- 1Department of Abdominal Cancer, Cancer Center, West China Hospital of Sichuan University, Chengdu, China
- 2Department of Biotherapy, Cancer Center, West China Hospital of Sichuan University, Chengdu, China
- 3West China Medical Publishers, West China Hospital of Sichuan University, Chengdu, China
- 4Lung Cancer Center, West China Hospital of Sichuan University, Chengdu, China
Background: Gallbladder squamous cell carcinoma (GSCC) is a rare carcinoma with limited evidence in literature, making it particularly difficult to study. Surveillance, Epidemiology, and End Results Database (SEER) were used to stress the clinicopathological features and outcomes associated with this tumor.
Methods: SEER registries were used to identify GSCC and gallbladder adenocarcinoma (GAC) cases from 2004 to 2015. The Propensity matching (PSM) method was used for minimized potential difference between the two types and the utmost. Patients with GSCC versus GAC were compared using the clinicopathological features and outcomes.
Results: There were 121 patients with GSCC and 6 580 patients had GAC. Compared with the GAC cohort, the GSCC cohort had a lower proportion of well-differentiated histology (3.3% vs. 12.1%, p < 0.001) and was diagnosed at a later T-stage (p < 0.001). Regarding treatment, patients treated with surgery, chemotherapy or radiation were associated with significantly better outcome than patients without undergoing these treatment modalities. In both univariate and multivariate analyses, GSCC histology was associated with worse prognosis than GAC histology.
Conclusions: Patients with GSCC were associated with a worse outcome than the GAC cohort. The independent risk factors for patients with GSCC are surgery and chemotherapy.
Introduction
Gallbladder carcinoma (GBC), a relatively uncommon malignancy, accounts for 4% of all gastrointestinal tract neoplasms (1). Most of the patients usually do not exhibit clinical symptoms or show some atypical symptoms with a 5-year survival rate of less than 10% (2, 3). About 97% of the diagnosed GBC in these patients is adenocarcinoma (AC), and its clinicopathological features and survival outcomes are relatively well-studied. Gallbladder squamous cell carcinoma (GSCC) is rarer, accounting for about 3%, which is unrecognized (4, 5). According to previous reports, squamous cell carcinoma variants of different anatomic sites exhibit completely different biology and clinical outcome compared to adenocarcinoma, depending on the primary tumor origin. For example, squamous cell carcinoma (SCC) of the cervix exhibit better results when compared with AC (6).
To date, most literatures regarding GBC consists of case reports/small case series, and very few studies have compared AC to SCC (7, 8). As with AC, surgical resection is the optimal therapeutic modality in patients with GSCC. And, patients with GSCC, diagnosed at early-stage, usually undergo simple cholecystectomy. In contrast, cholecystectomy, partial hepatectomy, and portal lymphadenectomy were usually conducted in patients with progressive disease (8–10). Regarding chemotherapy, there remains a controversy; some studies indicated that GSCC was resistant to chemotherapy, while others demonstrated that patients with GSCC can benefit from adjuvant radio-chemotherapy (8, 11, 12).
To better understand the clinicopathological features, treatment modalities, and survival of GSCC, especially comparing with gallbladder adenocarcinoma (GAC), we used Surveillance, Epidemiology, and End Results (SEER) Database (1975–2016) to provide comprehensive recognition concerning GSCC and survival outcomes between GSCC and GAC.
Material and Methods
Patients
All data of patients with GSCC and GAC were obtained from the SEER database 18 Regs (with additional treatment fields, 1975–2016 varying), using the SEER*Stat software (version 8.3.6; Surveillance Research Program, NCI, Bethesda, MD). Additional selection criteria for the SEER*Stat software used in identifying gallbladder cancer were as follows: 1) “Gallbladder” (C23.9) was limited to the site and 2) The histological subtype was defined as SCC and adenocarcinoma (AC) according to the International Agency for Research on Cancer classification using the International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) histology codes. The ICD-0-3 histology codes of histological subtypes were categorized into SCC (8070–8075) and AC (8140, 8144, 8310, 8480, and 8490). Patients diagnosed with more than one malignancy and those with information completely absent were excluded.
Variables
The following clinicopathological information (age, sex, race, marital status, SEER stage, grade, TNM stage, surgery, radiation, chemotherapy, survival months, and vital status) was obtained from each patient. The age at diagnosis was divided into three groups: ≤40, 40–60, and >60. All patients were staged on account of the SEER stage (localized, regional, and distant) and American Joint Commission on Cancer (AJCC) 6th Edition Staging System. Overall survival (OS) was defined as the time from confirmation diagnosis to death of any cause. Disease-specific-free survival (DSS) referred to the interval from definite diagnosis to death of these tumors.
Statistical Analysis
All categorical variables were presented with percentages. The χ2 test was used to compare demographic, clinical characteristics, and treatment of patients with GSCC/GAC. Because the uneven baseline features may potentially impact survival outcomes, a 1:2 propensity matching (PSM) method was used for removing the baseline variation to the utmost. The variables used for matching were age, sex, race, marital status, SEER stage, grade, t, n, and m stage, the metastatic status of bone, brain, liver, and lung, surgery, radiation, and chemotherapy. To ensure suitable matches, the nearest neighbor matching algorithm without replacement was used. Cumulative survival curves were shown using the Kaplan–Meier method, and the log-rank test was used to compare differences. Univariate and multivariate survival analyses were conducted using the Cox proportional hazard model, and the hazard ratios (HRs) with 95% confidence intervals (95% CI) were calculated. To determine the HRs in a matched population stratified by covariates, a subgroup analysis of gallbladder cancer were also conducted. All statistical analysis was conducted using R software version 3.6.2 (http://www.R-project.org) and SPSS 25.0 software e (IBM Corp., Armonk, NY, USA). The p-values < 0.05 were considered statistically significant.
