
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 16 March 2022
Sec. Genitourinary Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.834249
This article is part of the Research Topic Prognostic Factors and Novel Therapy in Urothelial Cancer View all 10 articles
 Hong-Yue Lai1,2
Hong-Yue Lai1,2 Chia-Chun Chiu3
Chia-Chun Chiu3 Yu-Hsuan Kuo4
Yu-Hsuan Kuo4 Hsin-Hwa Tsai1,2
Hsin-Hwa Tsai1,2 Li-Ching Wu1
Li-Ching Wu1 Wen-Hsin Tseng5
Wen-Hsin Tseng5 Chien-Liang Liu5,6
Chien-Liang Liu5,6 Chung-Hsi Hsing2,7,8
Chung-Hsi Hsing2,7,8 Steven K. Huang5,9*
Steven K. Huang5,9* Chien-Feng Li1,2,10,11,12,13*
Chien-Feng Li1,2,10,11,12,13*Background: Urothelial carcinoma (UC) patients often bear clinical and genetic heterogeneity, which may differ in management and prognosis. Especially, patients with advanced/metastatic UC generally have a poor prognosis and survive for only few months. The Wnt/β-catenin signaling is found to be highly activated in several cancers, including UC. However, accumulated evidence has shown discordance between the Wnt/β-catenin signaling and UC carcinogenesis. Accordingly, we aim to get a better understanding of the molecular characterization of UC, focusing on the Wnt signaling, which may add value to guiding management more precisely.
Patients and Methods: Clinical data and pathological features were retrospectively surveyed. The correlations of secreted Frizzled-related protein 2 (SFRP2) immunoexpression with clinicopathological features were analyzed by Pearson’s chi-square test. The Kaplan–Meier method with a log-rank test was employed to plot survival curves. All significant features from the univariate analysis were incorporated into the Cox regression model for multivariate analysis.
Results: Following data mining on a transcriptome dataset (GSE31684), we identified that 8 transcripts in relation to the Wnt signaling pathway (GO: 0016055) were significantly upregulated in advanced/metastatic bladder tumors. Among these transcripts, the SFRP2 level showed the most significant upregulation. Additionally, as SFRP2 is a putative Wnt inhibitor and may be expressed by stroma, we were interested in examining the immunoexpression and clinical relevance of stromal and tumoral SFRP2 in our urothelial carcinoma cohorts containing 295 urinary bladder UC (UBUC) and 340 upper urinary tract UC (UTUC) patients. We observed that high SFRP2 expression in stroma but not in tumors is significantly linked to aggressive UC features, including high tumor stage and histological grade, positive nodal metastasis, the presence of vascular and perineural invasion, and high mitotic activity in UBUC and UTUC. Moreover, high stromal SFRP2 expression significantly and independently predicted worse clinical outcomes in UBUC and UTUC. Utilizing bioinformatic analysis, we further noticed that stromal SFRP2 may link epithelial–mesenchymal transition (EMT) to UC progression.
Conclusion: Collectively, these results imply that stromal SFRP2 may exert oncogenic function beyond its Wnt antagonistic ability, and stromal SFRP2 expression can provide prognostic and therapeutic implications for UC patients.
Urothelial carcinoma (UC) is a malignancy derived from the transitional epithelium of the urinary tract, which is also known as transitional cell carcinoma. Although UC is mainly found in the urinary bladder (UBUC), the incidence of upper urinary tract UC (UTUC), including the ureter and renal pelvis, is increasing in Taiwan (1). UC patients often bear clinical and genetic heterogeneity, which may differ in management and prognosis. For UBUC, 70–80% of patients are non-muscle-invasive bladder cancer (NMIBC) at initial diagnosis, and the standard treatment is transurethral resection of bladder tumor (TURBT) with subsequent intravesical instillation (2). However, 50–70% of patients experience local recurrence, and 10–15% of patients progress to muscle-invasive or metastatic disease (3). In contrast, 20–30% of UBUC patients are initially diagnosed with muscle-invasive bladder cancer (MIBC) or metastatic disease, and radical cystectomy is indicated as the standard therapy (4). Nevertheless, recurrence and metastasis still occur in 15–50% of patients following radical surgery (4). For UTUC, curative nephroureterectomy with bladder cuff excision is recommended for most patients (5). In addition, cisplatin-based postoperative adjuvant chemotherapy is given for advanced/metastatic UBUC or UTUC patients, but the clinical outcomes remain disappointing due to chemoresistance (6). Since UBUC and UTUC are featured by clinical heterogeneity, the addition of genetic information may improve management and prognosis.
The Wingless-related integration site (Wnt)/β-catenin signaling pathway is involved in diverse physiological processes, including embryonic development, cell proliferation and differentiation, and tissue homeostasis and regeneration (7). The canonical Wnt/β-catenin signaling is triggered by the binding of Wnt ligands to the low density lipoprotein receptor-related protein 5/6 (LRP5/6) receptors and Frizzled (FZD) receptors on the cell surface. Subsequently, β-catenin is undegraded and increased in the cytosol and translocates to the nucleus and binds to T-cell factor (TCF)/lymphoid enhancer-binding factor (LEF) transcription factors, resulting in the upregulation of Wnt target genes (8). In addition to prominently described in colorectal cancer, the Wnt/β-catenin signaling is found to be highly activated in several cancers, including UC (9). This leads to the development of various Wnt/β-catenin inhibitors such as targeting Wnt ligand/receptor interface for cancer therapies (8). However, accumulated investigations have shown incompatible evidence between the Wnt/β-catenin signaling and UC carcinogenesis (10, 11). Additionally, the aberrant Wnt/β-catenin signaling is not only restricted to cancer cells but also implicated in dynamic interactions with the tumor microenvironment (TME) and immune system in UC (12). These observations further emphasize the molecular heterogeneity of UC, and a deeper understanding of the molecular characterization of UC may come up with more hints for how such pathways be therapeutically targeted.
The secreted Frizzled-related protein 2 (SFRP2) gene, which is located on chromosome 4q31.3 in humans, encodes a glycoprotein containing an Frizzled-like cysteine-rich domain. As this N-terminal cysteine-rich domain shares substantial sequence similarity with Wnt binding domain of FZD receptors, SFRP2 was initially considered to antagonize the Wnt signaling by preventing binding of Wnt ligands to FZD receptors, which may inhibit tumor development (13). However, recent study has indicated that SFRP2 can promote tumor angiogenesis via the noncanonical Wnt/Ca2+ signaling (14). Moreover, upon secreted to the extracellular matrix (ECM), SFRP2 has been linked to the fibronectin-integrin complex, which can promote cell adhesion and block apoptosis in canine mammary gland tumors (15). Also, high level of SFRP2 in serum has been correlated with a poor prognosis in breast cancer patients (16). A similar correlation between high SFRP2 protein expression and poor prognosis was observed in osteosarcoma, which usually develops in the osteoblast cells from bone (17). In addition, SFRP2 is also known as stromal cell-derived factor 5 (SDF5), which was identified by a cDNA screen for secreted proteins in bone marrow stromal cells (18). Impressively, it has been reported that SFRP2 expression in tumors may be conferred by stroma (19). These reflect the complicated regulation of SFRP2 in tumors and their microenvironment, which is composed of stromal cells, immune cells, and the ECM. The TME characteristics have also been comprehensively described in UC (20). Since SFRP2 is specifically expressed in the urinary bladder and the contribution of stromal cell–tumor cell communication to UC progression remained to be determined, we were interested in exploring the role of SFRP2 in UBUC and UTUC, thereby improving prognosis and therapy.
A transcriptome dataset (GSE31684), incorporating 93 bladder cancer patients who were managed by radical cystectomy, from the GEO database (NCBI) was used for data mining. The raw data of all probe sets were analyzed without preselection or filtering. To quantify the expression levels of all transcripts, we imported the raw CEL files into the statistical software Nexus Expression 3 (BioDiscovery, El Segundo, CA, USA). According to tumor invasion and metastasis determined by clinical assessment, a comparative analysis (invasive vs. noninvasive and metastatic vs. nonmetastatic) was conducted under supervision. We underscored differentially expressed genes with special interest to the Wnt signaling pathway (GO: 0016055) and further selected those with a log2-transformed expression fold change > 0.2 and a p-value less than 0.01 for further analysis.
This study was approved by the Institutional Review Board of Chi Mei Medical Center (10501005). We enrolled 340 UTUC and 295 UBUC patients who had curative surgery from 1996 to 2004, and all specimens with the informed consents were procured from our biobank. Clinical data, pathological features, and clinical outcomes were retrospectively obtained from the patients’ medical records. Patients with preoperative neoadjuvant chemotherapy, other malignancies, or missing clinical data were not included. With curative purpose, 10 UTUC patients received ureterectomy and 330 UTUC patients underwent nephroureterectomy with bladder cuff excision. Cisplatin-based postoperative adjuvant chemotherapy was given for 29 out of 106 UTUC patients with pT3 or pT4 status or nodal involvement. On the other hand, for patients with superficial UBUC (pTa or pT1), TURBT with or without intravesical Bacillus Calmette–Guérin (BCG) was performed. For those with tumor recurrence, radical cystectomy was further performed. Patients with muscle-invasive UBUC were subjected to radical cystectomy with bilateral pelvic lymph node dissection. Similarly, cisplatin-based postoperative adjuvant chemotherapy was given for UBUC patients with pT3 or pT4 stage disease or nodal metastasis. The median/mean follow-up duration was 38.9/44.7 and 23.1/30.8 months for UTUC and UBUC, respectively.
Tumor stages and histological grades were appraised on hematoxylin and eosin (H&E) staining of all cases according to the 7th edition of the American Joint Committee on Cancer (AJCC) staging system (21) and the World Health Organization (WHO) classification criteria (22), respectively. Tumor location and multifocality were used to evaluate clinical outcomes in UTUC but not in UBUC. Vascular invasion and perineural invasion were determined by the presence of tumor emboli in the vascular channels and tumor nests surrounding the nerve bundles, respectively. The mitotic rate was determined by calculating mitotic figures per 10 high-power fields (HPFs; 400x light microscopic magnification). We defined the mitotic rate less than 10/10 HPFs as low mitotic activity and the mitotic rate equal to or beyond 10/10 HPFs as high mitotic activity. Immunohistochemistry was performed based on our previous study (23), and slides were stained with an anti-SFRP2 antibody. Two independent pathologists (Chien-Feng Li and Wan-Shan Li) appraised SFRP2 immunoreactivity by integrating the percentage of stained tumor cells and intensity of staining of UC cells to produce the H-score as previously described (24). The H-score was determined with the following equation: H-score = ΣPi (i + 1), where i is the intensity of stained tumor cells (0 to 3+) and Pi is the percentage of staining for each intensity, ranging from 0% to 100%. According to the H-score, SFRP2 expression levels were divided into low (less than the median) and high (above or equal to the median) groups.
