
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 31 March 2022
Sec. Breast Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.835320
This article is part of the Research Topic Women in Breast Cancer: 2021 View all 31 articles
 Yirong Sim1,2,3*
Yirong Sim1,2,3* Cindy Lim4
Cindy Lim4 Nitar Phyu5
Nitar Phyu5 Kiat Tee Benita Tan1,2,3,6
Kiat Tee Benita Tan1,2,3,6 Lita Sui Tjien Chew7
Lita Sui Tjien Chew7 Chow Yin Wong2,3
Chow Yin Wong2,3 Preetha Madhukumar1,2,3
Preetha Madhukumar1,2,3 Wei Sean Yong1,2,3
Wei Sean Yong1,2,3 Sue Zann Lim1,2,3
Sue Zann Lim1,2,3 Julie Liana Bte Hamzah1,2,3
Julie Liana Bte Hamzah1,2,3 Si Ying Tan1,2,3
Si Ying Tan1,2,3 Wen Yee Chay8
Wen Yee Chay8 Fuh Yong Wong9
Fuh Yong Wong9 Puay Hoon Tan10
Puay Hoon Tan10 Veronique Kiak-Mien Tan1,2,3
Veronique Kiak-Mien Tan1,2,3Introduction: Statins, HMG-CoA reductase inhibitors, are commonly used cholesterol-lowering medications which are also increasingly recognized to have anti-cancer properties for various cancers, including breast cancer. Most clinical evidence supports a protective effect of statin on reducing breast cancer recurrence, particularly in hormone-receptor positive breast cancers.This study seeks to study the impact of statin use on breast cancer recurrence in an Asian population.
Methods: This is a retrospective study of patients diagnosed with breast cancer at the National Cancer Centre and Singapore General Hospital from 2005-2015. Statin use was defined as use after surgery. Associations between statin use, breast cancer recurrence and overall survival were estimated using Cox proportional hazards regression with adjustment for age, TNM stage, grade, ER/HER2 status, and co-morbidities. Associations between statin-use and disease-specific survival were estimated using competing risks regression.
Results: A total of 7858 females with breast cancer were studied, 1353(17.2%) were statin users, 6505(82.8%) were non-statin users, with a median follow-up of 8.67 years. Distribution of cancer stage, histology, molecular subtypes and grades were similar in both groups. Estrogen receptor(ER) positive (HR 0.57,95%CI 0.43-0.76,p<0.001) and HER2 negative (HR 0.74,95%CI 0.57-0.96,p=0.026) invasive cancers had a lower risk of recurrence in statin users. Statin users trended towards a long term recurrence-risk reduction (all subtypes,HR 0.48,p=0.002; ER-, HR 0.34,p=0.036; HER2+,HR 0.10,p=0.002). The risk-reduction benefit is not appreciated in statin users with DCIS, possibly due to small recurrence event numbers. Disease-specific survival benefit was seen in statin users with ER+ cancers (adjusted SHR 0.71,95%CI 0.53-0.96,p=0.027), especially ER+ invasive cancers (adjusted SHR 0.72, 95%CI 0.53-0.97,p=0.028), but with no statistically significant benefit in overall survival for statin users (all subtypes).
Conclusion: This is the first known retrospective study on the effect of statin use and breast cancer recurrence in an Asian population. Similar to previous international studies, statin use is associated with a risk reduction in breast cancer recurrence. This is especially beneficial in patients who have ER+ and HER2- invasive breast cancer. Statin use is also associated with a reduced risk of breast cancer recurrence in all subtypes of breast cancer in the long term (>6 years post diagnosis).
Breast cancer is the most commonly occurring cancer in women, and is generally subtyped according to its expression of three receptors - estrogen (ER), progesterone (PR) and HER2 receptors. The majority (67-80%) of breast cancer cells express the estrogen and/or progesterone hormone receptors (ER and PR) (1, 2). With increased screening, improved surgery and adjuvant treatment modalities (i.e. a combination of hormonal, chemo-, targeted and radiation- therapies), the long-term survival rate after the diagnosis of breast cancer is rising steadily. Each breast cancer survivor has a life-long risk (5-41%) of developing a breast cancer recurrence (3–5), and with an increasing number of breast cancer survivors, this translates to an important public health issue. Recurrence can be local, i.e. developing in or near the original site of cancer; regional, i.e. presenting as nodal involvement in the axillary, or supraclavicular anatomic locations; or distant, i.e. metastatic, commonly appearing in the bones, lung, liver, or brain.
Statins are 3-hydroxy-3-methylglutaryl–coenzyme A (HMG-CoA) reductase inhibitors and decrease cholesterol synthesis. They are commonly used as a cholesterol-lowering medication to reduce the risks of heart attacks and strokes. Statins have also been shown to have anti-cancer properties for various cancers, including breast cancer (6–11). Statin users showed a risk reduction in developing breast cancer (12), and breast cancer patients who used cholesterol lowering medicines had more favorable tumour characteristics and improved outcomes compared with nonusers (13). Most clinical evidence supports a protective effect of statins on reducing breast cancer recurrence (14–19).
This benefit appears to be strongest in younger patients (18) and early stage breast cancer (14, 18), suggesting a longitudinal influence of statin therapy (14, 16, 18). Some studies suggest the benefit of statins with hormone receptor positive breast cancers (18, 20), and not with the triple negative breast cancer (TNBC) cohort, i.e. breast cancer cells which do not express the estrogen, progesterone and HER2 receptors (21, 22). There is some suggestion of the lipophilic statins (such as simvastatin) being more effective at reducing breast cancer recurrence than the hydrophilic statins (15, 17, 19). To date, multiple meta-analyses yield conflicting results. Whilst some studies do not show an effect of statins on cancer incidence or survival (23–25), the majority of the meta-analyses support the finding that statins reduce the risk of breast cancer recurrence and breast cancer related mortality (17, 19, 26, 27), particularly during shorter term follow-up (27).
To date, most studies are performed in the Western population, hence there is an importance to perform this study in our local Asian population. The demographics of breast cancer in the East differ from the West, with a peak age of incidence of breast cancer in the East being 10 years younger than the West (28). There are also racial differences in the response to statins, due to genetic determinants and pharmacokinetic differences (29). Therefore, the aim of this study is to retrospectively review and explore the impact of statin use on breast cancer recurrence in the Asian population of Singapore.
This is a retrospective study of patients who were diagnosed with breast cancer at the National Cancer Centre Singapore and Singapore General Hospital from 2005 to 2015, inclusive. The data was extracted from the Joint Breast Cancer Registry (JBCR) and merged with the electronic drug prescriptions platform used by the public health institutions within the SingHealth cluster, which include the primary care physician clinics. This was done with approval by the SingHealth institutional ethics board (CIRB 2019/2419). Breast cancer patients with ductal carcinoma in situ (DCIS), Stage I to Stage III invasive cancers were included. Patients whose diagnosis date was before 1 Jan 2005, cancers which were stage IV or unknown/incomplete, and patients who did not undergo surgery were excluded. Patients who had recurrence or died or whose last follow-up date within a year from surgery were excluded (Figure 1). For patients who had synchronous bilateral cancers, the following criteria were used to select the side to be included in the analysis in this order: 1) higher TNM stage, 2) higher T stage, 3) more aggressive tumour biology (eg. HER2 positive or TNBC), 4) higher grade, 5) larger tumour size, 6) earlier diagnosis date. If all these 6 criteria were all identical, the left breast was selected by default.