Results
Demographic and Clinical Characteristics
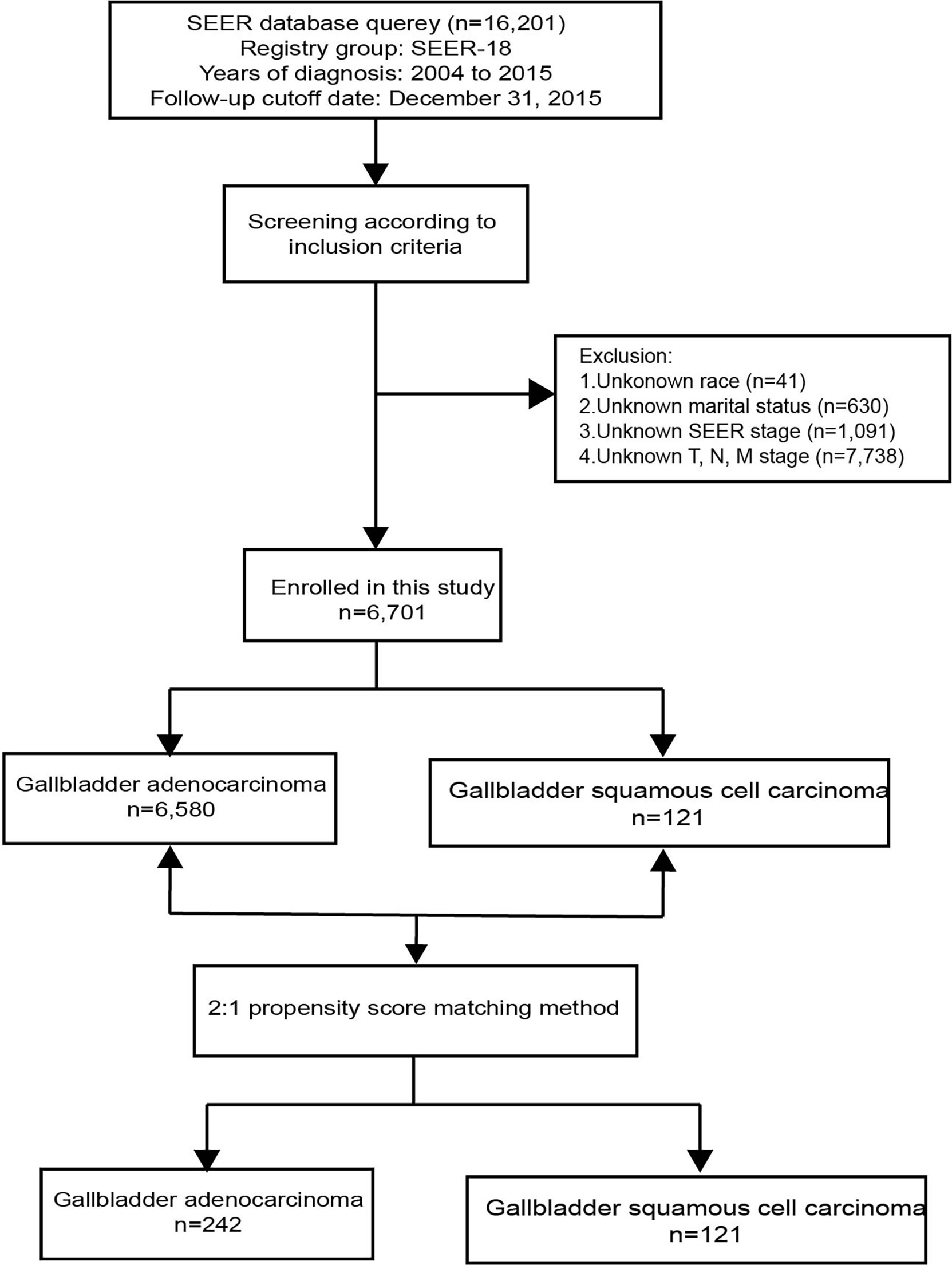
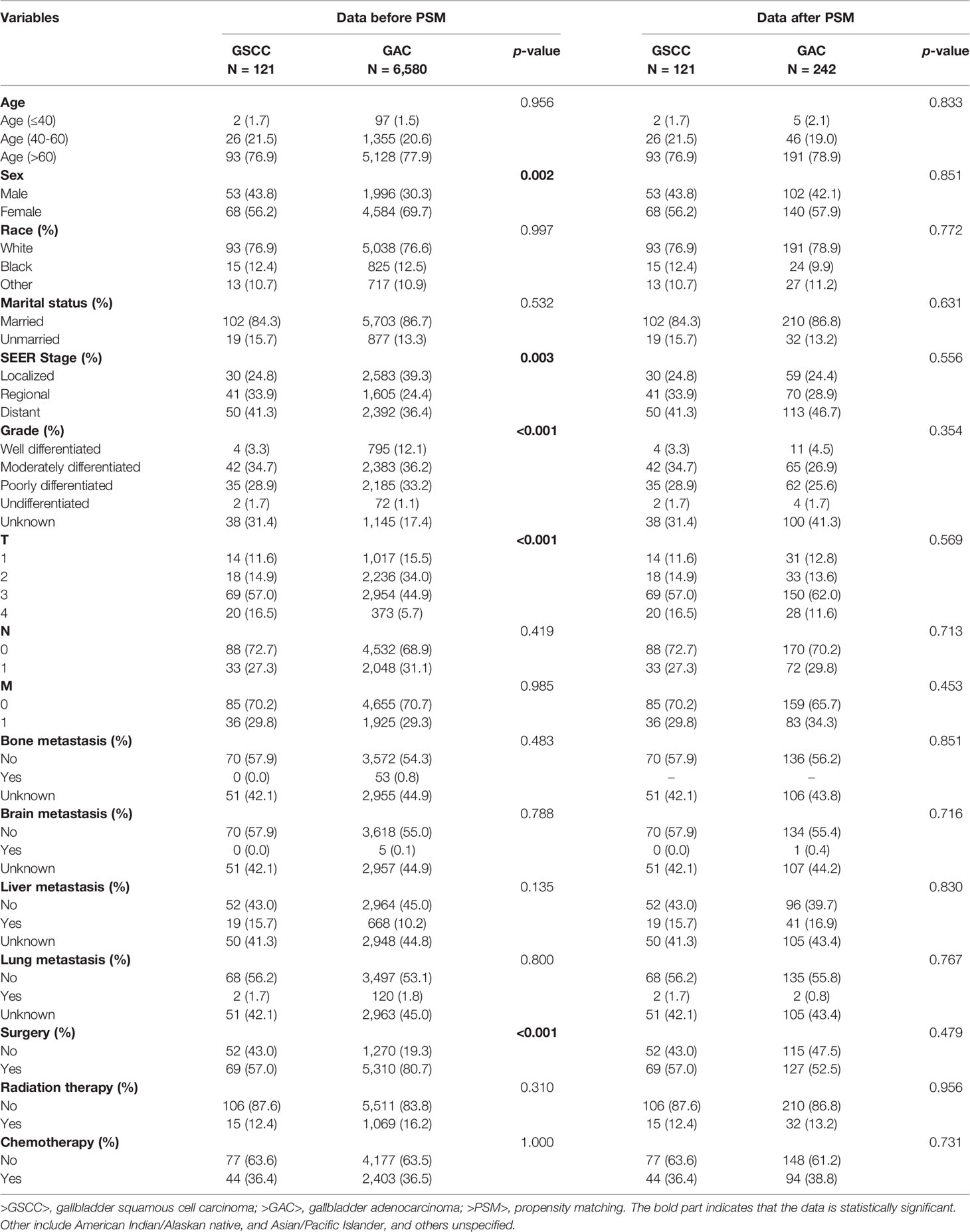
A total of 16,201 patients selected in the SEER database were eligible for inclusion from January 1st, 2004–December 31st, 2015, in this study. Ultimately 6,701 patients were selected for the final data analysis; 9,500 patients were excluded according to the predefined exclusion criteria (Figure 1). Among 6,701 patients, 121 (1.81%) had GSCC and 6,580 (98.19%) had GAC. After PSM, there were 121 patients with GSCC and 242 with GAC. Demographic and clinicopathological features of all eligible patients are described (Table 1). Similar to GAC patients, most GSCC patients were Caucasian and elderly females (>60 years old). The proportion of females was significantly higher in GAC patients than in GSCC patients (56.2% vs. 69.7%, p = 0.002). GSCC were likely to have a distant-stage disease. In contrast, a localized-stage disease was common in GAC patients. Compared to patients with GAC, patients with