Utilizing the cBioPortal web platform (http://cbioportal.org), the correlations of the mRNA level of SFRP2 with its coexpressed transcripts in the UTUC and UBUC datasets from the TCGA database were downloaded. To further realize the functional roles of SFRP2 in UC, the top 194 overlapping transcripts co-upregulated with SFRP2 between UTUC and UBUC were examined utilizing the Gene Ontology (GO) classification system (http://geneontology.org/) according to three functional groups (biological processes, molecular functions, or cellular components) and were ranked by fold enrichment. To plot representative GO terms, an R script with ggplot2 package was used.
All statistical analyses were executed in SPSS software version 22.0 (IBM Corporation, Armonk, NY, USA), and two-tailed tests with a p-value less than 0.05 were considered statistically significant. We analyzed three endpoints: disease-specific survival (DSS), metastasis-free survival (MeFS), and local recurrence-free survival (LRFS). DSS was measured from curative surgery to the time of cancer death, and MeFS and LRFS were measured from curative surgery to the first metastasis and local recurrence, respectively. Pearson’s chi-square test was used to appraise the correlations of clinicopathological variables with SFRP2 expression. The Kaplan–Meier method with a log-rank test was employed to plot survival curves. All significant characteristics from the univariate analysis were entered into the Cox regression model for multivariate analysis to find independent prognostic factors.
To identify promising genes related to UC progression, a public dataset (GSE31684), incorporating 93 bladder cancer patients who were managed by radical cystectomy, was used for data mining. Among these patients, 78 were diagnosed as muscle-invasive disease (pT2–pT4), and 15 were determined as having superficial disease (pTa or pT1). We identified 10 probes covering 8 transcripts zooming in the Wnt signaling pathway (GO: 0016055) (Table 1 and Figure 1). Phylogenetically, SFRP1, SFRP2, and SFRP5 belong to the same subfamily, whose members share homology in their cysteine-rich domain (25). In this study, we found that both the SFRP1 and SFRP2 transcripts are significantly increased in tumors with muscle invasiveness and distal metastasis, but the SFRP2 level showed the most prominent upregulation among all identified transcripts. Nevertheless, using the Gene Expression Profiling Interactive Analysis (GEPIA) database (http://gepia.cancer-pku.cn/detail.php?gene=SFRP1) (http://gepia.cancer-pku.cn/detail.php?gene=SFRP2), which incorporates the TCGA data, we noticed that both the SFRP1 and SFRP2 transcripts significantly decrease in bladder urothelial carcinoma (n = 404) compared to their paired normal tissue (n = 28). Intriguingly, the violin plots showed that both the mRNA levels of SFRP1 and SFRP2 significantly increase with the progression of bladder cancer (from stage II to stage IV) (Supplementary Figure 1A and Figure 2A). However, only bladder tumors with high mRNA level of SFRP2 (n = 101) significantly conferred inferior overall survival compared with those with low SFRP2 mRNA level (n = 100) (p = 0.00052) (Supplementary Figure 1B and Figure 2B).
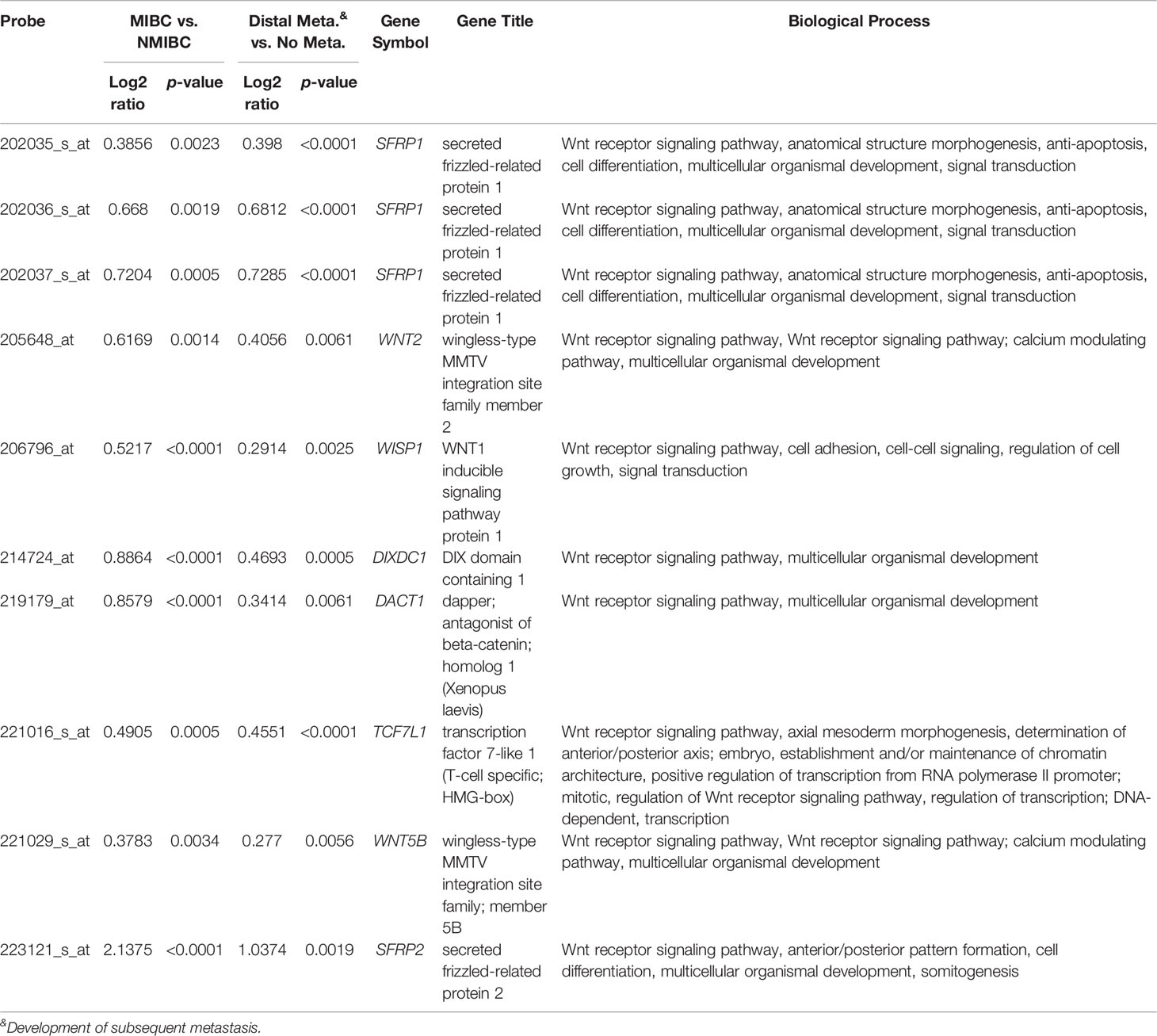
Table 1 Summary of 8 significantly altered genes associated with the Wnt signaling pathway (GO: 0016055) and UC invasion and metastasis (GSE31684).

Figure 1 Expression profiles of the top 8 genes correlated with the Wnt signaling pathway in advanced/metastatic bladder tumors. The expression levels of upregulated and downregulated genes are marked in red and green, respectively. A comparative analysis (invasive vs. noninvasive and metastatic vs. nonmetastatic) was conducted under supervision. We identified SFRP2 as the most considerably upregulated gene related to the Wnt signaling pathway (GO: 0016055) among bladder cancer patients with advanced/metastatic disease.
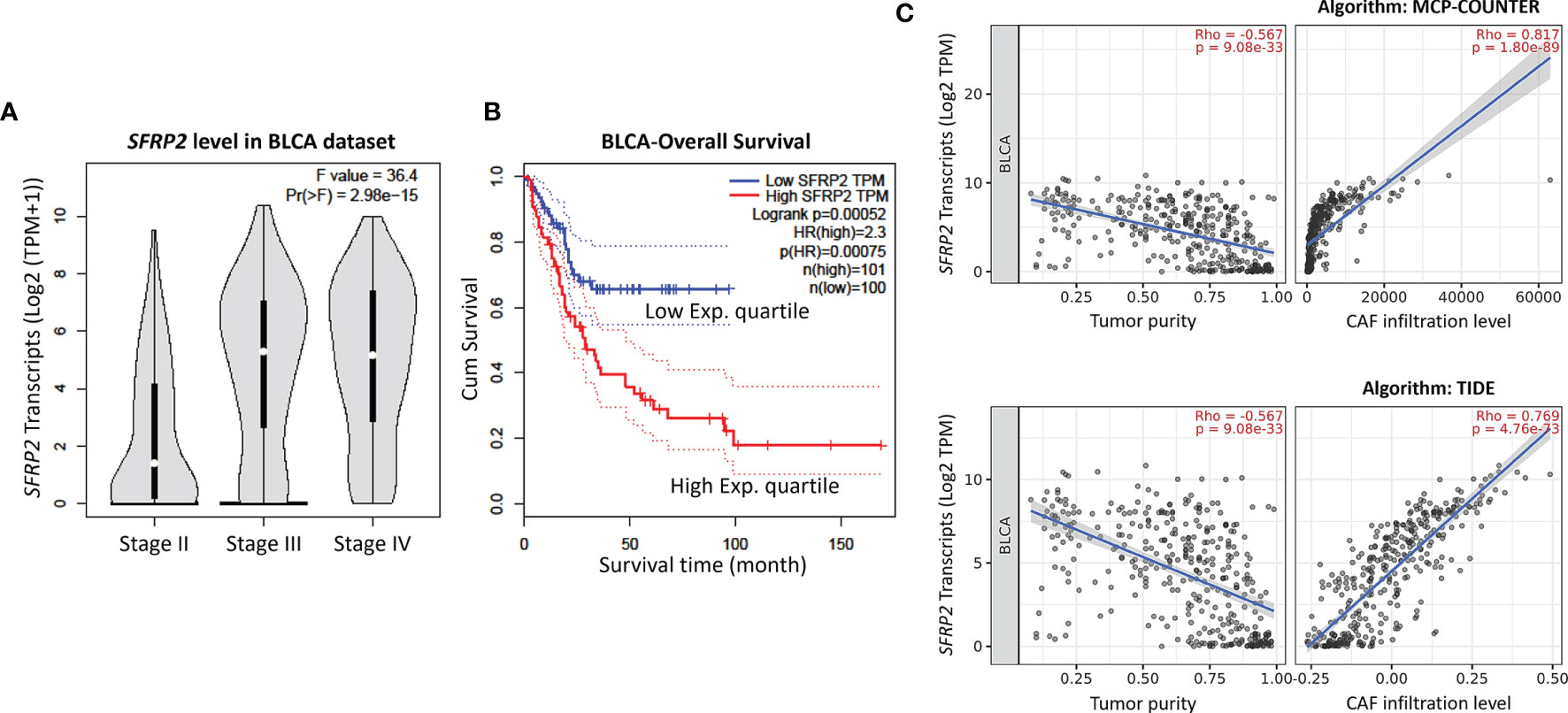
Figure 2 High SFRP2 mRNA level is correlated with advanced stage disease, inferior overall survival, and CAF infiltration. (A) The correlations between the mRNA levels of SFRP2 and bladder cancer progression. (B) The impact of SFRP2 mRNA levels on overall survival in bladder cancer. These data were acquired from the GEPIA database. (C) The correlations among the mRNA levels of SFRP2, tumor purity, and CAF infiltration in bladder cancer. These data were estimated using the MCP-COUNTER and TIDE algorithms from the TIMER2.0 database. BLCA, bladder urothelial carcinoma.