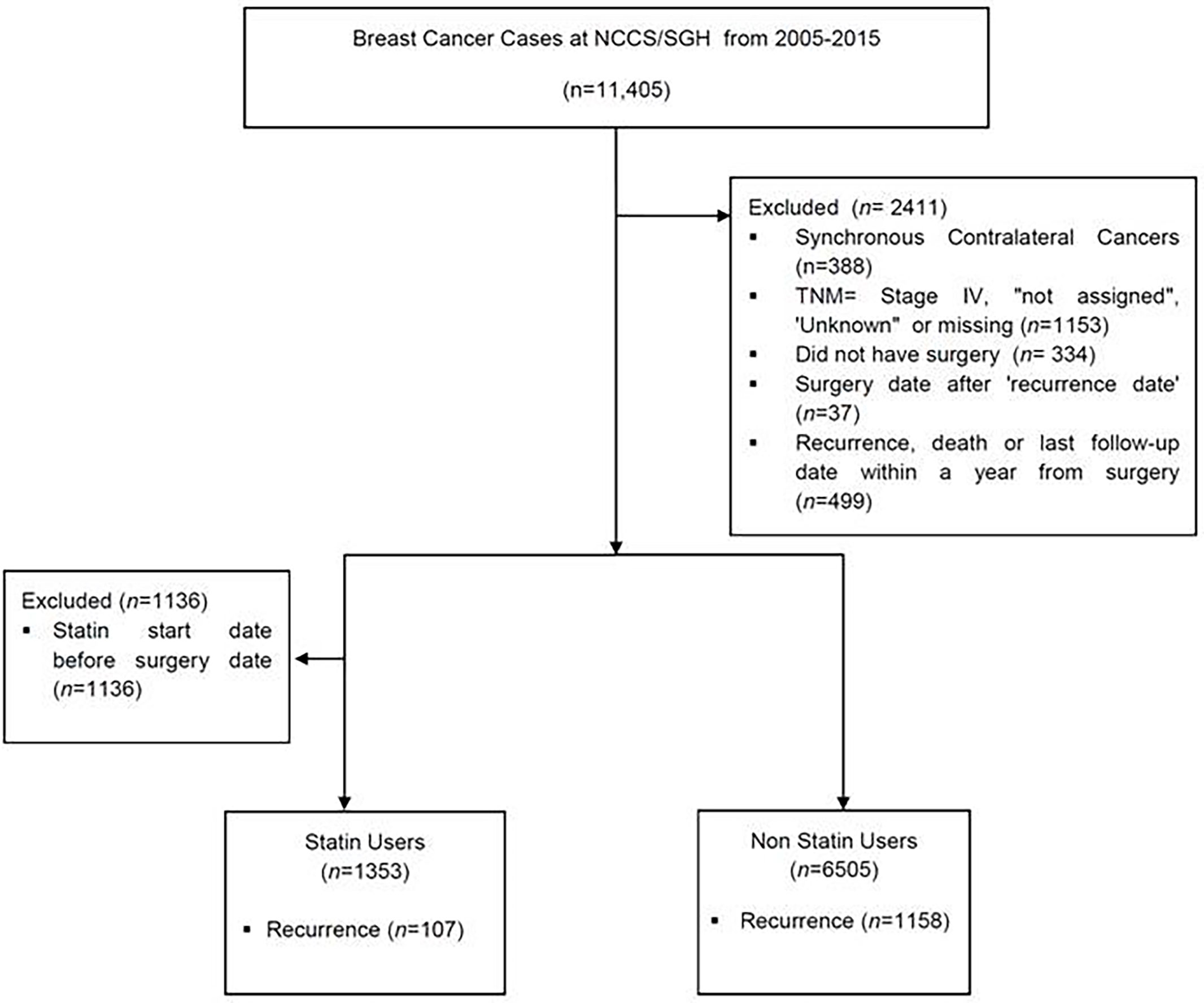
Figure 1 CONSOT Diagram to summarize the number of patients included and excluded in this study. NCCS, National Cancer Centre Singapore; SGH, Singapore General Hospital; TNM, Tumor Nodal Metastasis staging.
Statin use was defined as use after surgery, as represented by their prescription date. Patients who were on statins pre-surgery were excluded. Patients who developed a recurrence before their start date of statins were counted as statin non-users. The median duration from surgery to start of statins was 22.7 months (IQR 0.36-64.2).
Follow-up started 1 year from breast cancer surgery. Maximum length of follow-up was set to 10 years (or 11 years from surgery). Breast cancer recurrence was defined as any local, regional or distant recurrence, or contralateral breast cancer. Patients who did not have recurrence were censored at death, last follow-up, or when 10 years of follow-up was reached (whichever came first).
Patient and tumour characteristics were tabulated by statin use. Differences in characteristics between statin users and non-users were tested using Pearson’s chi-squared test or Fisher’s exact test (for variables with category frequencies of less than 5). Associations between statin use and breast cancer recurrence and overall survival were estimated using univariable and multivariable Cox proportional hazards regression models. Associations between statin use and disease-specific survival were estimated using univariable and multivariable competing risks regression models as described by Fine and Gray (30). Multivariable models were adjusted for age at diagnosis, TNM stage, histological grade, ER status, HER2 status, aspirin use (pre- and post-diagnosis) and the presence of the following co-morbidities at diagnosis: diabetes, hypertension, cardiac disease, neurological (CNS) disease, hyperlipidemia and renal disease. Statin and aspirin use were fitted as time-varying variables lagged by 1 year, i.e. patients only joined the statin group 1 year after they started on statins. Once started, these drugs were assumed to be taken for life. The proportional hazards assumption was tested using Schoenfeld residuals for Cox regression, and time-varying covariates for competing risks regression. Variables which were significantly non-proportional were modeled using a time-dependent interaction term which allowed the hazard ratio to change after 5 years. The hazard ratio for 6 to 10 years was obtained by multiplying the hazard ratio for 1 to 5 years by this interaction term.
The risk is evaluated according to time from enrolment. For statin users, they are in the nonuser group before statin use initiation and move to the statin group 1 year after statin initiation. The Cox model evaluates hazard rate at each timepoint, which is the risk of failure given the subject has survived up to that timepoint, hence the comparison is between statin users and nonusers who have survived for the same amount of time, and who have been on statins for at least 1 year (for the statin users). All statistical analyses were performed in Stata 16.1 (Statacorp, College Station, Texas). Two-sided p-values less than 0.05 were taken as significant.
In this study, there were a total of 7858 females with breast cancer were studied, 1353 (17.2%) were statin users, 6505 (82.8%) were non-statin users (Figure 1). The majority of the patients were prescribed a lipophilic statin (n=1303, 96.3%), with simvastatin and atorvastatin being the most common statins used. Hydrophilic statins contributed to a marked minority (n=50, 3.7%), with rosuvastatin being the most common hydrophilic statin used (n=47). The demographics and tumor characteristics of the study population is summarized in Table 1. The majority of the women in the statin group were postmenopausal. This is expected as the average age in the statin user group was higher, a reflection of statin prescription for cardiac protection in the older population. Nonetheless, the distribution of the cancer stage, histology, tumour molecular subtypes and grade are similar in both groups. As such the use of chemotherapy and hormone therapy are also similar in both groups. In terms of surgery, slightly more women in the statin group chose a mastectomy compared to the non-statin group (65.6 vs 55.6%, p<0.001), and hence the uptake of radiotherapy (which is coupled with breast conserving surgery) was lower in statin group (54.4% vs 61.5%, p<0.001). The median age in the mastectomy group was also higher (54 years, IQR 47-61), as compared to the median age in breast conserving surgery group (50 years, IQR 44-57), p<0.001; also a reflection of statin users being older on average.
Patients were followed up for a median of 8.74 years, with a 95% confidence interval of 8.59 to 8.88 years. Table 2 shows the unadjusted and adjusted hazard ratios for breast cancer recurrence by statin use in different subsets of patients.