GSCC had a lower proportion of well-differentiated histology (3.3% vs. 12.1%, p < 0.001). T3 and T4 stage diseases were more common in GSCC patients, while GAC patients were usually diagnosed with T2 and T3 stage diseases. Regarding treatment, the use of surgery was significantly lower in GSCC cohorts than in GAC cohorts (57.0% vs. 80.7%, P < 0.001). Radiation and chemotherapy did not show significant differences between the two groups. No statistical significance was observed in other variables like age, race, marital status, N stage, M stage, bone, brain, liver, or lung metastases. There was no significant difference in clinicopathological features after 1:2 matching between GSCC and GAC patients.
Prognostic Analysis of Patients With GSCC
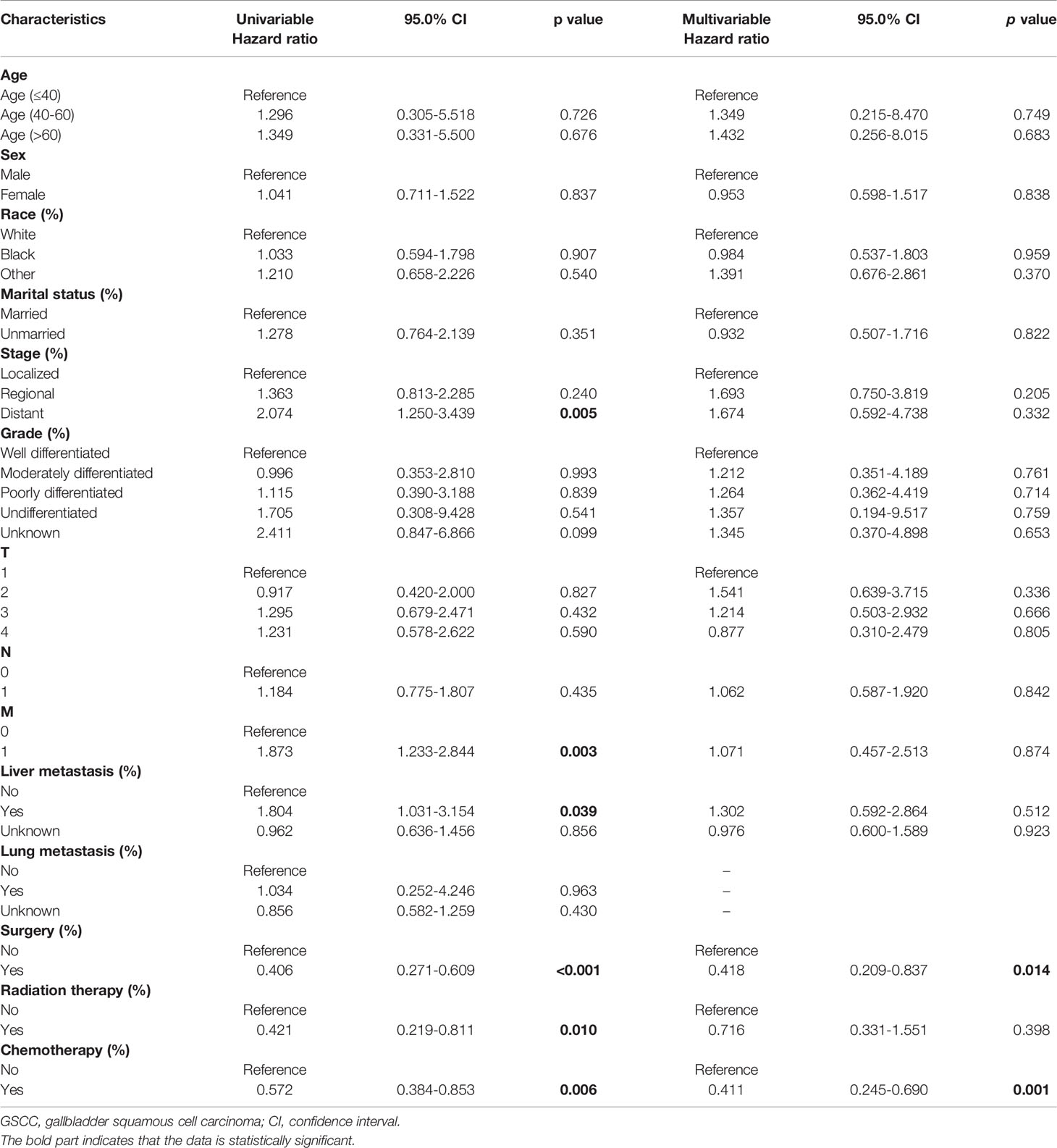
To identify potential predictors of OS in GSCC cohort, univariate and multivariate analyses were conducted regarding different clinicopathological variables. (Table 2) In the univariate analysis, the following variables were separately associated