In the bladder, stromal cells have been reported to be crucial for urothelial proliferation (26), and some MIBC patients are also featured by stroma-rich (smooth muscle cells and fibroblasts) subtype (27). However, little attention was paid to the crosstalk between stromal cells and tumors in UC. Using the Tumor Immune Estimation Resource (TIMER) database version 2.0 (28), which integrates the TCGA data, we observed that both the SFRP1 and SFRP2 transcripts are significantly negatively correlated with tumor purity (the percentage of cancer cells in a sample) and positively correlated with cancer-associated fibroblast (CAF) infiltration in bladder urothelial carcinoma, but the SFRP2 gene showed a more distinguished correlation (Supplementary Figure 2 and Figure 2C). These observations encouraged us to further survey the immunoexpression and clinical relevance of stromal and tumoral SFRP2 in our UTUC and UBUC cohorts.
We recruited 340 UTUC and 295 UBUC patients who had curative surgery from 1996 to 2004, and all samples were procured from our biobank (Table 2). In terms of UTUC, 62 (18.2%) patients showed multifocal tumors, and 49 (14.4%) patients had coexistent ureteral/renal pelvic tumors. There were 28 (8.2%) patients with lymph node metastasis, 159 (46.8%) patients with advanced stage disease, and 284 (83.5%) patients with high histological grade tumors. Perineural invasion was detected in 19 (5.6%) patients, and vascular invasion was found in 106 (31.2%) patients. Additionally, 167 (49.1%) samples were defined as high mitotic activity. In the context of UBUC, there were 29 (9.8%) patients with metastatic lymph nodes, 123 (41.7%) patients with muscle invasiveness, and 239 (81%) patients with high histological grade tumors. Vascular and perineural invasion were detected in 49 (16.6%) and 20 (6.8%) patients, respectively. Furthermore, 156 (52.9%) specimens were determined as having high mitotic activity.
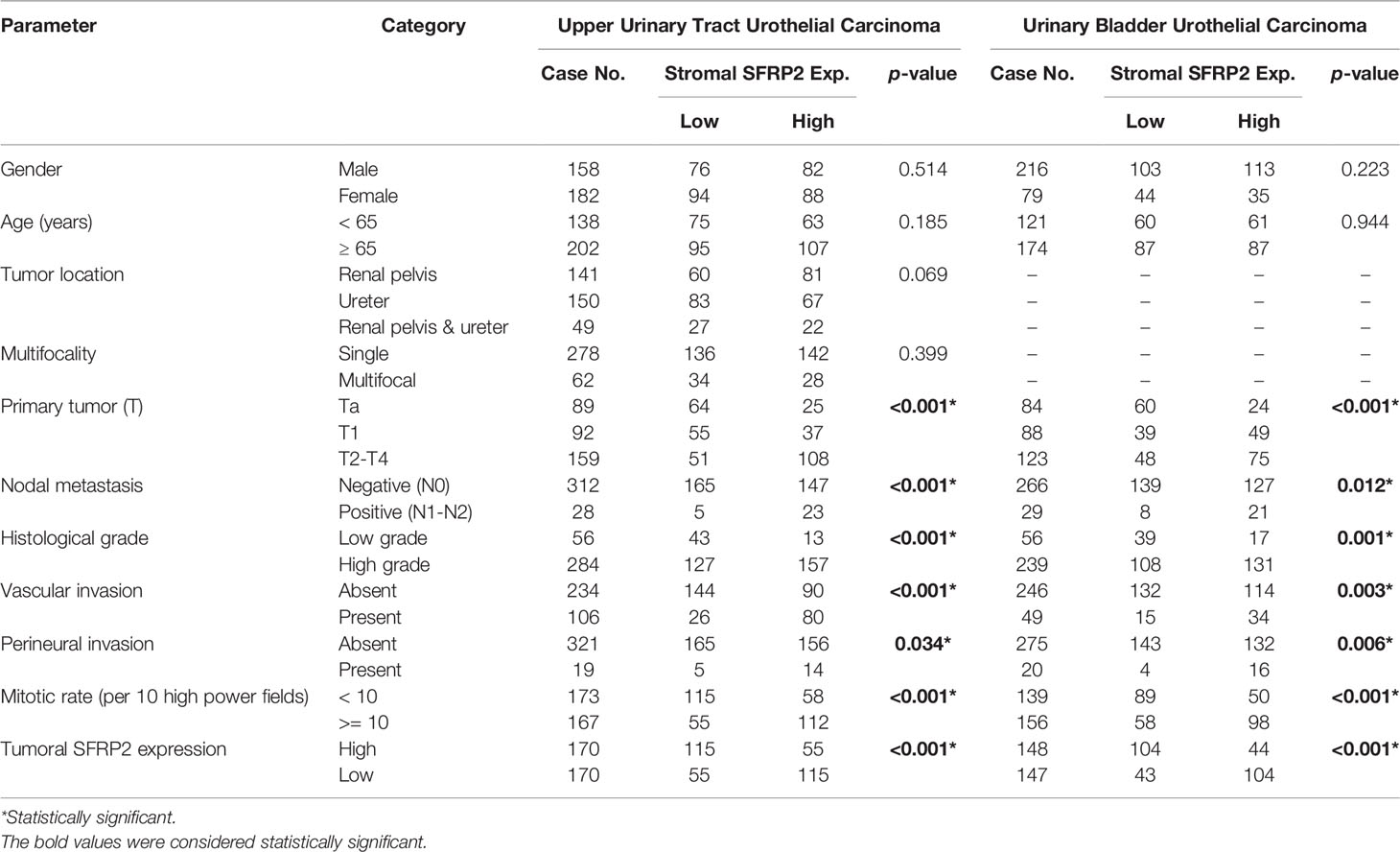
Table 2 Correlations between stromal SFRP2 expression and other important clinicopathological parameters in urothelial carcinomas.
Immunohistochemical staining revealed that SFRP2 immunoreactivity in stroma was progressively increased from early stage to advanced stage UC (Figures 3A, B). Table 2 displays stromal SFRP2 immunoexpression and its clinical significance in UTUC and UBUC. In the UTUC group, high stromal SFRP2 expression was remarkably linked to high tumor stage and histological grade (both p < 0.001), positive nodal metastasis (p < 0.001), the presence of vascular and perineural invasion (p < 0.001 and p = 0.034), high mitotic activity (p < 0.001), and low tumoral SFRP2 expression (p < 0.001). Likewise, in the UBUC group, we observed notable correlations between high stromal SFRP2 expression and high tumor stage and histological grade (p < 0.001 and p = 0.001), positive nodal metastasis (p = 0.012), the presence of vascular and perineural invasion (p = 0.003 and p = 0.006), high mitotic activity (p < 0.001), and low tumoral SFRP2 expression (p < 0.001).

Figure 3 High stromal SFRP2 immunoexpression was observed among bladder cancer patients with advanced stage disease. Immunohistochemistry was performed with an anti-SFRP2 antibody. SFRP2 immunoreactivity in stroma was gradually increased from (A) non-muscle-invasive to (B) muscle-invasive bladder cancer (200x, scale bar = 100 μm). Insect: 200x, scale bar = 25 μm.
In contrast, Table 3 exhibits tumoral SFRP2 immunoexpression and its clinical significance in UTUC and UBUC. Interestingly, in the UTUC group, low tumoral SFRP2 expression was remarkably linked to high histological grade (p = 0.041) and positive nodal metastasis (p = 0.006). In the UBUC group, we detected remarkable correlations between low tumoral SFRP2 expression and high tumor stage (p = 0.004), the presence of vascular invasion (p = 0.039), and high mitotic activity (p = 0.002). Accordingly, the disparate roles of SFRP2 seem to be dependent on cell type-specific contexts.
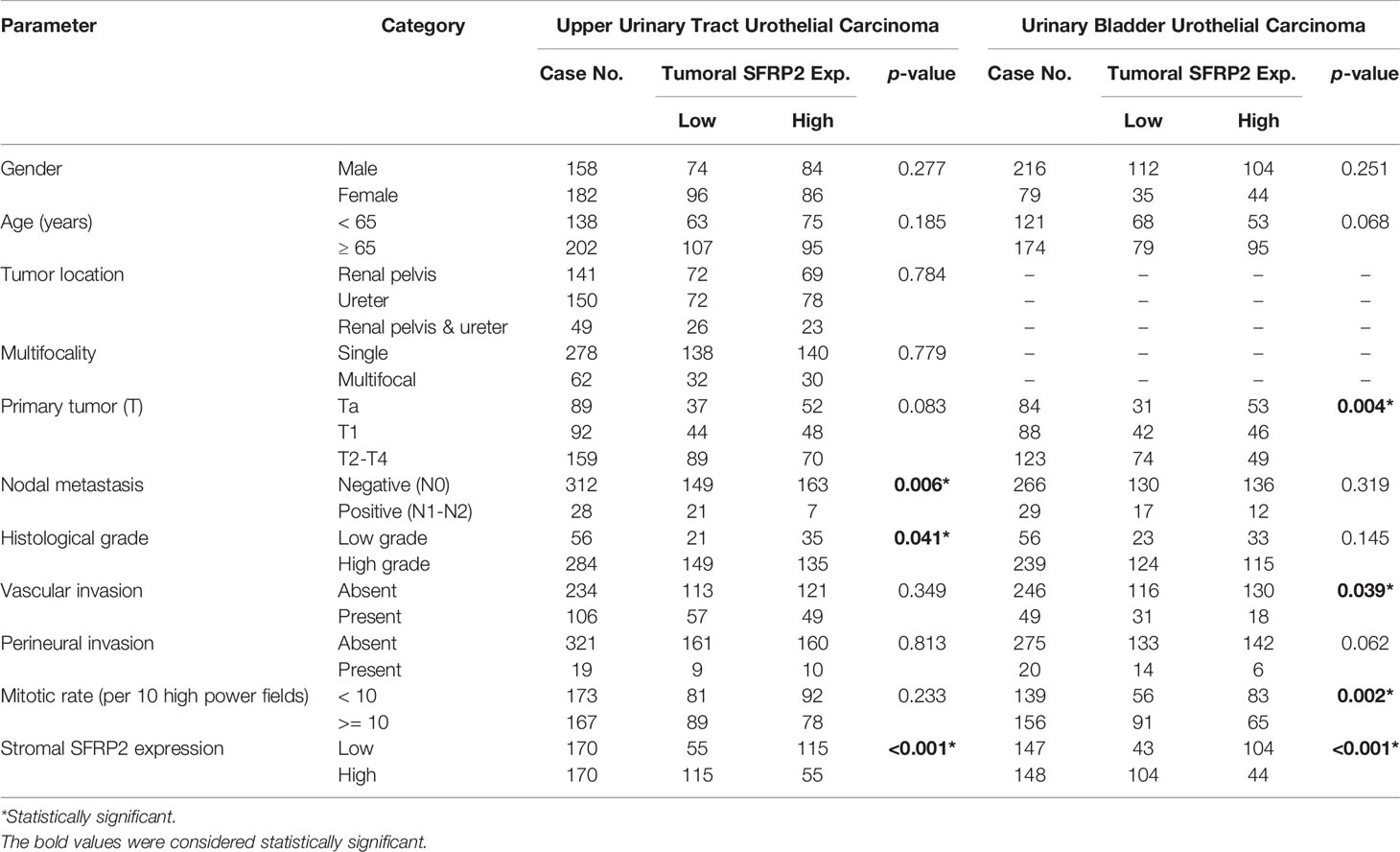
Table 3 Correlations between tumoral SFRP2 expression and other important clinicopathological parameters in urothelial carcinomas.