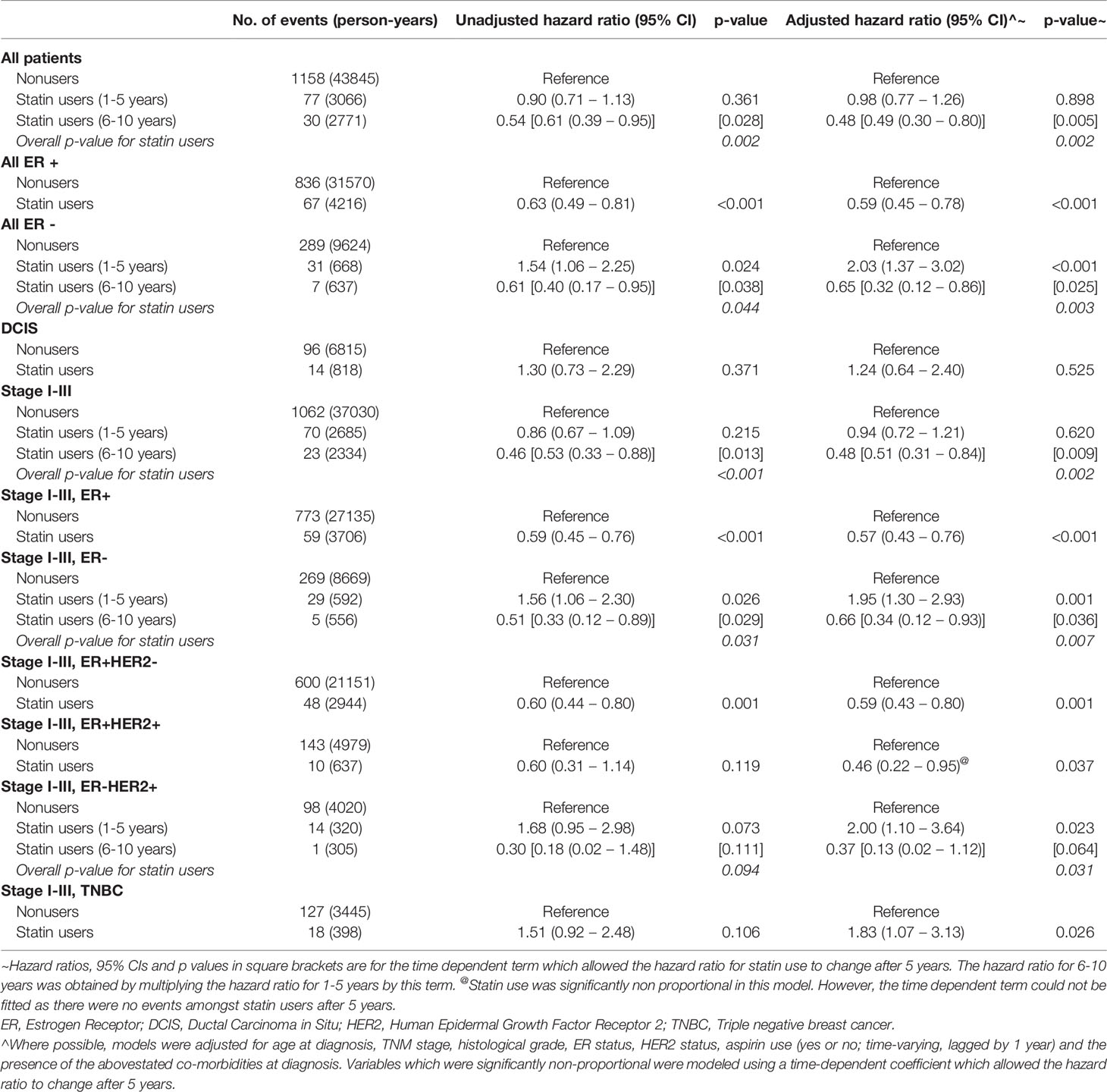
Table 2 Unadjusted and adjusted hazard ratios for breast cancer recurrence by statin use in different subsets of patients.
Comparing the statin user and non-user groups, when adjusted for age, and stage, statin users had similar risk of recurrence compared to non-statin users in the first 5 years of follow up (HR 0.98, 95%CI, 0.77-1.26). However, after 5 years, the risk of recurrence decreased significantly amongst statin users, with the hazard ratio estimated to be 0.48 after 5 years, p=0.002. This beneficial trend was seen in statin users with invasive cancers, beyond 6 years (HR 0.48, p= 0.002), but not so for statin users who had DCIS (HR 1.24, p=0.525).
ER positive cancers (DCIS inclusive) had a lower risk of recurrence in statin users (HR 0.59, 95%CI 0.45-0.78, p<0.001). On the contrary, there appeared to be an increased risk of recurrence in statin users who had ER negative cancers in the first 6 years post surgery (HR 2.03, p<0.001), but this risk of recurrence in statin users decreased significantly beyond the first 6 years (HR =0.65, p=0.003). It is uncertain if this increased risk was due to statin use itself, but it was more likely associated with the statin user not being accounted for in the model.
The benefit of statins in ER positive cancers was different between the in-situ and invasive groups. In the invasive cancer group, statin use was associated with a reduced risk of recurrence in the ER positive cancers (HR 0.57, 95%CI 0.43-0.76, p<0.001), and this is observed in both ER+ HER2-invasive cancers (HR 0.59, 95%CI 0.43-0.80,p=0.001) and ER+ HER2+ invasive cancers (HR 0.46, 95%CI 0.22-0.95, p=0.037). There appeared to be no statistically significant influence of statin in both the ER+ and ER- DCIS cancers.
Although there was no risk reduction benefit of statins on the ER- and HER2+ invasive cancers in the first 6 years, the risk of recurrence in both these ER- and HER2+ groups decreased significantly beyond the sixth year of diagnosis (HR 0.66, p=0.007 and HR 0.10, p=0.002 respectively).There was also an overall risk reduction benefit of statin in HER2- breast cancers, HR 0.74, p=0.026.
For disease-specific survival (Table 3), there was a benefit for statin users who were ER positive (adjusted SHR 0.71, 95%CI 0.53-0.96,p=.0.027), especially the ER+ invasive cancers (adjusted SHR 0.72, 95%CI 0.53-0.97, p=0.028) and ER+ HER2- invasive cancers (adjusted SHR 0.69, 95% CI 0.49-0.97, p=0.035). There was a suggestion of an improved overall survival in statin users who had ER+ cancers (HR=0.81) and ER+ invasive cancers (HR 0.79), but there was no statistically significant difference, albeit close (p=0.087 and p=0.061 respectively) There was no significant benefit in improved overall survival (Table 4) in all subtypes of breast cancer patients.
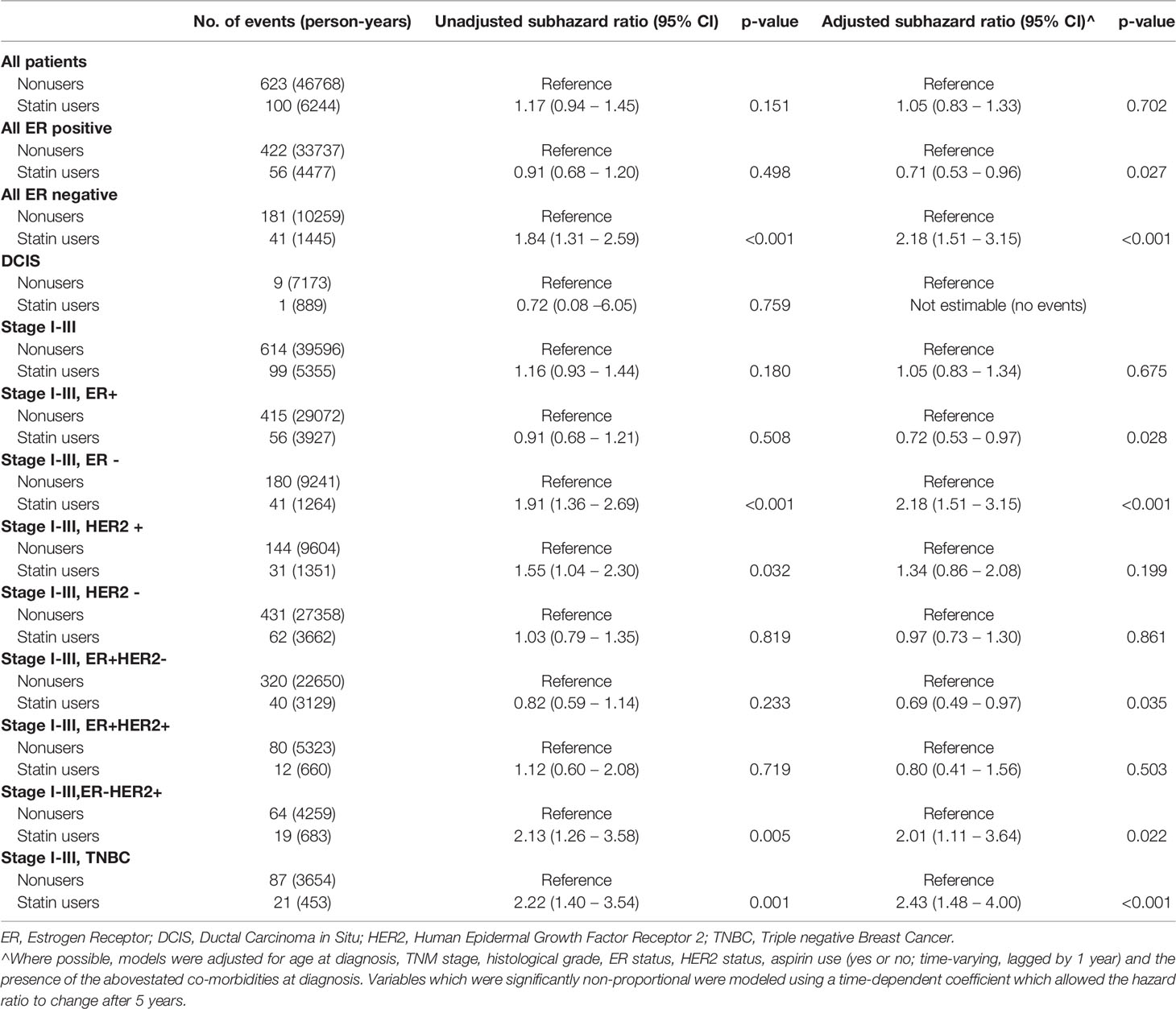
Table 3 Unadjusted and adjusted subhazard ratios for disease-specific survival by statin use in different subsets of patients.