with OS: distant-stage, status of metastasis, and liver metastasis. Regarding treatment, there was a significant difference in DSS (median DSS, 8.0 vs. 3.0; HR, 0.347; p < 0.001; Figure 2A) and OS (median OS, 5.0 vs 2.0; HR, 0.406; p < 0.001; Figure 2D) in patients treated with or without surgery. Similar trend was observed during chemotherapy (median DSS, 7.0 vs. 3.0; HR, 0.594; p = 0.014; Figure 2C; median OS, 6.0 vs 2.0; HR, 0.572; p = 0.006; Figure 2F) and radiation (median DSS, 12.0 vs 4.0; HR, 0.426; p = 0.013; Figure 2B median OS, 12.0 vs 3.0; HR, 0.421; p = 0.010; Figure 2E). As regarding to age, sex, race, marital status, grade, T and N stages and lung metastasis did not appear to significantly influence OS of patients with GSCC in both analyses.
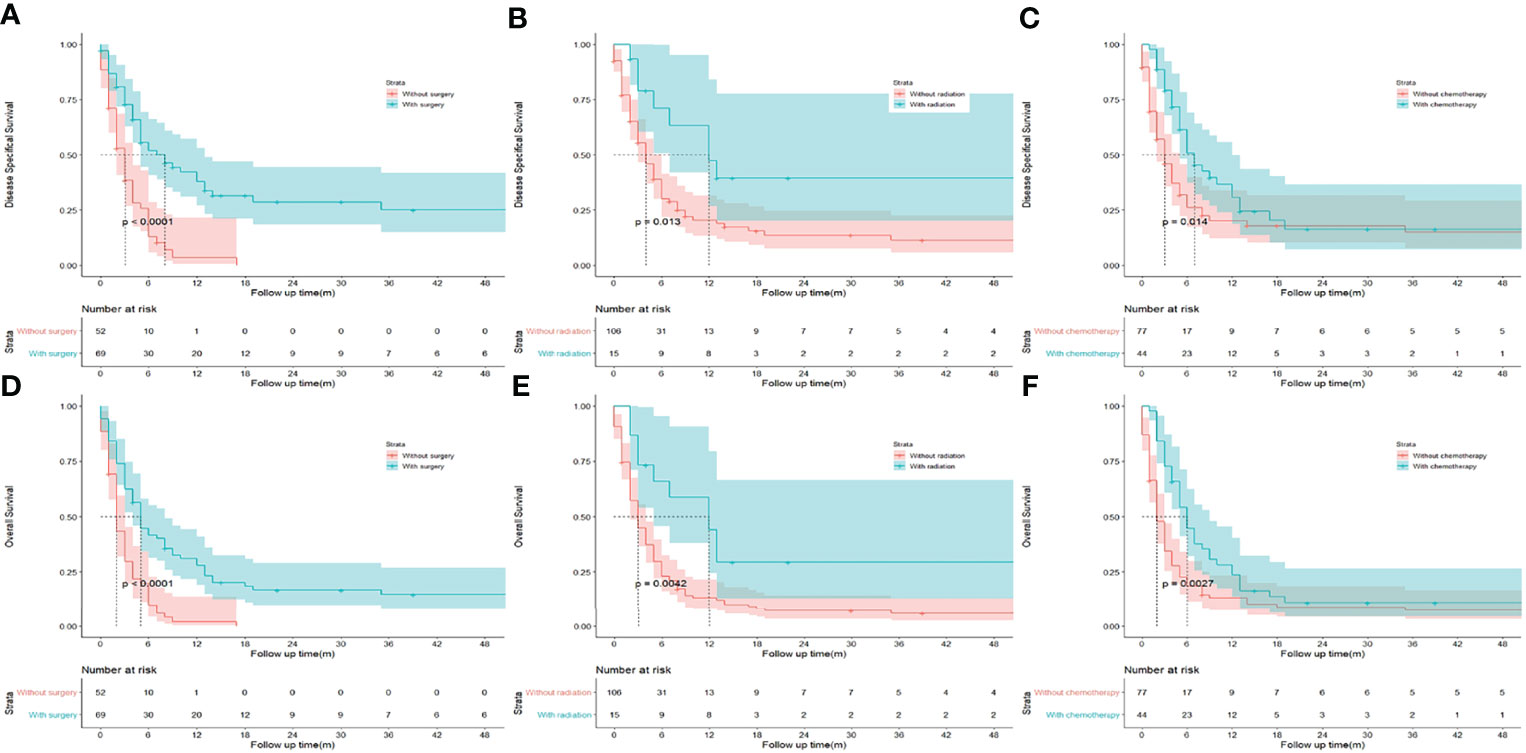
Figure 2 Kaplan-Meier analysis of disease specifical survival and overall survival between patients underwent surgery (A, D), radiation (B, E), and chemotherapy (C, F) or not in patients with gallbladder squamous cell carcinoma.