There were 113 deaths owing to UC, including 61 patients with UTUC and 52 patients with UBUC, in our cohorts. Additionally, a total of 146 patients, including 70 with UTUC and 76 with UBUC, had following distal metastasis. To appraise the prognostic implications of SFRP2 expression in patient death and distal metastasis in UC, univariate and multivariate analyses were utilized. In respect of UTUC, high SFRP2 expression in stroma but not in tumors was unfavorably prognostic for both disease-specific survival (DSS) and metastasis-free survival (MeFS) (both p < 0.0001) in the univariate analysis (Table 4 and Figures 4A–D). Also, multifocal tumors, high tumor stage and histological grade, positive nodal metastasis, and vascular and perineural invasion significantly conferred poor outcomes in both DSS and MeFS (all p < 0.0215). Following multivariate analysis, stroma with high SFRP2 expression and multifocality, positive nodal metastasis, and perineural invasion remained independently prognostic for poor DSS and MeFS (all p < 0.035). These results imply that high SFRP2 expression in stroma instead of tumors can act as an adverse prognostic indicator for UTUC patients.
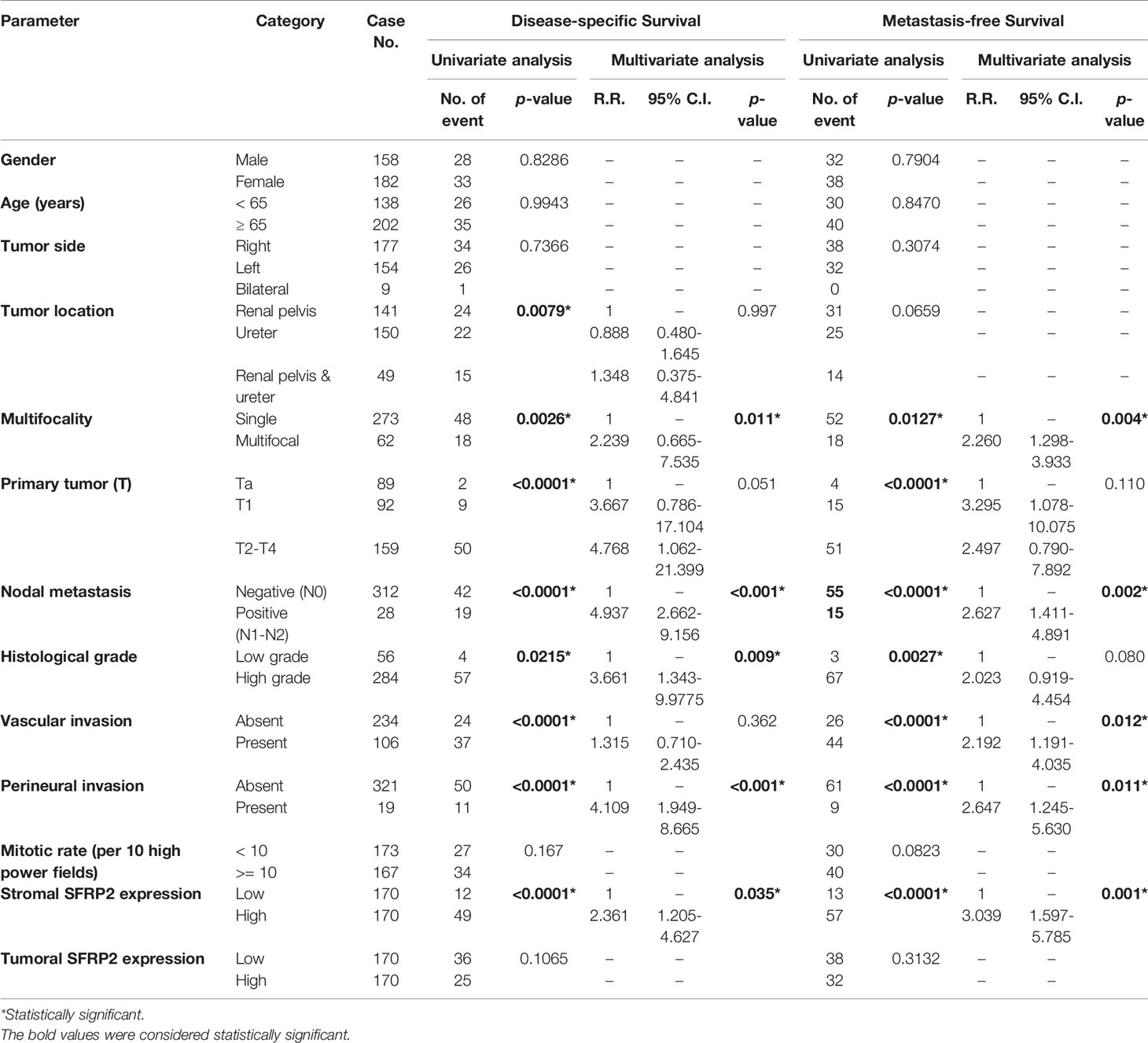
Table 4 Univariate log-rank and multivariate analyses for disease-specific and metastasis-free survivals in upper urinary tract urothelial carcinoma.
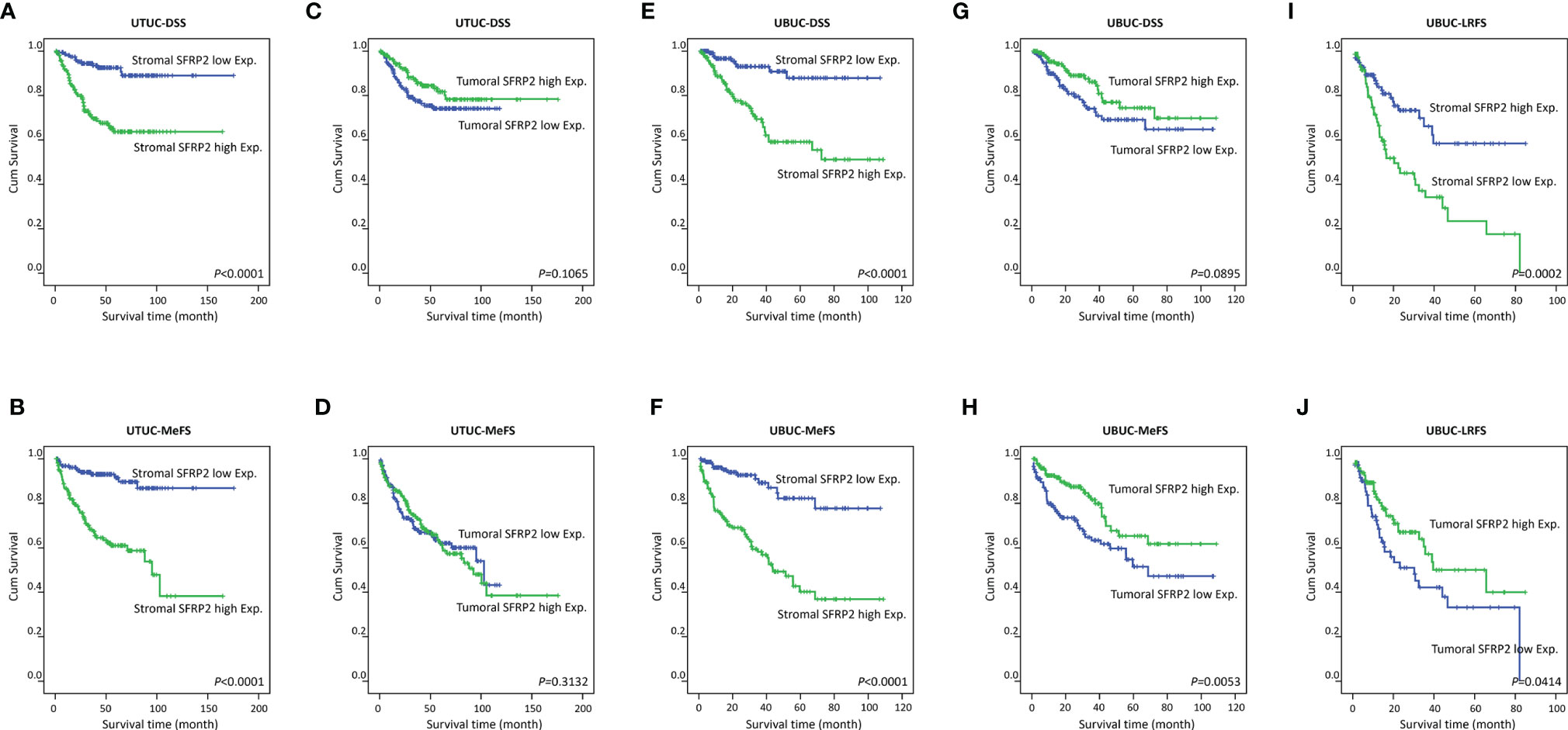
Figure 4 Survival analysis. Kaplan–Meier curves show that high stromal SFRP2 immunoexpression conferred unfavorable prognostic effects on disease-specific survival and metastasis-free survival in (A–D) UTUC and (E, F) UBUC patients. Low tumoral SFRP2 expression was adversely prognostic only for metastasis-free survival in (G, H) UBUC patients. Also, high SFRP2 immunoexpression in stroma and low SFRP2 immunoexpression in tumors were significantly correlated with poor local recurrence-free survival in (I, J) UBUC patients.
In terms of UBUC, high SFRP2 expression in stroma was unfavorably prognostic for both DSS and MeFS (both p < 0.0001) following univariate analysis (Table 5 and Figures 4E, F). In contrast, high SFRP2 expression in tumors was a favorable prognostic indicator and its prognostic effect was only on MeFS (p = 0.0053) (Figures 4G, H). In addition, high tumor stage and histological grade, positive nodal metastasis, vascular and perineural invasion, and high mitotic activity also significantly conferred worse outcomes in both DSS and MeFS (all p < 0.0024). In the multivariate analysis, stroma with high SFRP2 expression and high tumor stage were significantly prognostic for inferior DSS and MeFS (all p < 0.001). If NMIBC patients receiving TURBT develop local recurrence, radical cystectomy will be further performed, which may reduce quality of life. Accordingly, we also found that high stromal SFRP2 expression, low tumoral SFRP2 expression, and high tumor stage and histological grade were significantly correlated with poor local recurrence-free survival (LRFS) (all p < 0.0414) in the univariate analysis (Table 6 and Figures 4I, J). Furthermore, only stroma with high SFRP2 expression remained independently prognostic for inferior LRFS (p = 0.012) following multivariate analysis. Taken together, these results suggest that the prognostic effects of stromal and tumoral SFRP2 expression are distinct, and incorporation of these variables can more accurately guide management for UBUC patients.