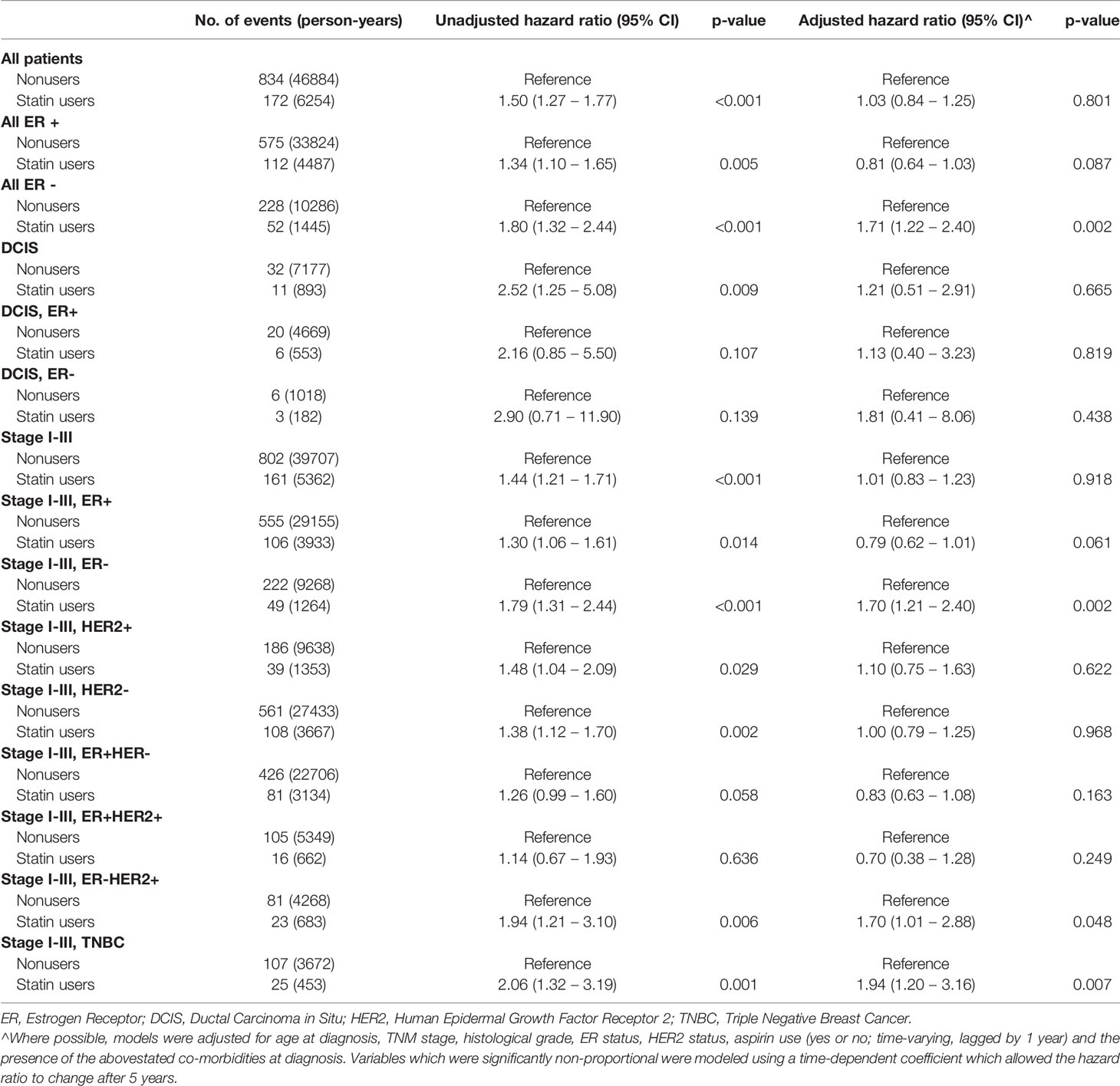
Table 4 Unadjusted and adjusted hazard ratios for overall survival by statin use in different subsets of patients.
To the best of our knowledge, this is the first retrospective study of the impact of statins on the risk of breast cancer recurrence in an Asian population. The results from this study demonstrate that statin use after the diagnosis of breast cancer can potentially reduce the risk of breast cancer recurrence. This is observed in patients who had invasive cancers which either expressed the estrogen receptor (ER) and/or who are HER2 negative. This is similar to previous studies done in Europe and in the US, that showed the beneficial effect of statins in reducing the risk of recurrence in Stage I-III invasive breast cancers (14, 15, 31–35), and the beneficial effect of statins in reducing the risk of recurrence in ER positive patients (13, 15, 35).
There are a few possibilities to explain this interesting phenomenon. One theory is the direct reduction of estrogen production, through the statin induced reduction of cholesterol (precursor of estrogen hormones). Although lipid concentrations (which are also decreased by statins) do not seem to have an impact on breast cancer risk overall (36), cholesterol levels may still modulate the action of estrogen receptors (37).
Another possibility is the synergism between statins and the adjuvant hormonal therapy (tamoxifen or aromatase inhibitor) which is also administered to ER+ breast cancer patients in the first 5 years post surgery (35). This is assuming patients with ER positive cancers (which are similar in proportion in both statin and non-statin groups) are compliant to adjuvant hormonal therapy as they are with their statin use. There are in vitro studies that demonstrate how statins attenuate the growth of ER+ breast cancer cell lines (MCF-7) (38–43), as well as the synergistic effect of statins with tamoxifen (44) or exemestane (an aromatase inhibitor) (45) to induce cell apoptosis, through the downregulation of the expression of suvivin proteins (43, 44).
Another theory is the direct impact of statins on the mevalonate pathway, which produces cholesterol, steroid hormones, and non-steroid isoprenoids, which are necessary for cell survival (46). HMGCR (3-hydroxy-3-methylglutaryl–coenzyme A reductase) is differentially expressed among breast cancers, with higher expression in the tumour cells (vs normal epithelial cells) (47), but the correlation with its expression and breast cancer prognosis and survival is controversial. One study (48) showed that HMGCR moderate/strong expression was associated with prognostically adverse tumour characteristics (higher histological grade, high Ki67, and ER negativity) and that neither HMGCR expression nor statin use was associated with breast cancer mortality (48). This was also seen in a Korean cohort study (49) which showed that a higher tumour expression of HMGCR was associated with poor disease free survival, particularly in the TNBC group. Yet, some studies showed that a stronger expression of HMGCR was associated with a less aggressive tumour profile (low histological grade, a small tumour size, oestrogen receptor (ER) positivity, and low proliferation) (50, 51), and HMGCR-positive cancers had longer recurrence-free survival, which was more pronounced in patients with ER-positive tumours (48, 51).
Interestingly, we also observed that statins appear to reduce the risk of recurrence in all patients with invasive breast cancer beyond the sixth year of diagnosis (HR 0.48, overall p=0.002). This is also observed in those with ER- (HR 0.66, overall p=0.007) and HER2+ (HR 0.10, overall p=0.002) invasive breast cancers. This is also supported by in vitro studies, where statins can also attenuate the growth of triple negative breast cell lines (MDA-MB231) (38–40, 52), and reduce breast cancer cell migration (42, 53, 54). These suggest more complex pathway(s) in which statins contribute to cancer risk reduction, beyond their direct impact on the ER receptor and its pathway.