Survival Analysis of Matched Patients
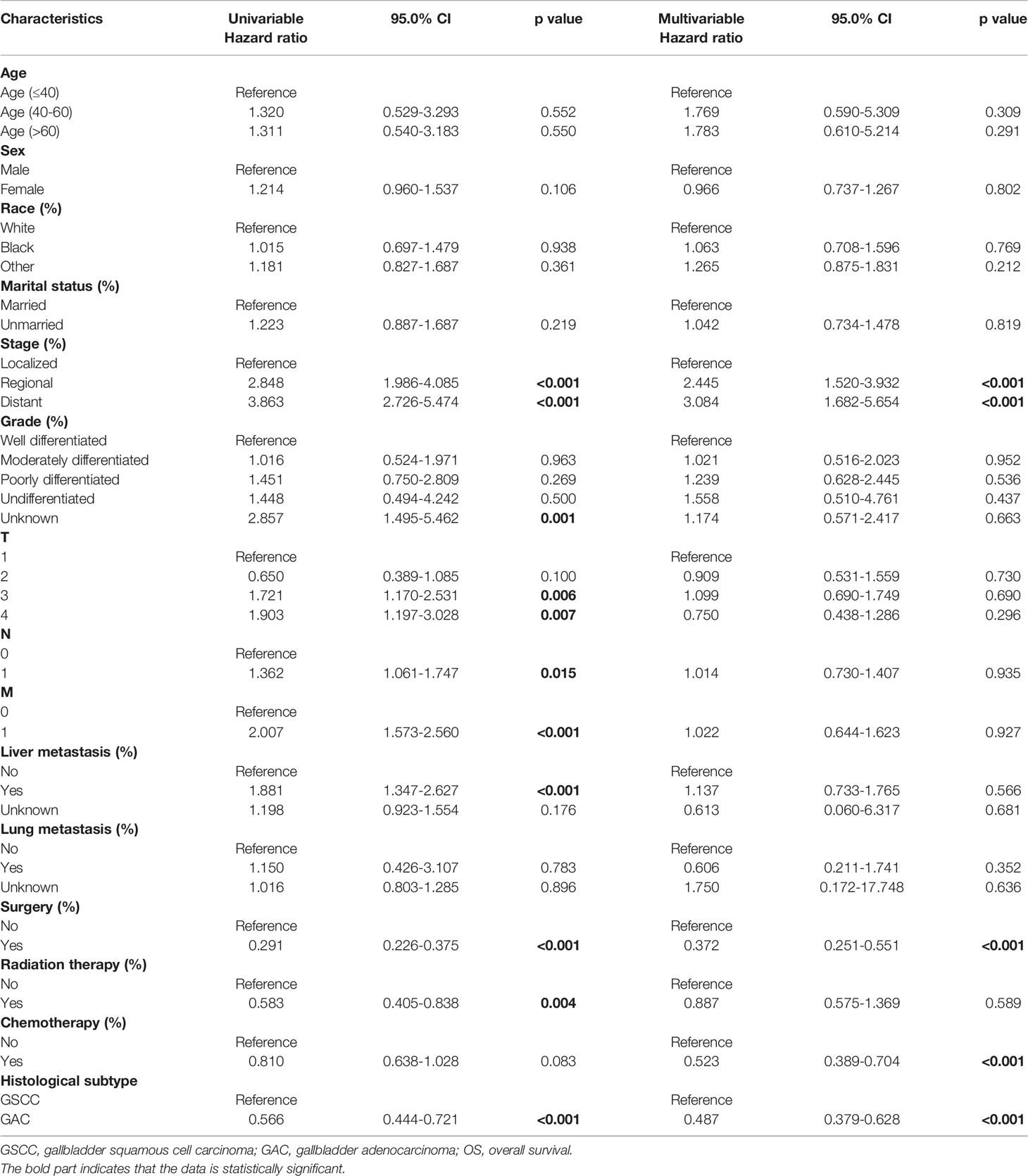
After PSM, GSCC was associated with significantly worse OS and DSS compared to GAC. (Figure 3) The DSS rates were 23.9% and 14.7% for the GSCC cohort at 1-year and 3-year, respectively, and 43.9% and 32.0% for the GAC cohort (HR, 0.614; 95%CI, 0.469–0.803; p < 0.001). Additionally, GSCC patients had 1-year and 3-year OS rates of 16.8% and 8.4%, respectively, while GAC patients were 38.4% and 24.4% (HR, 0.566; 95% CI, 0.444–0.721; p < 0.001). A significant difference was detected in the histological subtype, indicating that patients with GSCC presented a poorer outcome than patients with GAC in both univariate and multivariate analyses (Table 3).