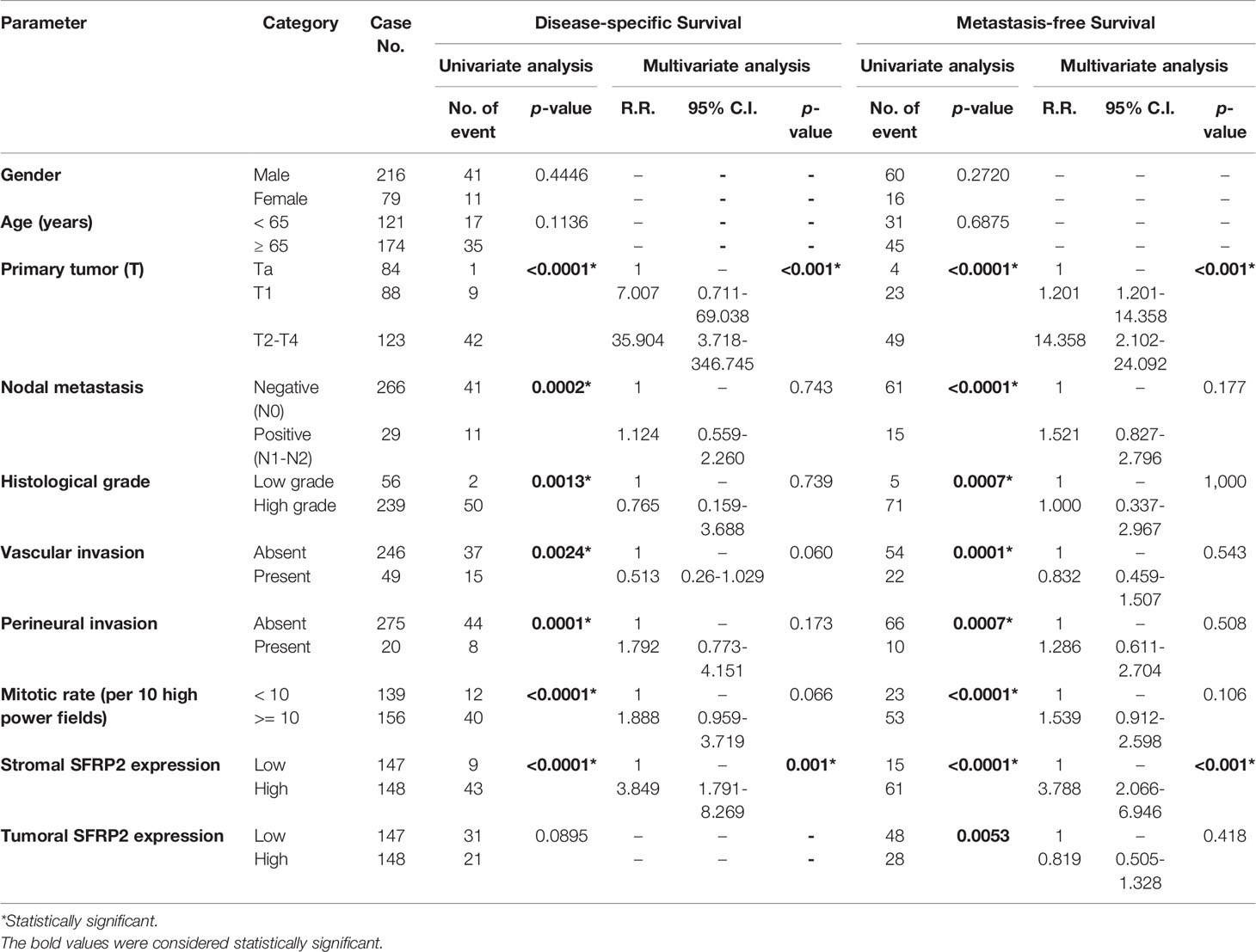
Table 5 Univariate log-rank and multivariate analyses for disease-specific and metastasis-free survivals in urinary bladder urothelial carcinoma.

Table 6 Univariate log-rank and multivariate analyses for local recurrence-free survivals in NMIBC post TURBT.
UTUC and UBUC are featured by etiological and clinical heterogeneity, while we identified SFRP2 linked to similar prognosis among UTUC and UBUC patients. To understand the functional roles of SFRP2 in UC, a gene coexpression network was examined. Employing the UTUC (n = 47) and UBUC (n = 411) datasets in the TCGA database, we appraised the top 194 overlapping transcripts (Figure 5A) that show positive correlations with SFRP2 between UTUC (Supplementary Table 1) and UBUC (Supplementary Table 2). Subsequently, we utilized the GO classification system for functional annotation. With regard to molecular functions, we found that these overlapping genes are mainly implicated in the composition of the ECM (Figure 5B). In respect of cellular components, we observed that these overlapping genes are mostly involved in the collagen trimer assembly (Figure 5C). Moreover, the fibulin 2 (FBLN2) gene, one of the top 194 overlapping genes co-upregulated with SFRP2 (Supplementary Figure 3A), has been identified as an unfavorable prognostic factor for UC in our previous study (29). Interestingly, the collagen family genes are also significantly positively correlated with FBLN2 (29), further supporting the important role of collagen in UC development. As to biological processes, we detected that these overlapping genes are largely implicated in the immune cell regulation (Figure 5D). Furthermore, expressed by macrophages, the cathepsin K (CTSK) gene, one of the top 194 overlapping genes co-upregulated with SFRP2 (Supplementary Figure 3B), has been suggested to be a promising therapeutic target for patients with high-risk MIBC (30). To connect key genes that were involved in the distinguished GO terms (fold enrichment > 50) of all three functional groups to each other, a weighted network was built using the GeneMANIA prediction server (31). The data showed that the top 2 predicted functions are mononuclear cell proliferation (false discovery rate: 1.85 x 10−7) and lymphocyte proliferation (false discovery rate: 1.85 x 10−7) (Figure 6). Additionally, annotated by the Molecular Signatures Database (MSigDB), several prominent pathways, including matrisome, epithelial–mesenchymal transition (EMT), and B lymphocyte, were identified. These observations further support the role of SFRP2 in the crosstalk among tumor cells, stromal cells, immune cells, and the ECM.
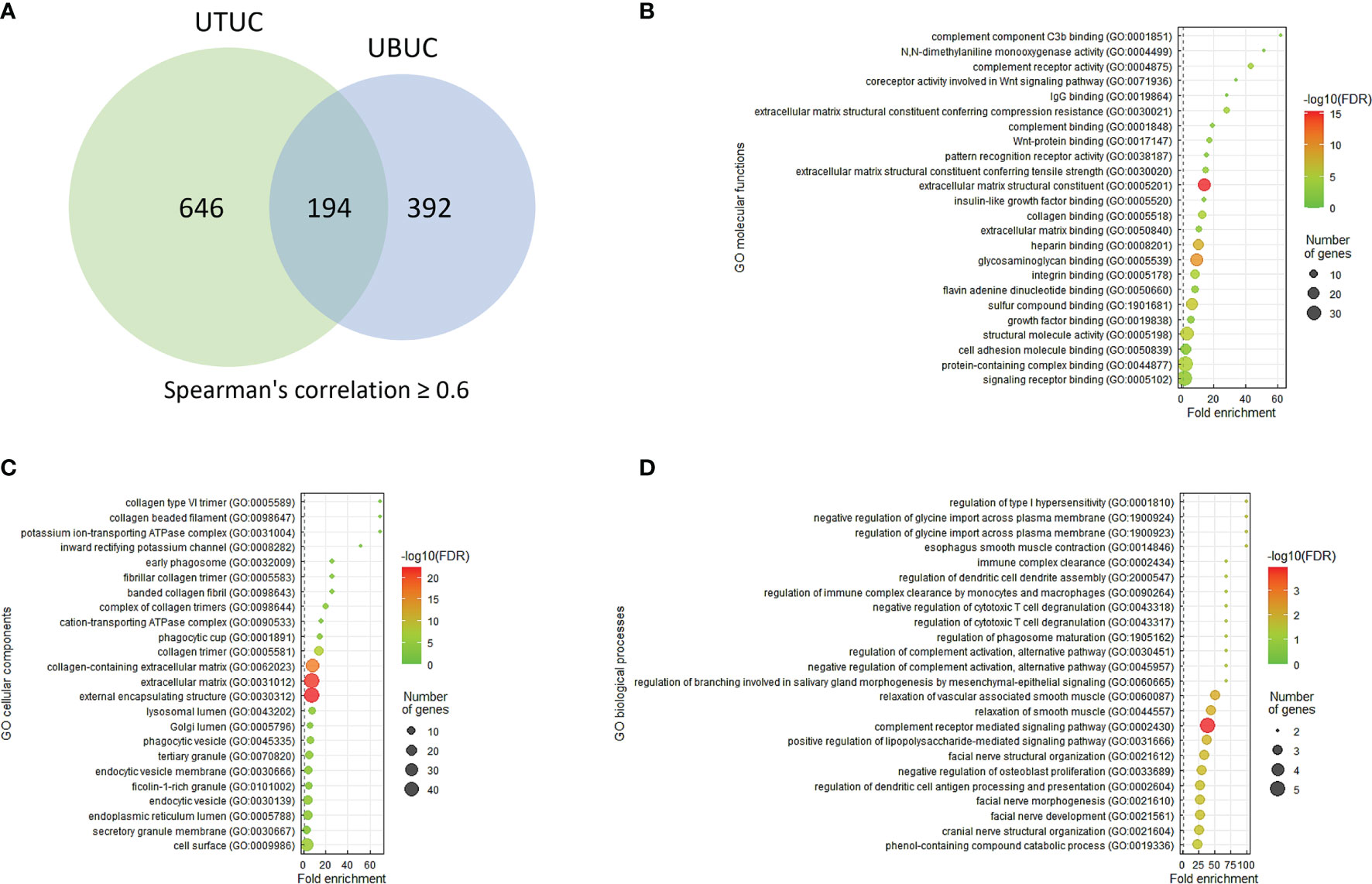
Figure 5 The prominent GO terms enriched in SFRP2 upregulation. (A) The top 194 overlapping genes (Spearman’s correlation ≥ 0.6) co-upregulated with SFRP2 between UTUC and UBUC were examined utilizing the GO classification system according to (B) molecular functions, (C) cellular components, or (D) biological processes and were ranked by fold enrichment for functional annotation. To plot representative GO terms, an R script with ggplot2 package was used.
