntosh AM, Russ TC, Deary IJ, Gale CR. Psychological Distress, Neuroticism, and Cause-Specific Mortality: Early Prospective Evidence From UK Biobank. J Epidemiol Commun Health (2016) 70(11):1136–9. doi: 10.1136/jech-2016-207267
10. Lahey BB. Public Health Significance of Neuroticism. Am Psychol (2009) 64(4):241–56. doi: 10.1037/a0015309
11. Biondi M, Peronti M, Pacitti F, Pancheri P, Pacifici R, Altieri I, et al. Personality, Endocrine and Immune Changes After Eight Months in Healthy Individuals Under Normal Daily Stress. Psychother Psychosom (1994) 62(3-4):176–84. doi: 10.1159/000288920
12. Kissen DM, Eysenck HJ. Personality in Male Lung Cancer Patients. J Psychosom Res (1962) 6:123–7. doi: 10.1016/0022-3999(62)90062-4
13. Jokela M, Batty GD, Hintsa T, Elovainio M, Hakulinen C, Kivimäki M. Is Personality Associated With Cancer Incidence and Mortality? An Individual-Participant Meta-Analysis of 2156 Incident Cancer Cases Among 42,843 Men and Women. Br J Cancer (2014) 110(7):1820–4. doi: 10.1038/bjc.2014.58
14. Lemogne C, Consoli SM, Geoffroy-Perez B, Coeuret-Pellicer M, Nabi H, Melchior M, et al. Personality and the Risk of Cancer: A 16-Year Follow-Up Study of the GAZEL Cohort. Psychosom Med (2013) 75(3):262–71. doi: 10.1097/PSY.0b013e31828b5366
15. Nakaya N, Tsubono Y, Hosokawa T, Nishino Y, Ohkubo T, Hozawa A, et al. Personality and the Risk of Cancer. J Natl Cancer Inst (2003) 95(11):799–805. doi: 10.1093/jnci/95.11.799
16. Hansen PE, Floderus B, Frederiksen K, Johansen C. Personality Traits, Health Behavior, and Risk for Cancer: A Prospective Study of Swedish Twin Court. Cancer (2005) 103(5):1082–91. doi: 10.1002/cncr.20871
17. Schapiro IR, Ross-Petersen L, Saelan H, Garde K, Olsen JH, Johansen C. Extroversion and Neuroticism and the Associated Risk of Cancer: A Danish Cohort Study. Am J Epidemiol (2001) 153(8):757–63. doi: 10.1093/aje/153.8.757
18. Nakaya N, Bidstrup PE, Saito-Nakaya K, Frederiksen K, Koskenvuo M, Pukkala E, et al. Personality Traits and Cancer Risk and Survival Based on Finnish and Swedish Registry Data. Am J Epidemiol (2010) 172(4):377–85. doi: 10.1093/aje/kwq046
19. Smith GD, Ebrahim S. ‘Mendelian Randomization’: Can Genetic Epidemiology Contribute to Understanding Environmental Determinants of Disease? Int J Epidemiol (2003) 32(1):1–22. doi: 10.1093/ije/dyg070
20. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian Randomization: Using Genes as Instruments for Making Causal Inferences in Epidemiology. Stat Med (2008) 27(8):1133–63. doi: 10.1002/sim.3034
21. Jiang X, Dimou NL, Al-Dabhani K, Lewis SJ, Martin RM, Haycock PC, et al. Circulating Vitamin D Concentrations and Risk of Breast and Prostate Cancer: A Mendelian Randomization Study. Int J Epidemiol (2019) 48(5):1416–24. doi: 10.1093/ije/dyy284
22. Johnson KE, Siewert KM, Klarin D, Damrauer SM, Chang KM, Tsao PS, et al. The Relationship Between Circulating Lipids and Breast Cancer Risk: A Mendelian Randomization Study. PLoS Med (2020) 17(9):e1003302. doi: 10.1371/journal.pmed.1003302
23. Murphy N, Carreras-Torres R, Song M, Chan AT, Martin RM, Papadimitriou N, et al. Circulating Levels of Insulin-Like Growth Factor 1 and Insulin-Like Growth Factor Binding Protein 3 Associate With Risk of Colorectal Cancer Based on Serologic and Mendelian Randomization Analyses. Gastroenterology (2020) 158(5):1300–12.e20. doi: 10.1053/j.gastro.2019.12.020
24. Zhou H, Zhang Y, Liu J, Yang Y, Fang W, Hong S, et al. Education and Lung Cancer: A Mendelian Randomization Study. Int J Epidemiol (2019) 48(3):743–50. doi: 10.1093/ije/dyz121