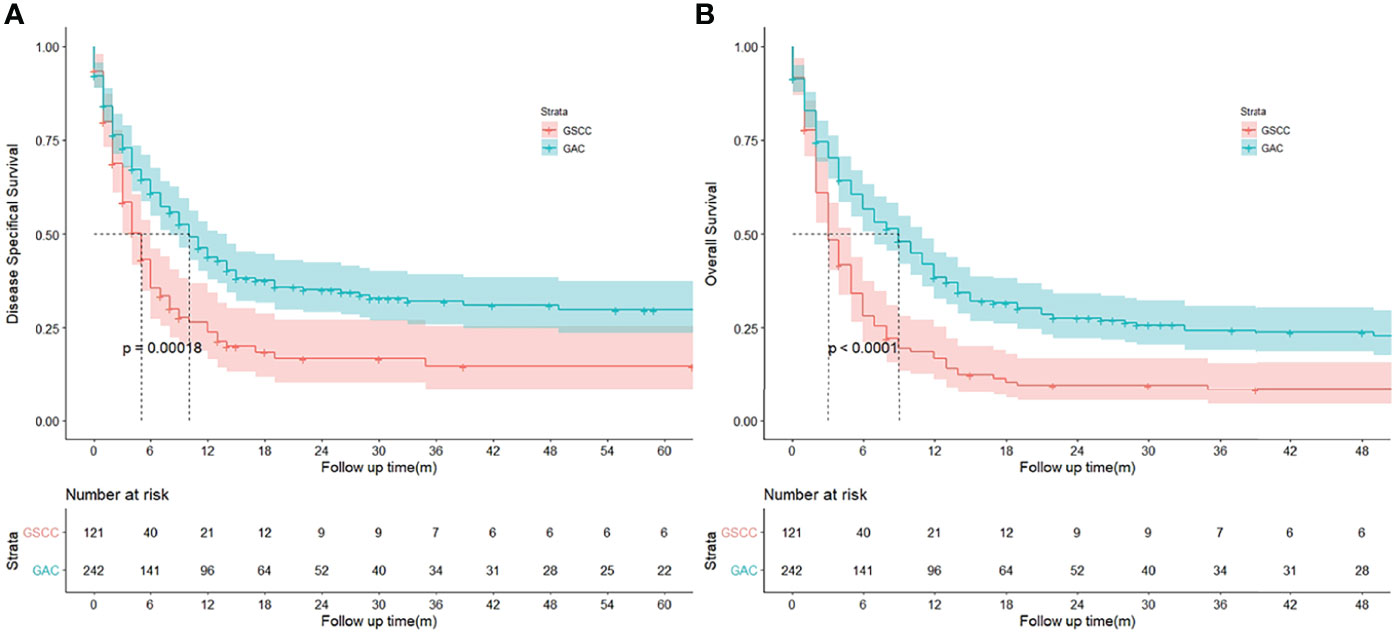
Figure 3 (A, B) Kaplan‐Meier curves to compare disease specifical survival (DSS) and overall survival (OS) of patients with gallbladder squamous cell carcinoma (GSCC) and gallbladder adenocarcinoma (GAC), respectively.
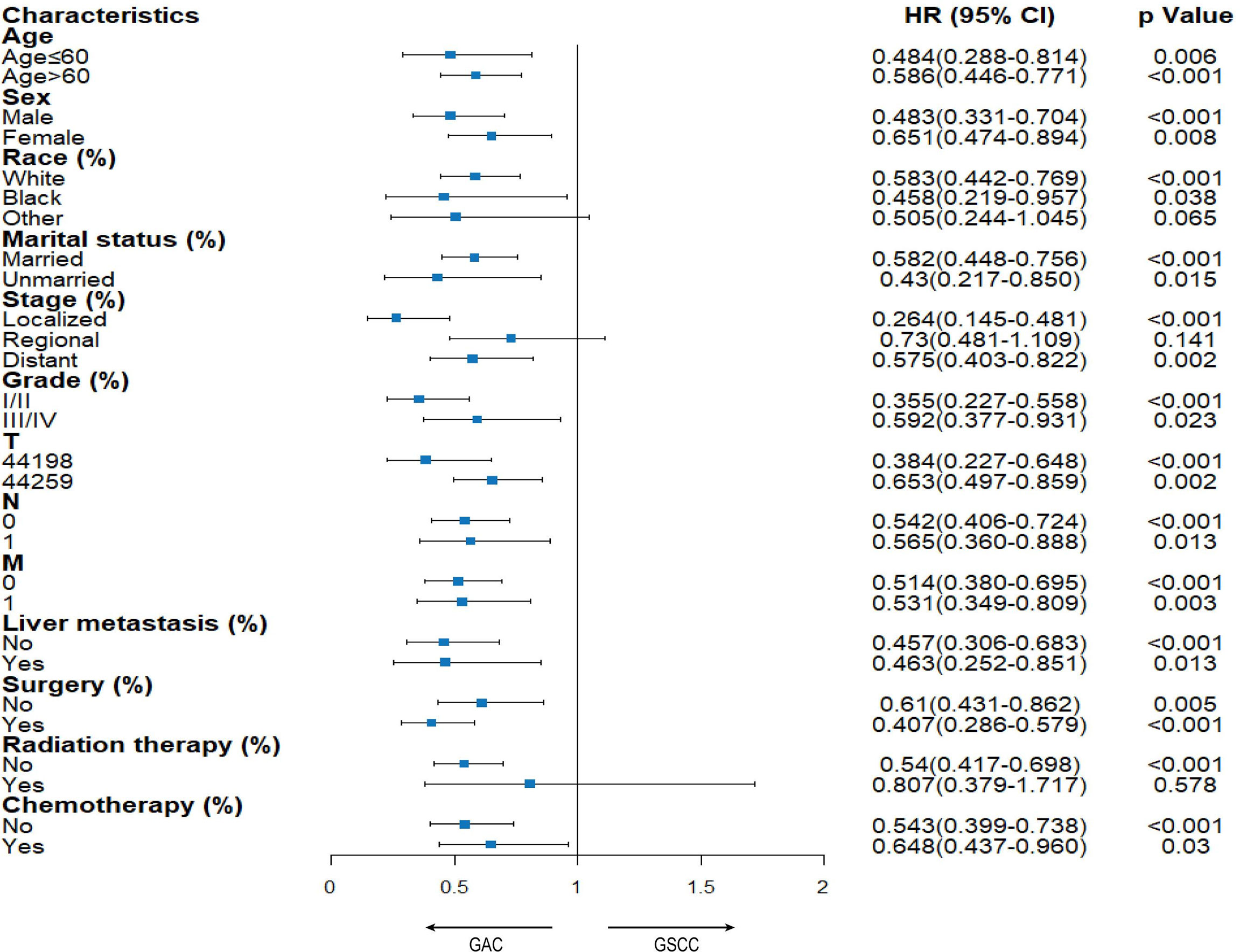
Stratified Analysis
A subgroup analysis was conducted to compare OS between patients diagnosed with the above two tumors (Figure 4). There was a significant difference in almost all variables, indicating that the GSCC group was associated with a significantly poorer OS than the GAC group. Although in multivariate analysis (Table 2), there was no significant difference in radiation of the GSCC cohort, patients receiving radiation exhibited comparable outcomes among GSCC and GAC cohorts in the stratified analysis.