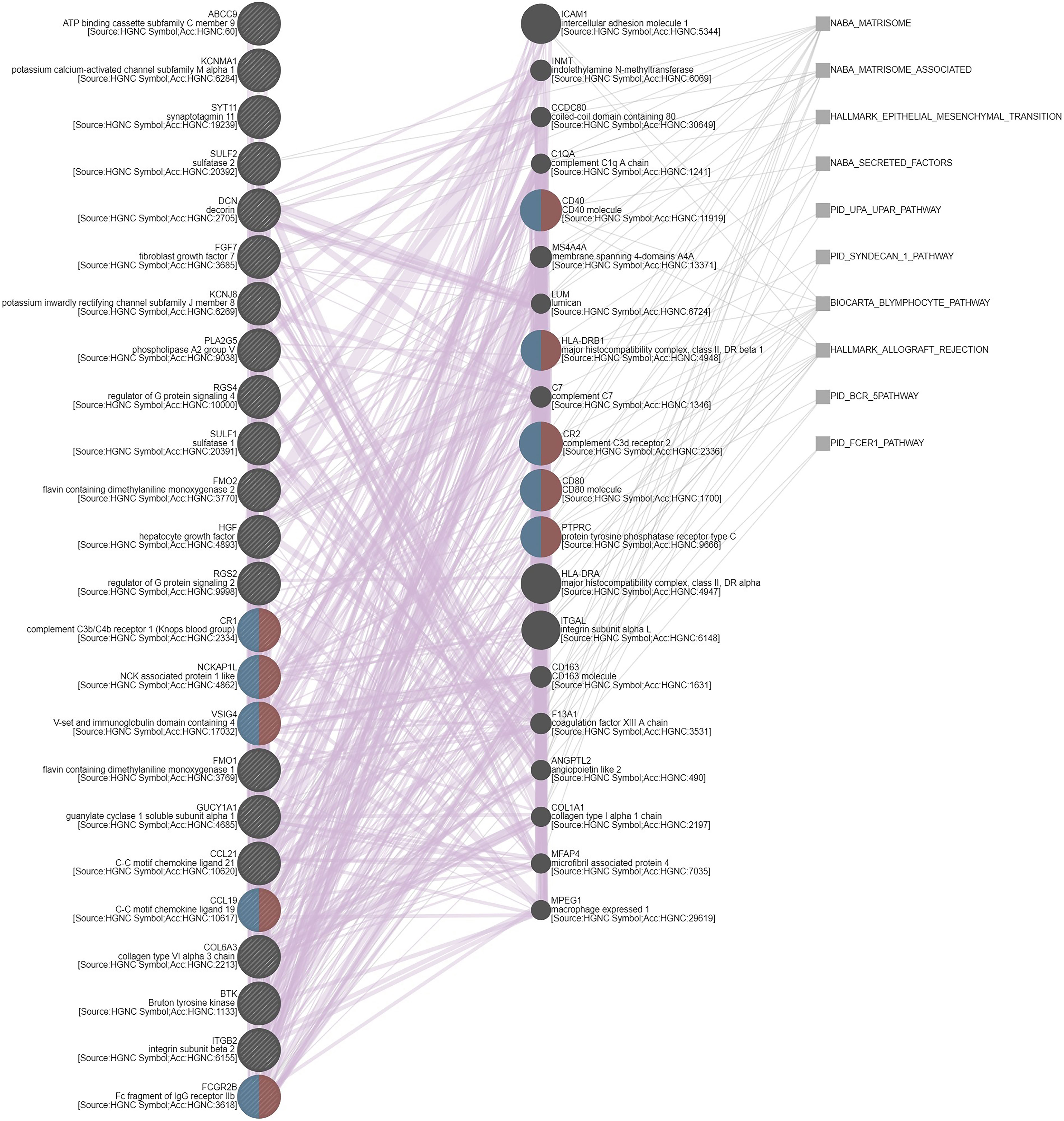
Figure 6 Gene coexpression network. A weighted network that connects key genes to each other was built using the GeneMANIA prediction server. Red and blue semicircles represent genes that belong to mononuclear cell proliferation and lymphocyte proliferation, respectively. Purple and grey lines indicate coregulation and pathways, correspondingly.
Furthermore, SFRP4 was also identified as one of the overlapping genes considerably co-upregulated with SFRP2 (Supplementary Figure 3C). It has been reported that SFRP2 and SFRP4 levels are tightly correlated with each other, which shares a common gene program across multiple cancers (19). Using the Human Protein Atlas database, in terms of single cell type specificity, we found that SFRP2 is specifically expressed in the fibroblasts (https://www.proteinatlas.org/ENSG00000145423-SFRP2/celltype), but SFRP4 is specifically expressed in the peritubular cells (https://www.proteinatlas.org/ENSG00000106483-SFRP4/celltype), suggesting that the contribution of tumor stroma to UC development is more likely to be mediated by SFRP2. We also identified fibroblast growth factor 7 (FGF7) and complement C1s (C1S) as the overlapping transcripts that are significantly positively correlated with SFRP2 (Supplementary Figures 3D, E). In our previous investigations, high FGF7 (32) and C1S (33) expression have been correlated with worse clinical outcomes in UC patients. Altogether, although UTUC and UBUC are clinically managed as distinct entities, they share common genomic landscape with similar actionable drivers and prognostic factors to improve precision therapy.
Composed of five secreted glycoproteins (SFRP1–5), the SFRP family is approximately 300 amino acids in length, which fold into two distinguishable domains: an N-terminal cysteine-rich domain and a C-terminal netrin domain (34). Because of their ability to antagonize the Wnt signaling and their frequent silencing by promoter methylation in many cancers, the SFRP proteins were initially described as tumor suppressors (35). Actually, one study found that the methylation level of SFRP2 in gastric cancer is higher than that in adjacent normal tissue (36), whereas a recent report showed that the mRNA level of SFRP2 is among the highest in advanced stages of gastric cancer and correlated with worse survival (37). Likewise, using the UALCAN platform (http://ualcan.path.uab.edu/cgi-bin/TCGA-methyl-Result.pl?genenam=SFRP1&ctype=BLCA) (http://ualcan.path.uab.edu/cgi-bin/TCGA-methyl-Result.pl?genenam=SFRP2&ctype=BLCA), we speculate that, compared to adjacent normal tissue, both the decreased SFRP1 and SFRP2 transcripts in bladder cancer may ascribe to their increased methylation levels, especially in patients with stage I disease. Interestingly, the violin plots showed that both the mRNA levels of SFRP1 and SFRP2 significantly increase with the progression of bladder cancer (from stage II to stage IV) (Supplementary Figure 1A and Figure 2A). These observed inconsistencies in the level of SFRP2 might be owing to differences in the progressive stage. However, we observed that both the SFRP1 and SFRP2 mRNA levels are not inversely correlated with their methylation status using the TCGA bladder cancer database (n = 413) (Supplementary Figures 4A, B). This implies that alternative mechanisms may contribute to increased SFRP1 and SFRP2 transcripts in UC patients with advanced disease.
The dynamic interplays between tumors and their microenvironment comprising immune cells (macrophages and lymphocytes), stromal cells (fibroblasts), and the ECM, supporting or limiting tumor growth. To further dissect the molecular characterization of tumor–immune interactions, the TIMER2.0 database was used. The data revealed that high CAF infiltration was significantly correlated with poor cumulative survival in bladder urothelial carcinoma (Supplementary Figures 5A, B). Moreover, we observed that both the SFRP1 and SFRP2 transcripts are significantly negatively correlated with tumor purity and positively correlated with CAF infiltration in bladder urothelial carcinoma, but the SFRP2 gene showed a more distinguished correlation (Supplementary Figure 2 and Figure 2C). These observations suggest that stromal cells may at least in part result in the increased SFRP2 transcripts in urothelial carcinoma with advanced stage. We further validated that high stromal SFRP2 expression evaluated by immunohistochemistry is significantly linked to an aggressive clinical course and inferior survival in our urothelial carcinoma cohorts containing 340 UTUC and 295 UBUC patients, highlighting the promising prognostic utility of stromal SFRP2 expression.
Initially regarded as transcriptional noise, non-coding RNAs (ncRNAs) have attracted wide attention for their involvement in epigenetic regulation and multiple biological functions, especially in cancer (38). Based on the length, ncRNAs can majorly be classified into microRNAs (miRNAs, about 22 nucleotides long) and long non-coding RNAs (lncRNAs, more than 200 nucleotides long) (39). miRNAs function by annealing to the three prime untranslated region (3′-UTR) of messenger RNA (mRNA) targets to negatively regulate gene expression. As competitive endogenous RNAs (ceRNAs) or miRNA sponges, lncRNAs with miRNA-complementary sites, which are also presented on mRNA targets, can compete with mRNA targets to bind to miRNAs, thereby reducing the availability of miRNAs. Accordingly, we aligned GSE31684 probe sequences with human lncRNA sequences from the LNCipedia database (https://lncipedia.org/) and created a lncRNA list. Utilizing the miRTarBase database (40), the regulatory networks among miRNAs, upstream regulators (lncRNA list), and downstream targets (gene list from Table 1) were analyzed, and the ZNF585B-6:1/miRNAs/SFRP2 network was identified (Supplementary Figure 6). Of these miRNAs, miR-218 has been reported to be downregulated in bladder cancer (41), which suggests that increased SFRP2 transcripts may attribute to miR-218 downregulation in UC. Consequently, we do not rule out the possibility that ncRNAs may contribute to increased SFRP2 transcripts in UC, and further analysis is needed.
A gene coexpression analysis was used to predict the functional roles of SFRP2 in UC, and we found that the collagen family genes, including collagen type I alpha 2 chain (COL1A2), COL3A1, COL6A3, COL8A1, COL10A1, and COL14A1, are significantly co-upregulated with SFRP2 (Supplementary Figures 7A–F). These collagen genes, such as COL1A2 and COL6A3, have been demonstrated to promote NMIBC progression to MIBC through EMT (42). We also found that EMT markers twist family basic helix-loop-helix (bHLH) transcription factor 1 (TWIST1) and zinc finger E-box binding homeobox 2 (ZEB2) are significantly co-upregulated with SFRP2 (Supplementary Figures 8A, B). However, whether SFRP2 promotes UC progression through EMT regulated by the above-mentioned collagen genes needs further confirmation. On the other hand, although the use of immunotherapy has improved outcomes in the management of UC, only approximately 20% of patients benefit from it (43), which warrants a better understanding of the mechanisms underlying immunotherapy resistance. It has been reported that the transforming growth factor beta (TGFβ) signaling from fibroblasts and collagen enriched in peritumoral stroma promote cytotoxic T cell exclusion and confer immunotherapy resistance among patients with metastatic urothelial cancer (44). Additionally, we also observed that TGFBI and CAF marker fibroblast activation protein (FAP) are significantly co-upregulated with SFRP2 (Supplementary Figures 9A, B). Accordingly, these observations suggest that SFRP2, a putative Wnt inhibitor, may function at the interactions between tumor cells and fibroblasts in UC development. Nevertheless, the Wnt/β-catenin signaling has also been suggested to drive cytotoxic T cell exclusion by modulating the crosstalk between tumor cells and tumor-associated macrophages (TAMs) and lead to immunotherapy resistance in UC treatment (12). These further highlight the complexity of the TME that creates a favorable niche to reduce treatment efficacy. Accordingly, SFRP2 may trigger immunotherapy resistance beyond its Wnt antagonistic ability, providing therapeutic implications for precisely selecting patients who can benefit from immunotherapy.