A possible explanation for this long term benefit of statins is that the triple negative (i.e. ER- PR-HER2-) breast cancers are generally more aggressive, have fewer available adjuvant systemic therapies, and have a higher risk of cancer recurrence within the first 5 years. This is opposed to ER positive cancers which tend to have a better prognosis, but longer periods of dormancy (55). Therefore, the beneficial effect of statins in reducing cancer recurrence in the longer-term may be attributed to their role in cancer dormancy. Tumour dormancy is a clinical phenomenon in which disseminated tumour cells remain occult, asymptomatic, and undetectable over a prolonged period of time. Tumour dormancy contributes to local recurrence or distal metastasis, up to years or decades after treatment (56). This may also explain why the protective role of statins in our DCIS population is not appreciated. DCIS refers to the proliferation of neoplastic epithelial cells within the tubulolobular system of the breast. Although DCIS shares morphological features of invasive breast carcinoma, they are confined by the myoepithelial cells and basement membrane of the ducts, without invasion of the stroma, lymphatic or blood vessels. DCIS, by definition is pre-invasive (Stage 0), and has a much lower risk of recurrence than invasive cancer.
This is further supported by the popular theory of the anti-inflammatory properties of statins, particularly on cytokines, in the tumour microenvironment. Statins have also been demonstrated in vitro to decrease interleukin-6 (IL6) production in breast cancer cell lines (57–60) and non-breast cancer cell lines (61–63). Lipophilic statins can also suppress the common cluster of genes (Hippo, Notch and Wnt pathways) governing the epithelial mesenchymal transition (EMT) process (64) - an important step in tumour dormancy, recurrence and metastasis. Other proposed anti-carcinogenic effects of statins include apoptosis (65–67) and inhibition of inflammation (66, 68, 69), proliferation, migration (39, 70, 71) and angiogenesis (72).
Majority of studies support an improvement in disease-specific survival (6, 16, 19, 24, 33, 73), but were without tumour subtype analysis. The findings in this study demonstrated a disease-specific survival benefit for statin users who were ER positive (HR 0.69, p=.0.023).This is appreciated in the ER+ invasive cancers (HR 0.70, p=0.024) and ER+ HER2- invasive cancers (HR 0.68, p=0.036), and is a reflection of the risk reduction benefit of statins on cancer recurrence in these ER+ invasive cancers. However, the benefit of statins reducing disease-specific survival in all breast cancer patients was not appreciated in this study, which may be in part due to small event numbers or an inadequately long enough follow-up (i.e.>10 years). There was a suggestion of an improved overall survival in statin users who had ER+ cancers (HR=0.81) and ER+ invasive cancers (HR 0.79), but there was no statistically significant difference, albeit close (p=0.087 and p=0.061 respectively). It is possible that the true benefit on overall survival may be better appreciated with a longer followup (i.e. > 10 years).
Similar to other studies (23, 33), there was no significant benefit in improved overall survival (Table 4) in all subtypes of breast cancer patients. This may be in part due to close surveillance and screening of breast cancer survivors, in picking up recurrence(s), if any, early, and the improved modality of systemic treatment. Also, statin users are also more likely to be older and have more comorbidities, which in themselves pose a risk of earlier mortality. Hence, after adjusting for these variables, there are no significant differences for overall and disease-specific survival between statin and non-statin users.
In this study, we chose to focus only on the post-diagnostic statin users, which is similar to most studies (14, 15, 22, 31–33, 74). This helps remove the confounders of statin use duration, and the bias that statin use in itself may reduce breast cancer risk (12, 31), and may have more favorable tumor characteristics and hence improved outcomes compared with statin nonusers (13).
The main limitation of this study is that it is a retrospective study in a single healthcare cluster. Nonetheless, SingHealth is the largest public healthcare cluster in Singapore. A large majority of our patients have long term follow up, with a median of 8.674 years, with a 95% confidence interval of 8.59 to 8.88 years. As such, the possible biases that might have been introduced due to a loss of follow-up is small. The probability of patients who were lost to follow up and had a greater proportion of statin users and recurrence than the rest of the patients who completed the study is also low.
Another assumption for this study is that the first electronic prescription of statins is the patients’ index prescription and hence the start date of statins. As the largest public healthcare cluster, we have a good electronic prescription record that also links up with the primary care physicians clinics within the cluster. All patients who undergo oncological surgery in hospital will have at least one record of their prescriptions on the day of their surgery when they are admitted, and hence any patients who are already on existing statin prescription will be detected and excluded from this study. Therefore, it is reasonable to assume that any prescription after the date of surgery will accurately represent the start of statins post surgery. We acknowledge that there may be a group of patients who are prescribed statins by their private practitioners or through another healthcare group post-diagnosis and this group of patients may contribute to a misclassification bias. This group is estimated to be small as the majority of patients tend to be followed up with the SingHealth primary health care, and/or have their usual prescriptions topped up during their oncologist visits. More importantly, patients who had a recurrence, and hence included in this study, would have been worked up in the treating institution at the point of recurrence, and have their electronic prescriptions reviewed, hence minimizing the chances of the misclassification bias. In addition, we have no reason to believe that the proportion of statin users erroneously classified would be too dissimilar in both outcome groups—those who recurred and those who remained free of disease. In that sense, misclassification errors should be non-differential and generally this tend to bias the study toward null hypothesis, not against it.
Another assumption of this study is the compliance of statins once the patient is started on it. We can only assume that a prescription represents compliance to consuming the medication, and that the patient complies life-long with this cardioprotective cholesterol-lowering statin. We can infer this through top-up prescriptions, but acknowledge that patients may also get their top-up prescription through other healthcare providers. There is also the assumption that patients who are more compliant with their medications, especially chronic medications, are more likely to be healthier and adherent to other prescribed drugs, such as the adjuvant hormonal therapeutic agents with tamoxifen or aromatase inhibitors. If this were the case, then a selection bias might have also played a role in this study.
Also, the vast majority of the patients in this study were prescribed a lipophilic statin (96.3%), with simvastatin and atorvastatin being the most common statins used. With this skewed preference for lipophilic statins in this population, a comparison between hydrophilic vs lipophilic statins and their impact on breast cancer recurrence was not feasible.
Another potential limitation of our study is that we could not adjust for body mass index and other lifestyle variables, such as smoking and alcohol use because the data collection for this was incomplete. A previously published study in an overlapping population showed that body mass index was associated only with distant breast cancer recurrences (75). Nonetheless, this paper has its strengths. It has a large number of patients (n=7858) with long follow-up median of 8.59 years. We were also able to analyze and compare the different subtypes of breast cancer - comparing the DCIS vs invasive breast cancers, as well as the different immunohistochemical subtypes of invasive breast cancer. Despite possible genetic differences in Asian breast cancer, pharmacokinetic differences in statin metabolism and lifestyle difference, we have demonstrated that, similar to previous international studies, statin use can help reduce a risk of breast cancer recurrence. This is especially beneficial in patients who have ER+ invasive breast cancer. We have demonstrated that statin use can potentially reduce breast cancer recurrence in all subtypes of breast cancer in the long term (> 6 years post diagnosis). The underlying mechanisms of its beneficial action is still yet to be fully elucidated.
We look forward to prospective clinical trials (such as NCT04601116, NCT03971019, NCT04705909, NCT00914017 and NCT02958852, and not exhaustive), to enlighten us on the true effects of statins in preventing breast cancer risk and recurrence; and if it outweighs the small, but known side effects, such as statin-associated muscle symptoms (SAMS) (76, 77), effect on glucose homeostasis and hepatic toxicity (77). Further in vitro and translation studies are also needed to understand the pathways in which statins exerts its anti-carcinogenic effects.
This is the first retrospective study in Asian population to study the effect of statin use and breast cancer recurrence. Despite possible genetic differences in Asian breast cancer, pharmacokinetic differences in statin metabolism and lifestyle difference, we have demonstrated that, similar to previous international studies, statin use can help reduce a risk of breast cancer recurrence. This is especially beneficial in patients who have ER+ and/or HER2- invasive breast cancer. We have also demonstrated that statin use potentially reduce breast cancer recurrence in all subtypes of breast cancer in the long term (> 6 years post diagnosis). This risk reducing effects of statins on breast cancer recurrence, coupled with their cardioprotective effect, demonstrate the underlying complexity in cancer pathways and metabolism, and may open up new potential anti-cancer targets for future therapeutics.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by SingHealth institutional ethics board (CIRB 2019/2419). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
YS contributed to the conception and design of the study, and the writing of the manuscript. NP contributed to the retrieval of data and organisation of the database and YS, CL, and NP contributed to the organization of the database. CL performed the statistical analysis and contributed to the design of the study. All authors contributed to manuscript revision, read and approved the submitted version.