Discussion
GSCC was a rare malignancy, accounting for less than 3% of all GBC (4).There was no accurate information on GSCC due to the rarity of this disease. In our study, the majority of GSCC patients were females, Caucasian, and age more than 60 years old worldwide. Furthermore, the GSCC cohort had a more distant-stage disease and a lower proportion of well-differentiated histology compared to the GAC cohort. Regarding treatment, GSCC patients could significantly benefit from surgery, chemotherapy, and radiation, which was similar to GAC cohorts.
Furthermore, we discovered that GSCC was correlated with poor prognosis. Compared to GAC, GSCC showed a higher SEER stage, higher grade, and a more advanced T-stage. Additionally, there were a lower proportion of patients who conducted adjuvant therapy like chemotherapy and radiation. It seems that these aggressive clinicopathological features and incomplete treatment may be the cause of the poor outcome of GSCC. We also used PSM to counterbalance the uneven clinicopathological features, and a significant difference in the prognosis between two subtypes was still observed. To our knowledge, no studies on GSCC have taken advantage of PSM. Additionally, a similar result can also be observed in stratified analysis for GSCC and GAC. Altogether, these results mean that GSCC had a worse outcome than GAC. However, a few studies have concentrated on molecular mechanisms of GSCC. To improve the prognosis of GSCC patients, there is an urgent need to conduct further fundamental and clinical studies to explore the potential molecular mechanism of GSCC.
A previous study conducted by Ayabe et al. (3) analyzed clinicopathological, and treatment characteristics between GSCC and GAC using another database, the National Cancer Database. In line with our study, they also concluded that patients with GSCC had a poorer outcome than patients with GAC (median OS, 9.0 months vs. 17.0 months; p < 0.001). However, they did not perform PSM to adjust the potential impact of different clinicopathologic values. In another study, Samuel et al. (13) also used the SEER database from 1988–2009 to compare clinicopathological characteristics and prognosis between different GBC histologic subtypes. They also demonstrated that SCC was associated with worse outcomes than other histological types covering papillary carcinoma and adenocarcinoma. But different from our study, they brought adenosquamous carcinoma (ASC) into SCC. This introduces the question of whether or not we should also consider ASC and SCC together. However, according to the World Health Organization’s Classification of Tumors and previous reports, ASC and SCC were different histological subtypes in the gallbladder (1, 7, 14). Therefore, based on the above reasons, we hold that ASC should not be considered together with SCC.
Besides gallbladder, adenocarcinoma (AC) and SCC was also compared in other locations like lung, esophagus, pancreas, cervix, rectum, and anus. A better OS of SCC was detected in the cervix (6, 15), rectum (16), and anus (17), while a worse OS of SCC was obtained in the esophagus (18) and pancreas (19). Current studies show different conclusions in survival advantages between AC and SCC of the lung, and it is difficult to differentiate whether prognosis is associated with pathologic patterns (20). Based on the above, we hold that SCC is not always related to worse clinical outcomes, which means histology is not the dangerous factor of prognosis, and location should also be considered.
There was also strength in this study. One is using data from the SEER database, which covers a wide geographic range of the United States, to provide a data set representative of the country’s diverse population. Another is using PSM and stratified analysis to counterbalance the potential influence of different baseline features. However, there are also some limitations in this study. First, vital information in the SEER database was not recorded. For example, patients treated with radiation or chemotherapy was associated with a better prognosis. However, records for some patients were unclear, which may underestimate the actual effect of treatment. Second, due to the rarity of GSCC, there were only small samples in the SEER database, which may result in selective bias. Consequently, prospective research with larger samples was needed to verify this conclusion.
Conclusion
Majority of patients with GSCC were Caucasian, elderly females, which were similar to patients with GAC. Additionally, more than 50% of patients with GSCC and GAC have undergone surgery. However, patients with GSCC present worse outcomes compared to patients with GAC.
Data Availability Statement
The data that support the findings of this study are available in Surveillance, Epidemiology, and End Results (SEER) Database 18 Regs (with additional treatment fields, 1975–2016 varying), using the SEER*Stat software (version 8.3.6; Surveillance Research Program, NCI, Bethesda, MD).
Author Contributions
XC collected data, reviewed the literature and wrote the manuscript. YZ collected data, wrote and revised the manuscript. DP wrote and revised the manuscript. XS collected data and rechecked the manuscript. GW assisted in drawing. MQ designed and revised the manuscript. QX revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Leigh N, Solomon D, Pletcher E, Sullivan B, Sarpel U, Labow DM, et al. Adeno-Squamous and Squamous Cell Carcinoma of the Gallbladder: The Importance of Histology in Surgical Management. Am J Surgery (2020) 220(5):1242–8. doi: 10.1016/j.amjsurg.2020.06.050
2. Shindoh J, de Aretxabala X, Aloia TA, Roa JC, Roa I, Zimmitti G, et al. Tumor Location Is a Strong Predictor of Tumor Progression and Survival in T2 Gallbladder Cancer: An International Multicenter Study. Ann Surg (2015) 261(4):733–9. doi: 10.1097/SLA.0000000000000728
3. Ayabe RI, Wach MM, Ruff SM, Diggs LP, Martin SP, Wiemken T, et al. Gallbladder Squamous Cell Carcinoma: An Analysis of 1084 Cases From the National Cancer Database. J Surg Oncol (2020). doi: 10.1002/jso.26066
4. D'Hondt M, Lapointe R, Benamira Z, Pottel H, Plasse M, Letourneau R, et al. Carcinoma of the Gallbladder: Patterns of Presentation, Prognostic Factors and Survival Rate. An 11-Year Single Centre Experience. Eur J Surg Oncol (2013) 39(6):548–53. doi: 10.1016/j.ejso.2013.02.010
5. Junior MAR, Favaro ML, Santin S, Silva CM, Iamarino APM. Giant Squamous Cell Carcinoma of the Gallbladder: A Case Report. World J Clin Cases (2019) 7(18):2787–93. doi: 10.12998/wjcc.v7.i18.2787
6. Pan X, Yang W, Wen Z, Li F, Tong L, Tang W. Does Adenocarcinoma Have a Worse Prognosis Than Squamous Cell Carcinoma in Patients With Cervical Cancer? A Real-World Study With a Propensity Score Matching Analysis. J Gynecol Oncol (2020) 31(6):e80. doi: 10.3802/jgo.2020.31.e80
7. Roa JC, Tapia O, Cakir A, Basturk O, Dursun N, Akdemir D, et al. Squamous Cell and Adenosquamous Carcinomas of the Gallbladder: Clinicopathological Analysis of 34 Cases Identified in 606 Carcinomas. Modern Pathol (2011) 24(8):1069–78. doi: 10.1038/modpathol.2011.68
8. Zou XP, Wang JY, Jiang YY, Chen G, Mo WJ, Feng ZB. Clinicopathological Features and Survival of Gallbladder Squamous Cell Carcinoma: Analysis of 121 Cases. Int J Clin Exp Pathology (2018) 11(7):3208–21.