Increased deposition of fibrous collagen is the most common feature of ECM remodeling in the primary tumor (45). Despite the fact that the collagen family genes are significantly positively correlated with SFRP2, it is unclear how SFRP2 regulates collagen homeostasis. Using structure prediction analysis, it has been showed that the C-terminal netrin domains of SFRP proteins and procollagen C-endopeptidase enhancer 1 (PCPE1 or PCOLCE) share sequence similarity with the N-terminal netrin domains of tissue inhibitor of metalloproteinases (TIMPs) (46). PCPE1 is suggested to be highly specific to collagen synthesis and fibrosis, and its upregulation is observed at sites of high collagen deposition (47). Moreover, TIMPs are considered to inhibit matrix metalloproteinases (MMPs)-induced collagen degradation (48). However, whether SFRP2 can promote collagen synthesis and prevent collagen degradation through its C-terminal netrin domain requires further analysis. Otherwise, due to its sensitivity to inflammatory perturbations, variation of collagen stainability may be of little utility in respect of the prognosis of UC (49). Consequently, SFRP2, a supposed upstream regulator of collagen homeostasis, may act as a reliable prognostic factor for UC.
Metastatic UC is an aggressive disease and generally has a poor prognosis with a median overall survival of 12–14 months (4). Following lymph nodes (69%), bone (47%) has been reported to be the second most common site of metastatic bladder cancer (50). Bone metastasis may be correlated with pain, bone loss, and functional impairment. Accordingly, recent study has reported that UC patients with bone as the only metastatic site are less likely to receive systemic chemotherapy owing to their lower Eastern Cooperative Oncology Group (ECOG) performance status (51). The current European Association of Urology (EAU) guideline recommends anti-osteoporotic drugs (zoledronic acid or denosumab) for supportive treatment in case of bone metastasis, but patients should be aware of potential side effects, such as osteonecrosis of the jaw and hypocalcaemia (4). As a result, these patients deserve a valuable prognostic factor and a specific therapeutic target. Using gene coexpression analysis, we found that CTSK is significantly co-upregulated with SFRP2 (Supplementary Figure 3B). Generally, CTSK, a lysosomal cysteine protease, is implicated in osteoclast-mediated bone degradation (52). Moreover, CTSK has also been described to be expressed by breast cancer (53) and prostate cancer (54) that metastasize to bone, where it functions in osteolysis that contributes to tumor invasiveness. Nevertheless, the correlations among the expression of SFRP2 and CTSK and metastatic bone disease in UC need further examination.
In this study, we identified that high stromal SFRP2 expression has a more significant impact on UC patient survival compared with that of low tumoral SFRP2 expression. This suggests that the distinct roles of SFRP2 seem to be dependent on cell type-specific contexts, and incorporation of these variables can more accurately guide management for UC patients. The current study has some limitations. First, further experiments are needed to validate the role of fibroblast SFRP2 in UC development and immunotherapy resistance. Second, in this study, UC patients who had curative surgery were analyzed retrospectively at a single institution; accordingly, the value of stromal SFRP2 expression should be prospectively verified by multi-center studies.
Because of their Wnt antagonistic ability and their frequent epigenetic silencing in many cancers, the SFRP proteins were initially described as tumor suppressors. In the current study, we validated that high stromal SFRP2 expression is considerably correlated with an aggressive clinical course and serves as an independent prognostic factor for worse survival in our well-characterized UBUC and UTUC cohorts. Utilizing bioinformatic analysis, we further observed that stromal SFRP2 may link EMT to UC progression. Collectively, stromal SFRP2 may exert oncogenic function beyond its Wnt antagonistic ability, and stromal SFRP2 expression evaluation can add value to guiding management more precisely for UC patients.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The studies involving human participants were reviewed and approved by Institutional Review Board of Chi Mei Medical Center (10501005). The patients/participants provided their written informed consent to participate in this study.
Conceptualization: H-YL and C-FL. Methodology: C-CC, Y-HK, H-HT, L-CW, W-HT, C-LL, and C-HH. Investigation: H-YL, C-CC, SH, and C-FL. Formal analysis: H-YL, C-CC, Y-HK, H-HT, and L-CW. Resources: W-HT, C-LL, and C-HH. Validation: Y-HK, H-HT, L-CW, W-HT, C-LL, and C-HH. Visualization: H-YL and C-CC. Writing - original draft: H-YL. Writing - review and editing: H-YL. Funding acquisition: SH and C-FL. Supervision: SH and C-FL. All authors contributed to the article and approved the submitted version.
Author C-CC is employed by Genetics Generation Advancement Corp.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.834249/full#supplementary-material
Supplementary Figure 1 | High SFRP1 mRNA level is correlated with advanced stage disease but not with inferior overall survival. (A) The correlations between the mRNA levels of SFRP1 and bladder cancer progression. (B) The impact of SFRP1 mRNA levels on overall survival in bladder cancer. These data were acquired from the GEPIA database. BLCA: bladder urothelial carcinoma.
Supplementary Figure 2 | SFRP1 transcripts are moderately negatively correlated with tumor purity and positively correlated with CAF infiltration. The correlations among the mRNA levels of SFRP1, tumor purity, and CAF infiltration in bladder cancer. These data were estimated using the MCP-COUNTER and TIDE algorithms from the TIMER2.0 database. BLCA, bladder urothelial carcinoma.
Supplementary Figure 3 | Correlations between the expression levels of SFRP2 and its co-upregulated genes. (A–E) Utilizing the cBioPortal web platform, these data were obtained from the TCGA database (n = 411).
Supplementary Figure 4 | Both the mRNA levels of SFRP1 and SFRP2 are not negatively correlated with their methylation status. (A) The correlations between the mRNA levels of SFRP1 and their methylation status. (B) The correlations between the mRNA levels of SFRP2 and their methylation status. Utilizing the cBioPortal web platform, these data were obtained from the TCGA database (n = 413).
Supplementary Figure 5 | High CAF infiltration is significantly correlated with poor cumulative survival. (A, B) The impact of CAF infiltration on cumulative survival in bladder cancer. These data were estimated using the MCP-COUNTER and TIDE algorithms from the TIMER2.0 database.
Supplementary Figure 6 | Regulatory networks among ZNF585B-6:1, miRNAs, and SFRP2. These data were obtained from the miRTarBase database.
Supplementary Figure 7 | Correlations between the expression levels of SFRP2 and collagen family genes. (A–F) Utilizing the cBioPortal web platform, these data were obtained from the TCGA database (n = 411).
Supplementary Figure 8 | Correlations between the expression levels of SFRP2 and EMT markers. (A, B) Utilizing the cBioPortal web platform, these data were obtained from the TCGA database (n = 411).
Supplementary Figure 9 | Correlations between the expression levels of SFRP2 and TGFB1 and CAF marker. (A, B) Utilizing the cBioPortal web platform, these data were obtained from the TCGA database (n = 411).
1. Yang MH, Chen KK, Yen CC, Wang WS, Chang YH, Huang WJ, et al. Unusually High Incidence of Upper Urinary Tract Urothelial Carcinoma in Taiwan. Urology (2002) 59(5):681–7. doi: 10.1016/S0090-4295(02)01529-7
2. Babjuk M, Burger M, Compérat EM, Gontero P, Mostafid AH, Palou J, et al. European Association of Urology Guidelines on Non-Muscle-Invasive Bladder Cancer (TaT1 and Carcinoma In Situ) - 2019 Update. Eur Urol (2019) 76(5):639–57. doi: 10.1016/j.eururo.2019.08.016
3. Taylor J, Becher E, Steinberg GD. Update on the Guideline of Guidelines: non-Muscle-Invasive Bladder Cancer. BJU Int (2020) 125(2):197–205. doi: 10.1111/bju.14915
4. Witjes JA, Bruins HM, Cathomas R, Compérat EM, Cowan NC, Gakis G, et al. European Association of Urology Guidelines on Muscle-Invasive and Metastatic Bladder Cancer: Summary of the 2020 Guidelines. Eur Urol (2021) 79(1):82–104. doi: 10.1016/j.eururo.2020.03.055
5. Rouprêt M, Babjuk M, Compérat E, Zigeuner R, Sylvester RJ, Burger M, et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2017 Update. Eur Urol (2018) 73(1):111–22. doi: 10.1016/j.eururo.2017.07.036
6. Kobayashi T, Ito K, Kojima T, Kato M, Kanda S, Hatakeyama S, et al. Risk Stratification for the Prognosis of Patients With Chemoresistant Urothelial Cancer Treated With Pembrolizumab. Cancer Sci (2021) 112(2):760–73. doi: 10.1111/cas.14762
7. MacDonald BT, Tamai K, He X. Wnt/beta-Catenin Signaling: Components, Mechanisms, and Diseases. Dev Cell (2009) 17(1):9–26. doi: 10.1016/j.devcel.2009.06.016
8. Zhang Y, Wang X. Targeting the Wnt/β-Catenin Signaling Pathway in Cancer. J Hematol Oncol (2020) 13(1):165. doi: 10.1186/s13045-020-00990-3
9. Garg M, Maurya N. WNT/β-Catenin Signaling in Urothelial Carcinoma of Bladder. World J Nephrol (2019) 8(5):83–94. doi: 10.5527/wjn.v8.i5.83
10. Ren J, Yang Y, Peng T, Xu D. Predictive Value of β-Catenin in Bladder Cancer: A Systematic Review and Meta-Analysis. Biosci Rep (2020) 40(9):BSR20202127. doi: 10.1042/BSR20202127
11. Zhu X, Kanai Y, Saito A, Kondo Y, Hirohashi S. Aberrant Expression of Beta-Catenin and Mutation of Exon 3 of the Beta-Catenin Gene in Renal and Urothelial Carcinomas. Pathol Int (2000) 50(12):945–52. doi: 10.1046/j.1440-1827.2000.01139.x