This project was supported by the National Cancer Centre Singapore (NCCS) Cancer fund.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
DCIS, Ductal Carcinoma in Situ; ER, Estrogen Receptor; HER2, Human Epidermal Growth Factor Receptor; HMG-CoA Reductase, HMGCR 3-hydroxy-3-methylglutaryl–coenzyme A reductase; HR, Hazard Ratio; SHR, Subhazard ratio; TNBC, Triple Negative Breast Cancer.
1. Dunnwald LK, Rossing MA, Li CI. Hormone Receptor Status, Tumor Characteristics, and Prognosis: A Prospective Cohort of Breast Cancer Patients. Breast Cancer Res (2007) 9(1):R6. doi: 10.1186/bcr1639
2. Kohler BA, Sherman RL, Howlader N, Jemal A, Ryerson AB, Henry KA, et al. Annual Report to the Nation on the Status of Cance-2011, Featuring Incidence of Breast Cancer Subtypes by Race/Ethnicity, Poverty, and State. J Natl Cancer Inst (2015) 107(6):djv048. doi: 10.1093/jnci/djv048
3. Brewster AM, Hortobagyi GN, Broglio KR, Kau SW, Santa-Maria CA, Arun B, et al. Residual Risk of Breast Cancer Recurrence 5 Years After Adjuvant Therapy. J Natl Cancer Inst (2008) 100(16):1179–83. doi: 10.1093/jnci/djn233
4. Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, et al. 20-Year Risks of Breast-Cancer Recurrence After Stopping Endocrine Therapy at 5 Years. N Engl J Med (2017) 377(19):1836–46. doi: 10.1056/NEJMoa1701830
5. van Maaren MC, de Munck L, Strobbe LJA, Sonke GS, Westenend PJ, Smidt ML, et al. Ten-Year Recurrence Rates for Breast Cancer Subtypes in the Netherlands: A Large Population-Based Study. Int J Cancer (2019) 144(2):263–72. doi: 10.1002/ijc.31914
6. Nielsen SF, Nordestgaard BG, Bojesen SE. Statin Use and Reduced Cancer-Related Mortality. N Engl J Med (2012) 367(19):1792–802. doi: 10.1056/NEJMoa1201735
7. Geybels MS, Wright JL, Holt SK, Kolb S, Feng Z, Stanford JL. Statin Use in Relation to Prostate Cancer Outcomes in a Population-Based Patient Cohort Study. Prostate (2013) 73(11):1214–22. doi: 10.1002/pros.22671
8. Wang A, Aragaki AK, Tang JY, Kurian AW, Manson JE, Chlebowski RT, et al. Statin Use and All-Cancer Survival: Prospective Results From the Women’s Health Initiative. Br J Cancer (2016) 115(1):129–35. doi: 10.1038/bjc.2016.149
9. Mei Z, Liang M, Li L, Zhang Y, Wang Q, Yang W. Effects of Statins on Cancer Mortality and Progression: A Systematic Review and Meta-Analysis of 95 Cohorts Including 555 1,111,407 Individuals. Int J Cancer (2017) 140(5):1068–81. doi: 10.1002/ijc.30526
10. Yang PR, Tsai YY, Chen KJ, Yang YH, Shih WT. Statin Use Improves Overall Survival of Patients With Gastric Cancer After Surgery and Adjuvant Chemotherapy in Taiwan: A Nationwide Matched Cohort Study. Cancers (Basel) (2020) 12(8):2055. doi: 10.3390/cancers12082055
11. Majidi A, Na R, Jordan SJ, De Fazio A, Webb PM. Statin Use and Survival Following a Diagnosis of Ovarian Cancer: A Prospective Observational Study. Int J Cancer (2021) 148(7):1608–15. doi: 10.1002/ijc.33333
12. Cauley JA, Zmuda JM, Lui LY, Hillier TA, Ness RB, Stone KL, et al. Lipid-Lowering Drug Use and Breast Cancer in Older Women: A Prospective Study. J Womens Health (Larchmt) (2003) 12(8):749–56. doi: 10.1089/154099903322447710
13. Borgquist S, Giobbie-Hurder A, Ahern TP, Garber JE, Colleoni M, Láng I, et al. Cholesterol, Cholesterol-Lowering Medication Use, and Breast Cancer Outcome in the BIG 1-98 Study. J Clin Oncol (2017) 35(11):1179–88. doi: 10.1200/jco.2016.70.3116
14. Kwan ML, Habel LA, Flick ED, Quesenberry CP, Caan B. Post-Diagnosis Statinuse and Breast Cancer Recurrence in a Prospective Cohort Study of Early Stage Breast Cancersurvivors. Breast Cancer Res Treat (2008) 109(3):573–9. doi: 10.1007/s10549-007-9683-8
15. Ahern TP, Lash TL, Damkier P, Christiansen PM, Cronin-Fenton DP. Statins and Breast Cancer Prognosis: Evidence and Opportunities. Lancet Oncol (2014) 15(10):e461–468. doi: 10.1016/s1470-2045(14)70119-6
16. Murtola TJ, Visvanathan K, Artama M, Vainio H, Pukkala E. Statin Use and Breast Cancer Survival: A Nationwide Cohort Study From Finland. PloS One (2014) 9(10):e110231. doi: 10.1371/journal.pone.0110231
17. Mansourian M, Haghjooy-Javanmard S, Eshraghi A, Vaseghi G, Hayatshahi A, Thomas J. Statins Use and Risk of Breast Cancer Recurrence and Death: A Systematic Review and Meta-Analysis of Observational Studies. J Pharm Pharm Sci (2016) 19(1):72–81. doi: 10.18433/j3202b
18. Sakellakis M, Akinosoglou K, Kostaki A, Spyropoulou D, Koutras A. Statins and Risk of Breast Cancer Recurrence. Breast Cancer (Dove Med Press) (2016) 8:199–205. doi: 10.2147/bctt.s116694
19. Liu B, Yi Z, Guan X, Zeng YX, Ma F. The Relationship Between Statins and Breast Cancer Prognosis Varies by Statin Type and Exposure Time: A Meta-Analysis. Breast Cancer Res Treat (2017) 164(1):1–11. doi: 10.1007/s10549-017-4246-0
20. Smith A, Murphy L, Zgaga L, Barron TI, Bennett K. Pre-Diagnostic Statin Use, Lymph Node Status and Mortality in Women With Stages I-III Breast Cancer. Br J Cancer (2017) 117(4):588–96. doi: 10.1038/bjc.2017.227
21. Botteri E, Munzone E, Rotmensz N, Cipolla C, De Giorgi V, Santillo B, et al. Therapeutic Effect of β-Blockers in Triple-Negative Breast Cancer Postmenopausal Women. Breast Cancer Res Treat (2013) 140(3):567–75. doi: 10.1007/s10549-013-2654-3
22. Shaitelman SF, Stauder MC, Allen P, Reddy S, Lakoski S, Atkinson B, et al. Impactof Statin Use on Outcomes in Triple Negative Breast Cancer. J Cancer (2017) 8(11):2026–32. doi: 10.7150/jca.18743
23. Wu QJ, Tu C, Li YY, Zhu J, Qian KQ, Li WJ, et al. Statin Use and Breast Cancer Survival and Risk: A Systematic Review and Meta-Analysis. Oncotarget (2015) 6(40):42988–3004. doi: 10.18632/oncotarget.5557
24. Mc Menamin Ú C, Murray LJ, Hughes CM, Cardwell CR. Statin Use and Breast Cancer Survival: A Nationwide Cohort Study in Scotland. BMC Cancer (2016) 16:600. doi: 10.1186/s12885-016-2651-0
25. Emilsson L, García-Albéniz X, Logan RW, Caniglia EC, Kalager M, Hernán MA. Examining Bias in Studies of Statin Treatment and Survival in Patients With Cancer. JAMA Oncol (2018) 4(1):63–70. doi: 10.1001/jamaoncol.2017.2752
26. Manthravadi S, Shrestha A, Madhusudhana S. Impact of Statin Use on Cancerrecurrence and Mortality in Breast Cancer: A Systematic Review and Meta-Analysis. Int J Cancer (2016) 139(6):1281–8. doi: 10.1002/ijc.30185