25. Zhang R, Chu M, Zhao Y, Wu C, Guo H, Shi Y, et al. A Genome-Wide Gene-Environment Interaction Analysis for Tobacco Smoke and Lung Cancer Susceptibility. Carcinogenesis (2014) 35(7):1528–35. doi: 10.1093/carcin/bgu076
26. Huang Y, Zhu M, Ji M, Fan J, Xie J, Wei X, et al. Air Pollution, Genetic Factors, and the Risk of Lung Cancer: A Prospective Study in the UK Biobank. Am J Respir Crit Care Med (2021) 204(7):817–25. doi: 10.1164/rccm.202011-4063OC
27. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med (2015) 12(3):e1001779. doi: 10.1371/journal.pmed.1001779
28. Smith DJ, Nicholl BI, Cullen B, Martin D, Ul-Haq Z, Evans J, et al. Prevalence and Characteristics of Probable Major Depression and Bipolar Disorder Within UK Biobank: Cross-Sectional Study of 172,751 Participants. PLoS One (2013) 8(11):e75362. doi: 10.1371/journal.pone.0075362
29. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base Platform Supports Systematic Causal Inference Across the Human Phenome. Elife (2018) 7. doi: 10.7554/eLife.34408
30. McKay JD, Hung RJ, Han Y, Zong X, Carreras-Torres R, Christiani DC, et al. Large-Scale Association Analysis Identifies New Lung Cancer Susceptibility Loci and Heterogeneity in Genetic Susceptibility Across Histological Subtypes. Nat Genet (2017) 49(7):1126–32. doi: 10.1038/ng.3892
31. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A Framework for the Investigation of Pleiotropy in Two-Sample Summary Data Mendelian Randomization. Stat Med (2017) 36(11):1783–802. doi: 10.1002/sim.7221
32. Burgess S, Small DS, Thompson SG. A Review of Instrumental Variable Estimators for Mendelian Randomization. Stat Methods Med Res (2017) 26(5):2333–55. doi: 10.1177/0962280215597579
33. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization With Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol (2016) 40(4):304–14. doi: 10.1002/gepi.21965
34. Bowden J, Davey Smith G, Burgess S. Mendelian Randomization With Invalid Instruments: Effect Estimation and Bias Detection Through Egger Regression. Int J Epidemiol (2015) 44(2):512–25. doi: 10.1093/ije/dyv080
35. Zhao Q, Wang J, Hemani G, Bowden J, Small DS. Statistical Inference in Two-Sample Summary-Data Mendelian Randomization Using Robust Adjusted Profile Score. Ann Stat (2020) 48(3):1742–1769, 28. doi: 10.1214/19-AOS1866
36. Burgess S, Thompson SG. Interpreting Findings From Mendelian Randomization Using the MR-Egger Method. Eur J Epidemiol (2017) 32(5):377–89. doi: 10.1007/s10654-017-0255-x
37. Bycroft C, Freeman C, Petkova D, Band G, Elliott LT, Sharp K, et al. The UK Biobank Resource With Deep Phenotyping and Genomic Data. Nature (2018) 562(7726):203–9. doi: 10.1038/s41586-018-0579-z
38. Arthur RS, Wang T, Xue X, Kamensky V, Rohan TE. Genetic Factors, Adherence to Healthy Lifestyle Behavior, and Risk of Invasive Breast Cancer Among Women in the UK Biobank. J Natl Cancer Inst (2020) 112(9):893–901. doi: 10.1093/jnci/djz241
39. Choi J, Jia G, Wen W, Shu XO, Zheng W. Healthy Lifestyles, Genetic Modifiers, and Colorectal Cancer Risk: A Prospective Cohort Study in the UK Biobank. Am J Clin Nutr (2021) 113(4):810–20. doi: 10.1093/ajcn/nqaa404
40. Assmann SF, Hosmer DW, Lemeshow S, Mundt KA. Confidence Intervals for Measures of Interaction. Epidemiology (1996) 7(3):286–90. doi: 10.1097/00001648-199605000-00012
41. Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc (1999) 94(446):496–509. doi: 10.1080/01621459.1999.10474144