9. Shimada M, Nishimura R, Nogawa T, Hatae M, Takehara K, Yamada H, et al. Comparison of the Outcome Between Cervical Adenocarcinoma and Squamous Cell Carcinoma Patients With Adjuvant Radiotherapy Following Radical Surgery: SGSG/TGCU Intergroup Surveillance. Mol Clin Oncol (2013) 1(4):780–4. doi: 10.3892/mco.2013.112
10. Aldossary MY, Alayed AA, Amr S, Alqahtani MS. Primary Squamous Cell Carcinoma of the Gallbladder: Report of a Rare Neoplasm From the Eastern Province of Saudi Arabia. Int J Surg Case Rep (2018) 51:186–9. doi: 10.1016/j.ijscr.2018.08.048
11. Soyama A, Tajima Y, Kuroki T, Tsuneoka N, Ohno S, Adachi T, et al. Radical Surgery for Advanced Pure Squamous Cell Carcinoma of the Gallbladder: Report of a Case. Hepatogastroenterology (2011) 58(112):2118–20. doi: 10.5754/hge10140
12. Lai HC, Chang SN, Lin CC, Chen CC, Chou JW, Peng CY, et al. Does Diabetes Mellitus With or Without Gallstones Increase the Risk of Gallbladder Cancer? Results From a Population-Based Cohort Study. J Gastroenterology (2013) 48(7):856–65. doi: 10.1007/s00535-012-0683-z
13. Samuel S, Mukherjee S, Ammannagari N, Pokuri VK, Kuvshinoff B, Groman A, et al. Clinicopathological Characteristics and Outcomes of Rare Histologic Subtypes of Gallbladder Cancer Over Two Decades: A Population-Based Study. PloS One (2018) 13(6):e0198809. doi: 10.1371/journal.pone.0198809
14. Nishihara K, Nagai E, Izumi Y, Yamaguchi K, Tsuneyoshi M. Adenosquamous Carcinoma of the Gallbladder: A Clinicopathological, Immunohistochemical and Flow-Cytometric Study of Twenty Cases. Japanese J Cancer Res Gann (1994) 85(4):389–99. doi: 10.1111/j.1349-7006.1994.tb02372.x
15. Biewenga P, van der Velden J, Mol BW, Stalpers LJ, Schilthuis MS, van der Steeg JW, et al. Prognostic Model for Survival in Patients With Early Stage Cervical Cancer. Cancer (2011) 117(4):768–76. doi: 10.1002/cncr.25658
16. Chiu MS, Verma V, Bennion NR, Bhirud AR, Li J, Charlton ME, et al. Comparison of Outcomes Between Rectal Squamous Cell Carcinoma and Adenocarcinoma. Cancer Med (2016) 5(12):3394–402. doi: 10.1002/cam4.927
17. Malakhov N, Kavi AM, Lee A, Adedoyin P, Sheth N, Lederman AJ, et al. Patterns of Care and Comparison of Outcomes Between Primary Anal Squamous Cell Carcinoma and Anal Adenocarcinoma. Dis Colon Rectum (2019) 62(12):1448–57. doi: 10.1097/DCR.0000000000001506
18. Kauppila JH, Mattsson F, Brusselaers N, Lagergren J. Prognosis of Oesophageal Adenocarcinoma and Squamous Cell Carcinoma Following Surgery and No Surgery in a Nationwide Swedish Cohort Study. BMJ Open (2018) 8(5):e021495. doi: 10.1136/bmjopen-2018-021495
19. Makarova-Rusher OV, Ulahannan S, Greten TF, Duffy A. Pancreatic Squamous Cell Carcinoma: A Population-Based Study of Epidemiology, Clinicopathologic Characteristics and Outcomes. Pancreas (2016) 45(10):1432–7. doi: 10.1097/MPA.0000000000000658
Keywords: squamous cell carcinoma, adenocarcinoma, propensity matching, SEER, outcome
Citation: Chen X, Zhou Y, Xu Q, Pu D, Shu X, Wei G and Qiu M (2022) Clinical Characteristics and Outcome Between Gallbladder Squamous Cell Carcinoma and Adenocarcinoma: A Propensity Matched Analysis Based on the Surveillance, Epidemiology, and End Results Database. Front. Oncol. 12:833447. doi: 10.3389/fonc.2022.833447
Received: 11 December 2021; Accepted: 30 March 2022;
Published: 02 May 2022.
Edited by:
Alessio G. Morganti, University of Bologna, ItalyReviewed by:
Baltasar Pérez Saborido, Hospital Universitario Río Hortega, SpainMilly Buwenge, University of Bologna, Italy
Copyright © 2022 Chen, Zhou, Xu, Pu, Shu, Wei and Qiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Meng Qiu, cWl1bWVuZ0B3Y2hzY3UuY24=
†These authors have contributed equally to this work
 Xiaorong Chen1†
Xiaorong Chen1† Dan Pu
Dan Pu Guixia Wei
Guixia Wei Meng Qiu
Meng Qiu