27. Lv H, Shi D, Fei M, Chen Y, Xie F, Wang Z, et al. Association Between Statin Use and Prognosis of Breast Cancer: A Meta-Analysis of Cohort Studies. Front Oncol (2020) 10:556243. doi: 10.3389/fonc.2020.556243
28. Leong SP, Shen ZZ, Tiu TJ, Agarwal G, Tajima T, Paik NS, et al. Is Breast Cancer the Same Disease in Asian and Western Countries? World J Surg (2010) 34(10):2308–24. doi: 10.1007/s00268-010-0683-1
29. Naito R, Miyauchi K, Daida H. Racial Differences in the Cholesterol-Lowering Effect of Statin. J Atheroscl Thromb (2017) 24(1):19–25. doi: 10.5551/jat.RV16004
30. Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc (1999) 94(446):496–509. doi: 10.1080/01621459.1999.10474144
31. Boudreau DM, Yu O, Miglioretti DL, Buist DS, Heckbert SR, Daling JR. Statin Use and Breast Cancer Risk in a Large Population-Based Setting. Cancer Epidemiol Biomarkers Prev (2007) 16(3):416–21. doi: 10.1158/1055-9965.epi-06-0737
32. Chae YK, Valsecchi ME, Kim J, Bianchi AL, Khemasuwan D, Desai A, et al. Reduced Risk of Breast Cancer Recurrence in Patients Using ACE Inhibitors, ARBs, and/or 456 Statins. Cancer Invest (2011) 29(9):585–93. doi: 10.3109/07357907.2011.616252
33. Cardwell CR, Hicks BM, Hughes C, Murray LJ. Statin Use After Diagnosis of Breast Cancer and Survival: A Population-Based Cohort Study. Epidemiology (2015) 26(1):68–78. doi: 10.1097/ede.0000000000000189
34. Borgquist S, Broberg P, Tojjar J, Olsson H. Statin Use and Breast Cancer Survival – a Swedish Nationwide Study. BMC Cancer (2019) 19(1):54. doi: 10.1186/s12885-018-5263-z
35. Harborg S, Heide-Jørgensen U, Ahern TP, Ewertz M, Cronin-Fenton D, Borgquist S. Statin Use and Breast Cancer Recurrence in Postmenopausal Women Treated With Adjuvant Aromatase Inhibitors: A Danish Population-Based Cohort Study. Breast Cancer Res Treat (2020) 183(1):153–60. doi: 10.1007/s10549-020-05749-5
36. His M, Dartois L, Fagherazzi G, Boutten A, Dupré T, Mesrine S, et al. Associations Between Serum Lipids and Breast Cancer Incidence and Survival in the E3N Prospective Cohort Study. Cancer Causes Control (2017) 28(1):77–88. doi: 10.1007/s10552-016-0832-4
37. Casaburi I, Chimento A, De Luca A, Nocito M, Sculco S, Avena P, et al. Cholesterol as an Endogenous Errα Agonist: A New Perspective to Cancer Treatment. Front Endocrinol (2018) 9:525. doi: 10.3389/fendo.2018.00525
38. Mueck AO, Seeger H, Wallwiener D. Effect of Statins Combined With Estradiol on the Proliferation of Human Receptor-Positive and Receptor-Negative Breast Cancer Cells. Menopause (2003) 10(4):332–6. doi: 10.1097/01.gme.0000055485.06076.00
39. Campbell MJ, Esserman LJ, Zhou Y, Shoemaker M, Lobo M, Borman E, et al. Breast Cancer Growth Prevention by Statins. Cancer Res (2006) 66(17):8707–14. doi: 10.1158/0008-5472.CAN-05-4061
40. Beckwitt CH, Shiraha K, Wells A. Lipophilic Statins Limit Cancer Cell Growth and Survival, via Involvement of Akt Signaling. PloS One (2018) 13(5):e0197422. doi: 10.1371/journal.pone.0197422
41. Göbel A, Breining D, Rauner M, Hofbauer LC, Rachner TD. Induction of 3-Hydroxy-3-Methylglutaryl-CoA Reductase Mediates Statin Resistance in Breast Cancer Cells. Cell Death Dis (2019) 10(2):91. doi: 10.1038/s41419-019-1322-x
42. Bytautaite M, Petrikaite V. Comparative Study of Lipophilic Statin Activity in 2D and 3D In Vitro Models of Human Breast Cancer Cell Lines MDA-MB-231 and MCF-7. Onco Targets Ther (2020) 13:13201–9. doi: 10.2147/ott.s283033
43. Huang SW, Chyuan IT, Shiue C, Yu MC, Hsu YF, Hsu MJ. Lovastatin-Mediated MCF-7 Cancer Cell Death Involves LKB1-AMPK-P38mapk-P53-Survivinsignalling Cascade. J Cell Mol Med (2020) 24(2):1822–36. doi: 10.1111/jcmm.14879
44. Moriai R, Tsuji N, Moriai M, Kobayashi D, Watanabe N. Survivin Plays as a Resistant Factor Against Tamoxifen-Induced Apoptosis in Human Breast Cancer Cells. Breast Cancer Res Treat (2009) 117(2):261–71. doi: 10.1007/s10549-008-0164-5
45. Shen Y, Du Y, Zhang Y, Pan Y. Synergistic Effects of Combined Treatment Withsimvastatin and Exemestane on MCF-7 Human Breast Cancer Cells. Mol Med Rep (2015) 12(1):456–62. doi: 10.3892/mmr.2015.3406
46. Mo H, Elson CE. Studies of the Isoprenoid-Mediated Inhibition of Mevalonate Synthesis Applied to Cancer Chemotherapy and Chemoprevention. Exp Biol Med (2004) 229(7):567–85. doi: 10.1177/153537020422900701
47. Elson CE, Peffley DM, Hentosh P, Mo H. Isoprenoid-Mediated Inhibition of Mevalonate Synthesis: Potential Application to Cancer. Proc Soc Exp Biol Med (1999) 221(4):294–311. doi: 10.1046/j.1525-1373.1999.d01-87.x
48. Bjarnadottir O, Feldt M, Inasu M, Bendahl PO, Elebro K, Kimbung S, et al. Statin Use, HMGCR Expression, and Breast Cancer Survival - The Malmö Diet and Cancer Study. Sci Rep (2020) 10(1):558. doi: 10.1038/s41598-019-57323-9
49. Kim H, Seol YM, Choi YJ, Shin HJ, Chung JS, Shin N, et al. HMG CoA Reductaseexpression as a Prognostic Factor in Korean Patients With Breast Cancer: A Retrospective Study. Med (Baltimore) (2019) 98(13):e14968. doi: 10.1097/md.0000000000014968
50. Borgquist S, Djerbi S, Pontén F, Anagnostaki L, Goldman M, Gaber A, et al. HMG- 417 CoA Reductase Expression in Breast Cancer Is Associated With a Less Aggressive Phenotype and Influenced by Anthropometric Factors. Int J Cancer (2008) 123(5):1146–53. doi: 10.1002/ijc.23597
51. Borgquist S, Jögi A, Pontén F, Rydén L, Brennan DJ, Jirström K. Prognosticimpact of Tumour-Specific HMG-CoA Reductase Expression in Primary Breast Cancer. Breast Cancer Res (2008) 10(5):R79. doi: 10.1186/bcr2146
52. Hu MB, Zhang JW, Gao JB, Qi YW, Gao Y, Xu L, et al. Atorvastatin Induces Autophagy in MDA-MB-231 Breast Cancer Cells. Ultrastruct Pathol (2018) 42(5):409–15. doi: 10.1080/01913123.2018.1522406
53. Kotamraju S, Williams CL, Kalyanaraman B. Statin-Induced Breast Cancer Cell Death: Role of Inducible Nitric Oxide and Arginase-Dependent Pathways. Cancer Res (2007) 67(15):7386–94. doi: 10.1158/0008-5472.can-07-0993
54. Sánchez CA, Rodríguez E, Varela E, Zapata E, Páez A, Massó FA, et al. Statin-Induced Inhibition of MCF-7 Breast Cancer Cell Proliferation Is Related to Cell Cycle Arrest and Apoptotic and Necrotic Cell Death Mediated by an Enhanced Oxidative Stress. Cancer Invest (2008) 26(7):698–707. doi: 10.1080/07357900701874658