42. Hänsel A, Hong S, Cámara RJ, von Känel R. Inflammation as a Psychophysiological Biomarker in Chronic Psychosocial Stress. Neurosci Biobehav Rev (2010) 35(1):115–21. doi: 10.1016/j.neubiorev.2009.12.012
43. Borella P, Bargellini A, Rovesti S, Pinelli M, Vivoli R, Solfrini V, et al. Emotional Stability, Anxiety, and Natural Killer Activity Under Examination Stress. Psychoneuroendocrinology (1999) 24(6):613–27. doi: 10.1016/S0306-4530(99)00016-5
44. Oswald LM, Zandi P, Nestadt G, Potash JB, Kalaydjian AE, Wand GS. Relationship Between Cortisol Responses to Stress and Personality. Neuropsychopharmacology (2006) 31(7):1583–91. doi: 10.1038/sj.npp.1301012
45. Sutin AR, Milaneschi Y, Cannas A, Ferrucci L, Uda M, Schlessinger D, et al. Impulsivity-Related Traits Are Associated With Higher White Blood Cell Counts. J Behav Med (2012) 35(6):616–23. doi: 10.1007/s10865-011-9390-0
46. Sutin AR, Terracciano A, Deiana B, Naitza S, Ferrucci L, Uda M, et al. High Neuroticism and Low Conscientiousness Are Associated With Interleukin-6. Psychol Med (2010) 40(9):1485–93. doi: 10.1017/S0033291709992029
47. Kiecolt-Glaser JK, Robles TF, Heffner KL, Loving TJ, R. Glaser. Psycho-Oncology and Cancer: Psychoneuroimmunology and Cancer. Ann Oncol (2002) 13(Suppl 4):165–9. doi: 10.1093/annonc/mdf655
48. Hakulinen C, Hintsanen M, Munafò MR, Virtanen M, Kivimäki M, Batty GD, et al. Personality and Smoking: Individual-Participant Meta-Analysis of Nine Cohort Studies. Addiction (2015) 110(11):1844–52. doi: 10.1111/add.13079
49. Larkins JM, Sher KJ. Family History of Alcoholism and the Stability of Personality in Young Adulthood. Psychol Addict Behav (2006) 20(4):471–7. doi: 10.1037/0893-164X.20.4.471
50. Sutin AR, Ferrucci L, Zonderman AB, Terracciano A. Personality and Obesity Across the Adult Life Span. J Pers Soc Psychol (2011) 101(3):579–92. doi: 10.1037/a0024286
51. Rhodes RE, Smith NE. Personality Correlates of Physical Activity: A Review and Meta-Analysis. Br J Sports Med (2006) 40(12):958–65. doi: 10.1136/bjsm.2006.028860
52. Ahlbom A, Alfredsson L. Interaction: A Word With Two Meanings Creates Confusion. Eur J Epidemiol (2005) 20(7):563–4. doi: 10.1007/s10654-005-4410-4
Keywords: lung cancer, neuroticism, genetic risk, prospective analysis, Mendelian randomization study
Citation: Wei X, Jiang X, Zhang X, Fan X, Ji M, Huang Y, Xu J, Yin R, Wang Y, Zhu M, Du L, Dai J, Jin G, Xu L, Hu Z, Hang D and Ma H (2022) Association Between Neuroticism and Risk of Lung Cancer: Results From Observational and Mendelian Randomization Analyses. Front. Oncol. 12:836159. doi: 10.3389/fonc.2022.836159
Received: 15 December 2021; Accepted: 25 January 2022;
Published: 14 February 2022.
Edited by:
Guangwen Cao, Second Military Medical University, ChinaReviewed by:
Shenying Fang, University of Texas MD Anderson Cancer Center, United StatesGuoqiao Zheng, Lund University, Sweden
Hongping Yu, Affiliated Tumor Hospital of Guangxi Medical University, China
Ding Ye, Zhejiang Chinese Medical University, China
Copyright © 2022 Wei, Jiang, Zhang, Fan, Ji, Huang, Xu, Yin, Wang, Zhu, Du, Dai, Jin, Xu, Hu, Hang and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongxia Ma, aG9uZ3hpYW1hQG5qbXUuZWR1LmNu; Dong Hang, aGFuZ2RvbmdAbmptdS5lZHUuY24=
†These authors have contributed equally to this work
 Xiaoxia Wei1†
Xiaoxia Wei1† Xiangxiang Jiang
Xiangxiang Jiang Mengmeng Ji
Mengmeng Ji Jing Xu
Jing Xu Rong Yin
Rong Yin Lingbin Du
Lingbin Du Juncheng Dai
Juncheng Dai Guangfu Jin
Guangfu Jin Zhibin Hu
Zhibin Hu Dong Hang
Dong Hang Hongxia Ma
Hongxia Ma