12. Chehrazi-Raffle A, Dorff TB, Pal SK, Lyou Y. Wnt/β-Catenin Signaling and Immunotherapy Resistance: Lessons for the Treatment of Urothelial Carcinoma. Cancers (Basel) (2021) 13(4):889. doi: 10.3390/cancers13040889
13. Chung MT, Lai HC, Sytwu HK, Yan MD, Shih YL, Chang CC, et al. SFRP1 and SFRP2 Suppress the Transformation and Invasion Abilities of Cervical Cancer Cells Through Wnt Signal Pathway. Gynecol Oncol (2009) 112(3):646–53. doi: 10.1016/j.ygyno.2008.10.026
14. van Loon K, Huijbers EJM, Griffioen AW. Secreted Frizzled-Related Protein 2: A Key Player in Noncanonical Wnt Signaling and Tumor Angiogenesis. Cancer Metastasis Rev (2021) 40(1):191–203. doi: 10.1007/s10555-020-09941-3
15. Lee JL, Lin CT, Chueh LL, Chang CJ. Autocrine/paracrine Secreted Frizzled-Related Protein 2 Induces Cellular Resistance to Apoptosis: A Possible Mechanism of Mammary Tumorigenesis. J Biol Chem (2004) 279(15):14602–9. doi: 10.1074/jbc.M309008200
16. Huang C, Ye Z, Wan J, Liang J, Liu M, Xu X, et al. Secreted Frizzled-Related Protein 2 Is Associated With Disease Progression and Poor Prognosis in Breast Cancer. Dis Markers (2019) 2019:6149381. doi: 10.1155/2019/6149381
17. Kim H, Yoo S, Zhou R, Xu A, Bernitz JM, Yuan Y, et al. Oncogenic Role of SFRP2 in P53-Mutant Osteosarcoma Development via Autocrine and Paracrine Mechanism. Proc Natl Acad Sci U S A (2018) 115(47):E11128–e37. doi: 10.1073/pnas.1814044115
18. Shirozu M, Tada H, Tashiro K, Nakamura T, Lopez ND, Nazarea M, et al. Characterization of Novel Secreted and Membrane Proteins Isolated by the Signal Sequence Trap Method. Genomics (1996) 37(3):273–80. doi: 10.1006/geno.1996.0560
19. Vincent KM, Postovit LM. A Pan-Cancer Analysis of Secreted Frizzled-Related Proteins: Re-Examining Their Proposed Tumour Suppressive Function. Sci Rep (2017) 7:42719. doi: 10.1038/srep42719
20. Meng J, Lu X, Zhou Y, Zhang M, Ge Q, Zhou J, et al. Tumor Immune Microenvironment-Based Classifications of Bladder Cancer for Enhancing the Response Rate of Immunotherapy. Mol Ther Oncolytics (2021) 20:410–21. doi: 10.1016/j.omto.2021.02.001
21. Edge SB, Compton CC. The American Joint Committee on Cancer: The 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Ann Surg Oncol (2010) 17(6):1471–4. doi: 10.1245/s10434-010-0985-4
22. Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours. Eur Urol (2016) 70(1):106–19. doi: 10.1016/j.eururo.2016.02.028
23. Chen TJ, Chou CL, Tian YF, Yeh CF, Chan TC, He HL, et al. High FRMD3 Expression is Prognostic for Worse Survival in Rectal Cancer Patients Treated With CCRT. Int J Clin Oncol (2021) 26(9):1689–97. doi: 10.1007/s10147-021-01944-6
24. Chou CL, Chen TJ, Tian YF, Chan TC, Yeh CF, Li WS, et al. Upregulated MUC2 Is an Unfavorable Prognostic Indicator for Rectal Cancer Patients Undergoing Preoperative CCRT. J Clin Med (2021) 10(14):3030. doi: 10.3390/jcm10143030
25. Jones SE, Jomary C. Secreted Frizzled-Related Proteins: Searching for Relationships and Patterns. BioEssays (2002) 24(9):811–20. doi: 10.1002/bies.10136
26. Shin K, Lee J, Guo N, Kim J, Lim A, Qu L, et al. Hedgehog/Wnt Feedback Supports Regenerative Proliferation of Epithelial Stem Cells in Bladder. Nature (2011) 472(7341):110–4. doi: 10.1038/nature09851
27. Tran L, Xiao JF, Agarwal N, Duex JE, Theodorescu D. Advances in Bladder Cancer Biology and Therapy. Nat Rev Cancer (2021) 21(2):104–21. doi: 10.1038/s41568-020-00313-1
28. Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q, et al. TIMER2.0 for Analysis of Tumor-Infiltrating Immune Cells. Nucleic Acids Res (2020) 48(W1):W509–w14. doi: 10.1093/nar/gkaa407
29. Li WM, Chan TC, Huang SK, Wu WJ, Ke HL, Liang PI, et al. Prognostic Utility of FBLN2 Expression in Patients With Urothelial Carcinoma. Front Oncol (2020) 10:570340. doi: 10.3389/fonc.2020.570340
30. Zhang PB, Huang ZL, Xu YH, Huang J, Huang XY, Huang XY. Systematic Analysis of Gene Expression Profiles Reveals Prognostic Stratification and Underlying Mechanisms for Muscle-Invasive Bladder Cancer. Cancer Cell Int (2019) 19:337. doi: 10.1186/s12935-019-1056-y
31. Franz M, Rodriguez H, Lopes C, Zuberi K, Montojo J, Bader GD, et al. GeneMANIA Update 2018. Nucleic Acids Res (2018) 46(W1):W60–w4. doi: 10.1093/nar/gky311
32. Fan EW, Li CC, Wu WJ, Huang CN, Li WM, Ke HL, et al. FGF7 Over Expression is an Independent Prognosticator in Patients With Urothelial Carcinoma of the Upper Urinary Tract and Bladder. J Urol (2015) 194(1):223–9. doi: 10.1016/j.juro.2015.01.073
33. Chang IW, Lin VC, Wu WJ, Liang PI, Li WM, Yeh BW, et al. Complement Component 1, s Subcomponent Overexpression is an Independent Poor Prognostic Indicator in Patients With Urothelial Carcinomas of the Upper Urinary Tract and Urinary Bladder. J Cancer (2016) 7(11):1396–405. doi: 10.7150/jca.15339
34. Chong JM, Uren A, Rubin JS, Speicher DW. Disulfide Bond Assignments of Secreted Frizzled-Related Protein-1 Provide Insights About Frizzled Homology and Netrin Modules. J Biol Chem (2002) 277(7):5134–44. doi: 10.1074/jbc.M108533200
35. Surana R, Sikka S, Cai W, Shin EM, Warrier SR, Tan HJ, et al. Secreted Frizzled Related Proteins: Implications in Cancers. Biochim Biophys Acta (2014) 1845(1):53–65. doi: 10.1016/j.bbcan.2013.11.004
36. Liu Y, Zhou Q, Zhou D, Huang C, Meng X, Li J. Secreted Frizzled-Related Protein 2-Mediated Cancer Events: Friend or Foe? Pharmacol Rep (2017) 69(3):403–8. doi: 10.1016/j.pharep.2017.01.001
37. Liu D, Sun C, Kim N, Bhan C, Tuason JPW, Chen Y, et al. Comprehensive Analysis of SFRP Family Members Prognostic Value and Immune Infiltration in Gastric Cancer. Life (Basel Switzerland) (2021) 11(6):522. doi: 10.3390/life11060522
38. Anastasiadou E, Jacob LS, Slack FJ. Non-Coding RNA Networks in Cancer. Nat Rev Cancer (2018) 18(1):5–18. doi: 10.1038/nrc.2017.99
39. Statello L, Guo CJ, Chen LL, Huarte M. Gene Regulation by Long Non-Coding RNAs and Its Biological Functions. Nat Rev Mol Cell Biol (2021) 22(2):96–118. doi: 10.1038/s41580-020-00315-9
40. Huang HY, Lin YC, Li J, Huang KY, Shrestha S, Hong HC, et al. miRTarBase 2020: Updates to the Experimentally Validated microRNA-Target Interaction Database. Nucleic Acids Res (2020) 48(D1):D148–54. doi: 10.1093/nar/gkz896
41. Yoshino H, Seki N, Itesako T, Chiyomaru T, Nakagawa M, Enokida H. Aberrant Expression of microRNAs in Bladder Cancer. Nat Rev Urol (2013) 10(7):396–404. doi: 10.1038/nrurol.2013.113
42. Zhu H, Chen H, Wang J, Zhou L, Liu S. Collagen Stiffness Promoted Non-Muscle-Invasive Bladder Cancer Progression to Muscle-Invasive Bladder Cancer. Onco Targets Ther (2019) 12:3441–57. doi: 10.2147/OTT.S194568
43. Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee JL, Fong L, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. N Engl J Med (2017) 376(11):1015–26. doi: 10.1056/NEJMoa1613683
44. Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFβ Attenuates Tumour Response to PD-L1 Blockade by Contributing to Exclusion of T Cells. Nature (2018) 554(7693):544–8. doi: 10.1038/nature25501
45. Winkler J, Abisoye-Ogunniyan A, Metcalf KJ, Werb Z. Concepts of Extracellular Matrix Remodelling in Tumour Progression and Metastasis. Nat Commun (2020) 11(1):5120. doi: 10.1038/s41467-020-18794-x
46. Bányai L, Patthy L. The NTR Module: Domains of Netrins, Secreted Frizzled Related Proteins, and Type I Procollagen C-Proteinase Enhancer Protein are Homologous With Tissue Inhibitors of Metalloproteases. Protein Sci (1999) 8(8):1636–42. doi: 10.1110/ps.8.8.1636
47. Lagoutte P, Bettler E, Vadon-Le Goff S, Moali C. Procollagen C-Proteinase Enhancer-1 (PCPE-1), a Potential Biomarker and Therapeutic Target for Fibrosis. Matrix Biol Plus (2021) 11:100062. doi: 10.1016/j.mbplus.2021.100062
48. Brew K, Nagase H. The Tissue Inhibitors of Metalloproteinases (TIMPs): An Ancient Family With Structural and Functional Diversity. Biochim Biophys Acta (2010) 1803(1):55–71. doi: 10.1016/j.bbamcr.2010.01.003
49. Brunner A, Tzankov A. The Role of Structural Extracellular Matrix Proteins in Urothelial Bladder Cancer (Review). Biomark Insights (2007) 2:418–27. doi: 10.4137/BMI.S294
50. Shinagare AB, Ramaiya NH, Jagannathan JP, Fennessy FM, Taplin ME, Van den Abbeele AD. Metastatic Pattern of Bladder Cancer: Correlation With the Characteristics of the Primary Tumor. AJR Am J Roentgenol (2011) 196(1):117–22. doi: 10.2214/AJR.10.5036
51. Necchi A, Pond GR, Pal SK, Agarwal N, Bowles DW, Plimack ER, et al. Bone Metastases as the Only Metastatic Site in Patients With Urothelial Carcinoma: Focus on a Special Patient Population. Clin Genitourin Cancer (2018) 16(2):e483–e90. doi: 10.1016/j.clgc.2017.10.012
52. Troen BR. The Regulation of Cathepsin K Gene Expression. Ann N Y Acad Sci (2006) 1068:165–72. doi: 10.1196/annals.1346.018
53. Littlewood-Evans AJ, Bilbe G, Bowler WB, Farley D, Wlodarski B, Kokubo T, et al. The Osteoclast-Associated Protease Cathepsin K is Expressed in Human Breast Carcinoma. Cancer Res (1997) 57(23):5386–90.
Keywords: urothelial carcinoma, bladder cancer, upper urinary tract cancer, SFRP2, collagen, stroma
Citation: Lai H-Y, Chiu C-C, Kuo Y-H, Tsai H-H, Wu L-C, Tseng W-H, Liu C-L, Hsing C-H, Huang SK and Li C-F (2022) High Stromal SFRP2 Expression in Urothelial Carcinoma Confers an Unfavorable Prognosis. Front. Oncol. 12:834249. doi: 10.3389/fonc.2022.834249
Received: 13 December 2021; Accepted: 22 February 2022;
Published: 16 March 2022.
Edited by:
Francesca Sanguedolce, University of Foggia, ItalyReviewed by:
Jian Lu, Peking University Third Hospital, ChinaCopyright © 2022 Lai, Chiu, Kuo, Tsai, Wu, Tseng, Liu, Hsing, Huang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chien-Feng Li, YW5nZWxvLnBAeWFob28uY29tLnR3; Steven K. Huang, NzIyNDgzN0BnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.