55. Waks AG, Winer EP. Breast Cancer Treatment: A Review. JAMA (2019) 321(3):288–300. doi: 10.1001/jama.2018.19323
56. Wang S-h, Lin S-Y. Tumor Dormancy: Potential Therapeutic Target in Tumor Recurrence and Metastasis Prevention. Exp Hematol Oncol (2013) 2:29–9. doi: 10.1186/2162-3619-2-29
57. Wang J, Kitajima I. Pitavastatin Inactivates NF-κb and Decreases IL-6 Production Through Rho Kinase Pathway in MCF-7 Cells. Oncol Rep (2007) 17(5):1149–54. doi: 10.3892/or.17.5.1149
58. Liu S, Uppal H, Demaria M, Desprez PY, Campisi J, Kapahi P. Simvastatin Suppresses Breast Cancer Cell Proliferation Induced by Senescent Cells. Sci Rep (2015) 5:17895. doi: 10.1038/srep17895
59. Shen YY, Yuan Y, Du YY, Pan YY. Molecular Mechanism Underlying the Anticancer Effect of Simvastatin on MDA-MB-231 Human Breast Cancer Cells. Mol Med Rep (2015) 12(1):623–30. doi: 10.3892/mmr.2015.3411
60. Mezquita B, Mezquita P, Pau M, Gasa L, Navarro L, Samitier M, et al. All-Trans-Retinoic Acid Activates the Pro-Invasive Src-YAP-Interleukin 6 Axis in Triple-Negative MDA-MB-231 Breast Cancer Cells While Cerivastatin Reverses This Action. Sci Rep (2018) 8(1):7047. doi: 10.1038/s41598-018-25526-1
61. Rezaie-Majd A, Maca T, Bucek RA, Valent P, Müller MR, Husslein P, et al. Simvastatin Reduces Expression of Cytokines Interleukin-6, Interleukin-8, and Monocyte Chemoattractant Protein-1 in Circulating Monocytes From Hypercholesterolemic Patients. Arterioscler Thromb Vasc Biol (2002) 22(7):1194–9. doi: 10.1161/01.atv.0000022694.16328.cc
62. Sakoda K, Yamamoto M, Negishi Y, Liao JK, Node K, Izumi Y. Simvastatin Decreases IL-6 and IL-8 Production in Epithelial Cells. J Dent Res (2006) 85(6):520–3. doi: 10.1177/154405910608500608
63. Gasbarrino K, Hafiane A, Zheng H, Daskalopoulou SS. Intensive Statin Therapy Compromises the Adiponectin-AdipoR Pathway in the Human Monocyte-Macrophage Lineage. Stroke (2019) 50(12):3609–17. doi: 10.1161/strokeaha.119.026280
64. Koohestanimobarhan S, Salami S, Imeni V, Mohammadi Z, Bayat O. Lipophilic Statins Antagonistically Alter the Major Epithelial-to-Mesenchymal Transition Signaling Pathways in Breast Cancer Stem-Like Cells via Inhibition of the Mevalonate Pathway. J Cell Biochem (2018) 120:2515–31. doi: 10.1002/jcb.27544
65. Wu J, Wong WW, Khosravi F, Minden MD, Penn LZ. Blocking the Raf/MEK/ERK Pathway Sensitizes Acute Myelogenous Leukemia Cells to Lovastatin-Induced Apoptosis. Cancer Res (2004) 64(18):6461–8. doi: 10.1158/0008-5472.can-04-0866
66. Jain MK, Ridker PM. Anti-Inflammatory Effects of Statins: Clinical Evidence and Basic Mechanisms. Nat Rev Drug Discov (2005) 4(12):977–87. doi: 10.1038/nrd1901
67. Cao Z, Fan-Minogue H, Bellovin DI, Yevtodiyenko A, Arzeno J, Yang Q, et al. MYC Phosphorylation, Activation, and Tumorigenic Potential in Hepatocellular Carcinoma Are Regulated by HMG-CoA Reductase. Cancer Res (2011) 71(6):2286–97. doi: 10.1158/0008-5472.can-10-3367
68. Ma Y, Chen Z, Zou Y, Ge J. Atorvastatin Represses the Angiotensin 2-Induced Oxidative Stress and Inflammatory Response in Dendritic Cells via the PI3K/Akt/Nrf 2 Pathway. Oxid Med Cell Longev (2014) 2014:148798. doi: 10.1155/2014/148798
69. McGuire TR, Kalil AC, Dobesh PP, Klepser DG, Olsen KM. Anti-Inflammatory Effects of Rosuvastatin in Healthy Subjects: A Prospective Longitudinal Study. Curr Pharm Des (2014) 20(7):1156–60. doi: 10.2174/1381612820666140127163313
70. Wejde J, Blegen H, Larsson O. Requirement for Mevalonate in the Control of Proliferation of Human Breast Cancer Cells. Anticancer Res (1992) 12(2):317–24.
71. Wong WW, Dimitroulakos J, Minden MD, Penn LZ. HMG-CoA Reductase Inhibitors and the Malignant Cell: The Statin Family of Drugs as Triggers of Tumor-Specific Apoptosis. Leukemia (2002) 16(4):508–19. doi: 10.1038/sj.leu.2402476
72. Dulak J, Józkowicz A. Anti-Angiogenic and Anti-Inflammatory Effects of Statins: Relevance to Anti-Cancer Therapy. Curr Cancer Drug Targets (2005) 5(8):579–94. doi: 10.2174/156800905774932824
73. Zhong S, Zhang X, Chen L, Ma T, Tang J, Zhao J. Statin Use and Mortality in Cancer Patients: Systematic Review and Meta-Analysis of Observational Studies. Cancer TreatRev (2015) 41(6):554–67. doi: 10.1016/j.ctrv.2015.04.005
74. Li YR, Ro V, Steel L, Carrigan E, Nguyen J, Williams A, et al. Impact of Long-Term Lipid-Lowering Therapy on Clinical Outcomes in Breast Cancer. Breast Cancer Res Treat (2019) 176(3):669–77. doi: 10.1007/s10549-019-05267-z
75. Ewertz M, Jensen MB, Gunnarsdóttir K, Højris I, Jakobsen EH, Nielsen D, et al. Effect of Obesity on Prognosis After Early-Stage Breast Cancer. J Clin Oncol (2011) 29(1):25–31. doi: 10.1200/jco.2010.29.7614
76. Stroes ES, Thompson PD, Corsini A, Vladutiu GD, Raal FJ, Ray KK, et al. Statin-Associated Muscle Symptoms: Impact on Statin Therapy-European Atherosclerosis Society Consensus Panel Statement on Assessment, Aetiology and Management. Eur Heart J (2015) 36(17):1012–22. doi: 10.1093/eurheartj/ehv043
77. Mach F, Ray KK, Wiklund O, Corsini A, Catapano AL, Bruckert E, et al. Adverse Effects of Statin Therapy: Perception vs. The Evidence - Focus on Glucose Homeostasis, Cognitive, Renal and Hepatic Function, Haemorrhagic Stroke and Cataract. Eur Heart J (2018) 39536(27):2526–39. doi: 10.1093/eurheartj/ehy182
Keywords: statin, HMG-CoA reductase inhibitor, breast cancer, recurrence, Asia
Citation: Sim Y, Lim C, Phyu N, Tan KTB, Chew LST, Wong CY, Madhukumar P, Yong WS, Lim SZ, Hamzah JLB, Tan SY, Chay WY, Wong FY, Tan PH and Tan VKM (2022) The Impact of Statin Use and Breast Cancer Recurrence - A Retrospective Study in Singapore. Front. Oncol. 12:835320. doi: 10.3389/fonc.2022.835320
Received: 14 December 2021; Accepted: 07 March 2022;
Published: 31 March 2022.
Edited by:
Angela Toss, University of Modena and Reggio Emilia, ItalyReviewed by:
Jerry Polesel, Aviano Oncology Reference Center (IRCCS), ItalyCopyright © 2022 Sim, Lim, Phyu, Tan, Chew, Wong, Madhukumar, Yong, Lim, Hamzah, Tan, Chay, Wong, Tan and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yirong Sim, c2ltLnlpcm9uZ0BzaW5naGVhbHRoLmNvbS5zZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.